Abstract
Aims
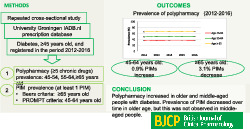
Polypharmacy is common in people with diabetes and is associated with the use of potentially inappropriate medication (PIM). This study aimed to assess trends in the prevalence of polypharmacy and PIM in older and middle‐aged people with diabetes.
Methods
A repeated cross‐sectional study using the University Groningen IADB.nl prescription database was conducted. All people aged 45 years and over who were treated for diabetes registered in the period 2012–2016 were included. Polypharmacy was assessed for three age groups. PIMs were assessed using Beers criteria for people ≥65 years old, and PRescribing Optimally in Middle‐aged People's Treatments (PROMPT) criteria for 45–64 years old. Chi‐square tests and regression analysis were applied.
Results
The prevalence of polypharmacy increased significantly in all age groups in the study period. In 2016, the prevalence of polypharmacy was 36.9% in patients aged 45–54 years, 50.3% in those aged 55–64 years, and 66.2% in those aged ≥65 years. The prevalence of older people with at least one PIM decreased by 3.1%, while in the middle‐aged group this prevalence increased by 0.9% from 2012 to 2016. The most common PIMs in both age groups were the use of long‐term high‐dose proton pump inhibitors, benzodiazepines and strong opioids without laxatives. Of those, only benzodiazepines showed a decreasing trend.
Conclusions
Polypharmacy increased in older and middle‐aged people with diabetes. While the prevalence of PIM decreased over time in older age, this trend was not observed in middle‐aged people with diabetes. Efforts are needed to decrease the use of PIMs in populations already burdened with many drugs, notably at middle age.
Keywords: diabetes, polypharmacy, potentially inappropriate medication (PIM), prevalence
What is already known about this subject
Polypharmacy is common in older people and associated with the use of potentially inappropriate medication (PIM), causing medication‐related harm. Significant increases in polypharmacy prevalence have also been observed in middle‐aged people.
There is an increasing prevalence of diabetes in middle‐aged people but little is known about trends of polypharmacy and PIM in people with diabetes.
What this study adds
The prevalence of polypharmacy increased in all people with diabetes in the period 2012–2016, with two‐thirds of those ≥65 years, half of those between 55 and 64 years, and one‐third of those between 45 and 54 years old being exposed to polypharmacy in 2016.
There was a decreasing trend in the prevalence of PIM in older people with diabetes and polypharmacy during the study period, with 25% of those ≥65 years receiving at least one of the assessed PIMs in 2016.
The was no decrease in the prevalence of PIM in middle‐aged people with diabetes and polypharmacy, with 38% of 45–64 year olds receiving at least one of the assessed PIMs in 2016.
1. INTRODUCTION
Polypharmacy is the concurrent use of multiple medications and is commonly defined as the chronic use of five or more medications daily.1 Older people are often seen as a high‐risk group but polypharmacy is not restricted to age. Significant increases in polypharmacy prevalence have also been observed in middle‐aged people.2, 3, 4 With increasing polypharmacy, the risk of medication‐related harm increases. On the other hand, the use of multiple medications can provide clinical benefit and may be needed, especially for people with comorbidities.5, 6 Polypharmacy is common in people with diabetes, and having diabetes is a risk factor for polypharmacy.7, 8 Many diabetes patients are prescribed more than eight different drugs to treat their diabetes and related diseases.5
In general, polypharmacy is associated with more use of potentially inappropriate medication (PIM).9 Not much is known about polypharmacy in relation to PIM in people with diabetes. It seems that PIM use is more common in older people with diabetes as compared to younger people with diabetes or older people with hypertension.10, 11 There is a worrying increase in the prevalence of diabetes in younger age groups.12 The extent of polypharmacy and PIM use have not been investigated in detail in this age group. Better insights into the trends of polypharmacy and PIM in older and middle‐aged people with diabetes are needed to guide the development of medication optimization interventions.
The aim of our study was to assess (1) the trends in polypharmacy in older and middle‐aged people treated for diabetes, and (2) the trends of PIM in those patients on polypharmacy.
2. METHODS
2.1. Study design and data source
We carried out a repeated cross‐sectional study over 5 years (2012–2016) to assess trends in polypharmacy and the prevalence of PIM in patients with polypharmacy in the Netherlands. The data were obtained from the University of Groningen community pharmacy database IADB.nl. This database includes all outpatient prescriptions that have been dispensed in more than 60 Dutch community pharmacies. The IADB.nl database has been validated and used for many studies in the pharmacoepidemiology field, and the prevalence of medication use in the IADB population was found to be representative of the Dutch population.13 This database contains anonymized information of dispensed prescriptions, including the Anatomical Therapeutic Chemical code (ATC code) of the drug, the date of dispensing, the number of units dispensed and the prescribed daily dose. The IADB.nl does not include information on medical conditions or on medication dispensed during hospitalization. Ethical approval is not required for studies using anonymous data in the Netherlands.
2.2. Study population
The study population consists of all adults known in the IADB.nl database, aged 45 years or older on 1 January of each study year and receiving at least two prescriptions for medication used for diabetes treatment (ATC codes: [A10A, insulin and analogues] and/or A10B [blood glucose lowering drugs, excluding insulin]) in the calendar year, and with follow‐up data for at least 12 months.
2.3. Polypharmacy definition
Polypharmacy was defined as the chronic use of at least five drugs concurrently.14 Chronic use was defined as a drug being dispensed for at least 90 days or at least three times in a four‐month period between 1 September and 31 December in the study year. Given the definition of the Dutch guideline,14 all drugs intended for topical use, contrast media, radiopharmaceuticals, surgical dressings and general nutrients (ATC codes belonging to D, V, Y and Z), as well as records with invalid ATC codes were excluded from the calculation of polypharmacy prevalence. To determine the number of different drugs for chronic use, the third level of the ATC code (ATC3), which describes pharmacological subgroups, was used following the Dutch guideline on polypharmacy.14 This implies that dispensing of different substances within the same pharmacological subgroup, e.g. beta‐blocking agents, are counted as one chronic drug.
2.4. Potentially inappropriate medication (PIM) criteria
We used the American Geriatrics Society (AGS) Beers 2015 criteria for assessment of PIM for patients aged 65 years and older,15 and the PRescribing Optimally in Middle‐aged People's Treatments (PROMPT) criteria for patients 45–64 years old.16 We included only those criteria for the assessment of a PIM that can be applied in a pharmacy database without clinical information. Beers criteria consist of a list of therapeutic classes and individual drug substances which should be avoided in people aged 65 and older in most circumstances or with some specified medical conditions.15 The PROMPT criteria were in part derived from the Beers criteria but in addition include criteria related to drug–drug interactions.16 Since such drug–drug interactions are not included in Beers, we excluded them from our PIM assessment. In total, 14 criteria from Beers and nine criteria from PROMPT were excluded because: (a) they could not be assessed with information available in the IADB database, (b) the drugs were not available on the Dutch market during the study period, or (c) they concerned drug–drug interactions. This resulted in 24 PIMs from Beers applied for patients aged 65 years and older, and 13 PIMs from PROMPT applied for patients between 45 and 64 years of age (Figure 1). Four of the PIMs from PROMPT were similar to PIMs from Beers. The other nine PIMs from PROMPT were either not part of the Beers criteria or defined in a stricter way in comparison to those of Beers. For comparison, these nine PIMs were also applied for patients aged 65 years and older (Table A1).
FIGURE 1.

Flow chart of included PIMs*Number according to the Beers list; additional substances belonging to these classes were on the Dutch market in the study period†1 therapeutic class (NSAIDs) included two different PIMs‡1 PIM included two drug substances (esomeprazole/omeprazole)**PIM criterion also assessed for ≥65 yearsBold in brackets are number of PIMs
2.5. Outcome
The primary outcome was polypharmacy prevalence per calendar year, stratified by age (45–54 years, 55–64 years and ≥65 years). Secondary outcomes were the prevalence of any PIM and the prevalence of the individual PIMs in polypharmacy patients. For the outcome of any PIM, patients were categorized as having no PIM or at least one PIM, using the 24 PIM criteria from Beers, and the 13 PIM criteria from PROMPT.
2.6. Analysis
The prevalence of polypharmacy and PIM were assessed for each year and expressed as percentages with 95% confidence intervals (CI). Additionally, absolute differences in these prevalences over the study period are presented. The prevalence of polypharmacy was calculated by dividing the number of people with diabetes on polypharmacy (numerator) by the total number of diabetes patients in each year (denominator). The prevalence of PIM was calculated by dividing the number of people with diabetes and polypharmacy who had at least one PIM by the total number of people with diabetes and polypharmacy. Linear‐by‐linear association with Chi‐square analysis and regression analysis were used to test for significant linear or quadratic trends of the prevalence rates. Statistical significance was evaluated at the two‐sided α = 0.05 level. All statistical analyses were carried out using SPSS software (version 23.0; IBM, Armonk, NY, USA).
3. RESULTS
3.1. Study population
The number of patients treated for diabetes included in this study ranged from 27 845 in 2012 to 24 809 in 2016. The percentage of people with diabetes out of the total population was around 8–9% throughout the study period. Among the people with diabetes, around 14% were 45–54 years, around 25% were 55–64 years, and around 61% were 65 years or older. From 2012 to 2016, the percentage in the oldest age group slightly increased from 59.1% to 61.5% (Table 1).
TABLE 1.
Number of people with diabetes stratified by age in IADB 2012–2016
| Years | Overall | Age 45–54 | Age 55–64 | Age ≥ 65 |
|---|---|---|---|---|
| No. of diabetes patients | No. of diabetes patients (%) | No. of diabetes patients (%) | No. of diabetes patients (%) | |
| 2012 | 27 845 | 3900 (14.0%) | 7487 (26.9%) | 16 458 (59.1%) |
| 2013 | 28 483 | 4007 (14.1%) | 7399 (26.0%) | 17 077 (60.0%) |
| 2014 | 28 804 | 4102 (14.2%) | 7263 (25.2%) | 17 439 (60.5%) |
| 2015 | 28 873 | 4122 (14.3%) | 7081 (24.5%) | 17 670 (61.2%) |
| 2016 | 24 809 | 3510 (14.1%) | 6032 (24.3%) | 15 267 (61.5%) |
3.2. Trends of polypharmacy in people with diabetes
Overall, the prevalence of polypharmacy increased from 56.5% (95% CI 55.9–57.1) in 2012 to 58.2% (95% CI 57.6–58.8) in 2016 (P‐value for trend <0.001), with the highest prevalence in 2015 of 58.8% (95% CI 58.2–59.3) (Figure 2). In the oldest age group, the prevalence was 66.2% in 2016 (95% CI 65.5–67.0). In the same year, the prevalence was 50.3% (95% CI 49.0–51.5) in the 55–64 years old group, and 36.9% (95% CI 35.3–38.5) in the 45–54 years old group (Figure 2, Table A2). The absolute increase over the study period was 1.0% in the ≥65 years old, 2.0% in the 55–64 years old, and 1.4% in the 45–54 years old (P‐values for linear trend for all age groups <0.001).
FIGURE 2.
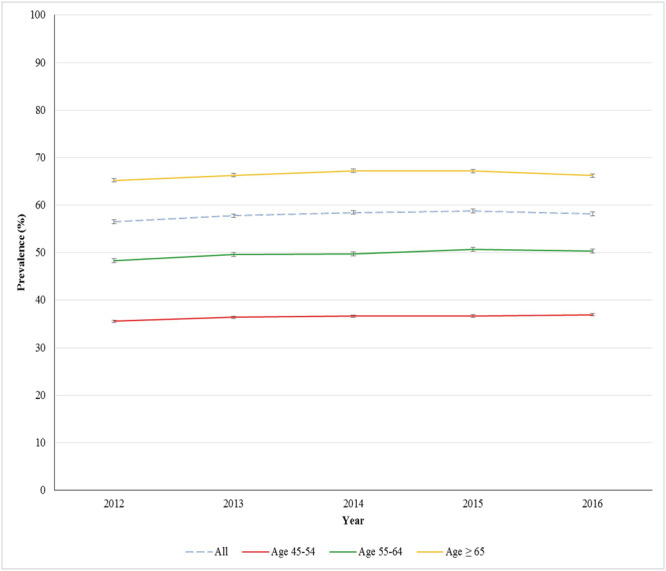
Trends in polypharmacy in people with diabetes 2012–2016
3.3. Trends in PIM in older people with diabetes and polypharmacy
In patients aged 65 years and older with polypharmacy, the percentage with at least one PIM according to the 24 Beers criteria decreased from 28.0% in 2012 to 24.9% in 2016 (P‐value for linear trend <0.001). The majority received one PIM (21–24%) and the highest number of PIMs was five (Table 2). The highest prevalence was observed for long‐term high‐dose use of proton pump inhibitors (PPI) (17–18%), for benzodiazepines (9–10%), for strong opioids without laxatives (7–8%) and for tricyclic antidepressants (TCAs) (6%) (Table 3).
TABLE 2.
Trends in PIM prevalence in people with diabetes and polypharmacy 2012–2016
| Age group | Year | Number of PIM (n, % within year) | At least 1 PIM (n, %) | |||||
|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | |||
| 45–64 years | 2012 | 3155 (63.1%) | 1379 (27.6%) | 366 (7.3%) | 87 (1.7) | 13 (0.3%) | 0 (0%) | 1845 (36.9%) |
| 2013 | 3143 (61.3%) | 1513 (29.5%) | 377 (7.4%) | 84 (1.6%) | 12 (0.2%) | 0 (0%) | 1986 (38.7%) | |
| 2014 | 3127 (61.1%) | 1526 (29.8%) | 362 (7.1%) | 93 (1.8%) | 9 (0.2%) | 0 (0%) | 1990 (38.9%) | |
| 2015 | 3114 (61.0%) | 1509 (29.6%) | 383 (7.5%) | 92 (1.8%) | 5 (0.1%) | 0 (0%) | 1989 (39.0%) | |
| 2016 | 2692 (62.2%) | 1233 (28.5%) | 304 (7%) | 91 (2.1%) | 8 (0.2%) | 1 (<0.1%) | 1637 (37.8%)* | |
| ≥65 years | 2012 | 7729 (72.0%) | 2544 (23.7%) | 412 (3.8%) | 46 (0.4%) | 2 (0.02%) | 0 (0%) | 3004 (28.0%) |
| 2013 | 8265 (73.0%) | 2563 (22.6%) | 433 (3.8%) | 56 (0.5%) | 4 (0.03%) | 1 (<0.1%) | 3057 (27.0%) | |
| 2014 | 8624 (73.6%) | 2590 (22.1%) | 467 (4%) | 41 (0.3%) | 0 (0%) | 0 (0%) | 3098 (26.4%) | |
| 2015 | 8817 (74.3%) | 2610 (22.0%) | 400 (3.4%) | 41 (0.3%) | 2 (0.02%) | 0 (0%) | 3053 (25.7%) | |
| 2016 | 7590 (75.1%) | 2160 (21.4%) | 326 (3.2%) | 32 (0.3%) | 0 (0%) | 0 (0%) | 2518 (24.9%)** | |
P‐value for quadratic trend <0.05,
P‐value for linear trend <0.001.
TABLE 3.
Prevalence of PIM in diabetes patients with polypharmacy, according to Beers and PROMPT criteria 2012–2016, stratified by age
| Year | 2012 | 2013 | 2014 | 2015 | 2016 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| PIM identification | Age 45–64 | Age ≥ 65 | Age 45–64 | Age ≥ 65 | Age 45–64 | Age ≥ 65 | Age 45–64 | Age ≥ 65 | Age 45–64 | Age ≥ 65 |
| Antihistamines, first generation (B) | x | 1.74 | x | 1.69 | x | 1.45 | x | 1.48 | x | 1.34 |
| Antihistamines, first generation>7 days (P, Po) | 0.16 | 0.01 | 0.12 | 0.03 | 0.18 | 0.00 | 0.10 | 0.01 | 0.23 | 0.02 |
| Corticosteroids long‐term without bisphosphonate (P, Po) | 2.30 | 3.11 | 2.53 | 3.01 | 2.64 | 2.87 | 2.53 | 2.97 | 2.63 | 2.45 |
| Tricyclic antidepressants (B, P) | 0.16 | 5.93 | 0.12 | 6.09 | 0.18 | 6.08 | 0.10 | 6.32 | 0.23 | 6.52 |
| Benzodiazepines (B) | x | 10.59 | x | 10.28 | x | 9.83 | x | 8.95 | x | 8.85 |
| Benzodiazepines long‐term (P, Po) | 11.36 | 10.34 | 11.25 | 10.24 | 10.71 | 9.85 | 10.88 | 9.71 | 10.97 | 9.35 |
| Nonbenzodiazepines (B) | x | 2.22 | x | 2.24 | x | 2.24 | x | 2.22 | x | 2.21 |
| Nonbenzodiazepines long‐term (P, Po) | 2.02 | 2.00 | 1.79 | 2.05 | 1.86 | 2.00 | 2.02 | 1.96 | 2.24 | 2.02 |
| Strong opioids without laxatives (P, Po) | 9.36 | 7.36 | 9.11 | 7.60 | 9.69 | 8.22 | 10.37 | 8.22 | 9.96 | 8.26 |
| Sulfonylureas, long‐acting (B, P) | 0.46 | 0.99 | 0.43 | 0.72 | 0.35 | 0.53 | 0.25 | 0.44 | 0.23 | 0.31 |
| PPI long‐term high‐dose (P, Po) | 22.62 | 17.23 | 23.92 | 17.6 | 24.12 | 17.78 | 23.79 | 17.70 | 23.31 | 17.72 |
| Stimulant laxatives no long‐term, except with opioid use (P, Po) | 0.50 | 0.56 | 0.49 | 0.52 | 0.45 | 0.48 | 0.45 | 0.4 | 0.35 | 0.47 |
| NSAIDs without PPI/misoprostol (B, P) | 3.48 | 1.2 | 3.96 | 0.86 | 3.63 | 0.96 | 3.23 | 0.82 | 3.05 | 0.81 |
| Dipyridamole (B, P) | 0.28 | 5.18 | 0.19 | 5.58 | 0.43 | 5.66 | 0.33 | 5.12 | 0.12 | 4.37 |
| Theophylline monotherapy (P, Po) | 0.04 | 0.01 | 0.08 | 0.01 | 0.04 | 0.03 | 0.00 | 0.00 | 0.00 | 0.00 |
| Acetylsalicylic acid >150 mg/day (P, Po) | 0.06 | 0.30 | 0.08 | 0.22 | 0.25 | 0.36 | 0.02 | 0.09 | 0.07 | 0.09 |
| Skeletal muscle relaxants (B) | x | 0.30 | x | 0.26 | x | 0.27 | x | 0.31 | x | 0.33 |
| Antispasmodics, anticholinergic (B) | x | 1.16 | x | 1.15 | x | 1.09 | x | 1.06 | x | 0.99 |
| Estrogen (B) | x | 0.18 | x | 0.16 | x | 0.14 | x | 0.14 | x | 0.10 |
| Insulin sliding scale (B) | x | 1.71 | x | 1.58 | x | 1.61 | x | 1.79 | x | 1.86 |
| Disopyramide (B) | x | 0.03 | x | 0.03 | x | 0.03 | x | 0.03 | x | 0.03 |
| Indomethacin (B) | x | 0.07 | x | 0.07 | x | 0.07 | x | 0.09 | x | 0.08 |
| Digoxin high‐dose (B) | x | 1.36 | x | 1.19 | x | 1.08 | x | 1.00 | x | 0.92 |
| Nifedipine, immediate release (B) | x | 0.07 | x | 0.05 | x | 0.07 | x | 0.06 | x | 0.05 |
| Pentazocine (B) | x | 0.02 | x | 0.02 | x | 0 | x | 0 | x | 0 |
B: Beers criteria
P: PROMPT criteria
Po: PROMPT criteria applied for older age group (shown in italic)
x: criteria not assessed for this age group
Barbiturates, Meprobamate, Ergoloid mesylates, Desiccated thyroid, Megestrol, Mineral oil, Meperidine, and Ketorolac were not dispensed in study period
At individual PIM level, also looking at the additional PROMPT criteria, absolute increases of at least 0.1% during the study period were seen for the use of strong opioids without laxatives (0.9%), TCAs (0.6%), long‐term high‐dose use of PPIs (0.5%), and sliding scale insulins (0.2%). Meanwhile absolute decreases of at least 0.1% were seen for the use of benzodiazepines (−1.7%), dipyridamole (−0.8%), long‐acting sulfonylureas (−0.7%), long‐term corticosteroids without biphosponates (−0.7%), high‐dose digoxin (−0.4%), first generation antihistamines (−0.4%), nonsteroidal anti‐inflammatory drugs (NSAIDs) without PPI or misoprostol (−0.4%), high‐dose acetylsalicylic acid (−0.2%), and antispasmodics (−0.2%) (Table 3).
3.4. Trends in PIM in middle‐aged people with diabetes and polypharmacy
In middle‐aged patients with polypharmacy, the percentage with at least one PIM from the 13 PROMPT criteria increased gradually from 36.9% in 2012 to 39% in 2015. In 2016, the percentage decreased to 37.8% (P‐value for quadratic trend <0.05). The majority received one PIM (27–30%), and the highest number of PIMs was five (Table 2). The highest prevalences were seen for the use of long‐term high‐dose PPI (22–23%), long‐term benzodiazepines (11%) and strong opioids without laxatives (9–10%) (Table 3).
At individual PIM level, absolute increases during the study period were seen for long‐term high‐dose use of PPIs (0.7%), strong opioids without laxatives (0.6%), long‐term corticosteroids without biphosponate (0.3%) and long‐term non‐benzodiazepines (0.2%). Conversely, NSAIDs long‐term without PPIs or misoprostol (0.4%), long‐term benzodiazepines (0.4%), long‐acting sulfonylureas (−0.2%), dipyridamole monotherapy (−0.2%), stimulant laxative no long‐term, except with opioid (−0.2%) showed absolute decreases in prevalence (Table 3).
4. DISCUSSION
4.1. Principal findings and their relationship with the literature
The prevalence of polypharmacy in older and middle‐aged people treated for diabetes increased from 56.5% in 2012 to 58.2% in 2016. Significant increases were seen in all age groups with the highest absolute increase in the 55–64 years old group. The prevalence of older people with at least one PIM decreased around 3% in the study period. In the middle‐aged group, the prevalence increased around 2% from 2012 to 2015, then it decreased 1% afterwards. The use of long‐term high‐dose PPI, benzodiazepines and strong opioids without laxatives were the most common PIMs in people with diabetes in both age groups, with gradual increases over time for PPIs and opioids without laxatives, and decreases for benzodiazepines. Furthermore, the use of TCAs was common in the older age group, with an increase over time.
The findings of this study confirm that polypharmacy is a concern in people with diabetes, with the highest rate in older‐age patients.8, 17, 18 The increasing trend of polypharmacy in the diabetes population was similar to the trend in the general population.2, 3 The polypharmacy prevalences in people with diabetes, ranging from 36% in the 45–54 years old group to 65% in those aged ≥65 years in 2012, were substantially higher than the prevalence seen in the general population at that time.2 Previously, an increase in polypharmacy was seen in middle‐aged people,2, 3 and likewise we observed this in people with diabetes. A noteworthy finding was that the absolute increase in the 55–64 years old group was larger than in the 65 years and older group. This may indicate an increase in the number of comorbidities in this age group, which is followed by an increase in prescribing of medication. Whether less increase in the older age group means less increase in comorbidities or more restrictive prescribing remains unknown. For middle‐aged patients, prescribers may not yet see polypharmacy as a large burden or problem. So far, attention for polypharmacy management is more focused on older than middle‐aged people.19
Our study showed that while polypharmacy increased for people with diabetes between 2012 and 2016, the prevalence of PIM in older patients with polypharmacy decreased in this period. This suggests that the continuing attention and many initiatives to decrease inappropriate polypharmacy in older patients have had an effect in this population.20 Studies in the general population using Beers criteria also showed decreasing trends in the annual PIM prevalence between 1995 and 2004 in France,21 and between 2007 and 2014 in the United States.22 Interestingly, a slight increase in PIM was observed in the beginning of our study period in middle‐aged people with polypharmacy, with the highest PIM prevalence of 39% in 2015. In 2016, there was a slight decrease and the significant quadratic trend may indicate the beginning of a decline in PIM in this middle‐aged group. Further data from recent years are needed to support this finding. The PIM prevalences of 37–39% we observed in middle‐aged diabetes patients with polypharmacy are of concern. Other studies which used PROMPT criteria in middle‐aged people showed PIM prevalences ranging from 21% to 43% in community‐dwelling people.23, 24 Although these numbers are difficult to compare due to differences in setting and assessment of the PROMPT criteria, they underline the importance of focusing on inappropriate polypharmacy in the middle‐aged group.
The use of long‐term high‐dose PPIs, benzodiazepines and strong opioids without laxatives was common in both age groups. The prevalence rates when applying the same PROMPT criteria were around 1–2% higher in the younger as compared to the older patients. Similar common PIMs have been found in a general middle‐aged population using PROMPT criteria.24 In the general older‐age population, benzodiazepines were also among the most prevalent PIMs, with the prevalence rates ranging from 0.74% to 3.9%.21, 25 The other most common PIMs when assessed using Beers criteria were from the opioid group, the antidepressant group and digoxin.21, 22, 25 Our findings indicate that prescribers appear not to be more cautious with prescribing these PIMs in older people with or without having diabetes. Apparently, substantial numbers of people with diabetes require these drugs according to their physicians. Chronic insomnia and anxiety are highly prevalent in people with diabetes,26, 27 and benzodiazepines can be prescribed to treat those problems. Proton pump inhibitors are considered as well‐tolerated drug classes for treating and preventing acid‐related disorders. However, the use of PPIs above maintenance dosage was identified to increase the risk of chronic kidney disease in the diabetic population.28 The reason for not adding a laxative in diabetes patients using opioids is also of concern, since they may already have a higher risk for constipation.29 A possible reason for not prescribing laxatives could be that patients are sufficiently managed with dietary measures or over‐the‐counter (OTC) drugs, or that patients experience insufficient benefits from laxatives.30 Benzodiazepines were frequently prescribed but the prevalence decreased in the study period. This may be part of a downward trend seen in the use of these drugs in the general population in the Netherlands.2 Since 2009, reimbursement for benzodiazepines has been restricted in the Netherlands to discourage overuse of these drugs. On the other hand, for the other common PIMs—i.e., long‐term high‐dose PPIs, strong opioids without laxatives and TCAs in older patients—some increases were seen, despite recommendations in the Dutch national guidelines against such use.31 It is disappointing that in people with diabetes, who already have a high medication burden, we do not see a more restricted use in the study period.
4.2. Strengths and limitations
This study is unique in providing detailed insights into the prevalence of polypharmacy and PIM in older and middle‐aged people with diabetes. A large pharmacy database was used which includes all outpatient prescriptions for people registered with the participating pharmacies. In the Netherlands, most people collect their medication from one pharmacy, where they are registered. There are some limitations. First of all, the different number and definitions of PIMs per age group implies that it is not meaningful to compare absolute prevalences between the age groups. Comparisons are thus restricted to trends seen within the age groups, where the denominator does not change. Second, direct comparisons of our PIM findings with other studies are limited. We excluded around a third of the criteria from Beers as well as from PROMPT. Particularly from the Beers list, we had to exclude several criteria that require clinical information, which is not available in a pharmacy database. From PROMPT, we excluded criteria focusing on drug–drug interactions. This is likely to lead to lower estimates of the overall PIM prevalence compared to other studies. On the other hand, given our objective, we calculated PIM prevalence amongst those with polypharmacy. This may lead to higher estimates of PIM prevalence compared to other studies. It should be noted that many different PIM lists have been developed, and usually adaptations and selections have to be made depending on the setting and the medications available in a country,32 complicating direct comparisons between studies. Third, the pharmacy database provides information about dispensed drugs, which may not reflect actual use and thus exposure to the PIM, and does not provide data on drugs used during hospitalization. Finally, we do not know whether patients used OTC drugs, which might include laxatives. We expect, however, that for chronic use such laxatives would be dispensed on prescription, allowing for reimbursement.
4.3. Implications for policy and practice
Polypharmacy can be considered as an indicator for medication burden, although it does not indicate inappropriate medication. In older people with diabetes, we observed a small decrease in patients with any PIM among an increasing population with polypharmacy. Despite this trend, more than a quarter of older people with diabetes and polypharmacy received at least one PIM. Notably, in the middle‐aged group the prevalence of PIM appears to be higher and did not decrease. One should be aware, however, that comparing the absolute prevalence rates between the groups is restricted by the use of different PIM lists for different age groups. Importantly, at individual PIM level, many PIMs were not decreasing in older nor middle‐aged people with diabetes.
It is therefore important not only to focus on older patients with PIM. To decrease inappropriate polypharmacy, attention should be paid to specific PIMs and to specific high‐risk populations, such as those with diabetes. Multifaceted, patient‐centred interventions have shown to be effective at reducing inappropriate medication in the general older population.33, 34 Developing such interventions for middle‐aged people with diabetes may be necessary. More research is needed to assess the benefits versus harms caused by PIMs in middle‐aged populations.
5. CONCLUSION
Polypharmacy is common in both older and middle‐aged people with diabetes, with increasing trends in the study period, particularly in patients between 55 and 64 years of age. Despite an increase in polypharmacy, we observed a decrease in PIM prevalence in patients aged 65 years and older with polypharmacy. No decrease was seen for middle‐aged patients with polypharmacy. The most common PIMs in people with diabetes were similar to those seen in the general population. Further efforts are needed to decrease the use of PIMs in populations already burdened with many drugs.
COMPETING INTERESTS
There are no competing interests to disclose.
CONTRIBUTORS
M.P.O. conceptualized the research, researched data, wrote and edited the manuscript. S.D.A validated data research and reviewed the manuscript. C.C.M.S‐V. conceptualized methods and reviewed the manuscript, H.J.B. validated data research and organized the database. K.T. conceptualized methods and reviewed the manuscript. E.H. supervised the research and reviewed the manuscript. P.D. conceptualized and supervised the research, and reviewed and edited the manuscript.
FUNDING INFORMATION
This research did not receive any specific grant from funding agencies in the public, commercial or not‐for‐profit sectors. M.P.O. reports grants (scholarship), grant number: S‐1535/LPDP.3/2017, to support her PhD program from the Indonesia Endowment Fund for Education (LPDP), during the conduct of the study.
ACKNOWLEDGEMENTS
M.P.O. would like to thank the Indonesia Endowment Fund for Education (LPDP) for their support of her PhD program. Financial support from LPDP has helped many Indonesian students to obtain higher education in order to build Indonesia development.
Appendix A. Appendices
TABLE A1.
Beers and PROMPT criteria definition according to IADB database
| PIM | Definition IADB | |
|---|---|---|
| Beers for ≥ 65 years | PROMPT for 45–64 years | |
|
Antihistamines, first generation ‐ Avoid in Beers ‐ No first line for >7 days in PROMPT |
R06AD01, N07CA52, N07CA02, R06AA04, R06AE03, R06AB03, N05BB01, R06AX17, R06AE05, R06AE55, R06AD08, R06AD02 Additionala: See PROMPT definition |
No first line: R06 first generation (see list ATC codes for Beers) at initiation, i.e. without R06 in the preceding 1 year, and > 7 days |
|
Corticosteroids ‐ No long term without biphosponate in PROMPT |
Additionala: See PROMPT definition | H02A or H02B or M01BA > 3 months, without M05BA or M05BB in the same period |
|
Tricyclic antidepressants, alone or in combination ‐ Avoid in Beers ‐ No first line in PROMPT |
N06AA | N06AA, without N06 in the preceding 1 year |
|
Benzodiazepines ‐ Avoid in Beers ‐ No long term in PROMPT |
N05BA, N05CD Additionala: See PROMPT definition |
N05BA or N05CD > 4 weeks |
|
Nonbenzodiazepine, benzodiazepine receptor agonist hypnotics ‐ Avoid in Beers ‐ No long term in PROMPT |
N05CF Additionala: See PROMPT definition |
N05CF > 4 weeks |
|
Strong opioids (e.g. buprenorphine, diamorphine, fentanyl, morphine, oxycodone) ‐ Not without laxatives in PROMPT |
Additionala: See PROMPT definition | N02A, without (A06AB or A06AC or A06AD) in overlapping period |
|
Sulfonylureas, long‐acting ‐ Avoid in Beers and PROMPT |
A10BB01, A10BB02 | A10BB01, A10BB02 |
|
Proton pump inhibitors ‐ No long term (>8 weeks) in high dose |
Additionala: See PROMPT definition with additional exclusion of chronic oral corticosteroids or NSAIDS, for which long term use is appropriate in elderly |
Omeprazol A02BC01 > 20 mg, >8 weeks Pantoprazol A02BC02 > 40 mg, >8 weeks Lansoprazol A02BC03 > 30 mg, >8 weeks Rabeprazole A02BC04 > 20 mg, >8 weeks Esomeprazole A02BC05 > 40 mg, >8 weeks |
|
Stimulant laxatives ‐ No long term (>4 weeks), except for opioid induced constipation |
Additionala: See PROMPT definition | A06AB > 4 weeks, excluding when N02A overlapping in the same period |
|
NSAIDs without PPI or misoprostol ‐ Avoid in Beers ‐ No long term (>3 months) in PROMPT |
M01A or N02BA01 dosage >325 mg/day, excluding M01AE52, without A02BC or A02BB01 in the same period | M01A, excluding M01AE52, > 3 months, without A02BC or A02BB01 in the same period) |
|
Dipyridamole, short acting ‐ Avoid in Beers ‐ No monotherapy in PROMPT |
B01AC07, excluding extended‐release combination with B01AC06 | B01AC07, without other B01A in same period |
|
Theophylline ‐ No monotherapy in PROMPT |
Additionala: See PROMPT definition | R03DA04, without other R03 in the same period |
|
Acetylsalicylic acid (aspirin) anti‐platelet ‐ Not >150 mg/day in PROMPT |
Additionala: See PROMPT definition | B01AC06, dosage >150 mg/day |
|
Skeletal muscle relaxants ‐ Avoid in Beers |
M03B | X |
|
Antispasmodics, anticholinergic ‐ Avoid in Beers |
A03A, A03B, A03C, A03D, A03E, A04AD | X |
|
Oestrogens with or without progestins ‐ Avoid in Beers |
G03CA, G03CB, G03CC, G03CX | X |
|
Insulins, sliding scale ‐ Avoid in Beers |
A10AB only, without other A10A in overlapping period | X |
|
Disopyramide ‐ Avoid in Beers |
C01BA03 | X |
|
Indomethacin ‐ Avoid in Beers |
M01AB01 | X |
|
Digoxin ‐ No high dose in Beers |
C01AA05, dosage >0.125 mg/day | X |
|
Nifedipine, immediate release ‐ Avoid in Beers |
C08CA05, immediate release | X |
|
Pentazocine ‐ Avoid in Beers |
N02AD01 | X |
|
Barbiturates ‐ Avoid in Beers |
N05CA | X |
|
Meprobamate ‐ Avoid in Beers |
N05BC01 | X |
|
Ergoloid mesylate ‐ Avoid in Beers |
C04AE01 | X |
|
Desiccated thyroid (thyroid extract) ‐ Avoid in Beers |
H03AA05 | X |
|
Megestrol ‐ Avoid in Beers |
G03AC05, G03DB02 | X |
|
Mineral oil, oral (wonderolie, castor oil) ‐ Avoid in Beers |
A06AB05 | X |
|
Meperidine (pethidine) ‐ Avoid in Beers |
N02AB02 | X |
|
Ketorolac ‐ Avoid in Beers |
M01AB15 | X |
For the ≥ 65 years, additional criteria were defined using the PROMPT definition.
X: not included in PROMPT
TABLE A2.
Trends in polypharmacy in people with diabetes 2012–2016 with confidence interval (CI)
| All | Age 45–54 | Age 55–64 | Age ≥ 65 | ||||
|---|---|---|---|---|---|---|---|
| % | 95% CI | % | 95% CI | % | 95% CI | % | 95% CI |
| 56.5 | 55.9–57.1 | 35.5 | 34.0–37.0 | 48.3 | 47.1–49.4 | 65.2 | 64.5–65.9 |
| 57.8 | 57.2–58.3 | 36.4 | 34.9–37.9 | 49.6 | 48.5–50.8 | 66.3 | 65.6–67.0 |
| 58.5 | 57.9–59.0 | 36.6 | 35.2–38.1 | 49.8 | 48.6–50.9 | 67.2 | 66.5–67.9 |
| 58.8 | 58.2–59.4 | 36.7 | 35.2–38.1 | 50.7 | 49.6–51.9 | 67.2 | 66.5–67.9 |
| 58.2 | 57.6–58.8 | 36.9 | 35.3–38.5 | 50.3 | 49.0–51.5 | 66.2 | 65.5–67.0 |
Oktora MP, Alfian SD, Bos HJ, et al. Trends in polypharmacy and potentially inappropriate medication (PIM) in older and middle‐aged people treated for diabetes. Br J Clin Pharmacol. 2021;87:2807–2817. 10.1111/bcp.14685
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the affiliations of the corresponding author upon reasonable request. The University Groningen Research and Data Management Plan (RDMP) only allows access to health care data via a password protected environment requested to Prof. dr. Bob Wilffert (email: b.wilffert@rug.nl), head of the unit PharmacoTherapy, ‐Epidemiology & ‐Economics and of the IADB.nl database.
REFERENCES
- 1.Masnoon N, Shakib S, Kalisch‐Ellett L, Caughey GE. What is polypharmacy? A systematic review of definitions. BMC Geriatr. 2017;17(1):230–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oktora MP, Denig P, Bos JHJ, Schuiling‐Veninga CCM, Hak E. Trends in polypharmacy and dispensed drugs among adults in the Netherlands as compared to the United States. PLoS ONE. 2019;14(3):e0214240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guthrie B, Makubate B, Hernandez‐Santiago V, Dreischulte T. The rising tide of polypharmacy and drug–drug interactions: population database analysis 1995–2010. BMC Med. 2015;13(1):74–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moriarty F, Hardy C, Bennett K, Smith SM, Fahey T. Trends and interaction of polypharmacy and potentially inappropriate prescribing in primary care over 15 years in Ireland: a repeated cross‐sectional study. BMJ Open. 2015;5(9):e008656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bauer S, Nauck MA. Polypharmacy in people with type 1 and type 2 diabetes is justified by current guidelines—a comprehensive assessment of drug prescriptions in patients needing inpatient treatment for diabetes‐associated problems. Diabet Med. 2014;31(9):1078‐1085. [DOI] [PubMed] [Google Scholar]
- 6.Payne RA, Abel GA, Avery AJ, Mercer SW, Roland MO. Is polypharmacy always hazardous? A retrospective cohort analysis using linked electronic health records from primary and secondary care. Br J Clin Pharmacol. 2014;77(6):1073‐1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Veehof L, Stewart R, Haaijer‐Ruskamp F, Jong BM. The development of polypharmacy. A longitudinal study. Fam Pract. 2000;17(3):261‐267. [DOI] [PubMed] [Google Scholar]
- 8.Alwhaibi M, Balkhi B, Alhawassi TM, et al. Polypharmacy among patients with diabetes: a cross‐sectional retrospective study in a tertiary hospital in Saudi Arabia. BMJ Open. 2018;8(5):e020852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tommelein E, Mehuys E, Petrovic M, Somers A, Colin P, Boussery K. Potentially inappropriate prescribing in community‐dwelling older people across Europe: a systematic literature review. Eur J Clin Pharmacol. 2015;71(12):1415‐1427. [DOI] [PubMed] [Google Scholar]
- 10.Formiga F, Vidal X, Agusti A, et al. Inappropriate prescribing in elderly people with diabetes admitted to hospital. Diabet Med. 2016;33(5):655‐662. [DOI] [PubMed] [Google Scholar]
- 11.Al Khaja KAJ, Ahmed Isa H, Veeramuthu S, Sequeira RP. Potentially inappropriate prescribing in older adults with hypertension or diabetes mellitus and hypertension in a primary care setting in Bahrain. Med Princ Pract. 2018;27(3):241‐249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.US Department of Health and Human Service , Centers for Disease Control and Prevention (CDC) . National diabetes statistics report 2020. Estimates of diabetes and its burden in the United States. 2020:2.
- 13.Visser ST, Schuiling‐Veninga CCM, Bos JH, de Jong‐van den Berg LT, Postma MJ. The population‐based prescription database IADB.nl: its development, usefulness in outcomes research and challenges. Expert Rev Pharmacoecon Outcomes Res. 2013;13(3):285‐292. [DOI] [PubMed] [Google Scholar]
- 14.Nederlands Huisartsen Genootschap (NHG) , Nederlandse Vereniging voor Klinische Geriatrie (NVKG) , Orde van Medisch Specialisten (OMS) . Multidisciplinaire richtlijn polyfarmacie bij ouderen; 2012. https://www.nhg.org/sites/default/files/content/nhg_org/uploads/polyfarmacie_bij_ouderen.pdf. Updated 2012. Accessed December 16, 2019.
- 15.American Geriatrics Society 2015 Beers Criteria Update Expert Panel . American Geriatrics Society 2015 updated Beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc 2015;63(11):2227‐2246. [DOI] [PubMed] [Google Scholar]
- 16.Cooper JA, Ryan C, Smith SM, et al. The development of the PROMPT (PRescribing Optimally in Middle‐aged People's Treatments) criteria. BMC Health Serv Res. 2014;14:484–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dobrica EC, Gaman MA, Cozma MA, Bratu OG, Pantea Stoian A, Diaconu CC. Polypharmacy in type 2 diabetes mellitus: insights from an internal medicine department. Medicina (Kaunas). 2019;55(8):436–445. 10.3390/medicina55080436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Noale M, Veronese N, Cavallo Perin P, et al. Polypharmacy in elderly patients with type 2 diabetes receiving oral antidiabetic treatment. Acta Diabetol. 2016;53(2):323‐330. [DOI] [PubMed] [Google Scholar]
- 19.Molokhia M, Majeed A. Current and future perspectives on the management of polypharmacy. BMC Fam Pract. 2017;18(1):70–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Santos NSD, Marengo LL, Moraes FDS, Barberato Filho S. Interventions to reduce the prescription of inappropriate medicines in older patients. Rev Saude Publica. 2019;53:7–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bongue B, Naudin F, Laroche ML, et al. Trends of the potentially inappropriate medication consumption over 10 years in older adults in the east of France. Pharmacoepidemiol Drug Saf. 2009;18(12):1125‐1133. [DOI] [PubMed] [Google Scholar]
- 22.Jirón M, Pate V, Hanson LC, Lund JL, Jonsson Funk M, Stürmer T. Trends in prevalence and determinants of potentially inappropriate prescribing in the United States: 2007 to 2012. J Am Geriatr Soc. 2016;64(4):788‐797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cooper JA, Moriarty F, Ryan C, et al. Potentially inappropriate prescribing in two populations with differing socio‐economic profiles: a cross‐sectional database study using the PROMPT criteria. Eur J Clin Pharmacol. 2016;72(5):583‐591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moriarty F, Cahir C, Bennett K, Hughes CM, Kenny RA, Fahey T. Potentially inappropriate prescribing and its association with health outcomes in middle‐aged people: a prospective cohort study in Ireland. BMJ Open. 2017;7(10):e016562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van der Hooft CS, Jong GW, Dieleman JP, et al. Inappropriate drug prescribing in older adults: the updated 2002 Beers criteria—a population‐based cohort study. Br J Clin Pharmacol. 2005;60(2):137‐144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bickett A, Tapp H. Anxiety and diabetes: innovative approaches to management in primary care. Exp Biol Med (Maywood). 2016;241(15):1724‐1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ogilvie RP, Patel SR. The epidemiology of sleep and diabetes. Curr Diab Rep. 2018;18(10):82–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang H, Juang SY, Liao KF. Proton pump inhibitors use and risk of chronic kidney disease in diabetic patients. Diabetes Res Clin Pract. 2019;147:67‐75. [DOI] [PubMed] [Google Scholar]
- 29.Ahmedzai SH, Boland J. Constipation in people prescribed opioids. BMJ Clin Evid. 2010;2010:2407–2420. [PMC free article] [PubMed] [Google Scholar]
- 30.Emmanuel A, Johnson M, McSkimming P, Dickerson S. Laxatives do not improve symptoms of opioid‐induced constipation: results of a patient survey. Pain Med. 2017;18(10):1932‐1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nederlands Huisartsen Genootschap (NHG) . NHG‐standaarden. https://www.nhg.org/nhg‐standaarden. Updated 2019. Accessed June 8, 2020.
- 32.Motter FR, Fritzen JS, Hilmer SN, Paniz EV, Paniz VMV. Potentially inappropriate medication in the elderly: a systematic review of validated explicit criteria. Eur J Clin Pharmacol. 2018;74(6):679‐700. [DOI] [PubMed] [Google Scholar]
- 33.Rieckert A, Reeves D, Altiner A, et al. Use of an electronic decision support tool to reduce polypharmacy in elderly people with chronic diseases: cluster randomised controlled trial. BMJ. 2020;m1822:369–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clyne B, Fitzgerald C, Quinlan A, et al. Interventions to address potentially inappropriate prescribing in community‐dwelling older adults: a systematic review of randomized controlled trials. J Am Geriatr Soc. 2016;64(6):1210‐1222. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the affiliations of the corresponding author upon reasonable request. The University Groningen Research and Data Management Plan (RDMP) only allows access to health care data via a password protected environment requested to Prof. dr. Bob Wilffert (email: b.wilffert@rug.nl), head of the unit PharmacoTherapy, ‐Epidemiology & ‐Economics and of the IADB.nl database.


