Abstract
Background
Sweat gland function deteriorates with aging, leading to loss of heat tolerance. However, it is unclear whether and how the structure of sweat glands changes during aging, because the 3D structure is complex and inaccessible.
Methods
To clarify age‐dependent changes in sweat glands, we developed a method for 3D structure analysis of sweat glands by means of X‐ray micro‐CT observation of human skin specimens followed by 3D digital reconstruction on computer (digital 3D skin).
Results
Comparison of eccrine sweat glands of abdominal skin from young and old subjects showed that the density and volume of sweat glands do not change with aging. In contrast, the depth of the secretory coil from the skin surface is decreased in the aged group. Surprisingly, the secretory ducts appear tortuous or meandering though their length is unchanged. The secretory coils are located at the dermal‐adipose layer boundary in both groups, but the thickness of the dermal layer decreases with aging, and the depth of the coils is correlated with the dermal thickness.
Conclusion
Our results suggest that sweat glands twist and rotate with aging to maintain the position of the coil at the dermal‐adipose boundary, causing an overall shift toward the skin surface.
Keywords: aging, digital 3D skin, sweat gland, three‐dimensional structure
1. INTRODUCTION
Secretion of sweat from sweat glands onto the skin surface contributes to the regulation of body temperature via the loss of heat of vaporization.1 It also provides water and natural moisturizer (urea and lactate) to prevent skin dryness, helping to maintain skin barrier function homeostasis.2 In addition, the acidic pH of the sweat provides an acidic mantle at the skin surface to regulate the skin's commensal organisms.3 Thus, sweat gland function is critical, and impairment can lead to skin diseases, such as atopic dermatitis and itchy skin.4, 5
There are three types of sweat glands in the human skin, eccrine, apoeccrine, and apocrine sweat glands.6 The eccrine sweat gland is the major type; there are 2‐4 million of them widely distributed over the surface of human skin, whereas apoeccrine sweat glands are mainly distributed in the axilla, and apocrine sweat glands are mainly distributed in the axilla, breasts, and perineum.7 Thus, eccrine sweat glands are the main contributors to the regulation of body temperature and skin surface condition.
Aging worsens eccrine sweat gland function, leading to loss of heat tolerance, which is associated with increased mortality due to heatstroke.8 Aged people have a reduced response to heat stimulation9 and also to stimuli from the nervous system.10 Further, deterioration of the sweating function proceeds from the lower limbs to the upper limbs.11 But, on the other hand, little is known about the morphological changes in eccrine sweat glands with aging, though this information would be helpful to find a suitable target for treatments to improve eccrine sweat gland function.
The main difficulty in studying the morphology of sweat glands is their complex structure. The sweat gland consists of two parts, the secretory coil and the secretory duct. The secretory coil has complex and tightly coiled structure, and generates the primary sweat from the blood. The secretory duct has long, tortuous structure that serves to resorb Na and Cl and deliver the final sweat to the skin surface.12 Conventional two‐dimensional (2D) histological observation is not readily applicable to analyze the morphology of this spatially complex organ. In order to understand the morphological changes, we need 3D structure information in a form that can be statistically analyzed.
Recently, X‐ray micro‐CT has been used for 3D imaging of complex organs at a resolution of micrometer order and has been applied to tracheal cartilage,13 bronchioles,14 and zebrafish gills.15 Therefore, in this study we first established a 3D analysis system for sweat gland morphology by means of X‐ray micro‐CT, followed by three‐dimensional (3D) digital reconstruction on computer. We then applied this methodology to investigate the age‐dependent changes in sweat gland morphology in human skin.
2. METHODS
2.1. Subjects
All studies were approved by the ethics committees of Shiseido Research Center and the International University of Health and Welfare. Twenty samples of healthy human abdominal skin, prepared from surplus skin excised during plastic surgery, were purchased from Biopredic International (Rennes, France). The donors were Caucasian females with no stretch marks in their skin, in the age range of 25‐71, and with a body mass index of 21 to 29 (Table 1).
TABLE 1.
Characteristics of subjects and the observed sweat glands
| (a) Subjects’ age and body weight | |||||||
|---|---|---|---|---|---|---|---|
| Number of subjects | Age | BMI | |||||
| Minimum | Maximum | Average | Minimum | Maximum | Average | ||
| Young | 10 | 25 | 39 | 33.4 | 21 | 29 | 25.2 |
| Old | 8 | 61 | 71 | 65.1 | 20 | 27 | 25.1 |
| (b) Observed skin specimens and number of sweat glands | ||||||
|---|---|---|---|---|---|---|
| Observed area (epidermis mm²) | Observed sweat gland number | |||||
| Minimum | Maximum | Average | Minimum | Maximum | Average | |
| Young | 12.3 | 24.2 | 18.6 | 8 | 30 | 17.1 |
| Old | 13.0 | 22.0 | 16.8 | 11 | 28 | 16.3 |
2.2. 3D imaging of sweat glands and reconstruction on computer
We chose abdominal skin for this study, as the abdomen has an intermediate density of sweat glands among various body sites.6 Skin specimens were cut into cubes of about 4 mm3 and subjected to X‐ray micro‐CT, D200RSS270 (Comscan Techno, Kanagawa, Japan) under the following conditions: voltage 80 kV, current 100 µA, rotation 360 degrees, integration time 200 msec, and resolution 2.6 µm. Sweat glands in the obtained images were segmented and reconstructed in 3D on a computer using Amira (Thermo Fisher Scientific, MA, USA).
Analysis of sweat glands.
The criteria for evaluating sweat glands were as follows: Sweat glands were included in the enumeration even if only a part of the gland lay within the measured area of epidermis. Sweat gland volume, length, and depth were calculated for sweat glands that lay wholly within the measurement area. The secretory coil region was defined morphologically as the tightly coiled region.
2.3. Histological observation
Skin specimens were fixed, embedded in paraffin, and sectioned serially at 5 μm vertically to the skin surface. The sections were deparaffinized, rehydrated through graded alcohols, and then stained with hematoxylin–eosin (HE).
2.4. Statistical analysis
All data were expressed as means ± SEM Differences between two groups were examined for statistical significance using the F test followed by Student's t test. Correlations were evaluated by calculating Pearson's correlation coefficient with Microsoft Excel. A P value of <.05 was considered to indicate a significant difference.
3. RESULTS
3.1. Digital 3D reconstruction of skin and identification of sweat glands
In order to reconstruct the structure of sweat glands and their distribution in the skin, skin specimens were cut into two parts from the skin surface to the adipose layer. One was used for X‐ray CT observation and the other subjected to conventional histological observation (HE staining). Sweat glands were identified based on their high brightness on CT images (Figure 1A,B) and directional continuity toward the epidermis in CT images of serial sections. This information was used to reconstruct 3D images of skin and segmented sweat glands on computer (Figure 1C,D). The reconstructed sweat glands were confirmed to show the characteristic structure observed in the HE‐stained reference skin specimen.
FIGURE 1.
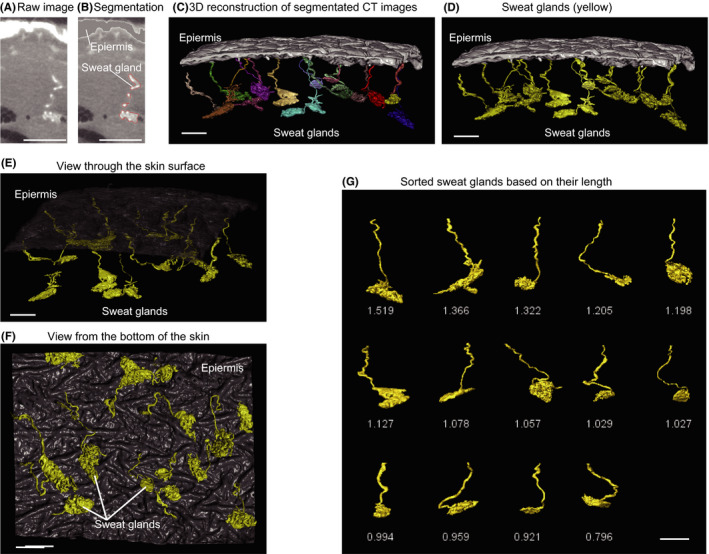
3D reconstruction of sweat glands and application of the methodology. A 4 mm3 skin specimen was observed by X‐ray micro‐CT. A, A raw CT image of a sweat gland. B, Segmented sweat gland on the same image. C, 3D reconstruction from segmented images, showing sweat glands. D, Sweat glands are colored yellow for clarity. E, Top view and (D) bottom view of sweat glands. G, Isolated sweat glands, ordered based on their length. These images were obtained from an abdominal skin specimen from a young (age 36) female subject. Scale bars indicate 500 µm
As every sweat gland in the reconstructed skin has its own spatial information, this skin can be manipulated in silico as required (Figure 1E,F). For example, each sweat gland is identified as an isolated structure, and can be separated, sorted, and quantified digitally (Figure 1G). Thus, we designated this 3D reconstructed skin as digital 3D skin.
3.2. Density and volume of sweat glands are maintained with aging
Using digital 3D skin methodology, we compared the sweat glands in abdominal skin of young (<40 years old) and old (>60 years old) female subjects (Table 1). The morphology of the sweat glands differed in the two groups (Figure 2A,B), but we found no statistically significant difference between the density of sweat glands in the two groups (Figure 2C). Furthermore, the total volumes of sweat glands and their components, that is, secretory duct and coil (Figure 3A), were the same in the two groups (Figure 3B‐D). These results suggest that aging may have little effect on the density or size of sweat glands in abdominal skin.
FIGURE 2.
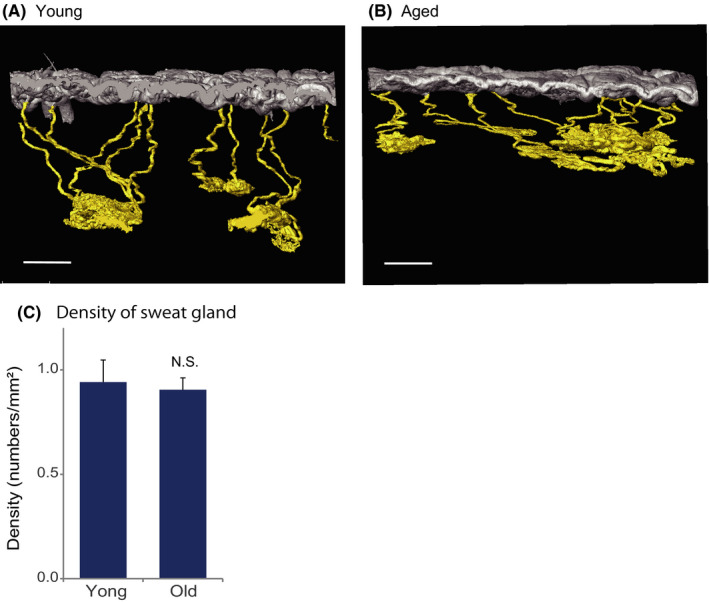
Density of sweat glands is maintained with aging. 3D images of sweat glands from abdominal skin of (A) young (age 37) and (B) old (age 64) subjects. Scale bars indicate 500 µm. C. There is no significant difference between the young (<40 years, n = 10) and old (>61 years, n = 8) groups. The density of sweat gland is expressed as mean ± SEM The statistical significance of differences between the groups was determined by means of Student's t test (NS, not significant)
FIGURE 3.
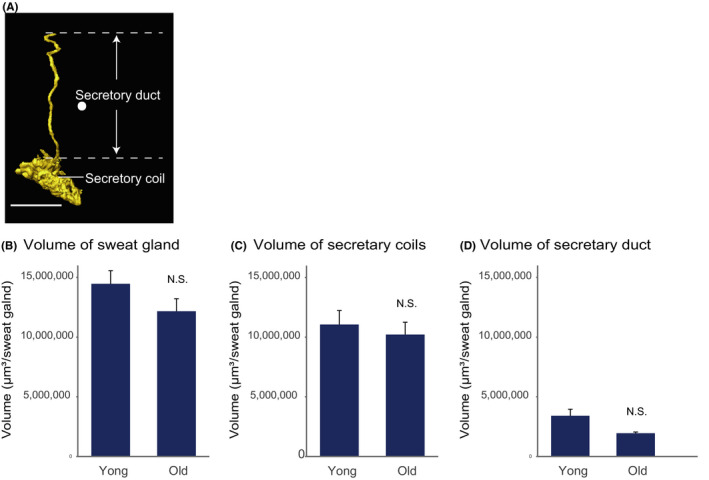
Sweat gland volume is stable during aging. A, 3D image of sweat gland and its components. Scale bars indicate 500 µm. b, Total volume, (C) secretory coil volume, and (D) secretory duct volume of young (<40 years, n = 10) and old (>61 years, n = 8) group are expressed as mean ± SEM There was no significant difference in any of these parameters between the groups as determined by means of Student's t test (NS, not significant)
3.3. Sweat glands shift toward the skin surface with aging
Next, we investigated whether the morphology of sweat glands changes with aging. Figure 4A shows representative morphology of sweat glands in the young group. In contrast, the secretory coil of sweat glands in the old group is located closer to the skin surface (Figure 4B,C). Specifically, the perpendicular depth of the secretory coil from the skin surface is significantly less than in the young group (Figure 4D), even though the length of the secretory duct does not change with aging (Figure 4E) (length was measured along the actual contour of the duct (dotted line).
FIGURE 4.
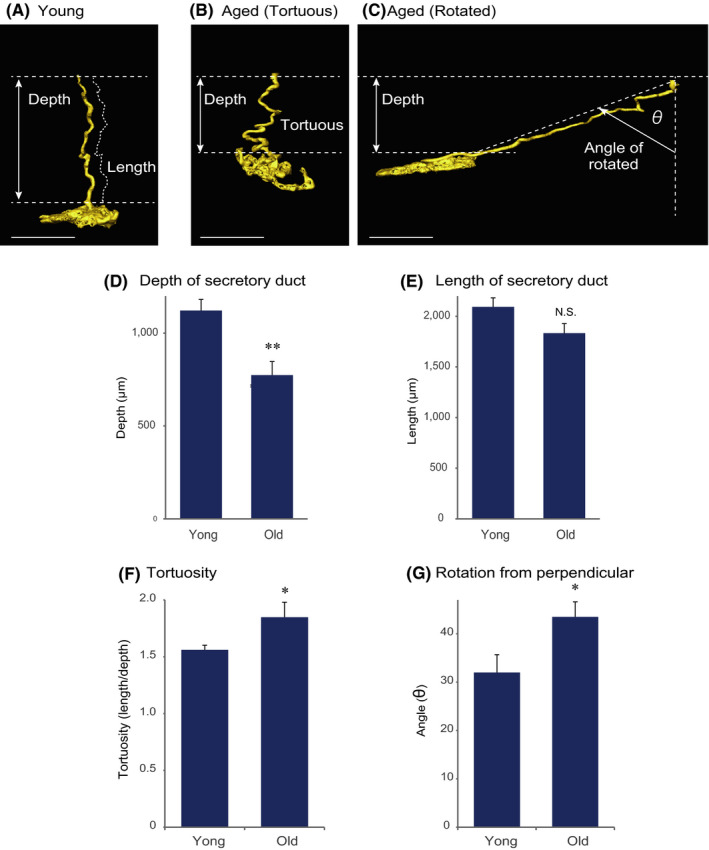
Sweat glands move toward the skin surface with aging. A, 3D reconstructed image of a representative sweat gland from a young subject (age 39). Representative sweat glands observed in an old subject (age 64): (B) tortuous or meandering form and (C) rotated form. Scale bars indicate 500 µm. D, The depth of the sweat gland was significantly decreased in the aged group (>61 years, n = 10) compared with the young group (<40 years, n = 8). E, There was no significant difference in the length of the secretory duct between the young and old groups. F, The tortuosity (length/depth) and (G) rotation angle (see Figure 4C) were significantly increased in the aged group (mean ± SEM, Student's t test (NS, not significant, *: P < .05, **: P < .01))
To understand the reason for this, we focused on the 3D structures. In the young group, the secretory coil was predominantly located perpendicularly beneath the duct outlet on the skin surface, as shown in Figure 4A. The aged group showed greater numbers of tortuous or meandering structures and rotated structures with the duct lying at an angle from the perpendicular (Figure 4B,C). The tortuosity and the angle from the perpendicular were significantly increased in the aged group (Figure 4F,g). Therefore, the combined effect of these morphological changes might account for the upward shift of sweat glands toward the skin surface with aging.
3.4. Decreased dermal layer thickness could contribute to the upward shift of sweat glands with aging
Secretory coils are located near the boundary of the dermal and adipose layers in both young skin (Figure 5A) and old skin (Figure 5B). Moreover, the thickness of the dermal layer is significantly decreased in aged skin (Figure 5C), and the depth of the secretory coil of sweat glands from the skin surface is positively correlated with the thickness of dermal layer (Figure 5D). Thus, because aging decreases the thickness of the dermal layer, and the sweat glands maintain their position at the dermal‐adipose boundary, the sweat glands are shifted upward, closer to the skin surface.
FIGURE 5.
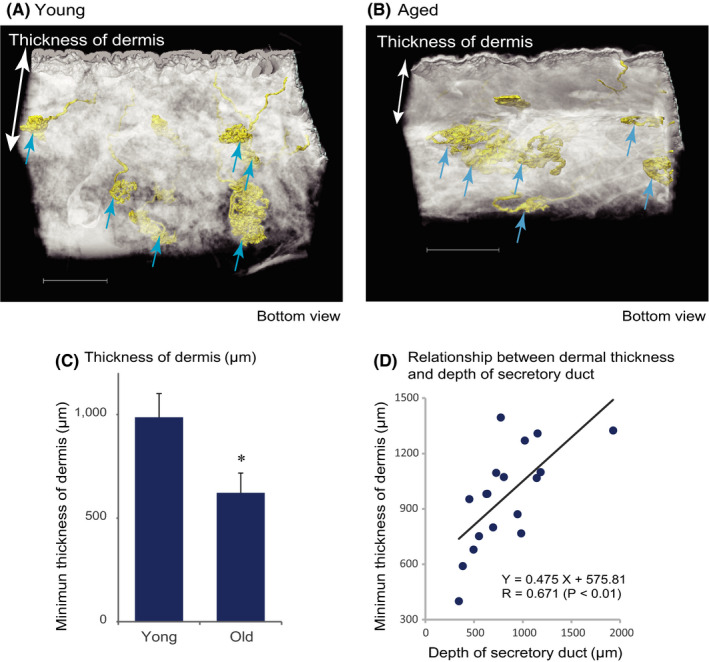
The depth of sweat glands from the skin surface decreases concomitantly with age‐related loss of dermal thickness. Representative 3D reconstructed images of skin of (A) young (age 37) and (B) old (age 64) subjects. The secretory coils of sweat glands (arrows) are located at the bottom of the dermal layer in each group. However, in the old subject, the dermal layer is thinner than in the young subject. Scale bars indicate 1000 µm. C, The minimum thickness of the dermal layer in the measured area, expressed as mean ± SEM, was significantly decreased in the old group (n = 8) compared with the young group (n = 10, *: P < .05). d) The depth of the sweat gland secretory duct was significantly positively correlated with the minimum thickness of the dermal layer (Pearson's correlation coefficient for all of the specimens, N = 18)
4. DISCUSSION
In this study, we developed methodology for 3D reconstruction of eccrine sweat glands (digital 3D skin) to enable precise statistical comparison of their properties in young and old subjects. This analysis showed that the density and volume of sweat glands did not change with aging, but the depth of the secretory coils from the skin surface decreased. Since the secretory coils remain fixed at the dermal‐adipose boundary independently of aging and the thickness of the dermal layer decreases with aging, their depth from the skin surface is decreased, that is, the sweat glands are shifted upward toward the skin surface.
Rittié16 and Poblet17 combined 2D histological images of sweat gland structure to build up 3D image data and demonstrated that sweat glands contribute to reepithelialization and are associated with hair follicles, respectively. Kurata used a whole‐mount immunostaining method to directly observe the 3D structure of sweat glands at the cellular level, describing the arrangement of component cells of sweat glands, blood vessels, and nerve fibers.18 However, to analyze the physiological changes in sweat glands, such as age‐dependent changes, we need to analyze the whole structure of sweat glands, as well as their distribution and location in the skin. We considered that this would require conversion of structural information about the whole skin, including sweat glands, to digital form that could be manipulated on computer. Our results confirm that the methodology described here is suitable for this purpose.
Sweat gland function has been reported to deteriorate with aging,19, 20 and the deterioration is local, not related to the central control of sweating.21 As sweating amount is related to the volume of sweat glands,22, 23 it has been considered that sweat gland volume decreases with aging. However, we found no significant difference in the volume of sweat glands or their component parts (secretory coil and duct) between the young and old groups. Furthermore, the density of sweat glands does not change with aging, as shown in Figure 2, in agreement with reported findings.1 Therefore, we considered that the age‐dependent morphological changes shown in Figure 4 might be related to the functional change.
The secretory coils of sweat glands are located near the boundary of the dermal and adipose layers.24 We found that this is so independently of age, and we also found a positive correlation between dermal thickness and the depth of sweat glands from the skin surface, defined as the perpendicular distance from the skin surface to the junction of the secretory coils and duct (Figure 5). These results suggest that sweat glands, especially the secretory duct, change their morphology as the dermal layer becomes thinner with aging. Indeed, we found that the secretory ducts become more tortuous or meandering, or displaced from the perpendicular, in older subjects. A recent study revealed that sweat glands are associated with other internal skin organs or hair follicles.25 Considering that age‐dependent changes occur in hair follicles,26 and dermal thinning has been reported in various sites of the body,27 it is plausible that interactions with other skin organs might influence the behavior of sweat glands.
In conclusion, our findings indicate that eccrine sweat glands move closer to the skin surface with aging due to the decrease in dermal thickness. The resulting age‐dependent change in their morphology may provide a physiological basis for understanding the deterioration of sweating function. This concept could open up new possibilities for treatments to improve poor skin condition in aged people, as well as to overcome loss of heat tolerance.
Ezure T, Amano S, Matsuzaki K. Aging‐related shift of eccrine sweat glands toward the skin surface due to tangling and rotation of the secretory ducts revealed by digital 3D skin reconstruction. Skin Res Technol.2021;27:569–575. 10.1111/srt.12985
REFERENCES
- 1.Baker LB. Physiology of sweat gland function: The roles of sweating and sweat composition in human health. Temperature (Austin). 2019;6(3):211‐259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shiohara T, Mizukawa Y, Shimoda‐Komatsu Y, Aoyama Y. Sweat is a most efficient natural moisturizer providing protective immunity at points of allergen entry. Allergol Int. 2018;67(4):442‐447. [DOI] [PubMed] [Google Scholar]
- 3.Schmid‐Wendtner MH, Korting HC. The pH of the skin surface and its impact on the barrier function. Skin Pharmacol Physiol. 2006;19(6):296‐302. [DOI] [PubMed] [Google Scholar]
- 4.Murota H, Yamaga K, Ono E, Murayama N, Yokozeki H, Katayama I. Why does sweat lead to the development of itch in atopic dermatitis? Exp Dermatol. 2019;28(12):1416‐1421. [DOI] [PubMed] [Google Scholar]
- 5.Hendricks AJ, Vaughn AR, Clark AK, Yosipovitch G, Shi VY. Sweat mechanisms and dysfunctions in atopic dermatitis. J Dermatol Sci. 2018;89(2):105‐111. [DOI] [PubMed] [Google Scholar]
- 6.Wilke K, Martin A, Terstegen L, Biel SS. A short history of sweat gland biology. Int J Cosmet Sci. 2007;29(3):169‐179. [DOI] [PubMed] [Google Scholar]
- 7.Sato K, Leidal R, Sato F. Morphology and development of an apoeccrine sweat gland in human axillae. Am J Physiol. 1987;252(1 Pt 2):R166‐180. [DOI] [PubMed] [Google Scholar]
- 8.Rittie L, Fisher GJ. Natural and sun‐induced aging of human skin. Cold Spring Harb Perspect Med. 2015;5(1):a015370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Inoue Y, Nakao M, Araki T, Murakami H. Regional differences in the sweating responses of older and younger men. J Appl Physiol. 1985;71(6):2453‐2459. [DOI] [PubMed] [Google Scholar]
- 10.Kenney WL, Fowler SR. Methylcholine‐activated eccrine sweat gland density and output as a function of age. J Appl Physiol. 1988;65(3):1082‐1086. [DOI] [PubMed] [Google Scholar]
- 11.Inoue Y, Shibasaki M. Regional differences in age‐related decrements of the cutaneous vascular and sweating responses to passive heating. Eur J Appl Physiol Occup Physiol. 1996;74(1–2):78‐84. [DOI] [PubMed] [Google Scholar]
- 12.Cui CY, Schlessinger D. Eccrine sweat gland development and sweat secretion. Exp Dermatol. 2015;24(9):644‐650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim IG, Park SA, Lee SH, et al. Transplantation of a 3D‐printed tracheal graft combined with iPS cell‐derived MSCs and chondrocytes. Sci Rep. 2020;10(1):4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tanabe N, Vasilescu DM, Kirby M, et al. Analysis of airway pathology in COPD using a combination of computed tomography, micro‐computed tomography and histology. Eur Respir J. 2018;51(2):1701245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Messerli M, Aaldijk D, Haberthur D, et al. Adaptation mechanism of the adult zebrafish respiratory organ to endurance training. PLoS One. 2020;15(2):e0228333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rittie L, Sachs DL, Orringer JS, Voorhees JJ, Fisher GJ. Eccrine sweat glands are major contributors to reepithelialization of human wounds. Am J Pathol. 2013;182(1):163‐171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Poblet E, Jimenez F, Escario‐Travesedo E, et al. Eccrine sweat glands associate with the human hair follicle within a defined compartment of dermal white adipose tissue. Br J Dermatol. 2018;178(5):1163‐1172. [DOI] [PubMed] [Google Scholar]
- 18.Kurata R, Futaki S, Nakano I, et al. Three‐dimensional cell shapes and arrangements in human sweat glands as revealed by whole‐mount immunostaining. PLoS One. 2017;12(6):e0178709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Larose J, Boulay P, Sigal RJ, Wright HE, Kenny GP. Age‐related decrements in heat dissipation during physical activity occur as early as the age of 40. PLoS One. 2013;8(12):e83148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Inoue Y, Kuwahara T, Araki T. Maturation‐ and aging‐related changes in heat loss effector function. J Physiol Anthropol Appl Human Sci. 2004;23(6):289‐294. [DOI] [PubMed] [Google Scholar]
- 21.Inoue Y, Shibasaki M, Ueda H, Ishizashi H. Mechanisms underlying the age‐related decrement in the human sweating response. Eur J Appl Physiol Occup Physiol. 1999;79(2):121‐126. [DOI] [PubMed] [Google Scholar]
- 22.Sato F, Owen M, Matthes R, Sato K, Gisolfi CV. Functional and morphological changes in the eccrine sweat gland with heat acclimation. J Appl Physiol. 1990;69(1):232‐236. [DOI] [PubMed] [Google Scholar]
- 23.Sato K, Sato F. Individual variations in structure and function of human eccrine sweat gland. Am J Physiol. 1983;245(2):R203‐R208. [DOI] [PubMed] [Google Scholar]
- 24.Bovell DL. The evolution of eccrine sweat gland research towards developing a model for human sweat gland function. Exp Dermatol. 2018;27(5):544‐550. [DOI] [PubMed] [Google Scholar]
- 25.Poblet E, Jimenez‐Acosta F, Hardman JA, Escario E, Paus R. Is the eccrine gland an integral, functionally important component of the human scalp pilosebaceous unit? Exp Dermatol. 2016;25(2):149‐150. [DOI] [PubMed] [Google Scholar]
- 26.Fenske NA, Lober CW. Structural and functional changes of normal aging skin. J Am Acad Dermatol. 1986;15(4 Pt 1):571‐585. [DOI] [PubMed] [Google Scholar]
- 27.Waller JM, Maibach HI. Age and skin structure and function, a quantitative approach (I): blood flow, pH, thickness, and ultrasound echogenicity. Skin Res Technol. 2005;11(4):221‐235. [DOI] [PubMed] [Google Scholar]


