Abstract
Antibiotic tolerance—the ability of bacteria to survive for an extended time in the presence of bactericidal antibiotics—is an understudied contributor to antibiotic treatment failure. Herein, I review the manifestations, mechanisms, and clinical relevance of tolerance to cell wall–active (CWA) antibiotics, one of the most important groups of antibiotics at the forefront of clinical use. I discuss definitions of tolerance and assays for tolerance detection, comprehensively discuss the mechanism of action of β‐lactams and other CWA antibiotics, and then provide an overview of how cells mitigate the potentially lethal effects of CWA antibiotic–induced cell damage to become tolerant. Lastly, I discuss evidence for a role of CWA antibiotic tolerance in clinical antibiotic treatment failure.
Keywords: antibiotic tolerance, phenotypic tolerance, beta lactams, autolysins, peptidoglycan
Herein, I review the manifestations, mechanisms, and clinical relevance of tolerance to cell wall–active (CWA) antibiotics, one of the most important groups of antibiotics at the forefront of clinical use. I discuss definitions of tolerance and assays for tolerance detection, comprehensively discuss the mechanism of action of β‐lactams and other CWA antibiotics, and then provide an overview of how cells mitigate the potentially lethal effects of CWA antibiotic–induced cell damage to become tolerant. Lastly, I discuss evidence for a role for CWA antibiotic tolerance in clinical antibiotic treatment failure.
Part I. Introduction
The discovery, development, and clinical implementation of antibiotics stands as one of the greatest achievements of the 20th century. They have enabled the treatment of myriad infectious diseases and facilitated important life‐saving advances in medicine, such as organ transplantation. Among the most important are cell wall–active (CWA) antibiotics, which selectively inhibit the synthesis or turnover of this essential bacterial structure. These agents include, inter alia, the β‐lactams (e.g., penicillins, cephalosporins, carbapenems, and monobactams), glycopeptides (e.g., vancomycin, teicoplanin, telavancin, oritavancin, and dalbavancin), and fosfomycin. The history describing their discovery and clinical development is the source of legend. These accounts range from the serendipitous, yet Argus‐eyed, discovery of penicillin by A. Fleming in the 1920s who noted the inhibitory activity on Staphylococcus aureus on an agar plate contaminated with the mold Penicillium notatum,1 to the exotic with the discovery by E. Kornfeld in the 1950s of compound 05865, that is, vancomycin (named from the word “vanquish”), produced by an isolate, Streptomyces orientalis (now Amycolatopsis orientalis), recovered from a soil sample from Borneo.2
However, these and related compounds have often been deployed with abandon and little consideration to the ease with which resistance can emerge. The emergence and increasing occurrence of antibiotic resistant organisms is a serious public health concern and places a substantial burden on the healthcare system and society in general. The Centers for Disease Control and Prevention estimates that more than 2 million antibiotic resistant infections occur in the United States each year, resulting in at least 23,000 deaths, $20 billion in excess direct healthcare costs, and $35 billion in lost productivity.3 Clearly, frank antibiotic resistance is a global threat; however, antibiotic tolerance (defined below) is believed to contribute to both treatment failure and the development of overt antibiotic resistance. Despite seminal work in the 1970s and 1980s that focused on CWA antibiotic tolerance, this remains underappreciated and understudied (e.g., a PubMed search for “antibiotic tolerance” yields 1/60th of the number of articles unearthed with the search term “antibiotic resistance”). Therefore, the primary motivating factor for this review is to illuminate the topic of tolerance of CWA antibiotics and to inspire the scientific community to address this important and fascinating subject.
Definitions and measurements of tolerance
Antibiotic tolerance remains a poorly defined term. Typically, it is used to express that bacteria remain viable (but unable to grow) for extended periods after exposure to an antibiotic typically considered bactericidal (Fig. 1). This is in clear contrast to resistance, which is defined as the ability to proliferate in the presence of an antibiotic (quantifiable by well‐defined measures, such as the zone of inhibition around an antibiotic‐containing disk, or the minimum inhibitory concentration, MIC). I herein treat tolerance as largely separate from persistence, which is defined as a small fraction of a bacterial population that is completely refractory to killing by bactericidal antibiotics. Numerous excellent reviews are available concerning persistence,4, 5, 6 which will not be discussed specifically in this review. However, as there is an overlap between persistence (small subpopulation of bacterial cells that survive antibiotic exposure) and tolerance (whole population of bacterial cells slowly dying), inclusion of persisters to some degree is necessary. Indeed, persistence has previously been defined as heterotolerance, that is, a subcategory of tolerance.4
Figure 1.
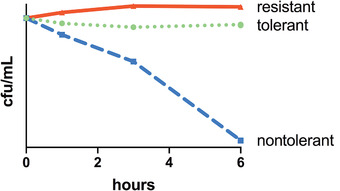
Time‐kill experiment illustrating the distinction between CWA antibiotic resistance, tolerance, and lack of tolerance at high inoculum. Shown are real data from an actual experiment where either a resistant isolate of Klebsiella pneumoniae (carrying the carbapenemase KPC‐1), a tolerant isolate of the same species (K. pneumoniae 1084), or a nontolerant E. coli isolate were exposed to the carbapenem antibiotic meropenem. The resistant isolate divides and thus increases in live cell counts, while the tolerant isolate neither grows nor significantly dies and the nontolerant isolate declines in viability by several orders of magnitude. Note that what is shown here represents exposure to antibiotics at high inoculum, where a very high number of tolerant cells remains viable.
Numerous attempts have been made to quantify tolerance in a clinically meaningful way. The earliest studies utilized some measure of the absolute survival numbers of a population after antibiotic exposure for a certain timeframe, with arbitrary cutoff points for what was considered tolerant; for example, >100 colony forming units (cfu)/mL after 24‐h incubation with antibiotic7 or 2% survival after 24 hours.8 A more widely established measure for tolerance is the minimum bactericidal concentration (MBC), typically reported in its relationship with the MIC. Tolerance breakpoints were often arbitrarily set at MBC/MIC >10–100 (Ref. 9). For MBC determinations, bacterial cultures are exposed to increasing concentrations of an antibiotic, followed by the determination of viability after some fixed time of incubation (typically 24 hours).10, 11 Usually, killing at first increases with antibiotic concentration, followed by a plateau where little additional killing is observed. In some instances, higher antibiotic concentrations effect less killing (termed the Eagle effect or paradoxical effect, which can be observed with certain β‐lactam antibiotics12). The MBC is then (again, somewhat arbitrarily) defined as the minimal concentration of antibiotic that kills 99.9% of bacteria. Several problems with the MBC metric for assessing tolerance were reported early on. Unlike MIC determinations, MBCs were found to be highly variable between different growth media and culture volumes used in these assays,9, 13, 14, 15, 16 adversely affecting its usefulness for clinical studies. Further, some bactericidal antibiotics never yield an MBC against some bacterial species (even if they are fully susceptible, but less than 99.9% of the population is killed).17, 18 Most importantly, when considering the phenomenology of tolerance, a bacterial isolate with a low MBC might still be considered tolerant if it takes an extended time to reach 99.9% of killing. For example, an isolate that experiences a 1000‐fold drop in viability within 1 h at a certain antibiotic concentration would arguably be less tolerant than an isolate that takes 24 h to reach the same level of killing, yet both might have the same MBC. As a potential way to circumvent this problem, another proposed tolerance measure uses the time needed to kill 99–99.9% of bacteria; that is, the minimum duration of killing (MDK99) as a readout for tolerance.19, 20 Lastly, Goessens et al. proposed the use of the tolerance percentage, which is measured by conducting an MBC experiment, but then reporting the percent survival of the bacterial population at the beginning of the plateau, that is, the survival fraction at the antibiotic concentration above which no further killing is observed.8 As such, the tolerance percentage would likely be a useful quantifier for persistence but not necessarily for tolerance since the latter is generally defined through a temporal aspect (“slower” killing). Therefore, the MDK99 is probably the most relevant measurement to capture the full breadth of tolerance phenotypes.
What almost all tolerance tests have in common is that unlike the gold standard MIC, they are too cumbersome and time‐consuming to be routinely conducted in a clinical laboratory. This problem has been recognized and two tests have thus been established to measure tolerance by the appearance of colonies in the zone of growth inhibition around an antibiotic‐containing disk upon prolonged incubation. This is accomplished either by adding, post‐incubation, an enzyme that degrades the antibiotic,21 or, in its more recent iteration from Nathalie Balaban's group, by adding nutrients and relying on antibiotic dissipation through diffusion to allow for regrowth of rare survivors (TDtest22). While these tests are only semiquantitative, for example, provide readouts of “no colonies” versus “few colonies” versus “many colonies,” they could potentially be compatible with a clinical microbiology laboratory workflow, opening up new avenues to include tolerance testing in the clinical practice.
Part II. Mechanisms of tolerance
Mechanism(s) of action of CWA antibiotics
To understand mechanisms of tolerance, one must first understand the mechanism of action of CWA antibiotics (summarized in Fig. 2); for that, an excursion into bacterial cell wall synthesis and turnover is necessary. Most bacteria surround themselves with a cell wall, which is either a thin layer (∼2–6 nm) covalently linked to an outer membrane (Gram‐negative bacteria) or a thick layer (∼50–60 nm) covalently linked with other polysaccharides (e.g., teichoic acids (TAs)) that often have regulatory functions.23, 24 In both Gram‐negative and ‐positive bacteria, the cell wall mainly comprises peptidoglycan (PG), a polymer of [N‐acetyl‐glucosamine]‐[N‐acetylmuramic acid] (NAG–NAM) residues that have short oligopeptide chains attached to the NAM residues. The NAG‐NAM disaccharide unit is almost invariant across bacterial species (with the exception of condition‐specific modifications like O‐acetylation25, 26), while the oligopeptide chain is highly variable depending on species and growth conditions.24 However, some commonalities, grounded in biochemical necessity, exist. For example, in all bacteria, the peptide sidechain contains d‐amino acids; the third position is occupied by a d‐amino acid that possesses free amine groups (typically diaminopimelic acid (DAP) or lysine (Lys)) and positions 4 and 5 consist of two d‐alanine (d‐Ala) residues in the vast majority of bacterial species.24, 27
Figure 2.

Summary of the main classes of CWA antibiotics and their mechanism of action. Antibiotics not used clinically are in parentheses. ROS, reactive oxygen species; GT, glycosyltransferase.
The basic building block for the cell wall is called lipid II and represents an NAG–NAM disaccharide with a pentapeptide sidechain and a C55 lipid anchor (undecaprenol phosphate (UP)). This precursor is produced in the cytoplasm by an assembly line of so‐called Mur enzymes, which first produce an NAM monosaccharide that is sequentially outfitted with amino acids to build the oligopeptide chain. The resulting NAM‐peptide is then attached to UP‐pyrophosphate (UPP) and subsequently joined with an NAG residue to form lipid II. Lipid II is translocated into the periplasm by dedicated flippase enzymes (MurJ and Amj28, 29, 30), where it is then used to assemble the final cell wall in two major reactions. The pyrophosphate bond in lipid II is used to provide energy for a glycosyltransferase reaction (GT reaction); that is, the polymerization of the disaccharides into polysaccharide strands of varying lengths.31 This reaction liberates the UPP carrier, which is first dephosphorylated by several phosphatases32, 33, 34, 35 and then recycled for another round of lipid II production in the cytoplasm.36, 37 The second reaction is transpeptidation (TP reaction), which exploits the energy of the d‐Ala–d‐Ala link to effect covalent linkage between a free amine group of DAP or Lys in the third position of the oligopeptide sidestem and the subterminal d‐Ala residue of an adjacent PG strand.31 Variations of this theme exist, with often elaborate peptide crossbridges between the Lys‐d‐Ala bond in Gram‐positive bacteria, as well as direct DAP–DAP crosslinks in both Gram‐negatives and ‐positives.24, 27
GT reactions can be catalyzed by three different types of synthases: the class A penicillin‐binding proteins (aPBPs), the shape, elongation, division, and sporulation (SEDS) proteins RodA and FtsW, as well as monofunctional transglycosylases with unclear physiological significance.38, 39, 40, 41, 42 TP reactions are catalyzed by aPBPs and monofunctional class B PBPs (bPBPs); the latter typically associate with SEDS proteins to catalyze cell wall assembly.39, 40, 41 A special type of TP reaction (e.g., catalyzing crosslinking between DAP–DAP) is mediated by l,d transpeptidases.43, 44, 45 Why bacteria encode separate biochemical entities catalyzing essentially the same reactions is poorly understood, but aPBPs appear to have a general (nonspecialized) PG synthesis and perhaps repair function,46, 47, 48 while bPBP–SEDS pairs have more specialized functions in cell elongation and division.39, 40, 41 A balance between aPBPs and bPBP‐SEDS activities is also important for cell width and cell wall turnover homeostasis in Bacillus subtilis.49, 50
While the PG cell wall is essential for maintenance of structural integrity and thus a relatively rigid structure, it is also remarkably dynamic. A large number of often biochemically redundant enzymes termed autolysins are able to cut and process PG. For a detailed discussion of these enzymes, several excellent reviews are available (e.g., see Refs. 51, 52, 53, 54). The physiological functions of these cutting processes include creating gaps for insertion of new PG during cell elongation,55, 56, 57 facilitating daughter cell separation after cell division,58, 59, 60, 61, 62, 63, 64 insertion of macromolecular transenvelope complexes into the cell wall,65, 66 and PG recycling.67 Autolysins are organized into four major families. The amidases cleave between the oligopeptide sidechain and the polysaccharide backbone. The endopeptidases (EPs) cut the intermolecular oligopeptide crosslinks between DAP3 and d‐Ala4 (d,d EPs), between DAP3–DAP3, as well as intramolecular DAP3‐d‐Glu2 bonds (d,l EPs), or within the peptide bridge elaborated by some Gram‐positive bacteria.68 The lytic transglycosylases (LTGs) cut within the polysaccharide backbone in a nonhydrolytic, intramolecular nucleophilic attack mechanism that generates characteristic saccharide‐anhydro residues,69 while the similar muramidases cut the backbone through a hydrolytic mechanism. Lastly, the carboxypeptidases (which cleave off terminal amino acids from the oligopeptide sidestems) are often grouped with the autolysins, though their cleavage activity does not directly result in cell wall integrity failure.54, 70
The amidases are active during cell division and have a function in daughter cell separation, which is understood in great mechanistic detail.61, 63 EPs have a demonstrated, yet less well‐understood role in cell elongation and division.55, 56, 57, 71, 72 The LTGs and muramidases facilitate insertion of transenvelope complexes, PG recycling, daughter cell separation, and toxin secretion.64, 65, 67, 73, 74 The carboxypeptidases have a poorly understood role in cell shape generation and in the regulation of PG synthesis.70, 75, 76, 77, 78 Since autolysin‐mediated cleavage processes are potential threats to cellular structural integrity, they are likely carefully controlled or balanced by efficient cell wall synthesis at all times, and this has indeed been demonstrated for amidases and to some degree for EPs.62, 63, 79, 80, 81, 82 Understanding the role and cleavage specificities of autolysins will be important to understand the mechanism of action of CWA antibiotics in the next sections.
Mechanism of action of β‐lactam antibiotics
The mechanism of action of β‐lactam antibiotics is simple: they inhibit the TP active site of PBPs. Following the resulting interruption of PG assembly, the cell wall is usually degraded, causing either lysis and death, or the emergence of viable, cell wall–deficient forms.17, 83, 84, 85, 86, 87, 88 However, considering the downstream consequences of β‐lactam–PBP interactions in more detail gives rise to considerable complexity. First, not all β‐lactams have the same affinity for all PBPs. In extreme cases, specific PBPs are exclusively inhibited by specific antibiotics (e.g., bPBP2 in many Gram‐negative bacteria, which is inhibited by mecillinam, or bPBP3, which in many bacteria is inhibited by aztreonam or cephalexin), but most β‐lactams bind to multiple PBPs with different affinities.89, 90, 91, 92, 93 Even at concentrations above the MIC, the consequences of β‐lactam exposure can thus be a function of the compound's concentration, rather than causing a simple dichotomy of growth versus death. For example, imipenem inhibits bPBP2 primarily at concentrations just above the MIC, but all PBPs at higher concentrations,94 favoring dramatically different cell fates (see below). Affinities can vary between bacterial species too. The Gram‐negative bPBP2 inhibitor mecillinam mentioned above specifically inhibits the class A PBPs in Streptococcus pneumoniae,92 and cefsulodin inhibits both aPBPs in Escherichia coli, but only aPBP1B in Vibrio cholerae.95 The physiological importance of specific β‐lactam–PBP affinities becomes apparent when considering the second complication of β‐lactam mechanism of action: inhibition of different PBPs can have drastically different consequences for cells. Inhibition of the cell division–specific bPBP3 by aztreonam results in filamentation and slow death, and inhibition of PBP2 results in continued cell wall synthesis, but an inability to divide and maintain rod shape and thus delayed lysis and death. By contrast, inhibition of the aPBPs generally results in catastrophic structural integrity failure and rapid cell lysis and death.90, 94, 96 In the model organism E. coli, where most work on β‐lactam susceptibility has been conducted, the most rapid lysis is observed when aPBPs plus one or more bPBPs are inhibited simultaneously.94
The last complication of β‐lactams is the historic observation that β‐lactam exposure can result in two separate death pathways, a lytic pathway and a nonlytic pathway. This was first observed by Alexander Tomasz's group in the 1970s where an autolysin‐deficient mutant of S. pneumoniae did not lyse in the presence of penicillin, but still died.97 Similarly, isolates of various Streptococcus species differ in their lytic responses: Streptococcus sanguis neither lyses nor dies (but is growth‐inhibited), Streptococcus pyogenes does not lyse, but dies, and S. pneumoniae both lyses and dies in the presence of penicillin.98 Thus, lysis and death induced by β‐lactam antibiotics are separable phenomena. The question of what kills lysis‐deficient, β‐lactam–exposed cells was reilluminated by Thomas Bernhardt's group in 2014. Here, the paradoxical observation that the bPBP2 inhibitor mecillinam still killed cells with genetic backgrounds where bPBP2 is nonessential, led to the realization that β‐lactam exposure does not merely result in PG synthesis inhibition and activation of autolysins. In addition to inducing cell wall degradation, inhibition of the TP active site promotes the continuous generation of long, uncrosslinked PG strands, which are immediately degraded by LTGs in a process termed futile cycling.99 This runaway process in turn likely results in precursor depletion (especially depletion of the limited UP membrane carrier, which is shared with other essential biochemical pathways) as well as unnecessary energy expenditure, and significantly contributes to cell death.99 Consistent with such a model, overexpression of aPBP mutants with inactivated TP domains induces lysis in E. coli,100 putatively also due to futile cycling.
Glycopeptide antibiotics
The glycopeptide antibiotics are high molecular weight antibiotics that are generally only active against Gram‐positive bacteria as due to their large size they do not significantly penetrate the outer memberane of most Gram‐negative bacteria.101 What all glycopeptides have in common is their ability to bind tightly to PG or PG precursors, though the specific binding region and thus killing potency can vary. Vancomycin and related glycopeptides (e.g., oritavancin) bind tightly to the d‐Ala‐d‐Ala end of stem oligopeptides and thus primarily inhibit the TP reaction through steric obstruction of PG synthase–substrate binding.102 However, vancomycin binding can also inhibit GT activity due to its affinity to the pentapeptide in lipid II.103, 104 Ramoplanin has a high affinity for the sugar portion of lipid II, essentially sequestering this essential building block from PG synthases and thereby inhibiting PG assembly (primarily by preventing GT activity).105 The newly discovered glycopeptides complestatin and corbomycin exhibit general PG binding properties (likely binding the polysaccharide backbone), which obstructs the activity of autolysins.106 Lastly, some newer generation glycopeptides (e.g., telavancin) may have additional mechanisms of action like membrane disruption and depolarization.107
Precursor synthesis and recycling inhibitors
Several clinically used antibiotics inhibit PG precursor synthesis and recycling. Fosfomycin, which is used to treat infections due to members of the Enterobacterales,108 covalently modifies and inhibits MurA, which catalyzes the first step of PG precursor synthesis.109 Inhibition ultimately results in precursor depletion, precluding cell wall assembly. d‐cycloserine is a structural d‐Ala analog that inhibits alanine racemase as well as d‐Ala‐d‐Ala ligase,110 resulting in the production of a lipid II species that lacks the d‐Ala‐d‐Ala cap and can thus not be used for canonical d,d crosslinking by the PBPs. Bacitracin and teixobactin are cyclic peptides with a unique mechanism of action: both bind to the UPP carrier molecule. Bacitracin sequesters the free form of UPP,111 thus inhibiting the dephosphorylation and recycling of this essential precursor. Ultimately, cell wall assembly fails due to the cell's inability to translocate precursors across the cytoplasmic membrane. Teixobactin likely interacts with UPP when linked with a saccharide residue (as found in lipid II and lipid III)112 and this compound inhibits PG synthesis as well as other UP‐dependent synthesis pathways,113 potentially through sequestration of building blocks in supramolecular complexes.114
Primary consequences of CWA antibiotic exposure
Why PG degradation and lysis is a consequence of exposure to CWA antibiotics is poorly understood in detail for most bacteria; however, autolysins are clearly the main effectors of this process,88, 115 and this was recognized as early as 1957.116 In S. pneumoniae, the amidase LytA is the sole contributor to penicillin‐induced lysis. Under normal growth conditions, LytA is sequestered by lipoteichoic acids (LTAs).23, 117 Exposure to penicillin results in the degradation of an LTA synthase, the lack of which then favors the production of cell wall–associated TAs (WTAs) rather than LTAs. WTA recruits LytA, resulting in cell wall degradation, lysis, and death.118 Consequently, a ∆lytA mutant fails to lyse in the presence of penicillin.118, 119, 120 Similarly, mutants in the major autolysin of S. aureus, the bifunctional muramidase/amidase Atl, exhibit reduced death and lysis in the presence of CWA antibiotics,121, 122 suggesting some commonality with the S. pneumoniae system. The identification of a single autolysin that is required for CWA antibiotic–induced lysis (like in S. pneumoniae) is unusual. Most bacteria contain a multitude of autolysins with their specific contributions to the lysis process unknown. Species‐specific differences may also exist. In E. coli, products of LTG and EP‐mediated PG degradation are observed after β‐lactam exposure,123, 124 and the amidases appear to be major contributors to lysis.61 In V. cholerae, EPs mediate initial cell wall degradation after exposure to penicillin, while the sole amidase is not required for the degradation process itself, but determines the location of its initiation (with a shift from septal blebbing to lateral wall blebbing in a ∆amiB mutant).17 Interestingly, exposure to the aPBP glycosyltransferase inhibitor moenomycin results in the degradation of the PG sacculus but does not yield LTG products in E. coli,124 suggesting that LTG activity is not actually required for sacculus destruction under all conditions. Likely, the appearance of LTG breakdown products thus depends on active GT activity (see “futile cycling” discussed above) by the Rod system and/or aPBPs.
Other downstream events of PG synthesis inhibition
All things considered, exposure to CWA antibiotics is a traumatic event for any bacterial cell, as these antibiotics eventually cause the destruction of the cell wall by autolysins. Thus, not surprisingly, exposure to such agents results in various sequelae that reflect distinct cellular responses as well as purely physical consequences of massive cell wall damage. Exposure to β‐lactams has been tied to the generation of reactive oxygen species (ROS),125 or not;126, 127 activation of iron uptake;128 changes in TCA cycle intermediate concentrations;129 induction of the heat shock response;130, 131 induction of the SOS DNA damage response;132, 133 RNA degradation;116, 134 and stringent response (SR) induction.135 In principle, any or all of these processes may contribute to some degree to β‐lactam–mediated cell death (or tolerance, as shall be considered later), but their detailed contributions have remained poorly understood.
Environmental factors promoting tolerance
A well‐recognized hallmark of antibiotic efficacy is its strong dependence on environmental factors,136, 137 and this is especially true for CWA antibiotic tolerance. For example, pH,138, 139 the presence of certain d‐amino acids in the growth medium140 or coculture with other bacteria,141 can drastically affect CWA antibiotic tolerance through poorly understood mechanisms. Tolerance can be a function of media composition as well: planktonic cells of Mycobacterium abscessus, for example, exhibit high tolerance to the β‐lactam antibiotic cefoxitin (and other antibiotics) in standard laboratory growth media, but not in artificial cystic fibrosis sputum medium.142
Environmental variables that affect growth are particularly relevant for CWA antibiotic tolerance. Since cell death and lysis induced by CWA antibiotics relies on active PG turnover as detailed above, a reduction or cessation of cell wall modifications should result in increased tolerance. One way of achieving this is to stop growing, and especially to stop cell division, which appears to be a particularly dynamic (and thus vulnerable) aspect of cell wall synthesis and turnover. Processes associated with cell division are, indeed, a major contributor to β‐lactam–induced lysis, at least in E. coli.143, 144, 145 Further, it is well established that the degree and rate of death induced by β‐lactam antibiotics strongly correlates with growth rate;146, 147 that is, slowly growing cells are killed less rapidly than fast growing cells. Not surprisingly then, tolerance is often enhanced by environmental conditions that favor slow growth. As such, entry into stationary phase, nutrient/metal depletion, and growth in biofilms are often associated with high tolerance to β‐lactams.142, 148, 149, 150, 151 In addition, reduced growth might be the underlying cause of the inoculum effect, that is, the observation that usage of high inocula for killing assays results in drastically increased MBCs.152 Lastly, slow growth may also underlie the observed tolerance of respiration‐defective small colony variants in multiple bacterial species.153
Slow growth is likely a major contributor to tolerance during infection, given the inhospitable environment infecting bacteria often find themselves in. Nutrient depletion, cell damage by immune functions, and transition metal sequestration affect growth rate in the host and not surprisingly, many infections indeed yield nongrowing or slowly growing cells.154, 155 This was demonstrated in model “tissue cage” infections, where a porous plastic body is inoculated with a bacterial suspension and inserted subcutaneously into experimental animals to emulate infections associated with medical devices.156 In a rat infection model of S. aureus, tissue cages were mostly populated by nongrowing cells (defined by their failure to resume growth within 2 h of subculture into Mueller Hinton Broth (MHB)) that were 1000‐ to 10,000‐fold more tolerant to oxacillin and vancomycin than bacteria grown solely in MHB.157 Salmonella typhimurium growing in macrophages becomes highly tolerant against a variety of antibiotics (including cell wall–active compounds),158 and this has also been proposed to be a consequence of slow/nongrowth under these nonoptimal conditions.149, 151, 158 Similarly, tolerance can also be caused simply by coadministration of antibiotics that stop or slow down growth.159 For example, antagonism between chloramphenicol (CHL) and penicillin is a well‐known in vivo and in vitro phenomenon20, 115, 160 and likely relies on the protective slowdown/shutdown of growth induced by inhibitors of translation. Likewise, erythromycin can treacherously induce vancomycin tolerance in S. pneumoniae, confounding the interpretation of tolerance assays.161, 162, 163
However, it is important to note that slow/nongrowth is actually neither necessary nor sufficient for tolerance to PG‐acting compounds. Slowly or nongrowing Borrelia burgdorferi cells (the etiological agent of Lyme disease) are effectively killed by vancomycin and ceftriaxone in stationary phase;164 conversely, V. cholerae fails to be appreciably killed even when exposed to β‐lactams during optimal growth in exponential phase.17, 165, 166 Growth rate also correlates imperfectly with killing activity in E. coli when grown in a minimal medium with a panel of nutrient sources,167 and a recent study has suggested that metabolic state (i.e., ATP availability) is more predictive of antibiotic‐mediated killing than growth rate.168 Likely, it is thus not growth/division rate per se that modulates killing by cell wall–active agents, but rather the specific nature and dynamics of PG turnover processes, which are imperfectly correlated with growth rate. Consistent with this idea, several β‐lactams have been identified that do kill nongrowing cells. Imipenem and some experimental β‐lactams can induce lysis in stationary phase cultures of E. coli and those that have stopped growing due to nutrient starvation.169, 170, 171 This might be due to the carbapenem's ability to inhibit l,d transpeptidases.172 In some bacteria, DAP–DAP crosslinks are upregulated under nongrowth conditions like stationary phase,45, 173 and at least in Mycobacterium tuberculosis, l,d TPs (rather than PBPs) contribute significantly to PG integrity maintenance.174 Lysis of nongrowing E. coli cells by carbapenems might reflect a similar enhanced reliance on l,d TPs under nongrowth conditions. Importantly, lysis protection by slow growth may also be overcome by simply increasing the concentration of β‐lactams,146 which may provide potential lessons for the treatment of infections caused by tolerant bacteria (see discussion below).
The complications surrounding slow growth and antibiotic tolerance are probably best illustrated by research on the SR. The SR is typically induced under conditions of nutrient limitation: amino acid, carbon or fatty acid starvation.175 Starvation is sensed by second messenger synthetases like RelA and SpoT. Upon sensing limitation of essential cellular building blocks via interaction with central hubs of metabolism like the ribosome (RelA), these proteins synthesize the second messenger guanosine penta/tetraphosphate ((p)ppGpp, henceforth, “ppGpp” for brevity). Accumulation of ppGpp results in massive transcriptional reprogramming of the cell,176 and, most importantly, induces transient growth arrest177, 178, 179 (this is the basis for the well‐established technique to isolate auxotrophic mutants using penicillin enrichment180, 181). Not surprisingly, the SR has been implicated in tolerance to CWA antibiotics in numerous studies with both Gram‐positive and ‐negative bacteria,178, 182, 183, 184, 185, 186, 187, 188 and in some cases, clinical mutants with enhanced tolerance due to background SR upregulation have been isolated.189 In a particularly intriguing example, the ppGpp synthetases RelP and RelQ of S. aureus are induced by antibiotic exposure itself, resulting in high ampicillin and vancomycin tolerance.135 The more interesting question surrounding the SR and antibiotic tolerance, however, is whether or not growth arrest is the only effector of SR‐induced tolerance.
A particularly counterintuitive early observation was that in E. coli, SR‐induced tolerance could be prevented (and lysis reinstated) by the addition of CHL to amino acid–starved cells.186, 187, 190 CHL addition was proposed to “relax” SR cells to prevent SR induction. Since SR induction by amino acid starvation depends on the interaction of uncharged tRNAs with the ribosome,191 inhibiting ribosome function could interrupt the continuous induction of RelA; ppGpp might then be rapidly degraded by the hydrolase SpoT, resulting in the resumption of ppGpp‐inhibited processes. CHL was used at high, growth‐inhibitory concentrations in SR experiments and the observation of CHL‐induced lysis in SR‐induced, β‐lactam–treated cells thus suggested that slow or nongrowth was not the only factor promoting SR‐induced tolerance.
How might SR protect cells from CWA antibiotic–mediated lysis independent of growth arrest? Early work from Edward Ishiguro's group and others suggested that ppGpp accumulation correlated with a decrease in PG synthesis functions192 (possibly at a step distal to precursor synthesis193, 194), and concomitant reduction in PG incorporation. These observations suggested that SR not only induced growth arrest, but also modulated PG synthesis and potentially turnover functions. Consistent with this idea, SR induction resulted in changes in PG architecture.195 Further, an increase in both RelA and l,d TPase activity is for an unknown reason required to reprogram E. coli cells from majority DAP‐d‐Ala to majority DAP–DAP crosslinks,196 also suggesting a PG modulatory function for the SR. Lastly, a potentially direct connection between the SR and cell wall turnover was described in S. pneumoniae, where amino acid deprivation changed the cell surface concentration of the autolysin LytA.185 As for the mechanistic link between SR and PG turnover, some studies suggested an involvement of fatty acid synthesis in the lysis process, since addition of the fatty acid inhibitor cerulenin could once again protect amino acid–starved, CHL‐treated cells from lysis.194, 197 As a caveat, cerulenin might have simply reverted these cells to a high ppGpp state, as fatty acid starvation was subsequently shown to induce SR.183, 198, 199 However, a connection between phospholipid synthesis and SR‐mediated ampicillin tolerance was also suggested by another experiment: Overexpression of the glycerol‐3‐phosphate acetyltransferase PlsB (the first dedicated step toward membrane phospholipid synthesis200 and a target of ppGpp201) reversed SR‐induced tolerance, suggesting that ppGpp‐mediated shutdown of PlsB (overcome by overexpressing it) might be a key step in tolerance development.188 In apparent contradiction to all that has been presented above, a recent study found no reduction in PG incorporation (and turnover after antibiotic treatment) in RelA overexpression strains when correcting for reduced growth rate,202 and the degree to which SR exerts a direct effect on PG turnover thus remains unclear. Taking all available evidence together, the exact mechanistic connection between SR induction and CWA antibiotic tolerance independent of a growth‐inhibitory effect thus remains poorly understood and might vary with bacterial species.
Genetic underpinnings of tolerance: loss of autolysin activity
A standard procedure in bacterial genetics is to generate mutants either defective or enhanced in a phenotype to interrogate its mechanistic underpinnings. This approach has been applied to tolerance research as well, both in the laboratory and by exploiting the power of natural selection (e.g., through the study of clinically selected mutations).203, 204 However, the analysis of mutants for tolerance pathways is not always straightforward. Highly tolerant mutants often include those with defects in important metabolic processes that probably confer CWA antibiotic tolerance solely as a secondary consequence of the slow growth rate associated with these mutations. For example, the small GTPase RsgA of S. aureus is required for ribosome assembly. An rsgA mutant exhibits reduced growth rate and is thus more tolerant to penicillin.205 Similarly, mutations in amino acid homeostasis (isoleucine tRNA synthetase) exhibit apparent vancomycin tolerance via slow growth.206 These types of mutants are thus less informative to elucidate mechanisms of tolerance, though they do often emphasize the significance of slow growth for tolerance development.
Early research using hypertolerant (both laboratory and clinically derived) Gram‐positive bacteria (S. pneumoniae, S. aureus, B. subtilis, and Lactobacillus species) led to the conclusion that these variants were defective in major autolysin activity,88, 115, 120, 207, 208 either due to direct mutational inactivation of cell wall hydrolases,204, 209, 210 by altering PG structure (potentially changing the substrate of autolysins)195, 211 or by disruption of regulatory pathways upstream of autolysin activity.88 For example, some highly tolerant mutants identified in Gram‐positive bacteria produced higher levels of TAs,204, 212 which can inhibit autolysin activity,23 by sequestration118 and/or protective coating of otherwise vulnerable PG species.122
In many cases, the regulatory pathways upstream of autolysin activity are still incompletely understood. Mutants in the lrgAB versus cidAB loci of S. aureus, for example, had opposing effects on tolerance to penicillin. In the absence of lrgAB, autolysin activity was high, and tolerance consequently low.213 In the absence of cidAB, autolysin activity was low, and tolerance was 100‐fold higher than the parental strain.214 LrgA and CidA exhibit similarities to antiholin/holin proteins of bacteriophages,215 and it was proposed that CidA (holin homolog) is somehow required for autolysin export, which is opposed by the antiholin homolog LrgA. The physiological function of these systems has been proposed to be DNA release during biofilm formation,216 but how they contribute to autolysin regulation is still unknown. What is conspicuously missing from the list of examples above are Gram‐negative bacteria. Indeed, it seems to be challenging to generate mutants in autolysins or their regulators that confer high tolerance, but this might simply be a consequence of using E. coli for most experiments, which is among the least tolerant Enterobacterales for unknown reasons.83 The relative paucity of autolysin‐deficient, highly tolerant mutants might be due to their thinner PG layer, which would not allow for the same degradation buffering capacity compared with the thicker Gram‐positive cell wall, or due to some differences in autolysin activity or regulation (e.g., a higher degree of functional redundancy in key autolysins in Gram‐negatives).
Nongenetic mechanisms of tolerance: stress response systems and damage repair
As described above, cell wall–acting agents tend to cause massive cell damage and thus induce complex responses. In principle, bacteria can become tolerant by suppressing those responses that are harmful to the cell, and/or by recognizing and repairing damage induced by the antibiotic (inducible responses promoting tolerance are summarized in Table 1). Bacterial responses to the environment are typically mediated by dedicated two‐component systems, alternative sigma factors and other stress‐sensing regulators, which sense specific signals (e.g., cellular damage) and induce damage repair regulons.217 Many stress‐sensing systems are induced by exposure to CWA antibiotics, and the diversity of transcriptional responses to these agents reflects the diversity of cell damage induced by CWA antibiotics. Not surprisingly, stress responses often contribute to survival (and thus tolerance) in the presence of CWA antibiotics. The cell envelope stress response alternative, sigma factor RpoE, for example, is activated by CWA antibiotics and required for β‐lactam tolerance in Burkholderia pseudomallei and V. cholerae.148, 165 RpoE controls general cell envelope health functions, such as periplasmic proteases and chaperones,218 and likely contributes to cell envelope strength when the cell wall is weakened. The stationary phase alternative sigma factor RpoS contributes to imipenem tolerance in P. aeruginosa,219 perhaps due to its involvement in maintaining the OM permeability barrier,220 or through its positive control over the superoxide dismutase SodB.221
Table 1.
Inducible mechanisms of CWA tolerance
| Species | Signal | Tolerance genes | Proposed tolerance mechanism | Citations |
|---|---|---|---|---|
| Streptococcus pneumoniae | Unknown | Unknown | Production of teichoic acids and downregulation of autolysin activity | 204, 212 |
| Vancomycin | ciaRH | Downregulation of autolysin activity | 232 | |
| Vancomycin | ptvR | Unknown | 235 | |
| Streptococcus pyogenes | Unknown | Stk | Unknown | 230 |
| Staphylococcus aureus | Unknown | Unknown | Production of teichoic acids and downregulation of autolysin activity | 204, 212 |
| Unknown | lrgAB/cidAB | Downregulation of autolysin activity | 213, 214 | |
| β‐lactam exposure | relP/relQ | Slow growth | 135 | |
| Unknown | sodA | Detoxification of ROS | ||
| Enterococcus faecalis | Unknown | sodA | Detoxification of ROS | 237 |
| Bacillus subtilis | Cell wall fragments | walRK | Inhibition of autolysin activity through IseA | 227 |
| Escherichia coli | pH | Unknown | Unknown | 138 |
| d‐amino acids | Unknown | Unknown | 140 | |
| Cell envelope/wall damage | Rcs | Unknown | 225 | |
| Vibrio cholerae | CWA exposure and autolysin overexpression | vxrAB | Upregulation of cell wall synthesis and remediation of toxic iron influx | 166 |
| Penicillin exposure/misfolded OMPs | rpoE | Cell envelope maintenance | 165 | |
| Penicillin exposure | sodB | Detoxification of ROS | 128, 165 | |
| Pseudomonas aeruginosa | Starvation/biofilm growth/stationary phase | sodB | Detoxification of ROS | 221, 238 |
| Entry into stationary phase | rpoS | Unknown | 219 | |
| Burkholderia pseudomallei | Unknown, likely CWA exposure | rpoE/degS | Unknown | 148 |
| Multiple species | Nutrient starvation | relA/spoT | Slow growth/reduced PG turnover | 180, 181 |
The most well‐studied pathogen in the context of Gram‐negative extreme β‐lactam tolerance is V. cholerae. Here, the VxrAB two‐component system is the major contributor to tolerance. VxrAB is induced by various forms of cell wall damage (CWA antibiotics and overexpression of endogenous PG EPs).166 A ∆vxrAB mutant exhibits ∼10,000‐fold decreased tolerance against CWA antibiotics compared with the wild‐type strain; importantly, this is not the consequence of enhanced lysis, but rather reflects reduced recovery of cell wall–deficient spheroplasts that are formed in response to CWA antibiotics in this organism.17 VxrAB positively controls genes encoding cell wall synthesis enzymes (including the entire intracellular precursor pathway), type VI secretion, and biofilm formation, and negatively controls motility and iron acquisition genes.128, 166, 222, 223 Both positive control over PG synthesis and negative control over iron acquisition contribute to CWA antibiotic tolerance.128 While PG synthesis induction is an intuitive way for a cell to recover from cell wall loss, control of iron acquisition was at first puzzling. Intriguingly, V. cholerae accumulates high intracellular iron levels upon β‐lactam exposure and this is exacerbated in the ∆vxrAB mutant.128 Further, β‐lactam exposure resulted in induction of the Fur iron starvation response, likely due to direct oxidation of its Fe2+ corepressor by β‐lactam–induced H2O2 production.128 It thus appears that VxrAB's role in tolerance is at least partially to tone down an out‐of‐control iron starvation response that would otherwise overload β‐lactam–stressed cells with iron, causing downstream toxic events like the generation of ROS. Consistent with this model, deleting iron transporters partially restored high tolerance levels to a ∆vxrAB mutant.128 The VxrAB system thus aptly illustrates the complex physiological consequences of β‐lactam exposure and tolerance pathways, where cells not only have to overcome cell wall loss, but also remediate toxic iron influx and potentially downstream accumulation of toxic ROS.
While the VxrAB system is only conserved among Vibrionaceae, other pathogens have potentially functionally analogous cell envelope stress response systems with roles in CWA antibiotic tolerance. The enterobacterial Rcs phosphorelay system negatively controls motility224 and is required for recovery from a cell wall–deficient state in E. coli,225 which is reminiscent of VxrAB phenotypes. In B. subtilis, the WalRK two‐component system plays a central role in cell wall homeostasis under normal growth, but also under CWA antibiotic–induced stress conditions.226 WalRK controls IseA/YoeB, a post‐translational inhibitor of EP activity, which effectively downregulates lytic activity upon exposure to CWA antibiotics.227 Interestingly, IseA is induced through WalRK specifically in response to the accumulation of PG degradation products resulting from d,l EP activity,228 which are likely to accumulate in CWA‐stressed cells. The Serine/Threonine protein kinase Stk that putatively feeds into the WalRK signaling cascade229 has been implicated in penicillin tolerance in S. pyogenes,230 which might further suggest a conserved role for WalRK in CWA antibiotic tolerance. In S. pneumoniae, the CiaRH two‐component system promotes tolerance to bacitracin, d‐cycloserine, and vancomycin.231, 232 This system is induced by CWA antibiotics (vancomycin)130 and controls several small RNAs233 with unclear targets. However, one of the consequences of activation is reduced autolysis through a process that requires capsular polysaccharide biosynthesis.232, 234 Lastly, and similarly poorly understood, S. pneumoniae also encodes the PtvR transcriptional repressor, which controls a regulon that slightly (∼5‐ to 10‐fold) enhances vancomycin tolerance upon sensing the antibiotic.235
In addition to obvious cell envelope stress functions, some less intuitive responses are also induced by CWA antibiotics, and these may similarly contribute to tolerance. Reactive oxygen species accumulate upon exposure to CWA antibiotics, purportedly due to an imbalance of respiratory processes125, 129, 236 that results from large‐scale perturbations of cell wall metabolism. ROS can potentially contribute to CWA antibiotic–induced lethality, and detoxification of ROS promotes CWA antibiotic tolerance in some bacteria; for example, superoxide dismutases (SODs) are effectors of β‐lactam tolerance in Enterococcus faecalis, V. cholerae, and Pseudomonas aeruginosa.165, 221, 237 In P. aeruginosa, a convergence of three stress response functions (SR, ROS detoxification, and an alternative sigma factor) promotes tolerance during growth in biofilms: the SR (see above) was implicated in tolerance to carbapenems and other antibiotics via its positive control over the Mn‐dependent SodB.238 A mutant incapable of producing ppGpp survived ∼10,000‐fold less than the wild‐type in the presence of high meropenem concentrations and an sodB mutant recapitulated this phenotype.221 Additionally, sodB was coregulated by the stationary phase alternative sigma factor RpoS. Overall, these observations suggest that an ability to detoxify ROS that accumulate after antibiotic exposure may be an important component of tolerance development. This is contrasted by observations in divergent bacteria that suggest ROS can actually promote the formation of persister cells, that is, a hypertolerant subpopulation.239, 240 It is, therefore, possible that ROS have contrary effects on heterogeneous pathogen populations and either promote or reduce tolerance/persistence depending on the specific physiological state or growth environment bacteria are in upon antibiotic exposure. Consistent with a growth modality component, the requirement for SODs appears to be most prominent during stationary phase or biofilm growth in both V. cholerae and P. aeruginosa,128, 221 rather than during exponential growth.
In both E. coli and S. aureus, exposure to β‐lactams results in induction of the SOS DNA damage response.132, 133 In E. coli, this is mediated specifically by inhibition of PBP3 in a sensing process requiring the two‐component system DpiAB.133 SOS induction results in inhibition of cell division through the FtsZ antagonist SulA. Since cell division is typically the location where β‐lactam–mediated lysis starts, at least in E. coli,145 preventing septum formation might delay lysis and thus promote temporary tolerance.
For unknown reasons, the heat shock response (via the alternative sigma factor RpoH) is induced by CWA antibiotics in phylogenetically diverse bacteria,130, 131, 241, 242, 243 suggesting a conserved pathway for induction. The signal for heat shock might be protein misfolding due to oxidation, which has been reported to occur in after exposure to β‐lactam antibiotics.128, 129 The exact degree to which heat shock contributes to tolerance is poorly understood; however, artificially upregulating RpoH prevented β‐lactam–induced lysis in E. coli,244 suggesting a potential role in surviving β‐lactam exposure. Further, in S. pneumoniae, the heat shock–induced protease ClpL enhances tolerance to oxacillin, likely by stabilizing the expression of bPBP2x,131 thus, promoting cell wall integrity maintenance.
Part III. Tolerance and antibiotic therapy
A special case of CWA antibiotic tolerance: L‐forms and spheroplasts
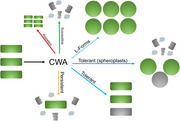
While shutting down growth is an effective means to resist antibiotic killing, some bacterial species and environmental conditions favor a different fate. Many bacteria are capable of fully enduring the destructive effects of CWA antibiotic exposure by converting to viable, cell wall–deficient forms (Fig. 3). Cell wall–deficient L‐forms (extensively and excellently reviewed elsewhere, see Refs. 84, 86, and 245) circumvent PG essentiality by proliferating via FtsZ‐independent stochastic membrane blebbing.246, 247 In addition, many Gram‐negative bacteria are capable of converting into a nondividing, cell wall–deficient spheroplast state upon β‐lactam exposure. Importantly, spheroplasts are distinct from the spherical cells that emerge upon inhibition of bPBP2. While the latter superficially resemble spheroplasts, they actually elaborate a full cell wall,83 while true spheroplasts are devoid of detectable PG material,17, 83 and thus presumably rely on the strength of their outer membrane to maintain structural integrity. The ability to do so appears to be widespread among clinically significant Gram‐negative pathogens (by current count Acinetobacter baumannii, Enterobacter species, Haemophilus influenzae, Klebsiella species, P. aeruginosa, Serratia marcescens, and V. cholerae 17, 83, 85, 87, 248) and is observed under in vivo‐like conditions (e.g., rabbit cecal fluid and human serum).17, 83 In V. cholerae and some Enterobacterales isolates, spheroplast formation results in an extreme form of tolerance, essentially converting β‐lactams to bacteriostatic antibiotics against these bacteria.17, 83, 165 Spheroplasts readily and quickly revert to rod‐shaped bacteria upon withdrawal of the antibiotic,83, 87 which would potentially enable them to repopulate an infection upon depletion of the offending antibiotic. Importantly, L‐forms and spheroplast‐like forms have been isolated from patients treated with CWA antibiotics,249, 250 and this form of tolerance might thus be an under‐recognized reason for antibiotic treatment failure.250 The exact mechanisms of spheroplast formation and maintenance are poorly understood, but physiological and genetic factors promoting this type of tolerance have been identified.87, 128, 165 In V. cholerae, survival of, and recovery from, the spheroplast state requires induction of PG synthesis functions, remediation of toxic iron influx, likely detoxification of ROS, and induction of cell envelope stress responses, as detailed above.128 An intriguing unresolved problem is how β‐lactam–tolerant spheroplasts survive futile cycling99 induced by β‐lactams and what makes their OM particularly resilient compared with nontolerant (lysis‐prone) Gram‐negatives.
Figure 3.
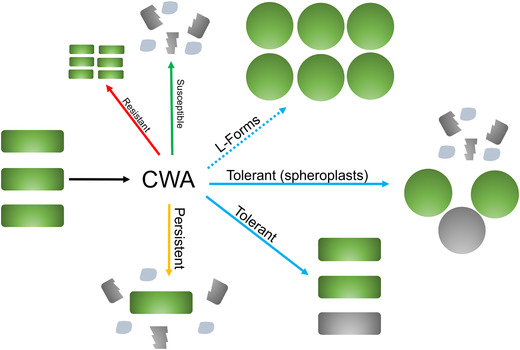
Variations of the morphological consequences of CWA antibiotic exposure. CWA antibiotics induce cell wall degradation in most growing bacteria. Resistant isolates can grow in the presence of these antibiotics. Susceptible, nontolerant populations lyse and die, while rare persisters may remain intact. In the absence of resistance mechanisms and in addition to persisters, CWA antibiotic–treated bacteria can present as L‐forms (Gram‐positive and ‐negative bacteria) that are able to divide in the absence of a cell wall, nondividing and cell wall–deficient spheroplasts (Gram‐negative bacteria), or tolerant cells of unknown morphology (here depicted as rods). Living cells are shaded in green; dead cells and debris are in gray.
Clinical relevance of CWA antibiotic tolerance
Intuitively, being able to survive exposure to normally lethal antibiotics might cause treatment failure if the offending pathogen is able to regrow after discontinuation of antibiotic therapy. However, just inhibiting pathogen growth is typically sufficient to give the immune system time to clear an infection. Whether for most infections bactericidal antibiotics perform superiorly to bacteriostatic ones is thus a matter of contention,251, 252 though in at least some cases they clearly do (e.g., in staphylococcal septicemia253). As far as direct treatment outcomes are concerned, tolerance might thus contribute adversely only under special circumstances, for example, in environments that are not easily penetrable for immune functions. Several peculiarities of the tolerance phenomenon compared with resistance (i.e., changes in MIC) have precluded studies rigorously testing clinical hypotheses surrounding tolerance. For example, tolerance can be more condition‐specific than resistance. MICs obtained in routine media used for antibiotic susceptibility testing, such as cation‐adjusted MHB, correlate with treatment outcome in vivo,254 suggesting that a concentration of antibiotic that inhibits growth of a pathogen in the laboratory is likely to do the same in the human body. Tolerance, on the other hand, varies dramatically with growth in very specific niches, as we have outlined above for Salmonella growing in macrophages and S. aureus isolated from model tissue cage infections. Removing a bacterium from this specific in vivo niche and allowing it to grow under optimal conditions in the laboratory could cause it to revert to a nontolerant state almost immediately. As discussed above, well‐established quantitative tolerance tests that can be incorporated into a clinical microbiology workflow are also lacking, preventing routine testing for tolerance in the healthcare setting. Existing studies that address the clinical impact of tolerance, therefore, suffer from the fact that quantitative definitions of tolerance are often arbitrary and cannot be used to clearly establish causation. Lastly, cryptic resistance mechanisms (e.g., resistance of a subpopulation that is not easily detectable by standard MIC assays) can create the appearance of antibiotic therapy failure in the absence of overt resistance. Treatment failure might in such a situation be interpreted as relying on something other than resistance (i.e., tolerance), while the real culprit might be heteroresistance,255 like heterogeneously vancomycin intermediate S. aureus (hVISA).256
Despite the above‐mentioned complications, numerous attempts have been made to quantitatively determine the extent to which tolerance affects outcome of antibiotic therapy and several animal model infections have supported the importance of tolerance. For example, imipenem treatment failed to clear an experimental Enterobacter cloacae thigh infection in a mouse model, despite high susceptibility of E. cloacae to the antibiotic.257 In a guinea pig model of foreign body infections by S. epidermidis, cure rates after vancomycin/teicoplanin therapy correlated more strongly with MBC than with MIC (both determined in MHB),258 suggesting that intrinsic (rather than condition‐specific) tolerance can indeed influence infection outcome. Similar results were observed in a rat model of foreign body infections.157 In an endocarditis rabbit model, higher tolerance in streptococci correlated with adverse outcome at low, but not high penicillin concentrations.259 Lastly, thigh infections in a mouse model using S. aureus were cleared at a lower rate when caused by highly tolerant variants compared with wild‐type strains exhibiting lower tolerance.260
Clinical evidence for a role of tolerance in antibiotic treatment outcomes remains scarce, although suggestive anecdotes and case reports abound.253, 261, 262, 263 Some established infections of S. aureus are known for their high rates of treatment failure despite their in vitro susceptibility (determined by MIC and MBC), and often require surgical intervention.263 Peterson et al. reported on three patients with S. aureus septicemia or endocarditis, where initial oxacillin, nafcillin, or vancomycin monotherapy failed to yield a favorable treatment outcome as defined by cessation of clinical symptoms and/or negative blood culture.16 The MICs and MBCs as determined in standard laboratory media were well below the blood serum levels of the antibiotics (which were measured in the same study), suggesting that clearance failure may be due to tolerance. A similar observation was made in another case study of S. aureus endocarditis, where vancomycin therapy failed to treat an infection caused by fully susceptible bacteria;264 however, note the discussion of hVISA strains above. Pediatric pharyngitis caused by S. pyogenes was found to exhibit a high degree of treatment failure after penicillin therapy, again despite apparent susceptibility of the involved pathogen.265 In another example, a prospective study conducted by Elaine Tuomanen's group found that infection with CWA antibiotic–tolerant S. pneumoniae isolates correlated with adverse outcomes in pediatric meningitis patients, where survival after treatment with CWA antibiotics was 49% for those infected with tolerant streptococci and 86% in those infected with nontolerant isolates.266 Tolerance was defined here via the magnitude of increased survival in the presence of vancomycin compared with the nontolerant reference strain R6.
In further support of the relevance of tolerance in the healthcare setting, CWA antibiotic–tolerant mutants have been isolated from patients after antibiotic therapy.267, 268, 269 The vancomycin‐tolerant S. pneumoniae Tupelo isolate was obtained from a patient with meningitis in which vancomycin/cephalosporin treatment had failed despite full vancomycin susceptibility of the isolate.270 Interestingly, this isolate was later found to contain a point mutation in the histidine kinase CiaH, which was hypothesized to activate the tolerance‐promoting ciaRH regulon (see above for details).234 Other vancomycin‐tolerant S. pneumoniae isolates have been tied to specific capsular serotypes,271 potentially supporting the poorly understood connection between capsule biosynthesis and tolerance, possibly via CiaRH.234 As is apparent from these examples, most clinical studies on tolerance have focused on the classical Gram‐positive model organisms of tolerance research, namely, members of the genera Staphylococcus and Streptococcus. Much less is known about the clinical significance of tolerance for Gram‐negatives, though case studies suggest instances of similar unexplained treatment failure despite the organism's in vitro susceptibility to antibiotics.249, 261, 272
Conversely, some studies have failed to show a clear correlation between tolerance and clinical outcomes; however, these studies are difficult to interpret due to their use of several antibiotics and the aforementioned problems with tolerance definitions and measurements. Mortality of a pediatric population with S. aureus bacteremia correlated more strongly with comorbidities than with tolerance (defined as MBC/MIC >10),212 but these infections were treated with combination therapy. Another study found no strong correlation between tolerance (defined as high MBC) and oxacillin/glycopeptide treatment outcomes in patients suffering from endocarditis caused by S. aureus.273
Overall, the connection between tolerance and treatment outcome is thus data deficient and requires—and deserves—further investigation. In addition, clinical studies have focused mainly on a direct influence of tolerance on clinical outcomes and neglected what is most likely a nefarious side effect of tolerance: its role as a stepping stone toward development of frank resistance. Several studies have shown that tolerant cells are a reservoir for the emergence of resistant variants.274, 275, 276, 277 This is at least in part due to the simple numerical fact that swiftly eradicating a pathogen population provides a lower chance of emergence of rare resistant variants. Furthermore, tolerant, damaged cells can actually exhibit enhanced mutation frequencies,278, 279 and this might be particularly true for CWA antibiotic–induced spheroplasts, which are metabolically active and accumulate oxidative damage. Thus, it is imperative that efforts be directed toward devising novel therapies aimed at eradicating tolerant pathogens, either through a combination therapy approach or via the development of novel antibiotics. The latter might be achieved through a recently developed, innovative antibiotic screening platform that relies on indicator‐enabled scoring of lethality in bacteria grown to stationary phase on filter disks.280 It seems that in some cases, CWA agent tolerance can be overcome by simply increasing the concentration of antibiotic, as even slow‐growing bacteria can be killed at higher concentrations of CWA agents.146 Optimization of antibiotic treatment length and dose during antibiotic therapy thus likewise hold great potential to improve the efficacy of existing antibiotics against tolerant cells.281, 282
Competing interests
The author declares no competing interests.
Acknowledgments
Research on tolerance in the Dörr laboratory is supported by the National Institutes of Health (NIH/NIAID) grant R01AI143704. I thank Dr. Lars F. Westblade (Weill Cornell Medicine, New York) and Dr. John Helmann (Cornell University, Ithaca) for helpful comments on the manuscript. The literature on tolerance is vast, and I apologize to those whose work might not have received the attention it deserves in this review due to space constraints.
References
- 1.Fleming, A.1929. On the antibacterial action of cultures of a penicillium, with special reference to their use in the isolation of B. influenzae . Br. J. Exp. Pathol. 10: 226–236. [Google Scholar]
- 2.Levine, D.P.2006. Vancomycin: a history. Clin. Infect. Dis. 42(Suppl. 1): S5–S12. [DOI] [PubMed] [Google Scholar]
- 3.CDC . 2019. Antibiotic resistance threats in the United States. Atlanta, GO: U.S. Department of Health and Human Services, CDC. [Google Scholar]
- 4.Balaban, N.Q.et al. 2019. Definitions and guidelines for research on antibiotic persistence. Nat. Rev. Microbiol. 17: 441–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lewis, K.2010. Persister cells. Annu. Rev. Microbiol. 64: 357–372. [DOI] [PubMed] [Google Scholar]
- 6.Conlon, B.P., Rowe S.E. & Lewis K.. 2015. Persister cells in biofilm associated infections. Adv. Exp. Med. Biol. 831: 1–9. [DOI] [PubMed] [Google Scholar]
- 7.Bradely, J.J., Mayhall C.G. & Dalton H.P.. 1978. Incidence and characteristics of antibiotic‐tolerant strains of Staphylococcus aureus . Antimicrob. Agents Chemother. 13: 1052–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goessens, W.H., Fontijne P., van Raffe M. & Michel M.F.. 1984. Tolerance percentage as a criterion for the detection of tolerant Staphylococcus aureus strains. Antimicrob. Agents Chemother. 25: 575–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sherris, J.C.1986. Problems in in vitro determination of antibiotic tolerance in clinical isolates. Antimicrob. Agents Chemother. 30: 633–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hacek, D.M., Dressel D.C. & Peterson L.R.. 1999. Highly reproducible bactericidal activity test results by using a modified National Committee for Clinical Laboratory Standards broth macrodilution technique. J. Clin. Microbiol. 37: 1881–1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anhalt, J.P. & Washington J.W.. 1985. Bactericidal tests. In Laboratory Procedures in Clinical Microbiology. Washington J.A., Ed.: 731–745. New York: Springer Verlag. [Google Scholar]
- 12.Eagle, H. & Musselman A.D.. 1948. The rate of bactericidal action of penicillin in vitro as a function of its concentration, and its paradoxically reduced activity at high concentrations against certain organisms. J. Exp. Med. 88: 99–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pelletier, L.L., Jr. & Baker C.B.. 1988. Oxacillin, cephalothin, and vancomycin tube macrodilution MBC result reproducibility and equivalence to MIC results for methicillin‐susceptible and reputedly tolerant Staphylococcus aureus isolates. Antimicrob. Agents Chemother. 32: 374–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goessens, W.H., Fontijne P. & Michel M.F.. 1982. Factors influencing detection of tolerance in Staphylococcus aureus . Antimicrob. Agents Chemother. 22: 364–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meylan, P.R., Francioli P. & Glauser M.P.. 1986. Discrepancies between MBC and actual killing of viridans group streptococci by cell‐wall‐active antibiotics. Antimicrob. Agents Chemother. 29: 418–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peterson, L.R., Gerding D.N., Hall W.H. & Schierl E.A.. 1978. Medium‐dependent variation in bactericidal activity of antibiotics against susceptible Staphylococcus aureus . Antimicrob. Agents Chemother. 13: 665–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dörr, T., Davis B.M. & Waldor M.K.. 2015. Endopeptidase‐mediated beta lactam tolerance. PLoS Pathog. 11: e1004850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fontana, R., Grossato A., Ligozzi M. & Tonin E.A.. 1990. In vitro response to bactericidal activity of cell wall‐active antibiotics does not support the general opinion that enterococci are naturally tolerant to these antibiotics. Antimicrob. Agents Chemother. 34: 1518–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brauner, A., Shoresh N., Fridman O. & Balaban N.Q.. 2017. An experimental framework for quantifying bacterial tolerance. Biophys. J. 112: 2664–2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jawetz, E., Gunnison J.B., Speck R.S. & Coleman V.R.. 1951. Studies on antibiotic synergism and antagonism; the interference of chloramphenicol with the action of penicillin. AMA Arch. Intern. Med. 87: 349–359. [DOI] [PubMed] [Google Scholar]
- 21.Peterson, L.R., Denny A.E., Gerding D.N. & Hall W.H.. 1980. Determination of tolerance to antibiotic bactericidal activity on Kirby–Bauer susceptibility plates. Am. J. Clin. Pathol. 74: 645–650. [DOI] [PubMed] [Google Scholar]
- 22.Gefen, O., Chekol B., Strahilevitz J. & Balaban N.Q.. 2017. TDtest: easy detection of bacterial tolerance and persistence in clinical isolates by a modified disk‐diffusion assay. Sci. Rep. 7: 41284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holtje, J.V. & Tomasz A.. 1975. Lipoteichoic acid: a specific inhibitor of autolysin activity in Pneumococcus. Proc. Natl. Acad. Sci. USA 72: 1690–1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vollmer, W., Blanot D. & de Pedro M.A.. 2008. Peptidoglycan structure and architecture. FEMS Microbiol. Rev. 32: 149–167. [DOI] [PubMed] [Google Scholar]
- 25.Bernard, E.et al. 2011. Characterization of O‐acetylation of N‐acetylglucosamine: a novel structural variation of bacterial peptidoglycan. J. Biol. Chem. 286: 23950–23958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sychantha, D., Brott A.S., Jones C.S. & Clarke A.J.. 2018. Mechanistic pathways for peptidoglycan O‐acetylation and De‐O‐acetylation. Front. Microbiol. 9. 10.3389/fmicb.2018.02332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rajagopal, M. & Walker S.. 2017. Envelope structures of Gram‐positive bacteria. Curr. Top. Microbiol. Immunol. 404: 1–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meeske, A.J.et al. 2015. MurJ and a novel lipid II flippase are required for cell wall biogenesis in Bacillus subtilis . Proc. Natl. Acad. Sci. USA 112: 6437–6442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sham, L.T.et al. 2014. Bacterial cell wall. MurJ is the flippase of lipid‐linked precursors for peptidoglycan biogenesis. Science 345: 220–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ruiz, N.2008. Bioinformatics identification of MurJ (MviN) as the peptidoglycan lipid II flippase in Escherichia coli . Proc. Natl. Acad. Sci. USA 105: 15553–15557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Egan, A.J., Biboy J., van't Veer I., et al. 2015. Activities and regulation of peptidoglycan synthases. Philos. Trans. R. Soc. Lond. B Biol. Sci. 370. 10.1098/rstb.2015.0031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bernard, R., El Ghachi M., Mengin‐Lecreulx D., et al. 2005. BcrC from Bacillus subtilis acts as an undecaprenyl pyrophosphate phosphatase in bacitracin resistance. J. Biol. Chem. 280: 28852–28857. [DOI] [PubMed] [Google Scholar]
- 33.El Ghachi, M., Bouhss A., Blanot D. & Mengin‐Lecreulx D.. 2004. The bacA gene of Escherichia coli encodes an undecaprenyl pyrophosphate phosphatase activity. J. Biol. Chem. 279: 30106–30113. [DOI] [PubMed] [Google Scholar]
- 34.El Ghachi, M., Derbise A., Bouhss A. & Mengin‐Lecreulx D.. 2005. Identification of multiple genes encoding membrane proteins with undecaprenyl pyrophosphate phosphatase (UppP) activity in Escherichia coli . J. Biol. Chem. 280: 18689–18695. [DOI] [PubMed] [Google Scholar]
- 35.Zhao, H.et al. 2016. Depletion of undecaprenyl pyrophosphate phosphatases disrupts cell envelope biogenesis in Bacillus subtilis . J. Bacteriol. 198: 2925–2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Manat, G.et al. 2014. Deciphering the metabolism of undecaprenyl‐phosphate: the bacterial cell‐wall unit carrier at the membrane frontier. Microb. Drug Resist. 20: 199–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bouhss, A., Trunkfield A.E., Bugg T.D. & Mengin‐Lecreulx D.. 2008. The biosynthesis of peptidoglycan lipid‐linked intermediates. FEMS Microbiol. Rev. 32: 208–233. [DOI] [PubMed] [Google Scholar]
- 38.Emami, K.et al. 2017. RodA as the missing glycosyltransferase in Bacillus subtilis and antibiotic discovery for the peptidoglycan polymerase pathway. Nat. Microbiol. 2. 10.1038/nmicrobiol.2016.253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Taguchi, A.et al. 2019. FtsW is a peptidoglycan polymerase that is functional only in complex with its cognate penicillin‐binding protein. Nat. Microbiol. 4: 587–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cho, H.et al. 2016. Bacterial cell wall biogenesis is mediated by SEDS and PBP polymerase families functioning semi‐autonomously. Nat. Microbiol. 1. 10.1038/nmicrobiol.2016.172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meeske, A.J.et al. 2016. SEDS proteins are a widespread family of bacterial cell wall polymerases. Nature 537: 634–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reed, P.et al. 2015. Staphylococcus aureus survives with a minimal peptidoglycan synthesis machine but sacrifices virulence and antibiotic resistance. PLoS Pathog. 11: e1004891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mainardi, J.L.et al. 2005. A novel peptidoglycan cross‐linking enzyme for a beta‐lactam‐resistant transpeptidation pathway. J. Biol. Chem. 280: 38146–38152. [DOI] [PubMed] [Google Scholar]
- 44.Magnet, S., Dubost L., Marie A., et al. 2008. Identification of the L,D‐transpeptidases for peptidoglycan cross‐linking in Escherichia coli . J. Bacteriol. 190: 4782–4785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cava, F., de Pedro M.A., Lam H., et al. 2011. Distinct pathways for modification of the bacterial cell wall by non‐canonical D‐amino acids. EMBO J. 30: 3442–3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wientjes, F.B. & Nanninga N.. 1991. On the role of the high molecular weight penicillin‐binding proteins in the cell cycle of Escherichia coli . Res. Microbiol. 142: 333–344. [DOI] [PubMed] [Google Scholar]
- 47.Straume, D.et al. 2020. Class A PBPs have a distinct and unique role in the construction of the pneumococcal cell wall. Proc. Natl. Acad. Sci. USA 117: 6129–6138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vigouroux, A.et al. 2020. Class‐A penicillin binding proteins do not contribute to cell shape but repair cell‐wall defects. elife 9: e51998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dion, M.F.et al. 2019. Bacillus subtilis cell diameter is determined by the opposing actions of two distinct cell wall synthetic systems. Nat. Microbiol. 4: 1294–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Patel, Y., Zhao H. & Helmann J.D.. 2020. A regulatory pathway that selectively up‐regulates elongasome function in the absence of class A PBPs. eLife 9: e57902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vermassen, A.et al. 2019. Cell wall hydrolases in bacteria: insight on the diversity of cell wall amidases, glycosidases and peptidases toward peptidoglycan. Front. Microbiol. 10. 10.3389/fmicb.2019.00331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee, T.K. & Huang K.C.. 2013. The role of hydrolases in bacterial cell‐wall growth. Curr. Opin. Microbiol. 16: 760–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Do, T., Page J.E. & Walker S.. 2020. Uncovering the activities, biological roles, and regulation of bacterial cell wall hydrolases and tailoring enzymes. J. Biol. Chem. 295: 3347–3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.van Heijenoort, J.2011. Peptidoglycan hydrolases of Escherichia coli . Microbiol. Mol. Biol. Rev. 75: 636–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Singh, S.K., SaiSree L., Amrutha R.N. & Reddy M.. 2012. Three redundant murein endopeptidases catalyse an essential cleavage step in peptidoglycan synthesis of Escherichia coli K12. Mol. Microbiol. 86: 1036–1051. [DOI] [PubMed] [Google Scholar]
- 56.Hashimoto, M., Ooiwa S. & Sekiguchi J.. 2012. Synthetic lethality of the lytE cwlO genotype in Bacillus subtilis is caused by lack of D,L‐endopeptidase activity at the lateral cell wall. J. Bacteriol. 194: 796–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dörr, T., Cava F., Lam H., et al. 2013. Substrate specificity of an elongation‐specific peptidoglycan endopeptidase and its implications for cell wall architecture and growth of Vibrio cholerae . Mol. Microbiol. 89: 949–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yamada, S.et al. 1996. An autolysin ring associated with cell separation of Staphylococcus aureus . J. Bacteriol. 178: 1565–1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cloud, K.A. & Dillard J.P.. 2004. Mutation of a single lytic transglycosylase causes aberrant septation and inhibits cell separation of Neisseria gonorrhoeae . J. Bacteriol. 186: 7811–7814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Garcia, D.L. & Dillard J.P.. 2006. AmiC functions as an N‐acetylmuramyl‐l‐alanine amidase necessary for cell separation and can promote autolysis in Neisseria gonorrhoeae . J. Bacteriol. 188: 7211–7221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Heidrich, C.et al. 2001. Involvement of N‐acetylmuramyl‐L‐alanine amidases in cell separation and antibiotic‐induced autolysis of Escherichia coli . Mol. Microbiol. 41: 167–178. [DOI] [PubMed] [Google Scholar]
- 62.Möll, A.et al. 2014. Cell separation in Vibrio cholerae is mediated by a single amidase whose action is modulated by two nonredundant activators. J. Bacteriol. 196: 3937–3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Uehara, T., Parzych K.R., Dinh T. & Bernhardt T.G.. 2010. Daughter cell separation is controlled by cytokinetic ring‐activated cell wall hydrolysis. EMBO J. 29: 1412–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Weaver, A.I.et al. 2019. Lytic transglycosylases RlpA and MltC assist in Vibrio cholerae daughter cell separation. Mol. Microbiol. 112: 1100–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Herlihey, F.A. & Clarke A.J.. 2017. Controlling autolysis during flagella insertion in Gram‐negative bacteria. Adv. Exp. Med. Biol. 925: 41–56. [DOI] [PubMed] [Google Scholar]
- 66.Scheurwater, E., Reid C.W. & Clarke A.J.. 2008. Lytic transglycosylases: bacterial space‐making autolysins. Int. J. Biochem. Cell Biol. 40: 586–591. [DOI] [PubMed] [Google Scholar]
- 67.Dik, D.A., Fisher J.F. & Mobashery S.. 2018. Cell‐wall recycling of the Gram‐negative bacteria and the nexus to antibiotic resistance. Chem. Rev. 118: 5952–5984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mainardi, J.L., Villet R., Bugg T.D., et al. 2008. Evolution of peptidoglycan biosynthesis under the selective pressure of antibiotics in Gram‐positive bacteria. FEMS Microbiol. Rev. 32: 386–408. [DOI] [PubMed] [Google Scholar]
- 69.Dik, D.A., Marous D.R., Fisher J.F. & Mobashery S.. 2017. Lytic transglycosylases: concinnity in concision of the bacterial cell wall. Crit. Rev. Biochem. Mol. Biol. 52: 503–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ghosh, A.S., Chowdhury C. & Nelson D.E.. 2008. Physiological functions of D‐alanine carboxypeptidases in Escherichia coli . Trends Microbiol. 16: 309–317. [DOI] [PubMed] [Google Scholar]
- 71.Priyadarshini, R., Popham D.L. & Young K.D.. 2006. Daughter cell separation by penicillin‐binding proteins and peptidoglycan amidases in Escherichia coli . J. Bacteriol. 188: 5345–5355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Heidrich, C., Ursinus A., Berger J., et al. 2002. Effects of multiple deletions of murein hydrolases on viability, septum cleavage, and sensitivity to large toxic molecules in Escherichia coli . J. Bacteriol. 184: 6093–6099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Herlihey, F.A., Moynihan P.J. & Clarke A.J.. 2014. The essential protein for bacterial flagella formation FlgJ functions as a β‐N‐acetylglucosaminidase. J. Biol. Chem. 289: 31029–31042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Geiger, T., Pazos M., Lara‐Tejero M., et al. 2018. Peptidoglycan editing by a specific LD‐transpeptidase controls the muramidase‐dependent secretion of typhoid toxin. Nat. Microbiol. 3: 1243–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nelson, D.E. & Young K.D.. 2001. Contributions of PBP 5 and DD‐carboxypeptidase penicillin binding proteins to maintenance of cell shape in Escherichia coli . J. Bacteriol. 183: 3055–3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Potluri, L.P., de Pedro M.A. & Young K.D.. 2012. Escherichia coli low‐molecular‐weight penicillin‐binding proteins help orient septal FtsZ, and their absence leads to asymmetric cell division and branching. Mol. Microbiol. 84: 203–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Potluri, L.et al. 2010. Septal and lateral wall localization of PBP5, the major D,D‐carboxypeptidase of Escherichia coli, requires substrate recognition and membrane attachment. Mol. Microbiol. 77: 300–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Peters, K.et al. 2016. The redundancy of peptidoglycan carboxypeptidases ensures robust cell shape maintenance in Escherichia coli . mBio 7. 10.1128/mBio.00819-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Meisner, J.et al. 2013. FtsEX is required for CwlO peptidoglycan hydrolase activity during cell wall elongation in Bacillus subtilis . Mol. Microbiol. 89: 1069–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dominguez‐Cuevas, P., Porcelli I., Daniel R.A. & Errington J.. 2013. Differentiated roles for MreB‐actin isologues and autolytic enzymes in Bacillus subtilis morphogenesis. Mol. Microbiol. 89: 1084–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shin, J.H.et al. 2020. Structural basis of peptidoglycan endopeptidase regulation. Proc. Natl. Acad. Sci. USA 117: 11692–11702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Singh, S.K., Parveen S., SaiSree L. & Reddy M.. 2015. Regulated proteolysis of a cross‐link‐specific peptidoglycan hydrolase contributes to bacterial morphogenesis. Proc. Natl. Acad. Sci. USA 112: 10956–10961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cross, T.et al. 2019. Spheroplast‐mediated carbapenem tolerance in Gram‐negative pathogens. Antimicrob. Agents Chemother. 63: e00756‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mercier, R., Kawai Y. & Errington J.. 2014. General principles for the formation and proliferation of a wall‐free (L‐form) state in bacteria. elife 3: e04629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zou, J.et al. 2020. Non‐walled spherical Acinetobacter baumannii is an important type of persister upon β‐lactam antibiotic treatment. Emerg. Microbes Infect. 9: 1149–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Claessen, D. & Errington J.. 2019. Cell wall deficiency as a coping strategy for stress. Trends Microbiol. 27: 1025–1033. [DOI] [PubMed] [Google Scholar]
- 87.Monahan, L.G.et al. 2014. Rapid conversion of Pseudomonas aeruginosa to a spherical cell morphotype facilitates tolerance to carbapenems and penicillins but increases susceptibility to antimicrobial peptides. Antimicrob. Agents Chemother. 58: 1956–1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tomasz, A.1974. The role of autolysins in cell death. Ann. N.Y. Acad. Sci. 235: 439–447. [DOI] [PubMed] [Google Scholar]
- 89.Kocaoglu, O. & Carlson E.E.. 2015. Profiling of β‐lactam selectivity for penicillin‐binding proteins in Escherichia coli strain DC2. Antimicrob. Agents Chemother. 59: 2785–2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Curtis, N.A., Orr D., Ross G.W. & Boulton M.G.. 1979. Affinities of penicillins and cephalosporins for the penicillin‐binding proteins of Escherichia coli K‐12 and their antibacterial activity. Antimicrob. Agents Chemother. 16: 533–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sutaria, D.S.et al. 2018. First penicillin‐binding protein occupancy patterns of β‐lactams and β‐lactamase inhibitors in Klebsiella pneumoniae . Antimicrob. Agents Chemother. 62: e00282‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kocaoglu, O., Tsui H.C., Winkler M.E. & Carlson E.E.. 2015. Profiling of β‐lactam selectivity for penicillin‐binding proteins in Streptococcus pneumoniae D39. Antimicrob. Agents Chemother. 59: 3548–3555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chambers, H.F. & Sachdeva M.. 1990. Binding of beta‐lactam antibiotics to penicillin‐binding proteins in methicillin‐resistant Staphylococcus aureus . J. Infect. Dis. 161: 1170–1176. [DOI] [PubMed] [Google Scholar]
- 94.Satta, G.et al. 1995. Target for bacteriostatic and bactericidal activities of beta‐lactam antibiotics against Escherichia coli resides in different penicillin‐binding proteins. Antimicrob. Agents Chemother. 39: 812–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dörr, T.et al. 2014. Differential requirement for PBP1a and PBP1b in in vivo and in vitro fitness of Vibrio cholerae . Infect. Immun. 82: 2115–2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chambers, H.F., Sachdeva M. & Kennedy S.. 1990. Binding affinity for penicillin‐binding protein 2a correlates with in vivo activity of beta‐lactam antibiotics against methicillin‐resistant Staphylococcus aureus . J. Infect. Dis. 162: 705–710. [DOI] [PubMed] [Google Scholar]
- 97.Moreillon, P., Markiewicz Z., Nachman S. & Tomasz A.. 1990. Two bactericidal targets for penicillin in pneumococci: autolysis‐dependent and autolysis‐independent killing mechanisms. Antimicrob. Agents Chemother. 34: 33–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Horne, D. & Tomasz A.. 1977. Tolerant response of Streptococcus sanguis to beta‐lactams and other cell wall inhibitors. Antimicrob. Agents Chemother. 11: 888–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cho, H., Uehara T. & Bernhardt T.G.. 2014. Beta‐lactam antibiotics induce a lethal malfunctioning of the bacterial cell wall synthesis machinery. Cell 159: 1300–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Meisel, U., Holtje J.V. & Vollmer W.. 2003. Overproduction of inactive variants of the murein synthase PBP1B causes lysis in Escherichia coli . J. Bacteriol. 185: 5342–5348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Delcour, A.H.2009. Outer membrane permeability and antibiotic resistance. Biochim. Biophys. Acta 1794: 808–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Walsh, C.1999. Deconstructing vancomycin. Science 284: 442–443. [DOI] [PubMed] [Google Scholar]
- 103.Patti, G.J.et al. 2009. Vancomycin and oritavancin have different modes of action in Enterococcus faecium . J. Mol. Biol. 392: 1178–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Schneider, T. & Sahl H.G.. 2010. An oldie but a goodie — cell wall biosynthesis as antibiotic target pathway. Int. J. Med. Microbiol. 300: 161–169. [DOI] [PubMed] [Google Scholar]
- 105.Fang, X.et al. 2006. The mechanism of action of ramoplanin and enduracidin. Mol. Biosyst. 2: 69–76. [DOI] [PubMed] [Google Scholar]
- 106.Culp, E.J.et al. 2020. Evolution‐guided discovery of antibiotics that inhibit peptidoglycan remodelling. Nature 578: 582–587. [DOI] [PubMed] [Google Scholar]
- 107.Blaskovich, M.A.T.et al. 2018. Developments in glycopeptide antibiotics. ACS Infect. Dis. 4: 715–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sastry, S. & Doi Y.. 2016. Fosfomycin: resurgence of an old companion. J. Infect. Chemother. 22: 273–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Silver, L.L.2017. Fosfomycin: mechanism and resistance. Cold Spring Harb. Perspect. Med. 7: a025262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lambert, M.P. & Neuhaus F.C.. 1972. Mechanism of D‐cycloserine action: alanine racemase from Escherichia coli W. J. Bacteriol. 110: 978–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Stone, K.J. & Strominger J.L.. 1971. Mechanism of action of bacitracin: complexation with metal ion and C 55‐isoprenyl pyrophosphate. Proc. Natl. Acad. Sci. USA 68: 3223–3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ling, L.L.et al. 2015. A new antibiotic kills pathogens without detectable resistance. Nature 517: 455–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Homma, T.et al. 2016. Dual targeting of cell wall precursors by teixobactin leads to cell lysis. Antimicrob. Agents Chemother. 60: 6510–6517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Shukla, R.et al. 2020. Mode of action of teixobactins in cellular membranes. Nat. Commun. 11: 2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rogers, H.J.1967. Killing of staphylococci by penicillins. Nature 213: 31–33. [Google Scholar]
- 116.Prestidge, L.S. & Pardee A.B.. 1957. Induction of bacterial lysis by penicillin. J. Bacteriol. 74: 48–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Giudicelli, S. & Tomasz A.. 1984. Attachment of pneumococcal autolysin to wall teichoic acids, an essential step in enzymatic wall degradation. J. Bacteriol. 158: 1188–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Flores‐Kim, J., Dobihal G.S., Fenton A., et al. 2019. A switch in surface polymer biogenesis triggers growth‐phase‐dependent and antibiotic‐induced bacteriolysis. elife 8: e44912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sanchez‐Puelles, J.M.et al. 1986. Searching for autolysin functions. Characterization of a pneumococcal mutant deleted in the lytA gene. Eur. J. Biochem. 158: 289–293. [DOI] [PubMed] [Google Scholar]
- 120.Tomasz, A., Albino A. & Zanati E.. 1970. Multiple antibiotic resistance in a bacterium with suppressed autolytic system. Nature 227: 138–140. [DOI] [PubMed] [Google Scholar]
- 121.Takahashi, J.et al. 2002. Molecular characterization of an atl null mutant of Staphylococcus aureus . Microbiol. Immunol. 46: 601–612. [DOI] [PubMed] [Google Scholar]
- 122.Schlag, M.et al. 2010. Role of staphylococcal wall teichoic acid in targeting the major autolysin Atl. Mol. Microbiol. 75: 864–873. [DOI] [PubMed] [Google Scholar]
- 123.Kitano, K., Tuomanen E. & Tomasz A.. 1986. Transglycosylase and endopeptidase participate in the degradation of murein during autolysis of Escherichia coli . J. Bacteriol. 167: 759–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kohlrausch, U. & Holtje J.V.. 1991. Analysis of murein and murein precursors during antibiotic‐induced lysis of Escherichia coli . J. Bacteriol. 173: 3425–3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Dwyer, D.J., Kohanski M.A. & Collins J.J.. 2009. Role of reactive oxygen species in antibiotic action and resistance. Curr. Opin. Microbiol. 12: 482–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Keren, I., Wu Y., Inocencio J., et al. 2013. Killing by bactericidal antibiotics does not depend on reactive oxygen species. Science 339: 1213–1216. [DOI] [PubMed] [Google Scholar]
- 127.Liu, Y. & Imlay J.A.. 2013. Cell death from antibiotics without the involvement of reactive oxygen species. Science 339: 1210–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Shin, J.H.et al. 2019. A multifaceted cellular damage repair and prevention pathway promotes high level tolerance to β lactam antibiotics. 10.1101/777375 [DOI] [PMC free article] [PubMed]
- 129.Belenky, P.et al. 2015. Bactericidal antibiotics induce toxic metabolic perturbations that lead to cellular damage. Cell Rep. 13: 968–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Haas, W., Kaushal D., Sublett J., et al. 2005. Vancomycin stress response in a sensitive and a tolerant strain of Streptococcus pneumoniae . J. Bacteriol. 187: 8205–8210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Tran, T.D.et al. 2011. Decrease in penicillin susceptibility due to heat shock protein ClpL in Streptococcus pneumoniae . Antimicrob. Agents Chemother. 55: 2714–2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Maiques, E.et al. 2006. Beta‐lactam antibiotics induce the SOS response and horizontal transfer of virulence factors in Staphylococcus aureus . J. Bacteriol. 188: 2726–2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Miller, C.et al. 2004. SOS response induction by beta‐lactams and bacterial defense against antibiotic lethality. Science 305: 1629–1631. [DOI] [PubMed] [Google Scholar]
- 134.McDowell, T.D. & Reed K.E.. 1989. Mechanism of penicillin killing in the absence of bacterial lysis. Antimicrob. Agents Chemother. 33: 1680–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Geiger, T., Kastle B., Gratani F.L., et al. 2014. Two small (p)ppGpp synthases in Staphylococcus aureus mediate tolerance against cell envelope stress conditions. J. Bacteriol. 196: 894–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Mueller, E.A., Egan A.J., Breukink E., et al. 2019. Plasticity of Escherichia coli cell wall metabolism promotes fitness and antibiotic resistance across environmental conditions. elife 8: e40754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Radlinski, L. & Conlon B.P.. 2018. Antibiotic efficacy in the complex infection environment. Curr. Opin. Microbiol. 42: 19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Goodell, E.W., Lopez R. & Tomasz A.. 1976. Suppression of lytic effect of beta lactams on Escherichia coli and other bacteria. Proc. Natl. Acad. Sci. USA 73: 3293–3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Venglarcik, J.S., 3rd, Blair L.L. & Dunkle L.M.. 1983. pH‐dependent oxacillin tolerance of Staphylococcus aureus . Antimicrob. Agents Chemother. 23: 232–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Tuomanen, E. & Tomasz A.. 1984. Protection by D‐amino acids against growth inhibition and lysis caused by beta‐lactam antibiotics. Antimicrob. Agents Chemother. 26: 414–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Radlinski, L.et al. 2017. Pseudomonas aeruginosa exoproducts determine antibiotic efficacy against Staphylococcus aureus . PLoS Biol. 15: e2003981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hunt‐Serracin, A.C., Parks B.J., Boll J. & Boutte C.C.. 2019. Mycobacterium abscessus cells have altered antibiotic tolerance and surface glycolipids in artificial cystic fibrosis sputum medium. Antimicrob. Agents Chemother. 63: e02488‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chung, H.S.et al. 2009. Rapid beta‐lactam‐induced lysis requires successful assembly of the cell division machinery. Proc. Natl. Acad. Sci. USA 106: 21872–21877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.de Pedro, M.A., Holtje J.V. & Schwarz H.. 2002. Fast lysis of Escherichia coli filament cells requires differentiation of potential division sites. Microbiology 148: 79–86. [DOI] [PubMed] [Google Scholar]
- 145.Yao, Z., Kahne D. & Kishony R.. 2012. Distinct single‐cell morphological dynamics under beta‐lactam antibiotics. Mol. Cell 48: 705–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Lee, A.J.et al. 2018. Robust, linear correlations between growth rates and β‐lactam‐mediated lysis rates. Proc. Natl. Acad. Sci. USA 115: 4069–4074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Tuomanen, E., Cozens R., Tosch W., et al. 1986. The rate of killing of Escherichia coli by beta‐lactam antibiotics is strictly proportional to the rate of bacterial growth. J. Gen. Microbiol. 132: 1297–1304. [DOI] [PubMed] [Google Scholar]
- 148.Held, K.et al. 2018. Determinants of extreme β‐lactam tolerance in the Burkholderia pseudomallei complex. Antimicrob. Agents Chemother. 62. 10.1128/AAC.00068-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Pontes, M.H. & Groisman E.A.. 2019. Slow growth determines nonheritable antibiotic resistance in Salmonella enterica . Sci. Signal. 12: eaax3938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ito, A., Taniuchi A., May T., et al. 2009. Increased antibiotic resistance of Escherichia coli in mature biofilms. Appl. Environ. Microbiol. 75: 4093–4100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Pontes, M.H. & Groisman E.A.. 2020. A physiological basis for nonheritable antibiotic resistance. mBio 11. 10.1128/mBio.00817-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kim, K.S. & Anthony B.F.. 1981. Importance of bacterial growth phase in determining minimal bactericidal concentrations of penicillin and methicillin. Antimicrob. Agents Chemother. 19: 1075–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kahl, B.C., Becker K. & Loffler B.. 2016. Clinical significance and pathogenesis of staphylococcal small colony variants in persistent infections. Clin. Microbiol. Rev. 29: 401–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Gold, B. & Nathan C.. 2017. Targeting phenotypically tolerant Mycobacterium tuberculosis . Microbiol. Spectr. 5. 10.1128/microbiolspec.TBTB2-0031-2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Brown, S.A., Palmer K.L. & Whiteley M.. 2008. Revisiting the host as a growth medium. Nat. Rev. Microbiol. 6: 657–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Nowakowska, J., Landmann R. & Khanna N.. 2014. Foreign body infection models to study host–pathogen response and antimicrobial tolerance of bacterial biofilm. Antibiotics (Basel) 3: 378–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Chuard, C.et al. 1991. Resistance of Staphylococcus aureus recovered from infected foreign body in vivo to killing by antimicrobials. J. Infect. Dis. 163: 1369–1373. [PubMed] [Google Scholar]
- 158.Helaine, S.et al. 2014. Internalization of Salmonella by macrophages induces formation of nonreplicating persisters. Science 343: 204–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Haaber, J.et al. 2015. Reversible antibiotic tolerance induced in Staphylococcus aureus by concurrent drug exposure. mBio 6: e02268‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Jawetz, E. & Speck R.S.. 1950. Joint action of penicillin with chloramphenicol on an experimental streptococcal infection of mice. Proc. Soc. Exp. Biol. Med. 74: 93–96. [DOI] [PubMed] [Google Scholar]
- 161.Haas, W., Sublett J., Kaushal D. & Tuomanen E.I.. 2004. Revising the role of the pneumococcal vex‐vncRS locus in vancomycin tolerance. J. Bacteriol. 186: 8463–8471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Novak, R., Henriques B., Charpentier E., et al. 1999. Emergence of vancomycin tolerance in Streptococcus pneumoniae . Nature 399: 590–593. [DOI] [PubMed] [Google Scholar]
- 163.Robertson, G.T.et al. 2002. Vancomycin tolerance induced by erythromycin but not by loss of vncRS, vex3, or pep27 function in Streptococcus pneumoniae . J. Bacteriol. 184: 6987–7000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Wu, X.et al. 2018. Identifying vancomycin as an effective antibiotic for killing Borrelia burgdorferi . Antimicrob. Agents Chemother. 62: e01201‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Weaver, A.I.et al. 2018. Genetic determinants of penicillin tolerance in Vibrio cholerae . Antimicrob. Agents Chemother. 62: e01326‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Dörr, T.et al. 2016. A cell wall damage response mediated by a sensor kinase/response regulator pair enables beta‐lactam tolerance. Proc. Natl. Acad. Sci. USA 113: 404–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Fung, D.K., Chan E.W., Chin M.L. & Chan R.C.. 2010. Delineation of a bacterial starvation stress response network which can mediate antibiotic tolerance development. Antimicrob. Agents Chemother. 54: 1082–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Lopatkin, A.J.et al. 2019. Bacterial metabolic state more accurately predicts antibiotic lethality than growth rate. Nat. Microbiol. 4: 2109–2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Tuomanen, E.1986. Phenotypic tolerance: the search for beta‐lactam antibiotics that kill nongrowing bacteria. Rev. Infect. Dis. 8(Suppl. 3): S279–S291. [DOI] [PubMed] [Google Scholar]
- 170.Tuomanen, E. & Schwartz J.. 1987. Penicillin‐binding protein 7 and its relationship to lysis of nongrowing Escherichia coli . J. Bacteriol. 169: 4912–4915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Tuomanen, E. & Tomasz A.. 1986. Induction of autolysis in nongrowing Escherichia coli . J. Bacteriol. 167: 1077–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Mainardi, J.L.et al. 2007. Unexpected inhibition of peptidoglycan LD‐transpeptidase from Enterococcus faecium by the beta‐lactam imipenem. J. Biol. Chem. 282: 30414–30422. [DOI] [PubMed] [Google Scholar]
- 173.Lavollay, M.et al. 2008. The peptidoglycan of stationary‐phase Mycobacterium tuberculosis predominantly contains cross‐links generated by L,D‐transpeptidation. J. Bacteriol. 190: 4360–4366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Baranowski, C.et al. 2018. Maturing Mycobacterium smegmatis peptidoglycan requires non‐canonical crosslinks to maintain shape. elife 7: e37516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Zhu, M., Pan Y. & Dai X.. 2019. (P)ppGpp: the magic governor of bacterial growth economy. Curr. Genet. 65: 1121–1125. [DOI] [PubMed] [Google Scholar]
- 176.Traxler, M.F.et al. 2008. The global, ppGpp‐mediated stringent response to amino acid starvation in Escherichia coli . Mol. Microbiol. 68: 1128–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Ferullo, D.J. & Lovett S.T.. 2008. The stringent response and cell cycle arrest in Escherichia coli . PLoS Genet. 4: e1000300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Kusser, W. & Ishiguro E.E.. 1985. Involvement of the relA gene in the autolysis of Escherichia coli induced by inhibitors of peptidoglycan biosynthesis. J. Bacteriol. 164: 861–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Zhu, M. & Dai X.. 2019. Growth suppression by altered (p)ppGpp levels results from non‐optimal resource allocation in Escherichia coli . Nucleic Acids Res. 47: 4684–4693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Lederberg, J. & Zinder N.. 1948. Concentration of biochemical mutants of bacteria with penicillin. J. Am. Chem. Soc. 70: 4267. [DOI] [PubMed] [Google Scholar]
- 181.Davis, B.D.1948. Isolation of biochemically deficient mutants of bacteria by penicillin. J. Am. Chem. Soc. 70: 4267. [DOI] [PubMed] [Google Scholar]
- 182.Hobbs, J.K. & Boraston A.B.. 2019. (p)ppGpp and the stringent response: an emerging threat to antibiotic therapy. ACS Infect. Dis. 5: 1505–1517. [DOI] [PubMed] [Google Scholar]
- 183.Germain, E.et al. 2019. YtfK activates the stringent response by triggering the alarmone synthetase SpoT in Escherichia coli . Nat. Commun. 10: 5763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Abranches, J.et al. 2009. The molecular alarmone (p)ppGpp mediates stress responses, vancomycin tolerance, and virulence in Enterococcus faecalis . J. Bacteriol. 191: 2248–2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Tuomanen, E. & Tomasz A.. 1990. Mechanism of phenotypic tolerance of nongrowing pneumococci to beta‐lactam antibiotics. Scand. J. Infect. Dis. Suppl. 74: 102–112. [PubMed] [Google Scholar]
- 186.Kusser, W., Pisabarro A.G., De Pedro M.A. & Ishiguro E.E.. 1990. Decay of the ampicillin‐induced lysis process in amino acid‐deprived Escherichia coli . Antimicrob. Agents Chemother. 34: 164–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Pisabarro, A.G., De Pedro M.A. & Ishiguro E.E.. 1990. Dissociation of the ampicillin‐induced lysis of amino acid‐deprived Escherichia coli into two stages. J. Bacteriol. 172: 2187–2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Rodionov, D.G. & Ishiguro E.E.. 1995. Direct correlation between overproduction of guanosine 3′,5′‐bispyrophosphate (ppGpp) and penicillin tolerance in Escherichia coli . J. Bacteriol. 177: 4224–4229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Bryson, D., Hettle A.G., Boraston A.B. & Hobbs J.K.. 2020. Clinical mutations that partially activate the stringent response confer multidrug tolerance in Staphylococcus aureus . Antimicrob. Agents Chemother. 64. 10.1128/aac.02103-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Rodionov, D.G. & Ishiguro E.E.. 1996. Effects of inhibitors of protein synthesis on lysis of Escherichia coli induced by beta‐lactam antibiotics. Antimicrob. Agents Chemother. 40: 899–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Irving, S.E. & Corrigan R.M.. 2018. Triggering the stringent response: signals responsible for activating (p)ppGpp synthesis in bacteria. Microbiology 164: 268–276. [DOI] [PubMed] [Google Scholar]
- 192.Ishiguro, E.E. & Ramey W.D.. 1976. Stringent control of peptidoglycan biosynthesis in Escherichia coli K‐12. J. Bacteriol. 127: 1119–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Ramey, W.D. & Ishiguro E.E.. 1978. Site of inhibition of peptidoglycan biosynthesis during the stringent response in Escherichia coli . J. Bacteriol. 135: 71–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Ehlert, K. & Holtje J.V.. 1996. Role of precursor translocation in coordination of murein and phospholipid synthesis in Escherichia coli . J. Bacteriol. 178: 6766–6771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Tuomanen, E., Markiewicz Z. & Tomasz A.. 1988. Autolysis‐resistant peptidoglycan of anomalous composition in amino‐acid‐starved Escherichia coli . J. Bacteriol. 170: 1373–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Hugonnet, J.E.et al. 2016. Factors essential for L,D‐transpeptidase‐mediated peptidoglycan cross‐linking and β‐lactam resistance in Escherichia coli . elife 5: e19469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Rodionov, D.G. & Ishiguro E.E.. 1996. Dependence of peptidoglycan metabolism on phospholipid synthesis during growth of Escherichia coli . Microbiology 142(Pt 10): 2871–2877. [DOI] [PubMed] [Google Scholar]
- 198.Sinha, A.K., Winther K.S., Roghanian M. & Gerdes K.. 2019. Fatty acid starvation activates RelA by depleting lysine precursor pyruvate. Mol. Microbiol. 112: 1339–1349. [DOI] [PubMed] [Google Scholar]
- 199.Battesti, A. & Bouveret E.. 2006. Acyl carrier protein/SpoT interaction, the switch linking SpoT‐dependent stress response to fatty acid metabolism. Mol. Microbiol. 62: 1048–1063. [DOI] [PubMed] [Google Scholar]
- 200.Cronan, J.E., Jr. & Bell R.M.. 1974. Mutants of Escherichia coli defective in membrane phospholipid synthesis: mapping of sn‐glycerol 3‐phosphate acyltransferase Km mutants. J. Bacteriol. 120: 227–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Heath, R.J., Jackowski S. & Rock C.O.. 1994. Guanosine tetraphosphate inhibition of fatty acid and phospholipid synthesis in Escherichia coli is relieved by overexpression of glycerol‐3‐phosphate acyltransferase (plsB). J. Biol. Chem. 269: 26584–26590. [PubMed] [Google Scholar]
- 202.Lai, G.C., Cho H. & Bernhardt T.G.. 2017. The mecillinam resistome reveals a role for peptidoglycan endopeptidases in stimulating cell wall synthesis in Escherichia coli . PLoS Genet. 13: e1006934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Tomasz, A. & de Vegvar M.L.. 1986. Construction of a penicillin‐tolerant laboratory mutant of Staphylococcus aureus . Eur. J. Clin. Microbiol. 5: 710–713. [DOI] [PubMed] [Google Scholar]
- 204.Sabath, L.D., Wheeler N., Laverdiere M., et al. 1977. A new type of penicillin resistance of Staphylococcus aureus . Lancet 1: 443–447. [DOI] [PubMed] [Google Scholar]
- 205.Corrigan, R.M., Bellows L.E., Wood A. & Grundling A.. 2016. ppGpp negatively impacts ribosome assembly affecting growth and antimicrobial tolerance in Gram‐positive bacteria. Proc. Natl. Acad. Sci. USA 113: E1710–E1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Singh, M.et al. 2017. In vitro tolerance of drug‐naive Staphylococcus aureus strain FDA209P to vancomycin. Antimicrob. Agents Chemother. 61: e01154‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Kim, K.S., Morrison J.O. & Bayer A.S.. 1982. Deficient autolytic enzyme activity in antibiotic‐tolerant lactobacilli. Infect. Immun. 36: 582–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Best, G.K., Best N.H. & Koval A.V.. 1974. Evidence for participation of autolysins in bactericidal action of oxacillin on Staphylococcus aureus . Antimicrob. Agents Chemother. 6: 825–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Chung, M., Borges V., Gomes J.P., et al. 2018. Phenotypic signatures and genetic determinants of oxacillin tolerance in a laboratory mutant of Staphylococcus aureus . PLoS One 13: e0199707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Rogers, H.J. & Forsberg C.W.. 1971. Role of autolysins in the killing of bacteria by some bactericidal antibiotics. J. Bacteriol. 108: 1235–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Filipe, S.R., Severina E. & Tomasz A.. 2002. The murMN operon: a functional link between antibiotic resistance and antibiotic tolerance in Streptococcus pneumoniae . Proc. Natl. Acad. Sci. USA 99: 1550–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Hilty, M.D., Venglarcik J.S. & Best G.K.. 1980. Oxacillin‐tolerant staphylococcal bacteremia in children. J. Pediatr. 96: 1035–1037. [DOI] [PubMed] [Google Scholar]
- 213.Groicher, K.H., Firek B.A., Fujimoto D.F. & Bayles K.W.. 2000. The Staphylococcus aureus lrgAB operon modulates murein hydrolase activity and penicillin tolerance. J. Bacteriol. 182: 1794–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Rice, K.C.et al. 2003. The Staphylococcus aureus cidAB operon: evaluation of its role in regulation of murein hydrolase activity and penicillin tolerance. J. Bacteriol. 185: 2635–2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Ranjit, D.K., Endres J.L. & Bayles K.W.. 2011. Staphylococcus aureus CidA and LrgA proteins exhibit holin‐like properties. J. Bacteriol. 193: 2468–2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Rice, K.C.et al. 2007. The cidA murein hydrolase regulator contributes to DNA release and biofilm development in Staphylococcus aureus . Proc. Natl. Acad. Sci. USA 104: 8113–8118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Poole, K.2012. Bacterial stress responses as determinants of antimicrobial resistance. J. Antimicrob. Chemother. 67: 2069–2089. [DOI] [PubMed] [Google Scholar]
- 218.Hews, C.L., Cho T., Rowley G. & Raivio T.L.. 2019. Maintaining integrity under stress: envelope stress response regulation of pathogenesis in Gram‐negative bacteria. Front. Cell. Infect. Microbiol. 9. 10.3389/fcimb.2019.00313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Murakami, K.et al. 2005. Role for rpoS gene of Pseudomonas aeruginosa in antibiotic tolerance. FEMS Microbiol. Lett. 242: 161–167. [DOI] [PubMed] [Google Scholar]
- 220.Mitchell, A.M., Wang W. & Silhavy T.J.. 2017. Novel RpoS‐dependent mechanisms strengthen the envelope permeability barrier during stationary phase. J. Bacteriol. 199. 10.1128/JB.00708-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Martins, D.et al. 2018. Superoxide dismutase activity confers (p)ppGpp‐mediated antibiotic tolerance to stationary‐phase Pseudomonas aeruginosa . Proc. Natl. Acad. Sci. USA 115: 9797–9802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Cheng, A.T., Ottemann K.M. & Yildiz F.H.. 2015. Vibrio cholerae response regulator VxrB controls colonization and regulates the type VI secretion system. PLoS Pathog. 11: e1004933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Teschler, J.K., Cheng A.T. & Yildiz F.H.. 2017. The two‐component signal transduction system VxrAB positively regulates Vibrio cholerae biofilm formation. J. Bacteriol. 199: e00139‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Wall, E., Majdalani N. & Gottesman S.. 2018. The complex Rcs regulatory cascade. Annu. Rev. Microbiol. 72: 111–139. [DOI] [PubMed] [Google Scholar]
- 225.Ranjit, D.K. & Young K.D.. 2013. The Rcs stress response and accessory envelope proteins are required for de novo generation of cell shape in Escherichia coli . J. Bacteriol. 195: 2452–2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Salzberg, L.I.et al. 2013. The WalRK (YycFG) and sigma(I) RsgI regulators cooperate to control CwlO and LytE expression in exponentially growing and stressed Bacillus subtilis cells. Mol. Microbiol. 87: 180–195. [DOI] [PubMed] [Google Scholar]
- 227.Salzberg, L.I. & Helmann J.D.. 2007. An antibiotic‐inducible cell wall‐associated protein that protects Bacillus subtilis from autolysis. J. Bacteriol. 189: 4671–4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Dobihal, G.S., Brunet Y.R., Flores‐Kim J. & Rudner D.Z.. 2019. Homeostatic control of cell wall hydrolysis by the WalRK two‐component signaling pathway in Bacillus subtilis . elife 8: e52088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Libby, E.A., Goss L.A. & Dworkin J.. 2015. The eukaryotic‐like Ser/Thr kinase PrkC regulates the essential WalRK two‐component system in Bacillus subtilis . PLoS Genet. 11: e1005275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Bugrysheva, J., Froehlich B.J., Freiberg J.A. & Scott J.R.. 2011. Serine/threonine protein kinase Stk is required for virulence, stress response, and penicillin tolerance in Streptococcus pyogenes . Infect. Immun. 79: 4201–4209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Zahner, D.et al. 2002. The ciaR/ciaH regulatory network of Streptococcus pneumoniae . J. Mol. Microbiol. Biotechnol. 4: 211–216. [PubMed] [Google Scholar]
- 232.Mascher, T., Heintz M., Zahner D., et al. 2006. The CiaRH system of Streptococcus pneumoniae prevents lysis during stress induced by treatment with cell wall inhibitors and by mutations in pbp2x involved in beta‐lactam resistance. J. Bacteriol. 188: 1959–1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Halfmann, A., Kovacs M., Hakenbeck R. & Bruckner R.. 2007. Identification of the genes directly controlled by the response regulator CiaR in Streptococcus pneumoniae: five out of 15 promoters drive expression of small non‐coding RNAs. Mol. Microbiol. 66: 110–126. [DOI] [PubMed] [Google Scholar]
- 234.Moscoso, M., Domenech M. & Garcia E.. 2010. Vancomycin tolerance in clinical and laboratory Streptococcus pneumoniae isolates depends on reduced enzyme activity of the major LytA autolysin or cooperation between CiaH histidine kinase and capsular polysaccharide. Mol. Microbiol. 77: 1052–1064. [DOI] [PubMed] [Google Scholar]
- 235.Liu, X.et al. 2017. Transcriptional repressor PtvR regulates phenotypic tolerance to vancomycin in Streptococcus pneumoniae . J. Bacteriol. 199. 10.1128/JB.00054-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Dwyer, D.J.et al. 2014. Antibiotics induce redox‐related physiological alterations as part of their lethality. Proc. Natl. Acad. Sci. USA 111: E2100–E2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Bizzini, A., Zhao C., Auffray Y. & Hartke A.. 2009. The Enterococcus faecalis superoxide dismutase is essential for its tolerance to vancomycin and penicillin. J. Antimicrob. Chemother. 64: 1196–1202. [DOI] [PubMed] [Google Scholar]
- 238.Nguyen, D.et al. 2011. Active starvation responses mediate antibiotic tolerance in biofilms and nutrient‐limited bacteria. Science 334: 982–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Rowe, S.E.et al. 2020. Reactive oxygen species induce antibiotic tolerance during systemic Staphylococcus aureus infection. Nat. Microbiol. 5: 282–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Wu, Y., Vulic M., Keren I. & Lewis K.. 2012. Role of oxidative stress in persister tolerance. Antimicrob. Agents Chemother. 56: 4922–4926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Cardoso, K.et al. 2010. DnaK and GroEL are induced in response to antibiotic and heat shock in Acinetobacter baumannii . J. Med. Microbiol. 59: 1061–1068. [DOI] [PubMed] [Google Scholar]
- 242.Kaldalu, N., Mei R. & Lewis K.. 2004. Killing by ampicillin and ofloxacin induces overlapping changes in Escherichia coli transcription profile. Antimicrob. Agents Chemother. 48: 890–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Utaida, S.et al. 2003. Genome‐wide transcriptional profiling of the response of Staphylococcus aureus to cell‐wall‐active antibiotics reveals a cell‐wall‐stress stimulon. Microbiology 149: 2719–2732. [DOI] [PubMed] [Google Scholar]
- 244.Powell, J.K. & Young K.D.. 1991. Lysis of Escherichia coli by beta‐lactams which bind penicillin‐binding proteins 1a and 1b: inhibition by heat shock proteins. J. Bacteriol. 173: 4021–4026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Errington, J.2017. Cell wall‐deficient, L‐form bacteria in the 21st century: a personal perspective. Biochem. Soc. Trans. 45: 287–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Mercier, R., Kawai Y. & Errington J.. 2013. Excess membrane synthesis drives a primitive mode of cell proliferation. Cell 152: 997–1007. [DOI] [PubMed] [Google Scholar]
- 247.Leaver, M., Dominguez‐Cuevas P., Coxhead J.M., et al. 2009. Life without a wall or division machine in Bacillus subtilis . Nature 457: 849–853. [DOI] [PubMed] [Google Scholar]
- 248.Horii, T., Kobayashi M., Sato K., et al. 1998. An in‐vitro study of carbapenem‐induced morphological changes and endotoxin release in clinical isolates of gram‐negative bacilli. J. Antimicrob. Chemother. 41: 435–442. [DOI] [PubMed] [Google Scholar]
- 249.Mickiewicz, K.M.et al. 2019. Possible role of L‐form switching in recurrent urinary tract infection. Nat. Commun. 10: 4379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Roberts, D., Higgs E., Rutman A. & Cole P.. 1984. Isolation of spheroplastic forms of Haemophilus influenzae from sputum in conventionally treated chronic bronchial sepsis using selective medium supplemented with N‐acetyl‐D‐glucosamine: possible reservoir for re‐emergence of infection. Br. Med. J. (Clin. Res. Ed.) 289: 1409–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Rhee, K.Y. & Gardiner D.F.. 2004. Clinical relevance of bacteriostatic versus bactericidal activity in the treatment of Gram‐positive bacterial infections. Clin. Infect. Dis. 39: 755–756. [DOI] [PubMed] [Google Scholar]
- 252.Pankey, G.A. & Sabath L.D.. 2004. Clinical relevance of bacteriostatic versus bactericidal mechanisms of action in the treatment of Gram‐positive bacterial infections. Clin. Infect. Dis. 38: 864–870. [DOI] [PubMed] [Google Scholar]
- 253.Denny, A.E., Peterson L.R., Gerding D.N. & Hall W.H.. 1979. Serious staphylococcal infections with strains tolerant to bactericidal antibiotics. Arch. Intern. Med. 139: 1026–1031. [PubMed] [Google Scholar]
- 254.CLSI . 2020. Performance standards for antimicrobial susceptibility testing. CLSI supplement M100. 30th ed. Wayne, PA: Clinical and Laboratory Standards Institute. [Google Scholar]
- 255.Band, V.I. & Weiss D.S.. 2019. Heteroresistance: a cause of unexplained antibiotic treatment failure? PLoS Pathog. 15: e1007726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Gomes, D.M., Ward K.E. & LaPlante K.L.. 2015. Clinical implications of vancomycin heteroresistant and intermediately susceptible Staphylococcus aureus . Pharmacotherapy 35: 424–432. [DOI] [PubMed] [Google Scholar]
- 257.Fantin, B., Leggett J., Ebert S. & Craig W.A.. 1991. Correlation between in vitro and in vivo activity of antimicrobial agents against gram‐negative bacilli in a murine infection model. Antimicrob. Agents Chemother. 35: 1413–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Widmer, A.F., Frei R., Rajacic Z. & Zimmerli W.. 1990. Correlation between in vivo and in vitro efficacy of antimicrobial agents against foreign body infections. J. Infect. Dis. 162: 96–102. [DOI] [PubMed] [Google Scholar]
- 259.Lowy, F.D., Neuhaus E.G., Chang D.S. & Steigbigel N.H.. 1983. Penicillin therapy of experimental endocarditis induced by tolerant Streptococcus sanguis and nontolerant Streptococcus mitis . Antimicrob. Agents Chemother. 23: 67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Goessens, W.H., Fontijne P. & Michel M.F.. 1984. Responses of tolerant and nontolerant Staphylococcus aureus strains to methicillin treatment in an experimental infection in mice. Antimicrob. Agents Chemother. 26: 829–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Peeters, P.et al. 2019. The impact of initial antibiotic treatment failure: real‐world insights in patients with complicated, health care‐associated intra‐abdominal infection. Infect. Drug Resist. 12: 329–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Meylan, S., Andrews I.W. & Collins J.J.. 2018. Targeting antibiotic tolerance, pathogen by pathogen. Cell 172: 1228–1238. [DOI] [PubMed] [Google Scholar]
- 263.Vaudaux, P. & Lew D.P.. 2006. Tolerance of staphylococci to bactericidal antibiotics. Injury 37(Suppl. 2): S15–S19. [DOI] [PubMed] [Google Scholar]
- 264.Gopal, V., Bisno A.L. & Silverblatt F.J.. 1976. Failure of vancomycin treatment in Staphylococcus aureus endocarditis. In vivo and in vitro observations. JAMA 236: 1604–1606. [PubMed] [Google Scholar]
- 265.Kaplan, E.L. & Johnson D.R.. 2001. Unexplained reduced microbiological efficacy of intramuscular benzathine penicillin G and of oral penicillin V in eradication of group a streptococci from children with acute pharyngitis. Pediatrics 108: 1180–1186. [DOI] [PubMed] [Google Scholar]
- 266.Rodriguez, C.A.et al. 2004. Tolerance to vancomycin in pneumococci: detection with a molecular marker and assessment of clinical impact. J. Infect. Dis. 190: 1481–1487. [DOI] [PubMed] [Google Scholar]
- 267.Henriques Normark, B.et al. 2001. Clinical isolates of Streptococcus pneumoniae that exhibit tolerance of vancomycin. Clin. Infect. Dis. 32: 552–558. [DOI] [PubMed] [Google Scholar]
- 268.Mulcahy, L.R., Burns J.L., Lory S. & Lewis K.. 2010. Emergence of Pseudomonas aeruginosa strains producing high levels of persister cells in patients with cystic fibrosis. J. Bacteriol. 192: 6191–6199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Schumacher, M.A.et al. 2015. HipBA‐promoter structures reveal the basis of heritable multidrug tolerance. Nature 524: 59–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.McCullers, J.A., English B.K. & Novak R.. 2000. Isolation and characterization of vancomycin‐tolerant Streptococcus pneumoniae from the cerebrospinal fluid of a patient who developed recrudescent meningitis. J. Infect. Dis. 181: 369–373. [DOI] [PubMed] [Google Scholar]
- 271.Fernebro, J.et al. 2004. Capsular expression in Streptococcus pneumoniae negatively affects spontaneous and antibiotic‐induced lysis and contributes to antibiotic tolerance. J. Infect. Dis. 189: 328–338. [DOI] [PubMed] [Google Scholar]
- 272.Silva, M.D. & Sillankorva S.. 2019. Otitis media pathogens — a life entrapped in biofilm communities. Crit. Rev. Microbiol. 45: 595–612. [DOI] [PubMed] [Google Scholar]
- 273.Pasticci, M.B.et al. 2011. Bactericidal activity of oxacillin and glycopeptides against Staphylococcus aureus in patients with endocarditis: looking for a relationship between tolerance and outcome. Ann. Clin. Microbiol. Antimicrob. 10. 10.1186/1476-0711-10-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Windels, E.M.et al. 2019. Bacterial persistence promotes the evolution of antibiotic resistance by increasing survival and mutation rates. ISME J. 13: 1239–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Levin‐Reisman, I., Brauner A., Ronin I. & Balaban N.Q.. 2019. Epistasis between antibiotic tolerance, persistence, and resistance mutations. Proc. Natl. Acad. Sci. USA 116: 14734–14739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Liu, J., Gefen O., Ronin I., et al. 2020. Effect of tolerance on the evolution of antibiotic resistance under drug combinations. Science 367: 200–204. [DOI] [PubMed] [Google Scholar]
- 277.Levin‐Reisman, I.et al. 2017. Antibiotic tolerance facilitates the evolution of resistance. Science 355: 826–830. [DOI] [PubMed] [Google Scholar]
- 278.Thi, T.D.et al. 2011. Effect of recA inactivation on mutagenesis of Escherichia coli exposed to sublethal concentrations of antimicrobials. J. Antimicrob. Chemother. 66: 531–538. [DOI] [PubMed] [Google Scholar]
- 279.Gutierrez, A.et al. 2013. β‐Lactam antibiotics promote bacterial mutagenesis via an RpoS‐mediated reduction in replication fidelity. Nat. Commun. 4: 1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Stokes, J.M.et al. 2019. A multiplexable assay for screening antibiotic lethality against drug‐tolerant bacteria. Nat. Methods 16: 303–306. [DOI] [PubMed] [Google Scholar]
- 281.Abel Zur Wiesch, P., Clarelli F. & Cohen T.. 2017. Using chemical reaction kinetics to predict optimal antibiotic treatment strategies. PLoS Comput. Biol. 13: e1005321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Martinecz, A. & Abel Zur Wiesch P.. 2018. Estimating treatment prolongation for persistent infections. Pathog. Dis. 76: 10.1093/femspd/fty065 [DOI] [PMC free article] [PubMed] [Google Scholar]


