Abstract
Riociguat is a first‐in‐class soluble guanylate cyclase stimulator, approved for the treatment of adults with pulmonary arterial hypertension (PAH), inoperable chronic thromboembolic pulmonary hypertension (CTEPH), or persistent or recurrent CTEPH after pulmonary endarterectomy. Approval was based on the results of the phase III PATENT‐1 (PAH) and CHEST‐1 (CTEPH) studies, with significant improvements in the primary endpoint of 6‐minute walk distance vs placebo of +36 m and +46 m, respectively, as well as improvements in secondary endpoints such as pulmonary vascular resistance and World Health Organization functional class. Riociguat acts as a stimulator of cyclic guanosine monophosphate synthesis rather than as an inhibitor of cGMP metabolism. As with other approved therapies for PAH, riociguat has antifibrotic, antiproliferative and anti‐inflammatory effects, in addition to vasodilatory properties. This has led to further clinical studies in patients who do not achieve a satisfactory clinical response with phosphodiesterase type‐5 inhibitors. Riociguat has also been evaluated in patients with World Health Organization group 2 and 3 pulmonary hypertension, and other conditions including diffuse cutaneous systemic sclerosis, Raynaud's phenomenon and cystic fibrosis. This review evaluates the results of the original clinical trials of riociguat for the treatment of PAH and CTEPH, and summarises the body of work that has examined the safety and efficacy of riociguat for the treatment of other types of pulmonary hypertension.
Keywords: drug information, pharmacotherapy, therapeutics
1. INTRODUCTION
Pulmonary hypertension (PH) is a condition that is classified by the World Health Organization (WHO) into 5 categories: group 1 includes pulmonary arterial hypertension (PAH); group 2, PH due to left‐heart disease; group 3, PH due to disorders of the respiratory system and chronic hypoxia; group 4, chronic thromboembolic PH (CTEPH); and group 5, PH with an unclear cause or multifactorial mechanism.1, 2 The term precapillary PH refers to a persistently elevated mean pulmonary arterial pressure (mPAP) of ≥25 mmHg at rest, with pulmonary arterial wedge pressure (PAWP) ≤15 mmHg and pulmonary vascular resistance (PVR) ≥3 Wood units (≥240 dyn·s·cm−5),1 assessed by right heart catheterisation. Recently, a new definition has been proposed: mPAP >20 mmHg and PVR ≥3 Wood units.2
PAH is a rare disease characterised by a proliferative vasculopathy and progressive remodelling of the pulmonary vasculature, leading to increased resistance to blood flow, primarily in the precapillary arterioles in the absence of other causes of precapillary PH such as lung disease, CTEPH or other rare diseases.1, 2, 3
CTEPH is an infrequent but life‐threatening sequela of pulmonary embolism characterised by obstruction of the pulmonary vasculature by organised thromboembolic material, leading to increased PVR and progressive PH.1, 4, 5 In addition to vascular obstruction, CTEPH is associated with a small‐vessel arteriopathy that further contributes to haemodynamic compromise, functional impairment and disease progression.6 The histology of small‐vessel disease in CTEPH is indistinguishable from that observed in PAH.7
Currently, CTEPH is the only form of PH that is potentially curable, with pulmonary endarterectomy (PEA) as the standard of care.1, 4, 8, 9, 10 However, up to 40% of patients are considered inoperable, and up to 51% of patients manifest persistent or recurrent PH after PEA,8, 11, 12, 13, 14, 15, 16, 17 in part because of small‐vessel disease that is not amenable to PEA. Such patients may require other treatments, namely medical therapy or balloon pulmonary angioplasty (BPA).
Patients with PAH or CTEPH experience debilitating symptoms including dyspnoea, fatigue, palpitations, chest pain, syncope, abdominal distension and lower extremity oedema.1 The chronic increases in right ventricular (RV) afterload and wall stress lead to RV remodelling. RV function can be maintained for a time, but ultimately maladaptive remodelling results in progressive RV dilatation, compromised function and, if untreated, RV failure.1, 9, 18
Endothelial dysfunction of the pulmonary vasculature plays a central role in the progression of PAH and is characterised by reduced production of vasodilators, such as nitric oxide (NO) and prostacyclin, and upregulation of vasoconstrictors, such as endothelin‐1.9, 19, 20, 21 Four types of targeted medical therapy are approved for PAH1, 22 (Figure 1): (i) prostacyclin analogues (e.g. epoprostenol, treprostinil and iloprost) and the prostaglandin I2 receptor agonist selexipag, which stimulate cyclic adenosine monophosphate production; (ii) endothelin receptor antagonists (ERAs; e.g. bosentan, ambrisentan and macitentan), which block endothelin receptors and mitigate the effects of excess endothelin; (iii) phosphodiesterase type‐5 inhibitors (PDE5i, e.g. sildenafil and tadalafil), which target the NO and natriuretic pathways; and (iv) the soluble guanylate cyclase (sGC) stimulator riociguat, which also targets the NO pathway.
FIGURE 1.
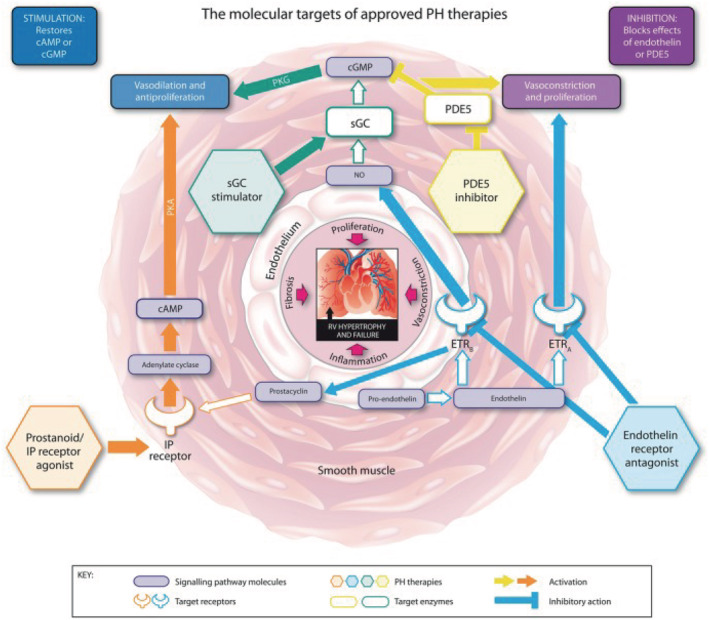
Key signalling pathways targeted by medical therapies for pulmonary hypertension (PH). Reproduced under a CC BY‐NC 4.0 license from Humbert et al.22 cAMP, cyclic adenosine monophosphate; cGMP, cyclic guanosine monophosphate; ETRA, endothelin receptor A; ETRB, endothelin receptor B; IP, prostacyclin; NO, nitric oxide; PDE5, phosphodiesterase type 5; PKA, phosphate kinase A; PKG, cGMP‐dependent protein kinase; RV, right ventricular; sGC, soluble guanylate cyclase
The latter 2 therapies enhance the biological effects of NO and natriuretic peptides, delaying degradation or enhancing the synthesis of cyclic guanosine monophosphate (cGMP; a secondary messenger of NO and natriuretic peptides, which mediates most of their biological properties in the pulmonary circulation). Phosphodiesterase type‐5 is the main enzyme responsible for the metabolism of cGMP in pulmonary vascular smooth muscle, and inhibition of this enzyme has been an effective approach for treating PAH. In the pulmonary circulation, NO produced by endothelial cells diffuses into vascular smooth muscle and binds to sGC, stimulating the enzyme and increasing cGMP synthesis.19, 20 However, NO synthesis may be deficient in patients with PAH,19 and could lead to insufficient cGMP signalling, despite inhibition of cGMP metabolism by PDE5i. This may explain why some patients do not achieve treatment goals with PDE5i therapy,23, 24 and may indicate that there is room for optimisation of therapy targeting the cGMP pathway. Riociguat has a dual mode of action that increases cGMP production by directly stimulating sGC via an NO‐independent binding site and by enhancing NO‐induced activation of sGC3, 19, 20, 25, 26, 27, 28 (Figure 2). In addition to being approved for the treatment of PAH, riociguat is the only medication currently approved for the treatment of CTEPH, with approvals in the USA and the EU, as well as many other countries including Canada, Australia and Japan.
FIGURE 2.
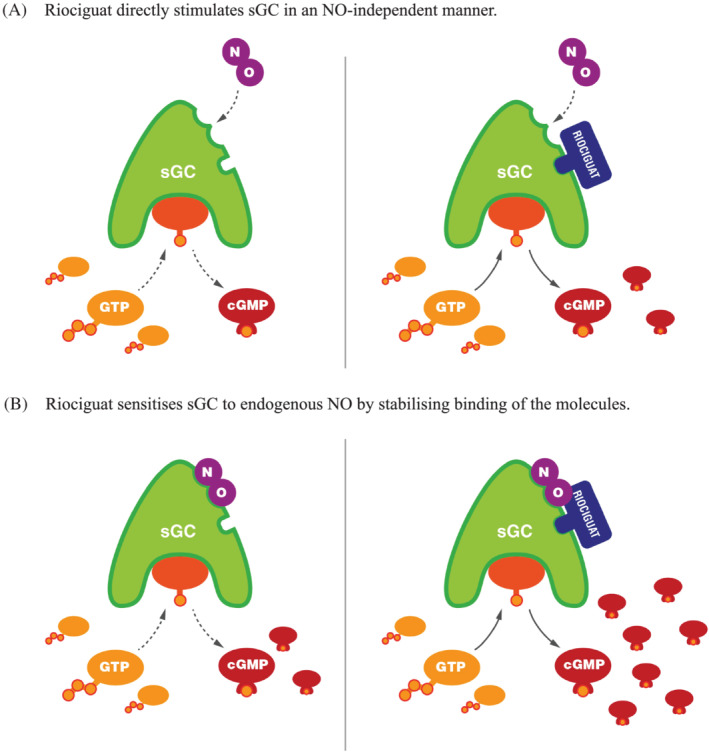
Mechanism of action of riociguat.29 (A) Riociguat directly stimulates soluble guanylate cyclase (sGC) in a nitric oxide (NO)‐independent manner. (B) Riociguat sensitises sGC to endogenous NO by stabilising binding of the molecules. Reproduced under a CC BY 4.0 license from Benza et al.29 cGMP, cyclic guanylate monophosphate; GTP, guanosine triphosphate
This review provides an overview of the data that led to the approval of riociguat for the treatment of PAH and CTEPH, and more recent data from studies examining the effects of switching from PDE5is to riociguat in PAH. We also review clinical trials of riociguat for the treatment of PH due to chronic heart and lung disease, and examine the efficacy of riociguat for the treatment of other clinical conditions. Finally, we discuss ongoing and future studies.
1.1. Preclinical data
sGC stimulators and activators have been shown to reverse PH, RV hypertrophy and pulmonary vascular remodelling in animal models,19, 20, 30, 31, 32 and riociguat and other sGC stimulators also have antifibrotic, antiproliferative and anti‐inflammatory effects.20, 33, 34, 35 Moreover, sGC stimulators and activators inhibit vascular smooth muscle cell proliferation and migration,36 and hypertrophy of cardiac myocytes, in vitro.37 The pulmonary vascular remodelling seen in PAH is associated with hyperproliferative/proinflammatory responses in pulmonary vascular endothelial cells, smooth muscle cells and fibroblasts. Furthermore, much of the RV failure that occurs in PAH is caused by maladaptive RV hypertrophic responses. Thus, the properties of sGC identified in these preclinical studies made them attractive candidates for treating PAH.
1.2. Phase I and II in PAH and CTEPH
In a single‐dose, phase I trial, riociguat reduced mean arterial blood pressure (BP) at 1 mg and 5 mg in 58 healthy male volunteers.38 In a subsequent single‐dose proof‐of‐concept study in 19 patients (12 with PAH, 6 with CTEPH and 1 with PH associated with interstitial lung disease [ILD]),39 riociguat (1 mg or 2.5 mg) caused a significant dose‐dependent reduction in mPAP and increased cardiac output, resulting in a significant decrease in PVR. The pulmonary haemodynamic effects were greater than that of inhaled NO given at 10–20 ppm for 10 minutes. In this short study, the drug was well tolerated, but the haemodynamic effects of riociguat were not selective for the pulmonary circulation and resulted in similar degrees of reduction in mean arterial pressure and systemic vascular resistance (SVR).
A multicentre, open‐label, uncontrolled, 12‐week, phase II study enrolled 33 patients with PAH and 42 patients with inoperable CTEPH (Table 1).28
TABLE 1.
Overview of completed riociguat clinical trials
| Study design | Patients | PAH‐specific therapy at baseline | Riociguat dose | Primary endpoint | |
|---|---|---|---|---|---|
| 12‐wk study and LTE28 | PAH (n = 33) | Bosentan (n = 6) | 1–2.5 mg tid |
(n = 75) AEs were reported in 65 (87%) patients; 42 (56%) were study drug related; 3 (4%) patients discontinued due to AEs. 96% of study drug‐related AEs considered mild or moderate. Most common AEs reported were dyspepsia, headache and hypotension. |
|
| CTEPH (n = 42) | |||||
| 6MWD (m), mean ± SD | |||||
| 12‐wk, double‐blind study (PATENT‐1) and open‐label extension study (PATENT‐2)40, 41, 42 | PAH (n = 443) |
ERAs (n = 194) Prostanoids (n = 28) None (n = 221) |
1.5 mg (max) or 2.5 mg (max) tid |
Placebo (n = 126) BL: 368 ± 75 Week 12 change from BL: −6 ± 86 |
Riociguat 2.5 mg – maximum (n = 254) BL: 361 ± 68 Week 12 change from BL: 30 ± 66 |
| Placebo‐corrected LS mean difference (95% CI): 36 (20–52), P < .001 | |||||
| 6MWD (m), mean ± SD | |||||
| 16‐wk, double‐blind study (CHEST‐1) and open‐label extension study (CHEST‐2)43, 44, 45 | CTEPH (n = 261) | None | 2.5 mg (max) tid |
Placebo (n = 88) BL: 356 ± 75 Week 16 change from BL: −6 ± 84 |
Riociguat ≤2.5 mg (n = 173) BL: 342 ± 82 Week 16 change from BL: 39 ± 79 |
| Placebo‐corrected LS mean difference (95% CI): 46 (25–67), P < .001 | |||||
| Supine SBP (mmHg), mean ± SD | |||||
| 12‐wk study and LTE (PATENT PLUS)46 | PAH (n = 18) | Sildenafil (n = 18) | 2.5 mg (max) tid | Placebo (n = 6) | Riociguat (n = 12) |
| BL: −7.6 ± 3.9 | BL: −20.2 ± 15.3 | ||||
| Week 12: −20.2 ± 12.9 | Week 12: −20.7 ± 18.0 | ||||
| 6MWD (m), mean ± SD; exploratory | |||||
| 24‐wk study (RESPITE)47, 48 | PAH (n = 61) | ERAs (n = 50) | 1–2.5 mg |
BL (n = 61): 357 ± 81 Week 24 change from BL (n = 51): 31 ± 63 |
|
| 95% CI: 13–49, P = .001 | |||||
| mPAP (mmHg), mean ± SD | |||||
| 16‐wk study (LEPHT)49 | PH associated with HFrEF (n = 201) | None | 0.5 mg, 1 mg or 2 mg tid |
Placebo (n = 56) BL: 40.4 ± 1.2 Week 16: 36.4 ± 1.4 |
Riociguat 2 mg (n = 54) BL: 38.1 ± 1.3 Week 16: 32.0 ± 1.6 |
| Placebo‐corrected LS mean difference (95% CI): −2.7 (−6.0–0.6), P = .10 | |||||
| mPAP (mmHg), mean ± SD | |||||
| Single‐dose study (DILATE)50 | PH associated with HFpEF (n = 39) | None | 0.5 mg, 1 mg or 2 mg |
Placebo (n = 11) BL: 34.9 ± 8.0 Peak change from BL: −6.3 ± 4.2 |
Riociguat 2 mg (n = 10) BL: 35.1 ± 8.8 Peak change from BL: −5.1 ± 4.7 |
| Difference vs placebo (95% CI): 1.2 ± 4.4 (−2.9–5.2), P = .60 | |||||
| 12‐wk pilot study (with 12‐month LTE)51 | PH‐ILD (n = 22) | None | 2.5 mg (max) tid |
104 AEs were reported across 22 patients; 86 of these treatment‐emergent; 70% considered drug related. 3 patients discontinued due to study drug. Most common AEs were dyspnoea (n = 6), peripheral oedema (n = 6), dyspepsia (n = 3), headache (n = 3) and feeling hot (n = 3). 25 SAEs were experienced by 16 patients; 8 SAEs were considered possibly study drug related (syncope, dyspnoea, pancytopenia, respiratory disorder and respiratory failure). |
|
| Single‐dose study52 | PH‐COPD (n = 23) | None | 1 mg or 2.5 mg | mPAP (mmHg), peak postbaseline effect, mean ± SD; exploratory | |
|
iNO 20 ppm (n = 8) −3.88 ± 2.90 |
Riociguat 2.5 mg (n = 12) −4.8 ± 4.17 |
||||
| P = .002 | |||||
| PVR (dyn·s·cm −5 ), peak postbaseline effect, mean ± SD; exploratory | |||||
|
iNO 20 ppm (n = 7) −57.14 ± 78.64 |
Riociguat 2.5 mg (n = 11) −123.80 ± 73.53 |
||||
| P = .0002 | |||||
| Digital blood flow (mean ± SD) | |||||
| Single‐dose study (DIGIT)53 | Raynaud's phenomenon (n = 20) | None | 2 mg |
Room temperature Placebo (n = 20): −5% (59) Riociguat (n = 20): +41% (109) |
Cold exposure Placebo (n = 10): +25% (114) Riociguat (n = 10): +15% (51) |
| 26‐wk study (RISE‐IIP) and LTE54 | Idiopathic interstitial pneumonias (n = 147) | None | 2.5 mg (max) tid | Change in 6MWD from baseline to week 26 (m) | |
| Placebo‐corrected LS mean difference (95% CI): +21 m (−9 to +52), P = .2074 | |||||
| The study was terminated during the LTE due to increased SAEs and mortality and an unfavourable risk:benefit ratio. | |||||
| 52‐wk study (RISE‐SSc)55 | Diffuse cutaneous systemic sclerosis (n = 121) | None | 2.5 mg (max) tid | Change in mRSS from baseline to week 52 (mean ± SD) | |
|
Placebo (n = 61) BL: 16.71 ± 4.06 Week 52: 15.73 ± 10.48 |
Riociguat (n = 60) BL: 16.88 ± 3.38 Week 52: 14.63 ± 6.56 |
||||
| Placebo‐corrected LS mean treatment difference (95% CI): −2.34 (−4.99–0.30), P = .08 | |||||
| Two‐part study (Rio‐CF)56, 57, 58 | Phe508del homozygous CF (n = 21) | None | Part 1: 0.5 mg tid for 14 days, then 1.0 mg tid for 14 days | Change in sweat chloride content in part 1 (n = 16 available for this analysis) | |
|
Placebo (n = 7) Day 14 (0.5 mg): +8.7 ± 8.2 mmol/L Day 28 (1.0 mg): +9.0 ± 12.7 mmol/L |
Riociguat (n = 9) Day 14 (0.5 mg): +7.1 ± 10.3 mmol/L Day 28 (1.0 mg): 3.4 ± 11.0 mmol/L |
||||
6MWD, 6‐min walking distance; AE, adverse event; BL, baseline; CF, cystic fibrosis; CI, confidence interval; COPD, chronic obstructive pulmonary disease; CTEPH, chronic thromboembolic pulmonary hypertension; ERA, endothelin receptor antagonist; HFpEF, heart failure with a preserved ejection fraction; HFrEF, heart failure with a reduced ejection fraction; iNO, inhaled nitric oxide; ILD, interstitial lung disease; LS, least squares; LTE, long‐term extension; max, maximum; mPAP, mean pulmonary artery pressure; mRSS, modified Rodnan Skin Score; PAH, pulmonary arterial hypertension; PH, pulmonary hypertension; PVR, pulmonary vascular resistance; SAE, serious adverse event; SBP, systolic blood pressure; SD, standard deviation; tid, 3 times daily.
The primary endpoints were safety and tolerability. In total, 87% of patients experienced adverse events (AEs), which were considered to be related to study treatment in 56% of patients. Overall, riociguat was generally well tolerated and was discontinued in only 3 patients (4%): 1 with pulmonary oedema, considered to be related to riociguat due to unmasking of pulmonary venous occlusive disease; 1 with progressive right heart failure unrelated to riociguat; and 1 (a patient with a history of multiple allergies) with drug‐related exanthema. Dyspepsia, headache and asymptomatic hypotension were the most common AEs, but only 2 of the 11 patients with hypotension (18%) required dose reduction. Secondary endpoints showed a significant increase from baseline to Week 12 in median 6‐minute walking distance (6MWD), improvement in WHO functional class (FC) and a reduction in PVR. In the open‐label long‐term extension (LTE) of the phase II trial, 68 of the 75 patients (91%) were followed for a median treatment period of 77 months.59 The most common AEs were nasopharyngitis (57%) and peripheral oedema (37%). Three patients (4%) had haemoptysis, including 1 severe event, but none were considered drug related. At 48 months, 6MWD increased from baseline by 69 ± 105 m. Three‐year survival was 91% and clinical worsening‐free survival was 49%.
PATENT PLUS (NCT01179334) was a phase II, randomised, double‐blind, placebo‐controlled study to assess the safety and efficacy of riociguat in combination with sildenafil in patients with PAH (Table 1).46 Eighteen patients with PAH receiving sildenafil 20 mg 3 times daily (tid) were randomised 2:1 to receive riociguat or placebo, with a primary endpoint of change in systolic BP at 12 weeks. No significant effect on BP was observed, and there were no signs of favourable effects on pulmonary haemodynamics or exercise capacity. One patient withdrew due to blurry vision that was considered drug related. The other 17 patients were entered into an LTE study and followed for an average of 305 days. In the LTE, 9 (53%) patients reported serious AEs (SAEs) and 3 patients (18%) died due to SAEs (subdural haematoma following a fall, cardiac arrest and right heart failure), which were considered by the investigator not to be drug related. There were also high rates of discontinuation due to hypotension in the LTE; 3 (18%) AEs and 1 (6%) SAE of arterial hypotension considered to be related to the study drug by the investigator led to discontinuation. Due to these unfavourable safety signals and no evidence of a positive risk:benefit ratio, the concomitant use of riociguat and a PDE5i is contraindicated.60, 61
2. PIVOTAL PHASE III: PAH
2.1. PATENT‐1
PATENT‐1 was a phase III, 12‐week, double‐blind, randomised, placebo‐controlled study (NCT00810693; Table 1).40 A total of 443 patients with symptomatic PAH entered the study and were randomised to receive placebo (n = 126), riociguat individually adjusted to a maximum of 2.5 mg tid (n = 254) or a maximum of 1.5 mg tid (n = 63; exploratory dose). In PATENT‐1, of the 254 patients in the riociguat 2.5 mg tid group, 48% were treatment‐naïve and 52% were pretreated with an ERA (44%) or nonintravenous prostanoid (7%). Fifty‐two percent of patients in the placebo group were treatment‐naïve and 48% were pretreated with an ERA (43%) or nonintravenous prostanoid (5%).
The primary endpoint was change from baseline to Week 12 in 6MWD. At the end of the treatment period, 6MWD increased by 30 m in the 2.5 mg tid riociguat group and decreased by 6 m in the placebo group (least squares mean difference between riociguat and placebo +36 m [95% confidence interval {CI}: 20–52; P < .001]). Significant improvements vs placebo were also seen in PVR, N‐terminal prohormone of brain natriuretic peptide (NT‐proBNP), WHO FC, time to clinical worsening, Borg dyspnoea score and quality of life measured by the Living with Pulmonary Hypertension (LPH) questionnaire.62 In a post hoc analysis of PATENT‐1, riociguat improved the proportion of patients achieving a number of clinically relevant responder thresholds.63
Based on the results of PATENT‐1, riociguat was approved for the treatment of patients with PAH to improve exercise capacity and WHO FC, and to delay clinical worsening.60, 61
2.2. PATENT‐2
Of the 405 patients who completed PATENT‐1, 396 (98%) enrolled in the LTE PATENT‐2 (NCT00863681) in which all patients received open‐label riociguat up to a maximum of 2.5 mg tid.41, 42 Of the 396 patients entering PATENT‐2, 199 (50%) patients were also receiving an ERA or a prostanoid, or both.
More than 80% of patients achieved the 2.5 mg tid maximum dose by Week 8, and 86% remained on this dose at 1 year. The median treatment duration was 139 weeks. At the final data cut‐off, 275 patients (69%) remained in the study. Overall, 340 patients (86%) received ≥2 years of riociguat treatment.
SAEs were recorded in 238 patients (60%) and drug‐related AEs were seen in 232 patients (59%). Most SAEs were not considered drug related. Forty‐five patients (11%) discontinued treatment due to ≥1 AE. There were 13 AEs of haemoptysis and pulmonary haemorrhage, 2 of which were fatal (1 in PATENT‐1 and 1 in PATENT‐2). These findings raised concern that the 3 cases of haemoptysis reported in the LTE of the phase II trial may have been drug related, even if reported not to be at the time of study. The functional improvements seen in PATENT‐1 were sustained at 2 years (Figure 3). Patients randomised to placebo in PATENT‐1 who were switched to riociguat in PATENT‐2 showed improvements in efficacy parameters comparable with those formerly randomised to riociguat.41, 42
FIGURE 3.
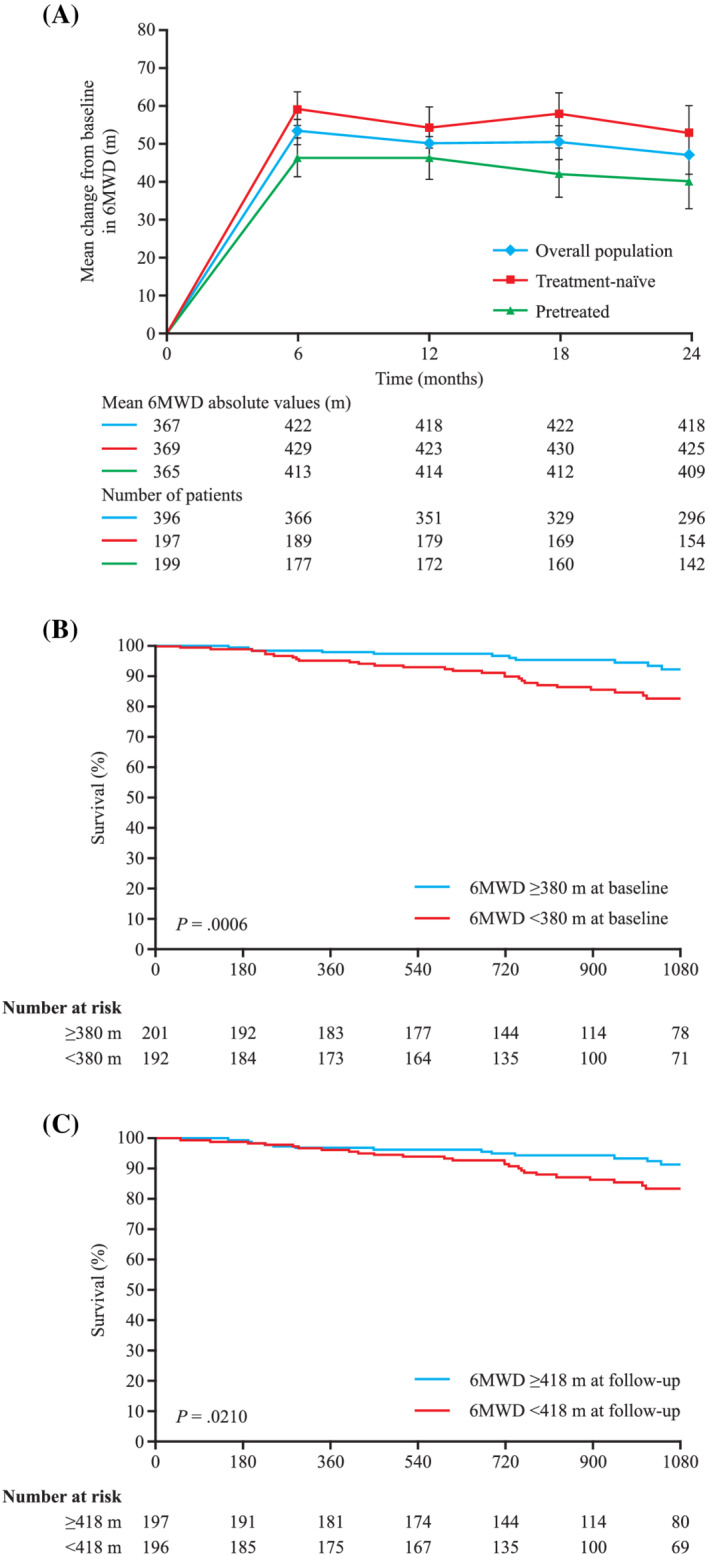
Six‐minute walking distance (6MWD) in PATENT‐2.41 (A) 6MWD in the overall population, and treatment‐naïve and pretreated subgroups of PATENT‐2. Graph shows mean ± standard error of the mean. (B) Kaplan–Meier analysis showing the association of 6MWD with survival based on median value at baseline. (C) Kaplan–Meier analysis showing the association of 6MWD with survival at follow‐up. Reprinted with permission from Elsevier from Ghofrani et al.41
The changes in 6MWD and WHO FC in PATENT‐1 and PATENT‐2 were comparable between treatment‐naïve patients, and patients receiving ERAs and prostanoids (Table 2).40, 68 The estimated overall survival at 2 years was 93% (95% CI: 90–95), and the rates of clinical worsening‐free survival were 84% (95% CI: 80–87) and 73% (95% CI: 68–77) at 1 and 2 years, respectively, assuming that patients who withdrew had experienced clinical worsening.41 Post hoc subgroup analyses from PATENT‐1 and PATENT‐2 revealed that riociguat was effective in the subgroups of patients with PAH associated with surgically corrected congenital heart disease (n = 35)69 and connective tissue disease (n = 111).70
TABLE 2.
Six‐minute walking distance (6MWD), N‐terminal prohormone of brain natriuretic peptide (NT‐proBNP) and World Health Organization functional class (WHO FC) in patients who were treatment‐naïve vs pretreated in PATENT studies, and patients who had inoperable chronic thromboembolic pulmonary hypertension and persistent/recurrent pulmonary hypertension in CHEST studies
| 6MWD (m), mean ± SD | NT‐proBNP (pg/mL), mean ± SD | WHO FC (improved/stabilised/worsened) | ||||
|---|---|---|---|---|---|---|
| Treatment‐naïve | Pretreated | Treatment‐naïve | Pretreated | Treatment‐naïve | Pretreated | |
| PATENT‐140, 64 | +38 (16–60)a (n = 123) | +34 (11–56)a (n = 131) | −443 ± 1233 (n = 113) | +43 ± 2071 (n = 115) | 15/80/4 (n = 123) | 26/71/3 (n = 131) |
| PATENT‐2 65 | +53 ± 84 (n = 154) | +40 ± 86 (n = 142) | −291 ± 1626 (n = 104) | +19 ± 1553 (n = 92) | 28/63/9 (n = 158) | 37/54/9 (n = 148) |
| Inoperable | Persistent/recurrent | Inoperable | Persistent/recurrent | Inoperable | Persistent/recurrent | |
| CHEST‐143, 66 | +44 ± 84 (n = 121) | +27 ± 68 (n = 52) | −364 ± 1868 (n = 108) | −102 ± 1247 (n = 42) | 31/64/5 (n = 121) | 38/56/6 (n = 52) |
| CHEST‐2 67 | +51 ± 70 (n = 119) | +45 ± 61 (n = 43) | −351 ± 1308 (n = 60) | −194 ± 816 (n = 20) | 38/59/2 (n = 128) | 43/52/5 (n = 42) |
CI, confidence interval; SD, standard deviation.
Data show improvement from baseline in efficacy endpoints.
Placebo‐corrected change (95% CI).
2.3. Patient risk assessment in the PATENT studies
A variety of risk assessment tools using clinical, echocardiographic, functional and haemodynamic parameters have been designed to predict outcomes in patients with PAH.1 Current European Society of Cardiology/European Respiratory Society (ESC/ERS) guidelines recommend that treatment of PAH should aim to achieve and maintain a low‐risk status as defined by 6MWD >440 m, WHO FC I or II, and NT‐proBNP <300 ng/L, right atrial pressure <8 mmHg, cardiac index ≥2.5 L/min/m2 and other criteria,1 and the role of risk stratification and the importance of achieving low‐risk status was highlighted in the recent proceedings of the 6th World Symposium on Pulmonary Hypertension.71 A post hoc analysis of PATENT‐1 showed that more patients achieved all low‐risk criteria at Week 12 with riociguat than with placebo (12 vs 5%). While the proportions of patients who met all criteria were small in both groups, those who met these criteria had better survival and clinical worsening‐free survival than those who did not.72
Recently, abbreviated versions of this ESC/ERS risk assessment tool were found to discriminate between prognostic groups in patients with newly diagnosed PAH in the French Pulmonary Hypertension Registry, Swedish Pulmonary Hypertension Association Registry and the Comparative, Prospective Registry of Newly Initiated Therapies for Pulmonary Hypertension.73, 74, 75, 76 A post hoc analysis applying these methods to the PATENT studies showed that with each of the models assessed, riociguat improved risk status at Week 12.77 Achieving 1 or more low‐risk criterion using the French noninvasive method or a low‐risk status, according to the SPAHR/COMPERA method, at Week 12 of PATENT‐1 conferred a significantly reduced risk of death or clinical worsening in PATENT‐2.
The REVEAL risk score (RRS) uses statistical modelling of validated parameters to assess the risk of 1‐year mortality in patients with PAH.78, 79, 80 Post hoc assessment of the PATENT database found that RRS and risk strata between baseline and PATENT‐1 Week 12 were significantly improved with riociguat treatment when compared with placebo. In PATENT‐1, 48% of patients treated with riociguat had an improved RRS (vs 39% with placebo) and 19% had a worsened RRS (vs 27% with placebo); the proportion of patients in the low‐risk stratum (score 1–7) increased from 58 to 71% with riociguat (and from 61 to 64% with placebo) and the proportion of patients in the high‐risk stratum (score ≥9) decreased from 23 to 16% (compared with a slight increase from 15 to 19% with placebo). RRS at baseline and Week 12, and change in RRS during PATENT‐1, were associated with improved survival (hazard ratios [HRs] for a 1‐point reduction in RRS: 0.675, 0.705 and 0.804, respectively) and clinical worsening‐free survival (HRs: 0.736, 0.716 and 0.753, respectively) over 2 years in PATENT‐2.81
3. PIVOTAL PHASE III: CTEPH
3.1. CHEST‐1
CHEST‐1 was a 16‐week, double‐blind, randomised, placebo‐controlled, phase III trial in treatment‐naïve patients with inoperable CTEPH (72%) or persistent/recurrent PH after PEA (28%; NCT00855465; Table 1).43 A total of 261 patients were enrolled, and randomised to receive placebo (n = 88) or riociguat individually adjusted to a maximum of 2.5 mg tid (n = 173).
The study met its primary endpoint of change in 6MWD from baseline to Week 16, with a mean increase of +39 m in the maximum riociguat 2.5 mg group and a decrease of −6 m in the placebo group (least squares mean difference +46 m [95% CI: 25–67]; P < .001). Riociguat significantly improved PVR, NT‐proBNP and WHO FC, as well as exploratory haemodynamic endpoints including mPAP and cardiac output. However, no significant difference was seen in the incidence of clinical worsening82 and, therefore, due to hierarchical statistical methodology, although Borg dyspnoea score, EuroQoL‐5D and LPH also improved, significance could not be claimed.
Riociguat improved primary and secondary endpoints in patients with inoperable CTEPH and patients with persistent/recurrent PH vs baseline, although a more marked effect was seen in inoperable patients69 (Table 2). In a post hoc analysis of PATENT‐1, riociguat improved the proportion of patients achieving several clinically relevant responder thresholds.83
Based on positive results from CHEST‐1, riociguat was approved for the treatment of inoperable CTEPH or persistent/recurrent PH after PEA, and remains the only approved medical treatment for this disease.60, 61
3.2. CHEST‐2
Of the 243 patients who completed CHEST‐1, 237 (98%) entered the open‐label CHEST‐2 LTE (NCT00910429) and received riociguat adjusted as tolerated up to a maximum dose of 2.5 mg tid.44, 45 At 1 year, 90% of patients were on the maximum dose of 2.5 mg tid. The median treatment duration was 116 weeks and 73% of patients had inoperable CTEPH, while 27% had persistent/recurrent PH following PEA. At the final data cut‐off, 172 patients (73%) remained in the study. Overall, 218 patients (90%) received treatment for >1 year and 147 (62%) received treatment for ≥2 years. Fifteen patients (10%) who were treated for ≥2 years were also receiving other PAH‐specific medications. The primary endpoints were safety and tolerability.
SAEs were reported in 129 patients (54%). The most common SAEs were syncope, hypotension, worsening PH and RV failure. The overall incidence of haemoptysis/pulmonary haemorrhage was 4 in 237 patients (2%; exposure‐adjusted rate: 0.7 cases per 100 patient‐years).
The improvement in 6MWD seen with riociguat in CHEST‐1 was sustained at 2 years in both inoperable and persistent/recurrent patients, but was more pronounced in the inoperable group (Figure 4; Table 2). Patients treated with placebo in CHEST‐1 also showed improvement in 6MWD after conversion to open‐label treatment with riociguat in CHEST‐2 (mean increase in 6MWD:C 36 ± 71 m).44 Estimated 2‐year survival was 93% (95% CI: 89–96), and the rates of clinical worsening‐free survival were 86% (95% CI: 81–90) and 78% (95% CI: 73–83) at 1 and 2 years, respectively, assuming that patients who withdrew had experienced clinical worsening.45 Higher 6MWD and lower NT‐proBNP values at baseline were associated with better overall survival and clinical worsening‐free survival in CHEST‐2. The change from baseline in 6MWD was also significantly associated with overall survival and clinical worsening‐free survival at 2 years (Figure 4).45
FIGURE 4.
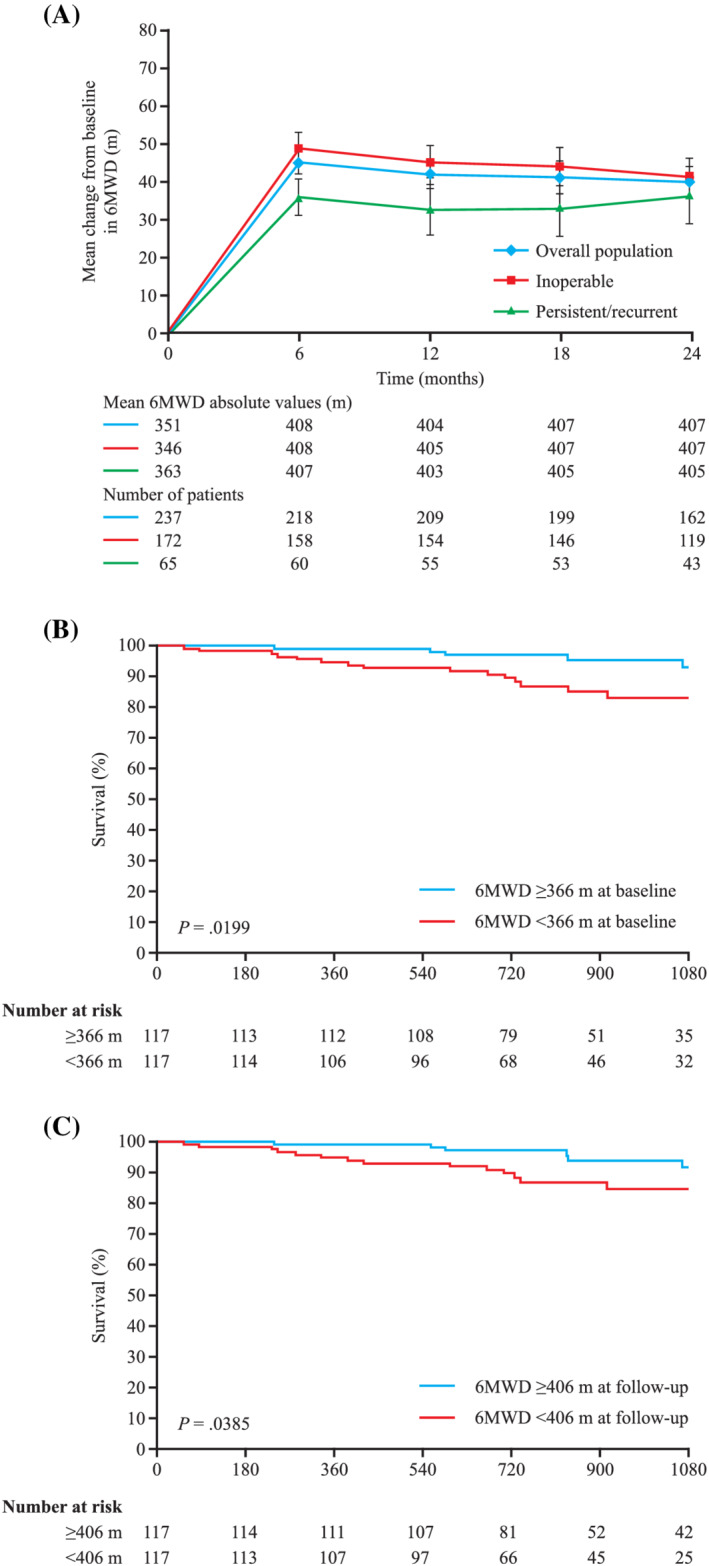
Six‐minute walking distance (6MWD) in CHEST‐2.45 (A) 6MWD in the overall population, and inoperable and persistent/recurrent subgroups of CHEST‐2. Graph shows mean ± standard error of the mean. (B) Kaplan–Meier analysis showing the association of 6MWD with survival based on median value at baseline. (C) Kaplan–Meier analysis showing the association of 6MWD with survival at follow‐up. Reprinted with permission from Elsevier from Simonneau et al.45
3.3. Patient risk assessment in the CHEST studies
Currently, there are no recommendations for risk assessment in CTEPH, and 1 tool may not be appropriate for all patients due to the differing treatment pathways for operable and inoperable patients. However, several abbreviated PAH risk assessment tools have been applied to several databases, including the CHEST study database, in exploratory post hoc analyses.77, 84 Application of the French invasive, French noninvasive and Swedish COMPERA methods to the CHEST database showed that treatment with riociguat was found to improve risk status from baseline to Week 16. Patients who had a greater number of low‐risk criteria or a low‐risk status at Week 16 of CHEST‐1 had lower mortality and fewer clinical worsening events in CHEST‐2.
Application of the RRS to the CHEST database also showed that riociguat improved RRS compared with placebo from baseline to Week 16. Furthermore, RRS at baseline and at Week 16, and the change in RRS during CHEST‐1, were associated with improved survival (HRs for a 1‐point reduction in RRS: 0.702, 0.692 and 0.682, respectively) and clinical worsening‐free survival (HRs: 0.697, 0.719 and 0.754, respectively) over 2 years in CHEST‐2.85
4. STUDIES OF SWITCHING TO RIOCIGUAT
Previous studies have suggested that endogenous NO production may be reduced in patients with PAH leading to lower cGMP levels in the pulmonary circulation.86, 87, 88, 89, 90 One of the rationales for developing sGC stimulators for the treatment of PAH is that a PDE5i may not be as effective under conditions where endogenous cGMP production is reduced.91 Some patients with PAH do not reach treatment goals on PDE5i therapy,23, 24 raising the question of whether they may respond better to enhanced cGMP production via sGC stimulators rather than decreased cGMP metabolism via a PDE5i.
RESPITE (NCT02007629) was a 24‐week, uncontrolled, open‐label, phase IIIb pilot study to determine the safety and efficacy of replacing a PDE5i with riociguat in patients with PAH who failed to respond to treatment with a PDE5i (Table 1).47, 48 Inclusion criteria were: 6MWD 165–440 m, WHO FC III, cardiac index <3.0 L/min/m2, mPAP >30 mmHg, PAWP ≤15 mmHg and PVR >400 dyn·s·cm−5 after ≥3 months of treatment with sildenafil or tadalafil. Of the 61 patients enrolled, 50 (82%) were receiving concomitant ERA treatment. For the 51 patients (84%) who completed 24 weeks of treatment, mean ± standard deviation (SD) 6MWD increased from baseline by +31 ± 63 m and NT‐proBNP decreased by −347 ± 1235 pg/mL. Improvements were also seen in haemodynamic parameters, including a decrease in PVR of −103 ± 296 dyn·s·cm−5 (95% CI: −188 to −18 dyn·s·cm−5; P = .0184), a decrease in mPAP of −2.8 ± 8.8 mmHg and an increase in cardiac index of +0.3 ± 0.5 L/min/m2 (95% CI: 0.2–0.5 L/min/m2; P = .0001). WHO FC improved in 28 patients (54%). Switching was generally well tolerated, and no SAEs occurred during the PDE5i treatment‐free period.
Based on the results of the RESPITE study, a larger, multicentre, randomised, controlled 24‐week open‐label study was conducted. The REPLACE study (NCT02891850) enrolled 226 patients with PAH who remained in WHO FC III with a 6MWD of 165–440 m despite treatment with stable doses of a PDE5i either alone or in combination with an ERA.92 Patients were randomised to remain on their PDE5i or to switch to riociguat (2.5 mg tid). Patients on concomitant ERA treatment prior to enrolment continued on this therapy throughout the study. The primary endpoint was clinical improvement at Week 24 defined as 2 of the following: ≥10%/≥30 m increase in 6MWD, WHO FC I/II, or ≥30% reduction in NT‐proBNP in the absence of clinical worsening (death from any cause, hospitalisation for worsening PAH or disease progression). Secondary endpoints included change from baseline at Week 24 in 6MWD, NT‐proBNP, WHO FC and time to first clinical worsening. Baseline demographics and disease characteristics were largely similar between the treatment groups. The primary endpoint was met in 45 patients (41%) treated with riociguat and 23 patients (20%) treated with a PDE5i (odds ratio 2.8; 95% CI: 1.5–5.1; P = .0007). Significant improvements in WHO FC and time to clinical worsening were observed with patients treated with riociguat compared with those receiving the PDE5i, and there were also trends toward greater improvements in 6MWD and NT‐proBNP. The frequency of AEs was similar between treatment groups, but in the PDE5i group, there were more deaths (3 vs 0%) and more patients reported serious AEs (17 vs 7%) compared with riociguat.92
The CTEPH early access study was an open‐label, uncontrolled, single‐arm, phase IIIb surveillance study. Of the 300 patients enrolled in the study, 84 (28%) had switched to riociguat from their prior off‐label PAH‐targeted therapy. Fifty‐eight (19%) of these patients switched from a PDE5i (most frequently sildenafil) and 44 (15%) switched from an ERA (most frequently bosentan). The safety and tolerability of riociguat were similar between patients who switched from other PAH‐targeted therapies and those who were treatment‐naïve.93
Together, the findings of the above studies suggest that switching from a PDE5i to riociguat is safe and, in some patients, can result in improved efficacy. REPLACE was the first randomised controlled study dedicated to the switch of oral PAH drugs including a head‐to‐head comparison. However, a common limitation of the mentioned studies is their open‐label nature, which allows for possible treatment bias. Attempts were made to mitigate this effect in the REPLACE study by requiring sites to have blinded investigators assess WHO FC and 6MWD and by central blinded adjudication of the primary endpoint and clinical worsening. However, the lack of blinding resulted in patients being aware that they had been randomised to a new therapy or to remain on their current therapy, and this may have impacted their symptom perception or even their performance in the 6MWD. None of the studies provide data on long‐term outcomes, although REPLACE was able to show a difference in time to clinical worsening during the 24‐week treatment period with cases of death in the control arm only.92 Randomisation and stratification worked in the study: apart from minor differences in baseline demographics (more elderly patients, males and patients with PAH associated with connective tissue disease, predictive of worse outcome), the only parameter with a clear difference at baseline was NT‐proBNP, with lower levels (that are associated with improved outcome) in the riociguat group compared with the PDE5i group.
5. FURTHER STUDIES IN PAH AND CTEPH
The MOTION study (NCT02191137) was a prospective, multicentre, single‐arm, open‐label, phase IV study to assess the impact of riociguat on patient‐reported outcomes in patients with PAH.94 The primary endpoint was change from baseline to Week 24 in the LPH questionnaire, a 21‐item, validated, disease‐specific health‐related quality of life assessment tool comprising physical (score 0–40) and emotional (score 0–25) domains; a reduction in score indicates an improvement in health‐related quality of life.95 The primary endpoint was met, with a mean ± SD change in LPH score from baseline to Week 24 of −5.4 ± 27.8 (n = 75, P = .048; the minimally important difference that indicates a clinically meaningful improvement was estimated to be a change from baseline of 7).95
RACE (NCT02634203) was a multicentre, randomised, controlled trial comparing riociguat with BPA in 105 newly diagnosed patients with inoperable CTEPH and PVR >320 dyn·s·cm−5.96 The primary endpoint was PVR at Week 26, expressed as a percentage of the baseline PVR, with secondary endpoints including changes from baseline in 6MWD, Borg dyspnoea index, WHO FC and NT‐proBNP, as well as time to clinical worsening and safety. After 26 weeks, PVR was significantly improved with BPA (41% of baseline) compared with riociguat (68% of baseline; P < .0001), although there was no improvement in 6MWD with BPA vs riociguat at Week 26 (+50 vs +44 m, respectively). BPA was also associated with significant improvements in Borg dyspnoea index, WHO FC, NT‐proBNP and a number of haemodynamic parameters, compared with riociguat. The frequency of treatment‐related SAEs, however, was substantially higher with BPA (42% of patients) compared with riociguat (9%). More data will become available once patients complete the extended 6‐month follow‐up period where crossovers were allowed.
6. RIOCIGUAT IN WHO GROUP 2 AND 3 PH
In addition to the treatment of PAH and CTEPH, several studies have examined the safety and efficacy of riociguat in the treatment of PH due to chronic heart and lung disease.
The phase II LEPHT study (NCT01065454) investigated the safety and efficacy of riociguat in 201 patients with PH and heart failure with reduced ejection fraction (≤40%).49 The primary endpoint, change from baseline to Week 16 in mPAP, was not met. In addition, although significant increases were observed in cardiac index and stroke volume index, with significant decreases in PVR and SVR in the riociguat treatment group, no significant difference was seen in 6MWD.49 In the smaller DILATE study (NCT01172756) in 39 patients with PH associated with heart failure with preserved ejection fraction, no change was seen in the primary endpoint of peak decrease in mPAP from baseline to 6 hours between 11 patients treated with placebo and 10 patients given 2 mg of riociguat.50 However, significant increases in stroke volume (+9 mL [95% CI: 0.4–17]; P = .04), and decreases in systolic BP (−12 mmHg [95% CI: −22 to −1]; P = .03) and RV end‐diastolic area (−5.6 cm2 [95% CI: −11 to −0.3]; P = .04), were seen without significant changes in heart rate, PVR or PAWP.50 This lack of statistically significant clinical effect is broadly consistent with studies of other PAH‐targeted therapies, which have also failed to meet primary endpoints in studies in patients with heart failure.97, 98 A recent study of the sGC stimulator vericiguat, however, showed a significant reduction in a composite endpoint of death from cardiovascular causes or first hospitalisation for heart failure compared with placebo in patients with heart failure with reduced ejection fraction.56 The 26‐week, randomised, double‐blind, multicentre, phase IIb DYNAMIC study, designed to test the efficacy of long‐term riociguat treatment in patients with PH associated with heart failure with preserved ejection fraction, is ongoing.57
Small open‐label pilot studies in PH associated with ILD51 and chronic obstructive pulmonary disease (COPD)52 have also been conducted. In a pilot study of 23 patients with PH‐COPD (NCT00640315), of whom 22 were evaluated for haemodynamics and lung function parameters, a single dose of riociguat 1 mg (10 patients) or 2.5 mg (12 patients) given during right heart catheterisation acutely reduced mPAP and PVR, without a change in lung function or gas exchange.52
The RISE‐IIP study (NCT02138825) was a phase II, 26‐week, multinational, randomised, double‐blind, placebo‐controlled trial that examined the safety and efficacy of riociguat in 147 patients with PH associated with idiopathic interstitial pneumonias (PH‐IIP), a subgroup of ILD (Table 1). RISE‐IIP was terminated on the recommendation of the independent Data Monitoring Committee due to increased rates of mortality and SAEs among patients receiving riociguat vs placebo, and no evidence of a positive risk:benefit ratio.54 This resulted in the contraindication of riociguat in patients with PH‐IIP.60, 61 Future studies in PH‐IIP could consider the use of centralised evaluation of baseline high‐resolution computed tomography data to better detect these patients.
7. RIOCIGUAT IN OTHER CONDITIONS
There is a significant unmet need in patients with diffuse cutaneous systemic sclerosis (dcSSc) for therapies that can halt or slow disease progression. As riociguat was shown to possess antifibrotic properties in preclinical studies,58, 99, 100 the safety and efficacy of riociguat for the treatment of dcSSc were investigated in RISE‐SSc (NCT02283762), a 52‐week, randomised, double‐blind, placebo‐controlled, phase II study in 121 patients (Table 1).55 The primary efficacy endpoint, mean change in modified Rodnan skin score (an assessment of skin fibrosis) at Week 52, was not met at predefined significance (P < .05). The small size of the study and a higher than expected skin fibrosis regression rate in the placebo arm may have resulted in decreased sensitivity for change in modified Rodnan skin score, potentially explaining the lack of a significant result. Exploratory analyses suggested beneficial effects of riociguat on a range of dcSSc parameters, such as a lower rate of skin fibrosis progression and reductions in Raynaud's phenomenon attacks.55 Analyses of the subgroups of patients with SSc‐ILD determined by medical history (n = 22), high‐resolution computed tomography (n = 21) or restrictive lung disease (n = 12), showed that worsening of forced vital capacity predicted (%) was less marked in the riociguat group than the placebo group. Fewer AEs (81 vs 92%) and SAEs (9 vs 25%) occurred in patients with ILD, with riociguat vs placebo.101 However, based on these results, the use of riociguat in SSc‐ILD cannot be recommended. Additional studies will be needed, particularly considering the increased rates of mortality and SAEs among patients receiving riociguat vs placebo in the RISE‐IIP study.
The DIGIT study (NCT01926847) assessed the safety, efficacy and pharmacokinetics of riociguat (single dose, 2 mg) in 20 patients with primary and secondary Raynaud's phenomenon (Table 1). Riociguat improved digital blood flow in 40% of patients at room temperature and 60% of patients under conditions of cold exposure.53
Riociguat has also been investigated in cystic fibrosis (CF) based on the observation that activity of the CF transmembrane conductance regulator (CFTR) protein, a transmembrane chloride channel, is modulated by accumulation of cGMP.102 In a mouse model of CF involving the ΔF508 CFTR mutation, the PDE5i sildenafil has been shown to increase CFTR function,103 and preclinical data have indicated that riociguat may have a potential disease‐modifying effect in CF by restoring chloride ion channel function (Bayer AG data on file). The Rio‐CF (NCT0202170025) study was a randomised, double‐blind, placebo‐controlled, multicentre, phase II study investigating the safety and efficacy of riociguat in 21 patients with CF who were homozygous for the ΔF508 mutation. Following Part 1 of the study, the independent Data Safety Monitoring Board provided a positive recommendation for continuation; however, due to a shift in the treatment landscape for patients with homozygous ΔF508 CF104 and difficulties adapting the study design, coupled with the limited efficacy seen in Part 1, Rio‐CF was terminated.
8. SAFETY OF RIOCIGUAT
Overall, riociguat has been well tolerated in clinical studies of PAH and CTEPH.40, 41, 43, 45 The most common AEs in PATENT and CHEST were headache, dizziness, dyspepsia, peripheral oedema, nasopharyngitis, nausea, vomiting, diarrhoea and hypotension.
In PATENT‐1, the most frequently occurring SAEs were syncope, worsening PH, chest pain and RV failure. Drug‐related SAEs in the maximum riociguat dose of 2.5 mg group included syncope (in 1% of patients) and single cases of increased hepatic enzyme levels, dizziness, presyncope, acute renal failure and hypotension (in a total of 0.4% of patients). Similar SAEs were seen in CHEST‐1, with the most frequently occurring SAEs being RV failure, syncope and haemoptysis. Drug‐related SAEs in the riociguat group included syncope (2% of patients), and gastritis, acute renal failure and hypotension (in 1 patient each, 1%).40, 43 At 2 years, the exposure‐adjusted frequency of common AEs was generally lower in the LTE studies, compared with PATENT‐1 or CHEST‐1.40, 41, 43, 45
Results from RESPITE47 and EXPosurE RegisTry riociguat in patients with PH (EXPERT; NCT02092818; a phase IV, prospective, noninterventional cohort study [registry] to investigate the long‐term safety of riociguat in clinical practice),105, 106 and observations in other studies49, 50 have revealed no new safety signals compared with PATENT and CHEST, and AEs of special interest remain infrequent. Although most of the AEs were not serious, serious haemoptysis and pulmonary haemorrhage, including cases with fatal outcomes, have been observed in patients with CTEPH or PAH treated with riociguat.61 These events may be driven by the significant vasodilator effect of riociguat on systemic vascular tone which could increase cardiac output resulting in greater blood flow through pulmonary capillaries. Due to the lack of selectivity for the pulmonary circulation, the dose of riociguat must be adjusted on a per‐patient basis to ensure that excessive effects on lowering BP do not occur.61
9. FUTURE DIRECTIONS OF RIOCIGUAT
New indications are being investigated in areas of unmet need: PATENT‐CHILD (NCT2562235) is an ongoing open‐label, single‐arm, individual dose‐titration study designed to assess the safety, tolerability, pharmacokinetics, pharmacodynamics and exploratory efficacy of riociguat plus standard of care (ERA and/or prostacyclin) in children and adolescents aged 6–17 years with PAH. Following safety review of an initial group of patients aged ≥12 years, recruitment has now also been opened to the age 6–11 years group. Patients who complete the initial 24‐week study are eligible for entry to the LTE.107 The planned enrolment of at least 20 patients on ERA therapy is ongoing.
Additionally, while conventional individual endpoints in PH trials, such as 6MWD and WHO FC, can provide valuable insight into the benefit of treatment for patients, there is a growing recognition that the impacts of these conditions are often multifaceted, and a more holistic approach incorporates different factors and endpoints being assessed together. This is now being considered in the inclusion of composite endpoints in recent and future trial designs, such as that evaluated in the recently reported REPLACE trial.92
10. CONCLUSIONS
Riociguat is approved for the treatment of PAH and CTEPH. It stimulates cGMP synthesis by enhancing the effect of endogenous NO on sGC activity and also stimulates sGC activity independently of NO. This makes it a unique agent to target the reduced NO activity contributing to the pathogenesis of PH. Following the sentinel studies that led to its approval for the treatment of PAH and CTEPH, the LTEs suggest that riociguat is generally well tolerated and that its beneficial effects on exercise capacity are maintained. Studies also suggest that riociguat can be effectively used as a monotherapy, or in combination with ERAs or prostanoids, but its use with PDE5i is contraindicated. Data demonstrating treatment efficacy of riociguat for PAH and CTEPH have been obtained from a small number of randomised controlled trials with a relatively low number of patients. This limitation is common to all currently approved medications for PAH due to the small number of patients with the disease and restricts the scientific rigour of the findings. However, data from LTEs and recently completed open‐label trials continue to add support for the efficacy of riociguat in the treatment of PH. Recent data suggest that riociguat may have beneficial effects in some patients with PAH who have not achieved a satisfactory response to a PDE5i. Initial safety concerns regarding systemic hypotension, syncope and haemoptysis have been rarely encountered in LTEs, but the effect of riociguat on lowering SVR needs to be considered when initiating treatment or when patients develop haemodynamic instability.
The beneficial effects of riociguat on PAH and CTEPH have not been seen in early studies of PH associated with chronic heart and lung disease, and at this time riociguat cannot be recommended for the treatment of PH associated with these diseases. Riociguat is contraindicated for use in PH‐IIP.
10.1. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org,108, 109, 110, 111 and are permanently archived in the Concise Guide to PHARMACOLOGY 2019/20.
COMPETING INTERESTS
J.R.K. reports research support to his institution from Actelion, Bayer AG, Lung Biotechnology and United Therapeutics. M.M.C. has received research support from Actelion, Eiger BioPharmaceuticals, GeNO LLC, Gilead, GlaxoSmithKline, Medtronic and Reata Pharmaceuticals; consulting fees from Actelion, Express Scripts Holding Company, Gilead, SteadyMed Therapeutics, United Therapeutics and WebMD LLC (Medscape); and honoraria for speaking for Bayer AG and Gilead. D.L. reports honoraria, consultation fees, research support and/or travel expenses from Actelion, Arena, Bayer AG, Northern Therapeutics, PhaseBio and United Therapeutics. S.R. reports grants and personal fees from Abbott, Actelion, Arena, Bayer AG, Ferrer, Gilead, GlaxoSmithKline, MSD, Novartis, Pfizer and United Therapeutics, and research support from Actelion, Bayer, Novartis, Pfizer and United Therapeutics. O.S. reports grants, personal fees and nonfinancial support from Actelion, Bayer AG, GlaxoSmithKline and Merck, and personal fees from Arena.
CONTRIBUTORS
All authors contributed to the writing of this review. Conceptualisation, J.R.K.; writing—original draft preparation, J.R.K.; writing—review and editing, J.R.K., M.M.C., D.L., S.R., O.S.; visualisation, J.R.K., M.M.C., D.L., S.R., O.S.; supervision, J.R.K. All authors have read and agreed to the published version of the manuscript.
ACKNOWLEDGEMENTS
The authors and Bayer AG would like to thank the patients and clinicians who participated in the riociguat clinical studies. Funding for medical writing services was provided by Bayer AG. Medical writing services were provided by Robyn Bradbury, PhD, of Adelphi Communications Ltd, Macclesfield, UK, in accordance with Good Publications Practice 3 guidelines.
Klinger JR, Chakinala MM, Langleben D, Rosenkranz S, Sitbon O. Riociguat: Clinical research and evolving role in therapy. Br J Clin Pharmacol. 2021;87:2645–2662. 10.1111/bcp.14676
Funding information Bayer AG.
REFERENCES
- 1.Galiè N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS) endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J. 2016;37(1):67‐119. [DOI] [PubMed] [Google Scholar]
- 2.Simonneau G, Montani D, Celermajer DS, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J. 2018;53(1):1801913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schermuly RT, Ghofrani HA, Wilkins MR, Grimminger F. Mechanisms of disease: pulmonary arterial hypertension. Nat Rev Cardiol. 2011;8(8):443‐455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim NH, Delcroix M, Jenkins DP, et al. Chronic thromboembolic pulmonary hypertension. J Am Coll Cardiol. 2013;62(25 Suppl):D92‐D99. [DOI] [PubMed] [Google Scholar]
- 5.Lang I. Chronic thromboembolic pulmonary hypertension: a distinct disease entity. Eur Respir Rev. 2015;24(136):246‐252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Simonneau G, Torbicki A, Kim N. The pathophysiology of chronic thromboembolic pulmonary hypertension. Eur Respir Rev. 2017;26(143):160112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bresser P, Pepke‐Zaba J, Jais X, Humbert M, Hoeper MM. Medical therapies for chronic thromboembolic pulmonary hypertension: an evolving treatment paradigm. Proc Am Thorac Soc. 2006;3(7):594‐600. [DOI] [PubMed] [Google Scholar]
- 8.Jenkins D. Pulmonary endarterectomy: the potentially curative treatment for patients with chronic thromboembolic pulmonary hypertension. Eur Respir Rev. 2015;24(136):263‐271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ghofrani HA, Humbert M, Langleben D, et al. Riociguat: mode of action and clinical development in pulmonary hypertension. Chest. 2017;151(2):468‐480. [DOI] [PubMed] [Google Scholar]
- 10.McLaughlin VV, Archer SL, Badesch DB, et al. ACCF/AHA 2009 expert consensus document on pulmonary hypertension: A report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association developed in collaboration with the American College of Chest Physicians; American Thoracic Society, Inc.; and the Pulmonary Hypertension Association. J Am Coll Cardiol. 2009;53(17):1573‐1619. [DOI] [PubMed] [Google Scholar]
- 11.Jenkins D, Madani M, Fadel E, D'Armini AM, Mayer E. Pulmonary endarterectomy in the management of chronic thromboembolic pulmonary hypertension (CTEPH). Eur Respir Rev. 2017;26(143):160111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Freed DH, Thomson BM, Berman M, et al. Survival after pulmonary thromboendarterectomy: effect of residual pulmonary hypertension. J Thorac Cardiovasc Surg. 2011;141(2):383‐387. [DOI] [PubMed] [Google Scholar]
- 13.Mayer E, Jenkins D, Lindner J, et al. Surgical management and outcome of patients with chronic thromboembolic pulmonary hypertension: results from an international prospective registry. J Thorac Cardiovasc Surg. 2011;141(3):702‐710. [DOI] [PubMed] [Google Scholar]
- 14.Pepke‐Zaba J, Delcroix M, Lang I, et al. Chronic thromboembolic pulmonary hypertension (CTEPH): results from an international prospective registry. Circulation. 2011;124(18):1973‐1981. [DOI] [PubMed] [Google Scholar]
- 15.Hurdman J, Condliffe R, Elliot CA, et al. ASPIRE registry: Assessing the Spectrum of Pulmonary hypertension Identified at a REferral centre. Eur Respir J. 2012;39(4):945‐955. [DOI] [PubMed] [Google Scholar]
- 16.Cannon JE, Su L, Kiely DG, et al. Dynamic risk stratification of patient long‐term outcome after pulmonary endarterectomy: results from the UK national cohort. Circulation. 2016;133(18):1761‐1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mayer E. Surgical and post‐operative treatment of chronic thromboembolic pulmonary hypertension. Eur Respir Rev. 2010;19(115):64‐67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Simonneau G, Delcroix M, Lang IM, Pepke‐Zaba J, Mayer E. Long‐term outcome of patients with chronic thromboembolic pulmonary hypertension: results of an international prospective registry comparing operated versus non operated patients. Am J Respir Crit Care Med. 2013;187:A5364. [Google Scholar]
- 19.Stasch JP, Evgenov OV. Soluble guanylate cyclase stimulators in pulmonary hypertension. Handb Exp Pharmacol. 2013;218:279‐313. [DOI] [PubMed] [Google Scholar]
- 20.Stasch JP, Pacher P, Evgenov OV. Soluble guanylate cyclase as an emerging therapeutic target in cardiopulmonary disease. Circulation. 2011;123(20):2263‐2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McLaughlin VV, McGoon MD. Pulmonary arterial hypertension. Circulation. 2006;114(13):1417‐1431. [DOI] [PubMed] [Google Scholar]
- 22.Humbert M, Ghofrani HA. The molecular targets of approved treatments for pulmonary arterial hypertension. Thorax. 2016;71(1):73‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oudiz RJ, Brundage BH, Galiè N, et al. Tadalafil for the treatment of pulmonary arterial hypertension: a double‐blind 52‐week uncontrolled extension study. J Am Coll Cardiol. 2012;60(8):768‐774. [DOI] [PubMed] [Google Scholar]
- 24.Rubin LJ, Badesch DB, Fleming TR, et al. Long‐term treatment with sildenafil citrate in pulmonary arterial hypertension: SUPER‐2. Chest. 2011;140(5):1274‐1283. [DOI] [PubMed] [Google Scholar]
- 25.Stasch JP, Hobbs AJ. NO‐independent, haem‐dependent soluble guanylate cyclase stimulators. Handb Exp Pharmacol. 2009;191:277‐308. [DOI] [PubMed] [Google Scholar]
- 26.Follmann M, Griebenow N, Hahn MG, et al. The chemistry and biology of soluble guanylate cyclase stimulators and activators. Angew Chem Int Ed Engl. 2013;52(36):9442‐9462. [DOI] [PubMed] [Google Scholar]
- 27.Ghofrani HA, Hoeper MM, Halank M, et al. Riociguat for chronic thromboembolic pulmonary hypertension and pulmonary arterial hypertension: first long‐term extension data from a phase II study. Am J Respir Crit Care Med. 2010;181:A6770. [DOI] [PubMed] [Google Scholar]
- 28.Ghofrani HA, Hoeper MM, Halank M, et al. Riociguat for chronic thromboembolic pulmonary hypertension and pulmonary arterial hypertension: a phase II study. Eur Respir J. 2010;36(4):792‐799. [DOI] [PubMed] [Google Scholar]
- 29.Benza RL, Raina A, Kanwar M, Nathan SD, Mathai SC. The soluble guanylate cyclase stimulator riociguat: Evidence in pulmonary hypertension and beyond. J Rare Dis. 2017;2(6):15‐22. [Google Scholar]
- 30.Dumitrascu R, Weissmann N, Ghofrani HA, et al. Activation of soluble guanylate cyclase reverses experimental pulmonary hypertension and vascular remodeling. Circulation. 2006;113(2):286‐295. [DOI] [PubMed] [Google Scholar]
- 31.Mittendorf J, Weigand S, Alonso‐Alija C, et al. Discovery of riociguat (BAY 63‐2521): a potent, oral stimulator of soluble guanylate cyclase for the treatment of pulmonary hypertension. ChemMedChem. 2009;4(5):853‐865. [DOI] [PubMed] [Google Scholar]
- 32.Lang M, Kojonazarov B, Tian X, et al. The soluble guanylate cyclase stimulator riociguat ameliorates pulmonary hypertension induced by hypoxia and SU5416 in rats. PLoS ONE. 2012;(7, 8 e43433):1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sandner P, Stasch JP. Anti‐fibrotic effects of soluble guanylate cyclase stimulators and activators: a review of the preclinical evidence. Respir Med. 2017;122(Suppl 1):S1‐S9. [DOI] [PubMed] [Google Scholar]
- 34.Masuyama H, Tsuruda T, Sekita Y, et al. Pressure‐independent effects of pharmacological stimulation of soluble guanylate cyclase on fibrosis in pressure‐overloaded rat heart. Hypertens Res. 2009;32(7):597‐603. [DOI] [PubMed] [Google Scholar]
- 35.Dunkern TR, Feurstein D, Rossi GA, Sabatini F, Hatzelmann A. Inhibition of TGF‐beta induced lung fibroblast to myofibroblast conversion by phosphodiesterase inhibiting drugs and activators of soluble guanylyl cyclase. Eur J Pharmacol. 2007;572(1):12‐22. [DOI] [PubMed] [Google Scholar]
- 36.Joshi CN, Martin DN, Fox JC, Mendelev NN, Brown TA, Tulis DA. The soluble guanylate cyclase stimulator BAY 41‐2272 inhibits vascular smooth muscle growth through the cAMP‐dependent protein kinase and cGMP‐dependent protein kinase pathways. J Pharmacol Exp Ther. 2011;339(2):394‐402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Irvine JC, Ganthavee V, Love JE, et al. The soluble guanylyl cyclase activator bay 58‐2667 selectively limits cardiomyocyte hypertrophy. PLoS ONE. 2012;7(11):e44481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Frey R, Mück W, Unger S, Artmeier‐Brandt U, Weimann G, Wensing G. Single‐dose pharmacokinetics, pharmacodynamics, tolerability, and safety of the soluble guanylate cyclase stimulator BAY 63‐2521: an ascending‐dose study in healthy male volunteers. J Clin Pharmacol. 2008;48(8):926‐934. [DOI] [PubMed] [Google Scholar]
- 39.Grimminger F, Weimann G, Frey R, et al. First acute haemodynamic study of soluble guanylate cyclase stimulator riociguat in pulmonary hypertension. Eur Respir J. 2009;33(4):785‐792. [DOI] [PubMed] [Google Scholar]
- 40.Ghofrani HA, Galiè N, Grimminger F, et al. Riociguat for the treatment of pulmonary arterial hypertension. N Engl J Med. 2013;369(4):330‐340. [DOI] [PubMed] [Google Scholar]
- 41.Ghofrani HA, Grimminger F, Grünig E, et al. Predictors of long‐term outcomes in patients treated with riociguat for pulmonary arterial hypertension: data from the PATENT‐2 open‐label, randomised, long‐term extension trial. Lancet Respir Med. 2016;4(5):361‐371. [DOI] [PubMed] [Google Scholar]
- 42.Rubin LJ, Galiè N, Grimminger F, et al. Riociguat for the treatment of pulmonary arterial hypertension: a long‐term extension study (PATENT‐2). Eur Respir J. 2015;45(5):1303‐1313. [DOI] [PubMed] [Google Scholar]
- 43.Ghofrani HA, D'Armini AM, Grimminger F, et al. Riociguat for the treatment of chronic thromboembolic pulmonary hypertension. N Engl J Med. 2013;369(4):319‐329. [DOI] [PubMed] [Google Scholar]
- 44.Simonneau G, D'Armini AM, Ghofrani HA, et al. Riociguat for the treatment of chronic thromboembolic pulmonary hypertension: a long‐term extension study (CHEST‐2). Eur Respir J. 2015;45(5):1293‐1302. [DOI] [PubMed] [Google Scholar]
- 45.Simonneau G, D'Armini AM, Ghofrani HA, et al. Predictors of long‐term outcomes in patients treated with riociguat for chronic thromboembolic pulmonary hypertension: data from the CHEST‐2 open‐label, randomised, long‐term extension trial. Lancet Respir Med. 2016;4(5):372‐380. [DOI] [PubMed] [Google Scholar]
- 46.Galiè N, Müller K, Scalise AV, Grünig E. PATENT PLUS: a blinded, randomised and extension study of riociguat plus sildenafil in PAH. Eur Respir J. 2015;45(5):1314‐1322. [DOI] [PubMed] [Google Scholar]
- 47.Hoeper MM, Simonneau G, Corris PA, et al. RESPITE: Switching to riociguat in PAH patients with inadequate response to PDE5i. Eur Respir J. 2017;50(3):1602425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hoeper MM, Klinger JR, Benza RL, et al. Rationale and study design of RESPITE: an open‐label, phase 3b study of riociguat in patients with pulmonary arterial hypertension who demonstrate an insufficient response to treatment with phosphodiesterase‐5 inhibitors. Respir Med. 2017;122(Suppl. 1):S18‐S22. [DOI] [PubMed] [Google Scholar]
- 49.Bonderman D, Ghio S, Felix SB, et al. Riociguat for patients with pulmonary hypertension due to systolic left ventricular dysfunction: a phase IIb double‐blind, randomized, placebo‐controlled, dose‐ranging hemodynamic study. Circulation. 2013;128(5):502‐511. [DOI] [PubMed] [Google Scholar]
- 50.Bonderman D, Pretsch I, Steringer‐Mascherbauer R, et al. Acute hemodynamic effects of riociguat in patients with pulmonary hypertension associated with diastolic heart failure (DILATE‐1): a randomized, double‐blind, placebo‐controlled, single‐dose study. Chest. 2014;146(5):1274‐1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hoeper MM, Halank M, Wilkens H, et al. Riociguat for interstitial lung disease and pulmonary hypertension: a pilot trial. Eur Respir J. 2013;41(4):853‐860. [DOI] [PubMed] [Google Scholar]
- 52.Ghofrani HA, Staehler G, Grünig E, et al. Acute effects of riociguat in borderline or manifest pulmonary hypertension associated with chronic obstructive pulmonary disease. Pulm Circ. 2015;5(2):296‐304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Huntgeburth M, Kießling J, Weimann G, et al. Riociguat for the treatment of Raynaud's phenomenon: A single‐dose, double‐blind, randomized, placebo‐controlled cross‐over study (DIGIT). Clin Drug Investig. 2018;38(11):1061‐1069. [DOI] [PubMed] [Google Scholar]
- 54.Nathan SD, Behr J, Collard HR, et al. Riociguat for idiopathic interstitial pneumonia‐associated pulmonary hypertension (RISE‐IIP): a randomised, placebo‐controlled phase 2b study. Lancet Respir Med. 2019;7(9):780‐790. [DOI] [PubMed] [Google Scholar]
- 55.Distler O, Allanore Y, Denton CP, et al. Riociguat in patients with early diffuse cutaneous systemic sclerosis: a randomized, double‐blind, placebo‐controlled phase IIb study (RISE‐SSc). Arthritis Rheumatol. 2018;70(Suppl. 9):A903. [DOI] [PubMed] [Google Scholar]
- 56.Armstrong PW, Pieske B, Anstrom KJ, et al. Vericiguat in patients with heart failure and reduced ejection fraction. N Engl J Med. 2020;382(20):1883‐1893. [DOI] [PubMed] [Google Scholar]
- 57.Mascherbauer J, Grünig E, Halank M, et al. Evaluation of the pharmacoDYNAMIC effects of riociguat in subjects with pulmonary hypertension and heart failure with preserved ejection fraction: Study protocol for a randomized controlled trial. Wien Klin Wochenschr. 2016;128(23–24):882‐889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sharkovska Y, Kalk P, Lawrenz B, et al. Nitric oxide‐independent stimulation of soluble guanylate cyclase reduces organ damage in experimental low‐renin and high‐renin models. J Hypertens. 2010;28(8):1666‐1675. [DOI] [PubMed] [Google Scholar]
- 59.Halank M, Hoeper MM, Ghofrani HA, et al. Riociguat for pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension: results from a phase II long‐term extension study. Respir Med. 2017;128:50‐56. [DOI] [PubMed] [Google Scholar]
- 60.Bayer AG. Adempas US prescribing information 2019. Available from: http://labeling.bayerhealthcare.com/html/products/pi/Adempas_PI.pdf. Accessed 19 March 2020.
- 61.Bayer AG. Adempas (riociguat tablets): EU summary of product characteristics 2019. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_‐_Product_Information/human/002737/WC500165034.pdf. Accessed 19 March 2020.
- 62.Mathai SC, Odufowora O, Minai OA, et al. Health outcome assessment in pulmonary arterial hypertension patients treated with riociguat: 2‐year results from the PATENT‐2 long term extension study. Am J Respir Crit Care Med. 2015;191:A4777. [Google Scholar]
- 63.Langleben D, Galiè N, He J, et al. Use of clinically relevant responder threshold criteria to evaluate the response to treatment in the phase III PATENT‐1 study. J Heart Lung Transplant. 2015;34(3):338‐347. [DOI] [PubMed] [Google Scholar]
- 64.Humbert M, Galiè N, Ghofrani HA, et al. Efficacy of riociguat in pretreated versus treatment‐naive patients with pulmonary arterial hypertension (PAH) In the phase III PATENT‐1 study. Am J Respir Crit Care Med. 2013;187:A3534. [Google Scholar]
- 65.McConnell JW, Engel P, Rischard F, et al. Effects of riociguat in treatment‐naive vs pretreated patients with pulmonary arterial hypertension: 2‐year efficacy results from the PATENT‐2 study. Chest. 2016;150(Suppl. 4):1162A.27832886 [Google Scholar]
- 66.Mayer E, D'Armini AM, Ghofrani HA, et al. Efficacy of riociguat in patients with inoperable CTEPH vs persistent/recurrent PH after pulmonary endarterectomy (PEA): results from the phase III CHEST‐1 study. Eur Respir J. 2013;42(Supp. 57):345s. [Google Scholar]
- 67.Kerr K, Hoeper M, Sood N, et al. Effects of riociguat in patients with inoperable chronic thromboembolic pulmonary hypertension versus persistent/recurrent pulmonary hypertension after pulmonary endarterectomy: 2‐year efficacy results from the CHEST‐2 study. Miami. FL, USA: Poster presented at PVRI; 26–29 January 2017. [Google Scholar]
- 68.Preston I, Hill N, Ghofrani HA, et al. Riociguat in combination with prostacyclin analogs for the treatment of pulmonary arterial hypertension (PAH): a subgroup analysis of the PATENT studies. Chest. 2015;148(4):922A. [Google Scholar]
- 69.Rosenkranz S, Ghofrani HA, Beghetti M, et al. Riociguat for pulmonary arterial hypertension associated with congenital heart disease. Heart. 2015;101(22):1792‐1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Humbert M, Coghlan JG, Ghofrani HA, et al. Riociguat for the treatment of pulmonary arterial hypertension associated with connective tissue disease: results from PATENT‐1 and PATENT‐2. Ann Rheum Dis. 2017;76(2):422‐426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Galiè N, Channick RN, Frantz RP, et al. Risk stratification and medical therapy of pulmonary arterial hypertension. Eur Respir J. 2019;1801889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Humbert M, Ghofrani HA, Busse D, de Olivereira PJ, Langleben D. Riociguat in pulmonary arterial hypertension: ERS/ESC risk assessment in PATENT. Eur Respir J. 2016;48(Suppl. 60):PA2401. [Google Scholar]
- 73.Boucly A, Weatherald J, Savale L, et al. Risk assessment, prognosis and guideline implementation in pulmonary arterial hypertension. Eur Respir J. 2017;50(2):1700889. [DOI] [PubMed] [Google Scholar]
- 74.Weatherald J, Boucly A, Sahay S, Humbert M, Sitbon O. The low‐risk profile in pulmonary arterial hypertension. time for a paradigm shift to goal‐oriented clinical trial endpoints? Am J Respir Crit Care Med. 2018;197(7):860‐868. [DOI] [PubMed] [Google Scholar]
- 75.Kylhammar D, Kjellström B, Hjalmarsson C, et al. A comprehensive risk stratification at early follow‐up determines prognosis in pulmonary arterial hypertension. Eur Heart J. 2018;39(47):4175‐4181. [DOI] [PubMed] [Google Scholar]
- 76.Hoeper MM, Kramer T, Pan Z, et al. Mortality in pulmonary arterial hypertension: prediction by the 2015 European pulmonary hypertension guidelines risk stratification model. Eur Respir J. 2017;50(2):1700740. [DOI] [PubMed] [Google Scholar]
- 77.Humbert M, Farber HW, Ghofrani HA, et al. Risk assessment in pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension. Eur Respir J. 2019;53(6):1802004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Benza RL, Gomberg‐Maitland M, Miller DP, et al. The REVEAL risk score calculator in newly diagnosed patients with pulmonary arterial hypertension. Chest. 2012;141(2):354‐362. [DOI] [PubMed] [Google Scholar]
- 79.Benza RL, Miller DP, Gomberg‐Maitland M, et al. Predicting survival in pulmonary arterial hypertension: insights from the Registry to Evaluate Early and Long‐Term Pulmonary Arterial Hypertension Disease Management (REVEAL). Circulation. 2010;122(2):164‐172. [DOI] [PubMed] [Google Scholar]
- 80.Benza RL, Miller DP, Foreman AJ, et al. Prognostic implications of serial risk score assessments in patients with pulmonary arterial hypertension: a Registry to Evaluate Early and Long‐Term Pulmonary Arterial Hypertension Disease Management (REVEAL) analysis. J Heart Lung Transplant. 2015;34(3):356‐361. [DOI] [PubMed] [Google Scholar]
- 81.Benza RL, Farber HW, Frost A, et al. REVEAL risk scores applied to riociguat‐treated patients in PATENT‐2: impact of changes in risk score on survival. J Heart Lung Transplant. 2018;37(4):513‐519. [DOI] [PubMed] [Google Scholar]
- 82.Kim N, D'Armini A, Grimminger F, et al. Haemodynamic effects of riociguat in inoperable/recurrent chronic thromboembolic pulmonary hypertension. Heart. 2017;103(8):599‐606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.D'Armini AM, Ghofrani HA, Kim NH, et al. Use of responder threshold criteria to evaluate the response to treatment in the phase III CHEST‐1 study. J Heart Lung Transplant. 2015;34(3):348‐355. [DOI] [PubMed] [Google Scholar]
- 84.Delcroix M, Staehler G, Gall H, et al. Risk assessment in medically treated chronic thromboembolic pulmonary hypertension patients. Eur Respir J. 2018;1800248. [DOI] [PubMed] [Google Scholar]
- 85.Benza RL, Farber HW, Frost A, et al. REVEAL risk score in patients with chronic thromboembolic pulmonary hypertension receiving riociguat. J Heart Lung Transplant. 2018;37(7):836‐843. [DOI] [PubMed] [Google Scholar]
- 86.Xu W, Kaneko FT, Zheng S, et al. Increased arginase II and decreased NO synthesis in endothelial cells of patients with pulmonary arterial hypertension. FASEB J. 2004;18(14):1746‐1748. [DOI] [PubMed] [Google Scholar]
- 87.Leopold JA, Cap A, Scribner AW, Stanton RC, Loscalzo J. Glucose‐6‐phosphate dehydrogenase deficiency promotes endothelial oxidant stress and decreases endothelial nitric oxide bioavailability. FASEB J. 2001;15(10):1771‐1773. [DOI] [PubMed] [Google Scholar]
- 88.Kaneko FT, Arroliga AC, Dweik RA, et al. Biochemical reaction products of nitric oxide as quantitative markers of primary pulmonary hypertension. Am J Respir Crit Care Med. 1998;158(3):917‐923. [DOI] [PubMed] [Google Scholar]
- 89.Kharitonov SA, Cailes JB, Black CM, du Bois RM, Barnes PJ. Decreased nitric oxide in the exhaled air of patients with systemic sclerosis with pulmonary hypertension. Thorax. 1997;52(12):1051‐1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pullamsetti S, Kiss L, Ghofrani HA, et al. Increased levels and reduced catabolism of asymmetric and symmetric dimethylarginines in pulmonary hypertension. FASEB J. 2005;19(9):1175‐1177. [DOI] [PubMed] [Google Scholar]
- 91.Zhao L, Mason NA, Strange JW, Walker H, Wilkins MR. Beneficial effects of phosphodiesterase 5 inhibition in pulmonary hypertension are influenced by natriuretic peptide activity. Circulation. 2003;107(2):234‐237. [DOI] [PubMed] [Google Scholar]
- 92.Hoeper MM, Ghofrani HA, Al‐Hiti H, et al. Switching from PDE5i to riociguat in patients with PAH: the REPLACE study. Abstract presented at: European Respiratory Society International Congress 2020; virtual meeting 7—9 September 2020.
- 93.McLaughlin V, Jansa P, Nielsen‐Kudsk JE, et al. Riociguat in patients with chronic thromboembolic pulmonary hypertension: results from an early access study. BMC Pulm Med. 2017;17(1):216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sood N, Aranda A, Platt D, LaRose A, Kleinjung F, O'Brien G. Riociguat improves health‐related quality of life for patients with pulmonary arterial hypertension: results from the phase 4 MOTION study. Pulm Circ. 2019;9(1):2045894018823715. 10.1177/2045894018823715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bonner N, Abetz L, Meunier J, Sikirica M, Mathai SC. Development and validation of the living with pulmonary hypertension questionnaire in pulmonary arterial hypertension patients. Health Qual Life Outcomes. 2013;11(1):161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jais X, Brenot P, Bouvaist H, et al. Balloon pulmonary angioplasty versus riociguat for the treatment of inoperable chronic thromboembolic pulmonary hypertension: results from the randomised controlled RACE study. Eur Respir J. 2019;54:RCT1885. [Google Scholar]
- 97.Packer M, McMurray JJV, Krum H, et al. Long‐term effect of endothelin receptor antagonism with bosentan on the morbidity and mortality of patients with severe chronic heart failure: primary results of the ENABLE trials. JACC Heart Fail. 2017;5(5):317‐326. [DOI] [PubMed] [Google Scholar]
- 98.Redfield MM, Chen HH, Borlaug BA, et al. Effect of phosphodiesterase‐5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: a randomized clinical trial. JAMA. 2013;309(12):1268‐1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dees C, Beyer C, Distler A, et al. Stimulators of soluble guanylate cyclase (sGC) inhibit experimental skin fibrosis of different aetiologies. Ann Rheum Dis. 2015;74(8):1621‐1625. [DOI] [PubMed] [Google Scholar]
- 100.Geschka S, Kretschmer A, Sharkovska Y, et al. Soluble guanylate cyclase stimulation prevents fibrotic tissue remodeling and improves survival in salt‐sensitive Dahl rats. PLoS ONE. 2011;6(7):1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Distler O, Allanore Y, Denton C, et al. Efficacy and safety of riociguat in patients with early diffuse cutaneous systemic sclerosis and interstitial lung disease (SSc‐ILD): results from the phase IIb RISE‐SSc Study. Am J Respir Crit Care Med. 2019;199:A4086. [Google Scholar]
- 102.Dhooghe B, Noel S, Bouzin C, Behets‐Wydemans G, Leal T. Correction of chloride transport and mislocalization of CFTR protein by vardenafil in the gastrointestinal tract of cystic fibrosis mice. PLoS ONE. 2013;8(10):e77314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dormer RL, Harris CM, Clark Z, et al. Sildenafil (Viagra) corrects DeltaF508‐CFTR location in nasal epithelial cells from patients with cystic fibrosis. Thorax. 2005;60(1):55‐59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wainwright CE, Elborn JS, Ramsey BW. Lumacaftor‐ivacaftor in patients with cystic fibrosis homozygous for Phe508del CFTR. N Engl J Med. 2015;373(18):1783‐1784. [DOI] [PubMed] [Google Scholar]
- 105.Hoeper MM, Gall H, Grünig E, et al. Safety of riociguat for the treatment of pulmonary arterial hypertension: Final data cut from the EXPERT registry. Paper presented at ATS International Conference; 17–22 May 2019; Dallas, TX, USA.
- 106.Ghofrani HA, Gall H, Grünig E, et al. Safety of riociguat for the treatment of chronic thromboembolic pulmonary hypertension: final data cut from the EXPERT registry. Poster presented at ATS International Conference; 17–22 May 2019; Dallas, TX, USA.
- 107.Beghetti M, Wirsching G, Bonnet D, et al. Riociguat in pediatric pulmonary arterial hypertension: PATENT‐CHILD study design and rationale. Poster presented at PHA's International PH Conference and Scientific Sessions; 29 June–1 July 2018; Orlando, FL, USA.
- 108.Alexander SPH, Christopoulos A, Davenport AP, et al. The Concise Guide to PHARMACOLOGY 2019/20: G protein‐coupled receptors. Br J Pharmacol. 2019;176(S1):S21‐S141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Alexander SPH, Fabbro D, Kelly E, et al. The Concise Guide to PHARMACOLOGY 2019/20: Enzymes. Br J Pharmacol. 2019;176(S1):S297‐S396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Alexander SPH, Fabbro D, Kelly E, et al. The Concise Guide to PHARMACOLOGY 2019/20: Catalytic receptors. Br J Pharmacol. 2019;176(S1):S247‐S296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Alexander SPH, Mathie A, Peters JA, et al. The Concise Guide to PHARMACOLOGY 2019/20: Ion channels. Br J Pharmacol. 2019;176(S1):S142‐S228. [DOI] [PMC free article] [PubMed] [Google Scholar]


