INTRODUCTION
The scope of the present article is the status of the research, development, and demonstrations of treatment technologies for per‐ and polyfluoroalkyl substance (PFAS)‐laden material that are funded by the Strategic Environmental Research and Development Program (SERDP) and the Environmental Security Technology Certification Program (ESTCP). Both SERDP and ESTCP are US Department of Defense (DoD) programs and are coordinated with the US Environmental Protection Agency and the US Department of Energy. The present article is 1 of a 3‐part series of Focus articles on the status of research, development, and demonstration efforts that will assist project delivery teams, within the DoD, in their efforts to manage an expansive portfolio of aqueous film‐forming foam (AFFF)‐impacted sites. The present article focuses on treatment technologies, and the second article in the series covers fate and transport. A third, overview, article briefly summarizes exposure pathways; analytical and environmental sampling methods; fate and transport; characterization; bioaccumulation, ecotoxicity, and ecological risk assessment; and treatment technologies.
The PFAS have been recognized as being one of the most persistent categories of anthropogenic chemicals found in the environment. In contrast to chlorinated solvents, these fluorinated compounds are largely impervious to common biological degradation processes and conventional chemical oxidation processes. They are a concern for the DoD and for municipal airports, due to the use of legacy AFFFs. They are also a concern for the community at large, due to the presence of PFAS in consumer products.
Ex situ groundwater treatment has become a common alternative for managing PFAS‐impacted groundwater. Although existing technologies are acceptable for ex situ treatment of PFAS‐impacted groundwater (e.g., relying on adsorptive media such as granular activated carbon [GAC]), operation of these pump‐and‐treat systems represents a considerable and growing expense, especially as more of these systems have to be installed across the United States. Technologies such as GAC and ion exchange continually generate residuals (e.g., spent media) that require off‐site treatment and/or disposal. Large quantities of investigation‐derived wastes (IDW) continue to be generated during characterization of PFAS‐impacted sites. More cost‐effective alternatives are needed for disposal of residuals, and PFAS‐laden IDW materials (e.g., drill cuttings, well development water).
Given the substantial number of pump‐and‐treat systems that are currently in operation, sizable cost savings can be realized via development of more cost‐effective treatment technologies. Advances may be realized in many different forms: improved media for groundwater treatment that require much less frequent replacement; media with improved capabilities for removing short‐chain PFAS constituents; destruction technologies to allow for on‐site treatment of groundwater, spent media, regenerant solutions, and/or IDW; and improved destruction technologies for off‐site treatment of residuals and IDW.
Development of effective in situ treatment technologies for PFAS‐contaminated groundwater represents another important goal. Considerable cost savings will be realized if a portion of the existing pump‐and‐treat systems can be replaced with passive, in situ treatment systems.
The treatment technologies we discuss are broadly classified as either ex situ or in situ (Figure 1 and Tables 1, 2, 3). For the ex situ treatment technologies, the projects are primarily subclassified under the following categories: aqueous media treatment, investigation‐derived wastes/soils, and residuals. Residuals includes materials such as spent treatment media (GAC and ion exchange resin), and concentrated brines derived from regeneration of ion exchange resin. Some of the treatment technologies under development do not necessarily fit within a single category (e.g., plasma‐based treatment processes can be applied to aqueous media, IDW water, and brine regenerant solutions). Also, some of the ex situ treatment technologies could potentially be applied in situ. The present article is not intended to provide comprehensive coverage of each and every PFAS treatment project being funded by SERDP and ESTCP, but rather to provide an overview, and to highlight a select list of representative treatment projects.
Figure 1.
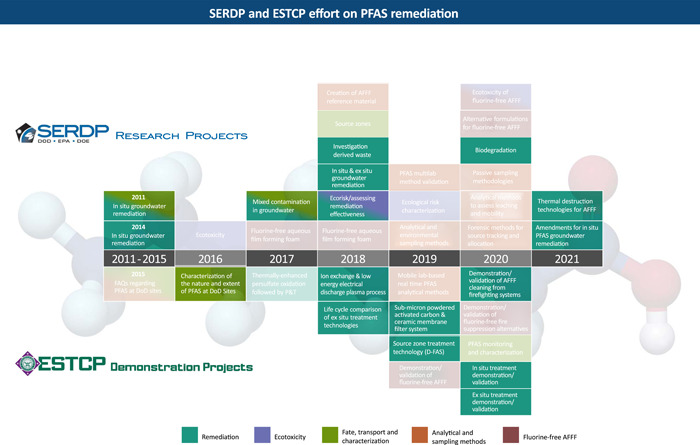
Chronology of Strategic Environmental Research and Development Program (SERDP) Statements of Need (SONs) and Environmental Security Technology Certification Program (ESTCP) projects related to the remediation of per‐ and polyfluoroalkyl substances (PFAS). See Tables 1, 2, and 3 for a description of each project. DoD = US Department of Defense; P&T = pump‐and‐treat; AFFF = aqueous fire‐fighting foam.
Table 1.
Description of funded projects related to ex situ treatment of per‐ and polyfluoroalkyl substance (PFAS)‐impacted aqueous media
| Project title | Primary objectives | Principal investigator (organization) | Treatment focus area | Start year | Status |
|---|---|---|---|---|---|
| Electrically assisted sorption and desorption of PFAS | Application of a scalable capacitive deionization to electrically enhance adsorption of key PFAS onto activated carbon and discharge them as a chemical‐free regeneration using a fraction of the energy of pressure‐driven membrane processes |
D. Call, (North Carolina State University, Raleigh, NC, USA) |
Sorption | 2018 |
Proof‐of‐concept (POC) in progress |
| An electrocoagulation and electro‐oxidation treatment train to degrade PFAS and other persistent organic contaminants in groundwater | Application of a novel treatment train combining electrocoagulation with electrochemical oxidation to remove and degrade PFAAs and comingled organics from groundwater |
D. Chiang (CDM Smith, New York, NY, USA) |
Electrochemical | 2018 | In progress |
| Combined in situ/ex situ treatment train for remediation of PFAS‐Impacted Groundwater | Evaluation of a range of combined situ/ex situ treatment trains for treatment of PFAS‐impacted groundwater and compare the scaled‐up cost and design challenges for implementation |
M. Crimi (Clarkson University, Potsdam, NY, USA) |
Abiotic oxidation/plasma/ion exchange | 2018 | In progress |
| Removal of complex mixtures of PFAAs from water using molecularly engineered coatings on sand and silica | Evaluation of reversible mesoporous organosilica sorbents for the treatment of a wide range of anionic, cationic, and nonionic forms of PFAS, while allowing for economical on‐site regeneration by solvent rinse | E. Edmiston (College of Wooster, Wooster, OH, USA) | Sorption | 2018 |
POC in progress |
| Ex situ treatment of PFAS‐impacted groundwater using ion exchange with regeneration | Evaluate one or more treatment trains for PFAS‐Impacted groundwater using novel ion exchange resins coupled with electrochemical and/or ultrasonic destruction of PFAS in regeneration solution waste streams |
M. Fuller (Aptim, Baton Rouge, LA, USA) |
Ion exchange | 2018 | In progress |
| Removal and destruction of PFAS and co‐occurring chemicals from groundwater via extraction and treatment with ion exchange media, and on‐site regeneration, distillation, and plasma destruction | Demonstrate a PFAS treatment train comprising ion exchange media, distillation and reuse of the regenerant solution, and on‐site destruction of the distillation waste with low‐energy plasma into existing co‐occurring chemical treatment systems | N. Hagelin (Wood, Portland, ME, USA) | Ion exchange | 2018 | In progress |
| Rational design and implementation of novel polymer adsorbents for selective uptake of PFAS from groundwater | Design, characterize, and evaluate novel regenerable mesoporous polymers of cyclodextrin adsorbents for in situ or ex situ remediation of PFAS‐impacted groundwater | D. Helbling (Cornell University, Ithaca, NY, USA) | Sorption | 2018 | In progress |
| Electrochemical oxidation (EO) of PFAAs in still bottoms from regeneration of ion exchange resins | Investigate how the major constituents in the ion exchange resin still bottoms (chloride and organic contents) may impact the EO treatment efficiency and the final water quality | Q. Huang [A] (University of Georgia, Athens, GA, USA) | Electrochemical | 2018 | POC follow‐on effort in progress |
| Treatment of legacy and emerging fluoroalkyl chemicals in groundwater with integrated approaches: Rapid and regenerable adsorption and UV‐induced defluorination | Develop an effective ex situ treatment train comprising oxidative pre and post treatment PFAS adsorption, and sorbent regeneration using reductive defluorination for rapid removal and complete destruction of PFAS and comingled organics (PHCs and CVOCs) in groundwater | J. Liu [A] (University of California, Riverside, CA, USA) | Sorption/UV | 2018 | In progress |
| Molecular design of effective and versatile adsorbents for ex situ treatment of AFFF‐impacted groundwater | Evaluate the propensity of PFAS to bind with proteins using a combination of molecular modeling and batch testing to verify whether PFAS–protein interactions could be tuned to efficiently adsorb a variety of PFAS | M. Michalsen (US Army Corps of Engineers, Vicksburg, MS, USA) | Sorption | 2018 |
POC follow‐on effort in progress |
| Evaluation and life cycle comparison of ex situ treatment technologies for PFAS in groundwater | Perform lifecycle cost assessment for established and emerging PFAS treatment approaches including GAC, ion exchange, GAC followed by ion exchange, and nanofiltration or reverse osmosis as well as assess technology readiness level for current and ongoing research on destructive treatment of residuals |
K. Ozekin (Water Research Foundation, Denver, CO, USA) |
Ion exchange/sorption/nanofiltration | 2018 | In progress |
| Improved longevity and selectivity of PFAS groundwater treatment using SPAC and ceramic membrane filter system | Demonstrate the long‐term effectiveness of SPAC‐CMF for the removal of a broad‐range of PFAS from groundwater at an impacted DoD site, and gather the necessary data to perform a detailed cost analysis | J. Quinnan [A] (Arcadis, Brighton, MI, USA) | Sorption | 2019 | In progress |
| Remediation of PFAS‐impacted groundwater using cationic hydrophobic polymers as ultra‐high‐affinity sorbents | Evaluate ultra‐high‐affinity cationic polyaniline and polypyrrole polymer‐based sorbents exploiting multiple bonding modes (e.g., electrostatic and hydrophobic interactions) for treatment of PFAS‐impacted groundwater | R. Sierra‐Alvarez (University of Arizona, Tucson, AZ, USA) | Sorption | 2018 | In progress |
| Regenerable resin sorbent technologies with regenerant solution recycling for sustainable treatment of PFAS | Develop a regenerable resin sorbent technology comprising commercially available ion exchange and nonionic resins for effective treatment of full diversity of PFAS present in groundwater impacted by AFFF as well as other cocontaminants | T. Strathmann (Colorado School of Mines, Golden, CO, USA) | Sorption | 2018 | In progress |
PFAS = per‐ and polyfluoroalkyl substance; PFAA = perfluoroalkyl acid; UV = ultraviolet; AFFF = aqueous foam‐forming film; PHC = petroleum hydrocarbon; CVOC = chlorinated volatile organic compound; GAC = granular activated carbon; SPAC–CMF = submicron powdered activated carbon–ceramic microfiltration; DoD = US Department of Defense.
Table 2.
Description of funded projects related to ex situ treatment of per‐ and polyfluoroalkyl substance (PFAS)‐impacted investigation‐derived wastes (IDW) and soils
| Project title | Primary objectives | Principal investigator (organization) | Treatment focus area | Start year | Status |
|---|---|---|---|---|---|
| Evaluation of indirect thermal desorption coupled with thermal oxidation technology to treat solid PFAS‐impacted IDW | Evaluate effectiveness of indirect thermal desorption coupled with thermal oxidation technology to treat PFAS‐impacted IDW solid media while addressing complete mass balance |
F. Barranco (EA Engineering, Science, and Technology, Hunt Valley, MD, USA) |
Thermal | 2018 |
Proof of concept (POC) complete |
| Enhanced oxidative destruction of PFAS in investigation derived waste soil and water | Evaluate a patented peroxone‐activated persulfate oxidation process for on‐site destruction of PFAS as well comingled chemicals (e.g., CVOCs, 1,4‐dioxane) from IDW residuals |
T. Boving (University of Rhode Island, South Kingston, RI, USA) |
Advanced oxidation | 2018 |
POC in progress |
| Pilot scale assessment of a deployable photocatalytic treatment system modified with BiPO4 catalyst particles for PFAS destruction in IDW | Evaluate the effectiveness of BiPO4, a polymorphic crystalline semiconductor material with photocatalytic properties, in the Purifics Photo‐Cat system for treatment of PFAS‐impacted groundwater |
E. Cates (Clemson University, Clemson, SC, USA) |
Photocatalytic | 2018 |
POC follow‐on effort in progress |
| Reactive electrochemical membrane (REM) reactors for the oxidation of perfluoroalkyl compound contaminated water | Evaluate cost‐effective REM for the remediation of PFAS in IDWs |
B. Chaplin (University of Illinois at Chicago, IL, USA) |
Electrochemical | 2018 |
POC in progress |
| Chemical decomposition combined with physical adsorption for the treatment of investigation‐derived waste containing PFAS | Integrate various treatment technologies, including adsorption, advanced oxidation and reductive defluorination, into one engineered system to synergistically remove and degrade PFAS in IDW under ambient conditions |
H. Cho (The University of Texas at Arlington, TX, USA) |
Sorption | 2018 |
POC in progress |
| Complete reductive defluorination of PFAS by hydrated electrons generated from 3‐indole‐acetic‐acid in chitosan‐modified montmorillonite | Develop a low‐cost, environmentally friendly “green chemistry” approach, using chitosan‐montmorillonite–based nanocomposite, for effectively degrading PFAS in IDW | H. Dong (Miami University, Oxford, OH, USA) | Sorption/advanced oxidation‐reduction | 2018 | POC in progress |
| Field demonstration of infrared thermal treatment of PFAS‐impacted soils from subsurface investigations | Demonstrate effective field treatment of PFAS‐impacted soil IDW with infrared thermal desorption and off‐gas capture, aiming at ultimate destruction | J. Hatton (CH2M Hill, Englewood, CO, USA) | Thermal | 2018 | POC complete |
| Effective destruction of PFAS in water by modified SiC‐based photocatalysts | Assess the ability of SiC‐based photocatalysts for complete molecular destruction of PFAS to achieve target water quality and reducing the time to remediation | Z. Hendren (Research Triangle Institute, Raleigh‐Durham, NC, USA) | Photocatalytic | 2018 | POC complete |
| Plasma based treatment processes for PFAS investigation derived waste | For aqueous IDW, evaluate a hybrid sorption/plasma reactor to remove PFAS onto ion exchange resin and then desorb and destroy the removed PFAS using plasma. For soil cuttings, evaluate soil washing followed by plasma‐aided PFAS destruction in the spent solution |
T. Holsen (Clarkson University, Potsdam, NY, USA) |
Thermal | 2018 |
POC in progress |
| A combined photo/electrochemical reductive pathway towards enhanced PFAS degradation | Develop a combined photo/electrochemical reduction process to treat recalcitrant PFAS and co‐occurring chemicals found in IDW generated during the study of impacted groundwater |
D. Jassby (University of California, Los Angeles, CA, USA) |
Electrochemical | 2018 |
POC in progress |
| Small‐scale thermal treatment of investigation‐derived wastes containing PFAS | Evaluate thermal decomposition of PFAS in IDW materials including use of Ca(OH)2 amendments to lower energy use and reduce VOF emissions produced during such decomposition |
P. Koster van Groos (Aptim, Princeton, NJ, USA) |
Thermal | 2018 |
POC in progress |
| High‐performance treatment of PFAS from investigation‐derived waste: Integrating advanced oxidation‐reduction and membrane concentration | Evaluate IDW treatment train approach comprising advanced oxidation, hydrated electron defluorination, and membrane‐based concentration for PFAS destruction under ambient conditions | J. Liu [B] (University of California, Riverside, CA, USA) | Advanced oxidation‐reduction | 2018 | POC in progress |
| Demonstration of smoldering combustion treatment of PFAS‐impacted investigation‐derived waste | Demonstrate the use of smoldering combustion to treat IDW (both liquid and solid) generated during investigation of PFAS‐impacted sites | D.W. Major (Geosyntec, Kingston, ON, Canada) | Thermal | 2018 | POC follow‐on effort in progress |
| Ex situ remediation of investigation‐derived wastes containing PFAS by electron beam technology | Investigate the utility of high‐energy electron beam technology as an innovative approach for on‐site treatment of IDW containing PFAS |
S.D. Pillai (Texas A&M University, College Station, TX, USA) |
Thermal | 2018 |
POC follow‐on effort in progress |
| Ex situ soil washing to remove PFAS adsorbed to soils from source zones | Demonstrate field‐scale soil washing as a cost‐effective mass removal technology to treat source zones soils containing PFAS |
J. Quinnan [B] (Arcadis, Brighton, MI, USA) |
Soil washing | 2020 | Awaiting contracting |
| Application of non‐thermal plasma technology for the removal of PFAS from Investigation‐Derived Wastes | Demonstrate the feasibility of applying dielectric barrier discharge to enhance the use of cold plasma to degrade PFAS in IDW |
C. Sales (Drexel University, Philadelphia, PA, USA) |
Thermal | 2018 |
POC follow‐on effort in progress |
| Hydrothermal technologies for on‐site destruction of site investigation wastes impacted with PFAS | Evaluate nascent hydrothermal conversion technologies coupled with low‐cost reactive amendments to destroy PFAS and co‐occurring chemicals present in IDW materials |
T. Strathmann (Colorado School of Mines, Golden, CO, USA) |
Thermal | 2018 |
POC in progress |
| Ex situ thermal treatment of perfluoroalkyl and polyfluoroalkyl substances | Field demonstration of thermal conduction heating to volatilize PFAS from ex situ soil stockpiles followed by vapor extraction and condensate treatment at Eielson Air Force Base |
J. Wehrmann (Paragon, Anchorage, AK, USA) |
Thermal | 2020 | Awaiting contracting |
| Destruction of PFAS and organic co‐occurring chemicals in water and soil present in investigation‐derived waste at DoD sites using novel adsorbent and ultrasound | Develop a low‐cost, simple to use technology using a cyclodextrin polymer and ionic liquid coated iron adsorbent for the removal of PFAS and co‐mingled organics from IDW containing groundwater and soil at DoD sites followed by ultrasonic destruction at 430 kHz |
H. Yu (Amriton, Norristown, PA, USA) |
Sorption | 2018 |
POC complete |
| A cost‐effective technology for destruction of PFAS from DoD subsurface investigation‐derived wastes using a new class of adsorptive photocatalysts | Evaluate a novel adsorptive photocatalyst comprising of activated charcoal/carbon and titanate nanotubes for adsorption and photocatalytic degradation of PFAS in IDW water; for IDW soil evaluate a dispersant for PFAS extraction followed by use of adsorptive photocatalyst to treat PFAS in the spent extractants | D. Zhao (Auburn University, Auburn, AL, USA) | Photocatalytic | 2018 |
POC follow‐on effort in progress |
PFAS = per‐ and polyfluoroalkyl substance; IDW = investigation‐derived wastes; CVOC = chlorinated volatile organic compound; SiC = silicon carbon; VOF = volatile organic fraction; DoD = US Department of Defense.
Table 3.
Description of funded projects related to in situ treatment of per‐ and polyfluoroalkyl substances (PFAS)‐impacted aqueous media, including source zone and plume
| Project title | Primary objectives | Principal investigator (organization) | Treatment focus area | Start year | Status |
|---|---|---|---|---|---|
| Combined in situ/ex situ treatment train for remediation of PFAS‐impacted groundwater | Evaluate a range of combined situ/ex situ treatment trains for treatment of PFAS‐impacted groundwater and compare the scaled‐up cost and design challenges for implementation | M. Crimi (Clarkson University, Potsdam, NY, USA) | Abiotic oxidation/plasma/ion exchange | 2018 | In progress |
| Validation of colloidal activated carbon for preventing the migration of PFAS in groundwater | Demonstrate and validate the field application of colloidal activated carbon for in situ sequestration of PFAS in source area groundwater for mitigating plume expansion | P. Hatzinger (Aptim, Lawrenceville, NJ, USA) | Sorption | 2020 | Awaiting contracting |
| Rational design and implementation of novel polymer adsorbents for selective uptake of PFAS from groundwater | Design, characterize, and evaluate novel regenerable mesoporous polymers of cyclodextrin adsorbents for in situ or ex situ remediation of PFAS‐impacted groundwater | D. Helbling (Cornell University, Ithaca, NY) | Sorption | 2018 | In progress |
| Remediation of perfluoroalkyl‐impacted aquifers using an in situ two‐layer barrier: Laboratory batch and column study | Assess the feasibility of using a permeable reactive barrier system to induce enzyme‐catalyzed humification reactions for in situ remediation of PFAS‐impacted groundwater | Q. Huang [B] (University of Georgia, Athens, GA, USA) | Bioremediation | 2011 | Proof of concept (POC) complete |
| In situ thermal treatment (ISTT) of PFAS in the vadose zone | Demonstrate the FlexHeater® thermal conduction heating system for in situ thermal treatment of PFAS in unsaturated soil coupled with destruction of PFAS condensate using UV‐sulfite and hydrothermal technologies | R. Iery (NAVFAC EXWC, Port Hueneme, CA, USA) | Thermal | 2020 | In progress |
| Quantification of in situ chemical reductive defluorination (ISCRD) of perfluoroalkyl acids in ground water impacted by AFFFs | Investigate the use of zero‐valent metals/bimetals (Pd/Fe, Mg, Pd/Mg) including Pd/Fe synthesized within clay interlayers as well as cosolvent‐assisted vitamin B12 defluorination in cost‐effective in situ treatment train for PFAS in aquifer systems | L. Lee (Purdue University, West Lafayette, IN, USA) | Abiotic reduction | 2014 | In progress |
| Anion exchange permeable adsorptive barriers (PABs) for in situ PFAS immobilization and removal | Pilot scale evaluation of PAB funnel and gate system utilizing both regenerable and nonregenerable anion exchange resins in 2 parallel gates to immobilize PFAS in situ | D. Lippincott (Aptim, Lawrenceville, NJ, USA) | Ion exchange | 2020 | Awaiting contracting |
| Bioaugmentation with vaults: Novel in situ remediation strategy for transformation of perfluoroalkyl compounds | Develop innovative in situ bioremediation technology using vault nanoparticles packaged with biodegradative enzymes to facilitate the degradation of PFAS, and potentially other chemicals in water | S. Mahendra (University of California Los Angeles, USA) | Bioremediation | 2014 | POC complete |
| In situ treatment of PFAS using D‐FAS technology | Demonstrate “D‐FAS” technology that exploits the preference for PFAS to accumulate at air/water interfaces by injecting gas bubbles at the base of the treatment well as a cost‐effective in situ solution to efficiently remove PFAS source zone mass | D. Reynolds (Geosyntec Consultants, Sydney, Australia) | Physical separation‐extraction | 2019 | In progress |
| Investigating electrocatalytic and catalytic approaches for in situ treatment of perfluoroalkyl substances in groundwater | Assess the use of electrocatalytic and catalytic approaches comprising ruthenium oxide‐coated titanium (Ti/RuO2) and other mixed metal oxide (MMO) anodes for in situ treatment of PFAS in groundwater under wide range of conditions | C. Schaefer (CDM Smith, Bellevue, WA, USA) | Electrochemical | 2014 | Complete |
| Coagulant‐enhanced sorption for in situ remediation of PFAS‐impacted groundwater systems | Develop a cost‐effective, in situ method using coagulants to sequester the 6 PFAS in the USEPA's UCMR3 list in groundwater systems to prevent their migration to drinking water supplies | M. Simcik [A] (University of Minnesota, Minneapolis, MN, USA) | Sorption | 2014 | In progress |
| In situ PFAS sequestration in AFFF‐impacted groundwater | Reduce PFAS flux from an AFFF‐impacted site to an adjacent surface water body by deploying a PAB in the saturated zone near the lake boundary | M. Simcik [B] (University of Minnesota, Minneapolis, MN, USA) | Sorption | 2020 | Awaiting contracting |
PFAS = per‐ and polyfluoroalkyl substances; AFFF = aqueous film‐forming foam; UV = ultraviolet; NAVFAC EXWC = Naval Facilities Engineering and Expeditionary Warfare Center; USEPA = US Environmental Protection Agency; UCMR3 = Third Unregulated Contaminant Monitoring Rule.
The stability of the C–F bond is believed to be due to the inherent bond strength, and the short length of the bond. The C–F bond is the strongest single bond known to organic chemistry, with a bond dissociation energy as high as 544 kJ/mol (for tetrafluoromethane). Because of the close proximity of the fluorine atoms that are bound to the carbon atoms, the outer fluorine atoms shield the underlying carbon backbone of PFAS constituents from reactive species. Owing to the extraordinary stability of the C–F bonds within PFAS, a considerable amount of energy is required to defluorinate PFAS constituents. Thus, destruction technologies for PFAS‐laden materials are relatively energy intensive, whereas nondestructive treatment processes are much less energy intensive. In general, the energy efficiency of destruction technologies for PFAS‐laden materials is greater for moderate or highly concentrated waste streams; however, most of the destruction technologies can be applied to either dilute or concentrated waste streams. Nondestructive processes may be coupled with destructive processes to achieve a complete treatment solution (e.g., use of ion exchange resin to treat groundwater, coupled with incineration of spent media). The development of complete and energy‐efficient treatment solutions is critical. Both lifecycle costs and greenhouse gas emissions will need to be taken into consideration, to compare and rank the merits of specific processes and combinations of processes.
Analysis of groundwater from AFFF fire training source areas has revealed that a large fraction of the total organic fluorine remains unmeasurable via conventional analytical methods (Schaefer et al. 2019). A groundwater sample from a DoD fire training source area was tested for the standard 24 PFAS analytes, total oxidizable precursors (TOPs), and also for total organic fluorine via combustion ion chromatography (TOF–CIC). The result from the TOP assay was added to the sum‐total result from the 24 measured PFAS analytes, and then compared with the result from TOF–CIC assay. The TOF‐CIC assay result was much higher, and comparison of the results indicated that approximately 65% of the organic fluorine present in the sample was not accounted for by adding the result from the TOP assay to the sum‐total result from the 24 measured PFAS analytes. This finding has important implications for PFAS treatment technologies. The presence of unmeasurable PFAS constituents (i.e., “dark matter”) has an important impact on the capacity of adsorbent media, and ion exchange resins. Also, the presence of unmeasurable PFAS constituents increases the energy requirements for PFAS destruction technologies and makes it more difficult to predict when change‐out of adsorbent media will be required. This is one reason why site‐specific column testing and/or pilot test data are critically important for design of full‐scale treatment systems, for estimating lifespan for treatment media, and for estimating electrical power costs for destruction technologies.
EX SITU REMEDIATION TECHNOLOGIES
Treatment of aqueous media
Aqueous media can be generally divided into 2 categories for the context of treatment: relatively dilute waste streams (e.g., groundwater and drinking water), and moderately concentrated waste streams (e.g., surface water impoundments that have been used to collect run‐off with relatively high concentrations of PFAS). Highly concentrated brines from regeneration processes are discussed later in the Treatment of residuals section.
Nondestructive treatment processes
Nondestructive treatment processes include GAC and ion exchange, which have become the default technologies for treatment of dilute aqueous media. Reverse osmosis and membrane filtration (RO/MF) processes are less widely used, but are also included in this category. Both GAC and ion exchange are generally favored over RO/MF processes because they are less energy intensive. The RO/MF processes require relatively high pressures to force the influent through nanoporous membranes.
Single‐use ion exchange resins are used more frequently than regenerable ion exchange resins. If the influent PFAS levels are high enough, then selection of a regenerable ion exchange resin may be justified. When the PFAS levels exceed a threshold concentration range (i.e., in the area of 10–100 μg/L total PFAS), then regenerable ion exchange resin may be more cost effective than single‐use. Below this range, single‐use ion exchange resin will generally be more cost‐effective (N. Hagelin [Purolite, King of Prussia, PA, USA], F. Boodoo [Purolite], and S. Woodard [ECT2, Portland, ME, USA], personal communication, 2020). An ion exchange pilot study and lifecycle comparison of costs for single‐use ion exchange versus regenerable ion exchange resin is being performed under an ESTCP project initiated in 2018 (N. Hagelin, Table 1).
Because powdered activated carbon has a much higher surface area than GAC, it also has a much greater adsorption capacity. However, use of an advanced filtration system is required for handling powdered activated carbon. The use of superfine, powdered activated carbon is being studied under an ESTCP project initiated in 2019 (J. Quinnan, Table 1). To develop modified adsorbents that are more effective for treatment of PFAS‐impacted waters, chemical modifications to organoclay‐based media are also under investigation (J. Liu [A], Table 1). Completely novel adsorbents are also under development. Examples of a novel adsorbent media include: a cyclodextrin‐based adsorbent (D. Helbling, Table 1), a mesoporous organosilica adsorbent (E. Edmiston, Table 1), and a protein‐based sorbent (M. Michalsen, Table 1). The goals of these projects are to develop adsorbents that have a higher adsorption capacity for PFAS, and also that are superior in terms of being able to remove short‐chain PFAS.
Batch test, isotherm studies are used to determine adsorption capacity (i.e., mass of chemical removed per mass of adsorbent, or mass of ion exchange resin). However, column studies must also be performed to determine the number of bed volumes of water that can be treated before breakthrough occurs. During treatment via either adsorption or ion exchange, the short‐chain PFAS are usually the first to break through. Thus, comparative column studies measuring the number of bed volumes until breakthrough of the high‐priority, short‐chain PFAS must be performed to compare different types of adsorbents. A comparative study of different types of ion exchange resins is being conducted (T. Strathmann, Table 1). In addition, a comparative assessment of lifecycle costs of several different treatment processes, including GAC, ion exchange, nanofiltration, and reverse osmosis, as well as superfine powdered activated carbon, is also underway (K. Ozekin, Table 1).
For moderately concentrated aqueous waste streams, a pretreatment step such as foam fractionation, polymer/coagulant addition, or electrocoagulation may need to be used to knock down high influent concentrations. Foam fractionation is one of a handful of treatment processes that takes advantage of the inherent attraction of PFAS to the air/water interface (AWI) to remove PFAS from the aqueous phase. This strategy is generally less effective for short‐chain PFAS constituents, which are not as strongly attracted to the AWI as longer chain‐length PFAS constituents. An electrocoagulation process is being investigated as a pretreatment step under a recent SERDP project (D. Chiang, Table 1). Coagulation processes are usually followed by sedimentation. A secondary treatment step (e.g., GAC or ion exchange) would typically be required following one of the above pretreatment steps.
Destructive treatment processes
Most of the destructive processes require electrical power to operate the treatment systems. Power is required to drive the oxidative and/or reductive processes, and in some cases, for ultraviolet (UV) light. The amount of power required is dependent on the influent PFAS concentrations, and also on a host of water quality parameters (e.g., dissolved organic carbon, total dissolved solids, and cocontaminants). This category includes the following treatment processes: nonthermal plasma, electrochemical, UV‐activated, and sonolysis. Although it doesn't necessarily require power, biological treatment is also included in this category. Ultimately, the various treatment technologies under development will have to be compared using consistent metrics for energy requirements (e.g., kilowatt hour/cubic meter of water treated [kWh/m3]). The energy metrics must also take into account the degree of PFAS destruction achieved (e.g., kWh/m3 per log reduction in PFAS concentration). Comparisons among plasma, electrochemical, UV‐persulfate, and sonolysis processes in terms of the energy efficiency for defluorination of perfluorooctanoic acid (PFOA) indicate that a high‐efficiency plasma process is 15% more efficient than an electrochemical process, 2.9 times more efficient than high‐rate plasma, 10 times more efficient than sonolysis, and 30 times more efficient than UV‐persulfate (Stratton et al. 2017). The high‐rate and high‐efficiency plasma processes are the same basic process; however, the high‐rate reactor allows for a higher flow rate.
Nonthermal plasma treatment processes rely on contact between rapid‐fire pulses of electrical discharge streamers and contaminated media. The electrical discharge plasma reactor developed by S. Mededovic‐Thagard and co‐workers at Clarkson University (Potsdam, NY, USA) operates by circulating a shallow layer of water through a reactor with a row of electrodes above the water surface. The reaction between contaminants and the arcs of electricity occurs at the AWI. Because the reaction occurs primarily at the surface, the amount of electrical power required for plasma treatment is believed to be relatively insensitive to water quality parameters that could cause adverse impacts in other types of treatment processes. Rows of fine‐bubble diffusers are used to move the PFAS constituents up to the surface. The PFAS are attracted to the AWI of the fine bubbles. In terms of level of development, the Clarkson plasma reactor that is being investigated under several projects (M. Crimi; N. Hagelin; T. Holsen, Table 1) is further along than most of the other ex situ treatment process being funded by SERDP. A similar plasma‐based treatment process is also being investigated and has recently successfully completed the first year proof‐of‐concept (C. Sales, Table 1).
Electrochemical treatment has been shown to be capable of destroying PFAS, and is capable of treating both dilute and relatively concentrated waste streams. Many different types of electrodes have been tested. Boron‐doped diamond electrodes have been shown to be an effective material for conventional electrochemical cell configurations. Other types of configurations and electrode materials are also under investigation. A system currently under development (B. Chaplin, Table 1) is configured such that the influent must flow through a porous electrode composed of titanium oxide (Ti4O7). This configuration ensures intimate contact between contaminants and the electrode surface. An electrochemical cell using solid Ti4O7 electrodes is also being tested (Q. Huang, Table 1). One advantage of electrochemical treatment is that it will not be adversely affected by the high ionic strength of brine regenerant solutions. A high level of ionic strength is desirable for conventional electrochemical cell systems because the effectiveness of treatment is dependent on the conductivity of the fluid within the electrochemical cell. However, if chloride is present, perchlorate may be generated, as an undesirable byproduct of electrochemical treatment.
Ultraviolet‐light activated processes are a component of several projects. The reaction between UV light (at 254 nm) and sulfite can be used to generate hydrated electrons and sulfite‐free radicals. The only byproduct of this reaction is sulfate. Destruction of PFAS constituents occurs due to reactions with hydrated electrons. Two SERDP projects are currently investigating UV‐sulfite treatment (J. Liu [A]; C. Schaefer, Table 1).
A BOHP UV reactor is currently being developed for PFAS treatment (E. Cates, Table 1). The reactor utilizes catalyst particles, consisting of Bi3O(OH)(PO4)2 microaggregates (BOHP) and bismuth phosphate (nano‐BiPO4 micro rods) The reactor design relies on mixing the influent with the catalyst suspension, which circulates through a set of tubes. The center of the tubes is occupied by a UV light source (a low‐pressure mercury lamp). After treatment, the catalyst suspension is separated from the treated water, and recovered for re‐use.
Photocatalytic processes are also being investigated in a recently completed proof‐of‐concept project (D. Zhao, Table 1). Researchers are developing a novel composite material with an activated carbon core and a photocatalyst shell of titanate nanotubes with high adsorption capacity and photochemical degradation toward PFAS and regeneration capability.
Sonolysis relies on use of ultra‐high‐frequency sound waves to induce formation of short‐lived microbubbles in solution. Violent collapse of the bubbles creates microsites with extremely high temperatures and pressures. Formation of free radicals also occurs during collapse of the microbubbles. The combination of high temperature, pressure, and free radicals allows for destruction of PFAS constituents. The sonolysis process benefits from the attraction of PFAS constituents to the AWI of the microbubbles. Sonolysis was used by one project for destruction of PFAS, following concentration and desorption steps (H. Yu, Table 2).
The majority of studies regarding fate and transport processes for PFAS have shown only limited biodegradation of some PFAS, or no biodegradation whatsoever (Pancras et al. 2016). Under aerobic conditions, some types of PFAS constituents (fluorotelomer alcohols and sulfonamides) can be biotransformed into dead‐end products such as carboxylates and sulfonates, for example, 8:2 fluorotelomer alcohol → PFOA, and perfluorooctane sulfonamide → perfluorooctane sulfonic acid (PFOS). However, researchers at Princeton University (Princeton, NJ, USA) have recently discovered a biodegradation process that may be capable of complete mineralization of PFOA and PFOS (Huang and Jaffé, 2019). Instead of electrical power, the Feammox process relies on a steady supply of iron (Fe) and ammonia. During the Feammox process, Fe(III) serves as the electron acceptor, and ammonia serves as the electron donor. The autotrophic bacterium that has been isolated, Acidimicrobiaceae sp. A6, is capable of respiring PFOA and PFOS, but only for a portion of its electron acceptor requirements. The study of Feammox as a PFAS treatment technology is in its infancy. Before Feammox can be considered economically viable, PFAS degradation rates would have to be shown to be rapid enough to demonstrate that it could be performed within a reasonably sized reactor. In theory, a Feammox‐based system similar to a constructed wetlands basin could be designed for treatment of PFAS‐impacted surface water. P.R. Jaffé and his team are continuing to work on this biodegradation process under a new SERDP project (P.R. Jaffé, Table 1).
Several projects are also investigating combinations of treatment processes in a treatment train approach. A set of combined in situ/ex situ treatment approaches is being investigated whereby in situ groundwater treatment via oxidation will be combined with ex situ treatment via regenerable ion exchange resin, while plasma is used for treatment of the regenerant solution (M. Crimi, Table 1). Another project team is developing a process combining photolysis and electrochemical reduction (D. Jassby, Table 1). A system combining physical adsorption, advanced oxidation, and reductive defluorination is being assessed (H. Choi, Table 2), and another team is examining a treatment train utilizing chemical oxidation followed by regenerable adsorption and defluorination via UV‐sulfite–generated hydrated electrons (J. Liu [A], Table 1).
Treatment of IDW and soils
Table 2 provides the description of all funded projects related to ex situ treatment of PFAS‐impacted IDW and soils under SERDP and ESTCP. The IDW may include both liquids and solids. The present discussion will focus on solids, as well as materials containing very high levels of solids (e.g., slurries, muds, and high‐turbidity liquids). However, some of the treatment processes we describe would also be suitable for liquids.
Thermal, hydrothermal, and supercritical water oxidation (SCWO) processes are being researched for treatment of IDW and/or soils. Low‐temperature thermal desorption (LTTD) is best suited for relatively low‐moisture‐content wastes (e.g., soils), whereas hydrothermal processes are more suitable for high‐moisture‐content wastes. Relatively high temperatures (at least 650 °C) are required for complete desorption of PFAS constituents from soil (Barranco et al. 2019). Any moisture present in the soil must first be driven off, to achieve such high temperatures. A great deal of energy is required to “burn” off the pre‐existing moisture. Hydrothermal and SCWO processes use a combination of heat and pressure for destruction of PFAS constituents, without the need to drive off moisture, thereby allowing for destruction to occur at lower temperatures. Data from a recent SERDP project indicate that destruction temperatures for hydrothermal treatment can be further reduced by elevating the pH (i.e., alkaline hydrothermal treatment; Wu et al. 2019).
The traditional thermal treatment technologies that have been used for soil treatment include incineration and low‐temperature thermal desorption. The LTTD process is used to move the PFAS constituents from the solid to the vapor phase, although a limited degree of thermal oxidation may occur, depending on operational temperatures. The PFAS‐laden vapors that are generated during LTTD can be either condensed or routed through a thermal oxidizer for on‐site destruction. During thermal treatment, when contaminants are collected in the condensate, they are typically concentrated by passing the condensate through GAC. The GAC would then be sent away for off‐site disposal. On‐site operation of a thermal oxidizer usually requires an air permit, or meeting the substantive requirements of an air permit. On‐site LTTD, with capture of condensate for off‐site disposal, is often deemed to be more readily implementable, because it usually eliminates the need to obtain an air permit, or to meet substantive requirements of an air permit.
A fluorine mass balance was conducted using data from a pilot‐scale thermal treatment system for PFAS‐impacted soil (Barranco et al. 2019). The system included a pilot‐scale indirect thermal desorption unit coupled with a thermal oxidizer for destruction of vapors from the thermal desorption unit. After thermal oxidation of the PFAS‐laden vapors, the fluorine mass balance indicated that nearly all the PFAS present in the spiked soil was converted into hydrogen fluoride (HF) gas. The 2 separate trial tests, with each trial test run in duplicate, resulted in fluorine mass recoveries (recovered as HF gas), that ranged from 84 to 114%, with a mean of 99%. The measured destruction and removal efficiency for the soil treatment system was 99.9997%, whereas the thermal desorption unit was operated at 650 °C with a residence time of 1 to 1.5 h. The thermal oxidizer was operated at 1000 °C, with a residence time of 2 s.
Blending of calcium hydroxide (Ca(OH)2) into PFAS‐impacted soil prior to thermal treatment is being tested (P. Koster van Groos, Table 2). The presence of Ca(OH)2 may allow for thermal destruction of PFAS at a lower temperature and may also reduce generation of HF emissions due to formation of fluorite residue. A smoldering combustion process is also being investigated for PFAS‐impacted soils and IDW (D.W. Major, Table 2). Smoldering combustion requires the presence of organic matter, which can be added to the soil, unless the natural organic matter content is already high enough. After ignition of the organic matter, a high‐temperature heat‐front slowly moves through the soil causing desorption and/or destruction of the PFAS constituents without the need for further addition of energy into the system. Due to the potential for volatilization, smoldering combustion may require an emissions control system.
Preliminary testing of high‐energy electron beam has been completed under a proof‐of‐concept study (S.D. Pillai, Table 2). The process relies on an electrically powered accelerator to generate high concentrations of electrons, which react with water to produce free radicals, hydrogen peroxide, hydrogen atoms, and hydrated protons. In theory, the beam gun would be positioned above the contaminated media, with a conveyance system to allow the media to pass under the beam. The beam causes unavoidable heating of the media, which may contribute to degradation reactions. Due to the heating, this type of process may require an emissions control system.
Several projects are also investigating combinations of the treatment processes. Studies combining adsorption and photolysis are being conducted in 2 projects, one of which was previously described in Destructive treatment processes (D. Zhao, Table 2). A chitosan‐modified montmorillonite nanocomposite will be used to adsorb both 3‐indole acetic acid (IAA) and PFAS constituents (H. Dong, Table 2). On exposure to UV light, hydrated electrons are released from IAA. The hydrated electrons are capable of defluorinating PFAS constituents.
Treatment of residuals
The term residuals includes materials such as spent treatment media (GAC, ion exchange resin), concentrated brines derived from regeneration of ion exchange resin, and rejectate from membrane filtration processes such as reverse osmosis. Many of the treatment processes previously discussed are also applicable to treatment of residuals. Energy content, moisture content, ionic strength, corrosivity, and foaming potential are all important properties for residuals, which can affect treatment/disposal alternatives. For GAC, the option or reactivation and reuse must also be considered. Interactions between PFAS constituents and GAC during thermal treatment are also worth reviewing because the presence of GAC can have profound, and unexpected, effects on the behavior and destruction of PFAS constituents.
Water content is a key aspect of residuals treatment. High water content residuals will be more amenable to processes such as hydrothermal and SCWO. Energy content of residuals is also an important factor for some types of treatment processes. The British thermal unit (BTU) content of the waste can, in some cases, off‐set the amount of energy required to drive the treatment process. For LTTD and incineration, low water content and high BTU content are desired. Ion exchange resin has a BTU content of approximately 12 000 BTU/lb, whereas GAC has a BTU content of 2000 to 4000 BTU/lb. However, during incineration of GAC, the majority of the combustion occurs in the primary combustion chamber, and only a limited amount of organic vapors are generated. For the waste material to be beneficial toward off‐setting the amount of fuel required to operate the secondary combustion chamber (SCC), the organic vapors must carry through to the SCC. Thus, GAC represents a low‐value feedstock for incinerators, whereas ion exchange resin represents a higher value feedstock because the organic vapors from ion exchange resin can significantly contribute to off‐setting the cost of fuel for operation of the SCC.
For cement kilns, the BTU content of spent GAC can significantly offset fuel requirements. Unlike most incineration facilities, where the primary combustion is indirectly fired, cement kilns are directly fired. Thus, the heating of the kiln, due to combustion of the GAC, contributes toward reducing fuel requirements.
Immediately after removal from adsorption vessels, spent GAC and spent ion exchange resin will have a high moisture content. The high moisture content can reduce the desirability of the material for incineration facilities and cement kilns because additional fuel will be required to drive off the moisture. Thus, the moisture content can partially off‐set the BTU content and reduce the value of the material as a feedstock for the thermal treatment facility.
During reactivation of spent GAC, destruction of PFAS can occur on the surface of the GAC (i.e., while the PFAS constituents remain in the adsorbed phase), or the PFAS constituents may move into the vapor phase before destruction occurs. Temperature can be used as a variable to control the extent of destruction on the surface of the GAC versus the extent to which transfer to the vapor phase occurs. Laboratory studies have shown that destruction of PFAS constituents on the surface of the GAC can be favored by limiting heating to 700 °C, or slowly increasing the temperature after reaching 700 °C (Watanabe et al. 2016). At 800 and 900 °C, an increased percentage of the adsorbed PFAS constituents were shown to move into the vapor phase. At 700 °C, that study indicated that a much greater degree of PFAS destruction occurred for PFAS that were adsorbed onto GAC, relative to PFAS in the absence of GAC. However, Watanabe et al. (2018) also showed that a temperature of 1000 °C was necessary for nearly complete destruction of vapor‐phase PFAS constituents. This conclusion was based on mass balance data for PFAS destruction, showing that volatile organic fluorine was <0.1% of adsorbed PFAS when the process was operating at 1000 °C. The authors therefore suggested that a primary combustion chamber temperature set to ramp up slowly after reaching 700 °C, in combination with an SCC temperature of 1000 °C, would be desirable for reactivation of PFAS‐laden GAC, in that it would maximize destruction of PFAS on the surface of the GAC, limit corrosive HF emissions, and minimize fuel consumption.
The extent to which the adsorbent media has been loaded with PFAS can also affect reuse/disposal options. Media that have been highly loaded with PFAS can be undesirable for thermal treatment processes because of the amount of corrosive HF gas that will be generated during treatment. Reactivation and reuse of spent GAC can be an option when the PFAS influent levels are relatively low. However, if the PFAS influent concentration approaches the area of 100 μg/L (based on the sum of the 24 commonly measured PFAS constituents), GAC vendors may no longer accept the spent material for reactivation, and it may have to be sent to an incinerator.
One difficulty that has been observed during treatment of concentrated brines is that, because the concentration of PFAS constituents is so high, foaming may interfere with the treatment process. The electrical discharge plasma reactor relies on fine‐bubble diffusers to move PFAS toward the surface, which has caused the foaming. During a treatability study (N. Hagelin, Table 1), it was found that the brine concentrate had to be diluted by a factor of 10, to prevent foaming from interfering with operation of the plasma reactor. During the same study, the Clarkson University team, led by T.M. Holsen, also found that improved removal of short‐chain PFAS constituents (e.g., perfluorobutane sulfonate [PFBS]) could be achieved by adding a cationic surfactant to the diluted brine solution. The complex that formed between PFBS and the cationic surfactant was more strongly attracted to the AWI of the bubbles than PFBS alone. Antifoaming agents often have to be mixed into concentrated PFAS residuals prior to incineration to prevent foaming. However, the high BTU content of the antifoaming agents may interfere with incinerator operations by causing rapid fluctuations in oxygen concentrations in the kiln, and the SCC. Treatment of concentrated brines is being investigated under 2 separate projects (M. Fuller; Q. Huang, Table 1).
IN SITU REMEDIATION TECHNOLOGIES
Table 3 provides a description of all funded projects related to in situ treatment of PFAS‐impacted aqueous media, including source zone and plume under SERDP and ESTCP. Injection of amendments for long‐term sorption of PFAS constituents is being investigated under 2 projects (P. Hatzinger; D. Helbling, Table 3). Colloidal activated carbon amendments have been commercially available for several years for in situ treatment of chloroethene‐contaminated groundwater, and these same materials are being tested for PFAS treatment. Polydiallyldimethylammonium chloride, a polymer‐based sequestrant commonly referred to as polyDADMAC, is also being investigated under 2 separate projects (M. Simcik [A]; M. Simcik [B], Table 3). Destruction of PFAS constituents does not occur via the use of any of these sorbent‐based amendments. However, they can be effective as a passive means for containment of groundwater plumes. To assess the long‐term effectiveness of sorbent‐based amendments, several years of monitoring at field test sites will be required.
Although they were originally developed under ESTCP for treatment of chlorinated solvents (Divine et al. 2017), horizontal flow reaction wells (HRX) may also be applied for in situ treatment of PFAS. The HRX wells are installed perpendicular to the direction of groundwater flow, and are designed to create a preferential flow conduit that draws in water from an upgradient area. Water that is drawn into the HRX well then flows through treatment cartridges, containing adsorbent media, before being discharged to the outlet of the HRX well. The treatment cartridges can be removed, and periodically replaced with new cartridges containing fresh media. Laboratory studies have been performed to assess design parameters for HRX wells and sorption of PFAS onto various types of GAC‐based media (M. Crimi [B], Table 3). An HRX well is currently being designed for PFAS‐impacted groundwater at Peterson Air Force Base (Colorado Springs, CO, USA). It may also be possible to use in‐well destructive processes (e.g., sonolytic reactors), connected to an above‐ground electrical power source, in conjunction with HRX wells.
Development of a new type of permeable adsorptive barrier (PAB) for PFAS treatment will be tested under a new ESTCP project (D. Lippincott, Table 3). Both regenerable and single‐use ion exchange resin will be tested for this in situ PFAS treatment as part of the project. A funnel and gate layout will be incorporated into the design of the PAB system.
In situ thermal treatment system for PFAS‐impacted soil in the vadose zone will also be tested (I. Iery, Table 3). The system will utilize thermal conduction heating (TCH), with a vapor extraction system coupled with ex situ destruction of PFAS condensate using UV‐sulfite and hydrothermal destruction technologies. The TCH system will be comprised of a coiled single wire heated to 900 °C and can deliver varying amounts of heat vertically in a steel heater well.
The term “D‐FAS” was coined to describe an in situ treatment process that is being tested to concentrate PFAS constituents from groundwater within treatment wells (D. Reynolds, Table 3). The treatment process involves injection of air and/or ozone, in the form of fine bubbles, near the bottom of the treatment well. The PFAS are attracted to the AWI of the bubbles. Air‐lift pumping within the treatment well creates a circulation cell that moves a portion of the surrounding groundwater toward the treatment well. During treatment, a foam concentrate forms at the surface of the water table, within the treatment well. Periodically, the foam concentrate is removed, and sent away for off‐site treatment. Geology is a critical factor for D‐FAS, and it is most likely to be viable only for high‐to‐moderate permeability settings.
CONCLUSIONS
As regulations evolve, treatment processes will need to be proved to be capable of treating PFAS constituents that were previously not measured, and previously unregulated. Also, improvements in capabilities for measuring total organic fluorine, volatile fluorinated constituents, and fluorinated transformation products will have to be taken into account during assessment of treatment performance. Anticipated changes in regulations governing treatment and disposal of media will have important impacts. The potential for new regulatory restrictions (e.g., increasingly stringent cleanup levels, landfilling restrictions, hazardous waste classification, and emissions criteria), and the need to limit greenhouse gas emissions will create new challenges. Developmental technologies must take all of these “moving targets” into consideration. Also, the presence of currently unmeasurable PFAS constituents will continue to present challenges for the design and implementation of treatment systems.
A wide array of new technologies is currently under development for treatment of PFAS‐laden materials. However, to be commercially viable, and receive widespread adoption, new technologies must be shown to be cost effective, energy efficient, and competitive with established technologies, capable of meeting stringent clean‐up criteria, and have a favorable greenhouse gas emission profile. It is critically important that standardized performance data be generated during development and demonstration of new technologies. Execution of experimental protocols that allow for consistent measurements is imperative for objective and consistent comparisons of treatment technologies. Standardized performance data must be obtained for both nondestructive (e.g., adsorption capacity and bed‐volumes until breakthrough) and destructive technologies (e.g., energy consumption and defluorination efficiency). For destructive technologies, fluorine mass balance data are essential for determining the extent to which complete mineralization is achieved.
The established, nondestructive technologies such as GAC and ion exchange are likely to continue to be the default choice for treatment of impacted groundwater, for at least the next several years. However, opportunities are emerging to couple existing nondestructive technologies with new types of destruction technologies. At the same time, new and improved nondestructive technologies are also rapidly emerging, in addition to new in situ treatment alternatives. Development of improved treatment trains, and innovative combinations of nondestructive and destructive technologies will eventually lead to advanced capabilities for remediation of PFAS‐laden materials.
Disclaimer
The views expressed in this article are those of the individual authors and do not necessarily reflect the views and policies of the US Army Corps of Engineers, or the US Army. Mention of trade names or commercial products does not constitute endorsement or recommendation for use.
[Correction added 18 February, 2021 after initial online publication. The article copyright was changed to Open Access.]
Data Availability Statement
Data, associated metadata, and calculation tools are available from the corresponding author (Charles.G.Coyle@usace.army.mil).
REFERENCES
- Barranco F, Caprio P, Harvey I, Hay G, Palmer C. 2019. Evaluation of indirect thermal desorption coupled with thermal oxidation (ITD/TO) technology to treat solid PFAS‐impacted investigation‐derived waste (IDW). Project ER18‐1572. Proceedings, SERDP ESTCP Symposium, 3–5 December, 2019, Washington, DC, USA.
- Divine CE, Roth T, Crimi M, DiMarco AC, Spurlin M, Gillow J, Leone G. 2017. The horizontal reactive media treatment well (HRX Well®) for passive in‐situ remediation. Ground Water Monit R 38:56–65. [Google Scholar]
- Huang S, Jaffé PR. 2019. Defluorination of perfluorooctanoic acid (PFOA) and perfluorooctane sulfonate (PFOS) by Acidimicrobium sp. strain A6. Environ Sci Technol 53:11410–11419. [DOI] [PubMed] [Google Scholar]
- Pancras T, Schrauwen G, Held T, Baker K, Ross I, Slenders H. 2016. Environmental fate and effects of poly and perfluoroalkyl substances (PFAS). Concawe, Brussels, Belgium.
- Schaefer C, Andaya C, Higgins C, Maizel A, Burant A, Strathmann T, Ferguson L, Choyke S. 2019. Investigating electrochemical and catalytic approaches for in situ treatment of PFAS in groundwater. Project ER‐2424. Proceedings, SERDP ESTCP Symposium, 3–5 December, 2019, Washington, DC, USA.
- Stratton GR, Dai F, Bellona CL, Holsen TM, Dickenson ERV, Mededovic‐Thagard S. 2017. Plasma‐based water treatment: Efficient transformation of perfluoroalkyl substances in prepared solutions and contaminated groundwater. Environ Sci Technol 51:1643–1648. [DOI] [PubMed] [Google Scholar]
- Watanabe N, Takata M, Takemine S, Yamamoto K. 2018. Thermal mineralization behavior of PFOA, PFHxA, and PFOS during reactivation of granular activated carbon (GAC) in nitrogen atmosphere. Environ Sci Pollut Res 25:7200–7205. [DOI] [PubMed] [Google Scholar]
- Watanabe N, Takemine S, Yamamoto K, Haga Y, Takata M. 2016. Residual organic fluorinated compounds from thermal treatment of PFOA, PFHxA and PFOS adsorbed onto granular activated carbon (GAC). J Mater Cycles Waste Manag 18:625–630. [Google Scholar]
- Wu B, Hao S, Choi Y, Higgins C, Deeb R, Strathmann T. 2019. Rapid destruction and defluorination of perfluorooctane sulfonate by alkaline hydrothermal reaction. Environ Sci Technol Lett 6:639–636. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data, associated metadata, and calculation tools are available from the corresponding author (Charles.G.Coyle@usace.army.mil).


