Abstract
Patients in sub‐Saharan Africa generally have poor anticoagulation control. We review the potential reasons for this poor control, as well as the potential solutions. Challenges include the affordability and centralisation of anticoagulation care, problems with access to medicines and international normalised ratio monitoring, the lack of locally validated standardized dosing protocols, and low levels of anticoagulation knowledge among healthcare workers and patients. Increasing numbers of patients will need anticoagulation in the future because of the increasing burden of noncommunicable disease in the region. We propose that locally developed “warfarin care bundles” which address multiple anticoagulation challenges in combination may be the most appropriate solution in this setting currently.
Keywords: anticoagulants, cardiovascular disease, drug utilization, medication safety
1. INTRODUCTION
Anticoagulation is used to treat and prevent venous thrombosis and to prevent intracardiac thrombosis due to some structural heart diseases or dysrhythmias. Depending on the indication, treatment may be lifelong. Over‐anticoagulation may result in bleeding and under‐coagulation in thrombotic complications, including stroke. With the advent of direct oral anticoagulants (DOACs), there is increased choice of drugs available. However, vitamin K antagonists such as warfarin remain the most widely used oral anticoagulants and form the focus of this article.
When using vitamin K antagonists, the degree of anticoagulation is measured by the international normalised ratio (INR), and a patient's longitudinal anticoagulation control can be described by the proportion of INRs which fall in the therapeutic range (PTR), or by the proportion of time spent in the therapeutic range (TTR), interpolating INR results for the time between actual INR measurements. Patients in sub‐Saharan Africa (SSA) have poor anticoagulation control as measured by their PTR/TTR. For example, under trial conditions South African participants in three large trials had mean TTRs of 55%,1 46%2 and 58%.3 In an atrial fibrillation (AF) registry mean TTR in nine African countries was 33% (vs 62% in 19 Western European countries)4 and in a rheumatic heart disease registry predominantly conducted in African countries PTR was 28%.5 A number of smaller observational studies from SSA are presented in Table 1; in all but one of these PTR or TTR ranged between 28% and 52%.
TABLE 1.
Observational studies from sub‐Saharan Africa which measured time in therapeutic range (TTR) or proportion of INR results in therapeutic range (PTR) among patients on anticoagulation
| Study, publication year | Setting | Sample characteristics | Sample size | Mean or median TTR | Mean or median PTR |
|---|---|---|---|---|---|
| Makubi,6 2008 |
Dar Es Salaam, Tanzania National referral hospital |
Patients with mechanical heart valves attending the ACC | 189 | 36% | |
| Manji,7 2011 |
Eldoret, Kenya Teaching hospital |
Adults attending ACC | 178 | 65% | |
| Menanga,8 2015 |
Yaoundé, Cameroon A general and a central hospital |
Adult inpatients and outpatients with AF on VKA | 27 | 48% | |
| Daba,9 2016 |
Addis Ababa, Ethiopia Teaching hospital |
Adults with VTE on anticoagulation | 91 | 34% | |
| Mariita,10 2016 |
Nairobi, Kenya Teaching hospital |
Adults attending cardiac, cardiothoracic or haemato‐oncology clinic for anticoagulation monitoring | 147 | 44% | |
| Sadhabiriss,11 2016 |
Durban, South Africa District‐level hospital |
Patients with nonvalvular AF or prosthetic heart valves attending outpatient department on warfarin for ≥12 mo | 177 | 30% | |
| Sonuga,12 2016 |
Cape Town, South Africa District hospital |
Adults attending ACC and on warfarin for ≥30 d | 136 | 49% | |
| Ahmed,13 2017 |
Khartoum, Sudan Specialist hospital |
Adults attending ACC and on warfarin for ≥1 yr | 135 | 52% | |
| Fenta,14 2017 |
Addis Ababa, Ethiopia Teaching hospital |
Adults attending cardiology or haematology outpatient clinics on warfarin for ≥3 mo | 360 | 29% | |
| Nyamu,15 2017 |
Nairobi, Kenya Teaching hospital |
Adults attending cardiac, cardiothoracic or haemato‐oncology clinics on warfarin | 102 | 28% | |
| Coulibaly,16 2018 |
Abidjan, Côte d'Ivoire Specialist hospital |
Adults with nonvalvular AF on VKA for ≥6 mo and with ≥6 INR measurements | 100 | 44% | |
| Ebrahim,17 2018 |
Cape Town, South Africa A specialist hospital and a community health centre |
Patients attending two ACCs and on warfarin for ≥27 mo with regular INR monitoring | 363 | 47% | |
| Mwita,18 2018 |
Gaborone, Botswana Tertiary hospital |
Patients attending medical outpatient clinics on warfarin for ≥1 mo with ≥4 INR measurements | 410 | 31% | |
| Jonkman,19 2019 |
Windhoek, Namibia Central hospital |
Patients attending ACC with ≥3 INR values | 215 | 29% | 25% |
| Karuri,20 2019 |
Nairobi, Kenya Teaching hospital |
Patients attending cardiac, cardiothoracic or haemato‐oncology clinics on warfarin for ≥1 mo with ≥2 INR measurements | 406 | 31% | |
| Botsile,21 2020 |
Gaborone, Botswana Tertiary hospital |
Adults with mechanical heart valves attending ACC on warfarin for ≥1 mo with ≥3 INR measurements | 142 | 30% | |
| Semakula,22 2020 |
Kampala, Uganda and Cape Town, South Africa A primary level health centre and four secondary and tertiary level hospitals |
Patients attending five ACCs | 229 | 41% |
ACC, anticoagulation clinic; AF, atrial fibrillation; INR, international normalised ratio; PTR, proportion of INR results in therapeutic range; TTR, time in therapeutic range; VKA, vitamin K antagonist; VTE, venous thromboembolism.
In this review, we explore potential reasons for this poor anticoagulation control in SSA and explore strategies to overcome these challenges.
2. METHODS
For this narrative (nonsystematic) review, we searched Pubmed and Africa‐Wide Information through EBSCOhost, using combinations of index terms (eg, “anticoagulants”, “Africa South of the Sahara”) and free text (eg, “anticoagulation”, “warfarin”, individual country names). We retained citations clustering around four themes: (1) burden of anticoagulation indications; (2) challenges accessing anticoagulation; (3) challenges with selecting and adjusting anticoagulant dose; and (4) patient‐related challenges including adherence, knowledge, attitudes and beliefs, and genetic factors. We also searched Google and dissertation databases (ProQuest Dissertations & Theses A&I, Open Access Theses and Dissertations (oatd.org)) for grey literature from SSA on these issues. Further reports were found by handsearching reference lists of included studies. Searches were conducted between March and May 2020.
3. CHANGING LIFE EXPECTANCY AND DISEASE BURDEN
SSA is undergoing an epidemiological transition: with increasing life expectancy the increasing burden of noncommunicable disease is colliding with the pre‐existing burden of infectious diseases. In the context of anticoagulation, this transition is evident in the increasing prevalence of (nonvalvular) AF, adding to the large number of people requiring anticoagulation for valvular heart disease, which in SSA is still mostly caused by rheumatic heart disease.23 This increase in indications for anticoagulation is occurring amidst an uneven distribution of limited resources. For example, there were only 57 centres in SSA capable of performing regular open‐heart operations in 2011/2012 (one per 15 million population); 35 of these were in South Africa.24 Figure 1 shows some examples of SSA anticoagulation studies mentioning resource limitations.
FIGURE 1.
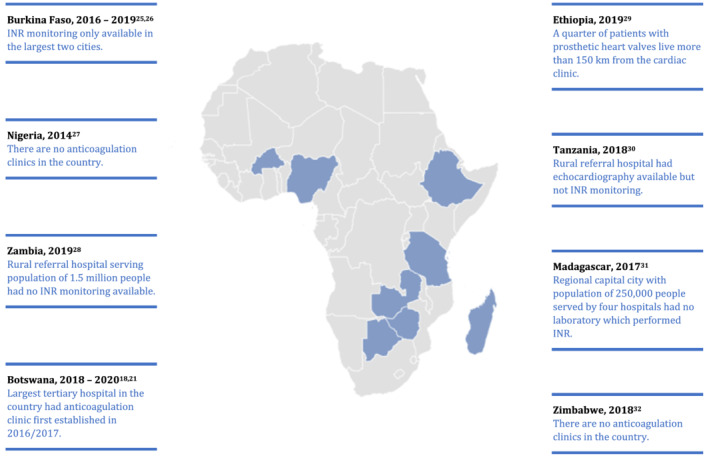
Example observational studies from sub‐Saharan Africa which describe resource limitations
A recent systematic review suggested that AF prevalence in SSA may be higher than previously thought,33 up to 4.3% in one Ethiopian community‐based survey.34 Patients with AF in SSA have high prevalence of concomitant stroke risk factors33, 35 and should therefore benefit from anticoagulation. However, despite clear indications for anticoagulation, it is not always implemented in SSA.26, 36, 37, 38, 39, 40, 41 Indeed, in the multiregional RELY‐AF registry,4 patients in Africa had the second‐lowest use of oral anticoagulation where indicated (19%, second only to China).
Rheumatic heart disease prevalence among school children in SSA is 1.5‐3.0%.42, 43, 44, 45, 46, 47 In rheumatic heart disease registries, only two‐thirds of patients with an indication for oral anticoagulation received it.5, 37, 48
Venous thromboembolism (VTE) epidemiology in SSA has not been well described,49 but HIV infection is a well‐established risk factor, associated with a 1.5‐fold increased hazard (95% confidence interval 1.1 to 2.0).50 As the HIV pandemic epicentre, HIV‐associated VTE is commonly seen in SSA. In all the studies in Table 2, HIV prevalence was higher among patients presenting with VTE than the background prevalence. Interestingly, in a Ugandan study 9% of patients on antiretroviral therapy attending routine outpatient follow‐up were found to have incidental deep venous thrombosis.51
TABLE 2.
Studies from sub‐Saharan Africa reporting the prevalence of HIV and tuberculosis among patients presenting to hospitals for venous thromboembolism
| Study | Setting | Population HIV prevalencea | Proportion HIV infected | Proportion TB infected |
|---|---|---|---|---|
| Mampuya52 | Regional/tertiary hospital, Kimberley, Northern Cape, South Africa, 2010‐2014 | 11.0% | 443/852 (52%) | 106/852 (12%) |
| Louw53 | Tertiary hospital, Johannesburg, Gauteng, South Africa, date NR | 16.9% | 11/24 (46%) | NR |
| Goldstein54 | Tertiary hospital emergency centre, Johannesburg, Gauteng, South Africa, 2012‐2013 | 17.8% | 35/70 (50%) | 21/70 (30%) |
| Awolesi55 | Urban district hospital, KwaZulu‐Natal, South Africa, 2013 | 27.0% | 42/81 (52%) | 29/81 (36%) |
| Olubanwo56 | Tertiary hospital, Mthatha, Eastern Cape, South Africa, 2010 | 18.0% | 81/102 (79%) | 42/102 (41%) |
| Alshehri57 | District hospital, Cape Town, Western Cape, South Africa, 2008‐2011 | 8.9% | 393/610 (64%) | 339/610 (56%) |
| Kamdem58 | Tertiary urban hospital, Douala, Cameroon, 2008‐2016 | 3.6% | 11/78 (14%) | 3/78 (3.8%) |
| Nkoke59 | Semi‐urban regional hospital, Buea, Cameroon, 2016‐2017 | 3.6% | 5/22 (23%) | 4/22 (18%) |
| Abah60 | Semi‐urban military hospital, Bamenda, Cameroon, 2010‐2013 | 3.6% | 17/79 (22%) | NR |
| Ogeng'o61 | National referral hospital, Nairobi, Kenya, date NR | 4.7% | 14/128 (11%) | 16/128 (13%) |
4. CHALLENGES ACCESSING ANTICOAGULATION
Vitamin K antagonists appear on most SSA countries' essential medicine lists.64 Warfarin is the most commonly used, followed by acenocoumarol.64, 65 Warfarin is cheap: a 5 mg tablet cost 8 US cents in rural Zambia59 or 14 US cents in Uganda.66 However, being essential and cheap does not necessarily make medicines available or accessible.67 Essential medicines' availability ranged from 25% in public facilities to 49% in private facilities in Cameroon,68 and from 49% in public facilities to 71% in retail pharmacies in Malawi.69 Anecdotally, frequent stock‐outs of warfarin have been mentioned in some SSA studies.22, 29, 70 To our knowledge, the only study to systematically evaluate warfarin availability in SSA was conducted in Uganda in 201766 and showed that warfarin was available on the survey day at 75/100 private pharmacies, 15/23 (65%) private hospitals and only 4/22 (18%) public hospitals, all randomly sampled.66
DOACs were only available in 14/33 African countries surveyed in 2018,71 and are often beyond the means of patients and public health services. In South Africa, which has statutory private sector medicine ceiling prices, 1 month's supply of dabigatran, apixaban or rivaroxaban costs the equivalent of approximately 60 hours' minimum wage.72 To our knowledge, no DOAC has yet been shown to be cost‐effective in any SSA country's public health sector.73
A weak medicines regulatory environment in much of SSA means that substandard and falsified medicines may be found on the market.74 We are not aware of any SSA data on the quality of warfarin on the market, but in a medicines quality assessment across 10 West and Central African countries, there was fortunately no evidence of poor quality acenocoumarol.75
Dedicated anticoagulation clinics using standardized approaches may achieve better anticoagulation control than routine models of care, where anticoagulation patients are seen as part of the general patient mix.76, 77, 78, 79, 80, 81 Such anticoagulation clinics are, however, not common in SSA; anticoagulation is often managed in outpatient cardiology, cardio‐thoracic surgery and haemato‐oncology clinics20, 32, 82 or by individual healthcare workers who may not use standardized approaches.27, 83 Prescribers of anticoagulation are often junior with limited practice experience.83
A few studies investigated SSA healthcare workers' knowledge, attitudes and beliefs about anticoagulation. In one such study 164 doctors and pharmacists at an Ethiopian tertiary hospital completed a self‐administered questionnaire.84 Participants' mean score on the warfarin knowledge section was 10/15 correct answers, yet only 7% identified their own lack of knowledge on warfarin as a barrier to effective patient counselling.84 Specific knowledge gaps identified included drug‐drug interactions and the target INR range appropriate to specific indications.84 In a second study at the same hospital, investigators directly observed the counselling pharmacists provided during warfarin dispensing and found that only 10% of patients were told what to do when they missed a dose, while interactions and side effects were discussed in only 9% and 3% of encounters, respectively.85 In addition, just 24% of patients were given an opportunity to ask questions at the dispensing encounter and only 40% of warfarin containers were labelled.85 At a South African academic hospital, in 86% of admissions for over‐anticoagulation with warfarin the cause of toxicity was not identified by attending clinicians.86 Drug‐drug interactions with warfarin, which went unrecognised by the attending clinicians, were retrospectively identified by study investigators in 77% of these patients.86
INR testing in centralised laboratories is the norm across SSA. As a result, people living in rural settings often have no access to INR monitoring. However, centralisation can even bypass people in urban settings, as illustrated in some of the examples in Figure 1. Centralised laboratory INR testing also means that results are delivered with a long turn‐around time, often a day or two,22, 27 and that patients may be required to attend separate blood sampling and INR monitoring visits,22, 87 driving up the expenses they incur.
The cost of INR testing has been identified as a barrier to anticoagulation therapy in Zambian88 and Ethiopian reports.29 We are aware of only one study systematically investigating the availability and cost of coagulation profile testing in SSA, conducted in Uganda in 2017.66 At randomly sampled facilities, coagulation profile testing was available at 13/22 (59%) private hospitals and 3/22 (14%) public hospitals,66 at a median price of US$8.30.
At least two different point‐of‐care INR monitors have been validated in South African patients,89, 90, 91 and there have been reports of using point‐of‐care INR monitors in anticoagulation clinics in Namibia,19 Kenya7, 70, 92, 93 and Nigeria.87 Considering only the cost per test, point‐of‐care testing is more expensive than laboratory monitoring; however, it may be cost‐effective in some settings, for example rural and remote settings.94 In SSA, most sites that reported using point‐of‐care testing received the monitors and test strips through donations, and offered point‐of‐care tests at a subsidised cost to patients.7, 87, 93
5. CHALLENGES WITH DOSE SELECTION AND DOSE ADJUSTMENTS
Warfarin dose selection and adjustment can be divided into an initiation phase, until a stable dose and INR is achieved, and a maintenance phase, during which further dose adjustments may be required for clinical and dietary reasons. Examples of dosing guidelines made by ministries/departments of health in SSA countries are given in Table 3. Except for the South African guideline, these are vague, with a large range of warfarin initiation doses and little detail about how often or by how much doses should be adjusted. Aside from these national guidelines, institution‐specific anticoagulation protocols, guidelines or algorithms may be in use: in one Nigerian survey 11% of clinicians reported using such guidelines in their institutions.95 At the time of our search, we did not find any warfarin dose initiation or dose adjustment algorithms in use in SSA that have been validated for the local population; our group subsequently published a validated dose initiation algorithm developed in South African and Ugandan patients.96
TABLE 3.
Examples of warfarin dose initiation and dose adjustment guidelines for venous thromboembolism by SSA countries' departments or ministries of health
| Guideline | Warfarin dose initiation guideline | Warfarin dose adjustment guideline |
|---|---|---|
| Ghana Standard Treatment Guidelines 201097 | “Warfarin, oral, adults: 10 mg daily at 6 pm for 2 days, then 5 mg daily” | “Regular dose adjustment and monitoring of INR until target of 2.0 and 3.0 is attained” |
| Namibia Standard Treatment Guidelines 201198 | “Warfarin: 5 to 10 mg orally; start at same time as heparin” | “Adjust dose according to INR” |
| Ethiopia Standard Treatment Guidelines for General Hospitals 201499 | “Warfarin (starting simultaneously with heparin), 5 mg orally, daily” | “Dose adjusted to achieve target INR of 2.0 to 3.0″ |
| Uganda Clinical Guidelines. National Guidelines for Management of Common Conditions 2016100 | “… plus warfarin 5 mg single dose given in the evening, commencing on the same day as heparin” | “Maintenance dose 2.5 to 7.5 mg single dose daily, adjusted according to the INR 2 to 3″ |
| Kenya National Guidelines for Cardiovascular Diseases Management 2018101 | “Recommended starting dose: 5 mg orally once a day” | “Typical maintenance dose: 2 to 10 mg orally once a day. Dosage must be individualized according to the patient's INR” |
| Standard Treatment Guidelines and Essential Medicines List for South Africa, Hospital Level, Adults 2019102 |
Day 1: 5 mg daily (2.5 mg daily for high sensitivity) 2‐3 d after initiation: • 5‐7.5 mg/d if INR < 1.5 • 2.5‐5 mg/d if INR 1.5 to 1.9 • 2.5 mg/d if INR 2.0 to 2.5 • hold warfarin if INR > 2.5 2‐3 d after last INR check: • 7.5‐10 mg/d if INR < 1.5 • 5‐10 mg/d if INR 1.5 to 1.9 • 2.5‐5 mg/d if INR 2.0 to 3.0 • hold warfarin if INR > 3 |
• Increase weekly dose by 10% if INR < 1.5 • Increase weekly dose by 5% if INR 1.5 to 1.9 • No change to warfarin dose if INR 2.0 to 3.0 • Decrease weekly dose by 5% if INR 3.1 to 4.0 • Decrease weekly dose by 10% if INR 4.1 to 5.0 • Decrease weekly dose by 20% if INR 5.1 to 9.0 |
INR, international normalised ratio.
Where detailed dose adjustment algorithms do not exist, clinicians may make erroneous or even paradoxical dose adjustments. Reviews from Cameroon,8 Ethiopia9, 14 and Namibia19 report dose increases after 4‐17% of supratherapeutic INRs and dose decreases after 4‐15% of subtherapeutic INRs. In addition, dose adjustments are often inappropriately large: While clinical trial evidence has shown that warfarin dose adjustments of 10‐15% were associated with better outcomes,103 the mean warfarin dose increase in one Ethiopian review was 58% in response to an INR of 1.5‐1.9 against a target of 2.0‐3.0.9 In the Namibian example, more than half of patients with an INR >4 were over‐corrected so that their subsequent INR was subtherapeutic.19
Warfarin formulations other than 5 mg are frequently not available in SSA,6, 22 making precision dosing difficult. Complicated weekly dosing schedules of alternating daily dosages which often require tablet splitting (sometimes into quarters) are used, potentially compromising the actual dose taken.104 This may in turn influence adherence and anticoagulation control. Reasons for the unavailability of alternative formulations seem to be market‐related as several SSA countries' essential medicines lists follow the WHO model list, which includes 1, 2 and 5 mg warfarin tablets. Even so, some Kenyan evidence suggests prescribers may simply be unaware of the market availability of alternatives to 5 mg tablets.83 Also, 1 or 2 mg formulations may be priced close to 5 mg tablets, making these alternative formulations less cost‐effective.
6. PATIENT‐RELATED CHALLENGES
The importance of pharmacogenetic variability on warfarin dose requirements in SSA was demonstrated in a recent systematic review.105 For example, three variants that are more prevalent in Black Africans than in other populations, CYP2C9*5, CYP2C9*6 and CYP2C9*11, affected warfarin dose requirements by −13, −8 and −5 mg/week, respectively.105
Potential drug‐drug interactions between warfarin and co‐prescribed medications hinder good anticoagulation control. In SSA, antiretroviral therapy is a significant source of potential drug‐drug interactions.18, 20, 22, 70, 92, 93, 106 Tuberculosis is also common in SSA, and rifampicin use induces multiple cytochrome P450 isoforms, resulting in a reduced warfarin effect. In a Kenyan case series of patients on concurrent rifampicin and warfarin, the median warfarin dose increase with rifampicin was 16%, but some patients required dose increases up to +441%.107 In a Ugandan case series, patients concomitantly prescribed rifampicin, antiretroviral therapy and warfarin had highly labile INRs and warfarin dose requirements, and it was not possible to predict the course of INR results in any individual patient.108 In Kenya, VTE patients with advanced HIV and tuberculosis required a median eight additional clinic visits to achieve or maintain a therapeutic INR.109
Despite widespread herbal medicine use in SSA110, 111 there is very little data on how this influences anticoagulation.112, 113 Nevertheless, it is plausible that some herbal medicines may interact with warfarin.
Anticoagulation patients in SSA are younger than those in high‐income settings. For example, in Uganda and South Africa the median age of patients attending five anticoagulation services was 56 years22 and in a Kenyan service the mean age was 43 years.20 Younger patients often show reduced adherence compared to older patients.114 Two possible reasons for this are that they are economically active and therefore may be unable to attend follow‐up appointments, and that they may have reproductive wishes and expectations and therefore intentionally reduce their intake of a potentially teratogenic medicine.115, 116
Four studies reporting patients' self‐reported adherence to anticoagulants in SSA are summarised in Table 4. Notably, from these, fewer than half of patients considered themselves highly adherent to warfarin. One study suggested that warfarin nonavailability may contribute to poor adherence.64 In an analysis of the “care cascade” of rheumatic heart disease patients in Uganda, retention in care was the stage with the highest patient drop‐out.48
TABLE 4.
Sub‐Saharan African studies of patients' self‐reported adherence to anticoagulation
| Study | Setting, sample | Adherence measure | Outcome |
|---|---|---|---|
| Chalachew29 |
Addis Ababa, Ethiopia, 2019 Children and young adults (11‐25 yr) with prosthetic valves on anticoagulation at a teaching hospital |
Not reported |
30/73 (41%) reported perfect adherence, 34/73 (47%) missed 1‐2 doses per week. 9/73 (12%) missed >2 doses per week Most common reasons were forgetfulness (30%) and unavailability of warfarin (23%) Association between adherence and INR control not reported |
| Mariita115 |
Nairobi, Kenya, 2015 Consecutive sample at cardiac, cardiothoracic and haemato‐oncology clinics of a teaching hospital |
MMAS‐8117 High adherence if score = 8, moderate adherence if score 6 or 7, low adherence if score <5 |
77/147 (52%) high adherence, 53/147 (36%) moderate adherence, 17/147 (12%) low adherence Association between adherence and INR control not reported |
| Iqbal118 |
Nairobi, Kenya, 2017 Convenience sample at cardiac, cardiothoracic haemato‐oncology and DVT clinics of a teaching hospital |
MMAS‐8117 High adherence if score = 8, moderate adherence if score 6 or 7, low adherence if score <5 |
13/45 (29%) high adherence, 32/45 (71%) low adherence Association between adherence and INR control not reported |
| Eltayeb119 |
Khartoum, Sudan, 2017 Convenience sample at cardiothoracic clinic of a teaching hospital |
Four‐item MMAS120 Considered adherent if score = 0, nonadherent if score >0 |
5/93 (5.4%) adherent Association between adherence and INR control not reported |
INR, international normalised ratio; MMAS‐8, eight‐item Morisky medication adherence scale.
We are not aware of any studies reporting SSA patients' attitudes and beliefs about anticoagulation. A few studies (Table 5) reported on patients' anticoagulation knowledge, with generally low levels of knowledge found. Only two studies investigated whether participants' anticoagulation knowledge correlated with their anticoagulation control and these reached conflicting results.10, 121 Levels of knowledge were generally associated with participants' level of education and with the provision of written educational materials. Topics on which participants' knowledge was low were drug and food interactions, the effect of missing a dose, the interpretation of INR values, recognizing the symptoms of over‐ or underdosing, contraception and pregnancy planning.10, 82, 122
TABLE 5.
Sub‐Saharan African studies of patients' knowledge about anticoagulation
| Study | Setting, sample | Knowledge measure | Outcome |
|---|---|---|---|
| Dwamena121 |
Accra, Ghana, 2012 Systematic sample of outpatients at anticoagulation clinic of a teaching hospital |
Own tool, adapted from Taylor,123 pass rate set at 70% |
112/175 (64%) passed Better knowledge was associated with better INR control |
| Assefa82 |
Addis Ababa, Ethiopia, 2014 Outpatients on warfarin at a teaching hospital |
Own tool, adapted from AKA,124 pass rate set at 75% |
18/130 (14%) passed Mean score was 59% Association between knowledge and INR control not reported |
| Mariita10 |
Nairobi, Kenya, 2016 Consecutive sample at cardiac, cardiothoracic and haemato‐oncology clinics of a teaching hospital |
Own tool, adapted from OAK,125 pass rate set at 75% |
15/147 (10%) passed Mean score was 57% Knowledge was not associated with INR control |
| Iqbal118 |
Nairobi, Kenya, 2017 Convenience sample at cardiac, cardiothoracic, haemato‐oncology and DVT clinics of a teaching hospital |
Own tool, adapted from OAK,125 “satisfactory knowledge” set at >70% |
12/45 (27%) had satisfactory knowledge pre‐intervention Association between knowledge and INR control not reported |
| Hutheram126 |
Gauteng, South Africa, 2016 Convenience sample at 10 private sector INR clinics attached to a private pathology company |
Own tool, adapted from OAK,125 pass rate set at 50% |
31/34 (91%) passed Association between knowledge and INR control not reported |
| Samadoulougou127 |
Ouagadougou, Burkina Faso, 2014 Convenience sample of patients in the cardiology clinic of a university hospital |
Own tool, adapted from Janoly‐Duménil128 |
Participants scored low in questions relating to their ability to anticipate and make decisions in risky situations Association between knowledge and INR control not reported |
| Maramba32 |
Harare, Zimbabwe, 2018 Convenience sample of outpatients with thrombophilia on long‐term warfarin |
Not clear |
29/47 (62%) were not aware of the need for regular check‐ups Association between knowledge and INR control not reported |
| Gregersen122 |
Johannesburg, South Africa, 2006 Convenience sample of women of childbearing age with valvular heart disease who had at least one pregnancy while on warfarin |
Own questionnaire |
38/124 (31%) were not using contraception; misperceptions about “the contraceptive effect of warfarin” were not uncommon Knowledge about effects of warfarin on the foetus was often inaccurate and not specific or detailed Association between knowledge and INR control not reported |
AKA, anticoagulation knowledge assessment; INR, international normalised ratio; OAK, oral anticoagulation knowledge test.
7. OVERCOMING THE CHALLENGES
Overcoming the multitude of challenges faced by anticoagulation services and patients requiring anticoagulation in SSA require a multifaceted approach. “Warfarin care bundles” are effective and viable strategies, as shown in a recent network meta‐analysis of anticoagulation interventions.129 In SSA such a warfarin care bundle must include both process‐centred and patient‐centred activities, the exact combination of which will be specific to each setting and will depend on cost‐effectiveness to guide rational allocation of limited resources. In making process changes, it will be important to leverage off existing successful systems, such as HIV treatment programmes,130 and to ensure these changes are embedded in a quality improvement framework, with regular feedback to patients, clinicians and managers.
Providing patient‐centred anticoagulation education and adherence support are two interventions possibly achievable over a shorter term. These interventions have shown benefit on patients' knowledge, adherence and INR control in a few individual SSA studies13, 14, 118 and may be of particular benefit in vulnerable populations.131 As pharmacists and doctors are a scarce resource in Africa, these education and support tasks can be successfully shifted to mid‐level healthcare workers.7 SSA has extensive expertise in providing adherence support to patients with HIV, and education and adherence support for patients with noncommunicable diseases should build on these existing systems.132
Process‐centred activities are likely to require a longer term, and more resources, to implement successfully. These activities may include decentralisation of anticoagulation services, setting up of anticoagulation clinics, improving access to warfarin (including formulations other than the 5 mg tablet), improving access to laboratory testing and/or scaling up point‐of‐care INR testing, task‐shifting of anticoagulation care to mid‐level healthcare workers, staff training, and implementing locally validated dose initiation and dose adjustment algorithms. Decentralised anticoagulation clinics can be successfully implemented in SSA, with improved outcomes (and better cost‐effectiveness) compared to those achieved at a central referral hospital.93 Point‐of‐care INR testing has also been successfully implemented in SSA, drastically reducing the number of visits patients have to make to the clinic.87 However, the high cost of test strips is problematic, and relying on donations and subsidies is not sustainable. Localised dose initiation and dose adjustment algorithms must consider the comorbidities and potential drug interactions that are prevalent in SSA, such as HIV, tuberculosis, antiretrovirals, antituberculosis therapy, co‐trimoxazole and herbal/traditional medicines. These algorithms must be easy to implement, for example be paper‐based, and should recommend small, percentage‐based dose adjustments.103
One example of an effective anticoagulation programme combining multiple interventions has come from Rwanda.133 In this programme, specialist noncommunicable disease nurses deliver postoperative care to valvular heart disease patients in decentralised clinics. Standard dosing algorithms are used, while nurses are supervised and supported by cardiologists, using mobile communications. Adherence support, as well as financial support, is offered to patients. While this small study did not report the effect of this programme on TTR, low mortality was described and there were no bleeding or thrombotic complications.133
DOACs may be a solution to some anticoagulation challenges in SSA: while they are still prohibitively expensive to most African patients and healthcare systems, they will in the future come off patent and generics may be more affordable. DOACs have the benefit of being used at fixed rather than individualised doses for the majority of patients and do not require routine monitoring.73 However, DOACs come with their own set of challenges. Fixed DOAC doses may not be appropriate for all patients, and some patients should be monitored for safety and efficacy.134, 135, 136 In addition, DOACs are contraindicated in valvular heart disease, a significant group of patients in SSA, and require dose adjustment/are contraindicated in severe renal impairment. DOACs have shorter half‐lives than warfarin, making strict adherence more critical; ironically, without regular monitoring, adherence problems may be missed in patients on DOACs.73 DOACs are also subject to drug‐drug interactions: notably for SSA, these include interactions with rifampicin and many antiretroviral agents.137 Finally, the management of major bleeding occurring with DOACs will require specific protocols, localised for SSA.73
8. CONCLUSION
There is significant room for improvement of anticoagulation control in countries across SSA. Increasing numbers of patients will need anticoagulation in the future because of the increasing burden of noncommunicable disease in the region. Despite the many challenges faced by patients, healthcare providers and health systems in these resource‐limited settings, several opportunities for improvement exist. Decentralisation of anticoagulation care, together with expanded access to medicines and monitoring, and enhanced support to practitioners and patients, are pivotal in achieving better control. Dose initiation and dose adjustment protocols that have been developed taking locally relevant factors into account can also contribute to better anticoagulation control. With the cost of DOACs still prohibitive, locally developed “warfarin care bundles” which address multiple anticoagulation challenges in combination, particularly when they leverage off systems that are already functional in SSA, currently appear to be the most appropriate strategy to improve anticoagulation control in this setting.
8.1. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY, and are permanently archived in the Concise Guide to PHARMACOLOGY 2019/20.138
COMPETING INTERESTS
M.P. receives research funding from various organisations including governmental bodies such as the MRC, the EU Commission and Health Education England. M.P. also received partnership funding for the following: MRC Clinical Pharmacology Training Scheme (co‐funded by MRC and Roche, UCB, Eli Lilly and Novartis), a PhD studentship jointly funded by EPSRC and Astra Zeneca, and grant funding from Vistagen Therapeutics. M.P. also received unrestricted educational grant support for the UK Pharmacogenetics and Stratified Medicine Network from Bristol‐Myers Squibb and UCB. M.P. held a joint grant from NIHR with MC Diagnostics for the development of an HLA panel. None of the funding M.P. received is related to the current paper.
The remaining authors declare no conflicts of interest.
CONTRIBUTORS
This manuscript was conceptualised by all the authors. J.P.M. conducted the search, analysed the data, interpreted the data and wrote the first draft of the manuscript. All authors critically revised the manuscript for important intellectual content.
ACKNOWLEDGEMENTS
This research was funded by the National Institute for Health Research (NIHR) (project ref: 16/137/101) using UK aid from the UK Government to support global health research. The views expressed in this publication are those of the author(s) and not necessarily those of the NIHR or the UK Department of Health and Social Care.
Mouton JP, Blockman M, Sekaggya‐Wiltshire C, et al. Improving anticoagulation in sub‐Saharan Africa: What are the challenges and how can we overcome them? Br J Clin Pharmacol. 2021;87:3056–3068. 10.1111/bcp.14768
REFERENCES
- 1.Singer DE, Hellkamp AS, Piccini JP, et al. Impact of global geographic region on time in therapeutic range on warfarin anticoagulant therapy: data from the ROCKET AF clinical trial. J am Heart Assoc. 2013;2(1):e000067. 10.1161/JAHA.112.000067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Connolly SJ, Pogue J, Eikelboom J, et al. Benefit of oral anticoagulant over antiplatelet therapy in atrial fibrillation depends on the quality of international normalized ratio control achieved by centers and countries as measured by time in therapeutic range. Circulation. 2008;118(20):2029‐2037. 10.1161/CIRCULATIONAHA.107.750000 [DOI] [PubMed] [Google Scholar]
- 3.Wallentin L, Yusuf S, Ezekowitz MD, et al. Efficacy and safety of dabigatran compared with warfarin at different levels of international normalised ratio control for stroke prevention in atrial fibrillation: An analysis of the RE‐LY trial. Lancet. 2010;376(9745):975‐983. 10.1016/S0140-6736(10)61194-4 [DOI] [PubMed] [Google Scholar]
- 4.Oldgren J, Healey JS, Ezekowitz M, et al. Variations in cause and management of atrial fibrillation in a prospective registry of 15 400 emergency department patients in 46 countries: The RE‐LY atrial fibrillation registry. Circulation. 2014;129(15):1568‐1576. 10.1161/CIRCULATIONAHA.113.005451 [DOI] [PubMed] [Google Scholar]
- 5.Zühlke L, Engel ME, Karthikeyan G, et al. Characteristics, complications, and gaps in evidence‐based interventions in rheumatic heart disease: The Global Rheumatic Heart Disease Registry (the REMEDY study). Eur Heart J. 2015;36(18):1115‐1122. 10.1093/eurheartj/ehu449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Makubi A, Lwakatare J, Nordrehaug J, Magesa P. Anticoagulant Control Results among Patients with Mechanical Heart Valves at Muhimbili National hospital, Tanzania. Tanzania Med J. 2008;23(1):12‐16. 10.4314/tmj.v23i1.39222 [DOI] [Google Scholar]
- 7.Manji I, Pastakia SD, Do AN, et al. Performance outcomes of a pharmacist‐managed anticoagulation clinic in the rural, resource‐constrained setting of Eldoret, Kenya. J Thromb Haemost. 2011;9(11):2215‐2220. 10.1111/j.1538-7836.2011.04503.x [DOI] [PubMed] [Google Scholar]
- 8.Menanga A, Sibetcheu A, Chelo D, et al. Surveillance du Traitement par Antivitamines K chez des Patients en Fibrillation Auriculaire à Yaoundé. Heal Sci Dis. 2015;16:1‐6. https://www.hsd-fmsb.org/index.php/hsd/article/view/487 [Google Scholar]
- 9.Daba FB, Tadesse F, Engidawork E. Drug‐related problems and potential contributing factors in the management of deep vein thrombosis. BMC Hematol. 2016;16(1):2. 10.1186/s12878-016-0043-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mariita K, Maina C, Nyamu D, et al. Patient factors impacting on oral anticoagulation therapy among adult outpatients in a Kenyan referral hospital. African J Pharmacol Ther. 2016;5:193‐200. http://journals.uonbi.ac.ke/ajpt/article/view/1534 [Google Scholar]
- 11.Sadhabiriss D.Warfarin: Time in Therapeutic Range, a Single Centre Study on Patients using Warfarin for Stroke Prevention in Non‐Valvular Atrial Fibrillation and Prosthetic Heart Valves. 2016. http://hdl.handle.net/10413/16021
- 12.Sonuga BO, Hellenberg DA, Cupido CS, Jaeger C. Profile and anticoagulation outcomes of patients on warfarin therapy in an urban hospital in Cape Town. South Africa African J Prim Heal Care Fam Med. 2016;8(1):a1032‐e8. 10.4102/phcfm.v8i1.1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahmed NO, Osman B, Abdelhai YM, el‐Hadiyah TMH. Impact of clinical pharmacist intervention in anticoagulation clinic in Sudan. Int J Clin Pharmacol. 2017;39(4):769‐773. 10.1007/s11096-017-0475-x [DOI] [PubMed] [Google Scholar]
- 14.Fenta TG, Assefa T, Alemayehu B. Quality of anticoagulation management with warfarin among outpatients in a tertiary hospital in Addis Ababa, Ethiopia: A Retrospective Cross‐Sectional Study. BMC Health Serv Res. 2017;17(1):389. 10.1186/s12913-017-2330-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nyamu DG, Guantai AN, Osanjo GO, et al. Predictors of adequate ambulatory anticoagulation among adult patients in a tertiary teaching and referral hospital in Kenya. African J Pharmacol Ther. 2017;6:20‐26. http://journals.uonbi.ac.ke/ajpt/article/view/1549 [Google Scholar]
- 16.Coulibaly I, Diatema S, Hauhouot‐Attoungbre M. Quality of oral anticoagulation with vitamin K antagonists assessed by the TTR in ambulatory non‐valvular atrial fibrillation patients (NVAF) at Abidjan Institute of Cardiology. Trop Cardiol Published Online First. 2018. http://tropical-cardiology.com/Accueil/index.php/fr/2013-08-10-06-44-55/n-153-juil-aout-sep-2018/356-qualite-de-l-anticoagulation-orale-par-antivitamine-k-evaluee-par-le-calcul-du-ttr-chez-des-patients-traites-en-ambulatoire-pour-fibrillation-auriculaire- [Google Scholar]
- 17.Ebrahim I, Bryer A, Cohen K, Mouton JP, Msemburi W, Blockman M. Poor anticoagulation control in patients taking warfarin at a tertiary and district‐level prothrombin clinic in Cape Town, South Africa. South African Med J. 2018;108(6):490‐494. 10.7196/SAMJ.2018.v108i6.13062 [DOI] [PubMed] [Google Scholar]
- 18.Mwita JC, Francis JM, Oyekunle AA, Gaenamong M, Goepamang M, Magafu MGMD. Quality of anticoagulation with warfarin at a tertiary hospital in Botswana. Clin Appl Thromb. 2018;24(4):596‐601. 10.1177/1076029617747413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jonkman LJ, Gwanyanya MP, Kakololo MN, Verbeeck RK, Singu BS. Assessment of anticoagulation management in outpatients attending a warfarin clinic in Windhoek, Namibia. Drugs Ther Perspect. 2019;35(7):341‐346. 10.1007/s40267-019-00630-y [DOI] [Google Scholar]
- 20.Karuri S, Nyamu D, Opanga S, et al. Factors associated with time in therapeutic range among patients on oral anticoagulation therapy in a tertiary teaching and referral hospital in Kenya. East Cent African J Pharm Sci. 2019;22:85‐95. http://uonjournals.uonbi.ac.ke/ojs/index.php/ecajps/article/view/293 [Google Scholar]
- 21.Botsile E, Mwita JC. Incidence and risk factors for thromboembolism and major bleeding in patients with mechanical heart valves: a tertiary hospital‐based study in Botswana. Cardiovasc J Afr. 2020;1‐5. 10.5830/CVJA-2020-006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Semakula JR, Mouton JP, Jorgensen A, et al. A cross‐sectional evaluation of five warfarin anticoagulation services in Uganda and South Africa. PLoS One. 2020;15(1):e0227458. 10.1371/journal.pone.0227458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rwebembera J, Manyilirah W, Zhu ZW, et al. Prevalence and characteristics of primary left‐sided valve disease in a cohort of 15,000 patients undergoing echocardiography studies in a tertiary hospital in Uganda. BMC Cardiovasc Disord. 2018;18:82. 10.1186/s12872-018-0813-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yankah C, Fynn‐Thompson F, Antunes M, et al. Cardiac surgery capacity in sub‐Saharan Africa: Quo vadis? Thorac Cardiovasc Surg. 2014;62(05):393‐401. 10.1055/s-0034-1383723 [DOI] [PubMed] [Google Scholar]
- 25.Mandi DG, Bamouni J, Naïbé DT, et al. Epidemiology and long‐term prognosis of atrial fibrillation in rural African patients. Egypt Hear J. 2019;71:6. 10.1186/s43044-019-0005-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yameogo AR, Kologo JK, Mandi G, et al. Use of vitamin K antagonists in non‐valvular atrial fibrillation thromboembolic risk prevention in Burkina Faso. Pan Afr Med J. 2016;24:108. 10.11604/pamj.2016.24.108.7100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anakwue R, Ocheni S, Madu A. Utilization of oral anticoagulation in a teaching hospital in Nigeria. Ann Med Health Sci Res. 2014;4(9):286‐290. 10.4103/2141-9248.141973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alcheikh A, Chawe A, Kowa SF, et al. A comprehensive approach to improving laboratory services in rural Zambia. Blood Adv. 2019;3:11‐15. 10.1182/bloodadvances.2019gs121633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chalachew T, Yadeta D, Tefera E. Factors associated with sub‐optimal control of anticoagulation in patients with prosthetic heart valves taking oral anticoagulants in a sub‐Saharan African setting. Cardiovasc J Afr. 2019;30(6):317‐320. 10.5830/cvja-2019-024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Raphael DM, Roos L, Myovela V, et al. Heart diseases and echocardiography in rural Tanzania: Occurrence, characteristics, and etiologies of underappreciated cardiac pathologies. PLoS One. 2018;13(12):e0208931. 10.1371/journal.pone.0208931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stenumgård PS, Rakotondranaivo MJ, Sletvold O, Follestad T, Ellekjær H. Stroke in a resource‐constrained hospital in Madagascar. BMC Res Notes. 2017;10(1):307. 10.1186/s13104-017-2627-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maramba A, Ncube S, Mandisodza A, da Silva Marques D, Matsikure T. An assessment of the effectiveness of warfarin therapy monitoring systems on thrombophilic patients in Zimbabwe. TH Open. 2018;02(03):e325‐e328. 10.1055/s-0038-1672186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Noubiap JJ, Nyaga UF. A review of the epidemiology of atrial fibrillation in sub‐Saharan Africa. J Cardiovasc Electrophysiol. 2019;30(12):3006‐3016. 10.1111/jce.14222 [DOI] [PubMed] [Google Scholar]
- 34.Tegene E, Tadesse I, Markos Y, Gobena T. Prevalence and risk factors for atrial fibrillation and its anticoagulant requirement in adults aged ≥40 in Jimma Town, Southwest Ethiopia: A community based cross‐sectional study. IJC Heart Vasc. 2019;22:199‐204. 10.1016/j.ijcha.2019.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stambler B, Ngunga L. Atrial fibrillation in Sub‐Saharan Africa: epidemiology, unmet needs, and treatment options. Int J Gen Med. 2015;8:231‐242. 10.2147/IJGM.S84537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ntep‐Gweth M, Zimmermann M, Meiltz A, et al. Atrial fibrillation in Africa: Clinical characteristics, prognosis, and adherence to guidelines in Cameroon. Europace. 2010;12:482‐487. 10.1093/europace/euq006 [DOI] [PubMed] [Google Scholar]
- 37.Zühlke L, Karthikeyan G, Engel ME, et al. Clinical outcomes in 3343 children and adults with rheumatic heart disease from 14 low‐ and middle‐income countries. Circulation. 2016;134(19):1456‐1466. 10.1161/CIRCULATIONAHA.116.024769 [DOI] [PubMed] [Google Scholar]
- 38.Getachew Erkabu S, Agedie Y, Mihretu DD, et al. Ischemic and hemorrhagic stroke in Bahir Dar, Ethiopia: A retrospective hospital‐based study. J Stroke Cerebrovasc Dis. 2018;27(6):1533‐1538. 10.1016/j.jstrokecerebrovasdis.2017.12.050 [DOI] [PubMed] [Google Scholar]
- 39.Shavadia J, Yonga G, Mwanzi S, Jinah A, Moriasi A, Otieno H. Clinical characteristics and outcomes of atrial fibrillation and flutter at the Aga Khan University Hospital, Nairobi. Cardiovasc J Afr. 2013;24(2):6‐9. 10.5830/CVJA-2012-064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pastakia SD, Fohl AL, Schellhase EM, Manji I, Ringenberg K. Needs assessment analysis for vitamin K antagonist anticoagulation in the resource‐constrained setting of Eldoret, Kenya. J am Pharm Assoc. 2010;50(6):723‐725. 10.1331/JAPhA.2010.09226 [DOI] [PubMed] [Google Scholar]
- 41.Sidibé S, Sako M, Sacko A, et al. Problématique de l'anticoagulation dans la fibrillation atriale non valvulaire du sujet âgé. Research. 2018;5:2618. 10.13070/rs.fr.5.2618 [DOI] [Google Scholar]
- 42.Moloi AH, Mall S, Engel ME, et al. The health systems barriers and facilitators for RHD prevalence: An epidemiological meta‐analysis from Uganda and Tanzania. Glob Heart. 2017;12(1):5‐15. 10.1016/j.gheart.2016.12.002 [DOI] [PubMed] [Google Scholar]
- 43.Zühlke LJ, Engel ME, Watkins D, et al. Incidence, prevalence and outcome of rheumatic heart disease in South Africa: A systematic review of contemporary studies. Int J Cardiol. 2015;199:375‐383. 10.1016/j.ijcard.2015.06.145 [DOI] [PubMed] [Google Scholar]
- 44.Keates AK, Mocumbi AO, Ntsekhe M, Sliwa K, Stewart S. Cardiovascular disease in Africa: epidemiological profile and challenges. Nat Rev Cardiol. 2017;14(5):273‐293. 10.1038/nrcardio.2017.19 [DOI] [PubMed] [Google Scholar]
- 45.Kane A, Mirabel M, Touré K, et al. Echocardiographic screening for rheumatic heart disease: Age matters. Int J Cardiol. 2013;168(2):888‐891. 10.1016/j.ijcard.2012.10.090 [DOI] [PubMed] [Google Scholar]
- 46.Longo‐Mbenza B, Bayekula M, Ngiyulu R, et al. Survey of rheumatic heart disease in school children of Kinshasa town. Int J Cardiol. 1998;63(3):287‐294. 10.1016/S0167-5273(97)00311-2 [DOI] [PubMed] [Google Scholar]
- 47.Engel ME, Haileamlak A, Zühlke L, et al. Prevalence of rheumatic heart disease in 4720 asymptomatic scholars from South Africa and Ethiopia. Heart. 2015;101(17):1389‐1394. 10.1136/heartjnl-2015-307444 [DOI] [PubMed] [Google Scholar]
- 48.Chang AY, Nabbaale J, Okello E, et al. Outcomes and care quality metrics for women of reproductive age living with rheumatic heart disease in Uganda. J am Heart Assoc. 2020;9(8):e015562. 10.1161/JAHA.119.015562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Danwang C, Temgoua MN, Agbor VN, Tankeu AT, Noubiap JJ. Epidemiology of venous thromboembolism in Africa: a systematic review. J Thromb Haemost. 2017;15(9):1770‐1781. 10.1111/jth.13769 [DOI] [PubMed] [Google Scholar]
- 50.Durand M, Sinyavskaya L, Jin YL, Tremblay CL, Ducruet T, Laskine M. Incidence of venous thromboembolism in patients living with HIV: A cohort study. AIDS Patient Care STDS. 2019;33(11):455‐458. 10.1089/apc.2019.0154 [DOI] [PubMed] [Google Scholar]
- 51.Vululi ST, Bugeza S, Zeridah M, et al. Prevalence of lower limb deep venous thrombosis among adult HIV positive patients attending an outpatient clinic at Mulago Hospital. AIDS Res Ther. 2018;15(1):3. 10.1186/s12981-018-0191-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mampuya FK, Steinberg WJ, Raubenheimer JE. Risk factors and HIV infection among patients diagnosed with deep vein thrombosis at a regional/tertiary hospital in Kimberley, South Africa. S Afr Fam Pract. 2018;60:107‐113. 10.1080/20786190.2018.1432135 [DOI] [Google Scholar]
- 53.Louw S, Jacobson BF, Büller H. Human immunodeficiency virus infection and acute deep vein thromboses. Clin Appl Thromb. 2008;14(3):352‐355. 10.1177/1076029607304411 [DOI] [PubMed] [Google Scholar]
- 54.Goldstein LN, Wu MT. A one year audit of patients with venous thromboembolism presenting to a tertiary hospital in Johannesburg, South Africa. African J Emerg Med. 2018;8(1):12‐15. 10.1016/j.afjem.2017.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Awolesi D, Naidoo M, Cassimijee MH. The profile and frequency of known risk factors or comorbidities for deep vein thrombosis in an urban district hospital in KwaZulu‐Natal. South Afr J HIV Med. 2016;17(1):a425. 10.4102/sajhivmed.v17i1.425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Olubanwo OO. The profile of HIV/AIDS patients admitted with deep venous thrombosis (DVT) at Nelson Mandela Hospital in Mthatha, South Africa. 2010. http://hdl.handle.net/10019.1/100702
- 57.Alshehri MF. Risk factors for deep vein thrombosis in a South African public hospital. 2013. http://hdl.handle.net/11427/2879
- 58.Kamdem F, Hugo BMN, Hamadou B, et al. Epidemiology, clinical presentations and in‐hospital mortality of venous thromboembolism at the Douala General Hospital: A cross‐sectional study in Cameroon, Sub‐Saharan Africa. World J Cardiovasc Dis. 2018;8(02):123‐132. 10.4236/wjcd.2018.82012 [DOI] [Google Scholar]
- 59.Nkoke C, Teuwafeu D, Mapina A, Nkouonlack C. A case series of venous thromboembolic disease in a semi‐urban setting in Cameroon. BMC Res Notes. 2019;12(1):40. 10.1186/s13104-019-4092-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Abah JP, Menanga A, Ngahane BHM, et al. Pattern of venous thromboembolic diseases in a resources‐limited setting in Cameroon. Pan Afr Med J. 2016;23:236. 10.11604/pamj.2016.23.236.7034 [DOI] [Google Scholar]
- 61.Ogeng'o JA, Obimbo MM, Olabu BO, et al. Pulmonary thromboembolism in an East African tertiary referral hospital. J Thromb Thrombolysis. 2011;32(3):386‐391. 10.1007/s11239-011-0607-4 [DOI] [PubMed] [Google Scholar]
- 62.Johnson LF, May MT, Dorrington RE, et al. Estimating the impact of antiretroviral treatment on adult mortality trends in South Africa: A mathematical modelling study. PLoS Med. 2017;14:e1002468. 10.1371/journal.pmed.1002468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.UNAIDS . AIDSinfo. 2018. aidsinfo.unaids.org (accessed 11 May 2020).
- 64.Persaud N, Jiang M, Shaikh R, et al. Global Essential Medicines Database. 2019. 10.6084/m9.figshare.7814246.v1 [DOI] [Google Scholar]
- 65.Medicines Patent Pool . Patented medicines that have clinical benefits but did not meet the EML Expert Review committee's comparative cost‐effectiveness criterion: Case study on novel oral anticoagulants. In: Exploring the Expansion of the Medicines Patent Pool's Mandate to Patented Essential Medicines: A Feasibility Study of the Public Health Needs and Potential Impact. Geneva: Medicines Patent Pool; 2015:64‐79. [Google Scholar]
- 66.Kibirige D, Atuhe D, Kampiire L, et al. Access to medicines and diagnostic tests integral in the management of diabetes mellitus and cardiovascular diseases in Uganda: Insights from the ACCODAD study. Int J Equity Health. 2017;16(1):154. 10.1186/s12939-017-0651-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Agyepong IA, Sewankambo N, Binagwaho A, et al. The path to longer and healthier lives for all Africans by 2030: the Lancet Commission on the future of health in sub‐Saharan Africa. Lancet. 2017;390(10114):2803‐2859. 10.1016/S0140-6736(17)31509-X [DOI] [PubMed] [Google Scholar]
- 68.Dzudie A, Njume E, Abanda M, et al. Availability, cost and affordability of essential cardiovascular disease medicines in the south west region of Cameroon: Preliminary findings from the Cameroon science for disease study. PLoS One. 2020;15:e0229307. 10.1371/journal.pone.0229307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Khuluza F, Haefele‐Abah C. The availability, prices and affordability of essential medicines in Malawi: A cross‐sectional study. PLoS One. 2019;14:e0212125. 10.1371/journal.pone.0212125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kanyi J, Karwa R, Pastakia SD, Manji I, Manyara S, Saina C. Venous thromboembolism requiring extended anticoagulation among HIV‐infected patients in a rural, resource‐constrained setting in western Kenya. Ann Pharmacother. 2017;51(5):380‐387. 10.1177/1060028016686106 [DOI] [PubMed] [Google Scholar]
- 71.Talle M, Bonny A, Scholtz W, et al. Status of cardiac arrhythmia services in Africa in 2018: a PASCAR Sudden Cardiac Death Task Force report. Cardiovasc J Afr. 2018;29(2):115‐121. 10.5830/CVJA-2018-027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.OpenUp . What should your medicines cost? mpr.code4sa.org (accessed 11 May 2020).
- 73.Bista D, Chalmers L, Bereznicki L, Peterson G. Potential use of NOACs in developing countries: Pros and cons. Eur J Clin Pharmacol. 2014;70(7):817‐828. 10.1007/s00228-014-1693-y [DOI] [PubMed] [Google Scholar]
- 74.Olsson S, Pal SN, Dodoo A. Pharmacovigilance in resource‐limited countries. Expert Rev Clin Pharmacol. 2015;8(4):449‐460. 10.1586/17512433.2015.1053391 [DOI] [PubMed] [Google Scholar]
- 75.Antignac M, Diop BI, Do B, et al. Quality assessment of 7 cardiovascular drugs in 10 Sub‐Saharan countries. JAMA Cardiol. 2017;2(2):223‐225. 10.1001/jamacardio.2016.3851 [DOI] [PubMed] [Google Scholar]
- 76.Rudd KM, Dier JG. Comparison of two different models of anticoagulation management services with usual medical care. Pharmacotherapy. 2010;30(4):330‐338. 10.1592/phco.30.4.330 [DOI] [PubMed] [Google Scholar]
- 77.Barnes GD, Kline‐Rogers E, Graves C, et al. Structure and function of anticoagulation clinics in the United States: an AC forum membership survey. J Thromb Thrombolysis. 2018;46(1):7‐11. 10.1007/s11239-018-1652-z [DOI] [PubMed] [Google Scholar]
- 78.Garrison SR, Allan GM. Do specialty anticoagulation clinics really outperform primary care at INR management? J Thromb Thrombolysis. 2014;38(3):420‐421. 10.1007/s11239-014-1113-2 [DOI] [PubMed] [Google Scholar]
- 79.McGuinn TL, Scherr S. Anticoagulation clinic versus a traditional warfarin management model. Nurse Pract. 2014;39(10):40‐46. 10.1097/01.NPR.0000451803.29453.0c [DOI] [PubMed] [Google Scholar]
- 80.Wilson SJ‐A, Wells PS, Kovacs MJ, et al. Comparing the quality of oral anticoagulant management by anticoagulation clinics and by family physicians: a randomized controlled trial. CMAJ. 2003;169:293‐298. https://www.cmaj.ca/content/169/4/293 [PMC free article] [PubMed] [Google Scholar]
- 81.Matchar DB. Do anticoagulation management services improve care? Implications of the Managing Anticoagulation Services Trial. Card Electrophysiol Rev. 2003;7(4):379‐381. 10.1023/B:CEPR.0000023144.60821.d1 [DOI] [PubMed] [Google Scholar]
- 82.Assefa T, Gedif T, Alemayehu B. Evaluation of patients' knowledge on warfarin therapy among outpatients receiving warfarin at Tikur Anbessa Specialized Hospital, Addis Ababa, Ethiopa. Ethiop Pharm J. 2014;30:133‐138. 10.4314/epj.v30i2.6 [DOI] [Google Scholar]
- 83.Nyamu D, Guantai A. Patterns of ambulatory anticoagulation practices in a county hospital in Nairobi. Pharm J Kenya. 2018;23:75‐82. https://psk.or.ke/journals/article-25 [Google Scholar]
- 84.Dejene F, Berihun D, Assefa T. Healthcare professionals' knowledge and counseling practice on warfarin therapy at tertiary care teaching hospital, Addis Ababa, Ethiopia. Cardiovasc Pharmacol Open Access. 2017;6(01):206. 10.4172/2329-6607.1000206 [DOI] [Google Scholar]
- 85.Tadesse TA, Alebachew M, Woldu A. Prevalence of warfarin drug interaction and warfarin education practice in outpatient setups of University Teaching Hospital: A retrospective chart review and an observational study. J Basic Clin Pharm. 2018;9:262‐266. https://www.jbclinpharm.org/articles/prevalence‐of‐warfarin‐drug‐interaction‐and‐warfarin‐educationpractice‐in‐outpatient‐setups‐of‐university‐teaching‐hospi.pdf [Google Scholar]
- 86.Jacobs A, Bassa F, Decloedt EH. A preliminary review of warfarin toxicity in a tertiary hospital in Cape Town, South Africa. Cardiovasc J Afr. 2017;28:346‐349. 10.5830/CVJA-2017-029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Abok II, Andeyaba B, Slusher T, Bode‐Thomas F. Point‐of‐care monitoring of international normalized ratio among patients with mechanical valves in Jos, North‐Central, Nigeria. Niger J Cardiol. 2019;16(2):98‐102. 10.4103/njc.njc_39_17 [DOI] [Google Scholar]
- 88.Goma F, Kalinchenko S. Atrial fibrillation in Lusaka – pathoaetiology, pathophysiology and clinical management challenges in primary care settings. Med J Zambia. 2015;42:31‐41. https://www.mjz.co.zm/index.php/mjz/article/view/275 [Google Scholar]
- 89.Benade EL, Jacobson BF, Louw S, Schapkaitz E. Validation of the CoaguChek XS international normalised ratio point‐of‐care analyser in patients at Charlotte Maxeke Johannesburg Academic Hospital, South Africa. South African Med J. 2016;106(3):280‐283. 10.7196/SAMJ.2016.v106i3.9422 [DOI] [PubMed] [Google Scholar]
- 90.Agyepong‐Yeboah AA. Comparison of the International Normalised Ratios obtained by the CoaguChek® XS coagulometer and by the Haematology Laboratory for patients on warfarin therapy at Dr George Mukhari Hospital. Gauteng Province; 2014https://repository.smu.ac.za/handle/20.500.12308/104 [Google Scholar]
- 91.Mbokota N, Schapkaitz E, Louw S. Verification of the qLabs international normalized ratio point‐of‐care device for monitoring of patients attending an anticoagulation clinic. Int J Lab Hematol. 2018;40(5):508‐514. 10.1111/ijlh.12849 [DOI] [PubMed] [Google Scholar]
- 92.Kamuren Z, Kigen G, Keter A, Maritim A. Characteristics of patients with thromboembolic disorders on warfarin therapy in resource limited settings. BMC Health Serv Res. 2018;18(1):723. 10.1186/s12913-018-3537-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nyandigisi EM. Comparative Effectiveness and Cost Analysis of an Anticoagulation Clinic Versus Laboratory Based Practice in Kenyan Tertiary Referral Hospitals. 2018. http://erepository.uonbi.ac.ke/handle/11295/104521
- 94.Systematic Review and Economic Analysis Canadian Agency for Drugs and Technologies in Health . Point‐of‐Care Testing of International Normalized Ratio for Patients on Oral Anticoagulant Therapy. Ottawa: The Agency; 2014. http://www.cadth.ca/media/pdf/OP0515_POCINR_Science_Report.pdf [PubMed] [Google Scholar]
- 95.Anakwue R, Nwagha T, Ukpabi O, et al. A survey of clinicians practice patterns in anticoagulation therapy & prophylaxis in Nigeria. Haematol Int J. 2018;2:1‐8. 10.23880/HIJ-16000125 [DOI] [Google Scholar]
- 96.Asiimwe IG, Waitt C, Sekaggya‐Wiltshire C, et al. Developing and validating a clinical warfarin dose‐initiation model for Black‐African patients in South Africa and Uganda. Clin Pharmacol TherPublished Online First: 6 December. 2020. 10.1002/cpt.2128 [DOI] [PubMed] [Google Scholar]
- 97.Ministry of Health Ghana . Standard Treatment Guidelines. Sixth edit ed. Accra: Ghana National Drugs Programme; 2010. [Google Scholar]
- 98.Ministry of Health and Social Services Namibia . Namibia Standard Treatment Guidelines. First edit ed. Windhoek: Ministry of Health and Social Services; 2011. [Google Scholar]
- 99.Food Medicine and Health Care Administration and Control Authority Ethiopia . Standard Treatment Guidelines for General Hospitals. Third edit ed. Addis Ababa: Food Medicine and Health Care Administration and Control Authority; 2014. [Google Scholar]
- 100.Ministry of Health Uganda , Uganda Clinical Guidelines . National Guidelines for Mangaement of Common Conditions. Ministry of Health: Kampala; 2016. 2016. [Google Scholar]
- 101.Division of Non‐Communicable Diseases Ministry of Health Kenya . Kenya National Guidelines for Cardiovascular Diseases Management. Nairobi: Ministry of Health; 2018. [Google Scholar]
- 102.National Department of Health South Africa . Standard Treatment Guidelines And Essential Medicines List for South Africa: Hospital Level, Adults. 5th ed. Pretoria: National Department of Health; 2019. [Google Scholar]
- 103.Van Spall HGC, Wallentin L, Yusuf S, et al. Variation in warfarin dose adjustment practice is responsible for differences in the quality of anticoagulation control between centers and countries: An analysis of patients receiving warfarin in the RE‐LY trial. Circulation. 2012;126(19):2309‐2316. 10.1161/CIRCULATIONAHA.112.101808 [DOI] [PubMed] [Google Scholar]
- 104.Hill SW, Varker AS, Karlage K, et al. Analysis of drug content and weight uniformity for half‐tablets of 6 commonly split medications. J Manag Care Pharm. 2009;15:253‐261. 10.18553/jmcp.2009.15.3.253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Asiimwe IG, Zhang EJ, Osanlou R, et al. Genetic factors influencing warfarin dose in Black‐African patients: A systematic review and meta‐analysis. Clin Pharmacol Ther. 2020;107(6):1420‐1433. 10.1002/cpt.1755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Teklay G, Shiferaw N, Legesse B, Bekele M. Drug‐drug interactions and risk of bleeding among inpatients on warfarin therapy: A prospective observational study. Thromb J. 2014;12(1):20. 10.1186/1477-9560-12-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Maina MW, Pastakia SD, Manji I, et al. Describing the profile of patients on concurrent rifampin and warfarin therapy in Western Kenya: A case series. Drugs R D. 2013;13:191‐197. 10.1007/s40268-013-0023-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sekaggya C, Nalwanga D, Von Braun A, et al. Challenges in achieving a target international normalized ratio for deep vein thrombosis among HIV‐infected patients with tuberculosis: a case series. BMC Hematol. 2016;16(1):16. 10.1186/s12878-016-0056-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tarus NK, Pau AK, Sereti I, et al. Challenges in management of warfarin anti‐coagulation in advanced HIV/AIDS patients with venous thrombotic events ‐ a case series from a research clinic in rural Kericho, Kenya. East Afr Med J. 2013;90:207‐213. https://www.ajol.info/index.php/eamj/article/view/108234 [PubMed] [Google Scholar]
- 110.Ahmed SM, Nordeng H, Sundby J, Aragaw YA, de Boer HJ. The use of medicinal plants by pregnant women in Africa: A systematic review. J Ethnopharmacol. 2018;224:297‐313. 10.1016/j.jep.2018.05.032 [DOI] [PubMed] [Google Scholar]
- 111.Liwa AC, Smart LR, Frumkin A, Epstein HAB, Fitzgerald DW, Peck RN. Traditional herbal medicine use among hypertensive patients in Sub‐Saharan Africa: A systematic review. Curr Hypertens Rep. 2014;16(6):437. 10.1007/s11906-014-0437-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cordier W, Steenkamp V. Herbal remedies affecting coagulation: A review. Pharm Biol. 2012;50(4):443‐452. 10.3109/13880209.2011.611145 [DOI] [PubMed] [Google Scholar]
- 113.Awortwe C, Makiwane M, Reuter H, Muller C, Louw J, Rosenkranz B. Critical evaluation of causality assessment of herb‐drug interactions in patients. Br J Clin Pharmacol. 2018;84(4):679‐693. 10.1111/bcp.13490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Salmasi S, Loewen PS, Tandun R, et al. Adherence to oral anticoagulants among patients with atrial fibrillation: A systematic review and meta‐analysis of observational studies. BMJ Open. 2020;10:1‐14. 10.1136/bmjopen-2019-034778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mariita K, Nyamu D, Maina C, et al. Patient associated factors that affect adherence to warfarin therapy in a tertiary referral hospital in Kenya. East Cent African J Pharm Sci. 2015;18:43‐50. https://www.ajol.info/index.php/ecajps/article/view/177867 [Google Scholar]
- 116.Chang AY, Nabbaale J, Nalubwama H, et al. Motivations of women in Uganda living with rheumatic heart disease: A mixed methods study of experiences in stigma, childbearing, anticoagulation, and contraception. PLoS One. 2018;13(3):e0194030. 10.1371/journal.pone.0194030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Morisky DE, Ang A, Krousel‐Wood M, Ward HJ. Predictive validity of a medication adherence measure in an outpatient setting. J Clin Hypertens. 2008;10(5):348‐354. 10.1111/j.1751-7176.2008.07572.x [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 118.Iqbal S.Effect of a Designed Warfarin Based Education Program on Patients' Knowledge and Anticoagulation Control Among Adult Outpatients Attending Clinics at Kenyatta National Hospital. 2017. http://erepository.uonbi.ac.ke/handle/11295/103355
- 119.Eltayeb TYM, Mohamed MS, Elbur AI, Elsayed ASA. Satisfaction with and adherence to warfarin treatment: A cross‐sectional study among Sudanese patients. J Saudi Heart Assoc. 2017;29(3):169‐175. 10.1016/j.jsha.2016.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self‐reported measure of medication adherence. Med Care. 1986;24(1):67‐74. 10.1097/00005650-198601000-00007 [DOI] [PubMed] [Google Scholar]
- 121.Dwamena JA. Knowledge Level and Anticoagulation Control Among Patient on Warfarin Therapy at the National Cardiothoracic Center, Korle Bu Teaching Hospital. 2012. http://ugspace.ug.edu.gh/handle/123456789/23702%09
- 122.Gregersen NE. The implications to women of childbearing age taking warfarin anticoagulation. 2006. http://hdl.handle.net/10539/1838
- 123.Taylor FC, Ramsay ME, Tan G, Gabbay J, Cohen H. Evaluation of patients' knowledge about anticoagulant treatment. Qual Health Care. 1994;3(2):79‐85. 10.1136/qshc.3.2.79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Briggs AL, Jackson TR, Bruce S, Shapiro NL. The development and performance validation of a tool to assess patient anticoagulation knowledge. Res Social Adm Pharm. 2005;1(1):40‐59. 10.1016/j.sapharm.2004.12.002 [DOI] [PubMed] [Google Scholar]
- 125.Zeolla MM, Brodeur MR, Dominelli A, et al. Development and validation of an instrument to determine patient knowledge: The oral anticoagulation knowledge test. Ann Pharmacother. 2006;40:633‐638. 10.1345/aph.1G562 [DOI] [PubMed] [Google Scholar]
- 126.Hutheram K.Investigating patients' knowledge and use of the patient information leaflet regarding their warfarin therapy. 2016. http://hdl.handle.net/10413/17693
- 127.Samadoulougou A, Temoua Naibe D, Mandi G, et al. Evaluation of the level of knowledge of patients on treatment with vitamin K antagonists in Ouagadougou cardiology department. Pan Afr Med J. 2014;19:286. 10.11604/pamj.2014.19.286.5411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Janoly‐Duménil A, Bourne C, Loiseau K, et al. Traitement anticoagulant oral ‐ Évaluation des connaissances des patients hospitalisés en services de médecine physique et réadaptation. Ann Phys Rehabil Med. 2011;54(3):172‐180. 10.1016/j.rehab.2011.02.007 [DOI] [PubMed] [Google Scholar]
- 129.Ng SS, Lai NM, Nathisuwan S, et al. Comparative efficacy and safety of warfarin care bundles and novel oral anticoagulants in patients with atrial fibrillation: a systematic review and network meta‐analysis. Sci Rep. 2020;10(1):662. 10.1038/s41598-019-57370-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Rabkin M, Melaku Z, Bruce K, et al. Strengthening health systems for chronic care: Leveraging HIV programs to support diabetes services in Ethiopia and Swaziland. J Trop Med. 2012;2012:137460‐137466. 10.1155/2012/137460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yiu A, Bajorek B. Patient‐focused interventions to support vulnerable people using oral anticoagulants: a narrative review. Ther Adv Drug Saf. 2019;10:1‐27. 10.1177/2042098619847423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kanters S, Park JJH, Chan K, et al. Interventions to improve adherence to antiretroviral therapy: a systematic review and network meta‐analysis. Lancet HIV. 2017;4(1):e31‐e40. 10.1016/S2352-3018(16)30206-5 [DOI] [PubMed] [Google Scholar]
- 133.Rusingiza EK, El‐Khatib Z, Hedt‐Gauthier B, et al. Outcomes for patients with rheumatic heart disease after cardiac surgery followed at rural district hospitals in Rwanda. Heart. 2018;104(20):1707‐1713. 10.1136/heartjnl-2017-312644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Chin PKL, Wright DFB, Patterson DM, Doogue MP, Begg EJ. A proposal for dose‐adjustment of dabigatran etexilate in atrial fibrillation guided by thrombin time. Br J Clin Pharmacol. 2014;78(3):599‐609. 10.1111/bcp.12364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Cohen D. Dabigatran: How the drug company withheld important analyses. BMJ. 2014;349(jul 23 12):g4670. 10.1136/bmj.g4670 [DOI] [PubMed] [Google Scholar]
- 136.Moore TJ, Cohen MR, Mattison DR. Dabigatran, bleeding, and the regulators. BMJ. 2014;349(jul 23 17):g4517. 10.1136/bmj.g4517 [DOI] [PubMed] [Google Scholar]
- 137.Wiggins BS, Dixon DL, Neyens RR, Page RL II, Gluckman TJ. Select drug‐drug interactions with direct oral anticoagulants: JACC review topic of the week. J Am Coll Cardiol. 2020;75(11):1341‐1350. 10.1016/j.jacc.2019.12.068 [DOI] [PubMed] [Google Scholar]
- 138.Alexander SPH, Fabbro D, Kelly E, et al. The Concise Guide to PHARMACOLOGY 2019/20: Enzymes. Br J Pharmacol. 2019;176(Issue S1):S297‐S396. [DOI] [PMC free article] [PubMed] [Google Scholar]


