Abstract
Introduction
Individuals in early dementia prevention trials may differ in how much they benefit from interventions depending on their initial risk level. Additionally, modifiable dementia risk scores might be used as surrogate/intermediate outcomes.
Methods
In the Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER), we investigated in post hoc analyses (N = 1207) whether the cognitive benefits of the 2‐year multi‐domain lifestyle intervention differed by baseline dementia risk measured with the “LIfestyle for BRAin Health” (LIBRA) score. We also investigated intervention effects on change in LIBRA score over time.
Results
Overall, higher baseline LIBRA was related to less cognitive improvement over time. This association did not differ between the intervention and control groups. The intervention was effective in decreasing LIBRA scores over time, regardless of baseline demographics or cognition.
Discussion
The cognitive benefit of the FINGER intervention was similar across individuals with different LIBRA scores at baseline. Furthermore, LIBRA may be useful as a surrogate/intermediate endpoint and surveillance tool to monitor intervention success during trial execution.
Keywords: Alzheimer's disease, cognitive impairment, dementia, intervention, lifestyle, multi‐domain, prevention, randomized controlled trial, risk factors, risk score
1. BACKGROUND
Despite extensive research efforts and financial investments, there is to date no effective disease‐modifying treatment for dementia. In parallel, there is accumulating evidence for the contribution of modifiable risk and protective factors to dementia risk.1, 2, 3, 4, 5 This has led to increased recognition that lifestyle interventions represent a key strategy for delaying or preventing dementia onset.3, 4, 6, 7 The majority of single‐domain prevention trials for cognitive decline and dementia have shown mainly negative results.8 Ideally, prevention trials would simultaneously target multiple health and lifestyle‐related risk factors early (eg, at midlife) before the onset of pathological processes leading to dementia, and follow participants over several decades to monitor dementia incidence.9 However, such studies do not exist given the methodological challenges (eg, attrition, maintaining adherence to long‐term lifestyle changes) and the requirement of substantial resources (eg, logistics, finance).10
The Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER) was the first large randomized controlled trial (RCT) showing beneficial effects on cognitive outcomes, measured through neuropsychological tests, after a 2‐year multi‐domain lifestyle intervention in 1260 at‐risk individuals from the general population aged 60 to 77 years.11 Participants were randomized to either a multi‐domain intervention (diet, exercise, cognition, and vascular risk management) or a control group (regular health advice).12, 13 These findings strongly support the idea that preventive strategies using multi‐domain lifestyle interventions might reduce dementia risk. Yet, heterogeneity in treatment effects (HTE) often dilutes stronger effects that exist in specific risk groups.14 If benefits are limited to such at‐risk groups, then interventions could be more effectively targeted. Also, modifiable risk scores might be useful for monitoring intervention effects on dementia risk reduction. The “LIfestyle for BRAin Health” (LIBRA) score is a promising tool consisting of 12 risk and protective factors for cognitive decline and dementia that can be targeted by lifestyle interventions and vascular risk management in primary care, with higher scores indicating higher dementia risk (ie, unhealthier lifestyle). LIBRA focuses exclusively on modifiable risk and protective factors, thereby capturing lifestyle‐based prevention potential. In contrast, other available risk scores for dementia have usually combined modifiable risk factors with non‐modifiable factors like age, sex, or genetics.15, 16, 17 LIBRA has been shown to predict cognitive decline and higher dementia risk in various general population‐ and patient‐based cohort studies.2, 18, 19, 20, 21, 22, 23 In addition, LIBRA has been previously used for HTE analysis in the Prevention of Dementia by Intensive Vascular Care (preDIVA) RCT24 and has recently been tested as a surrogate outcome in three multi‐domain lifestyle‐based intervention trials (preDIVA, Multidomain Alzheimer Preventive Trial [MAPT], Healthy Ageing Through Internet Counseling in the Elderly [HATICE]).25 Here, LIBRA was responsive to the interventions with almost 80% of the participants experiencing a change in LIBRA scores over time and moderate but significant between‐group differences. Notably, the number of available LIBRA factors in these trials varied between 6 and 11 out of 12 risk and protective factors.25
These initial findings suggest that LIBRA may be useful in selecting and monitoring individuals in lifestyle‐based prevention trials, taking into account different levels of initial risk. Therefore, the aim of the present study is two‐fold: (1) to investigate whether the intervention effects on cognition in FINGER differ across baseline LIBRA scores (based on all 12 LIBRA factors) and (2) to investigate the potential use of the LIBRA score as an outcome measure in early intervention studies.
RESEARCH IN CONTEXT
Systematic reviews: The authors reviewed the literature using traditional medical databases (eg, PubMed). One study using a modifiable dementia risk score to investigate heterogeneity in treatment effects in a multi‐domain dementia prevention trial was identified and one study using dementia risk scores as surrogate outcomes in multi‐domain prevention trials were identified. These studies are appropriately cited.
Interpretation: Overall, higher baseline “LIfestyle in BRAin Health” (LIBRA) score was related to less cognitive improvement over time. This association was not different between the intervention and control groups. The 2‐year multi‐domain lifestyle intervention was effective in decreasing LIBRA scores over time, regardless of demographic and socioeconomic factors, baseline cognition, or apolipoprotein E (APOE) ε4 carrier status.
Future directions: The current findings encourage future multi‐domain lifestyle interventions into dementia prevention to include a modifiable dementia risk score as an outcome measurement and to monitor risk modification over time.
2. METHODS
2.1. Study sample and design
The current study is a post hoc analysis of the FINGER trial. The study participants (N = 1260 aged 60–77 years) were recruited from earlier national health surveys at six study sites across Finland. Inclusion criteria were Cardiovascular Risk Factors, Ageing and Dementia (CAIDE) score of at least 6 points indicating presence of some modifiable risk factors,26 and cognitive performance at the mean level or slightly below mean of the respective age group based on the Consortium to Establish a Registry for Alzheimer's Disease (CERAD) cognitive screening test.27 Exclusion criteria were dementia or substantial cognitive impairment (Mini‐Mental State Examination [MMSE] <20 or clinical judgment),28 conditions preventing safe engagement in lifestyle trial, and participation in another ongoing intervention study. The present study included all FINGER participants randomly assigned in a 1:1 ratio to the intervention group (N = 631) or control group (N = 629). The intervention group received four intervention components: (1) a nutritional intervention, (2) a physical exercise training program, (3) cognitive training, and (4) monitoring and management of metabolic and vascular risk factors. The control group received regular health advice. More details of the sample characteristics,13 FINGER intervention, eligibility criteria, randomization procedure,12 and primary results11 have been previously described elsewhere. The FINGER trial was approved by the Coordinating Ethics Committee of the Hospital District of Helsinki and Uusimaa. Written informed consent was obtained from all participants at screening and baseline visit. The FINGER trial is registered with ClinicalTrials.gov (no. NCT01041989).
2.2. Demographics
Information on age and sex were derived from the Finnish national population register (https://dvv.fi/en), and years of education, marital status/cohabitation, and socioeconomic status were collected using standardized questionnaires administered at baseline. Socioeconomic status was based on annual household income and classified into low (0–20.000€), medium (20.001–50.000€) and high (>50.000€). Apolipoprotein E (APOE) genotype was determined by polymerase chain reactions using TaqMan genotyping assays.29 Participants were categorized as carriers of at least one ɛ4 allele versus non‐carriers.
2.3. LIBRA prevention index
LIBRA is a validated poly‐environmental risk score for both cognitive functioning and dementia risk,18, 19, 20, 21, 22, 23 developed as an instrument to show an individual's potential for dementia prevention after triangulation of results from a systematic literature review and an expert consensus study.2 LIBRA consists of a weighted sum score (theoretical range from −5.9 to +12.7; with higher scores indicating greater dementia risk) of 12 modifiable risk and protective factors for cognitive decline and dementia (Table 1). Risk factors are coronary heart disease, diabetes, hypercholesterolemia, hypertension, depression, obesity, smoking, physical inactivity, and renal disease. Protective factors are low‐to‐moderate alcohol use, high cognitive activity, and healthy diet. Details of the development of the LIBRA score have been described elsewhere.2
TABLE 1.
Baseline characteristics of study sample (N = 1207) by FINGER randomization group
| Randomization group | |||
|---|---|---|---|
| Variable | Participants with information available | Intervention (N = 609) | Control (N = 598) |
| Demographics | |||
| Age, mean (SD) | 1207 | 69.4 (4.6) | 69.1 (4.7) |
| Female, n (%) | 1207 | 274 (45.0) | 283 (47.3) |
| Years of education, mean (SD) | 1206 | 10.0 (3.5) | 10.0 (3.4) |
| Married or cohabiting, n (%) | 1203 | 448 (73.9) | 450 (75.4) |
| Socioeconomic status, n (%)* | 1155 | ||
| Low | 141 (24.2) | 124 (21.6) | |
| Medium | 341 (58.6) | 342 (59.7) | |
| High | 100 (17.2) | 107 (18.7) | |
| APOE ε4 gene carrier, n (%) | 1127 | 181 (31.8) | 189 (33.9) |
| Health‐ and lifestyle (LIBRA) factors† | |||
| Coronary heart disease, n (%) | 1207 | 83 (13.6) | 70 (11.7) |
| High blood sugar level, n (%) | 1207 | 28 (4.6) | 26 (4.4) |
| Hypercholesterolemia, n (%) | 1207 | 355 (58.3) | 344 (57.5) |
| Hypertension, n (%) | 1207 | 318 (52.2) | 311 (52.0) |
| Depression, n (%) | 1207 | 99 (16.3) | 112 (18.7) |
| Obesity, n (%) | 1207 | 182 (29.9) | 180 (30.1) |
| Smoking, n (%) | 1207 | 62 (10.2) | 49 (8.2) |
| Low‐to‐moderate alcohol use, n (%) | 1207 | 443 (72.7) | 411 (68.7) |
| Physical inactivity, n (%) | 1207 | 183 (30.1) | 169 (28.3) |
| High cognitive activity, n (%)‡ | 1207 | 290 (47.6) | 316 (52.8) |
| Healthy diet, n (%) | 1207 | 218 (35.8) | 217 (36.3) |
| Renal dysfunction, n (%) | 1207 | 63 (10.3) | 44 (7.4) |
| LIBRA score, mean (SD)§ | 1207 | 0.40 (2.6) | 0.21 (2.6) |
| Cognition¶ | |||
| NTB total score, mean (SD) | 1206 | −0.03 (0.56) | 0.02 (0.59) |
| Executive functioning, mean (SD) | 1205 | −0.03 (0.66) | 0.01 (0.70) |
| Processing speed, mean (SD) | 1206 | −0.03 (0.78) | 0.03 (0.85) |
| Memory, mean (SD) | 1206 | −0.03 (0.68) | 0.03 (0.66) |
| Abbreviated memory, mean (SD) | 1185 | −0.03 (0.79) | 0.03 (0.74) |
Abbreviations: APOE, apolipoprotein E; LIBRA, LIfestyle for BRAin Health; NTB, Neuropsychological Test Battery; SD, standard deviation.
Percentages may not sum up to 100 because of rounding.
See Table S1 in supporting information for the operationalization of the health‐ and lifestyle (LIBRA) factors.
High cognitive activity is based on self‐reported engagement in daily‐life leisure‐time activities. Participants were categorized as cognitively active or inactive based on the median distribution of reported activities (see Table S1 in supporting information).
LIBRA score theoretical range: −5.9 to 12.7; observed range: −5.9 to 7.9, with higher scores indicating higher dementia risk.
For the cognitive test scores, a high score indicates better performance.
In the FINGER trial, information was available for all 12 LIBRA factors at baseline, and at the 1‐ and 2‐year visits based on clinical (laboratory and anthropometric measurements) and self‐reported (dietary data and medical history) data from study visits. Each measure was dichotomized according to established cut‐offs as in Table S1 in supporting information.
2.4. Cognitive outcomes
Neuropsychological tests (an extended version of the Neuropsychological Test Battery [NTB])30 were administered at baseline and at the 1‐ and 2‐year visits by study psychologists who were blinded to the intervention allocation. Participants who dropped out during the study period were invited to the final cognitive assessment at the 2‐year visit. Test results for each time point were calculated on a standardized z‐scale (standardized to the baseline mean and standard deviation), with higher scores indicating better performance. Zero‐skewness log‐transformation was applied to skewed NTB components. In the present study, the following cognitive outcomes were used: (1) NTB composite z‐score (based on results from 14 individual cognitive tests; the primary outcome of the trial) and (2) domain‐specific NTB z‐scores for executive functioning, processing speed, memory, and abbreviated memory. Details of the specific cognitive test within each cognitive domain are described elsewhere.11, 31
2.5. Statistical analyses
Independent samples t‐tests and χ2‐tests were used to examine differences with regard to demographics and baseline cognition scores between participants with and without available LIBRA score data. Independent samples t‐tests and one‐way analysis of variance (ANOVA) were used to examine differences in baseline LIBRA scores among various demographic groups.
Linear mixed models with maximum likelihood estimation (following the intention‐to‐treat principle) tested the association between (1) baseline (continuous) LIBRA scores and change in cognition over time and (2) randomization group and change in LIBRA over time. The models included a random intercept and random slope with an unstructured covariance matrix, as suggested by likelihood ratio tests. Randomization group (dichotomous variable coded as 0 for control and 1 for intervention), time (first study aim: continuous variable coded as 0 for baseline, 1 for 1‐year visit, and 2 for 2‐year visit; second study aim: discrete time variable by using dummy variables for the two follow‐ups: 1 = baseline to 1‐year visit, 2 = baseline to 2 year‐visit to report the unstandardized regression coefficients of the change in LIBRA per intervention year), baseline LIBRA scores (continuous variable), and their possible interactions (Group x Time, Group x LIBRA, Time x LIBRA, and Group x Time x LIBRA) were included in the models.
For the first study aim, we report the unstandardized regression coefficients (and their 95% confidence intervals [CI]) of the best‐fitting model using Bayesian information criterion (BIC). The best‐fitting model (lowest BIC) was determined by comparing the full model (all interaction terms included) to alternative models excluding non‐significant interaction terms. Likelihood ratio tests were used to compare alternative models to the full model. Besides the Group x Time x LIBRA interaction (HTE analysis), the Group x Time (intervention effect) and Time x LIBRA (effect of baseline LIBRA on cognition over time) interactions are of main interest and were not excluded from the models. In addition to looking at the continuous LIBRA score, participants were also classified in three risk groups based on tertiles of the baseline LIBRA score (ie, tertile 1 = low risk, tertile 2 = intermediate risk, tertile 3 = high risk) to see whether specific risk groups benefited more from the FINGER intervention.
For the second study aim, we report the unstandardized regression coefficients (and their 95% CI) of the model that included the Group x Time (intervention effect on LIBRA) interaction. This model was additionally corrected for age, sex, years of education, and socioeconomic status. Next, several three‐way interactions (Group x Time x “Variable Z”) were added to the model to see whether this possible association was moderated by age, sex, years of education, socioeconomic status, being married/cohabitated, APOE ε4 carrier status and baseline cognitive performance (NTB total score). The distribution of the residuals of this model (change in LIBRA score as outcome) supported the assumption of normality.
All analyses were primarily adjusted for study site and were done in Stata/SE 15 (StataCorp, Texas), and the level of statistical significance was P < 0.05 in two‐sided tests.
3. RESULTS
3.1. Baseline population characteristics
After exclusion of individuals with an incomplete baseline LIBRA score (N = 53), the study sample consisted of 1207 individuals. Those with missing LIBRA score data did not differ from participants with available LIBRA score data regarding demographics and baseline cognition scores (Table S2 in supporting information). Baseline characteristics of the study sample are summarised by FINGER randomization group in Table 1.
Men had higher baseline LIBRA scores (ie, higher modifiable dementia risk) compared to women (mean = 0.59 [standard deviation = 2.65] versus −0.03 [2.56]; P < 0.001). Individuals with a low socioeconomic status had higher baseline LIBRA scores compared to participants with a medium or high socioeconomic status (low: 0.71 [2.82]; medium: 0.16 [2.56]; high: 0.25 [2.56]; P = 0.015). People who lived alone had higher LIBRA scores compared to people who were married or cohabiting (0.58 [2.69] versus 0.22 [2.60]; P = 0.039). Further, younger age (<70 years) was associated with higher LIBRA scores at baseline (0.48 [2.74] versus 0.10 [2.47]; P = 0.013). There were no significant differences in baseline LIBRA scores for APOE ε4 gene carrier status or years of education (<9 years versus 9 years or more).
3.2. Baseline LIBRA and cognition
At baseline, higher (continuous) LIBRA scores were cross‐sectionally associated with lower scores on all cognitive outcomes in the total sample. Higher LIBRA scores at baseline predicted less improvement on the NTB total score and in the memory and abbreviated memory domain over the 2‐year study period in the entire population (Table 2; Table‐S3 in supporting information). Further, the intermediate‐risk (LIBRA tertile 2) and high‐risk groups (LIBRA tertile 3) had lower scores on all cognitive outcomes at baseline compared to the low‐risk group (LIBRA tertile 1; except for memory and abbreviated memory in the intermediate‐risk versus the low‐risk group). Participants in the intermediate‐risk and high‐risk group also showed less improvement on the NTB total score and in the memory and abbreviated memory domain over the 2‐year study period (Table S4 in supporting information).
TABLE 2.
Associations between baseline LIBRA and baseline cognition, and baseline LIBRA and change in cognition over time in the total study sample (N = 1207). Estimates are from the best fitting linear mixed model
| Parameter | NTB total score | Executive functioning | Processing speed | Memory | Abbreviated memory |
|---|---|---|---|---|---|
| Estimate (95% CI), P‐value | |||||
| Baseline LIBRA and baseline cognition |
−0.029 (−0.041 to −0.017) P < 0.001 |
−0.023 (−0.038 to −0.009) P = 0.001 |
−0.045 (−0.062 to −0.028) P < 0.001 |
−0.025 (−0.039 to −0.010) P = 0.001 |
−0.019 (−0.034 to −0.003) P = 0.021 |
| Baseline LIBRA and change in cognition over time |
−0.007 (−0.011 to −0.003) P < 0.001 |
−0.004 (−0.009 to 0.000) P = 0.078 |
−0.005 (−0.010 to 0.000) P = 0.057 |
−0.011 (−0.017 to −0.004) P = 0.001 |
−0.009 (−0.016 to −0.003) P = 0.007 |
Abbreviations: CI, confidence interval; LIBRA, LIfestyle for BRAin Health; NTB, Neuropsychological Test Battery.
Other parameters included in the best fitting model: “Group”, “Time”, “Group x Time” (see Table S2 in supporting information).
Note: parameter for “Baseline LIBRA and baseline cognition” gives the baseline differences in cognition for each 1‐point increase in LIBRA; parameter for “Baseline LIBRA and change in cognition over time” gives the change in cognition per 1‐point increase in baseline LIBRA for each additional year in study.
However, LIBRA did not moderate the intervention effect on the FINGER primary outcome, ie, the effect of randomization group on change in NTB total scores (Group x Time x LIBRA interaction; B = −0.001, 95% CI −0.009 to 0.007; P = 0.804), showing that the intervention benefit on cognition was similar across LIBRA scores. Results did not change when testing LIBRA tertiles (Group x Time x LIBRA interaction; LIBRA tertile 2: B = −0.044, 95% CI −0.094 to 0.005; P = 0.079; LIBRA tertile 3: B = −0.010, 95% CI −0.059 to 0.040; P = 0.701). Also, LIBRA did not moderate the FINGER intervention effect on the secondary cognitive outcomes NTB executive functioning, processing speed, or memory domains (results not shown).
3.3. Intervention effects on change in LIBRA over time
At baseline, there was no difference in LIBRA scores between the two randomization groups (B = 0.134, 95% CI −0.151 to 0.419; P = 0.357). LIBRA scores declined more in the intervention group than in the control group after one (B = −0.44; 95% CI −0.72 to −0.16; P = 0.002) and two years (B = −0.35; 95% CI −0.64 to −0.07; P = 0.016; Figure 1). Results did not change after correcting for age, sex, years of education, and socioeconomic status. Age, sex, years of education, socioeconomic status, being married/cohabitated, APOE ε4 carrier status, and baseline cognitive performance (NTB total score) did not moderate the FINGER intervention effect on change in LIBRA over time (results not shown).
FIGURE 1.
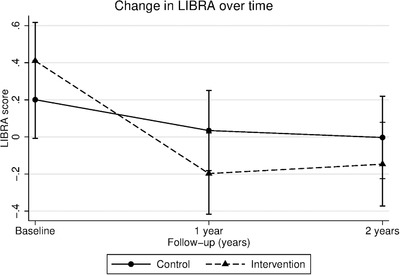
Change in LIBRA over time for individuals in the intervention and control group
Notes: Figure shows estimated mean LIBRA score at baseline, 1‐, and 2‐year visits (lower scores indicate lower dementia risk). Error bars are confidence intervals. Linear mixed models were used to assess differences in LIBRA change between intervention and control group over the 2‐year study period (Group x Time interaction).
Abbreviations: LIBRA, LIfestyle for BRAin Health
4. DISCUSSION
The multi‐domain FINGER intervention was effective in decreasing LIBRA scores over time. This decrease in LIBRA was more pronounced during the first year of the FINGER intervention, after which LIBRA stayed relatively stable during the second intervention year. Further, factors related to health inequalities (eg, low socioeconomic status and fewer years of education) did not have an impact on the intervention benefit on change in LIBRA over time.
4.1. LIBRA as surrogate outcome
Our findings give further weight that multi‐domain lifestyle interventions for dementia risk reduction are effective by showing that the lifestyle‐based risk score declined more in the intervention arm during the first year of intervention already and was sustained over 2 years. Yet, effects on cognition became more visible later in a time‐lagged fashion after 2 years.11 It could be suggested that this decrease in LIBRA during the first year of the intervention could be related to the intensity of the intervention components during this first year and that the stabilization of LIBRA can be explained by less adherence during the second year of the intervention. Alternatively, this could also be explained by an individual‐specific floor effect on how much one can change their risk (eg, their room for improvement was already tackled after 1 year of intervention). Hence, LIBRA might be used as an instrument to monitor progress in risk reduction within a relatively short timeframe during trial execution, upon which the intervention can be intensified or tailored toward specific risk factors, and as a surrogate/intermediate endpoint in future multi‐domain lifestyle interventions. These findings can be taken into account in the design and execution of future FINGER‐type interventions, eg, in the context of the World Wide FINGERS initiative.32, 33, 34
4.2. Heterogeneity in treatment effects on cognition
Further, LIBRA scores at baseline did not modify the previously reported FINGER intervention effect on the primary cognitive outcome NTB total score, meaning that the cognitive benefit of the FINGER intervention was similar across individuals with different LIBRA scores at baseline. This was most likely due to the fact that people were already selected in FINGER based on their elevated CAIDE dementia risk score, in addition to cognitive performance at the mean level or slightly lower than expected for age. Notably, there is some overlap between risk factors included in CAIDE and LIBRA (eg, blood pressure/hypertension, body mass index/obesity, total cholesterol/hypercholesterolemia, and physical activity). Alternatively, this finding can also imply that there was no HTE, meaning that the FINGER intervention benefits everybody.
4.3. LIBRA score, cognition, and sociodemographic factors
In the entire FINGER population, higher LIBRA scores (eg, an unhealthier lifestyle) at baseline were associated with lower baseline cognitive performance in all domains, and also less cognitive improvement over time in NTB total score and the memory domain. This is in line with findings from a previous LIBRA validation study in 2347 cognitive healthy middle‐aged individuals of the Doetinchem Cohort Study.20 In this observational study, higher baseline LIBRA scores predicted faster decline in verbal memory, cognitive flexibility, and mental speed. As in FINGER, men had higher LIBRA score at baseline compared to women.20 In a community‐dwelling prospective cohort study from the United Kingdom, individuals with a low socioeconomic status had higher LIBRA scores and a higher dementia risk compared to individuals with a high socioeconomic status. Mediation analysis showed that the difference in dementia risk between individuals with a high socioeconomic status and individuals with a low socioeconomic status could be for more than 50% explained by differences in modifiable health conditions and lifestyle factors (LIBRA).19 In the FINGER trial, participants with a low socioeconomic status also had higher LIBRA scores at baseline compared to participants with a medium or high socioeconomic status, but no significant difference in intervention benefit on change in estimated dementia risk (LIBRA) was found between socioeconomic groups or years of education. This means that less privileged groups (lower socioeconomic status and fewer years of education) benefited as much from the FINGER intervention in terms of change in estimated dementia risk as more privileged groups, thereby not widening the health gap between the rich and the poor. This finding is particularly important given a previous study reporting that lower income was associated with poorer adherence to the nutritional component of the FINGER intervention.35 The beneficial effect of the FINGER intervention on cognition regardless of sociodemographic (eg, age, sex, years of education), socioeconomic status, baseline cognitive performance, and cardiovascular risk factors (eg, body mass index, cholesterol, blood pressure) has already been reported.36
4.4. Strengths and limitations
Strengths of this study were the administration of a comprehensive neuropsychological test battery, the low drop‐out rate, and the thorough randomization and masking/blinding.35 Further, the FINGER population is representative for the at‐risk segment of the older general population without substantial cognitive impairment/dementia, who is also most likely to benefit from the intervention.13 Conventional subgroup analyses use single participant characteristics for stratification, but this approach is often unable to explain even large HTE.37 In contrast, multivariable risk scores, such as LIBRA, account for multiple relevant characteristics simultaneously and are more relevant for HTE analysis.38, 39 Yet, this study has some limitations. First, FINGER participants were recruited from persons who had earlier participated in population surveys. This might have led to a selection of individuals who were more willing to adhere to the intervention and were in general more interested in (improving) their personal health. Previous LIBRA studies had a prospective cohort design and the mean LIBRA score in these studies was somewhat higher compared to the present study.18, 19, 20 Yet, it has to be noted that in none of the previous studies all 12 LIBRA factors were available, making direct comparisons difficult (in most cases the protective factors high cognitive activity and healthy diet were not measured, which may have resulted in higher overall scores). Next, despite the relatively large sample size, there may be lack of statistical power for post hoc subgroup analyses such as three‐way interactions. Additionally, participants with missing data (incomplete LIBRA scores) were excluded from the analyses (4.2% of the total sample). However, subjects with missing LIBRA score data did not differ from the study sample with regard to demographics and baseline cognition scores. An extended follow‐up of FINGER participants is ongoing to investigate the long‐term effects of this multi‐domain intervention on dementia incidence.
5. CONCLUSIONS
In sum, the LIBRA score may be a suitable surrogate/intermediate endpoint to monitor and measure change in modifiable dementia risk in future multi‐domain lifestyle interventions. Further, these findings suggest that a composite risk score comprising unhealthy lifestyle and relatively poor health is associated with less cognitive improvement 2 years later. The multi‐domain FINGER intervention was equally effective in those at low, medium, or high modifiable dementia risk.
Conflicts of Interest
The authors report no conflicts of interest.
Funding Information
K. Deckers received funding from the Young European Research Universities (YERUN) Research Mobility Award and a travel fellowship from Alzheimer Nederland. T. Ngandu received research funding from EU Joint Programme–Neurodegenerative Disease Research (EURO‐FINGERS) and Finnish Cultural Foundation, Juho Vainio Foundation, and Jalmari and Rauha Ahokas Foundation, Finland. R. Antikainen received research funding from EVO/VTR grants of Oulu University Hospital and Oulu City Hospital (Finland). H. Soininen received funding from EU 7th framework collaborative project grant (HATICE), EU Joint Programme–Neurodegenerative Disease Research (MIND‐AD), UEF Strategic funding for UEFBRAIN (Finland), and EVO/VTR funding from Kuopio University Hospital (Finland). M. Kivipelto received research support from the Academy of Finland (317465), Finnish Social Insurance Institution, Finnish Ministry of Education and Culture, Juho Vainio Foundation (Finland), EU Joint Programme–Neurodegenerative Disease Research (MIND‐AD and EURO‐FINGERS), Alzheimer's Research and Prevention Foundation (US), Alzheimerfonden (Sweden), Swedish Research Council, Center for Innovative Medicine (CIMED) at Karolinska Institutet, Region Stockholm (ALF, NSV), Knut and Alice Wallenberg Foundation (Sweden), Stiftelsen Stockholms sjukhem (Sweden), Konung Gustaf V:s och Drottning Victorias Frimurarstiftelse (Sweden). A. Solomon received research funding from the European Research Council grant 804371, Academy of Finland (287490, 294061, 319318), Finnish Cultural Foundation, Yrjö Jahnsson Foundation (Finland), Alzheimerfonden, and Region Stockholm ALF (Sweden).
The funding sources had no role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Supporting information
Supporting information
Deckers K, Köhler S, Ngandu T, et al. Quantifying dementia prevention potential in the FINGER randomized controlled trial using the LIBRA prevention index. Alzheimer's Dement. 2021;17:1205–1212. 10.1002/alz.12281
The copyright line for this article was changed on 6 January 2021 after original online publication.
REFERENCES
- 1.Baumgart M, Snyder HM, Carrillo MC, Fazio S, Kim H, Johns H. Summary of the evidence on modifiable risk factors for cognitive decline and dementia: a population‐based perspective. Alzheimers Dement. 2015;11:718‐726. [DOI] [PubMed] [Google Scholar]
- 2.Deckers K, van Boxtel MP, Schiepers OJ, et al. Target risk factors for dementia prevention: a systematic review and Delphi consensus study on the evidence from observational studies. Int J Geriatr Psychiatry. 2015;30:234‐246. [DOI] [PubMed] [Google Scholar]
- 3.Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396:413‐446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Livingston G, Sommerlad A, Orgeta V, et al. Dementia prevention, intervention, and care. Lancet. 2017;390:2673‐2734. [DOI] [PubMed] [Google Scholar]
- 5.Norton S, Matthews FE, Barnes DE, Yaffe K, Brayne C. Potential for primary prevention of Alzheimer's disease: an analysis of population‐based data. Lancet Neurol. 2014;13:788‐794. [DOI] [PubMed] [Google Scholar]
- 6.Lincoln P, Fenton K, Alessi C, et al. The Blackfriars Consensus on brain health and dementia. Lancet. 2014;383:1805‐1806. [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization . Risk reduction of cognitive decline and dementia: WHO Guidelines. Geneva: World Health Organization; 2019. [PubMed] [Google Scholar]
- 8.Andrieu S, Coley N, Lovestone S, Aisen PS, Vellas B. Prevention of sporadic Alzheimer's disease: lessons learned from clinical trials and future directions. Lancet Neurol. 2015;14:926‐944. [DOI] [PubMed] [Google Scholar]
- 9.Solomon A, Mangialasche F, Richard E, et al. Advances in the prevention of Alzheimer's disease and dementia. J Intern Med. 2014;275:229‐250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Friedland RP, Nandi S. A modest proposal for a longitudinal study of dementia prevention (with apologies to Jonathan Swift, 1729). J Alzheimers Dis. 2013;33:313‐315. [DOI] [PubMed] [Google Scholar]
- 11.Ngandu T, Lehtisalo J, Solomon A, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at‐risk elderly people (FINGER): a randomised controlled trial. Lancet. 2015;385:2255‐2263. [DOI] [PubMed] [Google Scholar]
- 12.Kivipelto M, Solomon A, Ahtiluoto S, et al. The Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER): study design and progress. Alzheimers Dement. 2013;9:657‐665. [DOI] [PubMed] [Google Scholar]
- 13.Ngandu T, Lehtisalo J, Levalahti E, et al. Recruitment and baseline characteristics of participants in the Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER)‐a randomized controlled lifestyle trial. Int J Environ Res Public Health. 2014;11:9345‐9360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ioannidis JP, Lau J. The impact of high‐risk patients on the results of clinical trials. J Clin Epidemiol. 1997;50:1089‐1098. [DOI] [PubMed] [Google Scholar]
- 15.Hing Tang EY, Robinson L, Maree Stephan BC. Dementia risk assessment tools: an update. Neurodegener Dis Manag. 2017;7:345‐347. [DOI] [PubMed] [Google Scholar]
- 16.Licher S, Yilmaz P, Leening MJG, et al. External validation of four dementia prediction models for use in the general community‐dwelling population: a comparative analysis from the Rotterdam Study. Eur J Epidemiol. 2018;33:645‐655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tang EY, Harrison SL, Errington L, et al. Current Developments in Dementia Risk Prediction Modelling: an Updated Systematic Review. PLoS One. 2015;10:e0136181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deckers K, Barbera M, Köhler S, et al. Long‐term dementia risk prediction by the LIBRA score: a 30‐year follow‐up of the CAIDE study. Int J Geriatr Psychiatry. 2020;35:195‐203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deckers K, Cadar D, van Boxtel MPJ, Verhey FRJ, Steptoe A, Köhler S. Modifiable Risk Factors Explain Socioeconomic Inequalities in Dementia Risk: evidence from a Population‐Based Prospective Cohort Study. J Alzheimers Dis. 2019;71(2):549‐557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deckers K, Nooyens A, van Boxtel M, Verhey F, Verschuren M, Köhler S. Gender and educational differences in the association between lifestyle and cognitive decline over 10 years: the doetinchem cohort study. J Alzheimers Dis. 2018;70(s1):S31‐S41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pons A, LaMonica HM, Mowszowski L, Köhler S, Deckers K, Naismith SL. Utility of the LIBRA index in relation to cognitive functioning in a clinical health seeking sample. J Alzheimers Dis. 2018;62:373‐384. [DOI] [PubMed] [Google Scholar]
- 22.Schiepers OJG, Köhler S, Deckers K, et al. Lifestyle for Brain Health (LIBRA): a new model for dementia prevention. Int J Geriatr Psychiatr. 2018;33:167‐175. [DOI] [PubMed] [Google Scholar]
- 23.Vos SJB, van Boxtel MPJ, Schiepers OJG, et al. Modifiable Risk factors for prevention of dementia in midlife, late life and the oldest‐old: validation of the LIBRA Index. J Alzheimers Dis. 2017;58:537‐547. [DOI] [PubMed] [Google Scholar]
- 24.van Middelaar T, Hoevenaar‐Blom MP, van Gool WA, et al. Modifiable dementia risk score to study heterogeneity in treatment effect of a dementia prevention trial: a post hoc analysis in the preDIVA trial using the LIBRA index. Alzheimers Res The. 2018;10:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coley N, Hoevenaar‐Blom MP, van Dalen JW, et al. Dementia risk scores as surrogate outcomes for lifestyle‐based multidomain prevention trials‐rationale, preliminary evidence and challenges. Alzheimers Dement. 2020;16(12):1674–1685. [DOI] [PubMed] [Google Scholar]
- 26.Kivipelto M, Ngandu T, Laatikainen T, Winblad B, Soininen H, Tuomilehto J. Risk score for the prediction of dementia risk in 20 years among middle aged people: a longitudinal, population‐based study. Lancet Neurol. 2006;5:735‐741. [DOI] [PubMed] [Google Scholar]
- 27.Morris JC, Heyman A, Mohs RC, et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer's disease. Neurology. 1989;39:1159‐1165. [DOI] [PubMed] [Google Scholar]
- 28.Folstein MF, Folstein SE, McHugh PR. "Mini‐mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189‐198. [DOI] [PubMed] [Google Scholar]
- 29.De la Vega FM, Lazaruk KD, Rhodes MD, Wenz MH. Assessment of two flexible and compatible SNP genotyping platforms: taqMan SNP Genotyping Assays and the SNPlex Genotyping System. Mutat Res. 2005;573:111‐135. [DOI] [PubMed] [Google Scholar]
- 30.Harrison J, Minassian SL, Jenkins L, Black RS, Koller M, Grundman M. A neuropsychological test battery for use in Alzheimer disease clinical trials. Arch Neuro. 2007;64:1323‐1329. [DOI] [PubMed] [Google Scholar]
- 31.Solomon A, Turunen H, Ngandu T, et al. Effect of the Apolipoprotein E genotype on cognitive change during a multidomain lifestyle intervention: a subgroup analysis of a randomized clinical trial. JAMA Neurol. 2018;75:462‐470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kivipelto M, Mangialasche F, Ngandu T. Lifestyle interventions to prevent cognitive impairment, dementia and Alzheimer disease. Nat Rev Neurol. 2018;14:653‐666. [DOI] [PubMed] [Google Scholar]
- 33.Kivipelto M, Mangialasche F, Ngandu T. World Wide Fingers will advance dementia prevention. Lancet Neurol. 2018;17:27. [DOI] [PubMed] [Google Scholar]
- 34.Kivipelto M, Mangialasche F, Snyder HM, et al. World‐Wide FINGERS Network: a global approach to risk reduction and prevention of dementia. Alzheimers Dement. 2020;16:1078‐1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Coley N, Ngandu T, Lehtisalo J, et al. Adherence to multidomain interventions for dementia prevention: data from the FINGER and MAPT trials. Alzheimers Dement. 2019;15:729‐741. [DOI] [PubMed] [Google Scholar]
- 36.Rosenberg A, Ngandu T, Rusanen M, et al. Multidomain lifestyle intervention benefits a large elderly population at risk for cognitive decline and dementia regardless of baseline characteristics: the FINGER trial. Alzheimers Dement. 2018;14:263‐270. [DOI] [PubMed] [Google Scholar]
- 37.Kent DM, Hayward RA. Limitations of applying summary results of clinical trials to individual patients: the need for risk stratification. JAMA. 2007;298:1209‐1212. [DOI] [PubMed] [Google Scholar]
- 38.Dorresteijn JA, Visseren FL, Ridker PM, et al. Estimating treatment effects for individual patients based on the results of randomised clinical trials. BMJ. 2011;343:d5888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kent DM, Rothwell PM, Ioannidis JP, Altman DG, Hayward RA. Assessing and reporting heterogeneity in treatment effects in clinical trials: a proposal. Trials. 2010;11:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information


