Abstract
Purpose
To compare the efficacy of implanting a single Keraring segment according to a novel Q‐value‐based nomogram (QN) to that of segment implantation according to the manufacturer's standard nomogram (SN), for keratoconus treatment.
Methods
This was a prospective, randomized controlled trial of 104 patients (104 eyes) with Amsler‐Krumeich grade 1 or 2 keratoconus, and type 1 or 2 cone asymmetry determined according to manufacturer's classification. They were randomly distributed into two groups: group A patients (n = 52) underwent Keraring implantation according to the SN, and group B patients (n = 52) underwent implantation of a single (210° arc‐length) Keraring segment according to the QN. Both treatments were combined with accelerated transepithelial cross‐linking, and follow‐up was 6 months. Main outcome measures were preoperative and postoperative visual acuity, subjective refraction and corneal topography.
Results
At postoperative month 6, group B exhibited statistically significantly higher values of mean uncorrected distance visual acuity (UDVA), sphere, K2, K‐average, K‐max and Q‐anterior (p = 0.02, 0.01, 0.002, 0.001, 0.0001 and 0.03, respectively) compared to that of group A. However, group A exhibited better refractive cylindrical improvements (p = 0.04). In group A, we documented spontaneous extrusion of one Keraring segment.
Conclusion
Single 210° arc‐length segment implantation using our objective QN was more efficacious for keratoconus treatment than using the subjective SN. The nomograms were comparable when the Q‐anterior value was >−1.00; however, the QN was superior to the SN when the Q‐anterior value was ≤−1.00. The QN yielded greater postoperative UDVA and smoother corneal remodelling than did the SN for treatment of grade 1 and 2 keratoconic eyes.
Keywords: corneal asphericity, corneal cross‐linking, intracorneal rings, Kerarings, keratoconus, Q‐value nomogram
Introduction
Wollensak et al. (2003) introduced the first successful treatment of keratoconus using corneal cross‐linking (CXL). This technique, also known as the Dresden protocol, is able to halt disease progression and improves corneal biomechanical properties (Spoerl et al. 2011; Raiskap et al. 2015; Vinciguerra et al. 2017; Iqbal et al. 2019a; Iqbal et al. 2019b; Herber et al. 2020). Modified CXL techniques, such as accelerated epithelium‐off CXL (ACXL) and transepithelial accelerated epithelium‐on CXL (TCXL), have proven to be less efficient than standard CXL (Soeters et al. 2015; Bikbova & Bikbov 2016; Rush & Rush 2017; Mazzotta et al. 2019; Iqbal et al. 2020).
Intracorneal ring segment (ICRS) implantation flattens the central corneal curvature, thus reducing the refractive error and keratometry values, as well as improving visual outcomes (Kymionis et al. 2014; Tian et al. 2018; Iqbal et al. 2019c). Keraring segments (Mediphacos Inc., Belo Horizonte, Brazil), Ferrara ring segments (AJL Ophthalmic S.A., Vitoria‐Gasteiz, Spain) and INTACS ring segments (Addition Technology, Inc., Lombard, IL, USA) are widely used to correct refractive status in keratoconus patients, regularizing the anterior corneal surface to decrease both myopic and astigmatic components (Saleem 2015; Heikal et al. 2017; Guyot et al. 2019; Rocha et al. 2019; Zaky et al. 2020). The introduction of femtosecond laser surgery increased the popularity of ICRS implantation among cornea surgeons, as the procedure is simple, safe and accurate (Ibrahim et al. 2016; Al‐Tuwairqi et al. 2017; Park et al. 2019). When CXL is combined with refractive surgery, it may be referred to as ‘CXL‐Plus’ (Randleman et al. 2017); one example is the combination of CXL and ICRS implantation (Saleem et al. 2018).
The cornea has a steeper curvature in its centre than in its periphery, and normal corneas have a mean Q‐value (a coefficient of corneal asphericity) of −0.26 ± 0.18 and a range of −0.88 to +0.50 (Kiely et al. 1982; Safarzadeh & Nasiri 2016). There is an inverse relationship between keratoconus severity and the Q‐value, due to increased prolateness; however, this relationship is lost in advanced keratoconus, as it becomes difficult to obtain a reliable Q‐value (Torquetti et al. 2012).
Intracorneal ring segment implantation results in better visual outcomes in patients with advanced keratoconus and reduced preoperative visual acuity, than in patients with early keratoconus and good preoperative visual acuity (Alio et al. 2014; Vega‐Estrada & Alio 2016; Fariselli et al. 2020). As Keraring implantation outcomes in keratoconic eyes are still unpredictable, there is a great need for improved nomograms or computer algorithms (Alio et al. 2014; Vega‐Estrada & Alio 2016; Fariselli et al. 2020). Utine et al. 2018 noted that postoperative visual improvements seem to be related to improvements in corneal asphericity and that the ideal nomogram would allow one to choose ICRS parameters according to a target Q‐value. Therefore, we designed a Q‐value‐based nomogram (QN) and compared it to the manufacturer's standard nomogram (SN) for Keraring implantation in patients with keratoconus grades 1 and 2 (Amsler‐Krumeich classification) in a prospective, randomized controlled trial. Our primary outcome was to test the efficacy of the QN, while our secondary outcomes were to document the short‐term visual, refractive and topographic results and potential complications using both procedures.
Materials and Methods
The Institutional Review Board of the Sohag Faculty of Medicine, Sohag University, Egypt, approved the protocol for this prospective, multicentre, randomized controlled trial, which adhered to the tenets of the Declaration of Helsinki. This study was registered at the Pan African Clinical Trial Registry (PACTR201811613938575). Surgeries were performed in cooperation with two private eye centres, one in Sohag (Future Femtolaser Center) and one in Cairo (Durrah Specialized Eye Center), Egypt.
Our study included 104 keratoconic eyes of 104 patients with documented keratoconus progression (K‐max >1 D). The nature of the disease, its presentation, treatment options, Keraring nomogram options and potential consequences were fully explained to all patients, who subsequently provided informed consent before surgery. Inclusion criteria were as follows: grade 1 (mean keratometry [K‐average] value <48 D and myopic and astigmatic components <5 D) or grade 2 (K‐average value 48–53 D and myopic and astigmatic components 5–8 D) keratoconus based on the Amsler‐Krumeich classification; type 1 (100% of the cone on one side of the steepest meridian) or type 2 (80% of the cone on one side of the steepest meridian) cone asymmetry based on the standard manufacturer's classification; and ≥380 μm corneal thickness at the thinnest location (CTT). Exclusion criteria were as follows: previous corneal surgery; corneal opacities; history of, or concomitant, vernal keratoconjunctivitis (VKC); excessive eye rubbing; or dry eye syndrome.
All patients underwent ophthalmic examination, including preoperative and postoperative assessments of visual acuity, refractive status, slit‐lamp and fundus examinations, as well as topographic/tomographic parameters. Main outcome measures were as follows: logarithm of the minimum angle of resolution (logMAR) uncorrected distance visual acuity (UDVA); logMAR corrected distance visual acuity (CDVA); subjective refractive components, including sphere, refractive cylinder and spherical equivalent (SE); and topographic/tomographic outcomes, including keratometry (K) values, pachymetry, topographic cylinder and the corneal asphericity Q‐values on the anterior and posterior corneal surfaces (Q‐anterior and Q‐posterior, respectively).
Grouping of study participants
Patients were randomly assigned to one of two groups. Patients in each group underwent Keraring implantation combined with TCXL. Keraring implantation was performed according to the SN in 52 eyes (group A, the control group) or according to the QN in 52 eyes (group B, the experimental group).
Furthermore, we planned to conduct various subgroup analyses within each group. One subdivision was made according to Q‐anterior value: subgroup Q1 level had a Q‐anterior value >−1 and subgroup Q2 level had a Q‐anterior value ≤−1. The other subdivision was made according to keratoconus grade: subgroups Gr1 and Gr2. Thus, the eight subgroups were A‐Q1, A‐Q2, B‐Q1, B‐Q2, A‐Gr1, A‐Gr2, B‐Gr1 and B‐Gr2. Comparisons were made between groups and subgroups in order to determine the efficacy of each protocol.
Surgical procedures
We used the CSO SIRIUS Topographer (CSO, Florence, Italy) for corneal topography, the KXL System (Avedro Inc., Burlington, MA, USA) for TCXL and the iFS advanced femtosecond laser (Abbott Laboratories Inc., Abbott Park, IL, USA) for corneal tunnelling. All Keraring segments were the SI‐5 model, which is synthesized from poly(methyl methacrylate) and consists of a triangular, cross‐sectional design and a 5‐mm optical zone.
For corneal tunnelling, iFS parameters were as follows: corneal tunnel depth, fixed at 75% of the CTT; inner diameter, 5 mm; outer diameter, 5.9 mm; entry cut length, 1.40 mm; entry cut thickness, 1 mm; incision axis, according to the steepest meridian; ring energy, 1.95 μJ; and entry cut energy, 1.95 μJ.
Topical anaesthetic eye drops (0.4% benoxinate hydrochloride (BENOX Sterile Ophthalmic Solution, EIPICO, Tenth of Ramadan City, Egypt) were instilled into the eye 15 min prior to surgery. Patients were directed to fixate their eyes upon a flashing light, in order to mark the centre of the cornea. A suction ring was applied to fixate the eye during tunnelling. A spatula was introduced into the tunnel to ensure its patency. One or two Keraring segments were implanted into the tunnel according to the SN or the QN.
Standard nomogram
All eyes in group A were treated according to the SN (Keraring Calculation Guidelines 2009, version 5.2 available at Keraring Calculation Helpdesk at keraring@mediphacos.com; Mediphacos Inc., Belo Horizonte, Brazil). As we included only eyes with type 1 or 2 cone asymmetry, we used part A (i.e. nomogram A) of the SN to determine the number of segments to implant, in each case.
Q‐value nomogram
Table 1 contains our QN. The goal with the QN was to implant a single segment to reverse the protrusion of eyes with type 1 or 2 cone asymmetry, that is to lift the cones up and push them backwards and upwards towards the original direction from which they protruded downwards. The QN assists the practitioner in choosing the most suitable segment to achieve this. According to the QN, the segment arc‐length is fixed at 210° in all cases, while segment thickness is approximately 60% of the corneal thickness at the optical zone (CTO), where the segment is implanted.
Table 1.
The novel Q‐value‐based nomogram.
| Q‐anterior |
CTO < 450 µm Degree/µm |
CTO 450–500 µm Degree/µm |
CTO >500 µm Degree/µm |
|---|---|---|---|
| ˃−0.50 | 210/150 | 210/150 | 210/150 |
| −0.50 to ˃−1 | 210/150 | 210/200 | 210/250 |
| −1 to −1.50 | 210/200 | 210/250 | 210/300 |
| <−1.50 | 210/250 | 210/250 | 210/300 |
CTO = corneal thickness at the optical zone, Q‐anterior = asphericity of the anterior corneal surface.
Values are indicated as Keraring segment arc‐length/thickness.
During surgery, the cornea was marked along the 0°–180° axis during slit‐lamp examination. Thereafter, the steepest meridian was marked on table and femtosecond laser tunnelling was performed. The 210‐degree‐arc‐length segment was implanted into the tunnel and guided until its edge was 10° below the incision site. Accordingly, the segment straddled the cone, thereby reversing the protrusion and mimicking the pre‐ectatic condition.
Transepithelial CXL
Following implantation of Kerarings, TCXL was performed according to the manufacturer’s nomogram (Avedro Inc.). Two types of riboflavin were applied onto the intact epithelium of the cornea. The first type, 0.25% riboflavin with hydroxypropyl methylcellulose and benzalkonium chloride (ParaCel; Avedro Inc.), was instilled at 1.5‐min intervals for 4.5 min. The second type, an isotonic, dextran‐free solution of 0.22% riboflavin (VibeX Xtra; Avedro Inc.), was instilled at 1.5‐min intervals for 6 min. For UVA corneal irradiation, KXL System parameters were as follows: total UVA time, 2 min 40 seconds; total treatment time, 5 min 20 seconds; total energy delivered, 7.2 J/cm2, via 45 mW/cm2 power; and pulsed UVA mode, 1 second on and 1 second off. Finally, a bandage contact lens was applied to the cornea.
Postoperative medication and care
All patients received the same postoperative topical treatment, in the form of antibiotic eye drops (0.5% moxifloxacin hydrochloride; Vigamox, Alcon Laboratories, Inc., Fort Worth, TX, USA), steroidal eye drops (1% prednisolone acetate; Econopred Plus, Alcon Laboratories, Inc.) and lubricating eye drops (Systane Ultra, Alcon Laboratories, Inc.). All topical eye drops were instilled five times daily for the first week and three times daily for the second week. Bandage contact lenses were removed 24 hr after surgery, during the first follow‐up visit. All patients were followed up at postoperative day 1, week 1, as well as months 1, 3 and 6; however, corneal topography was measured only at postoperative month 6.
Statistical analysis
Data analysis and graph construction were performed using Stata 14.2 (Stata Statistical Software: Release 14.2; StataCorp LP, College Station, TX, USA). Quantitative data were represented as the mean ± standard deviation, or the median and range. Mean differences were calculated for paired data. Several different statistical tests were used to calculate the p values in this study. For comparing preoperative and postoperative outcomes within the same group, we used the paired t‐test for normally distributed data and the Wilcoxon matched‐pairs signed‐rank test for non‐normally distrusted data. For comparing postoperative differences between the two groups, we used Student’s t‐test for normally distributed data and the Mann–Whitney test for non‐normally distributed data. Regarding the postoperative differences among the two sets of subgroups, Student’s t‐test was used to compare two subgroups and one‐way analysis of variance (anova) with Bonferroni post hoc test to compare three subgroups or more for normally distributed data. For non‐normally distributed data, we used Mann–Whitney test to compare two subgroups and the Kruskal–Wallis test to compare three subgroups or more. A p value <0.05 was considered statistically significant.
Results
This study included one eye each of 104 keratoconus patients (59% male, 41% female). Patients in group A (n = 52) had a mean age of 28.06 ± 5.92 years, and patients in group B (n = 52) had a mean age of 28.73 ± 9.50 years. Patient characteristics and descriptive statistics of their eyes are summarized in Table 2. There were no statistically significant differences for these values between groups A and B, between the four Q‐value subgroups or between the four keratoconus‐grade subgroups.
Table 2.
Patient characteristics and descriptive statistics of their eyes.
| Variable |
Group A N = 52 eyes of 52 patients |
Group B N = 52 eyes of 52 patients |
p value |
|---|---|---|---|
| Age/years | |||
| Mean ± SD | 28.06 ± 5.92 | 28.73 ± 9.50 | 0.94 |
| Median (range) | 29 (15:41) | 29.5 (14:48) | |
| Gender | |||
| Total Patients (104) | 52 | 52 | 0.32 |
| Males (61) | 33 (63.46%) | 28 (53.85%) | |
| Females (43) | 19 (36.54%) | 24 (46.15%) | |
| Preoperative KC grading | |||
| Eyes (104) | 52 | 52 | 0.84 |
| Grade 1, mean K < 48 D (51 eyes) | 25 eyes (48.1%, A‐Gr1) | 26 eyes (50%, B‐Gr1) | |
| Grade 2, mean K 48–53 D (53 eyes) | 27 eyes (51.9%, A‐Gr2) | 26 eyes (50%, B‐Gr2) | |
| Preoperative Q‐value grading | |||
| Eyes (104) | 52 | 52 | 0.84 |
| Q1 value >−1 (51 eyes) | 26 eyes (50%, A‐Q1) | 25 eyes (48.1%, B‐Q1) | |
| Q2 value ≤−1 (53 eyes) | 26 eyes (50%, A‐Q2) | 27 eyes (51.9%, B‐Q2) | |
Visual, refractive and topographic outcomes
In group A, at postoperative month 6, there were improvements in all mean visual, refractive and keratometric outcomes, as well as in Q‐anterior (all p < 0.0001; Table 3). On the other hand, postoperative mean pachymetry, topographic cylinder and Q‐posterior were not statistically significantly different from that before surgery. Table 3 shows the summary of group A outcomes.
Table 3.
Visual, refractive and topographic data analysis of group A.
| Variable |
Preoperative Mean ± SD |
Postoperative 6th month Mean ± SD |
Difference (post‐pre) Mean ± SD (95 % CI) |
p value |
|---|---|---|---|---|
| UDVA (logMAR) | 1.13 ± 0.29 | 0.68 ± 0.23 | −0.44 ± 0.29 (−0.52:−0.36) | <0.0001 |
| CDVA (logMAR) | 0.58 ± 0.31 | 0.21 ± 0.21 | −0.37 ± 0.22 (−0.43:−0.31) | <0.0001 |
| Sphere (D) | −4.35 ± 1.51 | −1.75 ± 1.13 | 2.60 ± 1.23 (2.26:2.95) | <0.0001 |
| Refractive cylinder (D) | −3.18 ± 1.26 | −1.76 ± 1.14 | 1.41 ± 1.52 (0.99:1.84) | <0.0001 |
| SE (D) | −5.93 ± 1.54 | −2.65 ± 1.24 | 3.29 ± 1.46 (2.88:3.69) | <0.0001 |
| K1 (D) | 46.26 ± 2.75 | 43.68 ± 2.46 | −2.57 ± 1.90 (−3.10:−2.05) | <0.0001 |
| K2 (D) | 50.49 ± 3.44 | 47.46 ± 3.70 | −3.03 ± 2.17 (−3.63:−2.42) | <0.0001 |
| K‐average (D) | 48.37 ± 2.81 | 45.57 ± 2.89 | −2.80 ± 1.61 (−3.24:−2.35) | <0.0001 |
| K‐max (D) | 54.44 ± 3.84 | 50.96 ± 3.82 | −3.48 ± 2.44 (−4.16:−2.80) | <0.0001 |
| Topographic cylinder (D) | −4.23 ± 2.68 | −3.78 ± 2.47 | 0.45 ± 2.48 (−0.24:1.15) | 0.11 |
| Pachymetry (µm) | 449.79 ± 33.55 | 446.94 ± 36.91 | −2.85 ± 17.09 (−7.60:1.91) | 0.32 |
| Q‐anterior | −1.04 ± 0.41 | −0.59 ± 0.51 | 0.45 ± 0.39 (0.34:0.56) | <0.0001 |
| Q‐posterior | −0.92 ± 0.56 | −0.93 ± 0.66 | −0.01 ± 0.58 (−0.17:0.15) | 0.79 |
CDVA = corrected distance visual acuity, D = dioptre, K1, K2, K‐average and K‐max = keratometric readings, logMAR = logarithm of the minimum angle of resolution, Q‐anterior = asphericity of the anterior corneal surface, Q‐posterior = asphericity of the posterior corneal surface, SD = standard deviation, SE = spherical equivalent, UDVA = uncorrected distance visual acuity.
p values were calculated using either paired t‐test for normally distributed data or Wilcoxon matched‐pairs signed‐rank test for non‐normally distributed data.
In group B, at postoperative month 6, there were improvements in all mean visual, refractive and keratometric outcomes, as well as in Q‐anterior and topographic cylinder [all p < 0.0001, except for refractive and topographic cylinders (both p = 0.001); Table 4]. Meanwhile, postoperative mean pachymetry and Q‐posterior were not statistically significantly different from that before surgery. Table 4 shows the summary of group B outcomes.
Table 4.
Visual, refractive and topographic data analysis of group B.
| Variable |
Preoperative Mean ± SD |
Postoperative 6th month Mean ± SD |
Difference (post‐pre) Mean ± SD (95 % CI) |
p value |
|---|---|---|---|---|
| UDVA (logMAR) | 1.11 ± 0.30 | 0.53 ± 0.20 | −0.58 ± 0.30 (−0.67:−0.50) | <0.0001 |
| CDVA (logMAR) | 0.56 ± 0.16 | 0.18 ± 0.16 | −0.38 ± 0.17 (−0.47:−0.30) | <0.0001 |
| Sphere (D) | −4.27 ± 1.32 | −1.13 ± 0.79 | 3.13 ± 1.11 (2.83:3.44) | <0.0001 |
| Refractive cylinder (D) | −3.14 ± 1.63 | −2.35 ± 1.24 | 0.79 ± 1.50 (0.37:1.21) | 0.001 |
| SE (D) | −5.84 ± 1.51 | −2.29 ± 1.04 | 3.55 ± 1.45 (3.14:3.95) | <0.0001 |
| K1 (D) | 46.17 ± 2.37 | 42.98 ± 2.50 | −3.18 ± 1.62 (−3.64:−2.73) | <0.0001 |
| K2 (D) | 49.55 ± 2.57 | 45.41 ± 2.57 | −4.14 ± 1.82 (−4.64:−3.63) | <0.0001 |
| K‐average (D) | 47.86 ± 2.24 | 44.20 ± 2.42 | −3.66 ± 1.47 (−4.07:−3.25) | <0.0001 |
| K‐max (D) | 55.22 ± 4.21 | 50.09 ± 3.96 | −5.13 ± 3.17 (−6.01:−4.25) | <0.0001 |
| Topographic cylinder (D) | −3.38 ± 2.10 | −2.43 ± 1.49 | 0.95 ± 1.79 (0.45:−1.45) | 0.001 |
| Pachymetry (µm) | 447.25 ± 33.58 | 449.79 ± 34.03 | 2.54 ± 12.24 (−0.87:5.95) | 0.14 |
| Q‐anterior | −1.00 ± 0.35 | −0.45 ± 0.34 | −0.55 ± 0.40 (0.44:0.66) | <0.0001 |
| Q‐posterior | −1.20 ± 0.38 | −1.17 ± 0.40 | 0.03 ± 0.23 (−0.04:0.09) | 0.35 |
CDVA = corrected distance visual acuity, D = dioptre, K1, K2, K‐average and K‐max = keratometric readings, logMAR = logarithm of the minimum angle of resolution, Q‐anterior = asphericity of the anterior corneal surface, Q‐posterior = asphericity of the posterior corneal surface, SD = standard deviation, SE = spherical equivalent, UDVA = uncorrected distance visual acuity.
p values were calculated using either paired t‐test for normally distributed data or Wilcoxon matched‐pairs signed‐rank test for non‐normally distributed data.
Between‐group comparisons
We recorded no between‐group differences in any of the mean visual, refractive or topographic parameters at baseline, except for Q‐posterior (p = 0.002; Table 5). At postoperative month 6, we recorded significantly better postoperative improvements in the mean refractive in group A than in group B (p = 0.04, Table 5), and more significant improvements in the mean UDVA, sphere, K2, K‐average, K‐max and Q‐anterior in group B than in group A (p = 0.02, 0.01, 0.002, 0.001. 0.001 and 0.03, respectively; Table 5). Table 5 and Fig. 1 summarize the comparisons of the between‐group postoperative outcomes.
Table 5.
Comparative analysis of the visual, refractive and topographic postoperative differences between groups A and B.
| Postoperative Differences (Post 6ms‐Preoperative) |
Group A Mean ± SD Median (range) |
Group B Mean ± SD Median (range) |
p value |
|---|---|---|---|
| UDVA (logMAR) |
−0.44 ± 0.29 −0.4 (−1.3:0.1) |
−0.58 ± 0.30 −0.5 (−1.3:0) |
0.02 |
| CDVA (logMAR) |
−0.37 ± 0.25 −0.4 (−1:0) |
−0.38 ± 0.17 −0.4 (−0.7:0) |
0.14 |
| Sphere (D) |
2.60 ± 1.24 2.38 (0.75:6.25) |
3.13 ± 1.11 3.13 (0.75:5.25) |
0.01 |
| Refractive Cylinder (D) |
1.41 ± 1.52 1.25 (−2.5:3.5) |
0.79 ± 1.51 0.75 (−2.5:5) |
0.04 |
| SE (D) |
3.29 ± 1.46 3 (0.75:6.88) |
3.55 ± 1.45 3.56 (0.25:7) |
0.22 |
| K1 (D) |
−2.57 ± 1.90 −2.7 (−7.63:1.76) |
−3.18 ± 1.62 −3.00 (−7.59:−0.5) |
0.15 |
| K2 (D) |
−3.03 ± 2.17 −2.96 (−8.07:1.81) |
−4.14 ± 1.82 −4 (−8.25:0.17) |
0.002 |
| K‐average (D) |
−2.80 ± 1.61 −2.52 (−6.77:−0.21) |
−3.66 ± 1.47 −3.35 (−7.51:−0.17) |
0.001 |
| K‐max (D) |
−3.48 ± 2.44 −4.60 (−21.52:−0.04) |
−5.13 ± 3.17 −4.35 (−21.52:−1.19) |
0.001 |
| Pachymetry (µm) |
−2.85 ± 17.09 −1 (−60:35) |
2.53 ± 12.24 0.5 (−21:37) |
0.19 |
| Q‐anterior |
0.45 ± 0.39 0.38 (−0.14:1.36) |
0.55 ± 0.40 0.61 (−0.75:1.28) |
0.03 |
| Q‐posterior |
−0.01 ± 0.58 −0.11 (−1.25:1.25) |
0.03 ± 0.23 0.02 (−0.56:0.59) |
0.34 |
| Topographic Cylinder (D) |
0.45 ± 2.48 0.42 (−6.15:5.84) |
0.95 ± 1.79 1.01 (−2.48:5.57) |
0.29 |
CDVA = corrected distance visual acuity, D = dioptre, K1, K2, K‐average and K‐max = keratometric readings, logMAR = logarithm of the minimum angle of resolution, Q‐anterior = asphericity of the anterior corneal surface, Q‐posterior = asphericity of the posterior corneal surface, SD = standard deviation, SE = spherical equivalent, UDVA = uncorrected distance visual acuity.
p values were calculated using either Student’s t‐test for normally distributed data or Mann–Whitney test for non‐normally distrusted data.
Fig. 1.
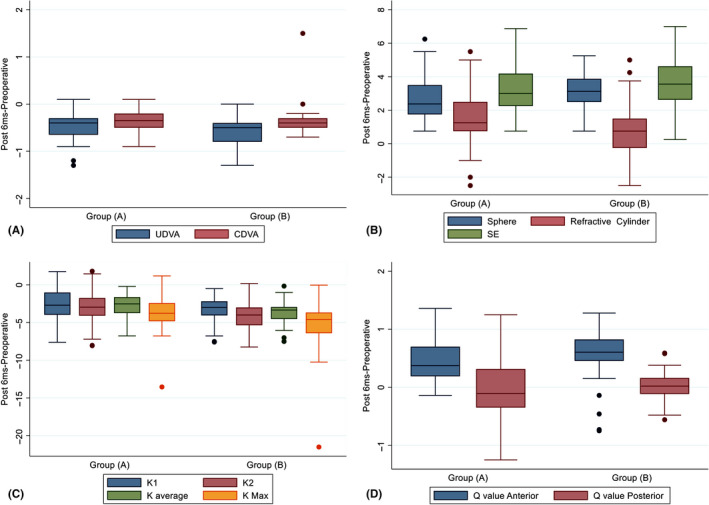
Analysis of the visual, refractive and topographic outcomes in groups A and B at postoperative month 6; (A) uncorrected distance visual acuity (UDVA) and corrected distance visual acuity (CDVA). (B) sphere, refractive cylinder and spherical equivalent (SE). (C) K1, K2, K‐average and K‐max. (D) asphericity of the anterior (Q‐anterior) and posterior (Q‐posterior) corneal surfaces. There were differences in UDVA, sphere, refractive cylinder, K2, K‐average, K‐max and Q‐anterior at postoperative month 6 (p = 0.02, 0.01, 0.04, 0.002, 0.001, 0.001 and 0.03, respectively).
Between‐subgroup comparisons based on Q‐value grading
Table 6 summarizes the postoperative between‐subgroup comparisons based on Q‐value grading. However, here, we present only the results of the comparisons between A‐Q1 and B‐Q1 (i.e. p2 in Table 6), as well as that between A‐Q2 and B‐Q2 (i.e. p5 in Table 6). We observed better improvements in mean K2, K‐average and K‐max values in subgroup B‐Q1 than in subgroup A‐Q1 (p = 0.009, 0.01 and 0.009, respectively). We observed better improvements in mean UDVA, sphere, SE, K‐max and Q‐anterior values in subgroup B‐Q2 than in subgroup A‐Q2 (p = 0.0004, 0.001, 0.04, 0.008 and 0.01, respectively).
Table 6.
Comparative analysis of the visual, refractive and topographic postoperative differences between A and B subgroups based on Q‐value grading.
| Postoperative Differences (Post 6ms‐Preop) |
A‐Q1 N = 26 eyes (Mean ± SD) |
A‐Q2 N = 26 eyes (Mean ± SD) |
B‐Q1 N = 25 eyes (Mean ± SD) |
B‐Q2 N = 27 eyes (Mean ± SD) |
P all | P1 | P2 | P3 | P4 | P5 | P6 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| UDVA (logMAR) | −0.37 ± 0.29 | −0.52 ± 0.29 | −0.34 ± 0.14 | −0.80 ± 0.24 | 0.0001 | 0.06 | 0.91 | 0.0001 | 0.03 | 0.0004 | 0.0001 |
| CDVA (logMAR) | −0.30 ± 0.19 | −0.43 ± 0.22 | −0.32 ± 0.41 | −0.44 ± 0.14 | 0.02 | 0.04 | 0.13 | 0.002 | 0.43 | 0.79 | 0.17 |
| Sphere (D) | 2.79 ± 1.23 | 2.41 ± 1.23 | 2.41 ± 1.02 | 3.48 ± 1.09 | 0.005 | 0.18 | 0.88 | 0.03 | 0.12 | 0.001 | 0.02 |
| Refractive Cylinder (D) | 1.18 ± 1.67 | 1.64 ± 1.35 | 0.61 ± 1.41 | 0.95 ± 1.60 | 0.13 | 0.41 | 0.18 | 0.49 | 0.02 | 0.12 | 0.47 |
| SE (D) | 3.38 ± 1.42 | 3.20 ± 1.51 | 3.07 ± 1.39 | 4 ± 1.39 | 0.07 | 0.45 | 0.53 | 0.10 | 0.81 | 0.04 | 0.01 |
| Topographic Cylinder (D) | −0.09 ± 2.52 | 0.99 ± 2.37 | 0.84 ± 1.62 | 1.06 ± 1.95 | 0.18 | 0.06 | 0.12 | 0.07 | 0.58 | 0.90 | 0.54 |
| K1 (D) | −2.54 ± 1.76 | −2.60 ± 2.06 | −3.07 ± 1.44 | −3.29 ± 1.80 | 0.52 | 0.65 | 0.49 | 0.38 | 0.30 | 0.16 | 0.96 |
| K2 (D) | −2.46 ± 2.12 | −3.60 ± 2.10 | −3.91 ± 1.98 | −4.35 ± 1.67 | 0.004 | 0.06 | 0.009 | 0.001 | 0.37 | 0.10 | 0.29 |
| K‐average (D) | −2.50 ± 1.49 | −3.10 ± 1.71 | −3.49 ± 1.53 | −3.82 ± 1.43 | 0.006 | 0.19 | 0.01 | 0.001 | 0.17 | 0.07 | 0.53 |
| K‐max (D) | −3.05 ± 2.99 | −3.90 ± 1.71 | −5.01 ± 4.06 | −5.25 ± 2.11 | 0.0008 | 0.03 | 0.009 | 0.0004 | 0.69 | 0.008 | 0.10 |
| Pachymetry (µm) | 0.85 ± 18.89 | −6.54 ± 14.5 | 7.56 ± 11.87 | −2.11 ± 10.8 | 0.004 | 0.10 | 0.15 | 0.15 | 0.001 | 0.52 | 0.003 |
| Q‐anterior | 0.47 ± 0.28 | 0.43 ± 0.48 | 0.38 ± 0.45 | 0.71 ± 0.27 | 0.01 | 0.32 | 0.82 | 0.003 | 0.80 | 0.01 | 0.01 |
CDVA = corrected distance visual acuity, D = dioptre, K1, K2, K‐average and K‐max = keratometric readings, logMAR = logarithm of the minimum angle of resolution, Q‐anterior = asphericity of the anterior corneal surface, SD = standard deviation, SE = spherical equivalent, UDVA = uncorrected distance visual acuity.
P1 compared A‐Gr1 & A‐Gr2, P2 compared A‐Gr1 & B‐Gr1, P3 compared A‐Gr1 & B‐Gr2, P4 compared A‐Gr2 & B‐Gr1, P5 compared A‐Gr2 & B‐Gr2 and P6 compared B‐Gr1 & B‐Gr2. p values were calculated using either Student’s t‐test to compare between 2 subgroups or anova test with Bonferroni post hoc test to compare between 3 subgroups or more for normally distributed data while we used Mann–Whitney test to compare two subgroups and Kruskal–Wallis test to compare between 3 subgroups or more for non‐normally distrusted data.
Between‐subgroup comparisons based on keratoconus grading
Table 7 summarizes the postoperative between‐subgroup comparisons based on keratoconus grading. Here, we present only the results of the comparisons between A‐Gr1 and B‐Gr1 (i.e. p2 in Table 7), as well as that between A‐Gr2 and B‐Gr2 (i.e. p5 in Table 7). We observed higher improvements in mean UDVA and K‐max values in subgroup B‐Gr1 than in subgroup A‐Gr1 (p = 0.01 and 0.004, respectively). We observed higher improvements in mean UDVA, sphere, K2 and K‐average values in subgroup B‐Gr2 than in subgroup A‐Gr2 (p = 0.04, 0.04, 0.002 and 0.003, respectively). However, subgroups A‐Gr2 and B‐Gr2 had similar mean K‐max improvements (p = 0.05). Figure 2 shows the postoperative differences among A and B subgroups in terms of UDVA and K‐max.
Table 7.
Comparative analysis of the visual, refractive and topographic postoperative differences between A and B subgroups based on keratoconus grading.
| Postoperative Differences (Post 6ms‐Preop) |
A‐Gr1 N = 25 eyes (Mean ± SD) |
A‐Gr2 N = 27 eyes (Mean ± SD) |
B‐Gr1 N = 26 eyes (Mean ± SD) |
B‐Gr2 N = 26 eyes (Mean ± SD) |
P all | P1 | P2 | P3 | P4 | P5 | P6 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| UDVA (logMAR) | −0.26 ± 0.21 | −0.61 ± 0.26 | −0.40 ± 0.18 | −0.77 ± 0.29 | 0.0001 | 0.0001 | 0.01 | 0.0001 | 0.002 | 0.04 | 0.0001 |
| CDVA (logMAR) | −0.29 ± 0.11 | −0.44 ± 0.26 | −0.29 ± 0.39 | −0.47 ± 0.15 | 0.002 | 0.02 | 0.11 | 0.0001 | 0.16 | 0.74 | 0.02 |
| Sphere (logMAR) | 2.01 ± 0.66 | 3.14 ± 1.40 | 2.40 ± 0.76 | 3.87 ± 0.90 | 0.0001 | 0.004 | 0.052 | 0.0001 | 0.06 | 0.04 | 0.0001 |
| Refractive Cylinder (D) | 0.99 ± 1.05 | 1.81 ± 1.78 | 0.36 ± 1.14 | 1.22 ± 1.71 | 0.008 | 0.03 | 0.10 | 0.60 | 0.001 | 0.17 | 0.07 |
| SE (D) | 2.51 ± 0.86 | 4.01 ± 1.53 | 2.58 ± 0.99 | 4.51 ± 1.18 | 0.0001 | 0.0004 | 0.43 | 0.0001 | 0.001 | 0.33 | 0.0001 |
| Topographic Cylinder (D) | 0.87 ± 1.97 | 0.06 ± 2.87 | 0.57 ± 1.41 | 1.34 ± 2.06 | 0.24 | 0.42 | 0.64 | 0.37 | 0.50 | 0.08 | 0.07 |
| K1 (D) | −2.21 ± 1.99 | −2.91 ± 1.78 | −2.93 ± 1.52 | −3.44 ± 1.71 | 0.25 | 0.25 | 0.22 | 0.051 | 0.96 | 0.34 | 0.41 |
| K2 (D) | −3.08 ± 1.82 | −2.98 ± 2.48 | −3.49 ± 1.86 | −4.78 ± 1.57 | 0.002 | 0.91 | 0.30 | 0.001 | 0.31 | 0.002 | 0.01 |
| K‐average (D) | −2.64 ± 1.63 | −2.94 ± 1.61 | −3.21 ± 1.54 | −4.11 ± 1.28 | 0.002 | 0.43 | 0.12 | 0.004 | 0.35 | 0.003 | 0.03 |
| K‐max (D) | −2.78 ± 1.93 | −4.12 ± 2.73 | −5.12 ± 4.15 | −5.14 ± 1.83 | 0.001 | 0.03 | 0.004 | 0.0002 | 0.40 | 0.05 | 0.44 |
| Pachymetry (µm) | 1.0 ± 17.4 | −6.41 ± 16.3 | 5.23 ± 11.5 | −0.15 ± 12.6 | 0.11 | 0.29 | 0.31 | 0.78 | 0.01 | 0.64 | 0.053 |
| Q‐anterior | 0.37 ± 0.36 | 0.54 ± 0.41 | 0.40 ± 0.47 | 0.70 ± 0.24 | 0.003 | 0.17 | 0.18 | 0.0001 | 0.56 | 0.11 | 0.01 |
CDVA = corrected distance visual acuity, D = dioptre, K1, K2, K‐average and K‐max = keratometric readings, logMAR = logarithm of the minimum angle of resolution, Q‐anterior = asphericity of the anterior corneal surface, SD = standard deviation, SE = spherical equivalent, UDVA = uncorrected distance visual acuity.
P1 compared A‐Gr1 & A‐Gr2, P2 compared A‐Gr1 & B‐Gr1, P3 compared A‐Gr1 & B‐Gr2, P4 compared A‐Gr2 & B‐Gr1, P5 compared A‐Gr2 & B‐Gr2 and P6 compared B‐Gr1 & B‐Gr2. p Values were calculated using either Student’s t‐test to compare between 2 subgroups or anova test with Bonferroni post hoc test to compare between 3 subgroups or more for normally distributed data while we used Mann–Whitney test to compare two subgroups and Kruskal–Wallis test to compare between 3 subgroups or more for non‐normally distrusted data.
Fig. 2.
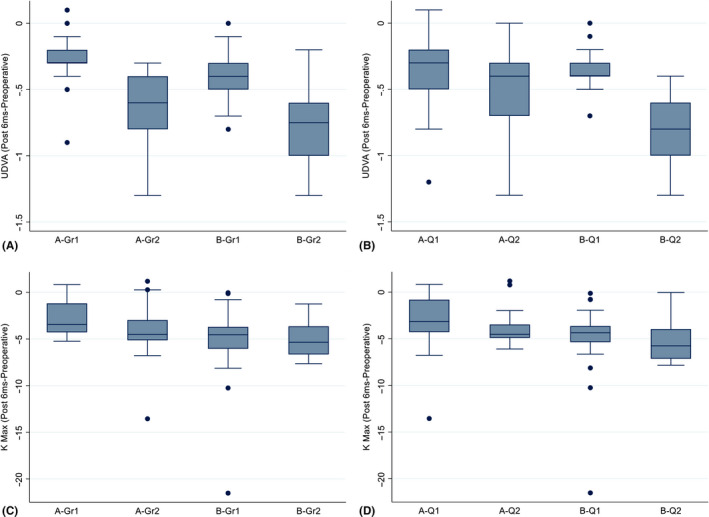
Analysis of UDVA and K‐max postoperative differences among A and B subgroups with regard to grades 1 and 2 keratoconus and levels 1 and 2 Q‐values at postoperative month 6; A and B: UDVA postoperative differences among the two sets of subgroups were statistically significant in favour of B subgroups; C and D: K‐max postoperative differences among the two sets of subgroups were statistically significant in favour of B subgroups.
Complications
In group A, we recorded spontaneous extrusion of one ring segment from one eye at postoperative month 6. We removed only this segment, and logMAR CDVA remained stable at 0.7. Furthermore, four eyes (8%) in group A and one eye (2%) in group B exhibited no postoperative UDVA and CDVA improvements, despite mild flattening of the cone and refractive improvements in three of these. However, we recorded no cases of postoperative keratoconus progression.
Discussion
Our study included 104 patients (104 keratoconus eyes) that underwent Keraring implantation combined with TCXL, in order to compare the SN and our novel QN. We decided to document our experience by performing this randomized controlled trial. We did not simply aim to flatten the cornea, but to mimic the pre‐ectatic corneal status as closely as possible by carefully restoring the cone to its original position. The key to success lay in achieving approximately normal corneal asphericity. In other words, we aimed for a target Q‐value, which can be measured objectively, maximally improving corneal refraction and visual acuity.
The main difference between the nomograms is that our QN relies on objective measurements, while the 2009 SN is subjective in nature. The SN depends on preoperative CDVA and subjective refraction to improve postoperative visual and refractive outcomes, which can be misleading in ectatic eyes, while the QN aims only to restore normal corneal asphericity. In addition, we believe that following the SN causes a flattening of the cornea, with redistribution of cone tissues in random directions, while our QN enables the repositioning of cone tissues as a unit, returning them to their pre‐ectatic positions.
We demonstrated that both nomograms improved visual, refractive and topographic outcomes more in grade 2 than in grade 1 keratoconic eyes, and more in eyes with a Q‐anterior value ≤−1.00 than in eyes with a Q‐anterior value >−1.00. However, the QN yielded statistically significantly better improvements regarding UDVA, sphere and K‐max values than the SN did, in eyes with grade 2 keratoconus and in eyes with a Q‐anterior value ≤−1.00. In addition, the QN yielded statistically significantly better improvements in keratometric values than the SN did in eyes with a Q‐anterior value >−1.00. Meanwhile, the SN was statistically significantly superior to the QN only in terms of improvement in refractive cylindrical measurements, and not when the groups were stratified by Q‐anterior value or keratoconus grade.
Regarding postoperative visual changes, 79% of eyes treated according to the QN, and 58% of eyes treated according to the SN, exhibited logMAR UDVA improvements ≥0.4. However, a similar proportion of eyes (54% with the QN and 52% with the SN) exhibited a postoperative improvement in logMAR CDVA ≥0.4. These outcomes signify that UDVA was more sensitive than CDVA in detecting the relative postoperative visual improvement when using the QN.
Moreover, when using the QN, 38.5% and 55.8% of eyes exhibited postoperative reductions ≥4 D in K‐average and K‐max, respectively; when using the SN, only 23.1% and 44.2% of eyes exhibited the corresponding postoperative reductions, respectively. These results indicate that the QN yielded better postoperative corneal flattening than the SN did, and the improvements in K‐average and K‐max could be correlated with the improvement in UDVA in the QN group.
In group A, 23 eyes were implanted with one Keraring segment, while 29 eyes were implanted with two Keraring segments according to standard nomogram. The 160 or 210 arc‐length segments were implanted in the 23 eyes of group A. Meanwhile, the 90, 120 or 160 arc‐length segments were implanted in the 29 eyes of group A. In group B, all 52 eyes were implanted with one Keraring segment, 210 arc‐length segment. We believe that the change in the number and size of ring segments were responsible for the differences between both nomogram outcomes. Therefore, we intended to change the SN and shift to QN by changing the number and size of ring segments to achieve better postoperative outcomes.
Utine et al. (2018) reported the results of Keraring implantation in 42 eyes according to the SN and concluded that postoperative improvements in UDVA and CDVA were related to postoperative improvements in Q‐anterior and K‐average values, but not related to postoperative improvements in SE or cylinder values. They also stated that corneal asphericity plays an important role in postoperative visual outcomes, which necessitates the development of new nomograms. Their results correspond with ours, as the QN yielded better improvements in Q‐anterior value and UDVA than the SN did. In other words, the improvement in UDVA may have resulted from the improvement in Q‐anterior values. Further, Ferrara & Torquetti (2010) concluded that nomograms may be improved by aiming for a Q‐anterior value of −0.23. In 2015, Seleet et al. reported on a case series of 10 keratoconic eyes with type 1 or 2 cone asymmetry, which were treated with implantation of a single Keraring segment with an arc‐length of 160°. They documented SE improvements in eight eyes, while two eyes exhibited deterioration in myopic and SE components, despite improvements in astigmatic components. The main differences between their study and ours were that we used a segment with a longer arc‐length (210°), and we recorded no deterioration in any of the refractive components.
Lisa et al. (2017) implanted a single, 210°‐arc‐length Ferrara segment (AJL Ophthalmic) each in 43 eyes with a Q‐anterior value ≥−1.00, which exhibited central hyper‐prolate keratoconus. Similar to our study, they revealed statistically significant postoperative improvements in UDVA, CDVA, SE and Q‐anterior values, which remained stable over a 3‐year follow‐up period. The main difference in our study was the comparison between the QN and the SN. Additionally, we operated upon eyes with type 1 and 2 cone asymmetry using a Keraring segment, while they operated upon eyes with central type 3 cones using a Ferrara ring segment. Moreover, they only included eyes with Q‐anterior values ≥‐1.00; however, their study had a longer follow‐up period than ours did.
Recently, Rocha et al. (2019) compared the Keraring and Ferrara ring nomograms, and concluded that the Keraring nomogram yielded better improvements in the topographic cylinder. However, our QN performed better in reducing the topographic cylinder than the SN, although the postoperative differences between both nomograms were not statistically significant (p = 0.29).
Prisant et al. (2020) evaluated the use of asymmetric Keraring segments to treat 104 keratoconic eyes, implanting one segment with a 160° arc‐length in all cases, with another asymmetric segment with arc‐length/thickness parameters of either 150°/250 μm or 200°/300 μm. They reported mean postoperative improvements of 0.36 logMAR UDVA, 1.94 D SE and 3.3 K‐max, which are similar to the corresponding outcomes in our study when using the SN, and lower than those when using the QN. In the same year, Fariselli et al. compared the use of an artificial neural network (ANN) to the use of the SN for Keraring implantation in 20 keratoconic eyes, each. They concluded that ANN yielded greater postoperative improvements in CDVA than did the SN.
Out of 104 eyes receiving Keraring implantation in the current study, only one (0.96%) exhibited a postoperative complication, in the form of segment extrusion, which required surgical removal. Mounir et al. (2020) reported a 2.1% Keraring‐extrusion rate in a study of 333 eyes implanted with Keraring segments using a femtosecond laser. The explanations they provided included segment migration, corneal melting and associated VKC. Similarly, Saleem et al. (2018) documented extrusion of a Keraring segment in only 1 of 43 eyes (2.3%) during a 3‐year follow‐up period. In a previous study, we (Iqbal et al. 2019c) reported a higher Keraring‐extrusion rate of 4.8% (3/63 eyes) in paediatric eyes, which was accompanied by severe postoperative VKC and vigorous eye rubbing. Kapitánová and Nikel (2018) reported a very high rate of Keraring‐extrusion (9/48 eyes, 18.75%) in all study participants and (2/15 eyes, 13.3%) in grade 2 keratoconus. They finally reiterated the importance of careful patient selection for ring implantation.
In the current study, 4.8% of eyes exhibited no postoperative visual improvements: four eyes treated according to the SN and one treated according to the QN. All of these were diagnosed with grade 1 keratoconus and exhibited either no improvement or slight improvement in refractive and keratometric values. This suggests that the QN is more efficacious and suitable for use with grade‐1 keratoconic eyes than the SN is. Other studies have also suggested avoiding use of the SN in low‐grade keratoconic eyes, especially in patients with higher preoperative UDVA, as it may reduce postoperative visual acuity; the authors went as far as to suggest that it might be wise to consider treatment options other than ICRS implantation (Alio et al. 2014; Vega‐Estrada & Alio 2016). In a previous study, we suggested postponing Keraring implantation in paediatric patients until adulthood (Iqbal et al. 2019c).
We recorded 37 patients (35.6%) in this study who used a contact lens (CL) before shifting to Keraring implantation due to their intolerance to CL wear. Despite being cheaper, CL is not well tolerated by many patients in our localities due to the nature of their work, for example construction workers and farmers, especially in our hot climate. In addition, CL requires good eye hygiene with daily CL care, which is not always maintained by all patients. Nevertheless, we believe that Keraring implantation is particularly suitable for those patients who cannot tolerate contact lenses as ICRS implantation is generally expensive.
We documented keratoconus progression (K‐max >1 D) in the study eyes before surgical intervention. Since our main purpose was to compare two different nomograms for Keraring segments implantation, we actually had two choices before the start of the study. The first choice was to implant Kerarings as a primary procedure to be followed sequentially by CXL treatment 6 months later. The advantage of this choice was to guarantee that CXL treatment would not interfere with the outcomes of both nomograms; however, it could potentially lead to further keratoconus progression in the study eyes within the 6‐month interval. The second choice was to combine CXL treatment with Keraring segment implantation, which was mostly guaranteed to halt keratoconus progression during the 6‐month interval. We finally went ahead with the second choice; however, there was no consensus regarding the type of the CXL treatment we should use in this study. In short, we believed that epithelium‐off CXL treatments would halt keratoconus progression but were aware of epithelial healing difficulties, especially with combined segment implantation, which were very likely to influence the nomogram outcomes in our short‐term 6‐month study period. Furthermore, successful epithelium‐off CXL treatments might be associated with unpredictable and unavoidable visual and/or refractive improvements that would definitely interfere with nomogram results. To avoid these obstacles, we chose TCXL to be combined with the Keraring segment implantations. Although we believe that standard CXL is more efficient and more advantageous and has longer stability than both ACXL and TCXL, we preferred to perform TCXL in our this study to avoid the potential hazards of the associated postoperative haze and/or delayed epithelial healing sequelae. Such complications may provide incorrect or misleading short‐term visual and/or refractive outcomes in terms of one or both of the two nomograms, thus masking the actual results of these nomograms that we mainly aimed to compare. Eventually, we observed no postoperative haze or keratoconus progression cases within the follow‐up period.
We discussed the different treatment options, including CXL treatment types and combination treatment modalities, with our patients who finally accepted our plan of treatment and were aware of the potential complications of the treatment procedures. Furthermore, all our patients were instructed to continue their regular follow‐up visits scheduled on a six monthly basis after the end of this study. In addition, they were also aware that if keratoconus progression is documented at any future follow‐up visit, they would be subjected to an epithelium‐off CXL retreatment.
A couple of important questions remains. Does the ring segment implantation itself have a stabilizing or a cross‐linking‐like effect on keratoconus? In addition, can the ring segment implantation halt keratoconus progression? The answers to both of these are still being debated. Flecha‐Lescún et al. (2018) used computational simulation to predict the effect of ICRS implantation on keratoconus and reported that the stromal depth of the implantation was the most important factor in determining the segments’ stabilizing effects on corneal optics. They concluded that, in theory, implantation in the anterior stroma could promote keratoconus progression, as it adds more stress on the posterior corneal surface, while implanting the segments in the posterior stroma could help in halting keratoconus progression due to relaxation of the posterior corneal surface (Flecha‐Lescún et al. 2018). A couple of long‐term studies reported the effectiveness of ICRS in stabilizing postoperative visual, refractive and/or topographic improvements upon ICRS implantation for treatment of the stable form of keratoconus (Vega‐Estrada et al. 2013; Kang et al. 2019). Furthermore, the results of other studies revealed the inability of ICRS implantation to halt the progression of actively progressive keratoconus; the authors of those studies recommended the postponement of ICRS implantation in young or paediatric patients until the keratoconus becomes stable (Vega‐Estrada et al. 2015; Saleem et al. 2018; Iqbal et al. 2019c). Therefore, we added TCXL treatment to ICRS implantation in our patients. Although there are no conclusive answers to the above‐mentioned questions, we believe that ICRS and CXL have different mechanisms of action. Whereas CXL treats the actual pathological aetiology of stromal weakness and thinning associated with keratoconus progression, we believe that ICRS action is mainly mechanical. However, a couple of studies demonstrated that ICRSs induce corneal remodelling via lipid deposition in the extracellular matrix, fibroblast migration and keratocyte apoptosis during the wound‐healing response (Ly et al. 2006; Samimi et al. 2007). Nevertheless, we also believe that ICRSs can be either supportive or traumatizing, depending on segment thickness and arc‐length; implantation site and depth; stability of the keratoconus; and combination with CXL treatment. Therefore, we performed this study to construct a better nomogram to enhance the supportive effect of ICRS implantation, bases on the above‐mentioned factors. In short, we believe that ICRS can help slow keratoconus progression if implanted appropriately, although keratoconus progression cannot be completely halted in this way. However, if implanted inappropriately, it may mask the actual postoperative keratoconus progression, especially when not combined with CXL treatment, resulting in apparent early postoperative improvements, due to corneal flattening, which will be lost over the long term. In the case of the latter, the stromal tissues will become progressively thinner and weaker, thus flattening until a breaking point is reached. Thereafter, the condition will deteriorate until the keratoconus progression becomes detectable in the corneal topography; however, the early opportunity to prevent this progression will have been missed.
We think that, despite its simplicity, the Amsler‐Krumeich classification has some deficits. It does not sharply differentiate numerically (e.g. mean K reading value and pachymetry) between the normal corneas and grade 1 keratoconus. However, the differentiation between both conditions is multifactorial clinically and in terms of corneal topography. In the context of corneal topography, grade 1 Amsler‐Krumeich shows eccentric corneal steepening, increased posterior surface keratometry, high posterior elevation, irregular astigmatism and asymmetry. Such topographic signs are not present in normal corneas. In addition, corneal examination on the slit‐lamp is helpful in the diagnosis of early keratoconus as it detects stromal thinning and/or posterior stress lines (Vogt’s striae) which suggest that the curvature of the posterior corneal surface is affected. In fact, we think that the recent Belin's ABCD grading system (Belin & Duncan 2016) is more advanced than the older Amsler‐Krumeich grading as it has the advantages of distinguishing early keratoconus from normal non‐ectatic corneas and also incorporating the posterior corneal surface examination. Unfortunately, the Belin's ABCD grading system was not available with our topographer devices that we had used for corneal topography in our study.
The main limitations of our study were the small sample size (104 eyes), the short follow‐up period (6 months) and the exclusion of eyes with grade 3 or 4 keratoconus. Another limitation was our use of an older version of the SN (2009) for comparison to our QN, as the newer version (2018) was published after commencement of our study. Therefore, we recommend that future studies should compare this new version of the SN to the QN.
In conclusion, we believe that no ICRS nomogram will be perfect for the treatment of all keratoconic eyes; however, clinical experience should be garnered to fine‐tune existing nomograms and develop new ones to improve postoperative outcomes. The QN we developed exhibited satisfactory efficacy and is simple to use, which means that it should be less time‐consuming in clinical practice than the SN and have lower cost of surgery. Furthermore, the QN yielded superior outcomes to the SN in eyes with low‐grade keratoconus and was more efficacious than the SN in eyes with a Q‐anterior value ≤−1.00, that is with increased Q‐value negativity. This may be attributed to the reliance of our QN on objective measurements, while the SN relies on subjective clinical parameters, such as CDVA and subjective refraction, which may vary between clinicians. Future randomized studies of larger sample size are recommended to evaluate our QN against the 2018 version of the SN, as well as against other nomograms.
The authors would like to thank Prof. Fouad Metry Yosef, the expert statistician who performed all statistical analyses. The authors are grateful for the assistance of Mr Hamza Mohammed, Mr Seif Mohammed and Ms Lina Mohammed with this research. They also appreciate the help and support of Dr Mona Abo‐Ali, as well as that of the EPK Group.
The corresponding author is a member of EVER only
References
- Alio JL, Vega‐Estrada A, Esperanza S, Barraquer RI, Teus MA & Murta J (2014): Intrastromal corneal ring segments: how successful is the surgical treatment of keratoconus? Middle East Afr J Ophthalmol 21: 3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al‐Tuwairqi WS, Osuagwu UL, Razzouk H, AlHarbi A & Ogbuehi KC (2017): Clinical evaluation of two types of intracorneal ring segments (ICRS) for keratoconus. Int Ophthalmol 37: 1185–1198. [DOI] [PubMed] [Google Scholar]
- Belin MW & Duncan JK (2016): Keratoconus: The ABCD Grading System. Keratokonus: Das ABCD‐System zur Stadieneinteilung. Klin Monbl Augenheilkd 233: 701–707. [DOI] [PubMed] [Google Scholar]
- Bikbova G & Bikbov M (2016): Standard corneal collagen crosslinking versus transepithelial iontophoresis‐assisted corneal crosslinking, 24 months follow‐up: randomized control trial. Acta Ophthalmol 94: e600–e606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fariselli C, Vega‐Estrada A, Arnalich‐Montiel F & Alio JL (2020): Artificial neural network to guide intracorneal ring segments implantation for keratoconus treatment: a pilot study. Eye Vis 7: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrara P & Torquetti L. (2010): The new Ferrara ring nomogram: The importance of corneal asphericity in ring selection. Vision Pan‐America Septiembre 2010: 92–95. Available at: https://ajlsa.com/wp‐content/uploads/2015/04/8‐The‐New‐Ferrara‐Ring‐Nomogram‐the‐importance‐of‐corneal‐asphericity‐in‐ring‐selection.pdf. (Accessed on 11 May 2020). [Google Scholar]
- Flecha‐Lescún J, Calvo B, Zurita J & Ariza‐Gracia MÁ (2018): Template‐based methodology for the simulation of intracorneal segment ring implantation in human corneas. Biomech Model Mechanobiol 17: 923–938. [DOI] [PubMed] [Google Scholar]
- Guyot C, Libeau L, Vabres B, Weber M, Lebranchu P & Orignac I (2019): Résultats réfractifs et facteurs pronostiques de succès du traitement du kératocône par anneaux intracornéens : étude rétrospective sur 75 yeux. J Fr Ophtalmol 42: 118–126. [DOI] [PubMed] [Google Scholar]
- Heikal MA, Abdelshafy M, Soliman TT & Hamed AM (2017): Refractive and visual outcomes after Keraring intrastromal corneal ring segment implantation for keratoconus assisted by femtosecond laser at 6 months follow‐up. Clin Ophthalmol 11: 81–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herber R, Francis M, Spoerl E, Pillunat LE, Raiskup F & Sinha Roy A. (2020): Comparison of waveform‐derived corneal stiffness and stress‐strain extensometry‐derived corneal stiffness using different cross‐linking irradiances: an experimental study with air‐puff applanation of ex vivo porcine eyes. Graefes Arch Clin Exp Ophthalmol. 10.1007/s00417-020-04792-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim O, Elmassry A, Said A, Abdalla M, El Hennawi H & Osman I (2016): Combined femtosecond laser‐assisted intracorneal ring segment implantation and corneal collagen cross‐linking for correction of keratoconus. Clin Ophthalmol 10: 521–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal M, Elmassry A, Badawi AE, Gharieb HM & Said OM (2019a): Visual and refractive long‐term outcomes following standard cross‐linking in progressive keratoconus management. Clin Ophthalmol 13: 2477–2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal M, Elmassry A, Saad H et al. (2020): Standard cross‐linking protocol versus accelerated and transepithelial cross‐linking protocols for treatment of paediatric keratoconus: a 2‐year comparative study. Acta Ophthalmol 98: e352–e362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal M, Elmassry A, Tawfik A et al. (2019b): Standard cross‐linking versus photorefractive keratectomy combined with accelerated cross‐linking for keratoconus management: a comparative study. Acta Ophthalmol 97: e623–e631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal M, Elmassry A, Tawfik A et al. (2019c): Analysis of the outcomes of combined cross‐linking with intracorneal ring segment implantation for the treatment of pediatric keratoconus. Curr Eye Res 44: 125–134. [DOI] [PubMed] [Google Scholar]
- Kang MJ, Byun YS, Yoo YS, Whang WJ & Joo CK (2019): Long‐term outcome of intrastromal corneal ring segments in keratoconus: five‐year follow up. Sci Rep 9: 315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapitánová K & Nikel J (2018): Femtosecond laser‐assisted intrastromal corneal segment implantation–our experience. Cesk Slov Oftalmol 74: 31–36. [PubMed] [Google Scholar]
- Kiely PM, Smith G & Carney LG (1982): The mean shape of the human cornea. Opt Acta 29: 1027–1040. [Google Scholar]
- Kymionis GD, Grentzelos MA, Portaliou DM, Kankariya VP & Randleman JB (2014): Corneal collagen cross‐linking (CXL) combined with refractive procedures for the treatment of corneal ectatic disorders: CXL plus. J Refract Surg 30: 566–576. [DOI] [PubMed] [Google Scholar]
- Lisa C, Fernández‐Vega Cueto L, Poo‐López A, Madrid‐Costa D & Alfonso JF(2017): Long‐term follow‐up of intrastromal corneal ring segments (210‐degree arc length) in central keratoconus with high corneal asphericity. Cornea 36: 1325–1330. [DOI] [PubMed] [Google Scholar]
- Ly LT, McCulley JP, Verity SM, Cavanagh HD, Bowman RW & Petroll WM (2006): Evaluation of intrastromal lipid deposits after Intacs implantation using in vivo confocal microscopy. Eye Contact Lens 32: 211–215. [DOI] [PubMed] [Google Scholar]
- Mazzotta C, Wollensak G, Raiskup F, Pandolfi AM & Spoerl E (2019): The meaning of the demarcation line after riboflavin‐UVA corneal collagen crosslinking. Expert Rev Ophthalmol 14: 115–131. [Google Scholar]
- Mounir A, Farouk MM, Abdellah MM & Mostafa EM (2020): Extrusion of femtosecond laser‐implanted intrastromal corneal ring segments in keratoconic eyes: prevalence, risk factors, and clinical outcomes. J Ophthalmol 2020: 8704219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SE, Tseng M & Lee JK (2019): Effectiveness of intracorneal ring segments for keratoconus. Curr Opin Ophthalmol 30: 220–228. [DOI] [PubMed] [Google Scholar]
- Prisant O, Pottier E, Guedj T & Hoang Xuan T (2020): Clinical outcomes of an asymmetric model of intrastromal corneal ring segments for the correction of keratoconus. Cornea 39: 155–160. [DOI] [PubMed] [Google Scholar]
- Raiskap F, Theuring A, Pillunat LE & Spoerl E (2015): Corneal collagen crosslinking with riboflavin and ultraviolet‐A light in progressive keratoconus: ten‐year results. J Cataract Refract Surg 41: 41–46. [DOI] [PubMed] [Google Scholar]
- Randleman JB, Santhiago MR, Kymionis GD & Hafezi F (2017): Corneal cross‐linking (CXL): standardizing terminology and protocol nomenclature. J Refract Surg 33: 727–729. [DOI] [PubMed] [Google Scholar]
- Rocha G, Silva LNP, Chaves LFOB, Bertino P, Torquetti L & de Sousa LB (2019): Intracorneal ring segments implantation outcomes using two different manufacturers' nomograms for keratoconus surgery. J Refract Surg 35: 673–683. [DOI] [PubMed] [Google Scholar]
- Rush SW & Rush RB (2017): Epithelium‐off versus transepithelial corneal collagen crosslinking for progressive corneal ectasia: a randomised and controlled trial. Br J Ophthalmol 101: 503–508. [DOI] [PubMed] [Google Scholar]
- Safarzadeh M & Nasiri N (2016): Anterior segment characteristics in normal and keratoconus eyes evaluated with a combined Scheimpflug/Placido corneal imaging device. J Curr Ophthalmol 28: 106–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleem MIHA (2015): Combined cross‐linking with femtosecond laser myoring implantation versus combined cross‐linking with femtosecond laser keraring implantation in the treatment of keratoconus. J Egypt Ophthalmol Soc 108: 140–147. [Google Scholar]
- Saleem MIH, Ibrahim Elzembely HAI, AboZaid MA, Elagouz M, Saeed AM, Mohammed OA & Kamel AG (2018): Three‐year outcomes of cross‐linking PLUS (combined cross‐linking with femtosecond laser intracorneal ring segments implantation) for management of keratoconus. J Ophthalmol 2018: 6907573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samimi S, Leger F, Touboul D & Colin J (2007): Histopathological findings after intracorneal ring segment implantation in keratoconic human corneas. J Cataract Refract Surg 33: 247–253. [DOI] [PubMed] [Google Scholar]
- Seleet MM, Soliman AH & Alaaeldin OM (2015): Femtosecond laser intracorneal ring segment implantation based on a nomogram modification in type 1 and type 2 ectasia. J Egypt Ophthalmol Soc 108: 1–5. [Google Scholar]
- Soeters N, Wisse RPL, Godefrooij DA, Imhof SM & Tahzib NG (2015): Transepithelial versus epithelium‐off corneal cross‐linking for the treatment of progressive keratoconus: a randomized controlled trial. Am J Ophthalmol 159: 821–828.e3. [DOI] [PubMed] [Google Scholar]
- Spoerl E, Terai N, Scholz F, Raiskup F & Pillunat LE (2011): Detection of biomechanical changes after corneal cross‐linking using Ocular Response Analyzer software. J Refract Surg 27: 452–457. [DOI] [PubMed] [Google Scholar]
- Tian M, Jian W, Sun L, Shen Y, Zhang X & Zhou X (2018): One‐year follow‐up of accelerated transepithelial corneal collagen cross‐linking for progressive pediatric keratoconus. BMC Ophthalmol 18: 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torquetti L, Ferrara G & Ferrara P (2012): Correlation of anterior segment parameters in keratoconus patients. Int J Keratoconus Ectatic Corneal Dis 1: 87–91. [Google Scholar]
- Utine CA, Ayhan Z & Engin CD (2018): Effect of intracorneal ring segment implantation on corneal asphericity. Int J Ophthalmol 11: 1303–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega‐Estrada A & Alio JL (2016): The use of intracorneal ring segments in keratoconus. Eye Vis 3: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega‐Estrada A, Alió JL, Brenner LF & Burguera N (2013): Outcomes of intrastromal corneal ring segments for treatment of keratoconus: five‐year follow‐up analysis. J Cataract Refract Surg 39: 1234–1240. [DOI] [PubMed] [Google Scholar]
- Vega‐Estrada A, Alió JL & Plaza‐Puche AB (2015): Keratoconus progression after intrastromal corneal ring segment implantation in young patients: five‐year follow‐up. J Cataract Refract Surg 41: 1145–52. [DOI] [PubMed] [Google Scholar]
- Vinciguerra R, Romano V, Arbabi EM, Brunner M, Willoughby CE, Batterbury M & Kaye SB. (2017): In vivo early corneal biomechanical changes after corneal cross‐linking in patients with progressive keratoconus. J Refract Surg 33: 840–846. [DOI] [PubMed] [Google Scholar]
- Wollensak G, Spoerl E & Seiler T (2003): Riboflavin/ultraviolet‐A‐induced collagen crosslinking for the treatment of keratoconus. Am J Ophthalmol 135: 620–627. [DOI] [PubMed] [Google Scholar]
- Zaky AG, KhalafAllah MT, Sarhan AE & Elsawy MF (2020): Evaluation of a tangential map‐based nomogram for intrastromal corneal ring segments' implantation in keratoconus: one year results. J Ophthalmol 2020: 3983508. [DOI] [PMC free article] [PubMed] [Google Scholar]


