Summary
Background
Silicone adhesive multilayer foam dressings are used as adjuvant therapy to prevent hospital‐acquired pressure ulcers (PUs).
Objectives
To determine whether silicone foam dressings in addition to standard prevention reduce the incidence of PUs of category 2 or worse compared with standard prevention alone.
Methods
This was a multicentre, randomized controlled medical device trial conducted in eight Belgian hospitals. At‐risk adult patients were centrally randomized (n = 1633) to study groups based on a 1 : 1 : 1 allocation: experimental groups 1 (n = 542) and 2 (n = 545) – pooled as the treatment group – and the control group (n = 546). The experimental groups received PU prevention according to hospital protocol, and a silicone foam dressing on the relevant body sites. The control group received standard of care. The primary endpoint was the incidence of a new PU of category 2 or worse at the studied body sites.
Results
In the intention‐to‐treat population (n = 1605), PUs of category 2 or worse occurred in 4·0% of patients in the treatment group and 6·3% in the control group [relative risk (RR) 0·64, 95% confidence interval (CI) 0·41–0·99, P = 0·04]. Sacral PUs were observed in 2·8% and 4·8% of the patients in the treatment group and the control group, respectively (RR 0·59, 95% CI 0·35–0·98, P = 0·04). Heel PUs occurred in 1·4% and 1·9% of patients in the treatment and control groups, respectively (RR 0·76, 95% CI 0·34–1·68, P = 0·49).
Conclusions
Silicone foam dressings reduce the incidence of PUs of category 2 or worse in hospitalized at‐risk patients when used in addition to standard of care. The results show a decrease for the sacrum, but no statistical difference for the heel and trochanter areas.
Short abstract
What is already known about this topic?
The incidence of hospital‐acquired pressure ulcers (PUs) remains high despite the implementation of best‐practice recommendations.
The concept of using silicone foam dressings as an additional prophylactic strategy in PU prevention has been investigated in previous studies but with some limitations.
Most RCTs were monocentric studies, restricted to either critically ill or acute care patients and did not observe more than two anatomical at‐risk skin sites, which limited the generalizability of the findings.
What does this study add?
This large pragmatic RCT suggests that it is beneficial to use silicone adhesive multilayer foam dressings on the sacrum, in addition to standard of care, to help prevent hospital‐acquired PUs.
Clinical decision making for heel dressings should be based on the clinical effectiveness of the intervention weighed against the potential risk of falling.
Linked Comment: F. Coyer. Br J Dermatol 2021; 185:4–5.
Pressure ulcers (PUs) or pressure injuries are localized injuries to the skin and/or underlying tissue, usually over a bony prominence, as a result of pressure, or pressure in combination with shear.1 The burden of hospital‐acquired PUs (HA‐PUs) is high on patients and healthcare systems.2 A systematic review presented the pooled prevalence of HA‐PUs among 1 366 848 patients as 12·8%, a pooled incidence rate of 5·4 per 10 000 patient‐days (n = 681 885), and the pooled rate of HA‐PUs among 1 893 593 patients as 8·4%.3 Two large studies determined that most category 2–4 PUs occur in the sacral area (45·0–51·7%) and the heels (26·7–40·7%), followed by the greater trochanter area (1·8–2·6%).4, 5 International guidelines recommend to reduce both the amount and the duration of pressure and shear, by implementing strategies that include regular and comprehensive risk assessment, patient repositioning, skincare, incontinence management, nutritional care, and the use of pressure redistribution surfaces.1, 6
There is growing international interest in the application of dressings covering areas at risk for PUs, to reduce the mechanical impact on the skin and underlying tissue. The clinical effectiveness has been summarized in five systematic reviews7, 8, 9, 10, 11 – covering six randomized controlled trials (RCTs)12, 13, 14, 15, 16, 17 with a total of 1985 recruited patients – and an additional RCT published recently.18 The RCTs were mostly monocentric studies and/or restricted to critically ill patients, and observed only one or two body sites (sacrum and/or heels).12, 13, 14, 15, 16, 17, 18 These studies were either funded by industry13, 15, 17, 18 or funders were not reported.12, 14, 16
Silicone foam dressings, depending on their construction, redistribute pressure over larger areas, mitigate external shearing forces on the skin (multiple layers), and might assist with maintaining the microclimate for the skin to function normally (foam structure or layers and film breathability).7, 8, 16, 19 Silicone‐based adhesives are incorporated into the dressings, which compared with traditional adhesives attach faster to uneven skin surfaces, are gentle to remove, and can be repositioned.20, 21 This allows skin visualization without replacing the dressing after lifting.
The aim of this study was to determine whether silicone adhesive multilayer foam dressings used in combination with standard of care would reduce the incidence of PUs of category 2, 3 or 4; unstageable PUs; and deep tissue injuries (referred to as PU category 2 or worse) on the sacrum, heels and greater trochanters of adult hospitalized patients in intensive care units (ICUs) and non‐ICU wards.
Patients and methods
Study design
This was a pragmatic, multicentre, randomized (1 : 1 : 1 allocation), open‐label, parallel‐group medical device trial performed in eight hospitals (three university or teaching hospitals and five general hospitals) in Belgium, including patients on both ICU and non‐ICU wards, with patients in ICU limited to < 25%.
Participants
The sample‐size calculation was based on the results of several randomized trials with an incidence of 6% of PU category 2 or worse on the sacrum, greater trochanter and heels in the standard of care (SOC) group,4 and the treatment group having a 50% reduction in incidence of PUs of category 2 or worse.2, 22 To have 80% power to detect a reduction in PU incidence from 6% to 3%, data had to be available for 1578 patients in total, of whom 526 had to be allocated to the control group and 1052 to the treatment group. Considering a 5% dropout rate, a total of 1662 patients had to be randomized to ensure enough patients would complete the trial without compromising the statistical power. The study was not powered to analyse subgroups.
Patients aged > 18 years who gave written informed consent (patient or proxy) were included if they were: (i) at risk for PU development based on Braden risk assessment23 (Braden score < 17); (ii) had been admitted to the hospital within the previous 48 h; (iii) had no PU of category 2 or worse present on the sacrum; and (iv) had no clinically relevant incontinence‐associated dermatitis or other skin condition that would be a contraindication for application of the study devices. The Braden scale was applied in all participating sites and in all units (both ICU and non‐ICU) because the scale is used nationally to assess risk for PU development (even in the ICU). The study team decided not to change the SOC. Patients could still be included if, at three of the other four sites (heel left/right and trochanter left/right), prevention could be applied or a PU category 2 or worse already existed. Full inclusion and exclusion criteria are available in the clinical study protocol (Appendix S1; see Supporting Information).
Randomization and blinding
Patients were centrally randomized to study groups based on a 1 : 1 : 1 allocation: experimental group 1 (Allevyn® brand), experimental group 2 (Mepilex® brand) and the control group (SOC). Experimental groups 1 and 2 were pooled in the analysis as the treatment group. Randomization was stratified by hospital and ICU vs. non‐ICU wards. The randomization was based on a permuted‐block randomization with varying block sizes. For each group of wards (ICU vs. non‐ICU) the randomization schedule ensured balance of the three study groups at the intended number of randomized patients. Patients, caregivers and study personnel were not blinded to the study procedures as blinding is not possible when using different types of dressings.
Intervention
The study interventions are summarized in Figure 1. Standard hospital protocols for prevention of PUs were used in the SOC and treatment groups, with addition of the silicone foam dressings as the only variable in the treatment group.
Figure 1.
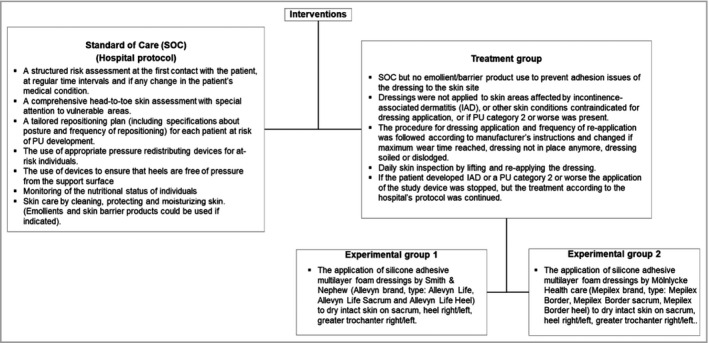
Interventions and procedures. IAD, incontinence‐associated dermatitis; PU, pressure ulcer.
Dressings were commercially available, purchased by the sponsor and delivered to the hospitals by the manufacturers. The university‐based study team did extensive training with the study sites regarding the PU prevention and study protocols, correct indication, and application of the study devices. In total 1192 nurses across 74 wards were trained during 124 sessions. Dressings were maintained on the treatable skin sites and were changed according to the manufacturer’s instructions for use. The study nurse inspected the skin beneath the dressing daily, by lifting the dressing and reapplying (not replacing) it.
If the patient developed a PU of category 2 or worse, or developed incontinence‐associated dermatitis, the application of the study device was stopped, and treatment of the PU was started according to the hospital’s wound care protocol. Photographs were taken whenever a PU of category 2 or worse developed and were transferred to the chief investigator for blinded central review.
Outcomes
The primary endpoint of this trial was the proportion of patients who developed at least one new PU of category 2 or worse on the sacrum, heels or greater trochanter as judged on site, during the trial period of the patient (maximum 14 days). These proportions were compared between the pooled treatment group and the SOC group as per the randomization scheme. An intention‐to‐treat (ITT) analysis including all patients randomized, and a per protocol (PP) analysis was performed. Exploratory analyses (ITT and PP) compared new PU incidence between the anatomical sites (sacrum, trochanters and heels) and the experimental investigational devices (experimental groups 1 and 2). Subgroup analyses of the primary endpoint were performed on patient characteristics: age, sex, type of ward, surgery, body mass index (BMI), diabetes and Braden score.
Statistical analysis
The primary analysis of the primary efficacy variable consisted of a superiority analysis that compared the incidence in the pooled treatment group vs. the control group, by means of the Cochran–Mantel–Haenszel (CMH) test controlled for type of ward (ICU or non‐ICU) on the ITT population. Superiority was concluded if the estimated impact of the treatment vs. control group was significant, based on a two‐sided test at 5% significance level. Patients with missing data were defined as those without any assessment of the primary endpoint after randomization. These patients were excluded from the ITT population.
For exploratory analysis, the primary endpoint was compared firstly between the treatment group and the control group and secondly between experimental group 1 and experimental group 2 (experimental investigational devices) by means of the CMH test, and controlled for type of ward (ICU or non‐ICU) in the ITT and PP populations (Appendix S2; see Supporting Information). Logistic regression models were used, adjusted for hospital, age, sex, type of ward and Braden score category (in the ITT and PP populations).
A sensitivity analysis of the primary endpoint of confirmed PU by blinded central review of photographs was conducted in the ITT population. All efficacy (primary and exploratory) analyses were reproduced in the PP population.
Descriptive safety analyses were performed, based on reported adverse device effects (ADEs). An ADE was defined as any adverse event related to the device used in the trial. Device deficiencies (DDs) were defined as the inadequacy of the study device related to its identity, quality, durability, reliability, safety or performance.
The safety population (n = 1077) was calculated after exclusion of patients in the ITT population who wanted their data excluded (n = 1), were not randomized (n = 46), did not receive at least one dressing (n = 10) or received only SOC (n = 546).
Statistical analyses were performed using the SAS statistical package, version 9·4 (IBM, Armonk, NY, USA).
Ethics
Approval was received from both central (Ghent University Hospital) and local ethics committees for the trial protocol, informed consent forms and other relevant documents. The study was registered at ClinicalTrials.gov (NCT03442777).
Results
Between February and December 2018, 1633 patients were centrally randomized to one of the study groups: 542 (33·2%) to experimental group 1, 545 (33·4%) to experimental group 2 and 546 (33·4%) to the SOC group.
Of the 1680 patients screened for eligibility, 46 patients were not randomized and one patient requested to have their data excluded from the analyses. Figure 2 summarizes the participant flow.
Figure 2.

Participant flowchart. PU, pressure ulcer.
Among the 1633 randomized patients, approximately 61·0% were > 80 years old (mean age 79·6 years, SD 12·2, range 28·3–103·7), and the majority were female (57·6%) and from non‐ICU wards (87·5%). Patients who were underweight (BMI < 18·5 kg m−2) accounted for 8·3% of the sample (n = 136), 29·7% were overweight (BMI 25·0–30·0 kg m−2) and 16·5% had obesity (BMI > 30 kg m−2). The patient characteristics were equally distributed across the three groups. The baseline demographics are displayed in Table 1.
Table 1.
Participants’ baseline data (frequencies and descriptive) and total Braden scores at baseline: by randomized arm, intention‐to‐treat population
| Randomized arm | Allevyn Life (n = 542) | Mepilex Border (n = 545) | Standard of care (n = 546) | Total (n = 1633) |
|---|---|---|---|---|
| Ward type at start of study | ||||
| ICU | 65 (12) | 67 (12) | 71 (13) | 203 (12) |
| Non‐ICU | 477 (88) | 478 (88) | 475 (87) | 1430 (88) |
| Age (years) | ||||
| < 60 | 46 (8) | 43 (8) | 45 (8) | 134 (8) |
| 60–69 | 50 (9) | 70 (13) | 56 (10) | 176 (11) |
| 70–79 | 106 (20) | 107 (20) | 117 (22) | 330 (20) |
| ≥ 80 | 340 (63) | 325 (59) | 328 (60) | 993 (61) |
| Median (IQR) | 83·1 (74·7–88·2) | 83·1 (72·6–88·3) | 82·7 (73·4–87·5) | 83·0 (73·7–87·9) |
| Sex | ||||
| Female | 320 (59) | 302 (55) | 319 (58) | 941 (58) |
| Male | 222 (41) | 243 (45) | 227 (42) | 692 (42) |
| BMI (kg m−2) | ||||
| Underweight (< 18·5) | 44 (8) | 53 (10) | 39 (7) | 136 (8) |
| Normal weight (18·5–25·0) | 234 (43) | 249 (45) | 258 (47) | 741 (45) |
| Overweight (25·0–30·0) | 161 (30) | 163 (30) | 162 (30) | 486 (30) |
| Obesity (≥ 30·0) | 103 (19) | 80 (15) | 87 (16) | 270 (17) |
| Median (IQR) | 24·8 (21·8–28·4) | 24·2 (21·3–27·6) | 24·5 (21·7–27·9) | 24·5 (21·5–28·0) |
| Diabetes | ||||
| No | 419 (77) | 427 (78) | 412 (75) | 1258 (77) |
| Yes | 123 (23) | 118 (22) | 134 (25) | 375 (23) |
| Total Braden score | ||||
| ≤ 11 | 129 (24) | 142 (26) | 126 (23) | 397 (24) |
| 12–16 | 392 (72) | 376 (69) | 403 (74) | 1171 (72) |
| 17 | 21 (4) | 27 (5) | 17 (3) | 65 (4) |
| Median (IQR) at baseline | 13 (12·0–15·0) | 14 (11·0–15·0) | 13 (12·0–15·0) | 13 (12·0–15·0) |
The data are presented as n (%) unless indicated otherwise. BMI, body mass index; ICU, intensive care unit; IQR, interquartile range.
Of the 1605 patients in the ITT population, 77 (4·8%) developed a PU of category 2 or worse: 4·0% in the treatment group and 6·3% in the SOC control group. The CMH test, controlled for type of ward (ICU or non‐ICU), showed a statistically significant reduction of the risk to develop a PU in the treatment group [relative risk (RR) 0·64, 95% confidence interval (CI) 0·41–0·99, P = 0·04], meaning that patients in the treatment group had a 36% risk reduction of developing a new PU compared with those in the SOC group (Table 2). This result was confirmed when using a logistic regression model, adjusted for hospital, age, sex, type of ward (ICU or non‐ICU) and Braden score at baseline (P = 0·01). The number needed to treat to prevent one new PU of category 2 or worse from developing was 43.
Table 2.
Estimated relative risks (RRs) and 95% confidence intervals (CIs) for pressure ulcers of category 2 or worse, in the experimental group compared with the standard‐of‐care group (intention‐to‐treat analyses)
| Experimental, n/N (%) | Standard of care, n/N (%) | RRa (95% CI) | P‐value | |
|---|---|---|---|---|
| Overall | 43/1066 (4·0) | 34/539 (6·3) | 0·64 (0·41–0·99) | 0·04 |
| Body site | ||||
| Sacrum | 30/1062 (2·8) | 26/539 (4·8) | 0·59 (0·35–0·98) | 0·04 |
| Any heel | 15/1063 (1·4) | 10/538 (1·9) | 0·76 (0·34–1·68) | 0·49 |
| Any trochanter | 1/1065 (0·1) | 0/539 (0) | n/a | n/a |
RR with reference to the standard‐of‐care group. n/a, not applicable.
With exploratory analyses, new PUs on the sacrum were observed in 2·8% and 4·8% of patients in the treatment and SOC groups, respectively. The risk of developing a new PU on the sacrum was statistically significantly reduced by 41% in the treatment group (RR 0·59, 95% CI 0·35–0·98, P = 0·04). The number needed to treat to prevent one new PU of category 2 or worse on the sacrum was 50. PUs on the heels occurred in 1·4% and 1·9% of patients in the treatment and SOC groups, respectively, and no statistical difference was identified (RR 0·76, 95% CI 0·34–1·68, P = 0·49). Only one patient (0·1%), in experimental group 1, developed a PU on the trochanter (Table 2). Exploratory data analyses did not demonstrate any major differences in effectiveness between the two brands, considering that the study was not powered to detect such differences.
The incidence of PUs increased with age (from 0·8% for < 60 years to 5·9% for ≥ 80 years) and was higher among women then among men (5·1% vs. 4·4%). The incidence of PUs decreased across Braden score categories (Table 3), from 6·7% (Braden score ≤ 11) to 4·3% (Braden score 12–16) and 1·6% (Braden score ≥ 17). In the high‐risk group (score ≤ 11), the incidence was higher in the experimental group (7·9%) than in the SOC group (4·0%), while it was the inverse in the mild risk category (2·8% in the experimental group vs. 7·3% in the SOC group).
Table 3.
Incidence of pressure ulcers of category 2 or worse, by intervention group stratified by patient characteristics (intention‐to‐treat analyses)
| Treatment, n = 1066 | Standard of care, n = 539) | Total, n = 1605 | |||
|---|---|---|---|---|---|
| Pressure ulcer | |||||
| All | Yes | 43 (4·0) | 34 (6·3) | 77 (4·8) | |
| No | 1023 (96·0) | 505 (93·7) | 1528 (95·2) | ||
| Age (years) | |||||
| < 60 | Yes | 0 (0) | 1 (2) | 1 (0·8) | |
| No | 81 (100) | 44 (98) | 125 (99·2) | ||
| 60–69 | Yes | 2 (1·7) | 2 (4) | 4 (2·3) | |
| No | 116 (98·3) | 53 (96) | 169 (97·7) | ||
| 70–79 | Yes | 8 (3·8) | 6 (5·2) | 14 (4·3) | |
| No | 205 (96·2) | 109 (94·8) | 314 (95·7) | ||
| ≥ 80 | Yes | 33 (5·0) | 25 (7·7) | 58 (5·9) | |
| No | 621 (95·0) | 299 (92·3) | 920 (94·1) | ||
| Sex | |||||
| Female | Yes | 27 (4·4) | 20 (6·3) | 47 (5·1) | |
| No | 581 (95·6) | 296 (93·7) | 877 (94·9) | ||
| Male | Yes | 16 (3·5) | 14 (6·3) | 30 (4·4) | |
| No | 442 (96·5) | 209 (93·7) | 651 (95·6) | ||
| Baseline ward | |||||
| ICU | Yes | 6 (4·8) | 3 (4) | 9 (4·6) | |
| No | 118 (95·2) | 68 (96) | 186 (95·4) | ||
| Non‐ICU | Yes | 37 (3·9) | 31 (6·6) | 68 (4·8) | |
| No | 905 (96·1) | 437 (93·4) | 1342 (95·2) | ||
| Surgery | |||||
| No | Yes | 39 (4·1) | 30 (6·2) | 69 (4·8) | |
| No | 913 (95·9) | 454 (93·8) | 1367 (95·2) | ||
| Yes | Yes | 4 (3·5) | 4 (7) | 8 (4·7) | |
| No | 110 (96·5) | 51 (93) | 161 (95·3) | ||
| BRADEN at baseline | |||||
| ≤ 11 | Yes | 21 (7·9) | 5 (4·0) | 26 (6·7) | |
| No | 244 (92·1) | 119 (96·0) | 363 (93·3) | ||
| 12–16 | Yes | 21 (2·8) | 29 (7·3) | 50 (4·3) | |
| No | 734 (97·2) | 369 (92·7) | 1103 (95·7) | ||
| 17 | Yes | 1 (2) | 0 (0) | 1 (2) | |
| No | 45 (98) | 17 (100) | 62 (98) | ||
| BRADEN – Sensory Perception | |||||
| Completely limited or very limited | Yes | 18 (7·6) | 7 (5·8) | 25 (7·0) | |
| No | 220 (92·4) | 113 (94·2) | 333 (93·0) | ||
| Slightly limited or no impairment | Yes | 25 (3·0) | 27 (6·4) | 52 (4·2) | |
| No | 803 (97·0) | 392 (93·6) | 1195 (95·8) | ||
| BRADEN – Activity | |||||
| Bedfast/chairfast | Yes | 42 (4·3) | 34 (6·8) | 76 (5·2) | |
| No | 928 (95·7) | 465 (93·2) | 1393 (94·8) | ||
| Walks occasionally or walks frequently | Yes | 1 (1) | 0 (0) | 1 (0·7) | |
| No | 95 (99) | 40 (100) | 135 (99·3) | ||
| BRADEN – Mobility | |||||
| Completely immobile/very limited | Yes | 39 (4·8) | 23 (5·5) | 62 (5·0) | |
| No | 778 (95·2) | 397 (94·5) | 1175 (95·0) | ||
| Slightly limited/no limitations | Yes | 4 (1·6) | 11 (9·2) | 15 (4·1) | |
| No | 245 (98·4) | 108 (90·8) | 353 (95·9) | ||
| BRADEN – Nutrition | |||||
| Very poor/probably inadequate | Yes | 34 (4·6) | 20 (5·4) | 54 (4·8) | |
| No | 709 (95·4) | 352 (94·6) | 1061 (95·2) | ||
| Adequate/excellent | Yes | 9 (2·8) | 14 (8·4) | 23 (4·7) | |
| No | 314 (97·2) | 153 (91·6) | 467 (95·3) | ||
ICU, intensive care unit.
A sensitivity analysis was conducted (ITT population) based on centrally confirmed PUs by blinded review of photographs by the chief investigator. Among the 77 new PUs of category 2 or worse reported by local assessment in the ITT population (n = 1065), 68 had photographs of sufficient quality and were assessed. Of these, 56 (82%) were confirmed as a PU of category 2 or worse by central review. All PP analyses confirmed the ITT analyses, as described in the clinical study report.24
No serious ADEs were reported in the safety population (n = 1077) during the study. Thirty‐three ADEs were reported in 28 patients. Most of them were mechanical skin injuries (skin tears or skin stripping, n = 11), PU formation (two of PU category 1, one of PU category 2) and blister formation at the edge of or underneath the dressing (n = 3). These injuries were anecdotally attributed to the dressing being ‘rolled up’, having ‘rolled edges’ or being ‘wrinkled up’, alluding to the inability of some of the dressings to stay in place, causing tension injuries on the skin. Heel dressings caused two patient falls, without significant harm, when the dressing was in direct contact with the floor (Table 4).
Table 4.
Number of adverse device effects and device deficiencies, safety population
| Treatment group | |||
|---|---|---|---|
| Allevyn Life, n = 539 | Mepilex Border, n = 538 | Total, n = 1077 | |
| Adverse device effect (33 in 28 patients) | |||
| All | 21 (3·9) | 12 (2·2) | 33 (3·1) |
| Pressure ulcer development | 1 (0·2) | 2 (0·4) | 3 (0·3) |
| Erythema | 4 (0·7) | 4 (0·7) | 8 (0·7) |
| Pruritus | 3 (0·6) | 1 (0·2) | 4 (0·4) |
| Blister formation | 2 (0·4) | 1 (0·2) | 3 (0·3) |
| Exacerbates athlete’s foot | 0 (0) | 1 (0·2) | 1 (0·1) |
| Mechanical skin injuries | 8 (1·4) | 3 (0·6) | 11 (1·0) |
| Patient fall | 2 (0·4) | 0 (0) | 2 (0·2) |
| Pain at sacrum | 1 (0·2) | 0 (0) | 1 (0·1) |
| Device deficiency (246 in 97 patients) | |||
| All | 168 (31·2) | 78 (14·5) | 246 (22·9) |
| Dressing layers separated | 20 (3·7) | 6 (1·1) | 26 (2·4) |
| Poor adhesion or adhesion failure | 75 (13·9) | 52 (9·7) | 127 (11·8) |
| Dressing causes floor to be slippery (increased fall risk) | 19 (3·5) | 7a (1·3) | 26 (2·4) |
| Adhesive residue | 10 (1·8) | 0 (0) | 10 (0·9) |
| Obstructs wearing footwear | 1 (0·2) | 2 (0·4) | 3 (0·4) |
| Backing film or liner: adhesive transfer or poor release | 10 (1·9) | 0 (0) | 10 (0·9) |
| Rolled‐up edges | 33 (6·1) | 11 (0·2) | 44 (4·1) |
The data are presented as n (%). aOne of these cases resulted in another person falling, without significant harms.
From the DD group in the safety population, 246 DDs were reported in 97 patients. Some of the seven categories identified related to the subsequent ADEs reported, namely poor adhesion or adhesion failure (n = 127), rolled‐up edges (n = 44) and dressing causing the floor to be slippery for others (n = 26); in one case of the latter this resulted in a fall, without significant harm. Other DDs noted were dressing layer separation, adhesive residue on the skin, adhesive transfer or poor release of the backing film or liner from the dressing, and footwear obstruction (Table 4).
Discussion
The use of silicone adhesive multilayer foam dressings for PU prevention at the sacrum, heels and trochanters significantly decreased the incidence of PUs of category 2 or worse from 6·3% to 4·0% in hospitalized at‐risk patients.
Exploratory analyses (per anatomical site and between experimental groups) were performed to investigate specific interactions on the primary outcome. Any apparent lack of effect should be regarded with caution as the trial was not specifically powered with interactions in mind. The results show a decrease in PUs of category 2 or worse for the sacrum (from 4·8% to 2·8%), but no statistically significant effect for the heels (decrease from 1·9% to 1·4%). The incidence of PUs on trochanters was too low to identify any effect. These results are consistent with those from previous trials using silicone foam dressings.7, 8, 9, 10, 11, 18 This study further expands the generalizability of the results as more than one dressing type was used, the study was performed in different disciplines, and multiple anatomical sites were included. There were no statistical differences between experimental groups 1 and 2 (detailed in the clinical study report).24
While no serious adverse events were reported, 33 ADEs in 28 patients, and 246 DDs in 97 patients were reported – including two incidents of patient falling, due to heel dressings being slippery on the floor. The surface of the dressing is designed to minimize friction (be slippery) and therefore advice was provided that the dressings should not be placed directly on the floor, and that shoes or antislip stockings should be worn, although three patients reported that their footwear did not fit over the bulk of the dressing. For both brands there were also reports that placing feet covered by heel dressings directly on the floor made the floors slippery and risked staff or other people slipping. In one case this resulted in a nonstudy individual falling, without significant harms. This observation appeared to be made more frequently when the ambient temperature was above 30 °C.
As the trial results showed no clear effect on the heels, a risk–benefit analysis should be considered when applying prophylactic dressings on the heels to determine whether any potential benefits of protecting the heels outweigh the reported risk of falls. The product specifications highlight differences in construction and adhesive properties between the two study dressings. This may explain the differences in the ADEs and DDs (Table 4). No known standards exist for this product class despite their widespread and growing use. This conclusion feeds the discussion about the need for performance standards for prophylactic dressings. In November 2020, the European Pressure Ulcer Advisory Panel and the National Pressure Injury Advisory Panel announced the establishment of an international task force to develop such performance standards (https://www.epuap.org/prophylactic‐dressing). These standards will generate critical information to guide (i) effective clinical product selection and practice, (ii) benchmarks for development purposes and (iii) reimbursement policies.
It should be reiterated that the use of silicone adhesive multilayer foam dressings might be an additional intervention to obtain adequate PU prevention, and that the current standard guidelines for PU prevention still remain the cornerstone of prevention.1 The risk exists that applying a prophylactic dressing on an at‐risk patient will create a false sense of security for the clinician, leading to the remaining ‘standard of care’ of PU prevention being ignored; however, there was no suggestion of this happening in this study. If prophylactic dressings are added to PU prevention strategies, the protocols should stress the importance of education, daily skin assessment underneath the dressing, and monitoring of the adherence to the protocol.
There were some limitations to this study. Performance and detection bias may have occurred because patients, caregivers and study personnel could not be blinded to the study procedures and devices. However, the results were consistent when based on confirmed PUs by review of photographs, all study nurses were trained, and for most hospitals their wound care teams were involved when a PU occurred, further strengthening the correct identification of skin injuries. As the organization of care and staff characteristics is setting specific, generalizability to other hospitals in other regions might be limited. However, there was a range of hospital types (university and general hospitals), the hospital standard prevention protocols corresponded to the international state‐of‐the‐art recommendations, and the treatment effect is consistent with the results of previous RCTs.
Strengths of this noncommercial study are firstly, its large size and the pragmatic nature of the trial setup and performance. Pragmatic studies can measure realistic treatment effects in daily clinical routines compared with highly standardized RCTs. Secondly, the effect estimate is based on category 2 or worse PUs. As category 1 PUs are not wounds, the clinical relevance of this outcome is questionable, and the measurement error of this outcome is high. Thirdly, the quality of data collection and high‐level education provided by the study team to the sites are a strength.
Future academic research priorities could include evaluating the cost and benefit of using prophylactic dressing to reduce HA‐PUs, and if prophylactic dressings are included within SOC for PU prevention, evaluating the adherence level to a newly implemented PU protocol.
In conclusion, our study confirmed previous reports from predominantly small single‐centre studies that multilayer dressings reduce the incidence of sacral PUs in addition to SOC, both in ICUs and in other wards. Given that on average 50 qualifying hospitalized patients would need to be treated with supplemental dressings in order to prevent one patient from developing a PU of category 2 or worse on the sacral area, a health‐economic analysis would be informative before such intervention is routinely implemented in hospitals.
Author Contribution
Dimitri Beeckman: Conceptualization (lead); Data curation (equal); Formal analysis (supporting); Funding acquisition (lead); Investigation (supporting); Methodology (lead); Project administration (equal); Supervision (lead); Validation (lead); Visualization (lead); Writing‐original draft (equal); Writing‐review & editing (equal). Anika Fourie: Data curation (equal); Formal analysis (equal); Methodology (equal); Project administration (equal); Resources (equal); Validation (equal); Writing‐original draft (lead); Writing‐review & editing (lead). Charlotte Raepsaet: Data curation (equal); Formal analysis (equal); Methodology (equal); Writing‐original draft (equal). Nele Van Damme: Data curation (equal); Investigation (equal); Project administration (equal); Writing‐original draft (lead). Bénédicte Manderlier: Data curation (equal); Investigation (equal); Project administration (equal); Writing‐original draft (equal). Dorien De Meyer: Data curation (equal); Investigation (equal); Project administration (equal); Writing‐original draft (equal). Hilde Beele: Data curation (equal); Investigation (equal). Steven Smet: Data curation (equal); Investigation (equal). Liesbet Demarré Data curation (equal); Investigation (equal). Rudi Vossaert: Data curation (equal); Investigation (equal). Annelies de Graaf: Data curation (equal); Investigation (equal). Liesa Verhaeghe: Data curation (equal); Investigation (equal). Nathalie Vandergheynst: Data curation (equal); Investigation (equal). Benoit Hendrickx: Data curation (equal); Investigation (equal). Valerie Hanssens: Data curation (equal); Investigation (equal). Hilde Keymeulen: Data curation (equal); Investigation (equal). Katrien Vanderwee: Data curation (equal); Investigation (equal). Johan Vandewoestijne: Data curation (equal); Investigation (equal). Sofie Verhaeghe: Conceptualization (supporting); Supervision (supporting); Writing‐original draft (supporting). Ann Van Hecke: Conceptualization (supporting); Supervision (supporting); Writing‐original draft (supporting). Isabelle Savoye: Formal analysis (lead); Methodology (equal); Resources (equal); Software (lead); Validation (equal); Writing‐review & editing (supporting). Jillian Harrison: Conceptualization (supporting); Data curation (supporting); Investigation (supporting); Methodology (supporting); Project administration (lead); Supervision (equal); Validation (equal); Writing‐review & editing (supporting). France Vrijens: Formal analysis (lead); Methodology (equal); Resources (equal); Software (equal); Validation (equal); Writing‐review & editing (supporting). Frank Hulstaert: Conceptualization (equal); Formal analysis (lead); Investigation (supporting); Methodology (equal); Resources (equal); Supervision (equal); Validation (equal); Visualization (equal); Writing‐original draft (supporting); Writing‐review & editing (supporting).
Supporting information
Appendix S1. Clinical study protocol.
Appendix S2. Statistical analysis plan.
Powerpoint S1 Journal Club Slide Set
Video S1 Author video
Acknowledgments
The study team at Ghent University developed the training material, and their high level of education to the sites and the commitment of the principal investigators and trial nurses should be commended.
Funding sources
The Belgian Health Care Knowledge Centre (KCE) funded and sponsored the trial via the KCE Trials Programme (study ID KCE16012), a national public funding programme of noncommercial trials. No funding from the manufacturers of the study devices was received. Involvement of the sponsor included refinement of the study design and statistical analyses but they were not involved in data collection. KCE members reviewed the manuscript for statistical correctness and interpretation. The corresponding author had the final responsibility for the decision to publish.
Conflicts of interest
The authors declare they have no conflicts of interest.
D.B. and A.F. contributed equally to the manuscript and are joint first authors.
Plain language summary available online
References
- 1.European Pressure Ulcer Advisory Panel, National Pressure Injury Advisory Panel and Pan Pacific Pressure Injury Alliance . Prevention and Treatment of Pressure Ulcers/Injuries: Clinical Practice Guideline. Available at: http://www.internationalguideline.com (last accessed 19 November 2020).
- 2.Demarré L, Verhaeghe S, Annemans Let al. The cost of pressure ulcer prevention and treatment in hospitals and nursing homes in Flanders: a cost‐of‐illness study. Int J Nurs Stud 2015; 52:1166–79. [DOI] [PubMed] [Google Scholar]
- 3.Li Z, Lin F, Thalib L, Chaboyer W. Global prevalence and incidence of pressure injuries in hospitalised adult patients: a systematic review and meta‐analysis. Int J Nurs Stud 2020; 105:103546. [DOI] [PubMed] [Google Scholar]
- 4.Vanderwee K, Defloor T, Beeckman Det al. Assessing the adequacy of pressure ulcer prevention in hospitals: a nationwide prevalence survey. BMJ Qual Saf 2011; 20:260–7. [DOI] [PubMed] [Google Scholar]
- 5.Schoonhoven L, Bousema MT, Buskens E. The prevalence and incidence of pressure ulcers in hospitalised patients in the Netherlands: a prospective inception cohort study. Int J Nurs Stud 2007; 44:927–35. [DOI] [PubMed] [Google Scholar]
- 6.Beeckman D, Matheï C, Van Lancker Aet al. A national guideline for the treatment of pressure ulcers – synthesis. Available at: https://kce.fgov.be/sites/default/files/atoms/files/KCE_203Cs_pressure_ulcers.pdf (last accessed 19 November 2020).
- 7.Clark M, Black J, Alves Pet al. Systematic review of the use of prophylactic dressings in the prevention of pressure ulcers. Int Wound J 2014; 11:460–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davies P. Role of multi‐layer foam dressings with Safetac in the prevention of pressure ulcers: a review of the clinical and scientific data. J Wound Care 2016; 25 (1 Suppl.):S1, S4–23. [PubMed] [Google Scholar]
- 9.Fulbrook P, Mbuzi V, Miles S. Effectiveness of prophylactic sacral protective dressings to prevent pressure injury: a systematic review and meta‐analysis. Int J Nurs Stud 2019; 100:103400. [DOI] [PubMed] [Google Scholar]
- 10.Moore ZE, Webster J. Dressings and topical agents for preventing pressure ulcers. Cochrane Database Syst Rev 2018; 12:CD009362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ramundo J, Pike C, Pittman J. Do prophylactic foam dressings reduce heel pressure injuries? J Wound Ostomy Continence Nurs 2018; 45:75–82. [DOI] [PubMed] [Google Scholar]
- 12.Aloweni F, Lim M, Chua Tet al. A randomised controlled trial to evaluate the incremental effectiveness of a prophylactic dressing and fatty acids oil in the prevention of pressure injuries. Wound Pract Res 2017; 25:24–34. [Google Scholar]
- 13.Forni C, D’Alessandro F, Gallerani Pet al. Effectiveness of using a new polyurethane foam multi‐layer dressing in the sacral area to prevent the onset of pressure ulcer in the elderly with hip fractures: a pragmatic randomised controlled trial. Int Wound J 2018; 15:383–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kalowes P, Messina V, Li M. Five‐layered soft silicone foam dressing to prevent pressure ulcers in the intensive care unit. Am J Crit Care 2016; 25:e108–e119. [DOI] [PubMed] [Google Scholar]
- 15.Lee YJ, Kim JY, Shin WY. Use of prophylactic silicone adhesive dressings for maintaining skin integrity in intensive care unit patients: a randomised controlled trial. Int Wound J 2019; 16 (Suppl. 1):36–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Santamaria N, Gerdtz M, Liu Wet al. Clinical effectiveness of a silicone foam dressing for the prevention of heel pressure ulcers in critically ill patients: Border II Trial. J Wound Care 2015; 24:340–5. [DOI] [PubMed] [Google Scholar]
- 17.Santamaria N, Gerdtz M, Kapp Set al. A randomised controlled trial of the clinical effectiveness of multi‐layer silicone foam dressings for the prevention of pressure injuries in high‐risk aged care residents: The Border III Trial. Int Wound J 2018; 15:482–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hahnel E, El Genedy M, Tomova‐Simitchieva Tet al. The effectiveness of two silicone dressings for sacral and heel pressure ulcer prevention compared with no dressings in high‐risk intensive care unit patients: a randomized controlled parallel‐group trial. Br J Dermatol 2020; 183:256–64. [DOI] [PubMed] [Google Scholar]
- 19.Gefen A, Alves P, Creehan Set al. Computer modelling of prophylactic dressings: an indispensable guide for health care professionals. Adv Skin Wound Care 2019; 32 (7 Suppl.):S4–13. [DOI] [PubMed] [Google Scholar]
- 20.Dykes PJ. The effect of adhesive dressing edges on cutaneous irritancy and skin barrier function. J Wound Care 2007; 16:97–100. [DOI] [PubMed] [Google Scholar]
- 21.Grove GL, Zerweck CR, Houser TPet al. A randomized and controlled comparison of gentleness of 2 medical adhesive tapes in healthy human subjects. J Wound Ostomy Continence Nurs 2013; 40:51–9. [DOI] [PubMed] [Google Scholar]
- 22.Nixon J, Nelson EA, Cranny Get al. Pressure relieving support surfaces: a randomised evaluation. Health Technol Assess 2006; 10:iii–iv, ix–x, 1–163. [DOI] [PubMed] [Google Scholar]
- 23.Bergstrom N, Braden B, Kemp Net al. Multi‐site study of incidence of pressure ulcers and the relationship between risk level, demographic characteristics, diagnoses, and prescription of preventive interventions. J Am Geriatr Soc 1996; 44:22–30. [DOI] [PubMed] [Google Scholar]
- 24.Belgian Health Care Knowledge Centre . KCE16012: a medical device trial to evaluate the use of a silicone adhesive multilayer foam dressing as prevention for pressure ulcer development in hospitalised patients. Available at: https://kce.fgov.be/en/kce16012‐a‐medical‐device‐trial‐to‐evaluate‐the‐use‐of‐a‐silicone‐adhesive‐multilayer‐foam‐dressing (last accessed 24 November 2020).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Clinical study protocol.
Appendix S2. Statistical analysis plan.
Powerpoint S1 Journal Club Slide Set
Video S1 Author video


