Abstract
Background
Chronic rhinosinusitis with nasal polyps (CRSwNP) is a type 2 inflammatory disease treated with sinus surgery when refractory to medical intervention. However, recurrence postsurgery is common. Dupilumab, a fully human monoclonal antibody, blocks the shared receptor for interleukin 4 (IL‐4) and IL‐13, key and central drivers of type 2 inflammation. We report the efficacy of dupilumab in patients with CRSwNP from the SINUS‐24/SINUS‐52 trials (NCT02912468/NCT02898454), by number of prior surgeries and time since last surgery.
Methods
Patients were randomized to placebo or dupilumab 300 mg every 2 weeks. Post hoc subgroup analyses were performed for patients with 0, ≥1, 1/2, or ≥3 prior surgeries, and for patients who had surgery within <3, 3 to <5, 5 to <10, or ≥10 years. Efficacy outcomes at 24 weeks included co‐primary endpoints nasal polyp score (NPS) and nasal congestion (NC), and Lund‐Mackay (LMK), 22‐item Sino‐Nasal Outcome Test (SNOT‐22), and smell scores.
Results
Of 724 patients randomized, 459 (63.4%) had ≥1 prior surgery. Baseline sinus disease (NPS, NC, LMK) and olfactory dysfunction (University of Pennsylvania Smell Identification Test [UPSIT] and loss of smell) scores were worse for patients with ≥3 prior surgeries vs no surgery. Baseline NPS and LMK were worse in patients with <3 years since last surgery than in patients with ≥5 years since last surgery. Dupilumab significantly improved all outcome measures vs placebo in all subgroups by number of surgeries and by time since last surgery. Improvements in NPS and LMK were greater in patients with <3 years since last surgery than patients with ≥5 years. Safety results were consistent with the known dupilumab safety profile.
Conclusion
Dupilumab improved CRSwNP outcomes irrespective of surgery history, with greater improvements in endoscopic outcomes in patients with shorter duration since last surgery.
Keywords: chronic disease, chronic rhinosinusitis, sinus surgery, subcutaneous immunotherapy, therapeutics
1.
Chronic rhinosinusitis with nasal polyps (CRSwNP) is a chronic inflammatory disease affecting the nose and paranasal sinuses, associated with a high symptom burden and poor health‐related quality of life.1 CRSwNP is characterized by symptoms of rhinosinusitis (nasal congestion [NC], anterior rhinorrhea/postnasal drip, reduction in or loss of smell, facial pain/headache) for at least 3 months, sinus obstruction, the presence of bilateral nasal polyps (NP) upon nasal endoscopy or evidence of sinus disease on a computed tomography (CT) scan, and by the presence of type 2 inflammation, with the involvement of type 2 cytokines interleukin 4 (IL‐4), IL‐13, and IL‐5 in its pathophysiology and high levels of tissue immunoglobulin E (IgE).2, 3, 4
Current international treatment guidelines recommend an incremental treatment approach determined by disease severity. Nasal saline irrigation and topical intranasal corticosteroids (INCS) are utilized at all severity levels of disease, with short courses of oral systemic corticosteroids (SCS) recommended for uncontrolled severe disease and sinus surgery to remove polyps if medical intervention fails.2, 5 Endoscopic sinus surgery (ESS) is recommended for the treatment of recalcitrant disease, with an estimated 46% to 79% of patients with CRSwNP undergoing at least 1 surgery.1, 6, 7 However, surgical procedures for CRSwNP lack uniformity, ranging from simple polypectomy to more extensive surgery options.8, 9, 10 Although patients commonly benefit symptomatically from surgery, postoperative recurrence of polyps is frequent. Around 40% of patients suffer clinically evident recurrence within 18 months postsurgery, with around 60% within 7 years and almost 80% within 12 years.11, 12, 13 Multiple rounds of surgery are therefore frequent, with a recent systematic review reporting that 14% to 24% of patients have undergone revision surgery due to recurrence.14 Patients with CRSwNP with a type 2 inflammatory signature—characterized by asthma and nonsteroidal anti‐inflammatory drug–exacerbated respiratory tract disease (NSAID‐ERD) or elevated eosinophil count and increased IL‐5 and IgE levels in the nasal or sinus tissues—show higher rates of polyp recurrence and earlier recurrence postsurgery,15, 16 and multiple procedures are particularly common in these patient groups.11, 17, 18
Dupilumab is a fully human VelocImmune®‐derived monoclonal antibody19, 20 that inhibits the signaling of both IL‐4 and IL‐13 (cytokines that are key and central drivers of type 2‐mediated inflammation) by binding to their shared receptor component.21, 22, 23 Dupilumab is approved as an add‐on maintenance treatment in adult patients with inadequately controlled CRSwNP.24, 25, 26 The phase 3 SINUS‐24 (Clinical Trials; https://clinicaltrials.gov/ct2/show/NCT02912468) and SINUS‐52 studies (Clinical Trials; https://clinicaltrials.gov/ct2/show/NCT02898454) demonstrated that dupilumab, on a background of INCS (mometasone furoate), significantly improved endoscopic, clinical, and patient‐reported outcomes in patients with severe CRSwNP uncontrolled by standard of care, and reduced SCS use and surgery.17 These studies also demonstrated the efficacy of dupilumab in patients who had undergone prior sinus surgery17; however, it remained unclear whether patients with multiple prior surgeries benefited as much as patients with 1 prior surgery.
Recurrence of polyps and symptoms following multiple surgeries may be an indicator of disease refractory to treatment, and it is not possible to assume that a patient with several prior surgeries will have the same response to a biologic as a patient with only a single surgery. Patients and clinicians may have concerns that the requirement for multiple surgeries indicates a patient with a more severe inflammatory burden who is refractory to all treatment options, or in whom scar tissue from previous surgeries may prevent benefit from biologic treatments. Investigation into the effects of prior surgical history on the efficacy of biologics may help to resolve uncertainties around value in healthcare settings where access to biologics is targeted to those in whom it has the greatest cost‐effectiveness.
This study aimed to support shared clinician and patient decision‐making in the use of dupilumab by analyzing whether patients who have undergone multiple surgeries can derive benefit from treatment with a biologic. This post hoc analysis examined a pooled population of patients with CRSwNP enrolled in the SINUS‐24 and SINUS‐52 studies to assess the efficacy of dupilumab compared with placebo at week 24 in patient subgroups according to history of prior sinus surgery (by number of surgeries and by the time since last surgery). The effect of dupilumab on the risk of rescue treatment, defined as treatment with SCS or need for sinus surgery,2 was also assessed.
2. PATIENTS AND METHODS
2.1. Study design
The SINUS‐24 and SINUS‐52 studies were randomized, multicenter, double‐blind, placebo‐controlled, parallel‐group studies that assessed dupilumab as an add‐on treatment to standard of care in adults with severe CRSwNP. The study designs have been described.17 In brief, in SINUS‐24, patients were randomized 1:1 to subcutaneous (SC) dupilumab 300 mg or placebo every 2 weeks (q2w) for 24 weeks. In SINUS‐52, patients were randomized 1:1:1 to SC dupilumab 300 mg q2w for 52 weeks, SC dupilumab 300 mg q2w for 24 weeks then every 4 weeks for 28 weeks, or placebo q2w for 52 weeks. There were prespecified study enrollment goals of 50% of patients with asthma and/or NSAID‐ERD based on patient history and 50% of patients with prior sinus surgery. Patients were stratified by history of prior sinus surgery, asthma/NSAID‐ERD, and country at randomization. Patients received 100 μg mometasone furoate nasal spray in each nostril twice daily throughout the trial period. Rescue treatment with SCS, sinus surgery, or nasal lavage with saline and/or systemic antibiotics were allowed per investigator's discretion.
2.2. Eligibility criteria
Adult patients with CRSwNP with either prior treatment with SCS within 2 years or contraindication/intolerance to SCS or prior surgery for NP were considered eligible for enrollment if they met the following criteria: bilateral NP despite INCS treatment for ≥2 months with nasal polyp score (NPS) ≥5 out of 8, and ≥2 for each nostril; ≥2 rhinosinusitis symptoms of nasal obstruction or discharge (symptom severity score ≥2) AND 1 of rhinorrhea (anterior/posterior) or reduction or loss of smell.
Patients were excluded if they had: been treated with monoclonal antibodies or immunosuppressive therapy treatment within 2 months, or had undergone anti‐IgE therapy (such as omalizumab) within 130 days of screening; undergone sinus surgery (including polypectomy) within 6 months before screening or sinus surgery that altered nasal lateral wall structure as to make NPS evaluation impossible; and forced expiratory volume (FEV1) ≤50% of predicted normal values.
In this post hoc analysis, the following subgroups were analyzed: patients who had no sinus surgery, ≥1, 1, 2, or ≥3 prior surgeries, and, among patients with prior surgery, <3, ≥3 to < 5, ≥5 to <10, and ≥10 years since their most recent surgery. Surgeries were categorized as either polypectomy or functional endoscopic sinus surgery (FESS). The type and extent of surgery were not accounted for in subgroup analyses.
2.3. Outcome measures
The effect of dupilumab was evaluated by subgroup by change in outcome scores, NPS, NC score, Lund‐MacKay (LMK) score, loss of smell score, University of Pennsylvania Smell Identification Test (UPSIT) score, 22‐item Sino‐Nasal Outcome Test (SNOT‐22) score from baseline to week 24, and the probability of patients requiring SCS or sinus surgery during the treatment period up to week 24. The procedures for assessment of outcome measures have been described.17 NPS and LMK scores were graded centrally and independently by masked review of endoscopy videos (NPS) and sinus CT images (LMK). Individual response thresholds were defined by NPS improvement from baseline ≥1 and ≥2, NC score improvement from baseline ≥1 (prespecified threshold levels in both studies), and SNOT‐22 improvement from baseline ≥8.9 (minimal clinically important difference; prespecified secondary efficacy endpoint).27 The European Quality of Life‐5D scale (0−100) was self‐reported by patients. Biomarkers of type 2 inflammation were assessed from blood samples taken at study baseline; eosinophil levels were assessed by cell count per mm3, and IgE, thymus and activation‐regulated chemokine (TARC), and periostin levels were determined by immunoassay.
Safety was assessed by pooling adverse event data up to week 24 for patients treated with placebo or dupilumab, by either no prior surgery or ≥1 prior surgery subgroups.
2.4. Statistical analyses
This pooled analysis of the SINUS‐24 and SINUS‐52 studies compared patients randomized to placebo with those randomized to dupilumab 300 mg q2w, to week 24.
Baseline characteristics were analyzed for differences between at least 2 subgroups and vs the no surgery subgroup (for patients by prior surgery) and vs the <3 years since last surgery subgroup (for patients by time since last surgery) by a chi‐square test for qualitative parameters or Kruskal‐Wallis test for quantitative parameters. As these post hoc tests were for exploratory purposes only, no adjustment on multiplicity was performed and nominal p values were provided. Variance was shown by standard deviation (SD).
The changes from baseline in outcome scores were analyzed separately in each subgroup with a hybrid of the worst observation carried forward (WOCF) and multiple imputation methods, followed by an analysis of covariance (ANCOVA) model with the baseline value of the corresponding endpoint, treatment, asthma or NSAID‐ERD status, region (pooled countries), and the study as covariates. For patients who received SCS or who underwent sinus surgery for any reason, data collected postsurgery or post‐SCS treatment were set to missing, and the worst postbaseline value on or before the time of surgery or SCS treatment was used to impute the week 24 values. For patients who discontinued treatment without rescue by surgery or SCS, a multiple imputation approach was used to impute missing values, using all patients who had not been rescued by surgery or were not receiving SCS. Statistical inference obtained from all imputed data was combined using Rubin's rule. Least squares (LS) mean differences vs placebo along with 95% confidence intervals (CIs) were then calculated in each subgroup.
To compare the effect between subgroups a similar ANCOVA model was carried out, with the addition of the subgroup covariate and the subgroup‐by‐treatment interaction. The interaction p value was calculated from this model.
The proportion of patients achieving response thresholds for NPS, NC, and SNOT‐22 was compared between dupilumab and placebo in each subgroup using a logistic regression, with treatment group, asthma/NSAID‐ERD status, prior surgery history, regions, and the study as covariates. Risk differences with 95% CIs were computed. Patients who were indicated for surgery for NP or received SCS for any reason were considered as nonresponders for time points after using SCS or surgery. Patients missing data at the visit of interest were considered as nonresponders. To compare the effect between subgroups, a similar model was carried out with the addition of the subgroup and the subgroup‐by‐treatment interaction as covariates, and the interaction p values derived.
The Cox proportional hazards model and the Kaplan‐Meier method were used to estimate the probability that a patient in each treatment group would require SCS or NP surgery (actual or planned) up to week 24, separately in each subgroup. The Cox model used the event as the dependent variable, and the treatment group, asthma or NSAID‐ERD status, the region (pooled country) and the study indicator (EudraCT Number EFC14280 = 0 and EFC14146 = 1) as covariates. Hazard ratios (HRs) and corresponding 95% CI and p values were estimated for dupilumab vs placebo. The Kaplan‐Meier method was used to derive the probability that a patient would have an event at week 24, with point probabilities and corresponding 95% CIs calculated. The HR was derived in each subgroup from a Cox proportional hazard model with the event of first SCS use and/or surgery as the response variable, and treatment, asthma/NSAID‐ERD status, region (pooled country), and study as covariates. In order to compare subgroups, a similar Cox model was performed, with the addition of the subgroup covariate and the subgroup‐by‐treatment interaction; the interaction p value was derived from this model. Variance was shown by 95% CI.
For all interaction p value calculations, the interaction p values compare the dupilumab vs placebo effect between subgroups, using the no prior surgery subgroup and the <3 years subgroup as references for their respective comparisons.
The incidence of treatment‐emergent adverse events (TEAEs) up to week 24 in patients with or without prior surgery was analyzed descriptively.
Across all analyses significance was attributed as p < 0.05. As all predictive analyses were post hoc, all p values should be considered nominal. Analyses were performed using SAS version 9.4 (SAS Institute, Inc., Cary, NC).
3. RESULTS
3.1. Patients and baseline characteristics
Overall, 724 patients randomized in the SINUS‐24 and SINUS‐52 studies were assessed and, of these, 459 (63.4%) had undergone ≥1 prior sinus surgery. The number of prior sinus surgery and time since last sinus surgery subgroups were balanced between the placebo and treatment groups (Table 1). The mean ± SD number of prior surgeries was 1.96 ± 1.56; the median time since last surgery was 5.3 years.
TABLE 1.
Sinus surgery history of patients with CRSwNP from SINUS‐24+SINUS‐52*
| Patients |
Placebo (n = 286) |
Dupilumab 300 mg q2w (n = 438) |
All patients (n = 724) |
|---|---|---|---|
| No prior surgeries, n (%) | 99 (34.6) | 166 (37.9) | 265 (36.6) |
| Number of prior surgeries, n (%) | |||
| ≥1 prior surgery | 187 (65.4) | 272 (62.1) | 459 (63.4) |
| 1 | 101 (54.0) | 153 (56.3) | 254 (55.3) |
| 2 | 39 (20.9) | 55 (20.2) | 94 (20.5) |
| ≥3 | 47 (25.1) | 64 (23.5) | 111 (24.2) |
| Number of prior surgeries, mean ± SD | 1.96 ± 1.45 | 1.96 ± 1.64 | 1.96 ± 1.56 |
| Time since last surgery, n (%)a | |||
| <3 years | 55 (29.4) | 81 (29.8) | 136 (29.6) |
| ≥3 and <5 years | 34 (18.2) | 47 (17.3) | 81 (17.6) |
| ≥5 and <10 years | 53 (28.3) | 80 (29.4) | 133 (29.0) |
| ≥10 years | 45 (24.1) | 63 (23.2) | 108 (23.5) |
| Time since last surgery (years), median (Q1:Q3) | 5.24 (2.62:9.83) | 5.33 (2.53:9.62) | 5.31 (2.56:9.62) |
*Percentages for patients with 1, 2, or ≥3 prior surgeries and for time since last surgery subgroups were calculated using the number of patients with ≥1 surgery as the denominator.
Data for time since last surgery were missing for 1 patient.
CRSwNP = chronic rhinosinusitis with nasal polyps; Q1 = first quartile; Q3 = third quartile; q2w = every 2 weeks; SD = standard deviation.
Baseline characteristics for patients by number of sinus surgeries and by time since last sinus surgery are outlined in Tables 2 and 3, respectively. Pairwise comparisons between subgroups by number of prior surgeries vs the no surgery subgroup showed that patients with a greater number of surgeries had a longer duration since diagnosis of CRSwNP, a younger age at onset of CRSwNP, a greater prevalence of asthma/NSAID‐ERD, and a shorter time duration since revision (Supplementary Fig. 1). Baseline sinus disease (LMK score) and olfactory dysfunction (loss of smell and UPSIT scores) were significantly worse with increased number of prior surgeries (Supplementary Fig. 1).
TABLE 2.
Baseline characteristics for patients by number of prior sinus surgeries
| Characteristic |
No surgery (n = 265) |
≥1 surgery (n = 459) |
1 surgery (n = 254) |
2 surgeries (n = 94) |
≥3 surgeries (n = 111) |
Overall p a |
|---|---|---|---|---|---|---|
| Age (years), mean ± SD | 51.4 ± 13.2 | 51.4 ± 12.6 | 52.3 ± 12.6 | 49.5 ± 13.2 | 50.9 ± 11.9 | 0.32 |
| Male, n (%) | 169 (63.8) | 268 (58.4) | 148 (58.3) | 50 (53.2) | 70 (63.1) | 0.25 |
| BMI (kg/m2), mean ± SD | 28.50 ± 5.73 | 27.60 ± 5.30 | 26.95 ± 4.87 | 27.87 ± 5.38 | 28.87 ± 5.93 | 0.004 |
| Time since first diagnosis of NP (years), mean ± SD | 5.53 ± 6.02 | 14.20 ± 9.62 | 12.29 ± 9.48 | 14.60 ± 8.73 | 18.37 ± 9.42 | <0.001 |
| Age at onset of nasal polyposis (years), mean ± SD | 45.98 ± 13.01 | 37.23 ± 13.18 | 40.02 ± 13.24 | 35.00 ± 12.19 | 32.60 ± 12.27 | <0.001 |
| Asthma/NSAID‐ERD from medical history, n (%) | 129 (48.7) | 322 (70.2) | 171 (67.3) | 66 (70.2) | 85 (76.6) | <0.001 |
| SCS use within 2 years, n (%) | 246 (92.8) | 292 (63.6) | 160 (63.0) | 57 (60.6) | 75 (67.6) | <0.001 |
| Time since last surgery (years), mean ± SD | — | 7.16 ± 6.44 | 8.12 ± 7.40 | 6.50 ± 4.80 | 5.50 ± 4.69 | 0.004 |
| Time since last surgery category, n (%)b | ||||||
| <3 years | — | 136 (29.7) | 70 (27.7) | 24 (25.5) | 42 (37.8) | |
| ≥3 and < 5 years | — | 81 (17.7) | 41 (16.2) | 19 (20.2) | 21 (18.9) | |
| ≥5 and < 10 years | — | 133 (29.0) | 70 (27.7) | 28 (29.8) | 35 (31.5) | |
| ≥10 years | — | 108 (23.6) | 72 (28.5) | 23 (24.5) | 13 (11.7) | |
| Assessment scores, mean ± SD | ||||||
| NPS (0–8) | 6.15 ± 1.20 | 5.86 ± 1.26 | 5.96 ± 1.20 | 5.62 ± 1.31 | 5.83 ± 1.35 | 0.005 |
| NC score, 0–3 | 2.33 ± 0.60 | 2.44 ± 0.56 | 2.44 ± 0.55 | 2.33 ± 0.60 | 2.54 ± 0.54 | 0.015 |
| LMK score, 0–24 | 17.15 ± 3.92 | 19.07 ± 3.97 | 18.57 ± 4.23 | 19.34 ± 3.50 | 19.95 ± 3.59 | <0.001 |
| UPSIT score, 0–40 | 16.02 ± 8.93 | 12.80 ± 7.52 | 12.87 ± 7.82 | 12.97 ± 7.89 | 12.49 ± 6.48 | <0.001 |
| Loss of smell score (0–3) | 2.65 ± 0.54 | 2.79 ± 0.52 | 2.76 ± 0.56 | 2.81 ± 0.43 | 2.83 ± 0.50 | <0.001 |
| SNOT‐22 total score (0–110) | 49.72 ± 21.44 | 51.63 ± 20.19 | 51.49 ± 20.60 | 51.97 ± 20.77 | 51.68 ± 18.89 | 0.69 |
| VAS for EQ‐5D (0–100) | 65.56 ± 22.15 | 65.37 ± 19.34 | 65.74 ± 18.47 | 64.03 ± 20.73 | 65.61 ± 20.23 | 0.82 |
| Biomarkers, mean ± SD | ||||||
| Blood eosinophils (1 × 109/L) | 0.37 ± 0.31 | 0.47 ± 0.36 | 0.47 ± 0.37 | 0.43 ± 0.32 | 0.50 ± 0.36 | <0.001 |
| Total IgE (IU/mL) | 241.22 ± 308.35 | 222.31 ± 323.75 | 239.02 ± 350.90 | 187.99 ± 297.47 | 213.07 ± 276.39 | 0.05 |
| Periostin (ng/mL) | 104.96 ± 40.67 | 116.65 ± 52.43 | 118.04 ± 51.15 | 109.83 ± 45.61 | 119.21 ± 60.16 | 0.04 |
| TARC (pg/mL) | 351.19 ± 243.42 | 361.32 ± 256.44 | 362.89 ± 254.80 | 345.58 ± 195.63 | 371.22 ± 303.76 | 0.78 |
aChi‐square test for qualitative parameters, Kruskal‐Wallis test for quantitative parameters; to compare if there was a difference between at least 1 subgroups among patients with no surgery (except for time since last surgery), 1, 2, or ≥3 surgeries.
bData for time since last surgery were missing for 1 patient.
BMI = body mass index; EQ‐5D = European Quality of Life‐5 Dimensions; IgE = immunoglobulin E; LMK = Lund‐Mackay; NC = nasal congestion; NPS = nasal polyp score; NSAID‐ERD = nonsteroidal anti‐inflammatory drug‐exacerbated respiratory tract disease; SCS = systemic corticosteroid; SD = standard deviation; SNOT‐22 = 22‐item Sino‐Nasal Outcome Test; TARC = thymus and activation‐regulated chemokine; UPSIT = University of Pennsylvania Smell Identification Test; VAS = visual analogue scale.
TABLE 3.
Baseline characteristics for patients with prior sinus surgery by time since last surgery
| Characteristic |
<3 years (n = 136) |
≥3 and <5 years (n = 81) |
≥5 and <10 years (n = 133) |
≥10 years (n = 108) |
Overall p a |
|---|---|---|---|---|---|
| Age (years) | 46.35 (12.51) | 50.38 (12.49) | 53.21 (12.13) | 56.19 (11.17) | <0.001 |
| Male, n % | 87 (64.0) | 48 (59.3) | 74 (55.6) | 59 (54.6) | 0.42 |
| BMI (kg/m2) | 27.64 (5.92) | 26.81 (4.77) | 28.04 (4.61) | 27.68 (5.65) | 0.41 |
| Time since first diagnosis of NP (years) | 8.56 (7.11) | 11.66 (8.11) | 15.32 (7.99) | 22.01 (9.88) | <0.001 |
| Age at onset of NP (years) | 37.82 (13.17) | 38.78 (13.93) | 38.08 (13.13) | 34.21 (12.40) | 0.10 |
| Asthma/NSAID‐ERD from medical history, n (%) | 87 (64.0) | 64 (79.0) | 101 (75.9) | 69 (63.9) | 0.02 |
| SCS use within 2 years, n (%) | 89 (65.4) | 51 (63.0) | 79 (59.4) | 72 (66.7) | 0.64 |
| Type of last surgery, n (%) | |||||
| FESS | 72 (52.9) | 47 (58.0) | 75 (56.4) | 54 (50.0) | |
| Polypectomy | 64 (47.1) | 34 (42.0) | 58 (43.6) | 54 (50.0) | |
| Number of prior sinus surgeries category, n (%) | 0.03 | ||||
| 1 surgery | 70 (51.5) | 41 (50.6) | 70 (52.6) | 72 (66.7) | |
| 2 surgeries | 24 (17.6) | 19 (23.5) | 28 (21.1) | 23 (21.3) | |
| ≥3 surgeries | 42 (30.9) | 21 (25.9) | 35 (26.3) | 13 (12.0) | |
| Assessment scores, mean (SD) | |||||
| NPS (0−8) | 5.41 (1.17) | 5.75 (1.18) | 6.05 (1.27) | 6.27 (1.27) | <0.001 |
| NC score (0−3) | 2.41 (0.53) | 2.37 (0.65) | 2.50 (0.55) | 2.46 (0.56) | 0.59 |
| LMK score (0−24) | 19.96 (3.78) | 19.75 (4.02) | 19.05 (3.76) | 17.38 (3.96) | <0.001 |
| UPSIT score (0−40) | 13.50 (7.70) | 12.20 (7.13) | 12.01 (7.14) | 13.34 (8.00) | 0.48 |
| Loss of smell score (0−3) | 2.80 (0.46) | 2.80 (0.44) | 2.84 (0.44) | 2.69 (0.71) | 0.36 |
| SNOT‐22 total score (0−110) | 51.56 (20.05) | 52.09 (21.47) | 52.62 (19.21) | 49.82 (20.56) | 0.80 |
| VAS for EQ‐5D (0−100) | 65.64 (19.84) | 69.45 (17.40) | 62.60 (20.41) | 65.61 (18.35) | 0.15 |
| Biomarkers, mean (SD) | |||||
| Blood eosinophils (1 × 109/L) | 0.45 (0.30) | 0.56 (0.47) | 0.45 (0.31) | 0.45 (0.39) | 0.31 |
| Total IgE (IU/mL) | 225.88 (271.55) | 241.24 (327.49) | 219.84 (342.49) | 202.69 (358.01) | 0.37 |
| Periostin (ng/mL) | 125.89 (60.60) | 127.23 (53.83) | 107.45 (44.52) | 107.89 (45.95) | 0.002 |
| TARC (pg/mL) | 373.04 (264.40) | 389.53 (277.67) | 308.49 (157.15) | 389.50 (314.23) | 0.17 |
Chi‐square test for qualitative parameters, Kruskal‐Wallis test for quantitative parameters; to compare if there was a difference between at least 2 subgroups among patients with < 3, ≥3 to <5, ≥5 to <10, and ≥10 years since last sinus surgery.
BMI = body mass index; EQ‐5D = European Quality of Life‐five Dimensions; FESS = functional endoscopic sinus surgery; IgE = immunoglobulin E; LMK = Lund‐Mackay; NC = nasal congestion; NP = nasal polyposis; NPS = nasal polyp score; NSAID‐ERD = nonsteroidal anti‐inflammatory drug–exacerbated respiratory tract disease; SCS = systemic corticosteroid; SD = standard deviation; SNOT‐22 = 22‐item Sino‐Nasal Outcome Test; TARC = thymus and activation‐regulated chemokine; UPSIT = University of Pennsylvania Smell Identification Test; VAS = visual analogue scale.
Pairwise comparisons between subgroups by time since last surgery vs the <3 years since last surgery subgroup showed that patients with a more recent surgery were generally younger, with a shorter time since last surgery and a lower NPS and higher LMK score than those with a longer time since last surgery (Supplementary Fig. 2).
The overall surgery types undergone by patients were similar across subgroups by time since last surgery (Table 3). Details for type of prior surgery by number of prior surgeries and most recent surgery type by time since last surgery are presented in Supplementary Tables 1 and 2, respectively.
3.2. Dupilumab demonstrated efficacy irrespective of the number or recency of prior sinus surgeries
The superiority of dupilumab vs placebo in the overall intent‐to‐treat (ITT) population for the outcome measures presented here has been reported.17 Subgroup analyses for the efficacy of dupilumab vs placebo by number of prior sinus surgeries, and time since last sinus surgery were consistent with the ITT results, with the LS mean difference in change from baseline favoring dupilumab for all outcome measures regardless of the number of prior sinus surgeries or the time since last surgery (Fig. 1).
FIGURE 1.
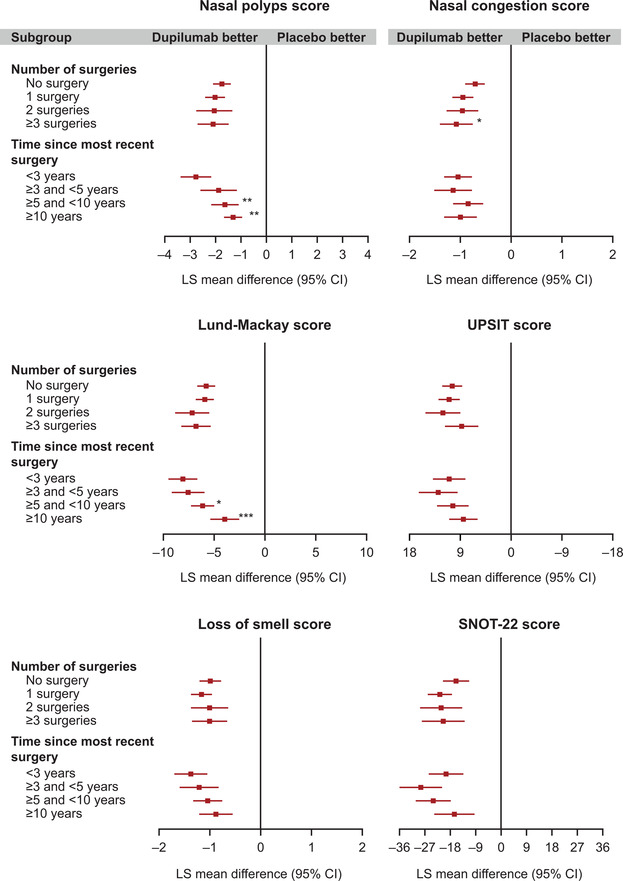
Dupilumab efficacy outcomes at week 24 by number of prior sinus surgeries, and by time since last sinus surgery. All panels show the LS mean difference in change from baseline, comparing the dupilumab vs placebo. *Subgroup‐by‐treatment interaction p < 0.05; ** p < 0.01; *** p < 0.001. LS mean: imputed complete data were analyzed by fitting an ANCOVA model with the corresponding baseline value, treatment group, asthma/NSAID‐ERD status, regions, and the study as covariates. Analysis was based on the same imputed dataset using WOCF/MI from primary analysis of the co‐primary endpoints. Interaction p value computed by fitting an ANCOVA model with the corresponding baseline value, treatment group, asthma/NSAID‐ERD status, and regions as covariates, plus the subgroup variable and the subgroup‐by‐treatment interaction and the study. The “no surgery” and <3 years subgroups were considered as references for the calculation of the interaction p values for the number of surgery and the time since most recent surgery subgroup analyses, respectively. ANCOVA = analysis of covariance; CI = confidence interval; LS mean = least squares mean; NSAID‐ERD = nonsteroidal anti‐inflammatory drug‐exacerbated respiratory tract disease; SNOT‐22 = 22‐item Sino‐Nasal Outcome Test; SE = standard error; UPSIT = University of Pennsylvania Smell Identification Test; WOCF/MI = worst observation carried forward/multiple imputation.
Analysis by number of prior sinus surgeries showed similar improvements with dupilumab vs placebo for patients with 1, 2, or ≥3 prior sinus surgeries across most endpoints (Fig. 1). For NC score, dupilumab was associated with a greater improvement vs placebo in patients with ≥3 prior sinus surgeries than in patients with no prior surgery (p < 0.05).
The magnitude of treatment effect of dupilumab vs placebo was generally greater in patients with more recent surgery, with a more marked improvement observed in patients who had surgery within 3 years compared with those with a longer duration since last surgery (Fig. 1). Significantly greater improvements with dupilumab vs placebo were observed in NPS and LMK score in the <3 years subgroup compared with the ≥5 to <10 and ≥10 years subgroups (NPS: LS mean differences [95% CI] vs placebo in <3 years subgroup, −2.76 [95% CI, −3.37 to −2.15]; ≥5 to <10 years subgroup, −1.66 [95% CI, −2.20 to −1.12], interaction p = 0.007 vs <3 years subgroup; ≥10 years subgroup, −1.31 [95% CI, −1.83 to −0.80], interaction p = 0.001 vs <3 years subgroup. LMK: <3 years subgroup, −8.06 [95% CI, −9.49 to −6.63]; ≥5 to <10 years subgroup, −6.08 [95% CI, −7.23 to −4.93], interaction p = 0.04 vs <3 years subgroup; ≥10 years subgroup, −3.94 [95% CI, −5.35 to −2.53], interaction p < 0.001 vs <3 years subgroup).
Analysis of efficacy by patients achieving response thresholds by number of prior sinus surgeries and time since last sinus surgery, as defined by NPS improvement ≥1 (prespecified threshold levels in the studies), NC score improvement ≥1, or SNOT‐22 improvement ≥8.9 (minimal clinically important difference; all prespecified threshold levels in the studies), demonstrated significantly greater risk difference (RD) with dupilumab vs placebo in all subgroups, with the exception of SNOT‐22 improvement ≥8.9 in patients with ≥10 years since last surgery (Fig. 2). RDs favoring dupilumab were observed across subgroups for the more stringent threshold of NPS improvement ≥2, albeit with wider 95% CI than with the lower thresholds of improvement due to the very low number of responders in the placebo group (Supplementary Fig. 3, Supplementary Table 3). Patients with <3 years since last sinus surgery were more likely to achieve NPS improvement ≥1 with dupilumab vs placebo compared with patients with ≥10 years since last sinus surgery (interaction p = 0.01; Fig. 2).
FIGURE 2.
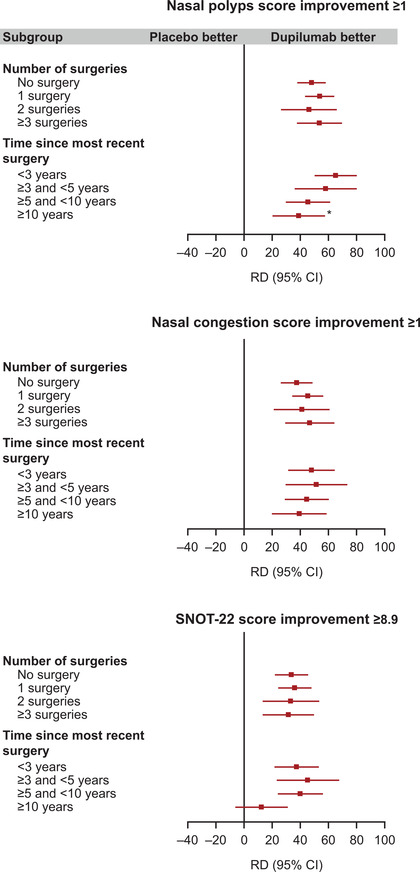
Differences in the proportion of patients achieving treatment response thresholds at week 24 by number of prior sinus surgeries, and by time since last sinus surgery. All panels show risk differences for response outcomes, comparing the dupilumab vs placebo groups. *Treatment‐by‐subgroup heterogeneity p < 0.05. RD derived in each subgroup using logistic regression with treatment group, asthma/NSAID‐ERD status, prior surgery history, regions, and the study; interaction p values derived using the same model, with the additional inclusion of the subgroup, and the subgroup‐by‐treatment interaction as covariates. The “no surgery” and <3 years subgroups were considered as references for the calculation of the heterogeneity p values for the number of surgery and the time since most recent surgery subgroup analyses, respectively. CI = confidence interval; NSAID‐ERD = nonsteroidal anti‐inflammatory drug‐exacerbated respiratory tract disease; RD = risk difference; SNOT‐22 = 22‐item Sino‐Nasal Outcome Test.
3.3. SCS use and/or sinus surgery rate
In the overall ITT population, dupilumab significantly reduced SCS use and/or rate of sinus surgery vs placebo during the treatment period (8.3% and 26.5% at week 24 for dupilumab vs placebo, respectively).17 In the post hoc analyses presented here, dupilumab consistently reduced rescue treatment with SCS and/or rate of sinus surgery vs placebo regardless of the number of prior sinus surgeries, with the difference being statistically significant in all but the smallest group (2 prior surgeries; Table 4). When assessed by time since last surgery, the differences favoring dupilumab were statistically significant in all subgroups except the ≥10 years group (Table 4).
TABLE 4.
Patients requiring SCS or sinus surgery up to week 24*
| Number of prior surgeries | |||||
|---|---|---|---|---|---|
| Parameter | No surgery | ≥1 | 1 | 2 | ≥3 |
| Patients with SCS/surgery, n/N (%) | |||||
| Placebo | 24/99 (24.2) | 51/187 (27.3) | 29/101 (28.7) | 9/39 (23.1) | 13/47 (27.7) |
| Dupilumab | 16/166 (9.6) | 20/272 (7.4) | 10/153 (6.5) | 5/55 (9.1) | 5/64 (7.8) |
| Probability of SCS use/surgery, (95% CI)a | |||||
| Placebo | 0.244 (0.165–0.332) | 0.276 (0.214–0.342) | 0.290 (0.205–0.381) | 0.232 (0.115–0.373) | 0.283 (0.162–0.417) |
| Dupilumab | 0.097 (0.058–0.148) | 0.074 (0.047–0.110) | 0.066 (0.034–0.113) | 0.092 (0.034–0.187) | 0.079 (0.029–0.162) |
| Hazard ratio (95% CI) | 0.338 (0.178–0.640) | 0.220 (0.130–0.372) | 0.187 (0.090–0.387) | 0.325 (0.099–1.060) | 0.246 (0.086–0.698) |
| Interaction p value vs no surgery | 0.29 | 0.22 | 0.89 | 0.53 | |
| Time since most recent surgery (years) | ||||
|---|---|---|---|---|
| Parameter | <3 | ≥3 and <5 | ≥5 and <10 | ≥10 |
| Patients with SCS/surgery, n/N (%) | ||||
| Placebo | 17/55 (30.9) | 10/34 (29.4) | 16/53 (30.2) | 8/45 (17.8) |
| Dupilumab | 7/81 (8.6) | 2/47 (4.3) | 4/80 (5.0) | 7/63 (11.1) |
| Probability of SCS use/surgery (95% CI)a | ||||
| Placebo | 0.315 (0.197–0.440) | 0.295 (0.154–0.451) | 0.302 (0.185–0.427) | 0.182 (0.085–0.308) |
| Dupilumab | 0.087 (0.038–0.160) | 0.047 (0.008–0.140) | 0.050 (0.016–0.113) | 0.111 (0.049–0.203) |
| Hazard ratio (95% CI) | 0.178 (0.069–0.463) | 0.078 (0.016–0.383) | 0.124 (0.041–0.380) | 0.590 (0.212–1.641) |
| Interaction p value vs < 3 years | 0.32 | 0.46 | 0.15 | |
*Hazard ratios derived from Cox proportional hazard model in each subgroup, with the event of first SCS use and/or surgery up to week 24 as the response variable, and treatment, asthma/NSAID‐ERD status, region (pooled country), and study indicator (EFC14280 = 0 and EFC14146 = 1) as covariates. Interaction p value, derived from Cox proportional hazard model with the event of first SCS use and/or surgery as the response variable, and treatment, asthma/NSAID‐ERD status, region (pooled country), study indicator (EFC14280 = 0 and EFC14146 = 1), and the subgroup and the subgroup‐by‐treatment interaction as covariates.
Kaplan‐Meier estimates.
CI = confidence interval; NSAID‐ERD = nonsteroidal anti‐inflammatory drug–exacerbated respiratory tract disease; SCS = systemic corticosteroids.
3.4. Safety
Dupilumab was generally well tolerated in patients with CRSwNP, with rates of TEAEs up to week 24 numerically lower in the dupilumab vs placebo groups for patients either with or without prior sinus surgery. The most common TEAEs were nasopharyngitis, NP, headache, and injection‐site erythema (Table 5). Overall, 26 patients (3.6%) experienced TEAEs leading to permanent treatment discontinuation: 15 patients in the placebo groups and 11 in the dupilumab groups. There were no deaths. Details of TEAEs have been described.17
TABLE 5.
Overview of treatment‐emergent adverse events: pooled analysis of SINUS‐24/SINUS‐52 up to week 24*
| No surgery | ≥1 prior surgery | |||
|---|---|---|---|---|
| Parameter |
Placebo (n = 97) |
Dupilumab 300 mg q2w (n = 167) |
Placebo (n = 185) |
Dupilumab 300 mg q2w (n = 273) |
| TEAEs, n (%) | ||||
| Any TEAE | 68 (70.1) | 110 (65.9) | 140 (75.7) | 195 (71.4) |
| Any serious TEAE | 3 (3.1) | 6 (3.6) | 13 (7.0) | 9 (3.3) |
| Any TEAE leading to death | 0 | 0 | 0 | 0 |
| Any TEAE leading to permanent treatment discontinuation | 5 (5.2) | 2 (1.2) | 10 (5.4) | 9 (3.3) |
| TEAEs occurring in ≥5% of patients in both subgroups, n (%)a | ||||
| Nasopharyngitis | 13 (13.4) | 20 (12.0) | 28 (15.1) | 35 (12.8) |
| Nasal polyps | 14 (14.4) | 5 (3.0) | 19 (10.3) | 7 (2.6) |
| Injection‐site erythema | 4 (4.1) | 10 (6.0) | 18 (9.7) | 18 (6.6) |
| Headache | 9 (9.3) | 9 (5.4) | 15 (8.1) | 23 (8.4) |
| Asthma | 6 (6.2) | 3 (1.8) | 14 (7.6) | 4 (1.5) |
| Epistaxis | 7 (7.2) | 10 (6.0) | 13 (7.0) | 15 (5.5) |
*Data are presented for the safety population (all patients who received ≥1 dose of a study drug).
According to MedDRA 21.0; nasal polyps refers to a worsening of nasal polyps, leading to surgery or systemic glucocorticoid use, and asthma refers to a worsening of asthma.
q2w = every 2 weeks; MedDRA = Medical Dictionary of Regulatory Activities; TEAE = treatment‐emergent adverse event.
4. DISCUSSION
The patients who participated in the SINUS‐24 and SINUS‐52 studies were representative of adults with severe CRSwNP, as demonstrated by their baseline characteristics of severe symptoms, extensive bilateral disease, severely impaired sense of smell, and impaired health‐related quality of life. These patients had inadequately controlled disease despite receiving standard of care therapy, including INCS, SCS, and/or sinus surgery. Almost two‐thirds of the patients evaluated had undergone 1 or more sinus surgery, with almost one‐quarter having undergone 3 or more surgeries. The distribution of patients in the time since last surgery subgroups, from <3 to ≥10 years, was relatively even, with a median duration since last surgery of 5.3 years. Patients with a history of prior sinus surgery generally had more pronounced signs and symptoms of CRSwNP with radiologically more extensive disease and greater olfactory dysfunction observed at study baseline than patients without prior surgery. These patients also had a younger age of disease onset and an increased prevalence of NSAID‐ERD, suggesting a more severe disease burden.
Dupilumab showed a consistent and clinically meaningful improvement in sinus outcome measures, as assessed by objective outcome measures (NPS, LMK score), by patient‐reported symptoms (NC and UPSIT scores), and by the proportion of patients achieving response thresholds (NPS improvement ≥1 and ≥2, NC score improvement ≥1) in patients with uncontrolled CRSwNP, regardless of whether they were surgery‐naïve, or had undergone 1, 2, or ≥3 prior sinus surgeries. Health‐related quality of life, as measured by SNOT‐22 and the proportion of patients achieving SNOT‐22 improvement ≥8.9, also improved with dupilumab vs placebo, regardless of surgery history.
Dupilumab generally also showed efficacy in patients regardless of time since prior sinus surgery. However, an incremental improvement was observed in measures of objective signs of disease severity (NPS and LMK score, and NPS improvement ≥1 and ≥2) in patients with surgery <3 years prior to study entry when compared with patients with surgeries ≥5 and <10 years, and ≥10 years prior to study entry. Though no framework currently exists for the assessment of therapeutic benefits over time following surgery, these data suggest that the time since last sinus surgery could influence the magnitude of response to dupilumab for these objective measures of disease.
In a study evaluating the prevalence of NP recurrence following ESS, a history of previous sinus surgery was identified as a significant risk factor for the recurrence of NP postsurgery,12 supporting the data presented here in which the number of prior surgeries was higher for patients with a more recent surgery. This suggests there may be an inverse relationship between the time since last surgery and the recurrence of polyps. It is possible that this group of patients, who have both more recent surgery and a higher number of surgeries, have a high type 2 inflammatory burden, as reflected by the increased prevalence of the type 2 inflammatory comorbidities of asthma/NSAID‐ERD and greater baseline blood eosinophil counts and periostin levels (markers of type 2 inflammation) compared with those with no surgery.11, 28
Recurrence of polyps is common in patients with high type 2 inflammation,15, 16 which is also linked to tissue remodeling associated with recalcitrant disease.29, 30 As dupilumab suppresses type 2 inflammation, this may potentially account for the greater improvements in objective CRSwNP outcomes seen in the subgroup of patients with <3 years since last sinus surgery vs the subgroups with ≥5 and <10 years and ≥10 years since last surgery. It is also possible that other mechanisms beyond the scope of this study may contribute to these findings. For example, although there is no evidence that fibrin deposition increases in NP with prolonged duration, if this is the case it may reduce response in patients who have had polyps for longer periods.31 Dupilumab has also been shown to suppress levels of leukotriene E4 (LTE4), a biomarker of mast cell activation,32 a cell type suggested to play a key role in the development of NP.33
Results presented here show the use of dupilumab reduced the incidence of future surgery, and in patients for whom surgery had been performed but the outcome was suboptimal, dupilumab efficacy was similar to that seen in surgery‐naïve patients. Although this study did not evaluate the time at which polyps recurred following surgery, at the time of trial enrolment all patients were considered as candidates for an advanced therapy. The occurrence of early and multiple revision surgeries suggests there is a patient population who have derived a less durable benefit from current standard of care treatments, and who might therefore also be expected to fail to benefit from other treatment options. The results presented here show that patients with multiple surgeries can benefit from treatment with dupilumab, and are in fact no less likely to do so than patients with 1 or no prior surgeries. The potential association between response to dupilumab and time since last surgery may contribute evidence for decisions around personalization of treatment and may aid decision‐making regarding treatment cost‐effectiveness.
The risk analyses presented here show that treatment with dupilumab reduced the rate of SCS and/or surgery over the 24‐week treatment period. SCS use has been linked to numerous health consequences,34, 35, 36 while surgery, although considered relatively safe, has been associated with both minor and major risks (reported incidence of 1.1% to 20.8% and 0% to 1.5%, respectively) such as severe bleeding, infection, or cranial damage.37, 38 Although assessment of long‐term exposure to dupilumab is ongoing in patients with CRSwNP, dupilumab has been well tolerated long term (up to 3 years) in patients with asthma or atopic dermatitis.39, 40, 41, 42 Therefore, treatment with dupilumab has the potential to reduce the risks associated with SCS treatment and surgery, while also improving signs and symptoms of CRSwNP, suggesting dupilumab may be suitable as a long‐term treatment option for patients with severe CRSwNP.
Potential limitations of this analysis include its post hoc nature, with neither study designed to specifically evaluate the efficacy of dupilumab by sinus surgery history. Alternate stratification of the time since last surgery subgroups may lead to different statistical results, which could in turn affect the conclusions drawn from these data. The study inclusion criteria were selected for a population with severe disease and may therefore not be fully representative of the spectrum of disease severities observed in the real‐world population of patients with CRSwNP. In addition, the prescription of rescue treatment with SCS and/or surgery was per the treating investigator's discretion, which introduces variability in the use of these treatments given the differing preferences, experience, and judgment of individual physicians. The lack of uniformity and retrospective reporting from case notes of surgical procedures is a limitation for this analysis but also reflects the real‐world challenges faced by patients with CRSwNP, as there is no standardization of the type and quality of surgery that they might receive. The variety of surgical procedures experienced by patients (Supplementary Tables 1 and 2) highlights current practices and subsequently a therapeutic need, and thus a potential benefit of treatment with a biologic may be that patients receive a consistent treatment that can be efficacious regardless of prior surgery history. The pooled analyses presented here show data only to 24 weeks and, while the sustained efficacy and tolerability of dupilumab have been demonstrated to 52 weeks,17 longer‐term data are needed for a chronic condition such as CRSwNP to ensure that the benefits of dupilumab treatment can be sustained.
The safety profile of dupilumab has been shown. When analyzed by surgery history, treatment was generally well tolerated, with no meaningful differences in TEAE profile between patients with or without prior sinus surgery.
5. CONCLUSION
In summary, the results presented here suggest that dupilumab is an effective treatment for patients with severe CRSwNP regardless of their number of prior sinus surgeries, and reduced SCS use, and/or surgery. Dupilumab treatment was associated with greater improvements in objective outcomes of CRSwNP in patients with a shorter duration since last sinus surgery that in these symptomatic patients, may be associated with high type 2 inflammation burden.
Supporting information
SUPPLEMENTARY FIGURE 1. Baseline characteristics by number of prior sinus surgeries, with pairwise comparisons vs the no surgery subgroup.
SUPPLEMENTARY FIGURE 2. Baseline characteristics by time since last prior sinus surgery, with pairwise comparisons vs the <3 years since last surgery subgroup.
SUPPLEMENTARY FIGURE 3. Differences in the proportion of patients achieving NPS improvement ≥2 at week 24 by number of prior sinus surgeries, and by time since last sinus surgery.
SUPPLEMENTARY TABLE 1. Description of prior surgeries by number of prior sinus surgeries.
SUPPLEMENTARY TABLE 2. Description of most recent prior surgery by time since last sinus surgery.
SUPPLEMENTARY TABLE 3. Proportion of patients achieving treatment response thresholds at week 24 by number of prior sinus surgeries, and by time since last sinus surgery.
ACKNOWLEDGMENTS
Medical writing assistance was provided under the direction of the authors by Joseph Hodgson, PhD, (Adelphi Group) in accordance with Good Publication Practice (GPP3) guidelines, funded by Sanofi Genzyme and Regeneron Pharmaceuticals, Inc.
Hopkins C, Wagenmann M, Bachert C, et al. Efficacy of dupilumab in patients with a history of prior sinus surgery for chronic rhinosinusitis with nasal polyps. Int Forum Allergy Rhinol. 2021;11:1087–1101. 10.1002/alr.22780
Additional Supporting Information may be found in the online version of this article.
Funding sources for the study: Sanofi (Cambridge, MA) and Regeneron Pharmaceuticals, Inc. (Tarrytown, NY) (NCT02912468 [SINUS‐24] and NCT02898454 [SINUS‐52]).
Potential conflict of interest: C.H.: GSK, Optinose, Sanofi Genzyme, Smith and Nephew—advisory board member. M.W.: ALK‐Abelló, Allakos, AstraZeneca, GSK, HAL Allergy, Meda Pharmaceuticals, Novartis, Otonomy, Roche, Sanofi‐Aventis, Stallergenes Greer, Strekin, Teva—member of national and international scientific advisory boards (consulting); fees for lectures; grants for research projects. C.B.: ALK, AstraZeneca, GlaxoSmithKline, Mylan, Novartis, Sanofi, Stallergenes Greer—advisory board member and speakers’ fees. M.D.: AstraZeneca, GSK, Probionase Therapies, Sanofi—clinical trial funding; Regeneron Pharmaceuticals, Inc., Sanofi—advisory board member; Probionase Therapies—equity holder. J.K.H.: Sanofi—advisory board member. P.W.H.: Regeneron Pharmaceuticals, Inc., Sanofi—advisory board member. S.L.: Allakos, AstraZeneca, Knopp Biosciences, Sanofi—clinical trial funding; Novartis, Regeneron Pharmaceuticals, Inc., Sanofi—advisory board member. J.M., P.R., L.P.M, R.R.: Sanofi—employee, may hold stock and/or stock options in the company. A.R., N.A., Y.D., B.O.: Regeneron Pharmaceuticals, Inc.—employee and shareholder.
Public clinical trial registration: SINUS‐24 (https://clinicaltrials.gov/ct2/show/NCT02912468) A Randomized, 24‐Week Treatment, Double‐blind, Placebo‐controlled Efficacy and Safety Study of Dupilumab 300 mg Every Other Week, in Patients With Bilateral Nasal Polyposis on a Background Therapy With Intranasal Corticosteroids. SINUS‐52 (https://clinicaltrials.gov/ct2/show/NCT02898454) A Randomized, Double‐blind, 52‐week, Placebo Controlled Efficacy and Safety Study of Dupilumab, in Patients With Bilateral Nasal Polyposis on a Background Therapy With Intranasal Corticosteroids
REFERENCES
- 1.Khan A, Vandeplas G, Huynh TMT, et al. The global allergy and asthma European network (GALEN) rhinosinusitis cohort: a large European cross‐sectional study of chronic rhinosinusitis patients with and without nasal polyps. Rhinology. 2019;57:32‐42. [DOI] [PubMed] [Google Scholar]
- 2.Fokkens WJ, Lund VJ, Hopkins C, et al. European position paper on rhinosinusitis and nasal polyps 2020. Rhinology. 2020;58(S29):1‐464. [DOI] [PubMed] [Google Scholar]
- 3.Tomassen P, Vandeplas G, Van Zele T, et al. Inflammatory endotypes of chronic rhinosinusitis based on cluster analysis of biomarkers. J Allergy Clin Immunol. 2016;137:1449‐1456.e4. [DOI] [PubMed] [Google Scholar]
- 4.Bachert C, Marple B, Hosemann W, et al. Endotypes of chronic rhinosinusitis with nasal polyps: pathology and possible therapeutic implications. J Allergy Clin Immunol Pract. 2020;8:1514‐1519. [DOI] [PubMed] [Google Scholar]
- 5.Orlandi RR, Kingdom TT, Hwang PH, et al. International consensus statement on allergy and rhinology: rhinosinusitis. Int Forum Allergy Rhinol. 2016;6(Suppl 1):S22‐S209. [DOI] [PubMed] [Google Scholar]
- 6.DeConde AS, Mace JC, Alt JA, et al. Investigation of change in cardinal symptoms of chronic rhinosinusitis after surgical or ongoing medical management. Int Forum Allergy Rhinol. 2015;5: 36‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.ENT UK. Royal College of Surgeons . Commissioning guide: chronic rhinosinusitis. London, UK: ENT UK; 2016. https://www.entuk.org/sites/default/files/files/Rhinosinusitis%20commissioning%20guide%20%20for%20REPUBLICATION(1).pdf. Accessed February 6, 2021. [Google Scholar]
- 8.Jankowski R, Rumeau C, Nguyen DT, Gallet P. Updating nasalisation: from concept to technique and results. Eur Ann Otorhinolaryngol Head Neck Dis. 2018;135:327‐334. [DOI] [PubMed] [Google Scholar]
- 9.Alsharif S, Jonstam K, van Zele T, et al. Endoscopic sinus surgery for type‐2 CRSwNP: an endotype‐based retrospective study. Laryngoscope. 2019;129:1286‐1292. [DOI] [PubMed] [Google Scholar]
- 10.Sharma R, Lakhani R, Rimmer J, Hopkins C. Surgical interventions for chronic rhinosinusitis with nasal polyps. Cochrane Database Syst Rev. 2014;(11):CD006990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Calus L, Van Bruaene N, Bosteels C, et al. Twelve‐year follow‐up study after endoscopic sinus surgery in patients with chronic rhinosinusitis with nasal polyposis. Clin Transl Allergy. 2019;9:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DeConde AS, Mace JC, Levy JM, et al. Prevalence of polyp recurrence after endoscopic sinus surgery for chronic rhinosinusitis with nasal polyposis. Laryngoscope. 2017;127:550‐555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hopkins C, Slack R, Lund V, et al. Long‐term outcomes from the English national comparative audit of surgery for nasal polyposis and chronic rhinosinusitis. Laryngoscope. 2009;119:2459‐2465. [DOI] [PubMed] [Google Scholar]
- 14.Loftus CA, Soler ZM, Koochakzadeh S, et al. Revision surgery rates in chronic rhinosinusitis with nasal polyps: meta‐analysis of risk factors. Int Forum Allergy Rhinol. 2020;10:199‐207. [DOI] [PubMed] [Google Scholar]
- 15.Vlaminck S, Vauterin T, Hellings PW, et al. The importance of local eosinophilia in the surgical outcome of chronic rhinosinusitis: a 3‐year prospective observational study. Am J Rhinol Allergy. 2014;28:260‐264. [DOI] [PubMed] [Google Scholar]
- 16.Lou H, Zhang N, Bachert C, Zhang L. Highlights of eosinophilic chronic rhinosinusitis with nasal polyps in definition, prognosis, and advancement. Int Forum Allergy Rhinol. 2018;8:1218‐1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bachert C, Han JK, Desrosiers M, et al. Efficacy and safety of dupilumab in patients with severe chronic rhinosinusitis with nasal polyps (LIBERTY NP SINUS‐24 and LIBERTY NP SINUS‐52): results from two multicentre, randomised, double‐blind, placebo‐controlled, parallel‐group phase 3 trials. Lancet. 2019;394:1638‐1650. [DOI] [PubMed] [Google Scholar]
- 18.Tokunaga T, Sakashita M, Haruna T, et al. Novel scoring system and algorithm for classifying chronic rhinosinusitis: the JESREC Study. Allergy. 2015;70:995‐1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Macdonald LE, Karow M, Stevens S, et al. Precise and in situ genetic humanization of 6 Mb of mouse immunoglobulin genes. Proc Natl Acad Sci U S A. 2014;111:5147‐5152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murphy AJ, Macdonald LE, Stevens S, et al. Mice with megabase humanization of their immunoglobulin genes generate antibodies as efficiently as normal mice. Proc Natl Acad Sci U S A. 2014;111:5153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Le Floch A, Allinne J, Nagashima K, et al. Dual blockade of IL‐4 and IL‐13 with dupilumab, an IL‐4Rα antibody, is required to broadly inhibit type 2 inflammation. Allergy. 2020;75:1188‐1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matsunaga K, Katoh N, Fujieda S, et al. Dupilumab: basic aspects and applications to allergic diseases. Allergol Int. 2020;69:187‐196. [DOI] [PubMed] [Google Scholar]
- 23.Gandhi NA, Bennett BL, Graham NM, et al. Targeting key proximal drivers of type 2 inflammation in disease. Nat Rev Drug Discov. 2016;15:35‐50. [DOI] [PubMed] [Google Scholar]
- 24.U.S. Food and Drug Administration (FDA) . FDA approves first treatment for chronic rhinosinusitis with nasal polyps. DUPIXENT FDA approval. Silver Spring, MD: FDA; 2019. https://www.fda.gov/news-events/press-announcements/fda-approves-first-treatment-chronic-rhinosinusitis-nasal-polyps#:~:text=The%20U.S.%20Food%20and%20Drug,the%20sinuses%20and%20nasal%20cavity) Accessed February 6, 2021. [Google Scholar]
- 25.European Medicines Agency (EMA) . Dupixent. Dupilumab. Dupixent II‐17 ‐ SMOP. Amsterdam, The Netherlands; EMA; 2019. https://www.ema.europa.eu/en/documents/smop/chmp-post-authorisation-summary-positive-opinion-dupixent-ii-17_en.pdf. Accessed February 6, 2021. [Google Scholar]
- 26.DUPIXENT PMDA Approval. (2019). Accessed Month X, 2021. https://www.pmda.go.jp/files/000235289.pdf. Accessed August 8, 2020.
- 27.Hopkins C, Gillett S, Slack R, et al. Psychometric validity of the 22‐item Sinonasal Outcome Test. Clin Otolaryngol. 2009;34:447‐454. [DOI] [PubMed] [Google Scholar]
- 28.Mendelsohn D, Jeremic G, Wright ED, Rotenberg BW. Revision rates after endoscopic sinus surgery: a recurrence analysis. Ann Otol Rhinol Laryngol. 2011;120:162‐166. [DOI] [PubMed] [Google Scholar]
- 29.Kato A. Immunopathology of chronic rhinosinusitis. Allergol Int. 2015;64:121‐130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Do TQ, Barham HP, Earls P, et al. Clinical implications of mucosal remodeling from chronic rhinosinusitis. Int Forum Allergy Rhinol. 2016;6:835‐840. [DOI] [PubMed] [Google Scholar]
- 31.Takabayashi T, Kato A, Peters AT, et al. Excessive fibrin deposition in nasal polyps caused by fibrinolytic impairment through reduction of tissue plasminogen activator expression. Am J Respir Crit Care Med. 2013;187:49‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bachert C, Cho SH, Laidlaw TM, et al. Dupilumab reduces blood, urine, and nasal biomarkers of type 2 inflammation in patients with chronic rhinosinusitis with nasal polyps in the phase 3 SINUS‐52 trial. [Poster presentation at AAAI 2020; Philadelphia, PA, USA]. J Allergy Clin Immunol. 2020;145(Supplement):AB185. [Abstract]. 10.1016/j.jaci.2019.12.324. Accessed February 6, 2021. [DOI] [Google Scholar]
- 33.Kim DW, Kulka M, Jo A, et al. Cross‐talk between human mast cells and epithelial cells by IgE‐mediated periostin production in eosinophilic nasal polyps. J Allergy Clin Immunol. 2017;139:1692‐1695.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Waljee AK, Rogers MA, Lin P, et al. Short term use of oral corticosteroids and related harms among adults in the United States: population based cohort study. BMJ. 2017;357:j1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Van Zele T, Gevaert P, Holtappels G, et al. Oral steroids and doxycycline: two different approaches to treat nasal polyps. J Allergy Clin Immunol. 2010;125:1069‐1076.e4. [DOI] [PubMed] [Google Scholar]
- 36.Hox V, Lourijsen E, Jordens A, et al. Benefits and harm of systemic steroids for short‐ and long‐term use in rhinitis and rhinosinusitis: an EAACI position paper. Clin Transl Allergy. 2020;10:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dessouky O, Hopkins C. Surgical versus medical interventions in CRS and nasal polyps: comparative evidence between medical and surgical efficacy. Curr Allergy Asthma Rep. 2015;15:66. [DOI] [PubMed] [Google Scholar]
- 38.Hosemann W, Draf C. Danger points, complications and medico‐legal aspects in endoscopic sinus surgery. GMS Curr Top Otorhinolaryngol Head Neck Surg. 2013;12:Doc06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Deleuran M, Thaçi D, Beck LA, et al. Dupilumab shows long‐term safety and efficacy in patients with moderate to severe atopic dermatitis enrolled in a phase 3 open‐label extension study. J Am Acad Dermatol. 2020;82:377‐388. [DOI] [PubMed] [Google Scholar]
- 40.Beck LA, Thaçi D, Deleuran M, et al. Dupilumab provides favorable safety and sustained efficacy for up to 3 years in an open‐label study of adults with moderate‐to‐severe atopic dermatitis. Am J Clin Dermatol. 2020;21:567‐577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Castro M, Corren J, Pavord ID, et al. Dupilumab efficacy and safety in moderate‐to‐severe uncontrolled asthma. N Engl J Med. 2018;378:2486‐2496. [DOI] [PubMed] [Google Scholar]
- 42.Wechsler ME, Ford LB, Maspero JF, et al. Late Breaking Abstract ‐ Dupilumab long‐term safety and efficacy in patients with asthma: LIBERTY ASTHMA TRAVERSE. Eur Respir J. 2020:56(Suppl 64):4613. 10.1183/13993003.congress-2020.4613. Accessed February 6, 2021. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SUPPLEMENTARY FIGURE 1. Baseline characteristics by number of prior sinus surgeries, with pairwise comparisons vs the no surgery subgroup.
SUPPLEMENTARY FIGURE 2. Baseline characteristics by time since last prior sinus surgery, with pairwise comparisons vs the <3 years since last surgery subgroup.
SUPPLEMENTARY FIGURE 3. Differences in the proportion of patients achieving NPS improvement ≥2 at week 24 by number of prior sinus surgeries, and by time since last sinus surgery.
SUPPLEMENTARY TABLE 1. Description of prior surgeries by number of prior sinus surgeries.
SUPPLEMENTARY TABLE 2. Description of most recent prior surgery by time since last sinus surgery.
SUPPLEMENTARY TABLE 3. Proportion of patients achieving treatment response thresholds at week 24 by number of prior sinus surgeries, and by time since last sinus surgery.


