Abstract
Objective
Identifying factors that control food intake is crucial to the understanding and treatment of eating disorders characterized by binge eating. In healthy individuals, stomach distension plays an important role in the development of satiation, but gastric sensations might be overridden in binge eating. The present study investigated the perception of gastric signals (i.e., gastric interoception) and gastric motility in patients experiencing binge‐eating episodes, that is, bulimia nervosa (BN) and binge‐eating disorder (BED).
Method
Twenty‐nine patients with BN or BED (ED group) and 32 age‐, sex‐, and BMI‐matched healthy controls (HC group) participated in the study. The onset of satiation and stomach fullness were assessed using a novel 2‐step water load test (WLT‐II). Gastric myoelectrical activity (GMA) was measured by electrogastrography (EGG) before and after ingestion of noncaloric water.
Results
Individuals in the ED group drank significantly more water until reporting satiation during the WLT‐II. The percentage of normal gastric myoelectrical power was significantly smaller in the ED group compared to HC, and negatively related to the number of objective binge‐eating episodes per week in patients with BN or BED. Power in the bradygastria range was greater in ED than in HC participants.
Discussion
Patients with EDs have a delayed response to satiation compared to HC participants, together with abnormal GMA. Repeated binge‐eating episodes may induce disturbances to gastric motor function.
Keywords: binge‐eating disorder, bulimia nervosa, electrogastrography, gastric interoception, gastric myoelectrical activity
1. INTRODUCTION
Among the key characteristics of bulimia nervosa (BN) and binge‐eating disorder (BED) are recurrent binge‐eating episodes, defined by the consumption of large amounts of food in a short period of time, accompanied by a sense of loss of control over eating (American Psychiatric Association, 2013). These episodes have been termed objective binge‐eating episodes (OBE; Fairburn & Cooper, 1993). A better understanding of the factors that control food intake and meal termination are crucial to the etiological models of BN and BED and their treatment. The present study focuses on the role of gastric sensations and activity.
The literature suggests that interoception, that is, the processing of afferent signals from visceral organs, plays an important role in the pathogenesis of BN and BED, in particular with respect to deficits in the perception of hunger and satiety (Kissileff et al., 1996; Sysko, Devlin, Walsh, Zimmerli, & Kissileff, 2007). Nevertheless, the empirical evidence is mixed, and measures used to assess interoceptive processing are heterogeneous (Klabunde, Collado, & Bohon, 2017). While research on interoceptive processes in BED remains scarce, studies on participants with BN show either attenuated interoceptive abilities compared to healthy controls (Klabunde, Acheson, Boutelle, Matthews, & Kaye, 2013) or no differences (Eshkevari, Rieger, Musiat, & Treasure, 2014; Pollatos & Georgiou, 2016). These inconsistencies might be due to the fact that all of these studies used measures of cardiac interoception as a proxy for interoception in general, which do not directly assess perceptions of hunger and satiety cues.
To investigate interoceptive processes in an organ system more directly associated with hunger and satiety (i.e., the gastric system), we recently developed a water load test (WLT) as a provocative test of gastric distention (van Dyck et al., 2016). Gastric distention during intake activates vagal and spinal mechanosensitive afferents, which transmit signals from the stomach to the brain and lead to the perception of satiation and fullness (Hellström et al., 2004). Reduced sensitivity to these signals may result in excessive food intake (Wang et al., 2008). The new WLT‐II is a two‐step drink test that measures water intake until eating‐related satiation, in addition to the experience of maximum stomach fullness. While satiation is associated with a positive feeling of satisfaction in healthy persons (Benelam, 2009), eating disordered or obese individuals tend to override these signals (Blundell & Finlayson, 2004). In addition to drinking periods, the WLT‐II comprises questionnaire items assessing self‐reported sensations in response to ingestion and gastric distention. The WLT‐II, therefore, constitutes a multidimensional measure that assesses different facets of interoception. Initial results in a nonclinical sample suggest that the WLT‐II is a simple, reliable test that distinguishes well between sensations of comfortable satiation and unpleasant fullness (van Dyck et al., 2016). Importantly, water volume ingested until satiation was positively associated with scores on the bulimia subscale of the Eating Disorder Inventory, corroborating the hypothesis of an altered development of satiation in eating disorders with binge eating.
In addition to provoking gastric distention and interoceptive signals of fullness, loading the stomach with water has been shown to stimulate the neuromuscular activity of the stomach (Koch & Stern, 2004). Stomach motility not only regulates food processing rates, but is also involved in the development of satiation. In healthy individuals, the stomach normally contracts approximately three times every minute (3 cycles per minute; cpm). A noninvasive measure to evaluate gastric motor activity is the electrogastrogram (EGG), which captures the myoelectric activity of gastric smooth muscles through electrodes attached to the abdominal wall. The frequency of the EGG signal is identical to the frequency of the postprandial stomach contractions and to the frequency of the signal recorded from the serosal surface (Levine, 2005). Water loads have been shown to increase normal gastric myoelectrical activity (GMA; normogastria) in healthy individuals, whereas gastric dysrhythmias (bradygastria and tachygastria) have been repeatedly associated with upper gastrointestinal symptoms, such as nausea, epigastric distress, and bloating (Koch et al., 1990).
Gastrointestinal disturbances have also been reported in patients with BN or BED. Early satiety, bloating, nausea, and upper abdominal discomfort are common symptoms in eating disorders (Hadley & Walsh, 2003). Furthermore, patients with BN have larger gastric capacities (Geliebter et al., 1992; Geliebter & Hashim, 2001), and show reduced sensitivity to gastric distention (Zimmerli, Walsh, Guss, Devlin, & Kissileff, 2006), delayed gastric emptying (Devlin et al., 1997), and a diminished gastric relaxation reflex (Walsh, Zimmerli, Devlin, Guss, & Kissileff, 2003). Patients with BED also have greater gastric capacities (Geliebter, Yahav, Gluck, & Hashim, 2004), and lower postprandial ghrelin, but gastric emptying rate is similar to control participants (Geliebter et al., 2004; Geliebter, Gluck, & Hashim, 2005).
There are only few studies investigating GMA in eating disordered patients. Ogawa et al. (2004) recorded the EGG from 36 patients with eating disorders before and after water ingestion. The percentage of normal 3 cpm gastric activity was significantly smaller for patients than for healthy controls. Diamanti et al. (2003) found that adolescents with BN displayed abnormal gastric activity, whereas patients with anorexia nervosa did not. Altered GMA in BN was also reported by Koch, Bingaman, Tan, and Stern (1998). These results suggest a role for gastric motility alterations in the development and/or maintenance of binge eating.
To the best of our knowledge there are no studies investigating binge‐eating behaviors and gastric motor function in combination with other measures of gastric interoception. Recent research has, however, suggested that objective physiological states (i.e., activity of the gastrointestinal system) should be considered as an additional level of processing of internal bodily signals (Forkmann et al., 2016). Furthermore, we are not aware of any studies using the WLT‐II in a sample of eating disordered patients. The present study was designed to fill these gaps in the literature. It was hypothesized that patients with BN or BED show altered perceptions of satiation and stomach fullness, together with abnormal gastric electric activity, as compared to healthy control participants. In addition, to examine if gastric motor function and gastric interoception in eating disordered patients are related to the severity of the disorder, correlational analyses were performed between the mean number of objective binge‐eating episodes per week within the 3 months prior to the study (OBE frequency), and WLT indices and EGG variables before and after water ingestion, respectively. Additional exploratory analyses were performed to test for differences between patients with and without regular purging behaviors (i.e., BN and BED).
2. METHODS
2.1. Participants
Thirty‐two individuals meeting DSM‐5 criteria for current BN or BED (ED group) and 32 healthy control participants without any previous history of eating disorders (HC group) were recruited through announcements in local newspapers, flyers, and on university campus. Participants were required to be at least 18 years of age, free from psychotropic medication, and without history of gastrointestinal illnesses or surgeries. Additional exclusion criteria included bipolar disorder, current or past psychotic disorder, current suicidal ideation, and physical conditions or treatments known to affect eating or weight. Based on these criteria, three eating disordered individuals were excluded (two had undergone bariatric surgery, one was diagnosed with type 2 diabetes), leaving a final sample of 29 eating disordered (18 BED, 11 BN) and 32 healthy control individuals.
Telephone screenings were conducted to determine initial eligibility, which was subsequently confirmed with face‐to‐face structured clinical interviews. Lifetime and present eating disorders were assessed using the Eating Disorder Examination (EDE; Fairburn & Cooper, 1993; Hilbert, Tuschen‐Caffier, & Ohms, 2004). All other diagnoses were determined using the Structured Clinical Interview for DSM‐IV Axis I (SCID; First, Spitzer, Gibbon, & Williams, 1995; Wittchen, Zaudig, & Fydrich, 1997). The study was approved by the Ethics Review Panel of the University of Luxembourg. All participants provided written informed consent and received compensation for their participation.
2.2. Water load test‐II
The WLT‐II was performed according to the procedure described in detail elsewhere (van Dyck et al., 2016). In short, participants were asked to drink noncarbonated water at room temperature, ad libitum over two consecutive 5‐min periods. During the first period, they were instructed to drink water until reaching the point of perceived satiation, that is, the sensation that determines meal termination. During the second period, participants were asked to drink again, this time until reaching the point of maximum stomach fullness. Water was administered in a nontransparent 5‐l flask from which participants drank through a straw to control for swallowing sizes. Unbeknownst to participants, the flask was filled with only 1.5 l of water. This procedure blinded participants to the amount they consumed while at the same time ensuring safety through the 1.5 l maximum. Absolute amounts of water ingested in milliliters and the percentage of satiation to maximum fullness were determined as measures of gastric interoception.
2.3. Questionnaires
2.3.1. WLT‐II questionnaire
The WLT‐II questionnaire (van Dyck et al., 2016) assesses sensations related to water ingestion. Participants are asked to concentrate on their current abdominal sensations, especially if their stomach feels full or empty. They then rate their momentary feelings of satiation and fullness, together with five items measuring negative affect (NA). The NA subscale assesses states of discomfort that can be related to food intake, including both psychological and physiological dimensions. All items are answered on a 7‐point scale ranging from 1 (no sensation/not at all) to 7 (extremely). Ratings are obtained before the first water intake (t0, baseline), and after the first (t1) and second (t2) drinking period. Internal consistency reliabilities for the subscale NA were .71, .77 and .81 for t0, t1 and t2, respectively.
2.3.2. Eating Disorder Inventory‐2
The Eating Disorder Inventory‐2 (EDI‐2; Garner, 1991; German version by Paul & Thiel, 2005) assesses the specific psychopathology of eating disorders. It consists of 91 items, each of which is answered along a scale ranging from 1 (never) to 6 (always). For the present study, only the subscales drive for thinness, bulimia, and body dissatisfaction are reported, as they assess core eating disorder criteria (Garner, 1991). The EDI‐2 has sound psychometric properties (Eberenz & Gleaves, 1994; Garner, 1991), with internal consistencies ranging between .81 and .91 (α's = .77–.92 in the current sample) and test–retest reliabilities between r = .81 and r = .89 (Thiel & Paul, 2006).
2.4. Educational level
Participants' educational level was coded according to the International Standard Classification of Education (ISCED; UNESCO, 1997), to enable valid comparisons between degrees obtained in different countries. The ISCED‐97 distinguishes between 7 levels of education, ranging from “not completed primary education” (= 0) to “second stage of tertiary education” (= 6).
2.5. Electrogastrography
GMA was measured by EGG in combination with the WLT‐II. Participants were tested in the morning, after an overnight fast of at least 8 hr. Compliance with the overnight fast was assessed with a face‐to‐face interview at the beginning of the session. The EGG signal was recorded by placing three disposable, ConMed Cleartrace cutaneous electrodes over the region of the antrum of the stomach. The two active electrodes were placed on the midpoint between the xiphoid notch and the umbiculus, and approximately 6 cm left from the abdominal midline, respectively. The reference electrode was placed approximately 10 cm to the right of the midline electrode (Koch & Stern, 2004). Electrode sites were prepared by gently abrading the skin (Nuprep, D.O. Weaver and Co., Aurora, CO) and cleaning the area with alcohol. EGG data was recorded on a hard disk with a BrainAmp ExG amplifier (Brain Products, Gilching, Germany) and sampled at 5,000 Hz, with a hardware low‐pass filter of 1,000 Hz and no high‐pass filter (DC recording). Participants were seated in a half‐reclining position at 30–45° in a comfortable chair, and instructed to minimize talking and movement during EGG recording. After signal stabilization, baseline tracing was performed for 15 min. This was followed by the WLT‐II, after which the recording continued for an additional 15 min.
EGG analysis was performed separately for the two 15‐min measurement segments (“pre” and “post” water ingestion) using WinCPRS 1.160 software (Absolute Aliens Oy, Turku, Finland). The raw EGG signal was visually inspected to determine its general quality. Only continuous, artifact‐free recordings with visually recognizable waveforms were submitted to computer analysis. The digitized signal was software‐filtered with 0.25–0.016 Hz (1–15 cpm; Koch & Stern, 2004) and down‐sampled to 10 Hz, before performing fast‐Fourier transformations of 240‐s runs with an overlap of 75% (Koch & Stern, 2004). Power spectral density was determined for bradygastria (1.0–2.5 cpm), normogastria (2.5–3.75 cpm); tachygastria (3.75–10.0 cpm) and the duodenal power band (10.1–15.0 cpm). Power in each EGG band was calculated as percentage of power in the respective frequency band relative to the total EGG band power.
2.6. Data analysis
After examination for normality, group differences in demographic characteristics and ingested water volumes were examined using independent samples t tests. To evaluate the effect of water ingestion on subjective ratings, we calculated three separate 3 (Time: t0, t1, t2) × 2 (Group: ED, HC) mixed‐design analyses of variance (ANOVAs) for each ratings of satiety, fullness and negative affect. To evaluate whether water ingestion caused changes in EGG activity, we conducted three separate 2 (Time: pre, post) × 2 (Group: ED, HC) mixed‐design ANOVAs, with the power bands as dependent variables. Significant effects and interactions were further analyzed with Bonferroni‐corrected post hoc independent and paired samples t tests. Where appropriate (i.e., Mauchlys Sphericity Test, p < .05), the Greenhouse–Geisser procedure was applied. Since the total amount of water ingested was not related to any outcome measure, it was not included as a covariate in the analysis. Pearson correlations were calculated between the frequency of binge eating, and WLT‐II indices and EGG power bands, respectively. To protect against Type I error, the Holm–Bonferroni method was applied, which is more powerful than the Bonferroni method (Eichstaedt, Kovatch, & Maroof, 2013). Hence, for correlations between OBE frequency and WLT‐II indices, the significance level was set at .0038 for the hypothesis with the smallest p‐value. For correlations between OBE frequency and EGG power bands, the significance level was set at .0063 for the hypothesis with the smallest p‐value. Group differences between eating disorder diagnoses (BN vs. BED) were examined using the same statistical tests as described above. Statistical software SPSS version 22.0 (SPSS Inc., Chicago, IL) was used for statistical data analysis.
3. RESULTS
3.1. Demographic characteristics
Demographic and clinical data for both groups are shown in Table 1. Groups did not differ in age, sex, BMI, fasting duration, or body dissatisfaction. The ED group had lower educational levels and reported a significantly higher drive for thinness and bulimia than the HC group. There were no group differences in OBE frequency or in EDI subscales between BN and BED.
TABLE 1.
Demographic characteristics
| ED (n = 29) | HC (n = 32) | t (59) | p | d | |
|---|---|---|---|---|---|
| Age, M (SD) | 39.39 (12.88) | 37.33 (13.59) | 0.61 | .547 | |
| Sex, female, n (%) | 26 (89.66) | 29 (90.63) | χ2 (1) = 0.02 | 1.00 | |
| BMI, M (SD) | 30.19 (8.34) | 29.02 (8.31) | 0.55 | .587 | |
| Education, M (SD) | 3.62 (1.05) | 4.38 (1.16) | −2.66 | .010 | 0.69 |
| Time since last meal (hr), M (SD) | 12.20 (2.72)a | 12.45 (1.54) | −0.44 | .664 | |
| Duration of illness (years), M (SD) | 13.05 (8.95)a | — | |||
| Current comorbid diagnoses, n (%) | |||||
| Any | 9 (31.0) | 2 (6.3) | |||
| Affective disorder | 4 (13.8) | 1 (3.1) | |||
| Anxiety disorder | 2 (6.9) | 1 (3.1) | |||
| Substance use disorder | 3 (10.3) | 0 | |||
| EDI, M (SD) | |||||
| Drive for thinness | 4.07 (1.15) | 2.92 (1.13) | 3.92 | <.001 | 1.01 |
| Bulimia | 3.57 (0.85) | 1.67 (0.51) | 10.70 | <.001 | 2.71 |
| Body dissatisfaction | 3.60 (1.19) | 3.49 (1.02) | 0.41 | .685 | |
| BN (n = 11) | BED (n = 18) | t (27) | p | |
|---|---|---|---|---|
| OBE frequency, M (SD) | 2.76 (1.00) | 2.72 (1.00) | −0.11 | .925 |
| Purging frequency, M (SD) | ||||
| Self‐induced vomiting | 3.34 (3.06) | — | ||
| Laxative use | 0.33 (0.65) | — | ||
| EDI, M (SD) | ||||
| Drive for thinness | 4.14 (1.13) | 4.02 (1.19) | −0.271 | .789 |
| Bulimia | 3.90 (0.69) | 3.37 (0.89) | −1.82 | .081 |
| Body dissatisfaction | 3.89 (1.37) | 3.43 (1.06) | −0.957 | .352 |
Abbreviations: BED, binge‐eating disorder; BMI, body mass index; BN, bulimia nervosa; ED, eating disordered patients; EDI, Eating Disorder Inventory; HC, healthy controls; OBE frequency, mean weekly frequency of objective binge eating during the past 3 months; purging frequency, mean weekly frequency of purging during the past 3 months.
Based on n = 28, because data was missing for one participant.
3.2. Ingested water volumes
Mean volumes of water ingested until satiation, additional water volumes consumed until maximum fullness, total water volumes ingested, and mean percentages of satiation to total volume are shown in Figure 1. Compared to HC individuals, participants with EDs ingested higher volumes until satiated (645 ± 311 ml vs. 395 ± 169 ml, p < .001, d = 1.00) and in total (1,031 ± 414 ml vs. 705 ± 273 ml, p = .001, d = 0.93), but not until maximum fullness (386 ± 196 ml vs. 309 ± 167 ml, p = .104). Patients with EDs had a higher percentages of satiation to total volume than HC individuals, but this difference was not significant (p = .091).
FIGURE 1.
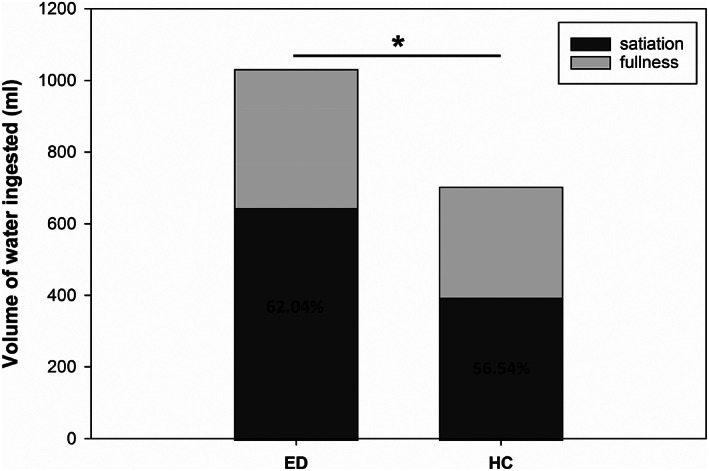
Mean volumes of water ingested until satiation and until maximum fullness in eating disordered individuals and in healthy controls. Percentages indicate mean percentages of satiation to total amount of water consumed. *p < .01, total water volumes ingested, eating disordered patients (ED) versus healthy controls (HC)
3.3. Self‐report ratings
Scores for satiety, fullness, and negative affect before the water load and after the first and second drinking period are shown in Figure 2. For satiety scores, significant main effects were found for Time, F(2, 59) = 182.85, p < .001, = .76, and Group, F(1, 59) = 4.85, p = .032, = .08. Satiety ratings significantly increased from t0 to t1 and from t1 to t2 in both groups (ps < .001), and were larger in the ED compared to the HC group (t0: 2.59 ± 1.35 vs. 2.13 ± 1.13; t1: 5.34 ± 1.34 vs. 4.47 ± 1.52; t2: 6.28 ± 1.53 vs. 5.88 ± 1.26). For fullness ratings, significant main effects occurred for Time, F(2, 59) = 205.16, p < .001, = .78 and Group, F(1, 59) = 4.82, p = .032, = .08. Again, fullness ratings increased from t0 to t1 and from t1 to t2 in both groups (ps < .001), and were larger in ED than in HC participants (t0: 2.24 ± 1.46 vs. 1.63 ± 1.13; t1: 5.17 ± 1.23 vs. 4.66 ± 1.68; t2: 6.38 ± 1.32 vs. 5.91 ± 1.23). For NA, results indicated significant main effects for Time, F(1.60, 94.11) = 22.50, p < .001, = .28 and Group, F(1, 59) = 8.14, p = .006, = .12, that were superseded by a significant Time × Group interaction, F(1.60, 94.11) = 4.85, p = .015, = .08. Paired samples t tests revealed a significant increase in NA from t0 to t1 in the ED group (p = .036), but not in HC participants (p = .177). NA significantly increased from t1 to t2 in both groups (ps < .001). Participants with EDs reported higher NA compared to HC at t1 (2.88 ± 1.11 vs. 2.09 ± 0.83, p = .002) and at t2 (3.59 ± 1.43 vs. 2.63 ± 0.95, p = .003), but not at baseline (2.55 ± 1.10 vs. 2.27 ± 0.92, p = .278).
FIGURE 2.

Satiety ratings, fullness ratings, and negative affect before water ingestion, and after the first and second drinking period, in eating disordered patients (ED) and in healthy controls (HC). Figures represent group means. Error bars are SE
3.4. Electrogastrographic response to water loads
Data from nine participants (5 BED, 4 HC) were excluded from EGG analysis because of poor signal quality. Figure 3 shows the mean percentage distributions of total EGG power in the four frequency ranges, for the ED (n = 24) and the HC group (n = 28). The mixed‐design ANOVA conducted on percentages of power in the bradygastric range revealed a significant main effect for Group, F(1, 50) = 12.98, p < .001, = .21. Differences between ED and HC participants were nonsignificant at baseline (41.62 ± 15.39 vs. 33.50 ± 16.83, p = .077), but significant after water ingestion (47.25 ± 11.65 vs. 32.25 ± 13.13, p < .001). Regarding percentages of normogastria power, there was a significant main effect for Group, F(1, 50) = 7.93, p = .007, = .14, and the Time × Group interaction showed a nonsignificant trend, F(1, 50) = 4.02, p = .050. Data from the fasting state showed no difference between ED and HC participants (36.45 ± 15.45 vs. 41.49 ± 16.52, p = .264), but 2.5–3.75 cpm activity was significantly lower in ED compared to HC participants after water ingestion (31.30 ± 11.55 vs. 46.45 ± 18.15, p < .001). The percentages of power in the tachygastric and duodenal‐respiratory ranges were similar for both groups throughout the study.
FIGURE 3.

Percentages of total EGG power in the four frequency ranges in the ED group and in healthy controls, before and after the WLT‐II. Figures represent group means. Error bars are SE. *p < .001, ED versus HC. ED, eating disordered patients; EGG, electrogastrography, HC, healthy controls; WLT‐II, water load test‐II
3.5. Correlations with binge‐eating frequency
OBE frequency was not related to mean volumes of water ingested until satiation, additional water volumes consumed until maximum fullness, total water volumes ingested, or mean percentages of satiation to total volume (all ps > .10). Subjective ratings of satiety, fullness and NA were not related to OBE frequency at t0, t1 or t2 (all ps > .10).
OBE frequency was not related to the percentages of EGG power in the four frequency bands before water ingestion (all ps > .10). After water ingestion, OBE frequency was negatively related to the percentage of normogastria (r = −.48, p = .017; see Figure 4); however, this result was nonsignificant after Holm‐Bonferroni correction. The correlation between OBE frequency and the percentage of bradygastria after the WLT did not reach significance (r = .35, p = .093). OBE frequency was not correlated with percentages of tachygastria or duodenal‐respiratory activity relative to total EGG power after the WLT‐II (all ps > .10).
FIGURE 4.
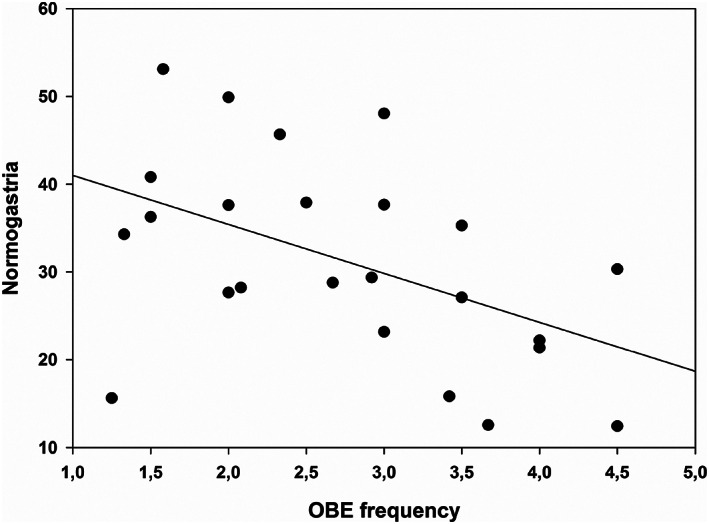
Correlation between the number of objective binge‐eating (OBE) episodes within the 3 months prior to the study and percentage of normogastric activity relative to the total power
3.6. Exploratory between‐ED‐group analyses
Additional analyses were performed to examine differences between eating disorder diagnoses (BED vs. BN). Neither were there differences between the two groups in ingested water volumes during the WLT, nor were there any group differences in ratings before or after water ingestion. There were no differences between participants with BN and BED in GMA before or after the WLT‐II (all ps > .05).
4. DISCUSSION
The first objective of this study concerned differences in perceptions of satiation and stomach fullness between patients with BN or BED and BMI‐matched healthy controls. Patients with EDs ingested more water until satiated, and the percentage of satiation to maximal fullness was larger compared to HCs, although the latter difference was not significant. These findings suggest that individuals experiencing regular binge‐eating episodes process the information on gastric distention differently and, therefore, may drink beyond this threshold. The larger percentage of satiation to maximum fullness in the ED group suggests that satiation occurred at a larger proportion of maximum stomach fullness. In other words, participants with EDs stopped drinking at a point, which was closer to their gastric fullness threshold compared to HC. These results add to previous evidence suggesting that patients with BN or BED show a loss of normal satiation, resulting in difficulties with meal termination. For example, Sysko et al. (2007) found individuals who binge eat to require substantially more food to reach similar levels of satiation compared to obese and normal‐weight controls. The difference between groups in total water volume ingested until maximum fullness may reflect a larger stomach capacity in patients with EDs, as suggested by studies using intragastric balloon methods (Geliebter et al., 1992, 2004; Geliebter & Hashim, 2001). Compared to intragastric balloon inflation, however, the WLT‐II has been developed as a physiologic and noninvasive test of distensibility of the entire upper gastric tract, not only the fundus (Koch, Hong, & Xu, 2000). Nevertheless, absolute water volume intakes should be interpreted with caution. A study using a barostat showed low correlations between drinking capacity and fundic accommodation or visceral hypersensitivity (Boeckxstaens, Hirsch, van den Elzen, Heisterkamp, & Tytgat, 2001), suggesting that drinking capacity is likely to also be affected by other factors than stomach volume, such as sensory (e.g., abnormal perception of the presence of nutrients) and psychological (e.g., disordered eating behaviors) determinants. In this study, however, a traditional one‐step WLT was used. Future studies should focus on further validating the WLT‐II against established gastric barostat procedures.
Perceptions of stomach fullness and satiety were significantly larger across the three time points in the ED group compared to the HC group. Thus, although eating disordered patients subjectively felt fuller and more satiated already at baseline, they still ingested larger water volumes compared to healthy controls. Koch et al. (1998) also observed increased perceptions of stomach fullness and satiety in individuals with BN at baseline and after water ingestion. Similar to the present study, self‐reported satiety and fullness increased at the same rate in ED and in HC, but their participants ingested a fixed amount of water (240 ml). Taken together, these findings raise the question of whether perceptions of stomach fullness and satiety may be affected by how much control eating disordered patients have over the amount consumed. In the present study, patients needed larger water volumes to induce the same increase in satiety and fullness perceptions, which is consistent with the idea of an altered development of satiety in individuals who binge eat. Kissileff et al. (1996) observed comparable changes in fullness over the course of a meal in BN and controls, but patients with BN consumed larger amounts of food than controls. Hence, the change in fullness per unit of food ingested was lower in BN than in controls, similar to the present findings with noncaloric water.
Patients with EDs reported a significant increase in NA after drinking water until satiated. In healthy individuals, satiation reflects a positive, comfortable sensation (Benelam, 2009), which concurs with findings from our control group. Patients with EDs, however, experienced water intake until satiated as uncomfortable, which supports the notion of disturbed sensory responses to intake in BN and BED.
Overall, the present findings add to the literature in that the WLT‐II is a useful clinical tool to measure gastric interoception at multiple levels in eating disordered patients. Further research is warranted to validate the WLT‐II in the context of established multidimensional models of interoception (Forkmann et al., 2016; Garfinkel, Seth, Barrett, Suzuki, & Critchley, 2015). Future studies should investigate whether (a) ingested water volumes are related to other measures of interoceptive accuracy, (b) WLT‐II questionnaire dimensions can be considered measures of interoceptive sensibility or interoceptive evaluation, (c) the concordance between self‐report ratings and ingested water volumes or EGG measures are related to other measures of interoceptive awareness, and (d) gastric functioning can be considered as an additional level of gastric interoception.
The second main objective of the present study concerned the assessment of GMA before and after the WLT‐II. The results show significantly higher bradygastria in the ED group compared to controls, together with significantly less normogastric activity. Mechanoreceptors in the proximal and distal stomach transmit sensory information regarding stomach distention and gastric contractions via vagal afferents to the brain (Hellström et al., 2004). Changes in EGG rhythm from normogastria to gastric dysrhythmia often result in epigastric distress, especially nausea (Koch & Stern, 2004). Thus, these altered gastric neuromuscular responses to the water load or abnormal visceral afferent sensory pathways may be related and contribute to the altered subjective sensations experienced by the patients.
The present EGG findings in patients with BN or BED concur with previous studies reporting altered GMA in BN (Diamanti et al., 2003; Koch et al., 1998; Ogawa et al., 2004). Yet, to the best of our knowledge, this is the first study investigating GMA in a sample including patients with BED. Ogawa et al. (2004) included eight patients with a DSM‐IV eating disorder not otherwise specified (EDNOS) diagnosis, which might have included some patients with BED. They observed predominant bradygastria in both patients with EDNOS and individuals with BN, yet there were no significant differences between patient groups, similar to the present lack of differences between BN and BED. This finding suggests that BN and BED share gastric rhythm abnormality, and that binge eating (i.e., the excessive stimulation with large volumes of food) may represent the key mechanism underlying altered gastric myoelectrical responses in BN and BED.
The degree of normal myoelectrical activity decreased with the number of binge‐eating episodes per week, but this result did not remain significant after correcting for multiple testing. Also, a nonsignificant positive association was found between the percentage of bradygastria and OBE frequency. Further studies are needed to investigate if there is a systematic relationship between the severity of the eating disorder and the extent of disturbances to gastric motor function.
The present results should be interpreted in combination with findings on other abnormalities in upper gastrointestinal tract functioning observed in patients with BN or BED. Several studies found diminished release of cholecystokinin (CCK; Devlin et al., 1997; Geracioti & Liddle, 1988), delayed gastric emptying (Geliebter et al., 1992, 2004) and diminished gastric relaxation reflex (Walsh et al., 2003) in patients with regular binge eating compared to controls. This raises the important question of how these abnormalities are related. It has been suggested that the abnormal gastric motor pattern in patients with BN might result in, or coincide with, delayed gastric emptying (Chen, Lin, Pan, & McCallum, 1996; Diamanti et al., 2003). The delayed gastric emptying may in turn result in reduced postprandial CCK release (Walsh et al., 2003), which has been demonstrated to affect satiation and enhance gastric relaxation. Another possibility is that these disturbances in gastrointestinal functioning are manifestations of an underlying physiological disturbance, such as impaired activation of the afferent vagus nerve that carries signals from the gut to higher brain areas (Faris et al., 2000; Wang et al., 2008). This hypothesis is supported by findings on increased pain thresholds in patients with BN or BED (Raymond et al., 1995). A growing body of literature suggests that altered interoception in eating disorders may be related to abnormal neural activation in response to interoceptive stimuli (Lutz et al., 2019; Quadt, Critchley, & Garfinkel, 2018). The current study's findings suggest that afferent interoceptive signals are also altered.
4.1. Limitations
Nine participants had to be excluded because of poor quality EGG signals. This might be due to the high number of overweight participants in our sample, because fat layer acts as an insulator and decreases the amplitude of the EGG signal (Farajidavar, 2018). An association between decreased detectability of the propagation of the gastric slow wave and the thickness of the abdominal wall has also been shown in a study using computer simulations (Liang & Chen, 1997). Nevertheless, as the number of participants excluded was almost identical between groups, and as groups were matched for BMI, it is unlikely that this would have affected the results on group differences in EGG patterns.
A second limitation concerns the small proportion of male participants. We recruited both women and men in an effort to increase ecological validity and generalizability of the results. Nevertheless, only few men volunteered, probably because more women are interested in, and seek treatment for, eating disorders. By rigorously matching groups for sex, however, we ensured that they were comparable.
Similarly, results on differences between participants with BN and BED need to be interpreted with caution because of the small sample sizes. Further research is required to examine the unique associations of binge eating, purging, and prolonged episodes of dietary restriction with GMA and gastric interoception.
Furthermore, the EGG recording periods in the presents study were comparatively short (i.e., 15 min before and after the WLT‐II). In line with our results, Koch et al. (1998) found less normogastria and more bradygastria in BN at baseline and during the first 20 min after water ingestion. By 21–30 min, however, the percentage of normogastric and bradygastric power were equivalent in both groups. Future studies should consider using longer recording periods of at least 30 min.
5. CONCLUSION
The present results show that patients with BN or BED differ from healthy controls in their gastric myoelectrical pattern and water load. Patients showed decreased sensitivity to gastric distention in response to the WLT‐II and more gastric dysrhythmias than control participants. These noninvasive measures may be a promising route to elucidate the pathophysiology of eating disorders, to develop effective therapeutic approaches, and to monitor the progress of treatments. Nevertheless, it remains a matter of debate whether impaired intestinal motility is a clinical manifestation of the eating disorder itself, a consequence thereof, or both.
CONFLICT OF INTEREST
All authors declare no conflict of interest for this study.
6.
van Dyck Z, Schulz A, Blechert J, Herbert BM, Lutz APC, Vögele C. Gastric interoception and gastric myoelectrical activity in bulimia nervosa and binge‐eating disorder. Int J Eat Disord. 2021;54:1106–1115. 10.1002/eat.23291
Action Editor: Guido Frank
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- American Psychiatric Association . (2013). Diagnostic and statistical manual of mental disorders (DSM‐5) (5th ed.). Washington, DC: Author. [Google Scholar]
- Benelam, B. (2009). Satiation, satiety and their effects on eating behaviour. Nutrition Bulletin, 34(2), 126–173. 10.1111/j.1467-3010.2009.01753.x [DOI] [Google Scholar]
- Blundell, J. E., & Finlayson, G. (2004). Is susceptibility to weight gain characterized by homeostatic or hedonic risk factors for overconsumption? Physiology and Behavior, 82(1), 21–25. 10.1016/j.physbeh.2004.04.021 [DOI] [PubMed] [Google Scholar]
- Boeckxstaens, G. E., Hirsch, D. P., van den Elzen, B. D., Heisterkamp, S. H., & Tytgat, G. N. (2001). Impaired drinking capacity in patients with functional dyspepsia: Relationship with proximal stomach function. Gastroenterology, 121(5), 1054–1063. 10.1053/gast.2001.28656 [DOI] [PubMed] [Google Scholar]
- Chen, J. D., Lin, Z., Pan, J., & McCallum, R. W. (1996). Abnormal gastric myoelectrical activity and delayed gastric emptying in patients with symptoms suggestive of gastroparesis. Digestive Diseases and Sciences, 41(8), 1538–1545. 10.1007/BF02087897 [DOI] [PubMed] [Google Scholar]
- Devlin, M. J., Walsh, B. T., Guss, J. L., Kissileff, H. R., Liddle, R. A., & Petkova, E. (1997). Postprandial cholecystokinin release and gastric emptying in patients with bulimia nervosa. American Journal of Clinical Nutrition, 65(1), 114–120. [DOI] [PubMed] [Google Scholar]
- Diamanti, A., Bracci, F., Gambarara, M., Ciofetta, G. C., Sabbi, T., Ponticelli, A., … Castro, M. (2003). Gastric electric activity assessed by electrogastrography and gastric emptying scintigraphy in adolescents with eating disorders. Journal of Pediatric Gastroenterology and Nutrition, 37(1), 35–41. 10.1097/00005176-200307000-00006 [DOI] [PubMed] [Google Scholar]
- Eberenz, K. P., & Gleaves, D. H. (1994). An examination of the internal consistency and factor structure of the Eating Disorder Inventory‐2 in a clinical sample. The International Journal of Eating Disorders, 16(4), 371–379. 10.1002/1098-108X(199412)16:43.0.CO;2-W [DOI] [PubMed] [Google Scholar]
- Eichstaedt, K. E., Kovatch, K., & Maroof, D. A. (2013). A less conservative method to adjust for familywise error rate in neuropsychological research: The Holm's sequential Bonferroni procedure. NeuroRehabilitation, 32(3), 693–696. 10.3233/NRE-130893 [DOI] [PubMed] [Google Scholar]
- Eshkevari, E., Rieger, E., Musiat, P., & Treasure, J. (2014). An investigation of interoceptive sensitivity in eating disorders using a heartbeat detection task and a self‐report measure. European Eating Disorders Review, 22(5), 383–388. 10.1002/erv.2305 [DOI] [PubMed] [Google Scholar]
- Fairburn, C. G., & Cooper, Z. (1993). The eating disorder examination. In Fairburn C. G. & Wilson G. T. (Eds.), Binge eating: Nature, assessment, and treatment (12th ed., pp. 317–360). New York, NY: Guilford Press. [Google Scholar]
- Farajidavar, A. (2018). Bioelectronics for mapping gut activity. Brain Research, 1693, 169–173. 10.1016/j.brainres.2018.03.004 [DOI] [PubMed] [Google Scholar]
- Faris, P. L., Kim, S. W., Meller, W. H., Goodale, R. L., Oakman, S. a., Hofbauer, R. D., … Hartman, B. K. (2000). Effect of decreasing afferent vagal activity with ondansetron on symptoms of bulimia nervosa: A randomised, double‐blind trial. Lancet, 355, 792–797. 10.1016/S0140-6736(99)09062-5 [DOI] [PubMed] [Google Scholar]
- First, M. B., Spitzer, R. L., Gibbon, M., & Williams, J. B. W. (1995). Structured clinical interview for DSM‐IV Axis I disorders. Washington, DC: American Psychiatric Press. [Google Scholar]
- Forkmann, T., Scherer, A., Meessen, J., Michal, M., Sch??chinger, H., V??gele, C., & Schulz, A. (2016). Making sense of what you sense: Disentangling interoceptive awareness, sensibility and accuracy. International Journal of Psychophysiology, 109, 71–80. 10.1016/j.ijpsycho.2016.09.019 [DOI] [PubMed] [Google Scholar]
- Garfinkel, S. N., Seth, A. K., Barrett, A. B., Suzuki, K., & Critchley, H. D. (2015). Knowing your own heart: Distinguishing interoceptive accuracy from interoceptive awareness. Biological Psychology, 104, 65–74. 10.1016/j.biopsycho.2014.11.004 [DOI] [PubMed] [Google Scholar]
- Garner, D. M. (1991). Eating disorders inventory 2: Professional manual. Odessa, FL: Psycho. [Google Scholar]
- Geliebter, A., Gluck, M. E., & Hashim, S. A. (2005). Plasma ghrelin concentrations are lower in binge‐eating disorder. The Journal of Nutrition, 135(5), 1326–1330. 10.1093/jn/135.5.1326 [DOI] [PubMed] [Google Scholar]
- Geliebter, A., & Hashim, S. A. (2001). Gastric capacity in normal, obese, and bulimic women. Physiology and Behavior, 74(4–5), 743–746. 10.1016/S0031-9384(01)00619-9 [DOI] [PubMed] [Google Scholar]
- Geliebter, A., Melton, P. M., McCray, R. S., Gallagher, D. R., Gage, D., & Hashim, S. A. (1992). Gastric capacity, gastric emptying, and test‐meal intake in normal and bulimic women. American Journal of Clinical Nutrition, 56(4), 656–661. [DOI] [PubMed] [Google Scholar]
- Geliebter, A., Yahav, E. K., Gluck, M. E., & Hashim, S. A. (2004). Gastric capacity, test meal intake, and appetitive hormones in binge eating disorder. Physiology and Behavior, 81(5), 735–740. 10.1016/j.physbeh.2004.04.014 [DOI] [PubMed] [Google Scholar]
- Geracioti, T. D., & Liddle, R. A. (1988). Impaired cholecystokinin secretion in bulimia nervosa. New England Journal of Medicine, 319(11), 683–688. 10.1056/NEJM198809153191105 [DOI] [PubMed] [Google Scholar]
- Hadley, S., & Walsh, B. (2003). Gastrointestinal disturbances in anorexia nervosa and bulimia nervosa. Current Drug Target—CNS & Neurological Disorders, 2(1), 1–9. 10.2174/1568007033338715 [DOI] [PubMed] [Google Scholar]
- Hellström, P. M., Geliebter, A., Näslund, E., Schmidt, P. T., Yahav, E. K., Hashim, S. A., & Yeomans, M. R. (2004). Peripheral and central signals in the control of eating in normal, obese and binge‐eating human subjects. British Journal of Nutrition, 92(S1), 47–57. 10.1079/BJN20041142 [DOI] [PubMed] [Google Scholar]
- Hilbert, A., Tuschen‐Caffier, B., & Ohms, M. (2004). Eating Disorder Examination: Deutschsprachige Version des strukturierten Essstörungsinterviews. Diagnostica, 50(2), 98–106. [Google Scholar]
- Kissileff, H. R., Wentzlaff, T. H., Guss, J. L., Walsh, B. T., Devlin, M. J., & Thornton, J. C. (1996). A direct measure of satiety disturbance in patients with bulimia nervosa. Physiology and Behavior, 60(4), 1077–1085 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/8884936 [DOI] [PubMed] [Google Scholar]
- Klabunde, M., Acheson, D. T., Boutelle, K. N., Matthews, S. C., & Kaye, W. H. (2013). Interoceptive sensitivity deficits in women recovered from bulimia nervosa. Eating Behaviors, 14(4), 488–492. 10.1016/j.eatbeh.2013.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klabunde, M., Collado, D., & Bohon, C. (2017). An interoceptive model of bulimia nervosa: A neurobiological systematic review. Journal of Psychiatric Research, 94, 36–46. 10.1016/j.jpsychires.2017.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch, K. L., Bingaman, S., Tan, L., & Stern, R. M. (1998). Visceral perceptions and gastric myoelectrical activity in healthy women and in patients with bulimia nervosa. Neurogastroenterology & Motility, 10(1), 3–10 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/9507247 [DOI] [PubMed] [Google Scholar]
- Koch, K. L., Hong, S. P., & Xu, L. (2000). Reproducibility of gastric myoelectrical activity and the water load test in patients with dysmotility‐like dyspepsia symptoms and in control subjects. Journal of Clinical Gastroenterology, 31(2), 125–129. 10.1097/00004836-200009000-00007 [DOI] [PubMed] [Google Scholar]
- Koch, K. L., & Stern, R. M. (2004). Handbook of electrogastrography. Oxford, England: Oxford University Press. [Google Scholar]
- Koch, K. L., Stern, R. M., Vasey, M., Botti, J. J., Creasy, G. W., & Dwyer, A. (1990). Gastric dysrhythmias and nausea of pregnancy. Digestive Diseases and Sciences, 35(8), 961–968. [DOI] [PubMed] [Google Scholar]
- Levine, M. E. (2005). Sickness and satiety: Physiological mechanisms underlying perceptions of nausea and stomach fullness. Current Gastroenterology Reports, 7(4), 280–288. 10.1007/s11894-005-0020-2 [DOI] [PubMed] [Google Scholar]
- Liang, J., & Chen, J. D. Z. (1997). What can be measured from surface electrogastrography: Computer simulations. Digestive Diseases and Sciences, 42(7), 1331–1343. 10.1023/A:1018869300296 [DOI] [PubMed] [Google Scholar]
- Lutz, A. P. C., Schulz, A., Voderholzer, U., Koch, S., van Dyck, Z., & Vögele, C. (2019). Enhanced cortical processing of cardio‐afferent signals in anorexia nervosa. Clinical Neurophysiology, 130(9), 1620–1627. 10.1016/j.clinph.2019.06.009 [DOI] [PubMed] [Google Scholar]
- Ogawa, A., Mizuta, I., Fukunaga, T., Takeuchi, N., Honaga, E., Sugita, Y., … Takeda, M. (2004). Electrogastrography abnormality in eating disorders. Psychiatry and Clinical Neurosciences, 58(3), 300–310. 10.1111/j.1440-1819.2004.01236.x [DOI] [PubMed] [Google Scholar]
- Paul, T., & Thiel, A. (2005). EDI‐2. Eating Disorder Inventory‐2. (Deutsche version). Göttingen, Germany: Hogrefe. [Google Scholar]
- Pollatos, O., & Georgiou, E. (2016). Normal interoceptive accuracy in women with bulimia nervosa. Psychiatry Research, 240, 328–332. 10.1016/j.psychres.2016.04.072 [DOI] [PubMed] [Google Scholar]
- Quadt, L., Critchley, H. D., & Garfinkel, S. N. (2018). The neurobiology of interoception in health and disease. Annals of the New York Academy of Sciences, 1428(1), 112–128. 10.1111/nyas.13915 [DOI] [PubMed] [Google Scholar]
- Raymond, N. C., de Zwaan, M., Faris, P. L., Nugent, S. M., Ackard, D. M., Crosby, R. D., & Mitchell, J. E. (1995). Pain thresholds in obese binge‐eating disorder subjects. Biological Psychiatry, 37(3), 202–204 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/7727630 [DOI] [PubMed] [Google Scholar]
- Sysko, R., Devlin, M. J., Walsh, B. T., Zimmerli, E., & Kissileff, H. R. (2007). Satiety and test meal intake among women with binge eating disorder. International Journal of Eating Disorders, 40(6), 554–561. 10.1002/eat [DOI] [PubMed] [Google Scholar]
- Thiel, A., & Paul, T. (2006). Test–retest reliability of the Eating Disorder Inventory 2. Journal of Psychosomatic Research, 61(4), 567–569. 10.1016/j.jpsychores.2006.02.015 [DOI] [PubMed] [Google Scholar]
- UNESCO . (1997). International standard classification of education. Paris, France: UNESCO. [Google Scholar]
- van Dyck, Z., Vögele, C., Blechert, J., Lutz, A. P. C., Schulz, A., & Herbert, B. M. (2016). The water load test as a measure of gastric interoception: Development of a two‐stage protocol and application to a healthy female population. PLoS One, 11(9), e0163574. 10.1371/journal.pone.0163574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh, B. T., Zimmerli, E., Devlin, M. J., Guss, J., & Kissileff, H. R. (2003). A disturbance of gastric function in bulimia nervosa. Biological Psychiatry, 54(9), 929–933. 10.1016/S0006-3223(03)00176-8 [DOI] [PubMed] [Google Scholar]
- Wang, G. J., Tomasi, D., Backus, W., Wang, R., Telang, F., Geliebter, A., … Volkow, N. D. (2008). Gastric distention activates satiety circuitry in the human brain. NeuroImage, 39(4), 1824–1831. 10.1016/j.neuroimage.2007.11.008 [DOI] [PubMed] [Google Scholar]
- Wittchen, H. U., Zaudig, M., & Fydrich, T. (1997). Strukturiertes klinisches Interview für DSM‐IV: Achse I. Göttingen, Germany: Hogrefe. [Google Scholar]
- Zimmerli, E. J., Walsh, B. T., Guss, J. L., Devlin, M. J., & Kissileff, H. R. (2006). Gastric compliance in bulimia nervosa. Physiology and Behavior, 87(2), 441–446. 10.1016/j.physbeh.2005.11.010 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


