Abstract
This report is the third in a series of studies that aimed to compile physiological parameters related to develop physiologically based pharmacokinetic (PBPK) models for drugs and environmental chemicals in food‐producing animals including swine and cattle (Part I), chickens and turkeys (Part II), and finally sheep and goats (the focus of this manuscript). Literature searches were conducted in multiple databases (PubMed, Google Scholar, ScienceDirect, and ProQuest), with data on relevant parameters including body weight, relative organ weight (% of body weight), cardiac output, relative organ blood flow (% of cardiac output), residual blood volume (% of organ weight), and hematocrit reviewed and statistically summarized. The mean and standard deviation of each parameter are presented in tables. Equations describing the growth curves of sheep and goats are presented in figures. When data are sufficient, parameter values are reported for different ages or production classes of sheep, including fetal sheep, lambs, and market‐age sheep (mature sheep). These data provide a reference database for developing standardized PBPK models to predict drug withdrawal intervals in sheep and goats, and also provide a basis for extrapolating PBPK models from major species such as cattle to minor species such as sheep and goats.
Keywords: blood flow, food animal residue avoidance databank, food safety, organ weight, physiologically based pharmacokinetic model
1. INTRODUCTION
Sheep and goats are important sources of dairy and meat products, especially for consumers in Asian, African, Caribbean, European, Latin American, and Oceanic countries (Gatzias et al., 2018; Montossi et al., 2013; Vacca et al., 2018). In the United States, the Food and Drug Administration (FDA) classifies sheep and goats as minor food‐producing animal species. Compared to the major species (e.g., cattle and swine), a substantially fewer number of medications are FDA approved for use in sheep and goats (FDA, 2020). As a result, veterinarians often need to prescribe medications in an extra‐label manner and scientific data are required for estimating an extended withdrawal interval in accordance with the Animal Medicinal Drug Use Clarification Act of 1994 (AMDUCA) (Martin et al., 2018; Riviere et al., 2017). This requirement for needing scientific data to establish a withdrawal interval after extra‐label drug use establishes the need to develop quantitative models to estimate withdrawal intervals after extra‐label drug use in sheep and goats.
Physiologically based pharmacokinetic (PBPK) modeling is a useful tool in the areas of drug development (Zhao et al., 2012), chemical risk assessment (Lin & Fisher, 2020; Tan et al., 2018), and drug tissue residue and withdrawal interval estimation in food animals for food safety assessment (Lautz et al., 2019; Lin et al., 2016). In the literature, there are about 40 published PBPK models in food animals, mainly in major species, but only three in sheep and goats (Craigmill, 2003; Lautz, Hoeks, et al., 2020; Leavens et al., 2012). These sheep/goat models primarily simulate drug pharmacokinetics in an average animal without characterizing population variabilities, in part, due to lack of data on the variabilities of physiological parameters within the population (Craigmill, 2003; Leavens et al., 2012). This is a critical data gap that prevents further development and application of population‐based PBPK models to estimate drug withdrawal intervals in small ruminants.
In light of the important role of physiological parameters in a PBPK model, it is necessary to compile physiological parameter data in different food‐producing animal species to facilitate PBPK model development and application. Such data have been compiled and widely cited for laboratory animal species and humans (Brown et al., 1997; Davies & Morris, 1993; ICRP, 2002). In terms of food animals, our group recently completed a comprehensive review on physiological parameters of body weight, organ/tissue weights, cardiac output, regional blood flow, and hematocrit in different species, including cattle, swine, chickens, turkeys, sheep, and goats. The manuscript describing the compiled data in cattle and swine has been published (Lin et al., 2020); the manuscript describing data in chickens and turkeys is under review (Wang et al., 2020); the present manuscript is the final part of this series and focuses on sheep and goats. The data are presented in the format of mean and standard deviation (SD) so that they can directly be used to characterize the variability of each physiological parameter and to create stochastic population PBPK models. This study also identifies data gaps. All raw data are provided in the “Supporting Information” section so that they can be updated when new data become available. This series of manuscripts provides a comprehensive data repository on physiological parameters for development of PBPK models of environmental chemicals and drugs in different food animal species to facilitate the use of PBPK models in the estimation of drug and chemical tissue residue and withdrawal intervals to ensure animal‐derived food safety.
2. METHODS AND MATERIALS
The methods on the literature search strategy, literature inclusion/exclusion criteria, data digitalization, standardization, and analysis, as well as the equations used in the calculation are the same as the methods described in detail in Parts I and II of this manuscript series (Lin et al., 2020; Wang et al., 2020). Readers are referred to these earlier publications for detailed methodology. Only methods that are unique to or relevant to understanding the present manuscript are described below. The process and outcome of the literature search, selection, and data analysis for the present study are shown in the flowchart of Figure 1.
Figure 1.
A brief flowchart for the process of literature search, inclusion, exclusion, data extraction, and analysis for physiological parameters in sheep and goats [Colour figure can be viewed at wileyonlinelibrary.com]
Regarding the definition of different production classes of sheep and goats, lamb refers to sheep younger than 14 months and having a cartilaginous break joint in at least one leg (USDA, 2019a); market‐age sheep refers to mature sheep that are older than 14 months; sheep refers to the sheep as a species, and when referencing production class, sheep represent a combination of market‐age sheep and lambs. Lamb meat is the meat from a sheep that is typically less than one year of age. Mutton is the meat from a sheep that is typically older than one year. In order to serve the purpose of modeling different production classes of sheep, the physiological parameters for sheep were categorized as for sheep, market‐age sheep, lamb, and fetus. Due to limited data for goats, their physiological parameters were not categorized for different ages or production classes. Data from animals of both sexes were included in our analysis.
For the first‐round literature search, general key words for animal species and physiological parameters were used in combinations. The key words for animal species included “Sheep,” “Lamb,” “Goat,” “Ewe,” “Ram,” and “Wether”; and the key words for physiological parameters included “Organ Weight,” “Tissue Volume,” “Blood Flow,” “Vascular Space,” and “Residual Blood Volume,” More specific key words on organ names in combination with parameter names were applied in the second‐round search due to limited data available for some of the physiological parameters.
Studies were included only for breeds of sheep and goats intended for food use, such as Merino, Clun Forest, Rambouillet, Suffolk and Dorset for sheep, and Boer, Spanish, Batina, Dhofari, and Toggenburg for goats. The selected studies must have implemented a method for randomization, and these studies must be a controlled trial with at least one control group for healthy animals. If the intervention had impacts on values of the physiological parameters, only the controlled groups were included into the calculation. Studies using sheep or goat breeds with gene mutations significantly affecting physiological parameters were excluded (He et al., 2018; Laville et al., 2004).
Physiological parameter data were extracted directly into an Excel® spreadsheet from papers reporting values in tables (refer to the Data Extraction tab in each of the Excel® files in the Supporting Information). When the relevant data were shown graphically, the graphic data were extracted and digitized with WebPlotDigitizer (version 4.1). Due to the variability of reported units from different studies, all data were converted into International System of Units (SI), and finally into the units commonly used in PBPK modeling. Briefly, gram (g) and kilogram (kg) were used as units for mass, minute (min) and hour (h) were used for time, and milliliter (ml) and liter (L) were used as units for volumes. Liter per hour per kilogram body weight (L hr−1 kg−1) was used as the unit for cardiac output and blood flow to individual tissue or organ. Tissue volume fractions and regional blood flow fractions are unitless.
3. RESULTS
3.1. Body weights for sheep and goats by different production classes
The average market weight for sheep, including both mature sheep and lambs, was calculated based on the data extracted from the weekly national lamb market summary from US Department of Agriculture (USDA) from May 2018 to May 2019 (USDA, 2019b). The average market weight of sheep was 62.88 kg based on the reported live weight of 115,000 animals (Table 1).
Table 1.
Average market weight for sheep
| Date | Number of animals | Live weight (lb) | Live weight (kg) |
|---|---|---|---|
| 4‐May‐19 | 39,000 | 136 | |
| 27‐Apr‐19 | 41,000 | 140 | |
| 5‐May‐18 | 35,000 | 140 | |
| All | 115,000 | 138.64 | 62.88 |
Data are from USDA (2019b). Sheep represent both mature sheep and lambs in this table.
Goats and sheep are marketed for meat over a wide age range, from a few weeks to several years of age. In order to classify values of physiological parameters into different age and production classes, data on ages, teeth, and body weights were extracted from a previous report (Machen, 2016). The details of ages and corresponding body weight ranges for different market categories of goats and sheep are shown in Table 2. The weight range for lambs is from 6.8 kg to ~36 kg. The original data and data analysis for the average market weight of sheep and market categories of goats and sheep are provided in Excel® File A1 of Supporting Information.
Table 2.
Market categories of goats and sheep and their respective weights
| Age | Teeth | Goats (kg) | Sheep (kg) | |||
|---|---|---|---|---|---|---|
| Beginning Weight | Final Weight | Beginning Weight | Final Weight | |||
| Kid, lamb | 0–11 months | Milk (baby) teeth | 6.8 | 31.75 | 6.8 | 36.28 |
| Yearling | 12–23 months | 1 pair permanent | 27.21 | 54.42 | 34.01 | 68.03 |
| Young | 24–36 months | 2–3 pair permanents | 40.82 | 81.63 | 40.82 | 90.7 |
| Mature | 4–6 years old | 4 pair permanents | 45.35 | 90.7 | 40.82 | 102.04 |
| Aged | 7+ years old | Worn, broken mouth | 45.35 | 40.82 | ||
Data are from Machen (2016).
3.2. Growth curves and body weight gain
The growth curves for fetal sheep and goats are important to develop gestational models to predict fetal exposure to drugs during pregnancy. Many factors, including genetic and environmental determinants, have impacts on the gestation time. The average lengths of gestation for sheep and goats are very similar (Sivachelvan et al., 1996), being reported as 148 days for sheep (Jainudeen & Hafez, 2000) and 146 days for goats (Devendra & McLeroy, 1982). Based on the available data, only the growth curves for fetal sheep, sheep from 2 months to 3 years, and goats from 0 to 6 months were generated. The growth curves of fetal sheep and sheep from 2 months to 3 years are based on data of different breeds and both sexes from different studies (Moss et al., 2005; Robillard & Weitzman, 1980; Singh et al., 2018). However, the growth curve of goats is based on data from two studies for Omani goats from the same research group (Mahgoub, 1997; Mahgoub & Lodge, 1998). The growth curve for sheep from gestational day (GD) 106–45 days after birth is:
| (1) |
where BW represents body weight in kg, Age_GD resembles gestational age values in days or days since the beginning of gestation, B0 = −5.4003, and B1 = 0.064 (R 2 = 0.846) (Figure 2).
Figure 2.
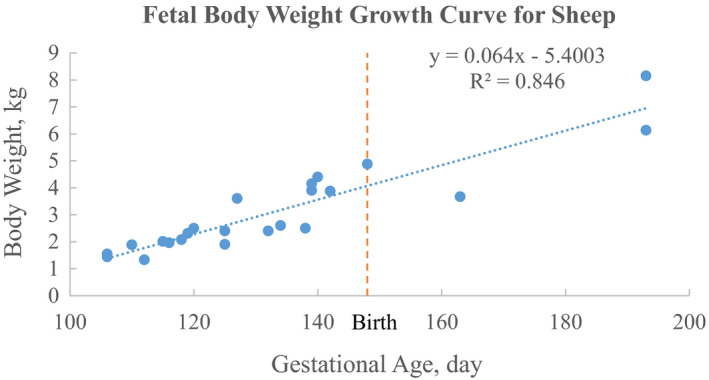
The growth curve of fetal body weight for sheep from 106 gestational days to 45 days after birth. The data of fetal body weights within 45 days of birth from the previous studies of Moss et al. (2005), Robillard and Weitzman (1980), and Singh et al. (2018) were pooled together to generate the growth curve. The reported 148 days as the average gestation days for sheep was considered as the time of birth [Colour figure can be viewed at wileyonlinelibrary.com]
The growth curve for sheep from around 2 months to 3 years old is:
| (2) |
where BW represents body weight in kg, age resembles age values in days after birth, “ln” stands for natural logarithm, B0 = −59.181, and B1 = 16.003 (R 2 = 0.8418) (Figure 3). Raw data and data analysis for Figures 2 and 3 are provided in Excel File B1 of Supporting Information.
Figure 3.
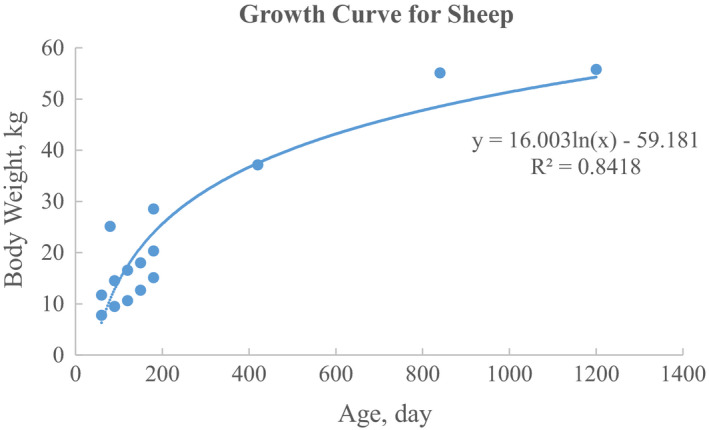
The growth curve for sheep from around 2 months to 3 years age. The data were pooled and calculated from previous studies of Moss et al. (2005) and Singh et al. (2018) [Colour figure can be viewed at wileyonlinelibrary.com]
The growth curve for goats from 0 month to around 6 months is:
| (3) |
where BW represents body weight in kg, age resembles age values in days after birth, B0 = 3.4623, and B1 = 0.0909 (R 2 = 0.9037) (Figure 4). Raw data and data analysis for Figure 4 are available in Excel File C1 of Supporting Information.
Figure 4.
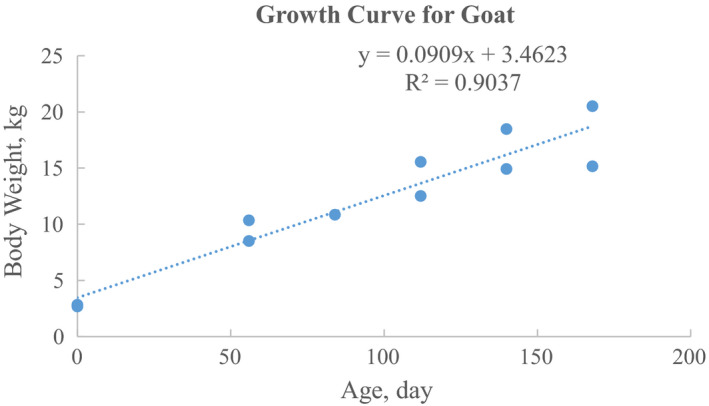
The growth curve for goats from 0 month to around 6 months age. The data were pooled and calculated from previous studies of Mahgoub (1997) and Mahgoub and Lodge (1998)
3.3. Organ weight
Relative organ weights in sheep based on all available data from both market‐age sheep and lambs are summarized in Table 3. The summary for relative organ weights in market‐age sheep was based on sheep with the body weight higher than 40 kg on both sexes in Table 4. The relative organ weights for lambs are listed in Table 5 using data from sheep with body weights from 7 to 40 kg. In Table 6, the relative organ weights in fetal and neonatal sheep are included, and these values were based on previous studies (Creasy et al., 1972; Greenwood et al., 2004; Hansard, 1956; Jobe et al., 1998; McDonald & Nathanielsz, 1991; Vonnahme et al., 2003). The average body weights of sheep for those data in Tables 4, 5, and 6 are 58.73, 28.34 and 3.40 kg, respectively. Most of the values are based on both sexes. The relative weights of udder tissues in non‐lactating and lactating female sheep are shown in Table 7, which were based on the data from the study of Thompson (1980). Original raw data for relative organ weights of market‐age sheep, lambs, and fetal and neonatal sheep are available in Excel File B2 in the Supporting Information. Due to limited data for organ weights in goats, the summary for relative organ weights in goats was based on both sexes and different breeds (shown in Table 8). The average body weight for goats used to calculate values in Table 8 was 25.56 kg ranging from 10.4 to 65 kg. For weights of blood in sheep and goats, detailed values for arterial blood and venous blood were not available. The raw data for relative organ weights in goats can be found in the Excel File C2 of the Supporting Information.
Table 3.
Relative organ weight (percent of body weight) or tissue volume for sheep based on all available data from market‐age sheep and lambs with the average body weight of 37.71 kg (body weights are higher than 7 kg)
| Mean | SD | Number of Animals | Number of Studies | References | |
|---|---|---|---|---|---|
| Adrenals | 0.007 | 0.002 | 37 | 4 | 1–4 |
| Adipose Tissue | 17.94 | 2.50 | 168 | 5 | 5–9 |
| Blood | 5.22 | 0.53 | 124 | 6 | 10–15 |
| Bone | 5.96 | 0.51 | 87 | 4 | 7, 8, 16, 17 |
| Brain | 0.26 | 0.03 | 21 | 2 | 2, 3 |
| GI tract | 4.83 | 1.15 | 57 | 3 | 11, 14, 18 |
| Stomach | 2.73 | 0.78 | 26 | 2 | 14, 18 |
| Rumen | 1.08 | 0.23 | 43 | 2 | 2, 18 |
| Reticulum | 0.25 | 0.05 | 43 | 2 | 2, 18 |
| Omasum | 0.22 | 0.06 | 52 | 3 | 1, 2, 18 |
| Abomasum | 0.42 | 0.14 | 80 | 4 | 1, 2, 14, 18 |
| Small intestine | 2.07 | 0.40 | 120 | 5 | 6, 14, 18–20 |
| Doudenum | 0.09 | 0.03 | 9 | 1 | 1 |
| Ileum | 0.16 | 0.09 | 9 | 1 | 1 |
| Jejunum | 0.56 | 0.09 | 9 | 1 | 1 |
| Large intestine | 1.15 | 0.26 | 120 | 5 | 6, 14, 18–20 |
| Heart | 0.42 | 0.08 | 146 | 8 | 1–3, 6, 10, 14, 18, 19 |
| Kidneys | 0.29 | 0.06 | 165 | 10 | 1–3, 6, 10, 11, 14, 18–20 |
| Liver | 1.66 | 0.24 | 249 | 13 | 1–4, 6, 10–14, 18–20 |
| Lungs | 1.72 | 0.22 | 201 | 7 | 3, 6, 10, 12, 14, 18, 19 |
| Muscle | 24.78 | 2.20 | 43 | 2 | 7, 16 |
| Pancreas | 0.10 | 0.02 | 31 | 4 | 1–3, 11 |
| Pituitary | 0.0016 | 0.0004 | 28 | 3 | 2–4 |
| Skin | 9.40 | 0.77 | 38 | 1 | 6 |
| Spleen | 0.31 | 0.13 | 73 | 6 | 1–3, 10, 11, 14 |
| Thyroid | 0.010 | 0.002 | 21 | 2 | 2, 3 |
| Uterus (female) | 3.52 | 1.05 | 7 | 1 | 4 |
| Udder (female) | 0.57 | 0.22 | 5 | 1 | 22 |
| Rest of body | 23.32 |
The studies involved in the organ weight or tissue volume calculations are: 1. Barnes et al. (1983); 2. Hales and Fawcett (1993); 3. Moss et al. (2005); 4. Vonnahme et al. (2003); 5. Gregory et al. (1986); 6. Joy et al. (2008); 7. Murray and Slezacek (1976); 8. Butterfield and Thompson (1983); 9. Pittroff et al. (2006); 10. Hansard (1956); 11. Macgregor and Gerrard (1980); 12. Riley et al. (1989); 13. Davison et al. (1965); 14. Kamalzadeh et al. (1998); 15. Anosa and Isoun (1976); 16. Elsley et al. (1964); 17. Perry et al. (1992); 18. McLeod and Baldwin (2000); 19. Rompala et al. (1988); 20. Burrin et al. (1990); 21. Tompson (1980); 22. Mahgoub and Lodge (1998).
Table 4.
Relative organ weight (percent of body weight) or tissue volume for market‐age sheep with the average body weight of 58.73 kg (body weights are higher than 40 kg)
| Mean | SD | Number of animals | Number of studies | References | |
|---|---|---|---|---|---|
| Adrenals | 0.006 | 0.002 | 13 | 2 | 1, 2 |
| Adipose Tissue | 20.98 | 2.68 | 14 | 2 | 3, 4 |
| Blood | 4.86 | 0.50 | 21 | 5 | 5–9 |
| Bone | 5.31 | 0.53 | 44 | 2 | 4, 10 |
| Brain | 0.21 | 0.02 | 6 | 1 | 1 |
| GI tract | 4.45 | 0.54 | 23 | 3 | 5, 8, 11 |
| Stomach | 2.17 | 0.42 | 10 | 1 | 12 |
| Rumen | 1.27 | 0.18 | 14 | 1 | 11 |
| Reticulum | 0.28 | 0.04 | 14 | 1 | 11 |
| Omasum | 0.26 | 0.05 | 14 | 1 | 11 |
| Abomasum | 0.43 | 0.14 | 22 | 2 | 8, 11 |
| Small intestine | 1.28 | 0.22 | 32 | 3 | 8, 11, 12 |
| Large intestine | 0.92 | 0.14 | 32 | 3 | 8, 11, 12 |
| Heart | 0.38 | 0.07 | 46 | 5 | 1, 6, 8–10 |
| Kidneys | 0.23 | 0.03 | 47 | 6 | 1, 5, 6, 8, 11, 12 |
| Liver | 1.27 | 0.24 | 54 | 8 | 1, 2, 5, 6–8, 11, 12 |
| Lungs | 1.07 | 0.24 | 46 | 5 | 1, 6, 8, 11, 12 |
| Pancreas | 0.10 | 0.02 | 7 | 2 | 1, 5 |
| Pituitary | 0.0018 | 0.0004 | 13 | 2 | 1, 2 |
| Spleen | 0.28 | 0.10 | 23 | 4 | 1, 5, 6, 8 |
| Thyroid | 0.0080 | 0.0002 | 6 | 1 | 1 |
| Uterus (female) | 3.52 | 1.05 | 7 | 1 | 2 |
| Udder (female) | 0.57 | 0.22 | 5 | 1 | 13 |
| Rest of body | 56.55 |
The studies involved in the organ weight or tissue volume calculations are: 1. Moss et al. (2005); 2. Vonnahme et al. (2003); 3. Gregory et al. (1986); 4. Butterfield and Thompson (1983); 5. Macgregor and Gerrard (1980); 6. Hansard (1956); 7. Davison et al. (1965); 8. Kamalzadeh et al. (1998); 9. Anosa and Isoun (1976); 10. Perry et al. (1992); 11. McLeod and Baldwin (2000); 12. Rompala et al. (1988); 13. Thompson (1980).
Table 5.
Relative organ weight (percent of body weight) or tissue volume for lambs with the average body weight of 28.34 kg (body weights are in the range of 7–40 kg)
| Mean | SD | Number of animals | Number of studies | References | |
|---|---|---|---|---|---|
| Adrenals | 0.008 | 0.002 | 24 | 2 | 1, 2 |
| Adipose Tissue | 17.67 | 2.48 | 154 | 3 | 3, 5, 12 |
| Blood | 5.29 | 0.54 | 103 | 3 | 6–8 |
| Bone | 6.64 | 0.48 | 43 | 2 | 5, 9 |
| Brain | 0.28 | 0.03 | 15 | 1 | 2 |
| GI tract | 5.08 | 1.42 | 34 | 2 | 8, 10 |
| Stomach | 3.09 | 0.94 | 16 | 1 | 11 |
| Rumen | 0.99 | 0.25 | 29 | 2 | 2, 10 |
| Reticulum | 0.24 | 0.05 | 29 | 2 | 2, 10 |
| Omasum | 0.21 | 0.06 | 38 | 3 | 1, 2, 10 |
| Abomasum | 0.42 | 0.14 | 58 | 4 | 1, 2, 8, 10 |
| Small intestine | 2.36 | 0.45 | 88 | 4 | 4, 8, 10, 11 |
| Doudenum | 0.09 | 0.03 | 9 | 1 | 1 |
| Ileum | 0.16 | 0.09 | 9 | 1 | 1 |
| Jejunum | 0.56 | 0.09 | 9 | 1 | 1 |
| Large intestine | 1.24 | 0.30 | 88 | 4 | 4, 8, 10, 11 |
| Heart | 0.44 | 0.09 | 100 | 6 | 1, 2, 4, 6, 8, 10 |
| Kidneys | 0.31 | 0.06 | 118 | 7 | 1, 2, 4, 6, 8, 10, 11 |
| Liver | 1.77 | 0.24 | 195 | 8 | 1, 2, 4, 6–8, 10, 11 |
| Lungs | 1.91 | 0.21 | 155 | 5 | 4, 6–8, 10 |
| Muscle | 24.78 | 2.20 | 43 | 2 | 5, 9 |
| Skin | 9.40 | 0.77 | 38 | 1 | 4 |
| Pancreas | 0.10 | 0.02 | 24 | 2 | 1, 2 |
| Pituitary | 0.0015 | 0.0003 | 15 | 1 | 2 |
| Spleen | 0.32 | 0.15 | 50 | 4 | 1, 2, 6, 8 |
| Thyroid | 0.010 | 0.002 | 15 | 1 | 2 |
| Rest of Body | 25.99 |
The studies involved in the organ weight or tissue volume calculations are as follows: 1. Barnes et al. (1983); 2. Hales and Fawcett (1993); 3. Gregory et al. (1986); 4. Joy et al. (2008); 5. Murray and Slezacek (1976); 6. Hansard (1956); 7. Riley et al. (1989); 8. Kamalzadeh et al. (1998); 9. Elsley et al. (1964); 10. McLeod and Baldwin (2000); 11. Burrin et al. (1990); 12. Pittroff et al. (2006).
Table 6.
Relative organ weight (percent of body weight) or tissue volume for fetal sheep with the average body weight of 3.40 kg (body weights are in the range of 2.5–5.1 kg)
| Mean | SD | Number of animals | Number of studies | References | |
|---|---|---|---|---|---|
| Adrenals | 0.02 | 0.02 | 55 | 5 | 1–5 |
| Blood | 9.30 | NA | 5 | 1 | 6 |
| Brain | 1.52 | 0.34 | 23 | 2 | 2, 5 |
| GI tract | 4.82 | 0.94 | 18 | 2 | 1, 5 |
| Stomach | 1.56 | 0.30 | 12 | 1 | 1 |
| Omasum | 0.15 | 0.03 | 11 | 1 | 4 |
| Abomasum | 0.28 | 0.07 | 11 | 1 | 4 |
| Small intestine | 2.67 | 0.78 | 12 | 1 | 1 |
| Large intestine | 0.79 | 0.17 | 12 | 1 | 1 |
| Heart | 0.73 | 0.12 | 23 | 3 | 1, 5, 6 |
| Kidneys | 0.56 | 0.28 | 51 | 5 | 1, 2, 4–6 |
| Liver | 2.10 | 0.92 | 60 | 6 | 1–6 |
| Lungs | 2.17 | 0.99 | 60 | 6 | 1–6 |
| Pancreas | 0.07 | 0.05 | 23 | 2 | 1, 4 |
| Spleen | 0.30 | 0.17 | 17 | 2 | 1, 6 |
| Thyroid | 0.02 | 0.01 | 18 | 2 | 1, 5 |
| Thymus | 0.42 | 0.12 | 18 | 2 | 1, 5 |
| Testes (male) | 0.11 | 0.03 | 12 | 1 | 1 |
| Rest of body | 77.85 | 0.02 |
Table 7.
Relative organ weight (percent of body weight) or tissue volume of udder tissues for non‐lactating and lactating sheep
| Non‐lactating | Lactating | |||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| Udder | 0.5672 | 0.2177 | 1.6248 | 0.3776 |
| Udder secretory tissue | 0.3444 | 0.1538 | 1.4101 | 0.3692 |
| Udder adipose tissue | 0.1594 | 0.0607 | 0.0994 | 0.0387 |
| Udder lymph tissue | 0.0075 | 0.0047 | 0.0119 | 0.0034 |
| Udder teat tissue | 0.0075 | 0.0014 | 0.0119 | 0.0035 |
| Udder skin tissue | 0.0485 | 0.0098 | 0.0915 | 0.0256 |
These values are based on a previous study (Thompson, 1980).
Table 8.
Relative organ weight (percent of body weight) or tissue volume for goats with the average body weight as 25.56 kg (body weights are in the range of 10.4–65 kg)
| Mean | SD | Number of animals | Number of studies | References | |
|---|---|---|---|---|---|
| Adipose tissue | 9.14 | 1.82 | 70 | 2 | 1, 2 |
| Blood | 5.49 | 0.51 | 55 | 2 | 3, 4 |
| Bone | 7.84 | 1.30 | 56 | 2 | 1, 6 |
| Brain | 0.32 | 0.03 | 16 | 1 | 6 |
| GI tract | 6.55 | 1.13 | 90 | 4 | 1–3, 6 |
| Rumen | 2.27 | 0.37 | 8 | 1 | 6 |
| Reticulum | 0.32 | 0.08 | 8 | 1 | 6 |
| Omasum | 0.24 | 0.08 | 20 | 2 | 3, 6 |
| Abomasum | 0.86 | 1.10 | 20 | 2 | 3, 6 |
| Small intestine | 1.96 | 0.70 | 28 | 2 | 3, 6 |
| Large intestine | 1.40 | 0.36 | 28 | 2 | 3, 6 |
| Cecum | 0.12 | 0.03 | 12 | 1 | 3 |
| Heart | 0.44 | 0.06 | 105 | 6 | 1–3, 5, 6 |
| Kidneys | 0.38 | 0.06 | 28 | 2 | 3, 6 |
| Liver | 1.89 | 0.29 | 82 | 3 | 1–3 |
| Lungs | 1.22 | 0.23 | 98 | 4 | 1–3, 6 |
| Muscle | 38.58 | 3.18 | 48 | 2 | 1, 6 |
| Skin | 6.77 | 1.11 | 82 | 3 | 1–3 |
| Spleen | 0.19 | 0.03 | 68 | 3 | 1, 3, 6 |
| Udder (female) | 1.78 | 0.54 | 7 | 1 | 7 |
| Rest of body | 19.42 |
3.3.1. Adrenals
The adrenal gland data of sheep were obtained from four different studies (Barnes et al., 1983; Hales & Fawcett, 1993; Moss et al., 2005; Vonnahme et al., 2003). The adrenal glands constitute approximately 0.007% of the body weight in sheep including both market‐age sheep and lambs (Table 3). The mean value for market‐age sheep is 0.006% (Table 4) and is 0.008% for lambs (Table 5). These values are comparable to the tissue weight of adrenal glands in beef cattle and dairy cows of 0.006% (Lin et al., 2020). The value of adrenal glands for fetal sheep is 0.022% (Table 6), which is much higher than those in market‐age sheep and lambs. All the values reported for sheep are in the range of previously reported values of 0.004%–0.14% in dogs, 0.02% in humans, and 0.01%–0.04% in mice (Brown et al., 1997). Data on the relative weight of adrenal glands are not available in goats.
3.3.2. Adipose tissue
The weight of adipose tissue reflects the weight of dissectible fat tissue only for sheep and goats. The mean relative tissue weight of adipose in sheep is 17.94% (Table 3), 20.98% for market‐age sheep (Table 4), and 17.67% for lambs (Table 5). The value is not available for fetal sheep. The summarized relative weights of adipose tissue in sheep and lambs are comparable to the values of 16.8% from previous studies in sheep (Craigmill, 2003; Upton, 2008) and 19.2% from another review article (Lautz et al., 2020) on physiological parameters in farm animals. The relative adipose tissue weights for goats were calculated based on two previous studies (Mahgoub, 1997; Mahgoub & Lu, 1998), and the average value is 9.14% (Table 8). The value in goats is close to the value of 8.5% in a previous PBPK model for goats (Leavens et al., 2012). All these values reported here for sheep and goats are within the range of ~7% to ~21.42% for mice, rats, and humans, and these values vary depending on the sex and the measurement method (Brown et al., 1997).
3.3.3. Blood
The calculated mean for relative blood weight in sheep is 5.22% in Table 3, and 4.86% and 5.29% for market‐age sheep and lambs, respectively, which are all comparable to the value of 4.31% in cattle (Lin et al., 2020) and close to the values of 5.7% (Upton, 2008) and 4.7% (Lautz, Dorne, et al., 2020) from previous reviews on sheep. Based on data reported in a previous study for neonatal sheep (Hansard, 1956), the average relative weight of the blood in fetal and neonatal sheep is approximately 9.30% of the body weight as shown in Table 6. The calculated relative blood weight for goats is 5.49% (Table 8). The values in sheep and goats are within the range of 4.9%–8.2% for the species of mice, rats, dogs, and humans (Brown et al., 1997), except the value in fetal sheep is slightly higher. The venous blood to arterial blood ratio is also an important parameter for PBPK modeling, especially when lung is included as an individual compartment and blood is separated into venous and arterial blood. However, no experimental study was identified for this parameter in sheep and goats.
3.3.4. Bone
The calculated mean relative weight value of bone is 5.96% for sheep (Table 3) based on data from four different studies (Butterfield & Thompson, 1983; Elsley et al., 1964; Murray & Slezacek, 1976; Perry et al., 1992). The average value for relative bone weight in market‐age sheep is 5.31% (Table 4), and in lambs is 6.64% (Table 5). These values for sheep are slightly lower than the value of 7% reported in a previous review paper for a standard size of 45 kg sheep (Upton, 2008). The relative bone weight for fetal sheep is not available. The mean relative bone weight for goats is 7.84% (Table 8), and this value was calculated based on values reported in two studies (Davis et al., 1975; Mahgoub, 1997). The calculated mean values for sheep and goats are comparable to the values of ~5.4% in mice, 5%–7% in rats, ~8.1% in dogs, and ~7.1% in humans (Brown et al., 1997).
3.3.5. Brain
The calculated mean value for relative brain weight in sheep is 0.26% (Table 3) based on data from two different studies (Hales & Fawcett, 1993; Moss et al., 2005), for market‐age sheep 0.21% (Table 4), for lambs 0.28% (Table 5), and for fetal sheep 1.52% (Table 6). The calculated value for relative brain weight in goats is 0.32% (Table 8). These values are close to the value of 0.2% reported for sheep in previous review articles (Lautz, Dorne, et al., 2020; Upton, 2008). Except for the value for fetal sheep, the values for relative brain weight in sheep and goats are much lower than the reported values in other species, which are in the range of 0.6% in rats to 2.0% in humans (Brown et al., 1997), but they are comparable to the value in swine of 0.22% (Lin et al., 2020). Our calculated relative brain weights of 0.26% for sheep and 0.32% for goats are also comparable to the reported values of 0.191% for 57.6 kg sheep and 0.416% for 31.3 kg goats from Boxe nbaum (1982). Using an allometric equation between brain weight and body weight (Boxe nbaum & D'Souza, 1990; R. D. Martin, 1981), the calculated relative brain weights are 0.395% for market‐age sheep (62.88 kg) and 0.361% for mature goats of 90.7 kg. This calculated percentage is slightly higher than our compiled value for sheep (0.26% vs. 0.395%), but very similar for goats (0.32% vs. 0.361%).
3.3.6. Gastrointestinal tract
The calculated values of total gastrointestinal (GI) tract and individual segments of the GI tract reported here already excluded the contents in GI tract. The mean value for relative tissue weight of total GI tract for sheep (pooled with market‐age sheep and lambs) is 4.83% (Table 3). The sum of individually reported relative tissue weights of stomach (2.73%), small (2.07%), and large intestines (1.15%) in Table 3 is 5.95%. The variability between these two values is due to the fact that they were calculated based on different studies. The relative tissue weights of GI tract for market‐age sheep are 4.45% (Table 4), for lambs 5.08% (Table 5), and for fetal sheep 4.82% (Table 6). The mean relative GI tract tissue weight is 6.55% for goats (Table 8). The relative GI tract tissue weight of sheep and goats are higher than the values reported for mammals without a ruminant digestive system (e.g., 4.2% for mice, 2.7% for rats, 3.7% for dogs, and 1.7% for humans) (Brown et al., 1997). However, these calculated values of sheep and goats are comparable to the values reported previously for sheep as 6.4% (Upton, 2008) and 5.4% (Lautz, Dorne, et al., 2020), and also close to 5.98% reported for cattle (Lin et al., 2020). The food or chemical residue transit time in each GI tract segment or gastric emptying time is important for the physiologically based model for GI tract. However, limited information is available for these parameters in sheep and goats. The average retention time in GI tract for sheep is in the range of 40.80–47.4 hr (Hadjigeorgiou et al., 2000; Kennedy et al., 1992; Tsiplakou et al., 2011) and for goats is 27.81 hr (Tsiplakou et al., 2011). The rumen retention time is 30.03 hr for sheep and much shorter for goats as 14.43 hr, and the cecum retention time is similar between sheep and goats as 1.91 hr for sheep and 1.89 hr for goats (Tsiplakou et al., 2011). In addition, previous studies have shown that intake food amounts (P. C. Gregory et al., 1985) and feeding practices (Tsiplakou et al., 2011) can significantly impact the food transit time in GI tract for sheep and goats.
3.3.7. Heart
The calculated mean relative organ weight of heart is 0.42% for sheep (pooled data from market‐age sheep and lambs) as shown in Table 3, 0.38% for market‐age sheep (Table 4), 0.44% for lambs (Table 5), and 0.73% for fetal sheep (Table 6). The relative organ weight of heart constitutes about 0.44% of the total body weight of goats (Table 8). The relative organ weights of heart for sheep and goats are comparable to the values of 0.37% (Upton, 2008) and 0.4% (Lautz, Dorne, et al., 2020) previously reported for sheep. All these values for sheep and goats are in the range of relative heart weight from 0.27% to 0.85% reported for mice, rats, dogs, and humans (Brown et al., 1997). However, the average value for fetal sheep, which is based on data from 3 different studies (Creasy et al., 1972; Greenwood et al., 2004; Hansard, 1956) with the range from 0.725% to 0.74%, is higher compared to values for market‐age sheep and lambs.
3.3.8. Kidneys
The relative organ weight for kidneys is reported as the sum of weight of both kidneys. The calculated mean relative organ weight of kidneys is 0.29% for sheep with data from both market‐age sheep and lambs (Table 3), 0.23% for market‐age sheep (Table 4), 0.31% for lambs (Table 5), and 0.56% for fetal sheep (Table 6). The kidneys constitute about 0.38% of the body weight in goats (Table 8). Except the value for fetal sheep, all other values for sheep and goats are close to the values reported in previous reviews as 0.46% (Upton, 2008) and 0.30% (Lautz, Dorne, et al., 2020) for sheep; and these values are also comparable to the reported values of 0.21% for adult cattle and 0.37% for swine (Lin et al., 2020). The higher relative organ weight value reported for fetal sheep may be due to the much lower body weight of fetal sheep. This explanation applies to the relative weights of a few other organs, such as brain, liver, blood, and lungs that are higher in the fetal sheep than in the market‐age sheep.
3.3.9. Liver
The calculated mean relative liver weight in sheep is 1.66% (Table 3), 1.27% for market‐age sheep (Table 4), 1.77% for lambs (Table 5), and 2.10% for fetal sheep (Table 6). The relative organ weight of liver in goats is 1.89% (Table 8). All these values are much lower than the values of 5.49%, 3.66%, 3.29%, and 2.57% reported for mice, rats, dogs, and humans, respectively (Brown et al., 1997). However, the values of relative liver organ weights for sheep and goats are close to the values of 1.6% (Upton, 2008) and 1.5% (Lautz, Dorne, et al., 2020) from previous reviews for sheep, and also comparable to the values of 1.23% in cattle and 2.04% in swine (Lin et al., 2020). The lower values of relative liver organ weights in food‐producing animals may be due to larger body size and body weight compared to laboratory animals.
3.3.10. Lungs
The calculated relative organ weight for lungs is 1.72% for sheep with data from market‐age sheep and lambs (Table 3), 1.07% for market‐age sheep (Table 4), 1.91% for lambs (Table 5), and 2.17% for fetal sheep (Table 6). The lungs constitute 1.22% of the body weight in goats (Table 8). All these values are comparable to the reported values of relative lung weight for sheep as 1.0% (Upton, 2008) and 1.1% (Lautz, Dorne, et al., 2020) from previous reviews for sheep, and also close to the values of 0.77% in adult cattle and 0.9% in swine (Lin et al., 2020).
3.3.11. Muscle
The calculated mean relative weight of muscle for sheep using all available data is 24.78% (Table 3), which is based on two studies with 43 lambs (Table 5) (Elsley et al., 1964; Murray & Slezacek, 1976). There are no available data on relative muscle weight for market‐age sheep and fetal sheep. As shown in Table 8, the weight of muscle for goats constitutes approximately 38.58% of the body weight. The value in sheep is close to the value of 27.7% reported in a previous review (Upton, 2008). The value in goats is close to the values for adult cattle as 36.10% and for swine as 36.32% of body weight (Lin et al., 2020).
3.3.12. Pancreas
The calculated mean value is 0.10% for relative weight of pancreas in sheep, market‐age sheep and lambs as shown in Tables 3, 4, 5, and 0.07% for fetal sheep (Table 6). These values are slightly lower than the reported values in the range of 0.14%–0.7% for mice, rats, dogs, and humans (Brown et al., 1997). There are no data available for tissue weight of the pancreas in goats. The relative organ weights of the pancreas for sheep, lambs and fetal sheep are close to the values of 0.09% in adult cattle and 0.15% in swine (Lin et al., 2020).
3.3.13. Pituitary
The calculated mean value for relative organ weight of pituitary in sheep pooled with market‐age sheep and lambs is 0.0016% (Table 3), 0.0018% for market‐age sheep (Table 4), and 0.0015% for lambs (Table 5). These values are not available in other species.
3.3.14. Spleen
Spleen constitutes about 0.31% of the body weight for sheep using pooled data from market‐age sheep and lambs (Table 3), 0.28% for market‐age sheep (Table 4), 0.32% for lambs (Table 5), and 0.30% for fetal sheep (Table 6). The average value of relative spleen weight for goats is 0.19% (Table 8). All these values in sheep and goats are comparable to the values in adult cattle as 0.18% and swine as 0.20% (Lin et al., 2020), and fall in or close to the range of 0.20%–0.35% for mice, rats, dogs, and humans (Brown et al., 1997).
3.3.15. Thymus
The mean relative weight of thymus is 0.42% in fetal sheep (Table 6). The data are very limited, and not available in other age groups of sheep, nor in goats. The value of relative organ weight of thymus for fetal sheep is comparable to the value of 0.23% in calves and 0.28% in swine (Lin et al., 2020).
3.3.16. Thyroid
The mean relative weight of the thyroid gland is 0.010% of body weight in sheep with pooled data from market‐age sheep and lambs (Table 3), 0.008% for market‐age sheep (Table 4), 0.010% for lambs (Table 5), and 0.02% for fetal sheep (Table 6). Relative weight data for thyroid glands are not available in goats. The relative weight of thyroid gland for sheep is comparable to the values of 0.005% in rats, 0.008% in dogs, and 0.03% in humans (Brown et al., 1997), as well as 0.011% in swine (Lin et al., 2020).
3.3.17. Mammary glands
The physiological parameters for mammary glands (i.e., udder) in sheep and goats are important for extrapolating PBPK models from meat sheep or goats to dairy sheep or goats. However, very limited data are available for mammary glands in sheep and goats. Available data for the weight of mammary glands were obtained from only one study in sheep (Thompson, 1980) and one study in lactating goats (Reynolds et al., 1968). The mammary glands constitute 0.57% of body weight for non‐lactating sheep (Table 3 and Table 4) and 1.62% for lactating sheep, and 1.78% for lactating goats (Table 8). The relative weights of different tissue components of mammary glands in non‐lactating and lactating sheep were obtained from the study by Thompson (1980), and are shown in Table 7. The relative weight of mammary glands for lactating goats is similar to the value of 1.67% for dairy cows (Lin et al., 2020), but the value for non‐lactating sheep is lower than lactating sheep, goats, and cows.
3.3.18. Reproductive organs
Limited data are available for reproductive organs in food‐producing animals. The relative weight of the uterus in pregnant sheep is 3.52% and shown in Tables 3 and 4 based on the data from one study of 7 animals (Vonnahme et al., 2003). The relative weight of the testes is 0.11% (Table 6), which is comparable to the value of 0.1% for beef cattle (Lin et al., 2020), but the relative weight of uterus in pregnant sheep is higher than the value in dairy cows as 1.09% (Lin et al., 2020), in part, because the uterus weight was measured in pregnant sheep (Vonnahme et al., 2003).
3.3.19. Mass balance
The value for the relative organ weight of the rest of body is included in the table to maintain the mass balance and to keep the sum of total relative organ weight fractions to be 100%. The value for rest of body in Table 3 for sheep is 23.32%, in Table 5 for lambs is 25.99%, and in Table 8 for goats is 19.42%, which counts for the ear, horn, eye, hoof, hair, some of the reproductive organs, and GI tract contents. As the value for relative muscle weight is not available for market‐age sheep, the rest of body value is 56.55% in Table 4, which includes around 24.78% of muscle weight. Without data for adipose tissue, bone, muscle, and skin, the value for rest of body for fetal sheep is 77.85% (Table 6), and according to Table 3 these four tissues should constitute around 58.08% of the total body weight in sheep.
3.4. Cardiac output
All cardiac output data in sheep and goats are from unanesthetized and resting animals only. Aesthesia and excise have impacts on the cardiac output in animals. The cardiac output values for sheep and goats are summarized in Tables 9 and 10, respectively. The value for cardiac output of sheep was calculated based on 11 different studies involving both lambs and adult sheep (Dodic et al., 2001; Evans et al., 1998; Gregory et al., 1986; Hales, 1973a, 1973b, 1973c; Hales et al., 1976; Hales & Fawcett, 1993; Schiffer et al., 1993; Talke et al., 2000; Ullman et al., 2001). The average value of cardiac output for sheep is 7.15 L hr−1 kg−1 body weight. The cardiac output value for goats was calculated based on data from three studies (Barcroft et al., 1934; Chaiyabutr et al., 1980; Kutter et al., 2006). The mean cardiac output value is 8.17 L hr−1 kg−1 body weight for goats. In the review paper by Upton (2008), the cardiac output of sheep was reported as 5,342 ml/min based on a standard sheep with body weight of 45 kg, which is equivalent to 7.12 L hr−1 kg−1 body weight after unit conversion. This value is similar to the cardiac output value of sheep reported in this manuscript. Cardiac output value in goats was not reported in previous review papers for physiological parameters (Brown et al., 1997; Upton, 2008). The raw data and data analysis for cardiac output for sheep are included in Excel File B3, and for goals are in Excel File C3 in the Supporting Information.
Table 9.
Cardiac output (L hr−1 kg−1 body weight) in unanesthetized sheep
| Mean | SD | Number of animals | Number of studies |
|---|---|---|---|
| 7.15 | 2.47 | 126 | 11 |
Table 10.
Cardiac output (L hr−1 kg−1 body weight) in unanesthetized goats
| Mean | SD | Number of animals | Number of studies |
|---|---|---|---|
| 8.17 | 1.79 | 29 | 3 |
3.5. Blood flow
Blood flow fractions are only reported for sheep in this manuscript (Table 11). Blood flow fractions are not summarized for goats due to insufficient data. Only blood flows to mammary glands (Linzell, 1960; Linzell & Fleet, 1966; Maltz et al., 1984) and brain (Pelligrino et al., 1984) were found for goats. Before all the experimentally measured blood flow fraction parameter values become available for goats, a similar strategy as used in a previous PBPK model in goats (Leavens et al., 2012) can be used to adapt values of organ blood flow fractions from sheep to goats.
Table 11.
Regional blood flow distribution percent of cardiac output in sheep
| Mean | SD | Number of animals | Number of studies | References | |
|---|---|---|---|---|---|
| Adrenals | 0.20 | 0.076 | 58 | 5 | 1–5 |
| Adipose Tissue | 6.18 | 4.756 | 16 | 1 | 6 |
| Brain | 2.95 | 1.187 | 54 | 5 | 1, 2, 4, 5, 7 |
| Heart | 5.59 | 3.000 | 61 | 6 | 1–5, 7 |
| Muscle | 10.09 | 4.206 | 46 | 4 | 4, 5, 7, 8 |
| Kidneys | 12.98 | 5.670 | 96 | 8 | 1–5, 7, 10, 11 |
| Liver | 1.20 | 0.445 | 9 | 1 | 3 |
| Pancreas | 2.35 | 0.865 | 24 | 2 | 3, 4 |
| Stomachs | |||||
| Rumen | 2.57 | 0.977 | 15 | 1 | 4 |
| Reticulum | 0.92 | 0.346 | 15 | 1 | 4 |
| Omasum | 0.93 | 0.328 | 24 | 2 | 3, 4 |
| Abomasum | 3.34 | 1.043 | 24 | 2 | 3, 4 |
| Small intestine | 10.70 | 1.263 | 41 | 3 | 1, 4, 5 |
| Large Intestine | 8.53 | 1.993 | 46 | 4 | 1, 2, 4, 5 |
| Pituitary | 0.010 | 0.003 | 41 | 3 | 1, 4, 5 |
| Skin | 10.93 | 3.124 | 18 | 2 | 2, 5 |
| Spleen | 4.54 | 2.944 | 58 | 5 | 1–5 |
| Thyroid | 0.36 | 0.196 | 49 | 4 | 1, 2, 4, 5 |
| Udder (female) | 0.35 | 0.168 | 5 | 1 | 12 |
| Rest of body | 15.28 | ||||
The regional blood flow fractions of sheep were calculated based following studies: 1. Hales (1973c); 2. Hales et al. (1976); 3. Barnes et al. (1983); 4. Hales and Fawcett (1993); 5. Hales (1973b); 6. Gregory et al. (1986); 7. Alexander et al. (1973); 8. Hales (1973a); 9. Gu et al. (1985); 10. Ullman et al. (2001); 11. Robillard and Weitzman (1980); 12. Thompson (1980). If no cardiac output reported in a specific study, the regional blood flow fractions were calculated using the average cardiac output of sheep reported in Table 9.
The units of blood flow for organs were standardized to percentage of cardiac output. The mean cardiac output reported in Table 9 was used for those studies that did not report values for cardiac output, including for non‐lactating and lactating sheep. The distribution of blood flow to different muscle groups may differ depending on the activities of those muscle groups (Brown et al., 1997). The present study selected hind limbs to represent the blood flow to muscle in sheep because it is a commonly selected area to measure muscle blood flow (Alexander et al., 1973; Hales & Fawcett, 1993). The blood flow fractions of udder tissues were calculated based on the data of five animals from a previously reported study using non‐lactating and lactating sheep (Thompson, 1980) and are shown in Table 12. The uterine and umbilical blood flows in pregnant sheep are reported in Table 13, and these data are based on pooled data from 21 pregnant animals from two different studies (Carver & Hay, 1995; Gu et al., 1985). All raw data and data calculations for blood flow fractions for sheep are provided in Excel File B4 of Supporting Information.
Table 12.
Regional blood flow distribution percent of cardiac output for udder tissues in lactating sheep
| Non‐Lactating | Lactating | |||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| Udder | 0.349 | 0.168 | 9.433 | 5.145 |
| Udder secretory tissue | 0.146 | 0.075 | 9.264 | 5.093 |
| Udder adipose tissue | 0.075 | 0.060 | 0.060 | 0.024 |
| Udder lymph tissue | 0.086 | 0.056 | 0.061 | 0.036 |
| Udder teat tissue | 0.005 | 0.003 | 0.009 | 0.004 |
| Udder skin tissue | 0.037 | 0.028 | 0.039 | 0.024 |
The values are based on the data of five animals from a previous study (Thompson, 1980).
Table 13.
Regional blood flow distribution percent of cardiac output for uterine and umbilical blood flow in pregnant sheep
| Mean | SD | |
|---|---|---|
| Uterine blood flow | 22.07 | 11.11 |
| Umbilical blood flow | 12.38 | 7.51 |
3.5.1. Adrenals
The blood flow fraction to adrenal glands in sheep is 0.20% of cardiac output (Table 11). This parameter was not reported in the two previous review articles on physiological parameters in sheep (Lautz, Dorne, et al., 2020; Upton, 2008). This value is similar to the values in other species, which are 0.3% in rats, 0.2% in dogs (Brown et al., 1997), and 0.06% in swine (Lin et al., 2020).
3.5.2. Adipose tissue
The blood flow fraction to adipose tissue in sheep constitutes 6.18% of total cardiac output (Table 11). This value falls in the range of 6%–15% reported in a previous PBPK model for sheep (Craigmill, 2003), and is comparable to the value of 7.0% in rats and 5.2% in humans (Brown et al., 1997). However, this value is higher than 2.3% reported in a recent review paper in sheep (Lautz, Dorne, et al., 2020).
3.5.3. Brain
The blood flow fraction to the brain is 2.95% of total cardiac output in sheep (Table 11). This value was based on data from 5 different studies with 54 animals (Alexander et al., 1973; Hales, 1973b, 1973c; Hales et al., 1976; Hales & Fawcett, 1993). This value is comparable to the value of 2% for dogs and rats, and 3.3% for mice (Brown et al., 1997), and is slightly higher than 1.9% for sheep reported in another review paper (Lautz, Nebbia, et al., 2020). This parameter value is much higher in humans, with a value of 11.4% (Brown et al., 1997).
3.5.4. Gastrointestinal tract
The blood flow fractions to the rumen, reticulum, omasum, abomasum, small intestine, and large intestine in sheep are 2.57%, 0.92%, 0.93%, 3.34%, 10.70%, and 8.53% of cardiac output, respectively (Table 11). The sum of the GI tract segmental blood flow fractions is 26.99%, which represents the blood flow fraction that travels to the liver through the portal vein. This value is very close to the mean value of 28% for the portal vein blood flow in calves, but lower than 39% for the adult cattle and slightly higher than 19.9% for swine (Lin et al., 2020).
3.5.5. Heart
The calculated average blood flow fraction to the heart in sheep of 5.64% (Table 11) is comparable to the values reported in previous review papers of 4.89% (Upton, 2008) and 4.5% (Lautz, Dorne, et al., 2020) for sheep. The blood flow fraction to the heart in sheep falls in the range of 4.0%–6.6% in mice, rats, dogs, and humans (Brown et al., 1997). It is similar to the value of 6% in calves, but larger than the value of 3% in swine (Lin et al., 2020).
3.5.6. Muscle
The calculated mean blood flow fraction to muscle in sheep is 10.09% of cardiac output as shown in Table 11. This value is lower than the range of 16.1%–29.7% in mice, rats, dogs, and humans (Brown et al., 1997), and less than 28% in adult cattle and 34.2% in swine (Lin et al., 2020), but it is comparable to the value of 13.74% in sheep reported in another review paper (Upton, 2008). However, in a recent review article, the fractional blood flow to muscle was reported as 33.2% of cardiac output (Lautz, Dorne, et al., 2020). The discrepancy of this parameter value between different studies could not be rectified.
3.5.7. Kidneys
The blood flow fraction to kidneys is 12.98% of cardiac output in sheep. This value is close to the values of 14.3% (Lautz, Dorne, et al., 2020) and 16.73% (Upton, 2008) from previous review papers on sheep. The value of blood flow fraction to kidneys in sheep falls in the range of 9.1%–17.5% for mice, rats, dogs, and humans (Brown et al., 1997). This value is also similar to the values of 10% in adult cattle and 11.4% in swine (Lin et al., 2020). The glomerular filtration rate (GFR) plays an important role in urine elimination. GFRs were reported in previous studies for sheep (Nesje et al., 1997; Parry & Taylor, 1956) and goats (Eriksson & Valtonen, 1982; Silanikove, 1984), and summarized in a review paper (Skotnicka et al., 2007) as 58 ml/min/m2 for sheep and 53 ml min−1 m−2 for goats. A previous PBPK modeling study of penicillin G reported that a lower urine elimination rate in heavy sows would lead to a longer withdrawal interval than that from market‐age swine (Li, Mainquist‐Whigham, et al., 2019). Similarly, the declined renal blood flow and decreased GFR were reported in aged female sheep (Lankadeva et al., 2014).
3.5.8. Liver
The liver blood flow fraction reported here is specific for the hepatic artery blood flow only, and it constitutes 1.20% of cardiac output in sheep (Table 11). However, the total blood flow to liver, represented as a combination of blood flow in the hepatic artery and portal vein, is commonly used in PBPK models. The total blood flow to liver can be obtained by the sum of hepatic artery blood flow (1.20%) and GI tract blood flow (26.99%) to be 28.19%. This value is higher than the value of 18.3% used in a previous PBPK model for sheep (Craigmill, 2003), and comparable to the total liver blood flow fraction of 24.3% in swine (Lin et al., 2020). The values of total blood flow fractions to liver are also in the range of 16.1%–29.7% in mice, rats, dogs, and humans (Brown et al., 1997). However, it is lower than the value of 46% from adult cattle (Lin et al., 2020) and the previously reported value of 47.40% for sheep (Upton, 2008).
3.5.9. Udder
The blood flow to the mammary gland (i.e., udder) in non‐lactating and lactating sheep was obtained from one study (Thompson, 1980), which was reported as 0.349% and 9.433% for non‐lactating and lactating sheep, respectively (Tables 11 and 12). Lactation increases the blood flow fraction to the mammary gland by ~27‐fold (Thompson, 1980). The blood flow to different components of mammary glands for non‐lactating and lactating sheep was also calculated and included in Table 12. The value of blood flow to mammary glands in lactating sheep is slightly higher than the value of 7.4% reported for sheep in another review paper (Lautz, Dorne, et al., 2020), and is smaller than the value of 13% reported for dairy cows (Lin et al., 2020).
3.5.10. Reproductive organs
There are limited data available for blood flow to reproductive organs in sheep. The values of blood flow to the uterus and through the umbilical cord of pregnant sheep were calculated based on data from two studies (Carver & Hay, 1995; Gu et al., 1985) and included in Table 13. The percentage of cardiac output is 22.07% and 12.38% flowing to the uterus and umbilical cord, respectively, in pregnant sheep.
3.5.11. Mass balance
The value for the blood flow to the rest of body is included in the table to maintain the mass balance. The blood flow fraction to the rest of body for sheep is 15.28% (Table 11), which accounts for the blood flow fractions to the ear, eye, bone, some reproductive organs, etc. As the values for individual organs are from different studies, the sum of these values may be higher or lower than the actual value due to variabilities between studies and between measurement methods.
3.6. Vascular space
Data for vascular fractions of blood in organs were obtained from only one available study reporting values from 6 sheep (Hansard, 1956). Data are not available for goats. Values of most key organs for PBPK modeling were reported in this study. This data set is comprised of two groups of sheep with different ages. One group was composed of 3 market‐age sheep with an average body weight of 50.49 kg, which is close to the average market weight for sheep (62.88 kg). The other group was composed of 3 lambs with an average body weight of 4.23 kg. The values of vascular fractions of blood in organs of sheep combining all data are summarized in Table 14, and the values for market‐age sheep and lambs are presented in Table 15 and Table 16, respectively. All raw data and data analysis for vascular space of organs in sheep are provided in Excel File B5 of Supporting Information.
Table 14.
Vascular space or volume fraction of blood (% of organ weight, unitless) in organs and tissues of sheep with different ages
| Mean | SD | |
|---|---|---|
| Spleen | 34.75 | 1.60 |
| Lungs | 22.42 | 5.54 |
| Liver | 8.83 | 1.28 |
| Kidneys | 5.62 | 0.60 |
| Pituitary | 2.12 | 0.21 |
| Adrenal | 2.53 | 0.95 |
| Heart | 5.53 | 0.69 |
| Pancreas | 3.18 | 0.77 |
| Loin muscle | 1.53 | 0.61 |
| Gastrocnemius Muscle | 1.40 | 0.43 |
Limited data were identified for the residual blood volume in organs of sheep. All data reported in this table were from the study by Hansard (1956) with values from six animals.
Table 15.
Vascular space or volume fraction of blood (% of organ weight, unitless) in organs and tissues of sheep at market age
| Mean | SD | |
|---|---|---|
| BW (kg) | 50.49 | 9.78 |
| Spleen | 34.67 | 1.94 |
| Lungs | 26.63 | 4.45 |
| Liver | 8.10 | 1.32 |
| Kidneys | 5.67 | 0.64 |
| Pituitary | 2.20 | 0.26 |
| Adrenal | 3.17 | 1.00 |
| Heart | 5.13 | 0.64 |
| Pancreas | 3.27 | 0.75 |
| Loin muscle | 1.17 | 0.31 |
| Gastrocnemius Muscle | 1.13 | 0.40 |
Limited data were identified for the residual blood volume in organs of sheep. All data reported in this table were from the study by Hansard (1956) with values from three animals.
Table 16.
Vascular space or volume fraction of blood (% of organ weight, unitless) in organs and tissues of lambs
| Mean | SD | |
|---|---|---|
| BW (kg) | 4.23 | 1.39 |
| Spleen | 34.83 | 1.63 |
| Lungs | 18.20 | 1.90 |
| Liver | 9.57 | 0.87 |
| Kidneys | 5.57 | 0.71 |
| Pituitary | 2.03 | 0.15 |
| Adrenal | 1.90 | 0.20 |
| Heart | 5.93 | 0.55 |
| Pancreas | 3.10 | 0.95 |
| Loin muscle | 1.90 | 0.66 |
| Gastrocnemius Muscle | 1.67 | 0.31 |
Limited data were identified for the residual blood volume in organs of sheep. All data reported in this table were from the study by Hansard (1956) with values from three animals.
3.7. Milk components and secretion
Besides meat production from sheep and goats, they are also important sources for dairy products. The milk components and physicochemical characteristics have been comprehensively reviewed in a recent article (Balthazar et al., 2017). The amount of different milk components relevant to PBPK modeling are summarized as following: water is 82.9 g/100 g in sheep's milk and 87.6 g/100 g in goats’ (Balthazar et al., 2017); fat weights 4.9–7.4 g/100 g in sheep's milk and 3.8–4.5 g/100 g in goats’ (Balthazar et al., 2017; Hadjipanayiotou, 1995; Larson, 1978); 5.5 g/100 g proteins are in sheep's milk and 3.7 g/100g in goats’ (Balthazar et al., 2017); casein weights 4.6–4.7 g/100 g in sheep's milk and 2.4–2.5 g/100 g in goats’ (Balthazar et al., 2017; Larson, 1978); and lactose is 4.8 g/100 g in sheep's milk and 4.1–4.6 g/100 g in goats’ (Balthazar et al., 2017; Hadjipanayiotou, 1995; Larson, 1978). The differences of milk components among different breeds of sheep and goats are reported in the previous review paper (Ferro et al., 2017).
The physiological parameters related to milk secretion in sheep and goats play an important role to establish a physiologically based model for milk production. The daily milk yield for sheep is 0.62–1.65 kg/day and for goats is 0.79–2.55 kg/day (Ferro et al., 2017; Salama et al., 2004), and milk yields in cistern and alveoli in goats were reported by Salama et al. (2004). The differences of milk yields among different breeds of sheep and goats were reported in the previous review (Ferro et al., 2017). The milk production in sheep, including total milk amount and the amount of milk in cistern and alveoli, was reported by Castillo et al. (2008). The milk productions in sheep and goats follow a linear regression (Castillo et al., 2008; Linzell, 1966; Salama et al., 2004) similar to the linear model used for milk production in cows from two recent studies (Woodward et al., 2020; Woodward & Whittem, 2019), which is different from the physiologically based milk secretion models using the Langmuir equation reported for dairy cows in earlier studies (Li et al., 2018; Whittem et al., 2012).
3.8. Hematocrit
The values of hematocrit for sheep of all ages, market‐age sheep, lambs, and fetal sheep are reported in Tables 17, 18, 19, and 20, respectively. The hematocrit for sheep of all different ages was calculated based on 24 studies (Alhidary et al., 2012; Anosa & Isoun, 1976; Bessho et al., 1997; Campbell et al., 2014; Carver & Hay, 1995; Ceï et al., 2018; Degen & Young, 1981; Gomes et al., 2013; Hart & Doyle, 1985; Horton & Burgher, 1992; Jobe et al., 1998; Maraba et al., 2018; Marsh et al., 2001; Matson et al., 1981; Mayland et al., 1986; Meyer‐Gesch et al., 2013; Rege et al., 2002; Robillard & Weitzman, 1980; Roghair et al., 2004; Saylor & Leach, 1980; Singh et al., 2018; Talke et al., 1985; Tran et al., 2000; Vannucchi et al., 2012), and the average value was 36.15% (Table 17). The value of hematocrit for goats was calculated based on five different studies (Anosa & Isoun, 1976; Courtice, 1943; McKean & Walker, 1974; Olsén et al., 2013; Olsson et al., 1994), and the average value was 29.38% (Table 21). All raw data and data analysis for hematocrit in sheep are provided in Excel File B6 of Supporting Information, and for hematocrit in goats are available in Excel File C4 of Supporting Information.
Table 17.
Hematocrit (%) for sheep of all ages
| Mean | SD | Number of animals | Number of studies |
|---|---|---|---|
| 36.15 | 7.50 | 11,683 | 24 |
The hematocrit of sheep with all ages was calculated based on 24 studies (Alhidary et al., 2012; Anosa & Isoun, 1976; Bessho et al., 1997; Campbell et al., 2014; Carver & Hay, 1995; Ceï et al., 2018; Degen & Young, 1981; Gomes et al., 2013; Hart & Doyle, 1985; Horton & Burgher, 1992; Jobe et al., 1998; Maraba et al., 2018; Marsh et al., 2001; Matson et al., 1981; Mayland et al., 1986; Meyer‐Gesch et al., 2013; Rege et al., 2002; Robillard & Weitzman, 1980; Roghair et al., 2004; Saylor & Leach, 1980; Singh et al., 2018; Talke et al., 1985; Tran et al., 2000; Vannucchi et al., 2012).
Table 18.
Hematocrit (%) for market‐age sheep
| Mean | SD | Number of animals | Number of studies |
|---|---|---|---|
| 34.69 | 3.09 | 174 | 5 |
Table 19.
Hematocrit (%) for lambs
| Mean | SD | Number of animals | Number of studies |
|---|---|---|---|
| 34.37 | 8.11 | 6,321 | 9 |
The hematocrit of lambs was calculated based on nine studies (Alhidary et al., 2012; Campbell et al., 2014; Degen & Young, 1981; Marsh et al., 2001; Matson et al., 1981; Meyer‐Gesch et al., 2013; Rege et al., 2002; Robillard & Weitzman, 1980; Roghair et al., 2004). The value of hematocrit for lambs is 34.37% based on these studies.
Table 20.
Hematocrit (%) for fetal sheep
| Mean | SD | Number of animals | Number of studies |
|---|---|---|---|
| 37.29 | 8.72 | 83 | 5 |
Table 21.
Hematocrit (%) for goats
| Mean | SD | Number of animals | Number of studies |
|---|---|---|---|
| 29.38 | 7.38 | 29 | 5 |
4. DISCUSSION
Based on available literature, this manuscript provides a comprehensive summary of PBPK‐related physiological parameters in sheep and goats, including relative tissue weights for market‐age sheep, lambs, fetal sheep and goats, cardiac outputs for sheep and goats, blood flow fractions for sheep, vascular space fractions of organs in sheep and lambs, as well as hematocrits for sheep and goats. For each parameter, the mean and standard deviation are reported in the tables. These compiled data serve as reference values for essential physiological parameters to facilitate the development of PBPK models in sheep and goats, and also allow one to characterize the population variability of the physiological and anatomical characteristics of sheep and goats. This study also identifies data gaps in this area, such as blood flow fractions to different organs in goats, providing a direction for future studies. This manuscript and our recently reported review articles on physiological parameters in cattle, swine, chickens and turkeys (Lin et al., 2020; Wang et al., 2020) together provide a centralized repository for physiological parameters needed to develop PBPK models in common food animal species, which is anticipated to accelerate the development of this field. A strength of this combined database is that all parameters were extracted and calculated using a consistent approach which should minimize errors when extrapolating across species.
Physiological parameters are an essential component of any PBPK model, and a centralized repository for physiological parameters provides a standard reference that will greatly facilitate the development and application of PBPK models. In the field of PBPK modeling, one of the most comprehensive reviews of physiological parameters was published by Brown et al. (1997), which focused on mice, rats, dogs, and humans. This publication represents a cornerstone for the development of PBPK modeling, has been extensively cited and greatly accelerated the advancement of this field, especially in the application of PBPK models for chemical risk assessment. Compared to the application of PBPK models in chemical risk assessment (Tan et al., 2018) and drug development (Shebley et al., 2018), applying PBPK modeling in the area of animal‐derived food safety assessment is still under development. It is well recognized that PBPK modeling has advantages over other modeling tools for chemical dosimetry predictions, and it can aid in the prediction of drug or chemical residues and withdrawal intervals in edible tissues (Lin et al., 2016). Despite this usefulness, there were only 2 or 3 PBPK models published per year in the food safety area before 2016 (Lin et al., 2016). An explanation for this could be the lack of robust computational tools and summarized or standardized physiological parameters for food‐producing animals. However, there were at least five papers published in 2017 for the application of PBPK modeling in food safety assessment (Lautz et al., 2019), and at least 9 papers (Lautz, Hoeks, et al., 2020; Lautz, Nebbia, et al., 2020; Li, Cheng, et al., 2019; Li, Mainquist‐Whigham, et al., 2019; Méda et al., 2020; Tebby et al., 2019; Xu et al., 2019; Yang et al., 2019; Zeng et al., 2019) published in this field during 2019 and 2020. The increasing development and application of PBPK modeling in the food safety area requires a standardized repository of physiological parameters in food‐producing animals. The present manuscript and our recently published manuscripts in other species (Lin et al., 2020; Wang et al., 2020) provide a timely resource to address this scientific need.
In the literature, there are two other review studies that report a summary of PBPK‐related physiological parameters in sheep, but not in goats (Lautz, Dorne, et al., 2020; Upton, 2008). The study by Upton (2008) focused on the application of sheep as animal models for biomedical research, and the reference body weight was 45 kg for sheep, which is lower than the average body weight for market‐age sheep in the United States as reported in the present study. Also, only mean values of relevant parameters were reported and the parameter variabilities were not available in the study be Upton (2008). Recently, another group of scientists reported a summary of PBPK‐related physiological parameters in swine, cattle, and sheep (Lautz, Dorne, et al., 2020). This study includes both means and coefficients of variance for relevant parameters. Overall, the parameter values presented in our manuscript are similar to the values reported in Lautz, Dorne, et al. (2020). However, the uniqueness of our study is that we categorized sheep into different age groups or production classes and provided physiological parameter values for each production class whenever data are available. Also, when data are sufficient, regression analysis was performed, and growth curve equations were reported for both sheep and goats in this manuscript. This allows for the development of age‐dependent PBPK models (Henri et al., 2017; Zeng et al., 2019) and is also important to develop PBPK models for drugs with longer withdrawal intervals for which body weight gain has to be considered (Xu et al., 2020). Also, this is the only report of PBPK‐related physiological parameters in goats currently in the literature.
Pharmacokinetic and tissue residue depletion data for drugs are more commonly available in major species, such as cattle, swine, chickens, and turkey, than in minor species, such as sheep and goats. Therefore, it is not unusual to extrapolate PBPK models from a major species to a minor species. The physiological parameter values for sheep and goats reported in this manuscript can help to extrapolate PBPK models from cattle to sheep and goats, especially to extrapolate from dairy cows to dairy goats. The venous blood to arterial blood ratio is not available for sheep and goats, and there is limited information for GI tract retention time of each segment for sheep and goats. This study also found that PBPK‐related physiological parameter data are relatively limited in goats compared to sheep, particularly with respect to individual organ blood flow rates and the vascular space for individual organs. The organ weights for adrenals, pancreas, pituitary, thyroid, and uterus are not available for goats. Until such data become available for goats, the corresponding parameter values may have to be adapted from sheep when developing a PBPK model in goats, similar to the approach used in the PBPK model for tulathromycin in goats (Leavens et al., 2012) provided that the data gaps and the potential impact of species difference in physiological parameters on model predictions are acknowledged in the study. However, due to differences between species, additional experimental studies are needed to fill the data gaps identified in this manuscript.
It is important to point out that other factors besides organ physiology will impact drug disposition and should be incorporated in PBPK models. These include species differences in biotransformation, excretory pathways, cellular transporters, and protein binding. Another caveat in this work is that the dividing line between lambs and market‐age sheep is related to long bone calcification of cartilaginous growth plates. In most pharmacological and toxicological research, maturity is related to either liver or kidney excretory and metabolic functions, weaning, reproductive maturity in ruminants, or maturation of the GI tract reflected in mature rumen function (dividing line between calves and cows). Thus, caution should be made when thinking about lambs versus sheep for pharmacological purposes for the difference is not correlated to such functions.
In conclusion, this manuscript reports a compiled summary on PBPK‐related physiological parameter values in sheep and goats. This manuscript and our recently published manuscripts on physiological parameters in cattle, swine (Lin et al., 2020), chickens, and turkeys (Wang et al., 2020) provide a comprehensive reference database to facilitate the development of standardized PBPK models for drugs and environmental chemicals in these common food animal species. These data provide a basis to create virtual animal populations for incorporation into commercial software programs or web‐based interactive PBPK model interfaces for rapid development and application to estimate chemical tissue residues and withdrawal intervals. Additional experimental or review studies on the expression and activities of key metabolic enzymes or transporters in major absorptive and excretory organs (e.g., intestine, liver, and kidney) at different life stages of these food animals are needed to create biologically realistic virtual populations. The methodology described in these review articles can also be used to review data in less common food animal species, such as duck, geese, pheasants, quails, rabbits, and deer.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTION
ZL, JER, LAT, REB, and JLD discussed and conceived this project. ML did the literature search, extracted the data into Excel files, analyzed the data, and presented the data as Tables and Figures based on advice from ZL. TEC contributed to the literature search and data analysis. YSW double checked all data presented in the Excel files. ZL double checked all data presented in the Tables of the manuscript. ML drafted the manuscript. ZL coordinated the project and comprehensively revised the manuscript. All authors contributed to data interpretation and provided critical comments on the manuscript. All authors approved the final manuscript.
Supporting information
Supinfo
ACKNOWLEDGMENT
This work was financially supported by the United States Department of Agriculture (USDA) National Institute of Food and Agriculture (NIFA) for the Food Animal Residue Avoidance Databank (FARAD) Program (Award No. 2017‐41480‐27310, 2018‐41480‐28805, and 2019‐41480‐30296). The authors would like to thank Dr. Thomas W. Vickroy at Department of Physiological Sciences, College of Veterinary Medicine, University of Florida for his contribution to the initial discussions on this project.
Li M, Wang Y‐S, Elwell‐Cuddy T, et al. Physiological parameter values for physiologically based pharmacokinetic models in food‐producing animals. Part III: Sheep and goat. J Vet Pharmacol Therap.2021;44:456–477. 10.1111/jvp.12938
REFERENCES
- Alexander, G., Bell, A. W., & Hales, J. R. S. (1973). Effects of cold exposure on tissue blood flow in the new‐born lamb. The Journal of Physiology, 234(1), 65–77. 10.1113/jphysiol.1973.sp010334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhidary, I. A., Shini, S., Al Jassim, R. A. M., & Gaughan, J. B. (2012). Effect of various doses of injected selenium on performance and physiological responses of sheep to heat load1. Journal of Animal Science, 90(9), 2988–2994. 10.2527/jas.2011-4908 [DOI] [PubMed] [Google Scholar]
- Anosa, V. O., & Isoun, T. T. (1976). Serum proteins, blood and plasma volumes in experimental Trypanosoma vivax infections of sheep and goats. Tropical Animal Health and Production, 8(1), 14–19. 10.1007/BF02383360 [DOI] [PubMed] [Google Scholar]
- Balthazar, C. F., Pimentel, T. C., Ferrão, L. L., Almada, C. N., Santillo, A., Albenzio, M., Mollakhalili, N., Mortazavian, A. M., Nascimento, J. S., Silva, M. C., Freitas, M. Q., Sant’Ana, A. S., Granato, D., & Cruz, A. G. (2017). Sheep milk: Physicochemical characteristics and relevance for functional food development. Comprehensive Reviews in Food Science and Food Safety, 16(2), 247–262. 10.1111/1541-4337.12250 [DOI] [PubMed] [Google Scholar]
- Barcroft, J., Flexner, L. B., & McClurkin, T. (1934). The output of the fetal heart in the goat. The Journal of Physiology, 82(4), 498–508. 10.1113/jphysiol.1934.sp003202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes, R. J., Comline, R. S., & Dobson, A. (1983). Changes in the blood flow to the digestive organs of sheep induced by feeding. Quarterly Journal of Experimental Physiology, 68(1), 77–88. 10.1113/expphysiol.1983.sp002704 [DOI] [PubMed] [Google Scholar]
- Bessho, T., Murata, Y., Ninomiya, Y., Ibara, S., Yamamoto, T., Miyake, Y., & Tyner, J. G. (1997). Effect of arginine vasopressin on breathing movements of chronically instrumented fetal lambs. Acta Obstetricia et Gynecologica Scandinavica, 76(2), 107–111. 10.3109/00016349709050063 [DOI] [PubMed] [Google Scholar]
- Boxenbaum, H. (1982). Interspecies scaling, allometry, physiological time, and the ground plan of pharmacokinetics. Journal of Pharmacokinetics and Biopharmaceutics, 10(2), 201–227. 10.1007/BF01062336 [DOI] [PubMed] [Google Scholar]
- Boxenbaum, H., & D'Souza, R. W. (1990). Interspecies pharmacokinetic scaling, biological design and neoteny. Advances in Drug Research, 19, 139–196. [Google Scholar]
- Brown, R. P., Delp, M. D., Lindstedt, S. L., Rhomberg, L. R., & Beliles, R. P. (1997). Physiological parameter values for physiologically based pharmacokinetic models. Toxicology and Industrial Health, 13(4), 407–484. 10.1177/074823379701300401 [DOI] [PubMed] [Google Scholar]
- Burrin, D. G., Ferrell, C. L., Britton, R. A., & Bauer, M. (1990). Level of nutrition and visceral organ size and metabolic activity in sheep. British Journal of Nutrition, 64(2), 439–448. 10.1079/BJN19900044 [DOI] [PubMed] [Google Scholar]
- Butterfield, R. M., & Thompson, J. M. (1983). Changes in body composition relative to weight and maturity of large and small strains of Australian merino rams. 4. Fat depots and bones. Animal Science, 37(3), 423–431. 10.1017/S000335610000204X [DOI] [Google Scholar]
- Campbell, E. S., Whitney, T. R., Taylor, C. A., & Garza, N. E. (2014). Effects of breed of sheep and dietary onions on bitterweed (Hymenoxys odorata DC). Toxicity, 29, 8. [Google Scholar]
- Carver, T. D., & Hay, W. W. (1995). Uteroplacental carbon substrate metabolism and O2 consumption after long‐term hypoglycemia in pregnant sheep. American Journal of Physiology‐Endocrinology and Metabolism, 269(2), E299–E308. 10.1152/ajpendo.1995.269.2.E299 [DOI] [PubMed] [Google Scholar]
- Castillo, V., Such, X., Caja, G., Salama, A. A., Albanell, E., & Casals, R. (2008). Changes in alveolar and cisternal compartments induced by milking interval in the udder of dairy ewes. Journal of Dairy Science, 91(9), 3403–3411. 10.3168/jds.2008-1097 [DOI] [PubMed] [Google Scholar]
- Ceï, W., Salah, N., Alexandre, G., Bambou, J. C., & Archimède, H. (2018). Impact of energy and protein on the gastro‐intestinal parasitism of small ruminants: A meta‐analysis. Livestock Science, 212, 34–44. 10.1016/j.livsci.2018.03.015 [DOI] [Google Scholar]
- Chaiyabutr, N., Faulkner, A., & Peaker, M. (1980). Effects of starvation on cardiovascular function (including the mammary circulation) and water balance in pregnant goats. Quarterly Journal of Experimental Physiology and Cognate Medical Sciences, 65(3), 207–216. 10.1113/expphysiol.1980.sp002507 [DOI] [PubMed] [Google Scholar]
- Courtice, F. C. (1943). The blood volume of normal animals. The Journal of Physiology, 102(3), 290–305. 10.1113/jphysiol.1943.sp004035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craigmill, A. L. (2003). A physiologically based pharmacokinetic model for oxytetracycline residues in sheep. Journal of Veterinary Pharmacology and Therapeutics, 26(1), 55–63. 10.1046/j.1365-2885.2003.00451.x [DOI] [PubMed] [Google Scholar]
- Creasy, R. K., Barrett, C. T., de Swiet, M., Kahanpää, K. V., & Rudolph, A. M. (1972). Experimental intrauterine growth retardation in the sheep. American Journal of Obstetrics and Gynecology, 112(4), 566–573. 10.1016/0002-9378(72)90317-1 [DOI] [PubMed] [Google Scholar]
- Davies, B., & Morris, T. (1993). Physiological parameters in laboratory animals and humans. Pharmaceutical Research, 10(7), 1093–1095. [DOI] [PubMed] [Google Scholar]
- Davis, C. N., Davis, L. E., & Powers, T. E. (1975). Comparative body compositions of the dog and goat. American Journal of Veterinary Research, 36(3), 309–311. [PubMed] [Google Scholar]
- Davison, K. L., McEntee, K., & Wright, M. J. (1965). Responses in pregnant ewes fed forages containing various levels of nitrate. Journal of Dairy Science, 48(7), 968–977. 10.3168/jds.S0022-0302(65)88370-9 [DOI] [PubMed] [Google Scholar]
- Degen, A. A., & Young, B. A. (1981). Response of lactating ewes to snow as a source of water. Canadian Journal of Animal Science, 61(1), 73–79. 10.4141/cjas81-011 [DOI] [Google Scholar]
- Devendra, C., & McLeroy, G. B. (1982). Goat and sheep production in the tropics. Longman. [Google Scholar]
- Dodic, M., Samuel, C., Moritz, K., Wintour, E. M., Morgan, J., Grigg, L., & Wong, J. (2001). Impaired cardiac functional reserve and left ventricular hypertrophy in adult sheep after prenatal dexamethasone exposure. Circulation Research, 89(7), 623–629. 10.1161/hh1901.097086 [DOI] [PubMed] [Google Scholar]
- Elsley, F. W. H., McDonald, I., & Fowler, V. R. (1964). The effect of plane of nutrition on the carcasses of pigs and lambs when variations in fat content are excluded. Animal Science, 6(2), 141–154. 10.1017/S0003356100021899 [DOI] [Google Scholar]
- Eriksson, L., & Valtonen, M. (1982). Renal urea handling in goats fed high and low protein diets. Journal of Dairy Science, 65(3), 385–389. 10.3168/jds.S0022-0302(82)82202-9 [DOI] [PubMed] [Google Scholar]
- Evans, W., Phernetton, T. M., & Magness, R. R. (1998). 17beta‐estradiol effect on critical cardiac output with reduction of cardiac output in oophorectomized sheep. The American Journal of Physiology, 275(1), H57–64. 10.1152/ajpheart.1998.275.1.H57 [DOI] [PubMed] [Google Scholar]
- FDA (2020). Minor use/minor species. Retrieved from https://www.fda.gov/animal‐veterinary/development‐approval‐process/minor‐useminor‐species [Google Scholar]
- Ferro, M. M., Tedeschi, L. O., & Atzori, A. S. (2017). The comparison of the lactation and milk yield and composition of selected breeds of sheep and goats. Translational Animal Science, 1(4), 498–506. 10.2527/tas2017.0056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatzias, I. S., Karabagias, I. K., Kontakos, S. P., Kontominas, M. G., & Badeka, A. V. (2018). Characterization and differentiation of sheep's milk from Greek breeds based on physicochemical parameters, fatty acid composition and volatile profile. Journal of the Science of Food and Agriculture, 98(10), 3935–3942. 10.1002/jsfa.8914 [DOI] [PubMed] [Google Scholar]
- Gomes, E., Louvandini, H., Dallago, B., Canozzi, M., Melo, C., MorenoBernal, F., & McManus, C. (2013). Productivity in ewes of different genetic groups and body sizes. Journal of Animal Science Advances, 3(5), 243. 10.5455/jasa.20130523111656 [DOI] [Google Scholar]
- Greenwood, P. L., Hunt, A. S., & Bell, A. W. (2004). Effects of birth weight and postnatal nutrition on neonatal sheep: IV. Organ growth. Journal of Animal Science, 82(2), 422–428. 10.2527/2004.822422x [DOI] [PubMed] [Google Scholar]
- Gregory, N. G., Christopherson, R. J., & Lister, D. (1986). Adipose tissue capillary blood flow in relation to fatness in sheep. Research in Veterinary Science, 40(3), 352–356. 10.1016/S0034-5288(18)30549-6 [DOI] [PubMed] [Google Scholar]
- Gregory, P. C., Miller, S. J., & Brewer, A. C. (1985). The relation between food intake and abomasal emptying and small intestinal transit time in sheep. British Journal of Nutrition, 53(2), 373–380. 10.1079/bjn19850044 [DOI] [PubMed] [Google Scholar]
- Gu, W., Jones, C. T., & Parer, J. T. (1985). Metabolic and cardiovascular effects on fetal sheep of sustained reduction of uterine blood flow. The Journal of Physiology, 368(1), 109–129. 10.1113/jphysiol.1985.sp015849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjigeorgiou, I., Dardamani, K., Goulas, C., & Zervas, G. (2000). The effect of water availability on feed intake and digestion in sheep. Small Ruminant Research, 37(1), 147–150. 10.1016/S0921-4488(99)00142-X [DOI] [PubMed] [Google Scholar]
- Hadjipanayiotou, M. (1995). Composition of ewe, goat and cow milk and of colostrum of ewes and goats. Small Ruminant Research, 18(3), 255–262. 10.1016/0921-4488(95)00697-3 [DOI] [Google Scholar]
- Hales, J. R. S. (1973a). Effects of exposure to hot environments on the regional distribution of blood flow and on cardiorespiratory function in sheep. Pflügers Archiv European Journal of Physiology, 344(2), 133–148. 10.1007/BF00586547 [DOI] [PubMed] [Google Scholar]
- Hales, J. R. S. (1973b). Effects of heat stress on blood flow in respiratory and non‐respiratory muscles in the sheep. Pflügers Archiv European Journal of Physiology, 345(2), 123–130. 10.1007/BF00585835 [DOI] [PubMed] [Google Scholar]
- Hales, J. R. S. (1973c). Radioactive microsphere measurement of cardiac output and regional tissue blood flow in the sheep. Pflugers Archiv: European Journal of Physiology, 344(2), 119–132. [DOI] [PubMed] [Google Scholar]
- Hales, J. R. S., Bennett, J. W., & Fawcett, A. A. (1976). Effects of acute cold exposure on the distribution of cardiac output in the sheep. Pflügers Archiv European Journal of Physiology, 366(2–3), 153–157. 10.1007/BF00585871 [DOI] [PubMed] [Google Scholar]
- Hales, J. R. S., & Fawcett, A. A. (1993). Wool production and blood supply to skin and other tissues in sheep. Journal of Animal Science, 71(2), 492–498. 10.2527/1993.712492x [DOI] [PubMed] [Google Scholar]
- Hansard, S. L. (1956). Residual organ blood volume of cattle, sheep and swine. Proceedings of the Society for Experimental Biology and Medicine, 91(1), 31–34. 10.3181/00379727-91-22160 [DOI] [PubMed] [Google Scholar]
- Hart, S. P., & Doyle, J. J. (1985). Adaptation of early‐weaned lambs to high‐concentrate diets with three grain sources, with or without sodium bicarbonate. Journal of Animal Science, 61(4), 975–984. 10.2527/jas1985.614975x [DOI] [Google Scholar]
- He, Z., Zhang, T., Jiang, L., Zhou, M., Wu, D., Mei, J., & Cheng, Y. (2018). Use of CRISPR/Cas9 technology efficiently targetted goat myostatin through zygotes microinjection resulting in double‐muscled phenotype in goats. Bioscience Reports, 38(6), 10.1042/BSR20180742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henri, J., Carrez, R., Meda, B., Laurentie, M., & Sanders, P. (2017). A physiologically based pharmacokinetic model for chickens exposed to feed supplemented with monensin during their lifetime. Journal of Veterinary Pharmacology and Therapeutics, 40(4), 370–382. 10.1111/jvp.12370 [DOI] [PubMed] [Google Scholar]
- Horton, G. M. J., & Burgher, C. C. (1992). Physiological and carcass characteristics of hair and wool breeds of sheep. Small Ruminant Research, 7(1), 51–60. 10.1016/0921-4488(92)90067-E [DOI] [Google Scholar]
- ICRP (2002). Basic anatomical and physiological data for use in radiological protection: Reference values: ICRP Publication 89. Annals of the ICRP, 32(3–4), 1–277. [PubMed] [Google Scholar]
- Jainudeen, M. R., & Hafez, E. S. E. (2000). Gestation, prenatal physiology, and parturition. In Hafez B., & Hafez E. S. E. (Eds.), Reproduction in farm animals, 7th edn., (pp. 140–155). Hoboken, New Jersey: Wiley‐Blackwell. [Google Scholar]
- Jobe, A. H., Wada, N., Berry, L. M., Ikegami, M., & Ervin, M. G. (1998). Single and repetitive maternal glucocorticoid exposures reduce fetal growth in sheep. American Journal of Obstetrics and Gynecology, 178(5), 880–885. 10.1016/S0002-9378(98)70518-6 [DOI] [PubMed] [Google Scholar]
- Joy, M., Ripoll, G., & Delfa, R. (2008). Effects of feeding system on carcass and non‐carcass composition of Churra Tensina light lambs. Small Ruminant Research, 78(1–3), 123–133. 10.1016/j.smallrumres.2008.05.011 [DOI] [Google Scholar]
- Kamalzadeh, A., Koops, W. J., van Bruchem, J., Tamminga, S., & Zwart, D. (1998). Feed quality restriction and compensatory growth in growing sheep: Development of body organs. Small Ruminant Research, 29(1), 71–82. 10.1016/S0921-4488(97)00111-9 [DOI] [Google Scholar]
- Kennedy, P. M., McSweeney, C. S., & Welch, J. G. (1992). Influence of dietary particle size on intake, digestion, and passage rate of digesta in goats and sheep fed wheaten (Triticum aestivum) hay. Small Ruminant Research, 9(2), 125–138. 10.1016/0921-4488(92)90191-6 [DOI] [Google Scholar]
- Kutter, A. P. N., Kastner, S. B. R., Bettschart‐Wolfensberger, R., & Huhtinen, M. (2006). Cardiopulmonary effects of dexmedetomidine in goats and sheep anaesthetised with sevoflurane. Veterinary Record, 159(19), 624–629. 10.1136/vr.159.19.624 [DOI] [PubMed] [Google Scholar]
- Lankadeva, Y. R., Singh, R. R., Hilliard, L. M., Moritz, K. M., & Denton, K. M. (2014). Impaired ability to modulate glomerular filtration rate in aged female sheep following fetal uninephrectomy. Physiological Reports, 2(1), e00208. 10.1002/phy2.208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson, B. L. (1978). The dairy goat as a model in lactation studies. Journal of Dairy Science, 61(7), 1023–1029. 10.3168/jds.S0022-0302(78)83682-0 [DOI] [PubMed] [Google Scholar]
- Lautz, L. S., Dorne, J. L. C. M., Oldenkamp, R., Hendriks, A. J., & Ragas, A. M. J. (2020). Generic physiologically based kinetic modelling for farm animals: Part I. Data collection of physiological parameters in swine, cattle and sheep. Toxicology Letters, 319, 95–101. 10.1016/j.toxlet.2019.10.021 [DOI] [PubMed] [Google Scholar]
- Lautz, L. S., Hoeks, S., Oldenkamp, R., Hendriks, A. J., Dorne, J. L. C. M., & Ragas, A. M. J. (2020). Generic physiologically based kinetic modelling for farm animals: Part II. Predicting tissue concentrations of chemicals in swine, cattle, and sheep. Toxicology Letters, 318, 50–56. 10.1016/j.toxlet.2019.10.008 [DOI] [PubMed] [Google Scholar]
- Lautz, L. S., Nebbia, C., Hoeks, S., Oldenkamp, R., Hendriks, A. J., Ragas, A. M. J., & Dorne, J. L. C. M. (2020). An open source physiologically based kinetic model for the chicken (Gallus gallus domesticus): Calibration and validation for the prediction residues in tissues and eggs. Environment International, 136, 105488. 10.1016/j.envint.2020.105488 [DOI] [PubMed] [Google Scholar]
- Lautz, L. S., Oldenkamp, R., Dorne, J. L., & Ragas, A. M. J. (2019). Physiologically based kinetic models for farm animals: Critical review of published models and future perspectives for their use in chemical risk assessment. Toxicology in Vitro, 60, 61–70. 10.1016/j.tiv.2019.05.002 [DOI] [PubMed] [Google Scholar]
- Laville, E., Bouix, J., Sayd, T., Bibé, B., Elsen, J. M., Larzul, C., Eychenne, F., Marcq, F., & Georges, M. (2004). Effects of a quantitative trait locus for muscle hypertrophy from Belgian Texel sheep on carcass conformation and muscularity. Journal of Animal Science, 82(11), 3128–3137. 10.2527/2004.82113128x [DOI] [PubMed] [Google Scholar]
- Leavens, T. L., Tell, L. A., Clothier, K. A., Griffith, R. W., Baynes, R. E., & Riviere, J. E. (2012). Development of a physiologically based pharmacokinetic model to predict tulathromycin distribution in goats. Journal of Veterinary Pharmacology and Therapeutics, 35(2), 121–131. 10.1111/j.1365-2885.2011.01304.x [DOI] [PubMed] [Google Scholar]
- Li, M., Cheng, Y.‐H., Chittenden, J. T., Baynes, R. E., Tell, L. A., Davis, J. L., Vickroy, T. W., Riviere, J. E., & Lin, Z. (2019). Integration of Food Animal Residue Avoidance Databank (FARAD) empirical methods for drug withdrawal interval determination with a mechanistic population‐based interactive physiologically based pharmacokinetic (iPBPK) modeling platform: Example for flunixin meglumine administration. Archives of Toxicology, 93(7), 1865–1880. 10.1007/s00204-019-02464-z [DOI] [PubMed] [Google Scholar]
- Li, M., Gehring, R., Riviere, J. E., & Lin, Z. (2018). Probabilistic physiologically based pharmacokinetic model for penicillin G in milk from dairy cows following intramammary or intramuscular administrations. Toxicological Sciences, 164(1), 85–100. 10.1093/toxsci/kfy067 [DOI] [PubMed] [Google Scholar]
- Li, M., Mainquist‐Whigham, C., Karriker, L. A., Wulf, L. W., Zeng, D., Gehring, R., Riviere, J. E., Coetzee, J. F., & Lin, Z. (2019). An integrated experimental and physiologically based pharmacokinetic modeling study of penicillin G in heavy sows. Journal of Veterinary Pharmacology and Therapeutics, 42(4), 461–475. 10.1111/jvp.12766 [DOI] [PubMed] [Google Scholar]
- Lin, Z., & Fisher, J. W. (2020). Chapter 1: A history and recent efforts of selected physiologically based pharmacokinetic modeling topics. In Fisher J. W., Gearhart J. M., & Lin Z. (Eds.), Physiologically based pharmacokinetic (PBPK) modeling: Methods and applications in toxicology and risk assessment (pp. 1–26). Elsevier Inc. [Google Scholar]
- Lin, Z., Gehring, R., Mochel, J. P., Lave, T., & Riviere, J. E. (2016). Mathematical modeling and simulation in animal health – Part II: Principles, methods, applications, and value of physiologically based pharmacokinetic modeling in veterinary medicine and food safety assessment. Journal of Veterinary Pharmacology and Therapeutics, 39(5), 421–438. 10.1111/jvp.12311 [DOI] [PubMed] [Google Scholar]
- Lin, Z., Li, M., Wang, Y.‐S., Tell, L. A., Baynes, R. E., Davis, J. L., Vickroy, T. W., & Riviere, J. E. (2020). Physiological parameter values for physiologically based pharmacokinetic models in foodproducing animals. Part I: Cattle and swine. Journal of Veterinary Pharmacology and Therapeutics, 43(5), 385–420. 10.1111/jvp.12861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linzell, J. L. (1960). Mammary‐gland blood flow and oxygen, glucose and volatile fatty acid uptake in the conscious goat. The Journal of Physiology, 153(3), 492–509. 10.1113/jphysiol.1960.sp006550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linzell, J. L. (1966). Measurement of udder volume in live goats as an index of mammary growth and function. Journal of Dairy Science, 49(3), 307–311. 10.3168/jds.S0022-0302(66)87853-0 [DOI] [PubMed] [Google Scholar]
- Linzell, J. L., & Fleet, I. R. (1966). Measurement of venous flow by continuous thermodilution and its application to measurement of mammary blood flow in the goat. Circulation Research, 18(6), 745–754. 10.1161/01.RES.18.6.745 [DOI] [PubMed] [Google Scholar]
- Macgregor, R., & Gerrard, F. (1980). The structure of the meat animals. A guide to their anatomy and physiology (3rd ed.). The Technical Press Ltd. [Google Scholar]
- Machen, R. (2016) Marketing goats & sheep. Texas A&M Agrilife Extension. [Google Scholar]
- Mahgoub, O. (1997). Meat production from the Omani Dhofari goat 1 liveweight growth and body composition. International Journal of Animal Sciences, 12, 25–30. [Google Scholar]
- Mahgoub, O., & Lodge, G. A. (1998). A comparative study on growth, body composition and carcass tissue distribution in Omani sheep and goats. The Journal of Agricultural Science, 131(3), 329–339. 10.1017/S0021859698005887 [DOI] [Google Scholar]
- Mahgoub, O., & Lu, C. D. (1998). Growth, body composition and carcass tissue distribution in goats of large and small sizes. Small Ruminant Research, 27(3), 267–278. 10.1016/S0921-4488(97)00055-2 [DOI] [Google Scholar]
- Maltz, E., Blatchford, D. R., & Peaker, M. (1984). Effects of frequent milking on milk secretion and mammary blood flow in the goat. Quarterly Journal of Experimental Physiology, 69(1), 127–132. 10.1113/expphysiol.1984.sp002773 [DOI] [PubMed] [Google Scholar]
- Maraba, K. P., Mlambo, V., Yusuf, A. O., Marume, U., & Hugo, A. (2018). Extra dietary vitamin E – selenium as a mitigation strategy against housing‐induced stress in Dohne Merino lambs: Effect on growth performance, stress biomarkers, and meat quality. Small Ruminant Research, 160, 31–37. 10.1016/j.smallrumres.2018.01.009 [DOI] [Google Scholar]
- Marsh, A. C., Gibson, K. J., Wu, J., Owens, P. C., Owens, J. A., & Lumbers, E. R. (2001). Chronic effect of insulin‐like growth factor I on renin synthesis, secretion, and renal function in fetal sheep. American Journal of Physiology‐Regulatory, Integrative and Comparative Physiology, 281(1), R318–R326. 10.1152/ajpregu.2001.281.1.R318 [DOI] [PubMed] [Google Scholar]
- Martin, K. L., Clapham, M. O., Davis, J. L., Baynes, R. E., Lin, Z., Vickroy, T. W., Riviere, J. E., & Tell, L. A. (2018). Extralabel drug use in small ruminants. Journal of the American Veterinary Medical Association, 253(8), 1001–1009. 10.2460/javma.253.8.1001 [DOI] [PubMed] [Google Scholar]
- Martin, R. D. (1981). Relative brain size and basal metabolic rate in terrestrial vertebrates. Nature, 293(5827), 57–60. 10.1038/293057a0 [DOI] [PubMed] [Google Scholar]
- Matson, J. R., Stokes, J. B., & Robillard, J. E. (1981). Effects of inhibition of prostaglandin synthesis on fetal renal function. Kidney International, 20(5), 621–627. 10.1038/ki.1981.185 [DOI] [PubMed] [Google Scholar]
- Mayland, H. F., Doyle, J. J., & Sharma, R. P. (1986). Effects of excess dietary selenite on lead toxicity in sheep. Biological Trace Element Research, 10(1), 65–75. 10.1007/BF02795319 [DOI] [PubMed] [Google Scholar]
- McDonald, T. J., & Nathanielsz, P. W. (1991). Bilateral destruction of the fetal paraventricular nuclei prolongs gestation in sheep. American Journal of Obstetrics and Gynecology, 165(3), 764–770. 10.1016/0002-9378(91)90325-L [DOI] [PubMed] [Google Scholar]
- McKean, T., & Walker, B. (1974). Comparison of selected cardiopulmonary parameters between the pronghorn and the goat. Respiration Physiology, 21(3), 365–370. 10.1016/0034-5687(74)90066-8 [DOI] [PubMed] [Google Scholar]
- McLeod, K. R., & Baldwin, R. L. (2000). Effects of diet forage:concentrate ratio and metabolizable energy intake on visceral organ growth and in vitro oxidative capacity of gut tissues in sheep. Journal of Animal Science, 78(3), 760. 10.2527/2000.783760x [DOI] [PubMed] [Google Scholar]
- Méda, B., Travel, A., Guyot, Y., Henri, J., Royer, E., Baéza‐Campone, E., & Jondreville, C. (2020). A PBPK model to study the transfer of α‐hexabromocyclododecane (α‐HBCDD) to tissues of fast‐ and slow‐growing broilers. Food Additives & Contaminants: Part A, 37(2), 316–331. 10.1080/19440049.2019.1681596 [DOI] [PubMed] [Google Scholar]
- Meyer‐Gesch, K. M., Sun, M. Y., Koch, J. M., Ramadoss, J., Blohowiak, S. E., Magness, R. R., & Kling, P. J. (2013). Ovine fetal renal development impacted by multiple fetuses and uterine space restriction. Journal of Developmental Origins of Health and Disease, 4(5), 411–420. 10.1017/S2040174413000329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montossi, F., Font‐i‐Furnols, M., del Campo, M., San Julián, R., Brito, G., & Sañudo, C. (2013). Sustainable sheep production and consumer preference trends: Compatibilities, contradictions, and unresolved dilemmas. Meat Science, 95(4), 772–789. 10.1016/j.meatsci.2013.04.048 [DOI] [PubMed] [Google Scholar]
- Moss, T. J. M., Doherty, D. A., Nitsos, I., Sloboda, D. M., Harding, R., & Newnham, J. P. (2005). Effects into adulthood of single or repeated antenatal corticosteroids in sheep. American Journal of Obstetrics and Gynecology, 192(1), 146–152. 10.1016/j.ajog.2004.06.065 [DOI] [PubMed] [Google Scholar]
- Murray, D. M., & Slezacek, O. (1976). Growth rate and its effect on empty body weight, carcass weight and dissected carcass composition of sheep. The Journal of Agricultural Science, 87(1), 171–179. 10.1017/S0021859600026721 [DOI] [Google Scholar]
- Nesje, M., Flåøyen, A., & Moe, L. (1997). Estimation of glomerular filtration rate in normal sheep by the disappearance of iohexol from serum. Veterinary Research Communications, 21(1), 29–35. 10.1023/b:verc.0000009698.28252.d1 [DOI] [PubMed] [Google Scholar]
- Ngwa, A. T., Dawson, L. J., Puchala, R., Detweiler, G. D., Merkel, R. C., Wang, Z., Tesfai, K., Sahlu, T., Ferrell, C. L., & Goetsch, A. L. (2009). Effects of breed and diet on growth and body composition of crossbred Boer and Spanish wether goats. Journal of Animal Science, 87(9), 2913–2923. 10.2527/jas.2009-1835 [DOI] [PubMed] [Google Scholar]
- Olsén, L., Olsson, K., Hydbring‐Sandberg, E., Bondesson, U., & Ingvast‐Larsson, C. (2013). Methadone in healthy goats – Pharmacokinetics, behaviour and blood pressure. Research in Veterinary Science, 95(1), 231–237. 10.1016/j.rvsc.2013.02.013 [DOI] [PubMed] [Google Scholar]
- Olsson, K., Hossaini‐Hilali, J., & Cvek, K. (1994). Discrepant effects of angiotensin II and phenylephrine on plasma volume in conscious goats. Acta Physiologica Scandinavica, 151(1), 83–90. 10.1111/j.1748-1716.1994.tb09723.x [DOI] [PubMed] [Google Scholar]
- Parry, H. B., & Taylor, W. H. (1956). Renal function in sheep during normal and toxaemic pregnancies. The Journal of Physiology, 131(2), 383–392. 10.1113/jphysiol.1956.sp005469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelligrino, D. A., Miletich, D. J., Hoffman, W. E., & Albrecht, R. F. (1984). Nitrous oxide markedly increases cerebral cortical metabolic rate and blood flow in the goat. Anesthesiology, 60(5), 405–412. 10.1097/00000542-198405000-00003 [DOI] [PubMed] [Google Scholar]
- Perry, D., Thompson, J. M., & Butterfield, R. M. (1992). Bone distribution patterns in sheep selected for high and low weaning weight. Animal Science, 55(1), 129–135. 10.1017/S0003356100037351 [DOI] [Google Scholar]
- Pittroff, W., Keisler, D. H., & Blackburn, H. D. (2006). Effects of a high‐protein, low‐energy diet in finishing lambs: 2. Weight change, organ mass, body composition, carcass traits, fatty acid composition of lean and adipose tissue, and taste panel evaluation. Livestock Science, 101(1), 278–293. 10.1016/j.livprodsci.2005.11.019 [DOI] [Google Scholar]
- Rege, J., Tembely, S., Mukasa‐Mugerwa, E., Sovani, S., Anindo, D., Lahlou‐Kassi, A., Nagda, S., & Baker, R. L. (2002). Effect of breed and season on production and response to infections with gastro‐intestinal nematode parasites in sheep in the highlands of Ethiopia. Livestock Production Science, 78(2), 159–174. 10.1016/S0301-6226(02)00088-X [DOI] [Google Scholar]
- Reynolds, M., Linzell, J., & Rasmussen, F. (1968). Comparison of four methods for measuring mammary blood flow in conscious goats. American Journal of Physiology‐Legacy Content, 214(6), 1415–1424. 10.1152/ajplegacy.1968.214.6.1415 [DOI] [PubMed] [Google Scholar]
- Riley, R. R., Savell, J. W., Shelton, M., & Smith, G. C. (1989). Carcass and offal yields of sheep and goats as influenced by market class and breed. Small Ruminant Research, 2(3), 265–272. 10.1016/0921-4488(89)90006-0 [DOI] [Google Scholar]
- Riviere, J. E., Tell, L. A., Baynes, R. E., Vickroy, T. W., & Gehring, R. (2017). Guide to FARAD resources: Historical and future perspectives. Journal of the American Veterinary Medical Association, 250(10), 1131–1139. 10.2460/javma.250.10.1131 [DOI] [PubMed] [Google Scholar]
- Robillard, J. E., & Weitzman, R. E. (1980). Developmental aspects of the fetal renal response to exogenous arginine vasopressin. American Journal of Physiology‐Renal Physiology, 238(5), F407–F414. 10.1152/ajprenal.1980.238.5.F407 [DOI] [PubMed] [Google Scholar]
- Roghair, R. D., Lamb, F. S., Bedell, K. A., Smith, O. M., Scholz, T. D., & Segar, J. L. (2004). Late‐gestation betamethasone enhances coronary artery responsiveness to angiotensin II in fetal sheep. American Journal of Physiology‐Regulatory, Integrative and Comparative Physiology, 286(1), R80–R88. 10.1152/ajpregu.00421.2003 [DOI] [PubMed] [Google Scholar]
- Rompala, R. E., Hoagland, T. A., & Meister, J. A. (1988). Effect of dietary bulk on organ mass, fasting heat production and metabolism of the small and large intestines in sheep. The Journal of Nutrition, 118(12), 1553–1557. 10.1093/jn/118.12.1553 [DOI] [PubMed] [Google Scholar]
- Salama, A. A., Caja, G., Such, X., Peris, S., Sorensen, A., & Knight, C. H. (2004). Changes in cisternal udder compartment induced by milking interval in dairy goats milked once or twice daily. Journal of Dairy Science, 87(5), 1181–1187. 10.3168/jds.S0022-0302(04)73267-1 [DOI] [PubMed] [Google Scholar]
- Saylor, W. W., & Leach, R. M.Jr (1980). Intracellular distribution of copper and zinc in sheep: Effect of age and dietary levels of the metals. Journal of Nutrition, 110(3), 448–459. 10.1093/jn/110.3.448 [DOI] [PubMed] [Google Scholar]
- Schiffer, E. R. C., Mentha, G., Schwieger, I. M., & Morel, D. R. (1993). Sequential changes in the splanchnic circulation during continuous endotoxin infusion in sedated sheep: Evidence for a selective increase of hepatic artery blood flow and loss of the hepatic arterial buffer response. Acta Physiologica Scandinavica, 147(3), 251–261. 10.1111/j.1748-1716.1993.tb09497.x [DOI] [PubMed] [Google Scholar]
- Shebley, M., Sandhu, P., Emami Riedmaier, A., Jamei, M., Narayanan, R., Patel, A., Peters, S. A., Reddy, V. P., Zheng, M., de Zwart, L., Beneton, M., Bouzom, F., Chen, J., Chen, Y., Cleary, Y., Collins, C., Dickinson, G. L., Djebli, N., Einolf, H. J., … Rowland, M. (2018). Physiologically based pharmacokinetic model qualification and reporting procedures for regulatory submissions: A consortium perspective. Clinical Pharmacology & Therapeutics, 104(1), 88–110. 10.1002/cpt.1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silanikove, N. (1984). Renal excretion of urea in response to changes in nitrogen intake in desert (black Bedouin) and non‐desert (Swiss Saanen) goats. Comparative Biochemistry and Physiology Part A: Physiology, 79(4), 651–654. 10.1016/0300-9629(84)90464-X [DOI] [PubMed] [Google Scholar]
- Singh, S. P., Dass, G., Natesan, R., Kushwah, Y., Sharma, N., & Kumar, A. (2018). Endocrine and hematobiochemical profile of lambs raised in a semiarid region with different growth potentials during the postweaning period. Turkish Journal of Veterinary and Animal Sciences, 10, 120–129. 10.3906/vet-1709-38 [DOI] [Google Scholar]
- Sivachelvan, M. N., Ali, M. G., & Chibuzo, G. A. (1996). Foetal age estimation in sheep and goats. Small Ruminant Research, 19(1), 69–76. 10.1016/0921-4488(95)00709-1 [DOI] [Google Scholar]
- Skotnicka, E., Muszczyñski, Z., Dudzinska, W., & Suska, M. (2007). A review of the renal system and diurnal variations of renal activity in livestock. Irish Veterinary Journal, 60(3), 161. 10.1186/2046-0481-60-3-161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talke, P., Dunn, A., Lawlis, L., Sziebert, L., White, A., Herndon, D., Flynn, J. T., & Traber, D. (1985). A model of ovine endotoxemia characterized by an increased cardiac output. Circulatory Shock, 17(2), 103–108. [PubMed] [Google Scholar]
- Talke, P., Traber, D., Richardson, C., Harper, D., & Traber, L. (2000). The effect of α2 agonist‐induced sedation and its reversal with an α2 antagonist on organ blood flow in sheep. Anesthesia & Analgesia, 90(5), 1060–1066. 10.1097/00000539-200005000-00011 [DOI] [PubMed] [Google Scholar]
- Tan, Y.‐M., Worley, R. R., Leonard, J. A., & Fisher, J. W. (2018). Challenges associated with applying physiologically based pharmacokinetic modeling for public health decision‐making. Toxicological Sciences, 162(2), 341–348. 10.1093/toxsci/kfy010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tebby, C., Brochot, C., Dorne, J.‐L., & Beaudouin, R. (2019). Investigating the interaction between melamine and cyanuric acid using a Physiologically‐Based Toxicokinetic model in rainbow trout. Toxicology and Applied Pharmacology, 370, 184–195. 10.1016/j.taap.2019.03.021 [DOI] [PubMed] [Google Scholar]
- Thompson, G. E. (1980). The distribution of blood flow in the udder of the sheep and changes brought about by cold exposure and lactation. The Journal of Physiology, 302(1), 379–386. 10.1113/jphysiol.1980.sp013249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran, N. D., Porada, C. D., Zhao, Y., Almeida‐Porada, G., Anderson, W. F., & Zanjani, E. D. (2000). In utero transfer and expression of exogenous genes in sheep. Experimental Hematology, 28(1), 17–30. 10.1016/S0301-472X(99)00133-2 [DOI] [PubMed] [Google Scholar]
- Tsiplakou, E., Hadjigeorgiou, I., Sotirakoglou, K., & Zervas, G. (2011). Differences in mean retention time of sheep and goats under controlled feeding practices. Small Ruminant Research, 95(1), 48–53. 10.1016/j.smallrumres.2010.09.002 [DOI] [Google Scholar]
- Ullman, J., Eriksson, S., & Rundgren, M. (2001). Effects of losartan, prazosin and a vasopressin V1‐receptor antagonist on renal and femoral blood flow in conscious sheep. Acta Physiologica Scandinavica, 171(1), 99–104. 10.1046/j.1365-201X.2001.00780.x [DOI] [PubMed] [Google Scholar]
- Upton, R. N. (2008). Organ weights and blood flows of sheep and pig for physiological pharmacokinetic modelling. Journal of Pharmacological and Toxicological Methods, 58(3), 198–205. 10.1016/j.vascn.2008.08.001 [DOI] [PubMed] [Google Scholar]
- USDA (2019a). U.S. National Residue Program for Meat, Poultry, and Egg Products: 2019 residue sampling plans. Retrieved from https://www.fsis.USDA.gov/wps/wcm/connect/394f0bd4‐2c5d‐47bc‐ba4f‐f65992972e43/2019‐blue‐book.pdf?MOD=AJPERES [Google Scholar]
- USDA (2019b). USDA Weekly National Lamb Market Summary. Retrieved from https://mymarketnews.ams.usda.gov/filerepo/sites/default/files/2878/2019‐05‐03/121803/LS20190503WLAMB.PDF [Google Scholar]
- Vacca, G. M., Stocco, G., Dettori, M. L., Pira, E., Bittante, G., & Pazzola, M. (2018). Milk yield, quality, and coagulation properties of 6 breeds of goats: Environmental and individual variability. Journal of Dairy Science, 101(8), 7236–7247. 10.3168/jds.2017-14111 [DOI] [PubMed] [Google Scholar]
- Vannucchi, C. I., Rodrigues, J. A., Silva, L. C. G., Lúcio, C. F., & Veiga, G. A. L. (2012). A clinical and hemogasometric survey of neonatal lambs. Small Ruminant Research, 108(1–3), 107–112. 10.1016/j.smallrumres.2012.05.013 [DOI] [Google Scholar]
- Vonnahme, K. A., Hess, B. W., Hansen, T. R., McCormick, R. J., Rule, D. C., Moss, G. E., Murdoch, W. J., Nijland, M. J., Skinner, D. C., Nathanielsz, P. W., & Ford, S. P. (2003). Maternal undernutrition from early‐ to mid‐gestation leads to growth retardation, cardiac ventricular hypertrophy, and increased liver weight in the fetal sheep. Biology of Reproduction, 69(1), 133–140. 10.1095/biolreprod.102.012120 [DOI] [PubMed] [Google Scholar]
- Wang, Y.‐S., Li, M., Tell, L. A., Baynes, R. E., Davis, J. L., Vickroy, T. W., Riviere, J. E., & Lin, Z. (2020). Physiological parameter values for physiologically based pharmacokinetic models in foodproducing animals. Part II: Chicken and Turkey. Journal of Veterinary Pharmacology and Therapeutics. (in press). 10.1111/jvp.12931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittem, T., Whittem, J. H., & Constable, P. D. (2012). Modelling the concentration–time relationship in milk from cattle administered an intramammary drug. Journal of Veterinary Pharmacology and Therapeutics, 35(5), 460–471. 10.1111/j.1365-2885.2011.01352.x [DOI] [PubMed] [Google Scholar]
- Woodward, A. P., Morin, D., & Whittem, T. (2020). Population physiologically based modeling of pirlimycin milk concentrations in dairy cows. Journal of Dairy Science, 103(11), 10639–10650. 10.3168/jds.2020-18760 [DOI] [PubMed] [Google Scholar]
- Woodward, A. P., & Whittem, T. (2019). Physiologically based modelling of the pharmacokinetics of three beta‐lactam antibiotics after intra‐mammary administration in dairy cows. Journal of Veterinary Pharmacology and Therapeutics, 42(6), 693–706. 10.1111/jvp.12812 [DOI] [PubMed] [Google Scholar]
- Xu, N., Li, M., Chou, W. C., & Lin, Z. (2020). A physiologically based pharmacokinetic model of doxycycline for predicting tissue residues and withdrawal intervals in grass carp (Ctenopharyngodon idella). Food and Chemical Toxicology, 137, 111127. 10.1016/j.fct.2020.111127 [DOI] [PubMed] [Google Scholar]
- Xu, N., Li, M., Fu, Y. U., Zhang, X., Dong, J., Liu, Y., Zhou, S., Ai, X., & Lin, Z. (2019). Effect of temperature on plasma and tissue kinetics of doxycycline in grass carp (Ctenopharyngodon idella) after oral administration. Aquaculture, 511, 734204. 10.1016/j.aquaculture.2019.734204 [DOI] [Google Scholar]
- Yang, F., Lin, Z., Riviere, J. E., & Baynes, R. E. (2019). Development and application of a population physiologically based pharmacokinetic model for florfenicol and its metabolite florfenicol amine in cattle. Food and Chemical Toxicology, 126, 285–294. 10.1016/j.fct.2019.02.029 [DOI] [PubMed] [Google Scholar]
- Zeng, D., Lin, Z., Zeng, Z., Fang, B., Li, M., Cheng, Y.‐H., & Sun, Y. (2019). Assessing global human exposure to T‐2 toxin via poultry meat consumption using a lifetime physiologically based pharmacokinetic model. Journal of Agricultural and Food Chemistry, 67(5), 1563–1571. 10.1021/acs.jafc.8b07133 [DOI] [PubMed] [Google Scholar]
- Zhao, P., Rowland, M., & Huang, S. M. (2012). Best practice in the use of physiologically based pharmacokinetic modeling and simulation to address clinical pharmacology regulatory questions. Clinical Pharmacology and Therapeutics, 92(1), 17–20. 10.1038/clpt.2012.68 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supinfo


