Abstract
Atrial fibrillation (AF), the most common arrhythmia, is a major cause of stroke and systemic embolism. Left atrial appendage closure (LAAC) has been proved to be noninferior to traditional Vitamin K antagonists (VKAs) as well as novel oral anticoagulants (NOACs), which is becoming an important alternative to prevent stroke in non‐valvular AF. Catheter‐based AF ablation (CA) is recommended to be a standard of care in patients with AF refractory to drug therapy due to a better rhythm control and improvement of life quality than antiarrhythmic drugs. Theoretically, the one‐stop combination with LAAC and CA tends to bring more benefits in patients with AF, as it not only relieves symptoms, but also reduces the risk of stroke significantly. However, several important questions still need to be considered in the combination procedure although quite a few attempts have already been made in clinical practice. This review provides a comprehensive update on the concept, technique, perioperative management, benefits and other critical issues of the “one‐stop” procedure.
Keywords: atrial fibrillation, catheter ablation, efficacy, left atrial appendage closure, safety, stroke
1. INTRODUCTION OF “ONE‐STEP” PROCEDURE IN AF RHYTHM CONTROL AND STROKE PREVENTION
Atrial fibrillation (AF) is the most common cardiac arrhythmia in both developed and developing countries. It's estimated that around 1%−2% in general population and nearly 25% among the age of over 40 are affected during their lifetime.1 AF is a manifestation of a variety of cardiovascular diseases, while at the same time, causing or aggravating the existences of cardiovascular abnormality. The most threatening complication of AF is ischemic stroke which accounts for 20%−25% of overall stroke, and its consequences are usually more severe than non‐AF stroke.2 It's well known that AF stroke is caused by thrombus formation in the left atrial appendage (LAA) falling off into circulation. Therefore, seeking a device via percutaneous access to cover or occlude the LAA to prevent ischemic stroke in AF is coming as a matter of course. Nowadays, the LAA closure (LAAC) procedure is becoming an important alternative mean to prevent the AF stroke. Current evidence shows noninferiority to traditional Vitamin K antagonists (VKAs) as well as novel oral anticoagulants (NOACs).3, 4
Catheter‐based AF ablation (CA) has become a standard of care in patients with AF refractory to drug therapy. Evidence has shown that CA achieves a significantly better rhythm control rate than antiarrhythmic drug treatment. The basic theory of CA is the isolation of pulmonary vein electric activity, so called the pulmonary vein isolation (PVI), with either hot (radio frequency) or cold energy (cryoballoon). However, the CA‐PVI model for AF treatment still carries high recurrent rate especially in non‐paroxysmal AF. It's reported that the 1‐year success rate of CA is 30%–75%, depending on the type of AF and comorbidities,5 while the long‐term (5 years) success rate may even decrease substantially.6 No randomized prospective trials have shown a significant reduction in thrombo‐embolic events after ablation, no matter how high the rhythm control rate. Due to the high recurrence rate and the absence of strong evidence in prevention of stroke, current guidelines recommend indefinite continuation of OAC therapy after ablation in AF patients at high risk of stroke, regardless of the rhythm outcome, based on a careful assessment of both stroke and bleeding risk.7
Based on the above mentioned, CA is not enough for stroke prevention and it naturally raises a question, can we add LAAC on the ablation procedure? Considering the same pathway of groin vein access, septal puncture and left atrium manipulation, it's easy for physicians to think of the combination of these two procedures. Here we suggest that the term “one‐stop” procedure strictly refers to the catheter‐based CA + LAAC, which targets one disease from two sides of AF treatment (rhythm control + stroke prevention), although many other procedures also have characteristics of combination.
2. IMPORTANT ISSUES TO BE CONSIDERED IN THE COMBINATION PROCEDURE
2.1. Does the one‐stop procedure affect the success rate of LAAC or CA?
2.1.1. CA‐first or LAAC‐first
The one‐stop procedure may be performed in different sequences with CA first or LAAC first, different anesthesia modes such as local anesthesia or general anesthesia, and different puncture sites of atrial septum and etc., which might affect the success rate of LAAC or CA.
As for the CA‐first procedure, since CA is performed without the influence of LAA occluder, the success rate of CA, theoretically, should be comparable to that of CA alone. However, for the CA‐first procedure, the acute tissue edema induced by radiofrequency (RF) heating and cryoballoon (CB) freezing could possibly change the shape and size of LAA ostium, which could affect physician's selection on device size and even the success rate of LAAC, and also increase the risk of peri‐device leak after edema subsided. Another problem is that the puncture site of atrial septum in routine ablation procedure is usually higher than that of its need for LAAC if without guidance of transesophageal echocardiography (TEE), which will bring some difficulties in the following LAAC procedure in some circumstances. In recent years, some small‐scale studies have reported the success rate of atrial fibrillation ablation in the CA‐first one‐stop procedure ranging from 49% to 70%,8, 9, 10, 11, 12 which seems comparable to CA alone.13, 14, 15 However, the long‐term success rate for maintaining sinus rhythm, either in paroxysmal or non‐paroxysmal AF is absent in most of these studies, and the power of these studies is limited due to their retrospective observatory nature with quite limited sample size. Besides, ablation methods, patient characteristics, drug therapy, follow‐up, and definition of success rate varied among these studies. Randomized controlled trials are required to further verify the results.
If the LAAC procedure is carried out before CA (LAAC first), it is easy to understand that the success rate of LAAC is not affected by the subsequent CA procedure. However, LAAC first may affect the following ablation procedure, mainly including PVI, LA linear ablation and sometimes LAA isolation. The influence on left PVI is obvious if the LAA occluder is “lobe and disk” structure such as ACP or LAmbre. Since the disk covers part of the LA ridge, which lies between LAA and left pulmonary vein, when RF catheter tip contacts the disk, discharge would be interrupted due to low impedance. Under such circumstance, catheter maneuver would be impeded, and it could be difficult to achieve satisfactory PVI. Linear ablation of mitral isthmus and LA anterior wall (left PV to mitral annulus) can also be affected for similar reason, hence increasing the risk of recurrent atrial tachycardia. Besides, when performing the CA procedure in the presence of occluder in the LAA, the operator must be more cautious when maneuvering the catheter. The Lasso circular mapping catheter might hawk the pre‐existing device especially a disc‐like occluder and lead to device dislocation, or ablation on the surface of device to cause perforation of the membrane and lead to the leak of device. But for a well‐experienced operator, the success rate is similar between the two strategies (CA first or LAAC first) according to recent reports.16, 17 However, most literatures regarding the combination procedure are CA first and it is more suitable for the logistic process in most catheter centers.
According to a prospective, non‐randomized multicenter study,8 349 patients with NVAF underwent the combined CA and LAAC procedure between 2009 and 2015, and Watchman implantation under TEE guidance and general anesthesia was subsequently carried out after CA completion in all patients. The results showed that the success rate of LAAC procedure was 100 percent in accordance with the definition of LAAC procedure success with no flow or minimal (<5 mm) residual flow with assessment by TEE. Besides, based on data from 149 patients underwent the one‐stop LAAC procedure after CA completion in EWOLUTION and WASP registries between 2013 and 2015, the LAAC success rate was also 100 percent by TEE assessment, and a residual flow over 5 mm was only found in one patient at 28 days post‐implantation.18 In other words, the LAAC success rate after CA in the one‐stop procedure is very high, which is similar to the success rate of LAAC alone as reported in EWOLUTION study.19 However, earlier report from Romanov A and colleagues20 in a small randomized clinical trial in 2015, which was to assess the impact of LAAC added to PVI in patients with high‐risk AF, showed that out of 45 patients underwent the one‐stop procedure with PVI + LAAC treatment, the success rate of LAAC was only 87%, and the combination procedure was significantly associated with a higher AF burden during the blanking period. A recent study from China revealed that the immediate success rate of LAAC after CA was 100 percent.21 What is more, a meta‐analysis of 18 studies showed that the one‐stop procedure had a 98% procedural success rate of LAAC.22 Different success rate of device implantation in the combination procedure may be associated with patient volume and operator's experience.
2.1.2. Comparison of RF ablation and cryoballoon ablation
RF energy results in hyperthermic cellular injury through a combination of coagulation and myocardial (coagulative) necrosis, and open irrigation electrode is now conventionally used to perform AF ablation on account of its reliable energy delivery and lesion extension with less thrombus formation, resulting in about 25% local atrial wall thickness increase by tissue edema. By contrast, cryoballoon ablation induces tissue damage via intra‐and extracellular ice crystal formation and enlargement during freezing and thawing phase. According to FIRE AND ICE trial,13 the efficacy of cryoballoon ablation for AF was comparable to that of RF ablation. However, how these two technics influence the following LAAC procedure remains unclear. As for LAAC, perfect seal and proper position are the key to successful occlusion, which could be affected by tissue edema, especially at LA ridge. Compared with RF electrode, reliable contact to the ridge is more accessible by means of cryoballoon. Moreover, compared with the tip‐endocardium interface of RF ablation, balloon‐endocardium interface yields more extensive contact area, which may cause more extensive tissue injury and edema. Therefore, compared with RF ablation, cryoballoon ablation is more likely to change LAA ostium shape, hence interfere with LAAC to a larger extent theoretically. However, according to recent studies, concomitant cryoballoon ablation and LAAC procedures appear safe and effective with an annualized stroke and bleeding rates of 1% and 2%, respectively, a 71% and 60% risk reduction in comparison to event rates predicted from CHA2DS2‐VASc and HAS‐BLED scores at 24 months post procedure.23 More and more evidence are accumulating for the combination of cryoballoon ablation plus LAAC.
2.1.3. Special consideration for left atrial appendage isolation (LAAI)
LAA has been identified as a potentially important source of AF triggers among persistent AF patients, therefore LAAI seems a potentially effective treatment.24 However, reduced LAA flow velocity and increased spontaneous contrast in LAA were observed after LAAI, indicating impaired systolic function of LAA, which was prone to thrombosis; and thromboembolism or asymptomatic thrombus was observed after LAAI.25 Moreover, LAA could be activated through both endocardial propagation and distal brunch of Bachmann bundle in epicardium, hence complete and durable isolation of LAA could be very difficult. Albeit LAAI combined with LAAC seems a feasible treatment to improve efficacy of CA as well as prevent ensuing LAA thrombus, physicians should consider thoroughly over the efficacy and feasibility of LAAI given the considerable risk of LAA reconnection.26 Accordingly, the 2019 EHRA/EAPCI expert consensus statement on catheter‐based left atrial appendage occlusion 63 recommended that the one‐stop LAAC may be a reasonable way to prevent stroke when patients underwent LAAI.
2.2. How does the one‐stop procedure affect the peri‐device leakage after implantation?
Human LAA has complex anatomy structures including at least four kinds of types, different lobes, sizes and depths, irregular and uneven ostium, and unevenly distributed pectinate muscles in LAA, all of which are disadvantages for complete closure of the LAA by percutaneous endocardium LAAC strategy, especially prone to cause the peri‐device leakage after implantation. Based on some observational studies, the incidence of residual peri‐device leakage after LAAC varies from 12.5%−34.3% detected by TEE and 31%−68.5% detected by CT.27, 28, 29, 30, 31 The residual leakage after LAAC detected by TEE or CT is generally less than 5 mm, but it is reported to be linked with increased risks of device‐related thrombosis (DRT) and stroke.32, 33, 34
The CA energy including RF heating or CB freezing immediately produce acute tissue edema in around ostium of pulmonary veins and adjacent tissue including pulmonary vein ridge and ostium of LAA,35, 36 which is the main cause of AF recurrence once tissue edema degrades and increases the risk of peri‐device leakage when patients undergo the following LAAC procedure after ablation.6 Considering the effect of edema on peri‐device leakage, LAAC first procedure may be one of the choices in some experienced operators, the disadvantage of this strategy is as above mentioned.17
To overcome the adverse effects of tissue edema induced by CA adjacent to LAA on peri‐device leakage in routine CA first procedure, a larger size of device with a higher compression ratio of at least 15% after deployment is recommended. Another recommendation is the deeper seated of the device, where the occluder's shoulder like Watchman should be just at the ostium of LAA marked with circumflex artery rather than protruded too much beyond the inferior rim of LAA ostium. In summary, one should pay more attention to the stabilization of the device with severe LAA ostium tissue edema in the CA first procedure.
2.3. Does the one‐stop procedure decrease the incidence of stroke or systemic thromboembolism more than the LAAC alone?
As is well known, CA is superior to antiarrhythmic drug therapy for maintaining sinus rhythm and reducing the burden of AF, so it is plausible that this approach could reduce stroke risk to some extent. According to a substudy of the AFFIRM trial, both oral anticoagulation (OAC) and maintenance of sinus rhythm were independently associated with a better survival and a 60% reduction in stroke risk.37 With regard to the outcome of stroke, a reduction was noted only in some observational studies,38, 39 but the limitations of observational design need to be borne in mind. More recently, however, the only largest randomized trial, the CABANA trial, addressed the question of whether AF ablation could impact on hard outcomes. The results revealed that the strategy of CA, compared with medical therapy, did not significantly reduce the primary composite end point of death, disabling stroke, serious bleeding, or cardiac arrest.40 In other words, whether elimination of AF or reduction of AF burden by CA reduces stroke risk is not yet answered according to current studies. In addition, CA is inadequate for patients with spontaneous echo contrast (SEC), or very large LA, which also increases the risk of stroke and often leads to a poor long‐term outcome.41 The newly released ESC guidelines clearly state that long‐term continuation of systemic anticoagulation beyond 2 months post ablation is based on the patient's stroke risk profile rather than the apparent success or failure of the ablation procedure.42
LAAC, especially with Watchman device, has been established with non‐inferior or superior effects on decreasing risks of stroke and bleeding compared with Warfarin on the basis of midterm and long‐term results in randomized PROTECT AF and PREVAIL trials and several prospective registration studies.43, 44, 45, 46, 47, 48, 49 Recently, data from several multinational registries indicated that significant decrease of annualized stroke rate was observed compared to the expected values based on CHA2DS2‐VASc score in patients underwent the one‐stop procedure with CA and LAAC.8, 50 According to a multinational registry with the largest combined procedure population sample (349 patients) to date, an annualized stroke rate of 0.9% for the cohort (78% risk reduction vs. expected based on the average CHA2DS2‐VASc score of 3.0) was documented during a mean follow up of 35 months, regardless of a 51% rate of arrhythmic recurrences and a 85% rate of OAC off.8 Plus, newly‐released data involved 142 subjects underwent a concomitant AF ablation and LAAC procedure at 11 centers, which were pooled from 1140 patients in two prospective, real‐world Watchman LAAC registries EWOLUTION and WASP between 2013 and 2015, revealed that although 92% of patients remained off OAC, the incidence of the composite endpoint of ischemic stroke/transient ischemic attack/systemic thromboembolism was only 1.09 per 100 patient‐years according to a mean follow‐up time of 726 ± 91 days, which represented a relative reductions of 84% than expected on the basis of the mean CHA2DS2‐VASc score of 3.4 ± 1.4.50
Generally, the one‐stop procedure does add some beneficial effects of sinus rhythm recovery by CA, however, the existing evidence does not support the standpoint that the one‐stop concomitant procedure with CA and LAAC produces additional decrease of stroke risk compared with the LAAC alone. In other words, it is urgent to carry out a comparative study between the one‐stop concomitant procedure and LAAC alone.
2.4. Other potential benefits from the one‐stop procedure
Atrial fibrillation often leads to a series of symptoms, adverse effects from medical treatment, and disabilities from AF‐related complications, which is associated with poor quality of life (QOL) in patients.51 Some of the early trials that examined the effects of catheter ablation on patients with AF found that ablation was more effective than drug therapy in improving QOL.52, 53, 54 The CABANA trial, which was designed to test the hypothesis that ablation therapy for AF was more effective than state‐of‐the‐art drug therapies in a broad population of symptomatic but inadequately treated participants with AF, indicated that the CA led to clinically important and significant improvements in QOL at 12 months although it did not significantly reduce the primary composite end point of death, disabling stroke, serious bleeding, or cardiac arrest.40, 55 The one‐stop concomitant procedure with CA and LAAC, which adds the beneficial effects of sinus rhythm recovery by CA, theoretically, may improve QOL in patients for this cohort more than LAAC alone because of more symptom relief and less adverse drug reactions from CA. So far, however, there is still no relevant studies comparing QOL differences between the one‐stop combination and LAAC alone. Besides, the one‐stop concomitant procedure may produce other potential benefits, such as one atrial septal puncture and femoral vein puncture, shorter hospital stays, and shortening of the duration of anticoagulation by merging the separated anticoagulation together, and etc., which can not only save materials and cost, e.g., the transseptal system, but also reduce procedure or treatment related complications. In some countries, however, reimbursement of this combined approach may be restricted due to financial concerns, which could to some extent influence clinical decisions.
Other potential benefits from the one‐stop procedure may include cognitive improvement since AF is associated with cognitive impairment, cognitive decline and dementia.56 Researches have shown that anticoagulation tends to bring cognitive benefit in patients with AF independent of stroke and TIA,57 while effects on cognition post LAAC or one‐stop procedure have not been reported, which needs further studies in the future.
2.5. What is the suitable antithrombotic regimen in preventing the device‐related thrombosis in patients with the one‐stop procedure?
In addition to residual peri‐device leakage after LAAC, DRT after LAAC is another concern. According to data from several multi‐center registries or randomized controlled trials,43, 58, 59, 60, 61 the incidence of DRT after LAAC varies from 3.7%−7.2%, which is established to increased risk of stroke.60 The exact mechanism of DRT is still not clear; however, multiple factors including lack or insufficiency of anticoagulation /antithrombotic treatment after LAAC, different types of device, operational procedures and skills, and patient's condition, are speculated to be associated to DRT formation after LAAC.
Antithrombotic treatment before complete endothelialization on the surface of device in the initial phase after LAAC is required to prevent DRT formation. So far, the antithrombotic regimen post‐LAAC is mainly based on the randomized trials on Watchman device in the early period with warfarin (on top of aspirin) for at least 45 days followed by 6‐month dual antiplatelet therapy (DAPT) and lifelong aspirin.46 Although nonrandomized data using NOACs and DAPT as the immediate post‐implantation therapy has revealed a comparable bleeding and thromboembolic event rates,48 these agents still need to be tested in further studies. Besides, patients also have varieties of conditions including different risk of bleeding, different tolerance of oral anticoagulants due to renal or hepatic insufficiency, and different concomitant diseases or treatments, which to some extent affect antithrombotic regimens and duration of treatment after LAAC. Therefore, the current guidelines or consensus including the 2019 update on the 2014 AHA/ACC/HRS guideline for the management of patients with AF,62 2019 HRS/EHRA/ECAS expert consensus statement63 and 2019 Chinese Society of Cardiology (CSC) expert consensus statement64 discussed recommendations regarding antithrombotic therapy to prevent DRT following LAAC, however, they did not recommend a unified antithrombotic regimen and duration of treatment following LAAC. In most cases, a regimen after LAAC with a 2‐month oral antithrombotic treatment with either warfarin or NOACs plus aspirin or clopidogrel, followed by a 4‐month DAPT and lifelong aspirin, is widely accepted and used for prevention of DRT. In clinical practices, individualized antithrombotic regimen post LAAC is also needed.
The one‐stop procedure with CA and LAAC combination is different from LAAC alone, which adds the beneficial effect of sinus rhythm recovery by CA on left atrial structures and left ventricle function compared with LAAC alone but is still a lack of convincing evidence. Some documented studies revealed that the restore of sinus rhythm by CA might be favorable for improvement of left ventricular ejection fraction (LVEF) and echocardiographic indices in AF patients with LV systolic dysfunction,65, 66, 67 which might be potential factors to decrease hemostasis in left atrium and thromboembolic events, but the CA process also led to acute injury to the atrial myocardium, as evidenced by post‐procedural troponin release and tissue edema, which might increase the risk of thrombosis formation in left atrium or on the surface of device in the early stage of post‐procedure.68 In addition, the prolonged procedure time of CA might be another adverse factor to increase intraoperative thrombosis during the subsequent LAAC stage if without timely monitoring activated clotting time (ACT) and appropriate heparin supplement. Based on data pooled from two prospective, real‐world Watchman LAAC registries running in parallel in Europe/Middle‐East/Russia (EWOLUTION) and Asia/Australia (WASP) between 2013 and 2015, of the 1140 patients, 142 subjects at 11 centers underwent the one‐stop procedure with CA and LAAC. At 28 days post‐procedure, 109 out of 141 patients took the first TEE follow‐up, and 3 of them (2.1%) were detected DRT formation but lacking the second TEE follow‐up data. All the 3 patients had no history of reduced LVEF or congestive cardiac failure and were identified to have appropriate positioning of the device and complete LAA seal under TEE imaging. In addition, all of them were taking NOACs at the time of DRT diagnosis.50 Except for this study, there are still no other studies to compare the differences of DRT incidence after implantation between the one‐stop procedure and the LAAC alone. In other words, for patients undergoing one‐stop procedure with CA and LAAC combination, the antithrombotic regimen and duration of treatment following LAAC are theoretically reasonable with reference to those of LAAC alone before new compelling evidence coming out.
3. WHO ARE THE CANDIDATES AND WHO REALLY BENEFIT FROM “ONE‐STOP” PROCEDURE?
The success rate of ablation varies widely among patients with different types of AF. An international and multicenter pilot study of 1391 patients,69 published in EHJ 2014, showed that the 1‐year success rate of patients with paroxysmal and persistent AF was 74.3% and 72.1% respectively, while the success rate for long‐term persistent was even lower, which was 57.8%, although 18.3% of the patients underwent the second ablation procedure. Besides of AF type, the left atrium diameter is also a risk factor of AF recurrence after ablation. Compared with those who remained sinus rhythm at one year after ablation, patients who had AF recurrences had significantly larger left atrium (42 vs. 44 mm, p = 0.001).
Recent guidelines and consensus of Europe and America unanimously recommended that anticoagulation was necessary for all indicated AF patients, no matter whether the ablation was successful or not. A national registry of Sweden published in 2017 showed that patients with CHA2DS2‐VASc score ≥ 2 points who suspended anticoagulation after ablation procedures had a significantly increased risk of stroke.70
In clinical practice, AF patients with longer course and larger left atrium tend to have lower success rate of atrial fibrillation ablation. Consequently, these patients benefit little from ablation but more from left atrial appendage closure for stroke prevention. Patients with extremely short AF course and normal cardiac structure, such as paroxysmal AF, are highly likely to remain sinus rhythm through ablation. Therefore, in clinical decision making, we can speculate that the higher the CHA2DS2‐VASc score and the larger LA size, the more benefit from LAAC, especially those with SEC or OAC intolerance, while the lower the CHA2DS2‐VASc score and the smaller LA size, the more benefit from PVI (Figure 1). However, there exists many patients with moderate AF duration and left atrium size who can benefit from both ablation procedure and LAAC.
FIGURE 1.
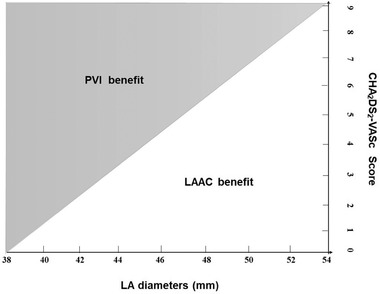
Clinical decision making in selection of AF procedures according to LA size and CHA2DS2‐VASc Score. The higher the CHA2DS2‐VASc score and the larger LA size, the more benefit from LAAC, while the lower the CHA2DS2‐VASc score and the smaller LA size, the more benefit from PVI. Abbreviations: LA, left atrium; LAAC, left atrial appendage closure; PVI, pulmonary vein isolation
At present, the specific candidates for one‐stop operations remains not fully settled. Figure 2 and Figure 3 are summaries of published clinical studies of one‐stop procedure, which show that most of the involved patients are between 62 to 68 years old and have CHA2DS2‐VASc scores ≥2. And the proportion of persistent or long‐standing persistent AF patients is 30%−60%, which indicates that potential candidates of one‐stop procedure are non‐elderly, non‐long‐term AF and anticoagulation‐indicated patients. Taking published studies and clinical experience together, it is speculated that the potential indications for one‐stop procedure are: (1) 60−70 years old age; (2) CHA2DS2‐VASc scores: male ≥2; female ≥3) left atrium diameter ≤48 mm; (4) duration of atrial fibrillation ≤3 years. Of course, with the accumulation of evidence, the characteristics of the indicated population will tend to be much clear and specific.
FIGURE 2.
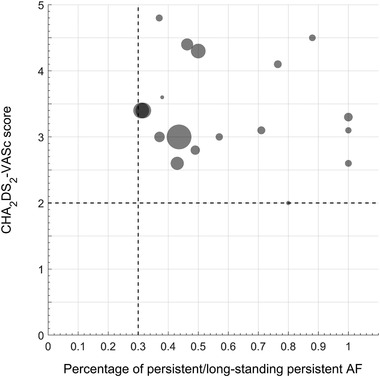
Relationship between AF type and CHA2DS2‐VASc Score in published “one‐stop procedure” studies. In patients underwent the one‐stop procedure with catheter ablation and left atrial appendage closure, the proportion of persistent or long‐standing persistent AF patients is 30%−60%
FIGURE 3.
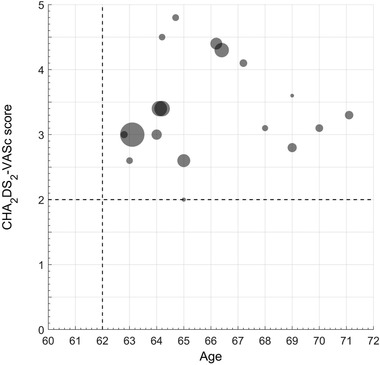
Relationship between Age and CHA2DS2‐VASc Score in published “one‐stop procedure” studies. Most of the involved patients underwent the one‐stop combination procedure with catheter ablation and left atrial appendage closure are between 62 to 68 years old with CHA2DS2‐VASc scores ≥2
CONFLICT OF INTEREST
The authors declare no conflict of interest.
FUNDING INFORMATION
National Natural Science Foundation of China, Grant number: 81830010 and 81330006; Science and Technology Commission of Shanghai Municipality, Grant number: 18411950400; Clinical Research Plan of SHDC, Grant number: 16CR1012A.
He B, Jiang L.‐S, Hao Z.‐Y, Wang H, Miao Y.‐T. Combination of ablation and left atrial appendage closure as “One‐stop” procedure in the treatment of atrial fibrillation: Current status and future perspective. Pacing Clin Electrophysiol. 2021;44:1259–1266. 10.1111/pace.14201
DATA AVAILABILITY STATEMENT
No data were used in this review.
REFERENCES
- 1.Schnabel R, Yin X, Gona P, et al. 50 year trends in atrial fibrillation prevalence, incidence, risk factors, and mortality in the Framingham Heart Study: a cohort study. Lancet. 2015;386:154‐162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chugh SS, Havmoeller R, Narayanan K, et al. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation. 2014;129:837‐847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holmes DR Jr, Doshi SK, Kar S, et al. Left atrial appendage closure as an alternative to warfarin for stroke prevention in atrial fibrillation: a patient‐level meta‐analysis. J Am Coll Cardiol. 2015;65:2614‐2623. [DOI] [PubMed] [Google Scholar]
- 4.Osmancik P, Herman D, Neuzil P, et al. Left atrial appendage closure versus direct oral anti‐ coagulants in high‐risk patients with atrial fibril‐lation. J Am Coll Cardiol. 2020;75:3122‐3135. [DOI] [PubMed] [Google Scholar]
- 5.Jiang RH, Po SS, Tung R, et al. Incidence of pulmonary vein conduction recovery in patients without clinical recurrence after ablation of paroxysmal atrial fibrillation: mechanistic implications. Heart Rhythm. 2014;11:969‐976. [DOI] [PubMed] [Google Scholar]
- 6.Calkins H, Hindricks G, Cappato R, et al. HRS/EHRA/ECAS/APHRS /SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. J Arrhythm. 2017. ;2017:369‐409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alkhouli M, Noseworthy PA, Rihal CS. Stroke prevention in nonvalvular atrial fibrillation: a stakeholder perspective. J Am Coll Cardiol. 2018;71:2790‐2801. [DOI] [PubMed] [Google Scholar]
- 8.Wintgens L, Romanov A, Phillips K, et al. Combined atrial fibrillation ablation and left atrial appendage closure: long‐term follow‐up from a large multicentre registry. Europace. 2018;20:1783‐1789. [DOI] [PubMed] [Google Scholar]
- 9.Alipour A, Swaans MJ, van Dijk VF, et al. Ablation for atrial fibrillation combined with left atrial appendage closure. JACC Clin Electrophysiol. 2015;1:486‐495. [DOI] [PubMed] [Google Scholar]
- 10.Calvo N, Salterain N, Arguedas H, et al. Combined catheter ablation and left atrial appendage closure as a hybrid procedure for the treatment of atrial fibrillation. Europace. 2015;17:1533‐1540. [DOI] [PubMed] [Google Scholar]
- 11.Du X, Chu H, Ye P, et al. Combination of left atrial appendage closure and catheter ablation in a single procedure for patients with atrial fibrillation: multicenter experience. J Formos Med Assoc. 2019;118:891‐897. [DOI] [PubMed] [Google Scholar]
- 12.Phillips KP, Walker DT, Humphries JA. Combined catheter ablation for atrial fibrillation and Watchman(R) left atrial appendage occlusion procedures: five‐year experience. J Arrhythm. 2016;32:119‐126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuck KH, Brugada J, Furnkranz A, et al. Cryoballoon or radiofrequency ablation for paroxysmal atrial fibrillation. N Engl J Med. 2016;374:2235‐2245. [DOI] [PubMed] [Google Scholar]
- 14.Andrade JG, Champagne J, Dubuc M, et al. Cryoballoon or radiofrequency ablation for atrial fibrillation assessed by continuous monitoring: a randomized clinical trial. Circulation. 2019;140:1779‐1788. [DOI] [PubMed] [Google Scholar]
- 15.Verma A, Jiang CY, Betts TR, et al. Approaches to catheter ablation for persistent atrial fibrillation. N Engl J Med. 2015;372:1812‐1822. [DOI] [PubMed] [Google Scholar]
- 16.Wintgens LIS, Klaver MN, Swaans MJ, et al. Left atrial catheter ablation in patients with previously implanted left atrial appendage closure devices. Europace. 2019;21:428‐433. [DOI] [PubMed] [Google Scholar]
- 17.Du X, Chu H, He B, et al. Optimal combination strategy of left atrial appendage closure plus catheter ablation in a single procedure in patients with nonvalvular atrial fibrillation. J Cardiovasc Electrophysiol. 2018;29:1089‐1095. [DOI] [PubMed] [Google Scholar]
- 18.Phillips KP, Pokushalov E, Romanov A, et al. Combining Watchman left atrial appendage closure and catheter ablation for atrial fibrillation: multicentre registry results of feasibility and safety during implant and 30 days follow‐up. Europace. 2018;20:949‐955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boersma LV, Schmidt B, Betts TR, et al. Implant success and safety of left atrial appendage closure with the WATCHMAN device: peri‐procedural outcomes from the EWOLUTION registry. Eur Heart J. 2016;37:2465‐2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Romanov A, Pokushalov E, Artemenko S, et al. Does left atrial appendage closure improve the success of pulmonary vein isolation? Results of a randomized clinical trial. J Interv Card Electrophysiol. 2015;44:9‐16. [DOI] [PubMed] [Google Scholar]
- 21.Li YG, Gong CQ, Zhao MZ, et al. Determinants of postoperative left atrial structural reverse remodeling in patients undergoing combined catheter ablation of atrial fibrillation and left atrial appendage closure procedure. J Cardiovasc Electrophysiol. 2019;30:1868‐1876. [DOI] [PubMed] [Google Scholar]
- 22.Jiang Y, Li F, Li D, et al. Efficacy and safety of catheter ablation combined with left atrial appendage occlusion for nonvalvular atrial fibrillation: a systematic review and meta‐analysis. Pacing Clin Electrophysiol. 2020;43:123‐132. [DOI] [PubMed] [Google Scholar]
- 23.Fassini G, Gasperetti A, Italiano G, et al. Cryoballoon pulmonary vein ablation and left atrial appendage closure combined procedure: a long‐term follow‐up analysis. Heart Rhythm. 2019;16:1320‐1326. [DOI] [PubMed] [Google Scholar]
- 24.Panikker S, Jarman JW, Virmani R, et al. Left atrial appendage electrical isolation and concomitant device occlusion to treat persistent atrial fibrillation: a first‐in‐human safety, feasibility, and efficacy study. Circ Arrhythm Electrophysiol. 2016;9:e003710. 10.1161/CIRCEP.115.003710. [DOI] [PubMed] [Google Scholar]
- 25.Rillig A, Tilz RR, Lin T, et al. Unexpectedly high incidence of stroke and left atrial appendage thrombus formation after electrical isolation of the left atrial appendage for the treatment of atrial tachyarrhythmias. Circ Arrhythm Electrophysiol. 2016;9:e003461. [DOI] [PubMed] [Google Scholar]
- 26.Turagam MK, Lavu M, Afzal MR, et al. Catheter ablation for atrial fibrillation in patients with watchman left atrial appendage occlusion device: results from a multicenter registry. J Cardiovasc Electrophysiol. 2017;28:139‐146. [DOI] [PubMed] [Google Scholar]
- 27.Qamar SR, Jalal S, Nicolaou S, et al. Comparison of cardiac computed tomography angiography and transoesophageal echocardiography for device surveillance after left atrial appendage closure. EuroIntervention. 2019;15:663‐670. [DOI] [PubMed] [Google Scholar]
- 28.Saw J, Tzikas A, Shakir S, et al. Incidence and clinical impact of device‐associated thrombus and peri‐device leak following left atrial appendage closure with the amplatzer cardiac plug. J Am Coll Cardiol Intv. 2017;10:391‐399. [DOI] [PubMed] [Google Scholar]
- 29.Lindner S, Behnes M, Wenke A, et al. Assessment of peri‐device leaks after interventional left atrial appendage closure using standardized imaging by cardiac computed tomography angiography. Int J Cardiovasc Imaging. 2019;35:725‐731. [DOI] [PubMed] [Google Scholar]
- 30.Nguyen A, Gallet R, Riant E, et al. Peridevice leak after left atrial appendage closure: incidence, risk factors, and clinical impact. Can J Cardiol. 2019;35:405‐412. [DOI] [PubMed] [Google Scholar]
- 31.Sivasambu B, Arbab‐Zadeh A, Hays A, Calkins H, Berger RD. Delayed endothelialization of watchman device identified with cardiac CT. J Cardiovasc Electrophysiol. 2019;30:1319‐1324. [DOI] [PubMed] [Google Scholar]
- 32.Aryana A, Singh SK, Singh SM, et al. Association between incomplete surgical ligation of left atrial appendage and stroke and systemic embolization. Heart Rhythm. 2015;12:1431‐1437. [DOI] [PubMed] [Google Scholar]
- 33.Kanderian AS, Gillinov AM, Pettersson GB, et al. Success of surgical left atrial appendage closure: assessment by transesophageal echocardiography. J Am Coll Cardiol. 2008;52:924‐929. [DOI] [PubMed] [Google Scholar]
- 34.Raphael CE, Friedman PA, Saw J, Pislaru SV, Munger TM. Residual leaks following percutaneous left atrial appendage occlusion: assessment and management implications. EuroIntervention. 2017;13:1218‐1225. [DOI] [PubMed] [Google Scholar]
- 35.Wright M, Harks E, Deladi S, et al. Characteristics of radiofrequency catheter ablation lesion formation in real time in vivo using near field ultrasound imaging. JACC Clin Electrophysiol. 2018;4:1062‐1072. [DOI] [PubMed] [Google Scholar]
- 36.Miyazaki S, Nakamura H, Kajiyama T, Watanabe T, Iesaka Y. Early tissue reaction after second‐generation cryoballoon ablation evaluated with intracardiac echocardiography. Int Heart J. 2019;60:618‐623.published correction appears in Int Heart J. 2019;60: 497‐8. [DOI] [PubMed] [Google Scholar]
- 37.Corley SD, Epstein AE, DiMarco JP, et al. Relationships between sinus rhythm, treatment, and survival in the Atrial Fibrillation Follow‐Up Investigation of Rhythm Management (AFFIRM) study. Circulation. 2004;109:1509‐1513. [DOI] [PubMed] [Google Scholar]
- 38.Bunch TJ, Crandall BG, Weiss JP, et al. Patients treated with catheter ablation for atrial fibrillation have longterm rates of death, stroke, and dementia similar to patients without atrial fibrillation. J Cardiovasc Electrophysiol. 2011;22:839‐845. [DOI] [PubMed] [Google Scholar]
- 39.Friberg L, Tabrizi F, Englund A. Catheter ablation for atrial fibrillation is associated with lower incidence of stroke and death: data from Swedish health registries. Eur Heart J. 2016;37:2478‐2487. [DOI] [PubMed] [Google Scholar]
- 40.Packer DL, Mark DB, Robb RA, et al. Effect of catheter ablation vs antiarrhythmic drug therapy on mortality, stroke, bleeding, and cardiac arrest among patients with atrial fibrillation: the CABANA randomized clinical trial. JAMA. 2019;321:1261‐1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yoo J, Song D, Baek JH, et al. Poor outcome of stroke patients with atrial fibrillation in the presence of coexisting spontaneous echo contrast. Stroke. 2016;47:1920‐1922. [DOI] [PubMed] [Google Scholar]
- 42.Hindricks G, Potpara T, Dagres N, et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio‐Thoracic Surgery (EACTS). Eur Heart J. 2020;00:1‐125. [Google Scholar]
- 43.Holmes DJ, Kar S, Price MJ, et al. Prospective randomized evaluation of the Watchman Left Atrial Appendage Closure device in patients with atrial fibrillation versus long‐term warfarin therapy: the PREVAIL trial. J Am Coll Cardiol. 2014;64:1‐12. [DOI] [PubMed] [Google Scholar]
- 44.Holmes DR, Reddy VY, Turi ZG, et al. Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: a randomised non‐inferiority trial. Lancet. 2009;374:534‐542. [DOI] [PubMed] [Google Scholar]
- 45.Reddy VY, Sievert H, Halperin J, et al. Percutaneous left atrial appendage closure vs warfarin for atrial fibrillation: a randomized clinical trial. JAMA. 2014;312:1988‐1998. [DOI] [PubMed] [Google Scholar]
- 46.Reddy VY, Doshi SK, Kar S, et al. 5‐year outcomes after left atrial appendage closure: from the PREVAIL and PROTECT AF trials. J Am Coll Cardiol. 2017;70:2964‐2975. [DOI] [PubMed] [Google Scholar]
- 47.Boersma LV, Ince H, Kische S, et al. Efficacy and safety of left atrial appendage closure with WATCHMAN in patients with or without contraindication to oral anticoagulation: 1‐Year follow‐up outcome data of the EWOLUTION trial. Heart Rhythm. 2017;14:1302‐1308. [DOI] [PubMed] [Google Scholar]
- 48.Boersma LV, Ince H, Kische S, et al. Evaluating real‐world clinical outcomes in atrial fibrillation patients receiving the WATCHMAN Left Atrial Appendage Closure Technology: final 2‐year outcome data of the EWOLUTION trial focusing on history of stroke and hemorrhage. Circ Arrhythm Electrophysiol. 2019;12:e006841. 10.1161/CIRCEP.118.006841. [DOI] [PubMed] [Google Scholar]
- 49.Lakkireddy DR, Turagam MK. NCDR left atrial appendage occlusion registry: the “Watch” Man has arrived. J Am Coll Cardiol. 2020;75:1519‐1522. [DOI] [PubMed] [Google Scholar]
- 50.Phillips KP, Romanov A, Artemenko S, et al. Combining left atrial appendage closure and catheter ablation for atrial fibrillation: 2‐year outcomes from a multinational registry. Europace. 2020;22:225‐231. [DOI] [PubMed] [Google Scholar]
- 51.Dorian P, Guerra P, Kerr C, et al. Validation of a new simple scale to measure symptoms in atrial fibrillation. Circ Arrhythm Electrophysiol. 2009;2:218‐224. [DOI] [PubMed] [Google Scholar]
- 52.Cosedis Nielsen J, Johannessen A, Raatikainen P, et al. Radiofrequency ablation as initial therapy in paroxysmal atrial fibrillation. N Engl J Med. 2012;367:1587‐1595. [DOI] [PubMed] [Google Scholar]
- 53.Morillo CA, Verma A, Connolly SJ, et al. Radiofrequency ablation vs antiarrhythmic drugs as first‐line treatment of paroxysmal atrial fibrillation (RAAFT‐2): a randomized trial. JAMA. 2014;311:692‐700. [DOI] [PubMed] [Google Scholar]
- 54.Wilber DJ, Pappone C, Neuzil P, et al. Comparison of antiarrhythmic drug therapy and radiofrequency catheter ablation in patients with paroxysmal atrial fibrillation: a randomized controlled trial. JAMA. 2010;303:333‐340. [DOI] [PubMed] [Google Scholar]
- 55.Mark DB, Anstrom KJ, Sheng S, et al. Effect of catheter ablation vs medical therapy on quality of life among patients with atrial fibrillation: the CABANA randomized clinical trial. JAMA. 2019;321:1275‐1285.published correction appears in JAMA. 2019; 321:2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hui DS, Morley JE, Mikolajczak PC, et al. Atrial fibrillation: a major risk factor for cognitive decline. Am Heart J. 2015;169:448‐456. [DOI] [PubMed] [Google Scholar]
- 57.Zeng D, Jiang CL, Su C, Tan Y, Wu J.Anticoagulation in atrial fibrillation and cognitive decline: a systematic review and meta‐analysis. Medicine (Baltimore). 2019;98: e14499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Reddy VY, Doshi SK, Sievert H, et al. Percutaneous left atrial appendage closure for stroke prophylaxis in patients with atrial fibrillation: 2.3‐year follow‐up of the PROTECT AF (Watchman Left Atrial Appendage System for embolic protection in patients with atrial fibrillation) Trial. Circulation. 2013;127:720‐729. [DOI] [PubMed] [Google Scholar]
- 59.Bergmann MW, Betts TR, Sievert H, et al. Safety and efficacy of early anticoagulation drug regimens after WATCHMAN left atrial appendage closure: three‐month data from the EWOLUTION prospective, multicentre, monitored international WATCHMAN LAA closure registry. EuroIntervention. 2017;13:877‐884. [DOI] [PubMed] [Google Scholar]
- 60.Fauchier L, Cinaud A, Brigadeau F, et al. Device‐related thrombosis after percutaneous left atrial appendage occlusion for atrial fibrillation. J Am Coll Cardiol. 2018;71:1528‐1536. [DOI] [PubMed] [Google Scholar]
- 61.Dukkipati SR, Kar S, Holmes DR, et al. Device‐related thrombus after left atrial appendage closure. Circulation. 2018;138:874‐885. [DOI] [PubMed] [Google Scholar]
- 62.January CT, Wann LS, Calkins H, et al. AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the management of patients with atrial fibrillation: a report of the American college of cardiology/American heart association task force on clinical practice guidelines and the heart rhythm society. J Am Coll Cardiol. 2019. ;2019:104‐132. [DOI] [PubMed] [Google Scholar]
- 63.Glikson M, Wolff R, Hindricks2 G, et al. EHRA/EAPCI expert consensus statement on catheter‐based left atrial appendage occlusion – an update. Europace. 2019;22:euz258. 10.1093/europace/euz258 [DOI] [PubMed] [Google Scholar]
- 64.Chinese Society of Cardiology (CSC), Editorial Board of Chinese Journal of Cardiology . 2019. Chinese expert consensus statement on left atrial appendage closure in the prevention of stroke in patients with atrial fibrillation. Chin. J. Cardiol. 2019:937‐955. [DOI] [PubMed] [Google Scholar]
- 65.Lutomsky BA, Rostock T, Koops A, et al. Catheter ablation of paroxysmal atrial fibrillation improves cardiac function: a prospective study on the impact of atrial fibrillation ablation on left ventricular function assess by magnetic resonance imaging. Europace. 2008;10:593‐599. [DOI] [PubMed] [Google Scholar]
- 66.Choi AD, Hematpour K, Kukin M, et al. Ablation vs medical therapy in the setting of symptomatic atrial fibrillation and left ventricular dysfunction. Congest Heart Failure. 2010;16:10‐14. [DOI] [PubMed] [Google Scholar]
- 67.Patel MR, Biviano AB. Atrial fibrillation ablation and left appendage closure in heart failure patients. Curr Opin Cardiol. 2015;30:259‐266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Packer M. Effect of catheter ablation on pre‐existing abnormalities of left atrial systolic, diastolic, and neurohormonal functions in patients with chronic heart failure and atrial fibrillation. Eur Heart J. 2019;40:1873‐1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Arbelo E, Brugada J, Hindricks G, et al. The Atrial fibrillation ablation pilot study: an European survey on methodology and results of catheter ablation for atrial fibrillation conducted by the European Heart Rhythm Association. Eur Heart J. 2014;35:1466‐1478. [DOI] [PubMed] [Google Scholar]
- 70.Katz DF, Maddox TM, Turakhia M, et al. Contemporary trends in oral anticoagulant prescription in atrial fibrillation patients at low to moderate risk of stroke after guideline‐recommended change in use of the CHADS2 to the CHA2DS2‐VASc score for thromboembolic risk assessment: analysis from the national cardiovascular data registry's outpatient practice innovation and clinical excellence atrial fibrillation registry. Circ Cardiovasc Qual Outcomes. 2017;10:e003476. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data were used in this review.


