Abstract
Objectives
We aimed to assess the safety and performance of the Magmaris sirolimus‐eluting bioresorbable magnesium scaffold in a large patient population.
Background
Magmaris has shown good outcomes in small‐sized controlled trials, but further data are needed to confirm its usability, safety, and performance.
Methods
BIOSOLVE‐IV is an international, single arm, multicenter registry including patients with a maximum of two single de novo lesions. Follow‐up is scheduled up to 5 years; the primary outcome is target lesion failure (TLF) at 12 months.
Results
A total of 1,075 patients with 1,121 lesions were enrolled. Mean patient age was 61.3 ± 10.5 years and 19.2% (n = 206) presented with non‐ST‐elevation myocardial infarction (NSTEMI). Lesions were 3.2 ± 0.3 mm in diameter and 14.9 ± 4.2 mm long; 5.1% (n = 57) were bifurcation lesions. Device success was 97.3% (n = 1,129) and procedure success 98.9% (n = 1,063). The Kaplan–Meier estimate of TLF at 12 months was 4.3% [95% confidence interval, CI: 3.2, 5.7] consisting of 3.9% target lesion revascularizations, 0.2% cardiac death, and 1.1% target‐vessel myocardial infarction. Definite/probable scaffold thrombosis occurred in five patients (0.5% [95% CI: 0.2, 1.1]), thereof four after early discontinuation of antiplatelet/anticoagulation therapy.
Conclusion
BIOSOLVE‐IV confirms the safety and performance of the Magmaris scaffold in a large population with excellent device and procedure success and a very good safety profile up to 12 months in a low‐risk population.
Keywords: bioresorbable scaffolds, coronary artery disease, magnesium, NSTEMI, sirolimus
1. INTRODUCTION
Bioresorbable scaffolds (BRS) have been developed to overcome shortcomings of permanent stents, avoiding long‐term effects such as late inflammation or mechanical failure, allowing restoration of vasomotion, adaptive remodeling, noninvasive imaging, and facilitating future interventions.1
The first marketed BRS was the Absorb polymeric scaffold (Abbott Vascular, Santa Clara, CA) that showed excellent initial results in the relatively simple lesions of the early ABSORB cohort A (Absorb version 1.0) and B (Absorb version 1.1) trials with absence of definite or probable scaffold thrombosis in 131 patients enrolled.2, 3 However, postmarket studies with less selected lesions showed increased scaffold thrombosis rates when compared to permanent drug‐eluting stents (DES), albeit it seems that these outcomes can be improved with optimized implantation technique.4, 5
In contrast to the Absorb BRS, Magmaris (Biotronik AG, Bülach, Switzerland) is magnesium‐based. The scaffold has been successfully used in 184 patients enrolled in the BIOSOLVE‐II and ‐III studies with good clinical outcomes and absence of definite or probable scaffold thrombosis up to 3 years.6, 7, 8 After gaining CE‐certification in June 2016, it was paramount to assess the device in a large number of centers. The device implantation is different from contemporary thin‐strut DES and outcomes might depend on the correct implantation technique.9 Furthermore, the implantation in more complex lesions might lead to increased thrombosis rates as observed for Absorb,4 and lastly, data on large patient populations are required to detect rare events. We herein report the outcomes of the first cohort of 1,075 patients followed for 12 months.
2. MATERIALS AND METHODS
2.1. Study design and population
BIOSOLVE‐IV is an international, single arm, multicenter registry to investigate the clinical performance and long‐term safety of Magmaris after CE‐mark. It is conducted in more than 80 centers in 23 countries in Europe, Asia, Africa, and Australia/New Zealand (Supporting Information, Table S1). The first patient was enrolled in September 2016.10 Initially planned to include a minimum of 1,065 patients, prior to enrolment completion, due to the alarming scaffold thrombosis rates detected for the Absorb scaffold, the registry was extended to 2,054 patients (second cohort) to include a powered objective performance outcome for definite or probable scaffold thrombosis.
Eligibility criteria aligned with the instructions for use. Main inclusion criteria were: a maximum of two single de novo lesions in two different major epicardial vessels, lesion length ≤21 mm, target lesion stenosis >50 and <100%, thrombolysis in myocardial infarction flow ≥1, and reference vessel diameter between 2.7 and 3.7 mm. Main exclusion criteria were left main disease, restenotic lesions, acute ST‐elevation myocardial infarction, important bifurcation lesions, and unsuccessful predilatation. The list of in‐ and exclusion criteria is available at ClinicalTrials.gov: NCT02817802.
Follow‐up was scheduled at 6 and 12 months, and annually thereafter until 5 years. It could be conducted by telephone or office visit and included assessments of adverse events and concomitant medications. The registry is performed in accordance with current guidelines such as Declaration of Helsinki and ISO14155, was approved by the ethics committees, and all patients provided written informed consent. Monitoring encompasses a minimum of 25% randomly selected subjects per center and an angiographic core laboratory is used to assess outcome‐related events and cases of device failure. All events for which a device relationship could not be ruled out were adjudicated by an independent clinical events committee member (CEC). In case of disagreement between CEC and study site, the case was reviewed by a second CEC member.
2.2. Device and procedure
Magmaris has been described previously.6, 7, 8, 11 In brief, it is a sirolimus‐eluting resorbable coronary magnesium scaffold system that consists of a balloon‐expandable scaffold with a strut thickness and width of 150 μm crimped on a delivery system. Its proBIO coating is the same as used for the Orsiro DES (Biotronik AG, Buelach, Switzerland), and consists of the active agent sirolimus in an absorbable poly‐L‐lactic acid polymer. The scaffold is available in diameters of 3.0 and 3.5 mm and lengths of 15, 20, and 25 mm. Its resorption time is 12 months (Figure 1).
FIGURE 1.
Magmaris resorption process. m, month; Mg, magnesium
Predilatation using a noncompliant balloon with a 1:1 balloon‐to‐artery ratio was mandatory and a 6F guiding catheter or larger ought to be used. The balloon should expand fully and the residual stenosis before Magmaris implantation should be <20%. The use of a drug‐coated balloon or rotational atherectomy device was not allowed. Furthermore, the scaffold implantation ought to follow standard of care and standard hospital practice and should follow the recommendation of the instructions for use and the consensus letter of Fajadet et al.9 Only one scaffold per lesion ought to be used. Postdilatation with a noncompliant balloon at high pressure (>16 atm) was recommended (in a later protocol version, it was specified that the postdilatation balloon should be up to 0.5 mm larger than the nominal scaffold size). In case of bailout situations, a second stent or scaffold could be used, preferably with proBIO coating to avoid electrochemical interaction.
Dual antiplatelet therapy (DAPT) was recommended for at least 6 months postprocedure.
2.3. Outcomes and definitions
The primary outcome was target lesion failure (TLF) at 12 months, defined as the hierarchical composite of cardiac death, target‐vessel myocardial infarction (TV‐MI), coronary artery bypass grafting, and clinically driven target lesion revascularization (TLR). Secondary outcomes were procedure success (final diameter stenosis <30% without death, myocardial infarction, or repeat TLR during the hospital stay) and device success (final diameter stenosis <30% using the assigned device only, successful delivery, and appropriate deployment of the scaffold and successful removal of the delivery system), clinically driven TLR, clinically driven target‐vessel revascularizations (TVR), cardiac death, TV‐MI, and scaffold thrombosis. Periprocedural myocardial infarctions were adjudicated using Society for Cardiovascular Angiography and Interventions (SCAI) definitions, spontaneous myocardial infarction using the extended historical definitions, and TLR, TVR, and scaffold thrombosis using the Academic Research Consortium definitions.12, 13, 14
2.4. Statistical analysis
The sample size was originally calculated based on the null‐hypothesis that Magmaris has a 12‐month TLF rate of ≥10%. A minimum of 1,065 patients were required including a 20% drop out rate and assuming an actual Magmaris TLF rate of 6.6% (based on Absorb data), 95% power, one‐sided, normal approximation of binominal test, and significance level α of 0.025.
For the second cohort, the sample size was calculated based on the null‐hypothesis that Magmaris has a 12‐month probable or definite scaffold thrombosis rate of ≥1.49% (based on Absorb data). A total of 2,054 patients were calculated including a 20% drop out rate and assuming an actual Magmaris probable or definite scaffold thrombosis rate of 0.65%, 90% power, one‐sided, exact binominal test, and significance level α of 0.025. Further details are provided in Tables S2 and S3.
The statistical analysis was performed for all enrolled subjects based on an intention‐to‐treat analysis. Subjects were considered enrolled after signature of written informed consent and after the device had entered the guiding catheter. Subjects in whom a Magmaris system entered the guiding catheter, but could not be implanted at the intended place, were considered for device and procedure success, but were excluded from other outcome analysis.
For quantitative variables, the mean values and SD were calculated and for qualitative variables, absolute and relative frequencies were calculated. For individual and combined clinical outcomes, the Kaplan–Meier estimator (and 95% confidence interval [CI]) was calculated. In a post‐hoc analysis, outcomes in specific high‐risk groups were compared using the log‐rank test. All analyses were conducted using SAS 9.4.
3. RESULTS
We herein report the primary outcome of the first cohort of 1,075 patients enrolled in the BIOSOLVE‐IV registry. Patients were 61.3 ± 10.5 years old, ranging from 29 to 86 years. The majority of patients were male (75.0%, n = 806) and had stable coronary artery disease, 19.2% (n = 206) presented with non‐ST‐elevation myocardial infarction (NSTEMI). Target lesions were 3.2 ± 0.3 mm in diameter and 14.9 ± 4.2 mm long with a diameter stenosis of 82.4 ± 10.6% (Table 1).
TABLE 1.
Baseline clinical and lesion characteristics
| Patients | N = 1,075 |
|---|---|
| Mean age, years | 61.3 ± 10.5 |
| Male | 806 (75.0%) |
| Hypertension | 724 (67.3%) |
| Hypercholesterolemia | 713 (66.3%) |
| Diabetes | 228 (21.2%) |
| Insulin‐dependent | 45 (19.7%) |
| Renal diseasea | 66 (6.1%) |
| History of myocardial infarction | 219 (20.4%) |
| Previous coronary surgeries/ interventions | 287 (26.7%) |
| Lesionsb | N = 1,121 |
|---|---|
| Lesion length, mm | 14.9 ± 4.2 |
| Reference vessel diameter, mm, N = 1,119 | 3.2 ± 0.3 |
| Diameter stenosis, % | 82.4 ± 10.6 |
| AHA/ACC classification type B2/C | 170 (15.2%) |
| Bifurcation lesion | 57 (5.1%) |
Note: Data are shown as mean ± SD or n (%)
site assessed, without specific definition.
Per site assessment.
The majority of patients had one lesion treated, and 46 patients (4.3%) two lesions. Magmaris could not be implanted in 0.9% (n = 10), thereof 4 patients did not receive any Magmaris scaffold. When multiple scaffolds were needed to treat a lesion, they were predominantly placed end‐to‐end (n = 23), or overlapping (n = 9), and in two lesions, there was a distance between the scaffolds. Postdilatation was performed in nearly all cases (96.4%, n = 1,081). Device and procedure success were 97.3% (n = 1,129) and 98.9% (n = 1,063), and periprocedural myocardial infarction occurred in 0.3% (n = 3) (Table 2).
TABLE 2.
Procedural characteristics
| N = 1,121 | |
|---|---|
| Predilatation performed | 1,118 (99.7%) |
| Maximum pressure applied, atm | 14.3 ± 3.4 |
| Scaffold length, mm, N = 1,150 | 19.6 ± 3.9 |
| Scaffold diameter, mm, N = 1,150 | 3.2 ± 0.3 |
| Maximum pressure applied, N = 1,145 | 14.4 ± 2.7 |
| Postdilatation performed, N = 1,121 | 1,081 (96.4%) |
| Maximum pressure applied, atm, N = 1,206 | 17.1 ± 3.3 |
| Device success, N = 1,160 stents | 1,129 (97.3%) |
| Procedure success, N = 1,075 patients | 1,063 (98.9%) |
Note: Data are shown as mean ± SD or n (%). Device success was defined as a final diameter stenosis of <30% using the assigned device only, successful delivery of the scaffold, appropriate scaffold deployment, and successful removal of the delivery system. Procedure success was defined final diameter stenosis <30% without the occurrence of death, myocardial infarction, or repeat target lesion revascularization during the hospital stay.
Follow‐up data at 6 months is available for 98.9% (1,059/1,071) of patients at 6 months and 97.9% (1,049/1,071) at 12 months (Figure 2). At baseline, all but one patient were symptomatic respective had silent ischemia, whereas at 6 months follow‐up, 89.9% were symptom‐free—a result that was sustained at 12 months with 91.8% symptom‐free patients (Figure 3). When returning for the 12‐month follow‐up, 85.1% (887/1042) were on DAPT.
FIGURE 2.
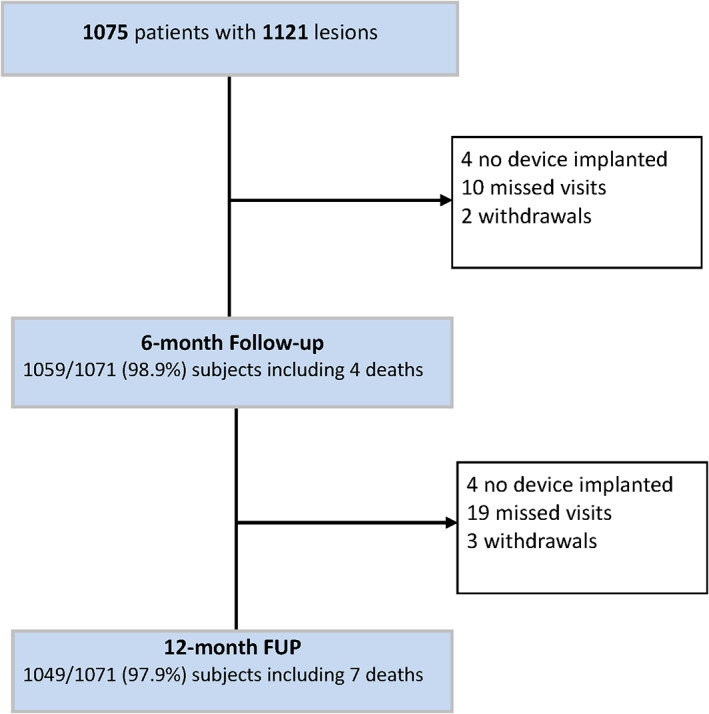
Subject disposition
FIGURE 3.
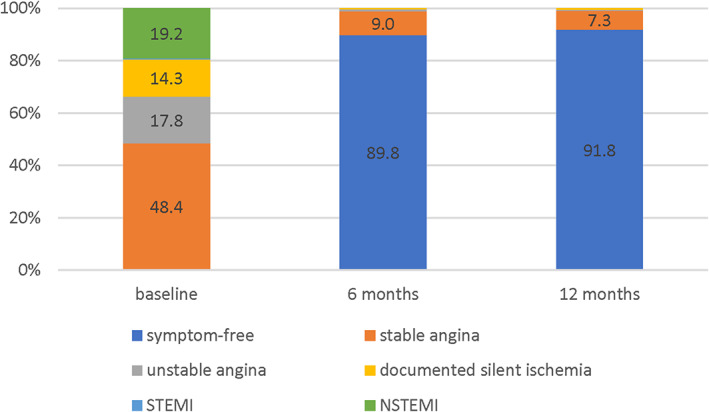
Ischemic status at baseline and follow‐up. Data were available for all patients at baseline, for 1,054 patients at 6 months, and for 1,041 patients at 12 months. NSTEMI, non‐ST‐elevation myocardial infarction
The primary outcome, the Kaplan–Meier estimator of TLF at 12 months, was 4.3% (n = 45, Figure 4, Table 3), consisting of 0.2% cardiac death, 1.1% TV‐MI, and 3.9% clinically driven TLR. Two patients died of cancer. There was no significant difference among outcomes in the subgroup analysis except that NSTEMI patients had significantly higher TV‐ and overall myocardial infarction rates (2.9 vs. 0.7%, p = 0.006 and 3.4 vs. 0.7%, p = 0.001, respectively). Of the nine patients with overlapping scaffolds, none had died and none experienced a definite or probable scaffold thrombosis, but one experienced a TV‐MI due to dissection and a TLR.
FIGURE 4.
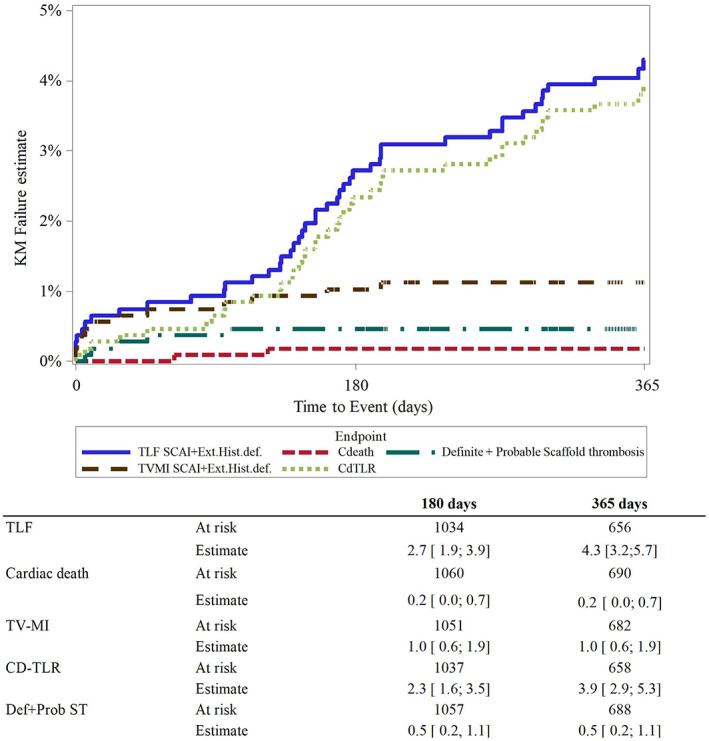
Estimates of events. CD‐TLR, clinically driven target lesion revascularization; Ext.Hist.Def, Extended historical definitions; SCAI, Society for Cardiovascular Angiography and Interventions; TLF, target lesion failure; TV‐MI, target‐vessel myocardial infarction
TABLE 3.
Kaplan–Meier estimates of clinical outcomes at 12 months
| Overall, N = 1,075 | NSTEMI, N = 206 | Diabetes, N = 228 | Type B2/C lesions, N = 164 | |
|---|---|---|---|---|
| TLF | 45 (4.3%) [3.2;5.7] | 12 (6.1%) [3.5;10.6] | 10 (4.7%) [2.5;8.7] | 9 (5.7%) [3.0;10.6] |
| Cardiac death | 2 (0.2%) [0.0;0.7] | 0 | 1 (0.4%) [0.1;3.1] | 1 (0.6%) [0.1;4.3] |
| TV‐myocardial infarction | 12 (1.1%) [0.6;2.0] | 6 (2.9%) [1.3;6.4]a | 4 (1.8%) [0.7;4.6] | 2 (1.3%) [0.3;4.9] |
| Clinically driven TLR | 39 (3.9%) [2.9;5.3] | 11 (5.6%) [3.1;10.0] | 8 (3.8%) [1.9;7.7] | 9 (5.7%) [3.0;10.6] |
| Death | 7 (0.7%) [0.3;1.4] | 1 (0.5%) [0.1;3.4] | 3 (1.3%) [0.4:4.0] | 2 (1.3%) [0.3;5.0] |
| Myocardial infarction | 13 (1.2%) [0.7;2.1] | 7 (3.4%) [1.6;7.0] | 4 (1.8%) [0.7:4.6] | 2 (1.3%) [0.3;4.9] |
| CD‐TVR | 44 (4.2%) [3.2;5.6] | 11 (5.6%) [3.1;10.0] | 8 (3.8%) [1.9;7.7] | 9 (5.7%) [3.0;10.6] |
| TVF | 48 (4.6%) [3.5;6.0] | 12 (6.1%) [3.5;10.6] | 10 (4.7%) [2.5;8.7] | 9 (5.7%) [3.0;10.6] |
| Stent thrombosis | ||||
| Definite | 5 (0.5%) [0.2;1.1] | 2 (1.0%) [0.2;3.8] | 2 (0.9%) [0.2;3.5] | 1 (0.6%) [0.1;4.3] |
| Definite/probable | 5 (0.5%) [0.2;1.1] | 2 (1.0%) [0.2;3.8] | 2 (0.9%) [0.2;3.5] | 1 (0.6%) [0.1;4.3] |
Note: Data are shown as n (Kaplan–Meier estimate in %) [95%CI].
Abbreviations: CABG, coronary artery bypass graft; DAPT, dual antiplatelet therapy; NSTEMI, non‐ST‐elevation myocardial infarction; TLF, target lesion revascularization; TLR, target lesion revascularization; TV, target vessel; TVF, target‐vessel failure; TVR, target‐vessel revascularization.
Significantly different to non‐NSTEMI patients.
Five definite or probable (0.5%) scaffold thromboses occurred on day 6, 10, 28, 46, and 95 postprocedure; thereof all but one occurred after early interruption of antiplatelet/anticoagulation therapy. Further details on these cases are provided in Table S4.
4. DISCUSSION
The presented outcomes confirm the favorable safety outcomes of the BIOSOLVE‐II and ‐III trials in a large patient population treated according to standard of care. With 4.3% TLF at 12 months, the predefined outcome for the first cohort of 1,075 patients was met and the null‐hypothesis rejected. These outcomes of BIOSOLVE‐IV are paramount as the recent European Society of Cardiology/European Association for Cardio‐Thoracic Surgery guidelines on myocardial revascularization acknowledge that initial results of magnesium scaffolds are encouraging, but more data are needed.15
4.1. Comparison to BIOSOLVE‐II and ‐III and other Magmaris data
Patient selection was performed carefully in the sense that in BIOSOLVE‐IV, predominantly simple lesions were treated. The only relevant difference was the inclusion of 19.2% NSTEMI patients that were excluded in BIOSOLVE‐II and ‐III.6, 7, 8
Pre‐ and postdilatation were performed in more than 95% of cases whereas in BIOSOLVE‐II and ‐III, predilatation was performed in 100% and postdilatation only in 69%.7 In the Magnesium‐2000 postmarket evaluation that included more than 2000 Magmaris implants from 356 hospitals across 45 countries, predilatation was performed in 95% and postdilatation in 82%.16
When several Magmaris were required (n = 34), the majority of scaffolds were placed end‐to‐end (n = 23), but nine were implanted overlapping, although the vascular effect of overlapping Magmaris scaffolds has not been tested and should be avoided according to the instructions for use.
In BIOSOLVE‐IV, the majority (85.1%) of patients were still on DAPT at the 12‐month visit, whereas DAPT was stopped prior to the 12‐month follow‐up in approximately half of BIOSOLVE‐II and ‐III patients (47.7%),7 This is likely attributed to the alerting scaffold thrombosis rates observed for the Absorb polymeric scaffold and the subsequent recommendations to prolong DAPT at least until the complete resorption of the device, which is approximately 12 months for Magmaris.1 Likewise, in the Magnesium 2000 postmarket evaluation, 14.2% of the users planned a DAPT‐time of at least 6 months and 83.2% of at least 12 months.16
The rate of TLF in our series was 4.3% [95% CI: 3.2, 5.7] at 12 months compared to 3.3% [95% CI: 1.2, 7.1] pooling BIOSOLVE‐II and BIOSOLVE‐III outcomes.6 As the confidence intervals overlap, this could be a by chance finding, but the difference might also be related to the inclusion of NSTEMI patients that exhibited higher myocardial infarction rates. Moreover, BIOSOLVE‐II and ‐III were controlled trials with a limited number of centers with experienced users whereas in BIOSOLVE‐IV, most centers were first‐time users.
Recently, case reports of early scaffold restenosis have been published.17, 18, 19 The terminology of the underlying failure is heterogeneous and warrants clear definitions as currently terms such as early or late recoil, compression, collapse, dismantling, and malapposition are used interchangeably. Underlying causes are discussed to be related to under‐ or overexpanded devices and/or to the mechanical properties of Magmaris. Noteworthy, although magnesium has several advantages similar to stainless steel stents, for example, low elastic recoil, minimal shortening after inflation, and higher initial radial strength than the polymeric Absorb scaffold,20 its radial strength is still lower than cobalt chromium. That is compensated with thicker struts, but still, situations such as calcified lesions should be avoided.11, 17 Undoubtedly, there is a trade‐off between scaffolding the vessel long enough to prevent restenosis versus fast resorption to allow restoration of vascular function, physiological vasomotion, and to prevent scaffold thrombosis18, 19, 21, 22; the optimal degradation time is still unknown.
Of utmost interest is the scaffold thrombosis rate as data from Absorb showed elevated thrombosis rates when tested outside small controlled trials.4 So far, no definite or probable scaffold thrombosis was observed for Magmaris in BIOSOLVE‐II and ‐III. While this winning streak ended with the first scaffold thrombosis in BIOSOLVE‐IV,6, 7, 8 the Kaplan–Meier estimate of 0.5% at 12 months is still low and within the expected range. Worthwhile to mention is that four out of five scaffold thromboses occurred after premature discontinuation of antiplatelet/anticoagulation therapy. Furthermore, they occurred between postprocedure day 6 and 95, hence prior to the resorption time of Magmaris. Results of the second cohort including 2,054 patients will be powered for definite and probable scaffold thrombosis and will be published once 12‐month outcomes are available.
4.2. Comparison of outcomes to polymeric absorbable scaffolds and contemporary permanent DES
Our 6‐month TLF rate (2.7%) is similar to the 2.4% of the German‐Austrian ABSORB registry GABI‐R,23 and our 12‐month TLF rate (4.3%) is comparable to the 3.2% of the ABSORB UK‐registry24 and the 3.9% of the ABSORB Italian RAI registry25; however, the definite scaffold thrombosis rate is lower (0.5% compared to 1.4 and 1.3% at 12 months and 1.0% at 6 months, respectively). Furthermore, our outcomes are similar to those of the DESolve novolimus‐eluting bioresorbable scaffold (Elixir, Milpitas, CA) that was tested in one multicenter postmarket evaluation in 102 patients that reported a TLF‐rate of 3.0% and a scaffold thrombosis rate of 1.0%.26 For the Fantom sirolimus‐eluting scaffold (REVA Medical, San Diego, California) no postmarket data are available, but the Fantom‐II premarket study reported a TLF‐rate of 4.2% and a scaffold thrombosis rate of 0.4% in 240 enrolled patients.27 Further details are provided in Table S5.
Our 12‐month outcomes are particularly relevant as they cover the resorption time of Magmaris, hence the period of the highest complication risk. They are also within the range of the objective performance criteria for new generation stents reported by the European Society of Cardiology‐European Association of Percutaneous Cardiovascular Interventions task force on the evaluation of coronary stents with 2.91% (IQR 1.67–5.94) TLR and 0.47% (IQR 0.28–0.72) definite stent thrombosis at 9‐ to 12‐month follow‐up.28 However, it has to be noted that the population of absorbable scaffolds in general is at lower risk as the common population that is treated with conventional DES.
Overall, it is encouraging that in a large patient population of more than 1,000 patients, the safety and performance of Magmaris has been confirmed. Although only randomized trials can proof noninferiority to DES, one might speculate that these would show superior short‐term TLR‐rates compared to Magmaris. Indeed, one small‐sized randomized trial comparing Magmaris with the sirolimus‐eluting Orsiro permanent stent in 150 STEMI patients showed—next to superior vasomotion—significantly higher TLR‐rates.29 Notably, in the delicate population of STEMI patients, scaffold sizing might be difficult with only 3.0 and 3.5 mm diameters available and predilatation might be performed more cautiously. Subsequently, Magmaris is currently not recommended for this indication.11 Finally, the benefit of bioresorbable technologies is meant to occur after the resorption period, through restoration of vascular function and avoidance of long‐term complication.1
Another potential advantage of magnesium‐based scaffolds is that they may result in decreased neoatherosclerosis progression. In an animal model, Magmaris showed significantly greater endothelial integrity than a 316‐L equivalent DES. Greater endothelial integrity is associated with lower macrophages infiltration and correspondingly, Magmaris showed lower neoatherosclerosis scores compared to the tested DES. This animal model has been confirmed in 21 patients implanted with Magmaris when at 3 years, optical coherence tomography follow‐up showed an excellent healing profile with little atherosclerosis progression (stable disease in 13 and disease regression in 5 patients considering a 5% change as relevant). However, larger studies are required for ultimate confirmation.30
BIOSOLVE‐IV has the common limitations of noninterventional registries. It is a single‐arm study and therefore comparisons with outcomes to other devices have to be interpreted with caution. The expert consensus advised that patients with a long life expectancy, stable angina, and patients with short de novo lesions are expected to benefit most of the treatment of BRS9—this is reflected in our patient population treating predominantly low‐risk patients with simple lesions in the clinical routine, but limiting the validity of the comparison to other studies. The strengths of BIOSOLVE‐IV are that outcome‐related events were independently adjudicated, that 25% of data were monitored, that a core laboratory was used, and the high follow‐up compliance. Its 5‐year follow‐up will show the behavior of the vessel beyond scaffold absorption. Ultimately, randomized controlled trials are needed to proof the usability of the Magmaris scaffold compared to state‐of‐the‐art DES.
5. CONCLUSION
Results from the BIOSOLVE‐IV registry confirm the low TLF‐rates from previous trials in a large, low‐risk patient population with simple lesions, reflecting the current use of BRS. Device and procedure success were good as were the safety outcomes with low cardiac death and myocardial infarction rates. Five definite/probable scaffold thrombosis occurred (0.5%), thereof all but one after early DAPT cessation prior to 6 months, emphasizing the need for strict DAPT adherence.
CONFLICT OF INTEREST
S.V. reports speaking fees from Biotronik, Elixir Medical, and Neovasc; M.H. reports study grants and personal fees from Biotronik, Abbott Vascular, Cardiac dimensions, and Philips, A.A.B.N. reports grants from Biotronik. J.B. has received research grants and speaking fees from Abbott Vascular and Biotronik AG. All other authors reported no conflict of interest.
Supporting information
Appendix S1. Supporting Information.
ACKNOWLEDGEMENTS
The authors thank MedStar Health Research Institute for angiographic core laboratory services and Beatrix Doerr for her help in drafting this manuscript, reimbursed by Biotronik AG. This study was funded by Biotronik AG, Buelach, Switzerland.
Verheye S, Wlodarczak A, Montorsi P, et al. BIOSOLVE‐IV‐registry: Safety and performance of the Magmaris scaffold: 12‐month outcomes of the first cohort of 1,075 patients. Catheter Cardiovasc Interv. 2021;98:E1–E8. 10.1002/ccd.29260
Funding information Biotronik
REFERENCES
- 1.Katagiri Y, Stone G, Onuma Y, Serruys P. State of the art: the inception, advent and future of fully bioresorbable scaffolds. EuroIntervention. 2017;13:734‐750. [DOI] [PubMed] [Google Scholar]
- 2.Serruys PW, Ormiston J, van Geuns RJ, et al. A polylactide bioresorbable scaffold eluting everolimus for treatment of coronary stenosis: 5‐year follow‐up. J Am Coll Cardiol. 2016;67:766‐776. [DOI] [PubMed] [Google Scholar]
- 3.Onuma Y, Dudek D, Thuesen L, et al. Five‐year clinical and functional multislice computed tomography angiographic results after coronary implantation of the fully resorbable polymeric everolimus‐eluting scaffold in patients with de novo coronary artery disease: the ABSORB cohort a trial. JACC Cardiovasc Interv. 2013;6:999‐1009. [DOI] [PubMed] [Google Scholar]
- 4.Lipinski MJ, Escarcega RO, Baker NC, et al. Scaffold thrombosis after percutaneous coronary intervention with ABSORB bioresorbable vascular scaffold: a systematic review and meta‐analysis. JACC Cardiovasc Interv. 2016;9:12‐24. [DOI] [PubMed] [Google Scholar]
- 5.Stone GW, Ellis SG, Gori T, et al. Blinded outcomes and angina assessment of coronary bioresorbable scaffolds: 30‐day and 1‐year results from the ABSORB IV randomised trial. Lancet. 2018;392:1530‐1540. [DOI] [PubMed] [Google Scholar]
- 6.Haude M, Ince H, Kische S, et al. Sustained safety and clinical performance of a drug‐eluting absorbable metal scaffold up to 24 months: pooled outcomes of BIOSOLVE‐II and BIOSOLVE‐III. EuroIntervention. 2017;13:432‐439. [DOI] [PubMed] [Google Scholar]
- 7.Haude M, Ince H, Kische S, et al. Safety and clinical performance of a drug eluting absorbable metal scaffold in the treatment of subjects with de novo lesions in native coronary arteries: pooled 12‐month outcomes of BIOSOLVE‐II and BIOSOLVE‐III. Catheter Cardiovasc Interv. 2018;92:E502‐E511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haude M, Ince H, Tölg R, et al. Safety and performance of the second‐generation drug‐eluting absorbable metal scaffold (DREAMS 2G) in patients with de novo coronary lesions: 3‐year clinical results and angiographic findings of the BIOSOLVE‐II first‐in‐man trial. EuroIntervention. 2020;15:e1375–e1382. 10.4244/EIJ-D-18-01000. [DOI] [PubMed] [Google Scholar]
- 9.Fajadet J, Haude M, Joner M, et al. Magmaris preliminary recommendation upon commercial launch: a consensus from the expert panel on 14 April 2016. EuroIntervention. 2016;12:828‐833. [DOI] [PubMed] [Google Scholar]
- 10.Verheye S, Wlodarczak A, Montorsi P, et al. Safety and performance of a resorbable magnesium scaffold under real‐world conditions: 12‐month outcomes of the first 400 patients enrolled in the BIOSOLVE‐IV registry. EuroIntervention. 2020;15:1383–1386. [DOI] [PubMed] [Google Scholar]
- 11.Cerrato E, Barbero U, Gil Romero JA, et al. Magmaris™ resorbable magnesium scaffold: state‐of‐art review. Futur Cardiol. 2019;15:267‐279. [DOI] [PubMed] [Google Scholar]
- 12.Cutlip DE, Windecker S, Mehran R, et al. Academic research consortium clinical end points in coronary stent trials: a case for standardized definitions. Circulation. 2007;115:2344‐2351. [DOI] [PubMed] [Google Scholar]
- 13.Vranckx P, Cutlip DE, Mehran R, et al. Myocardial infarction adjudication in contemporary all‐comer stent trials: balancing sensitivity and specificity. Addendum to the historical MI definitions used in stent studies. EuroIntervention. 2010;5:871‐874. [DOI] [PubMed] [Google Scholar]
- 14.Moussa ID, Stone GW. Myocardial infarction after percutaneous coronary intervention and coronary artery bypass graft surgery: time for a unifying common definition. JACC Cardiovasc Interv. 2017;10:1508‐1510. [DOI] [PubMed] [Google Scholar]
- 15.Neumann FJ, Sousa‐Uva M, Ahlsson A, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J. 2018;40:87‐165. [DOI] [PubMed] [Google Scholar]
- 16.Wlodarczak A, Garcia LAI, Karjalainen PP, et al. Magnesium 2000 postmarket evaluation: guideline adherence and intraprocedural performance of a sirolimus‐eluting resorbable magnesium scaffold. Cardiovasc Revasc Med. 2019;20:1140‐1145. [DOI] [PubMed] [Google Scholar]
- 17.Marynissen T, McCutcheon K, Bennett J. Early collapse causing stenosis in a resorbable magnesium scaffold. Catheter Cardiovasc Interv. 2018;92:310‐312. [DOI] [PubMed] [Google Scholar]
- 18.García‐Guimaraes M, Antuña P, Cuesta J, Alfonso F. Early restenosis of resorbable magnesium scaffolds: optical coherence tomography findings. Catheter Cardiovasc Interv. 2019;93:79‐81. [DOI] [PubMed] [Google Scholar]
- 19.Tovar Forero MN, van Zandvoort L, Masdjedi K, et al. Serial invasive imaging follow‐up of the first clinical experience with the Magmaris magnesium bioresorbable scaffold. Catheter Cardiovasc Interv. 2020;95(2):226‐231. 10.1002/ccd.28304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmidt W, Behrens P, Brandt‐Wunderlich C, Siewert S, Grabow N, Schmitz KP. In vitro performance investigation of bioresorbable scaffolds—standard tests for vascular stents and beyond. Cardiovasc Revasc Med. 2016;17:375‐383. [DOI] [PubMed] [Google Scholar]
- 21.Cubero‐Gallego H, Vandeloo B, Gomez‐Lara J, et al. Early collapse of a magnesium bioresorbable scaffold. JACC Cardiovasc Interv. 2017;10:e171‐e172. [DOI] [PubMed] [Google Scholar]
- 22.Brandt‐Wunderlich C, Ruppelt P, Zumstein P, et al. Mechanical behavior of in vivo degraded second generation resorbable magnesium scaffolds (RMS). J Mech Behav Biomed Mater. 2019;91:174‐181. [DOI] [PubMed] [Google Scholar]
- 23.Nef HM, Wiebe J, Kastner MJ, et al. Everolimus‐eluting bioresorbable scaffolds in patients with coronary artery disease: results from the German‐Austrian ABSORB RegIstRy (GABI‐R). EuroIntervention. 2017;13:1311‐1318. [DOI] [PubMed] [Google Scholar]
- 24.Baumbach A, Zaman A, West NEJ, et al. Acute and one‐year clinical outcomes following implantation of bioresorbable vascular scaffolds: the ABSORB UK Registry. EuroIntervention. 2018;13:1554‐1560. [DOI] [PubMed] [Google Scholar]
- 25.Ielasi A, Cortese B, Moscarella E, et al. One‐year clinical outcomes after unrestricted implantation of absorb bioresorbable scaffold (RAI registry). EuroIntervention. 2018;14:e546‐e553. [DOI] [PubMed] [Google Scholar]
- 26.Nef H, Wiebe J, Boeder N, et al. A multicenter post‐marketing evaluation of the elixir DESolve®Novolimus‐eluting bioresorbable coronary scaffold system: first results from the DESolve PMCF study. Catheter Cardiovasc Interv. 2018;92:1021‐1027. [DOI] [PubMed] [Google Scholar]
- 27.Chevalier B, Abizaid A, Carrié D, et al. Clinical and angiographic outcomes with a novel radiopaque Sirolimus‐eluting Bioresorbable vascular scaffold. Circ Cardiovasc Interv. 2019;12:e007283. 10.1161/CIRCINTERVENTIONS.118.007283. [DOI] [PubMed] [Google Scholar]
- 28.Byrne RA, Serruys PW, Baumbach A, et al. Report of a European Society of Cardiology‐European Association of Percutaneous Cardiovascular Interventions task force on the evaluation of coronary stents in Europe: executive summary. Eur Heart J. 2015;36:2608‐2620. [DOI] [PubMed] [Google Scholar]
- 29.Sabaté M, Alfonso F, Cequier A, et al. Magnesium‐based resorbable scaffold versus permanent metallic sirolimus‐eluting stent in patients with ST‐segment elevation myocardial infarction: the MAGSTEMI randomized clinical trial. Circulation. 2019;140:1904‐1916. 10.1161/CIRCULATIONAHA.119.043467. [DOI] [PubMed] [Google Scholar]
- 30.Joner M. Magmaris: Reducing the risk of neoatherosclerosis not only ST. Presented at: EuroPCR; May 22, 2018; Paris, France.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supporting Information.


