Abstract
Introduction
The introduction of the non‐invasive prenatal test (NIPT) has shifted the prenatal screening landscape. Countries are exploring ways to integrate NIPT in their national prenatal screening programs, either as a first‐ or second‐tier test. This study aimed to describe how the uptake of fetal aneuploidy screening changed after the introduction of NIPT as a second‐tier and as a first‐tier test within the national prenatal screening program of the Netherlands.
Material and methods
A population‐based register study in the Netherlands, recording uptake of fetal aneuploidy screening. Data from all pregnant women choosing to have the first‐trimester combined test (FCT) or first‐tier NIPT between January 2007 and March 2019 were retrospectively collected using national registration systems. Uptake percentages for fetal aneuploidy screening (FCT and NIPT) were calculated and stratified by region and maternal age. Statistical significance was determined using trend analysis and chi‐squared tests.
Results
Between 2007 and 2013 FCT uptake increased from 14.8% to 29.5% (P = .004). In April 2014 NIPT was introduced as a second‐tier test for high‐risk women after FCT (TRIDENT‐1 study). FCT uptake rose from 29.5% in 2013 to 34.2% in 2015 (P < .0001). After the introduction of NIPT as a first‐tier test for all women in April 2017 (TRIDENT‐2 study), FCT uptake declined significantly from 35.8% in 2016 to 2.6% in 2018 (P < .0001). NIPT uptake increased to 43.4% in 2018. Regionally, NIPT uptake ranged from 31.8% to 67.9%. Total uptake (FCT and NIPT) between 2007 and 2018 increased significantly from 14.8% to 45.9% (P < .0001). However, total uptake stabilized at 46% for both years of TRIDENT‐2 (April 2017‐March 2019).
Conclusions
An increase in total fetal aneuploidy screening uptake up to 45.9% was observed after the introduction of NIPT. Uptake appears to have stabilized within a year after introducing first‐tier NIPT.
Keywords: fetal aneuploidy screening, first‐trimester combined test, non‐invasive prenatal test, prenatal screening, register study, uptake
Abbreviations
- FCT
first‐trimester combined test
- NIPT
non‐invasive prenatal test
- TRIDENT
TRIals by Dutch laboratories for the Evaluation of Non‐invasive prenatal Testing
Key message.
After the introduction of first‐ and second‐tier NIPT in the Netherlands, uptake initially increased but has stabilized during the first 2 years of the TRIDENT‐2 study. Despite a centralized screening offer, regional and maternal age variations were observed.
1. INTRODUCTION
The emergence of the non‐invasive prenatal test (NIPT) using cell‐free fetal DNA for the detection of fetal aneuploidy has revolutionized prenatal screening worldwide. NIPT provides a safe and sensitive screening method to determine the risk for common fetal aneuploidies (Down, Edwards, and Patau syndromes).1 It has proven to be a more accurate test compared with other available serum screening tests, which have a higher proportion of false positives.2 As a result of the fewer false positives of NIPT, studies have shown a considerable reduction in invasive tests,3 which carry a small risk of procedure‐related miscarriage.4
After its introduction in 2011, NIPT was quickly adopted worldwide, with millions of tests performed yearly.5 Recently, the American College of Obstetricians and Gynecologists (ACOG) published new recommendations stating that prenatal genetic screening (including NIPT) should be discussed and offered to all women regardless of age or risk.6 Though NIPT is still primarily offered in a commercial setting, several countries have started initiatives to integrate NIPT into their national screening programs. However, because of high costs, NIPT is predominantly offered as a second‐tier (contingent) test after a high‐risk first‐trimester combined test (FCT) result.7 Currently, NIPT is being offered as a second‐tier test in several European countries including France, Switzerland, Italy, Denmark, Norway, Sweden, and Finland.8
Historically, the uptake of fetal aneuploidy screening in the Netherlands has been low (<30%), especially when compared with other European countries, where uptake is typically much higher (>70%).9 In the Netherlands, NIPT was first implemented as a second‐tier test as part of the TRIDENT‐1 study (TRIals by Dutch laboratories for the Evaluation of Non‐invasive prenatal Testing) in April 2014. NIPT became available to all Dutch pregnant women as an initial (first‐tier) test in April 2017, as part of the TRIDENT‐2 study. After counseling, women are offered a choice among NIPT, FCT, or no aneuploidy screening.
Due to its relative ease, accuracy, and non‐invasiveness, concerns have been raised regarding the considerable impact of NIPT on uptake rates, leading to potential uncritical use or routinization of screening. Moreover, the favorable NIPT characteristics might lead to women feeling pressure to test, hindering informed decision‐making.10 These factors could impede the aim of prenatal screening, which is to facilitate autonomous reproductive choices.11 In this study, we present how the introduction of NIPT within a national screening program changed the uptake of fetal aneuploidy screening in the Netherlands.
2. MATERIAL AND METHODS
This is a population‐based register study analyzing fetal aneuploidy screening uptake in the Netherlands between January 2007 and March 2019.
2.1. Prenatal screening in the Netherlands
In the Netherlands, prenatal screening is subject to the Population Screening Act. This act describes types of screening for which it is required to obtain a governmental license, to protect the population against possible harmful screening. Prenatal screening for fetal abnormalities consists of two programs: the screening for the three common fetal aneuploidies (Down, Edwards, and Patau syndromes), and the 20‐week fetal anomaly scan for structural defects. Eight Regional Centers are licensed to perform fetal aneuploidy screening.
Figure 1 shows a timeline of relevant events in fetal aneuploidy screening. In 2007, a public screening program was established for all pregnant women, with FCT being the standard test offered for Down syndrome screening. Before 2007, aneuploidy screening was not government regulated. In 2011, Edwards and Patau syndromes (trisomies 18 and 13) were added as target conditions. The costs of FCT were initially reimbursed through healthcare insurance for women 36 years and older. In 2015, this reimbursement was revoked, resulting in all women paying the same amount (€165 in 2015).
FIGURE 1.
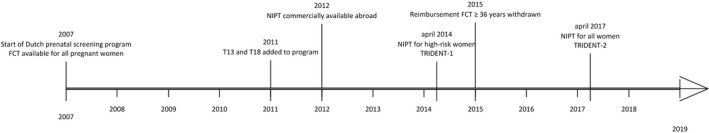
Timeline of fetal aneuploidy screening in the Netherlands. FCT, first‐trimester combined test; NIPT, non‐invasive prenatal test; T, trisomy; TRIDENT, TRIals by Dutch laboratories for the Evaluation of Non‐invasive prenatal Testing
In 2012, the arrival of commercially available NIPT in neighboring countries sparked “prenatal tourism”, with a proportion of pregnant women going abroad for NIPT.12 This has been estimated to be approximately 3‐5% of Dutch pregnancies.13 In April 2014, the Dutch NIPT Consortium was granted a license to implement NIPT for women with an increased risk for the common trisomies based on either FCT result (≥1:200) or medical history (TRIDENT‐1 study).3 In April 2017, an additional license was granted to offer NIPT to all pregnant women in the Netherlands on a trial basis (TRIDENT‐2 study).13
All Dutch women are offered information on prenatal screening for fetal abnormalities by their obstetric care provider, generally a midwife. Women expressing an interest in this information are offered a 30‐minute counseling session by a certified obstetric‐care professional. Since 2017, women are counseled for both FCT and NIPT, as well as the 20‐week fetal anomaly ultrasound scan. Women can choose to opt in or out for either or both screening programs. During counseling, women are given information regarding NIPT vs FCT, the common trisomies, the meaning of possible test results and diagnostic follow‐up testing. Women can choose between NIPT and FCT as a first‐tier aneuploidy test, both offered at comparable costs (€175 and €177 in 2019). The remaining costs for NIPT are subsidized by the government. Both NIPT and FCT are similarly accessible for women in physical distance. Currently, all women are offered a dating scan between 8 and 12 weeks and the 20‐week fetal anomaly scan. In September 2021, a nationwide research study will start to investigate the potential benefit of an additional 13‐week ultrasound scan for early diagnosis of fetal congenital anomalies in the Netherlands (IMITAS study).
At the time of this study, prenatal counseling is supported by a leaflet, available in 11 languages, by a website on fetal aneuploidy screening and a TRIDENT study website. Pregnancies with a previously identified vanishing or dichorionic twin, observed fetal ultrasound anomalies, including a nuchal translucency of ≥3.5 mm, or gestational age <10+0 weeks (TRIDENT‐2: <11+0 weeks), were excluded from the TRIDENT studies. Women included in the TRIDENT‐2 study only had a previous dating scan as there is currently no license to screen for structural fetal abnormalities in the first trimester of pregnancy. Therefore, the presence of fetal ultrasound anomalies (including increased nuchal translucency measurements) was often not known, because women were not routinely assessed regarding structural fetal abnormalities. Other exclusion criteria for both studies included: current maternal cancer, couples known to carry a (balanced) chromosomal abnormality (although Robertsonian translocations involving only chromosomes 13 or 21 were included in TRIDENT‐1), age <18 years old, and inability to give informed consent.3, 13 In addition, for TRIDENT‐2, women without a Dutch social security number or Dutch health insurance (mandatory for all Dutch residents) were excluded.13 These exclusion criteria were assessed by the prenatal counselor. Overviews of all the exclusion criteria for the TRIDENT studies have previously been published.3, 13 Women who do not meet the criteria for TRIDENT‐2 can still opt for FCT, and high‐risk women may opt for NIPT as part of TRIDENT‐1.3 Women who choose NIPT can choose whether or not to receive a report on additional findings (findings other than trisomy 21, 18, or 13).
2.2. Data collection
2.2.1. First‐trimester combined test
All data on the FCT uptake were collected from the FCT quality assessment data through the reference laboratory FCT of the Dutch Center for Population Screening of the Dutch National Institute for Public Health and the Environment (RIVM/CvB). Between 2007 and 2017, eight laboratories carried out the FCT blood analyses. Risk calculation was assessed at these laboratories or by ultrasound centers. However, because of a decrease in FCTs, all blood analyses were centralized to one laboratory from September 2017 onwards. An FCT risk calculation equal to or greater than 1:200 is considered high‐risk.
2.2.2. Non‐invasive prenatal test
First‐tier NIPT uptake information was retrieved from the national digital registration system for prenatal screening: Peridos. In this system, obstetric care professionals register counseling and NIPT applications. NIPT analysis was performed by clinical genetics laboratories from three university medical centers.13 Results were registered in Peridos as being either high‐risk or low‐risk starting from 1 April 2017.
2.2.3. Uptake of screening
Fetal aneuploidy screening data between January 2007 and March 2019 were retrieved from Peridos and the RIVM. Screening uptake was calculated as the number of tests (NIPT or FCT, corrected for repeated tests) performed divided by the number of pregnancies at 12 weeks of gestation. Monthly numbers of live births were obtained from the website of Statistics Netherlands (www.cbs.nl). The number of pregnancies was determined using numbers of live births 6 months after the sample period. The total number of live births was corrected for fetal loss at or after 12 weeks of gestation with a factor of 3.8%, as previously established and used by the Dutch prenatal screening program.14 Finally, the total number of pregnancies was corrected for multiple births by subtracting the number of twin births once and multiple births twice. As NIPT was introduced in the Netherlands as a first‐tier test in April 2017, we calculated uptake for the calendar years of 2017 and 2018 (from January until December), as well as the uptake of 2 years of NIPT in the Netherlands with TRIDENT‐2 (from April 2017 until March 2019). Calendar years were used as default unless stated otherwise. Data regarding the number of live births stratified by the Regional Center for Prenatal Screening were unavailable. Regional uptake was determined using the number of women receiving counseling for prenatal screening. Uptake by age group was calculated as the proportion of total tests performed, as the sizes of age groups remained stable over time.
2.3. Statistical analyses
Linear regression analyses were used to analyze time trends in the uptake of prenatal screening. Chi‐squared tests were used to investigate whether the proportions of prenatal screening tests decreased or increased significantly in between the two time periods. A value of P < .05 (two‐sided) was considered to be statistically significant. All statistical analyses were performed using IBM SPSS 24.0 (IBM Corp.).
2.4. Ethical approval
Approval for the TRIDENT‐2 study was granted by the Dutch Ministry of Health, Welfare and Sport (license 1017420‐153371‐PG) on 21 September 2016 and the Medical Ethics Committee of the Amsterdam UMC, VU University Medical Center (VUMC No. 2017.165) on 27 March 2017. See Supporting Information Appendix S1 for members of the Dutch NIPT Consortium.
3. RESULTS
Total prenatal screening uptake increased significantly in the Netherlands between January 2007 and December 2018 (regression coefficient 2.31; 95% CI 1.89‐2.73; P < .001) (Figure 2). At the start of the Dutch prenatal screening program in 2007, FCT uptake was 14.8%, rising to 29.5% in 2013 (regression coefficient 2.37, 95% CI CI 1.15‐3.59, P = .004). In April 2014, NIPT was introduced as a second‐tier test with TRIDENT‐1. FCT uptake increased from 29.5% in 2013, to 34.2% in 2015 (P < .001), and to 35.8% in 2016. After the introduction of first‐tier NIPT, FCT uptake in 2018 declined to 2.6%, and NIPT uptake increased to 43.4%.
FIGURE 2.
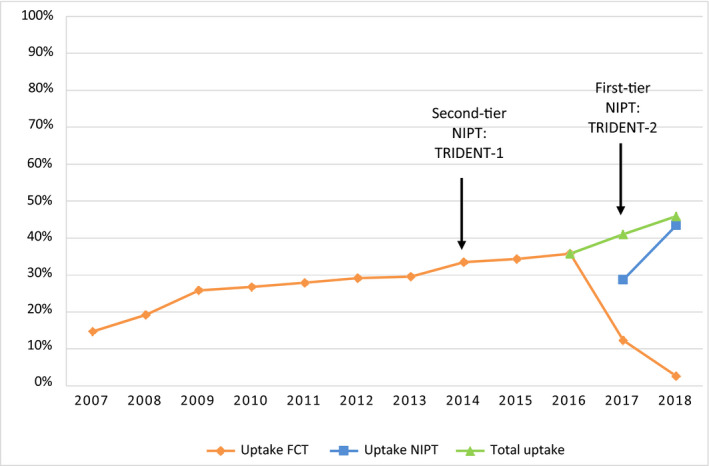
Nationwide uptake of fetal aneuploidy screening in the Netherlands 2007‐2018. FCT, first‐trimester combined test; NIPT, non‐invasive prenatal test; TRIDENT, TRIals by Dutch laboratories for the Evaluation of Non‐invasive prenatal Testing [Color figure can be viewed at wileyonlinelibrary.com]
During the first full year of NIPT in the Netherlands with TRIDENT‐2, beginning in April 2017, NIPT uptake was 42.3% and FCT uptake was 3.7%, with a total uptake of 46.0%. In the second year of TRIDENT‐2 (April 2018‐March 2019), NIPT uptake rose to 44.0%, and FCT uptake declined further to 1.9%. Total uptake stabilized at 45.9%.
In total, 499 077 FCTs were performed between 2007 and 2016. In the first quarter of 2017, 15 207 FCTs were performed, this decreased to 1996 FCTs in the second quarter of 2017: an 86% drop. During the second quarter of 2017, the first 18 595 NIPTs were performed. In total, 149 267 first‐tier NIPTs were performed between April 2017 and March 2019 (Figure S1).
In the first year of TRIDENT‐2, from 1 April 2017 until 31 March 2018, regional differences in prenatal screening uptake were observed (Figure 3). NIPT uptake ranged from 31.8% in the northeast, to 67.9% in the northwest and center of the Netherlands. FCT uptake ranged from 1.2% in the northwest to 6.0% in the southwest.
FIGURE 3.
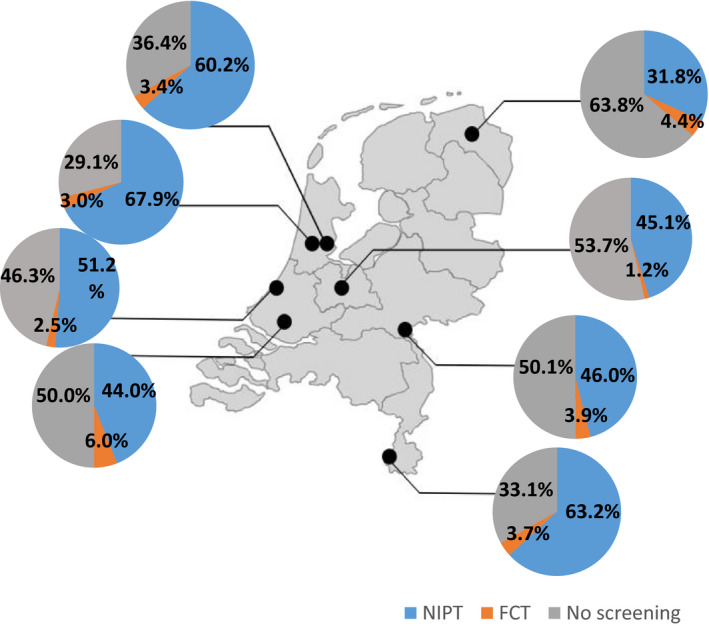
Regional uptake of non‐invasive prenatal test (NIPT) and first‐trimester combined test (FCT) in the Netherlands during the first year of TRIDENT‐2. Uptake for fetal aneuploidy screening in eight regions for prenatal screening in the Netherlands between 1 April 2017 and 31 March 2018 (first year of TRIDENT‐2). Each dot represents a region. Uptake was calculated using the number of women who received counseling for prenatal screening in that region as denominator. The number of pregnancies per region was not available. TRIDENT, TRIals by Dutch laboratories for the Evaluation of Non‐invasive prenatal Testing [Color figure can be viewed at wileyonlinelibrary.com]
Figure 4 shows the percentage of tests performed per age category between 2014 and 2018. For women aged 25 or younger, the percentage of total tests increased significantly from 4.8% in 2014 to 6.7% in 2018 (P < .001). A large increase was seen in the group of women between 26 and 30 years: the percentage of total tests increased from 23.7% in 2014 to 34.0% in 2018 (P < .001). For the 31‐ to 35‐year age group, the percentage of total tests remained stable: from 41.0% in 2014 to 41.5% in 2018 (P = .06). In 2014, 30.6% of the total tests were done for women aged 36 years and older, decreasing significantly to 17.8% in 2018 (P < .001).
FIGURE 4.
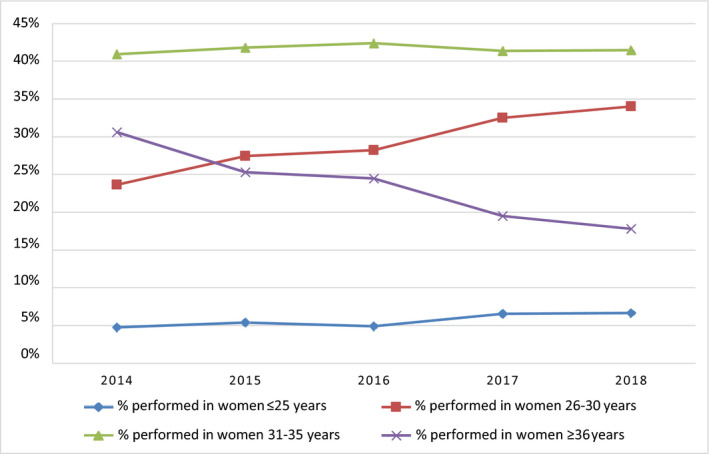
Percentages of prenatal aneuploidy screening tests performed per age category between 2014 and 2018. For the years 2017 and 2018, the number of non‐invasive prenatal tests and first‐trimester combined tests were combined to calculate the total percentages of tests performed [Color figure can be viewed at wileyonlinelibrary.com]
4. DISCUSSION
After the introduction of NIPT in the Netherlands an initial increase in uptake was observed. In the last 2 years, uptake appears to have stabilized at 46%. It should be emphasized that a change in uptake may or may not be desirable by itself; it is important to establish that this change is a reflection of an autonomous choice and free from pressure.11 Uptake should also not be considered a primary outcome measure for the performance of screening programs.11 Beginning in 2007, uptake significantly increased each year. It is not known how much of the increase in uptake can be specifically attributed to introducing NIPT. In contrast, an American study reported no significant difference in NIPT and FCT uptake between 2006 and 2013 after introducing NIPT.15 These authors concluded that it was unclear whether NIPT increases overall fetal aneuploidy screening uptake, or NIPT was simply preferred over FCT.
The increase in uptake of FCT corresponding with the availability of second‐tier NIPT (TRIDENT‐1) may be explained by the decreased need for invasive tests, which include a small risk of miscarriage.16 It was previously reported that some women decline first‐trimester screening because of the potential invasive follow‐up procedure.16 The initial increase in uptake after the introduction of first‐tier NIPT (TRIDENT‐2) may be due to the favorable test characteristics of NIPT (eg higher sensitivity and fewer false‐positives) compared with FCT. This was also suggested in earlier Dutch studies investigating women's hypothetical interest in NIPT including women who had declined FCT for test‐related reasons but who would be interested in having NIPT.16, 17
In 2018, the uptake of fetal aneuploidy screening in the Netherlands was 46%. Compared with other European countries, this number is relatively low. FCT uptake was 94% in Denmark between 2008 and 2013,18 88% in France in 2016,19 over 75% in Belgium in 2019,8 and 75% in England between April 2016 and March 2017.20 Several studies have attempted to explain the lower participation levels in the Netherlands. Crombag et al9 suggested that the framing of the offer of screening, emphasizing the “right not to know”, could explain the low uptake. In the Netherlands, women are first asked if they wish to receive information on prenatal screening, and only after a positive response does actual information provision occur. Other countries may offer screening as a routine part of prenatal care, resulting in higher uptake. Other factors associated with the low uptake are the positive attitudes towards Down syndrome, negative attitudes toward pregnancy termination and the costs of screening.9, 21, 22 These factors vary between and within countries and may result in differential uptake internationally and regionally.22 For example, though the Netherlands and Belgium both offer first‐tier NIPT, in Belgium the test is almost fully reimbursed. This may explain part of the differences in participation between the Netherlands and Belgium.
Uptake of FCT and NIPT showed regional variations. Previous studies in the Netherlands21, 23 and other countries with national fetal aneuploidy screening programs such as Sweden,24 England25 and Wales25 have also reported regional differences. Regional differences may be explained by variations in religious, social and cultural backgrounds,22, 23 but they may also be an indication of inequalities in access, due to physical distance or financial difficulties.26 A thorough understanding of factors affecting uptake is needed. Moreover, it is important to monitor regional variations, to ensure equal access to screening for all regions and to adapt to individual counseling needs.
An overall increase in the percentage of tests performed in women younger than 36 years was observed. This corresponds with Chen et al27 who reported a significant increase in the number of samples received from 65 countries for commercial NIPT from women aged 35 years and younger.27 One explanation for the increase among women under 36 years old is that in 2015 the reimbursement of FCT for women over 36 years was revoked. It is likely that the reimbursement policy based on maternal age may have given the impression to younger women that FCT was unnecessary, as they were at low‐risk.28 For women over 36 years, the costs of the test may have been a reason to refrain from testing, resulting in a decrease in uptake. Verweij et al29 suggested that personal costs are a significant factor in the decision‐making process regarding fetal aneuploidy screening. These authors also showed a decrease in uptake after revocation of reimbursements.29
When NIPT is part of routine procedure in prenatal screening, it might be accompanied with an increase in uptake by pregnant women feeling societal or provider pressure to test.10 Total uptake for both years of TRIDENT‐2 was 46%, this may imply that women experienced freedom to decline screening. Nevertheless, ongoing evaluation and development of counseling remain essential. Pre‐test counseling that provides couples with unbiased and accurate information and creates a meaningful dialog is crucial to facilitate autonomous and informed decision‐making.30 Further research should examine women's experiences of routinization of screening, and ways to mitigate potential negative effects. Additionally, there is a need for further development of education and training tools for counselors to promote informed decision‐making.31
Currently, there are two approaches for implementing NIPT. It can be offered as the primary screening method to all women, replacing FCT, or as a contingent test after a high‐risk assessment from FCT. A current benefit of the contingent model is lower costs for the healthcare system. A review comparing both approaches suggested that first‐tier NIPT was not cost‐effective.32 However, the costs of NIPT are likely to drop as sequencing technologies continue to develop. Uptake is an important variable when estimating the costs of implementing NIPT; a likely increase in uptake should be expected regardless of which implementation approach is chosen. A benefit of the first‐tier model is that screening can be performed earlier in pregnancy with fewer false positives. Second‐tier NIPT performance is partly determined by the primary test (FCT), which is associated with higher false‐positive rates and lower detection rates.1 It has been shown that FCT aimed at the common trisomies enriches for the detection of other chromosomal aberrations. Genome‐wide NIPT can also identify chromosomal abnormalities other than the common trisomies.13 It is not known exactly how the enrichment from contingent screening compares with the chromosomal aberrations detected by first‐tier genome‐wide NIPT. More research is needed into the detection, false‐positive rates, and clinical relevance of the additional findings for both approaches.
A strength of this study is the use of large nationwide data sets. The absence of commercial screening offers in the Netherlands allowed for an accurate report on uptake. The calculations of uptake rates carry a risk for imprecisions. However, as these errors remain stable over time, this is not likely to have affected the described trends. NIPT is not considered a suitable test in case of fetal abnormalities.33 Therefore, women with known fetal ultrasound abnormalities were excluded from the TRIDENT studies. It should be noted, however, that women were not routinely screened for structural fetal abnormalities because the first‐trimester ultrasound is not part of the current Dutch prenatal screening program. Some women with fetal abnormalities may therefore have had NIPT. Recent ACOG guidelines suggested that a baseline sonogram may be useful before NIPT.6 Before the introduction of NIPT as a first‐tier test, an estimated 3%‐5% of pregnant women went abroad to obtain NIPT commercially.13 Therefore, reported FCT uptake before 2017 might be an underrepresentation of actual uptake. Regional uptake was calculated using the number of women who received counseling for prenatal screening. This is a limitation, as the number of women who were registered as counseling recipients was lower than the number of pregnant women. Actual regional uptake may be lower. For 2016 and 2017, the difference between registered counseling recipients and actual pregnant women was approximately 14%.14 Furthermore, the reported increase in uptake could also be partly explained by other factors such as improved counseling, information provision, and education due to the presence of a study setting.
5. CONCLUSION
Though our findings are specific to the context of the Netherlands, they have important implications for other countries with or without national screening programs. The introduction of first‐tier NIPT did not lead to a major increase in uptake and despite a centralized offer, regional and maternal age variations were observed. These results highlight the importance of tailoring counseling to the diverse needs of pregnant women and a centralized approach ensuring access, quality and continuous monitoring. High‐quality counseling for aneuploidy screening is imperative to ensure that women are free to make decisions in line with their personal values. Further studies should investigate factors that influence the decision whether or not to participate in screening with NIPT or FCT and whether women experience routinization of prenatal screening with NIPT.
CONFLICT OF INTEREST
The authors have stated explicitly that there are no conflicts of interest in connection with this article.
Supporting information
Fig S1
Appendix S1
ACKNOWLEDGMENTS
This study was made possible in part using data from Peridos, the national digital registration system for prenatal screening and the Center for Population Screening of the Dutch National Institute for Public Health and the Environment. The authors would like to thank the Regional Centers for Prenatal Screening for their contribution in collecting the data used in this study.
van der Meij KRM, de Groot‐van Mooren M, Carbo EWS, et al. Uptake of fetal aneuploidy screening after the introduction of the non‐invasive prenatal test: A national population‐based register study. Acta Obstet Gynecol Scand. 2021;100:1265–1272. 10.1111/aogs.14091
Funding information
This study is supported by a grant from the Netherlands Organization for Health Research and Development (ZonMw grant no. 543002001).
REFERENCES
- 1.Gil MM, Accurti V, Santacruz B, Plana MN, Nicolaides KH. Analysis of cell‐free DNA in maternal blood in screening for aneuploidies: updated meta‐analysis. Ultrasound Obstet Gynecol. 2017;50:302‐314. [DOI] [PubMed] [Google Scholar]
- 2.Norton ME, Jacobsson BO, Swamy GK, et al. Cell‐free DNA analysis for noninvasive examination of trisomy. N Engl J Med. 2015;372:1589‐1597. [DOI] [PubMed] [Google Scholar]
- 3.Oepkes D, Page‐Christiaens GC, Bax CJ, et al. Trial by Dutch laboratories for evaluation of non‐invasive prenatal testing. Part I Clinical impact. Prenat Diagn. 2016;36:1083‐1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Salomon LJ, Sotiriadis A, Wulff CB, Odibo A, Akolekar R. Risk of miscarriage following amniocentesis or chorionic villus sampling: systematic review of literature and updated meta‐analysis. Ultrasound Obstet Gynecol. 2019;54:442‐451. [DOI] [PubMed] [Google Scholar]
- 5.Minear MA, Lewis C, Pradhan S, Chandrasekharan S. Global perspectives on clinical adoption of NIPT. Prenat Diagn. 2015;35:959‐967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rose NC, Kaimal AJ, Dugoff L, Norton ME. Screening for fetal chromosomal abnormalities: ACOG practice bulletin, Number 226. Obstet Gynecol. 2020;136:e48‐e69. [DOI] [PubMed] [Google Scholar]
- 7.Morris S, Karlsen S, Chung N, Hill M, Chitty LS. Model‐based analysis of costs and outcomes of non‐invasive prenatal testing for Down’s syndrome using cell free fetal DNA in the UK National Health Service. PLoS One. 2014;9:e93559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gadsbøll K, Petersen OB, Gatinois V, et al. Current use of noninvasive prenatal testing in Europe, Australia and the USA: a graphical presentation. Acta Obstet Gynecol Scand. 2020;99:722‐730. [DOI] [PubMed] [Google Scholar]
- 9.Crombag NMTH, Vellinga YE, Kluijfhout SA, et al. Explaining variation in Down's syndrome screening uptake: comparing the Netherlands with England and Denmark using documentary analysis and expert stakeholder interviews. BMC Health Serv Res. 2014;14:437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kater‐Kuipers A, de Beaufort ID, Galjaard R‐JH, Bunnik EM. Ethics of routine: a critical analysis of the concept of ‘routinisation’ in prenatal screening. J Med Ethics. 2018;44(9):626‐631. [DOI] [PubMed] [Google Scholar]
- 11.Dondorp W, de Wert G, Bombard Y, et al. Non‐invasive prenatal testing for aneuploidy and beyond: challenges of responsible innovation in prenatal screening. Eur J Hum Genet. 2015;23:1438‐1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Schendel RV, van El CG, Pajkrt E, Henneman L, Cornel MC. Implementing non‐invasive prenatal testing for aneuploidy in a national healthcare system: global challenges and national solutions. BMC Health Serv Res. 2017;17:670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van der Meij KRM, Sistermans EA, Macville MVE, et al. TRIDENT‐2: national implementation of genome‐wide non‐invasive prenatal testing as a first‐tier screening test in the Netherlands. Am J Hum Genet. 2019;105:1091‐1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liefers J, Cruijsberg J, Atsma F. Monitor 2017 Prenatale screening op down‐, edwards en patausyndroom en het Structureel EchoscopischOnderzoek. [Monitor 2017 Prenatal Screening for down, edwards and patau syndrome and fetal anomaly scan for structural defects] (in Dutch). Nijmegen: Scientific Center for Quality of Healthcare (IQ healthcare); 2018. [Google Scholar]
- 15.Larion S, Warsof SL, Romary L, Mlynarczyk M, Peleg D, Abuhamad AZ. Use of the combined first‐trimester screen in high‐ and low‐risk patient populations after introduction of noninvasive prenatal testing. J Ultrasound Med. 2015;34:1423‐1428. [DOI] [PubMed] [Google Scholar]
- 16.Crombag NMTH, van Schendel RV, Schielen PCJI, Bensing JM, Henneman L. Present to future: what the reasons for declining first‐trimester combined testing tell us about accepting or declining cell‐free DNA testing. Prenat Diagn. 2016;36:587‐590. [DOI] [PubMed] [Google Scholar]
- 17.Verweij EJ, Oepkes D, de Vries M, van den Akker ME, van den Akker ES, de Boer MA. Non‐invasive prenatal screening for trisomy 21: what women want and are willing to pay. Patient Educ Couns. 2013;93:641‐645. [DOI] [PubMed] [Google Scholar]
- 18.Ekelund CK, Petersen OB, Jørgensen FS, et al. The Danish Fetal Medicine Database: establishment, organization and quality assessment of the first trimester screening program for trisomy 21 in Denmark 2008–2012. Acta Obstet Gynecol Scand. 2015;94:577‐583. [DOI] [PubMed] [Google Scholar]
- 19.Blondel B, Coulm B, Bonnet C, Goffinet F, Le Ray C. Trends in perinatal health in metropolitan France from 1995 to 2016: results from the French National Perinatal Surveys. J Gynecol Obstet Hum. 2017;46:701‐713. [DOI] [PubMed] [Google Scholar]
- 20.Public Health England . NHS Fetal Anomaly Screening Programme: Screening Standards Data Report 1 April 2016 to 31 March 2017. London: PHE publications gateway number: GW‐151; 2019.
- 21.Bakker M, Birnie E, Pajkrt E, Bilardo CM, Snijders RJM. Low uptake of the combined test in the Netherlands ‐ which factors contribute? Prenat Diagn. 2012;32:1305‐1312. [DOI] [PubMed] [Google Scholar]
- 22.Hill M, Johnson J‐A, Langlois S, et al. Preferences for prenatal tests for Down syndrome: an international comparison of the views of pregnant women and health professionals. Eur J Hum Genet. 2015;24:968‐975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gitsels ‐ van der Wal JT, Verhoeven PS, Manniën J, et al. Factors affecting the uptake of prenatal screening tests for congenital anomalies; a multicentre prospective cohort study. BMC Pregnancy Childbirth. 2014;14:264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Petersson K, Lindkvist M, Persson M, Conner P, Åhman A, Mogren I. Prenatal diagnosis in Sweden 2011 to 2013—a register‐based study. BMC Pregnancy Childbirth. 2016;16:365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Souza E, Alberman E, Morris JK. Down's syndrome: screening and antenatal diagnosis regionally in England and Wales 1989–2008. J Med Screen. 2010;17:170‐175. [DOI] [PubMed] [Google Scholar]
- 26.Hayeems RZ, Campitelli M, Ma X, Huang T, Walker M, Guttmann A. Rates of prenatal screening across health care regions in Ontario, Canada: a retrospective cohort study. CMAJ Open. 2015;3:E236‐E243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen KM, White K, Shabbeer J, Schmid M. Maternal age trends support uptake of non‐invasive prenatal testing (NIPT) in the low‐risk population. J Matern Fetal Neonatal Med. 2019;32:4039‐4042. [DOI] [PubMed] [Google Scholar]
- 28.Engels MA, Bhola SL, Twisk JW, Blankenstein MA, van Vugt JM. Evaluation of the introduction of the national Down syndrome screening program in the Netherlands: age‐related uptake of prenatal screening and invasive diagnostic testing. Eur J Obstet Gynecol Reprod Biol. 2014;174:59‐63. [DOI] [PubMed] [Google Scholar]
- 29.Verweij EJ, Veersema D, Pajkrt E, Haak MC. Decision making in prenatal screening: money matters. Acta Obstet Gynecol Scand. 2015;94:212‐214. [DOI] [PubMed] [Google Scholar]
- 30.Metcalfe SA. Genetic counselling, patient education, and informed decision‐making in the genomic era. Semin Fetal Neonatal Med. 2018;23:142‐149. [DOI] [PubMed] [Google Scholar]
- 31.Benachi A, Caffrey J, Calda P, et al. Understanding attitudes and behaviors towards cell‐free DNA‐based noninvasive prenatal testing (NIPT): a survey of European health‐care providers. Eur J Med Genet. 2020;63:103616. [DOI] [PubMed] [Google Scholar]
- 32.Nshimyumukiza L, Menon S, Hina H, Rousseau F, Reinharz D. Cell‐free DNA noninvasive prenatal screening for aneuploidy versus conventional screening: a systematic review of economic evaluations. Clin Genet. 2018;94:3‐21. [DOI] [PubMed] [Google Scholar]
- 33.Beulen L, Faas BHW, Feenstra I, van Vugt JMG, Bekker MN. Clinical utility of non‐invasive prenatal testing in pregnancies with ultrasound anomalies. Ultrasound Obstet Gynecol. 2017;49:721‐728. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Appendix S1


