Abstract
The sense of hearing depends on a specialized sensory organ in the inner ear, called the cochlea, which contains the auditory hair cells (HCs). Noise trauma, infections, genetic factors, side effects of ototoxic drugs (ie, some antibiotics and chemotherapeutics), or simply aging lead to the loss of HCs and their associated primary neurons. This results in irreversible sensorineural hearing loss (SNHL) as in mammals, including humans; the inner ear lacks the capacity to regenerate HCs and spiral ganglion neurons. SNHL is a major global health problem affecting millions of people worldwide and provides a growing concern in the aging population. To date, treatment options are limited to hearing aids and cochlear implants. A major bottleneck for development of new therapies for SNHL is associated to the lack of human otic cell bioassays. Human induced pluripotent stem cells (hiPSCs) can be induced in two‐dimensional and three‐dimensional otic cells in vitro models that can generate inner ear progenitors and sensory HCs and could be a promising preclinical platform from which to work toward restoring SNHL. We review the potential applications of hiPSCs in the various biological approaches, including disease modeling, bioengineering, drug testing, and autologous stem cell based‐cell therapy, that offer opportunities to understand the pathogenic mechanisms of SNHL and identify novel therapeutic strategies.
Keywords: bioengineering, cell therapy, differentiation, drug testing, iPSCs, organoids, otic cell models, sensorineural hearing loss
Schematic of the various applications of induced pluripotent stem cell (IPSC) modular platform to challenge sensorineural hearing loss. IPSCs can be generated via genetic reprogramming of somatic cells from patient/healthy subject. In the inner ear, this technology has emerged as a promising tool for disease modeling, bioengineering, drug screening/validation and as an otic sensory progenitor source for autologous sensory cell replacement. In the cochlear epithelium, inner hair cells are arranged in a single row and outer hair cells are arranged in three rows (blue color). The supporting cells (yellow color) surround the sensory hair cells (HC).
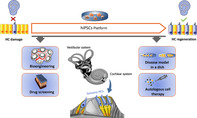
Significance statement.
Sensorineural hearing loss is the world's most common sensory deficit and affects nearly 470 million people. Discovery of human induced pluripotent stem cells (hiPSCs) has allowed the derivation of patient‐specific stem cells; they can self‐renew and have the capacity to differentiate into otic neurosensory cell derivatives, facilitating the study of disease mechanisms, bioengineering, and stem cell‐based cell replacement therapies either as individual cell types or as organoid three‐dimensional cultures. The authors highlight major achievements of, as well as challenges associated with, hiPSC‐based otic cell modeling as a human preclinical platform to understand pathogenesis and identify novel therapies.
1. INTRODUCTION
Sensorineural Hearing loss (SNHL) is the world's most common sensory deficit with high burden at the level of social and economic consequences. The World Health Organization (WHO) estimates SNHL affects nearly 470 million people and that this number will increase to 900 million people by 2050 (https://www.who.int/newsroom/fact-sheets/detail/deafness-and-hearing-loss).
The vast majority of SNHL originates from the cochlea, which represents the hearing component of the inner ear, reflecting currently incurable damage or loss to the delicate mechanosensory hair cells (HCs) and/or spiral ganglion neurons (SGNs) (Figure 1).
FIGURE 1.
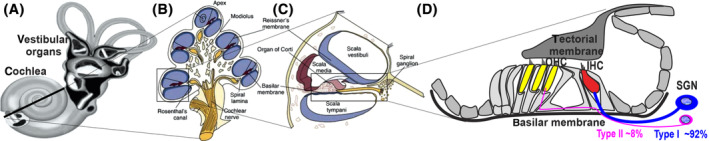
Schematic image of the inner ear. A, The inner ear is divided into the cochlea and the vestibular organs. B, A cross section (dotted line from A) shows the 2.5 turns containing the cochlea duct. C, A transverse section of the cochlea illustrating the three fluid compartments: the scala vestibuli, the scala media and the scala tympani. D, The organ of Corti contains the two types of sensory auditory hair cells‐a single row of inner hair cells (red) and three rows of outer hair cells (yellow)‐together with supporting cells. Peripheral fibers from spiral ganglion neurons (SGN) form synapses with the inner and outer hair cells. Note the fewer peripheral fibers that reach out type II SGN as compared with most type I SGN. IHC, inner hair cells; OHC, outer hair cells
Noise overexposure, ototoxic medications (such as aminoglycoside antibiotics, loop diuretics and platinum‐based chemotherapeutic agents), infections, genetic predisposition led to the loss of the sensory HCs in the cochlea. At birth, a human cochlea contains approximately 75 000 sensory HCs. The density of outer hair cells (OHCs) and inner hair cells (IHCs) decreases with age.1 Initially, OHC density in the inner ears is 300 per mm; by the time a person is 70 years old, this reduces to 65 per mm. Likewise, IHC density drops from 80 per mm to 30 per mm over time, and this is consistent with the longitudinal gradient base‐to‐apex loss of HCs in the cochlea and changes in hearing thresholds seen by 70 years of age.2, 3 The reduction in HC density and the subsequent effect on SNHL progresses differently in mice and humans during their lifespan3; both are worsened by noise trauma, ototoxic drugs, or genetic insults in older age.4, 5 Despite the widespread occurrence of SNHL in the world, there are no Food and Drug Administration (FDA)‐approved cell, molecular, or pharmacologic therapies.5, 6 Current treatments for human SNHL are largely limited to hearing aids and cochlear implants. Although these devices offer some relief of the symptoms of moderate SNHL by amplifying sound or directly electrically stimulating the auditory nerve, they fail to correct the underlying cause and have significant performance limitations.7 Thus, meeting the urgent, unmet medical need for novel therapies for human SNHL requires at least the generation of sensory HCs that can be used as tools to validate treatment strategies.5, 8 Given the complex cellular and molecular architecture of the human inner ear, combined with its inaccessibility to safe surgical biopsy and the small number of neurosensory cells, the generation of inner ear sensory cells from in vitro differentiation of pluripotent stem cells (PSCs), represent an obvious alternative. The human induced pluripotent stem cells (hiPSCs) are renewable and can differentiate into various tissue types, and their use avoids the ethical issues associated with human embryonic stem cells (hESCs).9 In addition, the derivation and biobanking of clinical‐grade personalized hiPSC lines10 would enable the development of a modular cell platform to challenge SNHL. Taken together, improvements in the hiPSC field bring opportunities to develop a human otic sensory cell‐based bioassay that would allow for exploring new biological approaches, including disease modeling, bioengineering, autologous stem cell based‐cell therapy, drugs testing and offers opportunities, to understand the pathogenic mechanisms of SNHL and identify novel therapeutic strategies.
This review provides an overview first of the genetics of developing mechanosensory HCs in the cochlea followed by highlights of the promise and challenges of using hiPSCs to develop effective, safe personalized restorative biological strategies for treatment of human SNHL.
2. THE KEY GENE EXPRESSION DURING INNER EAR NEUROSENSORY DEVELOPMENT
In the embryo, after the three germ layers are formed during the process of gastrulation, the definitive ectoderm commits to neural and non‐neural ectoderm, depending on BMP concentration gradient (Figure 2). Mutually opposing signals from BMP, WNT, and their antagonists act together to form the preplacodal ectoderm.11, 12 The preplacodal ectoderm is then induced to become the otic placode via the coordination between FGF and WNT signaling, specifically within the otic‐epibranchial domain.13 All cranial placodes, including the otic epibranchial placode (not represented in Figure 2), originate from the preplacodal ectoderm. The otic placode then invaginates and pinches off to form the otocyst or otic vesicle.11, 12, 13 The prosensory domain of the otic placode/vesicle gives rise to the vestibular/cochlear sensory epithelia and sensory neurons in response to NOTCH, SHH and Retinoic acid signaling pathways. Otic prosensory cells within the otic vesicle are defined by the combined regional expression of specific lineage gene markers that is, Pax2, Pax8, Ecad, Sox2, Lmx1a/b, and Jagged1.6, 13, 14 SOX2 is one of the earliest markers of the prosensory domain, the region containing cells that are specified to become either sensory HCs or supporting cells.15, 16 Upregulation of Neurog1 and Neurod1, two proneural basic helix‐loop‐helix factors, is in a subset of SOX2+ cells in the otocyst that leads to commitment of neuronal progenitors/neuroblasts, which delaminate from the otocyst to form the primary vestibular and cochlear ganglia.17 Final neurosensory development is regulated by multiple gene expression pattern.18 A crucial step is the transcription factor Atoh1 that defines the HCs, the supporting cell development is activated by the expression of Notch pathway mediator (Hes5) and Sox2 and sensory neurons are specified by the expression of Neurog1, Neurod1 and Pou4f1 proneural genes. The minimal essential gene expression profile required to generate inner ear HCs from PSCs is still unclear. Several genes downstream of Atoh1 are known to be important for normal development and can be used to generate initial HCs from mouse embryonic stem cells (mESCs) in vitro.19 Furthermore, combined overexpression of transcription factors including Six1 may provide efficient direct programming of mESCs toward a sensory HC fate.20 Despite progresses in inducing differentiation of mESCs into HC‐like cells, a different timetable in mice related to humans is clear: it declines at age 65 years old humans compared with 2 years in mice.3 In summary, we now know several critical steps are required to convert PSCs into HC‐like cells in vitro, but it remains unclear how long translational application of this approach will take, on which we can build future restorative biology strategies for human SNHL.
FIGURE 2.
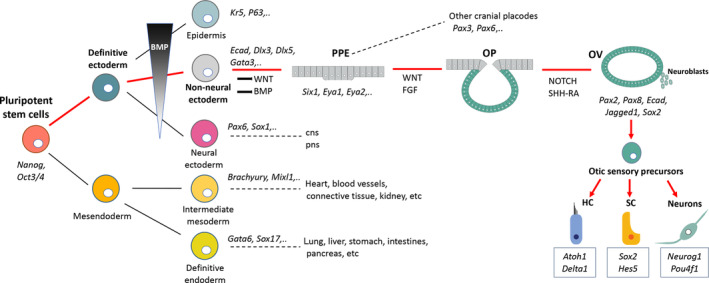
Schematic of cell lineage specification to illustrate otic development during early embryogenesis discussed in relation to the current methods for otic induction from pluripotent stem cells. CNS, central nervous system; HC, hair cell; OP, otic placode; OV, otic vesicle; PNS, peripheral nervous system; PPE, preplacodal ectoderm; SC, supporting cell
3. INNER EAR NEUROSENSORY CELL INDUCTION STRATEGIES FROM hiPSCs
Pluripotent stem cells can act as a model system via which to study early stages of human development that mimics the in vivo microenvironment. Understanding sequential transcriptional changes and signaling pathways that underlie in vivo development (Figure 2) is essential to drive efficient in vitro differentiation of PSCs into inner ear neurosensory cell types, such as HC, supporting cell, and neuron‐like cells.6, 21, 22 Various induction protocols have been developed to differentiate hiPSCs into HC‐like cells (Table S1, Supporting Information). Some of these protocols carried out completely in two‐dimensional (2D)‐cultures using treatments with a combination of small molecules and/or recombinant proteins to manipulate key signaling pathways of the inner ear development. The differentiation under 2D‐cultures can provide a relatively homogenous population of otic sensory progenitors and initial HC‐like cells in prolonged cell cultures.23, 24, 25, 26 In contrast to 2D‐culture system, the induction of PSCs under three‐dimensional (3D)‐organoid strategy allows for the generation of multiple cell types and recapitulates, to some extent, in vivo composition of an inner ear sensory epithelium containing HC, supporting cell and sensory neuron‐like cells.27, 28, 29 Before reaching the organoid phenotypes, otic signaling was induced by BMP activation and TGF‐β inhibition, whereas subsequent BMP inhibition and FGF activation induced a preotic cell fate. Then, combined treatment with LDN‐193189 (BMP inhibitor) and SBI (TGF‐β pathway inhibitor) resulted in the upregulation of the expression of neuroectodermal markers. After 40‐60 days in vitro, sensory epithelia developed from vesicles that expressed a subset of HC gene markers.27, 28, 29 A panel of hiPSC‐derived cells along the otic sensory lineage and HC‐like cells (Table S1, Supporting Information) have been evaluated for their morphological characteristics, expression of gene specific markers (Pax2, Myo7a, Pou4f3), and electrophysiological measurements.29, 30 Under physically defined environment cues mediated by extracellular matrices, the efficiency of differentiation, as well as the resulting complex cellular organization have been improved in 3D‐cultures.28, 29, 30, 31 For example, addition of Matrigel in the 3D‐inner ear organoids established by Koehler et al27, 28 facilitated the formation of fluid‐filled vesicles that harbors HC, supporting cell and neuron‐like cells. In contrast, vestibular tissue‐like organoids derived from hPSCs using the rotary cell culture system displayed neurosensory cells which express βIII tubulin within vesicles/organoids are positioned centrally while MYO7A immuno+ cells (ie, HC‐like cells) were mainly found on the external edge of the organoids, which is opposite to what occurs during in vivo development of the inner ear.31 These divergences may reflect the suboptimal growth conditions and microenvironment in in vitro model systems or their effects on tissue polarization. Currently, PSC‐derived HC‐like cells exhibit phenotypic and electrophysiological features of vestibular HCs and not cochlear HCs. A detailed mechanism that could reorient inner ear organoid differentiation toward a cochlear sensory epithelium phenotype from stepwise induction of PSCs as well as differentiation of IHCs vs OHCs are still needed. Next ameliorations will take place as we continue improving our understanding of the regulation of in vivo inner ear cell patterning signals and/or organoid systems. As with the HCs in general, inner ear sensory neurons and glial cells are derived from the otic placode.13 Therefore, some of the early neural induction protocols from PSCs were designed based on known developmental trajectories of the otic placode. Small molecules, such as BMPs, FGFs, and SHH are used to support neuronal outgrowth from the otic placode.32, 33 The neurotrophins BDNF and NT3 provide critical trophic support for inner ear primary sensory neurons in vivo34 and are also used to support survival and growth of PSC‐derived neurons in vitro.33, 35 In the previous protocols that have been devised to derive sensory neurons from PSCs, the Peripherin (type II SGNs) and POU4F1 are commonly used to confirm subtype‐specific inner ear neuronal derivatives.33, 35 On the other hand, neural innervation between coexisting HC‐like cells and sensory neuron‐like cells has been used to provide additional evidence as to the identity of the derived cell types. A recent study reported a protocol that efficiently generated cells that displayed the phenotypic characteristics of SGN‐like cells from hESCs and that were able to innervate both HCs and cochlear nucleus in organotypic explant brainstem slice cocultures.36 Other strategies have used either ectopic expression of Neurog1 and Neurod137 or the pluripotency factor Lin28a38 to reprogram inner ear glia toward SGN fate in a mouse model of auditory neuropathy. Alternatively, 3D inner ear organoids contain both sensory epithelia (HC and supporting cells) and sensory neuron‐like cells.28, 29 Although further characterization of sensory neuron‐like cells is still needed, the organoid system provides a useful tool to study sensory neural networks of the inner ear.
4. hiPSCs IN INNER EAR DISEASE MODELING
Modeling human inner ear disorders has relied heavily on insights obtained from mouse models.39 These models are powerful research tools to study development and pathologies. However, many fundamental disparities exist between mice and humans. In the case of the inner ear development, the mouse cochlea is still immature during early postnatal period, while human cochlea is completely mature at embryonic week‐20.3, 40 Indeed, inner ear organoids derived from hiPSCs or hESCs offer a unique opportunity approach to generate human‐specific models to study genetic defects associated with inner ear peripheral neurosensory cells. An example of such approach for investigating the molecular mechanisms underlying genetic inner ear disorders has been reported with mESC‐derived inner ear 3D‐organoids to study HC degeneration resulting from mutations in the transmembrane protease TMPRSS3.41 In this report, the functional consequences in defective Tmprss3 (associated with hearing loss in humans) were analyzed. The ESC‐derived organoids from Tmprss3 mutant line showed normal HC development followed by HC degeneration at differentiation day‐38 in vitro. In addition to recapitulating inner ear disease caused by genetic mutations, patient‐specific iPSC lines provide useful tools to assess the effects of correction of gene mutations to treat hereditary SNHL. Deleterious mutations in Myo7a and Myo15a are known human deafness genes.42 In two recent reports, investigators derived hiPSCs from somatic cells of deaf patients carrying Myo7a and Myo15 mutations, then the CRISPR/Cas9 technology is used to rectify these mutations. After correction of genetic mutations, the derived HC‐like cells from the corrected iPSCs exhibited, recovered organization of the stereociliary‐like structures, morphological and functional characteristics similar to controls.43, 44 Moreover, in another study, hiPSCs were derived from fibroblasts of a MERRF syndrome patient with A8344G mutation of mitochondrial DNA. These hiPSCs were induced by a panel of transcription factors ATOH1/RFX1/RFX3 that significantly increased their differentiation potential toward MYO7A+ cells. The differentiated HC‐like cells displayed expression of a subset of HC gene markers and promote HCs with more mature stereociliary‐like protrusions.45 While this review has focused on the generation of neurosensory cells from hiPSCs, associated cellular populations could be generated in culture, to allow characterization of genetic defects affecting other cell types of the inner ear. In particular, seeding on CX26GJ(+)‐vesicles derived from mouse iPCs on feeder cell layers has allowed the generation of cochlear supporting cell‐like cells in which to study mutations in Connexin 26, encoded by the GJB2 gene,46 that accounts for up to 50% of nonsyndromic SNHL in some populations.3 Although, the field is still in its infancy, the combination of hiPSC‐derived organoids and genome engineering has opened early investigation stages of the molecular mechanisms underlying genetic inner ear disorders, including the correction of mutations of in vitro differentiated hiPSCs.
5. hiPSCs‐OTIC CELL DERIVATIVES FOR OTOTOXICITY AND DRUG TESTING
Numerous drugs failed to translate into the clinical development mainly due to anatomic, molecular, and immunologic differences between rodents and humans that prevent the capacity to effectively mimic disease and to predict organ toxicity.47 In the inner ear field, a major limitation for drug development is the lack of bioassays based on human inner ear neurosensory cells that are available to test and validate in vitro promising drug candidates. This is a fundamental requirement for the transfer of findings between animal models and humans to speed up potential clinical translations. Human inner ear neurosensory cell‐based assays could evaluate a spectrum of drug‐induced ototoxic adverse effects, identify compounds that could prevent, delay, or reduce some degree of damage, and ultimately allow exploration of novel regenerative approaches. Today, either otoprotective or regenerative screens rely on models of zebrafish, primary cell cultures, and tissue culture explants that require microdissection by well‐trained investigators. Human inner ear sensory epithelia are largely inaccessible for surgical biopsy. For drug‐based therapies, human cell otic models need to develop artificial sensory epithelia or develop in vitro models, mimicking the in vivo sensory organs. The generation of inner ear sensory cells by in vitro differentiation of hiPSCs represents an obvious alternative option, as this could be scaled up and meet the need for robust characterization and exploitation for drug screening. Interestingly, HC and neuron‐like cells differentiated within epithelial patches in organoids exhibited some functionality.29, 30 These in vitro generated neurosensory patches should be suitable to evaluate ototoxicity and explore new regenerative strategies. Despite these advances, some of the current limitations of the organoid system could be further overcome by using microfluidic chips, as recently demonstrated by hiPSC‐derived models of the blood‐brain barrier or by controlled delivery of oxygen, nutrients, and waste removal.48 The integration of vascularization within the inner ear organoids (Figure 3) would allow for oxygen, nutrient, and metabolite exchange, which could enhance their semiphysiological state and maintain epithelial polarity in prolonged culture experiments. Other epithelial tissues (ie, gut, lung, kidney) derived from PSCs have been successfully tested using the microfluidic devices.49 Developing microfluidic inner ear organoids that can incorporate chip channels under dynamic growth conditions will enhance their utility in the detection of ototoxic and regenerative compounds. In addition, a robust human inner ear 3D culture on a microfluidic platform would allow the step of in vitro validation of drugs to be bypassed.
FIGURE 3.
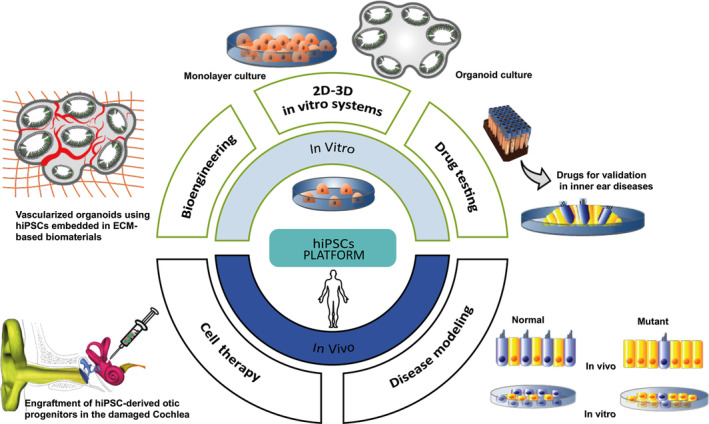
Application of hiPSC technological strategies to challenge inner ear neurosensory degeneration. Schematic categorization of the various biological approaches in hiPSC bioengineering, including disease modeling, autologous stem cell based‐cell therapy, and drug testing, as well as two‐dimensional (2D)/three‐dimensional (3D) organoid otic models, that offer opportunities to understand the pathogenic mechanisms of SNHL and identify novel therapeutic strategies. ECM, extracellular matrix; hiPSCs, human induced pluripotent stem cells
6. hiPSCs IN BIOENGINEERING FOR INNER EAR NEUROSENSORY CELL DIFFERENTIATION
The majority of the current understanding of stem cell differentiation toward inner ear neurosensory cells is based on 2D‐culture systems.23, 24, 25, 26 While few organoid cultures generated partial components of the inner ear tissue (ie, otic vesicle‐like structures) with the difficulty to generate cochlear sensory epithelium from PSCs.27, 28 In general, the 2D‐cultures, have ability to form stable, functional cell types but lack tissue‐specific architecture and cell‐to‐cell interactions.50 In these monolayer in vitro models, all cells are exposed to the same oxygen pressure, that makes their oxygen consumption rates stable. This contrasts with the 3D‐cultures in which oxygen diffuses into the complex structure with a concentration gradient depending on the cell density and the spatial location of cells within the 3D‐complex construct.51 However, the 3D‐in vitro structures should not increase above a certain level in diameter to allow nutrients and oxygen to diffuse within the tissue because the organoids lack a vascular network. The regions of the organoid mainly those in the core of the constructs that have poor oxygen/nutrients resources often exhibit limited survival and differentiation.52 In organoids, the niche components originated from the cells (ie, autocrine, paracrine, or juxtracrine signals) or exogenously added to the culture system, the extracellular matrix (ECM), small molecules, and growth factors. These interactions contribute to creating a dynamic environment that influences the differentiation in 3D‐cell cultures. To enhance the ability of 3D‐inner ear cultures on controlling differentiation, nutrients, and oxygen supply, it would be a useful approach to bioengineer the microenvironment signals by scaffold biomaterials. Inner ear organoids,6, 27, 28 like many others in organoid research, used Matrigel as a scaffold material for neurosensory differentiation. In addition to stem cell sources, Matrigel is often considered as a critical component of the organoid cultures. However, given its heterogeneous composition, this hydrogel does not allow proper morphogenetic processes, which are tightly governed in vivo by specific spatiotemporal cues, to be easily modulated under in vitro conditions. As an alternative, relevant types of ECM substrates could be selected, such as laminin and collagen both involved in inner ear development53 can be incorporated into customized biomimetic scaffolds to achieve the desired characteristics mimicking porosity and elasticity of the natural ECM. Interestingly, laminin and collagen IV were detected in the ECM after decellularization of the cochlea and were localized in vascular, neural and epithelial basal lamina material.54 The use of decellularizing strategies have become popular in tissue engineering applications as the natural ECM can provide the necessary physical cues for cell differentiation and tissue formation.55 In relation to cochlear tissue engineering,56 investigators demonstrated that decellularized cochlear tissues can act as a scaffold and support the incorporation of human exogenous Wharton's jelly cells suggesting that components of the cochlear ECM can affect development and behavior. Further work is necessary to seed hPSCs on the cochlea decellularized ECM to explore the potential of this inner ear natural scaffold to induce neurosensory differentiation and subsequent in vivo engraftment into damaged cochleae.
As previously mentioned, when organoids grow in size, their differentiation ability is limited due to hypoxic and hyponutrition conditions within deeper layers of the organoids.57 Applying proangiogenic factors, such as vascular endothelial growth factors (VEGFs) delivered using either microbeads or degradable vehicles within the scaffold could promote vascular network within the organoids.58 Another strategy to achieve this goal is to mix endothelial cells with PSCs to form a multicellular embryoid bodies that subsequently differentiates into vascularized organoids.59 Furthermore, vascularization is a crucial factor for the survival of organoids that not only promotes cell growth but also plays a role in reconstructing blood vessels in grafts after implantation in vivo.60 Thus, one can speculate that customized biomimetic scaffolds combined with appropriate proangiogenic factors may provide the right environment to produce hiPSCs‐derived inner ear vascularized organoids (Figure 3). Nevertheless, it is globally accepted that microfluidic devices can substitute to the lack of vascularization by integrating a controlled release of signal gradients and a constant supply of nutrients and oxygen. The drawback is that these microfluidic systems are still costly and hardly accessible to low‐resource setting labs. Altogether these strategies offer many opportunities to advances 3D‐culture systems bioengineering which has only been initiated in the inner ear biotechnology field.
7. hiPSC‐OTIC CELL DERIVATIVES FOR A CELL THERAPY APPROACH
Given the lack of endogenous regeneration and the limited therapeutic range available, the potential to develop a treatment based on the delivery of exogenous cells offers a promising therapeutic strategy.5, 8 Cell‐based approaches have been proposed for replacement or restoration of damaged HCs and/or SGNs. The hiPSC‐otic cell derivatives for a cell therapy approach are relevant candidates for biological implantation as they have a low risk of teratoma development and differentiate, both required features for a regenerative strategy option. Transplantation of mouse PSCs and adult stem cells as donor cells has been recently reviewed61 and hence, within the scope of this review, we will briefly focus on recent engraftment studies of human PSC (hESCs and hiPSCs) derived otic progenitors in the damaged cochleae. In contrast to the wealth of information available on transplanted PSC‐derived progenitors into other sensory placodes, for example, photoreceptor progenitors into the degenerative retina,62 only few reports aiming to inject inner ear progenitors into the cochleae.63, 64, 65, 66 Transplantation of partially differentiated progenitors from PSCs could be safer and more efficient since this approach is less prone to result in teratoma formation when compared with undifferentiated PSCs and is likely to generate differentiated HC or neuron‐like‐cells. Transplantation of hESC‐derived otic neural progenitors (ONPs) into the gerbils with selective ablation of SGNs has led to partial improvement in the auditory brainstem responses 10 weeks postengraftment. These investigators observed neuronal cell somas with processes that extend in opposite directions to both HCs and to cochlear nucleus.64 In another study, transplantation of hiPSC‐derived otic epithelial progenitors (OEPs) resulted in better survival and engraftment in the otocysts of Connexin30‐deleted mice when compared with transplanted cells into otocysts of wild‐type mice.63 Furthermore, hiPSC‐derived OEPs have been injected into the modiolus of gerbil cochleae and the migration and engraftment of transplanted OEPs have been reported.65 Recently our group has successfully engraft hiPSC‐derived OEPs into the cochlear sensory epithelium in an animal model of SNHL.66 In this study, partially differentiated OEPs infused into the scala tympani migrated throughout the cochlear turns, engrafted in nonsensory areas, and survived up to 4 weeks postengraftment. A subset of these engrafted OEPs responded to environmental cues and displayed immunophenotypes of sensory differentiation. The immunosuppression with cyclosporin improved the quantity of engrafted cells that migrated beyond the basilar membrane toward the scala media of the damaged cochleae. Despite the lack of restoration of auditory function, noticeable progress has been made and will need to be refined to allow optimal in vitro production of homogenous human OEPs and ONPs and to deliver these cells into the target, damaged area. Combining stem cells with biomaterial scaffolds can be used to improve the efficacy of cell transplantation.67 In the inner ear, neuronal induction of hiPSCs in a 3D‐collagen scaffold followed by transplantation into the scala tympani of the guinea‐pig cochleae successfully engrafted into the cochlear nerves.68 This transplantation paradigm resulted in glutamate transporter (VGlut1) expression in more than 50% of the transplanted ONPs, yet only about 2% of cells survived 2 weeks post‐transplantation. Furthermore, pharmacological agents that mimic the effects of matrix scaffolds (ie, heparan sulfate analogues) have been demonstrated to play an important role in promoting cell delivery to the inner ear of guinea pigs or to decellularized cochleae.69 Another study demonstrated that by using self‐assembling peptide amphiphile molecules to create a niche for hESC‐derived ONPs enhanced neural survival and differentiation after transplantation into X‐SCID rat cochlea.70 The same group used 3D‐spheroid system, ECM (ie, nanofibrillar cellulose hydrogel), and a neurotrophic factor delivery to artificially create a stem cell niche that allowed hESC‐derived ONP engraftment as well as neuronal differentiation.71 Despite the recent progress in cell transplantation to the inner ear, the optimization of this regenerative strategy is a phenomenal task, since there are multiple variables to consider in each experimental paradigm. Of critical importance is the technique for delivering stem cell derivatives to the right place within the damaged cochlea.
7.1. Complexity of injecting cells into the cochlea
The route for surgical delivery is another major technical factor when transplanting cells in the delicate tissues in the cochlea. The main objective for transplantation is to deliver the cells into the target. This targeting will depend on which type of degeneration has occurred after injury and whether the primary goal is to replace HCs and/or SGNs. Another challenge is to ensure precise cell injection throughout the length of cochlea while minimizing surgical trauma and hearing loss. A few articles have been published in the past 15 years showing different cell delivery approaches into rodent cochleae, which are summarized in the following section.
7.1.1. Intraperilymphatic injection approach
Perilymphatic transplantation via the scala tympani is the most used delivery technique so far. Positive features are the relative bigger volume when compared with the other cochlear compartments, together with its fluid runs along the cochlear length, making it the best vehicle to distribute injected cells throughout the cochlea. Moreover, surgical access to the perilymphatic space is believed to cause less trauma to the cochlea, and it can be realized either through the round window or cochleostomy in close proximity to it. Several transplantation experiments using different cell types delivered into the scala tympani found the cells localized to the perilymphatic space.65, 72, 73, 74, 75, 76 Kamiya et al75 have shown that it is possible to reach the cochlea perilymphatic compartment by injection via the posterior and lateral semicircular canals. There was evidence of cells being able to reach the modiolus and scala media by this intraperilymphatic injection approach.66, 72 Even though the number of cells in the scala media was small, it would suggest that they could migrate into the endolymphatic compartment from the perilymphatic space. However, it is possible that this movement of cells may have occurred through injury to the basilar membrane. How could cells operate to cross into a tightly sealed cochlear scala media compartment and survive in an environment with high potassium concentration, remains to be determined.
7.1.2. Intraendolymphatic injection approach
There have been few studies aiming to introduce undifferentiated stem cells and/or their derivatives directly into the scala media, and it remains the most technically challenging procedure. The main reason for this injection approach would be to target the organ of Corti area for replacement of HCs. However, several biological barriers would need to be overcome. The endolymph, the fluid located in this compartment, contains high levels of potassium (~150 mM) constitutes a hostile environment to many cell types, and it could lead to a very limited viability of transplanted cells as shown in the in vivo cochlea using HeLa cells.76 Moreover, the complex cytoarchitecture of the organ of Corti represents another challenge. The cochlear sensory epithelium is a tightly sealed barrier and, it will be difficult for the injected cells to break through the adherent and tight junction complexes between HCs and supporting cells in order to home and engraft. An initial attempt into scala media transplantation was described in mice, by delivering cells through the cochlear lateral wall of the second cochlear turn. This approach revealed a relative distribution of transplanted cells in all three cochlear compartments (Figure 1). There were no reports about differentiation and integration into the host tissue, neither confirmation of survival of transplanted cells in the endolymph.77 The access route via the cochlear lateral wall obviously damaged cochlear function, especially the structures needed to maintain homeostasis of K+ and/or endocochlear potential. It has been shown that by this surgical lateral wall approach to the scala media, it is possible to damage the stria vascularis and cochlear blood supply.78, 79 A different surgical access to the scala media was then developed by approaching through the basilar membrane with a cannula via the cochlear round window. This surgical approach was initially developed by Hildebrand et al80 to deliver mESCs and partially differentiated cell types in the scala media of deafened adult guinea pigs. There was no signs of mechanical damage to the organ of Corti or Reissner's membrane in any animal after surgical procedure. Some transplanted cells were localized close to the damaged organ of Corti; however, there was no evidence of differentiation and integration into the host tissue.
7.1.3. Modiolar and cochlear nerve injection approach
The modiolar and cochlear nerve trunk route for transplantation are mostly aimed to replace the degeneration of SGNs. Because of the complexity described above, HC replacement is still a long road ahead in the mature mammalian inner ear. However, targeting inner ear sensory neurons appears as far more realistic regenerative option in the short‐term. Moreover, a cell based‐therapy to reconstitute the nerve cells could be implemented in combination with the currently available cochlear implants. These electronic devices can substitute HC function but still require the presence of SGNs to function, as the bridge of signals to the central nervous system. For these reasons, some research groups have reoriented their interest to study the regeneration of SGNs and transplantation via cochlear nerve seem to be the most practical approach to deliver cells to the target location in Rosenthal's canal. Interesting initial results from mESCs transplantation via cochlear nerve trunk access have shown both peripheral and central migration along the cochlear nerve from the injection site. Transplanted cells were found close to the ventral cochlear nucleus but the number of cells that migrated to Rosenthal's canal was still limited.81 Although the cochlear nerve trunk approach seems to allow for a more targeted delivery of cells, it still unable to introduce the transplanted cells into their ultimate destination, the Rosenthal canal. Transplantation of hESC‐derived ONPs into ouabain‐lesioned gerbil modiolus with selective loss of SGNs has led to improvement in survival, cell migration and in auditory brainstem responses.64 Neuronal fibers project from ectopic, transplanted ONPs and penetrate into the Rosenthal canal. These are assumed to make synaptic connections at the base of HCs. However, more evidence is still needed to confirm that this kind of synaptic connection is sufficient for functional recovery, together with the establishment of central connections at the cochlear nucleus. Functional hearing measurements are also required to determine the level of damage from the surgical procedures that may further deteriorate the residual auditory nerve.
Although considerable information and technological knowledge is starting to gather about cochlear transplantation routes, more research is still needed to establish the optimal conditions to work. The balance between the appropriate intrinsic factors such as the stage of differentiation of grafted cells together with the extrinsic factors such as the host background, physical barriers within the cochlea and means of delivery, still needs to be optimized.
Additionally, comparing stem cell therapy using hiPSC‐derived inner ear otic sensory progenitors25 with gene editing approaches to induce directly novel gene expression using CRISPR/Cas9 are an interesting and underexplored future direction.82 The hiPSCs and their cell derivatives are providing a novel approach to help stem cell therapy in humans66 that may differentiate and expand the cochlear HCs.83 In contrast, the promises of CRISPR may eventually use the genetic defects that is just beginning.84, 85, 86 We would like to see in the near future, eventually, a combination to use CRISPR to maintain initial HCs and supplement lost HCs using cell‐based therapy approaches for SNHL.
8. CONCLUSION AND FUTURE PROSPECTS
Significant progress has been made in applications of hiPSCs in the field of inner ear therapies over the last decade and promises to make further major steps in the next few years, remaining an ambitious but relevant line of research to pursue for a potential treatment of SNHL. Current limitations to the use of hiPSCs to decipher inner ear neurosensory loss mechanisms and identifying novel therapies include extended period of in vitro culture, reproducibility, variable efficiency of tissue derivation, incapacity to generate cochlear tissues in 3D‐organoids. Today, scalable hiPSC‐derived human derived human otic sensory epithelium models allow for unprecedented possibilities for disease modeling, bioengineering, cell therapy and for the detection of ototoxic and regenerative compounds. In the next future, a possible generation of inner ear 3D‐organoids with vascularization network and their integration into microfluidic chips could improve culture and differentiation and to validate hiPSC‐based platform for SNHL preclinical applications.
CONFLICT OF INTEREST
The authors declared no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
A.Z.: conceived the manuscript, did literature review, drafted, revised, edited, read and approved the final manuscript; Y.M.: drafted and revised the figures and table, read and approved the final manuscript; B.F.: did the literature review, drafted and revised the manuscript, read and approved the final manuscript.
Supporting information
Table S1 Summary of the main recent accomplishments with otic cell models using hiPSCs for inner ear neurosensory cell differentiation. Abbreviations: HCs, hair cells; IHF, inactivated human feeders; LAP, low attachment plate; LCAW, low cell adhesion well; MCTFL, mitomycin C‐treated feeder layer; MTCEUSc, mitomycin‐treated chicken embryonic utricle stromal cell; OEP, otic epithelial progenitors; ONP, otic neural progenitors; OS, orbital shaker; SF, spinner flask; PAX2, PAX8, otic/placodal markers; MYO7A, Myosin7A, hair cell marker; POU4F3, hair cell marker; Synapsin I, synaptic marker; VGLUT1, vesicular glutamate transporter.
ACKNOWLEDGMENTS
We sincerely apologize for the articles that could not be referenced due to space limitations. This project was sponsored by the EU‐FP7 under the Health topic, grant number 603029 (to A.Z.) and NIH R01 AG060504 (to B.F.). The manuscript has been revised by an independent scientific English language editing service.
Zine A, Messat Y, Fritzsch B. A human induced pluripotent stem cell‐based modular platform to challenge sensorineural hearing loss. Stem Cells. 2021;39:697–706. 10.1002/stem.3346
Funding information EU‐FP7, Grant/Award Number: 603029; NIH, Grant/Award Number: R01 AG060504
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1.Lim R, Brichta AM. Anatomical and physiological development of the human inner ear. Hear Res. 2016;338:9‐21. [DOI] [PubMed] [Google Scholar]
- 2.Bowl MR, Dawson SJ. Age‐related hearing loss. Cold Spring Harb Perspect Med. 2019;9:a033217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yamoah EN, Li M, Shah A, et al. Using Sox2 to alleviate the hallmarks of age‐related hearing loss. Ageing Res Rev. 2020;59:101042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoffman HJ, Dobie RA, Losonczy KG, Themann CL, Flamme GA. Declining prevalence of hearing loss in US adults aged 20 to 69 years. JAMA Otolaryngol Head Neck Surg. 2017;143:274‐285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schilder AGM, Su MP, Blackshaw H, et al. Hearing protection, restoration, and regeneration. Otol Neurotol. 2019;40:559‐570. [DOI] [PubMed] [Google Scholar]
- 6.Roccio M, Edge ASB. Inner ear organoids: new tools to understand neurosensory cell development, degeneration and regeneration. Development. 2019;146:dev177188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kral A, Dorman MF, Wilson BS. Neuronal development of hearing and language: cochlear implants and critical periods. Annu Rev Neurosci. 2019;42:47‐65. [DOI] [PubMed] [Google Scholar]
- 8.Roccio M, Senn P, Heller S. Novel insights into inner ear development and regeneration for targeted hearing loss therapies. Hear Res. 2020;397:107859. [DOI] [PubMed] [Google Scholar]
- 9.Thomson JA. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145‐1147. [DOI] [PubMed] [Google Scholar]
- 10.Takahashi K, Okita K, Nakagawa M, Yamanaka S. Induction of pluripotent stem cells from fibroblast cultures. Nat Protoc. 2007;2:3081‐3089. [DOI] [PubMed] [Google Scholar]
- 11.Steventon B, Mayor R, Streit A. Neural crest and placode interaction during the development of the cranial sensory system. Dev Biol. 2014;389:28‐38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tambalo M, Anwar M, Ahmed M, Streit A. Enhancer activation by FGF signalling during otic induction. Dev Biol. 2020;457:69‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singh S, Groves AK. The molecular basis of craniofacial placode development. Wiley Interdiscip Rev Dev Biol. 2016;5:363‐376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chizhikov VV, Iskusnykh IY, Fattakhov N, Fritzsch B. Lmx1a and Lmx1b are redundantly required for the development of multiple components of the mammalian auditory system. Neuroscience. 2021;452:247‐264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dabdoub A, Puligilla C, Jones JM, et al. Sox2 signaling in prosensory domain specification and subsequent hair cell differentiation in the developing cochlea. Proc Natl Acad Sci USA. 2008;105:18396‐18401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dvorakova M, Macova I, Bohuslavova R, Anderova M, Fritzsch B, Pavlinkova G. Early ear neuronal development, but not olfactory or lens development, can proceed without SOX2. Dev Biol. 2020;457:43‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Evsen L, Sugahara S, Uchikawa M, et al. Progression of neurogenesis in the inner ear requires inhibition of Sox2 transcription by neurogenin1 and neurod1. J Neurosci. 2013;33:3879‐3890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Filova I, Dvorakova M, Bohuslavova R, et al. Combined Atoh1 and Neurod1 deletion reveals autonomous growth of auditory nerve fibers. Mol Neurobiol. 2020;57:5307‐5323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Costa A, Sanchez‐Guardado L, Juniat S, Gale JE, Daudet N, Henrique D. Generation of sensory hair cells by genetic programming with a combination of transcription factors. Development. 2015;142:1948‐1959. [DOI] [PubMed] [Google Scholar]
- 20.Menendez L, Trecek T, Gopalakrishnan S, et al. Generation of inner ear hair cells by direct lineage conversion of primary somatic cells. Elife. 2020;9:e55249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zine A, Lowenheim H, Fritzsch B. Toward translating molecular ear development to generate hair cells from stem cells. In: Kursad T, ed. Adult Stem Cells. New York: Springer; 2014:111‐161. [Google Scholar]
- 22.Czajkowski A, Mounier A, Delacroix L, et al. Pluripotent stem cell‐derived cochlear cells: a challenge in constant progress. Cell Mol Life Sci. 2018;76:627‐635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ohnishi H, Skerleva D, Kitajiri S, et al. Limited hair cell induction from human induced pluripotent stem cells using a simple stepwise method. Neurosci Lett. 2015;599:49‐54. [DOI] [PubMed] [Google Scholar]
- 24.Ealy M, Ellwanger DC, Kosaric N, Stapper AP, Heller S. Single‐cell analysis delineates a trajectory toward the human early otic lineage. Proc Natl Acad Sci USA. 2016;113:8508‐8513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lahlou H, Nivet E, Lopez‐Juarez A, Fontbonne A, Assou S, Zine A. Enriched differentiation of human otic sensory progenitor cells derived from induced pluripotent stem cells. Front Mol Neurosci. 2018;11:452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boddy SL, Romero‐Guevara R, Ji A‐R, et al. Generation of otic lineages from integration‐free human‐induced pluripotent stem cells reprogrammed by mRNAs. Stem Cells Int. 2020;2020:3692937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koehler KR, Hashino E. 3D mouse embryonic stem cell culture for generating inner ear organoids. Nat Protoc. 2014;9:1229‐1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koehler KR, Nie J, Longworth‐Mills E, et al. Generation of inner ear organoids containing functional hair cells from human pluripotent stem cells. Nat Biotechnol. 2017;35:583‐589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jeong M, O'Reilly M, Kirkwood NK, et al. Generating inner ear organoids containing putative cochlear hair cells from human pluripotent stem cells. Cell Death Dis. 2018;9:922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu X‐P, Koehler KR, Mikosz AM, Hashino E, Holt JR. Functional development of mechanosensitive hair cells in stem cell‐derived organoids parallels native vestibular hair cells. Nat Commun. 2016;7:11508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mattei C, Lim R, Drury H, et al. Generation of vestibular tissue‐like organoids from human pluripotent stem cells using the rotary cell culture system. Front Cell Dev Biol. 2019;7:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gunewardene N, Van BN, Crombie D, et al. Directing human induced pluripotent stem cells into a neurosensory lineage for auditory neuron replacement. Biores Open Access. 2014;3:162‐175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matsuoka AJ, Morrissey ZD, Zhang C, et al. Directed differentiation of human embryonic stem cells toward placode‐derived spiral ganglion‐like sensory neurons. Stem Cells Translational Medicine. 2017;6:923‐936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fritzsch B, Tessarollo L, Coppola E, et al. Neurotrophins in the ear: their roles in sensory neuron survival and fiber guidance. Prog Brain Res. 2004;146:265‐278. [DOI] [PubMed] [Google Scholar]
- 35.Shi F, Corrales CE, Liberman MC, Edge ASB. BMP4 induction of sensory neurons from human embryonic stem cells and reinnervation of sensory epithelium. Eur J Neurosci. 2007;26:3016‐3023. [DOI] [PubMed] [Google Scholar]
- 36.Hyakumura T, McDougall S, Finch S, et al. Organotypic cocultures of human pluripotent stem cell derived‐neurons with mammalian inner ear hair cells and cochlear nucleus slices. Stem Cells Int. 2019;2019:8419493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li X, Bi Z, Sun Y, Li C, Li Y, Liu Z. In vivo ectopic Ngn1 and Neurod1 convert neonatal cochlear glial cells into spiral ganglion neurons. FASEB J. 2020;34:4764‐4782. [DOI] [PubMed] [Google Scholar]
- 38.Kempfle JS, Luu N‐NC, Petrillo M, et al. Lin28 reprograms inner ear glia to a neuronal fate. Stem Cells. 2020;38:890‐903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wangemann P. Mouse models for pendrin‐associated loss of cochlear and vestibular function. Cell Physiol Biochem. 2013;32:157‐165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Johnson Chacko L, Wertjanz D, Sergi C, et al. Growth and cellular patterning during fetal human inner ear development studied by a correlative imaging approach. BMC Dev Biol. 2019;19:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tang P‐C, Alex AL, Nie J, et al. Defective Tmprss3‐associated hair cell degeneration in inner ear Organoids. Stem Cell Rep. 2019;13:147‐162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Libby RT, Steel KP. The roles of unconventional myosins in hearing and deafness. Essays Biochem. 2000;35:159‐174. [DOI] [PubMed] [Google Scholar]
- 43.Tang Z‐H, Chen J‐R, Zheng J, et al. Genetic correction of induced pluripotent stem cells from a deaf patient with MYO7A mutation results in morphologic and functional recovery of the derived hair cell‐like cells. Stem Cells Translational Medicine. 2016;5:561‐571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen J‐R, Tang Z‐H, Zheng J, et al. Effects of genetic correction on the differentiation of hair cell‐like cells from iPSCs with MYO15A mutation. Cell Death Differ. 2016;23:1347‐1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen Y‐C, Tsai C‐L, Wei Y‐H, et al. ATOH1/RFX1/RFX3 transcription factors facilitate the differentiation and characterisation of inner ear hair cell‐like cells from patient‐specific induced pluripotent stem cells harbouring A8344G mutation of mitochondrial DNA. Cell Death Dis. 2018;9:437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fukunaga I, Fujimoto A, Hatakeyama K, et al. In vitro models of GJB2‐related hearing loss recapitulate Ca(2+) transients via a gap junction characteristic of developing cochlea. Stem Cell Rep. 2016;7:1023‐1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Martignoni M, Groothuis GMM, de Kanter R. Species differences between mouse, rat, dog, monkey and human CYP‐mediated drug metabolism, inhibition and induction. Expert Opin Drug Metab Toxicol. 2006;2:875‐894. [DOI] [PubMed] [Google Scholar]
- 48.Vatine GD, Barrile R, Workman MJ, et al. Human iPSC‐derived blood‐brain barrier chips enable disease modeling and personalized medicine applications. Cell Stem Cell. 2019;24:995‐1005.e6. [DOI] [PubMed] [Google Scholar]
- 49.Schutgens F, Clevers H. Human organoids: tools for understanding biology and treating diseases. Annu Rev Pathol Mech Dis. 2020;15:211‐234. [DOI] [PubMed] [Google Scholar]
- 50.Nelson CM, Bissell MJ. Of extracellular matrix, scaffolds, and signaling: tissue architecture regulates development, homeostasis, and cancer. Annu Rev Cell Dev Biol. 2006;22:287‐309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Magliaro C, Mattei G, Iacoangeli F, Corti A, Piemonte V, Ahluwalia A. Oxygen consumption characteristics in 3D constructs depend on cell density. Front Bioeng Biotechnol. 2019;7:251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McMurtrey RJ. Analytic models of oxygen and nutrient diffusion, metabolism dynamics, and architecture optimization in three‐dimensional tissue constructs with applications and insights in cerebral organoids. Tissue Eng Part C Methods. 2016;22:221‐249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ishiyama A, Mowry SE, Lopez IA, Ishiyama G. Immunohistochemical distribution of basement membrane proteins in the human inner ear from older subjects. Hear Res. 2009;254:1‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Santi PA, Johnson SB. Decellularized ear tissues as scaffolds for stem cell differentiation. J Assoc Res Otolaryngol. 2013;14:3‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Agmon G, Christman KL. Controlling stem cell behavior with decellularized extracellular matrix scaffolds. Curr Opin Solid State Mater Sci. 2016;20:193‐201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mellott AJ, Shinogle HE, Nelson‐Brantley JG, Detamore MS, Staecker H. Exploiting decellularized cochleae as scaffolds for inner ear tissue engineering. Stem Cell Res Ther. 2017;8:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Grebenyuk S, Ranga A. Engineering organoid vascularization. Front Bioeng Biotechnol. 2019;7:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Balikov DA, Neal EH, Lippmann ES. Organotypic neurovascular models: past results and future directions. Trends Mol Med. 2020;26:273‐284. [DOI] [PubMed] [Google Scholar]
- 59.Takebe T, Wells JM. Organoids by design. Science (80–). 2019;364:956‐959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Daviaud N, Friedel RH, Zou H. Vascularization and engraftment of transplanted human cerebral organoids in mouse cortex. ENeuro. 2018;5:ENEURO.0219‐18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Takeda H, Dondzillo A, Randall JA, Gubbels SP. Challenges in cell‐based therapies for the treatment of hearing loss. Trends Neurosci. 2018;41:823‐837. [DOI] [PubMed] [Google Scholar]
- 62.West EL, Ribeiro J, Ali RR. Development of stem cell therapies for retinal degeneration. Cold Spring Harb Perspect Biol. 2020;12:a035683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Takeda H, Hosoya M, Fujioka M, et al. Engraftment of human pluripotent stem cell‐derived progenitors in the inner ear of prenatal mice. Sci Rep. 2018;8:1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen W, Jongkamonwiwat N, Abbas L, et al. Restoration of auditory evoked responses by human ES‐cell‐derived otic progenitors. Nature. 2012;490:278‐282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen J, Hong F, Zhang C, et al. Differentiation and transplantation of human induced pluripotent stem cell‐derived otic epithelial progenitors in mouse cochlea. Stem Cell Res Ther. 2018;9:230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lopez‐Juarez A, Lahlou H, Ripoll C, et al. Engraftment of human stem cell‐derived otic progenitors in the damaged cochlea. Mol Ther. 2019;27:1101‐1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mitrousis N, Fokina A, Shoichet MS. Biomaterials for cell transplantation. Nat Rev Mater. 2018;3:441‐456. [Google Scholar]
- 68.Ishikawa M, Ohnishi H, Skerleva D, et al. Transplantation of neurons derived from human iPS cells cultured on collagen matrix into Guinea‐pig cochleae. J Tissue Eng Regen Med. 2015;11:1766‐1778. [DOI] [PubMed] [Google Scholar]
- 69.Schulze J, Sasse S, Prenzler N, et al. Microenvironmental support for cell delivery to the inner ear. Hear Res. 2018;368:109‐122. [DOI] [PubMed] [Google Scholar]
- 70.Matsuoka AJ, Sayed ZA, Stephanopoulos N, et al. Creating a stem cell niche in the inner ear using self‐assembling peptide amphiphiles. PLoS One. 2017;12:e0190150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chang H‐T, Heuer RA, Oleksijew AM, et al. An engineered three‐dimensional stem cell niche in the inner ear by applying a nanofibrillar cellulose hydrogel with a sustained‐release neurotrophic factor delivery system. Acta Biomater. 2020;108:111‐127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lang H, Schulte BA, Goddard JC, et al. Transplantation of mouse embryonic stem cells into the cochlea of an auditory‐neuropathy animal model: effects of timing after injury. J Assoc Res Otolaryngol. 2008;9:225‐240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nishimura K, Nakagawa T, Sakamoto T, Ito J. Fates of murine pluripotent stem cell‐derived neural progenitors following transplantation into mouse cochleae. Cell Transplant. 2012;21:763‐771. [DOI] [PubMed] [Google Scholar]
- 74.Takeda H, Minoda R, Miwa T, Yamada T, Ise M. Transplanting mouse induced pluripotent stem cells into mouse otocysts in vivo. Neurosci Lett. 2017;647:153‐158. [DOI] [PubMed] [Google Scholar]
- 75.Kamiya K, Fujinami Y, Hoya N, et al. Mesenchymal stem cell transplantation accelerates hearing recovery through the repair of injured cochlear fibrocytes. Am J Pathol. 2007;171:214‐226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Park Y‐H, Wilson KF, Ueda Y, et al. Conditioning the cochlea to facilitate survival and integration of exogenous cells into the auditory epithelium. Mol Ther. 2014;22:873‐880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tateya I, Endo T, Kim T‐S, et al. Surgical techniques for cell transplantation into the mouse cochlea. Acta Otolaryngol. 2004;124:43‐47. [DOI] [PubMed] [Google Scholar]
- 78.Wangemann P. Supporting sensory transduction: cochlear fluid homeostasis and the endocochlear potential. J Physiol. 2006;576:11‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shibata SB, Di Pasquale G, Cortez SR, et al. Gene transfer using bovine adeno‐associated virus in the Guinea pig cochlea. Gene Ther. 2009;16:990‐997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hildebrand MS. Dahl H‐HM, Hardman J, et al. survival of partially differentiated mouse embryonic stem cells in the scala media of the Guinea pig cochlea. J Assoc Res Otolaryngol. 2005;6:341‐354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hu Z, Ulfendahl M, Olivius NP. Central migration of neuronal tissue and embryonic stem cells following transplantation along the adult auditory nerve. Brain Res. 2004;1026:68‐73. [DOI] [PubMed] [Google Scholar]
- 82.Zou B, Mittal R, Grati M, et al. The application of genome editing in studying hearing loss. Hear Res. 2015;327:102‐108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.He Z, Fang Q, Li H, et al. The role of FOXG1 in the postnatal development and survival of mouse cochlear hair cells. Neuropharmacology. 2019;144:43‐57. [DOI] [PubMed] [Google Scholar]
- 84.Mianné J, Chessum L, Kumar S, et al. Correction of the auditory phenotype in C57BL/6N mice via CRISPR/Cas9‐mediated homology directed repair. Genome Med. 2016;8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hou K, Jiang H, Karim MR, et al. A critical E‐box in Barhl1 3′ enhancer is essential for auditory hair cell differentiation. Cell. 2019;8:458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Li X, Yu X, Chen X, et al. Localization of TMC1 and LHFPL5 in auditory hair cells in neonatal and adult mice. FASEB J. 2019;33:6838‐6851. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Summary of the main recent accomplishments with otic cell models using hiPSCs for inner ear neurosensory cell differentiation. Abbreviations: HCs, hair cells; IHF, inactivated human feeders; LAP, low attachment plate; LCAW, low cell adhesion well; MCTFL, mitomycin C‐treated feeder layer; MTCEUSc, mitomycin‐treated chicken embryonic utricle stromal cell; OEP, otic epithelial progenitors; ONP, otic neural progenitors; OS, orbital shaker; SF, spinner flask; PAX2, PAX8, otic/placodal markers; MYO7A, Myosin7A, hair cell marker; POU4F3, hair cell marker; Synapsin I, synaptic marker; VGLUT1, vesicular glutamate transporter.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


