Abstract
Aims
Uterine fibroids are benign tumours that cause various complaints. These complaints may significantly compromise quality of life, necessitating a clinical intervention in 25–50% of the affected women. Hysterectomy, myomectomy or embolization may offer symptomatic relief, but are costly, include a recovery period, can cause serious side‐effects, sometimes fail to treat symptoms completely and are not always desired by patients. Ulipristal is a conservative long‐term treatment that has a fibroid‐volume decreasing effect, acceptable side‐effects while preserving fertility and may be an alternative to surgical alternatives. Currently, ulipristal is investigated by the European Medicine Agency and suspended from marketing authorization because it may cause drug‐induced liver injury (DILI). However, many drugs can cause severe DILI and prospective studies estimate 14–19 DILI cases/100 000 people.
Methods
This overview will discuss the risk–benefit balance between ulipristal and DILI, describe the safety–efficacy balance of ulipristal and its alternative treatments and the arguments that led to the suspension of its marketing authorization.
Results
Ulipristal may be associated with DILI resulting in a risk of severe liver injury in 1.5:100 000 patients and fatal liver injury in 0.1:100 000 patients. This risk needs to be weighed against the higher mortality risk of >1:1000 and higher incidence of severe complications after surgery.
Conclusion
The DILI risk of ulipristal is considerably lower than that of other medicines that are not suspended, nor need additional safety measures. When evaluating drugs and drug safety, risks that apply to the alternative nonpharmacological treatment options should be taken into consideration.
Keywords: leiomyoma, chemical and drug induced liver injury, clinical pharmacology, hepatopharmacology, hysterectomy, safety pharmacology, ulipristal acetate
1. INTRODUCTION
Uterine fibroids are benign tumours, most common in women of reproductive age.1 Depending on the amount, their volume and location, fibroids cause various complaints such as abnormal uterine bleeding, mechanical complaints, dyspareunia and subfertility. These complaints may compromise quality of life (QoL) and fertility/pregnancy related problems, necessitating clinical intervention in 25–50% of the affected women.2, 3 Hysterectomy is the most definitive option, myomectomy is recommended in case a future pregnancy is desired and e.g. uterine artery embolization (UAE) is an uterus‐sparing alternative. These options may offer symptomatic relief, but are costly, have recovery periods, have serious side effects and sometimes fail to treat symptoms completely. Also, many patients do not favour a surgical procedure. Pharmacological treatment such as oral contraceptives, can offer symptomatic relief for some patients, but do not diminish fibroid growth and ultimately fail for most women with significantly sized fibroids. Gonadotropin‐releasing hormone analogues (GnRHa) are used for short periods as pretreatment to reduce fibroid volume before surgery, due to their inherent hormonal suppression effect that causes menopausal symptoms and an increased risk of osteoporosis and cardiovascular disease.4, 5 As a corollary, fibroid treatment was in need of a conservative alternative that treats symptoms, has a fibroid volume‐decreasing effect (sustained after discontinuation) and has acceptable side effects while preserving fertility as an alternative to more invasive surgical options.
This treatment became available in February 2012. Ulipristal acetate was introduced as a promising new drug for symptomatic uterine fibroids. Initially ulipristal was prescribed as a single 12 week pretreatment course before surgery, but in 2015 this indication was expanded up to 4 12‐week courses. The thinking behind the use of subsequent courses was the anticipation that surgery would be postponed or even become unnecessary, although supporting clinical evidence was lacking.4, 5, 6 Nevertheless, ulipristal became the only registered pharmacological treatment to treat uterine fibroids over a longer period.
Since marketing authorization, it is estimated that >900 000 European and Canadian patients have been treated with ulipristal. However, postmarketing problems arose.7 It came to the attention of the European Medicine Agency (EMA) that 8 patients had developed acute liver failure while on ulipristal, which necessitated liver transplantation in 5 patients. This initiated an investigational procedure of ulipristal and subsequently marketing authorization was revoked in the European Union (EU).7
It was hypothesized that ulipristal could trigger a drug‐induced liver injury (DILI), that can be classified as intrinsic or idiosyncratic (see Table 1).8 Hy Zimmerman categorized symptoms in an effort to predict the risk of fatal DILI, which resulted in Hy's law.9 Diagnosing DILI requires diagnostic vigilance and is often missed in clinical practice because of the absence of specific diagnostic biomarkers for DILI. Therefore, the correct appraisal of suspected DILI depends on the judicious interpretation of serum liver biochemistry in combination with other routine laboratory and imaging to exclude alternative causes of liver disease. Regulatory bodies such as the EMA use Hy's law to identify DILI.10 In this paper we use the different DILI gradations, from the DILI‐Network, also given in Table 1.8, 11 The true incidence of DILI is uncertain due to differences in criteria and methodologies used to define DILI. In retrospective and case–control studies, the postmarketing annual incidence of DILI is estimated to range from 2.4 to 2.7/100 000 people.12, 13, 14 A cohort study suggested that ~1 in 100 hospitalized patients develop DILI, which is at odds with the often cited incidence data of 14–19/100 000 of some prospective studies.15, 16 Regardless, DILI has shown to be the most frequent cause of acute liver failure in the EU and USA.17, 18
In this overview we will discuss the risk–benefit balance of ulipristal and weigh this against the risk and benefits of its alternative treatments. Finally, we will compare our findings to the documented arguments that led to the suspension of its marketing authorization.
1.1. Ulipristal acetate
Ulipristal is an oral selective progesterone receptor modulator that has both agonistic and partial antagonistic activity, reducing fibroid volume, inducing amenorrhoea and thereby reducing fibroid related complaints. Its efficacy was evaluated in the PEARL and VENUS trials. PEARL‐I and VENUS‐I compared ulipristal 5 and 10 mg with placebo. PEARL‐I showed median fibroid volume reduction of 21% (IQR −41.1 to −1.1%) in the 5‐mg group and reduced excessive menstrual bleeding after a 12‐week pretreatment course prior to surgery.19 PEARL‐I did show adverse changes to the endometrium that were described as progesterone receptor modulator‐associated endometrial changes, which have been investigated in several clinical studies and the endometrium returned to normal after discontinuation of ulipristal, without any long‐term consequences.20, 21 The population of the VENUS‐I trial consisted of 69% African American patients and preliminary results showed superiority of ulipristal compared to placebo for amenorrhoea with 47.2% (97.5% confidence interval [CI] 31.6–63.2) and 1.8% (97.5% CI 0.0–10.9) for ulipristal 5 and the placebo‐treated group respectively (P < .001).22
PEARL‐II compared ulipristal with GnRHa prior to surgery and showed noninferiority on clinical endpoints (control of bleeding) but reported 11% hot flushes in the ulipristal compared to 40% in the GnRHa groups. However, the ulipristal group showed significantly smaller uterus volume reduction with −20% compared to −47% for GnRHa group. Fibroid volume reduction of the 3 largest fibroids did not differ significantly between groups.20 PEARL‐III and IV evaluated long‐term intermittent treatment with ulipristal up to 4 12‐week treatment courses, alternated with 8 weeks of medication free intervals. Donnez et al.23 concluded that ulipristal effectively reduced symptom severity scores with 40% and improved QoL with 40%. Also, fibroid volume reduced significantly up to 65% after the fourth treatment course. Amenorrhoea was stable over all the treatment courses with 89, 88 and 90% in women after courses 2, 3 and 4, respectively.23 Six months follow up in the PEARL‐II trial showed a sustained effect of ulipristal on fibroid volume after end of treatment, compared to rapid regrowth of fibroids in the GnRHa‐group.20 Similar results were shown for the African American population in the VENUS‐II trial. VENUS‐II compared ulipristal 5 and 10 mg with placebo in 2 intermittent 12‐week courses. Results showed significant improvement of fibroid symptoms in both ulipristal groups, compared to placebo with symptom severity scores reducing with −39.8 and −17.0 points from baseline for the ulipristal 5 mg and placebo groups, respectively. Also, treatment with 5‐mg ulipristal achieved amenorrhoea in 42.0% (97.5% CI 33.3–51.1; P < .001) of the patients respectively compared to 0% (97.5% CI 0.0–3.8) in the placebo‐treated group.24
Suggestions were made that in case of good response to ulipristal, surgery might be avoided or postponed in case a patient would wish to become pregnant first.4, 20, 25 Although the PEARL and VENUS trials were well conducted and peer reviewed, several questions remained. Were the patients from these trials representative of patients seen in clinical practice? And more importantly, were the appropriate outcomes evaluated? Also, long‐term follow up after discontinuation of the medication is lacking and no randomized comparisons have been made for the long‐term treatment.26 Currently, at least 1 trial has started to study the comparison of long‐term intermittent treatment of ulipristal with surgery (MYOMEX‐2 trial; Dutch Trial Register number: NTR6860; NL62638.029.18).27 Despite the lack of long‐term or comparative data, ulipristal quickly gained popularity and was enthusiastically welcomed by both patients and their gynaecologists. To illustrate: in the Netherlands, ulipristal prescriptions increased from 2067 in 2014 to 8023 in 2017.28
1.2. Safety issues concerning ulipristal acetate
The tide changed on 22 September 2017 (see Figure 1 for full timeline). A case of fulminant hepatitis led to hepatic transplantation and was reported to the French medicine agency (Agence Nationale de Sécurité du Médicament: ANSM). Subsequent investigation suggested a relation with ulipristal treatment and questions were raised whether ulipristal could be causing a DILI and lead to severe hepatic injury. As an outcome of this evaluation, the EMA requested their Pharmacovigilance Risk Assessment Committee (PRAC) to start an Article 20 procedure of ulipristal on 30 November 2017.29 This procedure was initiated to evaluate the possible causal relationship between ulipristal and acute liver injury and its impact on the benefit–risk balance of ulipristal. During this procedure, another case of fulminant liver injury requiring liver transplantation was reported. This patient died from transplantation‐related complications and in February 2018 physicians were recommended to stop prescribing ulipristal and advised to perform liver function tests (LFTs)—alanine aminotransferase, aspartate aminotransferase and serum total bilirubin—after stopping ulipristal.
FIGURE 1.
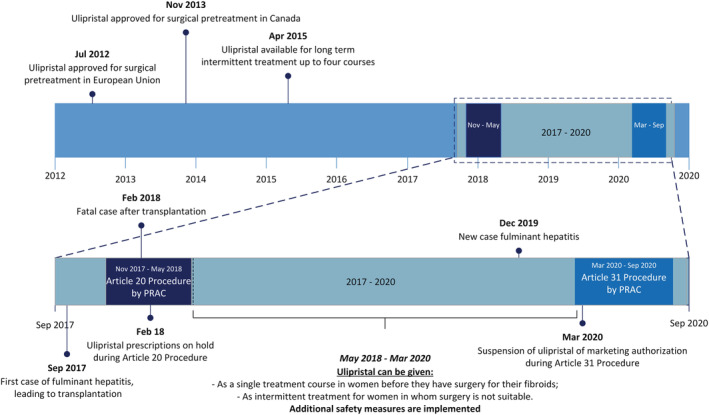
Ulipristal timeline. PRAC, Pharmacovigilance Risk Assessment Committee
In May 2018, the Article 20 procedure was completed. During the PEARL I‐IV trials, no signal of hepatic toxicity was identified. In the PEARL‐III and IV extension studies employing prolonged drug treatment consisting of up to 4 12‐week treatment courses, sporadic increases of liver transaminases were seen, but these were never associated with elevated total bilirubin.19, 20, 30 The PRAC‐report showed that postmarketing exposure was estimated to be around 765 000 patient prescriptions between registration in 2012 and May 2018, corresponding to approximately 175 000 patient‐years. A search for cases of hepatic disorders and ulipristal prescriptions in the Eudravigilance database retrieved 8 cases of severe liver injury with a possible contributing role of ulipristal and several other cases of hepatic disorders. Of these cases, 4 patients required liver transplantations, of whom, 1 had a fatal outcome. The PRAC concluded that ulipristal might have contributed to the development of idiosyncratic DILI, but that definite conclusions could not be drawn. The PRAC therefore recommended additional measures to minimize the DILI risk in women using ulipristal. From that moment onwards, ulipristal prescriptions were only allowed for long‐term intermittent treatment in women who were not eligible for surgery. Also, LFTs should be tested before, monthly during and 4 weeks after cessation of ulipristal treatment.31 Subsequently, this resulted in a 40% drop in ulipristal use in the EU and Canada from approximately 10 625 prescriptions a month (roughly calculated from 765 000 prescriptions from February 2012 to February 2018) to 6429 prescriptions a month between June 2018 and March 2020. In the Netherlands, this drop was about 70% with 8023 prescriptions in 2017 to 2347 prescriptions in 2018.28 In the UK, a similar drop was seen with ~20 000 prescriptions in 2017, decreasing with >80% to 2865 prescriptions in 2019 (see Figure 2).32, 33, 34
FIGURE 2.
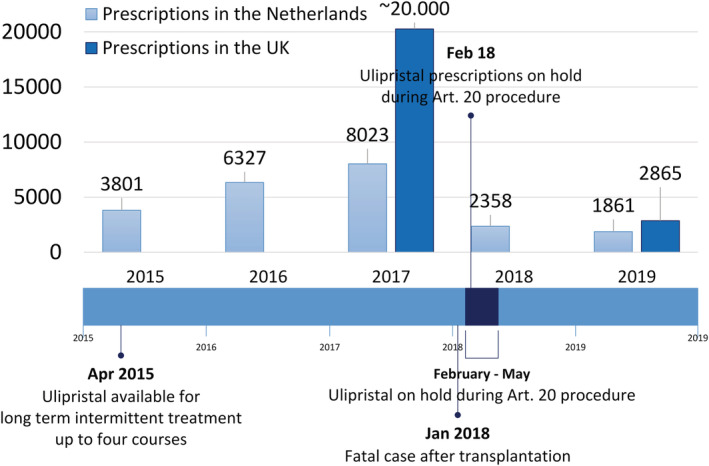
Ulipristal 5‐mg prescriptions in the UK and the Netherlands.28, 32, 33, 34
As a result of the increased vigilance by the community, a new case of possible DILI came to the attention of EMA in December 2019, 21 months after the completed article 21 procedure. In this case, exposure to ulipristal led to acute liver failure, requiring liver transplantation, making this the fifth known liver transplant case. This further supported the causal relation between ulipristal and DILI. In this specific case, the safety precautions had not resulted in prevention of development of severe liver injury and the subsequent liver transplantation, causing a major safety concern.35 The EMA decided to initiate an Article 31 Pharmacovigilance Referral of Directive, a referral that is initiated when the “interest of the EU is involved, following concerns relating to the quality, safety or efficacy of a medicine or class of medicines”.36 During this Referral procedure, all available information is reviewed, all patients on ulipristal are advised to stop and required to perform LFTs. As a temporary measure, all the ulipristal 5 mg medicinal products are suspended of the European market authorization until a final decision is made.
These findings were supported by a recent publication by Kang et al.38 They reviewed the postmarketing reports of the Food and Drug Administration (FDA) of possible DILI with ulipristal use from France, Germany, Spain, Canada and Portugal up to 31 January 2020. Their assessment showed the same 9 cases (the ninth case occurred in December 2019) of possible DILI, of which 5 resulted in a severe outcome (transplantation and/or death). They assessed severity according to the grading of the DILI‐Network, the National Institutes of Health and an FDA expert opinion. This severity was graded 1–5, with 1 being mild and 5 being fatal and includes liver transplantation and/or death.37, 38 They also assessed causality with the Roussel Uclaf Causality Assessment Method (RUCAM), which defines likelihood categories of a causal association of liver injury in 5 different categories (ranging from <24% being unlikely to >95% being definite). Kang et al. graded the 5 severe cases as probable likelihood and the remaining 4 cases to have a severity grade 2 and 3 (moderate and moderate to severe) and a RUCAM causality category of possible (n = 2) or probable (n = 2).38
1.3. Evaluation of DILI in the drug authorization process
A systematic review of 462 medicinal products withdrawn in the postmarketing phase (1953–2013), found that DILI was the most frequent cause of drug withdrawal. This makes DILI an important challenge for the pharmaceutical industry as well as regulatory bodies, because the hepatotoxic potential is not always clear from the preregistration Phase I–III trials, as with ulipristal.10
The National Institutes of Health provide an extensive database on hepatotoxicity: LiverTox, accessible through PubMed and it contains descriptions of >1200 agents, ranging from antibiotics to herbal products. The LiverTox database provides a summary of DILI frequency known for this agent and a likelihood score. Based upon published literature, a categorization of likelihood is made to assess the association of drugs and DILI. Consequently, this categorization is more reliable if more literature is available. For example, Category A likelihood score includes agents for which >50 cases series have been described that show a well‐known, well described cause to direct or idiosyncratic liver injury.39 If possible, representative and fully described case reports of hepatotoxicity of the agent are included. Sometimes insufficient literature data are available to rule out other sources of liver injury and to properly adjudicate the relationship. Furthermore, publication bias plays an important role. That is, if DILI is already a known adverse effect, a new case of DILI is not published as a case report as this is the case for agents such as amoxicillin and aspirin, which makes it difficult to assess the frequency of hepatotoxicity of these agents.37
To illustrate the difficulties that surround the evaluations in the drug authorization process, we will describe examples of several situations in which DILI did or did not influence the (post‐) marketing phase, which are shown in Table 2. The majority of the agents listed in LiverTox may still be prescribed, despite documented risk of DILI and DILI‐related fatal outcomes. For example diclofenac, a commonly used painkiller of the group nonsteroidal anti‐inflammatory drugs, has a category A likelihood score for DILI and an evident risk for fatal DILI (see also Table 2).
TABLE 2.
Several drugs and their evaluation in relation to marketing authorization and their potential to induce DILI
| Drug name | Indication | DILI‐risks described in literature | Marketing authorization |
|---|---|---|---|
| Diclofenac | Anti‐inflammatory pain medication |
Livertox37: ‐ 1‐5 cases/100 000 prescriptions ‐ Likelihood score A Björnsson et al.: Review of Swedish cases from 1966–200277: ‐ out of the 4687 DILI reports, 103 cases were fatal. ‐ 3.8% caused by diclofenac De Valle et al.: Retrospective study in Sweden from 1995–200514: ‐ 1164 liver disease cases; 77 due to DILI ‐ 18.2% caused by diclofenac. |
Authorized Widely used painkiller, although many same‐class drugs are available. |
| Tolvaptan | Long‐term intermittent treatment for autosomal dominant polycystic kidney disease |
TEMPO 3:4 registration trial; 961 participating patients41: ‐ 1.5% discontinued due to liver dysfunction ‐ 4.5% showed a clinically significant increase in aminotransferases ‐ 0.9% showed an increase in bilirubin levels ‐ 0% of acute liver failure All cases showed resolution in LFTs after tolvaptan discontinuation ‐ b1 case of acute liver failure, requiring transplantation |
Authorized with restrictions: Risk evaluation and mitigation strategy with frequent LFT: ‐ 2 and 4 weeks after start ‐ monthly in first 18 months ‐ thereafter every 3 months |
| Sitaxentan |
Endothelin receptor antagonist that had been authorized in the European Union for the treatment of PAH PAH is incurable; therapy is aimed at slowing the progression or improve symptoms |
Regulatory bodies, after registration trials47, 48, 50: ‐ known DILI risk; therefore, additional safety LFTs were performed during treatment Postmarketing, 2000 treated patients49: ‐ 4 cases of fatal liver injury ‐ 1 case of liver transplantation Cases are thought to be causally related to sitaxentan and did not resolve after discontinuation |
Authorized by regulatory bodies Withdrawn by pharmaceutical manufacturer |
| Ulipristal |
Selective progesterone receptor modulator for treatment of uterine fibroids Sole pharmaceutical treatment for long‐term intermittent use |
Registration trials; PEARL I‐IV19, 20, 23, 30: ‐ no hepatic toxicity was identified Postmarketing, 900 000 prescriptions: ‐ 91 possible adverse effects in the hepatic disorder spectrum ‐ including 8 reported cases of severe liver injury 5 resulted in liver transplantation 1 fatal outcome |
Ulipristal 5 mg tablets are suspended from marketing authorization until a final decision is made by the European Commission. |
DILI: drug‐induced liver injury; LFT: liver function test; PAH, pulmonary arterial hypertension.
Some drugs have triggered a response from the EMA and were investigated by the PRAC. As it was for ulipristal initially, these drugs required a similar LFT screening system. A good example is tolvaptan, a medicine used for autosomal dominant polycystic kidney disease. In prior studies, the potential for hepatotoxicity of tolvaptan was correctly predicted using DILIsym, a quantitative systems toxicology mathematical model of DILI.40, 41 Based on the FDA guidance, the identification of Hy's law cases indicates that tolvaptan had the potential to cause hepatic injury capable of progressing to liver failure in patients with autosomal dominant polycystic kidney disease.42, 43 To reduce the DILI risk in patients receiving long‐term tolvaptan, frequent monitoring of liver function tests has been recommended by the FDA.44, 45
Because DILI is a frequent reason for drug withdrawals or suspensions in the postmarketing phase, many examples are known.46 Agents can be retracted: (i) during registration trials due to clinically apparent DILI; (ii) during the assessment of marketing authorization; or (iii) after marketing authorization. Marketing authorization can be revoked by the regulatory bodies or by the pharmaceutical manufacturer. For example, sitaxentan is authorized in the EU for the treatment of pulmonary arterial hypertension, which is incurable and therapy is aimed to slow the progression or improve symptoms. Sitaxentan was known to cause DILI and additional safety LFTs had to be performed during treatment. Nevertheless, postmarketing experience showed 4 cases of fatal liver injury and 1 case of liver transplantation among 2000 treated patients (1:500). In this case, the regulatory bodies decided that the benefits of sitaxentan outweighed the risks of DILI, but the pharmaceutical manufacturer deemed that the DILI risk was too high.47, 48, 49
1.4. Ulipristal and risk of DILI
Current numbers indicate that the risk for severe or fatal DILI is low for ulipristal, with 8 cases of severe liver injury out of 900 000 prescriptions, 5 of which led to liver transplantation and 1 to a fatal outcome.7 In the current available information of the PRAC assessment in the article 31 procedure, from 1 March 2018 to 8 December 2019, 91 cases (including 6 nonvalid cases) were reported with possible serious adverse effects within the hepatic disorder spectrum. However, only 8 cases included sufficient or partially sufficient information for causality assessment and only in 5 cases with either sufficient or partially sufficient information did a causal relationship with ulipristal use seem possible (see also Figure 3).50 If we look at the DILI Network likelihood scores, ulipristal could be described as category C: “The drug is probably linked to idiosyncratic liver injury, but has been reported uncommonly and no characteristic signature has been identified; the number of identified cases is less than 12 without significant case series”.37 Björnsson et al. state that if there are <12 cases described, it is unclear whether there is an actual hepatotoxic relation. Of the drugs that are classified as category C, some agents did not even have documented fatal liver reactions or reports about a dechallenge–rechallenge test. As for ulipristal, these drugs lack well‐documented hepatotoxicity but DILI with their use also cannot be excluded. These category C agents have in common that the cases are generally poorly documented, have no complete assessment and have probable concomitant use of other medication, which makes it difficult to prove causality.51
FIGURE 3.
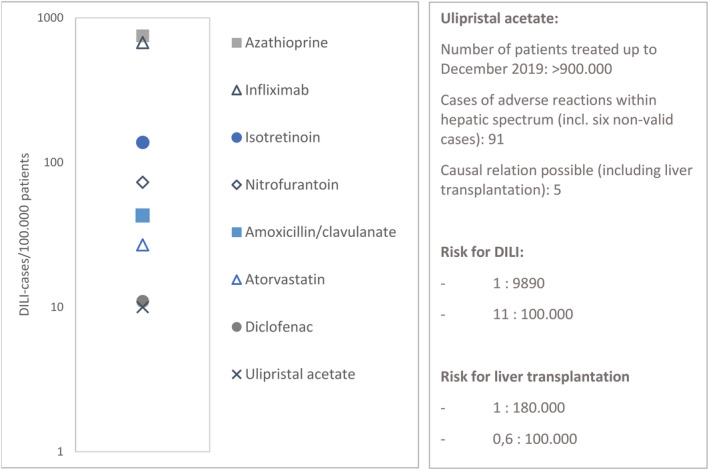
Semilogarithmic plot of diagnosed drug‐induced liver injury (DILI) per 100 000 treated patients for different agents. Adapted from Björnsson et al., study conducted in 2010–2011.16 Ulipristal numbers are derived from Pharmacovigilance Risk Assessment Committee assessment report from 1 March 2018 to 8 December 2019.50
Although it is likely that there are more cases of mild or moderate DILI (elevated LFTs below or meeting Hy's law) with ulipristal use, the known risk for severe DILI is currently 1.5/100 000 and 0.1/100.000 for fatal DILI. Based on prospective studies from Iceland and France, overall DILI incidence in inhabitants is about 14–19/100 000.16, 52 If Hy's law is met, about 10% of these DILI cases have a chance to progress towards liver failure and would consequentially be in need of liver transplantation (Table 1).53, 54 This would correspond with 1.4–1.9/100 000 in medication users in general, which is comparable to the current known risk of liver transplantation in ulipristal users.
TABLE 1.
Drug‐induced liver injury (DILI) explained: classification, definition according to Hy's law and gradation
| DILI classification8 | ||
|---|---|---|
| Intrinsic | Dose dependent and predictable (e.g. paracetamol) | |
| Idiosyncratic | Unexpected hepatotoxic reaction in a small population exposed to the agent | |
| Hy's law, based on 3 components9 | Definition | |
|---|---|---|
| 1 | Hepatocellular injury | Elevation of ≥3 times the ULN of: ALT and AST |
| 2 | Elevation of serum TB without findings of cholestasis | Elevation of serum TB ≥ 2 times the ULN |
| 3 | No other cause found for increased aminotransferases | Possible other causes: Viral hepatitis; alcohol abuses |
| European Medicine Agency cut‐off values: Combination of component 1 and 2.10 |
‐ ALT/AST ≥3 times ULN ‐ TB ≥2 times the ULN |
|
| DILI‐network grading scales8 | ||
|---|---|---|
| 1 | Mild |
‐ elevated ALT and/or AST ‐ but TB below Hy's law and INR < 1.5 |
| 2 | Moderate |
‐ all LFTs meeting Hy's law ‐ or INR ≥ 1.5 |
| 3 | Moderate–severe |
‐ LFTs meeting Hy's law ‐ hospitalization or ongoing hospitalization prolonged due to DILI |
| 4 | Severe* |
LFTs meeting Hy's law and at least 1 of the following criteria: ‐ hepatic failure (INR > 1.5, ascites or encephalopathy) ‐ other organ failure due to DILI |
| 5 | Fatal* | Death or liver transplantation due to DILI |
In most fatal cases, ALT and AST were 8–100× ULN9
Hy's law criteria are thought to indicate severe hepatocellular injury that is associated with at least 10% chance of severe liver failure and/or need for liver transplantation.53, 54
The DILI Network combines death and the need for liver transplantation in the fatal definition, which are identified separately in this paper as severe (in need of liver transplantation) and fatal (death).
ALT, alanine aminotransferase; AST, aspartate aminotransferase; INR, international normalized ratio; LFT, liver function tests; TB, total bilirubin; ULN, upper limit of normal
By contrast, Björnsson et al.16 conducted a prospective study from 2010–2011 in 96 patients with a diagnosed DILI. This study defined DILI as alanine aminotransferase >3× upper limit of normal and alkaline phosphatase >2× upper limit of normal. They presented the cases of diagnosed DILI resulting in different proportions per 100 000 patients, with e.g. amoxicillin–clavulanic acid DILI occurred in 1:2350 patients, giving a proportion of 43:100 000 patients (95% CI: 24–70).16 As can be seen in Figure 3, several other drugs were studied by Björnsson et al., showing different proportions of DILI risk. These drugs are prescribed for both acute and chronic diseases, for short‐term (nitrofurantoin, amoxicillin–clavulanic acid, diclofenac), intermittently (infliximab) and long‐term therapies (e.g. atorvastatin, azathioprine). Moreover, all these drugs have alternative (pharmaceutical) treatment options, which is not the case with ulipristal. If we assume that all the above mentioned 91 cases are in fact DILI related to ulipristal and that we are facing a true causal relationship, incidence of DILI is still comparable or lower than many drugs that are currently still available and do not need additional LFTs (see Figure 3).
Supposing that this is correct, we should have a look at the risks for patients when ulipristal is no longer available, thus when they choose alternative treatments. Therefore, the next question is: what alternatives do patients have when ulipristal is no longer available and how risky are these alternatives?
1.5. Alternative therapy for symptomatic uterine fibroids
Ulipristal is prescribed for moderate to severe symptoms caused by uterine fibroids as a pretreatment before surgery or as a long‐term intermittent treatment. Mild symptoms are not treated with ulipristal, but can be controlled with conservative therapies such as combined oral contraceptives, progestogens alone or tranexamic acid. Alternative to ulipristal as a pretreatment is GnRHas, alternative to the long‐term treatment is surgery: hysterectomy, myomectomy or UAE.
1.6. Ulipristal as pretreatment
As shown in PEARL‐II, GnRHa seems to have better fibroid and uterus volume reduction effects compared to ulipristal and similar effects on bleeding reduction and QoL. However, PEARL‐II showed a more sustained effect on the volume of the 3 largest uterine fibroids and uterine. At week 38 of follow‐up (treatment up to 12 weeks, cessation for 26 weeks), the change from baseline (CFB) for the 3 largest fibroids was −44.8% (IQR −75.1 to −12.0) compared to −16.5% (IQR −41.1 to 19.3) and −21.8% (IQR −37.7 to −5.6) compared to −11.1% (IQR −22.9 to −1.0) for the uterine volumes in the ulipristal and GnRHa‐groups respectively.20, 55 De Milliano et al. showed that pretreatment with GnRHa was more favourable than ulipristal in terms of fibroid volume reduction, intraoperative blood loss, haemoglobin decrease directly postoperatively, suturing time of the first fibroid and several subjective surgical ease parameters. However, this study was underpowered, so these results should be interpreted with caution.56 Table 3 shows a summary of the different advantages and limitations of both pretreatments.
TABLE 3.
Clinical comparison of ulipristal treatment and GnRHa treatment for controlling symptoms of uterine fibroids while awaiting surgical treatment.20, 56
| Therapy | Advantages | Limitations | Mild morbidity |
|---|---|---|---|
|
Ulipristal acetate 12 week treatment course |
Compared to GnRHa20: ‐ similar reduction of menstrual bleeding ‐ similar improved QoL ‐ similar fibroid volume reduction ‐ more sustained effect at long term follow up on fibroid/uterus volume with −45%/−22% compared to −17%/−11% for ulipristal and GnRHa respectively ‐ less (11%) hot flushes |
Frequent LFT PEARL trials: Fibroid reducing effect, in population with relatively small uterine fibroids Compared to GnRHa: Less (20%) uterus volume reduction |
Reversible endometrial changes Possible chance for mild/moderate DILI |
|
GnRHa 12 weeks to 6 months |
Compared to ulipristal: ‐ similar reduction of menstrual bleeding ‐ similar improved QoL ‐ similar fibroid volume reduction ‐ Favourable surgical parameters: Intra‐operative blood loss, suturing time and several subjective surgical ease parameters ‐ more (47%) uterus volume reduction |
Long‐term treatment correlated with increased risk of osteoporosis and cardiovascular symptoms Compared to ulipristal: ‐ more (40%) hot flushes ‐ chance on fibroid size regrowth |
Add‐back therapy possible to reduce the risk for osteoporosis and hot flushes |
GnRHa: gonadotropin releasing hormone agonists; QoL: quality of life; LFT: liver function test; DILI: drug‐induced liver injury.
Based on the above, efficacy of ulipristal is not convincingly superior to GnRHa. Thus, GnRHa seems to be a good (and maybe superior) alternative to ulipristal when it comes to pretreatment. The safety issues with ulipristal may consequently weigh GnRHa to be the favourable treatment when it comes to pretreatment before surgery. Since long‐term treatment with GnRHa is correlated to an increased risk of osteoporosis and cardiovascular symptoms, they are mostly only prescribed for 6 months. Moreover, after discontinuation, fibroids grow back to their original size within several weeks. Consequently, GnRHa treatment needs to be continued until menopause, which is seldom done. Therefore, GnRHa is not an optimal alternative compared to long‐term treatment with ulipristal.
1.7. Surgical alternatives for ulipristal
Moderate to severe symptoms are usually treated with surgical interventions. Hysterectomy is the most definite treatment, with absolute certainty that fibroids will not recur and related complaints will subside. When uterus preservation is desired, myomectomy or UAE are other alternatives. However, these procedures are not without risks: they can cause severe morbidity and even mortality. An overview of the different procedures compared to ulipristal is given in Table 4. A Cochrane review that analysed the surgical approaches of hysterectomy for benign indications, reported that complications occur whatever the type of surgery.57 Severe complications can occur in 1:100 patients during and after surgery, such as haemorrhage, vesicoperitoneal fistula, ureteral injury, rectal perforation or fistula.58, 59, 60 Altman et al., reported that hysterectomy for benign indications, independent of surgical technique, increases the risk for subsequent stress‐urinary‐incontinence surgery (hazard ratio 2.4; 95% confidence interval 2.3–3.5).61 A large case series of 664 229 women from the USA reports an overall mortality rate of 0.17% (1:588 women) associated to abdominal hysterectomy in the years 1998–2010. In that series, several complications were also registered.62
TABLE 4.
Clinical comparison of ulipristal with surgery, comparing advantages and limitations, also showing literature regarding morbidity and mortality
| Ulipristal acetate | Uterine artery embolization | Myomectomy | Hysterectomy | |
|---|---|---|---|---|
| Advantages |
‐ sole available long‐term treatment (up to 4 12 week treatment courses) ‐ sustained effect on fibroid/uterus volume ‐ Favourable side effects ‐ pregnancy not impaired |
Noninvasive surgical technique Reduction of symptoms Improvement of QoL |
Usually pregnancy after treatment is possible Reduction of symptoms Improvement of QoL |
Reduction of symptoms No fibroid complaints No menstrual bleeding Improvement of QoL Definite treatment option |
| Limitations |
‐ frequent LFT ‐ literature shows fibroid reducing effect, in population with relatively small uterine fibroids ‐ chance on symptom‐recurrence after cessation of therapy |
‐ risk for premature ovarian failure ‐ unknown effect on fertility, pregnancy after treatment is discouraged ‐ risk for procedure‐related complications ‐ risk for re‐intervention |
‐ risk for subfertility ‐ risk for procedure‐related complications |
Fertility impaired after treatment Risk for procedure‐related complications Altman et al.61: Increased risk for subsequent stress urinary incontinence (SUI) (HR 2.4; 95% CI 2.3–3.5) Overall surgical intervention rate due to SUI is more than doubled for women who had a hysterectomy |
| Mild morbidity |
‐ reversible endometrial changes ‐ possible chance for mild/moderate DILI |
‐ 1:33–1:20 re‐intervention ‐ 35% chance on secondary hysterectomy65, 66, 67, 68 ‐ 24% occurrence of perioperative and postdischarge complications71 |
‐ 29% occurrence of perioperative and postdischarge complications71 |
Cochrane Review57 1: 63 urinary tract injury (vaginal approach) 1: 42 urinary tract injury (laparoscopic approach) |
| Severe morbidity | 1.5: 100 000 for severe DILI |
Manyonda et al.71: ‐ 1:50 chance on major haemorrhage perioperative and predischarge 14% chance on postdischarge infection |
Manyonda et al.71: 1:20 chance on major haemorrhage perioperative and predischarge ‐ 17% chance on postdischarge infection 1:25 chance on general complications78: ‐ intraoperative haemorrhage requiring blood transfusion ‐ emergency hysterectomy |
Cochrane Review57 1:100 for haemorrhage, vesicoperitoneal fistula, ureteral injury, rectal perforation or fistula USA case series 1998–2010 (n = 664 229): 1:56–1:46 for haemorrhage 1:40–1:23 for respiratory failure 1:67–1:40 for infections German analysis ‘12 (n = 103 232)63: 1:100–1:25 for intra‐ and postoperative complication rates France survey 2006–2015 (n = 109 884)64: 1:13 severe complications in 60 months follow up |
| Mortality | 0.1: 100 000 for fatal DILI | Death is reported in cases due to e.g. infection or pulmonary embolism69, 70 |
USA case series 1998–2010 (n = 664 229): 1:588 (abdominal approach) German analysis 2012 (n = 103 232)63: 1:3700 |
QoL: quality of life; LFT: liver function test; SUI: stress urinary incontinence; HR: hazard ratio; CI: confidence interval; DILI: drug‐induced liver injury
In Germany, the national analysis of external hospital care quality assurance data for Gynaecological Operations evaluated intra‐ and postoperative complication rates of 103 232 hysterectomies performed for benign indications in 2012. In these data, the total complication rate was 1:19 and by calculation, the rate of death in 2012 was 27.1/100 000 hysterectomies for benign indications (1:3700 women).63 More recently, the Programme de médicalisation des systems d'information, performed a survey in France from 2006–2015, under 109.884 women who had a surgical intervention due to abnormal uterine bleeding. Of these women, 21% had a hysterectomy and 7.9% (1:13 patients) experienced severe complications in the 60‐month follow‐up period.64
Although myomectomy and UAE both preserve the uterus, they have an inherent complication risk and can cause subfertility. Presence of uterine scars after myomectomy forms a significant risk for developing postsurgical adhesions and increases the risk of uterine rupture during future pregnancies.4, 20 UAE induces ischaemic necrosis of the fibroids and presents a good treatment option for women with fibroids. However, premature ovarian failure can occur, sometimes resulting in early menopause and this strategy is therefore not a good alternative in women who want to preserve their fertility. Studies show that patients who underwent UAE need repeat UAE, additional surgery to solve complications and treatment failure leads to secondary hysterectomy in up to 35% of cases.65, 66, 67, 68 Also, death has been reported in a number of cases after UAE due to e.g. infection or pulmonary embolism.69, 70 Recent published outcomes from a randomized–controlled trial from Manyonda et al.71 compare UAE with myomectomy and find that irrespective of the assigned procedure, complications occur. These complications included both severe perioperative, pre‐ and postdischarge complications such as major haemorrhage and infections.71
2. DISCUSSION
In this paper, we deliberate on current developments around ulipristal, a selective progesterone receptor modulator that can be prescribed as a pretreatment and intermittent long‐term treatment for symptomatic uterine fibroids. We found that there have been over 900 000 ulipristal prescriptions since 2012, with 8 reported cases of severe liver injury, 5 liver transplantations and 1 fatal outcome. Although it is likely that there are more cases of liver injury or elevated liver transaminases (not requiring liver transplantation) with ulipristal use, the current known risk for severe DILI is 1.5/100 000 and the known mortality risk is 0.1/100 000. This risk estimation is supported by the recent published evaluation of Kang et al.38 In comparison to this, overall DILI incidence is reported to be 14–19 cases per 100 000 patients in EU and the USA, and severe liver failure or need for transplantation generally occurs in 10% of those cases if Hy's law is met.16, 52, 53 These numbers suggest that the risk of a liver transplantation in this ulipristal treated population is actually corresponding with the general risk on developing DILI with medicine use. Also, DILI risk with e.g. diclofenac use is higher than with ulipristal. Nevertheless, ulipristal is currently suspended from marketing authorization to further evaluate if there is a causal risk for DILI.
How should we interpret this DILI risk? It should be considered that ulipristal has a favourable effect on a major gynaecological problem. It offers women with a benign diagnosis that significantly impacts QoL a conservative long‐term treatment option. Alternatively, pretreatment can be done with a GnRHa and the gold standard long‐term alternative is hysterectomy. GnRHa as pretreatment might be superior to ulipristal, but is no optimal alternative for long‐term treatment because of bone mineral density loss and increased risk of heart and vessel disease when prescribed for longer than 6 months. Hysterectomy is a final and invasive treatment option, but with a disproportionately greater chance of both short‐ and long‐term minor and major complications than the current observed DILI risk of ulipristal. If we take the German study as an example study for the EU, in 2012 the rate of death was 27.1 per 100 000 hysterectomies for benign indications (i.e. 1:3700 women).63 To put this into perspective: if 1 patient dies in 900 000 prescriptions of ulipristal and 1:3700 women dies after a hysterectomy, that would mean that if all of these 900 000 women would have undergone a hysterectomy, 243 women would have died. Of course, it is questionable if these numbers can be extrapolated this way, but a higher mortality and morbidity after surgery seems apparent in comparison to ulipristal treatment and needs to be considered when future use of ulipristal is questioned and investigated by the EMA.
Long‐term treatment would even become more favourable if we could reduce the risk for severe DILI with frequent monitoring with LFTs or increase the chance to detect mild DILI in an early stage. This also means it should be accepted that occasionally this testing will show enhanced liver transaminases or mild DILI, after which ulipristal treatment can be stopped if the LFTs increase or reach Hy's criteria to prevent the development to severe DILI and liver transplantation. It can even be proposed to upscale the LFTs as a part of a risk evaluation and mitigation strategy comparable to tolvaptan use. So, to perform LFTs 2 and 4 weeks after start of treatment and monthly thereafter as the total treatment duration of 4 intermittent courses is 18 months. However, Kang et al. state that frequent and sustained LFTs are associated with high healthcare costs and that a liver monitoring programme is unlikely to succeed. They also suggest that the impact of an LFT programme would be small and the burden of implementation could be substantial.38 Although the risk of DILI and/or liver transplantation is serious, the alternative surgical treatments seem to have a higher risk of severe morbidity and mortality than ulipristal treatment.
The complications of this risk–benefit balance are shown by the outcomes of the Referral procedure. In September 2020, the PRAC formulated advice to withdraw ulipristal 5 mg from marketing authorization in the EU. However, the EMA's Committee for Medicinal Products for Human Use (CHMP), advised differently on 13 November. They concluded from the PRAC report that ulipristal should not be indicated as short‐term pretreatment anymore, but they do not advise to withdraw the authorization of long‐term treatment with ulipristal. Although they endorse the PRAC assessment of ulipristal's possible risk on DILI, they consider that the benefits of ulipristal treatment may outweigh the risks in women who are not suitable for surgical treatment or in whom surgery has failed. It is uncertain which patients are meant by not suitable for surgical treatment, but we interpret this as a strong advice not to prescribe ulipristal in general and eligible patients will be a highly selected group. Ulipristal will stay suspended of the European market authorization until a final decision by the European Commission is made.72 Eventually, if ulipristal is not banned from the market, more DILI might occur. However, if ulipristal is banned, a far higher incidence of mortality and morbidity will occur because of increasing numbers of alternative surgery. In a time where shared decision making is advocated, risks and benefits of all treatment options should be shared with patients.
3. CONCLUSION
Symptomatic fibroids are very common in women of reproductive age. Ulipristal had the potential of being a good alternative for surgery, considering its positive and sustained effect on fibroid size reduction and fibroid related symptoms in combination with minimal side‐effects. However, ulipristal may be associated with DILI resulting in a risk of severe liver injury in 1.5:100 000 patients and fatal liver injury in 0.1:100 000 patients. This needs to be weighed against the higher risk on major risks with mortality of up to >1:1000 and an even higher incidence of severe complications after surgical alternatives. Also, the DILI risk is considerably lower than the risk on severe DILI of other medicines such as several antibiotics and nonsteroidal anti‐inflammatory drugs that are not suspended, nor need additional LFTs. When evaluating drugs and drug safety, risks that apply to the alternative nonpharmacological treatment options should be taken into consideration. However, this requires a proper counselling of patients concerning pros and cons of all alternatives and thus also education of clinicians.
3.1. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, and are permanently archived in the Concise Guide to PHARMACOLOGY 2019/20.73, 74, 75, 76
COMPETING INTERESTS
The authors declare that they have no conflict of interest. The authors do not have any relation with Preglem SA, they do not participate in any advisory board related to Preglem SA and do not receive any funding from Gedeon Richter. M.A.M., W.J.F.H. and J.A.F.H. are currently performing a randomized–controlled trial that compares ulipristal in long‐term intermittent treatment with standard surgery (hysterectomy, myomectomy and uterine artery embolization): MYOMEX‐2 trial; Dutch Trial Register number: NTR6860; NL62638.029.18. This is a principle investigator initiated study, funded by NWO‐ZonMW.
ACKNOWLEDGEMENTS
Author contribution: J.A.F.H., M.A.M., P.M.B. and W.J.K.H. conceived the presented idea. M.A.M. and W.J.K.H. wrote the manuscript in consultation with P.M.B. and J.P.H.D. All authors discussed the content, provided critical feedback to shape the manuscript and contributed to the final manuscript.
Middelkoop M‐A, Bet PM, Drenth JPH, Huirne JAF, Hehenkamp WJK. Risk–efficacy balance of ulipristal acetate compared to surgical alternatives. Br J Clin Pharmacol. 2021;87:2685–2697. 10.1111/bcp.14708
REFERENCES
- 1.Baird DD, Dunson DB, Hill MC, Cousins D, Schectman JM. High cumulative incidence of uterine leiomyoma in black and white women: Ultrasound evidence. Am J Obstet Gynecol. 2003;188(1):100‐107. [DOI] [PubMed] [Google Scholar]
- 2.Stewart EA. Uterine fibroids. Lancet. 2001;357(9252):293‐298. [DOI] [PubMed] [Google Scholar]
- 3.Parazzini F, Tozzi L, Bianchi S. Pregnancy outcome and uterine fibroids. Best Pract Res Clin Obstet Gynaecol. 2016;34:74‐84. [DOI] [PubMed] [Google Scholar]
- 4.Donnez J, Dolmans M‐M. Uterine fibroid management: From the present to the future. Hum Reprod Update. 2016;22(6):665‐686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singh SS, Belland L, Leyland N, von Riedemann S, Murji A. The past, present, and future of selective progesterone receptor modulators in the management of uterine fibroids. Am J Obstet Gynecol. 2017:563–572. [DOI] [PubMed] [Google Scholar]
- 6.Donnez J, Donnez O, Dolmans MM. The current place of medical therapy in uterine fibroid management. Best Pract Res Clin Obstet Gynaecol. 2017:57–65. [DOI] [PubMed] [Google Scholar]
- 7.European Medicines Agency . Suspension of ulipristal acetate for uterine fibroids during ongoing ema review of liver injury risk. Press Release (2020): 25 March 2020. https://www.ema.europa.eu/en/documents/referral/ulipristal‐acetate‐5mg‐medicinal‐products‐article‐31‐referral‐review‐started_en.pdf [DOI] [PMC free article] [PubMed]
- 8.Sciences CfIOoM . Drug‐induced liver injury (dili): Current status and future directions for drug development and the post‐marketing setting. In: CIOMS , ed. CIOMS Working Group on Drug‐induced liver injury (DILI): consensus report. Geneva: CIOMS; 2020:160. [Google Scholar]
- 9.Zimmerman HJ. Hepatotoxicity. Dis Mon. 1993;39(10):675‐787. [PubMed] [Google Scholar]
- 10.Kaplowitz N. Chapter 1 ‐ drug‐induced liver injury: Introduction and overview. In: Kaplowitz N, DeLeve LD, eds. Drug‐induced liver disease (third edition). Boston: Academic Press; 2013:3‐14. [Google Scholar]
- 11.Aithal GP, Watkins PB, Andrade RJ, et al. Case definition and phenotype standardization in drug‐induced liver injury. Clin Pharmacol Ther. 2011;89(6):806‐815. [DOI] [PubMed] [Google Scholar]
- 12.de Abajo FJ, Montero D, Madurga M, Garcia Rodriguez LA. Acute and clinically relevant drug‐induced liver injury: A population based case‐control study. Br J Clin Pharmacol. 2004;58(1):71‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vega M, Verma M, Beswick D, et al. Drug Induced Liver Injury N: The incidence of drug‐ and herbal and dietary supplement‐induced liver injury: Preliminary findings from gastroenterologist‐based surveillance in the population of the state of delaware. Drug Saf. 2017;40(9):783‐787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Valle MB, Av Klinteberg V, Alem N, Olsson R, Bjornsson E. Drug‐induced liver injury in a swedish university hospital out‐patient hepatology clinic. Aliment Pharmacol Ther. 2006;24(8):1187‐1195. [DOI] [PubMed] [Google Scholar]
- 15.Meier Y, Cavallaro M, Roos M, et al. Incidence of drug‐induced liver injury in medical inpatients. Eur J Clin Pharmacol. 2005;61(2):135‐143. [DOI] [PubMed] [Google Scholar]
- 16.Bjornsson ES, Bergmann OM, Bjornsson HK, Kvaran RB, Olafsson S. Incidence, presentation, and outcomes in patients with drug‐induced liver injury in the general population of iceland. Gastroenterology. 2013;144(7):1419‐1425.e1411–1413; quiz e1419–1420 [DOI] [PubMed] [Google Scholar]
- 17.Mindikoglu AL, Magder LS, Regev A. Outcome of liver transplantation for drug‐induced acute liver failure in the united states: Analysis of the united network for organ sharing database. Liver Transpl. 2009;15(7):719‐729. [DOI] [PubMed] [Google Scholar]
- 18.Reuben A, Koch DG, Lee WM. Acute Liver Failure Study G: Drug‐induced acute liver failure: Results of a u.S Multicenter, prospective study. Hepatology. 2010;52(6):2065‐2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Donnez J, Tatarchuk TF, Bouchard P, et al. Ulipristal acetate versus placebo for fibroid treatment before surgery. N Engl J Med. 2012;366(5):409‐420. [DOI] [PubMed] [Google Scholar]
- 20.Donnez J, Tomaszewski J, Vazquez F, et al. Ulipristal acetate versus leuprolide acetate for uterine fibroids. N Engl J Med. 2012;366(5):421‐432. [DOI] [PubMed] [Google Scholar]
- 21.De Milliano I, Van Hattum D, Ket JCF, Huirne JAF, Hehenkamp WJK. Endometrial changes during ulipristal acetate use: A systematic review. Eur J Obstet Gynecol Reprod Biol. 2017;214:56‐64. [DOI] [PubMed] [Google Scholar]
- 22.Simon JA, Catherino W, Segars JH, et al. Ulipristal acetate for treatment of symptomatic uterine leiomyomas: A randomized controlled trial. Obstet Gynecol. 2018;131(3):431‐439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Donnez J, Vázquez F, Tomaszewski J, et al. Long‐term treatment of uterine fibroids with ulipristal acetate. Fert Ster. 2014;101(6):1565‐1573.e1518 [DOI] [PubMed] [Google Scholar]
- 24.Liu JH, Soper D, Lukes A, et al. Ulipristal acetate for treatment of uterine leiomyomas: A randomized controlled trial. Obstet Gynecol. 2018;132(5):1241‐1251. [DOI] [PubMed] [Google Scholar]
- 25.Donnez J, Arriagada P, Donnez O, Dolmans M‐M. Current management of myomas: The place of medical therapy with the advent of selective progesterone receptor modulators. Current Opinion in Obstetrics and Gynecology. 2015;27(6):422‐431. [DOI] [PubMed] [Google Scholar]
- 26.Middelkoop MA, Huirne JAF. Re: The past, present, and future of selective progesterone receptor modulators in the management of uterine fibroids. Am J Obstet Gynecol. 2018;219(4):424‐425. [DOI] [PubMed] [Google Scholar]
- 27.Middelkoop MA, Huirne JAF, van der Weide MCJ, Bosmans JE, Hehenkamp WJK. Myomex‐2 Trial G: A multi‐centre, randomized, non‐inferiority trial to compare ulipristal with standard surgical treatment in women with symptomatic uterine fibroids: Protocol of the myomex‐2 trial. Eur J Obstet Gynecol Reprod Biol. 2020;256:63‐69. [DOI] [PubMed] [Google Scholar]
- 28.Aantal uitgiftes 2014–2018 voor atc‐subgroep g03xb02: Ulipristal Zorginstituut Nederland/GIP. item=G03XB02
- 29.European Medicines Agency . Notification to the prac/ema secretariat of a referral under article 20 of regulation (ec)726/2004. Press Release 2017: 30‐11‐2017. https://www.ema.europa.eu/en/documents/referral/esmya‐article‐20‐procedure‐notification_en.pdf
- 30.Donnez J, Donnez O, Matule D, et al. Long‐term medical management of uterine fibroids with ulipristal acetate. Fertil Steril. 2016;105(1):165‐173.e164 [DOI] [PubMed] [Google Scholar]
- 31.European Medicine Agency . Esmya: New measures to minimise risk of rare but serious liver injury. Press Release (2018): 1 June 2018. http://www.ema.europa.eu/docs/en_GB/document_library/Referrals_document/Esmya_20/Opinion_provided_by_Committee_for_Medicinal_Products_for_Human_Use/WC500249905.pdf
- 32.GOV.UK . In: Agency MaHpR , ed. Esmya (ulipristal acetate) for uterine fibroids: Do not initiate or re‐start treatment; monitor liver function in current and recent users. Vol.11 Gov.UK; 2018. [Google Scholar]
- 33.GOV.UK . In: Agency MaHpR , ed. Esmya (ulipristal acetate) and risk of serious liver injury: New restrictions to use and requirements for liver function monitoring before, during, and after treatment. Vol.12 Gov.UK; 2018. [Google Scholar]
- 34.GOV.UK . In: Agency MaHpR , ed. Esmya (ulipristal acetate): Suspension of the licence due to risk of serious liver injury. Vol.13 Gov.UK; 2020. [Google Scholar]
- 35.European Medicines Agency. Assessment report on temporary measures esmya. Press Release (2020): 12 March 2020. https://www.ema.europa.eu/en/documents/referral/ulipristal‐acetate‐5mg‐medicinal‐products‐article‐31‐referral‐assessment‐report‐temporary‐measures_en.pdf
- 36.Referral procedures: ema.europa.eu
- 37.Livertox: Clinical and research information on drug‐induced liver injury [internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases, 2012. Available from: https://www.ncbi.nlm.nih.gov/books/NBK548196/ [PubMed]
- 38.Kang S, Brinker A, Jones SC, Dimick‐Santos L, Avigan MI. An evaluation of postmarketing reports of serious idiosyncratic liver injury associated with ulipristal acetate for the treatment of uterine fibroids. Drug Saf. 2020;43(12):1267‐1276. [DOI] [PubMed] [Google Scholar]
- 39.Hoofnagle JH, Bjornsson ES. Drug‐induced liver injury ‐ types and phenotypes. N Engl J Med. 2019;381(3):264‐273. [DOI] [PubMed] [Google Scholar]
- 40.Woodhead JL, Brock WJ, Roth SE, et al. Application of a mechanistic model to evaluate putative mechanisms of tolvaptan drug‐induced liver injury and identify patient susceptibility factors. Toxicol Sci. 2017;155(1):61‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Watkins PB, Lewis JH, Kaplowitz N, et al. Clinical pattern of tolvaptan‐associated liver injury in subjects with autosomal dominant polycystic kidney disease: Analysis of clinical trials database. Drug Saf. 2015;38(11):1103‐1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Khan MY, Rawala MS, Siddiqui M, Abid W, Aslam A. Tolvaptan‐induced liver injury: Who is at risk? A case report and literature review. Cureus. 2019;11(6):e4842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Endo M, Katayama K, Matsuo H, et al. Role of liver transplantation in tolvaptan‐associated acute liver failure. Kidney Int Rep. 2019;4(11):1653‐1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Risk evaluation and mitigation strategy (rems) document jynarque (tolvaptan) rems program. Press Release2018. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2018/204441Orig1s000REMS.pdf
- 45.Approved risk evaluation and mitigation strategies (rems): Tolvaptan: https://www.accessdata.fda.gov/scripts/cder/rems/index.cfm?event=IndvRemsDetails.page&REMS=380
- 46.Onakpoya IJ, Heneghan CJ, Aronson JK. Post‐marketing withdrawal of 462 medicinal products because of adverse drug reactions: A systematic review of the world literature. BMC Med. 2016;14(10):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.European Medicine Agency . Epar summary for the public: Thelin. Press Release (2010): 8–2010. https://www.ema.europa.eu/en/documents/overview/thelin‐epar‐summary‐public_en.pdf
- 48.European Medicine Agency . Thelin: Epar ‐ scientific discussion. Press Release 2007. https://www.ema.europa.eu/en/medicines/human/EPAR/thelin#product‐information‐section
- 49.Galie N, Hoeper MM, Gibbs JS, Simonneau G. Liver toxicity of sitaxentan in pulmonary arterial hypertension. Eur Respir J. 2011;37(2):475‐476. [DOI] [PubMed] [Google Scholar]
- 50.European Medicines Agency : Assessment report on temporary measures. Press Release 2020: 12 March 2020. https://www.ema.europa.eu/en/documents/referral/ulipristal‐acetate‐5mg‐medicinal‐products‐article‐31‐referral‐assessment‐report‐temporary‐measures_en.pdf
- 51.Bjornsson ES. Hepatotoxicity by drugs: The most common implicated agents. Int J Mol Sci. 2016;17(2):224–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sgro C, Clinard F, Ouazir K, et al. Incidence of drug‐induced hepatic injuries: A french population‐based study. Hepatology. 2002;36(2):451‐455. [DOI] [PubMed] [Google Scholar]
- 53.Zoubek ME, Pinazo‐Bandera J, Ortega‐Alonso A, et al. Liver injury after methylprednisolone pulses: A disputable cause of hepatotoxicity. A case series and literature review. United Eur Gastroenterol J. 2019;7(6):825‐837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Administration FUSFaD . In: (CDER) CfDEaR , (CBER) CfBEaR , eds. Guidance for industry drug‐induced liver injury: Premarketing clinical evaluation; 2009. [Google Scholar]
- 55.de Milliano I, Twisk M, Ket JC, Huirne JA, Hehenkamp WJ. Pre‐treatment with gnrha or ulipristal acetate prior to laparoscopic and laparotomic myomectomy: A systematic review and meta‐analysis. PLoS One. 2017;12(10):e0186158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.de Milliano I, Huirne JAF, Thurkow AL, et al. Ulipristal acetate vs gonadotropin‐releasing hormone agonists prior to laparoscopic myomectomy (myomex trial): Short term results of a double blind randomised controlled trial. Acta Obstet Gynecol Scand. 2019:89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Aarts JW, Nieboer TE, Johnson N, et al. Surgical approach to hysterectomy for benign gynaecological disease. Cochrane Database Syst Rev. 2015;8:CD003677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Clarke‐Pearson DL, Geller EJ. Complications of hysterectomy. Obstet Gynecol. 2013;121(3):654‐673. [DOI] [PubMed] [Google Scholar]
- 59.Forsgren C. Long‐term effects of hysterectomy: A focus on the aging patient. Aging Health. 2013;9(2):179‐187. [Google Scholar]
- 60.Forsgren C, Lundholm C, Johansson AL, Cnattingius S, Altman D. Hysterectomy for benign indications and risk of pelvic organ fistula disease. Obstet Gynecol. 2009;114(3):594‐599. [DOI] [PubMed] [Google Scholar]
- 61.Altman D, Granath F, Cnattingius S, Falconer C. Hysterectomy and risk of stress‐urinary‐incontinence surgery: Nationwide cohort study. Lancet. 2007;370(9597):1494‐1499. [DOI] [PubMed] [Google Scholar]
- 62.Wright JD, Ananth CV, Ojalvo L, et al. Failure to rescue after major gynecologic surgery. Am J Obstet Gynecol. 2013;209(5) 420:e421‐e428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Neis KJ, Zubke W, Fehr M, Romer T, Tamussino K, Nothacker M. Hysterectomy for benign uterine disease. Dtsch Arztebl Int. 2016;113(14):242‐249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fernandez H. Epidemiologie du fibrome uterin en france en 2010‐2012 dans les etablissements de sante ‐ analyse des donnees du programme medicalise des systemes d'information. J Gynecol Obstet Biol Reprod. 2014:616–628. [DOI] [PubMed] [Google Scholar]
- 65.Hehenkamp WJ, Volkers NA, Broekmans FJ et al. Loss of ovarian reserve after uterine artery embolization: A randomized comparison with hysterectomy. Hum Reprod. 2007; 22(7):1996–2005. [DOI] [PubMed] [Google Scholar]
- 66.Hirst A, Dutton S, Wu O, et al. A multi‐centre retrospective cohort study comparing the efficacy, safety and cost‐effectiveness of hysterectomy and uterine artery embolisation for the treatment of symptomatic uterine fibroids. The hopeful study. Health Technol Assess. 2008;12(5):1‐248.iii [DOI] [PubMed] [Google Scholar]
- 67.Carrillo TC. Uterine artery embolization in the management of symptomatic uterine fibroids: An overview of complications and follow‐up. Semin Intervent Radiol. 2008;25(4):378‐386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.de Bruijn AM, Ankum WM, Reekers JA, et al. Uterine artery embolization vs hysterectomy in the treatment of symptomatic uterine fibroids: 10‐year outcomes from the randomized emmy trial. Am J Obstet Gynecol. 2016;215(6):745 e741‐745 e712. [DOI] [PubMed] [Google Scholar]
- 69.Vashisht A, Studd J, Carey A, Burn P. Fatal septicaemia after fibroid embolisation. Lancet. 1999;354(9175):307‐308. [DOI] [PubMed] [Google Scholar]
- 70.Czeyda‐Pommersheim F, Magee ST, Cooper C, Hahn WY, Spies JB. Venous thromboembolism after uterine fibroid embolization. Cardiovasc Intervent Radiol. 2006;29(6):1136‐1140. [DOI] [PubMed] [Google Scholar]
- 71.Manyonda I, Belli AM, Lumsden MA, et al. Uterine‐artery embolization or myomectomy for uterine fibroids. N Engl J Med. 2020;383(5):440‐451. [DOI] [PubMed] [Google Scholar]
- 72.Agency EM. In: office EP , ed. Amsterdam Ulipristal acetate for uterine fibroids: Ema recommends restricting use; 2020. [Google Scholar]
- 73.Alexander SPH, Christopoulos A, Davenport AP, et al. The concise guide to pharmacology 2019/20: G protein‐coupled receptors. Br J Pharmacol. 2019;176(Suppl 1):S21‐S141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Alexander SPH, Cidlowski JA, Kelly E, et al. The concise guide to pharmacology 2019/20: Nuclear hormone receptors. Br J Pharmacol. 2019;176(Suppl 1):S229‐S246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Alexander SPH, Fabbro D, Kelly E, et al. The concise guide to pharmacology 2019/20: Enzymes. Br J Pharmacol. 2019;176(Suppl 1):S297‐S396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Alexander SPH, Kelly E, Mathie A, et al. The concise guide to pharmacology 2019/20: Transporters. Br J Pharmacol. 2019;176(Suppl 1):S397‐S493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bjornsson E, Jerlstad P, Bergqvist A, Olsson R. Fulminant drug‐induced hepatic failure leading to death or liver transplantation in sweden. Scand J Gastroenterol. 2005;40(9):1095‐1101. [DOI] [PubMed] [Google Scholar]
- 78.Pundir J, Krishnan N, Siozos A, et al. Peri‐operative morbidity associated with abdominal myomectomy for very large fibroid uteri. Eur J Obstet Gynecol Reprod Biol. 2013;167(2):219‐224. [DOI] [PubMed] [Google Scholar]


