Abstract
Introduction
Although the measurement of physical activity (PA) amongst people with haemophilia (PWH) has become increasingly widespread in recent years, the relationship between PA and bleeding phenotype remains poorly understood. In addition, the influence of various treatment regimens on this relationship has not been defined.
Aim
This review aimed to systematically assess the data that are available regarding PA levels amongst PWH, as well as the relationship between PA and bleeding.
Methods
A systematic search of the online databases EMBASE, Cochrane, MEDLINE Ovid, CINAHL and Web of Science was conducted by two independent reviewers. Quality assessment was undertaken using the AXIS Critical Appraisal Tool for Cross‐sectional Studies and the STROBE checklist.
Results
Of 1902 sources identified overall, 36 articles were included. Low‐to‐moderate transparency of reporting and various sources of bias were identified. PA levels varied amongst heterogeneous samples of PWH. The relationship between PA and bleeds was inconclusive, although there was evidence that improvements in treatment over recent decades have appeared to enable PWH to become more physically active.
Conclusion
Based upon the limited available evidence, the relationship between PA and bleeding phenotype in PWH remains unclear. However, with the development of improved prophylaxis treatment regimens in recent years, there is evidence that PA levels have increased, especially amongst people with severe haemophilia. The use of validated outcome measures of PA and more robust reporting of bleeds and treatment regimen are warranted in future research, especially in a rapidly evolving era of new treatments for PWH.
Keywords: bleeding, exercise, haemophilia, physical activity, prophylaxis, treatment
1. INTRODUCTION
The improvement in treatments over recent decades has increased the life expectancy of people with haemophilia (PWH) to be similar to that of the general population.1, 2 PWH were previously discouraged from leading a physically active lifestyle due to the perceived increased risk of bleeding.3, 4 However, the introduction of clotting factor concentrates (CFCs) has led to a change in attitude towards physical activity (PA).5 PA and exercise (generally of low impact and risk) are now recommended for PWH, and there has been some evidence to suggest it may reduce the incidence of bleeds and improve joint integrity.6, 7
Adequate levels of PA can reduce the risk of hypertension and type 2 diabetes and contribute towards weight maintenance or loss.8 These risk factors, including insufficient levels of PA, are amongst the leading causes of global mortality attributable to the development of non‐communicable diseases such as cardiovascular disease and certain types of cancer.9 An increase in cardiometabolic risk factors and disease, including hypertension and obesity, is becoming more prevalent in PWH.2, 10, 11 The potential for PA to aid the treatment and management of these comorbidities is becoming more pertinent in the context of chronic health, particularly in the ageing population with haemophilia. International PA guidelines recommend that aerobic PA of moderate intensity for 150–300 minutes per week (min/week) or vigorous intensity for 75–150 min/week, either combined or alone, results in substantial health benefits for adults in a dose‐response manner.8, 12
Although the life expectancy of PWH has increased, and some individuals may bleed less frequently than previously, the pain and disability of chronic haemophilic joint arthropathy (HJA) still persists for many with moderate‐to‐severe haemophilia, which negatively affects physical function and quality of life.13 Furthermore, the risk of bleeds and fear of joint damage have been identified as barriers to being active for some PWH.14, 15
Bleeding phenotype itself has been shown to vary even amongst people with severe haemophilia (PWSH) who, despite having factor VIII (FVIII) or factor IX (FIX) levels <0.01 IU/ml, approximately 10–15% exhibit a milder spontaneous bleeding tendency and lower usage of CFC.16 Further complicating phenotypic evaluation, the age of first joint bleed, pharmacokinetics of CFC clearance and development of HJA have also been shown to vary amongst PWSH.16, 17 Bleeding phenotype may be influenced by genetic factors, PA levels and obesity 16; however, the true relationship between bleeds and PA volume (ie frequency, intensity, type and duration) is not fully understood.
An accurate understanding of the relationship between bleeds, PA and the influence of treatment regimen on this relationship could be beneficial to PWH, in order to identify safe and optimal levels of PA without increasing the risk of bleeds. The primary objective of this review was to determine levels of PA amongst PWH. Secondary objectives were to determine current evidence of (1) the relationship between PA and bleeds in PWH and (2) the influence of treatment regimen on this relationship.
2. METHODS AND MATERIALS
2.1. Protocol and registration
The review protocol was registered with the National Institute of Health Research, International Prospective Register of Systematic Reviews (PROSPERO ID: CRD42018110106).
2.2. Search strategy
A search strategy including MeSH terminology related to “physical activity”; “exercise”; “h(a)emophilia”; “bleed”; “h(a)emorrhage”; and “h(a)emarthrosis” was created and tailored to each online database by a subject librarian (Appendix S1). The online databases of EMBASE, Cochrane, MEDLINE Ovid, CINAHL and Web of Science were searched between February and March 2018. The same search was updated in December 2020. A manual search of reference lists of relevant articles was also conducted.
2.3. Eligibility criteria
Studies were selected using PECOS criteria (Table 1) which was modified from the original PICOS (Participants, Intervention (Exposure), Comparators, Outcomes, Study Design) framework for formulating the research question, as per the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) guidelines.18 Both children and adults with mild, moderate or severe FVIII or FIX deficiency were included. Outcomes included any measurement of PA and any reporting of bleeds, where available. Any information on treatment regimen, where available, was viewed as the exposure which may influence the outcomes described. Interventional exercise/PA studies were not included as they influenced PA levels instead of capturing habitual PA. Only full‐text publications available in English were considered, and no date restrictions were placed on the search.
TABLE 1.
PECOS criteria
| Inclusion | Exclusion | |
|---|---|---|
| Participants |
|
|
| Exposures |
|
|
| Comparators |
|
|
| Outcomes |
|
|
| Study design |
|
|
2.4. Study selection and data extraction
Study screening and selection were performed by two independent reviewers. Conflicts were resolved via discussion with a third reviewer where necessary. Where full texts were unavailable, authors were contacted if correspondence details were available. Data were extracted using a standardised template pertaining to the PECOS criteria. Results were reported using a narrative synthesis.
2.5. Quality appraisal and risk of bias
The AXIS Critical Appraisal Tool for Cross‐sectional Studies was assessed by two independent reviewers.19 Conflicts were resolved via discussion with a third independent reviewer if necessary. Risk of bias was analysed and reported using a narrative synthesis. Quality and transparency of reporting was analysed by one reviewer using the ‘Strengthening the Reporting of Observational Studies in Epidemiology’ (STROBE) checklist and guidelines. STROBE is a standardised checklist of 22 items related to transparent reporting of observational studies.20 Four items were removed from the checklist as they were not applicable across the majority of studies, resulting in the highest score achievable being 30. These included items 6(b), 12(e), 14(c) and 16(c) related to reporting of participants in matched studies, sensitivity analysis, follow‐up time of cohort studies and the translation of estimates of relative risk to absolute risk for a meaningful time period, respectively. A ‘Completeness of Reporting’ (COR) score was calculated for each study. Higher scores indicated better transparency and quality of reporting. Mean ±standard deviation of COR score was calculated.
3. RESULTS
3.1. Study selection
The online search identified a total of 1902 sources (after duplicates), and seven additional articles were identified from the manual search.21, 22, 23, 24, 25, 26, 27 After the inclusion and exclusion criteria were applied, 36 articles were eligible.14, 15, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54 A PRISMA flow diagram of the screening and selection process is provided in Figure 1.
FIGURE 1.
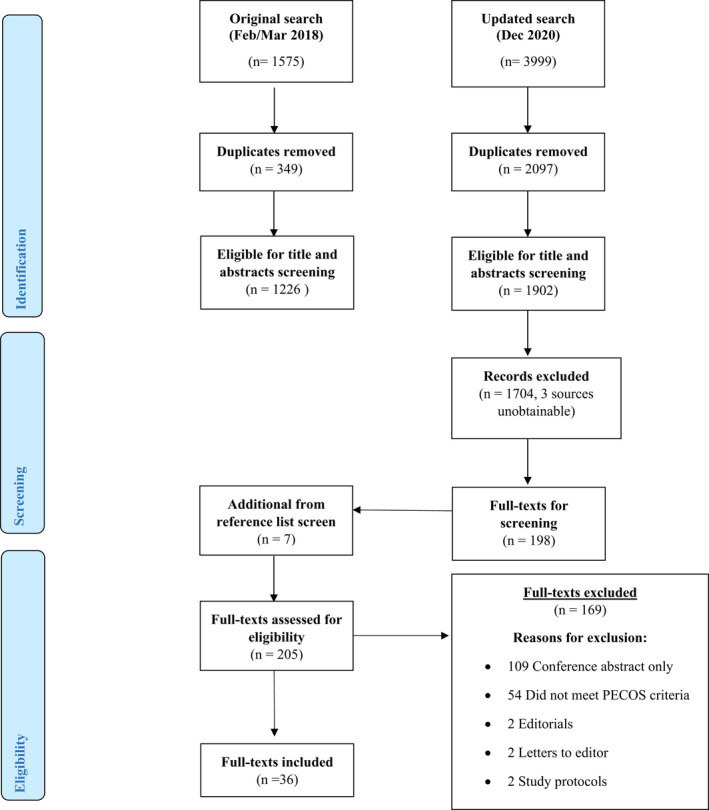
PRISMA flow diagram of systematic search
3.2. Quality appraisal and risk of bias
According to the STROBE analysis, the average COR score was 12 (±4.6) indicating low‐to‐moderate transparency and quality of reporting (Supporting Information: STROBE analysis). The Axis tool appraisal identified moderate‐to‐high risks of bias amongst studies (Table 2). Considerable rates of selection bias were evident due to convenience sampling methods, a lack of specificity in defining target populations in 32 studies 14, 15, 21, 49, 50, 52, 53, 54 and unclear inclusion and exclusion criteria in 21 studies.14, 15, 34, 36, 37, 38, 39, 40, 41, 43, 49, 50 Non‐response bias was also suspected for all except four studies,21, 33, 42, 51 methods to address non‐response were described by four studies 40, 47, 49, 53 and characteristics of non‐responders were provided in four studies.23, 40, 47, 49 Non‐specific reporting of psychometric properties of measurement tools (many which had not been validated in PWH), the lack of declaration of potential confounders and the majority use of self‐report methods raised concerns of measurement, social desirability and recall bias. Evidence of potential selective reporting was suspected in 13 studies as data were either not fully presented, or additional data which were not clearly described a priori in the methods were presented.21, 46, 51, 53, 54 How missing data were managed was also unclear in 20 studies.21, 22, 41, 42, 43, 47, 49, 50, 51, 53
TABLE 2.
AXIS critical appraisal
| Introduction | Janco et al. (1996)28 | Heijnen et al. (2000)21 | Van der Net et al. (2006) 29 | Nazzaro et al. (2006)30 | Fromme et al. (2007)31 | Tlacuilo‐Parra et al. (2008)22 | Tiktinsky et al. (2009)32 | Koiter et al. (2009)23 | Ross et al. (2009)33 | Sherlock et al. (2010)34 | Khawaji et al. (2010)35 | Buxbaum et al. (2010)36 | Groen et al. (2011)24 | Gonzalez et al. (2011)37 | Khair et al. (2012)38 | Broderick et al. (2012)39 | Baumgardner et al. (2013)40 | den Uijl et al. (2013)41 | Broderick et al. (2013)25 | Niu et al. (2014)26 | McGee et al. (2015)42 | Von Mackensen et al. (2016)27 | Cuesta‐Barriuso et al. (2016)43 | Bouskill et al. (2016)44 | Carneiro et al. (2017)45 | Baumann et al. (2017)14 | Flaherty et al. (2018)15 | Kempton et al. (2018)46 | Pinto et al. (2018)47 | Pinto et al. (2018)48 | Versloot et al. (2019)49 | Goto et al. (2019)50 | Zanon et al. (2020)51 | Timmer et al. (2020)52 | Taylor et al. (2020)53 | Bérubé et al. (2020)54 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Q1. | N | Y | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | Y | Y | N | Y | Y | Y | N | Y | Y | Y | Y | Y | Y | N | Y | N | Y | Y | Y | Y | Y | ||
| Methods | ||||||||||||||||||||||||||||||||||||||
| Q2. | Y | * | Y | Y | Y | Y | Y | Y | Y | Y | * | * | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | * | Y | Y | Y | * | ||
| Q3. | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | Y | N | N | N | N | N | N | N | Y | Y | N | N | N | N | N | N | N | N | N | N | N | ||
| Q4. | N | Y | Y | N | N | N | Y | Y | Y | N | Y | N | N | N | Y | Y | N | N | Y | Y | Y | Y | N | Y | Y | Y | N | N | Y | Y | Y | N | Y | N | Y | Y | ||
| Q5. | N | N | N | N | N | N | N | Y | N | Y | N | N | N | N | N | N | Y | N | N | N | N | N | N | N | N | N | N | N | Y | Y | N | N | Y | N | N | N | ||
| Q6. | N | N | N | N | N | N | Y | Y | Y | N | Y | N | N | N | N | N | N | N | N | Y | Y | N | N | Y | Y | N | N | Y | Y | Y | N | N | Y | Y | Y | Y | ||
| Q7. | N | NA | N | N | N | N | N | N | NA | N | N | N | N | N | N | N | Y | N | N | N | NA | N | N | N | N | N | N | N | Y | N | Y | N | N | N | Y | N | ||
| Q8. | N | N | Y | N | N | Y | Y | Y | N | Y | Y | N | Y | Y | Y | N | Y | Y | Y | Y | N | Y | Y | Y | Y | N | Y | Y | N | N | Y | Y | N | Y | N | N | ||
| Q9. | N | N | Y | N | N | Y | Y | Y | N | Y | Y | N | Y | Y | Y | N | Y | Y | N | Y | N | Y | Y | Y | Y | N | N | Y | N | N | Y | Y | N | Y | N | N | ||
| Q10. | N | N | Y | N | N | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | N | Y | N | Y | Y | N | Y | NA | N | Y | NA | Y | Y | Y | Y | N | Y | Y | ||
| Q11. | N | N | Y | N | N | Y | Y | Y | N | Y | N | Y | Y | Y | Y | Y | Y | N | N | Y | Y | Y | N | Y | Y | N | N | N | Y | Y | Y | N | Y | Y | Y | Y | ||
| Results | ||||||||||||||||||||||||||||||||||||||
| Q12. | N | N | Y | Y | N | Y | Y | Y | Y | Y | N | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | Y | N | N | Y | N | ||
| Q13. | Y | N | Y | Y | Y | Y | Y | Y | NA | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | NA | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | Y | Y | Y | ||
| Q14. | N | NA | N | N | N | N | N | Y | NA | N | N | N | N | N | N | N | Y | N | N | N | NA | N | N | N | N | N | N | N | Y | N | Y | N | N | N | N | N | ||
| Q15. | N | N | N | N | Y | N | N | Y | N | Y | N | N | Y | N | N | Y | Y | N | Y | Y | N | N | N | Y | Y | Y | Y | Y | N | Y | N | N | N | Y | N | Y | ||
| Q16. | N | N | N | Y | N | N | Y | Y | Y | Y | Y | N | Y | Y | N | Y | Y | Y | N | Y | Y | N | Y | Y | Y | Y | Y | N | Y | Y | Y | Y | N | Y | N | N | ||
| Discussion | ||||||||||||||||||||||||||||||||||||||
| Q17. | N | N | Y | Y | N | Y | Y | Y | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | Y | N | N | Y | Y | Y | Y | Y | N | N | Y | Y | Y | ||
| Q18. | N | Y | Y | Y | N | N | N | Y | Y | Y | N | Y | Y | Y | Y | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | Y | Y | Y | Y | ||
| Other | ||||||||||||||||||||||||||||||||||||||
| Q19. | Y | N | * | N | * | N | N | Y | * | N | N | Y | Y | N | N | Y | N | N | N | Y | N | Y | * | N | Y | Y | N | Y | N | N | Y | N | N | N | N | N | ||
| Q20a. | N | N | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | N | Y | Y | Y | Y | N | Y | Y | Y | Y | Y | ||
| Q20b. | Y | N | N | Y | N | Y | Y | Y | Y | Y | N | Y | Y | Y | Y | Y | Y | N | Y | N | N | Y | Y | Y | Y | N | N | Y | Y | Y | Y | N | Y | N | Y | Y | ||
Unable to Determine; ‘N’ = No; ‘NA’ = Not applicable; ‘Y’ = Yes. Q1. Were the aims/objectives clear? Q2. Was the study design appropriate for the stated aim (s)? Q3. Was the sample size justified? Q4. Was the target/reference population clearly defined? (Is it clear who the research was about?) Q5. Was the sample frame taken from an appropriate population base so that it closely represented the target/reference population under investigation? Q6. Was the selection process likely to select subjects/participants that were representative of the target/reference population under investigation? Q7. Were measures undertaken to address and categorise non‐responders? Q8. Were the risk factor and outcome variables measured appropriate to the aims of the study? Q9. Were the risk factor and outcome variables measured correctly using instruments /measurements that had been trialled, piloted or published previously? Q10. Is it clear what was used to determine statistical significance and/or precision estimates? (eg p‐values, confidence intervals) Q11. Were the methods (including statistical methods) sufficiently described to enable them to be repeated? Q12. Were the basic data adequately described? Q13. Does the response rate raise concerns about non‐response bias? Q14. If appropriate, was information about non‐ responders described? Q15. Were the results internally consistent? Q16. Were the results presented for all the analyses described in the methods? Q17. Were the authors’ discussions and conclusions justified by the results? Q18. Were the limitations of the study discussed? Q19. Were there any funding sources or conflicts of interest that may affect the authors’ interpretation of the results? Q20a. Was ethical approval attained? Q20b. Was consent of participants attained?
3.3. Participants
Data on PA were available for 3185 PWH. Approximately 1361 children and adolescents (0–18 years) and 1791 adults (18–85 years) were represented (estimated numbers due to heterogeneity of age category boundaries and four studies 23, 24, 46, 53 reported demographics of the total sample and not just those included in the PA analysis). Mixed samples of children, adolescents and adults with haemophilia were included in 10 studies,14, 51 with children and/or adolescents only included in 15 studies,22, 23, 24, 25, 42, 43, 44, 45, 54 and 11 studies involved adults only.15, 52, 53
Approximately 1701 and 887 participants had FVIII and FIX deficiency, respectively (estimated numbers as four studies did not report on type of haemophilia 24, 30, 31, 34 and four others did not provide numbers of FVIII versus FIX deficiency for PA data 21, 36, 41, 52). FVIII deficiency only was considered in five studies,15, 22, 29, 37, 51 whilst FIX deficiency only was considered in two.14, 26 Both types of haemophilia were included in 25 studies.21, 23, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 52, 53, 54
Approximately 732, 676 and 1876 people with mild, moderate and severe haemophilia were represented, respectively (estimated numbers as three studies did not provide a breakdown of mild versus moderate 26, 44, 52). Six studies focused on severe haemophilia only,29, 32, 35, 49, 51, 54 whilst five focused on moderate or severe haemophilia.25, 33, 39, 41, 45 The remaining 24 studies included all severities.14, 15, 30, 31, 34, 36, 37, 38, 40, 42, 43, 44, 46, 47, 48, 50, 52
3.4. PA in PWH
3.4.1. Self‐report of PA
PA was assessed using various self‐report methods including diaries, surveys, questionnaires and interviews in 11 studies.14, 48, 54 Full breakdown of PA levels was not reported by four studies, although activity was categorised as ‘strenuous’ 28 or by ‘risk’ of PA 33, 39, 54 and was investigated in the context of bleeds.
Lower levels of PA were found in children with mixed severities of FVIII deficiency compared to children without haemophilia in the study by Tlacuilo‐Parra et al.,22 whilst the study by van der Net et al.29 found variable levels of PA in children with severe FVIII deficiency compared with national guidelines (60%–180%). Five remaining studies reported on PA in children and/or adults with haemophilia; however, levels were not compared with normative data or guidelines 14, 15, 30, 47, 48 (Table 3).
TABLE 3.
Study sample characteristics, PA outcome measures and main findings
| Author and sample size | Age (years) | Type | Severity | PA outcome | Main findings | |
|---|---|---|---|---|---|---|
|
Janco et al. (1996)28 n = 96 |
4–17 (range) | Both | All | 6‐month daily checklist of PA, that is any strenuous or out of school activity; time spent with friend for 30 min not at school; house‐hold tasks. | Higher clotting factor levels reported higher levels of strenuous PA (p < .04). When controlled for factor level, higher levels of strenuous activity had higher rates of spontaneous joint bleeding (p < .05) and higher rates of trauma‐related soft tissue bleeding (p < .03) | |
|
Heijnen et al. (2000)21 n = 293 |
<6 to >29 (range) | Both | All | Self‐administered PA questionnaire. | Participated in 1+ sports: 74%; ‘Not active’ = 26%; Sev: ‘Active’ = 71% ‘Not active’ = 29%; ‘As active’ as Dutch male population (survey in 1990/91 ‐ 32% ‘not active’, 45% were semi‐active and 23% were active). | |
|
Van der Net et al. (2006)29 n = 13 |
6.6a (range 8–14.6) | FVIII | Sev | Self‐report: Hrs of PA at home; school; extra‐curricular sports; leisure time in 1 wk. | 245 (133.2; range: 90–540)a mins/wk, that is Between ±60% and ±180% of the Dutch PA guidelines (Moderate PA for at least 420 mins/wk, including twice a week vigorous sport activities.). | |
|
Nazzaro et al. (2006)30 n = 110 |
16.7a (range 13–21) | NS | All | Survey: 2 questions adapted from IPAQ on strenuous PA and 30 mins moderate PA over 1 wk. | Avoided or limited PA: 60%; Exercised as a preventative measure: 27%; Did not engage in regular strenuous/moderate PA: 27%. | |
|
Fromme et al. (2007)31 n = 71 i.e. 44 youths, 27 adults |
Youths 10.2 ± 3a Adults 29.2 ± 12.5a |
NS | All |
Self‐administered questionnaire (everyday activities/school sports/leisure sports) |
Regular participation in school sports: 79.6% youths; 37% adults did during school days (significant at p < .05). Excused due to risk of injury: 33.3% adults; 13.6% youths Youths: 88.6% performed one or more leisure sports; Adults: 66.7% performed one or more leisure sports. |
|
|
Tlacuilo‐Parra et al. (2008)22 n = 62 x 2 (HG and CG) |
HG 9.02 ± 3.7a CG 9.3 ± 3.7a |
FVIII | All | Self‐report on PA and inactivity (h/day spent in PA). |
HG vs. CG: Grouped sedentary and low PA significant; Inactive: 77% vs. 51% Sedentary: 33% vs. 11% Low PA: 44% vs. 40%; (grouped‐ p = .003, OR 3.24, 95% CI, 1.36–7.79); Moderate PA: 23% vs. 38%; Intense PA: 0 vs. 11%. |
|
|
Tiktinsky et al. (2009)32 n = 44 |
18±5a (range 12–25) | Both | Sev | G&SQ, 1 unit = Minimum 15 mins exercise outside PE and not associated with organised athletics. | Strenuous PA at least once/wka: 56.8%; 5.0 ± 6.9 units/wk; Moderate PAa: 4.5 ± 6.9 units/wk; Mild PAa: 3.0 ± 4.3 units/wk; G&SQ total scorea: 77.9 ± 80.2 i.e. 9 METS (strenuous units/wk) +5 METS (moderate units/wk) +3 METS (mild units/wk). | |
|
Koiter et al. (2009)23 n = 99 |
12.6a (range 8–18) | Both | All | The Movement and Sport Questionnaire: 12 questions on participation in PE, sports and active lifestyle and list 3 sports (including duration and freq/wk). |
1 sport minimum: All 99; 2+ sports: 80 (81%); Freq/wk: 5 ± 3.2a; Soccer (42%); swimming (22%); tennis (21%); gymnastics (13%); cardio‐fitness (13%). |
|
|
Ross et al. (2009)33 n = 37 |
6–21 (range) | Both | Sev | Medical chart audit of athletic participation with telephone interview if data missing regarding PA type, prophylaxis use and injuries. | Athletic activities were organised and supervised by adults; occurred at least x2/7, minimum 30 mins of PA. Athletic activities classified by likelihood of impact by NHF: High impact PA: 73%; Low impact PA: 27%. | |
|
Sherlock et al. (2010)34 n = 61 |
38a (range 16–63) | NS | All | IPAQ and questionnaire regarding participation in sport. | High PA: 46%; Moderate PA: 28%; Low PA: 16%; Sport: 51%; Moderate mins/wka: 152.7 (±167.2); Vigorous mins/wka: 141.1 (±145.6); Walking mins/wka: 444 (±156.5); Sitting mins/wka: 2262 (±1326.8); Half as much time in moderate and vigorous PA vs. EU average. | |
|
Khawaji et al. (2010)35 n = 30 |
30.5a (range 20–57) | Both | Sev | MAQ | Weight‐bearing PA: 96.6%; Vigorous PA: 56.6% (eg jogging, wood chopping, hunting); Non‐weight‐bearing PA: 60% (eg cycling, swimming, strength); Leisure walking: 63.3%; 4+ physical activities: 80%. | |
|
Buxbaum et al. (2010)36 n = 62 i.e. HG (17); CG (44) |
HG:13.71 ± 2.1a CG:13.28±2a |
Both (only FIX in severe group) | All | Biaxial accelerometer (ActiTraC; IM systems, Baltimore, MD, USA) on waist for 7 consecutive days. | HG vs. CG PA (h/wk)a: Low: 70.24 (±7.1) vs.75.0 2 (±6) [p = .010]; Moderate: 18.35 (±3.4) vs. 15.89 (±3.3) [p = .012]; High: 11.44 (±6.3) vs. 9.13 (±3.8) [p = .086]; Vigorous: 1.96 (±2.6) vs. 1.54 (±1.4) [p = .409]; Both spent >70%/day sedentary. | |
|
Groen et al. (2011)24 n = 36 |
12.5 ± 2.9a (range 8.2–17.4) | NS | All | MAQ compared with data from a previous study of the general Dutch population. |
1+ activities at competitive level: 83%; Met guidelines (1‐hr moderate PA/5‐8 METs/day): 27.8% (vs. 21% in general population); Inactivity: 8% (vs. 12% in general population). |
|
|
González et al. (2011)37 n = 66 i.e. HG (41); CG (25) |
HG:12.78 (0.48)a SEM CG:15.9 (0.18)a SEM |
FVIII | All |
Triaxial accelerometer (ActiGraph GT3X, Fort Walton Beach, FL, USA) on right hip for 7 consecutive days. |
HG vs. CG PA (mins/day) a SEM: Sedentary: 356.78 (16.6) vs. 479.41 (19.62) [p <.001] Light: 450.24 (18.68) vs. 479.41 (19.62) [p <.001]; Moderate: 8.48 (1.15) vs. 3.36 (0.86) [p = .001]; Vigorous: 0.25 (0.06) vs. 0.41 (0.10) (not significant); MVPA: 8.74 (1.19) vs. 3.77 (0.88) [p = .001]; Total PA (counts/min):652.63 (33.74) vs. 430.82 (30.63) [p <.001]. |
|
|
Khair et al. (2012)38 n = 84 |
11.52 ± 3.4a (range 5.83–17.86) |
Both | All | Questionnaire regarding sporting activities (freq and duration of sport/wk) |
Participation in sport: 90.5%; Number of sports per person: 4a; With friends: 80%; At school: 80%; Team/club sports: 40%; Golf course/gym: 50%; Total h/wka: 4.9 (range 1–13); 1 hr/wk: 2.6%; 2–5 h/wk: 59.2%; 6–9 h/wk: 35.5%; 10–13 h/wk: 2.6%; Freq/wk: x1: 21.1% x2: 48.7% x3 27.6% x > 3: 2.6%. |
|
|
Broderick et al. (2012)39 n = 104 |
9.5±4a (range 4–18) |
Both | Mod/Sev | Self‐reported PA 3 days before a bleed, PA categorised by risk of collision using NHF criteria. PA in 8‐h immediately before the bleed and two 8‐hr windows at 24 and 48 h before the bleed. |
Interviews conducted for 329 bleeds, there was exposure to: C2 ‐ Significant collisions might occur, for example basketball: 30.6% of bleed windows‐ 24.8% 1st control windows, 21.4% 2nd; C3‐ Significant collisions inevitable, for example wrestling: 7.0% of bleed windows‐ 3.4% 1st control windows, 4.6% 2nd. |
|
|
Baumgardner et al. (2013)40 n = 88 |
41 (31.9–52.4)b | Both | All | Framingham PAI | PAI score: 30.8 (27.7–35.8)b; ‘Active’ (score >38): 14%; ‘Sedentary’ (score <28): 25% | |
|
den Uijl et al. (2013)41 n = 199 i.e. HG (94); CG (105) |
HG 25–27 (20–33)b CG 24 (20–31)b |
Both | Mod/Sev | IPAQ and a self‐designed sport list specifying type of participation in sport during the preceding year. |
IPAQ results (METs)b: HG = 3276 (960–8640) vs. CG = 3023 (1493–6936) (p = .26). Participation in sport: HG: Sev: 47 (59%); Mod: 28 (70%) vs. CG: 92 (88%) (p < .01) High‐risk sport: HG: Sev: 27 (34%); Mod: 20 (50%) vs. CG: 64 (61%) (p < 0.01). |
|
|
Broderick et al. (2013)25 n = 104 (66 prospective diaries) |
9.5±4a (range 4–18) |
Both | Mod/Sev | MAQ (METs/wk for past year) and a random 1‐week prospective record of PA during year. PA categorised by risk of collision using NHF criteria. |
Total leisure‐time PAb: 7.9 (4.6–13.0) h/wk; Vigorous PA (>6 METs)b: 3.8 (1.6–6.4) h/wk; MVPA(>3METs)b: 6.4 (3.7–10.0) h/wk; 1 sport minimum: 45% for all and 61% for boys >10 years; Inactivity/day: 20.7 h (86.3%); C2 or C3 PA: 1.5 h (6.3%). Less than half met guidelines (43%) (less than children without haemophilia−57–67%). |
|
|
Niu et al. (2014)26 n = 122 (Adults‐ IPAQ (n = 69): children CPAQ (n = 53) |
5–14: 9.6 ± 2.6a 15–64:35.2 ± 15.5a |
FIX | All | IPAQ and CPAQ (parental proxy report). |
IPAQ: High PA: 62%; Moderate PA: 29%; Low PA: 9%; Walkingb: 210 mins; 79% achieved PA guidelines of 75–150 mins/wk MVPA. |
CPAQ: No engagement in PA = 2 (n). 79% of parents reported their child participated in PA on at least 4 days/wk. |
|
McGee et al. (2015)42 n = 48 |
14.3 ± 2.6a (range 10–18.8) | Both | All | Chart review of participation in organised sport (ie participating in sport at least x2/wk for 30 mins). PA categorised by risk of collision using adapted NHF criteria. |
Sport participation 1 season minimum of organised sport: 62.5% (30) Basketball: 12/30; Hockey: 2/30 (against the advice of the haemophilia treatment team); Number of sport participated in: 1 (0–3)c. |
|
|
von Mackensen et al. (2016)27 n = 50 |
35.12 ± 14.7a (range 17–66) |
Both | All | Questionnaire regarding sports (freq and duration/wk). | Participation in sport: 64%; Number of sports per person: 2a; With friends: 81.3%; Team/club sports: 37.5%; Golf course/gym: 50%; Total h/wk: 3.71 ± 1.7a‐ 1 hr: 12.5%; 2–3 h: 34.4%; 4 h: 25%; 5–8 h: 28.1%; Freq/wk: x1/7: 25% x2/7: 53.1% x3/7 18.8% x4/7: 3.1%. | |
|
Cuesta‐Barriuso et al. (2016)43 n = 104 i.e. HG (53); CG (51) |
HG 10.08 ± 1.36a CG 9.78 ± 1.22a |
Both | All | Participation in sport. | Days practicing sports (days/wk)a ( a ):1.29; HG: 1.81 ± 1.75 (0–5); CG: 2.18 ± 1.22 (0–5). No significant differences between groups; Sports played included swimming, cycling, tennis and football. | |
|
Bouskill et al. (2016)44 n = 66 |
11.52 ± 3.99a |
Both | All | Triaxial accelerometer (ActiGraph GT3X, ActiGraph Corp, Pensacola, FL, USA) on right hip for 7 consecutive days and 3DPAR. | ActiGraph data (mins/day) a: Sedentary: Sev: 633.4 (± 121.3); Mild/mod: 327 (±78.73); MVPA: Sev: 48 (±20); Mild/mod: 55 (±18); 3DPAR (METs/day) a: Sev: 3.54 (±2.17); Mild/mod: 4.71 (±2.86). Close to meeting guidelines of 1 hr/day. | |
|
Carneiro et al. (2017)45 n = 100 i.e. BrG (50); CaG (50) |
BrG 13 ± 2.9a CaG 12.1 ± 2.8a |
Both | Mod/Sev | IPAQ |
IPAQ vigorous METsb [BrG (n−10) vs. CaG]: 480 (960) vs. 1200 (3120) (p = .0017). Overall activity BrG vs. CaG (n): High 18 vs. 28 (p = 0.0045); Moderate 13 vs. 16; Low 9 vs. 6. |
|
|
Baumann et al. (2017)14 n = AG (299; 89/299 female); ChG (150; 29/150 female) |
AG 29 (18–70)c ChG 10 (0–18)c |
FIX | All | Survey on participation in recreational activities accounting for severity and treatment regimen, intensity and duration of activities. |
Most common current recreational activities: AG: walking (44%), dancing (26%), fishing (19%), and bicycling (16%); ChG: walking (49%), swimming (18%), bicycling (11%), jogging/running (11%), and martial arts (8%); *Intensity and mean/median duration of PA provided in article. |
|
|
Flaherty et al. (2018)15 n = 14 |
48a (range 24–77) |
FVIII | All | Semi‐structured interviews in person or by phone. | −11 reported daily PA, 2 reported being mostly sedentary; 2 reported current PA reduced from normal/desired routine due to injury—walking most common type of PA reported; 6 reported regular exercise, average 5 days/wk, 4 daily; large variety including walking, running, fitness class, cycling, hiking, kayaking, etc.; 8 infrequently exercised. | |
|
Kempton et al. (2018)46 n = 339 (IPAQ completed)/381 |
34 (26.3, 47.2)b |
Both | All | IPAQ |
166 (49.0%) reported PA in previous wk; Duration (mins/wk)b: walking: 60 (30, 240); moderate PA: 90 (60, 180); vigorous PA: 105 (60, 180); MET (mins/wk)b: walking: 346.5 (198.0, 660.0); moderate PA: 360.0 (160.0, 600.0); vigorous PA: 960.0 (360.0, 3360.0). |
|
|
Pinto et al. (2018)47 n = 146 AG (106); CTG (21); CPrG (6‐9y n = 11, 1‐5y n = 8) |
AG 43.49 (13.89)a CTG 14.00 (2.39)a ChG:6‐9y: 7.73 (1.01)a; 1‐5y: 3.38 (1.60)a |
Both | All | PA questionnaire which collected information on PA and sport participation. |
Regular participation (n): AG = 29 (27.4%), swimming (16), walking (5), cycling (3); CTG = 12 (57.1%), swimming (5), football (3), dance (2), gym (2); ChG 6‐9y = 9 (81.8%), swimming (7), hockey (1), dance (1); ChG 1‐5y = 4 (50.0%), swimming (4), football (1). |
|
|
Pinto et al. (2018)48 n = 102 |
43 (18–74)c | Both | All | Questionnaire on either regular or occasional PA (freq and types of PA). | 65 (63.7%) practiced PA, no detail on frequency and type provided in article. | |
|
Versloot et al. (2019)49 n = 144 i.e. DG (43); SG (28); DCG (46); SCG (27) |
26 (23–30)b |
Both | Sev | IPAQ and a questionnaire listing 23 sports played during last 12 months. Freq. of sport performed/wk in May also asked. PA categorised by risk as per NHF classification. |
High‐risk sports: 59.2% (DG 27.9%; SG 42.9%; p < .05); IPAQ DG vs. SG (×1000 METs/wk)b: 18‐22y: 5.8 (1.1–15.1) vs. 3.5 (1.2–7.9); 23‐29y: 5.0 (0.7–14.9) vs. 4.5 (1.3–12.0); 30‐40y: 2.6 (1.1–12.1) vs. 1.8 (0.5–12.6); Number and freq of sports per group provided in article. Similar participation in sport between peers and PWH (raw data available upon request). |
|
|
Goto et al. (2019)50 n = 106 |
40.8 (12.1)a (range 18–64) |
Both | All | IPAQ and sport participation questionnaire |
PA levels (MET‐mins/wk) = 1501.8 (3413.0)a; 693.0b Significantly lower PA than Irish patients p < .001 (Sherlock et al., (2010) had higher number of mild patients). Moderate PA (mins/wk) = 103.7 (372.1)a; <0.1b; Vigorous PA (mins/wk) = 53.4 (209.6)a; <0.1b; Walking (mins/wk) = 333.6 (1106.7)a; 122.5b; Low PA n = 63 (59.4%); Moderate PA n = 29 (27.4%); High PA n = 13 (12.3%). 0 mins/wk of vigorous PA, moderate PA and walking, n = 85 (80.2%), 81 (76.4%), and 32 (30.2%), respectively. Sport participation previous year n = 50 (47.2%). |
|
|
Zanon et al. (2020)51 n = 40, ChG (12); AdoG (9); AG (19) |
ChG = <12 AdoG = 12–18 AG = >18 |
FVIII | Sev | EPIC Norfolk PA Questionnaire |
More PA/sport participation noted in highly adherent patients on prophylaxis. A difference between adolescents and adults in type, freq, and impact of PA was noted (raw data NR). Type of sport by category of adherence (None‐High): Hobby/leisure: None = 3 (15%); Min = 2 (10%); Low = 2 (10%); Med = 2 (10%); High = 11 (55%); Endurance sports: None = 3 (14.3%); Min = 2 (9.9%); Low = 2 (9.9%); Med = 3 (14.3%); High = 11 (52.4%); Athletic sports: None = 2 (13.3%); Min = 2 (13.3%); Low = 1 (6.7%); Med = 1 (6.7%); High = 9 (60%); Ball sports: None = 2 (16.7%); Min = NA; Low = 1 (8.3%); Med = 2 (16.7%); High = 7 (58.3%). |
|
|
Timmer et al. (2020)52 n = 105 |
43 (30–54)b | Both | All | Activ8 accelerometer carried in trouser pocket for 7 consecutive days. |
Majority = Sedentary (n = 60); Walkers (n = 21); Bikers and runners (n = 24) Sitting (h/day): 9.2 (7.4–10.6)b; Standing (h/day): 2.8 (2.0–3.6)b; Walking (h/day): 1.9 (1.4–2.5)b; Biking (mins/day): 14.2 (5.8–28.7)b; Running (mins/day): 0.6 (0.2–1.9)b; Frequency of active bouts /day: 10.0 (7.1–12.7)b; Length active bout (mins): 11.8 (10.6–14.3)b. |
|
|
Taylor et al. (2020)53 n = 72 |
44.5 ± 15.5a (range 18–69) |
Both | All (mod excluded from analysis) | IPAQ and questionnaire on types of activities involved in |
High PA: Sev 17 (40%); Mild 15 (52%); Total 31 (43%); Moderate PA: Sev 19 (44%); Mild 9 (31%); Total 30 (42%); Low PA: Sev 7 (16%); Mild 5 (17%); Total 11 (15%) Total METa ( a ): Sev 3770 ± 3979 (219–20 739); Mild 4530 ± 4457 (33–18 339); Total 4075 ± 4164 (33–20 739); Vigorous and Moderate METa ( a ): Sev 2567 ± 3570 (0–18 660); Mild 3390 ± 3682 (0–16 260); Total 2899 ± 3613 (0–18 660); Self‐reported achieved UK guidelines for activity: Sev 15/43 (35%); Mild 19/29 (65%); Total 34/72 (47%); 85% met UK PA guidelines (higher than general population). |
|
|
Bérubé et al. (2020)54 n = 24 |
11.8 ± 3.3a (range 6–18) |
Both | Sev | Self‐report of PA/wk for safe and high‐risk PA in winter and summer‐ G&SQ wording used, parental proxy report taken for children <10y. |
When those who practiced high‐risk vs. low‐risk PA were compared, those in the high‐risk category practiced more high‐risk PA vs. those in the low‐risk category (p < .05) (2.6 vs. 0.6 days/wk). No significant differences between categories with regards practice of lower risk PA. |
|
Abbreviations: AdoG = adolescent group; AG = adult group; All = mild, moderate and severe; Both = FVIII and FIX deficiency; BrG = Brazilian group; C2 = category 2 activity; C3 = category 3 activity; CaG = Canadian group; CG = control group; ChG = children/caregivers of children group; CPAQ = Children's Physical Activity Questionnaire; CTG = children/teenager group; /day = Per day; DCG = Dutch control group; DG = Dutch group; FIX = FIX deficiency; freq = frequency; FVIII = FVIII deficiency; G&SQ = Godin & Shepard Physical Activity Questionnaire; HG = haemophilia group; hr(s) = hours; IPAQ = International Physical Activity Questionnaire; MAQ = Modifiable Activity Questionnaire; Med = medium; METs = metabolic equivalent of task; METs = metabolic equivalent of task; min = minimum; mins = minutes; Mod = moderate; MVPA = moderate‐vigorous physical activity; NA = not applicable; NHF = National Haemophilia Foundation; NR = not reported; NS = not specified; PA = physical activity; PAI = Physical Activity Index; PE = physical education; SCG = Swedish control group; SEM = standard error of the mean; Sev = severe; SG = Swedish group; 3DPAR = 3‐Day Physical Activity Recall Questionnaire; /wk = per week; /wk = per week HG = haemophilia group; y = years (age).
Mean±standard deviation
Median±(interquartile range)
Median±(range);
3.4.2. PA Questionnaires
Commonly used PA questionnaires were used in 15 studies. These included the Godin and Sheppard Questionnaire,32 the Framingham Physical Activity Index,40 the Three Day Physical Activity Recall Questionnaire,44 the International Physical Activity Questionnaire (IPAQ),26, 34, 41, 45, 46, 49, 50, 53 the Children's Physical Activity Questionnaire (CPAQ; parental proxy report),26 the Modifiable Activity Questionnaire (MAQ) 24, 25, 35 and the EPIC Norfolk Physical Activity Questionnaire.51
The most common questionnaire used was the IPAQ, and the reporting of results varied as some compared PA with guidelines,26 normative data 41 or a combination of both.34, 50, 53 Using the IPAQ, Sherlock et al.34 found adults with haemophilia spent less time engaged in moderate‐vigorous PA (MVPA) compared with normative data, whilst Goto et al. found their participants were less active compared with the sample assessed by Sherlock et al.50 Contrastingly, studies by Niu et al. and Taylor et al. found the majority of adults with haemophilia met PA guidelines26, 53 and no differences in PA were found between adults and children with haemophilia and controls in the study by den Uijl et al.41
Using the MAQ, Broderick et al.25 reported children with haemophilia were less active than their peers without haemophilia, differing from the study by Groen et al.24, who reported higher levels of PA in youths with haemophilia compared with the general population, although neither majority met PA guidelines. The remaining eight studies did not compare PA against guidelines or normative data (Table 3).26, 32, 35, 40, 45, 46, 49, 51
3.4.3. Objective Measures of PA
Three studies used different accelerometer devices worn on the waist or hip for one week to assess PA in heterogeneous samples of children with haemophilia.36, 37, 44 Two studies found children with haemophilia spent more time in moderate PA than controls 36, 37 and the remaining study found the average minutes spent in MVPA were close to meeting guidelines of one hour per day.44
One recent study assessed PA using an accelerometer carried in trouser pockets for one week in adults with haemophilia.52 A different method of classifying PA was used whereby PA behaviour was categorised as ‘sedentary’, ‘walkers’ or ‘bikers and runners,’ with the majority of participants categorised as sedentary.52
3.4.4. Participation in Sport
Participation in sport was described by 16 studies 14, 47, 49, 50, 51, 53 (as well as those studies who described types of PA using the MAQ 24, 25, 35). Considerable levels of engagement in sport (ie more than half the total sample engaged) were evident in 10 studies,21, 23, 27, 31, 33, 34, 38, 41, 42, 49 and a wide variety of sports (both high and low impact) were captured across all 19 studies (Table 3).
Lower rates of engagement in sport were found in PWH compared with normative data in the study by den Uijl e al.,41 whilst two studies found PWH were as engaged in sport compared with normative data.21, 43 Additionally, Versloot et al.49 found engagement in sport in adults with haemophilia was similar to their peers and trends in increasing age and reduced sport participation were noted in two studies.47, 50
3.4.5. PA by severity of haemophilia
PA or sports participation was compared by severity in heterogeneous samples of PWH in 15 studies (age range 4–69 years) 21, 44, 50, 52, 53 (Table 4). People with non‐severe haemophilia undertook more strenuous or higher levels of PA or sport in five studies.26, 28, 34, 41, 53 Contrastingly, no differences were found between severity of haemophilia and PA across 10 studies.21, 23, 24, 26, 36, 40, 42, 44, 50, 52 Additionally, two studies identified PWSH reported higher PA or sports participation than those with non‐severe haemophilia.27, 41
TABLE 4.
PA and sports participation by haemophilia severity
| Study | FVIII (n) | FIX (n) | Mild (n) | Mod (n) | Sev (n) | Treatment (n) | Findings |
|---|---|---|---|---|---|---|---|
| Janco et al. (1996)28 | 86 | 10 | 21 | 20 | 55 | OD: 96 | Higher levels of clotting factor reported higher daily strenuous PA (p < .04). |
| Heijnen et al. (2000)21 | Botha | Botha | 50 | 26 | 217 | NR | Sport participation of those with mod/sev haemophilia was similar to those with mild (73% vs. 85%, respectively). |
| Koiter et al. (2009)23 | 100 | 13 | 47 | 11 | 41 | OD: 56; PR: 43 | No significant differences in energy expenditure were found for haemophilia severity. |
| Sherlock et al. (2010)34 | NR | NR | 25 | 6 | 30 | NR | High PA levels were more commonly reported by people with mild/mod (64%) than sev (36%). Moderate PA levels were slightly more reported by people with sev (53%) than those with mild/mod (47%). Lower PA levels reported by people with sev (90%) compared with mild/mod (10%). |
| Buxbaum et al. (2010)36 | Botha | Botha | 7 | NA | 9 | OD: NR; PR: 9 | No significant differences were found in time spent in higher PA between mild and sev groups (moderate PA, p = .81, high PA, p = .18, and vigorous PA, p = .29). |
| Groen et al. (2011)24 | NR | NR | 19 | 7 | 21 | OD:22; PR: 25 | No statistically significant differences found in number of sports played by severity (p = .09). More non‐sev played football (p = .04), more with sev swam (p = .01). No differences between non‐sev vs. sev for total PA h/wk (8.7 ± 3.8 vs. 8.6 ± 7.8), MET h/wk (54.9 ± 30.3 vs. 55.6 ± 53.4) or vigorous h/wk (6.2 ± 4.2 vs. 5.4 ± 6.3). |
| Baumgardner et al. (2013)40 | 71 | 17 | 26 | 24 | 38 | OD: NR; PR: 20 | No relationship was identified between baseline factor VIII/IX levels and PA. |
| den Uijl et al. (2013)41 | 105c | 15c | NA | 34 | 60 | OD: NR; PR:90c | Patients with sev reported higher PA (median 4294 MET; IQR 1554–10,480) than those with mod (median 2484 MET; IQR 942–5660). Participation in sport and high‐risk sport was higher in those with mod (70%, 50%) than those with sev (59%, 34%). |
| Niu et al. (2014)26 | NA | 135 | NR | NR | 56 | NR | Adults with mild/mod were more active than those with sev (p = .0413). No significant differences found for severity (p = .095) between those adults who did/didn't achieve recommended PA guidelines. No statistically significant differences in PA by severity in children (p = .1639). |
| McGee et al. (2015)42 | 31 | 17 | 13 | 9 | 26 | OD: NR; PR: 32 | No statistically significant differences were found for sport participation by severity. |
| Von Mackensen et al. (2016)27 | 35 | 15 | 12 | 10 | 28 | OD: 23; PR: 27 | Significantly more with sev (78.6%) did sport vs. mild/mod (45.4%) (p < .017). |
| Bouskill et al. (2016)44 | 56 | 10 | (24)b | (24)b | 42 | OD:NR; PR: 47 | Severity was not significantly associated with time spent in MVPA (p adj = .32). |
| Goto et al. (2019)50 | 88 | 18 | 7 | 19 | 78 | OD: 37; PR: 70 | No significant relationship found between PA and severity of haemophilia (p = .783) |
| Timmer et al. (2020)52 | Botha | Botha | NR | NR | 73 | OD: NR; PR: 71 | No significant differences identified in the proportion of people with sev haemophilia in clusters of PA type (p = .28). |
| Taylor et al. (2020)53 | 67 | 6 | 29 | NA | 44 | OD: 42; PR: 31 | High PA levels more commonly reported by people with mild (52%) vs. sev (40%). Those with sev reported significantly less vigorous/moderate METs (2567 SD 3570) than those with mild (3390 SD 3682) (p <.001). No statistically significant differences found between severity for total PA METs. |
Abbreviations: FVIII = FVIII deficiency; FIX = FIX deficiency; hr(s) = hour(s); IQR = interquartile range; METs = Metabolic Equivalent of Task; mins = minutes; Mod = moderate; MVPA = moderate‐vigorous physical activity; NA = not applicable; NR = not reported; OD = on demand; PA = physical activity; SD = standard deviation; Sev = severe; /wk = per week.
Both FVIII and FIX deficiency assessed but breakdown of numbers not provided.
n represents 24 mixed mild/moderate group, breakdown of numbers not provided.
Overestimated n.
Information on treatment regimen was incomplete in nine studies,21, 26, 34, 36, 40, 41, 42, 44, 52 and the remaining six studies included participants who treated on demand or with prophylaxis 23, 24, 27, 28, 50, 53 (Table 4).
3.5. PA and bleeds
Data on bleeds were reported by 21 studies 25, 27, 45, 46, 47, 48, 49, 50, 51, 54 using various self‐report methods of PA and bleeds including diaries, questionnaires, phone interviews and retrospective chart audits. The relationship between bleeds and PA was assessed in 14 studies 25, 42, 49, 50, 54 (Table 5). Age varied amongst children and adults (4–66 years). Those with severe haemophilia only were assessed by four studies,32, 33, 49, 54 two studies assessed those with moderate and severe haemophilia,25, 39 and the eight remaining studies assessed mild, moderate and severe.27, 28, 31, 34, 37, 38, 42, 50 Seven studies collected data on bleeds and PA but did not carry out analysis between these variables.40, 41, 45, 46, 47, 48, 51
TABLE 5.
Study sample characteristics and main findings of bleeds, PA and treatment
|
Author Sample size (n) |
Age (y) | Type | Severity | Traumatic bleeds | Spontaneous bleeds | Other bleeds | Treatment | Bleeds and PA |
|---|---|---|---|---|---|---|---|---|
|
Janco et al. (1996)28 n = 96 |
4–17 | Both | All | Yes | Yes | NA | OD |
Spontaneous joint bleeds (p < .05) and traumatic soft tissue bleeds (p < .03) with strenuous PA when controlled for factor level. |
|
Fromme et al. (2007)31 n = 71 |
7–42 | NS | All | NS | NS | Exercise‐induced | NS |
17.6% of bleeds were exercise‐induced. 10.3% in youths, significantly less than adults (33.3%) (p < .05). Sports such as football, basketball and swimming were associated with bleeding complications. No statistically significant correlation between rate of bleeding complications and severity of haemophilia. |
|
Tiktinsky et al. (2009)32 n = 44 |
12–25 | Both | Sev | Yes | Yes | NA | PR excluded; Treatment NS |
Traumatic bleeds significantly associated with strenuous PA (p < .01). No significant differences between activity levels and mean number of bleeds. |
|
Ross et al. (2009)33 n = 37 |
6–21 | Both | Sev | NS | NS | Joint bleeds | PR |
Not associated with high‐impact PA (OR 0.32 (95% CI: 0.04–2.7, p = .3)) Median acute joint bleeds for high impact PA: 0.05 (0–4); low impact PA: 0.5 (0–2). |
|
Sherlock et al. (2010)34 n = 61 |
16–63 | NS | All | NS | NS | Sport‐related | NS |
55% of participants has sport‐associated bleeds. Bleeding episodes were reported in 7/8 patients with sev haemophilia. |
|
Gonzalez et al. (2011)37 n = 41 |
8–18 | FVIII | All | NS | NS |
‘a bleeding episode during the previous year’ |
OD & PR |
Patients who suffered from a bleeding episode during the previous year vs. those who did not did significantly more vigorous PA. (t39 = 3.41, p = .002, r = .28). |
|
Khair et al. (2012)38 n = 84 |
6–18 | Both | All | NS | NS | Total, joint and sport‐related bleeds in 6 months | OD & PR | Not associated with sedentary behaviour, sport participation, frequency or duration of sport. |
|
Broderick et al. (2012)39 n = 104 |
5–14 | Both | Mod/Sev | NS | NS | ‘An episode of bleeding requiring treatment with clotting factor’ | OD & PR |
Vigorous PA transiently associated with a moderate relative increase in risk of bleeding, thus absolute increase in risk associated with PA was low. |
|
Broderick et al. (2013)25 n = 104 |
4–18 | Both | Mod/Sev | NS | NS | NS | OD & PR |
Not associated with absolute or vigorous PA (r s = .05 and .07, respectively). |
|
McGee et al. (2015)42 n = 48 |
10–19 | Both | All | NS |
Excluded |
New target joints/injuries: soft tissue/haemarthrosis/muscle haemorrhage and head injury |
Most on PR | Mean number of ‘injuries’ not associated with sport participation (p = .44). Two subjects (mild/mod haemophilia) who did sport developed target joints compared to those who did not participate in sport. |
|
von Mackensen et al. (2016)27 n = 50 |
17–66 | Both | All | NS | NS | Total, joint and sport‐related bleeds in 6 months | OD & PR | Not associated with sedentary behaviour, sport participation, frequency or duration of sport. |
|
Versloot et al. (2019)49 n = 71 |
18–40 | Both | Sev | NS | NS | Joint bleeds (annual and 5‐year) | PR | Not associated with high‐risk sport participation (r s = −.25, p = .27/ r s = .08, p = .76) in either cohort. |
|
Goto et al. (2019)50 n = 106 |
18–64 | Both | All | NS | NS | Bleeding caused by sports; Intra‐articular bleeding | OD & PR |
84 (79.2%) experienced bleeding whilst playing sports. No significant association between intra‐articular bleeds during past 12 months and PA (r s = −.072, p = .466). |
|
Bérubé et al. (2020)54 n = 24 |
6–18 | Both | Sev | NS | NS | Bleeds in past year | PR or immune tolerance therapy |
No significant differences in number of bleeds episodes in past year between ‘Risk Profile’ and ‘Safe Profile’ categories (3.8 (6.3) vs. 4.7 (6.7), respectively). |
Abbreviations: All = Mild, moderate and severe; Both = FVIII and FIX deficiency; Mod = moderate; NA = not applicable; NS = not specified; OD = on demand; PA = physical activity; PR = prophylaxis; r = effect size; r s = Spearman's rho; Sev = severe; y = years (age).
With regard to prophylaxis, two studies did not present data on treatment regimen,31, 34 whilst the remaining 12 studies provided some indication to whether participants were taking prophylaxis and/or on demand treatment.25, 49, 50, 54
The studies by Fromme et al. and Sherlock et al. reported 17.6% and 55% experienced sport‐ or exercise‐related bleeds in heterogeneous samples of PWH, respectively,31, 34 and additionally, Fromme et al.31 did not identify any association between rate of bleeding complications and haemophilia severity. A higher prevalence of sport‐induced bleeding (79.2%) was identified by Goto et al.,50 although the association between PA and bleeds was not significant. Children and youths with haemophilia, who were not treated with prophylaxis (including those with severe haemophilia), both demonstrated a significant association between bleeds and strenuous PA in the studies by Janco et al.28 and Tiktinsky et al.32 However, there was no association between high impact PA or sport with bleeds or injury in PWH who treated either on demand or with prophylaxis across heterogeneous samples of PWH in seven studies.25, 27, 33, 38, 42, 49, 54 Contrastingly, Gonzalez et al.37 identified that patients (including those on prophylaxis) who suffered from a bleeding episode during the previous year undertook significantly more vigorous PA compared with those who did not suffer bleeding. Furthermore, Broderick et al.39 found that vigorous PA was transiently associated with a moderate increase in the relative risk of bleeding, but the absolute increase in risk associated with PA was low. Overall, variation in participant demographics and the definition and assessment of bleeds, PA and reporting of treatment regimens was found (Table 5).
3.6. PA and treatment regimen
Where data were reported, approximately 849 participants treated on demand and 1617 treated with prophylaxis. Details of treatment regimen for PA data were not reported by six studies 15, 21, 26, 31, 34, 41 and not reported in full by eight studies (ie data were only available for those regarding prophylaxis and no alternative treatment, if any, was specified for the remainder of participants).32, 36, 40, 41, 42, 44, 52, 54
Six studies provided detail on dosage or type of prophylaxis participants were using (i.e. primary, secondary, long‐term or short‐term) or indicated age at which treatment was commenced.27, 29, 35, 41, 45, 49 Prophylaxis was tailored to sport or PA in six studies.23, 27, 30, 34, 38, 50 A negative impact on engagement in activity in those with severe haemophilia or those taking routine treatment was reported in a large survey by Baumann et al.14 This impact included changes to treatment dosing and timing before vigorous PA.
More time spent in levels of high‐intensity PA was found in children who had more access to treatment in the study by Carneiro et al.45 Adults who treated with intermediate dose prophylaxis, in the study by Versloot et al.49, demonstrated an age‐related decline in sport participation (including high‐risk sports), compared with adults who treated with higher dose prophylaxis, although these groups were from two different countries (the Netherlands and Sweden). Lastly, a recent study by Zanon et al.51 reported that people with severe FVIII deficiency who were more adherent to prophylaxis engaged in more PA than those with lower adherence rates.
4. DISCUSSION
The objectives of this review were to determine levels of PA amongst PWH and, additionally, to determine the relationship between PA and bleeds and whether treatment regimen influences this relationship. Overall, we observed that levels of PA and participation in sport varied markedly amongst the heterogeneous samples of PWH reported in the literature.
When compared with normative data or PA guidelines, greater,24, 36, 37 similar,21, 26, 41, 43, 44, 49, 53 reduced 22, 25, 34, 41, 50 or variable 29 levels of more intensive PA or sports participation were identified across 15 studies. The remaining 21 studies did not compare PA to guidelines or normative data, limiting their interpretation.14, 15, 35, 38, 39, 40, 42, 45, 46, 47, 48, 51, 52, 54 Within studies, increased PA in lower age groups was apparent, which may be due to improvements in treatments, access to treatments and better promotion of PA from a young age in recent decades.14, 26, 27, 32, 34, 40, 44, 49 There were even considerable rates of participation in high‐risk sport in some youths.33, 41, 49 Although age‐related decline in PA is also a common trend seen in the general population,55 lower levels of PA amongst older adults with haemophilia may also be attributable to less promotion of PA when they were young due to less optimal treatments, resulting in more bleeds and joint damage. Factors other than age that have been suggested to impact PA levels, including socio‐economic, cultural, environmental, personality and behavioural influences,56 may also explain the variation in PA seen across the samples of PWH represented in this review.
A large variety of PA assessment methods were used with differing definitions of PA and inconsistent reporting of PA volume (frequency, intensity, type and duration). Many of the measurement tools used to assess PA have not been validated in PWH. The most commonly used PA questionnaire was the IPAQ; however, its validity and reliability have been reported to be poor or inconclusive in the general population.57, 58, 59 No studies have validated the IPAQ in adults with FVIII deficiency. Satisfactory reliability of the IPAQ was shown in adults with FIX deficiency from the B‐HERO‐S study by Buckner et al.60; however, construct validity was not assessed. The MAQ was also commonly used but little validation studies have been carried out on this questionnaire in the general population,61 and no studies have validated it in PWH. Self‐report questionnaires, such as the IPAQ and MAQ, are commonly chosen as convenient methods of PA assessment because they are time efficient and consider the various domains and dimensions of PA; however, they are largely susceptible to recall and social desirability bias and possess low levels of validity for the assessment of PA in the free‐living setting.62 Objective methods, including accelerometry, provide a more reliable assessment of frequency, intensity and duration of PA in the free‐living setting without being overly burdensome on the participant.62 A small number of studies used accelerometers to assess habitual PA in children with haemophilia,36, 37, 44 and one recent study was identified in adults.52 Objective measures of PA using accelerometry in children with haemophilia have been partially validated in mixed sample studies of children with chronic diseases, although different devices were examined and one study assessed the validity of accelerometry for sedentary behaviour.63, 64, 65 There is a need for more validation studies of objective measurements of PA in both children and adults with haemophilia, in addition to self‐report assessment tools. A combined approach of using self‐report and objective methods has the potential to provide the clearest, most feasible description of PA volume and type in this field of research.
The relationship between PA and bleed rate remains inconclusive. This was mostly due to heterogeneity in study sample characteristics, methods and the definition of bleeds and volume of PA. Bleeds in PWH tend to be spontaneous or traumatic, but the differentiation between types of bleeds was difficult to determine from some studies who classified bleeds as ‘joint bleeds’, ‘an episode of bleeding requiring treatment with clotting factor’ or ‘sport/exercise‐related bleeds or injury’. A milder bleeding phenotype has been described in a minority of PWSH, as well as those with FIX compared with FVIII deficiency.16, 66 Despite the fact that type and severity of haemophilia appear to be significant genetic modifiers of bleeding phenotype, small sample sizes may have prevented studies comparing bleeds and PA in these subgroups. Only one study was identified which compared sport‐associated bleeds and haemophilia severity 31 (Table 5). This study involved a heterogeneous sample and was limited by a lack of information on treatment regimen. Further investigation of the relationship between PA and bleeds in the context of haemophilia type and severity, and the influence of treatment regimen on these variables, is therefore warranted.
The sub‐analysis of PA levels by severity of haemophilia also revealed variable results whereby two studies carried out in the last 10 years have found PWSH were more active than those with non‐severe haemophilia,27, 41 although no differences in PA were found by severity in 10 studies.21, 23, 24, 26, 36, 40, 42, 44, 50, 52 This suggests that increased severity of haemophilia does not necessarily affect PA participation. The positive influence of prophylaxis appears to reduce the risk of bleeds associated with PA; however, it is challenging to establish the true relationship between specific volumes of PA and the exact levels of prophylaxis that are required to reduce even a transient increase in the risk of bleeding without modifying treatment regimen. Information regarding specific details of treatment regimen (ie age treatment was commenced, types, dosages and timing of prophylaxis, compliance to treatment) was inconsistent across studies, limiting the ability to draw conclusions on the influences of various aspects of treatment regimen on bleeds potentially related to PA. Broderick et al.39 proposed that considering vigorous PA is usually only a small proportion of overall activity, the relative and overall risk of bleeding is low if half‐life of prophylaxis maintains factor levels above 50% for approximately 6–12 hours. This highlights the need for more robust reporting of details of treatment regimen, especially factor half‐life and different types of newly available treatments in studies examining PA and bleeds.
The AXIS and STROBE analyses revealed low‐to‐moderate quality and transparency of reporting on average, as well as various sources of bias amongst studies. Selection and non‐response bias were common and are difficult to control for in observational research, especially for studies of rare genetic disorders such as haemophilia, where small sample sizes are a common limitation of research. Self‐report methods gave rise to potential measurement, social desirability and recall bias which limit the accuracy of study findings; thus, the development of validated objective measures in future research has the potential to overcome such sources of bias. Potential confounding factors that may influence the relationship between PA and bleeds, other than treatment regimen, including type of haemophilia, severity of HJA, pain, history of inhibitors and the prevalence of blood‐borne viruses, were described by some but not all studies and warrant consideration in future research.
Limitations of this review include possible omissions of studies due to the ambiguity of common terminology used in the search strategy; however, reference lists of full texts and other reviews were additionally screened for potential studies which were not detected during the online search. Abstracts from conferences were not included due to the lack of complete data reported; however, a proportion of these abstracts appeared to contain preliminary data from some of the final full texts included in this review.
5. CONCLUSION
In conclusion, this systematic review suggests that levels of PA vary markedly between individual adults and children with haemophilia. However, it is clear that the quality of the evidence available has inherent limitation. As a result, there is significant heterogeneity between different studies with respect to study samples and methodology, as well as the common use of self‐report methods. In addition, due to the inherent inter‐individual variability in bleeding tendency and varying treatment regimens amongst PWH, the relationship between bleeds and PA was difficult to elucidate. Longitudinal studies that encompass more rigorous assessment of PA and bleeds, along with the comparison between different treatment regimens, represent an important clinical unmet need. This is particularly important given the increasing life expectancy of the haemophilia community and the rapidly evolving era of new treatments for PWH.
CONFLICT OF INTERESTS
M. L. has received speaker's fees from Siemens Healthineers and served as on an advisory board to Tremeau Pharmaceuticals. She previously served on a speaker's bureau for Shire (now part of Takeda). N. M. O’C has received research support from SOBI; participated in advisory boards for F. Hoffmann‐La Roche Ltd, UniQure, SOBI, Freeline; and participated in speakers’ bureaus for F. Hoffmann‐La Roche Ltd, SOBI and Novo Nordisk. J. S. O’D has served on the speaker's bureau for Baxter, Bayer, Novo Nordisk, Boehringer Ingelheim, Leo Pharma, Takeda and Octapharma. He has also served on the advisory boards of Baxter, Bayer, Octapharma CSL Behring, Daiichi Sankyo, Boehringer Ingelheim, Takeda and Pfizer. J. S. O. D has received research grant funding awards from Baxter, Bayer, Pfizer, Shire (now part of Takeda), Takeda and Novo Nordisk. P. L. T. is full‐time employee of Baxalta Innovations GmbH, a member of the Takeda group of companies and shareholder of Takeda Pharmaceutical Company Limited. The remaining authors have no competing interests.
Supporting information
Supplementary Material
Supplementary Material
ACKNOWLEDGEMENTS
P. L. T, J. S. O’D, M. L., N. M. O’C. and J. G. all contributed to the research conception and design, data analysis and interpretation. M. K. was the principal reviewer involved in review data acquisition, analysis and interpretation. P. O’G. and A. M. were second and third independent reviewers, respectively, for data acquisition, analysis and interpretation. B. O’M. contributed to data interpretation. M. K. and J. G. wrote the paper. M. K., J. G., P. O’G., A. M., B. O’M., N. M. O’C., M. L., J. S. O’D. and P. L. T. all contributed to drafting and revising the paper and have approved the final version. A special acknowledgement and thank you is extended to David Mockler, from the Library of Trinity College Dublin, who assisted with the search strategy for this review. The iPATH study is supported in part by a research grant from Science Foundation Ireland (SFI) under the SFI Strategic Partnership Programme Grant (16/SPP/3303) and research support from Shire US Inc., a Takeda company, Lexington, MA, USA.
DATA AVAILABILITY STATEMENT
The data that support the findings of this review are available in the supplementary material of this article. Raw data are also available from the corresponding author upon reasonable request.
REFERENCES
- 1.Darby SC, Kan SW, Spooner RJ, et al. Mortality rates, life expectancy, and causes of death in people with hemophilia A or B in the United Kingdom who were not infected with HIV. Blood. 2007;110(3):815‐825. [DOI] [PubMed] [Google Scholar]
- 2.Canaro M, Goranova‐Marinova V, Berntorp E. The ageing patient with haemophilia. Eur J Haematol. 2015;94(Suppl 77):17‐22. [DOI] [PubMed] [Google Scholar]
- 3.Von Mackensen S. Quality of life and sports activities in patients with haemophilia. Haemophilia. 2007;13(SUPPL. 2):38‐43. [DOI] [PubMed] [Google Scholar]
- 4.Weigel N, Carlson BR. Physical activity and the hemophiliac: yes or no? Am Correct Ther J. 1975;29(6):197‐205. [PubMed] [Google Scholar]
- 5.Negrier C, Seuser A, Forsyth A, et al. The benefits of exercise for patients with haemophilia and recommendations for safe and effective physical activity. Haemophilia. 2013;19(4):487‐498. [DOI] [PubMed] [Google Scholar]
- 6.Gomis M, Querol F, Gallach JE, Gonzalez LM, Aznar JA. Exercise and sport in the treatment of haemophilic patients: a systematic review. Haemophilia. 2009;15(1):43‐54. [DOI] [PubMed] [Google Scholar]
- 7.Srivastava A, Santagostino E, Dougall A, et al. WFH guidelines for the management of Hemophilia, 3rd edition. Haemophilia. 2020;26(S6):1‐158. [DOI] [PubMed] [Google Scholar]
- 8.Piercy KL, Troiano RP, Ballard RM, et al. The Physical Activity Guidelines for Americans. JAMA. 2018;320(19):2020‐2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Collaborators, G.R.F. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1659‐1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Majumdar S, Morris A, Gordon C, et al. Alarmingly high prevalence of obesity in haemophilia in the state of Mississippi. Haemophilia. 2010;16(3):455‐459. [DOI] [PubMed] [Google Scholar]
- 11.Fransen van de Putte DE, Fischer K, Makris M, et al. Increased prevalence of hypertension in haemophilia patients. Thromb Haemost. 2012;108(4):750‐755. [DOI] [PubMed] [Google Scholar]
- 12.Department of Health and Children, H.S.E. The National Guidelines on Physical Activity for Ireland, in ISBN 978‐1‐906218‐27‐0. H.S.E. Department of Health and Children, Editor. 2009, The Department of Health and Children and The Health Service Executive. 1‐32. [Google Scholar]
- 13.Raffini L, Manno C. Modern management of haemophilic arthropathy. Br J Haematol. 2007;136(6):777‐787. [DOI] [PubMed] [Google Scholar]
- 14.Baumann K, Hernandez G, Witkop M, et al. Impact of mild to severe hemophilia on engagement in recreational activities by US men, women, and children with hemophilia B: The Bridging Hemophilia B Experiences, Results and Opportunities into Solutions (B‐HERO‐S) study. Eur J Haematol. 2017;98:25‐34. [DOI] [PubMed] [Google Scholar]
- 15.Flaherty LM, Schoeppe J, Kruse‐Jarres R, Konkle BA. Balance, falls, and exercise: Beliefs and experiences in people with hemophilia: A qualitative study. Res Pract Thromb Haemost. 2018;2(1):147‐154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Franchini M, Mannucci PM. Modifiers of clinical phenotype in severe congenital hemophilia. Thromb Res. 2017;156:60‐64. [DOI] [PubMed] [Google Scholar]
- 17.van den Berg HM, De Groot PH, Fischer K. Phenotypic heterogeneity in severe hemophilia. J Thromb Haemost. 2007;5(Suppl 1):151‐156. [DOI] [PubMed] [Google Scholar]
- 18.Moher D, Liberati A, Tetzlaff J, Altman DG, The PG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA Statement. PLoS Medicine. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Downes MJ, Brennan ML, Williams HC, Dean RS. Development of a critical appraisal tool to assess the quality of cross‐sectional studies (AXIS). BMJ Open. 2016;6(12):e011458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vandenbroucke JP, von Elm E, Altman DG, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. Int J Surg. 2014;12(12):1500‐1524. [DOI] [PubMed] [Google Scholar]
- 21.Heijnen L, Mauser‐Bunschoten EP, Roosendaal G. Participation in sports by Dutch persons with haemophilia. Haemophilia. 2000;6(5):537‐546. [DOI] [PubMed] [Google Scholar]
- 22.Tlacuilo‐Parra A, Morales‐Zambrano R, Tostado‐Rabago N, et al. Inactivity is a risk factor for low bone mineral density among haemophilic children. Br J Haematol. 2008;140(5):562‐567. [DOI] [PubMed] [Google Scholar]
- 23.Koiter J, van Genderen FR, Brons PP, Nijhuis‐van der Sanden MW. Participation and risk‐taking behaviour in sports in children with haemophilia. Haemophilia. 2009;15(3):686‐694. [DOI] [PubMed] [Google Scholar]
- 24.Groen WG, Takken T, van der Net J, Helders PJ, Fischer K. Habitual physical activity in Dutch children and adolescents with haemophilia. Haemophilia. 2011;17(5):e906‐912. [DOI] [PubMed] [Google Scholar]
- 25.Broderick CR, Herbert RD, Latimer J, van Doorn N. Patterns of physical activity in children with haemophilia. Haemophilia. 2013;19(1):59‐64. [DOI] [PubMed] [Google Scholar]
- 26.Niu X, Poon JL, Riske B, et al. Physical activity and health outcomes in persons with haemophilia B. Haemophilia. 2014;20(6):814‐821. [DOI] [PubMed] [Google Scholar]
- 27.von Mackensen S, Harrington C, Tuddenham E, et al. The impact of sport on health status, psychological well‐being and physical performance of adults with haemophilia. Haemophilia. 2016;22(4):521‐530. [DOI] [PubMed] [Google Scholar]
- 28.Janco RL, Maclean WE, Perrin JM, Gortmaker SL. A prospective study of patterns of bleeding in boys with haemophilia. Haemophilia. 1996;2(4):202‐206. [DOI] [PubMed] [Google Scholar]
- 29.van der Net J, Vos RC, Engelbert RH, et al. Physical fitness, functional ability and quality of life in children with severe haemophilia: a pilot study. Haemophilia. 2006;12(5):494‐499. [DOI] [PubMed] [Google Scholar]
- 30.Nazzaro A, Owens S, Hoots WK, Larson KL. Knowledge, attitudes, and behaviours of youths in the US hemophilia population: results of a national survey. Am J Public Health. 2006;96(9):1618‐1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fromme A, Dreeskamp K, Pollmann H, et al. Participation in sports and physical activity of haemophilia patients. Haemophilia. 2007;13(3):323‐327. [DOI] [PubMed] [Google Scholar]
- 32.Tiktinsky R, Kenet G, Dvir Z, et al. Physical activity participation and bleeding characteristics in young patients with severe haemophilia. Haemophilia. 2009;15(3):695‐700. [DOI] [PubMed] [Google Scholar]
- 33.Ross C, Goldenberg NA, Hund D, Manco‐Johnson MJ. Athletic participation in severe hemophilia: bleeding and joint outcomes in children on prophylaxis. Pediatrics. 2009;124(5):1267‐1272. [DOI] [PubMed] [Google Scholar]
- 34.Sherlock E, O'Donnell JS, White B, Blake C. Physical activity levels and participation in sport in Irish people with haemophilia. Haemophilia. 2010;16(1):e202‐209. [DOI] [PubMed] [Google Scholar]
- 35.Khawaji M, Astermark J, Åkesson K, Berntorp E. Physical activity for prevention of osteoporosis in patients with severe haemophilia on long‐term prophylaxis. Haemophilia. 2010;16(3):495‐501. [DOI] [PubMed] [Google Scholar]
- 36.Buxbaum NP, Ponce M, Saidi P, Michaels LA. Psychosocial correlates of physical activity in adolescents with haemophilia. Haemophilia. 2010;16(4):656‐661. [DOI] [PubMed] [Google Scholar]
- 37.González LM, Peiró‐Velert C, Devís‐Devís J, et al. Comparison of physical activity and sedentary behaviours between young haemophilia A patients and healthy adolescents. Haemophilia. 2011;17(4):676‐682. [DOI] [PubMed] [Google Scholar]
- 38.Khair K, Littley A, Will A, Von Mackensen S. The impact of sport on children with haemophilia. Haemophilia. 2012;18(6):898‐905. [DOI] [PubMed] [Google Scholar]
- 39.Broderick CR, Herbert RD, Latimer J, et al. Association between physical activity and risk of bleeding in children with hemophilia. JAMA. 2012;308(14):1452‐1459. [DOI] [PubMed] [Google Scholar]
- 40.Baumgardner J, Elon L, Antun A, et al. Physical activity and functional abilities in adult males with haemophilia: A cross‐sectional survey from a single US haemophilia treatment centre. Haemophilia. 2013;19(4):551‐557. [DOI] [PubMed] [Google Scholar]
- 41.den Uijl I, Biesma D, Grobbee D, Fischer K. Turning severe into moderate haemophilia by prophylaxis: are we reaching our goal? Blood Transfus. 2013;11(3):364‐369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McGee S, Raffini L, Witmer C. Organized sports participation and the association with injury in paediatric patients with haemophilia. Haemophilia. 2015;21(4):538‐542. [DOI] [PubMed] [Google Scholar]
- 43.Cuesta‐Barriuso R, Torres‐Ortuño A, Pérez‐Alenda S, et al. Sporting activities and quality of life in children with hemophilia: an observational study. Pediatr Phys Ther. 2016;28(4):453‐458. [DOI] [PubMed] [Google Scholar]
- 44.Bouskill V, Hilliard P, Stephens S, et al. An institutional pilot study to investigate physical activity patterns in boys with haemophilia. Haemophilia. 2016;22(5):e383‐389. [DOI] [PubMed] [Google Scholar]
- 45.Carneiro JDA, Blanchette V, Ozelo MC, et al. Comparing the burden of illness of haemophilia between resource‐constrained and unconstrained countries: the São Paulo‐Toronto Hemophilia Study. Haemophilia. 2017;23(5):682‐688. [DOI] [PubMed] [Google Scholar]
- 46.Kempton CL, Recht M, Neff A, et al. Impact of pain and functional impairment in US adults with haemophilia: Patient‐reported outcomes and musculoskeletal evaluation in the pain, functional impairment and quality of life (P‐FiQ) study. Haemophilia. 2018;24(2):261‐270. [DOI] [PubMed] [Google Scholar]
- 47.Pinto PR, Paredes AC, Pedras S, et al. Sociodemographic, clinical, and psychosocial characteristics of people with hemophilia in Portugal: findings from the First National Survey. TH Open. 2018;2(1):e54‐e57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pinto PR, Paredes AC, Moreira P, et al. Emotional distress in haemophilia: factors associated with the presence of anxiety and depression symptoms among adults. Haemophilia. 2018. [DOI] [PubMed] [Google Scholar]
- 49.Versloot O, Berntorp E, Petrini P, et al. Sports participation and physical activity in adult Dutch and Swedish patients with severe haemophilia: a comparison between intermediate‐ and high‐dose prophylaxis. Haemophilia. 2019;25(2):244‐251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Goto M, Haga N, Takedani H. Physical activity and its related factors in Japanese people with haemophilia. Haemophilia. 2019;25(4):e267‐e273. [DOI] [PubMed] [Google Scholar]
- 51.Zanon E, Tagliaferri A, Pasca S, et al. Physical activity improved by adherence to prophylaxis in an Italian population of children, adolescents and adults with severe haemophilia A: the SHAPE Study. Blood Transfus. 2020;18(2):152‐158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Timmer MA, Veenhof C, de Kleijn P, et al. Movement behaviour patterns in adults with haemophilia. Ther Adv Hematol. 2020;11:2040620719896959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Taylor S, Room J, Barker K. Physical activity levels in men with Haemophilia—a single centre UK survey. Haemophilia. 2020;26(4):718‐725. [DOI] [PubMed] [Google Scholar]
- 54.Berube S, Amesse C, Sultan S. Illness perceptions and their relation to physical activity in children and adolescents with hemophilia. Health Psychology and Behavioral Medicine. 2020;8(1):461‐474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sallis JF. Age‐related decline in physical activity: a synthesis of human and animal studies. Med Sci Sports Exerc. 2000;32(9). [DOI] [PubMed] [Google Scholar]
- 56.Seefeldt V, Malina RM, Clark MA. Factors affecting levels of physical activity in adults. Sports Med. 2002;32(3):143‐168. [DOI] [PubMed] [Google Scholar]
- 57.Kim Y, Park I, Kang M. Convergent validity of the International Physical Activity Questionnaire (IPAQ): meta‐analysis. Public Health Nutr. 2013;16(3):440‐452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Silsbury Z, Goldsmith R, Rushton A. Systematic review of the measurement properties of self‐report physical activity questionnaires in healthy adult populations. BMJ Open. 2015;5(9):e008430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ryan DJ, Wullems JA, Stebbings GK, et al. Reliability and validity of the international physical activity questionnaire compared to calibrated accelerometer cut‐off points in the quantification of sedentary behaviour and physical activity in older adults. PLoS One. 2018;13(4):e0195712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Buckner TW, Sidonio R, Guelcher C, et al. Reliability and validity of patient‐reported outcome instruments in US adults with hemophilia B and caregivers in the B‐HERO‐S study. Eur J Haematol. 2018;101(6):781‐790. [DOI] [PubMed] [Google Scholar]
- 61.Delshad M, Ghanbarian A, Ghaleh N, et al. Reliability and validity of the modifiable activity questionnaire for an Iranian urban adolescent population. Int J Prev Med. 2015;6(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Strath SJ, Leonard AK, Barbara EA, et al. Guide to the assessment of physical activity: clinical and research applications. Circulation. 2013;128(20):2259‐2279. [DOI] [PubMed] [Google Scholar]
- 63.Takken T, Stephens S, Balemans A, et al. Validation of the Actiheart activity monitor for measurement of activity energy expenditure in children and adolescents with chronic disease. Eur J Clin Nutr. 2010;64(12):1494‐1500. [DOI] [PubMed] [Google Scholar]
- 64.Walker RG, Obeid J, Nguyen T, et al. Sedentary time and screen‐based sedentary behaviors of children with a chronic disease. Pediatr Exerc Sci. 2015;27(2):219‐225. [DOI] [PubMed] [Google Scholar]
- 65.Timmer MA, Gouw SC, Feldman BM, et al. Measuring activities and participation in persons with haemophilia: A systematic review of commonly used instruments. Haemophilia. 2018;24(2):e33‐e49. [DOI] [PubMed] [Google Scholar]
- 66.Franchini M, Mannucci PM. Haemophilia B is clinically less severe than haemophilia A: further evidence. Blood Transfusion. 2018;16(2):121‐122. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Supplementary Material
Data Availability Statement
The data that support the findings of this review are available in the supplementary material of this article. Raw data are also available from the corresponding author upon reasonable request.


