Abstract
Posttransplant lymphoproliferative disorder (PTLD) is a serious complication of solid organ transplantation (SOT). Most PTLD cases are associated with Epstein–Barr virus (EBV) infection. The role of antiviral prophylaxis or rituximab therapy for prevention of PTLD in SOT recipients is controversial. In a nationwide cohort, we assessed the incidence, presentation, and outcome of histologically proven PTLD. We included 4765 patients with a follow‐up duration of 23 807 person‐years (py). Fifty‐seven PTLD cases were identified; 39 (68%) were EBV positive (EBV+ PTLD). Incidence rates for EBV+ PTLD at 1, 2, and 3 years posttransplant were 3.51, 2.24, and 1.75/1000 py and 0.44, 0.25, and 0.29/1000 py for EBV− PTLD. We did not find an effect of antiviral prophylaxis on early and late EBV+ PTLD occurrence (early EBV+ PTLD: SHR 0.535 [95% CI 0.199–1.436], p = .264; late EBV+ PTLD: SHR 2.213, [95% CI 0.751–6.521], p = .150). However, none of the patients (0/191) who received a rituximab‐containing induction treatment experienced PTLD, but 57 of 4574 patients without rituximab induction developed PTLD. In an adjusted restricted mean survival time model, PTLD‐free survival was significantly longer (0.104 years [95% CI 0.077–0.131]) in patients receiving rituximab as induction treatment. This study provides novel data on the association of rituximab induction and reduced risk for PTLD.
Keywords: clinical research/practice, complication: infectious, hematology/oncology, immunosuppressant –fusion proteins and monoclonal antibodies: B cell specific, infection and infectious agents – viral, infection and infectious agents – viral: Epstein‐Barr Virus (EBV), infectious disease, posttransplant lymphoproliferative disorder (PTLD)
Short abstract
In this nationwide cohort study of solid organ transplant recipients, rituximab given as part of the induction regimen, in contrast to antiviral prophylaxis, is associated with a lower incidence of posttransplant lymphoproliferative disorders.
Abbreviations
- ATG
antithymocyte globulin
- CMV
cytomegalovirus
- CNS
central nervous system
- D
donor
- EBER
EBV‐encoded RNA
- EBV
Epstein–Barr virus
- HSCT
hematologic stem cell transplant
- HSV
herpes simplex virus
- IQR
interquartile range
- LMP1
latent membrane protein 1
- MMF
mycophenolate mofetil
- PTLD
posttransplant lymphoproliferative disorder
- py
person‐years
- R
recipient
- RMST
restricted mean survival time analysis
- SHR
subdistribution hazard ratio
- SOT
solid organ transplantation
- STCS
Swiss Transplant Cohort Study
- VZV
varicella‐zoster virus
- WHO
World Health Organization
1. INTRODUCTION
Posttransplant lymphoproliferative disease (PTLD) is one of the most serious complications of solid organ transplantation (SOT). Epstein–Barr virus (EBV) is known to play a major role in the development of PTLD. However, EBV‐negative PTLD accounts for 20%–30% of cases.1 PTLD incidence ranges from 1% to 20% dependent on age,2 allograft type,3, 4, 5 type of induction treatment,3, 6 intensity of immunosuppressive therapy,3 time from transplant (early <1 year, late >1 year),7 and EBV serostatus of organ donor and recipient.8, 9 Several PTLD prevention strategies for EBV high‐risk SOT recipients (donor EBV positive, recipient EBV negative; D+/R−), such as EBV DNAemia surveillance with reduction of immunosuppression when DNAemia increases or administration of the cytolytic chimeric α‐CD20 monoclonal antibody rituximab10, 11, 12 have been proposed. Even though high EBV DNAemia is a risk factor for the onset of PTLD,13, 14 the time points for monitoring, source samples, and cutoff values for intervention are not standardized.7, 15
Antiviral prophylaxis by (val‐)acyclovir or (val‐)ganciclovir to prevent cytomegalovirus (CMV),16 herpes simplex virus (HSV), and varicella‐zoster virus (VZV) infection17, 18 has an inhibitory effect on lytic EBV replication and viral shedding.19 Nevertheless, the role of antiviral prophylaxis in prevention of EBV‐associated PTLD is controversial. A previous, retrospective study reported a reduced incidence of PTLD (3.9% vs. 0.5%) after introduction of antiviral prophylaxis20 and several other retrospective studies have supported its use.21, 22 These results have been challenged by more recent studies, which did not find a significant effect of antiviral prophylaxis on PTLD incidence.23, 24 In addition, a recent meta‐analysis reported no reduction in the rate of EBV‐associated PTLD in SOT recipients receiving antiviral prophylaxis by (val‐)acyclovir or (val‐)ganciclovir.25
In the hematologic stem cell transplant (HSCT) setting, the use of rituximab to prevent PTLD has become an initial preemptive intervention in the context of EBV replication.26 Moreover, rituximab used as prophylaxis pretransplant was associated with a reduction in EBV replication and EBV+ PTLD in high‐risk HSCT recipients.27 Less is known about the effect of rituximab on EBV+ PTLD occurrence in SOT recipients with EBV replication. Single center experiences have reported a reduction in PTLD rates with rituximab use in transplant recipients failing to respond to reducing immunosuppression compared with historical or contemporaneous controls.12, 28 To the best of our knowledge, the effect of rituximab, given as part of the induction regimen, on PTLD occurrence has not been studied yet.
The aim of this nationwide cohort study was to comprehensively describe the clinical characteristics of PTLD cases after SOT and to assess the effect of rituximab therapy and the use of antiviral prophylaxis on PTLD occurrence.
2. MATERIALS AND METHODS
2.1. Study design
We conducted a nested project based on data from the multicenter nationwide observational Swiss Transplant Cohort Study (STCS).29 In the current study, we included all SOT recipients enrolled in the STCS from May 2008 to June 2019. Only the first transplantation was analyzed. All six Swiss transplant centers participate in the STCS, and for the analyzed period, around 95% of all recipients of SOT performed in Switzerland consented to be included. The STCS and the current subproject were approved by the local ethics committee of each participating center (Ethics Commission of the Canton of Bern, Bern, Switzerland, Nr. 2019–00858).
2.2. Data collection
Clinical data on demographic characteristics, type of transplant, immunosuppressive regimen (induction and maintenance drugs, including rituximab), number of rejection episodes and rejection treatment (including rituximab), pretransplant donor and recipient CMV and EBV serostatus, administration and duration of antiviral prophylaxis (ganciclovir, valganciclovir, acyclovir, valacyclovir), occurrence of HSV, VZV, or CMV infection (number of episode per patient, use of antiviral therapy), and the occurrence of PTLD were prospectively collected and extracted from the STCS database. Additional data on PTLD (including localization, histopathological classification, and management) not captured in the STCS database were retrieved through a standardized data collection sheet from electronic medical records. Patient outcome (graft loss, death, death related to PTLD) were available from the STCS database. Treating physicians report systematically the most likely causes of death to the STCS using standardized data collection forms.
2.3. Clinical definitions
Histological confirmation was required for PTLD diagnosis. PTLD classification was based on World Health Organization criteria.30 EBV‐positive PTLD was identified by EBV‐encoded RNA (EBER) in situ hybridization or latent membrane protein 1 (LMP1) histochemical stains. PTLD localization was categorized as nodal and extra‐nodal or both. Early PTLD occurred in the first year after transplantation, late PTLD thereafter.
Viral infections are defined according to standard definitions generated by the Infectious Diseases Study Group of the STCS, as previously described.31 Each infection episode was validated by a Transplant Infectious Diseases specialist at each center. Furthermore, CMV infection and disease were classified according the definitions published by the American Society of Transplantation guidelines.32 We categorized SOT recipients to have rituximab‐containing induction regiments if rituximab was part of the induction treatment irrespective of the co‐administration of additional agents. Antiviral prophylaxis was initiated at the discretion of the treating physician in accordance with local protocols. For this study, antiviral prophylaxis was defined as the use of (val‐)ganciclovir or (val‐)acyclovir started within the first 2 weeks after transplantation.18 Acute rejection was defined for each organ following the standard international criteria.33
2.4. Statistical analyses
A descriptive analysis was performed to determine patients’ baseline characteristics, transplant outcome variables (acute rejection, graft loss, death), and episodes of CMV, HSV, and VZV (median number of episodes per patient). Cumulative PTLD incidence was calculated overall and by organ group. The impact of antiviral prophylaxis on EBV+ PTLD was analyzed using competing risk regression models (with death and graft loss as competing risk factors for EBV+ PTLD), adjusting for predefined confounding factors such as type of organ,3 EBV serostatus at transplantation,8 type of induction therapy,3 sex, and age.34 This was done separately for early and late EBV+ PTLD, since universal prophylaxis is given directly after transplantation and the treatment effect might be different for the two time periods.15 The impact of rituximab induction therapy on PTLD incidence was analyzed using restricted mean survival time analysis (RMST) adjusting for sex, age, transplanted organ, antithymocyte globulin (ATG) use, and EBV serostatus at transplantation. Since no development of PTLD occurred in patients with rituximab induction, competing‐risk regression models could not be used (violation of the proportional hazard assumption) for this analysis. RMST is a well‐established measure (based on differences in areas under Kaplan–Meier curves of two groups) that can be interpreted as the average event‐free survival time up to a prespecified time point.35 RMST is not dependent on the proportional hazards assumption.36 For this analysis, patients were censored at a maximum follow‐up duration of 9 years, death, graft loss or lost to follow‐up. The statistical analysis was conducted using STATA version 15.
3. RESULTS
3.1. Study population
A total of 4765 SOT recipients (57% kidney, 22% liver, 9% lung, 8% heart, 5% combined) were included. Median age at transplantation was 54 years (interquartile range [IQR] 42–62 years), and 36% (1711/4765) of patients were female. Median follow‐up time was 4.61 years (IQR 2.22–7.62). EBV high‐risk serostatus (D+/R−) was present in 6% (266/4765) of SOT recipients. Patient characteristics are detailed in Table 1.
Table 1.
Patient characteristics of recipients included in the analysis according to whether they developed PTLD, EBV associated or not
| No PTLD | PTLD | EBV+ PTLD | EBV− PTLD | |
|---|---|---|---|---|
| Characteristics | n = 4708 | n = 57 | n = 39 | n = 18 |
| Female, sex, n (%) | 1689 (36) | 22 (39) | 19 (49) | 3 (17) |
| Age at transplant, years, median (IQR) | 54 (42–62) | 47 (29–61) | 39 (20–59) | 61 (54–63) |
| Follow‐up, years, median (IQR) | 4.64 (2.22–7.64) | 2.09 (0.75–4.04) | 1.19 (0.65–2.74) | 4.74 (3.41–6.74) |
| EBV serostatus, n (%) | ||||
| EBV low‐risk (D−/R−) | 58 (1) | 1 (2) | 1 (3) | 0 (0) |
| EBV intermediate‐risk (R+) | 4326 (92) | 42 (74) | 24 (62) | 18 (100) |
| EBV high‐risk (D+/R−) | 253 (5) | 13 (23) | 13 (33) | 0 (0) |
| Missing | 71 (2) | 1 (2) | 1 (3) | 0 (0) |
| CMV serostatus, n (%) | ||||
| CMV low‐risk (D−/R−) | 925 (20) | 15 (26) | 12 (31) | 3 (17) |
| CMV intermediate‐risk (R+) | 2827 (60) | 31 (54) | 20 (51) | 11 (61) |
| CMV high‐risk (D+/R−) | 927 (20) | 10 (18) | 6 (15) | 4 (22) |
| Missing | 29 (1) | 1 (2) | 1 (3) | 0 (0) |
| Transplant, n (%) | ||||
| Kidney | 2674 (57) | 23 (40) | 16 (41) | 7 (39) |
| Liver | 1018 (22) | 16 (28) | 8 (21) | 8 (44) |
| Heart | 358 (8) | 4 (7) | 3 (8) | 1 (6) |
| Lung | 431 (9) | 11 (19) | 10 (26) | 1 (6) |
| Combined | 227 (5) | 3 (5) | 2 (5) | 1 (6) |
| Antiviral prophylaxis, n (%) | ||||
| 2097 (45) | 30 (53) | 25 (64) | 5 (28) | |
| (Val‐)ganciclovir, n (%) | 1872 (40) | 23 (40) | 18 (46) | 5 (28) |
| (Val‐)acyclovir, n (%) | 225 (5) | 7 (12) | 7 (18) | 0 (0) |
| Duration of acyclovir prophylaxis, days, median (IQR) | 94.5 (84.0–179.0) | 931.0 (8.0–1606.0) | 931.0 (8.0–1606.0) | 0 |
| Duration of ganciclovir prophylaxis, days, median (IQR) | 98.0 (79.0–173.0) | 61.0 (10.0–125.0) | 93.0 (10.0–170.0) | 27.0 (15.0–61.0) |
| Any CMV infection, n (%) | 1358 (29) | 16 (28) | 10 (26) | 6 (33) |
| Any VZV infection, n (%) | 158 (3) | 2 (4) | 0 (0) | 2 (11) |
| Any HSV infection, n (%) | 288 (6) | 4 (7) | 3 (8) | 1 (6) |
| Induction regiment contained, n (%) | ||||
| Basiliximab/Other | 3075 (65) | 41 (72) | 28 (72) | 13 (72) |
| ATG | 1016 (22) | 12 (21) | 9 (23) | 3 (17) |
| Rituximab | 191 (4) | 0 (0) | 0 (0) | 0 (0) |
| Unknown | 426 (9) | 4 (7) | 2 (5) | 2 (11) |
| Maintenance immunosuppression, n (%) | ||||
| Glucocorticosteroids | 4513 (97) | 55 (96) | 38 (97) | 17 (94) |
| MMF | 4054 (87) | 48 (84) | 32 (82) | 16 (89) |
| Azathioprin | 126 (3) | 3 (5) | 2 (5) | 1 (6) |
| Cyclosporin | 1047 (23) | 17 (30) | 14 (36) | 3 (17) |
| Tacrolimus | 3305 (71) | 38 (67) | 25 (64) | 13 (72) |
| Everolimus | 53 (1) | 1 (2) | 1 (3) | 0 (0) |
| Sirolimus | 23 (0) | 0 (0) | 0 (0) | 0 (0) |
| Unknown | 58 (1) | 0 (0) | 0 (0) | 0 (0) |
| Any rejection episode, n (%) | 1794 (38) | 21 (37) | 13 (33) | 8 (44) |
| Rejection episodes, n, median (IQR) | 1 (1–3) | 2 (1–2) | 2 (1–2) | 2 (2–2) |
| Treated rejection episodes, n, median (IQR) | 1 (1–2) | 1 (1–2) | 1 (1–2) | 2 (1–2) |
Abbreviations: ATG, antithymocyte globulin; CMV, cytomegalovirus; D, donor; HSV, herpes simplex virus; IQR, interquartile range; MMF, mycophenolate mofetil; PTLD, posttransplant lymphoproliferative disorder; R, recipient; VZV, varicella‐zoster virus.
3.2. Characteristics, treatment, and outcome of PTLD
Among 57 PTLD cases identified, 68% (39/57) were EBV+. Clinical characteristics, treatment, and outcome of PTLD cases are shown in Table 2.
Table 2.
PTLD incidence, classification, management, and outcome
| Total | EBV+ PTLD | EVB− PTLD | |
|---|---|---|---|
| n = 57 | n = 39 | n = 18 | |
|
Overall PTLD incidence rate /1000 py (IQR) according to organ transplant (IQR) |
2.39 (1.84–3.11) | 1.63 (1.19–2.24) | 0.75 (0.47–1.2) |
| Kidney | 1.59 (1.06–2.39) | 1.01 (0.68–1.81) | 0.48 (0.23–1.02) |
| Liver | 3.41 (2.09–5.57) | 1.71 (0.85–3.41) | 1.71 (0.85–3.41) |
| Heart | 2.41 (0.91–0.42) | 1.81 (0.58–5.61) | 0.60 (0.08–4.28) |
| Lung | 5.77 (3.19–10.42) | 5.24 (2.28–9.75) | 0.52 (0.07–3.72) |
| Combined | 2.68 (0.86–8.32) | 1.79 (0.44–7.16) | 0.89 (0.12–6.35) |
| PTLD WHO classification, n (%) | |||
| Early lesions | 6 (11) | 5 (13) | 1 (6) |
| Polymorphic PTLD | 13 (23) | 12 (31) | 1 (6) |
| Monomorphic PTLD | 38 (67) | 22 (56) | 16 (89) |
| B cell PTLD, n (%) | 54 (95) | 38 (97) | 16 (89) |
| T cell PTLD, n (%) | 3 (5) | 1 (3) | 2 (11) |
| CD20 status, n (%) | |||
| Positive | 43 (75) | 31 (79) | 12 (67) |
| Negative | 7 (12) | 5 (13) | 2 (11) |
| Unknown | 7 (12) | 3 (8) | 4 (22) |
| PTLD localization, n (%) | |||
| CNS | 8 (14) | 8 (21) | 0 (0) |
| Extra‐nodal | 48 (84) | 34 (87) | 14 (78) |
| Nodal | 8 (14) | 4 (10) | 4 (22) |
| Unknown | 1 (2) | 1 (3) | 0 (0) |
| PTLD management, n (%) | |||
| Reduction of immunosuppression alone | 7 (12) | 7 (18) | 0 (0) |
| Rituximab alone | 20 (35) | 14 (36) | 6 (33) |
| Chemotherapy alone | 5 (9) | 1 (3) | 4 (22) |
| Rituximab and chemotherapy | 19 (33) | 13 (33) | 6 (33) |
| Other | 6 (11) | 4 (10) | 2 (11) |
| PTLD outcome, n (%) | |||
| Died during follow‐up | 25 (44) | 16 (41) | 9 (50) |
| PTLD‐related death | 16 (28) | 10 (26) | 6 (33) |
| Time to PTLD‐related death, month (IQR) | 6.8 (1.3–12.9) | 7.3 (1.6–13.5) | 4.1 (0.3–8.6) |
Abbreviations: CNS, central nervous system; IQR, interquartile range; PTLD, posttransplant lymphoproliferative disorder; py; person‐years; WHO, World Health Organization.
The overall PTLD incidence was 2.39 per 1000 person‐years (py) and the highest incidence was found among lung transplant recipients (5.77/1000 py; Table 2). The incidence of EBV+ PTLD was highest in the first‐year posttransplant (3.51/1000 py), this was not the case for EBV− PTLD cases (Figure 1).
Figure 1.
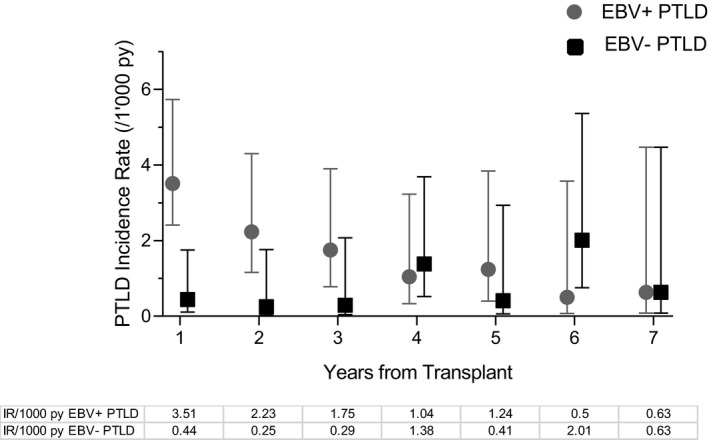
EBV+ and EBV− PTLD incidence per 1000 person‐years. Symbols represent point‐estimates, whiskers 95% confidence intervals. EBV, Epstein–Barr virus; IR, incidence rate; PTLD, posttransplant lymphoproliferative disorder; py, person‐years
Histopathological classification revealed early lesions in 11% (6/57), polymorphic in 23% (13/57), and monomorphic PTLD in 67% (38/57) of cases. Most PTLDs were of B cell origin (95%; 54/57). Extra‐nodal involvement was common (in 87% of EBV+ PTLD and 78% of EBV− PTLD; Figure 2) and central nervous system (CNS) involvement was exclusively found in EBV+ PTLD (21%; 8/39). PTLD lesions of the transplanted organ were detected in 29% (14/49) of patients with extra‐nodal involvement and most of these lesions were EBV+ (79%; 11/14).
Figure 2.
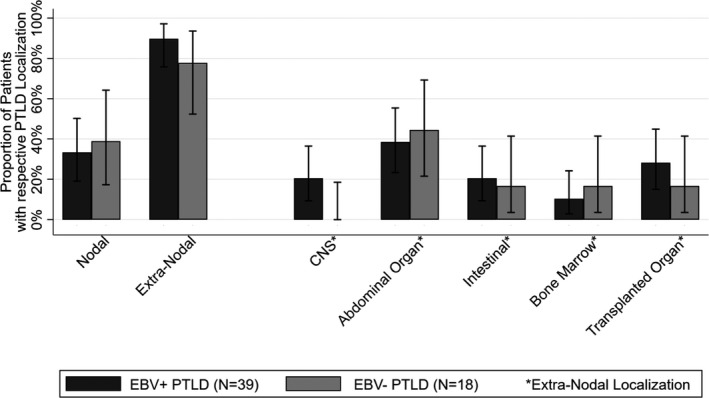
Localization of PTLD according to EBV association. Each extra‐nodal localization is counted separately (exceeds number of cases). CNS, central nervous system; EBV, Epstein–Barr virus; PTLD; posttransplant lymphoproliferative disorder
SOT recipients with EBV+ PTLD were younger compared to patients with EBV− PTLD (39 [IQR 20–59] vs. 61 years [IQR 54–63]; p < .01) and the median time from transplantation to PTLD diagnosis was shorter; 14.33 months (IQR 7.82–32.91) for EBV+ PTLD vs. 56.84 months (IQR 40.93–80.87; p < .001) for EBV− PTLD. EBV high‐risk serostatus (D+/R−) was more frequent in EBV+ PTLD (33%; 13/39) than in EBV− PTLD (0%; 0/18; p < .01).
Reducing immunosuppression alone was the treatment for 18% (7/39) of EBV+ PTLD cases while none of the EBV− PTLD cases were managed by reducing immunosuppression alone. Rituximab monotherapy was given in 35% (20/57) of patients.
Forty‐four percent (25/57) of patients diagnosed with PTLD died during follow‐up, with most deaths attributed to PTLD (64%; 16/25). PTLD‐related mortality was similar among EBV+ PTLD and EBV− PTLD cases (33% vs. 26%; p = .55, overall 28%) and the median time to PTLD‐related death did not differ among EBV+ PTLD (4.1 months [IQR 0.3–8.6]) and EBV− PTLD (7.3 months [IQR 1.6–13.5]; p = .28).
3.3. EBV+ PTLD and antiviral prophylaxis
Overall, 44.6% (2127/4765) of patients received antiviral prophylaxis with (val‐) ganciclovir (n = 1895) or with (val‐)acyclovir (n = 232) for a median duration of 97 days (IQR 79–173). The rate of recipients receiving antiviral prophylaxis was dependent on the type of transplant. Antiviral prophylaxis was more frequently given to lung transplant recipients (94% [414/442]) compared to kidney (45%; 1206/2697) heart (44%; 161/364), liver (22%; 230/1034), or combined transplant recipients (50%; 116/230). SOT recipients receiving antiviral prophylaxis were younger (median age: 53 [IQR 39–61] vs. 55 [IQR 43–62] years; p < .001) and more likely to have received ATG as part of the induction treatment (30% vs. 13%; p < .001) (Table S1). These patients also had a higher incidence of treatment requiring rejection episodes (221/1000 py [95% CI 209.4–233.9] vs. 268.5/1000 py [95% CI 254.1–283.8]). The crude EBV+ PTLD incidence rate in the first‐year posttransplant was 3.87 of 1000 py (95% CI 1.94–7.73) for patients receiving antiviral prophylaxis versus 3.21 of 1000 py (95% CI 1.61–6.41) for patients without antiviral prophylaxis (p = .35). A trend for a higher incidence rate was found for patients receiving (val‐)acyclovir (8.89/1000 py [95% CI 2.22–35.58]) compared to (val‐)ganciclovir (3.26/1000 py [95% CI 1.46–7.25]; p = .13) in the first‐year after transplant.
Crude incidence of EBV+ PTLD for the entire follow‐up duration was higher for patients receiving antiviral prophylaxis (2.31/1000 py [95% CI 0.64–1.82]) compared to patients not receiving prophylaxis (1.08/1000 py [95% CI 1.56–3.42]). In the adjusted risk regression model, we did not find an effect of antiviral prophylaxis on EBV+ PTLD incidence in the first‐year posttransplant or beyond this period (early EBV+ PTLD: SHR 0.535 [95% CI 0.199–1.436]; p = .264; late EBV+ PTLD: SHR 2.213 [95% CI 0.751–6.521]; Table 3). The results remained unchanged when comparing (val‐)ganciclovir, which is believed to be more active against EBV than (val‐) acyclovir,37 versus no antiviral prophylaxis (data not shown).
Table 3.
Risk factors of EBV+ PTLD early (<1 year) and late (>1 year) after transplantation
| Variable | Multivariable analysis | p‐value |
|---|---|---|
| SHR (95% CI) | ||
| Early EBV+ PTLD (<1 year after transplant) | ||
| Sex | ||
| Female | Reference | |
| Male | 1.030 (0.379–2.798) | .953 |
| Age | 0.992 (0.967–1.017) | .525 |
| Antiviral prophylaxis | ||
| No prophylaxis | Reference | |
| Antiviral prophylaxis | 0.535 (0.199–1.436) | .264 |
| EBV serostatus | ||
| Non high‐risk (D−/R−)/(R+) | Reference | |
| High‐risk (D+/R−) | 18.586 (5.540–62.355) | <.001 |
| Induction therapy | ||
| No ATG | Reference | |
| ATG | 1.284 (0.444–3.717) | .645 |
| Organ transplant | ||
| Kidney | Reference | |
| Liver | 1.256 (0.269–5.851) | .771 |
| Heart | 2.055 (0.476–8.881) | .334 |
| Lung | 5.979 (1.542–23.176) | .010 |
| Combined | 3.333 (0.470–23.604) | .228 |
| Late EBV+ PTLD (>1 year after transplant) | ||
| Sex | ||
| Female | Reference | |
| Male | 0.0543 (0.229–1.283) | .164 |
| Age | 0.965 (0.943–0.988) | .003 |
| Antiviral prophylaxis | ||
| No prophylaxis | Reference | |
| Antiviral prophylaxis | 2.213 (0.751–6.521) | .150 |
| EBV serostatus | ||
| Non high‐risk (D−/R−)/(R+) | Reference | |
| High‐risk (D+/R−) | 1.760 (0.605–5.120) | .178 |
| Induction therapy | ||
| No ATG | Reference | |
| ATG | 1.847 (0.519–6.571) | .343 |
| Organ transplant | ||
| Kidney | Reference | |
| Liver | 1.420 (0.502–4.015) | .508 |
| Heart | 0.421 (0.045–3.927) | .448 |
| Lung | 2.083 (0.650–6.669) | .217 |
| Combined | 0.893 (0.094–8.497) | .922 |
Abbreviations: ATG, antithymocyte globulin; EBV, Epstein–Barr virus; D, donor; IQR, interquartile range; PTLD, posttransplant lymphoproliferative disorder; R, recipient; SHR, subdistribution hazard ratio.
Variables significantly associated with a higher risk of EBV+ PTLD occurrence differed in early EBV+ PTLD (<1 year) compared to late (>1 year) EBV+ PTLD (Table 3).
3.4. PTLD and rituximab therapy
None of the 191 patients receiving rituximab as part of the induction treatment developed PTLD (Figure 3). Patients with rituximab induction therapy were younger (median age: 51 [IQR 41–61] vs. 54 [IQR 42–62] years; p < .001). The EBV serostatus distribution among both groups was similar. Most SOT recipients receiving rituximab as induction treatment were renal transplant recipients (95%; 182/191). The majority of these renal transplant recipients (88%; 161/182) received rituximab as part of their induction therapy for AB0 incompatible renal transplantation (Table S2). In the adjusted restricted mean survival time model (RMST), the mean PTLD‐free survival time at 9 years of follow‐up was significantly shorter (0.104 years [95% CI 0.077–0.131]) in patients not receiving rituximab as induction treatment (competing‐risk models were inappropriate due to violation of proportional hazard assumption; no PTLD occurred in patients receiving rituximab). Meaning that the average loss of PTLD‐free survival time at 9 years was 0.104 years in the group not receiving rituximab (Figure S1). In addition, none of the recipients receiving rituximab for treatment of rejection (n = 121) experienced PTLD during follow‐up.
Figure 3.
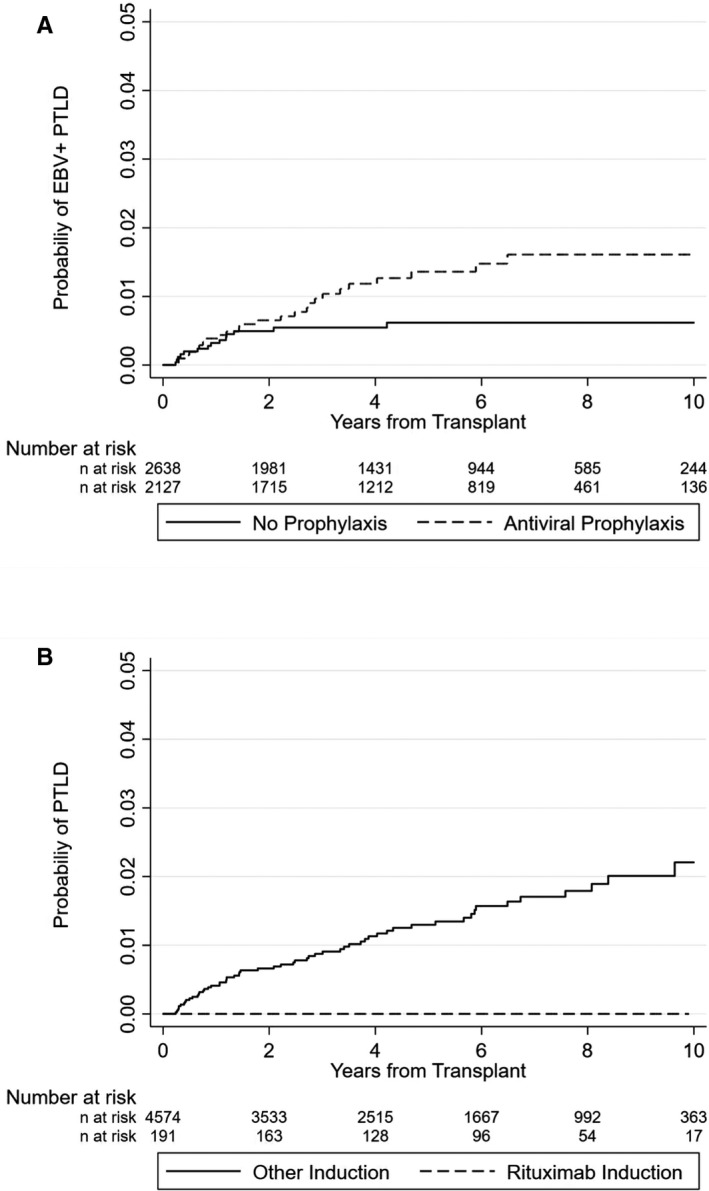
(A,B) Probability of EBV+ PTLD (A) or PTLD (B) occurrence according to antiviral prophylaxis (A) or rituximab therapy (B). EBV, Epstein–Barr virus; PTLD; posttransplant lymphoproliferative disorder
We performed a subanalysis restricted to renal transplant recipients to analyze if the effect of rituximab on PTLD occurrence is also verifiable in this subgroup. This analysis (adjusted RMST) confirmed the findings seen in the overall cohort that, patients not receiving rituximab induction had a significant shorter mean PTLD‐free survival time at 9 years follow‐up (0.067 years [95% CI 0.039–0.096]).
4. DISCUSSION
We assessed the clinical characteristics, incidence, and outcome of PTLD in a nationwide cohort (STCS). In addition, we explored the association of antiviral prophylaxis and rituximab induction therapy on PTLD occurrence. The major findings of our study are as follows: (1) the PTLD incidence rate among Swiss SOT recipients was low and depended on the type of transplant; (2) extra‐nodal involvement was common; (3) the mortality among SOT recipients with PTLD was high; (4) antiviral prophylaxis was not associated with a reduction of EBV+ PTLD occurrence; and (5) in contrast, rituximab induction therapy was associated with a reduced risk of PTLD occurrence.
The incidence rate of PTLD ranged from 1.59/1000 py to 5.77/1000 py dependent on type of transplant which is lower than previously reported in other cohorts.24, 38 This might be due to the effects of continuous improvement in prevention, such as screening for EBV DNAemia and preemptive reduction in immunosuppression, in the current era of transplant medicine.15 Incidence rates of EBV+ PTLD and EBV− PTLD differed over time. EBV+ PTLD incidence was highest in the first‐year posttransplant (3.51/1000 py [95% CI 2.14–5.72]) and decreased thereafter; this was not the case for EBV− PTLD (0.43/1000 py [95% CI 0.11–1.75]). The distinct temporal occurrence of EBV+ and EBV− PTLD potentially reflects the different biological entity of these two malignancies15, 39, 40 and may also explain the biphasic pattern of PTLD occurrence described in previous studies.41, 42, 43
In our study, most PTLDs had extra‐nodal localization. Nodal involvement was only found in about one third of cases. Interestingly, extra‐nodal involvement of lymphomas in the general population is less common44 and is associated with poor outcome.45 PTLD localization in the transplanted organ was mainly present in lung and liver recipients and more often found in EBV+ PTLD compared to EBV− PTLD (28% vs. 16%). Similar to our findings, previous studies also reported high rates of extra‐nodal PTLD.46 In our cohort, PTLD‐related mortality was around 30%. This is in line with findings of contemporary reports in pediatric47 and adult48 SOT PTLD patients. Present outcomes of patients, especially those with CD20+ PTLD, improved compared to historic reports, most likely as a result of the availability of rituximab and improved management of immunosuppression.15
Similar to previous reports, we identified different risk factors for early and late EBV+ PTLD.7 In our analysis, early EBV+ PTLD was associated with EBV high‐risk (D+/R−) serostatus (SHR 18.586 [95% CI 5.54–62.35]) and lung transplantation (SHR 5.98 [95% CI 1.54–23.17]). We did not find an association between young age or induction therapy with ATG and the risk for early EBV+ PTLD as reported by others.38 Most likely, this is due to the underrepresentation of pediatric patients in our cohort and the lower ATG doses used in the recent era compared to historical studies.15 Occurrence of late EBV+ PTLD was associated with young age at transplantation. However, this rather reflects an immortal person‐time bias than a real finding.49 In cohorts with an exclusively adult population, higher age is associated with occurrence of late PTLD.7
When correcting for known risk factors, EBV+ PTLD incidence rates were similar with or without antiviral prophylaxis. This is in line with the findings of the most recent meta‐analysis.25 There is a biologically plausible explanation for our findings. EBV‐active antiviral substances ((val‐)ganciclovir, (val‐)acyclovir)) are pro‐drugs and need to undergo phosphorylation by a viral thymidine kinase (TK), but EBV‐transformed proliferating B cells are latently infected and do not express EBV TK proteins.50, 51 Therefore, none of these antiviral agents can act on EBV‐driven cell proliferation of B cells.
In our cohort, rituximab given as part of the induction regimen was associated with a reduced risk for PTLD. The difference in average loss of PTLD‐free survival time at 9 years posttransplant (0.104 years [95% CI 0.077–0.131]) might appear small but the rarity of events and the large cohort have to be taken in to account when interpreting the data. The use of rituximab has become a common preemptive intervention strategy in EBV viremic HSCT recipients to reduce the risk of PTLD.26 Moreover, rituximab given prophylactically before or directly after HSCT was shown to reduce EBV replication52, 53 and EBV+ PTLD in high‐risk HSCT recipients.27 In SOT, reduced PTLD rates have been reported if rituximab was used preemptively in heart12 and renal28 transplant recipients, who failed to control EBV DNAemia despite reduction in immunosuppression. Rituximab is already used in the context of desensitization before AB0‐incompatible renal transplantation54 and has been given as sole induction therapy for renal transplantation.55 Side effects associated with rituximab mainly include an increased risk for infection56 and concerns about the emergence of CD20‐negative PTLD after receiving rituximab were previously expressed.57 The potential effect of rituximab on subsequent PTLD occurrence might be attributable to the depletion of CD20+ B cells58 which represent the major reservoir for latent EBV infection. The reduced abundance of these cells at risk for malignant transformation might be linked to a lower risk for PTLD.
Our study has several limitations. The relatively small number of PTLD cases potentially affects the power to identify factors associated with increased or reduced risk for development of PTLD. We cannot exclude underreporting of PTLD in our cohort, in particular late posttransplant, when patients were not exclusively followed at the transplant center. However, SOT recipients are to be expected to be referred to a tertiary transplant center upon PTLD diagnosis and all referral centers participate in the STCS. The duration of antiviral prophylaxis was not available for a relevant proportion of SOT recipients. In consequence, the effect of the duration of antiviral prophylaxis could not be adequately assessed. In addition, data on antiviral treatment duration for patients not receiving primary antiviral prophylaxis were not available. The majority of SOT recipients receiving rituximab as induction therapy were renal transplant recipients (95%). Therefore, the association of rituximab therapy and a reduction of PTLD incidence might not apply for other SOT recipients. Our findings regarding the effect of rituximab on PTLD occurrence are based on an observational study design, therefore we cannot exclude that this association is caused by confounding factors. However, we tried to address this by adjusting our multivariate model (restricted mean survival time model for rituximab use and development of PTLD) for confounding factors associated with PTLD including the type of transplanted organ, use of ATG, and EBV serostatus. To exclude that the effect of rituximab is solely caused by confounding due to a comparison of renal transplant patients with other transplant types which are associated with a higher PTLD risk, we performed a subgroup analysis restricted to renal transplant recipients. The association of a reduced PTLD incidence in patients with rituximab induction was also confirmed in this subgroup analysis. We therefore think that the reduced risk for PTLD development is rather associated with the use of rituximab and not by confounding factors.
In our study we did not address the potentially negative impact of rituximab induction. Therefore, we cannot provide an elaborated risk–benefit analysis of rituximab induction.
In summary, in this nationwide SOT cohort, PTLD incidence rate was low, but still associated with notable mortality. The incidence of EBV+ PTLD declined over time and was highest in the first‐year posttransplant, while EBV− PTLD incidence did not decline. There was no association between antiviral prophylaxis and PTLD incidence. We provide novel information that rituximab given as part of the induction regimen was associated with a decreased risk for PTLD occurrence. This finding needs to be confirmed in independent cohorts.
DISCLOSURE
The authors of this manuscript have no conflicts of interest as described by the American Journal of Transplantation.
Supporting information
AppendixS1
FigureS1
ACKNOWLEDGMENTS
This study has been conducted in the framework of the Swiss Transplant Cohort Study, supported by the Swiss National Science Foundation, the Swiss University Hospitals, and Transplant Centers (grant number: 33CS30_177522). The authors thank all patients for their willingness to participate in the STCS.
†The members of the Swiss Transplant Cohort Study are listed in the Appendix.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1.Leblond V, Davi F, Charlotte F, et al. Posttransplant lymphoproliferative disorders not associated with Epstein‐Barr virus: a distinct entity? J Clin Oncol. 1998;16(6):2052‐2059. [DOI] [PubMed] [Google Scholar]
- 2.Debray D, Baudouin V, Lacaille F, et al. De novo malignancy after solid organ transplantation in children. Transplant Proc. 2009;41(2):674‐675. [DOI] [PubMed] [Google Scholar]
- 3.Opelz G, Dohler B. Lymphomas after solid organ transplantation: a collaborative transplant study report. Am J Transplantat. 2004;4(2):222‐230. [DOI] [PubMed] [Google Scholar]
- 4.Steiner R, Kridel R, Giostra E, et al. Low 5‐year cumulative incidence of post‐transplant lymphoproliferative disorders after solid organ transplantation in Switzerland. Swiss Med Wkly. 2018;148:w14596. [DOI] [PubMed] [Google Scholar]
- 5.Green M, Michaels MG. Epstein‐Barr virus infection and posttransplant lymphoproliferative disorder. Am J Transplant. 2013;13(Suppl 3):41‐54; quiz 54. [DOI] [PubMed] [Google Scholar]
- 6.Hall EC, Engels EA, Pfeiffer RM, et al. Association of antibody induction immunosuppression with cancer after kidney transplantation. Transplantation. 2015;99(5):1051‐1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.San‐Juan R, Comoli P, Caillard S, et al. Epstein‐Barr virus‐related post‐transplant lymphoproliferative disorder in solid organ transplant recipients. Clin Microbiol Infect. 2014;20(Suppl 7):109‐118. [DOI] [PubMed] [Google Scholar]
- 8.Opelz G, Daniel V, Naujokat C, et al. Epidemiology of pretransplant EBV and CMV serostatus in relation to posttransplant non‐Hodgkin lymphoma. Transplantation. 2009;88(8):962‐967. [DOI] [PubMed] [Google Scholar]
- 9.Liu M, Husain S, Famure O, et al. Incidence, risk factors, clinical management, and outcomes of posttransplant lymphoproliferative disorder in kidney transplant recipients. Prog Transplant. 2019;29(2):185‐193. [DOI] [PubMed] [Google Scholar]
- 10.Kumar D, Patil N, Husain S, et al. Clinical and virologic outcomes in high‐risk adult Epstein‐Barr virus mismatched organ transplant recipients. Clin Transplant. 2017;31(7). [DOI] [PubMed] [Google Scholar]
- 11.Dharnidharka VR. Peripheral blood Epstein‐Barr viral nucleic acid surveillance as a marker for posttransplant cancer risk. Am J Transplant. 2017;17(3):611‐616. [DOI] [PubMed] [Google Scholar]
- 12.Choquet S, Varnous S, Deback C, et al. Adapted treatment of Epstein‐Barr virus infection to prevent posttransplant lymphoproliferative disorder after heart transplantation. Am J Transplant. 2014;14(4):857‐866. [DOI] [PubMed] [Google Scholar]
- 13.Holman CJ, Karger AB, Mullan BD, et al. Quantitative Epstein‐Barr virus shedding and its correlation with the risk of post‐transplant lymphoproliferative disorder. Clin Transplant. 2012;26(5):741‐747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bingler MA, Feingold B, Miller SA, et al. Chronic high Epstein‐Barr viral load state and risk for late‐onset posttransplant lymphoproliferative disease/lymphoma in children. Am J Transplant. 2008;8(2):442‐445. [DOI] [PubMed] [Google Scholar]
- 15.Allen UD, Preiksaitis JK. Post‐transplant lymphoproliferative disorders, Epstein‐Barr virus infection, and disease in solid organ transplantation: Guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin Transplant. 2019;33(9):e13652. [DOI] [PubMed] [Google Scholar]
- 16.Kotton CN, Kumar D, Caliendo AM, et al. The Third International Consensus guidelines on the management of cytomegalovirus in solid‐organ transplantation. Transplantation. 2018;102(6):900‐931. [DOI] [PubMed] [Google Scholar]
- 17.Pergam SA, Limaye AP, AST Infectious Diseases Community of Practice . Varicella zoster virus (VZV) in solid organ transplant recipients. Am J Transplant. 2009;9(Suppl 4):S108‐S115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martin‐Gandul C, Stampf S, Hequet D, et al. preventive strategies against cytomegalovirus and incidence of alpha‐herpesvirus infections in solid organ transplant recipients: a nationwide cohort study. Am J Transplant. 2017;17(7):1813‐1822. [DOI] [PubMed] [Google Scholar]
- 19.Cohen JI. Epstein‐Barr virus infection. N Engl J Med. 2000;343(7):481‐492. [DOI] [PubMed] [Google Scholar]
- 20.Darenkov IA, Marcarelli MA, Basadonna GP, et al. Reduced incidence of Epstein‐Barr virus‐associated posttransplant lymphoproliferative disorder using preemptive antiviral therapy. Transplantation. 1997;64(6):848‐852. [DOI] [PubMed] [Google Scholar]
- 21.Levine SM, Angel L, Anzueto A, et al. A low incidence of posttransplant lymphoproliferative disorder in 109 lung transplant recipients. Chest. 1999;116(5):1273‐1277. [DOI] [PubMed] [Google Scholar]
- 22.Malouf MA, Chhajed PN, Hopkins P, et al. Anti‐viral prophylaxis reduces the incidence of lymphoproliferative disease in lung transplant recipients. J Heart Lung Transplant. 2002;21(5):547‐554. [DOI] [PubMed] [Google Scholar]
- 23.Aris RM, Maia DM, Neuringer IP, et al. Post‐transplantation lymphoproliferative disorder in the Epstein‐Barr virus‐naive lung transplant recipient. Am J Respir Crit Care Med. 1996;154(6 Pt 1):1712‐1717. [DOI] [PubMed] [Google Scholar]
- 24.Opelz G, Daniel V, Naujokat C, et al. Effect of cytomegalovirus prophylaxis with immunoglobulin or with antiviral drugs on post‐transplant non‐Hodgkin lymphoma: a multicentre retrospective analysis. Lancet Oncol. 2007;8(3):212‐218. [DOI] [PubMed] [Google Scholar]
- 25.AlDabbagh MA, Gitman MR, Kumar D, et al. The role of antiviral prophylaxis for the prevention of Epstein‐Barr virus‐associated posttransplant lymphoproliferative disease in solid organ transplant recipients: a systematic review. Am J Transplant. 2017;17(3):770‐781. [DOI] [PubMed] [Google Scholar]
- 26.Rouce RH, Louis CU, Heslop HE. Epstein‐Barr virus lymphoproliferative disease after hematopoietic stem cell transplant. Curr Opin Hematol. 2014;21(6):476‐481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van Besien K, Bachier‐Rodriguez L, Satlin M, et al. Prophylactic rituximab prevents EBV PTLD in haplo‐cord transplant recipients at high risk. Leuk Lymphoma. 2019;60(7):1693‐1696. [DOI] [PubMed] [Google Scholar]
- 28.Martin SI, Dodson B, Wheeler C, et al. Monitoring infection with Epstein‐Barr virus among seromismatch adult renal transplant recipients. Am J Transplant. 2011;11(5):1058‐1063. [DOI] [PubMed] [Google Scholar]
- 29.Koller MT, van Delden C, Müller NJ, et al. Design and methodology of the Swiss Transplant Cohort Study (STCS): a comprehensive prospective nationwide long‐term follow‐up cohort. Eur J Epidemiol. 2013;28(4):347‐355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Swerdlow SH. WHO classification of tumours of haematopoietic and lymphoid tissues. WHO classification of tumours. 2008;22008:439. [PubMed] [Google Scholar]
- 31.van Delden C, Stampf S, Hirsch HH, et al. Burden and timeline of infectious diseases in the first year after solid organ transplantation in the Swiss Transplant Cohort Study. Clin Infect Dis. 2020;71(7):e159‐e169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Humar A, Michaels M, AST ID Working Group on Infectious Disease Monitoring . American Society of Transplantation recommendations for screening, monitoring and reporting of infectious complications in immunosuppression trials in recipients of organ transplantation. Am J Transplant. 2006;6(2):262‐274. [DOI] [PubMed] [Google Scholar]
- 33.Golshayan D, Wojtowicz A, Bibert S, et al. Polymorphisms in the lectin pathway of complement activation influence the incidence of acute rejection and graft outcome after kidney transplantation. Kidney Int. 2016;89(4):927‐938. [DOI] [PubMed] [Google Scholar]
- 34.Gibson TM, Engels EA, Clarke CA, et al. Risk of diffuse large B‐cell lymphoma after solid organ transplantation in the United States. Am J Hematol. 2014;89(7):714‐720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim DH, Uno H, Wei LJ. Restricted mean survival time as a measure to interpret clinical trial results. JAMA Cardiol. 2017;2(11):1179‐1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gregson J, Sharples L, Stone GW, et al. Nonproportional hazards for time‐to‐event outcomes in clinical trials: JACC review topic of the week. J Am Coll Cardiol. 2019;74(16):2102‐2112. [DOI] [PubMed] [Google Scholar]
- 37.Funch DP, Walker AM, Schneider G, et al. Ganciclovir and acyclovir reduce the risk of post‐transplant lymphoproliferative disorder in renal transplant recipients. Am J Transplant. 2005;5(12):2894‐2900. [DOI] [PubMed] [Google Scholar]
- 38.Caillard S, Dharnidharka V, Agodoa L, et al. Posttransplant lymphoproliferative disorders after renal transplantation in the United States in era of modern immunosuppression. Transplantation. 2005;80(9):1233‐1243. [DOI] [PubMed] [Google Scholar]
- 39.Morscio J, Tousseyn T. Recent insights in the pathogenesis of post‐transplantation lymphoproliferative disorders. World J Transplant. 2016;6(3):505‐516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dharnidharka VR, Webster AC, Martinez OM, et al. Post‐transplant lymphoproliferative disorders. Nat Rev Dis Primers. 2016;2:15088. [DOI] [PubMed] [Google Scholar]
- 41.Quinlan SC, Pfeiffer RM, Morton LM, et al. Risk factors for early‐onset and late‐onset post‐transplant lymphoproliferative disorder in kidney recipients in the United States. Am J Hematol. 2011;86(2):206‐209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dharnidharka VR. Comprehensive review of post‐organ transplant hematologic cancers. Am J Transplant. 2018;18(3):537‐549. [DOI] [PubMed] [Google Scholar]
- 43.L'Huillier AG, Dipchand AI, Ng VL, et al. Posttransplant lymphoproliferative disorder in pediatric patients: survival rates according to primary sites of occurrence and a proposed clinical categorization. Am J Transplant. 2019;19(10):2764‐2774. [DOI] [PubMed] [Google Scholar]
- 44.Zucca E, Cavalli F. Extranodal lymphomas. Ann Oncol. 2000;11(Suppl 3):219‐222. [DOI] [PubMed] [Google Scholar]
- 45.Zucca E, Conconi A, Cavalli F. Treatment of extranodal lymphomas. Best Pract Res Clin Haematol. 2002;15(3):533‐547. [DOI] [PubMed] [Google Scholar]
- 46.Ghobrial IM, Habermann TM, Maurer MJ, et al. Prognostic analysis for survival in adult solid organ transplant recipients with post‐transplantation lymphoproliferative disorders. J Clin Oncol. 2005;23(30):7574‐7582. [DOI] [PubMed] [Google Scholar]
- 47.L’Huillier AG, Dipchand AI, Ng VL, et al. Posttransplant lymphoproliferative disorder in pediatric patients: characteristics of disease in EBV‐seropositive recipients. Transplantation. 2019;103(11):e369‐e374. [DOI] [PubMed] [Google Scholar]
- 48.Caillard S, Porcher R, Provot F, et al. Post‐transplantation lymphoproliferative disorder after kidney transplantation: report of a nationwide French registry and the development of a new prognostic score. J Clin Oncol. 2013;31(10):1302‐1309. [DOI] [PubMed] [Google Scholar]
- 49.Ho A m‐h, Dion PW, Ng CSH, et al. Understanding immortal time bias in observational cohort studies. Anaesthesia. 2013;68(2):126‐130. [DOI] [PubMed] [Google Scholar]
- 50.Colby BM, Shaw JE, Elion GB, et al. Effect of acyclovir [9‐(2‐hydroxyethoxymethyl)guanine] on Epstein‐Barr virus DNA replication. J Virol. 1980;34(2):560‐568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gershburg E, Marschall M, Hong K, et al. Expression and localization of the Epstein‐Barr virus‐encoded protein kinase. J Virol. 2004;78(22):12140‐12146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dominietto A, Tedone E, Soracco M, et al. In vivo B‐cell depletion with rituximab for alternative donor hemopoietic SCT. Bone Marrow Transplant. 2012;47(1):101‐106. [DOI] [PubMed] [Google Scholar]
- 53.Burns DM, Rana S, Martin E, et al. Greatly reduced risk of EBV reactivation in rituximab‐experienced recipients of alemtuzumab‐conditioned allogeneic HSCT. Bone Marrow Transplant. 2016;51(6):825‐832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Morath C, Zeier M, Döhler B, et al. ABO‐incompatible kidney transplantation. Front Immunol. 2017;8:234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.van den Hoogen MW, Kamburova EG, Baas MC, et al. Rituximab as induction therapy after renal transplantation: a randomized, double‐blind, placebo‐controlled study of efficacy and safety. Am J Transplant. 2015;15(2):407‐416. [DOI] [PubMed] [Google Scholar]
- 56.Mikulska M, Lanini S, Gudiol C, et al. ESCMID Study Group for Infections in Compromised Hosts (ESGICH) Consensus Document on the safety of targeted and biological therapies: an infectious diseases perspective (Agents targeting lymphoid cells surface antigens [I]: CD19, CD20 and CD52). Clin Microbiol Infect. 2018;24(Suppl 2):S71‐S82. [DOI] [PubMed] [Google Scholar]
- 57.Comoli P, Basso S, Zecca M, et al. Preemptive therapy of EBV‐related lymphoproliferative disease after pediatric haploidentical stem cell transplantation. Am J Transplant. 2007;7(6):1648‐1655. [DOI] [PubMed] [Google Scholar]
- 58.Pescovitz MD. Rituximab, an anti‐cd20 monoclonal antibody: history and mechanism of action. Am J Transplant. 2006;6(5 Pt 1):859‐866. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
AppendixS1
FigureS1
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


