Abstract
Background and Aim
This study aimed to elucidate the clinical importance of muscle volume loss (pre‐sarcopenia) in patients receiving lenvatinib as treatment for unresectable hepatocellular carcinoma (u‐HCC).
Methods
Of 437 u‐HCC patients treated with lenvatinib at specific institutions in Japan between March 2018 and May 2020, 151 with available computed tomography imaging data from the time of lenvatinib introduction were enrolled. Pre‐sarcopenia was diagnosed based on a previously reported cut‐off value calculation formula [psoas muscle area at level of middle of third lumbar vertebra (cm2)/height (m)2]. Clinical features and prognostic factors for overall survival (OS) with inverse probability weighting were investigated retrospectively for their relationship with pre‐sarcopenia.
Results
Cox hazard multivariate analysis showed alpha‐fetoprotein (≥400 ng/mL) (hazard ratio [HR] 2.271, P < 0.001), Barcelona Clinic Liver Cancer stage (C and D) (HR 1.625, P = 0.018), and positive for pre‐sarcopenia (HR 1.652, P = 0.042) to be significant prognostic factors. OS rates for the pre‐sarcopenia group (n = 41) were worse than those for the non‐pre‐sarcopenia group (n = 110) (0.5‐, 1‐, and 1.5‐year OS: 72.5%, 27.9%, and 7.0% vs 80.7%, 56.7%, and 46.1%, respectively; P < 0.001), as was progression‐free survival (P = 0.025). Time to stopping lenvatinib or disease progression was better in the non‐pre‐sarcopenia group (0.5‐, 1‐, and 1.5‐year OS: 48.0%, 24.5%, and 8.4% vs 20.0%, 10.3%, and 4.2%, respectively; P < 0.001). Also, the frequency of the adverse event appetite loss (any grade) was greater in the pre‐sarcopenia group (43.9% vs 18.2%, P = 0.003).
Conclusion
Pre‐sarcopenia was shown to be a significant prognostic factor in patients treated with lenvatinib for u‐HCC.
Keywords: Hepatocellular carcinoma, Lenvatinib, Pre‐sarcopenia, Prognosis
Introduction
Hepatocellular carcinoma (HCC) is the most common primary malignancy of the liver and the fifth most common malignancy worldwide.1 Progression of surveillance and therapeutic modalities has improved the prognosis of affected patients,2, 3 with four different molecular targeting agents (MTAs), including sorafenib,4 regorafenib,5 lenvatinib,6 and ramucirumab,7 developed for unresectable HCC (u‐HCC) now available in Japan. Generally, tumor burden and hepatic reserve function have great influence on the prognosis of HCC patients.8, 9 To improve that of u‐HCC patients, sequential MTA treatment has an important role, and it has been shown that introduction of that in patients with good hepatic function is important for continued effective sequential MTA therapy.10, 11, 12, 13
Recent clinical studies have found that in addition to tumor burden, malignant potential, hepatic reserve function, and muscle volume depletion are important prognostic factors not only in patients undergoing curative treatment but also in those receiving palliative therapy.14 Muscle volume depletion is not rare in chronic liver disease (CLD) cases,15, 16, 17 and it has also been reported that worse prognosis was observed in u‐HCC patients with as compared with those without muscle volume loss who were receiving sorafenib treatment.18, 19, 20, 21 On the other hand, although lenvatinib has been used as a powerful MTA drug against u‐HCC in Japan,22 few reports have investigated the association between prognosis and muscle volume depletion in lenvatinib‐treated u‐HCC patients.23 The present study aimed to evaluate the clinical role of muscle volume depletion in u‐HCC patients undergoing treatment with lenvatinib.
Materials and methods
Patients
Of 437 patients with u‐HCC and being treated with lenvatinib at specific institutions in Japan between March 2018 and May 2020 (Ehime Prefectural Central Hospital, Ogaki Municipal Hospital, Okayama City Hospital, Himeji Red Cross Hospital, Kagawa University Hospital, Osaka Medical School, Nippon Medical School, Ehime University Graduate Hospital, Teine Keijinkai Hospital, Saiseikai Niigata Hospital, Kagawa Prefectural Central Hospital, Asahi General Hospital, Toyama University Hospital, Otakanomori Hospital, Tokushima Prefectural Central Hospital, Matsuyama Red Cross Hospital, Kagawa University Hospital, and Hamamatsu University School of Medicine Hospital), clinical features of 151, whose computed tomography (CT) imaging data at introducing lenvatinib were sent to Ehime Prefectural Central Hospital from each hospital, were evaluated in a retrospective manner. Those positive for hepatitis B virus surface antigen were judged to have HCC due to the presence of the hepatitis B virus, while those positive for the anti‐hepatitis C virus were judged to have HCC due to hepatitis C virus.
Hepatocellular carcinoma diagnosis
Based on an increasing course of alpha‐fetoprotein (AFP), as well as findings obtained in dynamic CT,24 magnetic resonance imaging,25, 26 contrast enhanced ultrasonography with perflubutane (Sonazoid®, Daiichi Sankyo Co., Ltd., Tokyo, Japan) examinations,27, 28 and/or pathological findings, HCC was diagnosed. To evaluate tumor progression, we used Barcelona Clinic Liver Cancer (BCLC) stage29 and tumor node metastasis (TNM) stage, determined as previously reported in a study for TNM staging of HCC conducted by the Liver Cancer Study Group of Japan (LCSGJ) 6th edition30 (TNM‐LCSGJ).
Assessment methods for hepatic reserve function and therapeutic response
Child‐Pugh classification31 and albumin–bilirubin (ALBI) grade were used for assessment of hepatic reserve function. ALBI grade was calculated based on serum albumin and total bilirubin values using the following formula: ALBI score = [(log10 bilirubin (μmol/L) × 0.66) + (albumin (g/L) × −0.085)], with the results defined by the following scores: ≤ −2.60, grade 1; > −2.60 to ≤ −1.39, grade 2; and > −1.39, grade 3.9, 32, 33 To perform more detailed evaluations of patients with the middle ALBI grade of 2, we used a revised grading system consisting of four levels that included sub‐grading for the middle grade of 2 (2a and 2b) based on an ALBI score of −2.27 as the cut‐off (modified ALBI grade [mALBI grade]), which was previously developed based on the value for indocyanine green retention after 15 min (ICG‐R15) of 30%.34, 35
Evaluation of muscle volume depletion
Muscle volume depletion was defined as “pre‐sarcopenia” following the definition provided by the European Working Group on Sarcopenia in Older People36 and evaluated based on psoas muscle area index (PSI) [psoas muscle area at level of middle of third lumbar vertebra (cm2)/height (m)2], which was a simple method to calculate from CT findings using the DICOM viewer personal computer software package (OsiriX 11.0®, https://www.osirix‐viewer.com) by manually hand‐tracing. Previously reported cut‐off values for muscle wasting in men and women (4.24 and 2.50 cm2/m2, respectively) were used.15 All patients underwent a CT examination within 1 month before starting lenvatinib; then the first follow‐up CT examination was performed at 4 weeks after starting treatment, whenever possible. For calculation of PSI, a hepatologist (AH) performed manual calculations using the DICOM software package, while other hepatologist (KM) confirmed the traced area of the bilateral psoas muscle in order to avoid human error or a mistake with tracing.
Lenvatinib treatment and assessment of adverse events
After obtaining written informed consent from each patient, lenvatinib treatment was started. Lenvatinib was orally administered at 8 mg/day in patients weighing <60 kg or 12 mg/day in those ≥60 kg and discontinued when any unacceptable or serious adverse event (AE) or clinical tumor progression was observed. According to the guidelines for administration of lenvatinib, the drug dose was reduced or treatment interrupted when a patient developed any grade 3 or more severe AE or if any unacceptable grade 2 drug‐related AE occurred. AEs were assessed according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0.37 AEs of grade 3 or more were defined as severe, and the worst grade for each AE during the present observation period was recorded. If a drug‐related AE was noted, dose reduction or temporary interruption was maintained until the symptom was resolved to grade 1 or 2, according to the guidelines provided by the manufacturer.
Evaluations of overall and progression‐free survival, and ethical approval
Patients were divided into those with (pre‐sarcopenia, n = 41) and without (non‐pre‐sarcopenia, n = 110) pre‐sarcopenia. Overall survival (OS) rate and progression‐free survival were analyzed according to the modified Response Evaluation Criteria In Solid Tumors criteria38, 39 based on results of dynamic CT examinations performed at intervals of 8–12 weeks and rate of discontinuation of lenvatinib medication.
Written informed consent for lenvatinib treatment was obtained from each patient. This was a retrospective analysis of records stored in a database, and official approval was received based on the Guidelines for Clinical Research issued by Ministry of Health and Welfare in Japan. All procedures complied with the Declaration of Helsinki.
Statistical analysis
Continuous variables are expressed as median values (the first–third quartile). Statistical analyses were performed using Welch's t‐test, Student's t‐test, Fisher's exact test, or Mann–Whitney's U test, as appropriate. Prognosis was analyzed by Cox hazard analysis, the Kaplan–Meier method, and a log–rank test.
Pre‐sarcopenia and non‐pre‐sarcopenia group probabilities (propensity) were calculated using logistic regression analysis with a set of covariates deemed likely to have effects on OS, including age, gender, AFP (tumor malignant potential), BCLC stage (tumor burden), and mALBI (hepatic reserve function). Inverse probability weighting (IPW) was defined as 1/(propensity score) for the pre‐sarcopenia group and 1/(1 − propensity score) for the non‐pre‐sarcopenia group. Prognostic factors for OS and difference in OS were tested using IPW‐adjusted Cox hazard analysis and an IPW‐adjusted log–rank test, respectively.40, 41
A P value less than 0.05 was considered to indicate statistical significance. All statistical analyses were performed using Easy R (EZR) version 1.42 (Saitama Medical Center, Jichi Medical University, Saitama, Japan),42 a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria).
Results
Clinical features of patients
Of 437 u‐HCC patients (mALBI 1:2a:2b:3 = 138:110:172:17) (median survival 1.4 years, 95% confidence interval [CI]: 1.2–1.5 years) (Fig. S1) with records in the databases of the participating institutions, 151 with CT imaging data available were evaluated for the present study. They were divided into the non‐pre‐sarcopenia (n = 110) and pre‐sarcopenia (n = 41) groups. Frequency of male gender was greater (P = 0.004), and serum level of albumin and body mass index were lower in the pre‐sarcopenia group (P = 0.012 and P < 0.001, respectively), while viral hepatitis, AFP level, Child‐Pugh classification, mALBI grade, BCLC stage, TNM‐LCSGJ, and past history of sorafenib and regorafenib treatments were not significantly different between the groups (Table 1). In addition, the calculated IPW scores of patients in the non‐pre‐sarcopenia and pre‐sarcopenia groups were 1.37 (1.08–1.66) and 3.61 (0.44–6.78), respectively (P < 0.001). During the observation period, 81 patients died.
Table 1.
Clinical features of unresectable hepatocellular carcinoma patients with and without pre‐sarcopenia
| Non‐pre‐sarcopenia group (n = 110) | Pre‐sarcopenia group (n = 41) | P value | |
|---|---|---|---|
| Age, years | 72 (65–79) | 74 (68–82) | 0.219 |
| Gender, male : female | 78:32 | 38:3 | 0.004 |
| Viral : non‐viral hepatitis (HCV : HBV : alcohol : others) | 70:40 (50:20:12:28) | 25:16 (21:4:12:4) | 0.850 |
| BMI, kg/m2 | 23.2 (16.4–42.2) | 20.6 (14.2–32.0) | < 0.001 |
| ECOG PS, 0:1:2:3 | 82:20:7:1 | 33:7:1:0 | 0.815 |
| Lenvatinib introduction at reduced dose | 19 (17.3%) | 9 (22.0%) | 0.491 |
| Platelets, ≥ 104/μL | 12.9 (10.6–17.0) | 13.1 (10.3–18.1) | 0.699 |
| AST, U/L | 44 (29–65) | 32 (29–50) | 0.147 |
| ALT, U/L | 30 (21–46) | 23 (18–33) | 0.075 |
| Prothrombin time, % | 87 (78–97) | 85 (77–96) | 0.627 |
| Total bilirubin, mg/dL | 0.8 (0.6–1.1) | 0.7 (0.6–1.0) | 0.137 |
| Albumin, g/dL | 3.7 (3.2–4.0) | 3.4 (3.1–3.7) | 0.012 |
| NH3, μg/dL | 45 (28–59) | 36 (24–53) | 0.152 |
| eGFR, mL/min/1.73 m2 | 70.0 (56.6–83.4) | 69.1 (54.1–86.0) | 0.771 |
| AFP, ng/mL | 30.7 (5.6–1454.5) | 134.5 (5.0–766.0) | 0.953 |
| Past history of sorafenib | 49 (44.5%) | 19 (46.3%) | 0.856 |
| Past history of regorafenib | 15 (13.6%) | 7 (17.1%) | 0.609 |
| Child‐Pugh class, A : B | 98:12 | 33:8 | 0.183 |
| mALBI, 1:2a:2b:3 | 38:25:44:3 | 7:11:21:2 | 0.160 |
| BCLC stage, A : B : C : D | 2:38:69:1 | 0:14:27:0 | 1.0 |
| TNM‐LCSGJ, I : II : III : IVa : IVb | 2:13:34:10:51 | 0:3:16:3:19 | 0.694 |
| Observation period, years | 1.0 (0.4–1.5) | 0.8 (0.4–1.3) | 0.186 |
| Best therapeutic response, mRECIST, CR; PR : SD : PD : NA/NE (ORR/DCR) | 11:44:31:15:9 (54.5%/85.1%) | 3:15:17:5:1 (45.0%/87.5%) | 0.751 |
| IPW | 1.37 (1.08–1.66) | 3.61 (0.44–6.78) | < 0.001 |
Median values (interquartile range) are shown as numbers, unless otherwise indicated. AFP, alpha‐fetoprotein; ALT, alanine aminotransferase; AST, aspartate transaminase; BCLC, Barcelona Clinic Liver Cancer stage; BMI, body mass index; CR, complete response; DCR, disease control rate; ECOG PS, Eastern Cooperative Oncology Group performance status; eGFR, estimated glomerular filtration rate; HBV, hepatitis B virus; HCV, hepatitis C virus; IPW, inverse probability weighting; mALBI, modified albumin–bilirubin grade; mRECIST, modified Response Evaluation Criteria In Solid Tumors; NA/NE, not available or not examined; ORR, objective response rate; PD, progression of disease; PR, partial response; SD, stable disease; TNM LCSGJ 6th, tumor node metastasis stage by Liver Cancer Study Group of Japan 6th edition.
Assessment of prognosis
Prognostic factors for OS were evaluated using Cox hazard univariate analysis adjusted for IPW, and the results showed body mass index (≥22 kg/m2), AFP (≥400 ng/mL), BCLC stage (C and D), and positive for pre‐sarcopenia as significant factors related to prognosis. Using multivariate analysis adjusted for IPW, AFP (≥400 ng/mL) (hazard ratio [HR] 2.271, 96% CI: 1.399–3.685, P < 0.001), BCLC stage (C and D) (HR 1.625, 95% CI: 1.089–2.427, P = 0.018), and positive for pre‐sarcopenia (HR 1.652, 95% CI: 1.017–2.686, P = 0.042) were chosen as significant prognostic factors (Table 2).
Table 2.
Cox hazard analysis adjusted with inverse probability weighting for prognostic factors of overall survival
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |
| Age (≥ 75 years) | 1.474 | 0.965–2.252 | 0.073 | — | — | — |
| Male gender | 1.256 | 0.819–1.924 | 0.296 | — | — | — |
| ECOG PS (≥ 2) | 0.933 | 0.314–2.729 | 0.900 | — | — | — |
| BMI (≥ 22 kg/m2) | 0.604 | 0.385–0.945 | 0.027 | 0.660 | 0.382–1.140 | 0.136 |
| Viral hepatitis | 0.953 | 0.613–1.481 | 0.830 | — | — | — |
| Lenvatinib introduction at reduced dose | 1.318 | 0.691–2.513 | 0.402 | — | — | — |
| ALT (≥ 40 U/L) | 1.259 | 0.804–1.971 | 0.314 | — | — | — |
| Platelet (≥ 104/μL) | 0.850 | 0.480–1.506 | 0.578 | — | — | — |
| AFP (≥ 400 ng/mL) | 2.254 | 1.427–3.562 | < 0.001 | 2.271 | 1.399–3.685 | < 0.001 |
| mALBI 2b or 3 | 1.355 | 0.774–2.370 | 0.288 | — | — | — |
| Past history of sorafenib | 1.222 | 0.8122–1.840 | 0.336 | — | — | — |
| Past history of regorafenib | 1.345 | 0.908–1.993 | 0.139 | — | — | — |
| BCLC stage C/D | 1.749 | 1.149–2.660 | 0.009 | 1.625 | 1.089–2.427 | 0.018 |
| Pre‐sarcopenia | 1.955 | 1.297–2.946 | 0.0014 | 1.652 | 1.017–2.686 | 0.042 |
AFP, alpha‐fetoprotein; ALT, alanine aminotransferase; BCLC, Barcelona Clinic Liver Cancer stage; BMI, body mass index; CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance status; HR, hazard ratio; mALBI, modified albumin–bilirubin grade.
When the Kaplan–Meier method was performed with adjustment for IPW, the 0.5‐, 1‐, and 1.5‐year OS rates for the pre‐sarcopenia group were worse than those for the non‐pre‐sarcopenia group (72.5%, 27.9%, and 7.0% vs 80.7%, 56.7%, and 46.1%, respectively, P < 0.001; log–rank test adjusted for IPW) (Fig. 1a), while progression‐free survival rates were better in patients without pre‐sarcopenia (55.3%, 25.5%, and 11.6% vs 45.5%, 8.5%, and 4.5%, respectively, P = 0.025; log–rank test adjusted for IPW) (Fig. 1b). When time to stopping lenvatinib or progression of disease was analyzed, that was better in patients without than in those with pre‐sarcopenia at 0.5, 1, and 1.5 years (48.0%, 24.5%, and 8.4% vs 20.0%, 10.3%, and 4.2%, respectively, P < 0.001; log–rank test for IPW adjusted) (Fig. 2). Objective response rate and disease control rate were not significantly different between the groups (Table 1).
Figure 1.
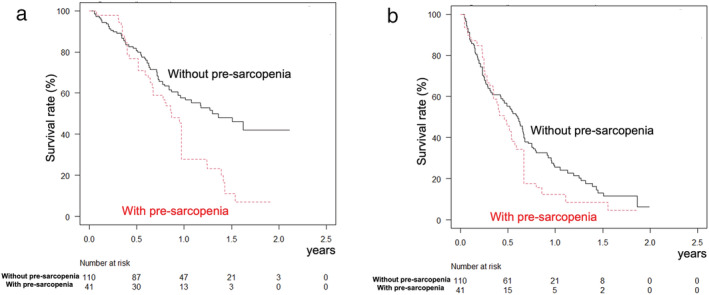
Overall and progression‐free survival adjusted for inverse probability weighting. (a) Overall survival was better in patients without (solid line) than those with (broken line) pre‐sarcopenia at 0.5, 1, and 1.5 years (80.7%, 56.7%, and 46.1% vs 72.5%, 27.9%, and 7.0%, respectively; P < 0.001). (b) Progression‐free survival was better in patients without (solid line) than those with (broken line) pre‐sarcopenia at 0.5, 1, and 1.5 years (55.3%, 25.5%, and 11.6% vs 45.5%, 8.5%, and 4.5%, respectively; P = 0.025).
Figure 2.
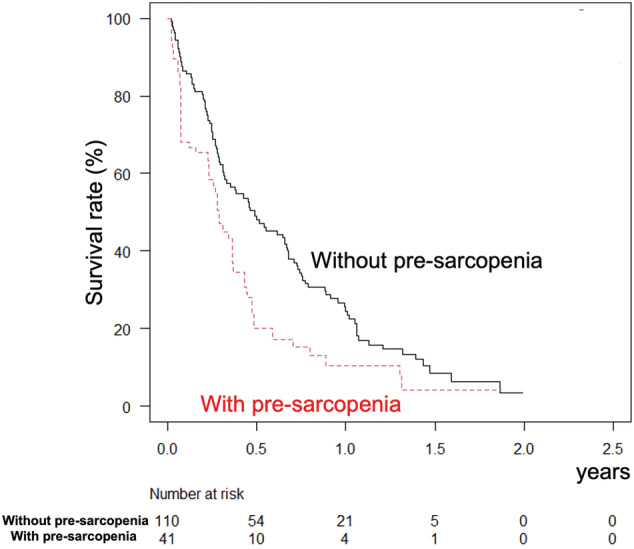
Lenvatinib medication period, after adjustment for inverse probability weighting. The lenvatinib medication period was better in patients without (solid line) than with (broken line) pre‐sarcopenia at 0.5, 1, and 1.5 years (48.0%, 24.5%, and 8.4% vs 20.0%, 10.3%, and 4.2%; P < 0.001).
Assessments of adverse events and relative changes in psoas muscle area index
There were no significant differences in regard to AEs between the pre‐sarcopenia and non‐pre‐sarcopenia groups, except for appetite loss (any grade) (P = 0.003) (Table 3). Although there was no difference for appetite loss (severe grade) between the groups (14.6% vs 4.5%, P = 0.070), patients with pre‐sarcopenia showed a greater frequency of severe grade in a sub‐analysis conducted after exclusion of patients whose initial dose was reduced (15.6% vs 3.3%, P = 0.028). In patients whose serial CT imaging examinations after introduction of lenvatinib could be evaluated (n = 106), the delta PSI per month values after beginning that therapy were not significantly different between the pre‐sarcopenia and non‐pre‐sarcopenia groups (−0.11 [−0.27 to 0.09] vs −0.13 [−0.38 to 0.08], P = 0.414).
Table 3.
Adverse events in unresectable hepatocellular carcinoma patients with and without pre‐sarcopenia
| Grade 3 or more | Any grade | |||||
|---|---|---|---|---|---|---|
| Non‐pre‐sarcopenia group | Pre‐sarcopenia group | P value | Non‐pre‐sarcopenia group | Pre‐sarcopenia group | P value | |
| Appetite loss | 5 (4.5%) | 6 (14.6%) | 0.070 | 20 (18.2%) | 18 (43.9%) | 0.003 |
| Fatigue | 7 (6.4%) | 0 (0%) | 0.190 | 27 (24.5%) | 11 (26.8%) | 0.834 |
| Hypertension | 5 (4.5%) | 1 (2.4%) | 1.0 | 17 (15.5%) | 5 (12.2%) | 0.797 |
| HFSR | 5 (4.5%) | 2 (4.9%) | 1.0 | 29 (26.4%) | 11 (26.8%) | 1.0 |
| Urine protein | 7 (6.4%) | 4 (9.8%) | 0.491 | 19 (17.3%) | 6 (14.6%) | 0.809 |
| Thyroid function abnormality | 3 (2.7%) | 1 (2.4%) | 1.0 | 22 (20.0%) | 13 (31.7%) | 0.136 |
| Diarrhea | 5 (4.5%) | 0 (0%) | 0.324 | 24 (21.8%) | 6 (14.6%) | 0.369 |
| Hepatic coma | 6 (5.5%) | 1 (2.4%) | 0.675 | 7 (6.4%) | 1 (2.4%) | 0.684 |
| Others | 29 (26.4%) | 13 (31.7%) | 0.544 | 47 (42.7%) | 18 (43.9%) | 1.0 |
HFSR, hand foot skin reaction.
Discussion
In this study, we applied IPW to the Kaplan–Meier method for hepatocarcinogenesis to adjust for potential imbalances, and the present results showed that muscle volume depletion is a prognostic factor for survival in u‐HCC patients undergoing treatment with lenvatinib. A previously reported meta‐analysis found that low skeletal muscle mass at the time of cancer diagnosis was associated with poor prognosis in patients with solid tumors and also noted that 19–74% of patients with advanced tumors were sarcopenic.43 Recently, muscle volume loss and muscle function decline have been reported to be not only primary but also secondary prognostic factors in patients with various chronic diseases.36 HCC often occurs due to CLD (e.g. chronic viral hepatitis, alcohol abuse, and non‐alcoholic steatohepatitis); thus, muscle volume depletion is not a rare finding in HCC patients. Several studies have shown a relationship of muscle volume loss with worse prognosis in u‐HCC patients treated with sorafenib,18, 19, 20, 21 with similar results seen in the present study. In addition, our present result of skeletal muscle loss during lenvatinib treatment after introduction is similar to past reports.18, 44 We should keep in mind that muscle mass will be maintained during the course of treatment, regardless of the amount of muscle mass at the time of introduction of the MTA therapeutic agent.
The frequency of appetite loss (any grade) was significantly greater in the pre‐sarcopenia group (43.9% vs 18.2%, P = 0.003). Although the difference in frequency of appetite loss (severe grade) was not significant (P = 0.070), the pre‐sarcopenia group showed a greater frequency of that in sub‐analysis findings after exclusion of patients whose initial dose was reduced (15.6% vs 3.3%, P = 0.028). On the other hand, there were no significant differences in the appetite loss between patients with and without previous MTA treatment history (any grade and severe grade: P = 0.145 and P = 0.980, respectively) (data not shown). The present findings indicate that muscle volume depletion might have an association with appetite loss, which has been revealed to be a significant prognostic factor in patients receiving lenvatinib treatment.12 Uojima et al. also reported that low skeletal muscle mass was associated with occurrence of severe AEs in patients undergoing lenvatinib therapy and considered that skeletal muscle mass is more important than bodyweight in those patients.23 For u‐HCC patients with pre‐sarcopenia, even a regular dosage based on bodyweight might actually be an overdose; thus, a dose reduction strategy for such cases should be considered.
In a previous study, Antoun et al. found that sorafenib treatment directly inhibits protein synthesis, because inhibition of VEGFR in a variety of cells by the drug was shown to result in downstream inhibition of PI3K, AKT, and mTOR, which are central for activation of muscle protein synthesis by amino acids.45 Because lenvatinib has greater potential for inhibition of VEGFR, it has been suggested that its antiangiogenic characteristic may have a relationship with muscle wasting, with the same speculated for sorafenib.45 In the present cohort as well, decreased muscle volume in association with lenvatinib treatment was common in both the pre‐sarcopenia and non‐pre‐sarcopenia groups.
In lenvatinib treatment, mALBI 2b or 3 at the time of starting the treatment has been reported as a prognostic factor for poor prognosis (HR 4.632, 95% CI: 1.649–13.02, P = 0.004) in our previous report.12 Although Kaplan–Meier curve of the 437 patients according to mALBI grade was thought to prove it (Fig. S1B), mALBI 2b or 3 was not significant factor in the analyzed cohort (n = 151). However, detailed assessment for hepatic function is very important in MTA treatments, because Kaplan–Meier curve of the present analyzed 151 from the 437 patients according to mALBI grade showed a similar result (median survival time of mALBI 1:2a:2b:3 = not reached:1.4:0.8:0.5 years) (P = 0.061) (Fig. S2). From the above, not only muscle volume loss but also decline of hepatic function, especially mALBI 2b or 3, should be kept in mind as prognostic factors for poor prognosis at the time of starting lenvatinib treatment. Introducing MTA should be considered in condition with better hepatic function to improve prognosis of u‐HCC patients.
Recently, Finn et al. reported that atezolizumab plus bevacizumab treatment had a positive therapeutic efficacy in u‐HCC patients, which was superior to that of sorafenib, used as a control arm, as first‐line therapy.46 Therefore, the combination of immunotherapy and MTA treatment is expected to be a highly effective first‐line treatment for u‐HCC available in the near future. In light of such dramatic changes in systemic chemotherapy for u‐HCC, maintaining muscle volume during the clinical course of CLD patients has become an important clinical matter. Assessment of muscle volume in CLD patients and intervention in those with a decline should be kept in mind before development of HCC47 as well as after starting treatment for HCC.48
The present study has some limitations, including its retrospective protocol. Furthermore, the number of patients analyzed was insufficient to obtain concrete conclusions. Finally, no data in regard to muscle strength (e.g. hand grip strength and walking speed) were available to evaluate its prognostic role. A prospective study with a greater number of patients should be planned to evaluate this clinical issue.
In conclusion, pre‐sarcopenia was a significant prognostic factor in patients receiving lenvatinib treatment for u‐HCC. Assessment of muscle volume is recommended, and attention should be given to severe appetite loss as an AE in order to improve the prognosis of pre‐sarcopenia u‐HCC patients.
Supporting information
Figure S1. A. Overall survival of all 437 patients treated with lenvatinib at participating institutions (median survival 1.4 years). B. Survival curve of all 437 patients according to modified ALBI grade. The median survival of patients with modified albumin‐bilirubin grade 1 was not reached, while that of those with 2a, 2b, and 3 was 1.6, 0.9, and 0.4 years, respectively (P < 0.001).
Figure S2. Survival curve of patients, whose muscle volume data were available, according to modified ALBI grade (n = 151). The median survival of patients with modified albumin‐bilirubin grade 1 was not reached, while that of those with 2a, 2b, and 3 was 1.4, 0.8, and 0.5, respectively (P = 0.061).
Acknowledgments
None to declare.
Hiraoka, A., Kumada, T., Kariyama, K., Tada, T., Tani, J., Fukunishi, S., Atsukawa, M., Hirooka, M., Tsuji, K., Ishikawa, T., Takaguchi, K., Itobayashi, E., Tajiri, K., Shimada, N., Shibata, H., Ochi, H., Kawata, K., Yasuda, S., Toyoda, H., Ohama, H., Nouso, K., Tsutsui, A., Nagano, T., Itokawa, N., Hayama, K., Arai, T., Imai, M., Koizumi, Y., Nakamura, S., Joko, K., Michitaka, K., Hiasa, Y., Kudo, M., and Real‐life Practice Experts for HCC (RELPEC) Study Group and HCC 48 Group (hepatocellular carcinoma experts from 48 clinics in Japan) (2021) Clinical importance of muscle volume in lenvatinib treatment for hepatocellular carcinoma: Analysis adjusted with inverse probability weighting. Journal of Gastroenterology and Hepatology, 36: 1812–1819. 10.1111/jgh.15336.
Declaration of conflict of interest: Atsushi Hiraoka, MD, PhD, received lecture fees from Bayer, Eisai, and Otsuka. Takashi Kumada, MD, PhD, received lecture fees from Eisai. Masatoshi Kudo, MD, PhD, received fees for advisory role from Eisai, Ono, MSD, Bristol‐Myers Squibb, and Roche; lecture fees from Eisai, Bayer, MSD, Bristol‐Myers Squibb, Eli Lilly, and EA Pharma; and research funding from Gilead Sciences, Taiho, Sumitomo Dainippon Pharma, Takeda, Otsuka, EA Pharma, Abbvie, and Eisai. None of the other authors have potential conflicts of interest to declare.
Author contribution: AH and TK conceived the study and participated in its design and coordination. AH, TK, Kka, TT, JT, SF, MA, MH, KTs, TI, KTak, EI, KTaj, NS, HS, HOc, KK, SY, HT, HOh, KN, AT, TN, NI, KH, TA, MI, YK, SN, KM, KJ, MK, and YH performed data curation. AH performed statistical analyses and interpretation. AH and TK drafted the text. All authors have read and approved the final version of the manuscript.
Ethical approval: The present study protocol was approved by the Institutional Ethics Committee of Ehime Prefectural Central Hospital (IRB No. 30‐66). The research was conducted in an ethical manner in accordance with the World Medical Association Declaration of Helsinki.
Financial support: None to declare.
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Estimating the world cancer burden: Globocan 2000. Int. J. Cancer 2001; 94: 153–156. [DOI] [PubMed] [Google Scholar]
- 2.Kudo M. Surveillance, diagnosis, treatment, and outcome of liver cancer in Japan. Liver Cancer. 2015; 4: 39–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kudo M. Management of hepatocellular carcinoma in Japan: current trends. Liver Cancer. 2020; 9: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Llovet JM, Ricci S, Mazzaferro Vet al. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 2008; 359: 378–390. [DOI] [PubMed] [Google Scholar]
- 5.Bruix J, Qin S, Merle Pet al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double‐blind, placebo‐controlled, phase 3 trial. Lancet (London, England) 2017; 389: 56–66. [DOI] [PubMed] [Google Scholar]
- 6.Kudo M, Finn RS, Qin Set al. Lenvatinib versus sorafenib in first‐line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non‐inferiority trial. Lancet (London, England) 2018; 391: 1163–1173. [DOI] [PubMed] [Google Scholar]
- 7.Zhu AX, Finn RS, Galle PR, Llovet JM, Kudo M. Ramucirumab in advanced hepatocellular carcinoma in REACH‐2: the true value of alpha‐fetoprotein. Lancet Oncol. 2019; 20: e191. [DOI] [PubMed] [Google Scholar]
- 8.Kudo M, Chung H, Osaki Y. Prognostic staging system for hepatocellular carcinoma (CLIP score): its value and limitations, and a proposal for a new staging system, the Japan Integrated Staging Score (JIS score). J. Gastroenterol. 2003; 38: 207–215. [DOI] [PubMed] [Google Scholar]
- 9.Hiraoka A, Kumada T, Michitaka Ket al. Usefulness of albumin‐bilirubin grade for evaluation of prognosis of 2584 Japanese patients with hepatocellular carcinoma. J. Gastroenterol. Hepatol. 2016; 31: 1031–1036. [DOI] [PubMed] [Google Scholar]
- 10.Finn RS, Merle P, Granito Aet al. Outcomes of sequential treatment with sorafenib followed by regorafenib for HCC: additional analyses from the phase III RESORCE trial. J. Hepatol. 2018; 69: 353–358. [DOI] [PubMed] [Google Scholar]
- 11.Hiraoka A, Kumada T, Atsukawa Met al. Early relative change in hepatic function with lenvatinib for unresectable hepatocellular carcinoma. Oncology 2019; 97: 334–340. [DOI] [PubMed] [Google Scholar]
- 12.Hiraoka A, Kumada T, Atsukawa Met al. Prognostic factor of lenvatinib for unresectable hepatocellular carcinoma in real‐world conditions—multicenter analysis. Cancer Med. 2019; 8: 3719–3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hiraoka A, Kumada T, Atsukawa Met al. Important clinical factors in sequential therapy including lenvatinib against unresectable hepatocellular carcinoma. Oncology 2019; 9: 1–9. [DOI] [PubMed] [Google Scholar]
- 14.Chang KV, Chen JD, Wu WT, Huang KC, Hsu CT, Han DS. Association between loss of skeletal muscle mass and mortality and tumor recurrence in hepatocellular carcinoma: a systematic review and meta‐analysis. Liver Cancer. 2018; 7: 90–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hiraoka A, Aibiki T, Okudaira Tet al. Muscle atrophy as pre‐sarcopenia in Japanese patients with chronic liver disease: computed tomography is useful for evaluation. J. Gastroenterol. 2015; 50: 1206–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hiraoka A, Otsuka Y, Kawasaki Het al. Impact of muscle volume and muscle function decline in patients undergoing surgical resection for hepatocellular carcinoma. J. Gastroenterol. Hepatol. 2018; 33: 1271–1276. [DOI] [PubMed] [Google Scholar]
- 17.Hiraoka A, Izumoto H, Ueki Het al. Easy surveillance of muscle volume decline in chronic liver disease patients using finger‐circle (yubi‐wakka) test. J. Cachexia. Sarcopenia Muscle 2019; 10: 347–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hiraoka A, Hirooka M, Koizumi Yet al. Muscle volume loss as a prognostic marker in hepatocellular carcinoma patients treated with sorafenib. Hepatol. Res. 2017; 47: 558–565. [DOI] [PubMed] [Google Scholar]
- 19.Takada H, Kurosaki M, Nakanishi Het al. Impact of pre‐sarcopenia in sorafenib treatment for advanced hepatocellular carcinoma. PLoS ONE 2018; 13: e0198812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Imai K, Takai K, Miwa Tet al. Rapid depletions of subcutaneous fat mass and skeletal muscle mass predict worse survival in patients with hepatocellular carcinoma treated with sorafenib. Cancer 2019; 11: 1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Labeur TA, van Vugt JL, Ten Cate DWet al. Body composition is an independent predictor of outcome in patients with hepatocellular carcinoma treated with sorafenib. Liver Cancer. 2019; 8: 255–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hiraoka A, Kumada T, Fukunishi Set al. Post‐progression treatment eligibility of unresectable hepatocellular carcinoma patients treated with lenvatinib. Liver Cancer. 2020; 9: 73–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Uojima H, Chuma M, Tanaka Yet al. Skeletal muscle mass influences tolerability and prognosis in hepatocellular carcinoma patients treated with lenvatinib. Liver Cancer. 2020; 9: 193–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology (Baltimore, Md) 2005; 42: 1208–1236. [DOI] [PubMed] [Google Scholar]
- 25.Di Martino M, Marin D, Guerrisi Aet al. Intraindividual comparison of gadoxetate disodium‐enhanced MR imaging and 64‐section multidetector CT in the detection of hepatocellular carcinoma in patients with cirrhosis. Radiology 2010; 256: 806–816. [DOI] [PubMed] [Google Scholar]
- 26.Sano K, Ichikawa T, Motosugi Uet al. Imaging study of early hepatocellular carcinoma: usefulness of gadoxetic acid‐enhanced MR imaging. Radiology 2011; 261: 834–844. [DOI] [PubMed] [Google Scholar]
- 27.Hiraoka A, Ichiryu M, Tazuya Net al. Clinical translation in the treatment of hepatocellular carcinoma following the introduction of contrast‐enhanced ultrasonography with Sonazoid. Oncol. Lett. 2010; 1: 57–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hiraoka A, Hiasa Y, Onji M, Michitaka K. New contrast enhanced ultrasonography agent: impact of Sonazoid on radiofrequency ablation. J. Gastroenterol. Hepatol. 2011; 26: 616–618. [DOI] [PubMed] [Google Scholar]
- 29.Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet (London, England) 2018; 391: 1301–1314. [DOI] [PubMed] [Google Scholar]
- 30.The Liver Cancer Study Group of Japan . The General Rules for the Clinical and Pathological Study of Primary Liver Cancer, 6th edn. Tokyo: Kanehara, 2015; 26. [Google Scholar]
- 31.Pugh RN, Murray‐Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br. J. Surg. 1973; 60: 646–649. [DOI] [PubMed] [Google Scholar]
- 32.Johnson PJ, Berhane S, Kagebayashi Cet al. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence‐based approach—the ALBI grade. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2015; 33: 550–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hiraoka A, Kumada T, Kudo Met al. Albumin‐bilirubin (ALBI) grade as part of the evidence‐based clinical practice guideline for HCC of the Japan Society of Hepatology: a comparison with the liver damage and Child‐Pugh classifications. Liver Cancer. 2017; 6: 204–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hiraoka A, Michitaka K, Kumada Tet al. Validation and potential of albumin‐bilirubin grade and prognostication in a nationwide survey of 46,681 hepatocellular carcinoma patients in Japan: the need for a more detailed evaluation of hepatic function. Liver Cancer. 2017; 6: 325–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hiraoka A, Kumada T, Tsuji Ket al. Validation of modified ALBI grade for more detailed assessment of hepatic function in hepatocellular carcinoma patients: a multicenter analysis. Liver Cancer. 2019; 8: 121–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cruz‐Jentoft AJ, Baeyens JP, Bauer JMet al. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010; 39: 412–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.National Cancer Institute. https://ctep.cancer.gov/protocolDevelopment/adverse_effects.htm (Accessed June 30, 2020).
- 38.Eisenhauer EA, Therasse P, Bogaerts Jet al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer (Oxford, England: 1990) 2009; 45: 228–247. [DOI] [PubMed] [Google Scholar]
- 39.Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin. Liver Dis. 2010; 30: 52–60. [DOI] [PubMed] [Google Scholar]
- 40.Kim HT. Cumulative incidence in competing risks data and competing risks regression analysis. Clin. Cancer Res. 2007; 13: 559–565. [DOI] [PubMed] [Google Scholar]
- 41.Xie J, Liu C. Adjusted Kaplan‐Meier estimator and log‐rank test with inverse probability of treatment weighting for survival data. Stat. Med. 2005; 24: 3089–3110. [DOI] [PubMed] [Google Scholar]
- 42.Kanda Y. Investigation of the freely available easy‐to‐use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013; 48: 452–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shachar SS, Williams GR, Muss HB, Nishijima TF. Prognostic value of sarcopenia in adults with solid tumours: a meta‐analysis and systematic review. Eur J Cancer (Oxford, England: 1990) 2016; 57: 58–67. [DOI] [PubMed] [Google Scholar]
- 44.Uchikawa S, Kawaoka T, Namba Met al. Skeletal muscle loss during tyrosine kinase inhibitor treatment for advanced hepatocellular carcinoma patients. Liver Cancer. 2020; 9: 148–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Antoun S, Birdsell L, Sawyer MB, Venner P, Escudier B, Baracos VE. Association of skeletal muscle wasting with treatment with sorafenib in patients with advanced renal cell carcinoma: results from a placebo‐controlled study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2010; 28: 1054–1060. [DOI] [PubMed] [Google Scholar]
- 46.Finn RS, Qin S, Ikeda Met al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N. Engl. J. Med. 2020; 382: 1894–1905. [DOI] [PubMed] [Google Scholar]
- 47.Hiraoka A, Michitaka K, Kiguchi Det al. Efficacy of branched‐chain amino acid supplementation and walking exercise for preventing sarcopenia in patients with liver cirrhosis Eur. J. Gastroenterol. Hepatol. 2017; 29: 1416–1423. [DOI] [PubMed] [Google Scholar]
- 48.Koya S, Kawaguchi T, Hashida Ret al. Effects of in‐hospital exercise on liver function, physical ability, and muscle mass during treatment of hepatoma in patients with chronic liver disease. Hepatol. Res. 2017; 47: E22–e34. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. A. Overall survival of all 437 patients treated with lenvatinib at participating institutions (median survival 1.4 years). B. Survival curve of all 437 patients according to modified ALBI grade. The median survival of patients with modified albumin‐bilirubin grade 1 was not reached, while that of those with 2a, 2b, and 3 was 1.6, 0.9, and 0.4 years, respectively (P < 0.001).
Figure S2. Survival curve of patients, whose muscle volume data were available, according to modified ALBI grade (n = 151). The median survival of patients with modified albumin‐bilirubin grade 1 was not reached, while that of those with 2a, 2b, and 3 was 1.4, 0.8, and 0.5, respectively (P = 0.061).


