Abstract
Preclinical studies have shown that mesenchymal stem cells have a positive effect in perinatal brain injury models. The mechanisms that cause these neurotherapeutic effects are not entirely intelligible. Mitochondrial damage, inflammation, and reactive oxygen species are considered to be critically involved in the development of injury. Mesenchymal stem cells have immunomodulatory action and exert mitoprotective effects which attenuate production of reactive oxygen species and promote restoration of tissue function and metabolism after perinatal insults. This review summarizes the present state, the underlying causes, challenges and possibilities for effective clinical translation of mesenchymal stem cell therapy.
Keywords: inflammation, intraventricular hemorrhage, mesenchymal stem cells, mitochondria, mitophagy, neonatal brain injury, neonatal hypoxia‐ischemia, reactive oxygen species
Mesenchymal stem cells have a positive effect in perinatal brain injury models. Mitochondrial damage, inflammation, and reactive oxygen species are critically involved in the development of injury. Mesenchymal stem cells have immunomodulatory action and exert mitoprotective effects which attenuate production of reactive oxygen species and promote restoration of tissue function and metabolism after perinatal insults. This review summarizes the present state, the underlying causes, challenges and possibilities for effective clinical translation of mesenchymal stem cell therapy. The image illustrates transfer of mitochondria from mesenchymal stem cells to damaged neurons and inflamed microglia via tunneling nanotubes.
Abbreviations
- AT‐MSCs
adipose tissue‐derived mesenchymal stem cells
- ATP
adenosine triphosphate
- BAG5
BCL2‐associated athanogene 5
- BAX
Bcl‐2‐associated X protein
- BDNF
brain‐derived neurotrophic factor
- BMSCs
bone marrow‐derived stem cells
- DC
dendritic cells
- ES
embryonic stem cells
- H2O2
hydrogen peroxide
- HI
hypoxia‐ischemia
- HIE
hypoxic‐ischemic encephalopathy
- HIF‐1α
hypoxia‐inducible factor‐1α
- IV
intravenous
- IVH
intraventricular hemorrhage
- LPS
lipopolysaccharide
- MAPK
mitogen‐activated protein kinase
- MCAO
middle cerebral artery occlusion
- MLKL
mixed lineage kinase domain like protein
- MSCs
mesenchymal stem cells
- NE
neonatal encephalopathy
- NK
natural killer
- NLRs
NOD‐like receptors
- O2•−
superoxide
- OGD
oxygen glucose deprivation
- OXPHOS
oxidative phosphorylation
- RLRs
RIG‐like receptors
- ROS
reactive oxygen species
- TLR
Toll‐like receptors
- TNTs
tunneling nanotubes
- TSG‐6
TNF‐α‐induced protein
- UCB
umbilical cord blood
1. INTRODUCTION
Intraventricular hemorrhage (IVH) in preterm neonates, stroke in preterm and term neonates, and neonatal encephalopathy (NE) in term neonates constitute three major destructive brain lesions observed during the perinatal period (Ferriero et al., 2019; Hill & Volpe, 1981). Treatment options for neonatal HI brain injury are limited to supportive care and therapeutic hypothermia (Hagberg et al., 2016; Jacobs et al., 2013). Hypothermia is only advised in term and late preterm infants with moderate‐to‐severe NE if identified before 6 hr of age (Jacobs et al., 2013). For cases of IVH unfortunately only supportive care can be provided. Pharmacological neuroprotective therapies have been thoroughly explored in multiple animal models of hypoxic‐ischemic encephalopathy (HIE) and IVH (Bel & Groenendaal, 2016). However, none have been successfully translated into a therapy to prevent or regenerate brain injury in newborn infants.
Inflammation is strongly recognized as a major cause leading to long‐term injury (Hagberg et al., 2015). Inflammation causes systemic up‐regulation of pro‐inflammatory cytokines and diffuse activation of microglia in the neonatal brain (Berger et al., 2012). Cytokine‐activated cells secrete toxic substances, such as ROS and toxic granules, including proteolytic enzymes and myeloperoxidase (Okazaki et al., 2006). This results in cell death causing white matter damage and long‐term neurological injury among pre‐term and term neonates (Leviton et al., 2005).
Cell death associated with HI and IVH can be classified as apoptotic, necrotic, or autophagic (Bobinger et al., 2018; Northington et al., 2011). Pro‐apoptotic proteins act directly (BAX and BAK) or indirectly (BAD, PUMA, BIM) as triggers of mitochondrial membrane permeabilization leading to activation of caspase activation and apoptosis‐inducing factor (AIF) translocation to nuclei which is believed to be crucial for injury to advance beyond the point of no return (Blomgren et al., 2001; Hagberg et al., 2009, 2014). Recruitment of mixed lineage kinase‐like protein (MLKL) to the necrosome results in mitochondria‐associated endoplasmic reticulum membranes triggering increased reactive oxygen species, fission, and necroptosis (Galluzzi et al., 2016). Death receptors trigger apoptotic death via caspase‐8 and necroptotic cell death through the formation of the necrosome, with both apoptotic and necrotic pathways converging at the mitochondria (Chavez‐Valdez et al., 2012, 2016; Galluzzi et al., 2011). Autophagy is increased in neuronal cells after neonatal HI and may represent a potential protective mechanism in the early stage of the brain injury (Carloni et al., 2008) but most data show that autophagy aggravates injury (Descloux et al., 2015; Xie et al., 2016).
Experimental results showing regeneration of the injured neonatal brain with stem cell‐based therapies are promising. Studies have shown that exogenous administration of stem cells significantly decreases brain injury in models of IVH (Ahn et al., 2013, 2015), HIE (Park et al., 2015), and neonatal stroke (Kim et al., 2012). These findings together with studies from adult neurological conditions (Caicedo et al., 2015) suggest that exogenous MSC transplantation might be a promising new therapy in the not too distant future for treating neonatal brain damage resulting from IVH, NE, or neonatal stroke.
2. MSCS—THE CELL OF CHOICE?
Major types of stem cells include embryonic stem cells, chemically induced pluripotent stem cells, neural stem cells, UCB cells, and MSCs. Embryonic stem (ES) cells have multiple characteristics such as self‐renewal, highly expandable, and possibility to derive neural stem cells and other neurogenic lineages (Svendsen & Smith, 1999) (Hynes & Rosenthal, 2000). Although ES seems an obvious choice for repairing brain injury, they can induce the formation of teratomas after transplantation (Bjorklund et al., 2002). Similarly, multipotent neural stem cells have the capacity to derive all neural lineages, but they also carry a significant risk of tumor formation (Comi et al., 2008; Fleiss et al., 2014). UCB contains MSCs, endothelial progenitor cells, and UCB‐mononuclear cells (Ingram et al., 2004). The advantages of UCB cells include easy access, limited ethical issues, and low immunogenicity. Administration of MSCs several days after neonatal HI markedly improves functional outcome, reduces infarct volume, and stimulates neurogenesis (Hawkins et al., 2018; van Velthoven et al., 2010a).
MSCs are characterized by their multi‐lineage differentiation capacity (Keating, 2012; Phinney et al., 2015; Pittenger et al., 1999), they express specific cell surface markers which include CD105 (Barry et al., 1999), CD73 (Barry et al., 2001), CD90 (Dominici et al., 2006), CD79 (Chu & Arber, 2001) and CD44 (L Ramos et al., 2016), but not CD79, CD19, CD14 (Wright et al., 1990), CD11b (Coxon et al., 1996), HLA‐DR (Dominici et al., 2006), and the hematopoietic marker CD45 (Irie‐Sasaki et al., 2001). They also lack co‐stimulatory proteins like CD80, CD40, and CD86 which makes them less immunogenic (Tse et al., 2003). Additionally, other non‐specific MSCs surface markers, like Stro‐1, SSEA‐4, CD271, and CD146, have also been proposed for positive characterization of MSCs (Lv et al., 2014). The International Society for Cellular Therapy has provided a consensus perspective on immune functional assays, functionally relevant surface markers etc., for MSCs as potency release criterion for clinical trials (Galipeau et al., 2016).
MSCs can be isolated from human placenta, umbilical cord (blood and Wharton's jelly), spleen, and adipose tissue‐derived mesenchymal stem cells (AT‐MSCs) (Gothard et al., 2014; Strioga et al., 2012) other than bone marrow. Other sites of MSC isolation include skin (Shih et al., 2005), dental pulp (Gronthos et al., 2000) and amniotic fluid (Nadri & Soleimani, 2007). Although these MSCs share common properties, they also exhibit differences in the expressed cell markers, in their differentiation potential and phenotypes (Gothard et al., 2014; Strioga et al., 2012).
Isolated MSCs from human submandibular skin tissues exhibit MSC characteristics and express a neural precursor marker, suggesting that human skin is a source of stem cells. However, the in vitro chemical neuronal induction of hMSC does not produce long‐lasting nerve cells and more studies are required before their use (Byun et al., 2012).
Recent studies have shown that tissue‐resident MSCs also express CD34, originally thought to be a hematopoietic marker as it is normally not found in culture‐expanded MSCs (Lin et al., 2012; Togarrati et al., 2017). CD146, a pericyte marker has been proposed to give rise to MSCs following blood vessel damage or inflammation (Bouacida et al., 2012; da Silva Meirelles et al., 2016). The secretome of MSCs has also been examined (although it varies by culture condition), and includes several growth factors such as vascular endothelial growth factor, brain‐derived neurotrophic factor, nerve growth factor, basic fibroblast growth factor as well as anti‐inflammatory cytokines (Qu et al., 2007). Growth factors secreted by MSCs mediates the proliferation and differentiation of progenitor cells (Ge et al., 2018; Martins et al., 2017).
MSCs have many favorable properties, they are non‐tumorigenic, have a positive safety profile, exhibit low immunogenicity and are easy to isolate/propagate reviewed in (Fleiss et al., 2014; Wagenaar et al., 2017). Also, MSCs have multiple therapeutic actions. They reduce inflammation and oxidative stress, restore energy failure, and decrease cell apoptosis (Li et al., 2012). MSCs boost the growth‐promoting environment for neural stem cells (Yoo et al., 2008). It has been shown that MSC mediated cell‐cell contacts provide additional signaling to improve adipogenic and osteogenic differentiation (Peng et al., 2012) through the increased gap junction or cadherin signaling (Lee et al., 2012; Tang et al., 2010). These effects of MSCs address many of the complex multifactorial factors driving tissue injury after severe IVH (Ballabh, 2010, 2014), or HIE (Aslam et al., 2019; Thornton et al., 2017; Wagenaar et al., 2018). A multifaceted therapeutic agent like MSCs may be suitable to improve outcomes of patients with these neonatal disorders (Park et al., 2018) (Figure 1).
FIGURE 1.
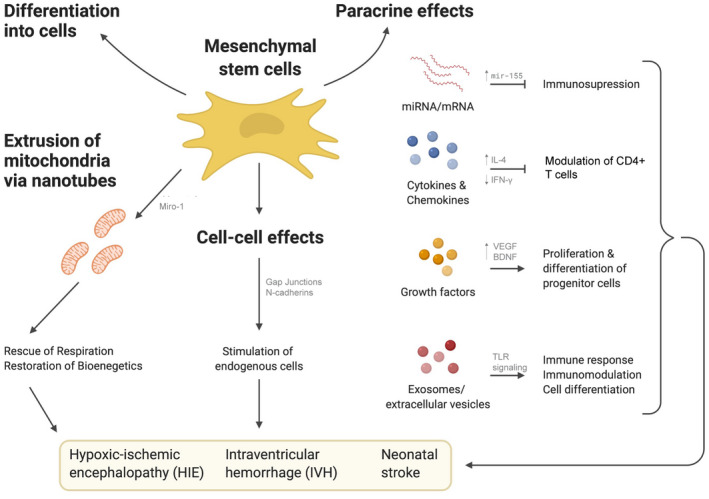
Mechanisms of neuroprotection offered by mesenchymal stem cells
3. EVIDENCE FOR MSC‐MEDIATED PROTECTION AND REPAIR OF PERINATAL BRAIN INJURY
The protective effects of MSCs, have been observed in various animal models of severe IVH (Ahn et al., 2013, 2015, 2017) and HIE (Gonzales‐Portillo et al., 2014; Pimentel‐Coelho & Mendez‐Otero, 2010). In an animal model of HIE, intranasal MSC transplantation at 10 days after the initial HI insult was still able to reduce brain damage (Donega et al., 2013), suggesting that MSCs also stimulate endogenous brain repair in addition to being solely neuroprotective early following injury (Donega et al., 2014; van Velthoven et al., 2013).
Ischemia‐reperfusion and/or infection‐inflammation are important factors in etiology of white matter injury (Hagberg et al., 2002). (Oppliger et al., 2016) showed that intranasal delivery of human Wharton's jelly‐MSC in rats prevented hypomyelination and microgliosis in a model of white matter injury in the premature rat pups. Following this another study showed the effect of human Wharton's jelly‐derived MSCs on white matter injury caused by intraperitoneal lipopolysaccharide (LPS) injection (0.1 mg/kg) in P3 rat pups followed by ligation of the left carotid artery and 40 min of hypoxia (8% O2) at P4. These animals then received intracranial MSC treatment at P11 which led to an improvement in locomotor activity, reduced myelin loss and astrocyte activation (Mueller et al., 2017). Several other studies have demonstrated that MSCs migrate to the ischemic boundary zone, induce changes in the brain environment, and support neurogenesis (van Velthoven et al., 2010a, 2011). It is also known that administration of MSCs after HI markedly induced cell proliferation in the hippocampus and cortex, stimulate neuronal cell differentiation, and formation of new neuroblasts within the subventricular zone (Chen et al., 2003; Donega et al., 2013; van Velthoven et al., 2010a; Yoo et al., 2008).
White matter involvement is recently realized as a common finding in mitochondrial disorders. Studies have shown that mitochondrial disorders play a principal role in the pathogenesis of white and grey matter injury (Galluzzi et al., 2009; Hagberg, 2004; Hagberg et al., 2014; Koning et al., 2019). Following HI, a primary phase of energy failure occurs because of ATP depletion, failure of mitochondrial respiration, increased excitotoxicity and associated influx of Ca2+ and Na+. Thereafter, a phase of recovery ensues usually referred to as the latent phase. This is followed by a secondary phase of energy depletion and tertiary phases of injury where inflammation, protein misfolding, increased ROS production, and disruption of mitochondrial integrity/function leading to cell death (Fleiss & Gressens, 2012; Hagberg et al., 2015).
4. MSC MECHANISMS OF ACTION
4.1. MSCs—neuroprotection via mitochondrial transfer
MSCs inhibit apoptosis and rejuvenate damaged cells by transferring mitochondria during reoxygenation after oxygen glucose deprivation (OGD) in vitro (Liu et al., 2014). This transfer of mitochondria was achieved through tunneling nanotubes (TNTs). Another in vitro study shows that besides mitochondria, TNTs also mediate cell‐to‐cell transfer of plasma membrane components, cytosolic molecules, and other cell organelles (Pasquier et al., 2013) (Figure 2). TNTs are membranous channels with a diameter of 50–200 nm and mediate continuity between the plasma membranes of connected cells (Gerdes et al., 2007; Rustom et al., 2004). Delivery of functional mitochondria via TNTs aid in the recovery of PC12 cells during early stages of apoptosis (Wang & Gerdes, 2015). A logical next step would be the development of a selective interference for TNT formation in vivo to unravel their functional role. TNTs are stated to be a potential defense response to stress since it is only stressed cells that develop TNTs (Zhang, 2011). In addition to OGD, described above, Wang et al. have shown that TNTs can be induced in rat hippocampal astrocytes and neurons by H2O2 or serum depletion (Wang et al., 2011).
FIGURE 2.
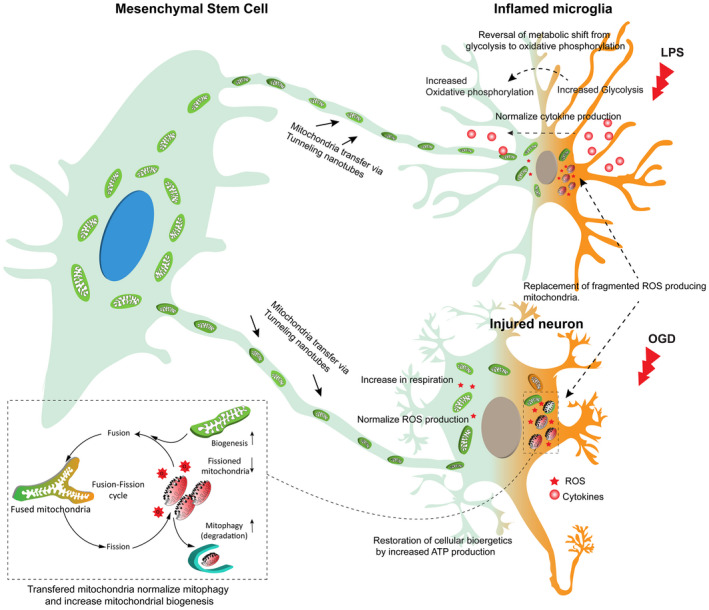
Mitochondrial transfer from mesenchymal stem cells via tunneling nanotubes for attenuation of brain inflammation and injury
Although the mechanisms associated with TNT formation have not been well studied, motor proteins, like calcium‐sensitive dynamin‐related Rho‐GTPases Miro1 and Miro2 (Ahmad et al., 2014; Fransson et al., 2006), KLF 5 kinesin motor protein (Falnikar et al., 2011), and accessory proteins like TRAK 1 and TRAK 2 (Glater et al., 2006; Stowers et al., 2002), and Myo 19 and Myo 10 (Bohil et al., 2006) facilitate efficient shipping of cargo between cells via an actin–myosin‐dependent mechanism (Nawaz & Fatima, 2017). Over‐expression of Miro1 in MSCs led to enhanced mitochondrial transfer and rescue of epithelial injury, while Miro1 knockdown led to reduced mitochondrial transfer (Ahmad et al., 2014). An important result of mitochondria transfer from MSCs is the restoration of cell functions such as mitochondrial respiration in the recipient cells (Caicedo et al., 2015). Mitochondria also have an innate ability to find their ways into a host cell. Isolated mitochondria can be incorporated in vitro into cells by a simple co‐incubation, without the need for transfection reagents or medium supplements. The incorporated mitochondria can increase ATP production and oxygen consumption inside the recipient cells (Kesner et al., 2016).
Mitochondria were isolated and transferred to target cells in a rabbit model of regional cardiac ischemia (Masuzawa et al., 2013). An injection of autologous mitochondria (~107 ) at the site of ischemia lead to their internalization within 8 hr of their administration and resulted in reduced apoptosis and infarct size, as detected 4 weeks later (Masuzawa et al., 2013). Chang et al have shown that allogeneic/xenogeneic injection of peptide‐labeled mitochondria into the medial forebrain bundle of adult rats restored mitochondrial functions and attenuated 6‐hydroxydopamine‐induced neurotoxicity in a model of Parkinson's disease (Chang et al., 2016). In a clinical study, it has been shown that healthy autologous mitochondria from non‐ischemic skeletal muscle in pediatric patients can be safely injected into damaged myocardium after ischemic injury for improvement of ventricular function (Emani et al., 2017). Similar studies have not been reported in neonatal models of brain injury or performed in patients with neonatal brain injury.
Besides improving the metabolic situation, transfer of healthy mitochondria to injured cells via TNTs can increase the turnover of damaged mitochondria by mitophagy (Li et al., 2017). Mitophagy is the selective autophagy of damaged mitochondria through the autophagosomal‐lysosomal pathway (Rodolfo et al., 2018; Thornton et al., 2018). Mitophagy plays a vital role in maintaining cellular homeostasis and defending against oxidative stress because this process controls mitochondrial quality and quantity by eliminating dysfunctional or damaged mitochondria (Hagberg et al., 2014). HI‐induced brain damage increases mitophagy and the inhibition of mitophagy aggravates brain damage in neonatal rats (Li et al., 2017). In stroke, most studies support the hypothesis that mitophagy favors neurons adapted to the stress by removing impaired mitochondria and suppressing cell death signaling cascades. In permanent MCAO, mitophagy was triggered at 1 hr and in the reperfusion phase (Zhang et al., 2013) (Zuo et al., 2014). Rapamycin treatment improves mitochondrial function after experimental ischemic stroke (Li et al., 2014). Infusion of MSCs restored PTEN‐induced kinase (Pink1)/ PTEN‐induced kinase 1 (Parkin)‐mediated mitophagy, improved mitochondrial dysfunction and attenuated apoptosis in endothelial cells in diabetic rats (Zhu et al., 2018).
4.2. MSCs—neuroprotection by modulation of immune‐inflammatory response
Inflammation is another important risk factor for injury in the developing brain (Bokobza et al., 2019; Hagberg et al., 2012, 2015). Intranasal administration of UC‐MSCs in postnatal day 10 Sprague‐Dawley rat pups after HI significantly reduced markers of neuroinflammation and significantly decreased the number of activated microglia, the brains innate immune cells (McDonald et al. 2019).
In clinical cases of IVH, microglial density, particularly IBA1/CD68 microglia, was higher within the affected brain regions when compared to healthy infants. Severe cases also had increased TNFa expression (Supramaniam et al., 2013). Following IVH, bleeding may extend into the intraventricular CSF, with cell‐free hemoglobin proposed as an inducer of inflammation, and hemoglobin metabolites inducing pro‐inflammatory cytokine expression (Gram et al., 2013) causing inflammation of the choroid plexus and ventricular ependymal lining (Simard et al., 2011). Inflammation and scarring may also prevent the flow of CSF reducing drainage (Strahle et al., 2012). Clinical studies have shown that the CSF inflammatory response to IVH is directly proportional to the extension of bleeding (Fam et al., 2017). Injury to the germinal matrix and HI, both possibly mediated by cytotoxins from blood products, are linked to the mechanisms of injury after IVH (du Plessis, 1998). Interestingly, brain‐derived neurotrophic factor (BDNF) secreted by MSCs, after contact with thrombin is an important factor in MSC therapy for IVH. MSCs with siRNA‐induced BDNF knockdown lose their therapeutic capacity in severe IVH‐induced brain injury in newborn rats (Ahn et al., 2017).
MSCs are capable of homing from the circulation to sites of injury and inflammation (Rustad & Gurtner, 2012). This can result in the resolution of the glial scar surrounding the lesion, decrease in reactive astrocytes and microglia and polarization of microglia toward the anti‐inflammatory phenotype (Donega et al., 2014). Lin et al. have shown that infusion of MSCs in a global cerebral ischemia mouse model increased release of TNF‐α‐induced protein (TSG‐6) thereby inhibiting local inflammation and improving function of the nervous system (Lin et al., 2013). Van Velthoven et al have demonstrated in the damaged area of the brain after MSC treatment, the up‐regulation of gene expression profiles associated with cell proliferation (Spp1 and IL17), neurogenesis (NRCAm and NGF), migration (CXCR4), and neuronal survival (glial‐derived neurotrophic factor) whereas genes involved in inflammation (e.g. IL1‐ β) were down‐regulated.
Since the immunomodulatory properties of MSCs were first reported by Bartholomew and colleagues (Bartholomew et al., 2002), subsequent studies have shown that MSCs mediate immunosuppression via up‐regulation of miR‐155 (Xu et al., 2013). MSCs have the capacity to interact with immune cells like B cells, T cells, dendritic cells (DCs), natural killer (NK) cells, neutrophil, and macrophages (Wang et al., 2014) and the MSCs secretome alters both adaptive and innate immune responses (Aslam et al., 2009; Bruno et al., 2015).
MSCs induce persistent peripheral T‐cell tolerance in a preterm sheep model and reduced invasion of T cells into the brain following global HI (Jellema et al., 2013). Besides T‐cell modulation, MSCs inhibit B cell proliferation, neutrophil and monocyte function, and NK toxicity (Duffy et al., 2011; Gerdoni et al., 2007; Jellema et al., 2013). Although these modulatory effects are only partially understood, direct cell‐to‐cell contact and soluble factors appear both to be relevant (Rahmat et al., 2013). MSCs recognize activated NK cells and inhibit their proliferation by downregulating their activation via IL‐2 or IL‐15 (Spaggiari & Moretta, 2012). MSCs inhibit CD14 + monocyte differentiation toward dendritic cells (DC) (Jiang et al., 2005). MSCs also suppress TNFα and IFN‐γ secretion by CD4 + T cells while promoting IL‐4 secretion (Aggarwal & Pittenger, 2005). Artificial transfer of isolated MSC‐derived mitochondria also affects inflammation via actions on adaptive immune cells. Specifically, transfer of mitochondria from MSCs to T cells increases the expression of mRNA transcripts involved in T‐cell activation and T regulatory cell differentiation including FOXP3, IL2RA, CTLA4, and TGFβ1, leading to an increase in a highly suppressive CD25 + FoxP3+ population (Court et al., 2020).
Besides the actions mentioned above, mitochondria also act as central hubs in the innate immune system by regulating RIG‐I‐like receptors (RLRs), NOD‐like receptors (NLRs), and Toll‐like receptors (TLR). The incorporation of mitochondria into this arm of immunity can result in increased cellular energy output and significant metabolic reprogramming (West et al., 2011). Microglia are the immune cells of the brain and our previous study demonstrated that their inflammatory activation is linked to changes in mitochondrial function (Nair et al., 2019). Specifically, a short exposure to a low dose of lipopolysaccharide (LPS) causes a transient increase in oxidative phosphorylation (OXPHOS) whereas prolonged exposure to high dose LPS causes suppression of OXPHOS and mitochondrial respiration. This suppression of OXPHOS forces a metabolic shift toward glycolysis (Nair et al., 2019), a phenomenon similar to the Warburg effect (Kelly & O'Neill, 2015). Although glycolysis results in less ATP production overall, the preferential use of glycolysis over OXPHOS enables immune cells to produce ATP at a faster rate (Marelli‐Berg et al., 2012) and to produce intermediates for cytokine production (Chang et al., 2013) (Figure 2).
MSCs alleviate inflammation and the anti‐inflammatory effects include a shift from pro‐inflammatory cytokines such as IL‐1β, IL‐6, TNFα, IFNγ, IL‐1Rα, and prostaglandin E2 to anti‐inflammatory cytokines like IL‐10 (Bartosh et al., 2010; Chaubey et al., 2018; Chou et al., 2016; Ding et al., 2017; Nemeth et al., 2009; Ortiz et al., 2007; Park et al., 2013) (Figure 2). IL‐10 inhibits LPS‐induced glucose uptake and glycolysis and promotes OXPHOS in macrophages. IL‐10 also eliminates dysfunctional mitochondria by promoting mitophagy (Ip et al., 2017) which may drive them toward an anti‐inflammatory phenotype.
MSCs impact the metabolic phenotype of primary macrophages in co‐cultures (Vasandan et al., 2016). However, we do not know if MSC‐mediated factors directly lead to a metabolic shift in brain cells such as microglia. Islam et al. demonstrated in a comprehensive study that transfer of mitochondria via gap junctional channels from MSCs to pulmonary alveolar epithelial cells in a murine model of LPS‐induced acute lung injury is protective (Islam et al., 2012). They demonstrated that the neuroprotective effect was because of transferred mitochondria by including studies using gap‐junction channel‐deficient MSCs. Gap‐junction channels mediate shorter range cell‐to‐cell interactions, whereas TNTs allow long‐range communication and mediate the exchange of larger organelles and vesicles (Gerdes et al., 2013; Okafo et al., 2017).
4.3. MSCs—neuroprotection by regulation of ros production
During HI, oxidative stress induced by ROS ‘bursts’ plays a vital role in mitochondrial dysfunction and subsequent cell death (Forman et al., 2004; Piantadosi & Zhang, 1996; Singh‐Mallah et al., 2019). Elevated levels of intracellular ROS caused by dysfunctional mitochondria attenuate global protein synthesis (Topf et al., 2018) and will indiscriminately attack phospholipids, proteins and DNA (Singh‐Mallah et al., 2019). Oxidative stress is an early feature after cerebral ischemia and experimental studies targeting the formation of free radicals demonstrate various degrees of protection after perinatal insults (Singh‐Mallah et al., 2019). The premature brain, in particular, is vulnerable to ROS‐induced damage because of the deficient antioxidant stores due of their insufficient ability to synthesize antioxidant enzymes at birth and impaired up‐regulation in response to oxidative stress (Lee & Davis, 2011; Singh‐Mallah et al., 2019).
The main species of ROS which include O2•− and H2O2 is produced from the dismutation of O2•− by MnSOD within the mitochondria (Singh‐Mallah et al., 2019). Ischemia also results in succinate accumulation in the immature brain (Koning et al., 2019) which is rapidly oxidized in the first minutes of reperfusion, generating large amounts of O2•(Sahni et al., 2018)−. The underdeveloped antioxidant system in the immature brain limits the inactivation of H2O2, which can cross lipid membranes and cause tissue damage. Mitochondrial ROS production and inflammation are increased after neonatal brain injury associated with altered Krebs cycle and succinate accumulation in the mitochondria (Koning et al., 2019). Excessive inflammation causes mitochondrial fragmentation and a metabolic switch in microglia cells, which is accompanied by increased ROS production (Nair et al., 2019).
MSCs reduce increased oxidative stress levels in pathophysiological conditions. Paliwal et al. (2017) investigated abilities of tissue‐specific MSCs (bone marrow, adipose tissue, dental pulp and umbilical cord), from young or old donors (<30 versus > 30 years of age) to reduce ROS and thereby mitigate cellular damage. They found that both donor's age and source of tissue affected the ability of MSCs to reduce increased ROS levels in injured cells, with young donor supplied dental pulp MSCs having the greatest efficacy (Paliwal et al., 2017). The importance of donor age in stem cell treatment has also been investigated in the use of UCB derived from term or preterm sheep, administered in a sheep model of HIE. Although both cell sources were protective, UCB from term (but not preterm) reduced markers of circulating oxidative stress (Li et al., 2018).
ROS levels can be efficiently reduced by stimulating mitophagy by rapamycin (Bin‐Umer et al., 2014; Kurihara et al., 2012). As damaged mitochondria are a primary source of ROS, failure to remove dysfunctional mitochondria results in increase ROS production. MSCs can ameliorate ROS overproduction by restoration of autophagic flux (Zhu et al., 2018). These findings indicate that MSCs are important regulators of oxidative stress production, enhance cell migration, and support their role to improve neuroprotection and brain recovery following injury.
5. HOW TO BUILD A BETTER MSC
Targeting metabolism is an important way to improve MSC function. Studies have shown that in a nutrient‐rich culture medium MSCs rapidly proliferate, and reconfigure their central energy metabolism to become significantly more dependent on oxidative phosphorylation (OXPHOS) (Pattappa et al., 2011). Whereas in their native (lower nutrient) environment MSCs exist in a quiescent state characterized by low proliferation and high multi‐potentiality (Yuan et al., 2019). In this state they are primarily glycolytic, with mitochondria maintained by active biogenesis‐mitophagy cycling (Katajisto et al., 2015; Sbrana et al., 2016). Dependence on OXPHOS‐fueled metabolic profile (in a nutrient‐rich environment) results in accumulation of reactive oxygen species (ROS) (Shyh‐Chang et al., 2013), that reduce basal autophagy and mitophagy rates while simultaneously increasing the fraction of senescent cells that have reduced clinical potency (Ho et al., 2017). Another important feature of MSCs for cell therapy is their immunomodulatory secretome—cytokines and cellular components such as microRNA and extracellular vesicles that affect their repair and regenerative capacities.
In vivo studies show that MSCs can home toward the ischemic lesion site via interaction between stromal cell‐derived factor‐1α and its receptor CXCR4 present on MSCs (Wang et al., 2008). The Stromal cell‐derived factor‐1α expression is upregulated near the lesion site and expressed for at least 14 d after induction of HI brain injury (Rosenkranz et al., 2010). The interaction of locally produced SDF‐1alpha and CXCR4 expressed on the MSC plays an important role in the migration of transplanted MSCs, suggests that it might be a potential approach to modulate the expression of the two molecules in order to promote the therapeutic effects of MSCs (Wang et al., 2008).
The characteristics of the secretome is significantly influenced by metabolism (Yuan et al., 2019), which itself is linked to MSC phenotype. MSC phenotype, is the state of the cell as a result of the interaction between the paracrine factors with receptors such as Toll‐like receptors (TLR) (Delarosa et al., 2012; Sangiorgi & Panepucci, 2016). (Waterman et al., 2010) has described two different MSC phenotypes distinguished as proinflammatory MSC phenotype (MSC1; induced by TLR4 activation) and anti‐inflammatory MSC phenotype (MSC2; induced by TLR3 activation).
Donald et al. have shown that MSCs manage intracellular oxidative stress by targeting depolarized mitochondria to the plasma membrane via arrestin domain‐containing protein 1‐mediated microvesicles (Phinney et al., 2015). Subsequently, these microvesicles are engulfed and re‐utilized via a process involving mitochondrial fusion by macrophages which results in enhanced bioenergetics (Phinney et al., 2015). In this process MSCs simultaneously shed micro RNA‐containing exosomes that inhibit macrophage activation by suppressing TLR signaling, thereby de‐sensitizing macrophages to the ingested mitochondria (Phinney et al., 2015).
Methods to manipulate MSCs to reduce ROS during their culture expansion phase and in the injured tissue microenvironment may also promote MSC engraftment and enhance tissue repair (Denu & Hematti, 2016). One potential method to reduce oxidative stress in MSCs is by modulating sirtuin expression and/or activity. Multiple studies have shown a protective role for SIRT1 in various models of neuronal injury and neurodegeneration (Della‐Morte et al., 2009). It is shown that SIRT1 over‐expression quenches age‐related MSCs senescence by enhancing TPP1 expression, telomerase activity, and reducing DNA damage. ROS levels in stem cells increase with age (Ito et al., 2006). Majority of ROS is produced in the mitochondria and SIRT3 is the major protein deacetylase in the mitochondria, where its actions decrease ROS production. In addition, SIRT3 initiates metabolic reprogramming toward more efficient electron transport and fuel usage away from carbohydrate catabolism, reducing excessive ROS (Denu, 2017). Data suggest that overexpressing SIRT3 (major mitochondrial deacetylase involved in reducing oxidative stress while preserving oxidative metabolism) might represent a strategy to increase the quality and quantity of MSCs that could be utilized for clinical applications (Denu, 2017).
6. CHALLENGES IN MSC‐BASED THERAPY
Researchers have made significant progress in enhancing the therapeutic properties of MSCs. Transplantation time and injected cell dose are key factors that determine the therapeutic effect of stem cell therapy (Wang et al., 2017). Studies with incomparable or conflicting results prevent the advancement of MSCs for clinical use. These discrepancies are mostly because of variabilities in dosage, administration route, timing, and the limited ability of animal models to mimic the extremely complex process of human physiology and etiology of encephalopathy. Possible routes for MSCs infusion in animals are, (i) systemic delivery [intra‐venous (IV) and intra‐arterial (IA) , intra‐peritoneal (IP), and intracardiac (IC) (ii) local/topical/regional delivery (cell‐spray, gel or subcutaneous injection with a carrier hydrogel intramuscular, intranasal, and intrathecal injection) or (iii) scaffold/bioengineered construct (cells embedded in a scaffold, such as vascular grafts and intra‐osseous injection), with each route having its advantages and disadvantages for specific disorders. Although intracerebral administration ensures direct and targeted delivery and a minimum loss of stem cells, it is an invasive procedure, especially as the sites for delivery are often deep in the brain. However, these approaches should not be considered inappropriate to develop, as invasive neurosurgical techniques have been developed for the treatment of severe IVH and these have had astounding positive effects on long‐term outcomes; a 23‐point increase in childhood IQ (Luyt et al., 2019). To avoid intracerebral injections, multiple studies have looked at systemic MSC administration routes: either intravenous (IV) or intra‐arterial or intranasal applications (Li et al., 2018, McDonald et al. 2019, Danielyan et al., 2009, van Velthoven et al., 2010b). IV injections by retro‐orbital injections, although may seem aesthetically distasteful, can be performed rapidly without requiring mechanical restriction or heat‐induced vasodilation. This route is ultimately more humane than alternative intravascular injection techniques in small animals (Leon‐Rico et al., 2015; Ukai et al., 2007; Yardeni et al., 2011).
Therapeutic modalities must not only be efficacious but also safe for the patient. While the majority of preclinical and clinical studies have found MSCs to be safe and well‐tolerated (Lalu et al., 2012), uncontrolled stem cell interventions have resulted in adverse incidents (Bauer et al., 2018; Moll et al., 2019). Systemic injections may be practical but they can activate the host innate immune cascade systems, such as complement and coagulation, termed the instant blood mediated inflammatory reaction (IBMIR) (Leibacher & Henschler, 2016; Moll et al., 2012, 2019). A meta‐analysis of preclinical studies of MSCs in ischemic stroke models found that although direct injection of MSCs provided the greatest benefit, although other methods of delivery such as IA and IV injections also demonstrated significant improvement in outcomes (Vu et al., 2014). Therefore, the present data, based on some preclinical studies and limited clinical trials, is not adequate to make any sound conclusions as to whether one delivery method is superior to another.
In addition to routes of administration, the delivery of sufficient MSCs is required to discern a significant therapeutic effect. Neuroprotective therapy for HIE such as hypothermia, needs to be started within 6 hr to be effective (Davidson et al., 2015). However, the therapeutic window of MSC treatment is probably wider in neonatal HI mice as it has been shown that MSCs improved functional outcome and lesion volume when administered at least until 10 days after induction of the insult (Donega et al., 2013). A single dose of 0.5 × 106 MSCs administered intranasally was identified as the minimally effective dose with a therapeutic window of at least 10 days but less than 17 days post‐HI was sufficient for a marked beneficial effect in neonatal mice (Donega et al., 2013). Velthoven et al have shown that intracranial administration of 100,000 MSCs at 3 and 10 days after neonatal HI markedly improves functional outcome, reduces infarct volume, and stimulates neurogenesis, with each injection having distinct effects on regenerative processes. Briefly, they find that a single injection of MSCs increased cell proliferation and differentiation of newly divided cells into NeuN‐ and olig2‐positive cells and reduced the percentage of BrdU+‐microglia, whereas a second additional injection with MSCs induced axonal outgrowth and remodeling, myelination and synaptogenesis which led to improved functional outcome (van Velthoven et al., 2010a).
7. CONCLUSION and FUTURE DIRECTIONS
MSCs secrete many paracrine factors which can impart pleiotropic effects in response to microenvironmental cues in the injured brain. MSCs attenuate pro‐inflammatory responses and the adverse effects of excessive ROS production. MSC mediated mitochondria transplantation could be a game‐changer for treating perinatal and neonatal brain injury. Future meticulous studies should be performed to delineate the mitochondria‐mediated protective mechanisms specifically for neonatal brain injury. For instance, the development of a selective inhibitor for TNT formation can help unravel its functional role. Another example is accelerated mitochondrial transfer by Miro1 over‐expression, as this may be a way to rejuvenate injured cells. Scientists are investigating the role of artificial transfer of isolated mitochondria as it can emerge as a regenerative or immunomodulatory therapy.
CONFLICT OF INTEREST
We report no conflict of interest.
ACKNOWLEDGEMENTS
The authors thank the support from the Swedish Medical Research Council (VR (2019‐01320; 2017‐01409, CM); the Swedish Governmental Grant to Researchers at University Hospitals (ALFGBG‐432291; ALFGBG‐722491, CM); Hjärnfonden (Brain Foundation FO2019‐0056; FO2019‐0270, CM); ERA‐NET (Contract: 0755101), and EU (contract: 874721 Horizon 2020), Ahlen Foundation (HH, CM, SN), Tore Nilsons Foundation (2018‐00594, SN), Jane and Dan Olssson Foundation (2020‐25, SN), Stiftelsen Fru Mary Von Sydows (3616, SN), Hasselblad Foundation (2020‐2021, ERF), Åke Wibergs Foundation (M19‐0660, ERF), Fondation pour le Recherche Médicale, Fondation Grace de Monaco, and an additional grant from Investissement d'Avenir (ANR‐11‐INBS‐0011) NeurATRIS, and the Cerebral Palsy Alliance (PG12518). Figure 1 has been re‐drawn by Marco Bazelmans in BioRender (https://biorender.com/) on the basis of a draft provided by the author.
Nair S, Rocha‐Ferreira E, Fleiss B, et al. Neuroprotection offered by mesenchymal stem cells in perinatal brain injury: Role of mitochondria, inflammation, and reactive oxygen species. J Neurochem.2021;158:59–73. 10.1111/jnc.15267
This is a Review for the special issue “Neuroimmune Metabolism”
REFERENCES
- Aggarwal, S., & Pittenger, M. F. (2005). Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood, 105, 1815–1822. 10.1182/blood-2004-04-1559 [DOI] [PubMed] [Google Scholar]
- Ahmad, T., Mukherjee, S., Pattnaik, B., Kumar, M., Singh, S., Kumar, M., Rehman, R., Tiwari, B. K., Jha, K. A., Barhanpurkar, A. P., Wani, M. R., Roy, S. S, Mabalirajan, U., Ghosh, B., & Agrawal, A. (2014). Miro1 regulates intercellular mitochondrial transport & enhances mesenchymal stem cell rescue efficacy. The EMBO Journal, 33, 994–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn, S. Y., Chang, Y. S., Sung, D. K., Sung, S. I., Ahn, J. Y., & Park, W. S. (2017). Pivotal role of brain‐derived neurotrophic factor secreted by mesenchymal stem cells in severe intraventricular hemorrhage in newborn rats. Cell Transplantation, 26, 145–156. 10.3727/096368916X692861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn, S. Y., Chang, Y. S., Sung, D. K., Sung, S. I., Yoo, H. S., Im, G. H., Choi, S. J., & Park, W. S. (2015). Optimal route for mesenchymal stem cells transplantation after severe intraventricular hemorrhage in newborn rats. PLoS One, 10, e0132919. 10.1371/journal.pone.0132919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn, S. Y., Chang, Y. S., Sung, D. K., Sung, S. I., Yoo, H. S., Lee, J. H., Oh, W. I., & Park, W. S. (2013). Mesenchymal stem cells prevent hydrocephalus after severe intraventricular hemorrhage. Stroke, 44, 497–504. 10.1161/STROKEAHA.112.679092 [DOI] [PubMed] [Google Scholar]
- Aslam, M., Baveja, R., Liang, O. D., Fernandez‐Gonzalez, A., Lee, C., Mitsialis, S. A., & Kourembanas, S. (2009). Bone marrow stromal cells attenuate lung injury in a murine model of neonatal chronic lung disease. American Journal of Respiratory and Critical Care Medicine, 180, 1122–1130. 10.1164/rccm.200902-0242OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aslam, S., Strickland, T., & Molloy, E. J. (2019). Neonatal encephalopathy: Need for recognition of multiple etiologies for optimal management. Frontiers in Pediatrics, 7, 142. 10.3389/fped.2019.00142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballabh, P. (2010). Intraventricular hemorrhage in premature infants: Mechanism of disease. Pediatric Research, 67, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballabh, P. (2014). Pathogenesis and prevention of intraventricular hemorrhage. Clinics in Perinatology, 41, 47–67. 10.1016/j.clp.2013.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry, F. P., Boynton, R. E., Haynesworth, S., Murphy, J. M., & Zaia, J. (1999). The monoclonal antibody SH‐2, raised against human mesenchymal stem cells, recognizes an epitope on endoglin (CD105). Biochemical and Biophysical Research Communications, 265, 134–139. 10.1006/bbrc.1999.1620 [DOI] [PubMed] [Google Scholar]
- Barry, F., Boynton, R., Murphy, M., Haynesworth, S., & Zaia, J. (2001). The SH‐3 and SH‐4 antibodies recognize distinct epitopes on CD73 from human mesenchymal stem cells. Biochemical and Biophysical Research Communications, 289, 519–524. 10.1006/bbrc.2001.6013 [DOI] [PubMed] [Google Scholar]
- Bartholomew, A., Sturgeon, C., Siatskas, M., Ferrer, K., McIntosh, K., Patil, S., Hardy, W., Devine, S., Ucker, D., Deans, R., Moseley, A., & Hoffman, R. (2002). Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Experimental Hematology, 30, 42–48. 10.1016/S0301-472X(01)00769-X [DOI] [PubMed] [Google Scholar]
- Bartosh, T. J., Ylostalo, J. H., Mohammadipoor, A., Bazhanov, N., Coble, K., Claypool, K., Lee, R. H., Choi, H., & Prockop, D. J. (2010). Aggregation of human mesenchymal stromal cells (MSCs) into 3D spheroids enhances their antiinflammatory properties. Proceedings of the National Academy of Sciences, 107, 13724–13729. 10.1073/pnas.1008117107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer, G., Elsallab, M., & Abou‐El‐Enein, M. (2018). Concise review: A comprehensive analysis of reported adverse events in patients receiving unproven stem cell‐based interventions. Stem Cells Translational Medicine, 7, 676–685. 10.1002/sctm.17-0282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bel, F., & Groenendaal, F. (2016). Drugs for neuroprotection after birth asphyxia: Pharmacologic adjuncts to hypothermia. Seminars in Perinatology, 40, 152–159. 10.1053/j.semperi.2015.12.003 [DOI] [PubMed] [Google Scholar]
- Berger, I., Peleg, O., & Ofek‐Shlomai, N. (2012). Inflammation and early brain injury in term and preterm infants. Israel Medical Association Journal, 14, 318–323. [PubMed] [Google Scholar]
- Bin‐Umer, M. A., McLaughlin, J. E., Butterly, M. S., McCormick, S., & Tumer, N. E. (2014). Elimination of damaged mitochondria through mitophagy reduces mitochondrial oxidative stress and increases tolerance to trichothecenes. Proceedings of the National Academy of Sciences, 111, 11798–11803. 10.1073/pnas.1403145111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorklund, L. M., Sanchez‐Pernaute, R., Chung, S., Andersson, T., Chen, I. Y. C., McNaught, K. S. P., Brownell, A.‐L., Jenkins, B. G., Wahlestedt, C., Kim, K.‐S., & Isacson, O. (2002). Embryonic stem cells develop into functional dopaminergic neurons after transplantation in a Parkinson rat model. Proceedings of the National Academy of Sciences of the United States of America, 99, 2344–2349. 10.1073/pnas.022438099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomgren, K., Zhu, C., Wang, X., Karlsson, J. O., Leverin, A. L., Bahr, B. A., Mallard, C., & Hagberg, H. (2001). Synergistic activation of caspase‐3 by m‐calpain after neonatal hypoxia‐ischemia: A mechanism of "pathological apoptosis"? Journal of Biological Chemistry, 276, 10191–10198. 10.1074/jbc.M007807200 [DOI] [PubMed] [Google Scholar]
- Bobinger, T., Burkardt, P., Huttner, B. H., & Manaenko, A.(2018). Programmed cell death after intracerebral hemorrhage. Current Neuropharmacology, 16, 1267–1281. 10.2174/1570159X15666170602112851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohil, A. B., Robertson, B. W., & Cheney, R. E. (2006). Myosin‐X is a molecular motor that functions in filopodia formation. Proceedings of the National Academy of Sciences, 103, 12411–12416. 10.1073/pnas.0602443103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokobza, C., Van Steenwinckel, J., Mani, S., Mezger, V., Fleiss, B., & Gressens, P. (2019). Neuroinflammation in preterm babies and autism spectrum disorders. Pediatric Research, 85, 155–165. 10.1038/s41390-018-0208-4 [DOI] [PubMed] [Google Scholar]
- Bouacida, A., Rosset, P., Trichet, V., Guilloton, F., Espagnolle, N., Cordonier, T., Heymann, D., Layrolle, P., Sensébé, L., & Deschaseaux, F. (2012). Pericyte‐like progenitors show high immaturity and engraftment potential as compared with mesenchymal stem cells. PLoS One, 7, e48648. 10.1371/journal.pone.0048648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno, S., Deregibus, M. C., & Camussi, G. (2015). The secretome of mesenchymal stromal cells: Role of extracellular vesicles in immunomodulation. Immunology Letters, 168, 154–158. 10.1016/j.imlet.2015.06.007 [DOI] [PubMed] [Google Scholar]
- Byun, J.‐H., Kang, E.‐J., Park, S.‐C., Kang, D.‐H., Choi, M.‐J., Rho, G.‐J., & Park, B.‐W. (2012). Isolation of human mesenchymal stem cells from the skin and their neurogenic differentiation in vitro. Jkaoms, 38, 343–353. [Google Scholar]
- Caicedo, A., Fritz, V., Brondello, J.‐M., Ayala, M., Dennemont, I., Abdellaoui, N., de Fraipont, F., Moisan, A., Prouteau, C. A., Boukhaddaoui, H., Jorgensen, C., & Vignais, M.‐L. (2015). MitoCeption as a new tool to assess the effects of mesenchymal stem/stromal cell mitochondria on cancer cell metabolism and function. Scientific Reports, 5, 9073. 10.1038/srep09073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carloni, S., Buonocore, G., & Balduini, W. (2008). Protective role of autophagy in neonatal hypoxia‐ischemia induced brain injury. Neurobiology of Diseases, 32, 329–339. 10.1016/j.nbd.2008.07.022 [DOI] [PubMed] [Google Scholar]
- Chang, C.‐H., Curtis, J. D., Maggi, L. B., Faubert, B., Villarino, A. V., O’Sullivan, D., Huang, S.‐C., van der Windt, G. J. W., Blagih, J., Qiu, J., Weber, J. D., Pearce, E. J., Jones, R. G., & Pearce, E. L. (2013). Posttranscriptional control of T cell effector function by aerobic glycolysis. Cell, 153, 1239–1251. 10.1016/j.cell.2013.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, J.‐C., Wu, S.‐L., Liu, K.‐H., Chen, Y.‐H., Chuang, C.‐S., Cheng, F.‐C., Su, H.‐L., Wei, Y.‐H., Kuo, S.‐J., & Liu, C.‐S. (2016). Allogeneic/xenogeneic transplantation of peptide‐labeled mitochondria in Parkinson's disease: Restoration of mitochondria functions and attenuation of 6‐hydroxydopamine–induced neurotoxicity. Translational Research, 170, 40–56.e43. 10.1016/j.trsl.2015.12.003 [DOI] [PubMed] [Google Scholar]
- Chaubey, S., Thueson, S., Ponnalagu, D., Alam, M. A., Gheorghe, C. P., Aghai, Z., Singh, H., & Bhandari, V. (2018). Early gestational mesenchymal stem cell secretome attenuates experimental bronchopulmonary dysplasia in part via exosome‐associated factor TSG‐6. Stem Cell Research & Therapy, 9, 173. 10.1186/s13287-018-0903-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavez‐Valdez, R., Flock, D. L., Martin, L. J., & Northington, F. J. (2016). Endoplasmic reticulum pathology and stress response in neurons precede programmed necrosis after neonatal hypoxia‐ischemia. International Journal of Developmental Neuroscience, 48, 58–70. 10.1016/j.ijdevneu.2015.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavez‐Valdez, R., Martin, L. J., & Northington, F. J. (2012). Programmed necrosis: A prominent mechanism of cell death following neonatal brain injury. Neurology Research International, 2012, 257563. 10.1155/2012/257563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, J., Li, Y., Katakowski, M., Chen, X., Wang, L., Lu, D., Lu, M., Gautam, S. C., & Chopp, M. (2003). Intravenous bone marrow stromal cell therapy reduces apoptosis and promotes endogenous cell proliferation after stroke in female rat. Journal of Neuroscience Research, 73, 778–786. 10.1002/jnr.10691 [DOI] [PubMed] [Google Scholar]
- Chou, H. C., Li, Y. T., & Chen, C. M. (2016). Human mesenchymal stem cells attenuate experimental bronchopulmonary dysplasia induced by perinatal inflammation and hyperoxia. American Journal of Translational Research, 8, 342–353. [PMC free article] [PubMed] [Google Scholar]
- Chu, P. G., & Arber, D. A. (2001). CD79: A review. Applied Immunohistochemistry & Molecular Morphology, 9, 97–106. 10.1097/00129039-200106000-00001 [DOI] [PubMed] [Google Scholar]
- Comi, A. M., Cho, E., Mulholland, J. D., Hooper, A., Li, Q., Qu, Y., Gary, D. S., McDonald, J. W., & Johnston, M. V. (2008). Neural stem cells reduce brain injury after unilateral carotid ligation. Pediatric Neurology, 38, 86–92. 10.1016/j.pediatrneurol.2007.10.007 [DOI] [PubMed] [Google Scholar]
- Court, A. C., Le‐Gatt, A., Luz‐Crawford, P., Parra, E., Aliaga‐Tobar, V., Bátiz, L. F., Contreras, R. A., Ortúzar, M. I., Kurte, M., Elizondo‐Vega, R., Maracaja‐Coutinho, V., Pino‐Lagos, K., Figueroa, F. E., & Khoury, M. (2020). Mitochondrial transfer from MSCs to T cells induces Treg differentiation and restricts inflammatory response. EMBO Reports, 21, e48052. 10.15252/embr.201948052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coxon, A., Rieu, P., Barkalow, F. J., Askari, S., Sharpe, A. H., von Andrian, U. H., Arnaout, M. A., & Mayadas, T. N. (1996). A novel role for the β2 integrin CD11b/CD18 in neutrophil apoptosis: A homeostatic mechanism in inflammation. Immunity, 5, 653–666. 10.1016/S1074-7613(00)80278-2 [DOI] [PubMed] [Google Scholar]
- da Silva Meirelles, L., Malta, T. M., Panepucci, R. A., & da Silva Jr, W. A. (2016). Transcriptomic comparisons between cultured human adipose tissue‐derived pericytes and mesenchymal stromal cells. Genomics Data, 7, 20–25. 10.1016/j.gdata.2015.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielyan, L., Schäfer, R., von Ameln‐Mayerhofer, A., Buadze, M., Geisler, J., Klopfer, T., Burkhardt, U., Proksch, B., Verleysdonk, S., Ayturan, M., Buniatian, G. H., Gleiter, C. H., & Frey, W. H. (2009). Intranasal delivery of cells to the brain. European Journal of Cell Biology, 88, 315–324. 10.1016/j.ejcb.2009.02.001 [DOI] [PubMed] [Google Scholar]
- Davidson, J. O., Wassink, G., van den Heuij, L. G., Bennet, L., & Gunn, A. J. (2015). Therapeutic hypothermia for neonatal hypoxic‐ischemic encephalopathy ‐ where to from here? Frontiers in Neurology, 6, 198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delarosa, O., Dalemans, W., & Lombardo, E. (2012). Toll‐like receptors as modulators of mesenchymal stem cells. Frontiers in Immunology, 3, 182. 10.3389/fimmu.2012.00182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Della‐Morte, D., Dave, K. R., DeFazio, R. A., Bao, Y. C., Raval, A. P., & Perez‐Pinzon, M. A. (2009). Resveratrol pretreatment protects rat brain from cerebral ischemic damage via a sirtuin 1‐uncoupling protein 2 pathway. Neuroscience, 159, 993–1002. 10.1016/j.neuroscience.2009.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denu, R. A. (2017). SIRT3 enhances mesenchymal stem cell longevity and differentiation. Oxidative Medicine and Cellular Longevity, 2017, 5841716. 10.1155/2017/5841716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denu, R. A., & Hematti, P. (2016). Effects of oxidative stress on mesenchymal stem cell biology. Oxidative Medicine and Cellular Longevity, 2016, 2989076. 10.1155/2016/2989076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Descloux, C., Ginet, V., Clarke, P. G., Puyal, J., & Truttmann, A. C. (2015). Neuronal death after perinatal cerebral hypoxia‐ischemia: Focus on autophagy‐mediated cell death. International Journal of Developmental Neuroscience, 45, 75–85. 10.1016/j.ijdevneu.2015.06.008 [DOI] [PubMed] [Google Scholar]
- Ding, H., Zhang, H., Ding, H., Li, D., Yi, X., Ma, X., Li, R., Huang, M., & Ju, X. (2017). Transplantation of placenta‐derived mesenchymal stem cells reduces hypoxic‐ischemic brain damage in rats by ameliorating the inflammatory response. Cellular & Molecular Immunology, 14, 693–701. 10.1038/cmi.2015.99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominici, M., Le Blanc, K., Mueller, I., Slaper‐Cortenbach, I., Marini, F. C., Krause, D. S., Deans, R. J., Keating, A., Prockop, D. J., & Horwitz, E. M. (2006). Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy, 8, 315–317. 10.1080/14653240600855905 [DOI] [PubMed] [Google Scholar]
- Donega, V., Nijboer, C. H., van Tilborg, G., Dijkhuizen, R. M., Kavelaars, A., & Heijnen, C. J. (2014). Intranasally administered mesenchymal stem cells promote a regenerative niche for repair of neonatal ischemic brain injury. Experimental Neurology, 261, 53–64. 10.1016/j.expneurol.2014.06.009 [DOI] [PubMed] [Google Scholar]
- Donega, V., van Velthoven, C. T., Nijboer, C. H., van Bel, F., Kas, M. J., Kavelaars, A., & Heijnen, C. J. (2013). Intranasal mesenchymal stem cell treatment for neonatal brain damage: Long‐term cognitive and sensorimotor improvement. PLoS One, 8, e51253. 10.1371/journal.pone.0051253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- du Plessis, A. J. (1998). Posthemorrhagic hydrocephalus and brain injury in the preterm infant: Dilemmas in diagnosis and management. Seminars in Pediatric Neurology, 5, 161–179. 10.1016/S1071-9091(98)80032-6 [DOI] [PubMed] [Google Scholar]
- Duffy, M. M., Ritter, T., Ceredig, R., & Griffin, M. D. (2011). Mesenchymal stem cell effects on T‐cell effector pathways. Stem Cell Research & Therapy, 2, 34. 10.1186/scrt75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emani, S. M., Piekarski, B. L., Harrild, D., Del Nido, P. J., & McCully, J. D. (2017). Autologous mitochondrial transplantation for dysfunction after ischemia‐reperfusion injury. Journal of Thoracic and Cardiovascular Surgery, 154, 286–289. 10.1016/j.jtcvs.2017.02.018 [DOI] [PubMed] [Google Scholar]
- Falnikar, A., Tole, S., & Baas, P. W. (2011). Kinesin‐5, a mitotic microtubule‐associated motor protein, modulates neuronal migration. Molecular Biology of the Cell, 22, 1561–1574. 10.1091/mbc.e10-11-0905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fam, M. D., Zeineddine, H. A., Eliyas, J. K., Stadnik, A., Jesselson, M., McBee, N., Lane, K., Cao, Y., Wu, M., Zhang, L., Thompson, R. E., John, S., Ziai, W., Hanley, D. F., & Awad, I. A. (2017). CSF inflammatory response after intraventricular hemorrhage. Neurology, 89, 1553–1560. 10.1212/WNL.0000000000004493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferriero, D. M., Fullerton, H. J., Bernard, T. J., Billinghurst, L., Daniels, S. R., DeBaun, M. R., deVeber, G., Ichord, R. N., Jordan, L. C., Massicotte, P., Meldau, J., Roach, E. S., & Smith, E. R. (2019). Management of stroke in neonates and children: A scientific statement from the American heart association/American stroke association. Stroke, 50, e51–e96. 10.1161/STR.0000000000000183 [DOI] [PubMed] [Google Scholar]
- Fleiss, B., & Gressens, P. (2012). Tertiary mechanisms of brain damage: A new hope for treatment of cerebral palsy? The Lancet Neurology, 11, 556–566. 10.1016/S1474-4422(12)70058-3 [DOI] [PubMed] [Google Scholar]
- Fleiss, B., Guillot, P. V., Titomanlio, L., Baud, O., Hagberg, H., & Gressens, P. (2014). Stem cell therapy for neonatal brain injury. Clinics in Perinatology, 41, 133–148. 10.1016/j.clp.2013.09.002 [DOI] [PubMed] [Google Scholar]
- Forman, H. J., Fukuto, J. M., & Torres, M. (2004). Redox signaling: Thiol chemistry defines which reactive oxygen and nitrogen species can act as second messengers. American Journal of Physiology. Cell Physiology, 287, C246–256. 10.1152/ajpcell.00516.2003 [DOI] [PubMed] [Google Scholar]
- Fransson, S., Ruusala, A., & Aspenström, P. (2006). The atypical Rho GTPases Miro‐1 and Miro‐2 have essential roles in mitochondrial trafficking. Biochemical and Biophysical Research Communications, 344, 500–510. 10.1016/j.bbrc.2006.03.163 [DOI] [PubMed] [Google Scholar]
- Galipeau, J., Krampera, M., Barrett, J., Dazzi, F., Deans, R. J., DeBruijn, J., Dominici, M., Fibbe, W. E., Gee, A. P., Gimble, J. M., Hematti, P., Koh, M. B. C., LeBlanc, K., Martin, I., McNiece, I. K., Mendicino, M., Oh, S., Ortiz, L., Phinney, D. G., … Sensebe, L. (2016). International Society for Cellular Therapy perspective on immune functional assays for mesenchymal stromal cells as potency release criterion for advanced phase clinical trials. Cytotherapy, 18, 151–159. 10.1016/j.jcyt.2015.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galluzzi, L., Blomgren, K., & Kroemer, G. (2009). Mitochondrial membrane permeabilization in neuronal injury. Nature Reviews Neuroscience, 10, 481–494. 10.1038/nrn2665 [DOI] [PubMed] [Google Scholar]
- Galluzzi, L., Bravo‐San Pedro, J. M., Blomgren, K., & Kroemer, G. (2016). Autophagy in acute brain injury. Nature Reviews Neuroscience, 17, 467–484. 10.1038/nrn.2016.51 [DOI] [PubMed] [Google Scholar]
- Galluzzi, L., Vanden Berghe, T., Vanlangenakker, N., Buettner, S., Eisenberg, T., Vandenabeele, P., Madeo, F., & Kroemer, G. (2011). Programmed necrosis from molecules to health and disease. International Review of Cell and Molecular Biology, 289, 1–35. [DOI] [PubMed] [Google Scholar]
- Ge, Q., Zhang, H., Hou, J., Wan, L., Cheng, W., Wang, X., Dong, D., Chen, C., Xia, J., Guo, J., Chen, X., & Wu, X. (2018). VEGF secreted by mesenchymal stem cells mediates the differentiation of endothelial progenitor cells into endothelial cells via paracrine mechanisms. Molecular Medicine Reports, 17, 1667–1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdes, H. H., Bukoreshtliev, N. V., & Barroso, J. F. (2007). Tunneling nanotubes: A new route for the exchange of components between animal cells. FEBS Letters, 581, 2194–2201. 10.1016/j.febslet.2007.03.071 [DOI] [PubMed] [Google Scholar]
- Gerdes, H.‐H., Rustom, A., & Wang, X. (2013). Tunneling nanotubes, an emerging intercellular communication route in development. Mechanisms of Development, 130, 381–387. 10.1016/j.mod.2012.11.006 [DOI] [PubMed] [Google Scholar]
- Gerdoni, E., Gallo, B., Casazza, S., Musio, S., Bonanni, I., Pedemonte, E., Mantegazza, R., Frassoni, F., Mancardi, G., Pedotti, R., & Uccelli, A. (2007). Mesenchymal stem cells effectively modulate pathogenic immune response in experimental autoimmune encephalomyelitis. Annals of Neurology, 61, 219–227. 10.1002/ana.21076 [DOI] [PubMed] [Google Scholar]
- Glater, E. E., Megeath, L. J., Stowers, R. S., & Schwarz, T. L. (2006). Axonal transport of mitochondria requires milton to recruit kinesin heavy chain and is light chain independent. Journal of Cell Biology, 173, 545–557. 10.1083/jcb.200601067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales‐Portillo, G. S., Reyes, S., Aguirre, D., Pabon, M. M., & Borlongan, C. V. (2014). Stem cell therapy for neonatal hypoxic‐ischemic encephalopathy. Frontiers in Neurology, 5, 147. 10.3389/fneur.2014.00147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gothard, D., Greenhough, J., Ralph, E., & Oreffo, R. O. (2014). Prospective isolation of human bone marrow stromal cell subsets: A comparative study between Stro‐1‐, CD146‐and CD105‐enriched populations. Journal of Tissue Engineering, 5, 2041731414551763. 10.1177/2041731414551763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gram, M., Sveinsdottir, S., Ruscher, K., Hansson, S. R., Cinthio, M., Akerström, B., & Ley, D. (2013). Hemoglobin induces inflammation after preterm intraventricular hemorrhage by methemoglobin formation. Journal of Neuroinflammation, 10, 100. 10.1186/1742-2094-10-100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronthos, S., Mankani, M., Brahim, J., Robey, P. G., & Shi, S. (2000). Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proceedings of the National Academy of Sciences, 97, 13625–13630. 10.1073/pnas.240309797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagberg, H. (2004). Mitochondrial impairment in the developing brain after hypoxia‐ischemia. Journal of Bioenergetics and Biomembranes, 36, 369–373. 10.1023/B:JOBB.0000041770.00567.4f [DOI] [PubMed] [Google Scholar]
- Hagberg, H., David Edwards, A., & Groenendaal, F. (2016). Perinatal brain damage: The term infant. Neurobiology of Diseases, 92, 102–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagberg, H., Gressens, P., & Mallard, C. (2012). Inflammation during fetal and neonatal life: Implications for neurologic and neuropsychiatric disease in children and adults. Annals of Neurology, 71, 444–457. 10.1002/ana.22620 [DOI] [PubMed] [Google Scholar]
- Hagberg, H., Mallard, C., Ferriero, D. M., Vannucci, S. J., Levison, S. W., Vexler, Z. S., & Gressens, P. (2015). The role of inflammation in perinatal brain injury. Nature Reviews. Neurology, 11, 192–208. 10.1038/nrneurol.2015.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagberg, H., Mallard, C., Rousset, C. I., & Thornton, C. (2014). Mitochondria: Hub of injury responses in the developing brain. The Lancet Neurology, 13, 217–232. 10.1016/S1474-4422(13)70261-8 [DOI] [PubMed] [Google Scholar]
- Hagberg, H., Mallard, C., Rousset, C. I., & Xiaoyang, W. (2009). Apoptotic mechanisms in the immature brain: Involvement of mitochondria. Journal of Child Neurology, 24, 1141–1146. 10.1177/0883073809338212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagberg, H., Peebles, D., & Mallard, C. (2002). Models of white matter injury: Comparison of infectious, hypoxic‐ischemic, and excitotoxic insults. Mental Retardation and Developmental Disabilities Research Reviews, 8, 30–38. 10.1002/mrdd.10007 [DOI] [PubMed] [Google Scholar]
- Hawkins, K. E., Corcelli, M., Dowding, K., Ranzoni, A. M., Vlahova, F., Hau, K.‐L., Hunjan, A., Peebles, D., Gressens, P., Hagberg, H., de Coppi, P., Hristova, M., & Guillot, P. V. (2018). Embryonic stem cell‐derived mesenchymal stem cells (MSCs) have a superior neuroprotective capacity over fetal MSCs in the hypoxic‐ischemic mouse brain. Stem Cells Translational Medicine, 7, 439–449. 10.1002/sctm.17-0260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill, A., & Volpe, J. J. (1981). Seizures, hypoxic‐ischemic brain injury, and intraventricular hemorrhage in the newborn. Annals of Neurology, 10, 109–121. 10.1002/ana.410100202 [DOI] [PubMed] [Google Scholar]
- Ho, T. T., Warr, M. R., Adelman, E. R., Lansinger, O. M., Flach, J., Verovskaya, E. V., Figueroa, M. E., & Passegué, E. (2017). Autophagy maintains the metabolism and function of young and old stem cells. Nature, 543, 205–210. 10.1038/nature21388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes, M., & Rosenthal, A. (2000). Embryonic stem cells go dopaminergic. Neuron, 28, 11–14. 10.1016/S0896-6273(00)00079-9 [DOI] [PubMed] [Google Scholar]
- Ingram, D. A., Mead, L. E., Tanaka, H., Meade, V., Fenoglio, A., Mortell, K., Pollok, K., Ferkowicz, M. J., Gilley, D., & Yoder, M. C. (2004). Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood, 104, 2752–2760. 10.1182/blood-2004-04-1396 [DOI] [PubMed] [Google Scholar]
- Ip, W. K. E., Hoshi, N., Shouval, D. S., Snapper, S., & Medzhitov, R. (2017). Anti‐inflammatory effect of IL‐10 mediated by metabolic reprogramming of macrophages. Science (New York, N.Y.), 356, 513–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irie‐Sasaki, J., Sasaki, T., Matsumoto, W., Opavsky, A., Cheng, M., Welstead, G., Griffiths, E., Krawczyk, C., Richardson, C. D., Aitken, K., Iscove, N., Koretzky, G., Johnson, P., Liu, P., Rothstein, D. M., & Penninger, J. M. (2001). CD45 is a JAK phosphatase and negatively regulates cytokine receptor signalling. Nature, 409, 349–354. 10.1038/35053086 [DOI] [PubMed] [Google Scholar]
- Islam, M. N., Das, S. R., Emin, M. T., Wei, M., Sun, L. I., Westphalen, K., Rowlands, D. J., Quadri, S. K., Bhattacharya, S., & Bhattacharya, J. (2012). Mitochondrial transfer from bone‐marrow–derived stromal cells to pulmonary alveoli protects against acute lung injury. Nature Medicine, 18, 759. 10.1038/nm.2736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito, K., Hirao, A., Arai, F., Takubo, K., Matsuoka, S., Miyamoto, K., Ohmura, M., Naka, K., Hosokawa, K., Ikeda, Y., & Suda, T. (2006). Reactive oxygen species act through p38 MAPK to limit the lifespan of hematopoietic stem cells. Nature Medicine, 12, 446–451. 10.1038/nm1388 [DOI] [PubMed] [Google Scholar]
- Jacobs, S. E., Berg, M., Hunt, R., Tarnow‐Mordi, W. O., Inder, T. E., & Davis, P. G. (2013). Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database of Systematic Reviews. 10.1002/14651858.CD003311.pub3 [DOI] [Google Scholar]
- Jellema, R. K., Wolfs, T. G. A. M., Lima Passos, V., Zwanenburg, A., Ophelders, D. R. M. G., Kuypers, E., Hopman, A. H. N., Dudink, J., Steinbusch, H. W., Andriessen, P., Germeraad, W. T. V., Vanderlocht, J., & Kramer, B. W. (2013). Mesenchymal stem cells induce T‐cell tolerance and protect the preterm brain after global hypoxia‐ischemia. PLoS One, 8, e73031. 10.1371/journal.pone.0073031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, X.‐X., Zhang, Y., Liu, B., Zhang, S.‐X., Wu, Y., Yu, X.‐D., & Mao, N. (2005). Human mesenchymal stem cells inhibit differentiation and function of monocyte‐derived dendritic cells. Blood, 105, 4120–4126. 10.1182/blood-2004-02-0586 [DOI] [PubMed] [Google Scholar]
- Katajisto, P., Dohla, J., Chaffer, C. L., Pentinmikko, N., Marjanovic, N., Iqbal, S., Zoncu, R., Chen, W., Weinberg, R. A., & Sabatini, D. M. (2015). Stem cells. Asymmetric apportioning of aged mitochondria between daughter cells is required for stemness. Science, 348, 340–343. 10.1126/science.1260384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keating, A. (2012). Mesenchymal stromal cells: New directions. Cell Stem Cell, 10, 709–716. 10.1016/j.stem.2012.05.015 [DOI] [PubMed] [Google Scholar]
- Kelly, B., & O'Neill, L. A. J. (2015). Metabolic reprogramming in macrophages and dendritic cells in innate immunity. Cell Research, 25, 771–784. 10.1038/cr.2015.68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesner, E. E., Saada‐Reich, A., & Lorberboum‐Galski, H. (2016). Characteristics of mitochondrial transformation into human cells. Scientific Reports, 6, 26057. 10.1038/srep26057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, E. S., Ahn, S. Y., Im, G. H., Sung, D. K., Park, Y. R., Choi, S. H., Choi, S. J., Chang, Y. S., Oh, W., Lee, J. H., & Park, W. S. (2012). Human umbilical cord blood‐derived mesenchymal stem cell transplantation attenuates severe brain injury by permanent middle cerebral artery occlusion in newborn rats. Pediatric Research, 72, 277–284. 10.1038/pr.2012.71 [DOI] [PubMed] [Google Scholar]
- Koning, G., Leverin, A.‐L., Nair, S., Schwendimann, L., Ek, J., Carlsson, Y., Gressens, P., Thornton, C., Wang, X., Mallard, C., & Hagberg, H. (2019). Magnesium induces preconditioning of the neonatal brain via profound mitochondrial protection. Journal of Cerebral Blood Flow & Metabolism, 39, 1038–1055. 10.1177/0271678X17746132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurihara, Y., Kanki, T., Aoki, Y., Hirota, Y., Saigusa, T., Uchiumi, T., & Kang, D. (2012). Mitophagy plays an essential role in reducing mitochondrial production of reactive oxygen species and mutation of mitochondrial DNA by maintaining mitochondrial quantity and quality in yeast. Journal of Biological Chemistry, 287, 3265–3272. 10.1074/jbc.M111.280156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos, T., Sánchez‐Abarca, L. I., Muntión, S., Preciado, S., Puig, N., López‐Ruano, G., Hernández‐Hernández, Á., Redondo, A., Ortega, R., Rodríguez, C., Sánchez‐Guijo, F., & del Cañizo, C. (2016). MSC surface markers (CD44, CD73, and CD90) can identify human MSC‐derived extracellular vesicles by conventional flow cytometry. Cell Communication and Signaling, 14, 2. 10.1186/s12964-015-0124-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalu, M. M., McIntyre, L., Pugliese, C., Fergusson, D., Winston, B. W., Marshall, J. C., Granton, J., & Stewart, D. J. (2012). Safety of cell therapy with mesenchymal stromal cells (SafeCell): A systematic review and meta‐analysis of clinical trials. PLoS One, 7, e47559. 10.1371/journal.pone.0047559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, E. J., Park, S. J., Kang, S. K., Kim, G. H., Kang, H. J., Lee, S. W., Jeon, H. B., & Kim, H. S. (2012). Spherical bullet formation via E‐cadherin promotes therapeutic potency of mesenchymal stem cells derived from human umbilical cord blood for myocardial infarction. Molecular Therapy, 20, 1424–1433. 10.1038/mt.2012.58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, J. W., & Davis, J. M. (2011). Future applications of antioxidants in premature infants. Current Opinion in Pediatrics, 23, 161–166. 10.1097/MOP.0b013e3283423e51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibacher, J., & Henschler, R. (2016). Biodistribution, migration and homing of systemically applied mesenchymal stem/stromal cells. Stem Cell Research & Therapy, 7, 7. 10.1186/s13287-015-0271-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon‐Rico, D., Fernandez‐Garcia, M., Aldea, M., Sanchez, R., Peces‐Barba, M., Martinez‐Palacio, J., Yanez, R. M., & Almarza, E. (2015). Comparison of haematopoietic stem cell engraftment through the retro‐orbital venous sinus and the lateral vein: Alternative routes for bone marrow transplantation in mice. Laboratory Animals, 49, 132–141. 10.1177/0023677214567915 [DOI] [PubMed] [Google Scholar]
- Leviton, A., Dammann, O., & Durum, S. K. (2005). The adaptive immune response in neonatal cerebral white matter damage. Annals of Neurology, 58, 821–828. 10.1002/ana.20662 [DOI] [PubMed] [Google Scholar]
- Li, C.‐J., Chen, P.‐K., Sun, L.‐Y., & Pang, C.‐Y. (2017). Enhancement of mitochondrial transfer by antioxidants in human mesenchymal stem cells. Oxidative Medicine and Cellular Longevity, 2017, 8510805. 10.1155/2017/8510805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, J., Li, D., Liu, X., Tang, S., & Wei, F. (2012). Human umbilical cord mesenchymal stem cells reduce systemic inflammation and attenuate LPS‐induced acute lung injury in rats. Journal of Inflammation (London), 9, 33. 10.1186/1476-9255-9-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, J., Yawno, T., Sutherland, A. E., Gurung, S., Paton, M., McDonald, C., Tiwari, A., Pham, Y., Castillo‐Melendez, M., Jenkin, G., & Miller, S. L. (2018). Preterm umbilical cord blood derived mesenchymal stem/stromal cells protect preterm white matter brain development against hypoxia‐ischemia. Experimental Neurology, 308, 120–131. 10.1016/j.expneurol.2018.07.006 [DOI] [PubMed] [Google Scholar]
- Li, M. X., Qu, Y., & Mu, D. Z. (2017). Role of mitophagy in neonatal rats with hypoxic‐ischemic brain damage. Zhongguo Dang Dai Er Ke Za Zhi, 19, 242–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Q., Zhang, T., Wang, J., Zhang, Z., Zhai, Y., Yang, G. Y., & Sun, X. (2014). Rapamycin attenuates mitochondrial dysfunction via activation of mitophagy in experimental ischemic stroke. Biochemical and Biophysical Research Communications, 444, 182–188. 10.1016/j.bbrc.2014.01.032 [DOI] [PubMed] [Google Scholar]
- Lin, C.‐S., Ning, H., Lin, G., & Lue, T. F. (2012). Is CD34 truly a negative marker for mesenchymal stromal cells? Cytotherapy, 14, 1159–1163. 10.3109/14653249.2012.729817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, Q.‐M., Zhao, S., Zhou, L.‐L., Fang, X.‐S., Fu, Y., & Huang, Z.‐T. (2013). Mesenchymal stem cells transplantation suppresses inflammatory responses in global cerebral ischemia: Contribution of TNF‐α‐induced protein 6. Acta Pharmacologica Sinica, 34, 784–792. 10.1038/aps.2012.199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, K., Ji, K., Guo, L., Wu, W., Lu, H., Shan, P., & Yan, C. (2014). Mesenchymal stem cells rescue injured endothelial cells in an in vitro ischemia‐reperfusion model via tunneling nanotube like structure‐mediated mitochondrial transfer. Microvascular Research, 92, 10–18. 10.1016/j.mvr.2014.01.008 [DOI] [PubMed] [Google Scholar]
- Luyt, K., Jary, S., Lea, C., Young, G. J., Odd, D., Miller, H., Kmita, G., Williams, C., Blair, P. S., Fernández, A. M., Hollingworth, W., Morgan, M., Smith‐Collins, A., Thai, N. J., Walker‐Cox, S., Aquilina, K., Pople, I., & Whitelaw, A. (2019). Ten‐year follow‐up of a randomised trial of drainage, irrigation and fibrinolytic therapy (DRIFT) in infants with post‐haemorrhagic ventricular dilatation. Health Technology Assessment, 23, 1–116. 10.3310/hta23040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv, F.‐J., Tuan, R. S., Cheung, K. M., & Leung, V. Y. (2014). Concise review: The surface markers and identity of human mesenchymal stem cells. Stem Cells, 32, 1408–1419. 10.1002/stem.1681 [DOI] [PubMed] [Google Scholar]
- Marelli‐Berg, F. M., Fu, H., & Mauro, C. (2012). Molecular mechanisms of metabolic reprogramming in proliferating cells: Implications for T‐cell‐mediated immunity. Immunology, 136, 363–369. 10.1111/j.1365-2567.2012.03583.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins, L. F., Costa, R. O., Pedro, J. R., Aguiar, P., Serra, S. C., Teixeira, F. G., Sousa, N., Salgado, A. J., & Almeida, R. D. (2017). Mesenchymal stem cells secretome‐induced axonal outgrowth is mediated by BDNF. Scientific Reports, 7, 4153. 10.1038/s41598-017-03592-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuzawa, A., Black, K. M., Pacak, C. A., Ericsson, M., Barnett, R. J., Drumm, C., Seth, P., Bloch, D. B., Levitsky, S., Cowan, D. B., & McCully, J. D. (2013). Transplantation of autologously derived mitochondria protects the heart from ischemia‐reperfusion injury. American Journal of Physiology. Heart and Circulatory Physiology, 304, H966–982. 10.1152/ajpheart.00883.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald, C. A., Djuliannisaa, Z., Petraki, M., Paton, M. C. B., Penny, T. R., Sutherland, A. E., Castillo‐Melendez, M., Novak, I., Jenkin, G., Fahey, M. C., & Miller, S. L. (2019). Intranasal delivery of mesenchymal stromal cells protects against neonatal hypoxic–ischemic brain injury. International Journal of Molecular Sciences, 20(10), 2449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll, G., Ankrum, J. A., Kamhieh‐Milz, J., Bieback, K., Ringdén, O., Volk, H. D., Geissler, S., & Reinke, P. (2019). Intravascular mesenchymal stromal/stem cell therapy product diversification: Time for new clinical guidelines. Trends in Molecular Medicine, 25, 149–163. 10.1016/j.molmed.2018.12.006 [DOI] [PubMed] [Google Scholar]
- Moll, G., Rasmusson‐Duprez, I., von Bahr, L., Connolly‐Andersen, A.‐M., Elgue, G., Funke, L., Hamad, O. A., Lönnies, H., Magnusson, P. U., Sanchez, J., Teramura, Y., Nilsson‐Ekdahl, K., Ringdén, O., Korsgren, O., Nilsson, B. O., & Le Blanc, K. (2012). Are therapeutic human mesenchymal stromal cells compatible with human blood? Stem Cells, 30, 1565–1574. 10.1002/stem.1111 [DOI] [PubMed] [Google Scholar]
- Mueller, M., Oppliger, B., Joerger‐Messerli, M., Reinhart, U., Barnea, E., Paidas, M., Kramer, B. W., Surbek, D. V., & Schoeberlein, A. (2017). Wharton's Jelly mesenchymal stem cells protect the immature brain in rats and modulate cell fate. Stem Cells and Development, 26, 239–248. 10.1089/scd.2016.0108 [DOI] [PubMed] [Google Scholar]
- Nadri, S., & Soleimani, M. (2007). Comparative analysis of mesenchymal stromal cells from murine bone marrow and amniotic fluid. Cytotherapy, 9, 729–737. 10.1080/14653240701656061 [DOI] [PubMed] [Google Scholar]
- Nair, S., Sobotka, K. S., Joshi, P., Gressens, P., Fleiss, B., Thornton, C., Mallard, C., & Hagberg, H. (2019). Lipopolysaccharide‐induced alteration of mitochondrial morphology induces a metabolic shift in microglia modulating the inflammatory response in vitro and in vivo. Glia, 67, 1047–1061. 10.1002/glia.23587 [DOI] [PubMed] [Google Scholar]
- Nawaz, M., & Fatima, F. (2017). Extracellular vesicles, tunneling nanotubes, and cellular interplay: Synergies and missing links. Frontiers in Molecular Biosciences, 4, 50. 10.3389/fmolb.2017.00050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Németh, K., Leelahavanichkul, A., Yuen, P. S. T., Mayer, B., Parmelee, A., Doi, K., Robey, P. G., Leelahavanichkul, K., Koller, B. H., Brown, J. M., Hu, X., Jelinek, I., Star, R. A., & Mezey, É. (2009). Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)‐dependent reprogramming of host macrophages to increase their interleukin‐10 production. Nature Medicine, 15, 42–49. 10.1038/nm.1905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northington, F. J., Chavez‐Valdez, R., & Martin, L. J. (2011). Neuronal cell death in neonatal hypoxia‐ischemia. Annals of Neurology, 69, 743–758. 10.1002/ana.22419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okafo, G., Prevedel, L., & Eugenin, E. (2017). Tunneling nanotubes (TNT) mediate long‐range gap junctional communication: Implications for HIV cell to cell spread. Scientific Reports, 7, 16660. 10.1038/s41598-017-16600-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okazaki, K., Nishida, A., Kato, M., Kozawa, K., Uga, N., & Kimura, H. (2006). Elevation of cytokine concentrations in asphyxiated neonates. Biology of the Neonate, 89, 183–189. 10.1159/000089180 [DOI] [PubMed] [Google Scholar]
- Oppliger, B., Joerger‐Messerli, M., Mueller, M., Reinhart, U., Schneider, P., Surbek, D. V., & Schoeberlein, A. (2016). Intranasal delivery of umbilical cord‐derived mesenchymal stem cells preserves myelination in perinatal brain damage. Stem Cells and Development, 25, 1234–1242. 10.1089/scd.2016.0027 [DOI] [PubMed] [Google Scholar]
- Ortiz, L. A., Dutreil, M., Fattman, C., Pandey, A. C., Torres, G., Go, K., & Phinney, D. G. (2007). Interleukin 1 receptor antagonist mediates the antiinflammatory and antifibrotic effect of mesenchymal stem cells during lung injury. Proceedings of the National Academy of Sciences, 104, 11002–11007. 10.1073/pnas.0704421104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paliwal, S., Kakkar, A., Sharma, R., Airan, B., & Mohanty, S. (2017). Differential reduction of reactive oxygen species by human tissuespecific mesenchymal stem cells from different donors under oxidative stress. Journal of Biosciences, 42, 373–382. 10.1007/s12038-017-9691-8 [DOI] [PubMed] [Google Scholar]
- Park, D., Lee, S. H., Bae, D. K., Yang, Y.‐H., Yang, G., Kyung, J., Kim, D., Choi, E.‐K., Hong, J. T., Shin, I. S., Kang, S. K., Ra, J. C., & Kim, Y.‐B. (2013). Transplantation of human adipose tissue‐derived mesenchymal stem cells restores the neurobehavioral disorders of rats with neonatal hypoxic‐ischemic encephalopathy. Cell Medicine, 5, 17–28. 10.3727/215517913X658936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, W. S., Ahn, S. Y., Sung, S. I., Ahn, J.‐Y., & Chang, Y. S. (2018). Strategies to enhance paracrine potency of transplanted mesenchymal stem cells in intractable neonatal disorders. Pediatric Research, 83, 214–222. 10.1038/pr.2017.249 [DOI] [PubMed] [Google Scholar]
- Park, W. S., Sung, S. I., Ahn, S. Y., Yoo, H. S., Sung, D. K., Im, G. H., Choi, S. J., & Chang, Y. S. (2015). Hypothermia augments neuroprotective activity of mesenchymal stem cells for neonatal hypoxic‐ischemic encephalopathy. PLoS One, 10, e0120893. 10.1371/journal.pone.0120893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquier, J., Guerrouahen, B. S., Al Thawadi, H., Ghiabi, P., Maleki, M., Abu‐Kaoud, N., Jacob, A., Mirshahi, M., Galas, L., Rafii, S., Le Foll, F., & Rafii, A. (2013). Preferential transfer of mitochondria from endothelial to cancer cells through tunneling nanotubes modulates chemoresistance. Journal of Translational Medicine, 11, 94. 10.1186/1479-5876-11-94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattappa, G., Heywood, H. K., de Bruijn, J. D., & Lee, D. A. (2011). The metabolism of human mesenchymal stem cells during proliferation and differentiation. Journal of Cellular Physiology, 226, 2562–2570. 10.1002/jcp.22605 [DOI] [PubMed] [Google Scholar]
- Peng, R., Yao, X., Cao, B., Tang, J., & Ding, J. (2012). The effect of culture conditions on the adipogenic and osteogenic inductions of mesenchymal stem cells on micropatterned surfaces. Biomaterials, 33, 6008–6019. 10.1016/j.biomaterials.2012.05.010 [DOI] [PubMed] [Google Scholar]
- Phinney, D. G., Di Giuseppe, M., Njah, J., Sala, E., Shiva, S., St Croix, C. M., Stolz, D. B., Watkins, S. C., Di, Y. P., Leikauf, G. D., Kolls, J., Riches, D. W. H., Deiuliis, G., Kaminski, N., Boregowda, S. V., McKenna, D. H., & Ortiz, L. A. (2015). Mesenchymal stem cells use extracellular vesicles to outsource mitophagy and shuttle microRNAs. Nature Communications, 6, 8472. 10.1038/ncomms9472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piantadosi, C. A., & Zhang, J. (1996). Mitochondrial generation of reactive oxygen species after brain ischemia in the rat. Stroke, 27, 327–331. discussion 332. 10.1161/01.STR.27.2.327 [DOI] [PubMed] [Google Scholar]
- Pimentel‐Coelho, P. M., & Mendez‐Otero, R. (2010). Cell therapy for neonatal hypoxic‐ischemic encephalopathy. Stem Cells and Development, 19, 299–310. 10.1089/scd.2009.0403 [DOI] [PubMed] [Google Scholar]
- Pittenger, M. F., Mackay, A. M., Beck, S. C., Jaiswal, R. K., Douglas, R., Mosca, J. D., Moorman, M. A., Simonetti, D. W., Craig, S., & Marshak, D. R. (1999). Multilineage potential of adult human mesenchymal stem cells. Science, 284, 143–147. 10.1126/science.284.5411.143 [DOI] [PubMed] [Google Scholar]
- Qu, R., Li, Y., Gao, Q., Shen, L., Zhang, J., Liu, Z., Chen, X., & Chopp, M. (2007). Neurotrophic and growth factor gene expression profiling of mouse bone marrow stromal cells induced by ischemic brain extracts. Neuropathology, 27, 355–363. 10.1111/j.1440-1789.2007.00792.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahmat, Z., Jose, S., Ramasamy, R., & Vidyadaran, S. (2013). Reciprocal interactions of mouse bone marrow‐derived mesenchymal stem cells and BV2 microglia after lipopolysaccharide stimulation. Stem Cell Research & Therapy, 4, 12. 10.1186/scrt160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodolfo, C., Campello, S., & Cecconi, F. (2018). Mitophagy in neurodegenerative diseases. Neurochemistry International, 117, 156–166. 10.1016/j.neuint.2017.08.004 [DOI] [PubMed] [Google Scholar]
- Rosenkranz, K., Kumbruch, S., Lebermann, K., Marschner, K., Jensen, A., Dermietzel, R., & Meier, C. (2010). The chemokine SDF‐1/CXCL12 contributes to the 'homing' of umbilical cord blood cells to a hypoxic‐ischemic lesion in the rat brain. Journal of Neuroscience Research, 88, 1223–1233. [DOI] [PubMed] [Google Scholar]
- Rustad, K. C., & Gurtner, G. C. (2012). Mesenchymal stem cells home to sites of injury and inflammation. Advances in Wound Care (New Rochelle), 1, 147–152. 10.1089/wound.2011.0314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rustom, A., Saffrich, R., Markovic, I., Walther, P., & Gerdes, H.‐H. (2004). Nanotubular highways for intercellular organelle transport. Science, 303, 1007–1010. 10.1126/science.1093133 [DOI] [PubMed] [Google Scholar]
- Sahni, P. V., Zhang, J., Sosunov, S., Galkin, A., Niatsetskaya, Z., Starkov, A., Brookes, P. S., & Ten, V. S. (2018). Krebs cycle metabolites and preferential succinate oxidation following neonatal hypoxic‐ischemic brain injury in mice. Pediatric Research, 83, 491–497. 10.1038/pr.2017.277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangiorgi, B., & Panepucci, R. A. (2016). Modulation of immunoregulatory properties of mesenchymal stromal cells by toll‐like receptors: Potential applications on GVHD. Stem Cells International, 2016, 9434250. 10.1155/2016/9434250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sbrana, F. V., Cortini, M., Avnet, S., Perut, F., Columbaro, M., De Milito, A., & Baldini, N. (2016). The role of autophagy in the maintenance of stemness and differentiation of mesenchymal stem cells. Stem Cell Reviews and Reports, 12, 621–633. 10.1007/s12015-016-9690-4 [DOI] [PubMed] [Google Scholar]
- Shih, D. T., Lee, D. C., Chen, S. C., Tsai, R. Y., Huang, C. T., Tsai, C. C., Shen, E. Y., & Chiu, W. T. (2005). Isolation and characterization of neurogenic mesenchymal stem cells in human scalp tissue. Stem Cells, 23, 1012–1020. 10.1634/stemcells.2004-0125 [DOI] [PubMed] [Google Scholar]
- Shyh‐Chang, N., Daley, G. Q., & Cantley, L. C. (2013). Stem cell metabolism in tissue development and aging. Development, 140, 2535–2547. 10.1242/dev.091777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simard, P. F., Tosun, C., Melnichenko, L., Ivanova, S., Gerzanich, V., & Simard, J. M. (2011). Inflammation of the choroid plexus and ependymal layer of the ventricle following intraventricular hemorrhage. Translational Stroke Research, 2, 227–231. 10.1007/s12975-011-0070-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh‐Mallah, G., Nair, S., Sandberg, M., Mallard, C., & Hagberg, H. (2019). The role of mitochondrial and endoplasmic reticulum reactive oxygen species production in models of perinatal brain injury. Antioxidants & Redox Signaling, 31, 643–663. 10.1089/ars.2019.7779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaggiari, G. M., & Moretta, L. (2012). Interactions between mesenchymal stem cells and dendritic cells. Mesenchymal Stem Cells‐Basics and Clinical Application II (pp. 199–208). Springer. [DOI] [PubMed] [Google Scholar]
- Stowers, R. S., Megeath, L. J., Górska‐Andrzejak, J., Meinertzhagen, I. A., & Schwarz, T. L. (2002). Axonal transport of mitochondria to synapses depends on milton, a novel Drosophila protein. Neuron, 36, 1063–1077. 10.1016/S0896-6273(02)01094-2 [DOI] [PubMed] [Google Scholar]
- Strahle, J., Garton, H. J., Maher, C. O., Muraszko, K. M., Keep, R. F., & Xi, G. (2012). Mechanisms of hydrocephalus after neonatal and adult intraventricular hemorrhage. Translational Stroke Research, 3, 25–38. 10.1007/s12975-012-0182-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strioga, M., Viswanathan, S., Darinskas, A., Slaby, O., & Michalek, J. (2012). Same or not the same? Comparison of adipose tissue‐derived versus bone marrow‐derived mesenchymal stem and stromal cells. Stem Cells and Development, 21, 2724–2752. 10.1089/scd.2011.0722 [DOI] [PubMed] [Google Scholar]
- Supramaniam, V., Vontell, R., Srinivasan, L., Wyatt‐Ashmead, J., Hagberg, H., & Rutherford, M. (2013). Microglia activation in the extremely preterm human brain. Pediatric Research, 73, 301–309. 10.1038/pr.2012.186 [DOI] [PubMed] [Google Scholar]
- Svendsen, C. N., & Smith, A. G. (1999). New prospects for human stem‐cell therapy in the nervous system. Trends in Neurosciences, 22, 357–364. 10.1016/S0166-2236(99)01428-9 [DOI] [PubMed] [Google Scholar]
- Tang, J., Peng, R., & Ding, J. (2010). The regulation of stem cell differentiation by cell‐cell contact on micropatterned material surfaces. Biomaterials, 31, 2470–2476. 10.1016/j.biomaterials.2009.12.006 [DOI] [PubMed] [Google Scholar]
- Thornton, C., Jones, A., Nair, S., Aabdien, A., Mallard, C., & Hagberg, H. (2018). Mitochondrial dynamics, mitophagy and biogenesis in neonatal hypoxic‐ischaemic brain injury. FEBS Letters, 592, 812–830. [DOI] [PubMed] [Google Scholar]
- Thornton, C., Leaw, B., Mallard, C., Nair, S., Jinnai, M., & Hagberg, H. (2017). Cell death in the developing brain after hypoxia‐ischemia. Frontiers in Cellular Neuroscience, 11, 248. 10.3389/fncel.2017.00248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Togarrati, P. P., Sasaki, R. T., Abdel‐Mohsen, M., Dinglasan, N., Deng, X., Desai, S., Emmerson, E., Yee, E., Ryan, W. R., da Silva, M. C. P., Knox, S. M., Pillai, S. K., & Muench, M. O. (2017). Identification and characterization of a rich population of CD34+ mesenchymal stem/stromal cells in human parotid, sublingual and submandibular glands. Scientific Reports, 7, 3484. 10.1038/s41598-017-03681-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topf, U., Suppanz, I., Samluk, L., Wrobel, L., Böser, A., Sakowska, P., Knapp, B., Pietrzyk, M. K., Chacinska, A., & Warscheid, B. (2018). Quantitative proteomics identifies redox switches for global translation modulation by mitochondrially produced reactive oxygen species. Nature Communications, 9, 324. 10.1038/s41467-017-02694-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse, W. T., Pendleton, J. D., Beyer, W. M., Egalka, M. C., & Guinan, E. C. (2003). Suppression of allogeneic T‐cell proliferation by human marrow stromal cells: Implications in transplantation. Transplantation, 75, 389–397. 10.1097/01.TP.0000045055.63901.A9 [DOI] [PubMed] [Google Scholar]
- Ukai, R., Honmou, O., Harada, K., Houkin, K., Hamada, H., & Kocsis, J. D. (2007). Mesenchymal stem cells derived from peripheral blood protects against ischemia. Journal of Neurotrauma, 24, 508–520. 10.1089/neu.2006.0161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Velthoven, C. T., Kavelaars, A., van Bel, F., & Heijnen, C. J. (2010a). Mesenchymal stem cell treatment after neonatal hypoxic‐ischemic brain injury improves behavioral outcome and induces neuronal and oligodendrocyte regeneration. Brain, Behavior, and Immunity, 24, 387–393. 10.1016/j.bbi.2009.10.017 [DOI] [PubMed] [Google Scholar]
- van Velthoven, C. T., Kavelaars, A., van Bel, F., & Heijnen, C. J. (2010b). Nasal administration of stem cells: A promising novel route to treat neonatal ischemic brain damage. Pediatric Research, 68, 419–422. 10.1203/PDR.0b013e3181f1c289 [DOI] [PubMed] [Google Scholar]
- van Velthoven, C. T., Kavelaars, A., van Bel, F., & Heijnen, C. J. (2011). Mesenchymal stem cell transplantation changes the gene expression profile of the neonatal ischemic brain. Brain, Behavior, and Immunity, 25, 1342–1348. 10.1016/j.bbi.2011.03.021 [DOI] [PubMed] [Google Scholar]
- van Velthoven, C. T., Sheldon, R. A., Kavelaars, A., Derugin, N., Vexler, Z. S., Willemen, H. L., Maas, M., Heijnen, C. J., & Ferriero, D. M. (2013). Mesenchymal stem cell transplantation attenuates brain injury after neonatal stroke. Stroke, 44, 1426–1432. 10.1161/STROKEAHA.111.000326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasandan, A. B., Jahnavi, S., Shashank, C., Prasad, P., Kumar, A., & Prasanna, S. J. (2016). Human Mesenchymal stem cells program macrophage plasticity by altering their metabolic status via a PGE2‐dependent mechanism. Scientific Reports, 6, 38308. 10.1038/srep38308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu, Q., Xie, K., Eckert, M., Zhao, W., & Cramer, S. C. (2014). Meta‐analysis of preclinical studies of mesenchymal stromal cells for ischemic stroke. Neurology, 82, 1277–1286. 10.1212/WNL.0000000000000278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagenaar, N., de Theije, C. G. M., de Vries, L. S., Groenendaal, F., Benders, M., & Nijboer, C. H. A. (2018). Promoting neuroregeneration after perinatal arterial ischemic stroke: Neurotrophic factors and mesenchymal stem cells. Pediatric Research, 83, 372–384. 10.1038/pr.2017.243 [DOI] [PubMed] [Google Scholar]
- Wagenaar, N., Nijboer, C. H., & van Bel, F. (2017). Repair of neonatal brain injury: Bringing stem cell‐based therapy into clinical practice. Developmental Medicine and Child Neurology, 59, 997–1003. 10.1111/dmcn.13528 [DOI] [PubMed] [Google Scholar]
- Wang, X., & Gerdes, H. H. (2015). Transfer of mitochondria via tunneling nanotubes rescues apoptotic PC12 cells. Cell Death & Differentiation, 22, 1181–1191. 10.1038/cdd.2014.211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y., Chen, X., Cao, W., & Shi, Y. (2014). Plasticity of mesenchymal stem cells in immunomodulation: Pathological and therapeutic implications. Nature Immunology, 15, 1009. 10.1038/ni.3002 [DOI] [PubMed] [Google Scholar]
- Wang, Y., Cui, J., Sun, X., & Zhang, Y. (2011). Tunneling‐nanotube development in astrocytes depends on p53 activation. Cell Death and Differentiation, 18, 732–742. 10.1038/cdd.2010.147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y., Deng, Y., & Zhou, G. Q. (2008). SDF‐1alpha/CXCR4‐mediated migration of systemically transplanted bone marrow stromal cells towards ischemic brain lesion in a rat model. Brain Research, 1195, 104–112. [DOI] [PubMed] [Google Scholar]
- Wang, Z., Wang, L., Su, X., Pu, J., Jiang, M., & He, B. (2017). Rational transplant timing and dose of mesenchymal stromal cells in patients with acute myocardial infarction: A meta‐analysis of randomized controlled trials. Stem Cell Research & Therapy, 8, 21. 10.1186/s13287-016-0450-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterman, R. S., Tomchuck, S. L., Henkle, S. L., & Betancourt, A. M. (2010). A new mesenchymal stem cell (MSC) paradigm: Polarization into a pro‐inflammatory MSC1 or an Immunosuppressive MSC2 phenotype. PLoS One, 5, e10088. 10.1371/journal.pone.0010088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- West, A. P., Shadel, G. S., & Ghosh, S. (2011). Mitochondria in innate immune responses. Nature Reviews Immunology, 11, 389–402. 10.1038/nri2975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright, S. D., Ramos, R. A., Tobias, P. S., Ulevitch, R. J., & Mathison, J. C. (1990). CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science, 249, 1431–1433. 10.1126/science.1698311 [DOI] [PubMed] [Google Scholar]
- Xie, C., Ginet, V., Sun, Y., Koike, M., Zhou, K., Li, T., Li, H, Wang, X, Uchiyama, Y., Truttmann, A. C., Kroemer, G., Puyal, J., Blomgren, K., & Zhu, C. (2016). Neuroprotection by selective neuronal deletion of Atg7 in neonatal brain injury. Autophagy, 12, 410–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, C., Ren, G., Cao, G., Chen, Q., Shou, P., Zheng, C., Du, L., Han, X., Jiang, M., Yang, Q., Lin, L., Wang, G., Yu, P., Zhang, X., Cao, W., Brewer, G., Wang, Y., & Shi, Y. (2013). miR‐155 regulates immune modulatory properties of mesenchymal stem cells by targeting TAK1‐binding protein 2. Journal of Biological Chemistry, 288, 11074–11079. 10.1074/jbc.M112.414862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yardeni, T., Eckhaus, M., Morris, H. D., Huizing, M., & Hoogstraten‐Miller, S. (2011). Retro‐orbital injections in mice. Lab Animal, 40(5), 155–160. 10.1038/laban0511-155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo, S. W., Kim, S. S., Lee, S. Y., Lee, H. S., Kim, H. S., Lee, Y. D., & Suh‐Kim, H. (2008). Mesenchymal stem cells promote proliferation of endogenous neural stem cells and survival of newborn cells in a rat stroke model. Experimental & Molecular Medicine, 40, 387–397. 10.3858/emm.2008.40.4.387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan, X., Logan, T. M., & Ma, T. (2019). Metabolism in human mesenchymal stromal cells: A missing link between hMSC biomanufacturing and therapy? Frontiers in Immunology, 10, 977. 10.3389/fimmu.2019.00977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, X., Yan, H., Yuan, Y., Gao, J., Shen, Z., Cheng, Y., Shen, Y., Wang, R.‐R., Wang, X., Hu, W.‐W., Wang, G., & Chen, Z. (2013). Cerebral ischemia‐reperfusion‐induced autophagy protects against neuronal injury by mitochondrial clearance. Autophagy, 9, 1321–1333. 10.4161/auto.25132 [DOI] [PubMed] [Google Scholar]
- Zhang, Y. (2011). Tunneling‐nanotube. Communicative & Integrative Biology, 4(3), 324–325. 10.4161/cib.4.3.14855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, W., Yuan, Y., Liao, G., Li, L., Liu, J., Chen, Y., Zhang, J., Cheng, J., & Lu, Y. (2018). Mesenchymal stem cells ameliorate hyperglycemia‐induced endothelial injury through modulation of mitophagy. Cell Death & Disease, 9, 837. 10.1038/s41419-018-0861-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo, W., Zhang, S., Xia, C. Y., Guo, X. F., He, W. B., & Chen, N. H. (2014). Mitochondria autophagy is induced after hypoxic/ischemic stress in a Drp1 dependent manner: The role of inhibition of Drp1 in ischemic brain damage. Neuropharmacology, 86, 103–115. 10.1016/j.neuropharm.2014.07.002 [DOI] [PubMed] [Google Scholar]


