Abstract
Aims
Combination therapy of 5α‐reductase inhibitor and α‐blocker is a guideline‐endorsed therapeutic approach for patients with moderate‐to‐severe lower urinary tract symptoms or benign prostatic hyperplasia (LUTS/BPH) who are at risk of disease progression. We aimed to disentangle the contribution of clinical and demographic baseline characteristics affecting the risk of acute urinary retention or BPH‐related surgery (AUR/S) from the effect of treatment with drugs showing symptomatic and disease‐modifying properties.
Methods
A time‐to‐event model was developed using pooled data from patients (n = 10 238) enrolled into six clinical studies receiving placebo, tamsulosin, dutasteride or tamsulosin‐dutasteride combination therapy. A parametric hazard function was used to describe the time to first AUR/S. Covariate model building included the assessment of relevant clinical and demographic factors on baseline hazard. Predictive performance was evaluated by graphical and statistical methods.
Results
An exponential hazard model best described the time to first AUR/S in this group of patients. Baseline International Prostate Symptom Score, prostate‐specific antigen, prostate volume and maximum urine flow were identified as covariates with hazard ratio estimates of 1.04, 1.08, 1.01 and 0.91, respectively. Dutasteride monotherapy and tamsulosin‐dutasteride combination therapy resulted in a significant reduction in the baseline hazard (56.8% and 66.4%, respectively). By contrast, the effect of tamsulosin did not differ from placebo.
Conclusions
Our analysis showed the implications of disease‐modifying properties of dutasteride and tamsulosin‐dutasteride combination therapy for the risk of AUR/S. It also elucidated the contribution of different baseline characteristics to the risk of these events. The use of tamsulosin monotherapy (symptomatic treatment) has no impact on individual long‐term risk.
Keywords: acute urinary retention, baseline risk factors, benign prostatic hyperplasia, disease‐modifying properties, dutasteride, lower urinary tract symptoms, tamsulosin, time‐to‐event modelling
Visual predictive checks. The line shows observed survival over time, whereas the shaded area describes the model predicted 95% CI for the survival. The different slopes of the survival curves for dutasteride monotherapy and combination therapy (relative to placebo) reflect the disease‐modifying properties of the intervention.

What is already known about this subject
Lower urinary tract symptoms (LUTS) in patients with benign prostatic hyperplasia (BPH) at risk of disease progression can deteriorate over time.
LUTS/BPH symptom deterioration is assessed by increasing International Prostate Symptom Scores (IPSS), reduction in maximum urine flow rate, episodes of acute urinary retention or the need for BPH‐related surgery (AUR/S).
Despite the evidence of the efficacy of combination therapy, limited attention has been given to the contribution of its disease‐modifying effects to the risk of AUR/S.
What this study adds
Availability of a time‐to‐event model allowed further characterization of the treatment effect on the risk of AUR/S in patients with moderate or severe LUTS/BPH symptoms.
In addition to quantifying the impact of the disease‐modifying properties of dutasteride and tamsulosin‐dutasteride combination therapy on the risk of AUR/S, our analysis showed the contribution of baseline characteristics (IPSS, prostate‐specific antigen, prostate volume and maximum urine flow) to instantaneous risk.
Combination therapy shows a hazard reduction of 66% relative to placebo. By contrast, tamsulosin monotherapy has no effect on baseline hazard rate and consequently does not alter or reduce the long‐term risk of AUR/S.
1. INTRODUCTION
Lower urinary tract symptoms (LUTS) are a common complaint in adult men with a major impact on quality of life. The understanding of the lower urinary tract as a functional unit, and the multifactorial aetiology of associated symptoms, means that LUTS now constitute the main focus of therapeutic intervention, including those patients who have confirmed diagnosis of benign prostatic hyperplasia (BPH).1 The progressive nature of BPH is reflected by the increase in LUTS severity over time, as measured by the International Prostate Symptom Score (IPSS), and reduction in maximum urine flow rate (Q max), which can lead to episodes of acute urinary retention, or the need for BPH‐related surgery (AUR/S).
Historically, the preferred treatment goal in LUTS/BPH was to reduce bothersome LUTS resulting from benign prostatic enlargement. This perspective has been amended over the past two decades, with clinical management now additionally aiming to alter disease progression and preventing long‐term complications like acute urinary retention or surgery in men at high progression risk.2, 3, 4 Treatment guidelines for patients at risk of progression centre on two drug classes, 5α‐reductase inhibitors (5‐ARI) and α1‐blockers.1
While disease progression is also reflected by deterioration of overt symptoms, such as voiding and storage symptoms and complications like infections, chronic obstruction or renal failure, AUR/S represents a critical complication for a subset of patients, with potentially long‐lasting impact on quality of life. Nevertheless, limited attention has been given to the contribution of clinical and demographic baseline characteristics known to be associated with disease progression to the overall risk of AUR/S. Similarly, an integrated evaluation is lacking with regard to the effect of pharmacological interventions on risk reduction and incidence of such events and potential influence of baseline characteristics on outcome. Data from large‐scale clinical studies indicate a significant reduction in risk of AUR and BPH‐related surgery with 5‐ARI.5, 6 Additionally, the 4‐year Combination of Avodart (dutasteride) and Tamsulosin (CombAT) study showed that the dutasteride‐tamsulosin combination was more effective than tamsulosin monotherapy in reducing the relative risk of AUR, BPH‐related surgery and BPH clinical progression in men with moderate‐to‐severe LUTS and larger prostates at increased risk of progression.7
Recently, a model‐based meta‐analysis including data from six clinical studies in LUTS/BPH patients with moderate or severe symptoms has been performed to characterize individual IPSS trajectories and disentangle disease progression rate from active treatment effect. The analysis has shown that in contrast to tamsulosin monotherapy, tamsulosin and dutasteride combination therapy has a significantly larger effect in men with large prostates, reducing not only IPSS, but also the underlying rate of progression (defined as the reduction in IPSS per month).8 These findings reflect the known disease‐modifying properties of 5‐ARIs and show the importance of pharmacological interventions that reduce or block the processes that determine symptom deterioration in a slowly progressive disease, such as BPH. They also highlight the limitations of treatment based on symptomatic improvement only, as such drugs do not alter the overall course of disease or underlying disease processes.9
Here we describe the development and evaluation of a time‐to‐event (TTE) model aimed at the characterisation of risk of AUR/S following administration of placebo (PLAC), watchful waiting with protocol‐defined initiation of tamsulosin (WW), immediate dutasteride monotherapy (DUT), immediate tamsulosin monotherapy (TAM) or tamsulosin and dutasteride combination therapy (CT) to patients with moderate or severe LUTS/BPH at risk of disease progression. Our analysis also provides insight into the contribution of clinical and demographic baseline characteristics to the underlying risk of AUR/S.
This approach presents a parametric representation of the event rate (incidence) along with the underlying hazard.10, 11 In addition, the availability a TTE model also facilitates a systematic evaluation of the impact of multiple contributing factors to the risk of AUR/S, enabling accurate prediction of the effect of recommended interventions in patients with moderate or severe symptoms at risk of disease progression.
2. METHODS
2.1. Data source
The data used for this analysis were obtained from six clinical trials (ARIA3001, ARIA3002, ARI40002, CombAT, CONDUCT and ARIB3003). The selection of these studies was based primarily on the fact that individual patient level data was available in LUTS/BPH patients showing moderate or severe symptoms who were considered at risk at disease progression. In addition, CombAT and CONDUCT reflect current clinical guidelines for the use of pharmacological interventions in LUTS patients.1 The pooled data included 140 733 clinical observations from 10 238 subjects who were randomised to receive PLAC, WW, TAM, DUT or CT over a period of up to 4 years. A total of 3790 subjects received 0.5 mg DUT over 2 or 4 years (studies ARIA3001, ARIA3002, CombAT and ARIB3003), whereas 2143 subjects received CT over a period of 2‐4 years (studies ARI40002, CombAT and CONDUCT). A total of 2158 subjects were exposed to PLAC for 2 years (studies ARIA3001, ARIA3002 and ARIB3003). An overview of the baseline demographic characteristics, along with the actual doses, regimens and eventual deviations, is shown in Table 1, where the efficacy population is summarised along with the original treatment details in the clinical study protocols. All patients enrolled into the selected clinical trials have given informed consent for participation. The terms of consent include the scope of the investigation presented here.
TABLE 1.
Overview of the studies identified for the proposed model‐based meta‐analysis
| Study no. | Informed consent for data reuse | Study description | Dose and administration* | Number of patients | Visits (months or weeks) | ||
|---|---|---|---|---|---|---|---|
| ARIA3001 | Yes | A multicentre randomised double‐blind, placebo‐controlled, 2‐year parallel group study with a 2‐year open label phase | 0.5 mg dutasteride for 2 years | 720 |
AUA‐SI/IPSS: 0, 1, 3, 6, 12, 18, 24, 30, 36, 42 and 48 Prostate volume: 0, 1, 6, 12, 24 and 48 Max. urinary flow: 0, 1, 3, 6, 12, 18, 24, 30, 36, 42 and 48 PSA: 0, 1, 3, 6, 12, 18, 24, 36 and 48 AUR and BPH‐related surgery monitored throughout study and censored at 4 years |
||
| placebo for 2 years | 720 | ||||||
| ARIA3002 | Yes | A multicentre randomised double‐blind, placebo‐controlled, 2‐year parallel group study with a 2‐year open label phase | 0.5 mg dutasteride for 2 years | 677 |
AUA‐SI/IPSS: 0, 1, 3, 6, 12, 18, 24, 30, 36, 42 and 48 Prostate volume: 0, 3, 6, 12, 24, 36 and 48 Max. urinary flow: 0, 1, 3, 6, 12, 18, 24, 30, 36, 42 and 48 PSA: 0, 1, 3, 6, 12, 18, 24, 36 and 48 AUR and BPH‐related surgery monitored throughout study and censored at 4 years |
||
| placebo for 2 years | 685 | ||||||
| ARI40002 | Yes | A pilot, multicentre, double‐blind, parallel group, 36 weeks randomised study | 0.5 mg dutasteride and 0.4 mg tamsulosin for 36 weeks | 164 |
AUA‐SI/IPSS: 0, 4, 12, 24, 30, 36 and 37 weeksPSA: 0 and 36 weeks AUR and BPH‐related surgery monitored throughout study and censored at 36 weeks |
||
| 0.5 mg dutasteride for 12 weeks after 24 weeks. combination therapy | 163 | ||||||
| CombAT | Yes | A multicentre, randomised, double‐blind, 4‐year parallel‐group study | 0.4 mg tamsulosin for 4 years | 1611 |
AUA‐SI/IPSS: 0, 3, 6, 9, 12, 15, 18, 21, 24, 27, 30, 33, 36, 39, 42, 45 and 48Prostate volume: 0, 12, 24, 36 and 48 Max. urinary flow: 0, 6, 12, 18, 24, 30, 36, 42 and 48PSA: 0, 12, 24, 36 and 48 AUR and BPH‐related surgery monitored throughout study and censored at 4 years |
||
| 0.5 mg dutasteride for 4 years | 1623 | ||||||
| 0.5 mg dutasteride and 0.4 mg tamsulosin therapy for 4 years | 1610 | ||||||
| CONDUCT | Yes | A multicentre, randomised, open‐label, 2‐year parallel‐group study | Watchful waiting with protocol‐defined initiation of 0.4 mg tamsulosin | 373 |
AUA‐SI/IPSS: 0, 1, 3, 6, 9, 12, 15, 18, 21 and 24 PSA: 0, 6, 12 and 24 AUR and BPH‐related surgery monitored throughout study and censored at 2 years |
||
| 0.5 mg dutasteride and 0.4 mg tamsulosin therapy for 2 years | 369 | ||||||
| ARIB3003 | Yes | A multicentre randomised double‐blind, placebo‐controlled, 2‐year parallel group study with a 2‐year open label phase | 0.5 mg dutasteride for 2 years | 770 |
AUA‐SI/IPSS: 0, 1, 3, 6, 12, 18, 24, 30, 36, 42 and 48 Prostate volume: 0, 6, 12, 18, 24 and 48Max. urinary flow: 0, 1, 3, 6, 12, 18 24, 30, 36, 42 and 48PSA: 0, 1, 3, 6, 12, 18, 24, 36 and 48 AUR and BPH‐related surgery monitored throughout study and censored at 4 years |
||
| placebo for 2 years | 753 | ||||||
Protocol title is shown along with details regarding treatment type, duration and the purpose of the study data during model building and validation procedures.
Abbreviations: AUA‐SI, American Urological Association‐Symptom Index; IPSS, International Prostate Symptom Score.
*All treatments were given as a once daily dosing regimen.
2.2. Analysis population
From a total pool of 10 238 subjects, 10 234 had accurate dosing records, IPSS and demographic data. The majority of the patients (n = 9268) were White (90.5%), 229 were Black (2.2%), 374 were Asian (3.7%) and 276 were Hispanic (2.7%). A total of 6031 patients were ≥65 years of age (58.9%). In addition, 402 subjects (229 in the tamsulosin step‐up arm of the CONDUCT study and 173 in the ARI40002 study who either switched from CT to DUT or did not complete the second phase of the trial) were excluded from the analysis to avoid potential bias in the estimation of the treatment effect on the baseline hazard.
Histograms describing the distribution of relevant baseline clinical and demographic characteristics were used to assess the homogeneity of the patient population across the different studies. Summaries of the distributions stratified by study and treatment arm are shown in Supporting Information Figures S1 and S2, respectively. Given the low proportion of missing data (10.5% for baseline Q max, <4% for baseline prostate volume, duration of BPH symptoms and time from BPH diagnosis), individual covariate values were imputed for the purposes of this analysis. For continuous covariates, missing values for an individual patient were imputed as the median value for the study population. Patients with missing categorical covariates were regrouped and defined “Other”. Subsequently, as per standard practice in nonlinear mixed effects modelling, the patients were randomly split into subgroups to ensure evaluation of the predictive performance of the model. An overview of the data sets is shown Figure 1. Data sets for internal validation were based on a random selection of 20% of subjects from the total population pool.
FIGURE 1.
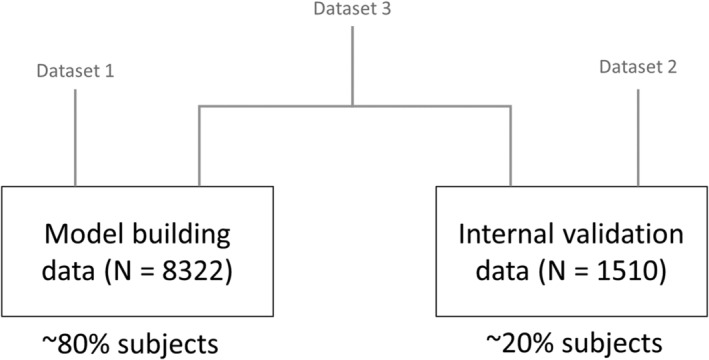
Diagram describing the different data sets used for model building (data set 1), internal validation (data set 2) and final data analysis (data set 3, N = 9832). Data set 3 comprises the total patient population from studies ARIA3001, ARIA3002, ARI40002, ARIB3003, CombAT and CONDUCT used for this analysis
2.3. Time‐to‐event model development
During a preliminary exploratory evaluation, a Kaplan‐Meier estimator along with different parametric and semiparametric models were considered.12 Subsequently, a parametric hazard model including only data from placebo‐treated patients was found to explain the time to first AUR/S accurately.
The probability density function that best described the observed time to first event was selected based on statistical and graphical criteria (ie, difference in log‐likelihood and goodness‐of‐fit plots). The probability density function was calculated as follows:
| (1) |
where h(t) represents the hazard function and S(t) is the survivor function. The following probability density functions were assessed:
exponential, λ · e −λ · t ;
proportional Weibull, ;
Gompertz, .
where λ represents the rate parameter and α and θ are shape parameters.
As a TTE model is used to calculate the probability distribution of events, no variance component (interindividual, interoccasion or residual variability) is obtained for model parameters. In this regard, differences in individual risk at baseline may be explained by individual patient characteristics. Therefore, following the structural model selection, demographic and clinical baseline covariates were tested using a stepwise forward addition‐backward elimination procedure.
Subject baseline demographics: age, race, weight, height, body mass index, smoking status, alcohol use, sexually active.
Baseline clinical characteristics: baseline IPSS, baseline serum concentration of prostate‐specific antigen (PSA), baseline prostate volume (PV), baseline maximum urine flow (Q max), duration of BPH symptoms, time since BPH diagnosis.
Concomitant medication and comorbidities or concurrent medical conditions were not accounted for as covariates. The rationale for the exclusion of these variables from the covariate analysis is based on the fact that concomitant drugs and concurrent conditions allowed in the protocols were not expected to have a direct effect on the risk of AUR/S.
For standardisation purposes, baseline measurements were defined as those collected on the last day of the placebo run‐in phase. WW was considered as a nonpharmacological intervention and handled as an active treatment arm, along with TAM, DUT and CT. Treatment was then evaluated as a discrete (covariate) effect on the underlying hazard parameters.
To ensure appropriate interpretation of the results, final model estimates were presented as hazard ratios. For continuous covariates, the hazard ratio (Equation 2) was calculated as the exponent of the coefficient of each parameter in the model. The ratio of the hazard of AUR/S is defined as follows:
| (2) |
where β in the fitted proportional hazard model is the estimated change in the logarithm of the hazard ratio when the value of x is increased by one unit.
For categorical variables the hazard ratio (Equation 3) for an individual in any group or category is relative to an individual in the first group or category. It is defined by the exponential of α j, where the parameter α is the logarithm of the relative hazard. The hazard function was calculated as follows:
| (3) |
Further details on the parameterisation of the hazard function along with the control stream file describing the final model are included in the Supporting Information.
Comparison of hierarchical models was based on the likelihood ratio test and standard error of the parameter estimates. Covariate model building was conducted in a stepwise manner and the likelihood ratio was used to test the effect of each covariate on model parameters with a significance level of 0.01. In the stepwise forward addition procedure, each covariate was individually included in the base model. If the reduction in the objective function value (OFV) between the base and more complex model was ≥3.84 (χ2 < 0.5 for 1 degree of freedom, df) then the covariate was considered statistically significant. All significant covariates were then added simultaneously into a full model. Subsequently, each covariate was independently removed from the full model. If the increase in the OFV was ≥6.64 (χ2 < 0.01 for 1 df), the covariate was considered to be significantly correlated with the model parameter and retained in the final model. It is worth mentioning that this analysis was implemented under the assumption that there is no statistically significant interaction between baseline covariates and treatment effect. In fact, there is no reason to believe that pharmacological effects would depend on or be correlated with any of the baseline covariates included in the model.
Internal validation procedures were implemented by splitting the full data set into an index data set (comprising ~80% of the data) and a reference data set (comprising the remaining portion of the data). Individual empirical Bayes estimates obtained from the index data set were then used to predict the reference data. The average relative error and average relative variance (mean square error) were used to assess the precision of parameter estimates and the robustness of the model obtained with the model building data set. The internal validation steps were considered as failed if an average relative error and average relative variance (mean square error) ≥30% was observed for at least one of the model parameters.
Visual predictive checks (VPC) were used to assess the adequacy of the parameter estimates of the final model, including the effects of statistically significant covariates. In the VPC, 1000 replicates of the original data set were simulated based on the final model obtained with each data set along with the 95% prediction intervals. The observed events (ie, AUR/S) were plotted over time along with the prediction intervals to visually assess the concordance between simulated and observed data (ie, Kaplan‐Meier survival curves). The final TTE model was assessed for its predictive performance to describe the time to first AUR/S based on stratification by treatment type, ie, PLAC, TAM, DUT or CT.
Model development and evaluation were implemented in NONMEM v.7.3 using the Laplacian estimation method. The analysis was run on the Model‐based Analyses Platform (MAP), a validated analysis platform entirely hosted on Amazon Web Services (AWS). The platform runs NONMEM 7.3 through gFortran compiler and Perl‐speaks‐NONMEM (PsN) 4.6.0. All required data manipulation, including graphical and statistical summaries were performed in R (version 3.2.5).13
2.4. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY, and are permanently archived in the Concise guide to PHARMACOLOGY 2019/20.14
3. RESULTS
3.1. Demographics and baseline characteristics
The age of the subjects across all studies included in the current analysis ranged from 47 to 94 years. At baseline, these patients had a median prostate volume and maximum urinary flow of 48.5 mL and 10.2 mL/s, respectively. Immediately prior to treatment initiation, 66% and 28.8% of the patients were found to have moderate or severe LUTS/BPH symptoms, respectively. A small proportion (5.2%) of patients who met the inclusion criteria at the screening visit showed mild LUTS (ie, IPSS < 8) at baseline. A summary of demographic and clinical baseline characteristics along with the studied covariates is presented in Table 2.
TABLE 2.
Demographics and clinical baseline characteristics of the pooled patient population included in the meta‐analysis
| Baseline demographics | n | Mean | SD | Median | Min. | Max. |
|---|---|---|---|---|---|---|
| Age (y) | 10 236 | 66.2 | 7.20 | 66 | 47 | 94 |
| Body weight (kg) | 10 206 | 83.2 | 13.60 | 82 | 37 | 179 |
| Height (cm) | 10 204 | 174.1 | 7.48 | 175 | 132 | 208 |
| Baseline PSA (ng/mL) | 10 206 | 3.98 | 2.10 | 3.4 | 0.6 | 23.2 |
| Baseline prostate volume (mL) | 9875 | 54.53 | 23.00 | 48.5 | 16.59 | 296.89 |
| Baseline IPSS | 10 228 | 16.48 | 6.10 | 16 | 1 | 35 |
| Baseline maximum urinary flow (mL/s] | 9163 | 10.49 | 3.59 | 10.20 | 2.2 | 36.2 |
| BMI (kg/m2) | 10 210 | 27.44 | 3.99 | 26.91 | 12.36 | 59.73 |
| Duration of BPH symptoms (y) | 9881 | 5.17 | 4.77 | 4.00 | 0 | 54.8 |
| Time from BPH diagnosis (y) | 10 080 | 2.65 | 4.26 | 2.3 | 0.58 | 52 |
| Alcohol use (yes/no) | 6198/3992 | |||||
| Sexually active (yes/no) | 7244/2984 | |||||
| Race (White/Black/Hispanic/Asian) | 9268/229/276/374 | |||||
| Smoking status (yes/no) | 1239/8998 | |||||
| Treatment information | Placebo | WW | Tamsulosin | Dutasteride | Combination therapy | |
| Number of subjects | 2158 | 373 | 1611 | 3790 | 2143 | |
| Treatment duration: | ||||||
| ≤12 mo | 475 | 44 | 180 | 659 | 610 | |
| ≤18 mo | 638 | 60 | 296 | 872 | 694 | |
| ≤24 mo | 2158 | 373 | 381 | 1181 | 940 | |
| <36 mo | 2158 | 373 | 536 | 1625 | 1125 | |
| <48 mo | 2158 | 373 | 1611 | 3790 | 2143 | |
Abbreviations: BMI, body mass index; BPH, benign prostatic hyperplasia; IPSS, International Prostate Symptom Score (questionnaire); PSA, prostate‐specific antigen; WW, watchful waiting.
3.2. Exploratory data analysis
Prior to model parameterisation, data integrity checks were performed with the objective of establishing the accuracy of the pooled data sets. The initial exploratory analysis showed no unexpected values or deviations regarding the dosing regimen, sampling times, PSA, prostate volume, Q max or observed IPSS.
No correlations or interactions were found between demographic and clinical baseline characteristics, other than those due to the known colinearity between variables for instance age and PSA (see Supporting Information Figure S3).
3.3. Time‐to‐event model building and validation
An exponential hazard model was found to best describe the time to first AUR/S in the overall patient population and across subgroups following stratification by treatment and baseline covariates. The final model consisted of a baseline hazard term and the associated covariate coefficients. All parameters were well estimated with good precision (relative standard error < 24%) and without statistically significant correlations between parameters.
To ensure biological plausibility and prevent overparameterisation, the evaluation of the demographic characteristics (eg, body mass index, body surface area, lean body weight or weight) was performed, taking into account colinearity. If a given covariate was identified as statistically significant, other descriptors displaying high collinearity were excluded in the subsequent steps.
At completion of the stepwise forward inclusion and backward exclusion procedures, the following baseline covariates showed statistical significance and were included in the final model: baseline IPSS, baseline PSA, baseline prostate volume, baseline Q max. These covariates reflect known factors associated with risk of disease progression and symptom severity, and were all found to significantly contribute to the underlying baseline hazard, independently of the pharmacological treatment effect.
Baseline covariates were parameterised as proportional terms to the baseline hazard parameter. It can be assumed that model structure and imputed covariate effects associated with the baseline hazard are sufficiently precise to disentangle the contribution of patient‐ and disease‐related factors from the effect of the intervention across the different treatment arms, WW, TAM, DUT or CT. Treatment‐specific terms were added to the model to characterise the changes associated with the active intervention. They were all parameterised as proportional to the baseline hazard rate. Final parameter estimates are summarised in Table 3.
TABLE 3.
Final parameter estimates for the TTE model
| Parameter | Parameter estimate | RSE (%) | 95% CI |
|---|---|---|---|
| Baseline hazard rate λ (per 100 000 subjects) [day −1 ] | 7.78 | (6%) | 6.86‐8.70 |
| Hazard ratios | |||
| Tamsulosin | 1 | … | … |
| Dutasteride | 0.432 | (7%) | 0.352‐0.512 |
| Combination | 0.336 | (7%) | 0.249‐0.423 |
| Baseline IPSS | 1.04 | (19%) | 1.022‐1.050 |
| Baseline PSA | 1.08 | (24%) | 1.041‐1.120 |
| Baseline prostate volume | 1.01 | (14%) | 1.007‐1.012 |
| Baseline maximum urinary flow | 0.91 | (14%) | 0.889‐0.935 |
Abbreviations: 95% CI, 95%‐confidence interval; IPSS, International Prostate Symptom Score; PSA, prostate‐specific antigen; RSE, relative standard error.
The final estimates for baseline hazard rates correspond to an incidence of 2.84% events per year. The parameter estimates for baseline covariate factors and treatments shown in Table 3 can be interpreted as follows: starting from the median value of the covariate factor a difference of 1 unit in the covariate value will correspond to a percentage increase or reduction in the baseline hazard of the magnitude of the parameter point estimate. For instance, for every unit increase in baseline IPSS (parameter estimate = 1.04), the instantaneous risk of AUR/S increases by 4%. Hence, a patient with baseline IPSS value of 26 (ie, 10 units higher than the median value) will have an instantaneous risk of AUR/S that is 40% higher than a patient with baseline IPSS of 16. Similarly, for every unit increase in PSA (parameter estimate = 1.08), the instantaneous risk of AUR/S increases by 8%, whereas a unit increase in maximum urinary flow (parameter estimate = 0.91) reduces the instantaneous risk by 9%. It is also worth mentioning that whilst the median prostate volume in this group of patients is relatively high, further enlargement of the prostate continues to affect the instantaneous risk of AUR/S, which increases by 1% for each unit of prostate volume (parameter estimate = 1.01).
In a comparable manner, the use of DUT (parameter estimate = 0.432) and CT (parameter estimate = 0.336) was found to significantly reduce the baseline hazard rate. On the other hand, the effect of treatment with TAM was not shown to be statistically significant relative to placebo. Its effect was therefore fixed to 1 to indicate no change to the baseline hazard rate. These results mean that at any point in time the risk AUR/S is 56.8% and 66.4% lower for patients receiving DUT and CT, respectively, as compared to patients receiving PLAC or TAM. Moreover, this treatment effect is independent of the actual baseline hazard rate, ie, irrespective of the contribution of baseline patient characteristics to the instantaneous risk of AUR/S. Reliable estimates of the effect of WW could not be obtained due to the small sample size and very low number of events in this group of patients.
The VPCs (Figures 2 and 3) showed that the observed TTE rate fell within the 95% confidence intervals of the simulated values, as depicted by the shaded area. Based on the VPCs, the final model was deemed to have acceptable predictive performance to describe the time to first AUR/S in this pool of patients with moderate or severe LUTS at risk of disease progression. The overall model performance was also evaluated by comparing predicted and observed events after stratification by selected baseline covariates.
FIGURE 2.
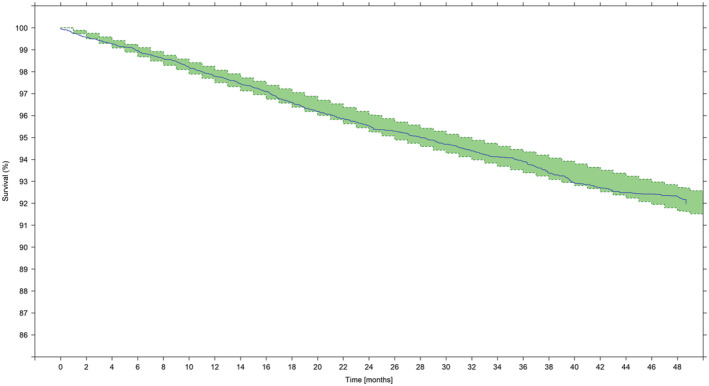
Visual predictive check showing the Kaplan‐Meier survival estimate over time. Survival (y axis) indicates the proportion of patients who have not had an event; at time zero the survival is 100% (ie, no patient has experienced an AUR/S). The solid line describes the observed time to first AUR/S over the period of 48 months across the overall population, irrespective of treatment. Shaded areas show the model‐predicted 95% confidence intervals of the survival
FIGURE 3.
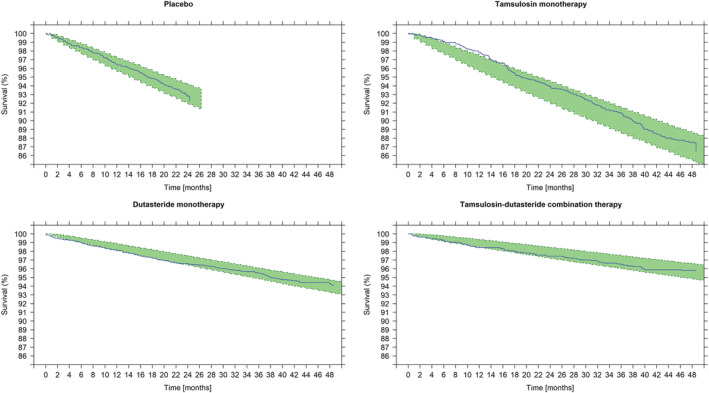
Visual predictive check showing the Kaplan‐Meier survival estimate over time stratified by treatment. Survival (y axis) indicates the proportion of patients who have not had an event; at time zero the survival is 100% (ie, no patient has experienced an AUR/S). The solid line describes the observed time to first AUR/S over the period of 48 months. Shaded areas show the model‐predicted 95% confidence intervals of the survival. The slope of survival curve for patients treated with tamsulosin (ie, a symptomatic treatment) does not differ from those receiving placebo. This contrasts with the slower incidence of events in patients treated with dutasteride (both as monotherapy and combination therapy), which shows disease‐modifying properties
3.4. Contribution of baseline covariate factors and treatment effect to the incidence of AUR/S
Even though no interaction has been identified between baseline covariates and treatment effect during model development, it is important to assess the overall effect of contributing factors to the changes in baseline hazard. Such an overview was obtained using simulations and subsequently stratifying the results by treatment arm and symptom severity, as assessed by IPSS. These simulations are summarised as heat maps in Figures 4–6, where the yearly incidence of events for patients receiving PLAC, TAM, DUT or CT is presented taking into account varying baseline IPSS, prostate volume, PSA and Q max. It is essential to highlight that the use of drugs with disease‐modifying properties affects the baseline hazard, irrespective of the magnitude of the effect of baseline covariates. Based on the heat maps, it becomes evident that DUT and CT effectively reduce the risks associated with known risk factors such as higher IPSS, prostate volume, PSA and lower Q max.
FIGURE 4.
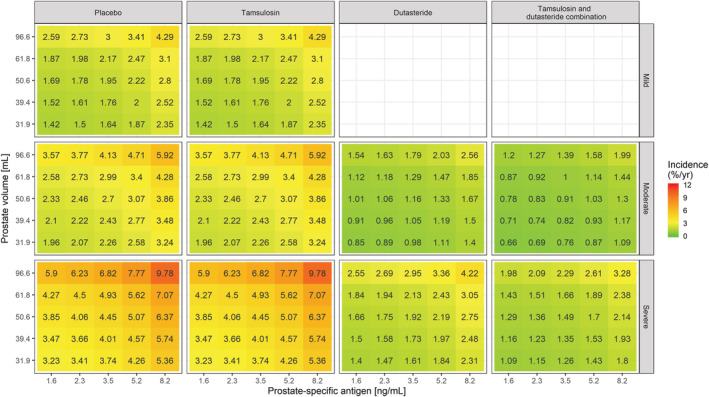
Heat map showing the incidence of AUR/S per year vs prostate‐specific antigen and prostate volume stratified by treatment and symptoms severity. The colour gradient from green to red reflects the increase in incidence of AUR/S in patients with higher symptom scores, larger PV and PSA values at baseline. Tamsulosin (ie, a symptomatic treatment) does not alter the underlying baseline hazard rate. Consequently, it does not modify the effect of baseline covariates on the incidence of events. This contrasts with dutasteride (both as monotherapy and combination therapy), which shows disease‐modifying properties. The midpoint for the colour gradient was set to 2.84%, which corresponds to the point estimate of the baseline hazard rate after placebo treatment. AUR/S incidence estimates were calculated taking into account the observed covariate distributions in Supporting Information Figure S1 and as such describe the effect of the different covariates in hypothetical LUTS/BPH patients across a clinically relevant range of values
FIGURE 6.
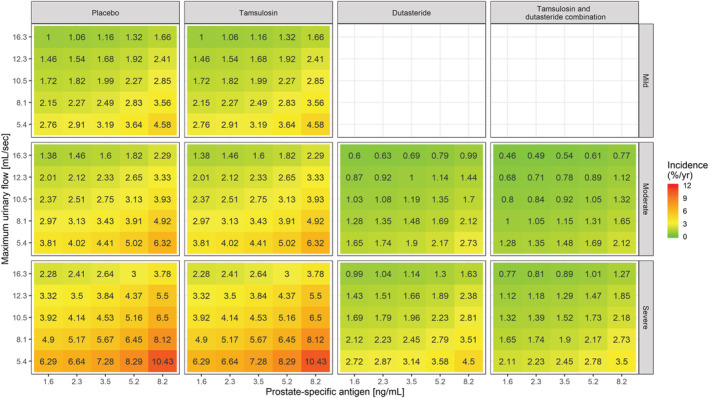
Heat map showing the incidence of AUR/S per year vs prostate‐specific antigen and maximum urinary flow stratified by treatment and symptoms severity. The colour gradient from green to red reflects the increase in incidence of AUR/Sin patients with higher symptom scores, larger PSA and lower Q max values at baseline. Tamsulosin does not alter the underlying baseline hazard rate. Consequently, it does not modify the effect of baseline covariates on the incidence of events. This contrasts with dutasteride (both as monotherapy and combination therapy), which shows disease‐modifying properties. The midpoint for the colour gradient was set to 2.84%, which corresponds to the point estimate of the baseline hazard rate after placebo treatment. AUR/S incidence estimates were calculated taking into account the observed covariate distributions in Supporting Information Figure S1 and as such describe the effect of the different covariates in hypothetical LUTS/BPH patients across a clinically relevant range of values
FIGURE 5.
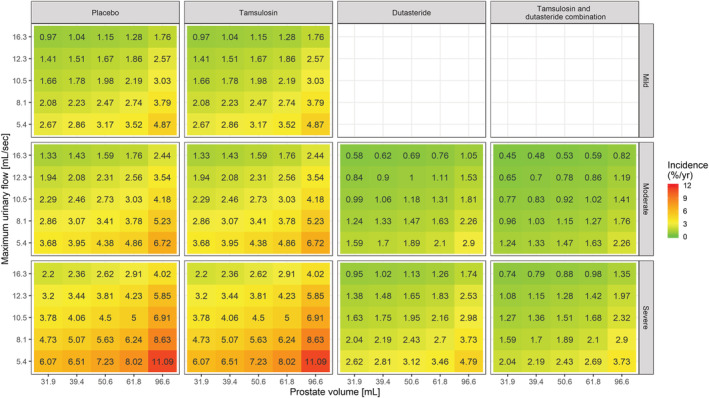
Heat map showing the incidence of AUR/S per year vs maximum urinary flow and prostate volume stratified by treatment and symptoms severity. The colour gradient from green to red reflects the increase in incidence of AUR/S in patients with higher symptom scores, larger PV and lower Q max values at baseline. Tamsulosin does not alter the underlying baseline hazard rate. Consequently, it does not modify the effect of baseline covariates on the incidence of events. This contrasts with dutasteride (both as monotherapy and combination therapy), which shows disease‐modifying properties. The midpoint for the colour gradient was set to 2.84%, which corresponds to the point estimate of the baseline hazard rate after placebo treatment. AUR/S incidence estimates were calculated taking into account the observed covariate distributions in Supporting Information Figure S1 and as such describe the effect of the different covariates in hypothetical LUTS/BPH patients across a clinically relevant range of values
4. DISCUSSION
Whereas the management of LUTS/BPH in patients with moderate or severe symptoms has changed significantly over the last two decades in response to the availability of new treatment options,1, 15, 16 AUR, which often presents as an emergency, remains an important complication. By contrast, BPH‐related surgery is usually a consequence of the perceived severity of the condition. In fact, despite considerable variation between studies in the reported incidence of AUR in the male population,17 AUR results in prostatectomy in only 24‐42% of men, while patients who avoid surgery through a successful trial without catheter have been found to be at high risk of requiring surgery within a year.18, 19, 20 Nevertheless, irrespective the fact that AUR and surgery are less common than overall symptomatic worsening, they are important progression events with financial, emotional and health‐related consequences.21
Even though clinical guidelines and supporting evidence highlight the benefits of tamsulosin and dutasteride combination therapy for patients with moderate and severe LUTS/BPH at risk of disease progression, in clinical practice patients are often treated initially with an α‐blocker.1 Such practice seems to overlook the concept of disease progression and the relevance of treatments with disease‐modifying properties.22, 23, 24 Our results clearly show that symptomatic intervention (ie, tamsulosin monotherapy) does not reduce the risk associated with AUR/S.
Notwithstanding the relevance of data generation in clinical trials, the use of drug‐disease models has been considered one of the most efficient approaches for knowledge integration. In fact, the use of a proportional hazard model to describe the time to first AUR/S can be compared to previous attempts in other therapeutic areas, such as the time to acute kidney injury in patients undergoing allogeneic stem cell transplantation.25 Another example of a similar application is the early identification of patients with febrile neutropenia for the initiation of adjuvant chemotherapy in breast cancer patients.26 Here we have shown the feasibility of a parametric approach to describe the time to first AUR/S taking into account baseline factors known to be associated with disease progression. Such an integrated analysis has allowed the effect of symptomatic interventions to be disentangled from those with disease‐modifying properties. The covariates identified during model development corroborate the available evidence from clinical trials and previous meta‐analyses.27, 28, 29 Another important feature of our analysis was the possibility of quantifying the magnitude of the effect of patient characteristics on baseline hazard, independently of treatment type. The availability of estimates of the effect of treatment and baseline characteristics on baseline hazard enable risk stratification along with prediction of long‐term consequences of disease progression.
Our results reveal that Q max is the most influential covariate factor among the statistically significant clinical baseline characteristics. These findings are in agreement with previous reports in which men with Q max < 12 ml/s have four times higher risk of AUR compared with those showing urinary flow rates > 12 ml/s (95% CI 2.3‐6.6).29, 30 Together with baseline IPSS, prostate volume and PSA levels, parameter estimates can be derived to predict changes in baseline hazard (ie, LUTS/BPH disease progression) and guide for patient stratification into risk groups. Most importantly, our results also show that treatment with tamsulosin monotherapy does not affect the baseline hazard and consequently does not alter the risk or incidence of AUR/S. The disease‐modifying properties of dutasteride, either as monotherapy or combination therapy, lead to a substantial decrease in the baseline hazard (point estimates correspond to reductions of 56.8% and 66.4%, respectively). Such treatment‐induced changes in hazard appear to reflect the known biological mechanisms by which dutasteride acts, ie, by converting testosterone to dihydrotestosterone, which is the androgen primarily responsible for the initial development and subsequent enlargement of the prostate. While the precise aetiological factors responsible for the development of AUR have not been fully characterised in LUTS/BPH patients,31 it seems that increased resistance to urine flow due to mechanical obstruction such as prostatic enlargement or an increase in either the smooth or striated muscle tone cannot be effectively counteracted by α1‐blockers only.32 Blockade of adrenoceptors and subsequent smooth muscle relaxation leads to symptom improvement but does not modify the progression of prostate enlargement or the long‐term occurrence of AUR/S. Indeed, the development of AUR and changes in bladder function that accompany benign prostatic obstruction are the subject of significant research interest. It should be noted that in addition to the macroscopic changes in bladder wall structure (eg, detrusor wall thickening),33 increasing obstruction is also associated with functional and ultrastructural changes, such as the interruption of crosstalk signalling for muscular coordination, changes in bladder muscle autonomic innervation, alterations in the spinal cord voiding reflex and changes in neurotransmitter levels.34 All of these likely contribute to the development of AUR, even though the role of each factor may vary among patients.35, 36
We anticipate, therefore, that the availability of a parametric model describing the time to first AUR/S will provide the basis for the evaluation of a range of clinical questions regarding the effect of different interventions on the risk of disease progression and deterioration of symptoms. In addition, this model offers the opportunity to assess the impact of an individual patient's clinical characteristics at baseline on the underlying hazard and predict the incidence of AUR/S for each subset or population.
4.1. Limitations
In any investigation, the appropriate model type depends greatly upon the problem at hand. Even though in statistical terms a parametric TTE model is a standard tool for the analysis of survival data, it can be parameterised to evaluate disease progression. In fact, survival or hazard functions describing discrete clinical endpoints can be linked to continuous disease progression models.22 In our analysis, the parameterisation of treatment as a discrete covariate is justified by the lack of pharmacokinetic data and standard use of a single dose level in all patients receiving an active pharmacological treatment.
From a methodological point‐of‐view, it is important to emphasise that some baseline variables, such as old age, are strongly correlated with symptom severity and have therefore not been selected as an independent factor during covariate model building. On the other hand, as information on postvoid residual urine, ultrasound estimated prostate weight and prostate transition zone volume were not available, these measurements could not be included in the analysis. In addition, we have assumed that parameter estimates obtained from the pooled database (n = 10 236) were sufficiently precise to describe the impact of baseline covariates and transition to a different treatment in a wider patient population.
We also need to underline that our analysis was aimed at characterising the mid‐ to long‐term risk of AUR/S. The contribution of covariate factors and different pharmacological interventions to instantaneous risk may not be clinically detectable when considering shorter intervals. This is particularly relevant when considering patients showing baseline characteristics which correlate with a lower risk of disease progression than those included in the available clinical trials.
5. CONCLUSIONS
In summary, our analysis showed the advantages of a parametric TTE model to characterise the incidence of AUR/S, disentangling the contribution of baseline covariates from the treatment effect on the underlying baseline hazard rate and disease progression. Known risk factors for AUR/S (ie, baseline IPSS, baseline PSA, baseline prostate volume and baseline Qmax) were found to have a statistically significant effect on the baseline hazard rate, which consequently alters the instantaneous risk of AUR/S. Their effect is independent of treatment type. Our analysis also reveals that the use of tamsulosin monotherapy (symptomatic treatment) does not reduce individual long‐term risk of AUR/S in LUTS/BPH patients with moderate or severe symptoms at risk of progression.
COMPETING INTERESTS
S.D.A. none to declare. M.O. has been a speaker, consultant and/or trial investigator for Apogepha, Astellas, Ferring, GSK, Pierre Fabre and Pfizer, and received research grants from Astellas and Pfizer. M.C.M. has been a speaker and consultant for Apogepha, Astellas, Dr. Willmar Schwabe, Ferring, GSK, Recordati and Velicept, and is a past employee of Boehringer Ingelheim and a current shareholder of Velicept. C.G.R. was previously employed as a consultant for GSK. C.C., M.M., J.M.P.M. and O.D.P. are GSK employees and hold stocks/shares in GSK.
CONTRIBUTORS
S.D.A. was involved in the design of the study, analysis and interpretation of study data, and the drafting and critical revision of the manuscript. M.M and J.M.P.M. were involved the design of the study, interpretation of study data, and the drafting and critical revision of the manuscript. C.C., M.O., M.C.M. and C.G.R. were involved in the interpretation of study data, and the drafting and critical revision of the manuscript. O.D.P. was involved in the conception/design and interpretation of study data, and the drafting and critical revision of the manuscript.
Supporting information
Supporting Information Figure S1 Baseline clinical and demographic characteristics (age, height, International Prostate Symptom Score, weight, prostate‐specific antigen, Q max, PV), stratified by study
Supporting Information Figure S2 Baseline clinical and demographic characteristics (age, height, International Prostate Symptom Score, weight, prostate‐specific antigen, Q max, PV), stratified by treatment
Supporting Information Figure S3 Correlation matrix between baseline demographic and clinical characteristics
ACKNOWLEDGEMENT
This investigation was funded by GlaxoSmithKline (GSK).
D'Agate S, Chavan C, Manyak M, et al. Model‐based meta‐analysis of the time to first acute urinary retention or benign prostatic hyperplasia‐related surgery in patients with moderate or severe symptoms. Br J Clin Pharmacol. 2021;87:2777–2789. 10.1111/bcp.14682
Funding information GlaxoSmithKline
DATA AVAILABILITY STATEMENT
Anonymized individual participant data and study documents can be requested for further research from www.clinicalstudydatarequest.com.
REFERENCES
- 1.Gravas S, Cornu JN, Gacci M, et al. EAU Guidelines: Treatment of non‐neurogenic male LUTS 2020. Available at: https://uroweb.org/guideline/treatment‐of‐non‐neurogenic‐male‐luts/
- 2.Emberton M, Cornel EB, Bassi PF, Fourcade RO, Gomez JM, Castro R. Benign prostatic hyperplasia as a progressive disease: a guide to the risk factors and options for medical management. Int J Clin Pract. 2008;62(7):1076‐1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McVary KT, Roehrborn CG, Avins AL, et al. Update on AUA guideline on the management of benign prostatic hyperplasia. J Urol. 2011;185(5):1793‐1803. [DOI] [PubMed] [Google Scholar]
- 4.O'Leary MP. LUTS, ED, QOL: alphabet soup or real concerns to aging men? Urology. 2000;56(5):7‐11. [DOI] [PubMed] [Google Scholar]
- 5.Debruyne F, Barkin J, van Erps P, et al. Efficacy and safety of long‐term treatment with the dual 5 alpha‐reductase inhibitor dutasteride in men with symptomatic benign prostatic hyperplasia. Eur Urol. 2004;46(4):488‐495. [DOI] [PubMed] [Google Scholar]
- 6.McConnell JD, Roehrborn CG, Bautista OM, et al. The long‐term effect of doxazosin, finasteride, and combination therapy on the clinical progression of benign prostatic hyperplasia. N Engl J Med. 2003;349(25):2387‐2398. [DOI] [PubMed] [Google Scholar]
- 7.Roehrborn CG, Siami P, Barkin J, et al. The effects of combination therapy with dutasteride and tamsulosin on clinical outcomes in men with symptomatic benign prostatic hyperplasia: 4‐year results from the CombAT study. Eur Urol. 2010;57(1):123‐131. [DOI] [PubMed] [Google Scholar]
- 8.D'Agate S, Wilson T, Adalig B, et al. Impact of disease progression on individual IPSS trajectories and consequences of immediate versus delayed start of treatment in patients with moderate or severe LUTS associated with BPH. World J Urol. 2020;38(2):463‐472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ploeger BA, Holford NH. Washout and delayed start designs for identifying disease modifying effects in slowly progressive diseases using disease progression analysis. Pharm Stat. 2009;8(3):225‐238. [DOI] [PubMed] [Google Scholar]
- 10.Frobel AK, Karlsson MO, Backman JT, et al. A time‐to‐event model for acute rejections in paediatric renal transplant recipients treated with ciclosporin A. Br J Clin Pharmacol. 2013;76(4):603‐615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lindauer A, Laveille C, Stockis A. Time‐to‐seizure modeling of lacosamide used in monotherapy in patients with newly diagnosed epilepsy. Clin Pharmacokinet. 2017;56(11):1403‐1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reporting and analysis plan for modelling and simulation of the time to acute urinary retention (AUR) or BPH‐related surgery after early vs. delayed onset of combination therapy with tamsulosin and dutasteride. GSK Study 209707, 2018 (available at: https://www.gsk‐studyregister.com/en/trial‐details/?id=209707).
- 13.R Core Team . R: A language and environment for statistical computing. In, Vienna, Austria R Foundation for Statistical Computing, 2013.
- 14.Alexander SPH, Kelly E, Mathie A, et al. The Concise Guide to PHARMACOLOGY 2019/20. Br J Pharmacol. 2019;176(S1):S1‐S493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fullhase C, Soler R, Gratzke C. New strategies in treating male lower urinary tract symptoms. Curr Opin Urol. 2014;24(1):29‐35. [DOI] [PubMed] [Google Scholar]
- 16.Shima Y, Kawano Y, Kobayashi A, et al. Comparison of the clinical effect of dutasteride therapy for benign prostatic hyperplasia when initiated at different time points: A multicentre, observational, retrospective chart review study. Int J Clin Pract. 2019;73:e13418. [DOI] [PubMed] [Google Scholar]
- 17.Speakman MJ, Desgrandchamps F, Mamoulakis C. Acute urinary retention rates in the general male population and in adult men with lower urinary tract symptoms participating in pharmacotherapy trials: a literature review. Urology. 2015;86:654‐665. [DOI] [PubMed] [Google Scholar]
- 18.Choong S, Emberton M. Acute urinary retention. BJU Int. 2000;85(2):186‐201. [DOI] [PubMed] [Google Scholar]
- 19.Desgrandchamps F, De La Taille A, Doublet JD, RetenFrance Study G. The management of acute urinary retention in France: a cross‐sectional survey in 2618 men with benign prostatic hyperplasia. BJU Int. 2006;97(4):727‐733. [DOI] [PubMed] [Google Scholar]
- 20.Pickard R, Emberton M, Neal DE, National Prostatectomy Audit Steering Group . The management of men with acute urinary retention. Br J Urol. 1998;81(5):712‐720. [DOI] [PubMed] [Google Scholar]
- 21.Kuiper JG, Bezemer ID, Driessen MT, et al. Rates of prostate surgery and acute urinary retention for benign prostatic hyperplasia in men treated with dutasteride or finasteride. BMC Urol. 2016;16(1):53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cook SF, Bies RR. Disease progression modeling: key concepts and recent developments. Curr Pharmacol Rep. 2016;2(5):221‐230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Djavan B. Treatment of symptomatic benign prostatic hyperplasia: current and future clinical practice in Europe ‐ What is really happening? Eur Urol Suppl. 2007;6(6):446‐453. [Google Scholar]
- 24.van Exel NJ, Koopmanschap MA, McDonnell J, et al. Medical consumption and costs during a one‐year follow‐up of patients with LUTS suggestive of BPH in six European countries: report of the TRIUMPH study. Eur Urol. 2006;49(1):92‐102. [DOI] [PubMed] [Google Scholar]
- 25.Pinana JL, Perez‐Pitarch A, Garcia‐Cadenas I, et al. A time‐to‐event model for acute kidney injury after reduced‐intensity conditioning stem cell transplantation using a tacrolimus‐ and sirolimus‐based graft‐versus‐host disease prophylaxis. Biol Blood Marrow Transplant. 2017;23(7):1177‐1185. [DOI] [PubMed] [Google Scholar]
- 26.Netterberg I, Karlsson MO, Nielsen EI, Quartino AL, Lindman H, Friberg LE. The risk of febrile neutropenia in breast cancer patients following adjuvant chemotherapy is predicted by the time course of interleukin‐6 and C‐reactive protein by modelling. Br J Clin Pharmacol. 2018;84(3):490‐500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pinto F, Racioppi M, Sacco E, et al. Progression, risk factors and subsequent medical management of symptomatic benign prostatic hyperplasia. Arch Ital Urol Androl. 2009;81:1‐8. [PubMed] [Google Scholar]
- 28.Roehrborn CG, Barkin J, Tubaro A, et al. Influence of baseline variables on changes in International Prostate Symptom Score after combined therapy with dutasteride plus tamsulosin or either monotherapy in patients with benign prostatic hyperplasia and lower urinary tract symptoms: 4‐year results of the CombAT study. BJU Int. 2014;113(4):623‐635. [DOI] [PubMed] [Google Scholar]
- 29.Zattoni F, Ficarra V, Novara G. Risk stratification for benign prostatic hyperplasia. Urologia. 2017;84(3):153‐157. [DOI] [PubMed] [Google Scholar]
- 30.Jacobsen SJ, Jacobson DJ, Girman CJ, et al. Natural history of prostatism: risk factors for acute urinary retention. J Urol. 1997;158(2):481‐487. [DOI] [PubMed] [Google Scholar]
- 31.Thomas K, Chow K, Kirby RS. Acute urinary retention: a review of the aetiology and management. Prostate Cancer Prostatic Dis. 2004;7(1):32‐37. [DOI] [PubMed] [Google Scholar]
- 32.Barendrecht MM, Abrams P, Schumacher H, de la Rosette JJ, Michel MC. Do alpha1‐adrenoceptor antagonists improve lower urinary tract symptoms by reducing bladder outlet resistance? NeurourolUrodyn. 2008;27(3):226‐230. [DOI] [PubMed] [Google Scholar]
- 33.Oelke M, Hofner K, Wiese B, Grunewald V, Jonas U. Increase in detrusor wall thickness indicates bladder outlet obstruction (BOO) in men. World J Urol. 2002;19(6):443‐452. [DOI] [PubMed] [Google Scholar]
- 34.Oelke M, Baard J, Wijkstra H, de la Rosette JJ, Jonas U, Hofner K. Age and bladder outlet obstruction are independently associated with detrusor overactivity in patients with benign prostatic hyperplasia. Eur Urol. 2008;54(2):419‐426. [DOI] [PubMed] [Google Scholar]
- 35.Cho SY, Bae J, Yoo C, Oh SJ. Establishment of a grading system for bladder trabeculation. Urology. 2013;81(3):503‐507. [DOI] [PubMed] [Google Scholar]
- 36.Chapple C, Abrams P. Male lower urinary tract symptoms. An international consultation on Male LUTS, Fukuoka, Japan. September 30‐October 4, 2012. In, Montreal, Canada: Société Internationale d'Urologie, 2013: 295‐315 (available at: https://www.siu‐urology.org/themes/web/assets/files/ICUD/pdf/Male Lower Urinary Tract Symptoms (LUTS).pdf).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information Figure S1 Baseline clinical and demographic characteristics (age, height, International Prostate Symptom Score, weight, prostate‐specific antigen, Q max, PV), stratified by study
Supporting Information Figure S2 Baseline clinical and demographic characteristics (age, height, International Prostate Symptom Score, weight, prostate‐specific antigen, Q max, PV), stratified by treatment
Supporting Information Figure S3 Correlation matrix between baseline demographic and clinical characteristics
Data Availability Statement
Anonymized individual participant data and study documents can be requested for further research from www.clinicalstudydatarequest.com.


