Abstract
Background
Cationic amphiphilic chitosan derivatives can form polymeric micelles, which are useful cosmetic materials, but they form polyion complexes with anionic polymers, which can cause formulation difficulties.
Aims
This study aimed to evaluate the usefulness of partially myristoylated carboxymethyl chitosan, an amphoteric‐amphiphilic chitosan derivative, as a new material for cosmetics in the absence of a surfactant comprising an anionic polymer.
Methods
An anionic polymer and 1,2‐decanediol (an antimicrobial agent)‐containing partially myristoylated carboxymethyl chitosan nanoemulsified lotion and glabridin (an antimelanogenic agent)‐containing partially myristoylated carboxymethyl chitosan polymeric micelle were prepared using a pressure homogenization method. The release of interleukin‐1α, cell viability, and melanogenesis inhibition was evaluated on a human skin model. Antimicrobial activity was evaluated using agar dilution method.
Results
A mixture of partially myristoylated carboxymethyl chitosan and carboxyvinyl polymer did not form a polyion complex, but it formed a hydrophilic gel. The anionic polymer‐containing partially myristoylated carboxymethyl chitosan nanoemulsified formulation was stable, with no decrease in cell viability and horny layer exfoliation, which are typically observed with Tween 60. Compared with the formulation with methyl paraben (0.2%), the formulation to which 1,2‐decanediol (0.05%) was added improved the antibacterial activity against methicillin‐resistant Staphylococcus aureus and Propionibacterium acnes; however, no interleukin‐1α upregulation was observed. The glabridin‐containing partially myristoylated carboxymethyl chitosan polymeric micelles enhanced melanogenesis inhibition and percutaneous glabridin delivery to the epidermis compared with conventional emulsified micelles.
Conclusions
These results suggest that partially myristoylated carboxymethyl chitosan‐forming polymeric micelles, in combination with 1,2‐decanediol and glabridin, may be useful for surfactant‐free cosmetic emulsions.
Keywords: Amphiphilic carboxymethyl chitosan, chitosan, cosmetic ingredient, human skin model, polymeric micelle
1. INTRODUCTION
Chitosan is a deacetylated product of chitin and a natural aminopolysaccharide with excellent biocompatibility. In the field of cosmetology and pharmaceutics, chitosan has been proposed to be useful owing to its moisture retention, wound healing, sustained release, and antibacterial properties, among others.1, 2, 3 Most cosmetic products for personal care are emulsions; in particular, low‐molecular‐weight surfactants are important components of emulsion formation. Some surfactants exhibit biotoxicity, which can impair skin barrier function through different mechanisms and can induce skin irritation.4 Development of safer alternatives for emulsion formation for the care of sensitive skin is required in the cosmetic and medical fields.
Numerous functional chitosan derivatives have been developed via the chemical modification of the amino group of chitosan. Amphiphilic chitosan derivatives with a long alkyl chain form polymeric micelles. Further, hydrophobic groups attached to a chitosan chain, aggregating to form polymeric micelles in water, are useful carriers for hydrophobic drugs.5 Glabridin (Glab) is an inhibitor of melanogenesis with a potent tyrosinase inhibitory activity.6 We developed a partially myristoylated cationic amphiphilic chitosan derivative. Because this derivative forms cationic polymeric micelles, the melanogenesis inhibition by Glab may be promoted by improving the penetration of a hydrophobic melanogenesis inhibitor into the epidermis.7
A polyion complex is formed by an electrostatic interaction between cationic and anionic polymers.8 Chitosan and cationic chitosan derivatives with amino groups form polyion complexes with anionic polymers,9 such as carboxyvinyl polymer (CVP) and hyaluronic acid, which are commonly used in cosmetics, and induce precipitation of these complexes, thereby inhibiting the stabilizing emulsions and causing difficulty in the formulation of skin care products.
Parabens, such as methyl paraben (MP), exhibit antibacterial activity and are widely used as preservatives in different commercial items, including cosmetics and pharmaceuticals. On the other hand, 1,2‐Alkanediols, such as 1,2‐decanediol (DD), exhibit antibacterial activity and can act as cosmetic water‐soluble antimicrobial agents.10 Nonionic low‐molecular‐weight surfactants, such as Tween 60 (INCI name: Polysorbate 60, which is used in pharmaceuticals and cosmetics), is a low‐toxicity surfactant and a emulsion that is considered to affect the lipid bilayers of the stratum corneum.11, 12 Tween 80 (INCI name: Polysorbate 80) affects the skin barrier and promotes the penetration of lorazepam.13
We developed a partially myristoylated carboxymethyl chitosan (PMCC, Figure 1) by adding a 12% myristoyl group to the amino group in the carboxymethyl chitosan. PMCC is an amphoteric‐amphiphilic chitosan derivative comprising an amino group as a cation and carboxymethyl group as an anion. In the present study, we reported our findings that suggested PMCC as a novel cosmetic material that may be useful for the application of surfactant‐free emulsification, which contains an anionic polymer and DD, and polymeric micelles that enhance the inhibition of Glab melanogenesis.
Figure 1.
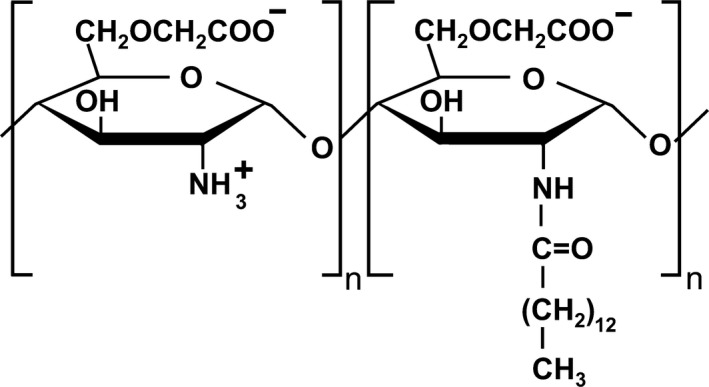
Structure of partially (12%) myristoylated carboxymethyl chitosan (PMCC)
2. MATERIALS AND METHODS
2.1. Materials
PMCC (MC‐Chitosan) was supplied by Pias Corp. Licorice root extract comprising 40% Glab (Glab‐40) was obtained from Maruzen Pharmaceuticals Co., Ltd. and used as a melanogenesis inhibitor.6 Moisturizing agent (eg, glycerin), emollient oil (eg, cetyl ethylhexanoate), and antimicrobial agents (MP and DD) used were cosmetic‐grade ingredients. Polyoxyethylene sorbitan monostearate (Tween 60) was purchased from Kao Corporation. CVP (Carbopol 940), acrylic acid alkyl copolymer (AACP) (PemulenTR‐1), and chitosan PCA (Kytamer PC) were purchased from Iwase Cosfa Co., Ltd. All other chemicals used were reagent grade. There was no requirement for ethics approval for the use of these human cells.
2.2. Measurement
The electron images of polymeric micelles and water‐based gel derived from PMCC were obtained using a transmission electron microscope (TEM) operating at 100 kV (Hitachi H7600, Hitachi, Tokyo, Japan). The specimens were negatively stained with 2.0% uranylacetate solution (Sigma‐Aldrich).
2.3. Preparation of mixture and aqueous gel from PMCC and anion polymer
Water solutions of chitosan PCA, PMCC, CVP, and AACP were dissolved in water at 50°C under continuous stirring for 60 minutes. Solution diluted to 0.1% (CVP and AACP) was added to those diluted 0.2% (chitosan PCA and PMCC) and mixed by stirring for 10 minutes. CVP solution (0.1%) was added to 0.4% PMCC solution; this mixture was further stirred at 40°C until a homogeneous gel was obtained.
2.4. Preparation of Glab‐containing PMCC polymeric micelle (Glab/PMCC‐PM)
Glab/PMCC‐PM was prepared by the following pressure homogenization method. An aqueous solution containing PMCC and 1,3‐butanediol was added to an oily solution of cetyl ethylhexanoate and Glab‐40 and homogenized using a homomixer (TK Homo Mixer, Primix, Osaka, Japan). The mixture was twice passed through a high‐pressure homogenizer (Nanomyzer; Yoshida Kikai Co., Ltd.) at 20 000 p.s.i. for producing a stable suspension of Glab/PMCC‐PM. The final concentrations of PMCC, Glab‐40, 1,3‐butanediol, and cetyl ethylhexanoate in the suspension were 0.20%, 0.01%, 5.00%, and 2.00%, respectively. For the preparation of Glab‐containing O/W nanomicelles, 0.5% Tween 60 was used. Both preparations were performed under same conditions.
2.5. Preparation of PMCC nanoemulsified lotion containing anionic polymers (PMCC/AP‐EL)
A nanoemulsion comprising a moisturizing agent and emollient oil derived from PMCC was prepared using the method described in 2.3. Final concentrations of PMCC, glycerin, dipropylene glycol, cetyl ethylhexanoate, and octyldodecanol in the nanoemulsion were adjusted to 0.20%, 5.00%, 8.00%, 5.00%, and 0.50%, respectively. Table 1 summarizes the composition of PMCC/AP‐EL. An aqueous phase comprising AACP, glycerin, dipropylene glycol, poly‐γ‐glutamine acid, and xanthan gum was dissolved in water under continuous stirring for 30 minutes. To prepare the nanoemulsified lotion, the prepared 10% nanoemulsion was added to the aqueous phase at 40°C under continuous stirring for 10 minutes. The stability of PMCC/AP‐EL was evaluated for any changes in appearance after 1 month of incubation at 45°C and 50°C. Viscosity was analyzed at 25°C using a rotational viscometer (DV‐1 viscometer, Brookfield, Middleborough, MA, USA). An O/W nanoemulsion comprising a low‐molecular‐weight surfactant was prepared using 4.0% Tween 60 under the same conditions used for the preparation of PMCC/AP‐EL.
Table 1.
PMCC nanoemulsified lotion formulations containing anionic polymer
| Ingredient | PMCC formulation (% w/w) | Tween 60 formulation (% w/w) |
|---|---|---|
| PMCC nanoemulsion | 10.00 | – |
| Tween 60 nanoemulsion | – | 10.00 |
| Glycerin | 5.00 | 5.00 |
| Dipropylene glycol | 8.00 | 8.00 |
| Butylene glycol | 3.00 | 3.00 |
| AACP | 0.10 | 0.10 |
| Poly‐γ‐glutamic acid | 0.04 | 0.04 |
| Xanthan gum | 0.10 | 0.10 |
| Distilled water | 100.00 | 100.00 |
Nanoemulsion system: cetyl ethylhexanoate 5.2%, octyldodecanol 0.5%, dipropylene glycol 8.0%, PMCC 0.2% or Tween 60 4.0%, and water adjusted to 100%.
Abbreviations: AACP, Acrylic acid alkyl copolymer; PMCC, Partially myristoylated carboxymethyl chitosan.
2.6. Skin safety evaluation of PMCC/AP‐EL using a human skin model
Safety of PMCC/AP‐EL was evaluated by an in vitro skin irritation test using TESTSKIN (LSE‐high, Toyobo Co. Ltd.), a commercial three‐dimensional human skin model comprising collagen gel‐containing epidermal cells, including the horny layer and fibroblasts. An aliquot of 100 μL of each sample lotion was applied to the surface of TESTSKIN, followed by incubation at 37°C under 5% CO2 humidity for 24 hours. The surface of the incubated skin model was rinsed 5 times with phosphate‐buffered saline (PBS; 1 mL) to remove the sample, the cells were incubated at 37°C under 5% CO2 humidity for 3 hours, and the cell viability was determined using the Cell Count Kit‐8 (CCK‐8) assay (Dojindo Laboratories, Kumamoto, Japan). Interleukin (IL)‐1α level in the medium was determined using an enzyme‐linked immunosorbent assay (Biotrak Human Interleukin‐1alpha ELISA system; Amersham Bioscience Corp.). Additionally, the skin tissue was cut at the midportion of the skin surface, following which each part was embedded in paraffin and stained with hematoxylin and eosin.
2.7. Assessment of antimicrobial activity against microorganisms
The microorganisms tested were methicillin‐resistant Staphylococcus aureus (MRSA), Propionibacterium acnes ATCC 6919, and Malassezia fungi NBRC 0656. MRSA, P acnes, and M fungi were incubated in soybean casein digest medium, Gifu anaerobic medium, and glucose peptone agar with lecithin and Polysorbate 80 medium, respectively. Following incubation at 35°C for 48 hours, the microorganisms were suspended in sterile PBS to adjust the colony‐forming units (CFUs) to approximately 108 CFU/mL. The nanoemulsified lotion was transferred to a sterile vial and inoculated with the test organism suspension for ensuring that it comprised approximately 1% suspension. The samples were incubated at 30°C for 0‐120 minutes, and viable cell count was determined by the agar dilution method.
2.8. Transdermal penetration assay for Glab using a human skin model
A reconstituted human skin was used as skin model (TESTSKIN LSE‐high). The test sample suspension was placed on the surface of the model and incubated at 37°C under 5% CO2 humidity for 24 hours. Following incubation, the surface of the skin model was rinsed 10 times with 1 mL PBS to remove the surplus test sample. For determining the Glab concentration absorbed into the interior of the skin model, the epidermis and dermis were separated from skin cells, and the isolated tissues were transferred to an Eppendorf tube containing 1 mL ethanol, followed by sonication for 1 hour. The Glab concentration in the supernatant of the cell‐free extract was measured using high‐performance liquid chromatography (4.6 × 256 mm TOSOH‐GEL column, TOSOH Co.; eluent: a mixture of water containing 2% acetic acid and acetonitrile (40:60 vol%); flow rate: 1.0 mL/min at 40°C) equipped with ultraviolet detector at 282 nm.
2.9. Cell pigmentation assay in the human skin model comprising functional melanocytes
A commercial three‐dimensional human skin equivalent (MEL‐300‐A; MatTek Corp.) was used for the cell pigmentation assay. MEL‐300‐A consists of human‐derived epidermal keratinocytes and melanocytes. The test sample suspension was placed on the surface of skin model and incubated at 37°C under 5% CO2 humidity. The cell culture was performed for 14 days, with the media being changed every second day. Following incubation, the tissue surface was rinsed 5 times with 1 mL PBS to remove the surplus tested sample. The brightness of the skin surface was measured using image editing application software (Photoshop, Adobe System).
2.10. Statistical analysis
Differences between the mean values of groups were assessed using one‐way analysis of variance (ANOVA) with the Tukey‐Kramer test or unpaired Student's t test. A P‐value of <.05 was considered to denote statistical significance.
3. RESULTS
3.1. Characteristics of the PMCC and anionic polymer mix
Chitosan and cationic chitosan derivatives comprising an amino acid group form insoluble polyion complexes when mixed with an anionic polymer. PMCC, despite having an amino group, remained in the transparent state when mixed with CVP or AACP, and no precipitation induced by the formation of a polyion complex was observed (Figure 2). In contrast, chitosan PCA formed insoluble polyion complexes. The addition of CVP enabled the formation of a hydrophilic gel in the PMCC aqueous solution. As observed on the TEM image, the hydrophilic gel comprised localized nanoscale fibers (Figure 3).
Figure 2.
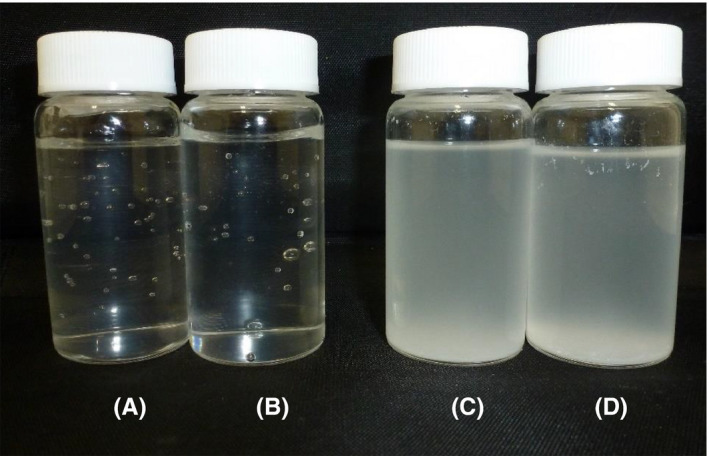
Appearance of 0.1% PMCC solution containing 0.05% anionic polymers. A, PMCC/CVP, (B) PMCC/AACP, (C) chitosan PCA/CVP, and (D) chitosan PCA/AACP
Figure 3.
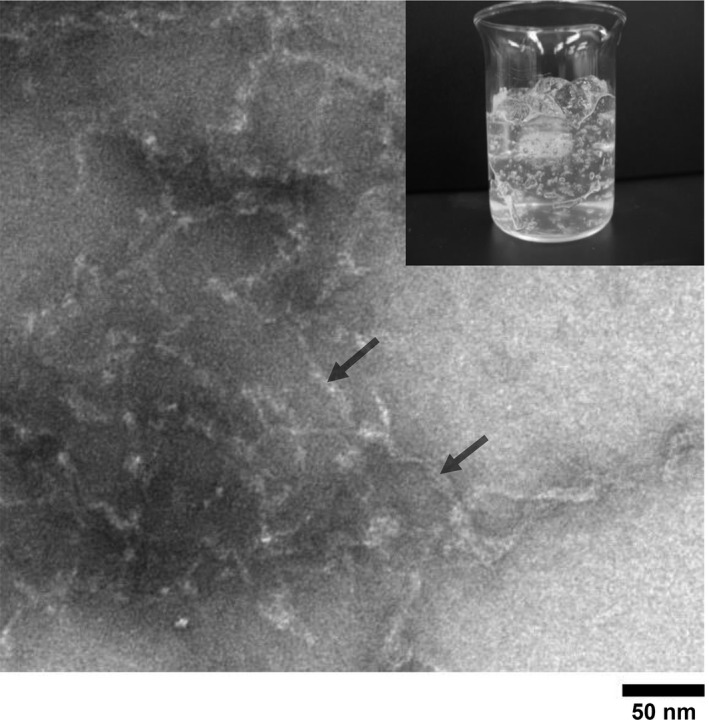
Morphology of PMCC/CVP water‐based gel. PMCC and CVP concentrations are 0.20% and 0.05%, respectively. A negatively stained electron microphotograph of PMCC micelle is shown
3.2. Skin safety and stability of surfactant‐free PMCC/AP‐EL
The TEM images of PMCC/AP‐EL are shown in Figure 4(a). Nanoscale spherical micelles were observed. In the in vitro skin irritation test of PMCC/AP‐EL and Tween 60‐nanoemulsified lotion (Tween 60/‐EL), a significant decrease was observed in the cell viability in the human skin model treated with Tween 60/‐EL (decrease by approximately 66% compared with the control); however, no decrease in cell viability was observed after treatment with PMCC/AP‐EL (Figure 5). The electron image of the hematoxylin and eosin‐stained skin tissue is shown in Figure 5. No difference was observed in the status of the horny cell layer and epidermis of the skin model treated with PMCC/AP‐EL compared with the control; however, in the model treated with Tween 60/‐EL, horny layer exfoliation and gaps between epidermal cells were observed. No changes in the appearance, such as separation and discoloration, of the PMCC/AP‐EL were observed after 1 month of incubation at 25°C, 45°C, and 50°C. After 1 month, the viscosity of the PMCC/AP‐EL was 1407 cps at 25°C, 1435 cps at 45°C, and 1287 cps at 50°C. These findings suggested that in the presence of anionic polymers, PMCC can be a surfactant‐free nanoemulsified formulation that is adequately stable and safe for the skin.
Figure 4.
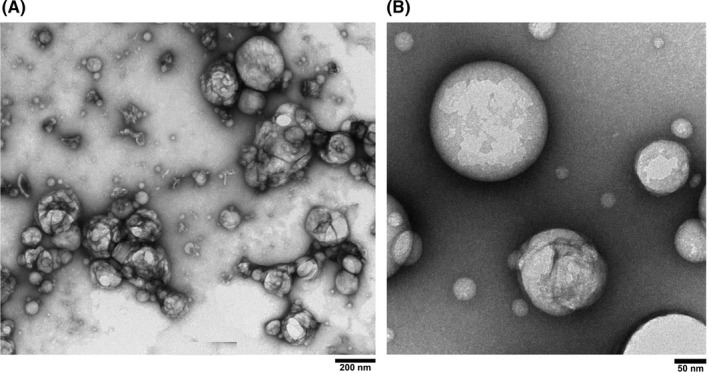
TEM image of (A) PMCC nanoemulsion and (B) Glab‐containing PMCC polymeric micelle
Figure 5.
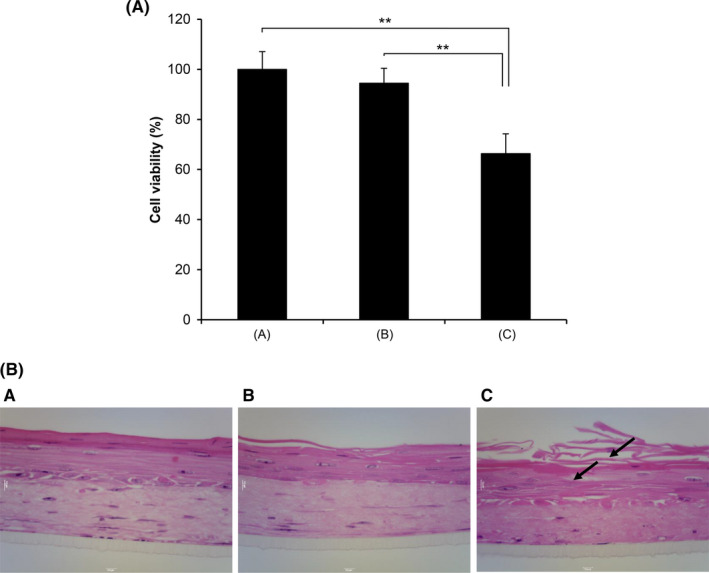
Cytotoxicity in human skin model treated with PMCC nanoemulsified formulation. (a) The cell viability rate in human skin model (TESTSKIN LSE‐high) was measured 72 h after test sample application. (b) Hematoxylin and eosin staining. (A) Control (water). (B) PMCC/nanoemulsified formulation. (C) Tween 60/emulsified formulation. Significant differences are indicated (**P<.01, ANOVA)
3.3. Effects of PMCC/AP‐EL containing antimicrobial agents on IL‐1α production and antibacterial activity
The release of the inflammatory mediator IL‐1α in the epidermis is increased in the early phase of inflammation.14 Figure 6 shows IL‐1α production in PMCC/AP‐EL (Table 1) containing MP or DD as an antimicrobial constituent. IL‐1α production with 0.2% MP and 0.1% DD were approximately 2.0 times and 2.5 times, respectively, higher than that without antimicrobial agent. In contrast, IL‐1α production was not upregulated when PMCC/AP‐EL containing 0.05% DD was added. Further, no reduction in cell viability was observed in any sample. Figure 7 presents the results of the in vitro antimicrobial test of PMCC/AP‐EL with 0.05% DD (PMCC/DD) and PMCC/AP‐EL with 0.2% MP (PMCC/MP). PMCC/DD sterilized almost all MRSA within 120 minutes after application; however, the cell viability in PMCC/MP was approximately 40%. PMCC/DD sterilized P acnes within approximately 30 minutes, whereas PMCC/MP sterilized it within 120 minutes. The antifungal activity against M fungi in PMCC/DD was higher than that in PMCC/MP.
Figure 6.
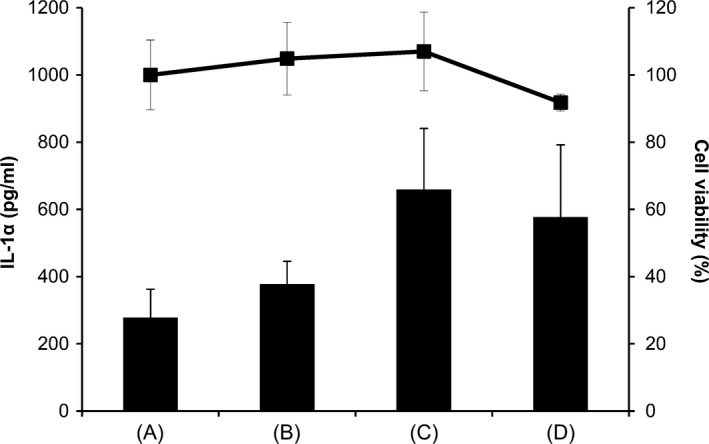
Release of IL‐1α and cytotoxicity in human skin model treated with PMCC emulsified formulation containing an antimicrobial agent. (A) PMCC emulsified formulation (control); (B) Control containing DD (0.05%); (C) Control containing DD (0.1%); and (D) Control containing MP (0.2%)
Figure 7.
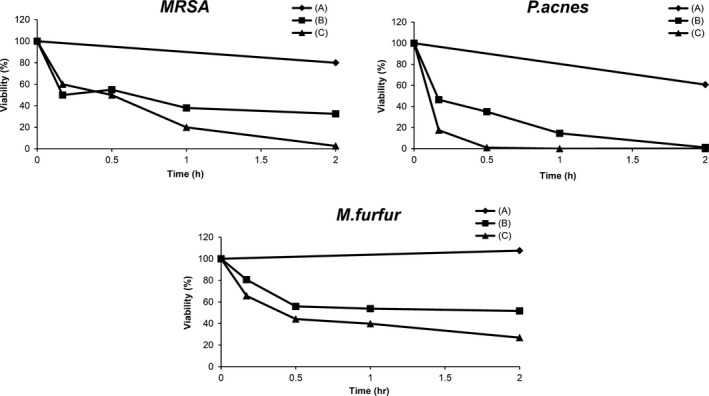
Antibacterial activity of PMCC emulsified formulation containing an antimicrobial agent. (A) Control; (B) PMCC formulation (0.20% MP); and (C) PMCC formulation (0.05% DD)
3.4. Glab/PMCC‐PM effect on the percutaneous transport Glab and melanogenesis inhibition
The TEM images of PM Glab/PMCC‐PM are shown in Figure 4(b). Nanoscale spherical micelles were observed, and the size was in the range of several tens of nanometers. The delivery of Glab from Glab/PMCC‐PM is expected. Glab/PMCC‐PM on the skin model was incubated, and the distribution of Glab in the skin model was examined (Figure 8). For Glab/PMCC‐PM, Glab was mainly present in the epidermis and Glab concentration was approximately > 3.0 times the concentration in the conventional O/W micelle. In contrast, almost no difference in Glab concentration in the dermis was observed between Glab/PMCC‐PM and conventional O/W micelle. Several studies have reported the use of a human skin model (MEL‐300) to evaluate melanogenesis inhibitors.15, 16 With regard to skin surface brightness (Figure 9), an increase in brightness indicates melanogenesis inhibition, thereby reducing the darkening due to melanin production. Glab/PMCC‐PM addition increased the skin surface brightness compared with conventional O/W micelle addition. Further, after 10 days of incubation (Figure 9), Glab/PMCC‐PM addition inhibited the skin surface darkening caused by melanin production.
Figure 8.
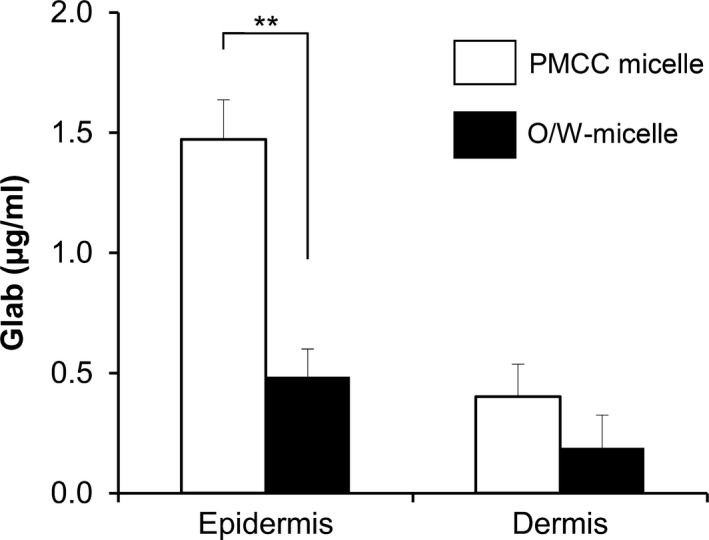
Glab distribution in a reconstituted human skin model. PMCC or O/W micelle was applied on the human skin model (TESTSKIN LSE‐high). Glab distribution of was monitored 24 h after sample application. Significant differences are indicated (**P<.01, t test)
Figure 9.
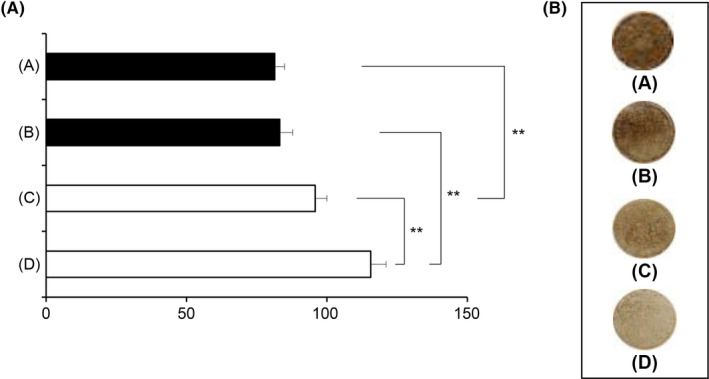
Melanogenesis inhibitory effects of PMCC polymeric micelles containing Glab in a reconstituted human skin model. (a) The test sample was incubated with the human skin model (MEL‐300A) for 14 d. The brightness of the skin surface was assayed. (b) Macroscopic views of a human skin model. (A) Placebo (O/W micelle without Glab). (B) placebo (PMCC micelle without Glab). (C) Control (O/W micelle). (D) PMCC micelle. Significant difference is indicated (**P<.01, ANOVA)
4. DISCUSSION
Chitosan and its derivatives have applications as digestible materials in the biomedical and biotechnological fields and are safe for topical use and as an ingredient in drugs or skin care cosmetics.17, 18 Cationic amphiphilic chitosan derivatives promote the penetration of the hydrophobic pharmaceutical ingredient into the skin as well as retain moisturizing and emulsifying properties; therefore, these derivatives are useful as new materials in cosmetics.19 However, chitosan and these cationic chitosan derivatives form polyion complexes when mixed with anionic polymers, such as CVP and alginate.8, 9 (commonly used in cosmetics), thereby leading to precipitation and de‐emulsification and causing issues in the formulation of skin care products. In contrast, the usefulness of amphoteric‐amphiphilic chitosan derivatives has rarely been evaluated. In the present study, we evaluated the efficacy of PMCC as a new material in cosmetics by assessing its emulsifying capacity in the absence of a surfactant comprising an anionic polymer.
Polyionic complexes of chitosan and CVP result in precipitation via electrostatic interactions between an amino group of the chitosan and carboxylate group of CVP.9 We verified that PMCC did not induce precipitation because of the formation of a polyion complex with the addition of CVP and that it formed a translucent hydrophilic gel. The nanoscale localized fibers was observed in the TEM image (Figure 3), suggesting the involvement of nanofiber formation in achieving viscosity. In this phenomenon, it is presumed that the repulsion of negative charges prevents polymer chain aggregation because the carboxymethyl group also exists in the PMCC molecule, although the amino group in the PMCC molecule and carboxyl group in the CVP electrostatistically interacts with each other. Further experiments are warranted to clarify the mechanism of hydrophilic gel formation.
We investigated the properties of a surfactant‐free PMCC/AP‐EL. No changes in the appearance or physical properties of PMCC/AP‐EL were observed after 1 month of incubation at 45°C and 50°C, indicating its excellent emulsification stability. Presumably, the mechanism of stabilization is that free PMCC, present in the external phase of emulsion particles, and anionic polymer interacts without forming an ion complex, thereby preventing the aggregation or coalescence of emulsion particles. The results of the in vitro safety test using the three‐dimensional human skin model demonstrated that PMCC/AP‐NEL did not decrease skin cell viability and horny layer exfoliation, which were observed when using Tween 60 (Figure 5). Nonionic surfactant exhibits lower toxicity than anionic and cationic surfactants.20 Although Tween 60 is a safe nonionic surfactant, it affects the lipid bilayers of the epidermis,11 causing damage to the skin tissue, such as a decrease in cell viability and horny exfoliation. In contrast, PMCC, which forms stable polymeric micelles, causes no disturbance to this lipid bilayer, suggesting that it has no damaging effect. The skin barrier function is reduced in sensitive skin and skin affected by atopic dermatitis, rendering them sensitive to stimulation.21 To address this issue, safer emulsified formulations should be developed. PMCC, which can be mixed with anionic polymers, is expected to be widely applied to surfactant‐free emulsified formulations for improving skin safety.
Parabens, such as MP, exhibit antibacterial activity and are used as preservatives in pharmaceutical and cosmetic products. However, recent studies that used cultured human skin cells reported that parabens have effects on the reduction of cell viability, induction of apoptosis, and reduction of collagen metabolism.22, 23 1,2‐Alkanediols, such as DD, exhibit antibacterial activity and can act as cosmetic water‐soluble antimicrobial agents because of their low toxicity and water solubility. In the present study, the human skin model test demonstrated that PMCC/AP‐EL with 0.05% DD did not upregulate IL‐1α production, which was observed in the presence of 0.2% MP (Figure 6). Nevertheless, its antibacterial activities against MRSA and P acnes were enhanced compared with the MP formulation (Figure 7). Reportedly, S aureus, such as MRSA, and P acnes are some of the causes of exacerbations in atopic dermatitis and inflammatory acne vulgaris, respectively.21, 24 The addition of DD to PMCC/AP‐EL may simultaneously achieve antibacterial activity against the microorganisms involved in the worsening of dermatitis. Further studies on the antimicrobial efficacy of DD are necessary, and their results may be useful for the treatment and prevention of dermatosis related to microbial infections.
Glab, a hydrophobic flavonoid obtained from licorice extract, is a powerful inhibitor of tyrosinase activity, which has antimelanogenic activity.6 The results of the testing on this human skin model comprising melanocytes in its basal layer suggest that the brightness of the skin is increased with polymeric micelles containing Glab formed from PMCC (Glab/PMCC‐PM) compared with the nanomicelles formed from Tween 60 (Figure 9). Therefore, Glab/PMCC‐PM appears to exhibit an excellent inhibitory activity against melanogenesis. Polymeric micelles formed from PMCC resulted in an approximately 3‐fold increase in Glab concentration in the epidermis (Figure 8), suggesting an enhanced capacity to reach melanocytes localized in the basal layer of the epidermis. At a physiological pH, the human stratum corneum is negatively charged and is cation permselective13; therefore, cationic polymer micelles with an interface rich in charged amino groups can enhance the percutaneous delivery of Glab to the human skin model.7 Recently, amphoteric carboxymethyl chitosan has been shown to exhibit excellent biocompatibility and controllable biodegradability.25 From the human skin model test, the affinity to the stratum corneum of PMCC was superior (data not shown). From these findings, it is assumed that the surfaces of polymeric micelles, formed from amphoteric PMCC, contain amino and carboxymethyl groups, possibly enhancing the penetration of Glab into the epidermis via the interaction of these groups with keratin in the stratum corneum. Further research is warranted to clarify the mechanism of Glab/PMCC‐PM‐mediated increased Glab penetration.
In conclusion, we suggest that the use of the amphoteric‐amphiphilic chitosan derivative PMCC may facilitate the development of a surfactant‐free emulsified formulation that is safe for the skin. PMCC could be widely used in cosmetics because of its ability to coexist with anionic polymers, such as CVP. Our results demonstrated that a polymeric emulsion prepared using PMCC, in combination with Glab as an antimelanogenic agent or DD as an antimicrobial agent, is an effective cosmetic product for skin care.
CONFLICT OF INTEREST
Pias Corporation manufactures and sells skin care products containing amphiphilic chitosan derivatives. A patent containing a part of this work is under application [2008‐222970 (Japan)]. There are no funder patents, product in development or marketed products to declare.
AUTHOR CONTRIBUTIONS
HS, NK, YA, and NN contributed to conceptualization. HS, NK, and YA contributed to data curation. HS, NK, YA, NO, and NN contributed to formal analysis. KH contributed to funding acquisition. HS, NK, YA, NO, and NN contributed to investigation. HS, NK, YA, and NN contributed to methodology. KH contributed to project administration. KH contributed to resources. HS contributed to supervision. HS, NK, and YA contributed to visualization. HS and NK contributed to roles/writing‐original draft. NO and NN contributed to writing‐review and editing.
ETHICAL APPROVAL
Research involving cell lines: Reconstituted commercial skin models (TESTSKIN LSE‐high) were purchased from Toyobo Co. Ltd., Osaka, Japan. A commercial three‐dimensional human skin equivalent (MEL‐300‐A) was purchased from MatTek Corp., Ashland, MA, USA There was no requirement for ethics approval for the use of these human cells.
ACKNOWLEDGMENTS
We thank Professor Hiroshi Uyama (Osaka University, Department of Applied Chemistry, Graduate School of Engineering) for the useful suggestions and discussions. We would like to thank Enago (www.enago.jp) for the English language review.
Seino H, Kawaguchi N, Arai Y, Ozawa N, Hamada K, Nagao N. Investigation of partially myristoylated carboxymethyl chitosan, an amphoteric‐amphiphilic chitosan derivative, as a new material for cosmetic and dermal application. J Cosmet Dermatol.2021;20:2332–2340. 10.1111/jocd.13833
Investigation of partially myristoylated carboxymethyl chitosan, an amphoteric‐amphiphilic chitosan derivative, as a new material for cosmetic and dermal application.
Contributor Information
Haruyoshi Seino, Email: hseino@pias.co.jp.
Norio Nagao, Email: nagao@pu-hiroshima.ac.jp.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1.Casadidio C, Peregrina DV, Gigliobianco MR, Deng S, Censi R, Di Martino P. Chitin and chitosans: characteristics, eco‐friendly processes, and applications in cosmetic science. Mar Drugs. 2019;17:369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hamedi H, Moradi S, Hudson SM, Tonelli AE. Chitosan based hydrogels and their applications for drug delivery in wound dressings: a review. Carbohydr Polym. 2018;199:445‐460. [DOI] [PubMed] [Google Scholar]
- 3.Verlee A, Mincke S, Stevens CV. Recent developments in antibacterial and antifungal chitosan and its derivatives. Carbohydr Polym. 2017;164:268‐283. [DOI] [PubMed] [Google Scholar]
- 4.Som I, Bhatia K, Yasir M. Status of surfactants as penetration enhancers in transdermal drug delivery. J Pharm Bioallied Sci. 2012;4:2‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang C, Ding Y, Yu LL, Ping Q. Polymeric micelle systems of hydroxycamptothecin based on amphiphilic N‐alkyl‐N‐trimethyl chitosan derivatives. Colloids Surf B Biointerfaces. 2007;55:192‐199. [DOI] [PubMed] [Google Scholar]
- 6.Yokota T, Nishio H, Kubota Y, Mizoguchi M. The inhibitory effect of glabridin from licorice extracts on melanogenesis and inflammation. Pigment Cell Res. 1998;11:355‐361. [DOI] [PubMed] [Google Scholar]
- 7.Seino H, Arai Y, Nagao N, Ozawa N, Hamada K. Efficient percutaneous delivery of the antimelanogenic agent glabridin using cationic amphiphilic chitosan micelles. PLoS One. 2016;11:e0164061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matsumoto T, Kawai M, Masuda T. Rheological properties and fractal structure of concentrated polyion complexes of chitosan and alginate. Biorheology. 1993;30:435‐441. [DOI] [PubMed] [Google Scholar]
- 9.Park SH, Chun MK, Choi HK. Preparation of an extended‐release matrix tablet using chitosan/carbopol interpolymer complex. Int J Pharm. 2008;347:39‐44. [DOI] [PubMed] [Google Scholar]
- 10.Lee E, An S, Cho SA, et al. The influence of alkane chain length on the skin irritation potential of 1,2‐alkanediols. Int J Cosmet Sci. 2011;33:421‐425. [DOI] [PubMed] [Google Scholar]
- 11.Endo M, Yamamoto T, Ijuin T. Effect of nonionic surfactants on the percutaneous absorption tenoxicam. Biorheology Chem Pharm Bull (Tokyo). 1996;44:865‐867. [DOI] [PubMed] [Google Scholar]
- 12.Moore J. Final Report on the Safety Assessment of Polysorbates 20, 21, 40, 60, 61, 65, 80, 81, and 85. J Am Coll Toxicol. 1984;3:1‐82. [Google Scholar]
- 13.Nokhodchi A, Shokri J, Dashbolaghi A, Hassan‐Zadeh D, Ghafourian T, Barzegar‐Jalali M. The enhancement effect of surfactants on the penetration of lorazepam through rat skin. Int J Pharm. 2003;250:359‐369. [DOI] [PubMed] [Google Scholar]
- 14.Han YP, Downey S, Garner WL. Interleukin‐1α‐induced proteolytic activation of metalloproteinase‐9 by human skin. Surgery. 2005;138:932‐939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goh MJ, Park JS, Bae JH, Kim DH, Kim HK, Na YJ. Effects of ortho‐dihydroxyisoflavone derivatives from Korean fermented soybean paste on melanogenesisin B16 melanoma cells and human skin equivalents. Phytother Res. 2012;26:1107‐1112. [DOI] [PubMed] [Google Scholar]
- 16.Fujimori H, Hisada M, Shibayama H, Kawase A, Iwaki M. Inhibitory effects of phytoncide solution on melanin biosynthesis. Biosci Biotech Biochem. 2010;74:918‐922. [DOI] [PubMed] [Google Scholar]
- 17.Aranaz I, Acosta N, Civera C, et al. Cosmetics and cosmeceutical applications of chitin, chitosan and their derivatives. Polymers. 2018;10:213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Afonso CR, Hirano RS, Gaspar AL, et al. Biodegradable antioxidant chitosan films useful as an anti‐aging skin mask. Int J Biol Macromol. 2019;132:1262‐1273. [DOI] [PubMed] [Google Scholar]
- 19.Seino H, Ozawa N, Akamasu E, Hamada K, Nonaka K, Yoshioka H. Usefulness of new chitosan derivative forming polymer emulsion as cosmetic ingredients. J Jpn Cosmet Sci Soc. 2002;26:71‐78. [Google Scholar]
- 20.Welss T, Basketter DA, Schroder KR. In vitro skin irritation: facts and future. State of the art review of mechanisms and models. Toxicol Vitro. 2004;18:231‐243. [DOI] [PubMed] [Google Scholar]
- 21.Nakatsuji T, Chen TH, Two AM, et al. Staphylococcus aureus exploits epidermal barrier defects in atopic dermatitis to trigger cytokine expression. J Invest Dermatol. 2016;136:2192‐2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Majewska N, Zareba I, Surazynski A, Galicka A. Methylparaben‐induced decrease in collagen production and viability of cultured human dermal fibroblast. J Appl Toxicol. 2017;37:1117‐1124. [DOI] [PubMed] [Google Scholar]
- 23.Dubey D, Chopra D, Singh J, et al. Photosensitized methyl paraben induces apoptosis via caspase dependent pathway under ambient UVB exposure in human skin cells. Food Chem Toxicol. 2017;108:171‐185. [DOI] [PubMed] [Google Scholar]
- 24.Nakatsuji T, Kao MC, Fang J‐Y, et al. Antimicrobial property of lauric acid against Propionibacterium acnes: its therapeutic potential for inflammatory acne vulgaris. J Invest Dermatol. 2009;129:2480‐2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shariatinia Z. Carboxymethyl chitosan: properties and biomedical applications. Int J Biol Macromol. 2018;120:1406‐1419. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


