Abstract
Aims
Arginine vasopressin (AVP) mediates deleterious effects via vascular V1a and renal V2 receptors in heart failure (HF). Despite positive short‐term decongestive effects in phase II HF studies, selective V2 receptor antagonism has shown no long‐term mortality benefit, potentially related to unopposed V1a receptor activation. We compared the novel dual V1a/V2 receptor antagonist pecavaptan with the selective V2 receptor antagonist tolvaptan in pre‐clinical HF models.
Methods and results
In vitro IC50 determination in recombinant cell lines revealed similar receptor selectivity profiles (V2:V1a) of tolvaptan and pecavaptan for human and dog AVP receptors, respectively. Two canine models were used to compare haemodynamic and aquaretic effects: (i) anaesthetised dogs with tachypacing‐induced HF, and (ii) conscious telemetric dogs with a non‐invasive cardiac output (CO) monitor. Tolvaptan and pecavaptan exhibited no differences in urinary output. In HF dogs, pecavaptan counteracted the AVP‐induced increase in afterload and decrease in CO (pecavaptan: 1.83 ± 0.31 L/min; vs. tolvaptan: 1.46 ± 0.07 L/min, P < 0.05). In conscious telemetric animals, pecavaptan led to a significant increase in CO (+0.26 ± 0.17 L/min, P = 0.0086 vs. placebo), in cardiac index (+0.58 ± 0.39 L/min/m2, P = 0.009 vs. placebo) and a significant decrease in total peripheral resistance (−5348.6 ± 3601.3 dyn × s/cm5, P < 0.0001 vs. placebo), whereas tolvaptan was without any significant effect.
Conclusions
Simultaneous blockade of vascular V1a and renal V2 receptors efficiently induces aquaresis and counteracts AVP‐mediated haemodynamic aggravation in HF models. Dual V1a/V2 antagonism may lead to improved outcomes in HF.
Keywords: Animal model, Heart failure, Pecavaptan, Vasopressin antagonist, V1a receptor, V2 receptor
Graphical abstract demonstrating the beneficial effect of dual vasopressin antagonism. AVP, arginine vasopressin; CO, cardiac output; TPR, total peripheral resistance; UV, urine volume.
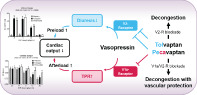
Introduction
Most hospitalisations for acute heart failure (HF) are due to signs and symptoms of congestion and fluid overload.1 Once patients are hospitalised for HF exacerbation, mortality and re‐admission rates are very high in the subsequent 60–90 days (up to 15% and 30%, respectively).2 Loop diuretics are the main treatment for patients with acute HF but are associated with neurohormonal stimulation and worsening renal function, and they do not improve long‐term outcomes.3 Therefore, adequate management of congestion while
offering renal protection remains a relevant target in the treatment of HF. Antagonists to arginine vasopressin (AVP) may provide an alternative decongestive strategy.
Arginine vasopressin acts via three different receptors: the V1a, V1b and V2 receptors. The vasopressin V2 receptor is located in the renal collecting duct, and binding of AVP mediates antidiuresis via the aquaporin‐2 system and blockade of the V2 receptor causes free water excretion (aquaresis).4, 5, 6 Tolvaptan, a selective V2 receptor antagonist given as adjunctive therapy to loop diuretics, produced a significant decongestive effect without worsening renal function while patients were hospitalised for HF. However, there was no benefit of tolvaptan on mortality or morbidity in the long‐term phase of the EVEREST phase III acute HF trial.7, 8 Given the selective V2 receptor blockade by tolvaptan, a compensatory increase in plasma AVP levels has been hypothesised to result in enhanced agonism at the vascular smooth muscle cell V1a receptor, with potentially adverse myocardial, renal and vascular effects.9 Thus, these unfavourable effects mediated by V1a receptor activation may have offset any favourable effects of renal protective decongestion by blocking the V2 receptor.
Recently, pecavaptan (BAY 1753011) was discovered as a novel, dual‐acting V1a/V2 receptor antagonist with oral bioavailability.10 Pecavaptan has almost identical affinities (Ki values) of 0.5 and 0.6 nM for the human V1a and V2 receptor, respectively, and thus a 1:1 receptor profile in vitro. We aimed to investigate the canine AVP receptor selectivity profile of pecavaptan in vitro before initiating in vivo studies in different pre‐clinical dog models. We subsequently investigated whether dual V1a/V2 blockade mediated by pecavaptan provides haemodynamic benefits in addition to aquaresis in two different large‐animal models.
Methods
Ethics statement
All study procedures conformed to the current national legislation [German protection of animals act (18 May 2006), last amended by article 4 paragraph 87 (BGBl. I S. 1666) on 18 July 2016] and EU directive 63/2010 (on the protection of animals used for scientific purposes). All performed studies were approved by the competent regional regulatory authority (LANUV NRW in Duesseldorf, Germany) and by the institutional animal care office of Bayer AG.
Cell‐based assays
Cell culture experiments describing cell lines and IC50 determination in recombinant CHO (Chinese Hamster Ovary, CHO K1; American Type Culture Collection, Manassas, VA, USA) cells stably expressing human V1a and V2 receptors are described in Kolkhof et al.10 Recombinant CHO cell lines expressing the cloned dog V1a and V2 receptors were established accordingly. In the case of the Gq‐coupled canine V1a receptor, cells were also stably transfected with a modified form of the calcium‐sensitive photoprotein obelin (dog V1a), which, after reconstitution with the cofactor coelenterazine, emits light when free intracellular calcium concentrations increase.11 The Gs‐coupled canine V2 receptor was stably transfected into a CHO cell line expressing the gene for firefly luciferase under control of a CRE‐responsive promoter. Activation of human and dog V2 receptors induces the activation of the CRE‐responsive promoter via cAMP increase, thereby inducing the expression of firefly luciferase. The light emitted by photoproteins of V1a cell lines as well as the light emitted by firefly luciferase of V2 cell lines corresponds to the activation or inhibition of the respective AVP receptor. The bioluminescence of the cell lines is detected using a suitable luminometer. All cell culture and test conditions were performed as described by Kolkhof et al.10
Canine tachypacing‐induced model of heart failure
Pecavaptan and tolvaptan were investigated in anaesthetised dogs with HF based on a previously reported protocol.12
On Day 0, six dogs were anaesthetised (initial anaesthesia: thiopental sodium 0.25–0.5 mg/kg, Trapanal®, Byk Gulden, Germany); maintenance: isoflurane (1.0–2.0%; Baxter, Germany), intubated and ventilated (Avance® anaesthesia ventilator, GE Healthcare, Germany; applying 30% oxygen) to implant a pacemaker lead (Setrox S60, Biotronik, Germany) into the right ventricle. After connecting the pacemaker lead to the pacemaker (Logos®, Biotronik, Germany), intracardiac signals were measured to ensure correct lead placement. For analgesia, fentanyl (10–40 µg/kg per hour; Mallinckrodt Inc.) was infused intravenously during the implantation procedure. In the wound‐healing phase, all animals were treated with parenteral antibiotic (Chlerobe, Pfizer, USA; subcutaneous) and analgesic (Metamizole WDT, WDT, Germany; 50 mg/kg; intramuscular). After wound healing (Day 14), the pacemakers were activated, and the right ventricles continuously stimulated with 220 bpm for 28 days. To investigate the tachypacing‐induced changes in heart function and dimensions, echocardiography (Vivid‐I, GE Healthcare, Germany) investigations were performed under healthy conditions and after 28 days of pacing.
After 28 days of pacing (Day 42), animals were studied to investigate systemic and cardiac haemodynamics and urine volume responses to an intravenous (i.v.) bolus of tolvaptan (0.01, 0.03, 0.1 and 0.3 mg/kg) or pecavaptan (0.01, 0.03, 0.1 and 0.3 mg/kg). Under sterile conditions the animals were instrumented with femoral artery access to measure arterial blood pressure via a NaCl 0.9%‐filled sheath introducer (Cordis®, Waterloo, Belgium), and left ventricular performance was assessed using electrocardiogram (ECG) and a 5F‐microtip catheter (Millar Instruments Inc., USA). Through an axillary vein, a Swan–Ganz catheter (CCOmbo® with Vigilance® monitor, Edwards Lifesciences, USA) was introduced to measure cardiac output (CO). All data were recorded and analysed with Ponemah software (Data Sciences International, USA). After completion of the instrumentation, AVP (Sigma‐Aldrich, Darmstadt, Germany) was given as an i.v. infusion at a rate of 4 mU/kg/min. The animals were further randomised to receive tolvaptan (n = 3) or pecavaptan (n = 3) (Figure 1).
Figure 1.
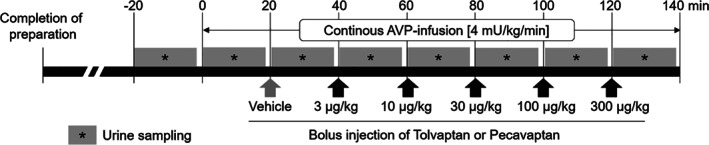
Study design using a canine tachypacing model of heart failure. AVP, arginine vasopressin.
Pecavaptan and tolvaptan were dissolved in dimethyl sulfoxide (1%, Sigma), polyethylene glycol (49.5%, Sigma) and transcutol (49.5%, Sigma). Vehicle (only solvent) and cumulative dosages of pecavaptan or tolvaptan were administrated by i.v. bolus injection 20 min after the AVP infusion was started. Haemodynamic parameters were recorded over 20 min after i.v. bolus injection.
Non‐invasive telemetric cardiac output monitoring in conscious dogs
A second study was performed to evaluate efficacy in the conscious state. Nine healthy beagle dogs (Marshall BioResources, USA) were instrumented with implantable telemetry sensors (Model L21, Data Sciences International, USA) for haemodynamic assessment. Therefore, animals were anaesthetised with thiopental sodium (0.25–0.5 mg/kg to effect; Trapanal, Byk Gulden, Germany). During implantation procedures, animals were intubated and mechanically ventilated with 30% oxygen (Avance® anaesthesia ventilator, GE Healthcare, Germany). Anaesthesia supplementation was provided by isoflurane (1.0–2.0%; Baxter, Germany) and administered via anaesthetic gas. For analgesia, fentanyl (10–40 µg/kg/h) was infused through the cephalic vein. After left‐sided thoracotomy, a pressure catheter was placed in the thoracic aorta to measure blood pressure. A second pressure catheter was inserted into the apex of the left ventricle to measure left ventricular pressure. Furthermore, electrodes were placed in apical and basal positions of the heart for continuous ECG recording. The electronic housing of the device was placed submuscular on the left thorax side. For telemetric data capture and data analysis Ponemah software (Version 5.2, Data Sciences International, USA) was used.
On the day of the study, additional surface sensors (CHEETAH NICOM™, USA) were placed for non‐invasive CO monitoring. The NICOM technology (Non‐Invasive Cardiac Output Monitoring) has been previously validated by Heerdt et al.13 for the measurement of drug‐induced changes of CO in dogs. Sensors were placed on the backs of the dogs and CO was measured continuously every 30 s. Additional bladder catheters were placed to investigate the V2‐mediated (diuretic) effects by determination of urine volume within each intervention (Figure 2). One hour before the telemetric measurements started, animals were orally pre‐treated [as solution in gelatine capsules (HGK 15‐30, Krüger, Germany)] with vehicle (0.5 mL/kg of PEG 400, Sigma‐Aldrich Chemie GmbH, Germany), pecavaptan (3 mg/kg in vehicle) or tolvaptan (10 mg/kg in vehicle). The respective doses of pecavaptan and tolvaptan were determined as matching aquaretic efficacy in previous pilot studies.
Figure 2.
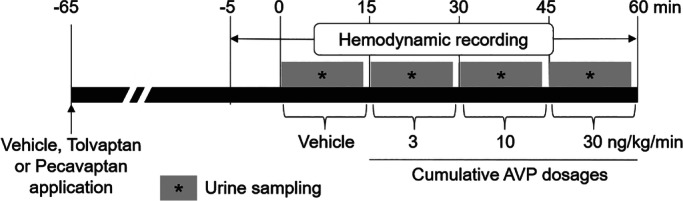
Study design for cardiac output monitoring in conscious telemetric dogs. AVP, arginine vasopressin.
Afterwards, all dogs were treated with vehicle (physiological NaCl, B. Braun, Germany) and cumulative dosages (3, 10 and 30 ng/kg/min; 15 min of i.v. infusion per dose) of AVP (Sigma‐Aldrich, Germany) to mimic elevated AVP levels in patients with HF. CO (L/min), cardiac index (CI; L/min/m2), total peripheral resistance (TPR, dyn × s/cm5), HR (bpm), blood pressure (mmHg) and contractility (+dP/dt, mmHg/s) were measured continuously. In this crossover‐designed study, each dog was treated on individual days with placebo, tolvaptan and pecavaptan. A washout period of one week was applied between experiments.
In both studies, urine and blood parameters were measured by standard methods by a clinical‐chemical analyser system (ADVIA 2400, Siemens).
Data analysis and statistical calculations
In the tachypacing‐induced HF model, for each animal and intervention (control, vehicle, tolvaptan or pecavaptan) haemodynamic data were analysed and averaged between 15 and 20 min post bolus injection. TPR was calculated as the product of mean blood pressure multiplied by 80, divided by CO. The percentage difference compared with the individual baseline measurements (controls) were calculated and expressed as mean data of n = 3 animals ± standard error of the mean (SEM). Urine was collected over a period of 20 min for every animal and intervention and expressed as mean data ± SEM. The corresponding dosage groups (tolvaptan and pecavaptan) were statistically analysed. Group differences (tolvaptan vs. pecavaptan) were analysed using a Student's unpaired t‐test. An alpha‐level of <0.05 was used to indicate statistical significance.
In conscious animals, haemodynamic data were analysed and averaged between 13 and 15 min post start of infusion for every AVP dose. In case of an inadequate online evaluation, the stored data could be evaluated offline. Data generated with signal noise, disrupted transmission or other artefacts were not considered for evaluation. TPR was calculated as described before and CI was analysed by the quotient of CO and the body surface area. Urine was collected over an individual period of 15 min, starting at the beginning of the individual interventions (control, placebo or AVP). All data were expressed as averaged absolute values of n = 9 animals ± SEM in graphics or standard deviation in text. Treatment groups were compared to corresponding individuals in the placebo group and were analysed using a repeated‐measure one‐way ANOVA and Tukey multiple comparison test. A P‐value of <0.05 was defined as demonstrating statistical significance.
In both studies, the pre‐defined study goal was a statistically significant improvement in CO and/or TPR in comparison to the reference group (placebo or tolvaptan).
Results
In vitro
IC50 determination at recombinant cell lines stably expressing the human and canine AVP receptors revealed similar individual receptor selectivity profiles for pecavaptan and tolvaptan. Table 1 shows that pecavaptan has a selectivity profile of 2:1 and 3:1 at human and dog receptors, respectively (i.e. slightly in favour of V2 receptors), based on IC50 values. In contrast, tolvaptan was found to possess a 19:1 selectivity profile at human V2:V1a receptors and has a 10:1 selectivity profile at dog V2:V1a receptors.10
Table 1.
IC50 values of pecavaptan and tolvaptan at human and canine vasopressin receptors determined in functional cell‐based assays
| Species | Pecavaptan | Tolvaptan |
|---|---|---|
| V1a IC50 (nM) | ||
| Human | 3.6 | 21 |
| Dog | 4.4 | 14 |
| V2 IC50 (nM) | ||
| Human | 1.7 | 1.1 |
| Dog | 1.3 | 1.4 |
IC50, half maximal inhibitory concentration.
In vivo
After confirmation of similar profiles at human and canine receptors, dual V1a/V2 receptor antagonism mediated by pecavaptan and selective V2 receptor antagonism mediated by tolvaptan were investigated in two pre‐clinical canine models: (a) an established experimental setup in tachypacing‐induced HF with application of exogenous AVP in anaesthetised dogs, and (b) oral application of pecavaptan and tolvaptan to conscious dogs with implantable and wearable devices under AVP infusion.
Tachypacing‐induced heart failure in dogs
Tachypaced dogs were investigated by invasive catheter‐based methods before drug treatment. Online supplementary Table S1 shows that all HF dogs developed a significant increase in filling pressure (left ventricular end‐diastolic pressure), as well as a decline in contractility (+dP/dt) and relaxation (−dP/dt) compared with healthy animals. Echocardiographic investigations (Table 2) confirmed the expected changes in heart function (decrease in ejection fraction and fractional shortening) and dimensions (increase in left ventricular end‐systolic and end‐diastolic volume) without any significant group differences.
Table 2.
Echocardiographic assessment at healthy condition and after 28 days of right ventricular pacing
| Characteristic | Healthy | Heart failure | ||
|---|---|---|---|---|
| Tolvaptan group | Pecavaptan group | Tolvaptan group | Pecavaptan group | |
| No. of animals | 3 | 3 | 3 | 3 |
| HR (bpm) | 88 ± 8 | 103 ± 6 | 94 ± 11 | 89.6 ± 8.0 |
| IVSd (cm) | 0.8 ± 0.1 | 0.8 ± 0.1 | 0.8 ± 0.1 | 0.8 ± 0.1 |
| LVPWd (cm) | 0.6 ± 0.1 | 0.7 ± 0.1 | 0.7 ± 0.1 | 0.7 ± 0.1 |
| LVIDd (cm) | 2.8 ± 0.2 | 2.7 ± 0.1 | 3.3 ± 0.1* | 3.3 ± 0.1* |
| LVIDs (cm) | 1.8 ± 0.2 | 1.8 ± 0.1 | 2.8 ± 0.2** | 2.7 ± 0.1*** |
| EDV (mL) | 29.3 ± 5.4 | 27.0 ± 2.9 | 45.6 ± 0.9* | 44.8 ± 0.6* |
| ESV (mL) | 10.7 ± 2.1 | 10.3 ± 0.5 | 30.1 ± 4.3* | 28.3 ± 1.3** |
| EF (%) | 64.0 ± 0.8 | 62.3 ± 1.7 | 34.0 ± 8.0* | 37.4 ± 3.9* |
| FS (%) | 33.3 ± 0.5 | 32.0 ± 1.6 | 15.8 ± 4.1* | 17.4 ± 1.7* |
| SV (mL) | 18.7 ± 3.4 | 15.7 ± 1.2 | 15.5 ± 3.4 | 16.9 ± 2.1 |
EDV, end‐diastolic volume; EF, ejection fraction; ESV, end‐systolic volume; FS, fractional shortening; HR, heart rate; IVSd, interventricular septum thickness at end diastole; LVPWd, left ventricular posterior wall thickness at end diastole; LVIDd, left ventricular internal dimensions at end diastole; LVIDs, left ventricular internal dimensions at end systole; SV, stroke volume.
Data are expressed as mean of n = 3 ± standard deviation. *P < 0.05, **P < 0.01, ***P < 0.001 compared to respective healthy group.
Intravenous bolus injection of tolvaptan and pecavaptan counteracted the antidiuretic activity of AVP infusion and induced dose‐dependent increases in urine volume with equal efficacy on urinary output (UOP) and without any significant difference between these two treatments (Figure 3A).
Figure 3.
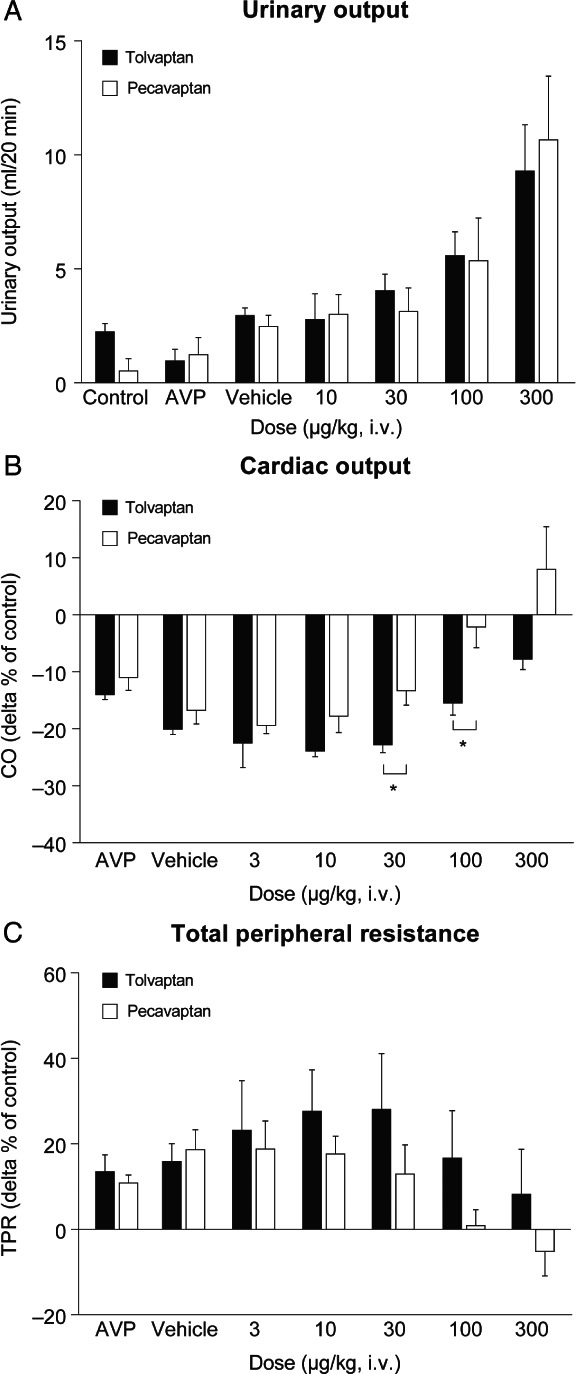
Diuretic (A) and haemodynamic (B, C) effects of pecavaptan and tolvaptan during continuous arginine vasopressin (AVP) infusion in anaesthetised heart failure dogs. All data are mean of n = 3 ± standard error of the mean. CO, cardiac output; i.v., intravenous; TPR, total peripheral resistance. *P < 0.05.
Intravenous infusion of AVP led to a CO decrease of −14.4 ± 0.90% in the tolvaptan group and −11.41 ± 3.35% in the pecavaptan group (compared with baseline measurements; Figure 3B). Pecavaptan fully counteracted the decrease in CO, whereas tolvaptan exhibited only a slight trend of improvement in the 100 and 300 µg/kg doses. In comparison with tolvaptan, pecavaptan was significantly (P < 0.05) superior in the 30 and 100 µg/kg doses in protecting from AVP‐mediated CO decrease. Similarly, infusion of AVP led to an increase in TPR (+13.9 ± 6.2%, tolvaptan group; +11.3 ± 2.5%, pecavaptan group) and cumulative dosages of pecavaptan counteracted these V1a‐mediated effects in a statistically significant manner (P = 0.011) vs. tolvaptan (tolvaptan: 4412.3 ± 358.8 dyn × s/cm5; pecavaptan: 3287.4 ± 247.8 dyn × s/cm5; 300 µg dose).
There were no differences between the tolvaptan group and the pecavaptan group in plasma sodium, osmolality, potassium and creatinine, indicating that neither plasma electrolyte homeostasis nor renal function was differentially modified (online supplementary Figure S1).
Conscious telemetric dogs with cardiac output monitor
Telemetry devices were successfully implanted, and all sensors delivered accurate physiological biopotential (ECG) or pressure (blood pressure and left ventricular pressure) signals during the whole study. Non‐invasive CO monitoring by bioimpedance measurement also delivered plausible results with respect to baseline data and during AVP infusion. All single experiments were in accordance with the study protocol and no dropouts were registered.
Oral pre‐treatment with placebo did not lead to any changes in urinary volume in the time course of the acute study. In comparison, pre‐treatment with pecavaptan as well as tolvaptan was able to significantly (P < 0.05, in comparison with placebo group) increase UOP during exogenous AVP infusion with 3 and 10 ng/kg/min, but not with 30 ng/kg/min (Figure 4A). In Figure 4B, the V1a‐mediated effects of AVP after placebo, pecavaptan or tolvaptan are shown. No differences during control conditions were observed. Increasing dosages of AVP led to a dose‐dependent decrease in CO (3 ng/kg/min: −14.7 ± 19.8%; 10 ng/kg/min: −33.0 ± 13.3%; 30 ng/kg/min: −36.2 ± 14.4%, compared with control measurements) in the placebo group. Tolvaptan led to a non‐significant trend towards higher CO values (3 ng/kg/min: +0.09 ± 0.19 L/min; 10 ng/kg/min: +0.15 ± 0.12 L/min; 30 ng/kg/min: +0.07 ± 0.13 L/min; compared with corresponding placebo measurements). In contrast, oral pre‐treatment with pecavaptan significantly counteracted the AVP‐induced changes in CO (3 ng/kg/min AVP: +0.19 ± 0.26 L/min, non‐significant; 10 ng/kg/min AVP: +0.32 ± 0.21 L/min, P = 0.002; 30 ng/kg/min AVP: + 0.26 ± 0.17 L/min, P = 0.0086; compared with corresponding placebo measurements). In the placebo group, AVP infusion led to a dose‐dependent decrease in CI (3 ng/kg/min AVP: −14.3 ± 20.3%, 10 ng/kg/min AVP: −32.5 ± 13.4%, 30 ng/kg/min AVP: −35.6 ± 13.4%; compared with control measurements), whereas pecavaptan — but not tolvaptan — could protect from a respective decline in CI (3 ng/kg/min AVP: +0.46 ± 0.64 L/min/m2; 10 ng/kg/min AVP: +0.75 ± 0.53 L/min/m2; 30 ng/kg/min AVP: +0.58 ± 0.39 L/min/m2; compared with corresponding placebo group). Similar to observations from the experimental setup in anaesthetised dogs, AVP infusion led to the expected increase in TPR in the placebo group, which peaked with 14 323 ± 4883.57 dyn × s/cm5 in the 30 ng/kg/min AVP dose. Pre‐treatment with pecavaptan led to a significant TPR decrease (3 ng/kg/min AVP: −2054.3 ± 2864.64 dyn × s/cm5; 10 ng/kg/min AVP: −4533.4 ± 3078.8 dyn × s/cm5, P < 0.0001; 30 ng/kg/min AVP: −5348.6 ± 3601.3 dyn × s/cm5, P < 0.0001; compared with corresponding placebo group), whereas tolvaptan was without a statistically significant effect here (3 ng/kg/min AVP: −1091.3 ± 1925.1 dyn × s/cm5; 10 ng/kg/min AVP: −2597.3 ± 2886.2 dyn × s/cm5; 30 ng/kg/min AVP: −2541.4 ± 2634.3 dyn × s/cm5; compared with corresponding placebo group).
Figure 4.
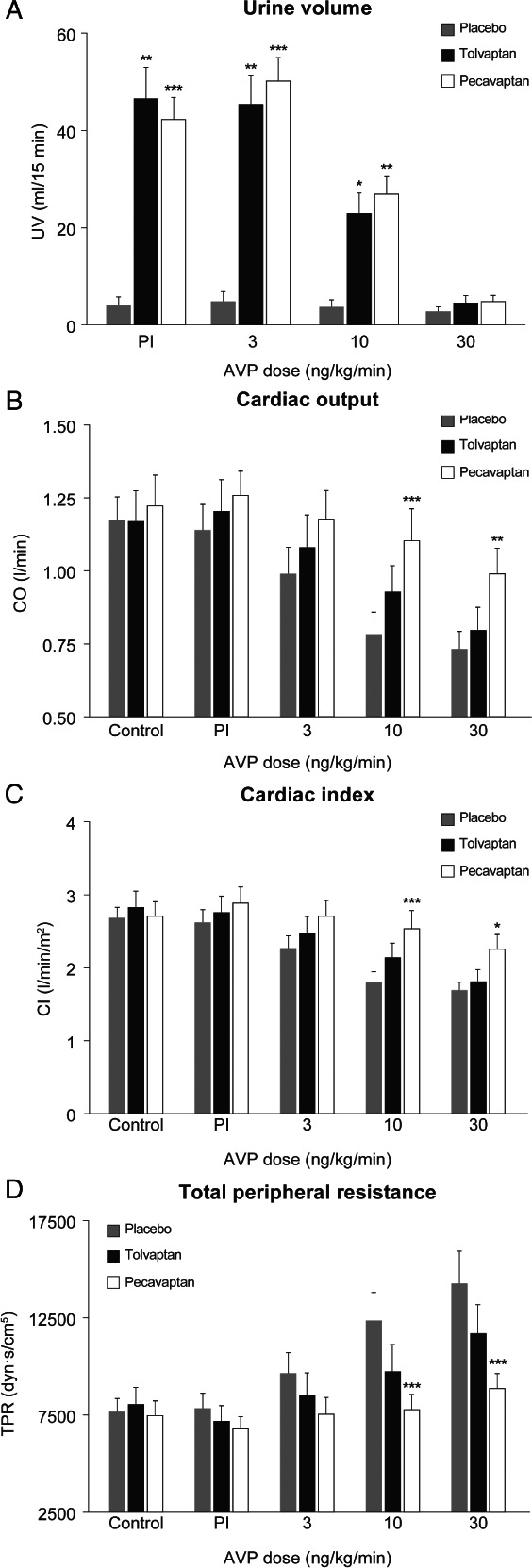
Diuretic (A) and haemodynamic (B–D) effects of oral treatment with pecavaptan or tolvaptan during infusion of increasing dosages of arginine vasopressin (AVP). All data are mean of n = 9 ± standard error of the mean. CI, cardiac index; CO, cardiac output; Pl, placebo; TPR, total peripheral resistance; UV, urine volume. *P < 0.05; **P < 0.01; ***P < 0.001.
There were no differences between the tolvaptan group and the pecavaptan group in urinary sodium, osmolality, potassium and creatinine, indicating that all urinary parameters were equally modified by the V2‐mediated increase in UOP (online supplementary Figure S2).
Discussion
Congestion is the most common reason for hospitalisation in HF and is associated with poor prognosis.14 Symptoms of congestion such as dyspnoea and oedema dramatically reduce the patient's exercise tolerance and quality of life.15 Loop diuretics remain some of the most commonly prescribed drugs in HF, but their use has major limitations such as worsening renal function, electrolyte depletion and renin–angiotensin–aldosterone system activation.3 Recently, selective vasopressin V2 receptor antagonism has been developed as an alternative aquaretic principle but no mortality benefit was demonstrated by tolvaptan in the large outcome trial EVEREST in patients with worsening chronic HF, potentially related to unopposed V1a receptor activation induced by compensatory AVP release.8
We previously determined the haemodynamic responses to conivaptan, a parenteral dual V1a/V2 antagonist, in tachypacing HF dogs under AVP infusion and observed a decline in TPR and an increase in CO.12 Since such a haemodynamic profile on top of a V2‐mediated decongestion should be an attractive goal for chronic congestive HF therapy, we developed the novel dual acting V1a/V2 receptor antagonist pecavaptan (BAY 1753011), which has identical affinities for the human V1a and V2 receptors, and a pharmacokinetic profile supporting once‐daily oral dosing in humans.
A major limitation of several vaptans are large species differences with respect to the V1a receptor. The affinity of several small molecule vaptans for the rodent V1a receptor is much reduced in comparison with respective affinities for the human receptor.16 In addition, large‐animal models are usually of particular use for translation to the clinical situation since they approximate human physiology more closely than do small‐animal models.17 However, a full pharmacological in vitro characterisation of novel drug candidates would necessitate cloning, expression and functional testing of the respective target protein from different species before conducting respective large‐animal studies. We found here that the IC50 values of pecavaptan for the human and dog vasopressin receptors are very similar and that the canine V2:V1a receptor profile was only slightly shifted in favour of the V2 receptor. Therefore, the dog represents a human‐like, non‐primate species for the in vivo characterisation of pecavaptan. Accordingly, we compared here for the first time (i) the acute haemodynamic performance of pecavaptan vs. tolvaptan in a tachypacing canine HF model (which is a well‐established pre‐clinical model of HF with documented neurohormonal activation18, 19), and (ii) the haemodynamic effects of pecavaptan vs. tolvaptan in conscious dogs instrumented with telemetric and surface sensors for non‐invasive CO monitoring (CHEETAH NICOM™, USA) as well as bladder catheterisation for UOP determination.
Pecavaptan and tolvaptan showed equipotent V2‐mediated effects on UOP, which is in accordance with the determined in vitro data. However, pecavaptan, but not tolvaptan, was able to protect from AVP‐mediated impairment in cardiac and systemic function. A statistically significant difference of the pre‐defined study goal was already reached with three animals per group. With regard to the predictive value of the chosen model, one should take into consideration that anaesthesia and surgical trauma might exert major biases on physiologic and pharmacologic interventions.20 To investigate if the observed effects also translate into a more predictive scenario, we developed a new model, in which we tested the haemodynamic and diuretic effects of pecavaptan vs. tolvaptan in conscious animals. Here, we used implantable telemetric sensors and wearable devices to measure relevant haemodynamics non‐invasively. Oral pre‐treatment with pecavaptan significantly protected from AVP‐induced haemodynamic impairment, whereas tolvaptan had only minor, non‐significant effects on these HF‐relevant parameters. Therefore, pecavaptan may serve to blunt V1a‐mediated effects seen with chronically elevated AVP levels (i.e. peripheral vasoconstriction) while maintaining the favourable effects of V2 antagonism (i.e. volume depletion). Based on its combined aquaretic and haemodynamic profile, pecavaptan could be useful in acute, worsening or chronic HF, either as adjunctive therapy or as a replacement for loop diuretics. In the latter case, pecavaptan might represent an equally effective but safer mode of achieving decongestion with potential additional benefit from acute haemodynamic and chronic anti‐remodelling effects mediated via V1a blockade in patients with HF.21 Moreover, pecavaptan might be invaluable for congested patients with late‐stage chronic kidney disease given that activation of renal vascular V1a receptors decreases renal blood flow and oxygenation in settings of increased vasopressin levels, at least in pre‐clinical animal models.22
Limitations of these studies are a result of the acute experimental setting and an uncertainty about the relevance of controlled AVP administration to the pathophysiological mechanism of neuro‐hormonally induced non‐osmotic AVP release. One limitation of UOP measurement in our studies is associated with the acute experimental setup with observational periods of 15 to 20 min per dosage, although we used bladder catheters for accurate UOP measurement. A further limitation concerns the measurement of CO/CI by the NICOM technology. The NICOM device measures the bioreactance or the phase shift in voltage across the thorax and there may be experimental situations (e.g. during periods of low flow) causing readings of decreased accuracy. Further future investigations should aim to differentiate against or on top of standard of care, as well as on potential effects on tissue remodelling in chronic studies, which was out of the scope of the current study.
The currently ongoing phase II AVANTI trial (NCT03901729) is assessing the safety and efficacy of pecavaptan, with or without furosemide, vs. furosemide alone in patients admitted to hospital with acutely decompensated HF and objective evidence of incomplete decongestion despite standard HF treatment.
Supporting information
Figure S1. Effect of pecavaptan or tolvaptan on plasma clinical chemistry in tachypaced dogs.
Figure S2. Effect of pecavaptan or tolvaptan on urine clinical chemistry in conscious telemetric dogs.
Table S1. Invasive haemodynamic investigation of heart failure dogs in comparison to healthy animals.
Acknowledgements
Portions of the results have been presented in the form of abstracts at the recent congresses of the European Society of Cardiology (ESC) in Rome in 2016 and ESC Heart Failure in Vienna in 2018.
Funding
This work was supported by Bayer AG.
Conflict of interest: At the time of study, the following authors were employees of Bayer AG: T.M., P.W., C.S., J.H., W.D., H.T., P.K.
References
- 1.Gheorghiade M, Filippatos G, De Luca L, Burnett J. Congestion in acute heart failure syndromes: an essential target of evaluation and treatment. Am J Med 2006;119(12 Suppl 1):S3–S10. [DOI] [PubMed] [Google Scholar]
- 2.Greene SJ, Fonarow GC, Vaduganathan M, Khan SS, Butler J, Gheorghiade M. The vulnerable phase after hospitalization for heart failure. Nat Rev Cardiol 2015;12:220–229. [DOI] [PubMed] [Google Scholar]
- 3.Felker GM, O'Connor CM, Braunwald E; Heart Failure Clinical Research Network Investigators . Loop diuretics in acute decompensated heart failure: necessary? Evil? A necessary evil? Circ Heart Fail 2009;2:56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goldsmith SR, Gheorghiade M. Vasopressin antagonism in heart failure. J Am Coll Cardiol 2005;46:1785–1791. [DOI] [PubMed] [Google Scholar]
- 5.Schrier RW, Gross P, Gheorghiade M, Berl T, Verbalis JG, Czerwiec FS, Orlandi C; SALT Investigators . Tolvaptan, a selective oral vasopressin V2‐receptor antagonist, for hyponatremia. N Engl J Med 2006;355:2099–2012. [DOI] [PubMed] [Google Scholar]
- 6.Gheorghiade M, Niazi I, Ouyang J, Czerwiec F, Kambayashi J, Zampino M, Orlandi C; Tolvaptan Investigators . Vasopressin V2‐receptor blockade with tolvaptan in patients with chronic heart failure: results from a double‐blind, randomized trial. Circulation 2003;107:2690–2696. [DOI] [PubMed] [Google Scholar]
- 7.Gheorghiade M, Konstam MA, Burnett JC Jr, Grinfeld L, Maggioni AP, Swedberg K, Udelson JE, Zannad F, Cook T, Ouyang J, Zimmer C, Orlandi C; Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study With Tolvaptan (EVEREST) Investigators . Short‐term clinical effects of tolvaptan, an oral vasopressin antagonist, in patients hospitalized for heart failure: the EVEREST clinical status trials. JAMA 2007;297:1332–1343. [DOI] [PubMed] [Google Scholar]
- 8.Konstam MA, Gheorghiade M, Burnett JC Jr, Grinfeld L, Maggioni AP, Swedberg K, Udelson JE, Zannad F, Cook T, Ouyang J, Zimmer C, Orlandi C; Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study With Tolvaptan (EVEREST) Investigators . Effects of oral tolvaptan in patients hospitalized for worsening heart failure: the EVEREST Outcome trial. JAMA 2007;297:1319–1331. [DOI] [PubMed] [Google Scholar]
- 9.Vaduganathan M, Goldsmith SR, Senni M, Butler J, Gheorghiade M. Contrasting acute and chronic effects of tolvaptan on serum osmolality in the EVEREST trial. Eur J Heart Fail 2016;18:185–191. [DOI] [PubMed] [Google Scholar]
- 10.Kolkhof P, Pook E, Pavkovic M, Kretschmer A, Buchmüller A, Tinel H, Delbeck M, Mondritzki T, Wasnaire P, Dinh W, Truebel H, Hüser J, Schmeck C. Vascular protection and decongestion without renin‐angiotensin‐aldosterone system stimulation mediated by a novel dual‐acting vasopressin V1a/V2 receptor antagonist. J Cardiovasc Pharmacol 2019;74:44–52. [DOI] [PubMed] [Google Scholar]
- 11.Illarionov BA, Bondar VS, Illarionova VA, Vysotski ES. Sequence of the cDNA encoding the Ca(2+)‐activated photoprotein obelin from the hydroid polyp Obelia longissima . Gene 1995;153:273–274. [DOI] [PubMed] [Google Scholar]
- 12.Mondritzki T, Kolkhof P, Sabbah HN, Gheorghiade M, Furstner C, Schmeck C, Siedentop H, Schaefer S, Truebel H. Differentiation of arginine vasopressin antagonistic effects by selective V2 versus dual V2/V1a receptor blockade in a preclinical heart failure model. Am J Ther 2011;18:31–37. [DOI] [PubMed] [Google Scholar]
- 13.Heerdt PM, Wagner CL, DeMais M, Savarese JJ. Noninvasive cardiac output monitoring with bioreactance as an alternative to invasive instrumentation for preclinical drug evaluation in beagles. J Pharmacol Toxicol Methods 2011;64:111–118. [DOI] [PubMed] [Google Scholar]
- 14.Costanzo MR, Negoianu D, Jaski BE, Bart BA, Heywood JT, Anand IS, Smelser JM, Kaneshige AM, Chomsky DB, Adler ED, Haas GJ, Watts JA, Nabut JL, Schollmeyer MP, Fonarow GC. Aquapheresis versus intravenous diuretics and hospitalizations for heart failure. JACC Heart Fail 2016;4:95–105. [DOI] [PubMed] [Google Scholar]
- 15.Malhotra R, Bakken K, D'Elia E, Lewis GD. Cardiopulmonary exercise testing in heart failure. JACC Heart Fail 2016;4:607–616. [DOI] [PubMed] [Google Scholar]
- 16.Schafer S, Kolkhof P. Failure is an option: learning from unsuccessful proof‐of‐concept trials. Drug Discov Today 2008;13:913–916. [DOI] [PubMed] [Google Scholar]
- 17.Dixon JA, Spinale FG. Large animal models of heart failure: a critical link in the translation of basic science to clinical practice. Circ Heart Fail 2009;2:262–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elsner D, Riegger GA. Experimental heart failure produced by rapid ventricular pacing in the dog. J Card Fail 1995;1:229–247. [DOI] [PubMed] [Google Scholar]
- 19.Yarbrough WM, Spinale FG. Large animal models of congestive heart failure: a critical step in translating basic observations into clinical applications. J Nucl Cardiol 2003;10:77–86. [DOI] [PubMed] [Google Scholar]
- 20.Vatner SF, Braunwald E. Cardiovascular control mechanisms in the conscious state. N Engl J Med 1975;293:970–976. [DOI] [PubMed] [Google Scholar]
- 21.Goldsmith SR, Udelson JE, Gheorghiade M. Dual vasopressin V1a/V2 antagonism: the next step in neurohormonal modulation in patients with heart failure? J Card Fail 2018;24:112–114. [DOI] [PubMed] [Google Scholar]
- 22.Perico N, Zoja C, Corna D, Rottoli D, Gaspari F, Haskell L, Remuzzi G. V1/V2 vasopressin receptor antagonism potentiates the renoprotection of renin‐angiotensin system inhibition in rats with renal mass reduction. Kidney Int 2009;76:960–967. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Effect of pecavaptan or tolvaptan on plasma clinical chemistry in tachypaced dogs.
Figure S2. Effect of pecavaptan or tolvaptan on urine clinical chemistry in conscious telemetric dogs.
Table S1. Invasive haemodynamic investigation of heart failure dogs in comparison to healthy animals.


