Abstract
After initial investigation of patients presenting with symptoms suggestive of neuropathy, a clinical decision is made for a minority of patients to undergo further assessment with nerve biopsy. Many nerve biopsies do not demonstrate a definitive pathological diagnosis and there is considerable cost and morbidity associated with the procedure. This highlights the need for appropriate selection of patients, nerves and neuropathology techniques. Additionally, concomitant muscle and skin biopsies may improve the diagnostic yield in some cases. Several advances have been made in diagnostics in recent years, particularly in genomics. The indications for nerve biopsy have consequently changed over time. This review explores the current indications for nerve biopsies and some of the issues surrounding its use. Also included are comments on alternative diagnostic modalities that may help to supplant or reduce the use of nerve biopsy as a diagnostic test. These primarily include extraneural biopsy and neuroimaging techniques such as magnetic resonance neurography and nerve ultrasound. Finally, we propose an algorithm to assist in deciding when to perform nerve biopsies.
Keywords: decision aid, indications for biopsy, nerve biopsy, neuropathology, vasculitic neuropathy
1. INTRODUCTION
Despite extensive investigation, no cause may be apparent in up to 10%‐35% of patients with peripheral neuropathy.1, 2 Clinicians may consider nerve biopsy in these cases, especially if there is rapid progression.3, 4 Separately, nerve biopsy in diseases such as vasculitis,5 amyloidosis,6 pure neuritic leprosy,7 neurosarcoidosis,8 and neurolymphomatosis9 enables a definitive diagnosis. The potential diagnostic benefits of nerve biopsy need to be considered in the context of the cost and complications of this procedure as well as the availability of alternative diagnostic tools. The present review will critically dissect the current needs for nerve biopsy as part of the clinical decision‐making process. Detailed reviews of each indication for nerve biopsy are provided below. They are categorized based on the relative importance of nerve biopsy for that indication: High, Moderate and Low. Tables 1, 2, 3 summarize the indications in each category.
TABLE 1.
Conditions in which nerve biopsies are of high importance
| Suspected diagnosis | When to biopsy? | When diagnosis can be made without biopsy | Biopsy findings | Other diagnostic tools |
|---|---|---|---|---|
| Vasculitic neuropathy |
|
|
Sensitivity: ~50% |
|
| NL |
|
|
Sensitivity: 88% in one study | Extraneural biopsy: Detection of extraneural malignancy may obviate the need for demonstrating malignant infiltration of the nerve. |
| Primary nerve/nerve sheath tumor/pseudo‐tumor |
|
Typical phenotype accompanied by characteristic MRI/US findings can establish diagnosis in some subtypes. | Not applicable | MRI and US: Can help in differential diagnosis and guiding biopsy. |
| Pure neuritic leprosy | Almost always (for definite diagnosis) | If fine needle aspiration cytology of the nerve demonstrates M. leprae bacilli or epithelioid granulomas | Sensitivity: 33.3%‐75.9% |
|
Abbreviations: CSF, cerebrospinal fluid; DNA, deoxyribonucleic acid; NL, neurolymphomatosis; M. leprae, Mycobacterium leprae; MRI, magnetic resonance imaging.
TABLE 2.
Conditions in which nerve biopsies are of moderate importance
| Suspected diagnosis | When to biopsy? | When diagnosis can be made without biopsy | Biopsy findings | Other diagnostic tools |
|---|---|---|---|---|
| Amyloid neuropathy | Other tissues not amenable to biopsy or have negative biopsy. | Amyloidosis pathologically demonstrated in other tissue. |
|
|
| SPN | No evidence of extraneural involvement or negative extraneural biopsy. | Probable SPN: Extraneural NCG in context of typical phenotype and evidence of neuroinflammation on CSF studies or MRI. | Sensitivity: 90.5% in one study. |
|
| IgG4 related perineural disease/neuropathy |
Most patients with neuropathy, especially if:
Poor steroid response |
Typical phenotype with tissue evidence of extraneural IgG4 related disorder, raised serum IgG4 levels and responsive to steroids. | No data |
|
Abbreviations: AL, light chain amyloid; ATTR, amyloid transthyretin; CSF, cerebrospinal fluid; ESC, electrochemical skin conductance; MRI, magnetic resonance imaging; SPN, sarcoid peripheral neuropathy.
TABLE 3.
Conditions in which nerve biopsies are of low importance
| Suspected diagnosis | When to biopsy? | When diagnosis can be made without biopsy | Biopsy findings | Other diagnostic tools |
|---|---|---|---|---|
| Para‐proteinemic neuropathy | Suspicion for diagnoses such as: vasculitic neuropathy, amyloid neuropathy or malignant infiltration |
|
Widened myelin lamellae: Sensitivity: 60%‐96% for anti‐MAG neuropathy. Very specific. | Bone marrow biopsy: to detect hematological malignancy |
| CIDP |
|
Most patients diagnosed based on clinical presentation, neurophysiology and other diagnostic tools. |
|
|
| Hereditary neuropathy | Not indicated | Most patients. | Certain findings are characteristic but not specific for particular neuropathies. |
|
| Adult polyglucosan body disease | High index of suspicion despite inconclusive genetic testing and/or GBE1 activity testing | Most patients diagnosed after genetic and/or GBE1 activity testing | Insufficient data | ‐ |
| Storage disorders | Atypical presentations or if high index of suspicion despite the usual tests being non‐diagnostic | Diagnosis obtained using relevant enzyme activity assays, genetic tests or biochemical tests | ‐ | ‐ |
| Motor neuropathy vs MND | Unable to distinguish between MND and motor neuropathy despite thorough testing | If UMN dysfunction can be proven clinically or with ancillary tests like EEG, PET, TT‐TMS | Overall sensitivity 95% in 1 study | MR Neurography and US: Higher nerve CSA in MMN compared to MND |
| Cryptogenic neuropathy/other etiology | Following other aetiologies are suspected despite extensive inconclusive workup:
|
Cryptogenic: Biopsy will not alter management for most patients. Other aetiologies: Usually diagnosed by other means. |
Cryptogenic neuropathy: Yield of useful information is 0%‐37% (studies published 1990‐2007) | ‐ |
Abbreviations: AAN, American Academy of Neurology; CANOMAD, chronic ataxic neuropathy, ophthalmoplegia, monoclonal IgM protein, cold agglutinins and disialosyl antibodies; CIDP, chronic inflammatory demyelinating polyneuropathy; CMV, cytomegalovirus; EEG, electroencephalogram; MND, motor neuron disease; MRI, magnetic resonance imaging; NCS, nerve conduction studies; POEMS, polyneuropathy, organomegaly, endocrinopathy, M‐protein, skin changes; PPV, positive predictive value; TT‐TMS, threshold tracking – transcranial magnetic stimulation; UMN; upper motor neuron.
2. METHODS
PubMed (1966 to June 2019) and Google Scholar (to June 2019) were searched using the following strategy: The term “nerve biopsy” was combined using the AND operator with one of the indications for nerve biopsy listed in this review or the terms “cost,” “yield,” or “utility.” Additional articles were identified by the co‐authors. Indications for nerve biopsy exclusive to the pediatric population were excluded. For each identified article, the bibliography and list of citing articles was examined for potentially relevant articles. Critical appraisal tools were used to assess the quality of articles.
2.1. Evolution of diagnostic modalities
With a rapidly expanding diagnostic armamentarium in recent decades, the most prominent reason for declining referrals for nerve biopsy relates to the availability of less invasive diagnostic modalities that can provide sufficient diagnostic certainty, particularly driven by advancements in molecular diagnostics.13 Advances in other diagnostic modalities include peripheral nerve imaging, neurophysiological investigations and skin biopsy. While magnetic resonance imaging (MRI) and ultrasound (US) are predominantly used for conditions that do not require nerve biopsy (entrapment neuropathy, traumatic neuropathy, space‐occupying nerve lesion), these modalities may provide information on nerve morphology, site, and extent of damage. This is especially useful in areas that are difficult to evaluate with neurophysiological tests. Several US‐based scoring systems have been developed and may be useful in patients with suspected inflammatory or hereditary neuropathies.14
2.2. Diagnostic yield of nerve biopsy
Previous studies investigating the utility of nerve biopsy determined that a significant proportion of nerve biopsies do not provide clinically useful information. In one study of 38 patients with peripheral neuropathy of unknown cause,15 nerve biopsy led to a confirmed diagnosis in 37%, usually among patients with asymmetric, non‐chronic phenotypes. In another study of 67 patients who underwent nerve biopsies, the results influenced the eventual diagnosis and management in 33% and 27% respectively.16 In the subset of patients referred for polyneuropathy of uncertain origin, a diagnosis was established in 24% and changed treatment in 20%. A prospective series of 50 cases found that sural nerve biopsy altered diagnosis in 14% and affected management in 60%.17 Similarly, another study reviewing 234 patients who underwent nerve biopsies determined that nerve biopsy was essential in 16%, and helpful in a further 22%.18 In one center, the diagnostic yield of nerve biopsy was noted to improve between 1981 and 2017 with the improvement attributed to improved patient selection and improved expertise in processing and examining nerve biopsies.13
2.3. Complications of nerve biopsy
The frequency of complications varies among studies involving commonly biopsied nerves, but in general includes persistent numbness (72%‐100%), persistent pain (0%‐58%), wound infection (5%‐20%), delayed wound healing (1%‐12%), dysesthesia (11%‐60%), paresthesia, hematoma, and neuroma.16, 19, 20 Potential complications from reversing anticoagulation remain a further consideration. Such complications and non‐diagnostic biopsies account for some of the dissatisfaction expressed by patients in post‐biopsy surveys.17 In about 4% of biopsies, the tissue contains blood vessel instead of nerve. For distal nerves, whole nerve biopsy is preferable to fascicular biopsy as the two approaches do not significantly differ in terms of post‐operative pain or other complications.17 It is recommended that 4‐5 cm of nerve be removed for sufficient diagnostic value, noting that the post‐biopsy neurological deficit is independent of specimen length.21, 22
2.4. Financial cost
Nerve biopsies are associated with significant costs. For instance, a German single center study evaluated the cost‐effectiveness of sural nerve biopsy in 80 patients and determined that guideline‐based laboratory workup, lumbar puncture and electrodiagnostic studies cost about 420 euros per patient.23 Nerve biopsy had diagnostic and therapeutic consequences in 36% and 23% of patients respectively and cost around 200 euros for surgery and 250 euros for neuropathology evaluation per patient. In the United States, Medicare reported that the average cost of a nerve biopsy was USD997 in an ambulatory surgical center (Medicare paid USD797) and USD1920 in a hospital outpatient department (Medicare paid USD1535).24 According to the Physician Fee Schedule,25 the national payment was USD240.36 for nerve analysis, USD232.42 for teased nerve preparations, USD394.10 for electron microscopy (EM) and additional costs for special stains and immunohistochemistry. In Australiaʼs government funded health system, basic examination of a nerve with light microscopy costs AUD274. Additional costs are associated with immunohistochemistry (AUD90), EM (at least AUD565), and specimen transport (~AUD300).26 This figure rises further with increased length of stay or complexity of the admission. Additional costs are associated with the management of potential complications. In contrast, nerve biopsy results that lead to change in management, especially where expensive therapy is consequently ceased or disability is reduced, will likely be cost‐saving.
2.5. Indications for nerve biopsy (high importance)
2.5.1. Vasculitic neuropathy
The evaluation for vasculitic neuropathy remains the most common indication for nerve biopsy.22 While definitive diagnosis of vasculitic neuropathy requires nerve biopsy, the Peripheral Nerve Society (PNS) suggests that nerve biopsy remains optional in patients with either clinicopathologically proven systemic vasculitis who develop a neuropathy phenotype typically seen in vasculitis or in patients with a diabetic radiculoplexus neuropathy phenotype. In contrast, nerve biopsy remains essential to the diagnosis of non‐systemic vasculitic neuropathy (NSVN).5
The typical presentation is an acute or subacute, painful, asymmetric, or multifocal sensorimotor (or pure sensory) neuropathy. From a histological perspective, diagnosis of definite vasculitic neuropathy requires inflammation within the vessel wall and associated vascular damage (Figure 1A). In the absence of such findings, the presence of certain histological features may support the diagnosis (see Table 4).5 Vasculitic neuropathies require involvement of the small vessels, ranging from small arteries and large arterioles (75‐300 μm) to small arterioles, capillaries, and venules (<40 μm). The former are typically involved in systemic vasculitic neuropathy. Involvement of the latter is usually observed in NSVN and its variants and is termed microvasculitis. Fibrinoid necrosis is not a feature of microvasculitis.27
FIGURE 1.
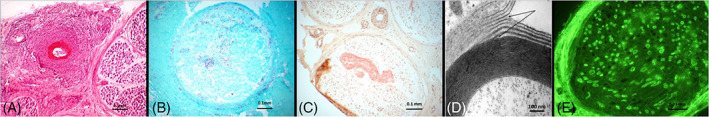
A, Vasculitic neuropathy. Intense vascular and perivascular inflammation of an epineurial blood vessel accompanied by fibrinoid necrosis is noted. B, Leprous neuropathy. Positive Ziehl‐Neelsen staining for acid fast bacilli is seen. C, Amyloid neuropathy. Amyloid deposits evident on Congo red staining. D, IgM paraproteinaemic neuropathy. Widening of the myelin outer lamella (arrows) is a characteristic finding. E, MAG neuropathy. Immunofluorescence demonstrates MAG antibodies bound to myelin
TABLE 4.
Nerve biopsy findings supportive for vasculitic neuropathy
| Predominantly axonal changes |
| Perivascular inflammation |
| Vascular deposition of complement, IgM, or fibrinogen demonstrated by direct immunofluorescence |
| Hemosiderin deposits |
| Asymmetric or multifocal nerve fiber loss or degeneration |
| Prominent active axonal degeneration |
| Perineurial thickening or degeneration |
| Injury neuroma |
| Neovascularization |
A meta‐analysis of patients with clinically suspected vasculitic neuropathy found that combined nerve and muscle biopsy increased the diagnostic yield by 5.1%,28 although this may be useful mainly when combined superficial peroneal nerve/peroneus brevis biopsy is undertaken rather than sural nerve/vastus lateralis.29 The PNS guidelines for vasculitic neuropathy recommend that concomitant muscle biopsy be undertaken if it can be obtained with the involved nerve through a single incision.5 As no independent reference standard for vasculitic neuropathy exists, the sensitivity of nerve biopsy for vasculitic neuropathy can only be estimated; around 50%.30
In terms of improving diagnostic yield, a small study performed combined nerve/muscle/skin biopsy through a single incision in patients suspected to have vasculitic neuropathy.31 Among patients without evidence of definite or probable vasculitis on nerve and muscle biopsy, cutaneous vasculitis was identified in 20%. Another small study of patients with biopsy‐proven NSVN found that quantification of perivascular macrophages, with a cutoff of 2.7 macrophages per vessel, on 5 mm skin punch biopsies had a sensitivity of 94% and specificity of 79% for NSVN.32 In a study of patients with peripheral neuropathies of various etiologies, quantifying scattered macrophages in skin biopsy using a cutoff of 13 per mm2 could differentiate vasculitic neuropathy from controls and other axonal neuropathies with a sensitivity of 71% and specificity of 79%.33 In patients with vasculitic neuropathy, a significant correlation was observed between the number of vessel‐bound T‐cells in the skin and the density of T‐cells in endoneurium and epineurium of the sural nerve.33 Together, these studies suggest that concomitant full thickness skin biopsy may increase diagnostic yield for vasculitic neuropathy and indeed, the presence of perivascular mononuclear inflammation in skin biopsies taken concurrently with nerve biopsies feature in the Brighton Collaborationʼs diagnostic criteria for histopathologically probable vasculitic neuropathy.34
Nerve US may provide evidence supporting a diagnosis of vasculitic neuropathy. There is incomplete overlap between findings on nerve US and the clinical and neurophysiological findings. Patients with vasculitic neuropathy tend to demonstrate focal nerve enlargement on US35 with one small study finding that the ultrasonographic presence of upper limb multifocal nerve enlargement proximal to sites of nerve compression separated vasculitic neuropathy from non‐inflammatory axonal polyneuropathies with 94% sensitivity and 88% specificity.36 US may improve diagnostic yield by influencing the selection of the most appropriate nerve to biopsy.37
2.5.2. Neuropathy related to hematological malignancy
Peripheral neuropathy may be the initial manifestation of hematological malignancy and in that setting, requires nerve biopsy for definite diagnosis.9 Neurolymphomatosis refers to neoplastic infiltration of the peripheral nervous system by a hematological malignancy, usually non Hodgkin lymphoma (rarely leukemia).9 It can be phenotypically heterogeneous.38 Neurolymphomatosis can be the initial presentation of a hematological malignancy (primary) or occur in the context of a known hematological malignancy (secondary), both associated with a poor prognosis.9 In the presence of a neuropathy (cranial or peripheral), radiculopathy or plexopathy, suggestive clinical findings include severe pain, asymmetric distribution and rapid evolution.9 The presence or suspicion of a hematological malignancy may heighten suspicion for this diagnosis. Biopsy is not required for secondary neurolymphomatosis unless there is diagnostic uncertainty. MRI and fluorodeoxyglucose positron emission tomography (PET) scans have estimated diagnostic yields of 80% and 88% respectively for neurolymphomatosis but findings can be non‐specific.9 Cerebrospinal fluid (CSF) cytology can confirm or support the diagnosis. In one study where diagnostic uncertainty persisted, nerve biopsy (often guided by imaging findings) was performed with 88% sensitivity for neurolymphomatosis.9 The imperfect sensitivity of nerve biopsy owes to the usually patchy distribution of malignant cells.39 In some patients with hematological malignancies that are latent or in remission, nerve biopsy may be the only way to link the malignancy with the neuropathy.40 Polymerase chain reaction (PCR) of the lymphoid infiltrate in nerve biopsies can prove monoclonality and help to distinguish between malignant and inflammatory infiltrates.41
2.5.3. Nerve and nerve sheath tumors and pseudotumors
Nerve biopsy is typically required to diagnose tumors of peripheral nerve; which may be benign or malignant. The most common benign tumors are schwannoma and neurofibroma, with perineurioma and ganglioneuroma less frequently encountered. Diagnosis can usually be made by characteristic histological appearances together with judicial use of immunohistochemistry with stains such as S100, EMA (epithelial membrane antigen), GFAP (glial fibrillary acidic protein), and CD34 (cluster of differentiation 34). Malignant tumors may arise from these or arise de novo and 37%‐60% of malignant peripheral nerve sheath tumors occur in patients with neurofibromatosis type 1.42, 43, 44, 45 Perineuriomas often present insidiously in young people with primarily motor and mild sensory deficits. Perineuriomas most commonly present as a mononeuropathy but can also present as a plexopathy.46
MRI and US are helpful in the assessment of peripheral nerve tumors and pseudotumors and allow confident diagnoses in patients with typical presentations of conditions such as traumatic neuroma, Mortonʼs neuroma, nerve sheath ganglion and lipomatosis of nerves.47
Pseudoneoplastic peripheral nerve lesions such as neuromuscular choristomas (NMC) and inflammatory pseudotumors are rare entities which require nerve biopsy for definitive diagnosis and to exclude malignancy. Inflammatory pseudotumors usually present as painful, progressive mononeuropathies with weakness and sensory loss. MRI findings tend to demonstrate heterogeneity in signal characteristics and contrast enhancement. Nerve biopsies usually demonstrate chronic inflammatory infiltrates, interstitial fibrosis, excess vascularity and increased lipocytes. The lesions are steroid‐responsive in some patients.48 NMCs are characterized by the presence of well‐differentiated muscle (usually skeletal) fibers admixed among mature nerve fascicles. While diagnostic, nerve biopsies are frequently complicated by subsequent aggressive fibromatosis. Given that characteristic findings on MRI have been shown to be very sensitive and specific in diagnosing NMCs, patients fitting the clinical picture with such findings can probably be diagnosed with NMC and followed up clinically and radiologically to check for stability.49
2.5.4. Pure neuritic leprosy
The cardinal symptom of leprosy is sensory loss, typically reflecting intracutaneous nerve damage.50 Skin biopsy or skin scrapings therefore remain the cornerstone of diagnosis. Pure neuritic leprosy accounts for 4%‐8% of leprosy cases with nerve biopsy being the gold standard for diagnosis. Definite diagnosis requires the presence of acid fast lepra bacilli (Figure 1B) usually within foam cells and Schwann cells.7 These bacilli primarily feature in lepromatous leprosy; they are rarely present in patients with tuberculoid leprosy which is characterized instead by epithelioid granulomas (sometimes with caseation).7 The sensitivity of nerve biopsy for leprosy varies across studies (33.3%‐75.9%)51, 52 but can be increased using quantitative PCR (qPCR) testing for Mycobacterium leprae deoxyribonucleic acid (DNA). In patients with pure neuritic leprosy (no skin lesions and negative slit skin smear bacilloscopy), it is important to perform qPCR for M. leprae DNA on slit skin smears and/or skin biopsies as this increases the sensitivity for diagnosing leprosy.50, 53 Fine needle aspiration cytology of the nerve can also demonstrate the lepra bacilli and epithelioid granulomas and has been shown to have comparable yields to nerve biopsy, while being less invasive.54
2.6. Indications for nerve biopsy (moderate importance)
2.6.1. Amyloid neuropathy
In amyloid neuropathy, the deposited amyloid fibrils are mainly composed of light chains (AL) or mutant transthyretin (ATTR) with other causes being rare.55 In patients with pathogenic TTR gene mutation, definitive diagnosis requires at least two amyloidosis related symptoms and presence of amyloid deposits (Figure 1C) in the biopsied tissue.6 While biopsy evidence may be sought from any involved organ, abdominal fat pad biopsy is less invasive. Non‐cardiac biopsies are less sensitive in ATTR amyloidosis compared to AL amyloidosis.56 Genetic testing can detect the specific TTR mutation. Sural nerve biopsy is about 80% sensitive57, 58 for detecting TTR amyloid and can establish amyloidosis as the cause for neuropathy. Concomitant muscle biopsy may increase the diagnostic yield.59, 60 Liver transplantation and therapies such as tafamidis, patisaran, and inotersen have been shown to reduce disease progression hence the importance of early (even pre‐symptomatic) diagnosis to preserve functional status.61, 62, 63 However, abnormalities on sural nerve biopsy may be a late manifestation. Phenotypic heterogeneity frequently leads to misdiagnosis (up to 32%) and delayed diagnosis. Chronic inflammatory demyelinating polyneuropathy (CIDP) is the most common misdiagnosis (about 20%) with up to 37% of patients having clinical findings meeting diagnostic criteria for CIDP.64
When AL amyloidosis is suspected, serum and urine immunofixation as well as serum free light chain assay is first undertaken with negative findings making the diagnosis unlikely. Definitive diagnosis requires histopathological demonstration of amyloid with further testing to confirm amyloid protein composition.65 Abdominal fat pad biopsy has a sensitivity of about 80%66 and exceeds 90% if combined with bone marrow testing. Nerve biopsy can be considered if both biopsies are negative but the index of suspicion remains high.67 The sensitivity of nerve biopsy is 30%‐100%.68 Rectal and renal biopsy can also be used to find amyloid deposits.55 Serial sections should be examined as the deposits tend to be focal. The typical pattern is one of severe nerve fiber loss, especially the small myelinated or unmyelinated fibers. Deposits of amyloid stained with Congo red or Hematoxylin and Eosin may be found within epineurial and endoneurial connective tissue and also thickened epineurial and endoneurial blood vessels. EM may detect deposits that cannot be seen with light microscopy.69
Amyloid protein composition is usually tested using immunohistochemistry due to its wider availability. Mass spectrometry offers higher sensitivity and specificity but is expensive and primarily found in specialized centers.70, 71, 72, 73, 74 This can help to guide management. For example, patients with presumed AL amyloidosis may instead have ATTR amyloidosis with coincidental monoclonal gammopathy on testing. Diagnosing AL amyloidosis is important as several treatment options are available including stem cell transplant and systemic chemotherapy.75
Novel diagnostic modalities are being evaluated to facilitate earlier diagnosis of amyloid neuropathy. Magnetic resonance neurography may identify peripheral nerve lesions in asymptomatic TTR mutation carriers. In the absence of monoclonal gammopathy, technetium‐labeled cardiac scintigraphy can diagnose cardiac ATTR amyloidosis with a positive predictive value of 100%. Other modalities include skin biopsy76 to evaluate for amyloid deposits and evidence of small fiber neuropathy (SFN),77 feet electrochemical skin conductance78 to measure early dysautonomia and periumbilical US for amyloid deposits.79
2.6.2. Sarcoid neuropathy
Among patients with sarcoidosis, 5%‐16% have neurological involvement,80 including 1% with neuropathy.81 Sarcoid neuropathy is phenotypically heterogeneous and rarely can be the sole (or initial) presentation of sarcoidosis.82 Various diagnostic criteria have been proposed for neurosarcoidosis, with just one including peripheral nervous system involvement.8, 83 In patients suspected to have neuroscarcoidosis on the basis of clinical manifestations and findings on MRI, CSF, and/or neurophysiological testing, a definitive diagnosis of sarcoid neuropathy requires demonstration of non‐caseating granulomas (NCG) on nerve biopsy which cannot be explained by other causes of granuloma formation, primarily tuberculosis and leprosy. Sarcoid granulomas are usually found in the epineurium and perineurium.82 Small fiber involvement can be demonstrated on skin biopsy with reduced intraepidermal nerve fiber density.84 A subset demonstrates evidence of concomitant vasculitis.82 If nerve biopsy is not appropriate or negative, diagnosis of probable neurosarcoidosis can be made using biopsy evidence of extraneural sarcoidosis when the clinical presentation is suggestive of neurosarcoidosis accompanied by typical findings on MRI, CSF and/or neurophysiological testing.8, 80, 85 However, studies have found concomitant central nervous system involvement in sarcoid neuropathy to be uncommon (0%‐21%).81, 82 One study81 diagnosed probable sarcoid neuropathy in the presence of extraneural NCG accompanied by symptoms/signs of limb neuropathy “judged to be related in time to histologic or radiologic evidence of active sarcoidosis.” In this series,81 nerve biopsy was performed in 21 patients with granulomatous reactions seen in 19. Concomitant muscle biopsy likely improves the yield for sarcoid neuropathy. Additionally, the presence of granulomas in muscle rules out tuberculoid leprosy which is muscle‐sparing.82 Nerve US and magnetic resonance neurography in sarcoid neuropathy patients may demonstrate higher cross‐sectional areas (CSA) in certain nerves.86 MRI may demonstrate nerve enlargement and enhancement of roots, plexus and nerves.81
2.6.3. Immunoglobulin G4 related peripheral neural involvement
Immunoglobulin (Ig) G4 related disease of a tissue is diagnosed when it is infiltrated by IgG4‐positive plasma cells with accompanying elevated serum IgG4 levels. In the rare cases of peripheral nervous system involvement, IgG4 related perineural disease usually occurs in the ocular and paravertebral regions and is mostly asymptomatic. It appears as well‐circumscribed soft‐tissue perineural masses on MRI that responds to steroids.87
This condition is distinguished from paranodopathies, which are mediated by autoantibodies (predominantly IgG4) against paranodal antigens such as neurofascin‐155 and contactin‐188 and are not characterized by IgG4 plasma cell infiltration.89
The context of raised serum IgG4 levels and the presence of IgG4 related disease in other organs suggests the diagnosis but definitive diagnosis requires the presence of IgG4‐positive plasma cell infiltrates on nerve biopsy.87 Five cases90, 91, 92, 93, 94 of peripheral neuropathy attributed to IgG4 related disease have been reported although the condition may be under‐diagnosed.95 The presentations were variable with mononeuritis multiplex seen in three cases. In two cases,90, 94 sural nerve biopsy showed IgG4‐positive plasma cells infiltrating the epineurium and reduction in myelinated nerve fibers. In the third case,91 features of axonal degeneration were seen with epineurial infiltration of lymphocytes and eosinophils. Although not demonstrated on sural nerve biopsy, IgG4 plasma cell infiltration was seen on inguinal lymph node biopsy. In the fourth case,92 neuropathy was preceded by pleural effusion. Sural nerve biopsy was normal with the IgG4 plasma cell infiltration demonstrated on pleural biopsy instead. Symptoms significantly improved with glucocorticoids in these four cases. The fifth case93 was steroid resistant. Sural nerve biopsy found “obstructive thromboangiitis with severe loss of myelin and axons” with IgG4 plasma cell infiltration diagnosed on bone marrow biopsy.
2.7. Indications for nerve biopsy (low importance)
2.7.1. Paraproteinaemic neuropathy
Paraproteins are present in up to 3%‐5% of patients with peripheral neuropathy although likely coincidental in many patients, especially the elderly.96 Although no specific tests can distinguish between coincidental and disease‐causing paraproteins, the use of indirect immunofluorescence may show binding to nerve components such as myelin, particularly in the case of IgM paraproteinaemia with anti‐myelin associated glycoprotein (MAG) antibodies.
In patients with a typical phenotype, detecting high titer of IgM antibodies to MAG or ganglioside Q1b (GQ1b) makes it highly probable that the paraprotein is responsible for the demyelinating neuropathy and in this setting, nerve biopsy is not usually required. With IgM antibodies directed towards other neural antigens (eg, GM1, GD1a, GD1b, GM2, sulfatide etc.), the association is weaker but remains possible. In this case or if IgG/IgA paraproteins are found, the presence on nerve biopsy of widely spaced myelin (Figure 1D) or Ig (Figure 1E) and/or complement bound to myelin strengthens the evidence for a causal relationship. Neuropathy in the context of Waldenstromʼs macroglobulinaemia, POEMS (polyneuropathy, organomegaly, endocrinopathy, M‐protein and skin changes) and CANOMAD (chronic ataxic neuropathy, ophthalmoplegia, monoclonal IgM protein, cold agglutinins, and disialosyl antibodies) syndrome does not require nerve biopsy for diagnosis.97
Regarding POEMS syndrome, it remains uncertain whether the M‐protein or cytokines such as vascular endothelial growth factor are pathogenic98; however, treatment is indicated regardless. The current literature is sparse concerning whether nerve biopsies contribute to management decisions or lead to improved clinical outcomes when paraproteinaemic neuropathy is suspected. The role of nerve biopsy is limited to situations when vasculitic neuropathy, amyloid neuropathy or malignant lymphoproliferative nerve infiltration is suspected; and in cases that are unresponsive to treatment where confirmation of the diagnosis would alter management.67, 99 If the patient has multiple myeloma, they may require systemic therapy regardless of the severity of neuropathy, thus biopsy may not change management.
Depending on the type of paraproteinemic neuropathy, features on biopsy can include widened myelin lamellae (which can have paraprotein deposits), demyelination, thinly myelinated fibers, tomacula, inflammatory infiltrates and axon de‐ and regeneration. In chronic paraproteinemic demyelinating neuropathy, features of chronic de‐ and remyelination like onion rings can be seen but the overall findings are heterogeneous.100, 101 EM is needed to demonstrate the presence of widely spaced myelin outer lamellae which is very sensitive and specific for anti‐MAG neuropathy.102, 103, 104 By comparison, widened lamellae are more common in the inner and middle myelin sheath layers in IgG/A paraproteinemic neuropathy.105, 106 Immuno‐EM may be used to demonstrate binding of monoclonal IgM or IgG to target antigens. Such studies implicate the Ig as pathologic and also show the injury mechanism.105, 106 In Waldenstromʼs macroglobulinemia associated neuropathy, demyelination and axon de‐ and regeneration can be seen with EM showing widely spaced myelin. In POEMS, axonal degeneration and segmental demyelination can be seen with scant (if any) inflammation107 and EM can show characteristic but non‐specific uncompacted myelin108 which may help differentiate POEMS from other demyelinating peripheral neuropathies. A retrospective analysis of nerve biopsies comparing patients with typical CIDP or POEMS found that nerve biopsies from POEMS patients demonstrated greater axonal degeneration and epineurial neovascularization while nerve biopsies from CIDP patients had greater endoneurial inflammation and onion‐bulb formations.109
Cryoglobulinemic neuropathy is usually clinically diagnosed in patients with peripheral neuropathy and mixed cryoglobulinemia. Nerve biopsy can help to confirm the diagnosis. There are no standardized or validated diagnostic criteria for cryoglobulinemic vasculitis.110 Nerve biopsy usually demonstrates predominantly large fiber axonal degeneration without regeneration and is commonly accompanied by vasculitis or features suggesting a vasculopathy.111 Intravascular cryoglobulin deposition without vasculitis can be seen in the vasa nervorum. Features of demyelination can be seen and EM may show myelin sheaths with vacuoles containing amorphous material.112 Where Ig deposits are seen in the context of monoclonal cryoglobulinemia, EM can identify if they are of similar composition.113
2.7.2. CIDP
CIDP can be a diagnostic challenge despite the availability of published criteria given its phenotypic heterogeneity. In patients meeting the European Federation of Neurological Societies (EFNS)/PNS 2010 diagnostic criteria for CIDP, definitive diagnosis relies on demonstrating prominent features of demyelination on neurophysiological testing of motor nerves.114 Where less prominent demyelinating features only suggest probable or possible CIDP, supportive criteria based on CSF analysis, MRI spine/plexus, sensory electrophysiology, response to immunomodulatory treatment, and nerve biopsy can be used to allow definitive diagnosis.114 In a retrospective analysis of 146 patients with definite CIDP based on the EFNS/PNS 2006 criteria, 25% of patients required supporting criteria for definite diagnosis. This included 12% of patients where nerve biopsy was the supportive criteria.115 Many of these patients, however, would likely satisfy the conditions for definite CIDP based on the additional supportive features in the newer EFNS/PNS 2010 criteria even without a nerve biopsy. This suggests that using the present criteria, where information from other supportive tests can be easily accessed, nerve biopsy would be rarely required to obtain definite diagnosis for CIDP.
Light microscopy findings (from distal nerves) typically seen in CIDP are neither sensitive nor specific. Demyelination and mononuclear cell infiltration are the main findings. Secondary axonal loss may be seen, usually accompanied by clusters of regenerating fibers.116 Onion bulbs can be seen in chronic cases. They are usually randomly distributed, lying adjacent to normally myelinated axons without onion bulbs. By contrast, onion bulbs involve nearly all nerve fibers in some inherited demyelinating neuropathies.117 There is some evidence that the pattern of endoneurial perivascular macrophage clusters118 and the extent of matrix metalloproteinase‐9 immunoreactivity119 can differentiate inflammatory from non‐inflammatory neuropathy. Ig and complement deposits may be seen in some patients.88 According to histopathologic criteria for CIDP proposed by the American Academy of Neurology, unequivocal evidence of demyelination and remyelination needs to be seen with greater than five demyelinated fibers on EM or evidence of demyelination/remyelination in at least 12% of 50 teased fibers containing a minimum of four internodes each.120 Evidence of macrophage‐mediated demyelination is characteristic of inflammatory neuropathy (acute and chronic) but is best seen on EM. It has been suggested that very severe CIDP may be misdiagnosed as chronic idiopathic axonal neuropathy in some patients and that EM is useful in detecting the demyelinating lesions in these cases.108
The utility of nerve biopsy is mainly for atypical CIDP, to rule out other differentials and when there is non‐response to treatment.121 Research has been conducted to identify non‐biopsy methods to achieve greater certainty in diagnosing CIDP. Neurophysiological testing of additional limbs/motor nerves significantly improves the diagnostic yield of definite CIDP.122 Since the pathological changes in polyradiculoneuropathies (CIDP and Guillain Barre Syndrome) particularly involve nerve roots and plexuses, it is important to test these regions using proximal stimulation, F‐waves, and evoked potentials. Novel neurophysiological methods have been suggested to increase the detection of demyelinating findings, an example being the triple‐stimulation test to evaluate for very proximal conduction blocks that cannot be detected with standard neurophysiological testing.123
Plexus MRI124 and nerve US125 can support a diagnosis of CIDP and help to differentiate it from multifocal motor neuropathy (MMN) and multifocal acquired demyelinating sensory and motor (MADSAM) neuropathy. In patients with atypical CIDP, a study found that supportive findings on plexus MRI could occur in the absence of supportive findings on distal nerve biopsy and vice versa.124 Abnormalities on MRI brachial plexus are usually symmetrical in CIDP and asymmetrical in MMN/MADSAM.126 Nerve US may help to differentiate CIDP from Charcot‐Marie‐Tooth (CMT).127 Immune‐mediated neuropathies usually have patchy nerve enlargement compared to diffuse enlargement in hereditary neuropathies,128 although diffuse enlargement can occur in patients with longstanding, untreated CIDP.129
2.7.3. Hereditary neuropathies
Nerve biopsy is not required for most patients with hereditary neuropathies, especially after the advent of next generation sequencing (NGS). Certain findings on nerve biopsy, usually EM, are suggestive of particular genetic mutations.130
2.7.4. Storage disorders
Lysosomal and perioxisomal disorders are uncommonly of adult onset and can be associated with neuropathy. Enzyme activity assays, genetic tests or biochemical tests are usually sufficient for diagnosis. Nerve biopsies for ultrastructural examination may be indicated with atypical presentations or if suspicion for the disease remains high despite the usual tests being non‐diagnostic.131 Accumulation of intralysosomal material may be found in neurons or Schwann cells forming characteristic structures such as Zebra or Tuff stone bodies or prismatic inclusions in metachromatic leukodystrophies and related sphingolipidoses. In Refsumʼs disease, marked onion bulb formation may occur.
2.7.5. Adult polyglucosan body disease
Adult polyglucosan body disease is an extremely rare late‐onset illness.132 Common clinical manifestations include neurogenic bladder, gait disturbance, distal lower limb sensory loss and mild cognitive impairment. Genetic testing is first‐line and if equivocal, an assay of glycogen branching enzyme (GBE) activity in skin fibroblasts or muscle is recommended. Nerve biopsy to demonstrate the polyglucosan bodies is recommended if the GBE activity assay is also equivocal.133, 134 In two studies of patients with this condition, polyglucosan bodies were seen in all patients in whom nerve biopsy was undertaken and occasionally found in patients without this condition.133, 135 Diagnosis allows for prognostication, genetic counseling and potential enrolment into clinical trials.
2.7.6. Pure motor neuropathies
In some patients with sporadic, recent onset lower motor neuron syndrome, differentiating motor neuropathy from progressive muscular atrophy (PMA) can be difficult especially with purely axonal electrophysiological findings and non‐response to immune‐mediated therapy. Motor nerve biopsy may aid this distinction. The motor branch of the obturator nerve has been studied for this indication,136 demonstrating a sensitivity of 95% in one small study of such patients136 with the histopathological diagnoses highly correlating with the final clinical diagnosis after 2 y of follow‐up. Combining their results with a previous study, the study investigators proposed a neuropathologic diagnostic criteria. Findings on motor nerve biopsy that suggest probable motor neurone disease are an axonal pathology combined with low regeneration activity (density of regenerating clusters less than 22.4/mm2 or cluster to fiber ratio less than 0.52). Conversely, a pathological diagnosis of motor neuropathy is suggested by high regeneration activity (density of regenerating clusters greater than 42.2/mm2 or cluster to fiber ratio greater than 0.89) and/or the presence of signs of demyelination/remyelination. Rarely, the presence of deposits such as amyloid or axonal inclusions such as polyglucosan bodies would also support a pathologic diagnosis of motor neuropathy.
Presence of upper motor neuron involvement can also be demonstrated with threshold tracking transcranial magnetic stimulation.137 Peripheral nerve imaging using high resolution US and MRI can help to discriminate between MMN and amyotrophic lateral sclerosis (ALS).138, 139
2.7.7. Other neuropathies
Neuropathological findings have been characterized for various other neuropathies, however none that rely on nerve biopsy for diagnosis.140, 141, 142 Toxic neuropathy secondary to some industrial agents or medications like amiodarone143 and chloroquine144 may produce characteristic findings. In the setting of occupational toxic exposure, the role of nerve biopsy is limited to clarifying ongoing diagnostic uncertainty despite extensive testing and assisting with prognostication.145 Human immunodeficiency virus infection can rarely cause diffuse infiltrative lymphocytosis syndrome (DILS) which is characterized by CD8 T‐lymphocytosis and CD8 T‐cell infiltration into multiple organs, including nerves. Nerve biopsy utility is limited to the rare situation where peripheral nerve involvement is the sole or initial manifestation. DILS usually improves with anti‐retroviral therapy.146
In patients with idiopathic distal symmetric polyneuropathy, a previous review was unable to identify any articles that could inform recommendations on the role of nerve biopsy.147 In most patients with chronic idiopathic sensory axonal neuropathy, sural nerve biopsies offer no benefit.148, 149 In the absence of clinical features suggesting a treatable cause, nerve biopsies may be considered in severe or progressive neuropathies to evaluate for potentially treatable etiologies.150 Finally, nerve biopsies have utility in research settings to investigate the pathological effect of novel autoantibodies in inflammatory neuropathies151 and to identify markers of pathogenicity or disease activity.152
2.8. Decision‐making
Optimal outcomes with nerve biopsy requires appropriate selection of patients,153 nerves and neuropathology techniques. No guidelines exist to inform patient selection for nerve biopsy. However, Dyck and colleagues154 counseled that nerve biopsy should be reserved for cases that have been carefully characterized by other clinical approaches first which have failed to provide a definite answer, and informed judgement is made that biopsy may be useful. To guide such a process, a decision aid for nerve biopsies has been constructed in the form of a flowchart (Figure 2) with the accompanying summary Tables 1, 2, 3.
FIGURE 2.
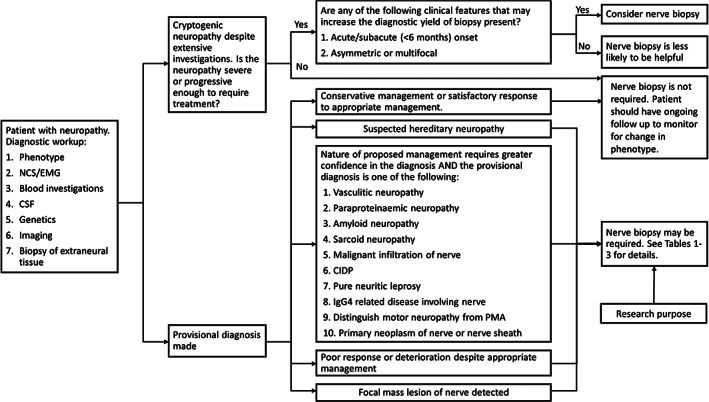
Proposed decision tree to facilitate decision‐making for nerve biopsy. EMG, electromyogram; NCS, nerve conduction studies
Nerve biopsy is more likely to be diagnostic when performed within 6 months from onset of symptoms.155 Diagnostic yield is also higher in patients with asymmetric or multifocal symptoms. In one study, diagnostic yield of biopsy was 32.7% in patients with asymmetric or multiple mononeuropathy compared to 17.7% in patients with symmetrical neuropathy.156 In another study, diagnostic yield was highest when there was clinical/neurophysiological evidence of asymmetry (60%) and multifocal distribution (75%).155 Diagnostic yield is also influenced by the pre‐biopsy provisional diagnosis with studies showing higher yields with provisional diagnoses of vasculitic neuropathy, inflammatory demyelinating polyneuropathy, and hereditary motor and sensory neuropathy.155, 156 The yield of nerve biopsies in cryptogenic neuropathies, while low, is not insignificant (0%‐37%).3, 15, 155, 157 Indeed, important diagnoses like vasculitis have been made in such patients.18
With regard to neuropathology techniques, there is debate regarding the value that teased fiber analysis adds in the evaluation of nerve biopsies.155, 158 A study including 102 patients with teased fiber analysis noted that it altered management in only four patients; three with hereditary neuropathy with pressure palsies and one with CIDP.155 The pattern of abnormality can indicate the underlying pathological process and is reviewed in detail elsewhere.135 Teased fiber preparations increase the sensitivity of identifying early demyelinating features compared to epoxy semithin sections. In some cases with initially non‐diagnostic paraffin sections, teased fiber abnormalities prompted requisition and examination of further paraffin sections leading to a pathological diagnosis.135 Examining serial frozen and paraffin/resin‐embedded sections has been shown to be useful.155 Immunohistochemistry can add diagnostic and prognostic value.118, 159, 160 One study found that EM added value in about 14% of cases.155 Diagnostic yield for pathologies known to have patchy involvement would be expected to improve with larger amount of nerve tissue for examination.
Apart from vasculitic neuropathy and neuropathy associated with amyloidosis and sarcoidosis, concomitant nerve‐muscle biopsy can occasionally help to diagnose cholesterol embolism,161 intra‐vascular lymphoma162 and mitochondrial disorders.59
With regards to appropriate nerve selection, identification of a focal lesion should ideally lead to a targeted biopsy of the lesion. Preference for the most clinically affected nerve needs to be balanced against the chance that severely affected nerves may have no residual nerve fiber to analyze. Sural nerves are most often biopsied, being easily accessible pure sensory nerves that are usually affected in length‐dependent neuropathies. The superficial branch of the radial nerve is preferred for upper limb dominant neuropathy. Motor nerves are occasionally considered for biopsy as discussed above with the procedures having reasonable safety profiles.136, 163, 164
Proximal nerve biopsies can be useful in certain clinical contexts. Retrospective reviews of patients selected to undergo targeted fascicular biopsy of the sciatic nerve (112 patients) or brachial plexus (74 patients) resulted in an overall diagnostic yield of 84.8% and 74.3% respectively with a wide range of diagnoses.165, 166 In the sciatic nerve biopsy cohort, 4.5% developed permanent complications including persistent numbness in a peroneal division distribution on the biopsy side. In the brachial plexus biopsy cohort, worsening of numbness or weakness occurred in four and three patients respectively. All patients had a thorough clinical workup, including neurophysiology and MRI of the relevant region. Positron emission tomography (PET)/computed tomography (CT) was done if malignancy was suspected. Some patients had previous skin, muscle or nerve (distal) biopsies. Biopsies were indicated when the patient had an idiopathic, atypical (particularly with focal deficits) neuropathy or a treatment‐refractory neuropathy; with the neuropathy deemed to localize proximally.
3. CONCLUSIONS
While the utility of nerve biopsy in the clinical setting has decreased over recent years, it continues to play a role in the diagnostic workup of highly selected patients. While nerve biopsy does not affect the diagnosis or management in a significant proportion of patients, it is important to consider factors that would maximize the diagnostic yield should a nerve biopsy be performed. This includes the clinical features of the neuropathy, appropriate nerve selection, consideration of combination tissue biopsies and the appropriate use of neuropathology techniques.
CONFLICT OF INTEREST
None of the authors has any conflict of interest to disclose.
ETHICAL PUBLICATION STATEMENT
We confirm that we have read the Journalʼs position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
ACKNOWLEDGMENTS
This work was supported by the National Health and Medical Research Council of Australia (NHMRC) program grant (#1037746).
Nathani D, Spies J, Barnett MH, et al. Nerve biopsy: Current indications and decision tools. Muscle & Nerve. 2021;64:125–139. 10.1002/mus.27201
Abbreviations: AL, amyloid light chain; ALS, amyotrophic lateral sclerosis; CANOMAD, chronic ataxic neuropathy, ophthalmoplegia, monoclonal IgM protein, cold agglutinins and disialosyl antibodies; CMT, Charcot‐Marie‐Tooth; CIDP, chronic inflammatory demyelinating polyneuropathy; CSA, cross‐sectional area; CSF, cerebrospinal fluid; CT, computed tomography; DNA, deoxyribonucleic acid; DILS, diffuse infiltrative lymphocytosis syndrome; EFNS, European Federation of Neurological Societies; EM, electron microscopy; EMA, epithelial membrane antigen; GBE, glycogen branching enzyme; GFAP, glial fibrillary acidic protein; GQ1b, ganglioside Q1b; Ig, immunoglobulin; MADSAM, multifocal acquired demyelinating sensory and motor; MAG, myelin associated glycoprotein; MGUS, monoclonal gammopathy of unknown significance; MMN, multifocal motor neuropathy; MRI, magnetic resonance imaging; NCG, non‐caseating granulomas; NG, next generation sequencing; NMC, neuromuscular choristoma; NSVN, non‐systemic vasculitic neuropathy; PCR/qPCR, polymerase chain reaction/qualitative polymerase chain reaction; PET, positron emission tomography; PMA, progressive muscular atrophy; PNS, Peripheral Nerve Society; POEMS, polyneuropathy, organomegaly, endocrinopathy, M‐protein, skin changes; SFN, small fiber neuropathy; TTR/ATTR, transthyretin/transthyretin amyloid; US, ultrasound.
The objectives of this activity are to: 1) Stratify neuropathic conditions according to importance of nerve biopsy to facilitate clinical decision‐making regarding whether to perform a nerve biopsy; 2) Understand and be able to assess the complications and financial costs of nerve biopsy; 3) Utilize an evidence‐based approach to determining when to order a nerve biopsy.
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1.Pasnoor M, Dimachkie MM, Barohn RJ. Cryptogenic sensory polyneuropathy. Neurol Clin. 2013;31(2):463‐476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hanewinckel R, van Oijen M, Ikram MA, van Doorn PA. The epidemiology and risk factors of chronic polyneuropathy. Eur J Epidemiol. 2016;31:5‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lubec D, Mullbacher W, Finsterer J, Mamoli B. Diagnostic work‐up in peripheral neuropathy: an analysis of 171 cases. Postgrad Med J. 1999;75(890):723‐726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.China L, Fernandez A, Lacroix C, Adams D, Planté V, Said G. Contribution of never biopsy findings to the diagnosis of disabling neuropathy in the elderly: a retrospective review of 100 consecutive patients. Brain. 1996;119(4):1091‐1098. [DOI] [PubMed] [Google Scholar]
- 5.Collins MP, Dyck PJB, Gronseth GS, et al. Peripheral Nerve Society Guideline on the classification, diagnosis, investigation, and immunosuppressive therapy of non‐systemic vasculitic neuropathy: executive summary. J Peripher Nerv Syst. 2010;15(3):176‐184. [DOI] [PubMed] [Google Scholar]
- 6.Adams D, Suhr OB, Hund E, et al. First European consensus for diagnosis, management, and treatment of transthyretin familial amyloid polyneuropathy. Curr Opin Neurol. 2016;29(suppl 1):S14‐S26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hui M, Uppin M, Challa S, Meena A, Kaul S. Pure neuritic leprosy: resolving diagnostic issues in acid fast bacilli (AFB)‐negative nerve biopsies: a single centre experience from South India. Ann Indian Acad Neurol. 2015;18(3):292‐297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stern BJ, Royal W, Gelfand JM, et al. Definition and consensus diagnostic criteria for neurosarcoidosis: from the neurosarcoidosis consortium consensus group. JAMA Neurol. 2018;75(12):1546‐1553. [DOI] [PubMed] [Google Scholar]
- 9.Grisariu S, Avni B, Batchelor TT, et al. Neurolymphomatosis: an International Primary CNS Lymphoma Collaborative Group report. Blood. 2010;115(24):5005‐5011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Breiner A, Brannagan TH. Comparison of sensitivity and specificity among 15 criteria for chronic inflammatory demyelinating polyneuropathy. Muscle Nerve. 2014;50(1):40‐46. [DOI] [PubMed] [Google Scholar]
- 11.Haq RU, Fries TJ, Pendlebury WW, Kenny MJ, Badger GJ, Tandan R. Chronic inflammatory demyelinating polyradiculoneuropathy: a study of proposed electrodiagnostic and histologic criteria. Arch Neurol. 2000;57(12):1745‐1750. [DOI] [PubMed] [Google Scholar]
- 12.Gasparotti R, Padua L, Briani C, Lauria G. New technologies for the assessment of neuropathies. Nat Rev Neurol. 2017;13:203‐216. [DOI] [PubMed] [Google Scholar]
- 13.Prada V, Massucco S, Venturi C, et al. Diagnostic value of sural nerve biopsy: retrospective analysis of clinical cases from 1981 to 2017. Front Neurol. 2019;10:1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grimm A, Rattay TW, Winter N, Axer H. Peripheral nerve ultrasound scoring systems: benchmarking and comparative analysis. J Neurol. 2017;264(2):243‐253. [DOI] [PubMed] [Google Scholar]
- 15.Schweikert K, Fuhr P, Probst A, Tolnay M, Renaud S, Steck AJ. Contribution of nerve biopsy to unclassified neuropathy. Eur Neurol. 2007;57(2):86‐90. [DOI] [PubMed] [Google Scholar]
- 16.Ruth A, Schulmeyer FJ, Roesch M, Woertgen C, Brawanski A. Diagnostic and therapeutic value due to suspected diagnosis, long‐term complications, and indication for sural nerve biopsy. Clin Neurol Neurosurg. 2005;107(3):214‐217. [DOI] [PubMed] [Google Scholar]
- 17.Gabriel CM, Howard R, Kinsella N, et al. Prospective study of the usefulness of sural nerve biopsy. J Neurol Neurosurg Psychiatry. 2000;69(4):442‐446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bilbao JM, Schmidt RE. Peripheral neuropathy and the role of nerve biopsy. In: Bilbao JM, Schmidt RE, eds. Biopsy Diagnosis of Peripheral Neuropathy. Cham: Springer International Publishing; 2015:1‐20. [Google Scholar]
- 19.Hart MG, Santarius T, Trivedi RA. Muscle and nerve biopsy for the neurosurgical trainee. Br J Neurosurg. 2013;27(6):727‐734. [DOI] [PubMed] [Google Scholar]
- 20.Hughes RAC. Complications from nerve biopsy. In: Vallat JM, Weis J, Gray F, Keohane K, eds. Peripheral Nerve Disorders: Pathology and Genetics. Hoboken, NJ: Wiley Blackwell; 2014. Accessed September 3, 2019. [Google Scholar]
- 21.Brandner S. The pathological diagnosis of nerve biopsies: a practical approach. Diagn Histopathol. 2016;22(9):333‐344. [Google Scholar]
- 22.Weis J, Brandner S, Lammens M, Sommer C, Vallat J‐M. Processing of nerve biopsies: a practical guide for neuropathologists. Clin Neuropathol. 2012;31(1):7‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kollewe K, Brandis A, Mohammadi B, Dengler R, Vogt P, Knobloch K. Cost‐effectiveness of sural nerve biopsies – a single center experience. Klin Neurophysiol. 2011;42(01):P259. [Google Scholar]
- 24.Centers for Medicare and Medicaid Services . Procedure Price Lookup 2020. https://www.medicare.gov/procedure-price-lookup/cost/64795. Accessed January 1, 2021.
- 25.Centers for Medicare and Medicaid Services . Physician Fee Schedule Search 2020. https://www.cms.gov/apps/physician-fee-schedule/search/search-criteria.aspx. Accessed January 1, 2021.
- 26.Commonwealth of Australia Department of Health . Medicare Benefits Schedule 2018. http://www.mbsonline.gov.au/internet/mbsonline/publishing.nsf/Content/Home. Accessed June 30, 2020.
- 27.Gwathmey KG, Tracy JA, Dyck PJB. Peripheral nerve vasculitis: classification and disease associations. Neurol Clin. 2019;37(2):303‐333. [DOI] [PubMed] [Google Scholar]
- 28.Vrancken AFJE, Gathier CS, Cats EA, Notermans NC, Collins MP. The additional yield of combined nerve/muscle biopsy in vasculitic neuropathy. Eur J Neurol. 2011;18(1):49‐58. [DOI] [PubMed] [Google Scholar]
- 29.Bennett DLH, Groves M, Blake J, et al. The use of nerve and muscle biopsy in the diagnosis of vasculitis: a 5 year retrospective study. J Neurol Neurosurg Psychiatry. 2008;79(12):1376‐1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Collins MP, Hadden RD. The nonsystemic vasculitic neuropathies. Nat Rev Neurol. 2017;13:302‐316. [DOI] [PubMed] [Google Scholar]
- 31.Masuda H, Misawa S, Arai K, et al. Combined nerve/muscle/skin biopsy could increase diagnostic sensitivity for vasculitic neuropathy. Clin Exp Neuroimmunol. 2015;6(3):312‐317. [Google Scholar]
- 32.Üçeyler N, Devigili G, Toyka KV, Sommer C. Skin biopsy as an additional diagnostic tool in non‐systemic vasculitic neuropathy. Acta Neuropathol. 2010;120(1):109‐116. [DOI] [PubMed] [Google Scholar]
- 33.Üçeyler N, Braunsdorf S, Kunze E, et al. Cellular infiltrates in skin and sural nerve of patients with polyneuropathies. Muscle Nerve. 2017;55(6):884‐893. [DOI] [PubMed] [Google Scholar]
- 34.Hadden RDM, Collins MP, Živković SA, et al. Vasculitic peripheral neuropathy: case definition and guidelines for collection, analysis, and presentation of immunisation safety data. Vaccine. 2017;35(11):1567‐1578. [DOI] [PubMed] [Google Scholar]
- 35.Grimm A, Décard BF, Bischof A, Axer H. Ultrasound of the peripheral nerves in systemic vasculitic neuropathies. J Neurol Sci. 2014;347(1):44‐49. [DOI] [PubMed] [Google Scholar]
- 36.Goedee HS, van der Pol WL, van Asseldonk J‐TH, et al. Nerve sonography to detect peripheral nerve involvement in vasculitis syndromes. Neurol Clin Pract. 2016;6(4):293‐303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chipman JN, Mott RT, Stanton CA, Cartwright MS. Ultrasonographic Tinel sign. Muscle Nerve. 2009;40(6):1033‐1035. [DOI] [PubMed] [Google Scholar]
- 38.Shree R, Goyal MK, Modi M, et al. The diagnostic dilemma of neurolymphomatosis. J Clin Neurol. 2016;12(3):274‐281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ramirez‐Zamora A, Morales‐Vidal S, Chawla J, Biller J. Autopsy proven peripheral nervous system neurolymphomatosis despite negative bilateral sural nerve biopsy. Front Neurol. 2013;4:197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Duchesne M, Mathis S, Corcia P, et al. Value of nerve biopsy in patients with latent malignant hemopathy and peripheral neuropathy: a case series. Medicine. 2015;94(3):e394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Duchesne M, Roussellet O, Maisonobe T, et al. Pathology of nerve biopsy and diagnostic yield of PCR‐based clonality testing in neurolymphomatosis. J Neuropathol Exp Neurol. 2018;77(9):769‐781. [DOI] [PubMed] [Google Scholar]
- 42.Ducatman BS, Scheithauer BW, Piepgras DG, Reiman HM, Ilstrup DM. Malignant peripheral nerve sheath tumors. A clinicopathologic study of 120 cases. Cancer. 1986;57(10):2006‐2021. [DOI] [PubMed] [Google Scholar]
- 43.Kourea HP, Bilsky MH, Leung DHY, Lewis JJ, Woodruff JM. Subdiaphragmatic and intrathoracic paraspinal malignant peripheral nerve sheath tumors. Cancer. 1998;82(11):2191‐2203. [DOI] [PubMed] [Google Scholar]
- 44.Bishop AJ, Zagars GK, Torres KE, Bird JE, Feig BW, Guadagnolo BA. Malignant peripheral nerve sheath tumors: a single institutionʼs experience using combined surgery and radiation therapy. Am J Clin Oncol. 2018;41(5):465‐470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zou C, Smith KD, Liu J, et al. Clinical, pathological, and molecular variables predictive of malignant peripheral nerve sheath tumor outcome. Ann Surg. 2009;249(6):1014‐1022. [DOI] [PubMed] [Google Scholar]
- 46.Mauermann ML, Amrami KK, Kuntz NL, et al. Longitudinal study of intraneural perineurioma—a benign, focal hypertrophic neuropathy of youth. Brain. 2009;132(pt 8):2265‐2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Abreu E, Aubert S, Wavreille G, Gheno R, Canella C, Cotten A. Peripheral tumor and tumor‐like neurogenic lesions. Eur J Radiol. 2013;82(1):38‐50. [DOI] [PubMed] [Google Scholar]
- 48.Mauermann ML, Scheithauer BW, Spinner RJ, et al. Inflammatory pseudotumor of nerve: clinicopathological characteristics and a potential therapy. J Peripher Nerv Syst. 2010;15(3):216‐226. [DOI] [PubMed] [Google Scholar]
- 49.Niederhauser BD, Spinner RJ, Jentoft ME, Everist BM, Matsumoto JM, Amrami KK. Neuromuscular choristoma: characteristic magnetic resonance imaging findings and association with post‐biopsy fibromatosis. Skeletal Radiol. 2013;42(4):567‐577. [DOI] [PubMed] [Google Scholar]
- 50.Santos DF, Mendonça MR, Antunes DE, et al. Revisiting primary neural leprosy: clinical, serological, molecular, and neurophysiological aspects. PLoS Negl Trop Dis. 2017;11(11):e0006086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Garbino JA, Ura S, Belone AD, Marciano LH, Fleury RN. Clinical and diagnostic aspects of the primarily neural leprosy. Hansenol Int. 2004;29:130‐136. [Google Scholar]
- 52.Suneetha S, Arunthathi S, Kurian N, Chacko CJ. Histological changes in the nerve, skin and nasal mucosa of patients with primary neuritic leprosy. Acta Leprol. 2000;12(1):11‐18. [PubMed] [Google Scholar]
- 53.Menicucci LA, Miranda A, Antunes SLG, et al. Microscopic leprosy skin lesions in primary neuritic leprosy. J Am Acad Dermatol. 2005;52(4):648‐652. [DOI] [PubMed] [Google Scholar]
- 54.De A, Hasanoor Reja A, Aggarwal I, et al. Use of fine needle aspirate from peripheral nerves of pure‐neural leprosy for cytology and polymerase chain reaction to confirm the diagnosis: a follow‐up study of 4 years. Indian J Dermatol. 2017;62(6):635‐643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Loavenbruck AJ, Singer W, Mauermann ML, et al. Transthyretin amyloid neuropathy has earlier neural involvement but better prognosis than primary amyloid counterpart: an answer to the paradox? Ann Neurol. 2016;80(3):401‐411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gertz MA, Benson MD, Dyck PJ, et al. Diagnosis, prognosis, and therapy of transthyretin amyloidosis. J Am Coll Cardiol. 2015;66(21):2451‐2466. [DOI] [PubMed] [Google Scholar]
- 57.Cappellari M, Cavallaro T, Ferrarini M, et al. Variable presentations of TTR‐related familial amyloid polyneuropathy in seventeen patients. J Peripher Nerv Syst. 2011;16(2):119‐129. [DOI] [PubMed] [Google Scholar]
- 58.Koike H, Hashimoto R, Tomita M, et al. Diagnosis of sporadic transthyretin Val30Met familial amyloid polyneuropathy: a practical analysis. Amyloid. 2011;18(2):53‐62. [DOI] [PubMed] [Google Scholar]
- 59.Vital A, Vital C. Clinical neuropathology practice guide 3‐2014: combined nerve and muscle biopsy in the diagnostic work‐up of neuropathy – the Bordeaux experience. Clin Neuropathol. 2014;33(3):172‐178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vital C, Lagueny A, Mercie P, Viallard JF, Delabrousse‐Mayoux JP, Vital A. Usefulness of combined nerve and muscle biopsy in the diagnosis of amyloid neuropathy—a study of 6 new cases. Clin Neuropathol. 2010;29(2):59‐64. [DOI] [PubMed] [Google Scholar]
- 61.Coelho T, Maia LF, da Silva AM, et al. Long‐term effects of tafamidis for the treatment of transthyretin familial amyloid polyneuropathy. J Neurol. 2013;260(11):2802‐2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Adams D, Gonzalez‐Duarte A, OʼRiordan WD, et al. Patisiran, an RNAi therapeutic, for hereditary transthyretin amyloidosis. N Engl J Med. 2018;379(1):11‐21. [DOI] [PubMed] [Google Scholar]
- 63.Benson MD, Waddington‐Cruz M, Berk JL, et al. Inotersen treatment for patients with hereditary transthyretin amyloidosis. N Engl J Med. 2018;379(1):22‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cortese A, Vegezzi E, Lozza A, et al. Diagnostic challenges in hereditary transthyretin amyloidosis with polyneuropathy: avoiding misdiagnosis of a treatable hereditary neuropathy. J Neurol Neurosurg Psychiatry. 2017;88(5):457‐458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vaxman I, Gertz M. Recent advances in the diagnosis, risk stratification, and management of systemic light‐chain amyloidosis. Acta Haematol. 2019;141(2):93‐106. [DOI] [PubMed] [Google Scholar]
- 66.Fernández de Larrea C, Verga L, Morbini P, et al. A practical approach to the diagnosis of systemic amyloidoses. Blood. 2015;125(14):2239‐2244. [DOI] [PubMed] [Google Scholar]
- 67.DʼSa S, Kersten MJ, Castillo JJ, et al. Investigation and management of IgM and Waldenström‐associated peripheral neuropathies: recommendations from the IWWM‐8 consensus panel. Br J Haematol. 2017;176(5):728‐742. [DOI] [PubMed] [Google Scholar]
- 68.Simmons Z, Blaivas M, Aguilera AJ, Feldman EL, Bromberg MB, Towfighi J. Low diagnostic yield of sural nerve biopsy in patients with peripheral neuropathy and primary amyloidosis. J Neurol Sci. 1993;120(1):60‐63. [DOI] [PubMed] [Google Scholar]
- 69.Vital C, Vital A, Bouillot‐Eimer S, Brechenmacher C, Ferrer X, Lagueny A. Amyloid neuropathy: a retrospective study of 35 peripheral nerve biopsies. J Peripher Nerv Syst. 2004;9(4):232‐241. [DOI] [PubMed] [Google Scholar]
- 70.Klein CJ, Vrana JA, Theis JD, et al. Mass spectrometric–based proteomic analysis of amyloid neuropathy type in nerve tissue. Arch Neurol. 2011;68(2):195‐199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vrana JA, Gamez JD, Madden BJ, Theis JD, Bergen HR 3rd, Dogan A. Classification of amyloidosis by laser microdissection and mass spectrometry‐based proteomic analysis in clinical biopsy specimens. Blood. 2009;114(24):4957‐4959. [DOI] [PubMed] [Google Scholar]
- 72.Holub D, Flodrova P, Pika T, Flodr P, Hajduch M, Dzubak P. Mass spectrometry amyloid typing is reproducible across multiple organ sites. Biomed Res Int. 2019;2019:3689091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vaxman I, Dispenzieri A, Muchtar E, Gertz M. New developments in diagnosis, risk assessment and management in systemic amyloidosis. Blood Rev. 2020;40:100636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gilbertson JA, Theis JD, Vrana JA, et al. A comparison of immunohistochemistry and mass spectrometry for determining the amyloid fibril protein from formalin‐fixed biopsy tissue. J Clin Pathol. 2015;68(4):314‐317. [DOI] [PubMed] [Google Scholar]
- 75.Gertz MA. Immunoglobulin light chain amyloidosis: 2016 update on diagnosis, prognosis, and treatment. Am J Hematol. 2016;91(9):947‐956. [DOI] [PubMed] [Google Scholar]
- 76.Huang C‐Y, Wang W‐J, Wong C‐K. Skin biopsy gives the potential benefit in the diagnosis of systemic amyloidosis associated with cardiac involvement. Arch Dermatol. 1998;134(5):643‐645. [DOI] [PubMed] [Google Scholar]
- 77.Ebenezer GJ, Liu Y, Judge DP, et al. Cutaneous nerve biomarkers in transthyretin familial amyloid polyneuropathy. Ann Neurol. 2017;82(1):44‐56. [DOI] [PubMed] [Google Scholar]
- 78.Lefaucheur J‐P, Zouari HG, Gorram F, Nordine T, Damy T, Planté‐Bordeneuve V. The value of electrochemical skin conductance measurement using Sudoscan® in the assessment of patients with familial amyloid polyneuropathy. Clin Neurophysiol. 2018;129(8):1565‐1569. [DOI] [PubMed] [Google Scholar]
- 79.Misumi Y, Ueda M, Yamashita T, et al. Novel screening for transthyretin amyloidosis by using fat ultrasonography. Ann Neurol. 2017;81(4):604‐608. [DOI] [PubMed] [Google Scholar]
- 80.Ibitoye RT, Wilkins A, Scolding NJ. Neurosarcoidosis: a clinical approach to diagnosis and management. J Neurol. 2017;264(5):1023‐1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Burns TM, Dyck PJB, Aksamit AJ, Dyck PJ. The natural history and long‐term outcome of 57 limb sarcoidosis neuropathy cases. J Neurol Sci. 2006;244(1):77‐87. [DOI] [PubMed] [Google Scholar]
- 82.Said G, Lacroix C, Planté‐Bordeneuve V, et al. Nerve granulomas and vasculitis in sarcoid peripheral neuropathy: a clinicopathological study of 11 patients. Brain. 2002;125(2):264‐275. [DOI] [PubMed] [Google Scholar]
- 83.Bagnato F, Stern BJ. Neurosarcoidosis: diagnosis, therapy and biomarkers. Expert Rev Neurother. 2015;15(5):533‐548. [DOI] [PubMed] [Google Scholar]
- 84.Tavee JO, Karwa K, Ahmed Z, Thompson N, Parambil J, Culver DA. Sarcoidosis‐associated small fiber neuropathy in a large cohort: clinical aspects and response to IVIG and anti‐TNF alpha treatment. Respir Med. 2017;126:135‐138. [DOI] [PubMed] [Google Scholar]
- 85.Lacomis D. Neurosarcoidosis. Curr Neuropharmacol. 2011;9(3):429‐436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kerasnoudis A, Woitalla D, Gold R, Pitarokoili K, Yoon MS. Sarcoid neuropathy: correlation of nerve ultrasound, electrophysiological and clinical findings. J Neurol Sci. 2014;347(1):129‐136. [DOI] [PubMed] [Google Scholar]
- 87.Inoue D, Zen Y, Sato Y, et al. IgG4‐related perineural disease. Int J Rheumatol. 2012;2012:401890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mathey EK, Park SB, Hughes RAC, et al. Chronic inflammatory demyelinating polyradiculoneuropathy: from pathology to phenotype. J Neurol Neurosurg Psychiatry. 2015;86(9):973‐985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ogata H, Yamasaki R. Anti‐neurofascin 155 antibody‐related neuropathy. Clin Exp Neuroimmunol. 2018;9(1):54‐64. [Google Scholar]
- 90.Baptista B, Casian A, Gunawardena H, DʼCruz D, Rice CM. Neurological manifestations of IgG4‐related disease. Curr Treat Options Neurol. 2017;19(4):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yokoi S, Kawagashira Y, Ohyama K, et al. Mononeuritis multiplex with tumefactive cellular infiltration in a patient with reactive lymphoid hyperplasia with increased immunoglobulin G4–positive cells. Hum Pathol. 2014;45(2):427‐430. [DOI] [PubMed] [Google Scholar]
- 92.Waheed W, Nickerson J, Ambaye AB, Babi M‐A, Tandan R. IgG4‐related neuromyopathy associated with recurrent pleural effusion. J Clin Neuromuscul Dis. 2015;16(4):210‐219. [DOI] [PubMed] [Google Scholar]
- 93.Yu S, Makoto S, Hiroshi Y, Masato D, Masayuki K, Yasuhiro H. A case of IgG4‐related peripheral neuropathy due to significant axonal injury and resistant to treatment. Clin Neurol. 2016;56(5):323‐327. [Google Scholar]
- 94.Zhan L, Fan M, Cai N, Cai B. Combination of autoimmune pancreatitis and peripheral neuropathy on an IgG4‐related disease patient with 4 years following‐up. J Neuroimmunol. 2020;348:577378. [DOI] [PubMed] [Google Scholar]
- 95.Ohyama K, Koike H, Takahashi M, et al. Immunoglobulin G4‐related pathologic features in inflammatory neuropathies. Neurology. 2015;85(16):1400‐1407. [DOI] [PubMed] [Google Scholar]
- 96.Chaudhry HM, Mauermann ML, Rajkumar SV. Monoclonal gammopathy–associated peripheral neuropathy: diagnosis and management. Mayo Clin Proc. 2017;92(5):838‐850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hadden RDM, Nobile‐Orazio E, Sommer CL, et al. European Federation of Neurological Societies/Peripheral Nerve Society Guideline on management of paraproteinemic demyelinating neuropathies. Report of a Joint Task Force of the European Federation of Neurological Societies and the Peripheral Nerve Society – first revision. J Peripher Nerv Syst. 2010;15(3):185‐195. [DOI] [PubMed] [Google Scholar]
- 98.Sekiguchi Y, Misawa S, Shibuya K, et al. Ambiguous effects of anti‐VEGF monoclonal antibody (bevacizumab) for POEMS syndrome. J Neurol Neurosurg Psychiatry. 2013;84(12):1346‐1348. [DOI] [PubMed] [Google Scholar]
- 99.Mathis S, Franques J, Richard L, Vallat J‐M. Monoclonal gammopathy of undeterminated significance and endoneurial IgG deposition: a case report. Medicine. 2016;95(36):e4807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kawagashira Y, Koike H, Tomita M, et al. Morphological progression of myelin abnormalities in IgM‐monoclonal gammopathy of undetermined significance anti‐myelin‐associated glycoprotein neuropathy. J Neuropathol Exp Neurol. 2010;69(11):1143‐1157. [DOI] [PubMed] [Google Scholar]
- 101.Bleasel AF, Hawke SH, Pollard JD, McLeod JG. IgG monoclonal paraproteinaemia and peripheral neuropathy. J Neurol Neurosurg Psychiatry. 1993;56(1):52‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Magy L, Kabore R, Mathis S, et al. Heterogeneity of polyneuropathy associated with anti‐MAG antibodies. J Immunol Res. 2015;2015:450391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Vital A, Vital C, Julien J, Baquey A, Steck AJ. Polyneuropathy associated with IgM monoclonal gammopathy. Acta Neuropathol. 1989;79(2):160‐167. [DOI] [PubMed] [Google Scholar]
- 104.Ellie E, Vital A, Steck A, Boiron J‐M, Vital C, Julien J. Neuropathy associated with “benign” anti‐myelin‐associated glycoprotein IgM gammopathy: clinical, immunological, neurophysiological pathological findings and responses to treatment in 33 cases. J Neurol. 1996;243(1):34‐43. [DOI] [PubMed] [Google Scholar]
- 105.Vallat J‐M, Tabaraud F, Sindou P, Preux P‐M, Vandenberghe A, Steck A. Myelin widenings and MGUS‐IgA: an immunoelectron microscopic study. Ann Neurol. 2000;47(6):808‐811. [PubMed] [Google Scholar]
- 106.Vallat J‐M, Magy L, Sindou P, Magdelaine C, Cros D. IgG neuropathy: an Immunoelectron microscopic study. J Neuropathol Exp Neurol. 2005;64(5):386‐390. [DOI] [PubMed] [Google Scholar]
- 107.Vital C, Vital A, Ferrer X, et al. Crow–Fukase (POEMS) syndrome: a study of peripheral nerve biopsy in five new cases. J Peripher Nerv Syst. 2003;8(3):136‐144. [DOI] [PubMed] [Google Scholar]
- 108.Vallat JM, Magy L, Richard L, Sturtz F, Couratier P. Contribution of electron microscopy to the study of neuropathies associated with an IgG monoclonal paraproteinemia. Micron. 2008;39(2):61‐70. [DOI] [PubMed] [Google Scholar]
- 109.Cerri F, Falzone YM, Riva N, Quattrini A. An update on the diagnosis and management of the polyneuropathy of POEMS syndrome. J Neurol. 2019;266(1):258‐267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ramos‐Casals M, Stone JH, Cid MC, Bosch X. The cryoglobulinaemias. Lancet. 2012;379(9813):348‐360. [DOI] [PubMed] [Google Scholar]
- 111.Nemni R, Corbo M, Fazio R, Quattrini A, Comi G, Canal N. Cryoglobulinaemic neuropathy. A clinical, morphological and immunocytochemical study of 8 cases. Brain. 1988;111(3):541‐552. [DOI] [PubMed] [Google Scholar]
- 112.Ciompi ML, Marini D, Siciliano G, et al. Cryoglobulinemic peripheral neuropathy: neurophysiologic evaluation in twenty‐two patients. Biomed Pharmacother. 1996;50(8):329‐336. [DOI] [PubMed] [Google Scholar]
- 113.Vallat JM, Desproges‐Gotteron R, Leboutet MJ, Loubet A, Gualde N, Treves R. Cryoglobulinemic neuropathy: a pathological study. Ann Neurol. 1980;8(2):179‐185. [DOI] [PubMed] [Google Scholar]
- 114.Van den Bergh PYK, Hadden RDM, Bouche P, et al. European Federation of Neurological Societies/Peripheral Nerve Society Guideline on management of chronic inflammatory demyelinating polyradiculoneuropathy: report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society – first revision. J Peripher Nerv Syst. 2010;15(1):1‐9. [DOI] [PubMed] [Google Scholar]
- 115.Viala K, Maisonobe T, Stojkovic T, et al. A current view of the diagnosis, clinical variants, response to treatment and prognosis of chronic inflammatory demyelinating polyradiculoneuropathy. J Peripher Nerv Syst. 2010;15(1):50‐56. [DOI] [PubMed] [Google Scholar]
- 116.Luigetti M, Romano A, Di Paolantonio A, et al. Pathological findings in chronic inflammatory demyelinating polyradiculoneuropathy: a single‐center experience. Brain Sci. 2020;10(6):383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tracy JA, Dyck PJ, Klein CJ, Engelstad JK, Meyer JE, Dyck PJB. Onion‐bulb patterns predict acquired or inherited demyelinating polyneuropathy. Muscle Nerve. 2019;59(6):665‐670. [DOI] [PubMed] [Google Scholar]
- 118.Sommer C, Koch S, Lammens M, Gabreels‐Festen A, Stoll G, Toyka KV. Macrophage clustering as a diagnostic marker in sural nerve biopsies of patients with CIDP. Neurology. 2005;65(12):1924‐1929. [DOI] [PubMed] [Google Scholar]
- 119.Jann S, Bramerio MA, Beretta S, et al. Diagnostic value of sural nerve matrix metalloproteinase‐9 in diabetic patients with CIDP. Neurology. 2003;61(11):1607‐1610. [DOI] [PubMed] [Google Scholar]
- 120.Cornblath DR, Asbury AK, Albers JW, et al. Research criteria for diagnosis of chronic inflammatory demyelinating polyneuropathy (CIDP). Neurology. 1991;41(5):617‐618. [PubMed] [Google Scholar]
- 121.Rajabally YA, Stettner M, Kieseier BC, Hartung H‐P, Malik RA. CIDP and other inflammatory neuropathies in diabetes — diagnosis and management. Nat Rev Neurol. 2017;13:599‐611. [DOI] [PubMed] [Google Scholar]
- 122.Vo ML, Hanineva A, Chin RL, Carey BT, Latov N, Langsdorf JA. Comparison of 2‐limb versus 3‐limb electrodiagnostic studies in the evaluation of chronic inflammatory demyelinating polyneuropathy. Muscle Nerve. 2015;51(4):549‐553. [DOI] [PubMed] [Google Scholar]
- 123.Attarian S, Franques J, Elisabeth J, et al. Triple‐stimulation technique improves the diagnosis of chronic inflammatory demyelinating polyradiculoneuropathy. Muscle Nerve. 2015;51(4):541‐548. [DOI] [PubMed] [Google Scholar]
- 124.Lozeron P, Lacour M‐C, Vandendries C, et al. Contribution of plexus MRI in the diagnosis of atypical chronic inflammatory demyelinating polyneuropathies. J Neurol Sci. 2016;360(suppl C):170‐175. [DOI] [PubMed] [Google Scholar]
- 125.Kerasnoudis A, Pitarokoili K, Gold R, Yoon MS. Bochum ultrasound score allows distinction of chronic inflammatory from multifocal acquired demyelinating polyneuropathies. J Neurol Sci. 2015;348(1–2):211‐215. [DOI] [PubMed] [Google Scholar]
- 126.Jongbloed BA, Bos JW, Rutgers D, van der Pol WL, van den Berg LH. Brachial plexus magnetic resonance imaging differentiates between inflammatory neuropathies and does not predict disease course. Brain Behav. 2017;7(5):e00632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Niu J, Cui L, Liu M. Multiple sites ultrasonography of peripheral nerves in differentiating Charcot–Marie–Tooth type 1A from chronic inflammatory demyelinating polyradiculoneuropathy. Front Neurol. 2017;8:181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Shen J, Cartwright MS. Neuromuscular ultrasound in the assessment of polyneuropathies and motor neuron disease. J Clin Neurophysiol. 2016;33(2):86‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Grimm A, Vittore D, Schubert V, et al. Ultrasound aspects in therapy‐naive CIDP compared to long‐term treated CIDP. J Neurol. 2016;263(6):1074‐1082. [DOI] [PubMed] [Google Scholar]
- 130.Duchesne M, Mathis S, Richard L, et al. Nerve biopsy is still useful in some inherited neuropathies. J Neuropathol Exp Neurol. 2018;77(2):88‐99. [DOI] [PubMed] [Google Scholar]
- 131.Mrak RE. The Big Eye in the 21st century: the role of electron microscopy in modern diagnostic neuropathology. J Neuropathol Exp Neurol. 2002;61(12):1027‐1039. [DOI] [PubMed] [Google Scholar]
- 132.Hellmann MA, Kakhlon O, Landau EH, et al. Frequent misdiagnosis of adult polyglucosan body disease. J Neurol. 2015;262(10):2346‐2351. [DOI] [PubMed] [Google Scholar]
- 133.Mochel F, Schiffmann R, Steenweg ME, et al. Adult polyglucosan body disease: natural history and key magnetic resonance imaging findings. Ann Neurol. 2012;72(3):433‐441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Klein CJ. Adult polyglucosan body disease. 2009. In: GeneReviews [Internet]. Seattle, WA: University of Washington, Seattle. https://www.ncbi.nlm.nih.gov/books/NBK5300/. Accessed September 30, 2019.
- 135.Xu M, Pinto M, Sun C, et al. Expanded teased nerve fibre pathological conditions in disease association. J Neurol Neurosurg Psychiatry. 2019;90(2):138‐140. [DOI] [PubMed] [Google Scholar]
- 136.Riva N, Iannaccone S, Corbo M, et al. Motor nerve biopsy: clinical usefulness and histopathological criteria. Ann Neurol. 2011;69(1):197‐201. [DOI] [PubMed] [Google Scholar]
- 137.Garg N, Park SB, Vucic S, et al. Differentiating lower motor neuron syndromes. J Neurol Neurosurg Psychiatry. 2017;88(6):474‐483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Jongbloed BA, Haakma W, Goedee HS, et al. Comparative study of peripheral nerve Mri and ultrasound in multifocal motor neuropathy and amyotrophic lateral sclerosis. Muscle Nerve. 2016;54(6):1133‐1135. [DOI] [PubMed] [Google Scholar]
- 139.Grimm A, Décard BF, Athanasopoulou I, Schweikert K, Sinnreich M, Axer H. Nerve ultrasound for differentiation between amyotrophic lateral sclerosis and multifocal motor neuropathy. J Neurol. 2015;262(4):870‐880. [DOI] [PubMed] [Google Scholar]
- 140.Katona I, Weis J. Chapter 31 ‐ Diseases of the peripheral nerves. In: Kovacs GG, Alafuzoff I, eds. Handbook of Clinical Neurology. Vol 145. Amsterdam: Elsevier; 2017:453‐474. [DOI] [PubMed] [Google Scholar]
- 141.Weis S, Büttner A. Chapter 14 ‐ Neurotoxicology and drug‐related disorders. In: Kovacs GG, Alafuzoff I, eds. Handbook of Clinical Neurology. Vol 145. Amsterdam: Elsevier; 2017:181‐192. [DOI] [PubMed] [Google Scholar]
- 142.Little AA, Albers JW. Chapter 16 ‐ Clinical description of toxic neuropathies. In: Lotti M, Bleecker ML, eds. Handbook of Clinical Neurology. Vol 131. Amsterdam: Elsevier; 2015:253‐296. [DOI] [PubMed] [Google Scholar]
- 143.Pellissier JF, Pouget J, Cros D, De Victor B, Serratrice G, Toga M. Peripheral neuropathy induced by amiodarone chlorhydrate: a clinicopathological study. J Neurol Sci. 1984;63(2):251‐266. [DOI] [PubMed] [Google Scholar]
- 144.Estes ML, Ewing‐Wilson D, Chou SM, et al. Chloroquine neuromyotoxicity. Clinical and pathologic perspective. Am J Med. 1987;82(3):447‐455. [DOI] [PubMed] [Google Scholar]
- 145.Rutchik JS. Occupational medicine physicianʼs guide to neuropathy in the workplace, part 2: electromyography and cryptogenic and toxic neuropathy. J Occup Environ Med. 2009;51(5):622‐625. [DOI] [PubMed] [Google Scholar]
- 146.Chahin N, Temesgen Z, Kurtin PJ, Spinner RJ, Dyck PJB. HIV lumbosacral radiculoplexus neuropathy mimicking lymphoma: diffuse infiltrative lymphocytosis syndrome (DILS) restricted to nerve? Muscle Nerve. 2010;41(2):276‐282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.England JD, Gronseth GS, Franklin G, et al. Practice parameter: the evaluation of distal symmetric polyneuropathy: the role of autonomic testing, nerve biopsy, and skin biopsy (an evidence‐based review). PM R. 2009;1(1):14‐22. [DOI] [PubMed] [Google Scholar]
- 148.Wolfe GI, Baker NS, Amato AA, et al. Chronic cryptogenic sensory polyneuropathy: clinical and laboratory characteristics. Arch Neurol. 1999;56(5):540‐547. [DOI] [PubMed] [Google Scholar]
- 149.Notermans NC, Wokke JH, Franssen H, et al. Chronic idiopathic polyneuropathy presenting in middle or old age: a clinical and electrophysiological study of 75 patients. J Neurol Neurosurg Psychiatry. 1993;56(10):1066‐1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Traub R. Neuropathies, idiopathic. In: Aminoff MJ, Daroff RB, eds. Encyclopedia of the Neurological Sciences. 2nd ed.Oxford: Academic Press; 2014:485‐488. [Google Scholar]
- 151.Querol L, Devaux J, Rojas‐Garcia R, Illa I. Autoantibodies in chronic inflammatory neuropathies: diagnostic and therapeutic implications. Nat Rev Neurol. 2017;13:533‐547. [DOI] [PubMed] [Google Scholar]
- 152.Ubogu EE. Inflammatory neuropathies: pathology, molecular markers and targets for specific therapeutic intervention. Acta Neuropathol. 2015;130(4):445‐468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Nathani D, Barnett MH, Spies J, Pollard J, Wang M‐X, Kiernan MC. Vasculitic neuropathy: comparison of clinical predictors with histopathological outcome. Muscle Nerve. 2019;59(6):643‐649. [DOI] [PubMed] [Google Scholar]
- 154.Dyck PJ, Dyck PJB, Engelstad J. Chapter 32 ‐ pathologic alterations of nerves. In: Dyck PJ, Thomas PK, eds. Peripheral Neuropathy. 4th ed.Philadelphia: W.B. Saunders; 2005:733‐829. [Google Scholar]
- 155.Deprez M, Ceuterick‐de Groote C, Gollogly L, Reznik M, Martin JJ. Clinical and neuropathological parameters affecting the diagnostic yield of nerve biopsy. Neuromuscul Disord. 2000;10(2):92‐98. [DOI] [PubMed] [Google Scholar]
- 156.Anish L, Nagappa M, Mahadevan A, Taly AB. Neuropathy in elderly: lessons learnt from nerve biopsy. Age Ageing. 2015;44(2):312‐317. [DOI] [PubMed] [Google Scholar]
- 157.Rappaport WD, Valente J, Hunter GC, et al. Clinical utilization and complications of sural nerve biopsy. Am J Surg. 1993;166(3):252‐256. [DOI] [PubMed] [Google Scholar]
- 158.Mellgren SI, Lindal S. Nerve biopsy – some comments on procedures and indications. Acta Neurol Scand. 2011;124(s191):64‐70. [DOI] [PubMed] [Google Scholar]
- 159.Eurelings M, Notermans N, Wokke J, Bosboom W, Van den Berg L. Sural nerve T cells in demyelinating polyneuropathy associated with monoclonal gammopathy. Acta Neuropathol. 2002;103(2):107‐114. [DOI] [PubMed] [Google Scholar]
- 160.Eurelings M, van den Berg LH, Wokke JHJ, Franssen H, Vrancken AFJE, Notermans NC. Increase of sural nerve T cells in progressive axonal polyneuropathy and monoclonal gammopathy. Neurology. 2003;61(5):707‐709. [DOI] [PubMed] [Google Scholar]
- 161.Bendixen BH, Younger DS, Hair LS, et al. Cholesterol emboli neuropathy. Neurology. 1992;42(2):428‐430. [DOI] [PubMed] [Google Scholar]
- 162.Vital C, Heraud A, Vital A, Coquet M, Julien M, Maupetit J. Acute mononeuropathy with angiotropic lymphoma. Acta Neuropathol. 1989;78(1):105‐107. [DOI] [PubMed] [Google Scholar]
- 163.Dy CJ, Lange DJ, Jones KJ, Garg R, DiCarlo EF, Wolfe SW. Diagnostic biopsy of the pronator teres and a motor branch of the median nerve: indications and technique. J Hand Surg Am. 2012;37(12):2570‐2575. [DOI] [PubMed] [Google Scholar]
- 164.DʼAmico RS, Winfree CJ. Diagnostic biopsy of a motor branch of the superficial peroneal nerve to the peroneus longus: a convenient alternative for motor nerve biopsy. World Neurosurg. 2017;103(suppl C):526‐s. [DOI] [PubMed] [Google Scholar]
- 165.Capek S, Amrami KK, Dyck PJB, Spinner RJ. Targeted fascicular biopsy of the sciatic nerve and its major branches: rationale and operative technique. Neurosurg Focus. 2015;39(3):E12. [DOI] [PubMed] [Google Scholar]
- 166.Laumonerie P, Capek S, Amrami KK, Dyck PJB, Spinner RJ. Targeted fascicular biopsy of the brachial plexus: rationale and operative technique. Neurosurg Focus. 2017;42(3):E9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


