Abstract
Purpose
To describe the methodology and pilot data of the Shanghai Child and Adolescent Large‐scale Eye Study (SCALE‐HM).
Methods
This is a population‐based, prospective, examiner‐masked study with annual follow‐up. Patients are 4‐ to 18‐year‐olds with high myopia. The participants will fill out questionnaires and then undergo visual acuity, axial length (AL), intraocular pressure, ophthalmologist assessment, microperimetry, cycloplegic refraction, Pentacam, wavefront aberration, fundus, blood and saliva examinations. To describe the pilot data, intergroup differences were assessed with t‐tests or analysis of variance and a logistic regression model was used to determine the independent factors associated with peripapillary atrophy (PPA).
Results
Overall, 134 eyes of 79 participants met the pilot study recruitment criteria. The mean AL and spherical equivalent were 26.91 ± 1.07 mm and −9.40 ± 1.77 D, respectively. Peripapillary atrophy (PPA) (N = 112) and tessellated fundus (N = 67) were the most common fundus changes. The mean AL was significantly longer in PPA (27.08 ± 0.93 mm) than in non‐PPA eyes (26.06 ± 1.31 mm; p < 0.001). Axial length (AL) (p = 0.041) was the only independent factor associated with PPA. Axial length (AL) was significantly longer in eyes with diffuse chorioretinal atrophy (N = 11; 28.02 ± 1.31 mm) than without myopic retinal lesions (N = 56; 26.48 ± 0.91 mm, p < 0.001) or with tessellated fundus (N = 67; 27.09 ± 0.97 mm, p = 0.012). The myopic degree was higher in eyes with diffuse chorioretinal atrophy than without myopic retinal lesions (−10.51 ± 2.76 D versus −9.06 ± 1.58 D, p = 0.039).
Conclusion
Peripapillary atrophy and tessellated fundus were common in children and adolescents with high myopia. Results from this prospective study will help to understand the mechanisms, development and prognosis of these changes and can guide early myopia screening.
Keywords: children, fundus change, high myopia, registration study
Introduction
Myopia is a common eye disease in the world (Morgan et al. 2018) and has a particularly high incidence and prevalence, as myopia and high myopia, in developed countries and regions of East and Southeast Asia (Matsumura & Hirai 1999; He et al. 2004; Morgan et al. 2012; Jung et al. 2012; Wu et al. 2013b; Lee et al. 2013; Koh et al. 2014; Morgan et al. 2018). Current predictions are that the number of persons with myopia will reach 5 billion globally by 2050 and that one billion will have high myopia (Holden et al. 2016). One study in Taiwan showed that more than 80% of adolescents suffered from myopia by the time they graduated from high school and 10% of these had high myopia (Lin et al. 2004). Another study performed in coastal East China showed that about 14% of the 17‐year‐olds were highly myopic, and 80% were myopic (Wu et al. 2013a). The economic burden of myopia is increasingly heavy, and the complications of high myopia are extremely severe; therefore, both the economic costs and the severity of myopia need greater attention.
The aetiology and pathogenesis of myopia and high myopia are still unclear, although the involvement of both environmental and genetic factors is suspected in the development of these eye conditions. At present, most studies have indicated a direct association between a higher educational level and a higher prevalence and worse degree of myopia (Saw et al. 2007; Ip et al. 2008; Mirshahi et al. 2014), and increasing outdoor time can reduce the incidence of myopia (Wu et al. 2013b; French et al. 2013; He et al. 2015; Xiong et al. 2017b). Moreover, one longitudinal study showed that more time spent on reading and close work and less on outdoor activities in childhood were associated with adulthood high myopia (Pärssinen & Kauppinen 2018). But whether high myopia that is caused by environmental factors gradually develops into pathologic myopia remains unclear. The occurrence of myopia and high myopia may also have a genetic cause, as at least 19 myopia loci have been identified through family studies and twin studies (Yamashiro 2014). However, the pathogenic myopia genes are not fully characterized, nor have genes associated with fundus lesions been identified. One GWAS meta‐analysis identified 161 common variants, explaining only approximately 8% of the phenotypic variance of myopia (Tedja et al. 2019). The question of whether high myopia and pathologic myopia are two different diseases at the genetic level or whether they represent different states controlled by the same genes has also not been established.
Studies focused on the morphological changes and visual functional changes in children with high myopia are limited, and long‐term follow‐up data are rare. Morphological studies have suggested that β‐peripapillary atrophy (β‐PPA) and optic disc tilt are common fundus changes in children with high myopia, but the incidence of posterior staphyloma and retinochoroidal atrophy is low in childhood (Kobayashi et al. 2005; Samarawickrama et al. 2011). Previous studies have also suggested that diffuse retinochoroidal atrophy around the optic disc in childhood could easily progress to pathological myopia in adulthood (Yokoi et al. 2016). However, this conclusion may not be applicable to the general condition, as the children included in this study attended a high myopia clinic very early in life and may cause potential bias. Children with myopia and high myopia also present with thinning of the retina, choroid and sclera (Nagasawa et al. 2013; Read et al. 2013; Jin et al. 2016; Xiong et al. 2017a; Deng et al. 2018; Deng et al. 2019). Besides the changes in the posterior pole, peripheral retinal abnormalities may also exist (Byer 1965; Karlin & Curtin 1976; Pierro et al. 1992; Bansal & Hubbard 2010). One early study showed that approximately one third of highly myopic children had peripheral retinal findings, and lattice degeneration, white‐without‐pressure and retinal tears are common peripheral retinal lesions in children with high myopia (Bansal & Hubbard 2010). The structural changes seen in patients with high myopia are also associated with changes in microperimetry and electrophysiology (Luu et al. 2006; Wolsley et al. 2008; Qin et al. 2010; Gella et al. 2011; Ho et al. 2012; Park et al. 2013; Zaben et al. 2015; Alzaben et al. 2017). Additionally, one longitudinal study reported that visual field defects may develop in high myopes, while the visual ability is only occasionally affected (Fledelius et al. 2019). However, many unknowns still exist with respect to the early morphological and visual functional changes, as well as their developmental processes in children with high myopia.
Based on a large‐scale eye study involving more than 2 million children and adolescents (He et al. 2018), the aim of the present study was to conduct a long‐term prospective study for further exploration of the fundus changes, as well as the accompanying visual functional changes in children with high myopia. Susceptibility genes related to high myopia and myopic fundus changes were also tested to elucidate the mechanisms behind these changes. Differences in living quality, psychology, behaviour and social activities in children with high myopia were also studied, as these features are rarely considered by clinicians.
Materials and Methods
The SCALE‐HM study is a population‐based, prospective, examiner‐masked study. The database of the SCALE study (the methodology of which has been published elsewhere) (He et al. 2018) will be used to select children and adolescents, aged between 4 and 18 years, with high myopia for participation in the study. The trial protocol was approved by the Shanghai General Hospital Ethics committee and adhered to the tenets of the Declaration of Helsinki (ClinicalTrials.gov Identifier: NCT03666052). The study was initiated in 2018 and is expected to continue until 2038. The follow‐up frequency is set at once per year. Given that pathological changes may appear later in life, the follow‐up time may be extended in the future if possible.
Sample size and study recruitment
Because myopia develops with age, children with differing myopia levels will be enrolled based on their ages. To include children who may progress into high myopia with ageing and to avoid bias during sampling, we decided to set our inclusion criteria as follows: For children aged between 4 and 5 years, at least one eye of spherical equivalent (SE, defined as spherical power + 0.5*cylindrical power) ≤−0.5 D will be included; for those aged between 6 and 8 years, at least one eye of SE ≤ −3.0 D will be included; for those aged between 9 and 12 years, at least one eye of SE ≤ −5.0 D will be included; for those aged ≥ 13 years, at least one eye of SE ≤ −6.0 D will be included. More than two million children and adolescents are currently registered in the system, and more than 10 000 meet our recruitment requirements. Assuming a 30% response and this inclusion proportion, the expected initial registration number is about 3000.
Eligibility criteria
Prior to the study commencement, the study rationale will be explained to all children and their parents/guardians. Written informed consent will be obtained from all parents/guardians and from children 12 or more years of age, while oral consent will be obtained from children younger than 12 years. Children who meet the recruitment criteria, who have no organic eye diseases, are in good general condition and reside in Shanghai will be included in the study. Children with organic eye diseases, including amblyopia, strabismus, moderate–severe ptosis, congenital cataract or glaucoma, fundus diseases other than myopia‐related fundus lesions and other cases not suitable for the study, will be excluded. Those who have undergone intraocular or refractive surgeries will also be excluded.
Preparation before data collection
The SCALE‐HM Registration Information System, which included the registration, real‐time collection of data, online feedback of results, daily contact and data management, was established by an information technology company (Gaussinfomad, Beijing, China). All researchers will be trained before taking part in the study. The training contents will be printed as work manuals and distributed to researchers. The ophthalmologists, optometrists and technicians need to learn the working procedures and instruments operations, and to pass relevant tests before formal appointment. Items that will be carried out involving different operators will undergo consistency tests before the study.
Data collection
The process of data collection will be as follows: (a) registration of basic information; (b) filling out the questionnaires; (c) performing examinations and data collection; and (d) obtaining examination result feedback. The following items will be included in the examination programme: visual acuity (VA), axial length (AL), intraocular pressure (IOP), ophthalmologist examination, microperimetry, cycloplegia, autorefraction, subjective refraction, Pentacam examination, optical coherence tomography (OCT)/optical coherence tomography angiography (OCTA), fundus photography, ultrawide‐field fundus photography, wavefront aberration, and blood and saliva sample collection. Among these items, VA, AL, IOP and microperimetry will be measured before cycloplegia. Detailed information about the examination programme is described below, and the flow chart is displayed in Fig. 1.
Fig. 1.
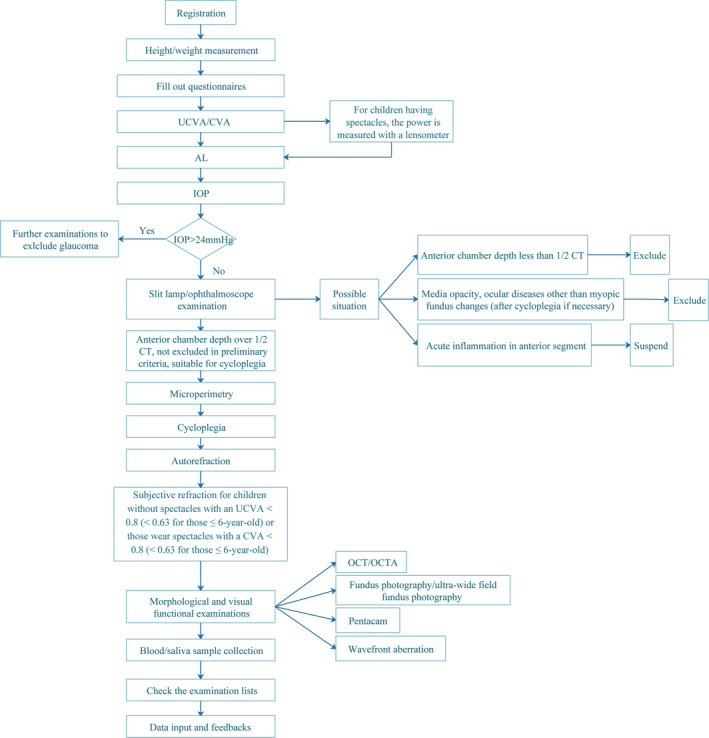
Flow chart of the examination programme.
Registration of basic information
After obtaining consent from both the children and their guardians, the basic information about each child, including ID number, name, birth date, gender and other information regarding the child’s general physical condition will be recorded in the information system.
Questionnaire
Prior to data collection, the participating children, together with their parents or guardians, will be required to fill out questionnaires. Children older than 10 years of age will fill out the questionnaires with the help of their parents or guardians, whereas the questionnaires of children 10 years of age or younger will be filled out by their parents or guardians. The questionnaires will mainly collect information on the following items: general characteristics, family history of myopia, child's medical history of myopia, child's past medical history, treatment and correction for myopia, eye care habits, early‐life health, behaviours and emotions, social ability, academic performance, quality of life and treatment costs. The questionnaire contains 124 items in total and will take about 30 min to fill out.
A number of questionnaires and checklists were consulted during the design of our own questionnaire; these included the National Eye Institute Visual Function Questionnaire (NEI‐VFQ) (Mangione et al. 1998), Achenbach Child Behavior Checklist (CBCL) (Achenbach & Ruffle 2000), Strengths and Difficulties Questionnaire (SDQ), Child Health Questionnaire (CHQ) and Child Vision Care Related Behavior Assessment Scale.
VA, AL and IOP measurements
The VA will be tested with a retro‐illuminated Early Treatment of Diabetic Retinopathy Study (ETDRS) chart (Guangzhou Xieyi Weishikang, Guangzhou, China) at a 4 m distance and tumbling E will be served as optotype. The VA examination will include two parts: uncorrected visual acuity (UCVA) for each participant and corrected visual acuity (CVA, using their habitual correction) for children who wear spectacles, and the results will be recorded as Snellen decimals. For those children with spectacles, the power will be measured with a lensometer (CL‐300, Topcon, Japan). The AL will be measured with an IOL Master 700 (Carl Zeiss Meditec, Germany). Each eye will be measured three times, and if the differences between any two measurements are larger than 0.05 mm, the procedure will be repeated until the differences are on par. The IOP will be measured with a non‐contact tonometer (NT‐510, Nidek, Japan). Each eye will be measured three times, and the mean value will be recorded; the differences between each measurement should be less than 5 mmHg.
Microperimetry
Microperimetry will be performed with a microperimetry device (MP‐3, Nidek, Japan), and the Goldman III, 4‐2‐1 mode will be selected to detect retinal light sensitivity within 10 degrees of the macula. A total of 40 stimulation points will be used (1–8 stimulation points, 3–16 stimulation points, 5–16 stimulation points, shown in Fig. 2), and the stimulation intensity of each point will be ranged from 20 decibels (dB) (equivalent to 20 asb) to 0 dB (equivalent to 400 asb). All participants will receive at least five light stimuli before the formal test to familiarize them with the examination process and to minimize the impact of learning effects. The system will project a super‐threshold stimulus on the physiological blind spot every 60 s to monitor false positive reactions. If a false positive reaction occurs, the examination will be repeated. This item will be optional for children under 10 years old.
Fig. 2.
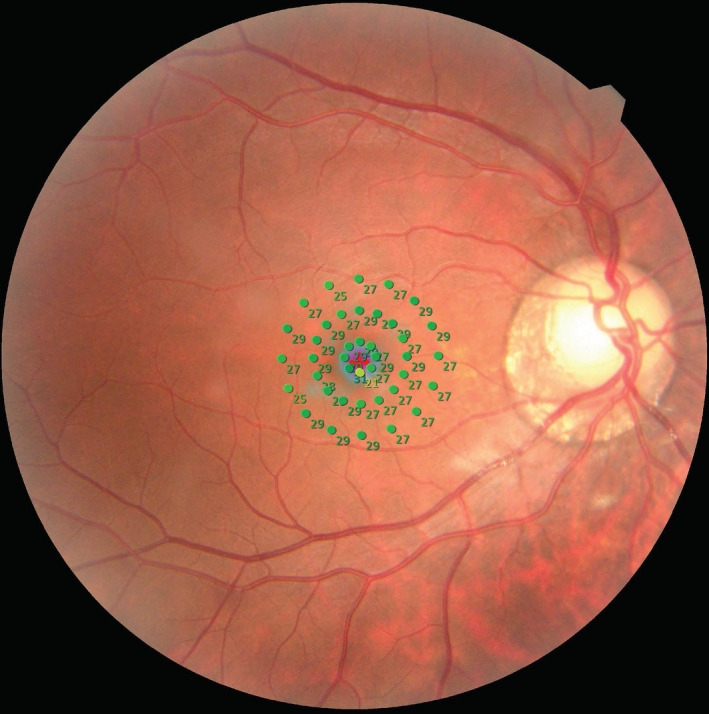
Microperimetry examination results for one participant. The numbers located within 10 degrees of the macula represent the retinal light sensitivity.
Ophthalmologist examinations
Ophthalmologist examinations will include slit lamp and ophthalmoscopy examinations (66 Vision Tech, Suzhou, China). Slit lamp examinations will be performed before cycloplegia, and ophthalmoscopy examinations will be performed after cycloplegia. For participants with peripheral anterior chamber depths less than one half the corneal thickness, with acute anterior segment inflammation, or with other diseases that are not suitable for cycloplegia, the ophthalmologist will record the relevant information and the subsequent examinations will be delayed or cancelled. After cycloplegia, the ophthalmologist will use an ophthalmoscope to determine the presence of any fundus lesions and will record them in detail.
Cycloplegia
The cycloplegia procedure will be performed as follows: one drop of 0.5% proparacaine (Alcaine, Alcon) will be administered to each eye, followed by two drops of 1% cyclopentolate (Cyclogyl, Alcon), with each drop 5 min apart. Light reflex will be checked at least 30 min after the last drop of cyclopentolate is administered. The absence of light reflex and a pupil diameter larger than 6 mm indicate the completion of cycloplegia.
Measurement of refractive status
The refractive status will be measured with an autorefractor (KR‐8900, Topcon, Japan) after cycloplegia. Each eye will be measured three times, and if the differences between any two measurements are larger than 0.5 dioptre (D), the procedure will be repeated. The BCVA will be acquired by subjective refraction examinations for children without spectacles with an UCVA < 0.8 (< 0.63 for children 6 years of age or younger) or those who wear spectacles with a CVA < 0.8 (<0.63 for children 6 years of age or younger).
Pentacam and wavefront aberration
The Pentacam examination (Oculus Optikgeräte GmbH, Wetzlar, Germany) will be conducted after cycloplegia. Measurements will include corneal thickness, corneal diameter and curvature, anterior chamber depth and volume, anterior chamber angle, pupil diameter and lens thickness. Image quality that displays 'OK' and availability of lens thickness are requirements for a qualifying image. The IOP value will then be adjusted according to corneal thickness, particularly for children with extremely thin or thick cornea. This item will be optional for children under 6 years of age. Wavefront aberration will be measured after cycloplegia with a wavefront aberrometer (VX 120, Luneau SAS, Prunay le Gillon, France). Images influenced by eyelashes or eyelids will be retaken.
OCT and OCTA examinations
Swept‐source optical coherence tomography (SS‐OCT, DRI OCT Triton, Topcon, Tokyo, Japan) will be used for OCT examination after cycloplegia. This will be performed from 10:00 AM to 3:00 PM each day to minimize the influence of diurnal variation. Spherical power, cylindrical power, AL and cornea curvature radius will be input into the OCT system before OCT image collection to compensate for the magnification factors. A signal strength ≥ 60 and image quality ≥ 90 will be required for each qualified OCT image. Each SS‐OCT examination will include 12 radial scan lines focused on the centre of the fovea or the optic disc. Each scan line will be 9 mm long and will separate from the adjacent lines by 15° (Fig. 3A,B). Sixteen B‐scan OCT images will be obtained on each scan line and will be averaged with built‐in software to create an averaged image.
Fig. 3.
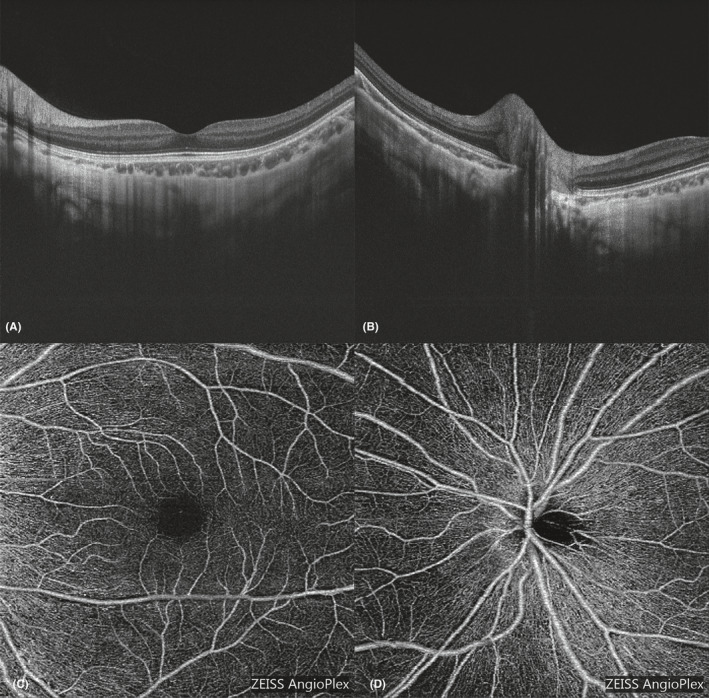
Optical coherence tomography (OCT) and optical coherence tomography angiography (OCTA) images of one child with high myopia. (A and C) show the OCT and OCTA images, respectively, focused on the fovea, while Fig. 3B,D show the OCT and OCTA images, respectively, focused on the optic disc.
Optical coherence tomography angiography examinations will be performed after cycloplegia with a Cirrus HD‐OCT (Zeiss 5000, Carl Zeiss Meditec, Germany). Each eye will undergo two OCTA examinations centred on the fovea and optic disc (6 mm*6 mm, shown in Fig. 3C,D). A signal strength ≥ 6 will be required for each qualified scan. These items will be optional for children under 6 years of age.
Fundus photography and fundus autofluorescence
Regular fundus images (Fig. 4A) and fundus autofluorescence (FAF) images (Fig. 4B) of each eye will be collected with a fundus camera (TRC‐50DX, Topcon, Japan) after cycloplegia. Each fundus image will be focused on the fovea. The exposure intensity will be 30 ws for regular fundus photography and 300 ws for FAF. Ultrawide‐field fundus photography will be performed with a panoramic ophthalmoscope (Daytona P200T, Optos) after cycloplegia. The regular pseudocolour image (Fig. 4C) and FAF image (Fig. 4D) will be collected in each eye. The procedure will be repeated if images are of substandard quality (incorrect location, covered by an eyelid or eyelash, large areas of dark field, etc.).
Fig. 4.
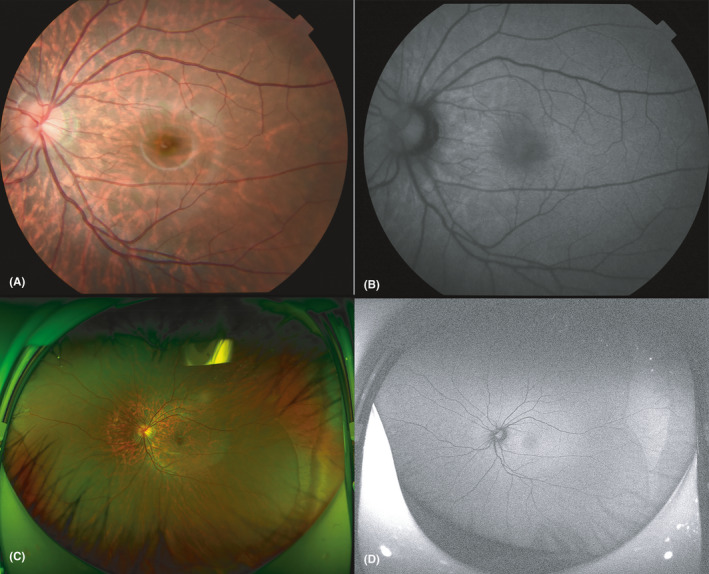
Fundus photography and ultrawide‐field fundus photography of one of the participating children. (A) Shows the regular fundus image, and B shows the fundus autofluorescence (FAF) image. Each fundus image is focused on the fovea. The exposure intensity was 30 ws for regular fundus photography and 300 ws for FAF. (C) shows the regular pseudocolour image, and (D) Shows the FAF image.
Blood and saliva sample collection
Fasting whole blood samples will be collected in EDTA‐treated tubes and will be stored at 0°C until testing for levels of growth hormone, insulin‐like growth factor‐1 (IGF‐1), oestradiol, testosterone and several micronutrients. Buccal cell samples will be collected with buccal swabs as follows: all participants will be asked to rinse the mouth for at least 3 min before the collection. The investigators will then rub swabs up and down twenty times against the inside of each participant’s cheek. The swabs will be stored in tubes containing DNA preservation solution until DNA extraction and genotyping. Two replicate specimens of blood and buccal cells will be obtained for each participant.
Organization and Supervision
The programme was drafted and organized by the Shanghai Eye Disease Prevention and Treatment Center, Shanghai General Hospital, while the Shanghai three‐level system (municipal‐district‐community) for eye disease prevention and control jointly guaranteed the implementation of this research. The draft was reviewed, discussed, revised and finalized by a group of epidemiologists and ophthalmologists. The research plan was implemented after approval by the ethics committee. If major revisions to the research plan were required, they were resubmitted to the ethics committee for approval before further implementation.
The researchers responsible for quality control will be required to make regular checks of the data for each item. The quality control standards will strictly follow the details of the data collection guideline. For examiners with high failure rates, retraining and reassessment will be required before re‐employment. The study committee will also invite experts to conduct field supervision and quality control at least once per season during the study period. To ensure the quality of data collection, all researchers will only know the information that is relevant to the examinations they are responsible for and will remain blinded to other irrelevant information. In addition, the instruments will be calibrated each day before examinations. Instruments that require upgrading during follow‐up will be evaluated for comparability.
Data management
The data acquisition and management system and study application were developed by a third party (Gaussinfomad, Beijing, China). Data from the study instruments will be uploaded into the data system and the accuracy and completeness of the data will be checked automatically by the data system. Any changes to the data will be recorded by the system, and this operation will be authorized by the supervisor. The study application will allow parents to access various tools, such as appointments, examination report downloads, online consultations and blank questionnaires.
Data analysis
Before data analysis, each image will be checked and fundus grading diagnosis will be made independently by two experts in the field. The experts will be masked to the personal information of the participants. Any discrepancy between the two experts will be recorded and later discussed by an expert panel. The latest classification and grading system for myopic maculopathy‐ATN system will be adopted in the study (Ruiz‐Medrano et al. 2019).
Statistical analysis for pilot data from baseline was performed using SAS 9.3 (SAS Institute, Cary, NC, USA) and SPSS (IBM SPSS Statistics, Inc., version 22.0, Chicago, IL, USA). The data distribution was examined using the Kolmogorov–Smirnov test. Intergroup differences were assessed with t‐tests or analysis of variance, and the Bonferroni method was used for post hoc tests. Simple linear regression models were used to show the relationship between the right eyes and the left eyes, and a logistic regression model was used to determine the independent factors associated with PPA. Statistical significance was defined as p < 0.05 (two‐tailed). During data analysis, staff members were blinded to the personal information and only relevant information was revealed to them.
Results
General characteristics of the participants
The pilot study was performed from August to September 2018. A total of 79 children and adolescents (37 boys and 42 girls) aged 4 to 18 years were recruited. Among the 79 participants, 71 right eyes and 63 left eyes met the recruitment criteria. The general characteristics of the 79 participants and the 134 involved eyes are shown in Table 1. The mean UCVA, AL and SE were 0.09 ± 0.06, 26.91 ± 1.07 mm and −9.40 ± 1.77 D, respectively. Among the involved eyes, peripapillary atrophy (N = 112) and tessellated fundus (N = 67) were the most common fundus changes. White‐without‐pressure was the most common change in the peripheral retina (N = 56). The number of other fundus changes was small: 11 eyes had diffuse chorioretinal atrophy, 12 eyes had dark‐without‐pressure, 5 eyes had pigmentary degeneration, 1 eye had retinal detachment and 3 eyes had other peripheral changes.
Table 1.
General characteristics of the participants in the pilot study.
| Parameters | Mean ± SD | Maximum | Minimum |
|---|---|---|---|
| Age, year (N = 79) | 12.32 ± 3.14 | 18 | 4 |
| Height, cm (N = 79) | 156.7 ± 17.7 | 187.0 | 96.0 |
| Weight, kg (N = 79) | 49.2 ± 18.3 | 90.8 | 15.3 |
| UCVA (N = 132)† | 0.09 ± 0.06 | 0.4 | 0.01 |
| AL, mm (N = 134) | 26.91 ± 1.07 | 31.06 | 23.10 |
| SE, dioptre (N = 134) | −9.40 ± 1.77 | −4.00 | −18.00 |
AL = axial length; SE = spherical equivalent; UCVA = uncorrected visual acuity.
The visual acuity data of a 5‐year‐old girl are missing, as this examination was beyond her understanding.
Axial length was the only independent factor associated with PPA
We divided the eyes into two groups: a peripapillary atrophy group and a group without peripapillary atrophy. The general characteristics of the two groups are listed in Table 2. The mean AL was significantly longer in the eyes with peripapillary atrophy than without peripapillary atrophy (27.08 mm versus 26.06 mm, p < 0.001). The myopic degree was also significantly higher (−9.56 D versus −8.56 D, p = 0.014), and the UCVA was worse (0.08 versus 0.13, p = 0.041) in the eyes with peripapillary atrophy than without peripapillary atrophy. Eyes with peripapillary atrophy were more common in older children. Further logistic regression modelling (Table 3) showed that AL (p = 0.041) was the only independent factor associated with PPA in high myopic eyes.
Table 2.
Comparisons between eyes with and without peripapillary atrophy.
| Eyes with PPA (N = 112) | Eyes without PPA (N = 22) | T | p value | |
|---|---|---|---|---|
| UCVA | 0.08 ± 0.05† | 0.13 ± 0.10 | −2.166 | 0.041 |
| AL, mm | 27.08 ± 0.93 | 26.06 ± 1.31 | 4.356 | <0.001 |
| SE, dioptre | −9.56 ± 1.75 | −8.56 ± 1.68 | −2.489 | 0.014 |
| Age, year | 12.68 ± 3.15 | 10.86 ± 2.38 | 2.559 | 0.012 |
AL = axial length, PPA = peripapillary atrophy, SE = spherical equivalent, UCVA = uncorrected visual acuity.
The number of eyes with peripapillary atrophy was 110 for UCVA.
Table 3.
Logistic regression model for eyes with and without peripapillary atrophy.
| B | SE | Wald | p | Exp(B) | 95% CI of Exp(B) | ||
|---|---|---|---|---|---|---|---|
| Lower limit | Upper limit | ||||||
| Age | 0.119 | 0.103 | 1.339 | 0.247 | 1.126 | 0.921 | 1.377 |
| UCVA | −7.272 | 3.981 | 3.336 | 0.068 | 0.001 | 0.000 | 1.701 |
| AL | 0.781 | 0.382 | 4.179 | 0.041* | 2.184 | 1.033 | 4.620 |
| SE | −0.199 | 0.211 | 0.882 | 0.348 | 0.820 | 0.542 | 1.241 |
| Constant | −21.724 | 9.191 | 5.587 | 0.018 | 0.000 | ||
AL = axial length; SE = spherical equivalent; UCVA = uncorrected visual acuity.
p < 0.05.
Eyes with diffuse chorioretinal atrophy had longer AL and higher myopic degree
We then classified the 134 eyes into three groups according into myopic maculopathy categories of ‘no myopic retinal lesions’ (group 1, N = 56), ‘tessellated fundus’ (group 2, N = 67) and ‘diffuse chorioretinal atrophy’ (group 3, N = 11). The detailed information for these three groups is shown in Table 4.
Table 4.
Comparisons among eyes with different levels of myopic maculopathy.
| UCVA | AL, mm | SE, dioptre | Age, year | |
|---|---|---|---|---|
| Group 1 (N = 56) | 0.09 ± 0.07† | 26.48 ± 0.91 | −9.06 ± 1.58 | 11.93 ± 3.16 |
| Group 2 (N = 67) | 0.09 ± 0.07 | 27.09 ± 0.97 | −9.50 ± 1.67 | 12.75 ± 3.17 |
| Group 3 (N = 11) | 0.08 ± 0.04 | 28.02 ± 1.31 | −10.51 ± 2.76 | 12.45 ± 2.21 |
| F | 0.370 | 13.799 | 3.391 | 1.063 |
| p | 0.691 | <0.001* | 0.037* | 0.348 |
| Group 1 versus Group 2, p | 1.000 | 0.002* | 0.518 | 0.444 |
| Group 1 versus Group 3, p | 1.000 | <0.001* | 0.039* | 1.000 |
| Group 2 versus Group 3, p | 1.000 | 0.012* | 0.225 | 1.000 |
AL = axial length, Group 1 = no myopic retinal lesions, Group 2 = tessellated fundus, Group 3 = diffuse chorioretinal atrophy, SE = spherical equivalent, UCVA = uncorrected visual acuity.
The number of eyes without myopic retinal lesions was 54 for UCVA.
p < 0.05.
The AL was significantly longer in group 3 (28.02 ± 1.31 mm) than in group 1 (26.48 ± 0.91 mm; p < 0.001) and group 2 (27.09 ± 0.97 mm; p = 0.012). The AL was also significantly longer in group 2 than in group 1 (p = 0.002). In terms of the degree of myopia, the SE was significantly smaller in group 3 (−10.51 ± 2.76 D) than in group 1 (−9.06 ± 1.58 D; p = 0.039), while no significant difference was observed between group 3 and group 2 (−9.50 ± 1.67 D; p = 0.225) or between group 1 and group 2 (p = 0.518). No significant difference was observed among these three groups in terms of UCVA or age.
Discussion
At present, studies focused on children and adolescents with high myopia have been limited, and large‐scale, long‐term follow‐up reports are rare. This gap in knowledge prompted our group to launch this SCALE‐HM study, with the primary aim of providing basic information about the early evolution of high myopic eyes especially for acquired high myopia during the paediatric stage, and obtaining a multidirectional and multidimensional understanding of the potential risk factors for the early pathological changes. In addition, the results were anticipated to provide valuable insights into the development and progression of pathological changes in high myopic eyes with increasing age.
As mentioned above, our current study focuses mainly on children with relatively high degree of myopia, which is different from several other studies. One follow‐up study performed in Singapore recently reported that children with early onset and high baseline myopia progress rapidly by adolescence to their more severe adult SE, and early onset of myopia is associated with thinner choroid and tessellated fundus in young adulthood (Li et al. 2020). However, the mean myopic degree of their study at baseline was lower than ours. Therefore, the myopic degree and the pathological changes are expected to be various at the end of the study. Several studies performed in Guangzhou suggested that older subjects tended to have more severe myopia and longer ALs (Chen et al. 2018). Children with early‐onset high myopia have an increased risk of developing clinically significant myopic maculopathy (Xiao et al. 2018). As compared, the participants of our study are younger and therefore we are expected to provide more information about the early development process of pathological changes in high myopes. Several other studies performed in Japan showed that parapapillary diffuse choroidal atrophy in children is associated with thinning of the choroid in the parapapillary region (Yokoi et al. 2017) and the presence of peripapillary diffuse chorioretinal atrophy in children may be an indicator for the eventual development of pathologic myopia in adults (Yokoi et al. 2016). Different from their sampling strategy, we recruit participants from the school‐based population, which may better reflect the general development process of high myopia.
So why studies focused on children and adolescents with high myopia have been limited? One of the main reasons for the lack of high myopia research in children and adolescents may be the difficulty in obtaining samples. Usually, research can be conducted more easily in areas with a high prevalence of myopia and high myopia, as well as in populations with complete systems that allow ready enrolment of participants. The prevalence of children with myopia in mainland China is highest, at 52.2%, in Shanghai, a coastal metropolis in East China (Ma et al. 2016). The prevalence of high myopia is also extremely high in Shanghai, at up to 19.5% in young university‐age adults (Sun et al. 2012). Our research group commenced the Shanghai Child and Adolescent Large‐scale Eye (SCALE) Study in 2012‐2013 and, to date, the non‐cycloplegic autorefraction data for over 2 million children and adolescents have been registered in the information system (He et al. 2018). The difference between non‐cycloplegic and cycloplegic refraction was considered acceptable in children with myopia with SE less than −3.0 D (Sankaridurg et al. 2017); therefore, we estimated that about 10 000 children and adolescents would meet the enrolment requirements of the current SCALE‐HM study, indicating that a source of subjects was guaranteed in Shanghai. Moreover, Shanghai's unique three‐level network for primary eye care ensures that we are able to inform parents of studies through the school system. Given the 30% response and the proportion of inclusion based on the pilot study, we anticipated being able to recruit the expected number (3,000) of samples. We determined whether potential recruitment bias existed by analysing the refraction distribution differences between responders and non‐responders.
As mentioned above, studies focused on early fundus changes are limited in children and adolescents with myopia. Structural changes, such as PPA, may be common in the early stage of high myopia, while visually threatening complications, like chorioretinal atrophy or staphyloma, are rare (Samarawickrama et al. 2011). How these early‐stage structural changes gradually evolve into severe lesions is unknown, and the nature of the internal relations among these changes in the different stages of high myopia has not been established. These issues form key aspects of our ongoing SCALE‐HM study.
One study carried out on animals suggested that myopia‐related visual stimuli and changes in choroidal blood flow may be potential factors for myopia development (Wu et al. 2018); and another animal study found that choroidal thickness and choroidal blood perfusion were significantly decreased in myopic animal models, and they both increased during recovery. Changes in choroidal thickness were positively correlated with changes in choroidal blood perfusion (Zhang et al. 2019). However, these have not yet been confirmed in patients with high myopia. For those limited studies performed in myopic patients, the results were quite different. One study suggested that the area of flow deficit in the choriocapillaris is increased in eyes with greater myopia (Al‐Sheikh et al. 2017), while another study showed that choriocapillary blood flow retained a constant level with increasing physiological myopia (Scherm et al. 2019). Such discrepancy may arise from the differences in quantitative methods. Nevertheless, these clues imply that changes in choroidal blood flow may be associated with the development and progress of high myopia and more accurate quantitative methods to calculate choroidal blood flow are required in the future. Moreover, the choroid is the only source of blood for the avascular fovea (Spaide 2014). One study in adults showed that macular light sensitivity decreased significantly in patients with high myopia with normal corrected visual acuity (Qin et al. 2010), and electroretinogram examinations have indicated decreased b‐wave amplitudes in patients with nonpathologic myopia (Kawabata & Adachi‐Usami 1997; Luu et al. 2006). These discoveries have suggested that early injury of the cones may occur in high myopic eyes, even in those with good visual acuity and without visible pathological changes. Our study will focus on whether these functional changes are already present in children and adolescents with high myopia and whether they are correlated with early changes in choroidal blood flow.
In our current study, we used advanced devices, such as SS‐OCT, OCTA and microperimetry, to record the tiny structural and functional fundus changes in children and adolescents with high myopia. Optical coherence tomography angiography (OCTA) can assess fundus blood flow changes, which are rarely mentioned with respect to high myopia in children, and the follow‐up data may provide us with abundant clues for understanding the relationship between fundus lesions and blood flow changes. The blood flow of the choroid cannot be accurately measured with OCTA, but choroidal vessel segmentation based on SS‐OCT images and an artificial intelligence technique developed by our study group could be an alternative approach (Liu et al. 2019). Additionally, unlike the previous studies that focused mainly on morphological changes, this pilot study investigated the use of microperimetry, a technique commonly used for quadrant‐specific assessment of macular light sensitivity, to assess the visual functional changes in children and adolescents with high myopia.
This pilot study revealed that peripapillary atrophy and tessellated fundus were the most common fundus changes in children and adolescents with high myopia, while other lesions, like posterior staphyloma or chorioretinal atrophy, were rare in these cases. This finding agreed with similar results from two previous studies (Kobayashi et al. 2005; Samarawickrama et al. 2011). For eyes with peripapillary atrophy, the AL was longer, the myopic degree was higher and the UCVA was worse when compared with eyes without peripapillary atrophy. However, the logistic regression model indicated that AL was the only independent factor. These results suggest that AL may be a critical influencing factor for the development of peripapillary atrophy. Similar findings have been reported in other paediatric and adult populations (Nonaka et al. 2011; Liu et al. 2017). Age was excluded by the regression model, which suggested that peripapillary atrophy may occur even at a very early stage of high myopia and that prevention and control of fundus complications should be emphasized in children with high myopia who have long AL. However, due to the relatively small number of participants in this pilot study, we were unable to further divide the peripapillary atrophy into different subgroups according to their size, location or pattern. More sophisticated categories of peripapillary atrophy are needed in the future to determine its detailed relationship with AL and other parameters, such as retinal, choroidal and scleral morphologies and function‐related indicators. How peripapillary atrophy gradually evolves into lesions that influence visual functions and the cut‐off point for these changes for the development of high myopia are questions that need to be addressed in follow‐up studies.
We also found that the AL was significantly longer in eyes with diffuse chorioretinal atrophy than without myopic retinal lesions or with tessellated fundus, and that the degree of myopia was also much higher. However, these differences were smaller between the eyes with tessellated fundus and the eyes without myopic retinal lesions. These preliminary findings suggest that the progression of fundus lesions from a tessellated fundus to diffuse chorioretinal atrophy may be a cut‐off point for morphological and functional development in children with high myopia; however, this requires longer term research with extensive follow‐up to confirm. The outcomes after transformation from a tessellated fundus to diffuse chorioretinal atrophy in childhood are also unknown and deserve further exploration. Previous studies have suggested that diffuse retinochoroidal atrophy around the optic disc in childhood could progress to pathologic myopia in adulthood (Yokoi et al. 2016). The prognosis for visual function in children and adolescents with macular diffuse retinochoroidal atrophy is uncertain, raising the question of whether those lesions will also progress to a higher level at a relatively young age in adulthood. These issues are expected to be resolved during the long‐term follow‐up in future research.
Our current study has several limitations. One was that this is an observational study designed for long‐term follow‐up starting with children and adolescents. Some ophthalmic changes may take a long time to detect, and the long observation process will in all likelihood result in high loss rate of subjects, especially when these children and adolescents grow up and move to other locations. Therefore, optimizing the service process and improving satisfaction to maintain patient compliance and a good follow‐up rate are appreciable challenges.
A second limitation is that microperimetry was adopted to assess the early visual functional changes in children with high myopia. However, young children are limited by their comprehensive ability so they cannot accurately undergo microperimetry examinations. Multifocal electroretinograms might have been a good alternative for young children who cannot understand microperimetry examinations, but we did not use that technique due to its complicated operation and potential risks. In addition, observation of peripheral fundus lesions depended mainly on ultrawide‐field fundus photography and ophthalmoscopy examinations by the ophthalmologists. The resolution of ultrawide‐field fundus photography and the subjectivity of ophthalmologists may have meant that tiny peripheral fundus lesions were overlooked. However, these oversights could be controlled to a minimum level by having at least two experts make independent diagnoses. A further limitation is that the results from our study may not be applicable to children from other regions, other ethnicities and other age groups.
In summary, despite its limitations, this preliminary study indicates that the long‐term observational SCALE‐HM study could be of great importance for understanding the development, progression and prognosis of high myopia at its very early stages. We hope that the SCALE‐HM study will provide insights into the mechanisms underlying fundus changes, help to understand the multidimensional aspects of myopia and aid in the establishment of a systematic early prediction index system for pathological myopic changes. Myopic fundus complications are quite difficult to treat and control at present. However, increased follow‐up frequency and increased education on eye health care may help to slow down the progress of myopia, which is important to the prevention and control of advanced complications caused by high myopia. This study and relevant practice may guide the management of high myopia and address the burden of vision loss caused by this disease.
Xiangui He and Junjie Deng are equal first authors.
We gratefully acknowledge the expert guidance provided by the following individuals: Ohno‐Matsui Kyoko, Padmaja Sankaridurg, Ian Morgan, Kathryn Rose, Serge Resnikoff and Seang Mei Saw. We also acknowledge the support of the Shanghai 16 district‐level eye disease prevention and control branch centres and related community health service centres; the involved children and their family members in the study; and the contributions of many other individuals on this project. There is no conflict of interest to declare, and the sponsor or funding organization had no role in the design or conduct of this research.
Funding sources: (1) Municipal Human Resources Development Program for Outstanding Young Talents in Medical and Health Sciences in Shanghai (Grant No.2017YQ019); (2) National Key R&D Program of China (2016YFC0904800, 2019YFC0840607); (3) National Science and Technology Major Project of China (2017ZX09304010); (4) National Natural Science Foundation of China (No.81900911); (5) Shanghai Sailing Program (18YF1420200).
References
- Achenbach TM & Ruffle TM (2000): The Child Behavior Checklist and related forms for assessing behavioral/emotional problems and competencies. Pediatr Rev 21: 265–271. [DOI] [PubMed] [Google Scholar]
- Al‐Sheikh M, Phasukkijwatana N, Dolz‐Marco R, Rahimi M, Iafe NA, Freund KB, Sadda SR & Sarraf D (2017): Quantitative OCT angiography of the retinal microvasculature and the Choriocapillaris in myopic eyes. Invest Ophthalmol Vis Sci 58: 2063–2069. [DOI] [PubMed] [Google Scholar]
- Alzaben Z, Cardona G, Zapata MA & Zaben A (2017): Interocular asymmetry in choroidal thickness and retinal sensitivity in high myopia. Retina 38: 1620–1628. [DOI] [PubMed] [Google Scholar]
- Bansal AS & Hubbard GB 3rd (2010): Peripheral retinal findings in highly myopic children < or =10 years of age. Retina 30: S15–S19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byer NE (1965): Clinical study of lattice degeneration of the retina. Trans Am Acad Ophthalmol Otolaryngol 69: 1065–1081. [PubMed] [Google Scholar]
- Chen Y, Xiao O, Guo X, Wang D, Sankaridurg P, Morgan I & He M (2018): Methodology of the ZOC‐BHVI High Myopia Cohort Study: The onset and progression of myopic pathologies and associated risk factors in highly myopic Chinese. Ophthalmic Epidemiol 25: 31–38. [DOI] [PubMed] [Google Scholar]
- Deng J, Jin J, Lv M et al. (2019): Distribution of scleral thickness and associated factors in 810 Chinese children and adolescents: a swept‐source optical coherence tomography study. Acta Ophthalmol 97: e410–e418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng J, Li X, Jin J et al. (2018): Distribution pattern of choroidal thickness at the posterior pole in Chinese children with myopia. Invest Ophthalmol Vis Sci 59: 1577–1586. [DOI] [PubMed] [Google Scholar]
- Fledelius HC, Jacobsen N, Li XQ & Goldschmidt E (2019): The Longitudinal Danish High Myopia Study Cohort 1948: at age 66 years visual ability is only occasionally affected by visual field defects. Acta Ophthalmol 96: 36–43. [DOI] [PubMed] [Google Scholar]
- French AN, Ashby RS, Morgan IG & Rose KA (2013): Time outdoors and the prevention of myopia. Exp Eye Res 114: 58–68. [DOI] [PubMed] [Google Scholar]
- Gella L, Raman R & Sharma T (2011): Evaluation of in vivo human retinal morphology and function in myopes. Curr Eye Res 36: 943–946. [DOI] [PubMed] [Google Scholar]
- He M, Xiang F, Zeng Y et al. (2015): Effect of time spent outdoors at school on the development of myopia among children in China: a randomized clinical trial. JAMA 314: 1142–1148. [DOI] [PubMed] [Google Scholar]
- He M, Zeng J, Liu Y, Xu J, Pokharel GP & Ellwein LB (2004): Refractive error and visual impairment in urban children in southern China. Invest Ophthalmol Vis Sci 45: 793–799. [DOI] [PubMed] [Google Scholar]
- He X, Zhao R, Sankaridurg P et al. (2018): Design and methodology of the Shanghai child and adolescent large‐scale eye study (SCALE). Clin Exp Ophthalmol 46: 329–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho WC, Kee CS & Chan HH (2012): Myopia progression in children is linked with reduced foveal mfERG response. Invest Ophthalmol Vis Sci 53: 5320–5325. [DOI] [PubMed] [Google Scholar]
- Holden BA, Fricke TR, Wilson DA et al. (2016): Global prevalence of myopia and high myopia and temporal trends from 2000 through 2050. Ophthalmology 123: 1036–1042. [DOI] [PubMed] [Google Scholar]
- Ip JM, Saw SM, Rose KA, Morgan IG, Kifley A, Wang JJ & Mitchell P (2008): Role of near work in myopia: findings in a sample of Australian school children. Invest Ophthalmol Vis Sci 49: 2903–2910. [DOI] [PubMed] [Google Scholar]
- Jin P, Zou H, Zhu J et al. (2016): Choroidal and retinal thickness in children with different refractive status measured by swept‐source optical coherence tomography. Am J Ophthalmol 168: 164–176. [DOI] [PubMed] [Google Scholar]
- Jung SK, Lee JH, Kakizaki H & Jee D (2012): Prevalence of myopia and its association with body stature and educational level in 19‐year‐old male conscripts in Seoul, South Korea. Invest Ophthalmol Vis Sci 53: 5579–5583. [DOI] [PubMed] [Google Scholar]
- Karlin DB & Curtin BJ (1976): Peripheral chorioretinal lesions and axial length of the myopic eye. Am J Ophthalmol 81: 625–635. [DOI] [PubMed] [Google Scholar]
- Kawabata H & Adachi‐Usami E (1997): Multifocal electroretinogram in myopia. Invest Ophthalmol Vis Sci 38: 2844–2851. [PubMed] [Google Scholar]
- Kobayashi K, Ohno‐Matsui K, Kojima A et al. (2005): Fundus characteristics of high myopia in children. Jpn J Ophthalmol 49: 306–311. [DOI] [PubMed] [Google Scholar]
- Koh V, Yang A, Saw SM et al. (2014): Differences in prevalence of refractive errors in young Asian Males in Singapore between 1996–1997 and 2009–2010. Ophthalmic Epidemiol 21: 247–255. [DOI] [PubMed] [Google Scholar]
- Lee JH, Jee D, Kwon JW & Lee WK (2013): Prevalence and risk factors for myopia in a rural Korean population. Invest Ophthalmol Vis Sci 54: 5466–5471. [DOI] [PubMed] [Google Scholar]
- Li J, Lanca C, Htoon HM, Wong YL, Nyunt SZ, Tan D, Sabanayagam C & Saw SM (2020): High myopes in Singapore: 19‐year progression from childhood to adulthood. Ophthalmology S0161–6420(20): 30473–5. [DOI] [PubMed] [Google Scholar]
- Lin LL, Shih YF, Hsiao CK & Chen CJ (2004): Prevalence of myopia in Taiwanese schoolchildren: 1983 to 2000. Ann Acad Med Singapore 33: 27–33. [PubMed] [Google Scholar]
- Liu X, Bi L, Xu Y, Feng D, Kim J & Xu X (2019): Robust deep learning method for choroidal vessel segmentation on swept source optical coherence tomography images. Biomed Opt Express 10: 1601–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Gong L, Li Y, Zhu X, Stewart JM & Wang C (2017): Peripapillary atrophy in high myopia. Curr Eye Res 42: 1308–1312. [DOI] [PubMed] [Google Scholar]
- Luu CD, Lau AM & Lee SY (2006): Multifocal electroretinogram in adults and children with myopia. Arch Ophthalmol 124: 328–334. [DOI] [PubMed] [Google Scholar]
- Ma Y, Qu X, Zhu X et al. (2016): Age‐specific prevalence of visual impairment and refractive error in children aged 3–10 years in Shanghai, China. Invest Ophthalmol Vis Sci 57: 6188–6196. [DOI] [PubMed] [Google Scholar]
- Mangione CM, Lee PP, Pitts J, Gutierrez P, Berry S & Hays RD (1998): Psychometric properties of the National Eye Institute Visual Function Questionnaire (NEI‐VFQ). Arch Ophthalmol 116: 1496–1504. [DOI] [PubMed] [Google Scholar]
- Matsumura H & Hirai H (1999): Prevalence of myopia and refractive changes in students from 3 to 17 years of age. Surv Ophthalmol 44(Suppl 1): S109–115. [DOI] [PubMed] [Google Scholar]
- Mirshahi A, Ponto KA, Hoehn R, Zwiener I, Zeller T, Lackner K, Beutel ME & Pfeiffer N (2014): Myopia and level of education: results from the Gutenberg health study. Ophthalmology 121: 2047–2052. [DOI] [PubMed] [Google Scholar]
- Morgan IG, French AN, Ashby RS, Guo X, Ding X, He M & Rose KA (2018): The epidemics of myopia: aetiology and prevention. Prog Retin Eye Res 62: 134–149. [DOI] [PubMed] [Google Scholar]
- Morgan IG, Ohno‐Matsui K & Saw SM (2012): Myopia. Lancet 379: 1739–1748. [DOI] [PubMed] [Google Scholar]
- Nagasawa T, Mitamura Y, Katome T et al. (2013): Macular choroidal thickness and volume in healthy pediatric individuals measured by swept‐source optical coherence tomography. Invest Ophthalmol Vis Sci 54: 7068–7074. [DOI] [PubMed] [Google Scholar]
- Nonaka A, Hangai M, Akagi T, Mori S, Nukada M, Nakano N & Yoshimura N (2011): Biometric features of peripapillary atrophy beta in eyes with high myopia. Invest Ophthalmol Vis Sci 52: 6706–6713. [DOI] [PubMed] [Google Scholar]
- Park S, Kim SH, Park TK & Ohn YH (2013): Evaluation of structural and functional changes in non‐pathologic myopic fundus using multifocal electroretinogram and optical coherence tomography. Doc Ophthalmol 126: 199–210. [DOI] [PubMed] [Google Scholar]
- Pärssinen O & Kauppinen M (2018): Risk factors for high myopia: a 22‐year follow‐up study from childhood to adulthood. Acta Ophthalmol 97: 510–518. [DOI] [PubMed] [Google Scholar]
- Pierro L, Camesasca FI, Mischi M & Brancato R (1992): Peripheral retinal changes and axial myopia. Retina 12: 12–17. [DOI] [PubMed] [Google Scholar]
- Qin Y, Zhu M, Qu X, Xu G, Yu Y, Witt RE & Wang W (2010): Regional macular light sensitivity changes in myopic Chinese adults: an MP1 study. Invest Ophthalmol Vis Sci 51: 4451–4457. [DOI] [PubMed] [Google Scholar]
- Read SA, Collins MJ, Vincent SJ & Alonso‐Caneiro D (2013): Choroidal thickness in myopic and nonmyopic children assessed with enhanced depth imaging optical coherence tomography. Invest Ophthalmol Vis Sci 54: 7578–7586. [DOI] [PubMed] [Google Scholar]
- Ruiz‐Medrano J, Montero JA, Flores‐Moreno I, Arias L, García‐Layana A & Ruiz‐Moreno JM (2019): Myopic maculopathy: current status and proposal for a new classification and grading system (ATN). Prog Retin Eye Res 69: 80–115. [DOI] [PubMed] [Google Scholar]
- Samarawickrama C, Mitchell P, Tong L, Gazzard G, Lim L, Wong TY & Saw SM (2011): Myopia‐related optic disc and retinal changes in adolescent children from Singapore. Ophthalmology 118: 2050–2057. [DOI] [PubMed] [Google Scholar]
- Sankaridurg P, He X, Naduvilath T et al. (2017): Comparison of non‐cycloplegic and cycloplegic autorefraction in categorizing refractive error data in children. Acta Ophthalmol 95: e633–e640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saw SM, Cheng A, Fong A, Gazzard G, Tan DT & Morgan I (2007): School grades and myopia. Ophthalmic Physiol Opt 27: 126–129. [DOI] [PubMed] [Google Scholar]
- Scherm P, Pettenkofer M, Maier M, Lohmann CP & Feucht N (2019): Choriocapillary blood flow in myopic subjects measured with OCT angiography. Ophthalmic Surg Lasers Imaging Retina 50: e133–e139. [DOI] [PubMed] [Google Scholar]
- Spaide RF (2014): The choroid. In: Pathologic Myopia., (Spaide RF, Ohno‐Matsui K & Yannuzzi LA eds.). New York: Springer‐Verlag, pp. 113–131. [Google Scholar]
- Sun J, Zhou J, Zhao P et al. (2012): High prevalence of myopia and high myopia in 5060 Chinese university students in Shanghai. Invest Ophthalmol Vis Sci 53: 7504–7509. [DOI] [PubMed] [Google Scholar]
- Tedja MS, Haarman AEG, Meester‐Smoor MA et al. (2019): IMI – myopia genetics report. Invest Ophthalmol Vis Sci 60: M89–M105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolsley CJ, Saunders KJ, Silvestri G & Anderson RS (2008): Investigation of changes in the myopic retina using multifocal electroretinograms, optical coherence tomography and peripheral resolution acuity. Vision Res 48: 1554–1561. [DOI] [PubMed] [Google Scholar]
- Wu JF, Bi HS, Wang SM et al. (2013a): Refractive error, visual acuity and causes of vision loss in children in Shandong, China. The Shandong Children Eye Study. PLoS One 8:e82763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu PC, Tsai CL, Wu HL, Yang YH & Kuo HK (2013b): Outdoor activity during class recess reduces myopia onset and progression in school children. Ophthalmology 120: 1080–1085. [DOI] [PubMed] [Google Scholar]
- Wu H, Chen W, Zhao F et al. (2018): Scleral hypoxia is a target for myopia control. Proc Natl Acad Sci U S A 115: E7091–E7100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao O, Guo X, Wang D et al. (2018): Distribution and severity of myopic maculopathy among highly myopic eyes. Invest Ophthalmol Vis Sci 59: 4880–4885. [DOI] [PubMed] [Google Scholar]
- Xiong S, He X, Deng J et al. (2017a): Choroidal thickness in 3001 Chinese children aged 6 to 19 years using swept‐source OCT. Sci Rep 7: 45059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong S, Sankaridurg P, Naduvilath T et al. (2017b): Time spent in outdoor activities in relation to myopia prevention and control: a meta‐analysis and systematic review. Acta Ophthalmol 95: 551–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashiro K (2014): Genes involved in the development of myopia. In: Pathologic Myopia (Spaide RF, Ohno‐Matsui K & Yannuzzi LA eds.). New York: Springer‐Verlag, pp. 13–24. [Google Scholar]
- Yokoi T, Jonas JB, Shimada N, Nagaoka N, Moriyama M, Yoshida T & Ohno‐Matsui K (2016): Peripapillary diffuse chorioretinal atrophy in children as a sign of eventual pathologic myopia in adults. Ophthalmology 123: 1783–1787. [DOI] [PubMed] [Google Scholar]
- Yokoi T, Zhu D, Bi HS et al. (2017): Parapapillary diffuse choroidal atrophy in children is associated with extreme thinning of parapapillary choroid. Invest Ophthalmol Vis Sci 58: 901–906. [DOI] [PubMed] [Google Scholar]
- Zaben A, Zapata MÁ & Garcia‐Arumi J (2015): Retinal sensitivity and choroidal thickness in high myopia. Retina 35: 398–406. [DOI] [PubMed] [Google Scholar]
- Zhang S, Zhang G, Zhou X et al. (2019): Changes in Choroidal Thickness and Choroidal Blood Perfusion in Guinea Pig Myopia. Invest Ophthalmol Vis Sci 60: 3074–3083. [DOI] [PubMed] [Google Scholar]


