Abstract
Background
Previous studies showing an association between chronic use of proton pump inhibitor (PPI) and gastric cancer are limited by confounding by indication. This relationship has not been studied in patients receiving PPI for prophylaxis, such as those undergoing percutaneous coronary intervention (PCI).
Method
This was a retrospective cohort study including 14 hospitals under the Hospital Authority of Hong Kong between 1 January 2004 and 31 December 2017. Participants were patients who underwent first-ever PCI, were not on PPI prescription within 30 days before admission for PCI, had no known malignancy and survived for 365 days after PCI. Propensity score matching was used to balance baseline characteristics and other prescription patterns. The primary outcome was diagnosis of gastric cancer made >365 days after PCI as a time-to-first-event analysis. The secondary outcome was death from gastric cancer.
Results
Among the 13 476 patients (6738 pairs) matched by propensity score, gastric cancer developed in 17 (0.25%) PPI users and 7 (0.10%) PPI non-users after a median follow-up of 7.1 years. PPI users had a higher risk of gastric cancer (HR 3.55; 95% CI 1.46 to 8.66, p=0.005) and death from gastric cancer (HR 4.18; 95% CI 1.09 to 16.08, p=0.037), compared with non-users. The association was duration-dependent and patients who took PPI for ≥365 days were at increased risk.
Conclusions
Chronic use of PPI was significantly associated with increased risk of gastric cancer and death from gastric cancer in patients for whom it was prescribed as prophylaxis. Physicians should judiciously assess the relevant risks and benefits of chronic PPI use before prescription.
Keywords: gastric cancer, proton pump inhibition, gastric acid
Summary box.
What is already known about this subject?
Previous studies showing an association between chronic use of proton pump inhibitor (PPI) and gastric cancer are limited by confounding by indication.
What are the new findings?
Chronic use of PPI was associated with increased risk of gastric cancer and death from gastric cancer in patients for whom it was prescribed as prophylaxis.
How might it impact on clinical practice in the foreseeable future?
Physicians should judiciously assess the relevant risks and benefits of chronic PPI use before prescription.
Background
Proton pump inhibitors (PPI) are one of the most widely prescribed medications worldwide.1 These potent gastric acid suppressors are used in treatment and prevention of peptic ulcer disease, gastro-oesophageal reflux disease (GERD) and eradication of Helicobacter pylori infection. Several large observational studies have demonstrated an association between prolonged use of PPI and gastric cancer.2–6 The main limitations of these studies were confounding by indication and protopathic bias, and similar risks have not been detected in randomised controlled trials (RCTs).7 However, RCT may be inadequately powered to detect cancer risks given the limitations in duration of follow-up and sample size.6 There is insufficient equipoise to justify large-scale RCT to examine the cancer risks of PPI.
In patients who undergo percutaneous coronary interventions (PCI), dual antiplatelet therapy (DAPT) with aspirin and a P2Y12 inhibitor, such as clopidogrel, is mandatory for a prolonged period to prevent the catastrophic event of stent thrombosis.8 9 The ulcerogenicity of aspirin compounded with concurrent use of P2Y12 inhibitors exposes this group of patients to higher risk of gastrointestinal (GI) bleeding.10 11 PPI has been shown to be an effective prophylaxis to mitigate such risk,12 13 and is prescribed in up to 40% patients on DAPT in certain localities.14 These patients on DAPT with new prescription of PPI after PCI provided a unique opportunity to examine the PPI-cancer relationship because confounding by indication could be minimised. We hypothesised that chronic use of PPI started after PCI is associated with an increased incidence of gastric cancer.
Methods
Study population and design
Data from all patients who underwent first-ever PCI between 1 January 2004 and 31 December 2017 from all 14 public hospitals that performed PCI and recorded in a territorial-wide PCI registry were reviewed. Patients’ baseline characteristics, exposures and outcomes were retrieved from the PCI Registry and Clinical Data and Analysis Reporting System.
We included all adult patients (18 years of age or older) who underwent first-ever PCI and survived for at least 365 days after PCI, which was landmarked as time zero for outcome observation (figure 1). Exclusion criteria were patients who were prescribed PPI within 30 days before hospital admission for PCI or had any known malignancy before PCI.
Figure 1.
Study timeline. Timeline showing key definitions for the study. PCI, percutaneous coronary intervention; PPI, proton pump inhibitor.
Definitions of exposure and outcome variables
PPI users were defined as patients who had any PPI prescription that started from hospital admission to 30 days after PCI and continued for >180 consecutive days, with reference to definitions used previously.2 The comparator group of PPI non-users were defined as patients whose total duration of PPI prescription was <14 days during the entire 365-day period after PCI. All PPI were prescribed at the discretion of clinicians. As PPI are prescription-only medications and payment contribution by patients is trivial in this hospital system, prescription can be assumed for actual usage. The primary outcome was the first diagnosis of gastric cancer (excluding lymphoma) after time zero (ie, 365 days after PCI). The secondary outcome was death from gastric cancer after time zero. Events occurring before 365 days after PCI were excluded to control for protopathic bias.15
Statistical analysis
All analyses were performed with prespecified outcome and statistical methods. Using logistic regression, we constructed a propensity score for the likelihood of PPI use with variables selected a priori based on data in the published literature and biological plausibility: gender, age, Chinese ethnicity, tobacco use, diabetes mellitus, metformin use,16 hypertension, dyslipidaemia, cerebrovascular disease, chronic obstructive pulmonary disease, previous myocardial infarction, previous coronary artery bypass grafting, previous heart failure, atrial fibrillation or flutter, cirrhosis, estimated glomerular filtration rate <50 mL/min/m2, anaemia (haemoglobin <130 g/L for men, <120 g/L for women), GERD, peptic ulcer disease, history of H. pylori eradication, presentation with acute myocardial infarction, PCI urgency, year of PCI, metformin on discharge, aspirin on discharge,17 P2Y12 inhibitor on discharge, anticoagulation therapy on discharge, statin on discharge18 19 and drop of haemoglobin >2 g/dL after PCI. The study cohort consisted of two comparison groups—‘PPI user group’ and ‘PPI non-user group’—generated by 1:1 propensity score matching using a calliper of 0.2 times SD of the logit of propensity score.20
Unadjusted analyses were made using χ2 tests for categorical variables and Student’s t-test or Wilcoxon rank-sum tests for continuous variables. Cox proportional hazards regression was performed to evaluate the relationship between PPI use and study outcome, as a time-to-first-event analysis starting from 365 days after PCI, accounting for competing risk of death, loss to follow-up or end of study period (30 June 2020).
Sensitivity analyses
As to control for bias that may result from differences in healthcare-seeking behaviour, we repeated the primary analysis on patients who had at least one upper endoscopy at any time after PCI. We also performed another sensitivity analysis to include all patients before propensity score matching. A multivariable Cox regression model, adjusting for the same set of variables in the construction of the propensity score, was used to examine the association between PPI use and the primary outcome. Furthermore, to assess whether the duration of PPI usage was associated with increased risk of the primary outcome, a separate Cox regression model with a 3-level exposure (defined as 0–14 days for minimal duration, 14–364 days for short duration, 365 days or more after PCI for long duration)21 was used with adjustment for the same set of variables.
To assess for any residual confounding by treatment selection, we performed falsification testing with two clinical outcomes: new diagnosis of lung cancer after time zero. It was selected based on their association with mortality but was biologically unlikely to be causally related to PPI use.
The complete case method was adopted to address missing data in the primary analysis. To test the robustness of our results, the multivariable Cox regression analysis was repeated with the entire cohort using the technique of multiple imputations by chained equations.
Data management and statistical analyses were performed in Stata software, V.16 (StataCorp). A two-tailed p value of <0.05 was considered statistically significant.
Results
Patients and characteristics
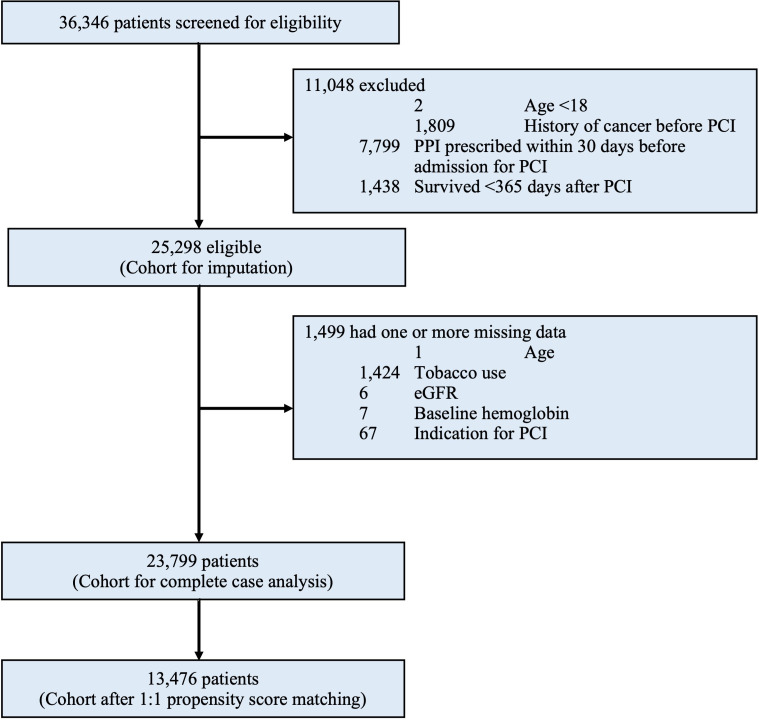
Between January 2004 and December 2017, a total of 36 346 patients were considered for inclusion: 11 048 (30.4%) were excluded after application of exclusion criteria. Of the remaining 25 298 patients, a total of 1499 (5.9%) were excluded from complete case analysis due to missing values in any of the variables used in the propensity score matching model (figure 2). The median follow-up period was 7.1 years. The characteristics of the entire cohort before propensity score matching are shown in online supplemental table S1 and online supplemental table S2 of the online supplemental appendix. After 1:1 propensity score matching, a total of 6738 pairs were generated. Table 1 shows the baseline characteristics of the matched study population. All variables in table 1 were included in the propensity score model, and were balanced between groups (standardised difference <0.1) except for acute myocardial infarction and urgency of PCI. Table 2 shows the medications on discharge and postprocedural characteristics after PCI.
Figure 2.
Study profile. eGFR, estimated glomerular filtration rate; PCI, percutaneous coronary intervention.
Table 1.
Baseline characteristics of patients after propensity score matching
| Characteristics | PPI users | PPI non-users | P value | Standardised difference |
| N=6738 | N=6738 | |||
| Female gender | 1488 (22.1%) | 1430 (21.2%) | 0.23 | −0.021 |
| Age, mean (SD) | 64.2 (11.5) | 63.2 (11.1) | <0.001 | −0.092 |
| Chinese | 6353 (94.3%) | 6353 (94.3%) | 1.00 | <0.001 |
| Tobacco use | 3117 (46.3%) | 3223 (47.8%) | 0.067 | 0.032 |
| Diabetes mellitus | 1941 (28.8%) | 2142 (31.8%) | <0.001 | 0.065 |
| Hypertension | 3835 (56.9%) | 3987 (59.2%) | 0.008 | 0.046 |
| Dyslipidaemia | 3902 (57.9%) | 4078 (60.5%) | 0.002 | 0.053 |
| Cerebrovascular disease | 500 (7.4%) | 521 (7.7%) | 0.49 | 0.012 |
| Chronic obstructive pulmonary disease | 122 (1.8%) | 135 (2.0%) | 0.41 | 0.014 |
| Previous myocardial infarction | 335 (5.0%) | 477 (7.1%) | <0.001 | 0.089 |
| Previous CABG | 44 (0.7%) | 68 (1.0%) | 0.023 | 0.039 |
| Congestive heart failure | 312 (4.6%) | 356 (5.3%) | 0.081 | 0.030 |
| Atrial fibrillation or flutter | 228 (3.4%) | 230 (3.4%) | 0.92 | 0.002 |
| Cirrhosis | 11 (0.2%) | 8 (0.1%) | 0.49 | −0.012 |
| Baseline eGFR <50 mL/min/m2 | 570 (8.5%) | 510 (7.6%) | 0.057 | −0.033 |
| Baseline anaemia | 2025 (30.1%) | 1760 (26.1%) | <0.001 | −0.088 |
| GERD | 56 (0.8%) | 40 (0.6%) | 0.10 | −0.028 |
| Peptic ulcer disease | 239 (3.5%) | 175 (2.6%) | 0.001 | −0.055 |
| Helicobacter pylori eradication | 214 (3.2%) | 155 (2.3%) | 0.002 | −0.054 |
| Acute myocardial infarction | 4997 (74.2%) | 4330 (64.3%) | <0.001 | −0.216 |
| Urgent or emergency PCI | 4452 (66.1%) | 3366 (50.0%) | <0.001 | −0.331 |
| Year of PCI | <0.001 | −0.6803 | ||
| 2004–2008 | 358 (5.3%) | 702 (10.4%) | ||
| 2009–2012 | 1037 (15.4%) | 2554 (37.9%) | ||
| 2013–2016 | 2336 (34.7%) | 2346 (34.8%) | ||
| 2016–2017 | 3007 (44.6%) | 1136 (16.9%) |
CABG, coronary artery bypass grafting; eGFR, estimated glomerular filtration rate; GERD, gastro-oesophageal reflux disease;; PCI, percutaneous coronary intervention; PPI, proton pump inhibitor.
Table 2.
Medications on hospital discharge and postprocedure characteristics of patients after propensity score matching
| Characteristics | PPI users | PPI non-users | P value | Standardised difference |
| N=6738 | N=6738 | |||
| Duration of PPI after PCI, median (IQR)—days | 1314 (718–1901) | 0 (0–0) | <0.001 | −2.580 |
| On PPI >30 days after cessation of P2Y12 inhibitor | 5736 (85.3%) | 0 (0.0%) | <0.001 | −3.411 |
| Aspirin on discharge | 6614 (98.2%) | 6591 (97.8%) | 0.16 | −0.024 |
| P2Y12 inhibitors on discharge | 6722 (99.8%) | 6705 (99.5%) | 0.015 | −0.042 |
| Potent P2Y12 inhibitors on discharge* | 1793 (26.6%) | 659 (9.8%) | <0.001 | −0.447 |
| Duration of P2Y12 inhibitor after PCI (IQR)—days | 366 (365, 425) | 365 (184, 387) | <0.001 | −0.147 |
| Anticoagulation on discharge | 258 (3.8%) | 153 (2.3%) | <0.001 | −0.091 |
| Metformin on discharge | 1483 (22.0%) | 1512 (22.4%) | 0.55 | 0.010 |
| Statin on discharge | 6514 (96.7%) | 6403 (95.0%) | <0.001 | −0.083 |
| Angiotensin blockade on discharge | 4930 (73.2%) | 4680 (69.5%) | <0.001 | −0.082 |
| Beta-blocker on discharge | 4975 (73.8%) | 5166 (76.7%) | <0.001 | 0.066 |
| Drop in haemoglobin >2 g/dL after PCI | 1497 (22.2%) | 1301 (19.3%) | <0.001 | −0.072 |
| Gastrointestinal bleeding during follow-up | 362 (5.4%) | 336 (5.0%) | 0.31 | −0.017 |
| Upper endoscopy during follow-up | 1076 (16.0%) | 957 (14.2%) | 0.004 | −0.049 |
*Potent P2Y12 inhibitors=ticagrelor or prasugrel.
PCI, percutaneous coronary intervention; PPI, proton pump inhibitor.;
bmjgast-2021-000719supp001.pdf (117.7KB, pdf)
Primary and secondary outcomes
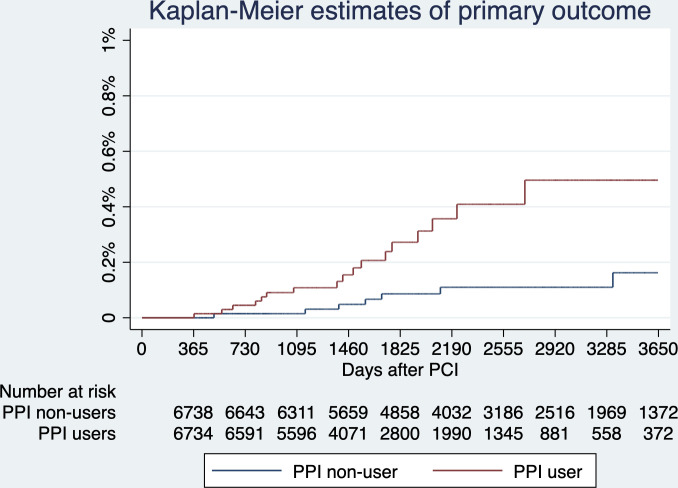
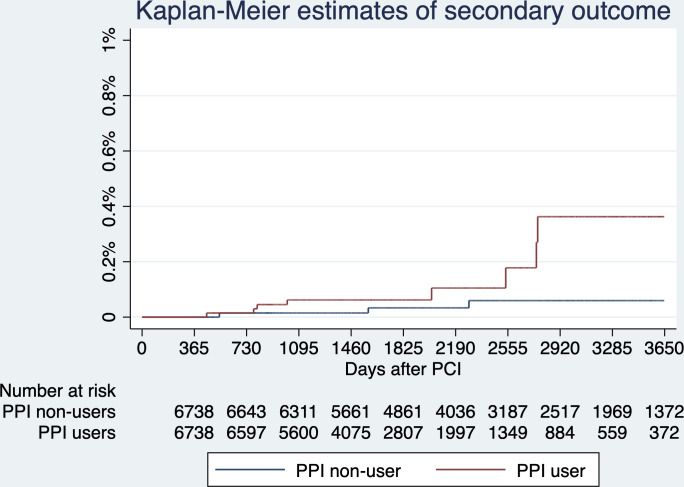
The primary outcome, gastric cancer diagnosed >365 days after PCI, developed in 17 (0.25%) PPI users and 7 (0.10%) PPI non-users. The risk of gastric cancer was higher in PPI users (HR 3.55; 95% CI 1.46 to 8.66, p=0.005), an absolute between-group difference of 0.21 percentage points (95% CI 0.05 to 0.36) and the number needed to harm to cause one gastric cancer was 476. The secondary outcome, death from gastric cancer >365 days after PCI, developed in 8 (0.12%) PPI users and 3 (0.04%) PPI non-users. The risk of death from gastric cancer was higher in PPI users (HR 4.18; 95% CI 1.09 to 16.08, p=0.037), an absolute between-group difference of 0.07 percentage points (95% CI −0.02 to 0.17); and the number needed to harm to cause one death from gastric cancer was 1429. Results are shown in figures 3–4 and table 3. The histological information of the gastric cancers in patients who were treated in 2 of the 14 hospitals (Queen Mary Hospital and Grantham Hospital) were reviewed, and yielded 100% specificity of the diagnostic codes.
Figure 3.
Primary outcome—estimated probabilities of gastric cancer stratified by proton pump inhibitor (PPI) use. The risks of gastric cancer diagnosed after time zero (ie, 365 days after percutaneous coronary intervention (PCI)) were higher in PPI users (HR 3.55; 95% CI 1.46 to 8.66, p=0.005). Noteworthily, four patients, all being PPI users, developed gastric cancer between 0 and 365 days after PCI, which were not considered as primary outcome, but reduced the number at risk at 365 days by 4.
Figure 4.
Secondary outcome—estimated probabilities of death from gastric cancer stratified by proton pump inhibitor (PPI) use. The risk of death from gastric cancer after time zero (ie, 365 days after percutaneous coronary intervention (PCI)) was significantly higher with PPI use (HR 4.18; 95% CI 1.09 to 16.08, p=0.037).
Table 3.
Primary and secondary outcomes stratified by PPI use
| Outcomes | Event rates per 100 000 patient-year | HR | 95% CI | P value | |
| PPI users | PPI non-users | ||||
| Primary | |||||
| Gastric cancer | 60.2 | 16.9 | 3.55 | 1.46 to 8.66 | 0.005 |
| Secondary | |||||
| Death from gastric cancer | 28.3 | 7.22 | 4.18 | 1.09 to 16.08 | 0.037 |
PPI, proton pump inhibitor.
Sensitivity and falsification analyses
First, we repeated the primary analysis on 2033 patients who had at least one upper endoscopy any time after PCI, the risk of gastric cancer diagnosed after time zero was higher in PPI users (HR 3.09; 95% CI 1.20 to 7.94, p=0.019). Next, another sensitivity analysis was performed on all patients before propensity score matching and with complete information of variables used in the primary analysis model. After adjustment by Cox regression model, the risk of gastric cancer was higher for PPI users compared with non-users (adjusted HR 2.38; 95% CI 1.20 to 4.76, p=0.014) (online supplemental table S3 and S4 in the online supplemental appendix). Finally, patients were divided into three groups according to the duration of PPI use, only those with long duration (≥365 days) of PPI use had excess risk of gastric cancer compared with minimal duration (adjusted HR 2.06; 95% CI 1.01 to 4.18; p=0.046) (table 4).
Table 4.
Association between duration of PPI use and gastric cancer after adjustment
| Duration of PPI use after PCI | N | HR | 95% CI | P value |
| Minimal duration (<14 days) | 13 454 | Reference | ||
| Short duration (14–364 days) | 3552 | 1.75 | 0.94 to 3.26 | 0.08 |
| Long duration (≥365 days) | 6793 | 2.06 | 1.01 to 4.18 | 0.046 |
PCI, percutaneous coronary intervention; PPI, proton pump inhibitor.
Falsification testing showed that PPI use was not associated with increased risk of lung cancer diagnosed after time zero (HR 1.00; 95% CI 0.70 to 1.43; p=0.98).
A total of five variables in the Cox regression model had missing data. Tobacco use, the variable which had the largest amount of missing data, had 1424 (5.6%) missing values. Multiple imputation was conducted, and the imputed cohort included all 1499 (5.9%) patients who were excluded due to missing values in any of the variables used in the model. The association between PPI use and gastric cancer in the imputed dataset remained significant after propensity score adjustment (adjusted HR 1.98; 95% CI 1.07 to 3.66; p=0.030).
Discussion
In this cohort of 25 298 adult patients who were not on PPI therapy at baseline, PPI prescription of >180 days for prophylaxis after PCI was associated with a 2.5-fold increase in risk of gastric cancer and 3-fold increase in risk of death from gastric cancer over a median follow-up of 7.1 years. The association was duration-dependent and patients who took PPI for ≥365 days were at highest risk. The association remained consistent after adjustment for factors potentially affecting PPI prescription and multiple sensitivity analyses, including falsification testing.
Chronic PPI use has been shown to be associated with increased incidence of gastric cancer in several large observational studies, but confounding by indication and protopathic bias remains a major limitation. Brusselaers et al used a national cohort from Sweden (n=797 067) and found a twofold increase in risk of gastric cancer among PPI users.2 However, 25% of the PPI users had unidentified indication for PPI, and those with longest PPI use paradoxically had lower excess risk of gastric cancer, leading to concerns of protopathic bias. Ahn et al analysed three observational studies and found a 40% excess risk of gastric cancer in PPI users.3 Interestingly, the authors of all three original studies attributed the findings to confounding by indication.22–24 In a cohort study by Poulsen et al, the association between PPI and gastric cancer disappeared after a 1-year lag time was introduced to control for protopathic bias. Therefore, alternative patient populations who do not present with GI symptoms could be better suited for the examination of cancer risk due to chronic PPI use. To our best knowledge, our cohort is one of the first large-scale observational studies in patients with new PPI prescription for prophylactic purposes, and the effect of PPI can be examined in isolation of potential underlying GI conditions substantially reducing concerns of confounding by indication. To further safeguard against protopathic bias, we defined time zero as 365 days after PCI.15
In a meta-analysis of six RCT (n=785), chronic PPI use was not associated with higher risk of gastric premalignancy lesions.7 This was not surprising as these RCTs had limited sample sizes and follow-up periods (6–36 months), given the low incidence of gastric cancer and the longer lag phase between exposure to causative factors and cancer diagnosis, these studies were likely underpowered to detect a statistically significant difference.25 In comparison, our data including a substantially larger sample size and longer follow-up period (median 7.1 years) provided the unique opportunity to examine this association. It is unlikely that future RCT be ethical or cost-effective in analysing this rare long-term outcome.
There are several potential carcinogenic mechanisms for PPI. Potent acid suppression has been linked to the development of gastric cancer through worsening gastric atrophy, causing hypergastrinaemia via negative feedback and bacterial overgrowth in the stomach.6 26 27 Gastrin is shown to promote gastric cancer development due to its progrowth effect on enterochromaffin-like (ECL) cells in the oxyntic mucosa and stimulation of the release of signal substances (eg, histamine, PEG protein) from the ECL cells.6 28–30 Overgrowth of non-H. pylori bacteria that possesses nitrate reductase could increase the production of N-nitroso compounds from food nitrates, which are known carcinogens.31 These mechanisms are duration-dependent, in agreement with our observation and previous reports.5 23
Another research question is the interaction of aspirin on the PPI-gastric cancer association. Cheung et al found a 1.4-fold increase in risk of gastric cancer among chronic PPI users (n=63 397) after H. pylori eradication.5 A post hoc analysis suggested that aspirin could mitigate the excess risk associated with PPI use, which was in agreement with published data on the protective effect of aspirin on gastric cancer.32–34 Our findings add to existing knowledge that the excess risk of gastric cancer was significant even in a cohort with almost universal use of aspirin.
Current American guidelines recommended PPI prophylaxis in patients on DAPT who have high GI bleeding risk.35 The main drawback of PPI considered in the guidelines was the drug interaction between PPI and P2Y12 inhibitors.35 Our results should be considered in alerting potential prescribers of PPI therapy to use a lowest effective dose over the shortest time period possible. Notably, 7053 (27.9%) patients in our entire cohort were maintained on PPI therapy beyond 30 days after cessation of P2Y12 inhibitors, suggesting that continuous review of the appropriateness for chronic PPI therapy is necessary.36 Nonetheless, the absolute increase in the risk of gastric cancer is quite small, thereby the decision of prophylactic PPI should take into account of the risk of upper GI bleeding and associated risk of premature interruption of DAPT and thrombotic events.37–39
This study had several strengths. It included users of PPI for purposes unrelated to GI manifestations, minimising the risk of confounding by indication. The large cohort size with complete electronic healthcare records minimised selection, information and recall biases, and the long follow-up period was well-suited to studying cancer incidence.
This study had some limitations. First, the observational nature of the study conferred risk of unmeasured confounding and bias, but the findings were consistent in many sensitivity analyses including falsification analysis. Chart review of the diagnostic codes for the primary outcome yielded 100% specificity in the subset with accessible histological information. The incidence rate of gastric cancer for the current cohort was 49.8 per 100 000 patient year, similar to the age-adjusted incidence rate of 41.1 for the Hong Kong population during the same period,40 suggestive of accurate reporting. Second, we only collected prescription data and could not ascertain drug adherence, which could have biased towards the null. Third, almost all patients were taking aspirin concurrently after PCI, and the study findings may not be directly applicable to other patient groups. Fourth, the details of cancer histology were not available in many patients, precluding exploration of the relationship between PPI use and specific subtypes of gastric cancer.
Conclusion
In conclusion, we showed that chronic PPI use started for ulcer prophylaxis after PCI was significantly associated with increased risk of gastric cancer and death from gastric cancer. While awaiting prospective data to better ascertain the causal relationship, physicians should judiciously assess the risks and benefits of chronic PPI use on prescription, and minimise the duration of exposure as far as possible.
Footnotes
Twitter: @drandrewkyng
Contributors: AK-YN, K-SC and C-WS was responsible for the conception and design of the study. AK-YN analysed the data collected by AI. AK-YN interpreted the data. AK-YN and PYN drafted the manuscript. All authors revised and approved the final manuscript, and are accountable for the accuracy and integrity of the work.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available on reasonable request. Please contact the corresponding author for details.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
The study was approved by the Institutional Review Board of the University of Hong Kong/Hospital Authority.
References
- 1.Daniels B, Pearson S-A, Buckley NA, et al. Long-term use of proton-pump inhibitors: whole-of-population patterns in Australia 2013-2016. Therap Adv Gastroenterol 2020;13:1756284820913743. 10.1177/1756284820913743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brusselaers N, Wahlin K, Engstrand L, et al. Maintenance therapy with proton pump inhibitors and risk of gastric cancer: a nationwide population-based cohort study in Sweden. BMJ Open 2017;7:e017739. 10.1136/bmjopen-2017-017739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahn JS, Eom C-S, Jeon CY, et al. Acid suppressive drugs and gastric cancer: a meta-analysis of observational studies. World J Gastroenterol 2013;19:2560–8. 10.3748/wjg.v19.i16.2560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chien L-N, Huang Y-J, Shao Y-HJ, et al. Proton pump inhibitors and risk of periampullary cancers--A nested case-control study. Int J Cancer 2016;138:1401–9. 10.1002/ijc.29896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheung KS, Chan EW, Wong AYS, et al. Long-term proton pump inhibitors and risk of gastric cancer development after treatment for Helicobacter pylori: a population-based study. Gut 2018;67:28–35. 10.1136/gutjnl-2017-314605 [DOI] [PubMed] [Google Scholar]
- 6.Cheung KS, Leung WK. Long-term use of proton-pump inhibitors and risk of gastric cancer: a review of the current evidence. Therap Adv Gastroenterol 2019;12:1756284819834511. 10.1177/1756284819834511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eslami L, Nasseri-Moghaddam S. Meta-analyses: does long-term PPI use increase the risk of gastric premalignant lesions? Arch Iran Med 2013;16:449–58. doi:0013168/AIM.004 [PubMed] [Google Scholar]
- 8.Levine GN, Bates ER, Bittl JA. ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American College of Cardiology/American heart association Task force on clinical practice guidelines. J Am Coll Cardiol 2016;2016:1082–115. [DOI] [PubMed] [Google Scholar]
- 9., Windecker S, Kolh P, et al. , Authors/Task Force members . 2014 ESC/EACTS Guidelines on myocardial revascularization: The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS)Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J 2014;35:2541–619. 10.1093/eurheartj/ehu278 [DOI] [PubMed] [Google Scholar]
- 10., Baigent C, Blackwell L, et al. , Antithrombotic Trialists' (ATT) Collaboration . Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet 2009;373:1849–60. 10.1016/S0140-6736(09)60503-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grove EL, Würtz M, Schwarz P, et al. Gastrointestinal events with clopidogrel: a nationwide population-based cohort study. J Gen Intern Med 2013;28:216–22. 10.1007/s11606-012-2208-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhatt DL, Cryer BL, Contant CF, et al. Clopidogrel with or without omeprazole in coronary artery disease. N Engl J Med 2010;363:1909–17. 10.1056/NEJMoa1007964 [DOI] [PubMed] [Google Scholar]
- 13.Chin MWS, Yong G, Bulsara MK, et al. Predictive and protective factors associated with upper gastrointestinal bleeding after percutaneous coronary intervention: a case-control study. Am J Gastroenterol 2007;102:2411–6. 10.1111/j.1572-0241.2007.01460.x [DOI] [PubMed] [Google Scholar]
- 14.Lam AS, Yan BP, Lee VW. Trends of prescribing adherence of antiplatelet agents in Hong Kong patients with acute coronary syndrome: a 10-year retrospective observational cohort study. BMJ Open 2020;10:e042229. 10.1136/bmjopen-2020-042229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tamim H, Monfared AAT, LeLorier J. Application of lag-time into exposure definitions to control for protopathic bias. Pharmacoepidemiol Drug Saf 2007;16:250–8. 10.1002/pds.1360 [DOI] [PubMed] [Google Scholar]
- 16.Zhou X-L, Xue W-H, Ding X-F, et al. Association between metformin and the risk of gastric cancer in patients with type 2 diabetes mellitus: a meta-analysis of cohort studies. Oncotarget 2017;8:55622–31. 10.18632/oncotarget.16973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheung KS, Chan EW, Wong AYS, et al. Aspirin and risk of gastric cancer after Helicobacter pylori eradication: a Territory-Wide study. J Natl Cancer Inst 2018;110:743–9. 10.1093/jnci/djx267 [DOI] [PubMed] [Google Scholar]
- 18.Singh PP, Singh S. Statins are associated with reduced risk of gastric cancer: a systematic review and meta-analysis. Ann Oncol 2013;24:1721–30. 10.1093/annonc/mdt150 [DOI] [PubMed] [Google Scholar]
- 19.Cheung KS, Chan EW, Wong AYS, et al. Statins Were Associated with a Reduced Gastric Cancer Risk in Patients with Eradicated Helicobacter Pylori Infection: A Territory-Wide Propensity Score Matched Study. Cancer Epidemiol Biomarkers Prev 2020;29:493–9. 10.1158/1055-9965.EPI-19-1044 [DOI] [PubMed] [Google Scholar]
- 20.Austin PC. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat 2011;10:150–61. 10.1002/pst.433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wennerström ECM, Simonsen J, Camargo MC, et al. Acid-suppressing therapies and subsite-specific risk of stomach cancer. Br J Cancer 2017;116:1234–8. 10.1038/bjc.2017.84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tamim H, Duranceau A, Chen L-Q, et al. Association between use of acid-suppressive drugs and risk of gastric cancer. A nested case-control study. Drug Saf 2008;31:675–84. 10.2165/00002018-200831080-00004 [DOI] [PubMed] [Google Scholar]
- 23.García Rodríguez LA, Lagergren J, Lindblad M. Gastric acid suppression and risk of oesophageal and gastric adenocarcinoma: a nested case control study in the UK. Gut 2006;55:1538–44. 10.1136/gut.2005.086579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Poulsen AH, Christensen S, McLaughlin JK, et al. Proton pump inhibitors and risk of gastric cancer: a population-based cohort study. Br J Cancer 2009;100:1503–7. 10.1038/sj.bjc.6605024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Song H, Ekheden IG, Zheng Z, et al. Incidence of gastric cancer among patients with gastric precancerous lesions: observational cohort study in a low risk Western population. BMJ 2015;351:h3867. 10.1136/bmj.h3867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Waldum HL, Sandvik AK, Brenna E, et al. Gastrin-histamine sequence in the regulation of gastric acid secretion. Gut 1991;32:698–701. 10.1136/gut.32.6.698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Joo MK, Park J-J, Chun HJ. Proton pump inhibitor: the dual role in gastric cancer. World J Gastroenterol 2019;25:2058–70. 10.3748/wjg.v25.i17.2058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Waldum HL, Sandvik AK, Idle JR. Gastrin is the most important factor in ECL tumorigenesis. Gastroenterology 1998;114:1113–4. 10.1016/S0016-5085(98)70346-4 [DOI] [PubMed] [Google Scholar]
- 29.Hayakawa Y, Chang W, Jin G, et al. Gastrin and upper GI cancers. Curr Opin Pharmacol 2016;31:31–7. 10.1016/j.coph.2016.08.013 [DOI] [PubMed] [Google Scholar]
- 30.Cheung K-S, Leung WK. Risk of gastric cancer development after eradication of Helicobacter pylori. World J Gastrointest Oncol 2018;10:115–23. 10.4251/wjgo.v10.i5.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parsons BN, Ijaz UZ, D'Amore R, et al. Comparison of the human gastric microbiota in hypochlorhydric states arising as a result of Helicobacter pylori-induced atrophic gastritis, autoimmune atrophic gastritis and proton pump inhibitor use. PLoS Pathog 2017;13:e1006653. 10.1371/journal.ppat.1006653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cheung KS, Leung WK. Modification of gastric cancer risk associated with proton pump inhibitors by aspirin after Helicobacter pylori eradication. Oncotarget 2018;9:36891–3. 10.18632/oncotarget.26382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.García Rodríguez LA, Soriano-Gabarró M, Vora P, et al. Low-Dose aspirin and risk of gastric and oesophageal cancer: a population-based study in the United Kingdom using the health improvement network. Int J Cancer 2020;147:2394–404. 10.1002/ijc.33022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brusselaers N, Lagergren J. Maintenance use of non-steroidal anti-inflammatory drugs and risk of gastrointestinal cancer in a nationwide population-based cohort study in Sweden. BMJ Open 2018;8:e021869. 10.1136/bmjopen-2018-021869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abraham NS, Hlatky MA, Antman EM, et al. ACCF/ACG/AHA 2010 expert consensus document on the concomitant use of proton pump inhibitors and thienopyridines: a focused update of the ACCF/ACG/AHA 2008 expert consensus document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use: a report of the American College of cardiology Foundation Task force on expert consensus documents. Circulation 2010;122:2619–33. 10.1161/CIR.0b013e318202f701 [DOI] [PubMed] [Google Scholar]
- 36.Safety of long-term PPi use. JAMA 2017;318:1177–8. 10.1001/jama.2017.13272 [DOI] [PubMed] [Google Scholar]
- 37.van Werkum JW, Heestermans AA, Zomer AC, et al. Predictors of coronary stent thrombosis: the Dutch stent thrombosis registry. J Am Coll Cardiol 2009;53:1399–409. 10.1016/j.jacc.2008.12.055 [DOI] [PubMed] [Google Scholar]
- 38.Eisenberg MJ, Richard PR, Libersan D, et al. Safety of short-term discontinuation of antiplatelet therapy in patients with drug-eluting stents. Circulation 2009;119:1634–42. 10.1161/CIRCULATIONAHA.108.813667 [DOI] [PubMed] [Google Scholar]
- 39.Mehran R, Baber U, Steg PG, et al. Cessation of dual antiplatelet treatment and cardiac events after percutaneous coronary intervention (Paris): 2 year results from a prospective observational study. Lancet 2013;382:1714–22. 10.1016/S0140-6736(13)61720-1 [DOI] [PubMed] [Google Scholar]
- 40.Hong Kong Cancer Registry . Website of Hong Kong cancer registry, hospital authority, 2021. Available: www3.ha.org.hk/cancereg
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjgast-2021-000719supp001.pdf (117.7KB, pdf)
Data Availability Statement
Data are available on reasonable request. Please contact the corresponding author for details.