Abstract
We report on a unique case of a 7-year-old girl with new onset ocular myasthenia gravis shortly after recovery from multisystem inflammatory syndrome in children (MIS-C) temporally associated with SARS-CoV-2 infection. The diagnosis of myasthenia gravis was based on suggestive symptoms of fatigable bilateral orbital ptosis, diplopia, positive ocular cold compression test and serum acetylcholine receptor antibody positivity, as well as a favourable treatment response to pyridostigmine. The addition of corticosteroids and methotrexate resulted in complete resolution of the ocular signs.
Keywords: COVID-19, neurology (drugs and medicines), paediatrics (drugs and medicines), neuroimaging, neuromuscular disease
Background
Neuromuscular complications of COVID-19 are well reported as a result of either the infection itself or the treatment thereof. However, there are emerging reports postulating an autoimmune mechanism for certain manifestations of COVID-19 such as interstitial pneumonia, Guillain-Barre syndrome, Miller-Fisher syndrome, among others.1 Multisystem inflammatory syndrome in children (MIS-C) is recognised by the WHO and Centers of Disease Control (CDC) as a rare, but severe, condition associated with a delayed, dysregulated B-cell and T-cell immune response, cytokine storm and endothelial damage in response to a preceding COVID-19 infection.2 Several cases of MIS-C have been reported in South Africa.3 To the best of our knowledge, this is the first case report of acetylcholine receptor-related myasthenia gravis post MIS-C.
Case presentation
A previously well 7-year-old girl presented with a 4-day history of fever, sore throat, cough, diarrhoea and headaches. There was no history of contact with a patient with COVID-19; however, the family reported upper respiratory tract symptoms of sore throat and cough 2 weeks preceding the child’s presentation. Clinical examination revealed a prostrated and dehydrated child in shock (hypotensive—blood pressure 65/53 mm Hg, tachycardic 135 beats/min, cold extremities and a capillary refill time >3 s). Additional findings observed were a temperature of 39.5°C, bilateral non-suppurative conjunctivitis, an erythematous desquamating rash on the abdomen, back and neck, and swelling of the hands and feet. An emergent assessment of circulatory shock was made requiring multiple fluid boluses and an immediate transfer to the paediatric intensive care unit (PICU).
In the PICU, the multiorgan failure (respiratory, cardiac and renal) necessitated ventilatory support, inotropes (epinephrine and norepinephrine) and close monitoring of fluid balance, respectively. Based on the clinical presentation, the multiorgan failure, raised inflammatory markers (see results in the Investigations section) and a positive SARS-CoV-2 antibody test, MIS-C was diagnosed. This was in accordance with the Royal College of Paediatrics and Child Health case definition of MIS-C.4 The intensive and intricate management steered a 10-day stay in PICU.
Following discharge from the PICU, myalgia and marked proximal muscle weakness were noted. The weakness was more pronounced in the lower limbs (proximal 2/5, distal 3/5) compared with the upper limbs (proximal 3/5, distal 4/5). Proximal muscle weakness was evidenced by a positive Gower sign, waddling gait and compensatory lumbar lordosis. The deep tendon reflexes were preserved.
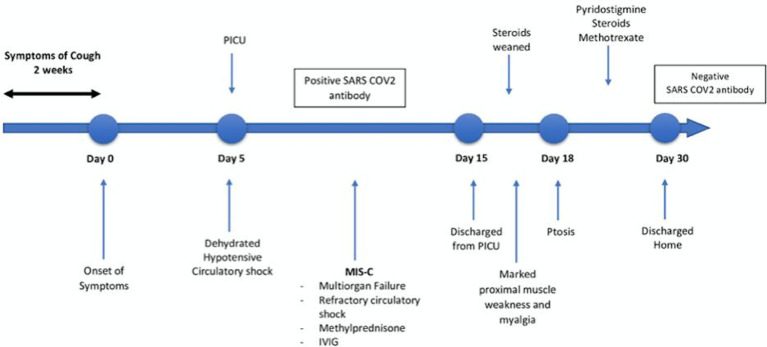
Subsequently, 72 hours later, fluctuating and fatigable bilateral ptosis emerged (figure 1) with impairment of upward gaze, mild diplopia and a positive curtain and Cogan eyelid twitch test. Pupillary reflexes were preserved. The clinical signs as well as a positive ocular cold compression test led to a suspected diagnosis of ocular myasthenia gravis. The rest of the systemic examination proved unremarkable. Figure 2 summarises the timeline of events from admission to discharge.
Figure 1.
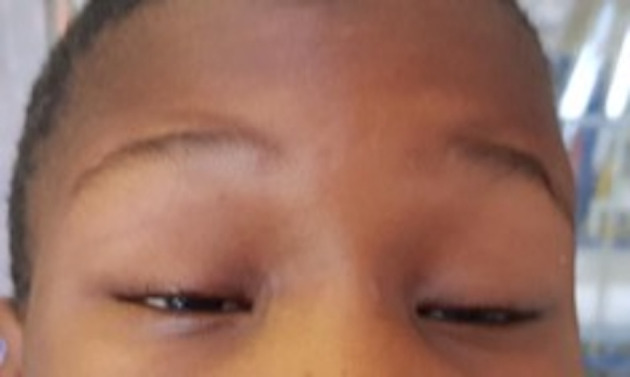
Severe bilateral symmetric eyelid ptosis with compensatory brow elevation and elongated margin fold distance.
Figure 2.
Timeline of events from onset of symptoms to discharge from the hospital. IVIG, intravenous immunoglobulin; MIS-C, multisystem inflammatory syndrome in children; PICU, paediatric intensive care unit.
Investigations
Relevant investigations, which aided in the diagnosis of MIS-C, yielded the following: SARS-CoV-2 PCR (negative), SARS-CoV-2 IgG antibodies to N protein (positive), full blood count (leucocytosis with a neutrophilia and lymphopenia—white cell count 24.0×109/L, neutrophil 94.2% and lymphocyte 2.4%, haemoglobin 10.5 g/dL, platelets 122×109/L), raised inflammatory markers (C reactive protein 292 mg/L, ferritin 1022 μg/L, D-dimer 11.65 mg/L, lactate dehydrogenase 517 u/L), renal function (urea 22.1 mmol/L, creatinine 259 μmol/L, sodium 126 mmol/L, potassium 3.2 mmol/L) and markedly elevated cardiac enzymes (troponin T 477 ng/L, pro-BNP (pro B-type natriuretic peptide) 98 900 ng/L). Additionally, the echocardiogram displayed reduced left ventricular function with mildly dilated left ventricle with a markedly reduced ejection fraction of 38%. No coronary aneurysms were noted.
The creatine kinase was elevated at 6617 u/L. Neurophysiological studies were withheld as the child was not cooperative. The acetylcholine receptor antibody tested positive with a titre of 0.51 nm/L (0.39). Due to lack of availability, the muscle-specific tyrosine kinase antibody test was not done.
Differential diagnosis
Toxic shock syndrome (TSS) and incomplete Kawasaki disease (KD) were initial diagnostic considerations. Although the CDC criteria for TSS were met, the absence of a bacterial source of infection and blood cultures made the diagnosis less likely. Similarly, the patient also matched the diagnostic criteria for incomplete KD; however, the age of the patient (older than 5 years), absence of cervical lymphadenopathy, absence of oral mucous membrane changes and subsequent absence of coronary artery aneurysms (on serial echocardiography) argued against it. Identification of a source of infection (positive SARS-CoV-2—N protein-driven antibody test) and echocardiographic findings (reduced ejection fraction representing global cardiac involvement) favoured a diagnosis of MIS-C. Other diagnostic considerations were vasculitides and systemic lupus erythematosus; however, laboratory results were not supportive.
Differential diagnosis for the severe muscle weakness included critical illness myopathy (CIM), critical illness neuropathy (CIN) or post COVID-19 myositis. Risk factors for CIM included the prolonged intensive care unit stay, use of corticosteroids and the limb weakness. However, neuromuscular-blocking agents were never prescribed and the weaning of ventilatory support proved uneventful. The preservation of deep tendon reflexes argued against a CIN.
Treatment
Intravenous methylprednisolone, 30 mg/kg/day over 3 days, and intravenous immunoglobulin (IVIG), 2 g/kg over 2 days, were given for the MIS-C. This was followed by 2 mg/kg/day of oral prednisone that was gradually weaned over a 4-week period. The response to treatment was positive with improvements seen in blood pressure, ejection fraction and fall in inflammatory markers (C reactive protein, ferritin and erythrocyte sedimentation rate). Additional supportive treatment included low-dose molecular-weight heparin and low-dose aspirin (for coronary artery protection and antiplatelet cover to prevent risk of thromboembolic events associated with MIS-C), calcium, vitamin D and zinc supplementation.
On diagnosis of myasthenia gravis, pyridostigmine was initiated. However, since the response was suboptimal, low-dose oral corticosteroids and methotrexate were initiated. Since IVIG was administered in PICU for the MIS-C, it was not repeated due to its long half-life. Moreover, intensive physiotherapy and occupational therapy were provided for the muscle weakness. On discharge, the patient was ambulatory with normal vital signs.
Outcome and follow-up
One-month post-discharge, the ejection fraction had returned to normal and no coronary aneurysms were noted. There was complete recovery of renal, liver and haematological function. Repeat SARS-CoV-2 antibody and acetylcholine receptor antibody testing proved negative, which suggests fading B cell immunity. On clinical review, the ptosis had completely resolved, along with marked improvement in the proximal muscle weakness. The pyridostigmine and corticosteroids were slowly discontinued. She continues to receive physiotherapy as an outpatient and regular clinical follow-up by the multidisciplinary team.
Discussion
This is the first report of transient acetylcholine receptor-related myasthenia gravis occurring shortly after MIS-C. In addition, our patient also had features of myositis, which has been previously reported as a complication of severe COVID-19 disease, suggesting a contributory autoimmune mechanism. Furthermore, there is also a direct effect of SARS-CoV-2 evidenced by the presence of ACE 2 receptors on muscle fibres.5
A few adult case reports have been reported of either myasthenia exacerbation or post-COVID-19 myasthenia gravis. All patients with post-COVID-19 myasthenia gravis had positive acetylcholine receptor antibodies and responded to pyridostigmine alone or in combination with IVIG and low-dose steroids. The symptoms of myasthenia gravis occurred within weeks after the primary COVID-19 infection. These findings were similar in our patient.6–8
Infections are known to trigger a crisis in patients with myasthenia gravis via molecular mimicry between the acetylcholine receptor and the viral protein.9 However, there is no clear consensus whether viral infections can cause myasthenia gravis in otherwise healthy children. Thymus overgrowth has been shown to play a role, as more than 80% of patients with early-onset myasthenia gravis have thymic hyperplasia and about 10%–15% have thymomas or benign thymic tumours. It has been proposed that thymus inflammation caused by viruses may contribute to autoimmunity. The most common viruses implicated are varicella, parvovirus B19 and West Nile virus.10
MIS-C is now a recognised disease associated with SARS-CoV-2 occurring a few weeks after the peak of COVID-19, with the majority of cases having a negative antigen test but a positive antibody test, which was the case in this patient. The precise reason of how SARS-CoV-2 causes MIS-C is still unknown. Possible mechanisms reported in the literature include antibodies causing an increase in viral entry into the cells and therefore enhancing disease and antibody/T-cell-mediated damage.11 Severity of infection has also been associated with a dysregulated immune response and subsequent cytokine storm and endothelial damage. This results in hyperinflammation and may cause the multisystem inflammatory syndrome.12
We postulate that the hyperinflammation could have triggered thymic inflammation, leading to formation of acetylcholine receptor antibodies. Alternatively, there could be presence of molecular mimicry with COVID-19 antibodies against the SARS-CoV-2 protein cross-reacting with acetylcholine receptor subunits due to similarity between SARS-CoV-2 epitopes and components of the neuromuscular junction. With both hypotheses, one can expect this to be a transient phenomenon as once the inflammation subsides, antibody production should cease, and if the molecular mimicry theory applies, the repeat antibody test should become negative (as was the case), which should lead to reversal of symptoms.
Neuromuscular complications have been associated with either COVID-19 infection or its treatments. A particular trigger for myasthenia is azithromycin, which is commonly used in patients infected with COVID-19.13 However, this treatment was not used in our patient. Treatment of myasthenia gravis during the COVID-19 pandemic is aimed at reversal of symptoms with immunosuppressive therapies. Current guidelines recommend tailoring treatment on an individual basis.14
Learning points.
Multisystem inflammatory syndrome in children (MIS-C) in the era of COVID-19 requires a high index of suspicion and prompt treatment for good outcomes.
Critical illness/steroid myopathy and post-COVID-19 myositis are important considerations in patients with prolonged intensive care unit stay and COVID-19 infection/MIS-C.
Transient ocular myasthenia gravis is a possible complication of MIS-C.
Footnotes
Contributors: Dr FE, Professor RS and Professor RVT were the neurologists insole with the case. Professor PG is the pulmonologist. Dr Abraham and Dr Lishman were the infectious disease and immunologist involved. All were involved in writing the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Parents/guardian consent obtained.
References
- 1.Ehrenfeld M, Tincani A, Andreoli L, et al. Covid-19 and autoimmunity. Autoimmun Rev 2020;19:102597. 10.1016/j.autrev.2020.102597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2..’Centers for Disease Control and Prevention (CDC), . Health advisory on multisystem inflammatory syndrome in children (MIS-C) associated with coronavirus disease 2019.
- 3.Webb K, Abraham DR, Faleye A, et al. Multisystem inflammatory syndrome in children in South Africa. Lancet Child Adolesc Health 2020;4:e38. 10.1016/S2352-4642(20)30272-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Royal College of Paediatrics and Child Health . Guidance: paediatric multisystem inflammatory syndrome temporally associated with COVID-19. Available: https://www.rcpch.ac.uk/sites/default/files/2020-05/COVID-19-Paediatric-multisystem-%20inflammatory%20syndrome-20200501.pdf [DOI] [PubMed]
- 5.Beydon M, Chevalier K, Al Tabaa O, et al. Myositis as a manifestation of SARS-CoV-2. Ann Rheum Dis 2021;80:e42. 10.1136/annrheumdis-2020-217573 [DOI] [PubMed] [Google Scholar]
- 6.Sriwastava S, Tandon M, Kataria S. New onset of ocular myasthenia gravis in a patient with COVID-19: a novel case report and literature review. J Neurol 2020:1–7. 10.1007/s00415-020-10263-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aksoy E, Oztutgan T. COVID-19 presentation in association with myasthenia gravis: a case report and review of the literature. Case Rep Infect Dis 2020;2020:1–4. 10.1155/2020/8845844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moschella P, Roth P. Isolated COVID-19 infection precipitates myasthenia gravis crisis: a case report. Clin Pract Cases Emerg Med 2020;4:524–6. 10.5811/cpcem.2020.9.49049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Felice KJ, DiMario FJ, Conway SR. Postinfectious myasthenia gravis: report of two children. J Child Neurol 2005;20:441–4. 10.1177/08830738050200051501 [DOI] [PubMed] [Google Scholar]
- 10.Gong L, Li Y, Li X, et al. Detection of human parvovirus B19 infection in the thymus of patients with thymic hyperplasia-associated myasthenia gravis. Clin Microbiol Infect 2019;25:109.e7–12. 10.1016/j.cmi.2018.03.036 [DOI] [PubMed] [Google Scholar]
- 11.Jiang L, Tang K, Levin M, et al. COVID-19 and multisystem inflammatory syndrome in children and adolescents. Lancet Infect Dis 2020;20:e276–88. 10.1016/S1473-3099(20)30651-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kest H, Kaushik A, DeBruin W, et al. Multisystem inflammatory syndrome in children (MIS-C) associated with 2019 novel coronavirus (SARS-CoV-2) infection. Case Rep Pediatr 2020;2020:1–4. 10.1155/2020/8875987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guidon AC, Amato AA. COVID-19 and neuromuscular disorders. Neurology 2020;94:959–69. 10.1212/WNL.0000000000009566 [DOI] [PubMed] [Google Scholar]
- 14.International MG/COVID-19 Working Group, Jacob S, Muppidi S, et al. Guidance for the management of myasthenia gravis (MG) and Lambert-Eaton myasthenic syndrome (LEMS) during the COVID-19 pandemic. J Neurol Sci 2020;412:116803. 10.1016/j.jns.2020.116803 [DOI] [PMC free article] [PubMed] [Google Scholar]