Abstract
Background
Patients with misophonia suffer from anger or disgust confronted with specific sounds such as smacking or breathing. Avoidance of cue‐related situations results in social isolation and significant functional impairment. This is the first randomized, controlled cognitive behavioral therapy (CBT) trial for misophonia, evaluating the short‐ and long‐term efficacy.
Methods
The evaluator‐blinded, randomized clinical trial was conducted from May 2017 until December 2018 at an academic outpatient clinic. Misophonia patients were randomly assigned to 3 months of weekly group‐CBT or a waiting list and tested at baseline, 3 months (following CBT or waiting list), 6 months (after cross‐over), and 15/18 months (1‐year follow‐up). CBT consisted of task concentration and arousal reduction, positive affect labeling, and stimulus manipulation. Co‐primary outcomes were symptom severity assessed by the Amsterdam Misophonia Scale‐Revised (AMISOS‐R) and improvement on the Clinical Global Impression‐Improvement (CGI‐I). Secondary outcomes were self‐assessed ratings of general psychopathology (Symptom Checklist‐90‐Revised [SCL‐90‐R]) and quality of life (five‐dimensional EuroQol [EQ5‐D], Sheehan Disability Scale [SDS], WHO Quality of Life‐BREF [WHOQoL‐BREF]).
Results
In all, 54 out of 71 patients were included (mean age, 33.06 [SD, 14.13] years; 38 women [70.4%]) and 46 (85%) completed the study. In the randomized phase, CBT resulted in statistically significant less misophonia symptoms in the short‐term (−9.7 AMISOS‐R; 95% CI, −12.0 to −7.4; p < .001, d = 1.97). The CBT group had an observed clinical improvement (CGI‐I < 3) in 37% compared to 0% in the waiting list group (p < .001). The effect of CBT was maintained at 1‐year follow‐up on primary and secondary outcomes.
Conclusions
This first randomized control trial shows both short‐term and long‐term efficacy of CBT for misophonia.
Keywords: cognitive behavioral therapy, misophonia, psychotherapy, randomized clinical trial, treatment
1. INTRODUCTION
Patients with misophonia suffer from irritation, anger, or disgust confronted with specific sounds, such as eating sounds or breathing. Avoidance of cue‐related situations and preoccupation with possible triggers result in social isolation and significant functional impairment. Misophonia patients are often not able to eat, sleep, or work in company, and most social and family relations are negatively affected. Especially when avoidance (e.g., walking away or wearing earplugs) is not possible, patients suffer. For example, detecting an apple at the desk during a meeting can already cause an extreme emotional response.
A growing scientific interest in misophonia has emerged within the last two decades (Brout et al., 2018). Research has mainly focused on clinical features, leading to the proposal of diagnostic criteria (Dozier et al., 2017; Jager et al., 2020; Schröder et al., 2013). There is no consensus about the nature of the disorder, other research groups emphasize its audiological or neurological nature. Incidence and prevalence are unknown, though a Chinese student sample indicates an incidence of 6% (Zhou et al., 2017). Misophonia is often found among family members, suggesting a hereditary component (Jager et al., 2020; Sanchez & Silva, 2018). The exact etiology of misophonia still remains unclear, but misophonia is possibly associated with neurodevelopmental conditions. Jager et al. (2020) found comorbid DSM‐IV Axis I diagnoses in 28%, most commonly, mood disorders (10%) and anxiety disorders (9%). Autism spectrum disorders and attention‐deficit/(hyperactivity) disorder (AD(H)D) were both common comorbid disorders, and important differential diagnoses for misophonia. Specific sample studies for misophonia prevalence among these neurodevelopmental conditions are yet to come. Though the nosography is not yet established, misophonia is widely recognized as an impairing condition (Taylor, 2017).
There are little well‐established, empirically supported treatments for misophonia. A few case studies were published in which misophonia patients were treated with cognitive behavioral therapy (CBT) using various techniques (Bernstein et al., 2013; Dozier, 2015; McGuire et al., 2015) and with dialectical behavior therapy (Kamody & Del, 2017). Our research group conducted an open‐label trial involving 90 patients with misophonia (Schröder et al., 2017), which showed promising results for CBT. In this trial, 48% of patients improved after eight sessions of CBT on the Clinical Global Impression‐Improvement (CGI‐I), and misophonia symptoms improved by 4.5 points on the Amsterdam Misophonia Scale; range from 0 to 24. Besides this trial, three case reports (total n = 4) showed anecdotal evidence for CBT. Bernstein et al. (2013) showed six sessions of CBT improved social and occupational functioning. Dozier (2015) showed after 14 sessions of counterconditioning misophonia symptoms were decreased. McGuire et al. (2015) showed 10–18 sessions of CBT (exposure) reduced misophonia symptoms in two youths and improved school and family functioning. No randomized clinical trials for any treatment have been published yet.
The current study is the first randomized controlled trial of CBT for misophonia and examines the efficacy of CBT compared to a waiting list control group. CBT is mainly concentrated on the preoccupation and associated arousal with misophonia triggers, since hyperfocus is considered a core symptom. Interventions also target the associated negative response by overwriting this and examining underlying assumptions. The study has two goals: first, to examine the short‐term efficacy and second, to examine the effect of CBT at follow‐up. We hypothesized a reduction in symptoms and improvement in quality of life (QoL) in the CBT group compared to the waiting list control group and the effects to persist at 1‐year follow‐up.
2. MATERIALS AND METHODS
The study site was the outpatient clinic of the department of psychiatry of the Amsterdam University Medical Center (Amsterdam UMC, location AMC). The study was registered in the Netherlands Trial Register (www.trialregister.nl) under number NL6304. The authors assert that all procedures contributing to this study comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. All procedures involving patients were approved by AMC Medical Ethics Committee. Written informed consent was obtained from all patients.
2.1. Participant selection, recruitment, and enrollment
A total of 71 patients were recruited from the outpatient clinic at the Amsterdam UMC. All patients were referred by their General Practitioners for treatment of impairing misophonia symptoms. All had received a psychiatric assessment and were on the waiting list for treatment. A research assistant approached all patients on the waiting list by telephone to inform them about the study, screened them, and sent written information by email or post.
Inclusion criteria were the presence of misophonia (as proposed by Schröder et al., 2013) diagnosed by a psychiatrist during a structured diagnostic interview at the intake of our psychiatric center and aged between 18 and 70 years. Exclusion criteria were the presence of major depression or anxiety disorder as primary diagnosis, bipolar disorder, autism spectrum disorders, schizophrenia, or any other psychotic disorder, substance‐related disorder during the past 6 months, any structural central nervous system disorder or stroke within the last year, currently taking benzodiazepines or stimulants, patients at risk for suicide, and patients with language barriers or illiteracy.
2.2. Randomization
Patients were randomized to treatment condition groups using a computerized randomization procedure (www.randomizer.org) with a 1:1 allocation ratio. Patients learned their treatment assignment directly after randomization. The independent researcher (IJ) was naive to randomization status. We assessed the fidelity of masking, which was found to be poor (78% of the assessments of treatment allocation were correctly guessed at the second clinical interview).
2.3. Treatment condition
The intervention was a manualized group treatment fairly similar to CBT used in our previous open‐label study (Schröder et al., 2017). The treatment manual was refined for this study with the elaborate input of the participating therapists. The manual (Van Loon et al., 2019) had specific instructions for each session (e.g., with a set time for each intervention, fully written exercises, and instruction videos for the therapists) to optimize equivalence among the different treatment groups. All interventions were checked after applying.
CBT was given in combined psychotherapy and Psychomotor Therapy (PMT) and consisted of four components: task concentration exercises, positive affect labeling, stimulus manipulation, and arousal reduction. Two elements were added: re‐evaluating (eating) norms and stress reduction. Family and friends were seen in groups in one separate session for psychoeducation and sharing experiences, and one family session for practicing the learned techniques together (see Table S1 and Appendix S1).
Group therapy was conducted in a closed group of nine patients with seven weekly meetings of 1.5 h of psychotherapy and 1.5 h of PMT, and one follow‐up meeting of 1.5 h after 3 weeks.
2.4. Waiting list condition
Patients in the waiting list condition received no treatment in the first 3 months. After 3 months, they received the same treatment as patients in the treatment condition, as described above.
2.5. Therapist training and quality assurance
Therapists for group CBT were licensed clinical psychologists with extensive training and experience in CBT for obsessive–compulsive and related disorders and misophonia in particular. Co‐therapists were licensed clinical psychologists, registered psychiatric nurses, and psychomotor therapists with CBT training. The department has so far diagnosed 1800 misophonia patients and treated over 1100 in this team. Therapists were provided with ongoing peer supervision throughout the randomized control trial (RCT) every 2 weeks. A research assistant attended the team‐meetings of each therapy group before and after sessions 1, 4, 7, and 8 to evaluate treatment adherence with detailed feedback, to maintain treatment fidelity and to ensure all measures were taken.
All raters (therapists and the independent investigator) were trained in April 2017 in scoring the clinical interview measuring misophonia severity and improvement. This interview was co‐rated until they demonstrated at least a 0.80 inter‐rater reliability.
2.6. Assessments
Patients were assessed at baseline (T1), 3 months (T2, post‐CBT or waiting list), 6 months (T3, 3 months after cross‐over), and 15/18 months (T4, 1‐year follow‐up). At T1, T2, and T3 the blinded investigator assessed all patients in a clinical interview by phone. In addition to the study assessments, two symptom‐questionnaires were administered after CBT sessions 4 and 7.
2.7. Primary outcomes
Misophonia symptoms were measured using the Amsterdam Misophonia Scale‐Revised (AMISOS‐R; see the Supplementary Appendix; Jager et al., 2020). This improved version of the A‐MISO‐S (Schröder et al., 2013) is in the process of validation; it consists of 10 items with scores ranging from 0 to 40. Higher scores indicate more severe misophonia; 0–10: normal to subclinical misophonia; 11–20: mild misophonia; 21–30: moderate–severe misophonia; 31–40: severe to extreme misophonia. Preliminary results of the validation show reliability of the scale was good (α = .84), as well as its validity (r = .87, p < .01). The co‐primary outcome was the CGI‐I (Guy, 1976) as blinded observer ratings. The CGI‐I is a clinical interview to answer the question: “Compared to the patient's condition at admission to the project this patient's condition is 1 = very much improved; 2 = much improved; 3 = minimally improved; 4 = no change from baseline (the initiation of treatment); 5 = minimally worse; 6 = much worse; 7 = very much worse since the initiation of treatment.”
2.8. Secondary outcomes
Secondary outcomes included the CGI Severity scale (CGI‐S), which was scored by a blinded rater and independently by group therapists who were not blind to treatment allocation. The CGI‐S score ranges from 1 to 7 with higher scores indicating more severe illness. General mental and physical dysfunction was assessed with the Symptom Checklist‐90‐Revised (SCL‐90‐R; Arrindell & Ettema, 1986; Derogatis et al., 1973). The total score is 90–450, with higher scores indicating more general psychopathology. QoL and impairment was assessed with three questionnaires: the five‐dimensional EuroQol (EQ5‐D; Lamers et al., 2006), Sheehan Disability Scale (SDS; Sheehan, 1983), and WHO Quality of Life‐BREF (WHOQoL‐BREF; Trompenaars et al., 2005; WHOQoL Group, 1998). The EQ5‐D score represents a health state between 0 (worst imaginable condition) and 1 (perfect health). The SDS has three domains; Work, Social, and Family and the range per domain is 0–10. The total score is 0–30, with higher scores indicating more impairment. The WHOQoL‐BREF has four domain scores: physical health, psychological health, social relationships, and environment, each with scores ranging from 4 to 20, and a score for general health, ranging from 1 to 10, with higher scores indicating a higher perceived QoL. At last, post‐treatment, all diagnostic criteria of misophonia were examined systematically by the blinded investigator.
2.9. Statistical analysis
Treatment groups were compared on baseline characteristics using χ 2 tests for binary and categorical variables and two‐tailed t tests for continuous variables.
We tested symptom severity in the randomized phase with a linear mixed model, which is suitable for longitudinal data and can handle missing values appropriately (Molenberghs et al., 2004). The dependent variable was AMISOS‐R total score; independent fixed factors were assessments (T1, T2), condition (CBT, waiting list), and their interaction, with a random intercept with subject as grouping variable. Clinical improvement (CGI‐I scores < 3) were tested with a χ 2 test, with condition as the independent variable.
Continuous secondary outcomes in the randomized phase were analyzed with linear mixed models similar to the one used for AMISOS‐R, except for the dependent variable (SCL‐90 [after log transformation to ensure normality of residuals], SDS, EQ5‐D, and WHOQoL scales). The CGI‐S was analyzed with a Mann–Whitney U test with condition as independent variable.
To test long‐term efficacy, we used linear mixed models with AMISOS‐R, log‐transformed SCL‐90, EQ5‐D, SDS, or the WHOQOL domains as dependent variables. Independent fixed factors were Assessment (baseline [T1], post‐CBT [i.e., T2 in the CBT group and T3 in the waiting list group] and 1‐year follow‐up [T4]), Condition (CBT or waiting list), and their interaction, and a random intercept with subject as grouping factor was included. CGI‐S was analyzed with a Mann–Whitney U test with time as the independent variable.
The sample size was set to be able to detect 4 points' mean difference in the AMISOS score, with an assumed SD of 3.5 points (based on previous research) and a power of 90%. After accounting for expected dropout rates, we aimed to recruit 45 patients in total. To account for possible small differences of the revised version (the AMISOS‐R), we planned to enroll 27 patients in each group before the start of the study. Power calculations and all analyses were all based on two‐tailed t tests. For co‐primary outcomes p < .025 was considered to be statistically significant, because of multiple testing (conform Bonferroni). For secondary outcomes we considered p < .05 to be statistically significant. Data were analyzed using IBM SPSS Statistics version 25 and R Version 3.5.
3. RESULTS
3.1. Participant flow and characteristics
A total of 54 patients (38 [70.4%] female; mean [SD] age, 33.06 [14.13] years) were included (Table 1). No significant differences were found between the two groups at baseline on main characteristics. Comorbid medical disorders were comparable amongst the two groups, including irritable bowel syndrome (n = 3), migraine (n = 2), and hypothyroidism (n = 2). Three patients reported tinnitus, previously confirmed by an otorhinolaryngologist. Hyperacusis, hearing loss, or other hearing problems were not reported. In each group one patient used medication (venlafaxine 225 mg daily and risperidone 0.5 mg daily) for comorbid obsessive–compulsive disorder and depressive disorder. Doses of medication were stable for at least 3 months at time of inclusion and were not changed during treatment.
Table 1.
Demographic and clinical baseline characteristics of the ITT group by condition
| CBT Group | WL Group | Total | |
|---|---|---|---|
| Variable | (n = 27) | (n = 27) | (N = 54) |
| Gender (female) | 21 (77.8) | 17 (63) | 38 (70.4) |
| Age, mean (SD), year | 31.30 (12.80) | 34.81 (15.38) | 33.06 (14.13) |
| Marital status (in a relationship) | 15 (55.6) | 17 (63) | 32 (59.3) |
| Level of education | |||
| Low (primary or secondary) | 10 (37) | 14 (51.9) | 24 (44.4) |
| High (college or university) | 17 (63) | 13 (58.1) | 30 (55.6) |
| Employment | |||
| Employed or studying | 24 (88.9) | 25 (92.6) | 49 (90.7) |
| Unemployed | 3 (11.1) | 2 (7.4) | 5 (9.3) |
| Axis I disorder | |||
| Mood | 3 (11.1) | 1 (3.7) | 4 (7.4) |
| Anxiety | 1 (3.7) | 1 (3.7) | 2 (3.7) |
| Axis II disorder | |||
| OCPD | 1 (3.7) | 0 (0) | 1 (1.9) |
| Obsessive–compulsive traits | 8 (29.6) | 7 (25.9) | 15 (27.8) |
| Other traits | 1 (3.7) | 2 (7.4) | 3 (5.6) |
| Axis III disorder | |||
| Tinnitus | 1 (3.7) | 2 (7.4) | 3 (5.6) |
| Other | 7 (25.9) | 7 (25.9) | 14 (25.9) |
| Age of onset misophonia | 10.7 (4.00) | 11.89 (5.40) | 11.3 (4.74) |
| Primary outcomes | |||
| CGI‐S score, mean (SD) | 5.56 (0.75) | 5.00 (0.83) | 5.28 (0.83) |
| AMISOS‐R score, median (95% CI) | 30.00 (20–36) | 27.50 (19–38) | 29.00 (19–38) |
Abbreviations: AMISOS‐R, Amsterdam Misophonia Scale‐Revised; CBT, cognitive behavioral therapy; CGI‐S, CGI Severity scale; ITT, intention‐to‐treat; OCPD, obsessive–compulsive personality disorder; WL, waiting list.
Twenty‐seven patients (50.9%) were randomized to the treatment group (CBT) and 26 patients (49.1%) randomized to the waiting list control group (WL). All of them completed 1 or more post‐baseline assessment (Figure 1). All completers attended at least six CBT sessions. There was no difference in treatment participation between the two groups.
Figure 1.
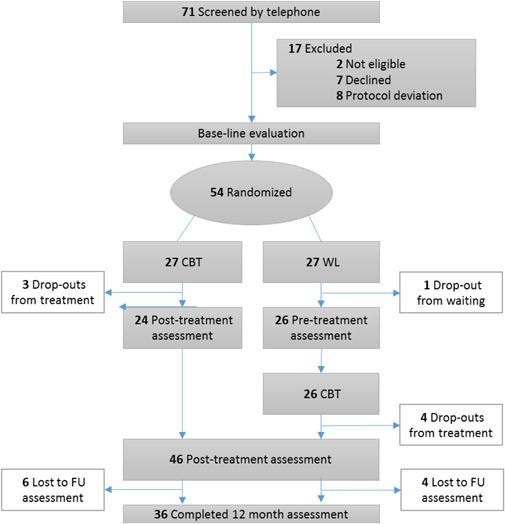
CONSORT diagram of participants in a study of CBT for misophonia. CBT, cognitive behavioral therapy; FU, follow‐up; WL, waiting list
During treatment/waiting period (T1–T3) eight patients dropped out (n = 3 in the CBT, n = 5 in WL, χ 2(1) = 0.6, p = .70). Reasons for dropout were comorbid psychiatric disorders, for example, relapse depression (3), stressors, for example, deceased family member (2), comorbid somatic disorders (1), and planning problems (1). At last follow‐up (T4) nine patients had dropped out in each condition (18 in total). Partly due to dropout some scores are missing, see Tables 2 and 3 for the actual number of observations. No difference in number of missing values was found between conditions (see Table 2, biggest difference: 8 vs. 5 missings: χ 2(1) = 0.9, p = .53).
Table 2.
Outcome measures of ITT by condition T2–T1
| WL group | CBT group | ||
|---|---|---|---|
| Co‐primary variables | (n = 27) | (n = 27) | d |
| AMISOS‐R (MD; 95% CI) | −0.8 (−2.1 to 0.4) | −9.7 (−12.0 to −7.4)*** | 1.97 |
| n missing | 0 | 1 | |
| CGI‐I (n) | |||
| Very much improved | 0 | 1*** | NA |
| Much improved | 0 | 9*** | NA |
| Minimally improved | 4 | 8 | |
| No change | 14 | 8 | |
| Minimally worse | 9 | 1 | |
| Much worse | 0 | 0 | |
| Very much worse | 0 | 0 | |
| n missing | 0 | 0 | |
| Secondary variables | |||
| CGI‐S (MD; 95% CI) | 0.0 (−0.1 to 0.2) | −1.0 (−1.4 to −0.6)*** | 1.39 |
| n missing | 1 | 3 | |
| SCL‐90‐R (MD; 95% CI) | −1.2 (−13.6 to 11.2) | −30.9 (−46.6 to −15.1)*** | 0.86 |
| n missing | 4 | 4 | |
| EQ5‐D (MD; 95% CI) | 0.0 (−0.1 to 0.1) | 0.0 (0.0 to 0.1) | −0.13 |
| n missing | 7 | 5 | |
| SDS Total (MD; 95% CI) | 0.2 (−2.1 to 2.6) | −6.0 (−7.9 to −4.2)*** | 1.33 |
| Work | −0.3 (−1.5 to 0.8) | −1.8 (−2.7 to −0.8) | 0.60 |
| Social | 0.9 (−0.2 to 2.0) | −2.0 (−2.7 to −1.3)*** | 1.42 |
| Family | −0.3 (−1.3 to 0.6) | −2.3 (−2.9 to −1.7)*** | 1.12 |
| n missing | 8 | 5 | |
| WHOQoL (MD; 95% CI) | |||
| Social relationships | 0.8 (0.2 to 1.3) | 1.0 (0.0 to 1.9) | −0.11 |
| Environment | 0.3 (−0.4 to 1.0) | 1.0 (0.0 to 1.9) | −0.33 |
| Physical health | 0.1 (−0.9 to 1.0) | 1.3 (0.5 to 2.1) | −0.60 |
| Psychological health | 0.1 (−0.7 to 1.0) | 1.1 (0.5 to 1.6) | −0.59 |
| General health | 0.2 (−0.3 to 0.7) | 0.0 (−0.7 to 0.7) | 0.14 |
| n missing | 7 | 5 |
Abbreviations: AMISOS‐R, Amsterdam Misophonia Scale‐Revised; CBT, cognitive behavioral therapy; CGI‐I, Clinical Global Impression‐Improvement; CGI‐S, CGI Severity scale; EQ5‐D, five‐dimensional EuroQol; ITT, intention‐to‐treat; MD, mean difference; NA, not applicable; QoL, quality of life; SCL‐90‐R, Symptom Checklist‐90‐R; SDS, Sheehan Disability Scale; WL, waiting list; WHOQoL‐BREF, WHO Quality of Life‐BREF.
p < .001.
Table 3.
Treatment efficacy at posttreatment and 12‐month follow‐up
| Mean (SD) [95% CI]a | Mean (SD) [95% CI] | Time effect | Within‐group p value | ||||
|---|---|---|---|---|---|---|---|
| Outcome and time | N | WL | CBT | F (df) | Pre to Post | Pre to FU | Post to FU |
| Primary outcome | |||||||
| AMISOS‐R | 48.76 (2, 86.6)*** | *** | *** | .383 | |||
| Pre | 54 | 27.5 (4.8) [25.6–29.3] | 30.4 (4.2) [28.8–32.0] | ||||
| Post | 48 | 19.7 (6.8) [16.8–22.6] | 21.9 (7.0) [19.2–24.6] | ||||
| Follow‐up | 37 | 22.3 (8.3) [18.4–26.1] | 22.2 (8.4) [18.4–26.0] | ||||
| Secondary outcomes | |||||||
| CGI‐S | NaN | ||||||
| Pre | 54 | 5.0 (0.8) [4.7–5.3] | 5.6 (0.8) [5.3–5.8] | ||||
| Post | 49 | 3.5 (1.4) [2.9–4.0] | 4.7 (1.0) [4.3–5.0] | ||||
| Follow‐up | b | b | b | ||||
| CGI‐S therapist | NaN | ||||||
| Pre | 53 | 5.7 (0.7) [5.4–5.9] | 5.7 (0.6) [5.5–5.9] | ||||
| Post | 48 | 2.8 (1.0) [2.3–3.2] | 3.5 (1.4) [2.9–4.0] | ||||
| Follow‐up | b | b | b | ||||
| SCL‐90 | 31.00 (2, 81.9)*** | *** | *** | .107 | |||
| Pre | 54 | 147.0 (42.5) [130.8–163.1] | 182.8 (62.7) [159.0–206.5] | ||||
| Post | 47 | 116.5 (22.8) [106.7–126.3] | 156.5 (75.0) [127.6–185.5] | ||||
| Follow‐up | 37 | 133.6 (55.1) [108.0–159.1] | 142.2 (54.9) [117.4–167.0] | ||||
| EQ5‐D | 1.57 (2, 84.9) | .431 | .229 | .659 | |||
| Pre | 53 | 0.8 (0.2) [0.8–0.9] | 0.8 (0.1) [0.7–0.8] | ||||
| Post | 44 | 0.8 (0.2) [0.7–0.9] | 0.8 (0.2) [0.7–0.9] | ||||
| Follow‐up | 37 | 0.9 (0.1) [0.8–0.9] | 0.8 (0.2) [0.7–0.9] | ||||
| SDS Work | 10.76 (2, 82.6)*** | *** | *** | .914 | |||
| Pre | 54 | 4.0 (3.0) [2.8–5.1] | 6.0 (2.3) [5.1–6.9] | ||||
| Post | 46 | 2.6 (2.3) [1.6–3.6] | 4.2 (2.7) [3.1–5.3] | ||||
| Follow‐up | 37 | 2.3 (2.4) [1.2–3.4] | 4.3 (2.7) [3.1–5.5] | ||||
| SDS Social | 27.60 (2, 80.6)*** | *** | *** | .841 | |||
| Pre | 54 | 6.0 (2.0) [5.2–6.7] | 6.9 (1.7) [6.2–7.5] | ||||
| Post | 46 | 3.4 (2.1) [2.5–4.3] | 5.0 (2.7) [4.0–6.1] | ||||
| Follow‐up | 37 | 4.6 (3.0) [3.2–5.9] | 4.6 (3.1) [3.2–6.0] | ||||
| SDS Family | 29.20 (2, 82.7)*** | *** | *** | * | |||
| Pre | 54 | 6.4 (2.8) [5.4–7.5] | 8.0 (1.4) [7.5–8.6] | ||||
| Post | 46 | 4.0 (2.9) [2.8–5.3] | 6.1 (1.9) [5.4–6.9] | ||||
| Follow‐up | 37 | 4.4 (3.3) [2.9–6.0] | 4.6 (2.8) [3.4–5.9] | ||||
| SDS Total | 37.00 (2, 80.3)*** | *** | *** | .310 | |||
| Pre | 54 | 16.4 (5.8) [14.2–18.6] | 20.9 (3.9) [19.4–22.3] | ||||
| Post | 46 | 10.1 (5.9) [7.6–12.6] | 15.4 (6.1) [13.0–17.8] | ||||
| Follow‐up | 36 | 10.9 (7.0) [7.6–14.3] | 13.5 (8.0) [9.9–17.2] | ||||
| WHOQoL Social relationships | 4.44 (2, 79.1)* | * | .074 | .767 | |||
| Pre | 53 | 14.3 (2.8) [13.2–15.3] | 12.9 (2.5) [12.0–13.9] | ||||
| Post | 43 | 15.3 (2.2) [14.4–16.2] | 14.1 (3.0) [12.8–15.3] | ||||
| Follow‐up | 37 | 13.9 (2.9) [12.6–15.3] | 14.0 (2.8) [12.8–15.3] | ||||
| WHOQoL Environment | 4.11 (2, 80.3)* | * | *** | .140 | |||
| Pre | 53 | 16.7 (1.9) [16.0–17.5] | 14.9 (2.1) [14.1–15.7] | ||||
| Post | 43 | 16.8 (1.9) [16.0–17.6] | 15.8 (2.6) [14.7–16.8] | ||||
| Follow‐up | 37 | 16.8 (1.9) [15.9–17.7] | 16.4 (2.3) [15.4–17.5] | ||||
| WHOQoL Physical health | 13.07 (2, 79.1)*** | ** | *** | .216 | |||
| Pre | 53 | 14.9 (2.5) [13.9–15.9] | 13.1 (2.5) [12.1–14.0] | ||||
| Post | 44 | 16.3 (1.6) [15.6–17.0] | 14.1 (2.7) [13.0–15.3] | ||||
| Follow‐up | 37 | 16.3 (2.4) [15.3–17.4] | 14.9 (3.3) [13.5–16.4] | ||||
| WHOQoL Psychological health | 12.74 (2, 79.4)*** | ** | *** | .225 | |||
| Pre | 53 | 13.6 (2.0) [12.8–14.4] | 12.1 (2.3) [11.3–13.0] | ||||
| Post | 44 | 14.7 (1.2) [14.2–15.3] | 13.1 (2.6) [12.0–14.1] | ||||
| Follow‐up | 37 | 14.0 (2.6) [12.7–15.2] | 13.6 (2.5) [12.5–14.8] | ||||
| WHOQoL General health | 1.99 (2, 78.9) | .968 | .420 | .453 | |||
| Pre | 53 | 7.5 (1.4) [7.0–8.0] | 7.1 (1.5) [6.6–7.7] | ||||
| Post | 44 | 8.2 (1.1) [7.7–8.7] | 7.1 (1.9) [6.4–7.9] | ||||
| Follow‐up | 36 | 8.2 (1.3) [7.6–8.8] | 7.5 (1.9) [6.6–8.3] | ||||
| CGI‐I | NaN | ||||||
| Pre | NaN | NaN | NaN | ||||
| Post | 49 | 2.3 (0.8) [2.0–2.6] | 3.0 (1.0) [2.6–3.3] | ||||
| Follow‐up | b | b | b | ||||
| CGI‐I therapist | NaN | ||||||
| Pre | NaN | NaN | NaN | ||||
| Post | 48 | 2.0 (0.8) [1.7–2.4] | 2.3 (0.9) [1.9–2.7] | ||||
| Follow‐up | b | b | b | ||||
Abbreviations: AMISOS‐R, Amsterdam Misophonia Scale‐Revised; CBT, cognitive behavioral therapy; CGI‐I, Clinical Global Impression‐Improvement; CGI‐S, CGI Severity scale; EQ5‐D, five‐dimensional EuroQol; FU, follow‐up; NaN, Not a Number; QoL, quality of life; SCL‐90, Symptom Checklist‐90; SDS, Sheehan Disability Scale; WL, waiting list; WHOQoL‐BREF, WHO Quality of Life‐BREF.
For the waiting list condition in “Pre” data of T2 was used.
There was no CGI assessment at FU.
p < .05.
p < .01.
p < .001.
3.2. Short‐term efficacy; primary outcomes
Compared with baseline (T1), the mean scores decreased after 3 months (T2) by 9.7 (95% CI, −12.0 to −7.4) in the CBT group and by 0.8 (95% CI, −2.1 to 0.4) in the waiting list control group (Table 2). The standardized effect‐size was very large (d = 1.97).
The CGI‐I showed a significant difference between the two groups: 37% of the CBT group and 0% of the WL group was much or very much improved (CGI‐I < 3) in the intention‐to‐treat group (χ 2(4) = 19.37, p < .001).
Compared to the waiting list condition, patients in the CBT condition showed a significantly larger decrease of AMISOS‐R scores (F(1, 48.6) = 49.8, p < .001).
3.3. Secondary outcomes
A Mann–Whitney U test showed that the CGI‐S (scored blinded) was significantly reduced by CBT, U = 507.00, p = .000. In addition, CBT significantly reduced the SCL‐90‐R score with −30.9 (−46.6 to −15.1) points mean difference (95% CI) versus a decrease of −1.2 (−13.6 to 11.2) in the waiting list condition (F(1, 44.8) = 14.3, p < .001). Results for the blinded CGI‐S showed an effect of d = 1.39, and results for the SCL‐90 showed an effect size of d = 0.86.
CBT significantly increased SDS total score compared to WL, especially in two subscales (Social: F(1, 45.3) = 21.8, p < .001 and Family life: F(1, 39.1) = 13.02, p < .001). No significant differences were found between CBT and WL on other quality of life scales (EQ5‐D and WHOQoL‐BREF).
The course of decrease in misophonia symptoms during treatment was similar for both groups and is shown in Figure S1. The SCL‐90‐R showed a comparable pattern of decrease during treatment.
A structured diagnostic interview showed 37% of the completers did not meet diagnostic criteria for misophonia any more post‐treatment. Most patients (70.6%) failed to meet more than one criterion. Improvement was found particularly in experiencing more self‐control (38.9% no longer meeting criterion B) and less problems in day‐to‐day life (25% no longer meeting criterion E).
3.4. 1‐year follow‐up
The analysis of all data (CBT and delayed CBT combined) for three time points: baseline (T1), after CBT (T2/T3), and 1‐year follow‐up (T4), showed a main effect of time, but no condition or interaction effects were found for the AMISOS‐R scores (Time: F(2, 86.6) = 48.76, p < .0001). Improvement on the primary outcome was sustained, since no significant changes were found at follow‐up compared to post‐treatment (see Table 3 and Figure 2).
Figure 2.
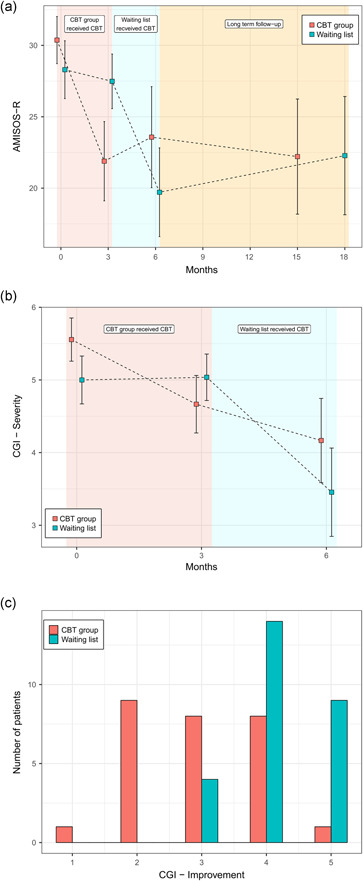
Results for co‐primary outcomes at all assessments (PP). AMISOS‐R, Amsterdam Misophonia Scale‐Revised; CBT, cognitive behavioral therapy; CGI‐I, Clinical Global Impression‐Improvement; PP, per‐protocol
A Mann–Whitney U test revealed significant differences in CGI‐S scores pre‐ (MD = 5, n = 54) and posttreatment (MD = 4, n = 49), U = 631.50, p = .000.
For the other secondary outcomes group‐by‐time interaction effect was not statistically significant, except for the SCL‐90 (p = .028) and WHOQol‐BREF Environment (p = .032). There were significant between‐group differences on the SCL‐90 (p = .042), SDS Work (p = .004), SDS Total (p = .003), and WHOQoL‐BREF Physical and Psychological health scores (p = .014 and p = .042).
Main effect of time was significant for all variables, except for EQ5‐D and WHOQoL‐BREF General health. The pattern of scores was similar to the primary outcomes; differences between pre‐ and post‐intervention were significant, except for the WHOQoL‐BREF Environment. Improvement on all secondary outcomes was sustained at follow‐up, with no significant differences between post‐treatment and follow‐up, except for SDS subscale Family. At follow‐up the SDS subscale Family was further improved.
3.5. Adverse events and treatment acceptability
Five patients expressed their concern to adopt trigger‐sounds from fellow group members at the start of CBT. At the end of treatment none of the patients reported to have obtained new trigger‐sounds. During treatment/waiting period (T1–T3) eight patients dropped out. One reported an adverse event as a cause of dropout. This subject had increased misophonia symptoms and anxiety and explained this by an inability to open up in a group. No serious adverse events were reported.
Treatment acceptability (n = 43) was measured by satisfaction and a report mark for the therapy. Post‐treatment, 65.1% were (very) satisfied, 25.6% were neutral, 7% were not satisfied, and 2.3% were very dissatisfied (n = 1). Treatment was rated by patients with a mean (SD) of 6.72 (1.59).
4. DISCUSSION
This first randomized trial with CBT for misophonia showed both short‐ and long‐term efficacy. Three months of CBT reduced misophonia symptoms compared to a waiting list. Clinical improvement was found in 56% of all completers (37% intention‐to‐treat) compared to 0% in the waiting list. General mental and physical dysfunction decreased and patients reported less disabilities in family and social functioning after CBT than after waiting list as well. Importantly, 12 months after the end of treatment, the considerable improvement in misophonia symptoms was sustained. On top of these measurements, group therapists rated 74% of all completers clinically improved (CGI < 3).
These results confirm the positive effect of CBT previously found in case reports and a previous open‐label trial in our center (Bernstein et al., 2013; Dozier, 2015; McGuire et al., 2015; Schröder et al., 2017) and extends its findings. This RCT provides evidence for the efficacy of CBT for misophonia and can serve as a stepping stone to implement CBT in clinical practice. We have published our protocol, so more misophonia patients can benefit from this treatment.
In clinical practice and future trials, these results could be improved. Patients gradually improved in time. Since there was no plateau effect (see Figure S1), there is room for further improvement. By adding more sessions, we could possibly even reach a better outcome. This notion is supported by detailed feedback of patients, who endorsed a prolonged treatment. Future studies should investigate whether additional sessions lead to additional improvement.
As opposed to the strong symptom improvement after CBT, we did not find a meaningful effect on QoL in the primary analysis. There are several reasons; first of all, a ceiling effect is probable, given the high pretreatment score on both questionnaires. We doubt misophonia has no effect on QoL, so these two questionnaires are probably not suitable for this population. Possibly, a different questionnaire, the Manchester Short Assessment of Quality of Life (MANSA; Priebe et al., 1999), would have been more sensitive, since patients with misophonia score low on the MANSA (Jager et al., 2020). Misophonia has a clear impact on social and family functioning. The MANSA has more focus on the social domain (e.g., with several items concerning the quality of relationships with friends, family, and colleagues) than the EQ5‐D or WHOQoL‐BREF.
In this first RCT for misophonia, the highly controlled intervention (Van Loon et al., 2019) is a major strength. The interventions were highly comparable in all treatment groups, because the study was situated in one center. Furthermore, only a few experienced therapists were involved and monitored by regular intervision. Another strength are the assessments. Assessments were thorough with the use of blinded observer ratings, clinical interviews by both therapists and observer, diverse self‐reports, multiple measures during treatment, and a measure at 1‐year follow‐up.
4.1. Limitations
However, this study has several limitations. A first potential study limitation is the lack of a fully validated misophonia questionnaire. Although the AMISOS‐R is a good scale and is almost fully validated (publication in preparation), we used the CGI‐I as a co‐primary outcome to aid interpretation of the clinical results. Second, there were missing data, especially at 1‐year follow‐up (33%), even though we went to great lengths to complete the data. This is common in treatment studies and percentages of dropout in other CBT studies with 1‐year follow‐up were comparable to our dropout rates. For instance, Segal et al. (2020) had a dropout of 23%–33% at 1 year follow‐up (n = 460), and Wiltink et al. (2017) dropout rates at 1 year follow‐up were 25%–36% (n = 109). Third, our study missed a condition controlling for nonspecific effects of treatment. Future research should compare the current CBT to an active control group, for example, a support group. Because of this comparison with a waiting list, the fidelity of masking was poor. The assessor guessed the majority correctly based on the reported improvement, despite the fact that patients managed to keep their allocation secret during the blinded assessment (merely two patients used terms obviously learned in therapy). This could have led to a bias.
4.2. Conclusions
This RCT evaluating the immediate and long‐term effects of manualized CBT compared with a waiting list control group demonstrated CBT is effective for reducing misophonia symptoms in adults and for improving social and family functioning. Future work should include an active control group.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Inge J. Jager: conceptualization, data curation, formal analysis, investigation, methodology, and writing—original draft. Nienke C. C. Vulink: conceptualization, methodology, supervision, and writing—review and editing. Isidoor O. Bergfeld: data curation, formal analysis, and writing—review and editing. Arnoud J. J. M. van Loon: conceptualization and investigation. Damiaan A. J. P. Denys: conceptualization, methodology, supervision, and writing—review and editing.
Supporting information
Supporting information.
Supporting information.
ACKNOWLEDGMENTS
We are very grateful to our colleagues from the AMC misophonia treatment team. We thank Michel H.P. Hof of the AMC Dept. Clinical Epidemiology, Biostatistics, and Bioinformatics for his advice at the beginning of this study. Finally, we thank all misophonia patients for their contribution.
Jager IJ, Vulink NCC, Bergfeld IO, van Loon AJJM, Denys DAJP. Cognitive behavioral therapy for misophonia: A randomized clinical trial. Depression Anxiety. 2021;38:708–718. 10.1002/da.23127
Trial Registration: www.trialregister.nl Identifier NL6304
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Arrindell, W. A., & Ettema, J. H. M. (1986). SCL‐90: Handleiding bij een multidimensionele psychopathologie‐indicator. Swets Test Publishers. [Google Scholar]
- Bernstein, R. E., Angell, K. L., & Dehle, C. M. (2013). A brief course of cognitive behavioural therapy for the treatment of misophonia: A case example. The Cognitive Behaviour Therapist, 6, e10. [Google Scholar]
- Brout, J. J., Edelstein, M., Erfanian, M., Mannino, M., Miller, L. J., Rouw, R., Kumar, S., & Rosenthal, M. Z. (2018). Investigating misophonia: A review of the empirical literature, clinical implications, and a research agenda. Frontiers in Neuroscience, 12, 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derogatis, L. R., Lipman, R. S., & Covi, L. (1973). The SCL‐90: An outpatient psychiatric rating scale—Preliminary report. Psychopharmacology Bulletin, 9, 13–18. [PubMed] [Google Scholar]
- Dozier, T. H. (2015). Counterconditioning treatment for misophonia. Clinical Case Studies, 14, 374–387. [Google Scholar]
- Dozier, T. H., Lopez, M., & Pearson, C. (2017). Proposed diagnostic criteria for misophonia: A multisensory conditioned aversive reflex disorder. Frontiers in Psychology, 8, 1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy, W. B. R. R. (1976). CGI, Clinical global impressions. ECDEU assessment manual for psychopharmacology. [Google Scholar]
- Jager, I. J., De Koning, P. P., Bost, T. J. M., Vulink, N. C. C., & Denys, D. A. J. P. (2020). Misophonia: Phenomenology, comorbidity and demographics in a large sample. PLOS One, 15(4), e0231390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamody, R. C., & Del, G. C. (2017). Using dialectical behavior therapy to treat misophonia in adolescence. The Primary Care Companion for CNS Disorders, 19(5), 17l02105. [DOI] [PubMed] [Google Scholar]
- Lamers, L. M., Bouwmans, C. A. M., van Straten, A., Donker, M. C. H., & Hakkaart, L. (2006). Comparison of EQ‐5D and SF‐6D utilities in mental health patients. Health Economics, 15, 1229–1236. [DOI] [PubMed] [Google Scholar]
- McGuire, J. F., Wu, M. S., & Storch, E. A. (2015). Cognitive‐behavioral therapy for 2 youths with misophonia. The Journal of Clinical Psychiatry, 76(5), 573–574. [DOI] [PubMed] [Google Scholar]
- Molenberghs, G., Thijs, H., Jansen, I., Beunckens, C., Kenward, M. G., Mallinckrodt, C., & Carroll, R. J. (2004). Analyzing incomplete longitudinal clinical trial data. Biostatistics, 5(3), 445–464. [DOI] [PubMed] [Google Scholar]
- Priebe, S., Huxley, P., Knight, S., & Evans, S. (1999). Application and results of the Manchester Short Assessment of Quality of Life (MANSA). International Journal of Social Psychiatry, 45(1), 7–12. [DOI] [PubMed] [Google Scholar]
- Sanchez, T. G., & da Silva, F. E. (2018). Familial misophonia or selective sound sensitivity syndrome: Evidence for autosomal dominant inheritance? Brazilian Journal of Otorhinolaryngology, 84(5), 553–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schröder, A., Vulink, N., & Denys, D. (2013). Misophonia: Diagnostic criteria for a new psychiatric disorder. PLOS One, 8(1), e54706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schröder, A. E., Vulink, N. C., van Loon, A. J., & Denys, D. A. (2017). Cognitive behavioral therapy is effective in misophonia: An open trial. Journal of Affective Disorders, 217, 289–294. [DOI] [PubMed] [Google Scholar]
- Segal, Z. V., Dimidjian, S., Beck, A., Boggs, J. M., Vanderkruik, R., Metcalf, C. A., Gallop, R., Felder, J. N., & Levy, J. (2020). Outcomes of online mindfulness‐based cognitive therapy for patients with residual depressive symptoms: A randomized clinical trial. JAMA Psychiatry, 77(6), 563–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan, D. V. (1983). Sheehan disability scale. Handbook of psychiatric measures, 2, 100–102. [Google Scholar]
- Taylor, S. (2017). Misophonia: A new mental disorder? Medical Hypotheses, 103, 109–117. [DOI] [PubMed] [Google Scholar]
- Trompenaars, F. J., Masthoff, E. D., Van Heck, G. L., Hodiamont, P. P., & De Vries, J. (2005). Content validity, construct validity, and reliability of the WHOQOL‐Bref in a population of Dutch adult psychiatric outpatients. Quality of Life Research, 14(1), 151–160. [DOI] [PubMed] [Google Scholar]
- Van Loon, A., Van der Pol, M., Slaghekke, S., Van der Meer, C., Schekman, E., Nieuwendijk, E., De Wit, I., & Jager, J. (2019). Misofonie: Behandelprotocol in 8 sessies [Misophonia: Treatment protocol of 8 sessions]. Boom Publishers. [Google Scholar]
- WHOQoL Group . (1998). Development of the World Health Organization WHOQOL‐BREF quality of life assessment. Psychological Medicine, 28(3), 551–558. [DOI] [PubMed] [Google Scholar]
- Wiltink, J., Ruckes, C., Hoyer, J., Leichsenring, F., Joraschky, P., Leweke, F., Pöhlmann, K., & Beutel, M. E. (2017). Transfer of manualized Short Term Psychodynamic Psychotherapy (STPP) for social anxiety disorder into clinical practice: Results from a cluster‐randomised controlled trial. BMC Psychiatry, 17(1), 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, X., Wu, M. S., & Storch, E. A. (2017). Misophonia symptoms among Chinese university students: Incidence, associated impairment, and clinical correlates. Journal of Obsessive‐Compulsive and Related Disorders, 14, 7–12. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Supporting information.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


