Abstract
A 60-year-old patient presented with respiratory distress, after recently being tested COVID-19 positive and was mechanically ventilated for 15 days. After cessation of sedation, he remained in deep comatose state, without any reaction on pain stimuli (Glasgow Coma Score 3). MRI of the brain showed diffuse leukoencephalopathy and multiple (>50) microbleeds. Diffuse COVID-19-associated leukoencephalopathy with microhaemorrhages is associated with a poor prognosis. However, 3 months later, our patient showed a remarkable recovery and was able to walk independently. This case report shows COVID-related leukoencephalopathy and intracerebral microbleeds, even with persistent comatose state, may have a favourable clinical outcome and prolonged treatment should be considered in individual cases.
Keywords: COVID-19, coma and raised intracranial pressure
Background
In March 2020, WHO declared the COVID-19 a global pandemic, with almost 200 million infections and over 4 million deaths in July 2021.1 Neurological complications of COVID-19 have been well recognised and include acute ischaemic stroke due to large vessel occlusions, intracranial haemorrhages, acute haemorrhagic encephalopathy, Guillain-Barré syndrome and anosmia.2 Diffuse COVID-19-associated encephalopathy with microhaemorrhages is a rare complication and associated with a poor prognosis.3 4 We describe a patient with a remarkable recovery after a severe COVID-19 encephalopathy.
Case presentation
A 60-year-old man with only a history of diabetes mellitus type 2 presented with fever, dyspnoea, headache and arthralgia and was tested COVID-19 positive. On day 5, he developed progressive dyspnoea. Chest X-ray and CT demonstrated bilateral predominantly peripheral ground glass opacities, consistent with an interstitial viral COVID-19 pneumonia. Laboratory results showed an increased ferritin level at admission (2951 µg/L), increased D-dimer (range 0.5–2.8 mg/L), slightly lowered haemoglobin (10.56 g/dL) and haematocrit (range 0.35–0.45 L/L).
Despite dexamethasone (6 mg once a day during 10 days) and additional high-flow oxygen support sedation (isoflurane, maximum 5 mL/hour), mechanical ventilation was needed until day 22. During the admission, there was no relevant hypertension or hypotension (mean blood pressure 130/70 mm Hg), no renal replacement therapy or extracorporeal membrane oxygenation. Medication included nadroparin 5700IE once per day (thrombotic prophylaxis).
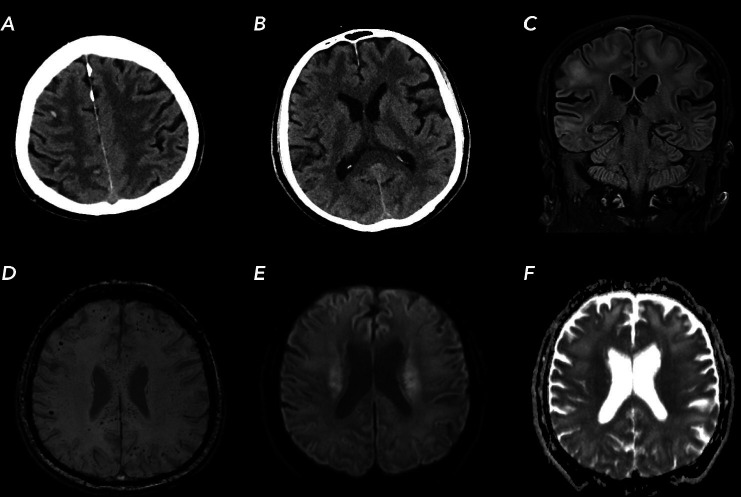
After sedation cessation, he remained comatose without any reaction to pain stimuli (Glasgow Coma Score (GCS) 3), but preserved brainstem reflexes. A non-contrast CT of the head showed extensive, fairly symmetrical hypodense areas in the supratentorial white matter with accentuation of grey-white matter differentiation and several subcortical small haemorrhages in the supratentorial hemispheres. MRI showed extensive confluent T2-hyperintensies within these areas in the periventricular and subcortical white matter with sparing of the U-fibres. Besides the subcortical haemorrhages, MRI showed widespread microbleeds (>50) in the subcortical white matter, basal ganglia, cerebellum and in particularly involvement of the splenium of the corpus callosum (figure 1). At that time, neurological prognosis was uncertain and appeared to be poor, but ventilation and maximal supportive care were continued.
Figure 1.
Brain CT and MR images of our patient with COVID-19 infection. (A, B) axial CT images showing subcortical small haemorrhages and symmetrical hypodense areas in the supratentorial white matter. (C) MR turbo inversion recovery magnitude T2-weighted image showing subcortical T2-hyperintensies in the subcortical areas. (D) MR susceptibility weighted images showing widespread microbleeds in white matter and splenium. (E, F) paired diffusion-weighted imaging and apparent diffusion coefficient showing restricted diffusion in the corona radiata.
Outcome and follow-up
Ten days later (day 32), our patient gradually recovered, obeyed commands and was only slightly disoriented in time and place (GCS 14). He was able to use both arms and legs (Medical Research Council score of 4). Seven weeks after admission to the hospital, he was discharged to a rehabilitation centre. Within 1 month after discharge, he was also able to walk independently.
Discussion
We report a COVID-19 case with persistent comatose state due to diffuse leukoencephalopathy and parenchymal (micro)haemorrhages with a good clinical outcome.
Pathophysiological mechanisms for COVID-19-related diffuse leukoencephalopathy and microbleeds have been proposed.3–5 SARS-CoV-2 virus has been shown to enter cells via ACE-II, expressed in endothelial brain cells. Subsequent endothelial cell damage, capillary leakage and inflammatory response may cause diffuse leukoencephalopathy and microbleeds. In addition, pulmonary COVID-19 infection associated general and cerebral hypoxaemia may cause myelin sheath damage through dysfunction of the adenosine triphosphate-dependent enzymes (responsible for myelin secretion and maintenance). This could result in diffuse leukoencephalopathy, similar to delayed posthypoxic leukoencephalopathy (DPHL) which is believed to be related to oligodendroglial cell death and subsequent demyelination.
The predilection of abnormalities in the deep white matter and sparing of the juxtacortical white matter and deep-grey nuclei on neuroimaging are typical of DPHL. Other causes of diffuse leukoencephalopathy in critically ill patients include posterior reversible encephalopathy, acute disseminated encephalomyelitis, sepsis-associated encephalopathy and various toxic and metabolic causes. The pattern of microhaemorrhages particularly involving the subcortical white matter and the corpus callosum described in DPHL are similar to the patterns in high-altitude cerebral oedema and acute respiratory distress syndrome (ARDS) and in diffuse axonal injury. However, these conditions obviously have a different clinical presentation.3 4
It is of importance to consider whether the cerebral microbleeds (over 50) could have been pre-existent before the COVID-19 infection. In a series of 159 participants without stroke of dementia (median age, 67 years), all participants with ≥5 incident microbleeds had baseline hypertension.6 A population-based study in Rotterdam showed a prevalence of 3.4% of multiple cerebral microbleeds in people between the ages of 50 and 60 years old and less than 0.5% of the people had over 20 microbleeds. These microbleeds were associated with cardiovascular risk factors, that is, systolic blood pressure, hypertension, smoking, but not with diabetes.7 Others emphasise an important role of microbleeds in patients with diabetes mellitus and vascular cognitive impairment8
Our patient had no history of hypertension, including normal electrocardiography without signs of left ventricular hypertrophy, and no other known risk factors for cerebral microbleeds, except diabetes mellitus. Although the relationship with diabetes and cerebral microbleeds is not fully understood, a role of diabetes in our patient cannot completely be ruled out. In this respect, our patient was not known with cognitive complaints or impairment before admission.
We believe the radiological features, including the microbleeds, and the concomitant clinical presentation without vascular history or earlier cognitive and physical complaints are most likely, if not almost certainly, related to COVID-19.
The clinical improvement of our patient is remarkable, particularly because of persistent coma. In a previous study 35 out of 115 COVID-19 patients in whom MRI was performed showed leukoencephalopathy and/or cerebral microbleeds.3 These 35 patients had a lower GCS at the time of MRI, required longer ventilator support, longer hospital stay and had a higher mortality rate compared with patients without these MRI findings. The mean Modified Ranking Scale in this group at the time of discharge was 5, indicative of severe disability and the need of constant nursing care in most patients. Twenty per cent of these patients died. We do not know if individual cases with persistent coma and comparable radiological features to our patient had a favourable outcome.
In a series of 11 patients with COVID-19, MRI showed leukoencephalopathy only in four patients, microbleeds in one and both leukoencephalopathy and microbleeds in six patients.4 Five weeks later, 6 of 11 patients had died, and 5 continued to receive critical care.
Recently six patients were reported with reversible unconsciousness following COVID-19 respiratory failure. MRI of the brain showed diffuse leukoencephalopathy without any microbleeds. All six patients were awake and showed slow improvement of their tetraparesis, but were not able to walk independently like our patient.9
Against the background of these earlier case series the clinical improvement of our patient is remarkable, also considering his prolonged state of deep coma and impressive MRI-findings with multiple (>50) microbleeds. In contrast with previous reports prognosis of diffuse leukoencephalopathy with parenchymal (micro)haemorrhages in COVID-19 appears sometimes surprisingly good, even after persistent deep comatose state. In previous series, we do not know if selection bias played a role to withdraw life sustaining therapies too early in patients with the presumption of poor clinical outcome. It is possible in individual patients with apparent COVID-19-related microbleeds the presumption of poor outcome lead to a self-fulfilling prophecy; withdrawal of life-sustaining therapies to avoid severe and persistent neurological handicap. In that case good outcome in our patient would only be the result of extended care, in earlier cases wrongly withdrawn based on misinformation. Even if so, the present case is of great importance to correct this presumption.
Availability of intensive care and life support is scarce and decisions on whether or not to discontinue treatment have to be made in every day practice. Our case shows life-sustaining therapy should not be withheld beforehand in patients with COVID-19-related diffuse leukoencephalopathy and microbleeds because the prognosis is uncertain and may sometimes be good.
Conclusion
We present a patient with COVID-19 with persistent coma and leukoencephalopathy and multiple microbleeds in need of full life support. In contrast to earlier limited reports, our patient showed a remarkable improvement and was able to walk independently 2 months after initial presentation. Patients with similar clinical and radiological features deserve prolonged life support, especially in the early stages of the disease, as the prognosis might be good in individual cases.
Learning points.
Diffuse leukoencephalopathy with microbleeds is a serious complication of COVID-19 infection and may cause persistent coma.
Although the prognosis is uncertain and often poor, a favourable outcome is possible, even after weeks of prolonged comatose state.
Patients with COVID-19-related leukoencephalopathy and microbleeds deserve and need prolonged intensive care treatment.
Acknowledgments
We thank Sebastiaan Hammer, MD PhD for his writing assistance and proofreading.
Footnotes
Contributors: EHW initiated the project, drafted and revised the paper. FYJ drafted and revised the papier. WL drafted and revised the paper. SFTMdB initiated the project and revised the paper.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Consent obtained from next of kin.
References
- 1.Worldometer . COVID-19 Coronavirus pandemic [Internet]. Available: https://www.worldometers.info/coronavirus/ [Accessed 28 Jul 2021].
- 2.Ahmad I, Rathore FA. Neurological manifestations and complications of COVID-19: a literature review. J Clin Neurosci 2020;77:8–12. 10.1016/j.jocn.2020.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agarwal S, Jain R, Dogra S, et al. Cerebral microbleeds and leukoencephalopathy in critically ill patients with COVID-19. Stroke 2020;51:2649–55. 10.1161/STROKEAHA.120.030940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Radmanesh A, Derman A, Lui YW, et al. COVID-19-associated diffuse leukoencephalopathy and microhemorrhages. Radiology 2020;297:E223–7. 10.1148/radiol.2020202040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang H, Eichelberger H, Chan M, et al. Pearls & Oy-sters: Leukoencephalopathy in critically ill patients with COVID-19. Neurology 2020;95:753–7. 10.1212/WNL.0000000000010636 [DOI] [PubMed] [Google Scholar]
- 6.Xia Y, Wang Y, Yang L, et al. Incident cerebral microbleeds and hypertension defined by the 2017 ACC/AHA guidelines. Ann Transl Med 2021;9:314. 10.21037/atm-20-5142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Poels MMF, Vernooij MW, Ikram MA, et al. Prevalence and risk factors of cerebral microbleeds: an update of the Rotterdam scan study. Stroke 2010;41:S103–6. 10.1161/STROKEAHA.110.595181 [DOI] [PubMed] [Google Scholar]
- 8.Zhou H, Yang J, Xie P, et al. Cerebral microbleeds, cognitive impairment, and MRI in patients with diabetes mellitus. Clin Chim Acta 2017;470:14–19. 10.1016/j.cca.2017.04.019 [DOI] [PubMed] [Google Scholar]
- 9.Lang M, Buch K, Li MD, et al. Leukoencephalopathy associated with severe COVID-19 infection: sequela of hypoxemia? AJNR Am J Neuroradiol 2020;41:1641–5. 10.3174/ajnr.A6671 [DOI] [PMC free article] [PubMed] [Google Scholar]