Abstract
Background and purpose
The main cause of death in COVID-19 pneumonia is acute respiratory distress syndrome which is preceded by massive cytokine release. Low-dose radiation therapy (LDRT) has anti-inflammatory and immunomodulatory effects that can interfere with the inflammatory cascade, reducing the severity of associated cytokine release.
Material & methods
25 patients with RT-PCR proven COVID-19 pneumonia were enrolled between November 2020 and May 2021. All patients had SpO2 < 94 % on room air, respiratory frequency > 24/min and SpO2/FiO2 ratio (SF ratio) of >89 but <357. Patients were treated according to standard COVID-19 management guidelines along with single fraction LDRT of 0.5 Gy to bilateral whole lungs within 10 days of symptom onset and 5 days of hospital admission.
Results
LDRT was well tolerated by all patients. There was a statistically significant improvement in oxygenation as given by the SF ratio between pre-RT and day 2 (p < 0.05), day 3 (p < 0.001) and day 7 (p < 0.001) post RT. Demand for supplemental oxygen showed statistically significant reduction between pre-RT and day 2 (p < 0.05), day 3 (p < 0.001), day 7 (p < 0.001) post RT. 88 % patients attained clinical recovery within 10 days post LDRT and median time to hospital discharge from day of LDRT was 6 days. Three patients deteriorated and died.
Conclusion
As per our initial experience, LDRT appears to be a promising modality of treatment with rapid relief of respiratory distress in selected patients with moderate to severe COVID-19 pneumonia. This translates to early clinical recovery and hospital discharge in the selected patient group.
Keywords: Low dose radiotherapy, Immunomodulatory therapy, Anti-inflammatory therapy, COVID-19 therapy, Viral pneumonia, COVID-19 pneumonia
As of May 31, 2021, COVID-19 has been confirmed in more than 170 million individuals worldwide and has resulted in over 3.5 million deaths. India is the second worst country in the world in terms of case numbers with over 28 million cases cumulatively and third worst in terms of mortality reporting over 329,000 deaths at the time of writing [1].
Amongst the several pharmacological therapies, apart from corticosteroids [2], most of the other widely used pharmacologic therapies like remdesivir, hydroxychloroquine, lopinavir/ritonavir, interferon had little or no effect on overall mortality, initiation of ventilation or duration of hospital stay in hospitalized patients [3]. Recently, Indian Council of Medical Research had recommended against the use of plasma therapy for COVID patients in the country. As the pandemic continues to cause large number of casualties worldwide, there is a desperate need for a life-saving modality of treatment for those severely affected by the disease.
Low dose radiation therapy (LDRT) is being explored around the world in many institutions for terminally ill COVID-19 patients. The potential benefits of such an approach is well documented in literature [4], [5]. LDRT acts via polarization of macrophages to a M2 phenotype, which is the basis for its anti-inflammatory effects in COVID associated pneumonia [6]. Based on pre-clinical observations, a dose range of 0.5–1 Gy was suggested for clinical use. This low dose range was observed to induce human lung macrophage reprogramming through production of the immunosuppressive IL-10 cytokine and the suppression of the inflammatory signals from IFNγ [7].
The need for this research is to evaluate this novel treatment option as an anti-inflammatory/immunomodulatory approach in moderate to severely diseased COVID-19 patients.
Methods
Patient selection
All patients with moderate to severe COVID-19 pneumonia were evaluated by a multidisciplinary board (including specialties such as Radiation Oncology, Internal Medicine, Pulmonology, Critical Care and Anesthesia) to determine the benefits and risks of their inclusion in the study.
Study design
This single institutional prospective study was approved by the Institutional Ethics Committee registered with the Central Drugs Standard Control Organization, India (Registration number ECR/926/Inst/TN/2017/RR-20). The study was planned to be conducted in 2 phases:
-
i)
An initial phase enrolling 10 patients, which assessed the feasibility and efficacy of low-dose bilateral whole lung irradiation, evaluated according to an increase in SpO2/FiO2 ratio by at least 20 % at 48 h with respect to the pre-irradiation value in minimum 30 % of the patients treated.
-
ii)
ii) Upon achieving the minimum efficacy, the study proceeded to the Randomized comparative phase conducted in two groups: a control group, which only received standard pharmacological treatment, and an experimental arm with pharmacological treatment and LDRT. We had planned to include 51 patients, the allocation being 1: 2, that is, 17 in the control arm and 34 in the experimental arm. At the time of writing this manuscript, 25 patients (10 in first phase and 15 in second phase) had received LDRT and there were 8 patients in the control group. The preliminary results of these 25 patients who underwent LDRT are presented in this manuscript.
Inclusion criteria
-
1.
Adult patients above the age of 40 with RT-PCR proven COVID-19 with fewer than 10 days of symptom onset that warranted hospitalization and currently receiving standard medication for COVID-19 at appropriate doses (including corticosteroids, antivirals, antibiotics, anticoagulants, oral supplements and others) as per the Ministry of Health and Family Welfare standard COVID-19 management guidelines
And
-
2.
And moderate to severe dyspnea with respiratory frequency ≥ 24/min, oxygen saturation on room air SpO2 < 94 % and SpO2/FiO2 ratio > 89 and < 357.
Exclusion criteria
-
1.
Actual or planned Pregnancy
-
2.
Prior lobectomy or pneumonectomy
-
3.
Prior thoracic radiotherapy resulting in a maximum lung dose of 100 cGy or higher within 14 days of enrollment
-
4.
Prior chemotherapy or other systemic therapy with potential for pulmonary toxicity or radio sensitization within 14 days or 5 half-lives, whichever is greater, of enrollment, e.g., bleomycin, gemcitabine
-
5.
Prior cancer immunotherapy with an immune checkpoint inhibitor within 60 days of enrollment
-
6.
Severe pre-existing heart disease, e.g., New York Heart Association (NYHA) functional class ≥ 3 congestive heart failure
-
7.
History of bone marrow or solid organ transplantation
-
8.
Known history of autoimmune collagen vascular disease, e.g., scleroderma
-
9.
Known hereditary syndrome with increased sensitivity to ionizing radiation, e.g., Ataxia-telangiectasia or Fanconi anemia
Treatment procedure
Patients were positioned supine on the Linear Accelerator (6MV) couch with their hands over the head and neck in a slightly extended position. Two equally weighted fields- Antero-posterior and Postero-anterior were used. A dose of 0.5 Gy in a single fraction was delivered for all patients at the prescribed mid-depth, each field delivering 0.25 Gy.
End points
Primary endpoint:
-
•
Efficacy of LDRT based on an improvement in SpO2/FiO2 (SF) ratio, defined as the ratio of Oxygen saturation by pulse oximetry to that of fraction of inspired oxygen, measured at 48 h, 72 h and 7 days from the time of LDRT compared to the pre-irradiation measurement.
Secondary endpoints:
-
•
Reduction in the need for Oxygen supplementation measured at 48 h, 72 h and 7 days from the time of LDRT compared to the pre-irradiation measurement
-
•
Time to clinical recovery, defined as time to wean from supplemental oxygen and remain off supplemental oxygen for at least 12 consecutive hours
-
•
Time to Hospital discharge post LDRT
Criteria for Hospital Discharge:
-
•
No fever for prior 48 consecutive hours without the use of anti-pyretic medication
-
•
Oxygen saturation ≥ 95 % on room air and ability to maintain the same without the use of supplemental oxygen for more than 48 h
-
•
Mild symptoms requiring minimal supportive care like oral medication
-
•
Ability to adhere to home isolation recommendations
Statistical analysis
The data was analyzed using SPSS Software version 23. Descriptive statistics were performed for demographic data and clinical characteristics at baseline and post-intervention. Frequency was reported for categorical variables and mean (±SD) or median (Interquartile range) for continuous variables as appropriate. Boxplots were used to visualize the distribution of clinical parameters such as oxygen requirement (L/min), SpO2/FiO2 (SF) ratio and absolute lymphocyte counts at various time points before and after the intervention, and also assess the presence of outliers. Normality was assessed for these distributions at each time point by use of the Shapiro-Wilk test. Upon violation of the assumption of normality, a Friedman test was run to determine the difference in clinical parameters pre- and post- intervention. Post hoc analysis was performed with a Bonferroni correction applied for multiple comparisons. A two-sided p value less than 0.05 was considered to be statistically significant.
Pharmacological treatment
All 25 irradiated patients received standard pharmacological therapy at appropriate doses which included corticosteroids (Methyl prednisolone/Dexamethasone), anti-coagulants (Enoxaparin sodium), Vitamin C and Zinc supplementation. Notably, all patients were initiated on corticosteroids at a median (IQR) of 24 h (12–84 h) prior to irradiation, given for a median of 7 days (Range 5–10 days). Dose of corticosteroids were 1 mg/kg/day of methylprednisolone in two divided doses (or an equivalent dose of dexamethasone) for moderate cases and 1.5 mg/kg/day of methylprednisolone in two divided doses (or an equivalent dose of dexamethasone) for severe cases. Eight patients received Remdesivir 200 mg IV on the first day of admission followed by 100 mg IV once daily for the next 4 days.
Results
25 patients with a mean (±SD) age of 57 (±13) were treated between November 2020 and May 2021. The clinical characteristics of the patients are listed in Table 1 . Fever was the most common presenting symptom seen in 88 % of patients followed by cough (64 %). All patients had less than 10 days of first symptom onset during admission. LDRT was given at a median of 2 days of hospital admission (Range 12 h to 5 days). Average time taken for LDRT between patients’ entry and exit from the LINAC room was 7–10 minutes
Table 1.
Clinical characteristics of the study participants (n = 25).
| Characteristic | Number of study participants, n (%) |
|---|---|
| Age in years | |
| 40–59 | 16 (64) |
| 60–79 | 8 (32) |
| ≥80 | 1 (4) |
| Sex | |
| Male | 16 (64) |
| Female | 9 (36) |
| Presence of comorbidity | |
| Co-morbidity | 20 (80) |
| Diabetes mellitus | 16(64) |
| Systemic Hypertension | 12(48) |
| Chronic Liver disease | 4(16) |
| Bronchial Asthma | 1(4) |
| Non-comorbid | 5 (20) |
| Baseline SpO2 in room air, % | |
| 70–79 | 3 (12) |
| 80–89 | 20 (80) |
| ≥90 | 2 (8) |
| CT Severity Score (out of 25) | |
| ≤12 | – |
| 13–19 | 22 (88) |
| ≥20 | 3 (12) |
| Baseline lymphophenia grade | |
| No lymphopenia | 8 (32) |
| Grade 1 lymphopenia | 4 (16) |
| Grade 2 lymphopenia | 8 (32) |
| Grade 3 lymphopenia | 5 (20) |
All patients completed the single fraction of 0.5 Gy LDRT and tolerated well without any significant acute toxicity. We performed serial blood tests on day 1, day 3, day 7 and day 14 after RT and compared with the pre-RT value to observe the change in lymphocyte counts. WHO grading of lymphopenia was used to monitor the lymphocyte counts. The median lymphocyte counts pre-RT (788) corresponded to WHO grade 2 lymphopenia which is defined as a lymphocyte count between 500–799/µL. A Friedman test determined statistically significant reduction (p = 0.001) in median lymphocyte count between pre-RT (median = 788) and day 7 (median = 558). But this did not correlate with worsening of clinical grade of lymphopenia between those measured timepoints. Five patients who had grade 3 lymphopenia (Lymphocyte count between 200–499/µL) at pre-RT did not worsen to grade 4 lymphopenia (<200/µL) at any time point. After reaching nadir at day 7 post RT, the lymphocyte counts recovered by day 14 post RT. The pattern of lymphocyte count change with respect to pre-RT values is represented in Table 2 and compared using a box plot in Fig. 3.
Table 2.
Pre-post comparison of lymphocyte count in patients receiving radiotherapy.
| Time point | Absolute Lymphocyte Count in cells/µL |
p value | ||
|---|---|---|---|---|
| Minimum | Maximum | Median (IQR) | ||
| Pre-RT | 368 | 2158 | 788 (583–1210) | Reference |
| Day 1 | 383 | 2526 | 759 (540–1072) | 1.000 |
| Day 3 | 306 | 2278 | 624 (542–808) | 0.701 |
| Day 7 | 336 | 1296 | 558 (515–714) | 0.001* |
| Day 14 | 647 | 1852 | 1035 (825–1202) | 0.701 |
Statistically significant at p < 0.05.
Fig. 3.
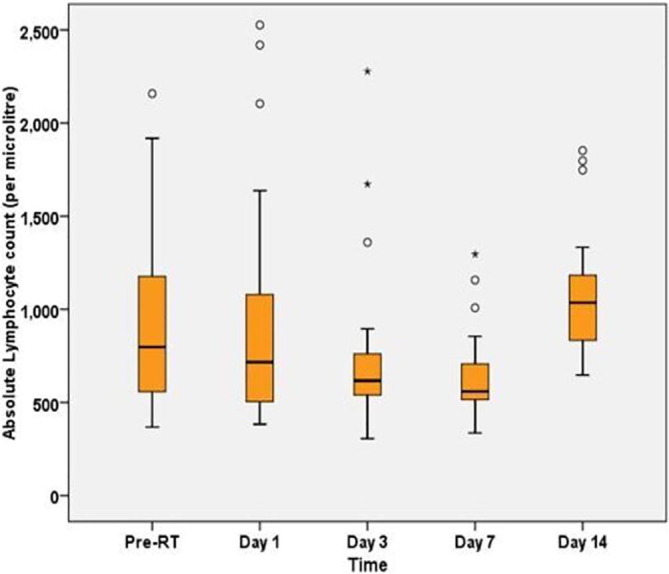
Boxplot showing Absolute Lymphocyte Count in patients pre- and post-LDRT (n = 25).
There was an improvement in hypoxia as given by statistically significant increase in SF ratio before and after radiotherapy, χ2 (3) = 57.18, p < 0.001. Post hoc analysis revealed that LDRT elicited a statistically significant increase in median SF ratio from pre-RT (median = 200) to 48 h post RT (median = 314) (p = 0.025), which further significantly increased on days 3 and 7 post-RT. This is depicted in Fig. 1 and Table 3 . All pairwise comparisons showed a significant difference in SF ratio except between day 2 (median = 314) and day 3 (median = 376) which was not found to be significant (p = 0.690).
Fig. 1.
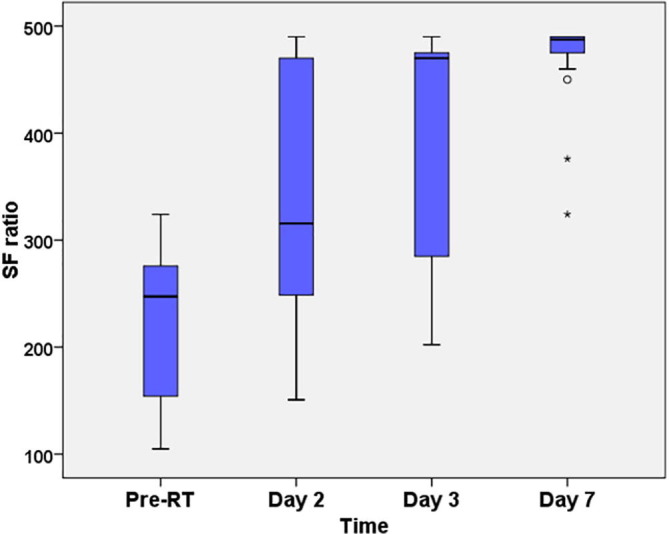
Boxplot showing distribution of SpO2/FiO2 (SF) ratio in patients receiving LDRT: Pre-post comparison (n = 25).
Table 3.
Summary of statistics and post-hoc analysis of SpO2/FiO2 (SF) ratio in patients receiving radiotherapy: Pre-post comparison.
| Time point | SF Ratio |
p value* | ||
|---|---|---|---|---|
| Minimum | Maximum | Median (IQR) | ||
| Pre-RT | 105 | 324 | 200 (151–276) | Reference |
| Day 2 | 130 | 490 | 314 (198–425) | 0.025 |
| Day 3 | 105 | 490 | 376 (217–472) | 0.000 |
| Day 7 | 324 | 490 | 488 (475–490) | 0.000 |
Statistically significant at p < 0.05.
Median (IQR) Time to Clinical Recovery was 3 (2–6) days in our study (Min: 1 day, Max: 9 days). Median (IQR) Length of hospital stay was 6 (5–9) days (Min: 4 days, Max: 13 days). 22 out of 25 patients, that is 88 %, attained clinical recovery and were discharged from the hospital within 10 days of LDRT.
A Friedman test determined that median oxygen requirement in patients decreased in a statistically significant manner before and after radiotherapy, χ2 (3) = 52.1, p < 0.001. Post hoc analysis with a Bonferroni correction applied revealed that low-dose radiotherapy elicited a significant reduction in required supplemental oxygen from pre-RT to 48 h, 72 h and 7 days post RT (p < 0.05). This is depicted in Fig. 2 and Table 4 . However, there was only a slight reduction in oxygen requirement between Days 2 and 3 (p = 0.479) and Days 3 and 7 (p = 0.690) post-RT, which was not statistically significant.
Fig. 2.
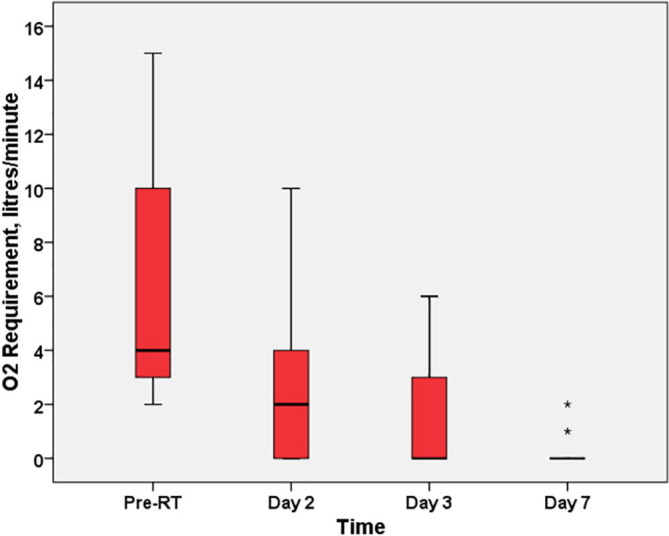
Boxplot showing distribution of oxygen requirement in patients receiving LDRT: Pre-post comparison (n = 25).
Table 4.
Summary of statistics and post-hoc analysis of oxygen requirement in patients receiving radiotherapy: Pre-post comparison.
| Time point | Oxygen Requirement in liters/minute |
p value* | ||
|---|---|---|---|---|
| Minimum | Maximum | Median (IQR) | ||
| Pre-RT | 2 | 15 | 6 (3–10) | Reference |
| Day 2 | 0 | 12 | 2 (0.5–6.5) | 0.017 |
| Day 3 | 0 | 15 | 1 (0–5.5) | 0.000 |
| Day 7 | 0 | 2 | 0 (0–0) | 0.000 |
Statistically significant at p < 0.05.
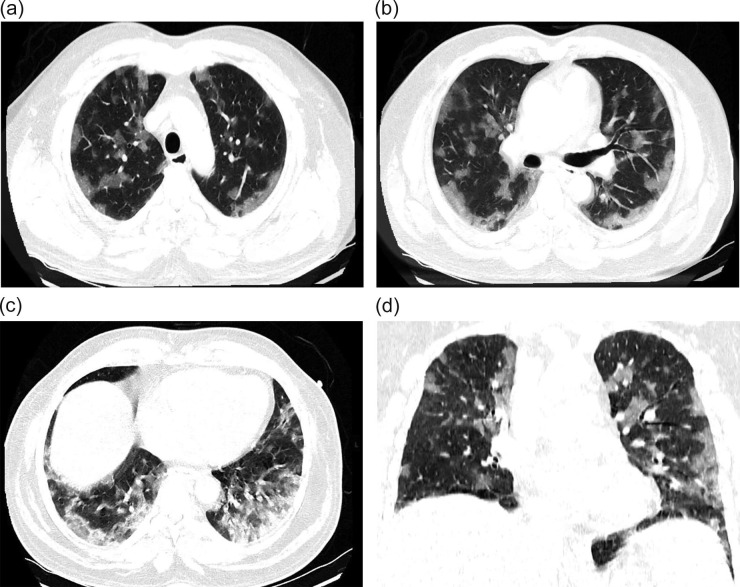
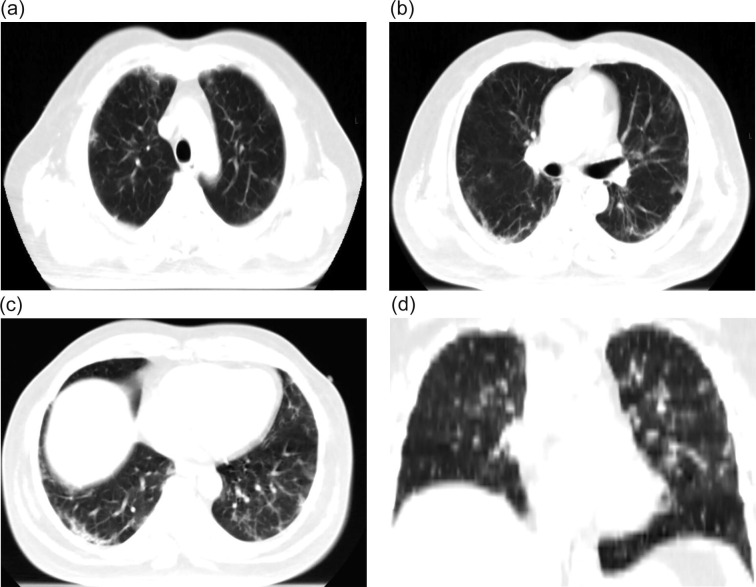
There was evident radiological resolution correlating with clinical improvement as seen on Chest radiographs taken on day 3 and day 7 post RT compared to the pre-RT radiograph that was taken 2 h before RT. One of the patients’ comparative radiograph series is represented in Fig. 4A, Fig. 4B, Fig. 4C . A representative CT image series pre-RT (taken 1 hour before LDRT) and 14 days post LDRT is shown in Fig. 5, Fig. 6 . CT severity scores were assigned based on the method proposed by Li et al and depicted in Table 5 [8].
Fig. 4A.
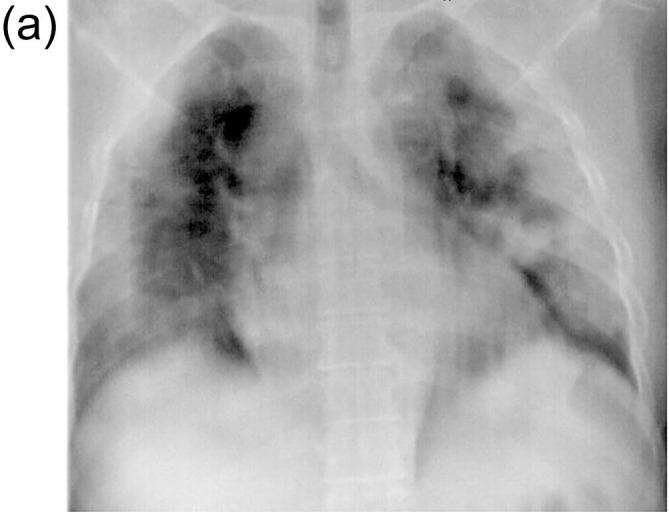
Chest X-ray Pre-LDRT showing multifocal diffuse patchy consolidation in bilateral lung fields; Loss of vascular markings; Obliteration of right costophrenic angle.
Fig. 4B.
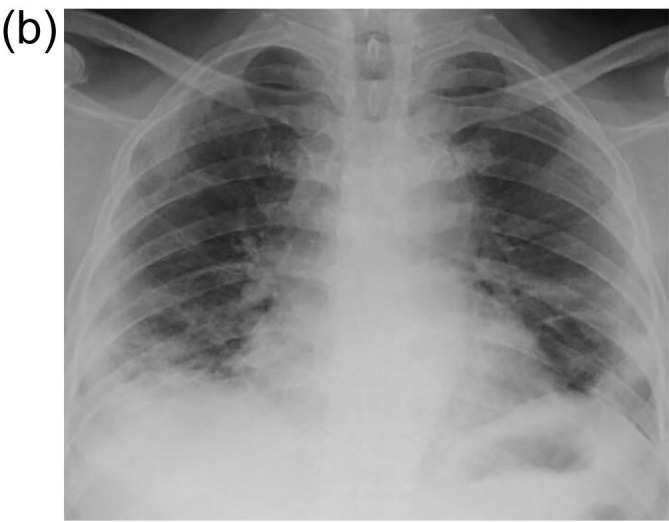
Chest X-ray on day 3 post LDRT showing evidence of significant resolution of opacities in bilateral upper zones and left lower zones; Subpleural patchy air space opacities in bilateral lower zones, right mid zone and left upper zone.
Fig. 4C.
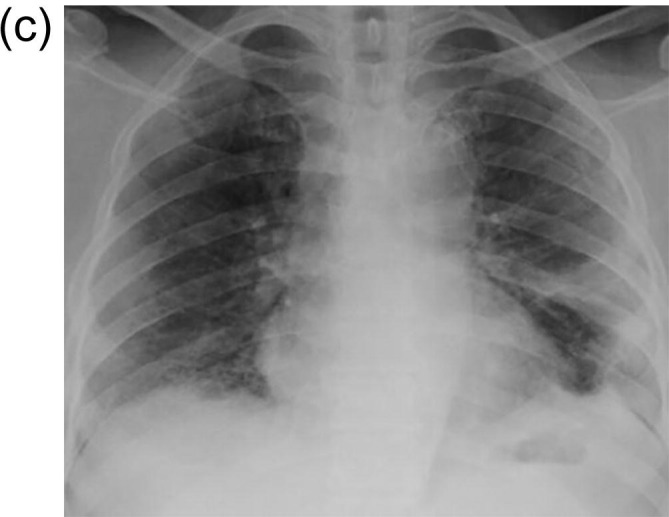
Chest X[HYPHEN]ray on Day 7 post LDRT. Showing further resolution of opacities in right lower zone.
Fig. 5.
A-D (Pre-LDRT CT scan) showing Multifocal asymmetric subpleural and intraparenchymal patchy air space opacities noted diffusely in bilateral lung fields CT severity score 19/25.
Fig. 6.
A-D (Day 14 post LDRT CT scan) showing significant clearance of intraparenchymal air space opacities; clearance of evolving consolidation in postero-basal segments of both lower lobes; Areas of ground glass opacities replaced by fibrosis CT severity score 12/25.
Table 5.
CT severity scores pre-RT and Day 14 post RT.
| Lobe | % of involvement |
Score |
||
|---|---|---|---|---|
| Pre-RT | Day 14 post RT |
Pre-RT | Day 14 post RT |
|
| Right upper lobe | 50% | 40% | 4/5 | 3/5 |
| Right middle lobe | 60% | 20% | 4/5 | 2/5 |
| Right lower lobe | 50% | 40% | 4/5 | 3/5 |
| Left upper lobe | 40% | 15% | 3/5 | 2/5 |
| Left lower lobe | 60% | 20% | 4/5 | 2/5 |
| TOTAL SCORE | 19/25 | 12/25 | ||
At the time of writing, all patients had a follow-up of at least 14 days from LDRT. Three patients (2 male and 1 female) had clinical deterioration and required mechanical ventilation. All of them succumbed to the disease. Hence, the all-cause mortality rate was 12 % at 2 weeks from LDRT. The clinical characteristics of these patients are given in Table 6 .
Table 6.
Clinical characteristics of patients who succumbed to the disease.
| Age/sex | Comorbid condition | Pre-RT Spo2 (ra) |
Pre-RT O2 req. (L/min) |
CTSS | Spo2/Fio2 ratio |
Days from LDRT to ventilator support | Days from LDRT to death | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre-RT | D1 | D2 | D3 | D4 | D5 | ||||||||
| 1 | 75/M | Diabetic | 75% | 10 | 21/25 | 148 | 144 | 158 | 107 | 95 | – | 4 | 4 |
| 2 | 70/F | Nil | 80% | 1 | 22/25 | 186 | 118 | 130 | 132 | 109 | 105 | 6 | 6 |
| 3 | 43/M | Diabetic | 80% | 6 | 20/25 | 200 | 151 | 148 | 105 | 92 | – | 4 | 4 |
CTSS- CT severity score.
Discussion
The utilization of low dose radiotherapy for the treatment of viral pneumonia is documented in literature since the first half of 20th century. It had shown great promise with cure rates up to 80 % as observed by several authors [9]. The treatment was replaced by wide availability of antibiotics and was never again used for this indication for several decades until the emergence of COVID-19. As of date, there are several published preliminary reports examining various aspects on the use of LDRT [10], [11], [12], [13], [14]. The key parameters are outlined and compared with our study in Table 7 .
Table 7.
Review of published LDRT studies & their comparison with this study.
| Author & Place | Sample size | Median age | % Comorbidities in sample | Interval to LDRT from admission (Avg) | RT Dose (Gy) | RT Fields | Response rate | Acute toxicities |
|---|---|---|---|---|---|---|---|---|
| Sharma et al. [9], India | 10 | 51 | 30% | 3 days | 0.7 | AP-PA | 90% | Nil |
| Hess et al. [10], USA | 10 | 90 | 90% | 5 days | 1.5 | AP-PA | 90% | Nil |
| Ameri et al. [11], Iran | 10 | 72 | 80% | 2 days | 0.5 | AP-PA | 60% | Nil |
| Sanmamed et al. [12], Spain | 9 | 66 | 55% | – | 1 | 3DCRT | 78% | Lymphopenia |
| Papachristofilou et al. [13], Switzerland | 11 | 75 | 54% | 2 days | 1 | AP only | 64% | Lymphopenia |
| This study | 25 | 56 | 80% | 2 days | 0.5 | AP-PA | 88% | Nil |
Abbreviations: AP -Anteroposterior; PA-Posteroanterior; 3DCRT- Three-Dimensional conformal radiotherapy.
We concur with the authors describing the transition from viral pulmonary phase to hyper-inflammatory phase as the “hypothetical therapeutic window of opportunity” for LDRT [15] and accordingly timed the treatment for our patients, i.e., within 10 days of symptom onset and an average of 2 days of hospital admission (Range 12 h to 5 days).
For the determination and monitoring of oxygenation status, we used SpO2/FiO2 ratio which is a simple, faster and non-invasive alternative to PaO2/FiO2 ratio. SF Ratio of >213 but <357 corresponds to PaO2/FiO2 ratio (PF ratio) of 200–300 indicating mild to moderate respiratory distress and value of >89 but <214 corresponds to PF ratio of 100–200 indicating severe respiratory distress. Patients having SF ratio < 89 (PF ratio < 100) indicating critical respiratory distress were not included in the study [16]. Sanmamed et al [14] showed a statistically significant difference between pre-RT and day 3, day 7 SpO2/FiO2 ratios which our study corresponds with.
Three patients who died had pre-RT SF ratio values between 148–200 along with CT severity score of ≥20 out of 25. Five patients with <150 (100–149) pre-RT SF ratio scores had recovered, albeit with comparatively longer duration to wean O2 and hospital discharge. These 5 patients had an average CT severity score of 17 out of 25. Careful selection of patients is required for them to benefit from LDRT. In our institute, we are no longer enrolling patients with CT severity score ≥ 20 (out of 25).
Probably the most prominent finding in our study was the dramatic reduction in demand for oxygen supplementation, with reduction up to 66.6 % at 48 h compared to pre-LDRT levels. Given the current situation in India, this finding is of great importance as it addresses the core issue of oxygen shortage.
Our median time to clinical recovery of 3 days is comparable with that of Hess et al. [11]. Median duration of hospital admission post RT in our study was 6 days (5–9 days). Hess et al. [11] quoted the median time from first COVID-19 intervention to hospital discharge as 12 days (7–25 days) in their LDRT patient cohort whereas Sanmamed et al. [14] reported a median hospitalization time of 13 days after RT (4–77 days).
In our study, the median lymphocyte counts pre-RT corresponded to WHO grade 2 lymphopenia. Some degree of lymphopenia is expected, as both COVID-19 infection and concurrent administration of corticosteroids can cause reduction in lymphocyte counts during the early disease course. Sanmamed et al. [14] reported worsening of clinical grade of lymphopenia in two patients on day 3 and in one patient on day 7 following a LDRT dose of 1 Gy. In our study group, the median lymphocyte counts reached nadir by day 7 post RT but we did not observe a worsening of clinical grade of lymphopenia post RT compared to pre-RT. The lethal dose required for 50 % reduction in surviving fraction of lymphocytes was reported to be 2 Gy by Nakamura et al. [17]. Our LDRT dose of 0.5 Gy could have played a part in reduction in lymphocyte counts but had not resulted in clinically significant toxicity. Its noteworthy that previously published studies that utilized the dose range of 0.5–0.7 Gy did not report any acute toxicity [10], [12].
Chance of cancer induction at later life is the chief concern, both for physicians and patients while deciding for LDRT. The lifetime excess absolute risk of cancer induction, by the Preston formula was 0.4 % for a whole-body dose of 0.5 Gy [18]. A whole lung dose of 0.5 Gy will reduce this percentage further. But, a recent review suggested that the lifetime attributable risk of cancer for a dose of 0.5 Gy for ages 20–80 can range from 0.29-1.7 % for males and 0.5–4.9 % for females [19]. The physician should discuss the benefits and risks in detail with the patient and take an informed decision about LDRT on a case-to-case basis. Till date, there is no evidence to suggest LDRT will induce selective pressure and cause new viral mutations [7].
There are some limitations in this study. Absence of early inflammatory response evaluation of patients makes the study lacking in terms of correlation between oxygenation, radiological and inflammatory response. A short follow-up of 2 weeks chosen for these preliminary results precludes comparison with the control group to achieve solid conclusions. Mortality data will especially be more valid if patients are followed up longer. Time to clinical recovery is defined in a subjective manner.
Conclusion
LDRT has the potential to prevent selected patients with moderate COVID pneumonia from deteriorating to severe and critical stage in majority of the cases. It has the ability to reduce the demand for oxygen supplementation by up to two-thirds (66.6 %) within 48 h of treatment. This, combined with earlier time to clinical recovery and hospital discharge makes it an attractive treatment option in carefully selected patients. This needs to be validated by larger prospective randomized trials.
Conflicts of interest
None.
Financial support
The authors declare that there has been no significant financial support from any fund source(s) that could have influenced the outcome of the study.
Acknowledgements
The Authors would like to acknowledge the following people for their inputs and assistance in this research: Dr Manoharan, Dr Nishanth M, Dr Deepan Chakravarthi, Dr Shruthee Deepan, Mr Manimaran R, Dr Pugazhenthan T, Dr GS Harsha Vardhanan, Ms Muthulakshmi K, Mr Selva Kumar K, Ms Aarthi A, Ms Jothimeena K, Ms Joshuasahay J and Ms Uma Maheshwari.
References
- 1.WHO Corona virus dashboard [Internet] Available from: https://covid19.who.int.
- 2.RECOVERY Collaborative Group Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO Solidarity Trial Consortium Repurposed antiviral drugs for COVID-19—interim WHO SOLIDARITY trial results. N Engl J Med. 2021;384:497–511. doi: 10.1056/NEJMoa2023184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dhawan G., Kapoor R., Dhawan R., Singh R., Monga B., Giordano J., et al. Low dose radiation therapy as a potential lifesaving treatment for COVID-19-induced acute respiratory distress syndrome (ARDS) Radiother Oncol. 2020 doi: 10.1016/j.radonc.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rödel F, Arenas M, Ott OJ, Fournier C, Georgakilas AG, Tapio S, et al., Low-dose radiation therapy for COVID-19 pneumopathy: what is the evidence? Strahlentherapie und Onkologie. 2020:1-4. [DOI] [PMC free article] [PubMed]
- 6.Calabrese E.J., Dhawan G., Kapoor R., Kozumbo W.J. Radiotherapy treatment of human inflammatory diseases and conditions: optimal dose. Hum Exp Toxicol. 2019;38:888–898. doi: 10.1177/0960327119846925. [DOI] [PubMed] [Google Scholar]
- 7.Meziani L., Robert C., Classe M., Da Costa B., Mondini M., Clémenson C., et al. Low doses of radiation increase the immunosuppressive profile of lung macrophages during viral infection and pneumonia. Int J Radiat Oncol Biol Phys. 2021 doi: 10.1016/j.ijrobp.2021.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li K., Wu J., Wu F., Guo D., Chen L., Fang Z., et al. The clinical and chest CT features associated with severe and critical COVID-19 pneumonia. Invest Radiol. 2020 doi: 10.1097/RLI.0000000000000672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Calabrese E.J., Dhawan G. How radiotherapy was historically used to treat pneumonia: could it be useful today? Yale J Biol Med. 2013;86:555. [PMC free article] [PubMed] [Google Scholar]
- 10.Sharma DN, Guleria R, Wig N, Mohan A, Rath GK, Subramani V, Bhatnagar S, et al., Low dose radiation therapy for covid-19 pneumonia: a pilot study. medRxiv. 2020. [DOI] [PMC free article] [PubMed]
- 11.Hess C.B., Nasti T.H., Dhere V.R., Kleber T.J., Switchenko J.M., Buchwald Z.S., et al. Immunomodulatory low-dose whole-lung radiation for patients with coronavirus disease 2019-related pneumonia. Int J Radiat Oncol Biol Phys. 2021;109:867–879. doi: 10.1016/j.ijrobp.2020.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Papachristofilou A., Finazzi T., Blum A., Zehnder T., Zellweger N., Lustenberger J., et al. Low dose radiation therapy for severe COVID-19 pneumonia: a randomized double-blind study. Int J Radiat Oncol Biol Phys. 2021 doi: 10.1016/j.ijrobp.2021.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ameri A., Rahnama N., Bozorgmehr R., Mokhtari M., Farahbakhsh M., Nabavi M., et al. Low-dose whole-lung irradiation for COVID-19 pneumonia: short course results. Int J Radiat Oncol Biol Phys. 2020;108:1134–1139. doi: 10.1016/j.ijrobp.2020.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sanmamed N., Alcantara P., Cerezo E., Gaztañaga M., Cabello N., Gómez S., et al. Low-dose radiation therapy in the management of coronavirus disease 2019 (COVID-19) pneumonia (LOWRAD-Cov19): preliminary report. Int J Radiat Oncol Biol Phys. 2021;109:880–885. doi: 10.1016/j.ijrobp.2020.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prasanna P.G., Woloschak G.E., DiCarlo A.L., Buchsbaum J.C., Schaue D., Chakravarti A., et al. Low-dose radiation therapy (LDRT) for COVID-19: benefits or risks? Radiat Res. 2020;194:452–464. doi: 10.1667/RADE-20-00211.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pandharipande P.P., Shintani A.K., Hagerman H.E., St Jacques P.J., Rice T.W., Sanders N.W., et al. Derivation and validation of SpO2/FiO2 ratio to impute for PaO2/FiO2 ratio in the respiratory component of the Sequential Organ Failure Assessment (SOFA) Score. Crit Care Med. 2009;37:1317. doi: 10.1097/CCM.0b013e31819cefa9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakamura N., Kusunoki Y., Akiyama M. Radiosensitivity of CD4 or CD8 positive human T-lymphocytes by an in vitro colony formation assay. Radiat Res. 1990;123:224–227. [PubMed] [Google Scholar]
- 18.Preston D.L., Ron E., Tokuoka S., Funamoto S., Nishi N., Soda M., et al. Solid cancer incidence in atomic bomb survivors: 1958–1998. Radiat Res. 2007;168:1–64. doi: 10.1667/RR0763.1. [DOI] [PubMed] [Google Scholar]
- 19.García-Hernández T., Romero-Expósito M., Sánchez-Nieto B. Low dose radiation therapy for COVID-19: Effective dose and estimation of cancer risk. Radiother Oncol. 2020;1:289–295. doi: 10.1016/j.radonc.2020.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]