Abstract
The article describes the possible pathophysiological origin of COVID-19 and the crucial role of renin-angiotensin system (RAS), providing several “converging” evidence in support of this hypothesis. SARS-CoV-2 has been shown to initially upregulate ACE2 systemic activity (early phase), which can subsequently induce compensatory responses leading to upregulation of both arms of the RAS (late phase) and consequently to critical, advanced and untreatable stages of COVID-19 disease. The main and initial actors of the process are ACE2 and ADAM17 zinc-metalloproteases, which, initially triggered by SARS-CoV-2 spike proteins, work together in increasing circulating Ang 1–7 and Ang 1–9 peptides and downstream (Mas and Angiotensin type 2 receptors) pathways with anti-inflammatory, hypotensive and antithrombotic activities. During the late phase of severe COVID-19, compensatory secretion of renin and ACE enzymes are subsequently upregulated, leading to inflammation, hypertension and thrombosis, which further sustain ACE2 and ADAM17 upregulation. Based on this hypothesis, COVID-19-phase-specific inhibition of different RAS enzymes is proposed as a pharmacological strategy against COVID-19 and vaccine-induced adverse effects. The aim is to prevent the establishment of positive feedback-loops, which can sustain hyperactivity of both arms of the RAS independently of viral trigger and, in some cases, may lead to Long-COVID syndrome.
Keywords: Renin-angiotensin system, ADAM17, Zinc-metalloproteases, Zinc-chelating agents, Spike N-Terminal domain, RAS inhibitors
1. Introduction
COVID-19 is caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, for which vaccination strategies and neutralising antibodies specific to SARS-CoV-2 antigens are currently the only approved treatments. Because of their “exclusive” effectiveness and specificity in preventing COVID-19 disease, these immunotherapies have received the emergency use authorization. Nevertheless, there are several other treatments (including hydroxychloroquine, ivermectin, cyclooxygenase inhibitors, corticosteroids, bismuth-based drugs and convalescent plasma) that have been shown to exert some protective effects against COVID-19 (Casciola-Rosen et al., 2020; De Rosa et al., 2021; Franchini et al., 2021; Suter et al., 2021; Yagisawa et al., 2021; Yuan et al., 2020). However, the protective activity of these treatments was often limited to some cohorts and/or to a phase of the disease and no clear cause-effect relationship was consistently confirmed because the complete mechanism of action and/or side effects in COVID-19 patients have not been fully elucidated yet. For this reason, these therapies are not “officially” considered effective against COVID-19, allowing the emergency use authorization of a new generation of vaccines, whose long-term adverse effects are still unknown (Zamai and Rocchi, 2021).
Differently from other reports, our approach was aimed to determine, first of all, the possible pathophysiological mechanisms that, triggered by SARS-CoV-2, produced the severe COVID-19 symptoms in a specific subset of the human population. Subsequently, after having disclosed the probable mechanisms involved in COVID-19 pathogenesis, it has been looked for a pharmacotherapy that could rationally and safely counter the pathophysiological mechanisms activated by SARS-CoV-2. In doing so, several data on SARS-CoV-1 infection were collected over the past 18 years. The initial and crucial event of SARS-CoV as well as SARS-CoV-2 infection is the binding to human ACE2, which works as a surface receptor for viral entry. Initial reports (2005) showed that SARS-CoV-1 induced ACE2 cell surface down-modulation and suggested that ACE2 was internalized, leading to its loss of function (Imai et al., 2005; Kuba et al., 2005). ACE2 is a zinc-metalloprotease that, processing plasma angiotensin (Ang) I and II respectively into Ang (1–9) and Ang (1–7), can mediate several systemic effects through the Mas and Ang type 2 (AT2R) receptors, respectively (Zamai, 2020a). Moreover, ACE2 is believed to produce several protective effects that compensate the deleterious activity of ACE/Ang II. Based on these initial considerations, an explosion of articles with the same mainstream hypothesis proposed that pathophysiology of COVID-19 could be related to an ACE2 downregulation, indicating that recombinant ACE2 (rACE2) and/or ACE pathway inhibitors may exert a protective effect against COVID-19 (see (Vaduganathan et al., 2020), as an example). Indeed, the therapeutic activity of rACE2 was supposed not only to work by inhibiting viral spike binding to cell surface and viral infection, but also to compensate ACE2 loss of function and the consequent (hypothesised) increase of Ang II (Pang et al., 2020; Pucci et al., 2020; Zhang et al., 2020a). In this regard, human rACE2 has been shown to inhibit SARS-CoV-2 infection in vitro in human organoids (Monteil et al., 2020) and a pilot study using rACE2 protein for COVID-19 started at the end of February 2020 (ClinicalTrials.gov number, NCT04287686). However, despite the initial expectations, the clinical trial with rACE2 was abandoned without further details and the treatment with rACE2 produced only a case report of a single survivor of COVID-19 (Zoufaly et al., 2020). On the other hand, hypertensive patients, most of which are “pretreated” with ACE/Ang II/AT1R pathway inhibitors, had an increased risk to develop severe SARS-CoV-2 infection (see (Zamai, 2020a)), suggesting that these inhibitors were not expected to protect from COVID-19. Indeed, the only randomised trial with hospitalized patients under ACE inhibitors (ACEIs) or Ang type 1 receptor blockers (AT1RBs) showed no difference between those that randomly continued or discontinued the antihypertensive therapies (Cohen et al., 2021), suggesting that factors other than ACE2 loss of function and relative increase of ACE activity were involved in COVID-19 development. In this regard, other reports clearly showed that SARS-CoV-1-induced ACE2 down-modulation was due to ACE2 cell surface shedding, which was mediated by the activation of extracellular ADAM17 zinc-metalloprotease (Glowacka et al., 2010; Haga et al., 2008). Interestingly, after shedding, the soluble form of ACE2 (sACE2) retained not only its binding ability for spike SARS-CoV-1 proteins thus protecting against cell (but not organism) infection but also its enzymatic activity (Haga et al., 2008; Jia et al., 2009; Li et al., 2005). Therefore, even if SARS-CoV-induced ACE2 shedding can locally repress ACE2 function, this process would increase ACE2 systemic activity, a condition that was proposed to be on the origin of COVID-19 (Zamai, 2020a, 2021). Indeed, results from differential gene expression analysis of RAS genes in cells from bronchoalveolar lavage samples from patients with severe COVID-19 identified an increase in ACE2 expression that likely affects RAS balance (Garvin et al., 2020).
2. SARS-CoV-induced ACE2 (and ADAM17) zinc-metalloprotease systemic hyperactivity as a possible pathophysiological origin of COVID-19
A confirmation that SARS-CoVs can induced an ACE2 hyperactivity comes from recent reports showing a progressive increase of systemic ACE2 activity in COVID-19 patients and in accordance to disease severity (Nagy et al., 2021; Patel et al., 2021; Reindl-Schwaighofer et al., 2021). Similarly, high circulating levels of sACE2 were significantly associated with worse or longer COVID-19 outcome in hospitalized patients (Kragstrup et al., 2021; Lundström et al., 2021; Reindl-Schwaighofer et al., 2021). Notably, in healthy individuals circulating ACE2 activity is usually low to undetectable (Patel et al., 2021; Lew et al., 2008), instead pre-existing high levels of circulating ACE2 protein/activity are typical of patients with comorbidities (e.g. male sex, age, cardiopathies, hypertension, diabetes) associated to severe COVID-19 (Anguiano et al., 2015; Epelman et al., 2009; Kornilov et al., 2020; Kragstrup et al., 2021; Narula et al., 2020; Ortiz-Pérez et al., 2013; Ramchand et al., 2018; Sama et al., 2020; Soro-Paavonen et al., 2012; Úri et al., 2014, 2016; Wallentin et al., 2020; Walters et al., 2017), suggesting that an elevated baseline activity of ACE2 in circulation would predispose to severe COVID-19 because SARS-CoV infections would further increase ACE2 systemic activity. Altogether this evidence strongly supports the hypothesis that the main and initial cause of COVID-19 would be an excessive activity of circulating ACE2 zinc-metalloprotease, initially triggered by SARS-CoV infection (Zamai, 2020a, 2021). Interestingly, sACE2 has also been shown to bind to integrins and to increase their adhesive function (Clarke et al., 2012), a feature that might expand virus tropism to non-ACE2 positive tissues and might contribute to the complex pattern of COVID-19. In this regard, sACE2-mediated endocytosis of SARS-CoV-2 via AT1R or vasopressin receptor in transfected cell lines has been recently shown, highlighting a new cell entry mechanism of SARS-CoV-2 infection that may also occur in vivo (Yeung et al., 2021). Notably, the addition of physiological concentrations of rACE2, mimicking the sACE2, increased SARS-CoV-2 infectivity in a renal cell line (Yeung et al., 2021), indicating that rACE2 therapeutic approach against COVID-19, if not detrimental, was a useless therapy. Instead, in order to inhibit SARS-CoV-2 infection, the authors proposed to target ACE2 shedding pathway, i.e. to inhibit ADAM17 activity, (Yeung et al., 2021), a pharmacological treatment that we have already proposed (Zamai, 2020a, 2021) and it was originally proposed by Haga and colleagues for SARS-CoV-1 infection (Haga et al., 2010). In this regard, the initial binding event to ACE2-expressing cells is shared by different coronaviruses, i.e. SARS-CoVs and HCoV-NL63; however, HCoV-NL63 is only responsible of symptoms typical of common cold. Instead, both severe respiratory symptoms and ADAM17-mediated cell surface shedding of ACE2 are exclusive features of SARS-CoVs (Glowacka et al., 2010; Haga et al., 2008; Yeung et al., 2021), further suggesting that ACE2 surface receptor cleavage and pathway hyperactivation downstream of ACE2 activity may be key players in COVID-19 disease development. Notably, in order to induce ACE2 shedding, SARS-CoV virions need to recruit ADAM17 close to ACE2, a mechanism that could possibly be mediated by the N-terminal domain (NTD) of the SARS-CoV-2 spike S1 subunit. In this regard, the NTD is the highest variable region of the spike protein in SARS-CoV-2 variants and its frequency of mutation is even higher than the receptor binding domain (RBD) present in the C-terminal domain (CTD) of the S1 (McCallum et al., 2021; Thomson et al., 2021). It is well known that the ACE2-mediated viral entry is allowed by the RBD of the spike protein and this domain is target of the neutralising antibody induced by SARS-CoV-2 vaccine or infection. This is the reason why mutations in the RBD that confer resistance to neutralising antibodies as well as increase the binding affinity to ACE2 have selective advantage and are preferentially expressed by SARS-CoV-2 spike variants (Thomson et al., 2021). However, single mutations in the RBD are often associated with deletions in the NTD that drive antibody escape (McCarthy et al., 2021; Thomson et al., 2021), suggesting that NTD may participate in the infection of SARS-CoV-2.
It is known that most coronaviruses have the RBD in the CTD of S1; however, in these cases, the NTD have been suggested to enhance the binding of the virus to the cell surface by recognizing specific sugar moieties (Krempl et al., 1997; Künkel and Herrler, 1993; Li, 2016; Lu et al., 2015; Awasthi et al., 2020; Park et al., 2019). Indeed, NTDs of the S protein of betacoronaviruses fold into a galectin-like structure, a typical carbohydrate-recognition domain, that can be utilized as an alternate receptor, possibly affecting viral attachment and tropism (Li et al., 2016). For example, attachment to host sialic acid receptors has been suggested to be a key step in regulating MERS-CoV infection (Park et al., 2019). Interestingly, both MERS-CoV and SARS-CoV sialic acid binding pockets (galectin-like structures) have a short helix that characterise and distinguish them from other coronaviruses, thus suggesting that they may share similar carbohydrate specificities (Awasthi et al., 2020; Park et al., 2019; Yuan et al., 2017). On the other hand, circulating activity of dipeptidyl-peptidase-4 (DPP-4), the membrane receptor for MERS-CoV entry, is mediated by zinc-metalloprotease shedding activity and it is induced by hypoxia (Röhrborn et al., 2014), thus further sharing features with ACE2-mediated SARS-CoV infection. In addition, neutralising antibodies to NTD of the spike protein have been shown to be highly potent against infectious virus and to protect from diseases, by inhibiting not only attachment but also post-attachment steps of both MERS-CoV and SARS-CoV-2 infections (Zhou et al., 2019; McCallum et al., 2021; Suryadevara et al., 2021; Chi et al., 2020; Graham et al., 2021), indicating that NTD of spike proteins may participate at different stages in inducing infections. In particular, these antibodies were able to block SARS-CoV-2 S-mediated cell-cell fusion, suggesting that they could prevent interaction with an auxiliary receptor, possibly mediating proteolytic activation and/or membrane fusion (McCallum et al., 2021). In this regard, it has already been suggested that cleavage of ACE2 might be a prerequisite for the fusion of spike proteins (cleaved at the S1/S2 boundary site) with membrane (cleaved) ACE2, finally leading to spike-ACE2 fusion proteins and viral infection (Song et al., 2018; Zamai, 2020a). Therefore, the auxiliary receptor mediating the proteolytic activation and membrane fusion might be ADAM17. It is known that ADAM17 is a heavily glycosylated protein and it is able to cleave its substrates at a fixed distance close to the cell membrane (Calligaris et al., 2021). Therefore, NTD specific affinity for sugar moieties on ADAM17 and RBD affinity for ACE2 might drive the correct distance to induce ACE2 shedding and consequent infection. In line with this hypothesis, some cell lines (e.g. HepG2 and 293T cells) expressing membrane ACE2 but unable to release sACE2 were not susceptible to SARS-CoV-2 infection (Yeung et al., 2021), further indicating that the recruitment/activity of ADAM17 may be crucial for SARS-CoV-2 infectivity. In addition, sugar moieties on ADAM9, a membrane-anchored protease member of the ADAM family, have been already shown to have a role in promoting viral infection (Baggen et al., 2019). Therefore, neutralising anti-NTD of spike proteins might act by inhibiting ADAM17 recruitment/activity and possibly by shifting SARS-CoV-2 to a common cold-like infection, such as that induced by HCoV-NL63. If this is the case, NTD of spike protein might facilitate not only the binding of SARS-CoV-2 virion to ACE2-ADAM17 expressing cells but also the ADAM17-mediated ACE2 shedding, which would lead to both fusion protein formation and systemic sACE2 release (see Fig. 1 ).
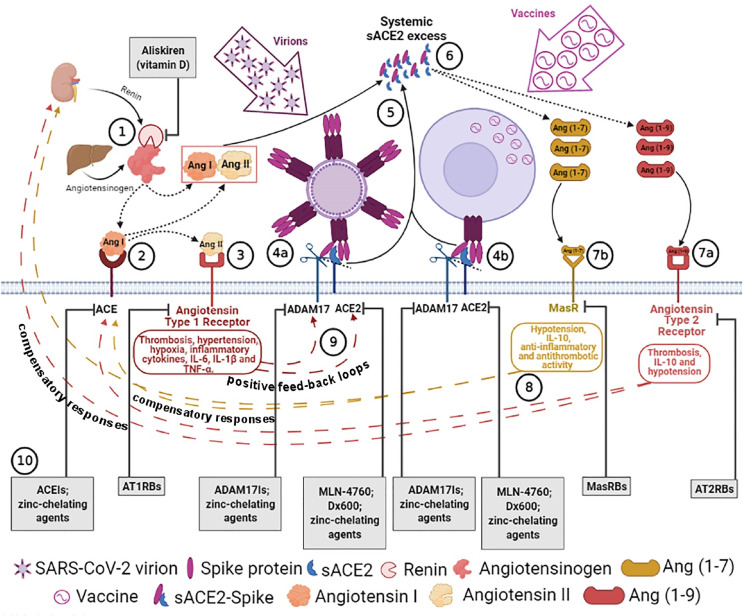
Fig. 1.
Putative physiopatological mechanisms leading to COVID-19 after SARS-CoV-2 infection or possibly to mild adverse reactions following vaccination and treatment proposals. 1) Renin secreted, by the kidney, cleaves angiotensinogen, produced by liver, to form Ang I, 2) Ang I is converted to Ang II by pulmonary ACE zinc-metalloprotease. 3) Ang II binds to angiotensin type 1 receptors (AT1Rs) or it is transformed into Ang (1–7) by ACE2 zinc-metalloprotease (see 6). 4a) SARS-CoV-2 spikes of virions or 4b) of “vaccinated” cells bind to ACE2, inducing ADAM17-mediated ACE2 shedding, which is possibly driven by spike N-terminal domain binding/recruitment of ADAM17 close to ACE2. 5) ADAM17-regulated ectodomain shedding of ACE2 results in increased amount of soluble and active circulating ACE2 (sACE2). 6) sACE2 can transform both Ang I and Ang II into Ang (1–9) and Ang (1–7), respectively. 7) The excess of Ang 1–9 and Ang 1–7 signalling via the AT2Rs (7a) and MasRs (7b) can induce hypotension, anti-inflammatory (IL-10), antithrombotic or prothrombotic pathway activation. 8) Differently from vaccination, in susceptible subjects (expressing high baseline levels of sACE2 activity), the persistent activation of these pathways caused by viral expansion will, in turn, produce late compensatory responses that upregulate both renin and ACE expression, leading to an excess of Ang II. 9) The excess of Ang II through AT1R hyperactivation can cause thrombosis, hypertension, hypoxia, inflammation, IL-6, IL-1β and TNF-α, which establish positive feedback loops by inducing further ACE2 and ADAM17 upregulation (for further details, see the text). 10). In the grey bottom boxes, drugs inhibiting RAS enzymes that can potentially stop compensatory responses and/or positive feedback loops (depending on COVID-19 phase) are indicated (for further details, see the text). Receptor Blockers: AT1RBs, AT2RBs, MasRBs and enzymatic inhibitors: ACEIs, ACE2Is, ADAM17Is, are indicated. Dotted arrows indicate an enzymatic transformation, dashed arrows represent compensatory responses and positive feedback loops, and full arrows indicate releases in systemic circulation. Created in Biorender.com.
Interestingly, soluble ACE2 protein production can also be generated by alternative mRNA splicing (Rehman and Tabish, 2020). However, this ACE2 isoform may have not only different expression/localisation (soluble vs membrane bound) but also function. In this regard, the human ACE2 enzyme consists of two main domains: the extracellular peptidase catalytic domain (residues 19 to 615) that includes the critical residues responsible for the binding of the SARS-CoV-2 spike protein and the collectrin-like domain (residues 615 to 768) that contains the neck (residues 616 to 726), the transmembrane helix (residues 741 to 764, encoded by exon E17) and an intracellular segment (Towler et al., 2004; Rehman and Tabish, 2020; Yan et al., 2020). The full-length ACE2 protein of 805 amino acids is encoded by 18 coding exons and a soluble isoform of 555 amino acids is truncated after exon 12 (Rehman and Tabish, 2020). Notably, the ACE2 cleavage site mediated by ADAM17 has been predicted between amino acids 716 and 741 (Jia et al., 2009), which corresponds to neck-transmembrane boundary of ACE2 collectrin-like domain, while the soluble ACE2 isoform is “cleaved” at the level of the peptidase domain, raising the possibility that this ACE2 secreted form might be enzymatically inactive and/or unable to bind to surface receptors/integrins (Clarke et al., 2012; Yeung et al., 2021). On the other hand, this sACE2 isoform may maintain its binding affinity for SARS-CoV-2 spike protein, and consequently may provide protection against infection, because the interaction between the SARS-CoV-2 spike protein and human ACE2 is mediated by amino acid residues (Gln 24, Asp 30, His 34, Tyr 41, Gln 42, Met 82, Lys 353, and Arg 357) that are all present in soluble isoform of 555 amino acids (Rehman and Tabish, 2020). In line with this hypothesis, differently from ADAM17-mediated ACE2 release (Yeung et al., 2021), transcription of the soluble ACE2 isoform has been shown to protect from SARS-CoV-2 infection in the upper respiratory tract, while transcription of transmembrane ACE2 (that can be subsequently shed by ADAM17) positively correlated with SARS-CoV-2 viral RNA loads (Nikiforuk et al., 2021). In this regard, catalytically inactive forms of recombinant or chemically synthesized sACE2 were shown to potently inhibit SARS-CoV-1 infection (Han et al., 2006; Moore et al., 2004), further suggesting that events downstream of ACE2 enzymatic activity and/or its shedding may be crucial in favouring the viral infection and COVID-19 development.
For the sake of completeness, several alternative SARS-CoV-2 host cell receptors/routes (such as neuropilin-1, dipeptidyl peptidase 4, CD147 and clathrin-mediated endocytosis) which may impact tropism of SARS-CoV-2 infection independently of ACE2 have been also described (Cantuti-Castelvetri et al., 2020; Li et al., 2020a; Masre et al., 2020; Puray-Chavez et al., 2021; Radzikowska et al., 2020). These observations suggest that there are additional factors that may participate in COVID-19 progression, which may not be solely reconciled by evaluating ADAM17/ACE2 activities. However, the actual involvement of these pathways in vivo and consequently in COVID-19 pathogenesis has to be confirmed. A further degree of complexity on the impact of SARS-CoV-2 tropism/infection comes from the observation that ACE2-expressing exosomes are released/present in plasma samples (El-Shennawy et al., 2020), raising the possibility of an exosome-mediated intercellular ACE2 transfer. A possible further mechanism (never been documented so far) that might not only make ACE2 negative recipient cells susceptible to virus entry (Hassanpour et al., 2020) but also increase ACE2 systemic activity; a scenario that would deserve an investigation.
Therefore, after SARS-CoV-2 challenge, ACE2-positive cells can release soluble (and possibly exosomal) forms of ACE2, which may mediate either virus import into several tissues (even ACE2-negative ones) or systemic ACE2 gain of function. It is therefore proposed that high circulating level of ACE2 cannot be considered a mere biomarker of COVID-19 or other diseases characterised by increased plasma ACE2 protein/activity, rather it represents an active (pre-existing) factor with a crucial role in determining the pathological conditions and the probability to develop severe COVID-19. However, the above-described “converging” evidence raises the question whether an excessive ACE2 activity can lead to manifestations compatible with the complex pattern of COVID-19 disease, such as inflammation, thrombosis, cardiac dysfunction and respiratory syndrome.
3. Pathological effects of ACE2 pathway hyperactivity and RAS-mediated positive feed-back loops triggered by SARS-CoV-2 infection
The renin-angiotensin system (RAS) is composed of several angiotensin peptides with pleiotropic and interconnected activity, whose biogenesis is mediated by three main enzymes: renin and two zinc metalloproteases, angiotensin converting enzyme (ACE) and ACE2. Renin is secreted in the blood by kidney pericytes of the afferent arterioles of the juxtaglomerular apparatus in response to various stimuli, such as low Na+ concentration and hypotension (Patel et al., 2017). In the plasma, renin processes hepatic angiotensinogen to generate angiotensin (Ang) I, which is further transformed by endothelial ACE into Ang II (Hunyady and Catt, 2006; Patel et al., 2017). Finally, ACE2 can transform Ang I and II into Ang (1–9) and Ang (1–7), respectively (Jia, 2016; Kuba et al., 2010; Magalhaes et al., 2018; Ocaranza et al., 2020; Ocaranza and Jalil, 2012; Passos-Silva et al., 2013; Santos et al., 2013; Sharma et al., 2019; Turner, 2015; Xu et al., 2011; Zamai, 2020a). Ang II exerts its activity by binding to AT1R that produces vasoconstriction and stimulates aldosterone and antidiuretic hormone secretion respectively by zona glomerulosa cells of the adrenal cortex and hypothalamic-neurohypophyseal axis, finally leading to sodium/water retention and hypertension. It is well known that excessive Ang II/AT1R pathway activation induces not only hypertension but also inflammation, hypertrophy, thrombosis and fibrosis that lead to dysfunction of several organs (Hunyady and Catt, 2006; Walters et al., 2017). Instead, Ang (1–7) and Ang (1–9) peptides binding to MasR and AT2R, respectively, induce endothelium-dependent NO-mediated effects and oppose the Ang II/AT1R pathway, exerting hypotensive, anti-inflammatory, antithrombotic and antiproliferative/apoptotic activities (Jia, 2016; Kuba et al., 2010; Magalhaes et al., 2018; Ocaranza et al., 2020; Ocaranza and Jalil, 2012; Passos-Silva et al., 2013; Santos et al., 2013; Sharma et al., 2019; Turner, 2015; Xu et al., 2011; Zamai, 2020a). Several studies report the beneficial effects of ACE2 pathway activation in models in which the ACE pathway is upregulated, therefore balancing an unbalanced situation. However, there is a range of normality for every biological parameter, and this is likely true for ACE2 as well. In this regard, some experimental models in the context of ACE2 hyperactivity and/or Ang (1–7) infusion have also been associated to pathological conditions involving heart, liver, intestine, lung and coagulation and these studies were already extensively described in previous works, for details see (Zamai, 2020a, 2021). Intriguingly, some of the pathological effects induced by excessive activation of the ACE2/Ang (1–7)/MasR pathway resemble those produced by hyperactivation of the Ang II/AT1R pathway, making it difficult to understand what the original cause and the cause-effect relationship of the phenomenon are. Nevertheless, it was suggested that the establishment of viral-independent positive feedback loops sustaining hyperactivation of both arms of RAS were responsible for the critical stages of COVID-19 disease (Zamai, 2020a, 2021) (see Fig. 1). Notably, increase of circulating sACE2 activity depends on an increased ACE2 cell “hypertranscription”, synthesis and consequently ACE2 surface membrane expression, which leads to more ACE2 receptors available for SARS-CoV-2 infection.
The sequential activation of different key actors downstream of ACE2 hyperactivity that leads to manifestations consistent with COVID-19 has been already disclosed (see (Zamai, 2021; 2020a)). In particular, in some predisposed individuals (with high basal activity of circulating ACE2, a prognostic marker), ACE2-induced excessive and persistent anti-inflammatory cytokines and hypotension (early disease phase) have been proposed to subsequently induce a compensatory upregulation of renin and ACE (antagonist) zinc-metalloprotease, leading to inflammatory cytokine secretion and systemic thrombosis/hypoxia (late disease phase) that further induce ACE2 synthesis/upregulation (final disease phase). Notably, this hypothesis was practically confirmed by a recent work describing the plasma concentrations of sACE2, Ang (1–7) and Ang II (Reindl-Schwaighofer et al., 2021), in which it was reported that both Ang II and Ang (1–7) [~1.0 pmol/L in healthy individuals (Lawrence et al., 1990)] concentrations were significantly higher in hospitalized patients with severe versus mild COVID-19. Interestingly, plasma levels of Ang II in mild COVID-19 patients (early 0–3 days of hospitalisation, median 47.7 pmol/L, interquartile range, IQR, 16.3–131.7 and late 9–11 days, median 32.9 pmol/L, IQR, 9.3–78.2 pmol/L) were basically lower than healthy subjects [median 147 pmol/L, IQR, 67–227 pmol/L (Kovarik et al., 2015)], suggesting an increased ACE2-mediated metabolisation of Ang II that is what is expected during the first phase of COVID-19. Differently, in severe COVID-19, plasma concentrations of Ang II have been shown to be significantly higher than healthy controls (see (Liu et al., 2020b)), and they were particularly higher during the initial days of hospitalisation as compared with those observed during late days [median 165.7 pmol/L, IQR, 61.2–680.6 vs median 95.7, IQR, 37.5–250.0 (Reindl-Schwaighofer et al., 2021),], suggesting that during the early days of hospitalisation, severe COVID-19 patients had a disease progression characterised by upregulation of both renin and ACE (a feature of the second dangerous phase of COVID-19). Indeed, COVID-19 patients (compared with healthy individuals) initially had lower levels of the soluble form of ACE, which significantly increase at follow-up (four months after the first test) (Lundström et al., 2021). Therefore, the reduction of Ang II levels observed during the late days of hospitalisation may be consistent with a further increase of ACE2 activity (a feature of the final phase of COVID-19). In line with this hypothesis, in patients with severe COVID-19, sACE2, Ang (1–7) and Ang (1–7)/Ang II ratio increased over time, indicating a progressive increase of sACE2-mediated formation of Ang (1–7) (early 0–3 days of hospitalisation, median 10.8 pmol/L, IQR, 5.1–50.7 and late 9–11 days, median 49.8 pmol/L, IQR, 14.6–132.0 pmol/L), which reached peak values similar to those observed in acute respiratory distress syndrome (Khan et al., 2017; Reddy et al., 2019; see Zamai, 2020a) during the “final” and worse phase of the disease (days 9–11 of hospitalisation) (Reindl-Schwaighofer et al., 2021). Nevertheless, in this report, ACE2 protein levels were similar between influenza and mild COVID-19 patients. In this regard, ACE2 can be upregulated during inflammatory phase of viral infections, such as human influenza, because it is an interferon stimulated gene (Ziegler et al., 2020), thus accounting for the increase of circulating ACE2 protein/activity in influenza infection as compared with healthy subjects (in which ACE2 activity is usually low to undetectable (Patel et al., 2021; Lew et al., 2008). Moreover, there was no a clear correlation between increased circulating ACE2 protein and Ang (1–7) levels (Reindl-Schwaighofer et al., 2021). For example, in non-severe COVID-19 patients, Ang (1–7) levels were similar in the early and late phases despite circulating ACE2 is more than 2-fold higher at the late (virus eradicating) phase. Moreover, despite ACE2 levels were similar between patients with late phase non-severe COVID-19 and those with influenza, Ang (1–7) was at significant higher levels in patients with influenza. Similarly, there was no significant difference between ACE2 protein levels in the plasma of COVID-19 positive and negative patients (Kragstrup et al., 2021). In this regard, it has been already highlighted (see (Zamai, 2020a)) that in order to draw conclusions from blood sample analyses of circulating levels of ACE2 protein, substrates, products and activity, it is important to consider that:
-
(1)
circulating S1-sACE2 and SARS-CoV-2-sACE2 complexes (that still maintain ACE2 enzymatic activity), which are formed by viral-induced ACE2 shedding, might not be detectable by anti-ACE2 antibodies in ELISA because the complexes may prevent sACE2 antibody sandwich/recognition. Therefore, differently from normal or other pathologic conditions (e.g. influenza, cardiopathies, hypertension, diabetes), sACE2 protein detection by ELISA (and its real protein concentration) in plasma samples of COVID-19 patients might not be reliable because its detection is affected by systemic viral loads. In this regard, late phases of COVID-19 are expected to have lower viral loads either because of the recovery from COVID-19 in mild infection or because severe forms of COVID-19 are later sustained independently of SARS-CoV-2 infection; consequently, the observed increase of ACE2 protein in late COVID-19 phases (see (Reindl-Schwaighofer et al., 2021)) could be, at least in part, apparent;
-
(2)
the evaluation of circulating ACE2 activity using fluorogenic substrates can be different because it can be determined using different substrates and by two different methods: directly on untouched plasma samples (thus with the actual plasma Zn2+ levels and with the endogenous ACE2 inhibitor present in plasma (Lew et al., 2008)) or by adding ZnCl2 (which is able to induce ACE2 maximal activity (Anguiano et al., 2015)). Moreover, part of sACE2 (and ACE2-expressing exosomes) in plasma might bind to recipient cells, thus reducing circulating ACE2 protein; however, it may maintain its “systemic” activity;
-
(3)
circulating concentrations ACE2 products (e.g. Ang (1–7) and Ang (1–9)) depend not only on ACE2 enzymatic activities but also on availability of substrates (which are affected by renin and ACE activities) and on the level of expression of the respective receptors (e.g. AT2R and MasR) that, by binding ACE2 products, remove them away from circulation.
For example, plasma Ang (1-7) concentration is dependent not only on ACE2 enzymatic activity but also on both amount of the (antagonistic) Ang II (and its precursor, Ang I) and on MasR expression on surface of cells in the organs. In the light of these considerations, the recent reports determining plasma ACE2 activity by fluorimetric evaluation, which showed a marked and progressive increase of circulating ACE2 activity that correlates with COVID-19 severity (Nagy et al., 2021; Patel et al., 2021), clearly indicate the crucial role of ACE2 systemic activity in COVID-19. In line with this hypothesis, a recent report showed that SARS-CoV-2 trimeric spike protein significantly increased ACE2 enzymatic activity and that the enhancement was mediated by the binding of SARS-CoV-2 spike RBD domain (Lu and Sun, 2020). Moreover, SARS-CoV-2 spike protein binding has been shown to selectively accelerate substrate-specific catalytic activity of ACE2 and, in particular, the cleavage of des-Arg(9)-bradykinin (leading to an inactive bradykinin product) and Ang I (to Ang (1–9) product) substrate analogs, while angiotensin II analog cleavage was minimally affected by the binding of spike protein (Kiseleva et al., 2021). In this regard, the detection of inactive products of ACE2 activity, such as that derives from des-Arg(9)-bradykinin, could be a more reliable marker than Ang (1–7) to evaluate ACE2 systemic activity. Moreover, data obtained by adding human recombinant ACE2 ex vivo in plasma samples (thus mimicking an acute increase of systemic ACE2 activity (Ye et al., 2012), showed an expected strong reduction of Ang II, a moderate reduction of Ang I and a significant increase of Ang (1–9), Ang (1–7) and its ACE-induced product, Ang (1–5) (Ye et al., 2012), suggesting that Ang (1–5) might be an interesting surrogate marker of ACE-ACE2 combined activity in severe forms of COVID-19.
Sequential anti-inflammatory/proinflammatory pathway activation. Based on the above-described pathophysiological mechanism, SARS-CoV-2 would initially induce excessive and persistent anti-inflammatory, hypotensive and antithrombotic activities, which can subsequently drive rebound compensatory responses, leading to increase of the antagonist RAS pathway. As an example, longitudinal studies of cytokine production reveal that immune inhibitory cytokines (such as IL-10 and IL-1 receptor antagonist) are upregulated earlier than inflammatory cytokines (such as IL-6) in plasma of severe COVID-19 patients (Zhao et al., 2020). Differently from inflammatory cytokines, IL-10 is associated with a reduced immune proliferation and response (Schülke, 2018) and consistent with eosinopaenia and lymphopaenia, both early markers of COVID-19 (Zamai, 2020a, 2021). In this regard, IL-10 has been shown to antagonise type I interferons (IFN–I) signalling by suppressing phosphorylation of STAT1 (Ito et al., 1999), which is responsible for the transcription of IFN-I genes, one of the major innate immune mechanisms against viral pathogens. Indeed, IFN-I gene transcription is inhibited by SARS-CoV-2 infection (Blanco-Melo et al., 2020), suggesting that delayed inflammatory signalling could be mediated by early production of IL-10. Notably, IL-10 secretion can be induced by compounds that mimic Ang (1–7)/MasR or Ang (1–9)/AT2R pathways in mouse models (Fatima et al., 2021; Rodrigues-Machado et al., 2013), thus suggesting a link between ACE2 activity and (downstream) IL-10 secretion. Among the major producers of IL-10, myeloid-derived suppressor cells (MDSCs) massively expand during the early phase of severe COVID-19 (Agrati et al., 2020), suggesting that early increase of IL-10 could be related to their expansion. In this regard, RAS has been shown to play an important role in myelopoiesis (Lin et al., 2011), raising the possibility of a possible link between RAS imbalance and massive expansion of MDSC. Indeed, ACE-deficient mice had an increased number of cells with a MDSC phenotype (Lin et al., 2011), while a mouse bone marrow cell line overexpressing ACE showed a reduced MDSC production (Shen et al., 2014); findings that are consistent with the possibility that a relative increase of ACE2/ACE activity ratio might contribute to early expansion of IL-10-producing MDSCs during COVID-19 disease. However, chronic upregulation of IL-10 has also been shown to contribute to inflammatory diseases (Lauw et al., 2000; Lu et al., 2021; Yaseen et al., 2020), thus suggesting that an initial and persistent secretion of immunosuppressive IL-10 could trigger a subsequent compensatory inflammatory response, as it may occur in the late phase of severe COVID-19. In line with this hypothesis, in COVID-19 patients, sACE (but not sACE2), which is initially lower than healthy individuals, has been shown to significantly increase with time and to display positive correlations with IL-6 and TNF-α inflammatory cytokines (Lundström et al., 2021). In this regard, the so called cytokine storm is mediated by increased levels of several proinflammatory cytokines, among which TNF-α and IL-1β have been shown to induce ACE2 synthesis and/or shedding (Clarke et al., 2014; Jia et al., 2009) that further increase sACE2 systemic release and activity, which, in this case, are independent of SARS-CoV-2 binding. Along this line of thinking, since ACE2 is an interferon stimulated gene (Ziegler et al., 2020), ACE2 could be further upregulated independently of SARS-CoV-2 during late inflammatory phase of severe disease (Ziegler et al., 2020). However, the increase in ACE2 expression may also be a non-specific response to an initial inflammatory milieu (as it may occur during human influenza infection (Reindl-Schwaighofer et al., 2021), rather than a COVID-19 specific associated condition. For example, the increased expression of circulating ACE2 during influenza infection (Reindl-Schwaighofer et al., 2021) may suggest that endothelial cells express interferon-inducible membrane bound ACE2 that is subsequently shed. Similarly, induction of membrane ACE2 expression by SARS-CoV-2 infection in a subpopulation of epithelial ciliated epithelia has been associated with IFN secretion by immune cells (Chua et al., 2020) and this evidence may correlate with cough, one of the most common early symptoms of COVID-19 (Docherty et al., 2020). Nevertheless, differently form influenza, SARS-CoV-2 induces early IL-10 secretion (Zhao et al., 2020) that might inhibit interferon-inducible gene expression (Ito et al., 1999; Blanco-Melo et al., 2020), such as ACE2, and “runny nose” is an uncommon early symptom of SARS-CoV-2 infection that has a lower frequency than gastrointestinal symptoms (Docherty et al., 2020). In this regard, recent studies suggest that full-length ACE2 expression that is independent on IFN-responsive elements is preferentially transcribed in the gastrointestinal tract, while interferons and viruses do not induce the full-length ACE2 receptor in the upper aerodigestive tract but rather a novel truncated and unstable ACE2 isoform, lacking domains required for SARS-CoV-2 binding and peptidase activity (Ng et al., 2020; Onabajo et al., 2020). It is therefore unlikely that this isoform, lacking part of the extracellular ACE2 domain, could contribute to SARS-CoV-2 infection through membrane bound ACE2 (or indirectly via ACE2 enzymatic hyperactivity) at least in the upper aerodigestive track, rather it might protect from SARS-CoV-2 infection and/or from ACE2 hyperactivity. The discovery of this tissue-specific new isoform of ACE2 that is generated by co-option of intronic retroelements as promoter and alternative exon, indicates a further degree of tissue-specific adaptation of both regulatory (IFN-inducible elements) and protein-coding gene isoforms of ACE2. In this regard, it has been recently described how the majority of protein-coding and regulatory gene sequences can be “edited” by non-random intronic retrotransposon-mediated mechanisms (resembling that of immunoglobulin somatic hypermutation) and non-random retrotransposon transposition, respectively (see (Zamai, 2020b)). In particular, this occurs when genes are transcribed at their maximum rate (“hyper-transcribed”) and yet still are unable to meet environmental demands, i.e., their protein products are inadequate. Through these non-random mechanisms, new coding and regulatory sequences can incidentally generate more adequate and functional proteins (Zamai, 2020b). However, the majority of genetic attempts can generate dysfunctional proteins and/or proteins that can be recognised as extraneous (non-self) by the immune system, ultimately leading to autoimmunity (Zamai, 2020b). It is now emerging that during late phases of severe SARS-CoV-2 infection can appear various autoimmune and inflammatory diseases (see (Casciola-Rosen et al., 2020; Zamai, 2021)). It is tempting to speculate that, during COVID-19, hypertranscription of ACE2 may induce the generation of several new gene attempts encoding non-functional ACE2 proteins, which can be sensed as non-self by the immune system. Indeed, anti-ACE2 non-neutralising (IgM) auto-antibodies have been recently associated with autoimmunity and severe inflammatory complications in some COVID-19 patients (Casciola-Rosen et al., 2020). In this regard, it was recently proposed that anti-ACE2 non-neutralising (IgM) auto-antibodies may mimic SARS-CoV-2 virions by inducing membrane ACE2 shedding and thus sustaining the systemic sACE2 enzymatic activity even in the absence of viral load (Zamai, 2021).
Sequential hypotensive/hypertensive pathway activation. Similarly to the anti-inflammatory activities, initial hypotension in SARS-CoV patients (Yu et al., 2006) is likely induced by both ACE2-mediated Ang II hydrolysis and hypotensive activity of both Ang (1–7)/MasR and/or Ang (1–9) axes (see (Ocaranza et al., 2020; Santos et al., 2013; Xu et al., 2011; Zamai, 2020a)). Indeed, although the ACE2 enzymatic activity against Ang I is about 400-fold lower than that against Ang II (Vickers et al., 2002), high levels of circulating ACE2 may be able to increase concentrations of Ang (1–9), which possesses a hypotensive activity via AT2R induction of NO production and natriuresis (Ocaranza et al., 2020). These multiple hypotensive effects, if persistent, can subsequently induce upregulation of renin and ACE, and consequently increased production of the hypertensive Ang II. However, Ang II can be further processed by sACE2 excess into hypotensive Ang (1–7), thus establishing a positive feedback loop. On the other hand, Ang II/AT1R axis has been shown to increase myocardial ADAM17 expression and activity (Patel et al., 2014), thus inducing further ACE2 shedding and sACE2 systemic activity that is again independent of viral infection. Therefore, both Ang (1–7) and Ang II facilitate the systemic activity of their negative regulators (ACE and ACE2), which finally induce a progressive hyperactivity of both arms of RAS through a viral-independent positive feedback loop.
Sequential antithrombotic/prothrombotic pathway activation. Finally, also antithrombotic/prothrombotic activities may follow a dual pattern of disease progression along the different phases of severe COVID-19. Indeed, sequential activations of RAS pathways that resembles those described for anti-flammatory/proinflammatory and hypotensive/hypertensive responses can account for the complex coagulopathy pattern characterised by concomitant thrombocytopenia and thrombosis (Bhattacharjee and Banerjee, 2020). ACE2/Ang-(1–7)/MasR axis, which is likely induced early during disease progression, has been shown to have an antithrombotic effect (Fraga-Silva et al, 2008, 2010; Kucharewicz et al., 2002). For example, Ang (1–7)/MasR axis has been shown to induce an antithrombotic activity mediated by platelet release of NO (Fraga-Silva et al., 2008). In contrast, Ang II/AT1R axis, which is supposed to be upregulated at late stages of severe COVID-19, has a well-known prothrombotic activity mediated by either reduction of fibrinolytic activity or induction of microvascular thrombosis by promoting thrombin generation (Mogielnicki et al., 2005; Senchenkova et al., 2010; Vaughan et al., 1995). However, in rat models of thrombosis, even Ang (1–9) has been shown to induce both arterial thrombotic platelet aggregation and impairment of fibrinolysis (enhancing venous thrombosis) via Ang II/AT1R axis (Kramkowski et al., 2010; Mogielnicki et al., 2014), thus suggesting that ACE2-produced Ang (1–9) could synergistically work with Ang II in inducing thrombotic/ischemic processes at late and severe stages of COVID-19. In addition, recent reports (Abassi et al., 2021; Zhang et al., 2020b), showing that ACE2 is expressed by platelets, suggest that SARS-CoV-2 virions can bind platelets, finally inducing platelet activation that may participate not only in thrombus formation but also in systemic sACE2 release.
RAS-mediated liver dysfunction promoting deficit of anticoagulant proteins and/or elevation of procoagulant factors (e.g., C-reactive protein) was also recently proposed to be relevant for thrombotic ischemic events (Zamai, 2021). Moreover, reduced level of hepatic albumin (the major transporter of zinc ions in plasma (Coverdale et al., 2019) was suggested to increase availability of free zinc ions (see (Zamai, 2021; 2020a), which have been shown to promote platelet-dependent fibrin formation (Kiran Gotru et al., 2019), thus revealing a procoagulant role of zinc ions and conversely an “anticoagulant” activity of albumin. Intriguingly, chloroquine has been shown to both enhance zinc uptake in the lysosomes (Xue et al., 2014) and inhibit platelet activation and their zinc ion release (Belizna et al., 2018), raising the possibility that in early phase of COVID-19, chloroquine might act reducing circulating zinc availability and zinc systemic functions. Similarly, ivermectin has been indicated to have the ability to both increase prothrombin time by disrupting vitamin K–dependent clotting factors (Gilbert and Slechta, 2018) and bind metal cations, thus suggesting a possible antithrombotic mechanism of action for this drug against COVID-19, which might be mediated by cation sequestration (Rizzo, 2020).
RAS activation under hypoxic conditions consequent to systemic thrombosis. When pulmonary circulation is involved in the thrombotic complications, this event causes severe acute respiratory syndrome (SARS) and hypoxemia, which is known to upregulate both arms of the RAS including renin, ACE and ACE2 syntheses and secretion/shedding in different tissues (Burrell et al., 2005; Clarke et al., 2014; Hampl et al., 2015; Joshi et al., 2019; Kramer et al., 1998; Paizis et al., 2005; Zamai, 2020a; Zhang et al., 2009), what seems to happen during the late (hospitalized) phase of COVID-19 (Reindl-Schwaighofer et al., 2021). Hypoxic conditions, in turn, would induce further ACE2 cell membrane expression and consequently an increased probability of both SARS-CoV-2 infection and ACE2 shedding/systemic activity, finally generating positive feedback loops that sustain COVID-19. In this regard, since ACE2 is undetectable or transcribed at low levels in normal lung epithelium (Carithers and Moore, 2015), it is possible that the lung microvasculature of severe patients could be infected late after hypoxic induction of ACE2 expression (Garvin et al., 2020). Interestingly, chronic hypoxia induced a relative increase of ACE2-MasR/ACE-AT1R activity ratio in lungs of pulmonary hypertensive (Ren-2) transgenic rats (expressing higher baseline levels of renin) but not in normotensive transgene-negative control rats (Hampl et al., 2015), suggesting that renin inhibition might be useful to inhibit not only both arms of RAS but also the relative increase of ACE2 pathway activity, which may occur in the final phase (characterised by severe hypoxic conditions) during COVID-19 progression (Reindl-Schwaighofer et al., 2021). Moreover, in myocardial ischemia/reperfusion-induced injury, Ang (1–7) has been shown to exhibit cardioprotective effects, which were inhibited by indomethacin, a cyclooxygenase (COX) inhibitor (Liao et al., 2011), thus suggesting that COX activation is downstream of ACE2/Ang (1–7)/MasR pathway and accounting for protective activity of COX inhibitors when administered early during the course of SARS-CoV-2 infection (Suter et al., 2021).
Possible new antithrombotic strategies and similarities between COVID-19 early phase and vaccine-induced mild adverse effects. Notably, the occurrence of thrombotic events in severe COVID-19 patients despite high heparin doses indicates the need to search for a better antithrombotic strategy (Longhitano et al., 2020; Zanza et al., 2021). In this regard, in a mouse model of microvascular thrombosis, Ang II-induced blood flow cessation was not inhibited by heparin pre-treatment; differently, antithrombin III pre-treatment was highly effective in preventing the acceleration of blood flow cessation elicited by Ang II infusion (Senchenkova et al., 2010). Therefore, this last antithrombotic strategy might prove more effective than heparin in treatment of the late phase of COVID-19, when both arms of the RAS are activated increasing both Ang II and the risk for thrombus formation. Moreover, administration of arginine (the NO precursor) has also been proposed to recover from coagulopathy in severe fever associated with thrombocytopenia syndrome (Li et al., 2018). In this syndrome, expansion of MDSCs, which are able not only to secrete anti-inflammatory cytokines but also to express arginase, was associated with decreased NO concentration in platelet, leading to platelet hyperactivation and thrombocytopenia (Li et al., 2018); curiously, both of which can occur either following SARS-CoV-2 infection (Bhattacharjee and Banerjee, 2020; Zanza et al., 2021) or, in some rare cases, after vaccination (Lee et al., 2021; Tobaiqy et al., 2021). Indeed, despite the current vaccines do not contain the inactivated or attenuated virus, but only the nucleic acid sequence of the SARS-CoV-2 spike glycoprotein (a small fragment of the entire viral sequence) (Kyriakidis et al., 2021), SARS-CoV-2 vaccinated people may exhibit either mild to moderate COVID-19-like symptoms or, rarely, severe thrombotic-related adverse reactions (Lee et al., 2021; Tobaiqy et al., 2021). Notably, the envelope of the vaccine determines the cells in which the genetic content is inserted and expressed (the vaccine tropism); differently from traditional vaccines, novel spike-based vaccines can insert the spike nucleotide sequence into different cell tissues independently of their ACE2 surface expression. Moreover, spike protein expression (induced by vaccination) on the cell surface can favour not only immune protective responses but also the binding of spike-expressing cells to ACE2 cell surface receptors, which, as for binding to SARS-CoV-2 virions, can be subsequently shed from the cell surface (see Fig. 1). In the case in which spike proteins are expressed by blood cells, they might induce the release of ACE2 from the surface of endothelial cells into the systemic circulation. This possibility could lead to an increase of ACE2 systemic activity similar to that induced by SARS-CoV-2 virions during the early and mild phase of COVID-19 (see Fig. 1), thus justifying the similarities between (early-phase) COVID-19 symptoms and vaccine-mediated mild adverse effects. The lack of replication activity of vaccines likely limits the severity of adverse side reactions that usually produce symptoms similar to those induced during the early phase of COVID-19. It is therefore tempting to speculate that systemic and/or local ACE2 pathway activation might lead to both mild/moderate adverse symptoms and/or severe coagulation disorders that are similar (except in frequency) in both SARS-COV-2 infection and vaccination. However, if this is the case, systemic ACE2 protein/activity is expected to increase after vaccination and ACE2/ADAM17 inhibitors should work in protecting from severe forms of both COVID-19 and vaccine-induced adverse effects. Finally, persistent elevated activity of plasma ACE2 may be at the origin also of Long-COVID (Phetsouphanh et al., 2021), a syndrome characterised by prolonged illness lasting longer than 3 months after SARS-CoV-2 infection. However, as suggested above, both arms of RAS are likely upregulated in the late phases of severe COVID-19. In this regard, levels of follow-up sACE2 (four months after hospitalisation), but not those of sACE, correlated with several risk factors for severe COVID-19 infection, the total number of comorbidities and treatment with inhibitors (ACEIs and/or AT1RBs) of RAS pathway (Lundström et al., 2021). Unfortunately, the levels of ACE and renin activities in Long-COVID patients were not evaluated. Therefore, it is not clear whether inhibition of only ACE2 or rather of both arms of RAS might help to recover (or protect) from Long-COVID symptoms.
Although in the present work it was supposed that most of the COVID-19 symptoms may derive from Ang (1–7), Ang II and Ang (1–9) peptide upregulation, the involvement of other pathways mediated by processing of ACE2 substrates such as bradykinins, apelins, casomorphins and dynorphins (Kuba et al., 2010; Vickers et al., 2002; Garvin et al., 2020) cannot be rule out. In particular, kinin-kallikrein system which can be affected by enzymes of RAS could also be involved in COVID-19 pathophysiology.
Possible involvement of kinin-kallikrein system in COVID-19. The kinin-kallikrein system (KKS) plays a role in several physiological processes through different peptides (bradykinins), which are generated by enzymatic activation from two inactive kininogens. These two inactive proteins are the result of a tissue-specific alternative splicing, which generates the high molecular weight kininogen that is cleaved by plasma kallikrein into bradykinins (BKs) and the low molecular weight kininogen that is cleaved by tissue kallikreins into lysyl-BKs (De Maat et al., 2020; Garvin et al., 2020; Kalinska et al., 2016; Schmaier, 2003; Su, 2014; van de Veerdonk et al., 2020). The main active peptides of the plasma kallikrein-kinin system are BK and des-Arg(9)-BK. BK can activate the constitutively expressed bradykinin B2 receptor (BKB2R), which exerts hypotensive effects, while des-Arg(9)-BK can exerts inflammatory activities through the activation of the inducible BKB1R. It is well-known that there is a multilayered interaction between the RAS and plasma KKS systems, which reciprocally affect each other in regulating blood pressure, coagulation and inflammation (Schmaier, 2003; Su, 2014). For example, ACE has the bifunctional activity being able not only of converting Ang I to the biologically active Ang II but also of inactivating BK (which represents the preferred substrate over Ang I) to form an inactive bradykinin peptide. ACE2 is another enzyme shared by these two systems, being able to metabolise and inactivate des-Arg(9)-BK. Therefore, ACE and ACE2 activities can downregulate the hypotensive and inflammatory effects mediated by BKB2R and BKB1R, respectively (Schmaier, 2003; Su, 2014). On the other hand, a kallikrein, a kinin-generating enzyme, has been proposed as a prorenin-activator (Schmaier, 2003; Su, 2014). Finally, the plasma KKS and RAS systems also interact at other levels, since BK synergise with Ang (1–7) in inducing hypotensive effects and the BKB2R can form heterodimers with the AT1R (Schmaier, 2003; Su, 2014). Notably, BKB1R is hardly expressed at baseline but it is highly inducible by proinflammatory cytokines, tissue injury and anoxia (Su, 2014), which are markedly upregulated during SARS-CoV-2 infection. Moreover, activation of BKB1R mediated by attenuation of pulmonary ACE2 activity has been shown to exacerbate inflammation by inducing further release of proinflammatory cytokines and promoting lung neutrophil infiltration (Sodhi et al., 2018). For these reasons, several reports have suggested the involvement of the KKS in COVID-19 (Abassi et al., 2021; De Maat et al., 2020; Garvin et al., 2020; Tolouian et al., 2020; van de Veerdonk et al., 2020). However, most reports hypothesised an enhanced Arg(9)-BK/BKB1R signalling due to a SARS-CoV-2-induced downregulation of ACE2 activity (Abassi et al., 2021; De Maat et al., 2020; Tolouian et al., 2020). Instead, ACE2 upregulation was recently revealed; therefore, an increased inactivation of Arg(9)-BK should lead to switch off BKR1R inflammatory pathway, at least during the early phase of COVID-19. On the other hand, gene expression data from cells in bronchoalveolar lavage fluid from COVID-19 patients identified an increased expression of kininogen, many kallikrein enzymes, both bradykinin receptors, ACE2, renin, angiotensin and RAS receptors, in combination with decrement in ACE expression, suggesting that the elevate bradykinin levels could cause increases in vasodilation, vascular permeability and hypotension (Garvin et al., 2020). Indeed, a decrease of both Ang I and BK metabolisation due to downregulation of ACE would produce a relative decrease activity of Ang II/AT1R axis and a relative increase of BK/BKB2R pathway activity, both leading to hypotensive effects. Consistent with this hypothesis, in COVID-19 patients, the concentrations of the soluble form of ACE were initially lower than healthy controls (Lundström et al., 2021) and hypotension in SARS-CoV patients has been documented (Yu et al., 2006). Interestingly, inhaled BK has been reported to induce cough response via a BK/BKB2R axis. Activation of BKB2R has been proposed to stimulate the release of both COX and 12-lipoxygenase enzymes that produce metabolites, enhancing both cough and airway obstruction (Al-Shamlan and El-Hashim, 2019). These symptoms are common during the early phase of COVID-19 (Docherty et al., 2020) and therefore they might be related, at least in part, to activation of BK/BKB2R axis. Although the above observations suggest that the KKS system, as well as the RAS, might induce an anti-inflammatory and hypotensive effects during the early phase of COVID-19, compensatory effects similar to those described for the RAS during late phase of COVID-19 could also be hypothesised for the KKS system. Unfortunately, analyses of circulating levels of bradykinin peptides and plasma kallikrein activities are lacking to address this possibility. Therefore, the role of a dysregulated KKS in COVID-19 remains to be elucidated. Nevertheless, the dysregulation of RAS enzymes, suggested in the present work, and the reciprocal interactions between KKS and RAS pathways indicate that both systems may be involved in COVID-19 manifestations. For example, the contribution of plasma kallikrein to prorenin activation might participate in hyperactivity of both arms of RAS during late phase of COVID-19.
The many layers of interaction between the KKS and RAS suggest a fine tuning between these systems that contributes to maintain physiological homeostasis. In some cases, the changes in both the RAS and KKS seem to occur in the same direction. However, for other aspects, activation of one system is counterbalanced by the other system that is depressed (Schmaier, 2003; Su, 2014). In this regard, it is interestingly to note that simple cations such as free Zn2+, have been shown to inhibit kallikreins (Kalinska et al., 2016), while the same cation is known to activate the RAS pathway, indicating the importance of free Zn2+ concentrations in regulating both plasma KKS and RAS systems at a post-translational level possibly in the opposite way. This evidence adds further complexity to the regulation of RAS and KKS systems, whose activities do not depend only on their translated enzymes but also on the microenvironment in which the KKS/RAS enzymes work. In this regard, the total zinc concentration in the sera of healthy adults is mainly bound to albumin (approximately 75–90 %), while about 10–20 % bound to α2-macroglobulin (Craig et al., 1990; Foote and Delves, 1984). For this reason, there is a significant positive correlation between the serum zinc and albumin levels in the sera of healthy adults and in elderly hospital patients (Craig et al., 1990; Foote and Delves, 1984). Therefore, albumin is a “site” of Zn2+ storage in plasma, while α2-macroglobulin is known to inhibit the activity of proteinases including some kallikreins (Kalinska et al., 2016). On the other hand, labile-bound pool of Zn2+ (labile complexes, e.g., with AA) and free Zn2+ (present at low nanomolar concentrations) are the bioavailable forms of zinc in blood for (endothelial) cell internalization and/or enzymatic activities (Coverdale et al., 2019). Therefore, albumin, by binding most of the Zn2+ in plasma, reduces its bioavailability to endothelial cells. However, COVID-19 patients suffer from hypoalbuminaemia (Ramadori, 2020; Zamai, 2020a), which can lead to both reduction of total plasma Zn2+ concentrations and increase of bioavailable forms of plasma Zn2+, which, imported into endothelial cells, can ultimately sustain the upregulation of zinc-dependent RAS functions induced by SARS-CoV-2 infection. In this regard, the SARS-CoV-2-mediated increase of circulating sACE2, which is a zinc-binding enzyme, is expected to partially compensate the zinc reduction due to hypoalbuminemia. For this reason, we are now evaluating whether the ratio between plasma zinc and albumin could be used as a surrogate marker of circulating ACE2 and possibly as a prognostic marker of severe forms of COVD-19. Preliminary data indicate a significant increase of Zn/albumin ratio in plasma of COVID-19 patients as compared with healthy subjects (manuscript in preparation), suggesting that the disease induces a relative increase of albumin-unbound forms of zinc, such as free/bioavailable Zn2+ and/or Zn2+ bound to ACE2.
Altogether the above observations suggest how SARS-CoV-2 infection may trigger several different positive feedback loops downstream of ACE2 shedding/activity (see also (Zamai, 2021; 2020a)), which sustain viral-independent COVID-19 symptoms and may justify the lack of correlation between SARS-CoV-2 viral loads and COVID-19 symptoms (Amiral, 2020; Casciola-Rosen et al., 2020; Huang et al., 2020; Lavezzo et al., 2020). Moreover, the same aetiological agent, SARS-CoV-2, can produce a variety of different clinical manifestations, which, therefore, mainly depend on the subject's predisposition. Interestingly, COVID-19 symptom progression consists of two main phases consistent with the sequential steps described above: an early “latency” (slow disease development) and a late severe and sudden progression of the disease. Indeed, the initial phase is commonly characterised by mild/moderate symptoms in most of the infected individuals (possibly corresponding to viral infection and ACE2 upregulation) and it is followed by either a progressive disease resolution or a second phase characterised by sudden worsening of symptoms, usually in comorbid patients characterised by high basal ACE2 activity (possibly concomitant to upregulation of both arms of RAS).
Based on the above observations, in order to block the RAS-induced positive feedback loops, a COVID-19-phase-specific inhibition the RAS pathways with different pharmacological drugs can be hypothesised and pursued alone or in combination. This approach will be proposed and discussed in the following section.
4. COVID-19-phase-specific inhibition of RAS pathways, as possible targets for COVID-19 treatment
In the present work, it has been proposed that COVID-19 can be distinguished in three different phases: early, late and final. During the early “latent” phase of COVID-19, SARS-CoV-2 acts as a “pharmacological” activator of both ADAM17 and ACE2 zinc-metalloproteases, which ultimately increases ACE2 systemic activity, thus unbalancing the RAS toward the alternative ACE2 axis. In some individuals with high baseline levels of ACE2 activity (prognostic marker), a compensatory phase subsequent to persistent systemic ACE2 activation characterised by renin and ACE upregulation is supposed to occur and lead to severe forms of COVID-19. A third final phase, during which ACE2 activity is further upregulated, is also suggested by increase of circulating Ang (1–7)/Ang II ratio at day 9–11 of hospitalisation (Reindl-Schwaighofer et al., 2021).
Based on this phase distinction, it is possible to propose different combinations of existing inhibitors (drug repurposing) specific for one or both arms of RAS pathways in a COVID-19 phase-specific manner, as COVID-19 therapies.
Inhibition of specific RAS pathways during the early phase of COVID-19. In the early phase of COVID-19 or after vaccination, ADAM17 and ACE2 are likely the enzymes that are directly activated by spike SARS-CoV-2 protein binding. Specific inhibitors that target these two zinc-metalloproteases are expected to prevent at the origin the “pharmacological” ACE2 activation induced by spike SARS-CoV-2 proteins and therefore to work during the initial phase of COVID-19 and possibly against adverse effects induced by vaccines. The hypothesis that vaccination may induce SARS-CoV-like pathways/symptoms, if confirmed, might allow to prevent vaccine-induced side effects by a pre-treatment with RAS inhibitors, just before a scheduled vaccination. A more “safe” vaccination would hopefully lead to a higher compliance and less vaccine hesitancy, in particular in elderly and more vulnerable people for which risk/benefit balance is more favourable and opportune (Zamai and Rocchi, 2021).
Inhibition of ADAM17 zinc-metalloprotease activity. The pharmacological inhibition of ADAM17 responsible of ACE2 shedding is expected to prevent the establishment of the positive feedback loops induced downstream of systemic ACE2 enzyme hyperactivity. The use of ADAM17 inhibitors (ADAM17Is) has been already proposed against SARS-CoV-1 (Haga et al., 2010) and recently against SARS-CoV-2 infection (Yeung et al., 2021; Zamai, 2020a, 2021; Palau et al., 2020). In this regard, the targeting of ADAM17 activity has been considered especially in the context of cancer and inflammatory diseases (Calligaris et al., 2021), and in cancer therapy has been shown to have low toxicity (Bandsma et al., 2015; Blaydon et al., 2011); therefore, administration of ADAM17Is might be a safe and effective treatment against COVID-19. In fact, although ADAM17Is are expected to increase ACE2 membrane expression and therefore the probability of viral binding (and possibly viral entry) to ACE2-positive cells, the inhibition of ACE2 release in the systemic circulation during the early phase of COVID-19 should be sufficient to limit upregulation of pathways downstream of systemic ACE2 activity and therefore the development of severe COVID-19. Among the pathways activated by ACE2, the ADAM17Is should be able to inhibit the IL-10-mediated immune suppression, thus allowing the re-establishment of the correct immune responses, which are expected to be effective against SARS-CoV-2 as well as against HCoV-NL63 infection.
MLN4760 and Dx600 ACE2 Inhibitors. MLN-4760 and DX600 are both potent and selective human ACE2 inhibitors (ACE2Is) (Dales et al., 2002; Joshi et al., 2016; Ye et al., 2012; Zamai, 2020a). MLN-4760 is able to strongly inhibit rhACE2 activity (near maximal inhibition at 10−8 M), preventing rhACE2-mediated Ang II metabolisation, whereas DX600 inhibits rhACE2 at relatively higher concentrations ((Ye et al., 2012) see (Zamai, 2020a)). Both inhibitors are more effective in inhibiting soluble forms of ACE2 than membrane-bound ones expressed on human mononuclear or CD34+ cells (Joshi et al., 2016). Moreover, MLN-4760 inhibitor has been shown to retain its inhibitory effects on sACE2 bound to spike proteins (Li et al., 2005). Therefore, these inhibitors at opportune (low) concentrations are expected to preserve local, membrane-associated ACE2 activity, while preferentially reducing systemic sACE2 activity induced by SARS-COV-2 binding. However, MLN-4760 inhibitor has been shown to induce the closed ACE2 conformation (Towler et al., 2004), which seems to be the preferential conformer for virus binding (Hadi-Alijanvand and Rouhani, 2020; Yan et al., 2020; Zamai, 2021). For this reason, MLN-4760 is expected to not prevent viral entry; nevertheless, its inhibitory activity on sACE2 is expected to impair the establishment of positive feedback loops, ultimately allowing a quick eradication of SARS-CoV-2, as it happens in HCoV-NL63 infection. However, the inhibition of Ang II metabolisation by ACE2 could lead to an increase of blood pressure, as a potential risk. Nevertheless, the data from chronic administration of MLN-4760 do not reveal significant adverse effects or mortality in several rodent experimental models in vivo (Byrnes et al., 2009; Evans et al., 2020; Kim et al., 2010; Shenoy et al., 2013; Tikellis et al., 2008; Trask et al., 2010) nor in a clinical Phase I trial in humans (http://oreholdings.com/wp-content/uploads/2013/06/09.10.09-425.pdf). Similarly, chronic administration of Dx600 inhibitor in mice suggests that its use is safe (Sodhi et al., 2018). Therefore, data indicate that chronic inhibition of systemic ACE2 activity is safe, and this is in line with the observation that in healthy individuals, circulating ACE2 activity is usually low to undetectable due to an endogenous ACE2I (Lew et al., 2008). However, in a diabetic mouse model, chronic administration of MLN-4760 led to increase of ACE, albuminuria and glomerular injury, indicating a possible adverse effect of ACE2 inhibition in a diabetic background (Soler et al., 2007). On the other hand, a report showed data consistent with a protective role of a prolonged ACE2 inhibition in post-myocardial infarction (Kim et al., 2010), see also (Zamai, 2020a). Therefore, inhibition of ACE2 activity using MLN-4760 might be helpful not only for COVID-19 but also for other chronic pathologies associated with a persistent ACE2 hyperactivity, such as cardiovascular diseases, hypertension, metabolic syndrome, and inflammatory bowel disease (Anguiano et al., 2015; Epelman et al., 2009; Garg et al., 2015; Kornilov et al., 2020; Narula et al., 2020; Ortiz-Pérez et al., 2013; Ramchand et al., 2018; Sama et al., 2020; Soro-Paavonen et al., 2012; Úri et al., 2014, 2016; Walters et al., 2017). As an example, inhibition of ACE2 by GL1001 (an old name of MLN-4760) surprisingly showed to produce anti-inflammatory activity in a colitis mouse model (Byrnes et al., 2009), further suggesting that the well-known anti-inflammatory and protective activity of ACE2 in an acute phase of disease may lead to deleterious health effects when ACE2 is chronically activated. Unfortunately, a Phase Ib/IIa clinical trial using oral capsules (300 mg of ORE1001, the “clinical” name of MLN-4760) was abandoned (https://clinicaltrials.gov/ct2/show/NCT01039597). For further details (safety concerns, administration routes and dosages) on these two ACE2-specific inhibitors, see (Zamai, 2020a).
Inhibition of pathways downstream of Ang (1–7)/MasR and/or Ang (1–9)/AT2R axes. Inhibition of specific pathways downstream of ACE2 activity, such as Ang (1–7)/MasR and/or Ang (1–9)/AT2R pathways, can also be pursued through specific MasR (MasRBs) and/or AT2R blockers/antagonists [AT2RBs, e.g. PD12319, (Senchenkova et al., 2010; Simões E Silva et al., 2013; Xu et al., 2011), see Fig. 1]. However, Ang (1–7)/MasR axis, the main pathway activated downstream of ACE2, is composed of, at least, two distinct MasR subtypes. In fact, D-Ala7-Ang-(1–7) (also known as A779), and D-Pro7-Ang-(1–7), two distinct MasRBs, have been shown to have differential ability to fully prevent some biological actions of Ang-(1–7) (Simões E Silva et al., 2013; Xu et al., 2011). Therefore, in order to inhibit Ang (1–7)/MasR pathway, MasRBs should be administered in combinations. In rodent models, MasRBs has been shown to be safe in acute and chronic in vivo studies (for further details see (Zamai, 2020a), Box 3); however, there are no data on administration in humans, therefore their administration in COVID-19 patients is not actionable at the moment. Finally, differently from ACE2Is, these pathway-specific inhibitors may only partially work against COVID-19, since they do not inhibit the pathways affected by other ACE2 substrates/products, such as some bradykinins, apelins, casomorphins and dynorphins (Garvin et al., 2020; Kuba et al., 2010; Vickers et al., 2002).
Inhibition of ADAM-17 and ACE2 zinc-metalloproteases by metal chelating agents. Based on zinc-metalloprotease mechanism of action, there are alternative ways to impair ADAM-17 and ACE2 hyperactivity, for example by interfering with their zinc-dependent catalytic activity and/or limiting bioavailability of zinc ions. Cells of COVID-19 patients need high amounts of bioavailable Zn2+ in order to synthesise high amounts of functional ACE2 and ADAM17 (and ACE) zinc-metalloproteases. Since zinc-metalloproteases are activated by high concentrations of free Zn2+ (Anguiano et al., 2015; Towler et al., 2004), bioavailable plasma Zn2+ acts as “fuel” for ADAM17, ACE2 (and ACE) zinc-metalloprotease activities. Therefore, zinc chelating agents, by limiting zinc cellular availability, may affect zinc-dependent enzymes at different levels from the synthesis of functional proteins to their conformation, which may not only impair zinc-metalloprotease activities but also change their (permissive) cell surface structure for viral entry (see (Zamai, 2021; 2020a)). In this regard, zinc displacement by bismuth-based drugs has been shown to exhibit anti-SARS-CoV-2 activity in vivo golden Syrian hamster model (Yuan et al., 2020), suggesting that reduction of zinc-mediated functions can be effective against COVID-19. Altogether these observations suggest that zinc-metalloprotease activities, such as ACE2 shedding and ACE2 enzymatic function, which are strongly upregulated by SARS-CoV-2, are expected to be sensitive to reduction of bioavailable Zn2+ concentrations in plasma. Therefore, drugs, such as metal-chelating agents that remove metal ions and in particular zinc from the body, are expected to work against severe forms of COVID-19. In this regard, both EDTA and citrate have been proposed as zinc-chelating agents able to reduce zinc bioavailability and therefore to work against COVID-19 (Zamai, 2020a, 2021). Indeed, CaNa2EDTA and citrate, as well as other zinc-chelating agents (e.g., phytates, phytates folic acid, nicotianamine and zeolites), have been already proposed as anti-SARS-CoV-2 agents whose administration routes dosages and safety concerns have been widely discussed in previous works (Zamai, 2020a, 2021). Briefly, citrate can sequestrate both extracellular and intracellular Zn2+ (Sul et al., 2016) see (Zamai, 2021), while EDTA is an extracellular zinc chelator that is also able to directly inhibit ACE2 (and ACE) activity (Anguiano et al., 2015; Vickers et al., 2002). Similarly, nicotianamine, a metal chelator extracted from soybean, has been shown to inhibit the activity of both ACE2 (and ACE) zinc-metalloproteases (Takahashi et al., 2015), suggesting that it is able to accommodate into the catalytic pocket of ACE2 (and ACE) and bind to zinc. However, EDTA is poorly absorbed by the gastrointestinal wall; nevertheless, the small adsorbed fraction diffuses in extracellular fluids and it is subsequently excreted by kidney, while the non-adsorbed fraction can inhibit intestinal zinc absorption, thus reducing plasma zinc levels (see (Zamai, 2020a)). The differences in both affinities for zinc ions and whole-body bio-distributions between EDTA and citrate suggest that their combination treatment is expected to be more effective than each drug alone (Zamai, 2020a, 2021). Altogether, these observations suggest that zinc-chelating agents may affect zinc-dependent RAS pathways upregulated by SARS-CoV-2 and, consequently, susceptibility to SARS-CoV-2 infection.
Alternative agents for inhibition of the zinc-metalloproteases activated by SARS-CoV-2 infection. A bismuth citrate-based drug has also been shown to work against SARS-CoV-2 infection in vivo in a golden model hamster (Yuan et al., 2020). Bismuth-induced zinc displacement and inhibition of the zinc-dependent SARS-CoV-2 elicase has been claimed as the cause of protection from COVID-19 and mechanism of action (Yuan et al., 2020). However, when administered systemically, bismuth can induce zinc displacement in several different zinc-dependent proteins/enzymes including those of the host organism (Zamai, 2021). In this regard, it has been proposed that the actual targets of bismuth citrate were the zinc-metalloproteases activated by SARS-CoV-2 infection, suggesting that its potential mechanism against COVID-19 might be mediated by RAS pathway inhibition (see (Zamai, 2021)). However, it is not clear whether bismuth systemic administration is feasible and safe in COVID-19 patients.
Of interest, an endogenous small basic peptide has been shown to compete with ACE2 substrates and specifically inhibit ACE2 activity (Lew et al., 2008). Among the possible inhibitors, the chemical structure of agmatine (decarboxylated arginine) resembles that of a specific ACE2I, NAAE (Huentelman et al., 2004), raising the possibility that agmatine may represent the endogenous inhibitor of ACE2 (see (Zamai, 2020a)). Alternatively, a (basic) amino acid (AA), which can chelate zinc, forming labile zinc (forming labile zinc complexes), could be capable to accommodate into the catalytic site of ACE2 and inhibit its activity (Zamai, 2020a). Indeed, most natural AAs can form complexes with Zn2+ and formulations of Zn-AA complexes have been shown to be absorbed by enterocytes, suggesting that zinc chelates of AA may contribute to cell uptake of zinc by AA transporter (Sauer et al., 2017). In this regard, B0AT1 neutral AA transporter, which is associated with ACE2 (Yan et al., 2020), might also be involved in zinc-AA uptake, possibly supplying ACE2-positive cells with zinc for biosynthesis ACE2 zinc-metalloprotease (Zamai, 2020a). Interestingly, pancreatic β-cells express ACE2 (Fignani et al., 2020) and their insulin secretory granules have extremely high concentration of Zn2+ [see (Coverdale et al., 2019)], possibly suggesting a mechanism of zinc uptake for insulin biosynthesis similar to that hypothesised for ACE2. Indeed, Zn2+ is necessary to stabilise the inactive form of insulin [see (Coverdale et al., 2019)]. On the other hand, active monomeric form of insulin requires zinc dissociation, which is promoted by plasma albumin that acts as a scavenger of zinc, by reducing plasma free Zn2+ [see (Coverdale et al., 2019)]. However, glycation of albumin reduces its zinc-binding ability [see (Coverdale et al., 2019)], thus increasing plasma free zinc concentrations. Finally, this complex interplay among Zn2+, albumin and insulin may indicate a rational for the diabetogenic effect of COVID-19 (Lim et al., 2021; Rubino et al., 2020; Sathish et al., 2021), for example it may be a consequence of SARS-COV-2-induced persistent hypoalbuminemia and/or increase free zinc levels in some susceptible individuals.
Inhibition of both arms of RAS pathways during the late phase of COVID-19. When both arms of the RAS are upregulated, inhibition of ADAM17 and ACE2 may have a limited action and, by shifting the balance of ACE/ACE2 ratio in favour of ACE, their exclusive inhibition might be even dangerous. Instead, inhibition of both ACE2 and ACE and/or renin enzymatic activities may effectively work in blocking the compensatory responses that are established later in severe COVID-19 patients. Therefore, among the inhibitors of the RAS pathways, different strategy can be pursued involving not only ACE2 but also renin and/or ACE enzymatic activities, which are upstream of ACE2 pathway. Interestingly, enzymatic activity of both ACE and ACE2 enzymes seems significantly upregulated in ARDS as well as in pulmonary arterial hypertension (PAH) and coronary atherosclerosis (see (Hemnes et al., 2018; Li et al., 2016; Reddy et al., 2019; Zamai, 2020a). In this regard, both ACE2 and ACE zinc-metalloproteases have been shown to be activated by high concentrations of zinc (and chloride) ions (Anguiano et al., 2015; Towler et al., 2004), Intriguingly, inhalation of fumes containing zinc induces fever, fatigue, muscle ache, cough, dyspnoea, and a cytokine storm, which are symptoms similar to those observed in COVID-19 patients (Bleidorn et al., 2019; Riccelli et al., 2020). Moreover, inhalation of smoke bombs containing ZnCl2/ZnO/hexachloroethane induces ARDS with lung radiographic and angiographic pictures that strongly resemble those of COVID-19 (Hjortsø et al., 1988; Plum et al., 2010; Xie et al., 2017). It is therefore possible that ARDS induced by smoke bombs might originate from zinc-mediated activation of both arms of the RAS, as it may occur for SARS in COVID-19 patients (for further details see (Zamai, 2020a).
Inhibition of renin enzymatic activity. Renin inhibitors can be useful “tools” to reduce production of Ang I, which is the necessary “fuel” to allow both ACE and ACE2 pathway hyperactivities and their detrimental effects. Among renin inhibitors, which are able to inhibit the RAS pathway at its origin, aliskiren is the sole compound that was approved and commercialized for the management of hypertension (Ramya et al., 2020). Therefore, when hypoxia and other systemic conditions upregulate both arms of the RAS, aliskiren may be used alone or in combination with other drugs to face severe forms of COVID-19. Alternatively, modulation of renin levels could be also pursued through vitamin D supplementation. Indeed, vitamin D is a regulator of RAS, as vitamin D metabolites mediate suppression of renin expression and RAS activity (Vaidya and Williams, 2012). Moreover, vitamin D deficiencies have been associated with severity of illness in COVID-19 patients (Carpagnano et al., 2021) and, in general, patients deficient in Vitamin D have an increased risk to develop ARDS (Dancer et al., 2015). Since the sun is the best source of vitamin D, its down-regulatory function on renin expression suggests a link between summer environmental conditions and susceptibility to severe COVID-19.
However, the sole inhibition of renin and RAS pathways is not able to work on other pathways that can be affected by the enzymatic activities of ACE and ACE2, which have been shown to have broad substrate specificities (Kuba et al., 2010; Skidgel and Erdös, 1987; Vickers et al., 2002), and might not be able to restore homeostasis and recover from COVID-19 pathological conditions. Indeed, a double-blind, randomised, placebo-controlled trial with a single high dose of vitamin D3 did not show significant reduction in length of hospitalisation, thus not supporting the use of vitamin D3 for treatment of moderate to severe COVID-19 ((Murai et al., 2021), ClinicalTrials.gov: NCT04449718.). Therefore, a combination of RAS inhibitors specific for all three RAS enzymes may be more effective.
Inhibition of the classical RAS pathway - ACE/Ang II/AT1R by ACE zinc-metalloprotease inhibitors or AT1R blockers. Hypertension, cardiovascular diseases and diabetes, which are the most frequent comorbidities for COVID-19, are often treated with ACEIs and/or with AT1RBs, suggesting a possible positive correlation (Bosso et al., 2020; Brown, 2020; Fang et al., 2020a, 2020b). In this regard, individuals on therapy with AT1RBs or oral antidiabetic agents have an increased ACE2 activity in circulation, while treatment with ACEIs and vitamin D have no significant influence on circulating ACE2 activity (Anguiano et al., 2015). In line with these observations, an AT1RB, but not an ACEI, has been shown to upregulate ACE2 activity (Ferrario et al., 2005). However, a report showed that plasma Ang (1–7) (a product of ACE2 activity) was increased in subjects treated with both ACEIs and AT1RBs, but ACEIs produced an increase of Ang (1–7) that was higher than that of AT1RBs (Binder et al., 2019). Moreover, both ACEIs and AT1RBs have been shown to upregulate ACE2 protein expression under hypoxic conditions (Zhang et al., 2009), a condition that occurs during the late phase of COVID-19. Therefore, hypoxia together with ACEI or AT1RB treatments might upregulate membrane ACE2 expression and therefore susceptibility to SARS-CoV-2 infection. On the other hand, ACEIs and AT1RBs can reduce the deleterious effects of Ang II/AT1R activation that occurs during the late COVID-19 phase and ACEIs might indirectly limit the persistent activation of Ang (1–7)/MasR pathway (typical of COVID-19), by reducing Ang II (an ACE2 substrate for Ang (1–7) production); nevertheless, ACEI-induced decrease of ACE/ACE2 activity ratio together with hypoxia-induced renin upregulation can abnormally increase Ang I concentrations and consequently ACE2-mediated production of Ang (1–9), leading to hyperactivation of AT2R pathway. Moreover, non-RAS peptide metabolisation by ACE and/or ACE2 enzymatic activities (Garvin et al., 2020; Kuba et al., 2010; Skidgel and Erdös, 1987; Vickers et al., 2002) or transformation of Ang II (when increased by AT1RB treatments) by non-RAS enzymes into RAS peptides (see (Velez et al., 2012)) might lead to even more unbalanced conditions, when ACEIs and/or AT1RBs are administered. Nevertheless, at the present time, several studies, meta-analyses and one randomised controlled trial have not confirmed a significant association between the use of classical RAS inhibitors and severity of COVID-19 (Bean et al., 2020; Chan et al., 2020; Cohen et al., 2021; Ghosal et al., 2020; J. Li et al., 2020; X. Li et al., 2020; Mackey et al., 2021; Reynolds et al., 2020; Yang et al., 2020). Actually, some reports show that administration of ACEIs and/or AT1RBs are associated with a lower risk of severe COVID-19 (Liu et al., 2020a; Meng et al., 2020; Pirola and Sookoian, 2020; Ren et al., 2020; Semenzato et al., 2021; P. Zhang et al., 2020; Xue Zhang et al., 2020); however, results were not always consistent between ACEI and AT1RB treatments (see (Zamai, 2020a)), thus suggesting that some of these reports did not adjust for confounders and/or there was a variability of responses depending on subject/cohort predisposition.
Altogether the results suggest that the sole inhibition of the classical RAS pathway is not sufficient to recover from severe COVID-19, rather a pharmacological targeting of both arms of RAS may be more effective against late and severe phase of COVID-19. Indeed, during the late phase of COVID-19, when both arms of RAS are strongly activated by hypoxemia, a combined inhibition of the classical (by ACEIs/AT1RBs) and the alternative (by ACE2Is) RAS pathways together with that of renin activity, is expected to be a most effective treatment.
Inhibition of zinc-metalloproteases and coagulation cascade by metal chelating agents. Alternatively to above-mentioned combination therapy, cation chelating agents such as CaNa2EDTA, citrate and nicotianamine might also work against severe COVID-19, by inhibiting both arms of the RAS. Indeed, zinc chelation may be an effective strategy to inhibit not only ADAM17 and ACE2 but also ACE, which is upregulated during late and severe phase of COVID-19. In addition, EDTA and citrate are also well-known for their anticoagulant activities, by chelating (although at different affinities) both Ca2+ and Zn2+ (see (Kiran Gotru et al., 2019; Zamai, 2021, 2020a)). Thrombotic complications in late and severe phase of COVID-19 and, in some rare cases after vaccination are well-known; therefore, the activity of chelating agents may involve different targets that affect a range of functions from Zn2+-dependent RAS enzymatic activities to Ca2+- and/or Zn2+-dependent coagulation processes.
5. Conclusions
At the moment, specific pharmacological therapies against COVID-19 are lacking. In the present work, we have shown a body of evidence suggesting that targeting one or both arms of RAS pathways with existing inhibitors (drug repurposing) may be effective against this complex disease. The proposed pharmacological treatments based on a specific mechanism of action have been developed after having disclosed the most probable mechanisms involved in COVID-19 pathogenesis. The underlying mechanism of disease progression described in the current work is also consistent with the observation that some treatments can lead to opposite effects depending on the disease phase (early or late). On the other hand, one of the take-home messages of all these findings is that the same response (e.g ACE2 shedding/systemic activity) that is protective in an acute phase of a disease may become even deleterious in chronic conditions. This is an evolutionary functional mechanism since it tends to select organisms that are able to quickly solve the challenge (e.g. SARS-CoV-2) posed by “environmental issues”, while eliminating those inadequate to properly respond to the new and persistent environmental conditions (see (Zamai, 2020b) paragraph 5.5).
Among the COVID-19-phase-specific treatments, chelating agent supplementation during the SARS-CoV-2 pandemic as an adjunct treatment to reduce the risk of infection and severe disease progression is a feasible treatment because oral supplementation of chelating agents is well-tolerated, safe, promptly available, easily deliverable and storable, inexpensive and practically useable also in developing countries. Unfortunately, clinical trials employing chelating agents in COVID-19 are currently absent and urgently needed in order to shed more light on the efficacy of zinc chelators against SARS-CoV-2 infection in vivo. For further details (safety concerns, administration routes and dosages) on EDTA and citrate, see (Zamai, 2020a, 2021). However, metal chelating agents might be helpful not only against COVID-19 and side effects of SARS-CoV-2 vaccines but also against other chronic pathologies associated with upregulation of both arms of the RAS, such as ARDS, PAH, coronary atherosclerosis (see (Zamai, 2020a)). New treatment possibilities that would deserve further investigations.
Finally, the proposed therapies for COVID-19, working downstream of the host pathways activated by SARS-CoV-2 infection, are expected both to protect from development of severe COVID-19 forms independently of SARS-CoV-2 variants and to allow the development of a natural and strong immunity against SARS-CoV-2 infection.
Funding
This research received no external funding.
CRediT authorship contribution statement
Mariele Montanari: Supervision, Validation, Writing – review & editing. Barbara Canonico: Validation, Writing – review & editing. Evelyn Nordi: Writing – review & editing. Daniela Vandini: Writing – review & editing. Simone Barocci: Writing – review & editing. Serena Benedetti: Writing – review & editing. Eugenio Carlotti: Writing – review & editing. Loris Zamai: Conceptualization, Writing – original draft.
Declaration of competing interest
The authors declare that they have no competing interests.
Acknowledgments
We have to thank Prof Lucio Ildebrando Cocco and Giuseppe Remuzzi for their support and encouragement. We really hope that this work could become decisive in order to face this pandemic. We would also like to apologize to those whose relevant contributions could not be cited because of our limits.
Footnotes
Data sharing is not applicable to this article because no new data were created or analysed in this study.
References
- Abassi Z., Skorecki K., Hamo-Giladi D.B., Kruzel-Davila E., Heyman S.N. Kinins and chymase: the forgotten components of the renin-angiotensin system and their implications in COVID-19 disease. Am. J. Physiol. Lung Cell Mol. Physiol. 2021 doi: 10.1152/AJPLUNG.00548.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrati C., Sacchi A., Bordoni V., Cimini E., Notari S., Grassi G., Casetti R., Tartaglia E., Lalle E., D'Abramo A., Castilletti C., Marchioni L., Shi Y., Mariano A., Song J.W., Zhang J.Y., Wang F.S., Zhang C., Fimia G.M., Capobianchi M.R., Piacentini M., Antinori A., Nicastri E., Maeurer M., Zumla A., Ippolito G. Expansion of myeloid-derived suppressor cells in patients with severe coronavirus disease (COVID-19) Cell Death Differ. 2020;27:3196–3207. doi: 10.1038/s41418-020-0572-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Shamlan F., El-Hashim A.Z. Bradykinin sensitizes the cough reflex via a B2 receptor dependent activation of TRPV1 and TRPA1 channels through metabolites of cyclooxygenase and 12-lipoxygenase. Respir. Res. 2019;20 doi: 10.1186/s12931-019-1060-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amiral J. Can COVID-19 induce an autoimmune disease associated with long-lasting symptoms and delayed complications? Ann. Clin. Immunol. Microbiol. 2020;2:1014. [Google Scholar]
- Anguiano L., Riera M., Pascual J., Valdivielso J.M., Barrios C., Betriu A., Mojal S., Fernández E., Soler M.J., Faura A., Castro E., María V., Molí T., Soria M., Aladrén Regidor M.J., Almirall J., Ponz E., Arteaga Coloma J., Bajo Rubio M.A., Belart Rodríguez M., Bielsa-García S., Bover Sanjuan J., Bronsoms Artero J., Cabezuelo Romero J.B., Muray Cases S., Calviño Varela J., Caro Acevedo P., Carreras Bassa J., Cases Amenós A., Massó Jiménez E., Castilla Pérez J., Cigarrán Guldris S., López Prieto S., Comas Mongay L., Comerma I., Compte Jové M.T., Cuberes Izquierdo M., De Álvaro F., Hevia Ojanguren C., De Arriba De La Fuente G., Del Pino Pino M.D., Diaz-Tejeiro Izquierdo R., Dotori M., Duarte V., Estupiñan Torres S., Fernández Reyes M.J., Fernández Rodríguez M.L., Fernández G., Galán Serrano A., García Cantón C., García Herrera A.L., García Mena M., Gil Sacaluga L., Aguilar M., Górriz J.L., Huarte Loza E., Lerma J.L., Liebana Cañada A., Marín Álvarez J.P., Martín Alemany N., Martín García J., Martínez Castelao A., Martínez Villaescusa M., Martínez I., Moina Eguren I., Moreno Los Huertos S., Mouzo Mirco R., Munar Vila A., Muñoz Díaz A.B., Navarro González J.F., Nieto J., Carreño A., Novoa Fernández E., Ortiz A., Fernandez B., Paraíso V., Pérez Fontán M., Peris Domingo A., Piñera Haces C., Prados Garrido M.D., Prieto Velasco M., Puig Marí C., Rivera Gorrín M., Rubio E., Ruiz P., Salgueira Lazo M., Martínez Puerto A.I., Sánchez Tomero J.A., Sánchez J.E., Sans Lorman R., Saracho R., Sarrias M., Prat O., Sousa F., Toran D., Tornero Molina F., Usón Carrasco J.J., Valera Cortes I., Vilaprinyo Del Perugia M.M., Virto Ruiz R.C. Circulating angiotensin-converting enzyme 2 activity in patients with chronic kidney disease without previous history of cardiovascular disease. Nephrol. Dial. Transplant. 2015;30:1176–1185. doi: 10.1093/ndt/gfv025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awasthi M., Gulati S., Sarkar D.P., Tiwari S., Kateriya S., Ranjan P., Verma S.K. 2020. The Sialoside-Binding Pocket of SARS-CoV-2 Spike Glycoprotein Structurally Resembles MERS-CoV. Viruses 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baggen J., Jan Thibaut H., Hurdiss D.L., Wahedi M., Van Vliet A.L.W., Van Kuppeveld F.J.M. Identification of the cell-surface protease adam9 as an entry factor for encephalomyocarditis virus. mBio. 2019;10 doi: 10.1128/mBio.01780-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandsma R.H.J., Van Goor H., Yourshaw M., Horlings R.K., Jonkman M.F., Schölvinck E.H., Karrenbeld A., Scheenstra R., Kömhoff M., Rump P., Koopman-Keemink Y., Nelson S.F., Escher J.C., Cutz E., Martín M.G. Loss of ADAM17 is associated with severe multiorgan dysfunction. Hum. Pathol. 2015;46:923–928. doi: 10.1016/j.humpath.2015.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean D.M., Kraljevic Z., Searle T., Bendayan R., Kevin O., Pickles A., Folarin A., Roguski L., Noor K., Shek A., Zakeri R., Shah A.M., Teo J.T., Dobson R.J. Angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers are not associated with severe COVID-19 infection in a multi-site UK acute hospital trust. Eur. J. Heart Fail. 2020;22:967–974. doi: 10.1002/ejhf.1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belizna C., Pregnolato F., Abad S., Alijotas-Reig J., Amital H., Amoura Z., Andreoli L., Andres E., Aouba A., Apras Bilgen S., Arnaud L., Bienvenu B., Bitsadze V., Blanco P., Blank M., Borghi M.O., Caligaro A., Candrea E., Canti V., Chiche L., Chretien J.M., Cohen Tervaert J.W., Damian L., Delross T., Dernis E., Devreese K., Djokovic A., Esteve-Valverde E., Favaro M., Fassot C., Ferrer-Oliveras R., Godon A., Hamidou M., Hasan M., Henrion D., Imbert B., Jeandel P.Y., Jeannin P., Jego P., Jourde-Chiche N., Khizroeva J., Lambotte O., Landron C., Latino J.O., Lazaro E., de Leeuw K., Le Gallou T., Kiliç L., Limper M., Loufrani L., Lubin R., Magy-Bertrand N., Mahe G., Makatsariya A., Martin T., Muchardt C., Nagy G., Omarjee L., Van Paasen P., Pernod G., Perrinet F., Pïres Rosa G., Pistorius M.A., Ruffatti A., Said F., Saulnier P., Sene D., Sentilhes L., Shovman O., Sibilia J., Sinescu C., Stanisavljevic N., Stojanovich L., Tam L.S., Tincani A., Tollis F., Udry S., Ungeheuer M.N., Versini M., Cervera R., Meroni P.L. HIBISCUS: hydroxychloroquine for the secondary prevention of thrombotic and obstetrical events in primary antiphospholipid syndrome. Autoimmun. Rev. 2018 doi: 10.1016/j.autrev.2018.05.012. [DOI] [PubMed] [Google Scholar]
- Bhattacharjee S., Banerjee M. Immune thrombocytopenia secondary to COVID-19: a systematic review. SN Compr. Clin. Med. 2020;2:2048–2058. doi: 10.1007/s42399-020-00521-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder C., Poglitsch M., Agibetov A., Duca F., Zotter-Tufaro C., Nitsche C., Aschauer S., Kammerlander A.A., Oeztuerk B., Hengstenberg C., Mascherbauer J., Bonderman D. Angs (angiotensins) of the alternative renin-angiotensin system predict outcome in patients with heart failure and preserved ejection fraction. Hypertension. 2019;74:285–294. doi: 10.1161/HYPERTENSIONAHA.119.12786. [DOI] [PubMed] [Google Scholar]
- Blanco-Melo D., Nilsson-Payant B.E., Liu W.C., Uhl S., Hoagland D., Møller R., Jordan T.X., Oishi K., Panis M., Sachs D., Wang T.T., Schwartz R.E., Lim J.K., Albrecht R.A., tenOever B.R. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell. 2020;181:1036–1045. doi: 10.1016/j.cell.2020.04.026. e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaydon D.C., Biancheri P., Di W.-L., Plagnol V., Cabral R.M., Brooke M.A., van Heel D.A., Ruschendorf F., Toynbee M., Walne A., O'Toole E.A., Martin J.E., Lindley K., Vulliamy T., Abrams D.J., MacDonald T.T., Harper J.I., Kelsell D.P. Inflammatory skin and bowel disease linked to ADAM17 deletion. N. Engl. J. Med. 2011;365:1502–1508. doi: 10.1056/nejmoa1100721. [DOI] [PubMed] [Google Scholar]
- Bleidorn J., Alamzad-Krabbe H., Gerhards B., Kraus T., Brand P., Krabbe J., Martin C. The pro-inflammatory stimulus of zinc- and copper-containing welding fumes in whole blood assay via protein tyrosine phosphatase 1B inhibition. Sci. Rep. 2019;9 doi: 10.1038/s41598-018-37803-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosso M., Thanaraj T.A., Abu-Farha M., Alanbaei M., Abubaker J., Al-Mulla F. The two faces of ACE2: the role of ACE2 receptor and its polymorphisms in hypertension and COVID-19. Mol. Ther. - Methods Clin. Dev. 2020 doi: 10.1016/j.omtm.2020.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J.D. Antihypertensive drugs and risk of COVID-19? Lancet Respir. Med. 2020 doi: 10.1016/S2213-2600(20)30158-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrell L.M., Risvanis J., Kubota E., Dean R.G., MacDonald P.S., Lu S., Tikellis C., Grant S.L., Lew R.A., Smith A.I., Cooper M.E., Johnston C.I. Myocardial infarction increases ACE2 expression in rat and humans. Eur. Heart J. 2005;26:369–375. doi: 10.1093/eurheartj/ehi114. [DOI] [PubMed] [Google Scholar]
- Byrnes J.J., Gross S., Ellard C., Connolly K., Donahue S., Picarella D. Effects of the ACE2 inhibitor GL1001 on acute dextran sodium sulfate-induced colitis in mice. Inflamm. Res. 2009;58:819–827. doi: 10.1007/s00011-009-0053-3. [DOI] [PubMed] [Google Scholar]
- Calligaris M., Cuffaro D., Bonelli S., Spanò D.P., Rossello A., Nuti E., Scilabra S.D. Strategies to target ADAM17 in disease: from its discovery to the iRhom revolution. Mol. 2021. 2021;26 doi: 10.3390/MOLECULES26040944. Page 944 26, 944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantuti-Castelvetri L., Ojha R., Pedro L.D., Djannatian M., Franz J., Kuivanen S., van der Meer F., Kallio K., Kaya T., Anastasina M., Smura T., Levanov L., Szirovicza L., Tobi A., Kallio-Kokko H., Österlund P., Joensuu M., Meunier F.A., Butcher S.J., Winkler M.S., Mollenhauer B., Helenius A., Gokce O., Teesalu T., Hepojoki J., Vapalahti O., Stadelmann C., Balistreri G., Simons M. Neuropilin-1 facilitates SARS-CoV-2 cell entry and infectivity. Science. 2020;370 doi: 10.1126/science.abd2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carithers L.J., Moore H.M. The genotype-tissue expression (GTEx) project. Biopreserv. Biobanking. 2015 doi: 10.1089/bio.2015.29031.hmm. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpagnano G.E., Di Lecce V., Quaranta V.N., Zito A., Buonamico E., Capozza E., Palumbo A., Di Gioia G., Valerio V.N., Resta O. Vitamin D deficiency as a predictor of poor prognosis in patients with acute respiratory failure due to COVID-19. J. Endocrinol. Invest. 2021;44:765–771. doi: 10.1007/s40618-020-01370-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casciola-Rosen L., Thiemann D.R., Andrade F., Trejo Zambrano M.I., Hooper J.E., Leonard E., Spangler J., Cox A.L., Machamer C., Sauer L., Laeyendecker O., Garibaldi B.T., Ray S.C., Mecoli C., Christopher-Stine L., Gutierrez-Alamillo L., Yang Q., Hines D., Clarke W., Rothman R.E., Pekosz A., Fenstermacher K., Wang Z., Zeger S.L., Rosen A. IgM autoantibodies recognizing ACE2 are associated with severe COVID-19. medRxiv Prepr. Serv. Heal. Sci. 2020 doi: 10.1101/2020.10.13.20211664. [DOI] [Google Scholar]
- Chan C.K., Huang Y.S., Liao H.W., Tsai I.J., Sun C.Y., Pan H.C., Chueh J.S., Wang J.T., Wu V.C., Chu T.S. Renin-angiotensin-aldosterone system inhibitors and risks of severe acute respiratory syndrome coronavirus 2 infection: a systematic review and meta-analysis. Hypertension. 2020;76:1563–1571. doi: 10.1161/HYPERTENSIONAHA.120.15989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi X., Yan R., Zhang Jun, Zhang G., Zhang Y., Hao M., Zhang Z., Fan P., Dong Y., Yang Y., Chen Z., Guo Y., Zhang Jinlong, Li Y., Song X., Chen Y., Xia L., Fu L., Hou L., Xu J., Yu C., Li J., Zhou Q., Chen W. A neutralizing human antibody binds to the N-terminal domain of the Spike protein of SARS-CoV-2. Science. 2020;369:650–655. doi: 10.1126/science.abc6952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua R.L., Lukassen S., Trump S., Hennig B.P., Wendisch D., Pott F., Debnath O., Thürmann L., Kurth F., Völker M.T., Kazmierski J., Timmermann B., Twardziok S., Schneider S., Machleidt F., Müller-Redetzky H., Maier M., Krannich A., Schmidt S., Balzer F., Liebig J., Loske J., Suttorp N., Eils J., Ishaque N., Liebert U.G., von Kalle C., Hocke A., Witzenrath M., Goffinet C., Drosten C., Laudi S., Lehmann I., Conrad C., Sander L.E., Eils R. COVID-19 severity correlates with airway epithelium–immune cell interactions identified by single-cell analysis. Nat. Biotechnol. 2020;38:970–979. doi: 10.1038/s41587-020-0602-4. [DOI] [PubMed] [Google Scholar]
- Clarke N.E., Fisher M.J., Porter K.E., Lambert D.W., Turner A.J. Angiotensin converting enzyme (ACE) and ACE2 bind integrins and ACE2 regulates integrin signalling. PloS One. 2012;7:34747. doi: 10.1371/journal.pone.0034747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke N.E., Belyaev N.D., Lambert D.W., Turner A.J. Epigenetic regulation of angiotensin-converting enzyme 2 (ACE2) by SIRT1 under conditions of cell energy stress. Clin. Sci. 2014;126:507–516. doi: 10.1042/CS20130291. [DOI] [PubMed] [Google Scholar]
- Cohen J.B., Hanff T.C., William P., Sweitzer N., Rosado-Santander N.R., Medina C., Rodriguez-Mori J.E., Renna N., Chang T.I., Corrales-Medina V., Andrade-Villanueva J.F., Barbagelata A., Cristodulo-Cortez R., Díaz-Cucho O.A., Spaak J., Alfonso C.E., Valdivia-Vega R., Villavicencio-Carranza M., Ayala-García R.J., Castro-Callirgos C.A., González-Hernández L.A., Bernales-Salas E.F., Coacalla-Guerra J.C., Salinas-Herrera C.D., Nicolosi L., Basconcel M., Byrd J.B., Sharkoski T., Bendezú-Huasasquiche L.E., Chittams J., Edmonston D.L., Vasquez C.R., Chirinos J.A. Continuation versus discontinuation of renin–angiotensin system inhibitors in patients admitted to hospital with COVID-19: a prospective, randomised, open-label trial. Lancet Respir. Med. 2021;9:275–284. doi: 10.1016/S2213-2600(20)30558-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coverdale J.P.C., Khazaipoul S., Arya S., Stewart A.J., Blindauer C.A. Crosstalk between zinc and free fatty acids in plasma. Biochim. Biophys. Acta Mol. Cell Biol. Lipids. 2019 doi: 10.1016/j.bbalip.2018.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig G.M., Evans S.J., Brayshaw B.J. An inverse relationship between serum zinc and C-reactive protein levels in acutely ill elderly hospital patients. Postgrad. Med. 1990;66:1025–1028. doi: 10.1136/pgmj.66.782.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dales N.A., Gould A.E., Brown J.A., Calderwood E.F., Guan B., Minor C.A., Gavin J.M., Hales P., Kaushik V.K., Stewart M., Tummino P.J., Vickers C.S., Ocain T.D., Patane M.A. Substrate-based design of the first class of angiotensin-converting enzyme-related carboxypeptidase (ACE2) inhibitors. J. Am. Chem. Soc. 2002;124:11852–11853. doi: 10.1021/ja0277226. [DOI] [PubMed] [Google Scholar]
- Dancer R.C.A., Parekh D., Lax S., D'Souza V., Zheng S., Bassford C.R., Park D., Bartis D.G., Mahida R., Turner A.M., Sapey E., Wei W., Naidu B., Stewart P.M., Fraser W.D., Christopher K.B., Cooper M.S., Gao F., Sansom D.M., Martineau A.R., Perkins G.D., Thickett D.R. Vitamin D deficiency contributes directly to the acute respiratory distress syndrome (ARDS) Thorax. 2015;70:617–624. doi: 10.1136/thoraxjnl-2014-206680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Maat S., De Mast Q., Danser A.H.J., Van De Veerdonk F.L., Maas C. Impaired breakdown of bradykinin and its metabolites as a possible cause for pulmonary edema in COVID-19 infection. Semin. Thromb. Hemost. 2020 doi: 10.1055/s-0040-1712960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Rosa F.G., Palazzo A., Rosso T., Shbaklo N., Mussa M., Boglione L., Borgogno E., Rossati A., Mornese Pinna S., Scabini S., Chichino G., Borrè S., Del Bono V., Garavelli P.L., Barillà D., Cattel F., Di Perri G., Ciccone G., Lupia T., Corcione S. Risk factors for mortality in COVID-19 hospitalized patients in piedmont, Italy: results from the multicenter, regional, CORACLE registry. J. Clin. Med. 2021;10:1951. doi: 10.3390/jcm10091951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docherty A.B., Harrison E.M., Green C.A., Hardwick H.E., Pius R., Norman L., Holden K.A., Read J.M., Dondelinger F., Carson G., Merson L., Lee J., Plotkin D., Sigfrid L., Halpin S., Jackson C., Gamble C., Horby P.W., Nguyen-Van-Tam J.S., Ho A., Russell C.D., Dunning J., Openshaw P.J.M., Baillie J.K., Semple M.G. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020;369 doi: 10.1136/bmj.m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Shennawy L., Hoffmann A.D., Dashzeveg N.K., Mehl P.J., Yu Z., Tokars V.L., Nicolaescu V., Ostiguin C., Jia Y., Li L., Furlong K., Mao C., Wysocki J., Batlle D., Hope T.J., Shen Y., Luo Y., Chae Y., Zhang H., Swaminathan S., Randall G.C., Demonbreun A.R., Ison M.G., Fang D., Liu H. Circulating ACE2-expressing exosomes block SARS-CoV-2 infection as an innate antiviral mechanism. bioRxiv 2020. 2020 doi: 10.1101/2020.12.03.407031. 12.03.407031. [DOI] [Google Scholar]
- Epelman S., Shrestha K., Troughton R.W., Francis G.S., Sen S., Klein A.L., Wilson Tang W.H. Soluble angiotensin-converting enzyme 2 in human heart failure: relation with myocardial function and clinical outcomes. J. Card. Fail. 2009;15:565–571. doi: 10.1016/j.cardfail.2009.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans C.E., Miners J.S., Piva G., Willis C.L., Heard D.M., Kidd E.J., Good M.A., Kehoe P.G. 2020. Correction to: ACE2 activation protects against cognitive decline and reduces amyloid pathology in the Tg2576 mouse model of Alzheimer's disease. Acta Neuropathol. 2020;139(3):485–502. doi: 10.1007/s00401-020-02229-4. 10.1007/s00401-019-02098-6). Acta Neuropathol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang L., Karakiulakis G., Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir. Med. 2020 doi: 10.1016/S2213-2600(20)30116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang L., Karakiulakis G., Roth M. Antihypertensive drugs and risk of COVID-19? – authors' reply. Lancet Respir. Med. 2020 doi: 10.1016/S2213-2600(20)30159-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatima N., Patel S., Hussain T. Angiotensin AT2 receptor is anti-inflammatory and reno-protective in lipopolysaccharide mice model: role of IL-10. Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.600163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrario C.M., Jessup J., Chappell M.C., Averill D.B., Brosnihan K.B., Tallant E.A., Diz D.I., Gallagher P.E. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation. 2005;111:2605–2610. doi: 10.1161/CIRCULATIONAHA.104.510461. [DOI] [PubMed] [Google Scholar]
- Fignani D., Licata G., Brusco N., Nigi L., Grieco G.E., Marselli L., Overbergh L., Gysemans C., Colli M.L., Marchetti P., Mathieu C., Eizirik D.L., Sebastiani G., Dotta F. SARS-CoV-2 receptor angiotensin I-converting enzyme type 2 (ACE2) is expressed in human pancreatic β-cells and in the human pancreas microvasculature. Front. Endocrinol. 2020;11 doi: 10.3389/fendo.2020.596898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foote J.W., Delves H.T. Albumin bound and α2-macroglobulin bound zinc concentrations in the sera of healthy adults. J. Clin. Pathol. 1984;37:1050–1054. doi: 10.1136/jcp.37.9.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraga-Silva R.A., Pinheiro S.V.B., Gonçalves A.C.C., Alenina N., Bader M., Souza Dos Santos R.A. The antithrombotic effect of angiotensin-(1-7) involves Mas-mediated NO release from platelets. Mol. Med. 2008;14:28–35. doi: 10.2119/2007-00073.Fraga-Silva. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraga-Silva R.A., Sorg B.S., Wankhede M., deDeugd C., Jun J.Y., Baker M.B., Li Y., Castellano R.K., Katovich M.J., Raizada M.K., Ferreira A.J. ACE2 activation promotes antithrombotic activity. Mol. Med. 2010;16:210–215. doi: 10.2119/molmed.2009.00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franchini M., Glingani C., Morandi M., Corghi G., Cerzosimo S., Beduzzi G., Storti A., Di Stasi V., Rastrelli G., Vignozzi L., Mengoli C., Garuti M., Beccaria M., Inglese F., Caruso B., Petilino R.A., Amato M., Nicchio M., Pagani M., Bellani A., Castelli G., Casari S., De Donno G. Safety and efficacy of convalescent plasma in elderly COVID-19 patients: the RESCUE trial. Mayo Clin. Proc. Innov. Qual. Outcomes. 2021;5:403–412. doi: 10.1016/j.mayocpiqo.2021.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg M., Burrell L.M., Velkoska E., Griggs K., Angus P.W., Gibson P.R., Lubel J.S. Upregulation of circulating components of the alternative renin-angiotensin system in inflammatory bowel disease: a pilot study. JRAAS - J. Renin-Angiotensin-Aldosterone Syst. 2015;16:559–569. doi: 10.1177/1470320314521086. [DOI] [PubMed] [Google Scholar]
- Garvin M.R., Alvarez C., Miller J.I., Prates E.T., Walker A.M., Amos B.K., Mast A.E., Justice A., Aronow B., Jacobson D. A mechanistic model and therapeutic interventions for covid-19 involving a ras-mediated bradykinin storm. Elife. 2020;9:1–16. doi: 10.7554/eLife.59177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosal S., Mukherjee J., Sinha B., Gangopadhyay K. 2020. The effect of angiotensin converting enzyme inhibitors and angiotensin receptor blockers on death and severity of disease in patients with coronavirus disease 2019 (COVID-19): a meta-analysis. medRxiv. 2020 doi: 10.1101/2020.04.23.20076661. 04.23.20076661. [DOI] [Google Scholar]
- Gilbert B.W., Slechta J. A case of ivermectin-induced warfarin toxicity: first published report. Hosp. Pharm. 2018;53:393–394. doi: 10.1177/0018578718758972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glowacka I., Bertram S., Herzog P., Pfefferle S., Steffen I., Muench M.O., Simmons G., Hofmann H., Kuri T., Weber F., Eichler J., Drosten C., Pöhlmann S. Differential downregulation of ACE2 by the spike proteins of severe acute respiratory syndrome coronavirus and human coronavirus NL63. J. Virol. 2010;84:1198–1205. doi: 10.1128/jvi.01248-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham C., Seow J., Huettner I., Khan H., Kouphou N., Acors S., Winstone H., Pickering S., Galao R.P., Dupont L., Lista M.J., Jimenez-Guardeño J.M., Laing A.G., Wu Y., Joseph M., Muir L., van Gils M.J., Ng W.M., Duyvesteyn H.M.E., Zhao Y., Bowden T.A., Shankar-Hari M., Rosa A., Cherepanov P., McCoy L.E., Hayday A.C., Neil S.J.D., Malim M.H., Doores K.J. Neutralization potency of monoclonal antibodies recognizing dominant and subdominant epitopes on SARS-CoV-2 Spike is impacted by the B.1.1.7 variant. Immunity. 2021;54:1276–1289. doi: 10.1016/j.immuni.2021.03.023. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadi-Alijanvand H., Rouhani M. Studying the effects of ACE2 mutations on the stability, dynamics, and dissociation process of SARS-CoV-2 S1/hACE2 complexes. J. Proteome Res. 2020;19:4609–4623. doi: 10.1021/acs.jproteome.0c00348. [DOI] [PubMed] [Google Scholar]
- Haga S., Yamamoto Norio, Nakai-Murakami C., Osawa Y., Tokunaga K., Sata T., Yamamoto Naoki, Sasazuki T., Ishizaka Y. Modulation of TNF-α-converting enzyme by the spike protein of SARS-CoV and ACE2 induces TNF-α production and facilitates viral entry. Proc. Natl. Acad. Sci. U.S.A. 2008;105:7809–7814. doi: 10.1073/pnas.0711241105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haga S., Nagata N., Okamura T., Yamamoto Norio, Sata T., Yamamoto Naoki, Sasazuki T., Ishizaka Y. TACE antagonists blocking ACE2 shedding caused by the spike protein of SARS-CoV are candidate antiviral compounds. Antivir. Res. 2010;85:551–555. doi: 10.1016/j.antiviral.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampl V., Herget J., Bíbová J., Banasová A., Husková Z., Vańourková Z., Jíchová Kujal P., Vernerová Z., Sadowski J., Červenka L. Intrapulmonary activation of the angiotensin-converting enzyme type 2/angiotensin 1-7/g-protein-coupled mas receptor axis attenuates pulmonary hypertension in ren-2 transgenic rats exposed to chronic hypoxia. Physiol. Res. 2015;64:25–38. doi: 10.33549/physiolres.932861. [DOI] [PubMed] [Google Scholar]
- Han D.P., Penn-Nicholson A., Cho M.W. Identification of critical determinants on ACE2 for SARS-CoV entry and development of a potent entry inhibitor. Virology. 2006;350:15–25. doi: 10.1016/j.virol.2006.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassanpour M., Rezaie J., Nouri M., Panahi Y. The role of extracellular vesicles in COVID-19 virus infection. Infect. Genet. Evol. 2020 doi: 10.1016/j.meegid.2020.104422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemnes A.R., Rathinasabapathy A., Austin E.A., Brittain E.L., Carrier E.J., Chen X., Fessel J.P., Fike C.D., Fong P., Fortune N., Gerszten R.E., Johnson J.A., Kaplowitz M., Newman J.H., Piana R., Pugh M.E., Rice T.W., Robbins I.M., Wheeler L., Yu C., Loyd J.E., West J. A potential therapeutic role for angiotensin-converting enzyme 2 in human pulmonary arterial hypertension. Eur. Respir. J. 2018;51 doi: 10.1183/13993003.02638-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjortsø E., Ovist J., Bud M.I., Thomsen J.L., Andersen J.B., Wiberg-Jørgensen F., Jensen N.K., Jones R., Reid L.M., Zapol W.M. ARDS after accidental inhalation of zinc chloride smoke. Intensive Care Med. 1988;14:17–24. doi: 10.1007/BF00254116. [DOI] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huentelman M.J., Zubcevic J., Hernández Prada J.A., Xiao X., Dimitrov D.S., Raizada M.K., Ostrov D.A. Sructure-based discovery of a novel angiotensin-converting enzyme 2 inhibitor. Hypertension. 2004;44:903–906. doi: 10.1161/01.HYP.0000146120.29648.36. [DOI] [PubMed] [Google Scholar]
- Hunyady L., Catt K.J. Pleiotropic AT1 receptor signaling pathways mediating physiological and pathogenic actions of angiotensin II. Mol. Endocrinol. 2006 doi: 10.1210/me.2004-0536. [DOI] [PubMed] [Google Scholar]
- Imai Y., Kuba K., Rao S., Huan Y., Guo F., Guan B., Yang P., Sarao R., Wada T., Leong-Poi H., Crackower M.A., Fukamizu A., Hui C.C., Hein L., Uhlig S., Slutsky A.S., Jiang C., Penninger J.M. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436:112–116. doi: 10.1038/nature03712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito S., Ansari P., Sakatsume M., Dickensheets H., Vazquez N., Donnelly R.P., Larner A.C., Finbloom D.S. Interleukin-10 inhibits expression of both interferon – and interferon γ– induced genes by suppressing tyrosine phosphorylation of STAT1. Blood. 1999;93:1456–1463. doi: 10.1182/blood.v93.5.1456. [DOI] [PubMed] [Google Scholar]
- Jia H. Pulmonary angiotensin-converting enzyme 2 (ACE2) and inflammatory lung disease. Shock. 2016 doi: 10.1097/SHK.0000000000000633. [DOI] [PubMed] [Google Scholar]
- Jia H.P., Look D.C., Tan P., Shi L., Hickey M., Gakhar L., Chappell M.C., Wohlford-Lenane C., McCray P.B. Ectodomain shedding of angiotensin converting enzyme 2 in human airway epithelia. Am. J. Physiol. Lung Cell Mol. Physiol. 2009;297:84–96. doi: 10.1152/ajplung.00071.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi S., Balasubramanian N., Vasam G., Jarajapu Y.P.R. Angiotensin converting enzyme versus angiotensin converting enzyme-2 selectivity of MLN-4760 and DX600 in human and murine bone marrow-derived cells. Eur. J. Pharmacol. 2016;774:25–33. doi: 10.1016/j.ejphar.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi S., Wollenzien H., Leclerc E., Jarajapu Y.P.R. Hypoxic regulation of angiotensin-converting enzyme 2 and Mas receptor in human CD34+ cells. J. Cell. Physiol. 2019;234:20420–20431. doi: 10.1002/jcp.28643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalinska M., Meyer-Hoffert U., Kantyka T., Potempa J. Kallikreins - the melting pot of activity and function. Biochimie. 2016 doi: 10.1016/j.biochi.2015.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan A., Benthin C., Zeno B., Albertson T.E., Boyd J., Christie J.D., Hall R., Poirier G., Ronco J.J., Tidswell M., Hardes K., Powley W.M., Wright T.J., Siederer S.K., Fairman D.A., Lipson D.A., Bayliffe A.I., Lazaar A.L. A pilot clinical trial of recombinant human angiotensin-converting enzyme 2 in acute respiratory distress syndrome. Crit. Care. 2017;21 doi: 10.1186/s13054-017-1823-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M.A., Yang D., Kida K., Molotkova N., Yeo S.J., Varki N., Iwata M., Dalton N.D., Peterson K.L., Siems W.E., Walther T., Cowling R.T., Kjekshus J., Greenberg B. Effects of ACE2 inhibition in the post-myocardial infarction heart. J. Card. Fail. 2010;16:777–785. doi: 10.1016/j.cardfail.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiran Gotru S., van Geffen J.P., Nagy M., Mammadova-Bach E., Eilenberger J., Volz J., Manukjan G., Schulze H., Wagner L., Eber S., Schambeck C., Deppermann C., Brouns S., Nurden P., Greinacher A., Sachs U., Nieswandt B., Hermanns H.M., Heemskerk J.W.M., Braun A. Defective Zn2+ homeostasis in mouse and human platelets with α- and δ-storage pool diseases. Sci. Rep. 2019;9 doi: 10.1038/s41598-019-44751-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiseleva A.A., Troisi E.M., Hensley S.E., Kohli R.M., Epstein J.A. SARS-CoV-2 spike protein binding selectively accelerates substrate-specific catalytic activity of ACE2. J. Biochem. 2021 doi: 10.1093/jb/mvab041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornilov S.A., Lucas I., Jade K., Dai C.L., Lovejoy J.C., Magis A.T. Plasma levels of soluble ACE2are associated with sex, Metabolic Syndrome, and its biomarkers in a large cohort, pointing to a possible mechanism for increased severity in COVID-19. Crit. Care. 2020 doi: 10.1186/s13054-020-03141-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovarik J.J., Antlanger M., Domenig O., Kaltenecker C.C., Hecking M., Haidinger M., Werzowa J., Saemann C.K.M.D. Molecular regulation of the renin-Angiotensin system in haemodialysis patients. Nephrol. Dial. Transplant. 2015;30:115–123. doi: 10.1093/ndt/gfu265. [DOI] [PubMed] [Google Scholar]
- Kragstrup T.W., Søgaard Singh H., Grundberg I., Langkilde-Lauesen Nielsen A., Rivellese F., Mehta A., Goldberg M.B., Filbin M., Qvist P., Martin Bibby B., Wenzel Kragstrup T. 2021. Plasma ACE2 levels predict outcome of COVID-19 in hospitalized patients. medRxiv. 2021 doi: 10.1101/2021.03.08.21252819. 03.08.21252819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer B.K., Ritthaler T., Schweda F., Kees F., Schricker K., Holmer S.R., Kurtz A. Kidney International, Supplement. Nature Publishing Group; 1998. Effects of hypoxia on renin secretion and renal renin gene expression. [DOI] [PubMed] [Google Scholar]
- Kramkowski K., Mogielnicki A., Leszczynska A. Angiotensin-(1-9), the product of angiotensin I conversion in platelets, enhances arterial thrombosis in rats - PubMed. J. Physiol. Pharmacol. 2010;61:317–324. [PubMed] [Google Scholar]
- Krempl C., Schultze B., Laude H., Herrler G. Point mutations in the S protein connect the sialic acid binding activity with the enteropathogenicity of transmissible gastroenteritis coronavirus. J. Virol. 1997;71:3285–3287. doi: 10.1128/jvi.71.4.3285-3287.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuba K., Imai Y., Rao S., Gao H., Guo F., Guan B., Huan Y., Yang P., Zhang Y., Deng W., Bao L., Zhang B., Liu G., Wang Z., Chappell M., Liu Y., Zheng D., Leibbrandt A., Wada T., Slutsky A.S., Liu D., Qin C., Jiang C., Penninger J.M. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat. Med. 2005;11:875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuba K., Imai Y., Ohto-Nakanishi T., Penninger J.M. Trilogy of ACE2: a peptidase in the renin-angiotensin system, a SARS receptor, and a partner for amino acid transporters. Pharmacol. Ther. 2010 doi: 10.1016/j.pharmthera.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucharewicz I., Pawlak R., Matys T., Pawlak D., Buczko W. Antithrombotic effect of captopril and losartan is mediated by angiotensin-(1-7) Hypertension. 2002;40:774–779. doi: 10.1161/01.HYP.0000035396.27909.40. [DOI] [PubMed] [Google Scholar]
- Künkel F., Herrler G. Structural and functional analysis of the surface protein of human coronavirus oc43. Virology. 1993;195:195–202. doi: 10.1006/viro.1993.1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyriakidis N.C., López-Cortés A., González E.V., Grimaldos A.B., Prado E.O. 2021. SARS-CoV-2 Vaccines Strategies: a Comprehensive Review of Phase 3 Candidates. npj Vaccines. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauw F.N., Pajkrt D., Hack C.E., Kurimoto M., van Deventer S.J.H., van der Poll T. Proinflammatory effects of IL-10 during human endotoxemia. J. Immunol. 2000;165:2783–2789. doi: 10.4049/jimmunol.165.5.2783. [DOI] [PubMed] [Google Scholar]
- Lavezzo E., Franchin E., Ciavarella C., Cuomo-Dannenburg G., Barzon L., Del Vecchio C., Rossi L., Manganelli R., Loregian A., Navarin N., Abate D., Sciro M., Merigliano S., De Canale E., Vanuzzo M.C., Besutti V., Saluzzo F., Onelia F., Pacenti M., Parisi S.G., Carretta G., Donato D., Flor L., Cocchio S., Masi G., Sperduti A., Cattarino L., Salvador R., Nicoletti M., Caldart F., Castelli G., Nieddu E., Labella B., Fava L., Drigo M., Gaythorpe K.A.M., Ainslie K.E.C., Baguelin M., Bhatt S., Boonyasiri A., Boyd O., Cattarino L., Ciavarella C., Coupland H.L., Cucunubá Z., Cuomo-Dannenburg G., Djafaara B.A., Donnelly C.A., Dorigatti I., van Elsland S.L., FitzJohn R., Flaxman S., Gaythorpe K.A.M., Green W.D., Hallett T., Hamlet A., Haw D., Imai N., Jeffrey B., Knock E., Laydon D.J., Mellan T., Mishra S., Nedjati-Gilani G., Nouvellet P., Okell L.C., Parag K.V., Riley S., Thompson H.A., Unwin H.J.T., Verity R., Vollmer M.A.C., Walker P.G.T., Walters C.E., Wang H., Wang Y., Watson O.J., Whittaker C., Whittles L.K., Xi X., Ferguson N.M., Brazzale A.R., Toppo S., Trevisan M., Baldo V., Donnelly C.A., Ferguson N.M., Dorigatti I., Crisanti A. Suppression of a SARS-CoV-2 outbreak in the Italian municipality of Vo’. Nature. 2020;584:425–429. doi: 10.1038/s41586-020-2488-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence A.C., Evin G., Kladis A., Campbell D.J. An alternative strategy for the radioimmunoassay of angiotensin peptides using amino-terminal-directed antisera: measurement of eight angiotensin peptides in human plasma. J. Hypertens. 1990;8:715–724. doi: 10.1097/00004872-199008000-00005. [DOI] [PubMed] [Google Scholar]
- Lee E.J., Cines D.B., Gernsheimer T., Kessler C., Michel M., Tarantino M.D., Semple J.W., Arnold D.M., Godeau B., Lambert M.P., Bussel J.B. Thrombocytopenia following pfizer and moderna SARS-CoV-2 vaccination. Am. J. Hematol. 2021 doi: 10.1002/ajh.26132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew R.A., Warner F.J., Hanchapola I., Yarski M.A., Manohar J., Burrell L.M., Smith A.I. Angiotensin-converting enzyme 2 catalytic activity in human plasma is masked by an endogenous inhibitor. Exp. Physiol. 2008;93:685–693. doi: 10.1113/expphysiol.2007.040352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F. Structure, function, and evolution of coronavirus spike proteins. Annu. Rev. Virol. 2016 doi: 10.1146/annurev-virology-110615-042301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Xu S., Yu M., Wang K., Tao Y., Zhou Y., Shi J., Zhou M., Wu B., Yang Z., Zhang C., Yue J., Zhang Z., Renz H., Liu X., Xie J., Xie M., Zhao J. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J. Allergy Clin. Immunol. 2020;146:110–118. doi: 10.1016/j.jaci.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Zhang C., Sui J., Kuhn J.H., Moore M.J., Luo S., Wong S.K., Huang I.C., Xu K., Vasilieva N., Murakami A., He Y., Marasco W.A., Guan Y., Choe H., Farzan M. Receptor and viral determinants of SARS-coronavirus adaptation to human ACE2. EMBO J. 2005;24:1634–1643. doi: 10.1038/sj.emboj.7600640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Li J., Hao P., Chen W., Meng X., Li H., Zhang Y., Zhang C., Yang J. Imbalance between angiotensin II and angiotensin-(1-7) in human coronary atherosclerosis. JRAAS - J. Renin-Angiotensin-Aldosterone Syst. 2016;17 doi: 10.1177/1470320316659618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X.K., Lu Q. Bin, Chen W.W., Xu W., Liu R., Zhang S.F., Du J., Li H., Yao K., Zhai D., Zhang P.H., Xing B., Cui N., Yang Z.D., Yuan C., Zhang X.A., Xu Z., Cao W.C., Hu Z., Liu W. Arginine deficiency is involved in thrombocytopenia and immunosuppression in severe fever with thrombocytopenia syndrome. Sci. Transl. Med. 2018;10 doi: 10.1126/scitranslmed.aat4162. [DOI] [PubMed] [Google Scholar]
- Li J., Wang X., Chen J., Zhang H., Deng A. Association of renin-angiotensin system inhibitors with severity or risk of death in patients with hypertension hospitalized for coronavirus disease 2019 (COVID-19) infection in wuhan, China. JAMA Cardiol. 2020;5:825–830. doi: 10.1001/jamacardio.2020.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Zhang Z., Yang L., Lian X., Xie Y., Li S., Xin S., Cao P., Lu J. The MERS-CoV receptor DPP4 as a candidate binding target of the SARS-CoV-2 spike. iScience. 2020;23 doi: 10.1016/j.isci.2020.101160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao X., Wang L., Yang C., He J., Wang X., Guo R., Lan A., Dong X., Yang Z., Wang H., Feng J., Ma H. Cyclooxygenase mediates cardioprotection of angiotensin-(1-7) against ischemia/reperfusion-induced injury through the inhibition of oxidative stress. Mol. Med. Rep. 2011;4:1145–1150. doi: 10.3892/mmr.2011.570. [DOI] [PubMed] [Google Scholar]
- Lim S., Bae J.H., Kwon H.S., Nauck M.A. COVID-19 and diabetes mellitus: from pathophysiology to clinical management. Nat. Rev. Endocrinol. 2021 doi: 10.1038/s41574-020-00435-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C., Datta V., Okwan‐Duodu D., Chen X., Fuchs S., Alsabeh R., Billet S., Bernstein K.E., Shen X.Z. Angiotensin‐converting enzyme is required for normal myelopoiesis. Faseb. J. 2011;25:1145–1155. doi: 10.1096/fj.10-169433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Huang F., Xu J., Yang P., Qin Y., Cao M., Wang Z., Li X., Zhang S., Ye L., Lv J., Wei J., Xie T., Gao H., Xu K.-F., Wang F., Liu L., Jiang C. Anti-hypertensive Angiotensin II receptor blockers associated to mitigation of disease severity in elderly COVID-19 patients. medRxiv 2020. 2020 doi: 10.1101/2020.03.20.20039586. 03.20.20039586. [DOI] [Google Scholar]
- Liu Y., Yang Y., Zhang C., Huang F., Wang F., Yuan J., Wang Z., Li Jinxiu, Li Jianming, Feng C., Zhang Z., Wang L., Peng L., Chen L., Qin Y., Zhao D., Tan S., Yin L., Xu J., Zhou C., Jiang C., Liu L. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci. China Life Sci. 2020;63:364–374. doi: 10.1007/s11427-020-1643-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longhitano Y., Racca F., Zanza C., Muncinelli M., Guagliano A., Peretti E., Minerba A.C., Mari M., Boverio R., Salio M., Chichino G., Franceschi F., Piccioni A., Abenavoli L., Salvini M., Artico M. Venous thrombo-embolism in hospitalized sars-cov-2 patients treated with three different anticoagulation protocols: prospective observational study. Biology. 2020;9:1–12. doi: 10.3390/biology9100310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J., Sun P.D. High affinity binding of SARS-cov-2 spike protein enhances ACE2 carboxypeptidase activity. J. Biol. Chem. 2020;295:18579–18588. doi: 10.1074/jbc.RA120.015303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu G., Wang Q., Gao G.F. Bat-to-human: spike features determining “host jump” of coronaviruses SARS-CoV, MERS-CoV, and beyond. Trends Microbiol. 2015 doi: 10.1016/j.tim.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L., Zhang H., Dauphars D.J., He Y.W. A potential role of interleukin 10 in COVID-19 pathogenesis. Trends Immunol. 2021 doi: 10.1016/j.it.2020.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundström A., Ziegler L., Havervall S., Rudberg A.S., von Meijenfeldt F., Lisman T., Mackman N., Sandén P., Thålin C. Soluble angiotensin-converting enzyme 2 is transiently elevated in COVID-19 and correlates with specific inflammatory and endothelial markers. J. Med. Virol. 2021 doi: 10.1002/jmv.27144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackey K., Kansagara D., Vela K. Update alert 8: risks and impact of angiotensin-converting enzyme inhibitors or angiotensin-receptor blockers on SARS-CoV-2 infection in adults. Ann. Intern. Med. 2021;174:W54. doi: 10.7326/l21-0223. –W55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magalhaes G.S., Barroso L.C., Reis A.C., Rodrigues-Machado M.G., Gregório J.F., Motta-Santos D., Oliveira A.C., Perez D.A., Barcelos L.S., Teixeira M.M., Santos R.A.S., Pinho V., Campagnole-Santos M.J. Angiotensin-(1-7) promotes resolution of eosinophilic inflammation in an experimental model of asthma. Front. Immunol. 2018;9 doi: 10.3389/fimmu.2018.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masre S.F., Jufri N.F., Ibrahim F.W., Abdul Raub S.H. Classical and alternative receptors for SARS-CoV-2 therapeutic strategy. Rev. Med. Virol. 2020 doi: 10.1002/rmv.2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCallum M., De Marco A., Lempp F.A., Tortorici M.A., Pinto D., Walls A.C., Beltramello M., Chen A., Liu Z., Zatta F., Zepeda S., di Iulio J., Bowen J.E., Montiel-Ruiz M., Zhou J., Rosen L.E., Bianchi S., Guarino B., Fregni C.S., Abdelnabi R., Foo S.Y.C., Rothlauf P.W., Bloyet L.M., Benigni F., Cameroni E., Neyts J., Riva A., Snell G., Telenti A., Whelan S.P.J., Virgin H.W., Corti D., Pizzuto M.S., Veesler D. N-terminal domain antigenic mapping reveals a site of vulnerability for SARS-CoV-2. Cell. 2021;184:2332–2347. doi: 10.1016/j.cell.2021.03.028. e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy K.R., Rennick L.J., Nambulli S., Robinson-McCarthy L.R., Bain W.G., Haidar G., Paul Duprex W. Recurrent deletions in the SARS-CoV-2 spike glycoprotein drive antibody escape. Science. 2021;371:1139–1142. doi: 10.1126/science.abf6950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng J., Xiao G., Zhang J., He X., Ou M., Bi J., Yang R., Di W., Wang Z., Li Z., Gao H., Liu L., Zhang G. Renin-angiotensin system inhibitors improve the clinical outcomes of COVID-19 patients with hypertension. Emerg. Microb. Infect. 2020 doi: 10.1080/22221751.2020.1746200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogielnicki A., Chabielska E., Pawlak R., Szemraj J., Buczko W. Angiotensin II enhances thrombosis development in renovascular hypertensive rats. Thromb. Haemostasis. 2005;93:1069–1076. doi: 10.1160/TH04-10-0701. [DOI] [PubMed] [Google Scholar]
- Mogielnicki A., Kramkowski K., Hermanowicz J.M., Leszczynska A., Przyborowski K., Buczko W. Angiotensin-(1-9) enhances stasis-induced venous thrombosis in the rat because of the impairment of fibrinolysis. JRAAS - J. Renin-Angiotensin-Aldosterone Syst. 2014;15:13–21. doi: 10.1177/1470320313498631. [DOI] [PubMed] [Google Scholar]
- Monteil V., Kwon H., Prado P., Hagelkrüys A., Wimmer R.A., Stahl M., Leopoldi A., Garreta E., Hurtado del Pozo C., Prosper F., Romero J.P., Wirnsberger G., Zhang H., Slutsky A.S., Conder R., Montserrat N., Mirazimi A., Penninger J.M. Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2. Cell. 2020;181:905–913. doi: 10.1016/j.cell.2020.04.004. e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore M.J., Dorfman T., Li W., Wong S.K., Li Y., Kuhn J.H., Coderre J., Vasilieva N., Han Z., Greenough T.C., Farzan M., Choe H. Retroviruses pseudotyped with the severe acute respiratory syndrome coronavirus spike protein efficiently infect cells expressing angiotensin-converting enzyme 2. J. Virol. 2004;78:10628–10635. doi: 10.1128/jvi.78.19.10628-10635.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murai I.H., Fernandes A.L., Sales L.P., Pinto A.J., Goessler K.F., Duran C.S.C., Silva C.B.R., Franco A.S., MacEdo M.B., Dalmolin H.H.H., Baggio J., Balbi G.G.M., Reis B.Z., Antonangelo L., Caparbo V.F., Gualano B., Pereira R.M.R. Effect of a single high dose of vitamin D3on hospital length of stay in patients with moderate to severe COVID-19: a randomized clinical trial. JAMA, J. Am. Med. Assoc. 2021;325:1053–1060. doi: 10.1001/jama.2020.26848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy B., Fejes Z., Szentkereszty Z., Sütő R., Várkonyi I., Ajzner É., Kappelmayer J., Papp Z., Tóth A., Fagyas M. A dramatic rise in serum ACE2 activity in a critically ill COVID-19 patient. Int. J. Infect. Dis. 2021;103:412–414. doi: 10.1016/j.ijid.2020.11.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narula S., Yusuf S., Chong M., Ramasundarahettige C., Rangarajan S., Bangdiwala S.I., van Eikels M., Leineweber K., Wu A., Pigeyre M., Paré G. Plasma ACE2 and risk of death or cardiometabolic diseases: a case-cohort analysis. Lancet. 2020;396:968–976. doi: 10.1016/S0140-6736(20)31964-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng K.W., Attig J., Bolland W., Young G.R., Major J., Wrobel A.G., Gamblin S., Wack A., Kassiotis G. Tissue-specific and interferon-inducible expression of nonfunctional ACE2 through endogenous retroelement co-option. Nat. Genet. 2020;52:1294–1302. doi: 10.1038/s41588-020-00732-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikiforuk A.M., Kuchinski K.S., Twa D.D.W., Lukac C.D., Sbihi H., Basham C.A., Steidl C., Prystajecky N.A., Jassem A.N., Krajden M., Patrick D.M., Sekirov I. The contrasting role of nasopharyngeal angiotensin converting enzyme 2 (ACE2) transcription in SARS-CoV-2 infection: a cross-sectional study of people tested for COVID-19 in British Columbia, Canada. EBioMedicine. 2021 doi: 10.1016/j.ebiom.2021.103316. 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ocaranza M.P., Jalil J.E. Protective role of the ACE2/Ang-(19) axis in cardiovascular remodeling. Int. J. Hypertens. 2012 doi: 10.1155/2012/594361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ocaranza M.P., Riquelme J.A., García L., Jalil J.E., Chiong M., Santos R.A.S., Lavandero S. Counter-regulatory renin–angiotensin system in cardiovascular disease. Nat. Rev. Cardiol. 2020 doi: 10.1038/s41569-019-0244-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onabajo O.O., Banday A.R., Stanifer M.L., Yan W., Obajemu A., Santer D.M., Florez-Vargas O., Piontkivska H., Vargas J.M., Ring T.J., Kee C., Doldan P., Tyrrell D.L., Mendoza J.L., Boulant S., Prokunina-Olsson L. Interferons and viruses induce a novel truncated ACE2 isoform and not the full-length SARS-CoV-2 receptor. Nat. Genet. 2020;52:1283–1293. doi: 10.1038/s41588-020-00731-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz-Pérez J.T., Riera M., Bosch X., De Caralt T.M., Perea R.J., Pascual J., Soler M.J. Role of circulating angiotensin converting enzyme 2 in left ventricular remodeling following myocardial infarction: a prospective controlled study. PloS One. 2013;8:61695. doi: 10.1371/journal.pone.0061695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paizis G., Tikellis C., Cooper M.E., Schembri J.M., Lew R.A., Smith A.I., Shaw T., Warner F.J., Zuilli A., Burrell L.M., Angus P.W. Chronic liver injury in rats and humans upregulates the novel enzyme angiotensin converting enzyme 2. Gut. 2005;54:1790–1796. doi: 10.1136/gut.2004.062398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palau V., Riera M., Soler M.J. ADAM17 inhibition may exert a protective effect on COVID-19. Nephrol. Dial. Transplant. 2020;35:1071–1072. doi: 10.1093/ndt/gfaa093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang X., Cui Y., Zhu Y. Recombinant human ACE2: potential therapeutics of SARS-CoV-2 infection and its complication. Acta Pharmacol. Sin. 2020 doi: 10.1038/s41401-020-0430-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park Y.J., Walls A.C., Wang Z., Sauer M.M., Li W., Tortorici M.A., Bosch B.J., DiMaio F., Veesler D. Structures of MERS-CoV spike glycoprotein in complex with sialoside attachment receptors. Nat. Struct. Mol. Biol. 2019;26:1151–1157. doi: 10.1038/s41594-019-0334-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passos-Silva D.G., Verano-Braga T., Santos R.A.S. Angiotensin-(1-7): beyond the cardio-renal actions. Clin. Sci. 2013 doi: 10.1042/CS20120461. [DOI] [PubMed] [Google Scholar]
- Patel V.B., Clarke N., Wang Z., Fan D., Parajuli N., Basu R., Putko B., Kassiri Z., Turner A.J., Oudit G.Y. Angiotensin II induced proteolytic cleavage of myocardial ACE2 is mediated by TACE/ADAM-17: a positive feedback mechanism in the RAS. J. Mol. Cell. Cardiol. 2014;66:167–176. doi: 10.1016/j.yjmcc.2013.11.017. [DOI] [PubMed] [Google Scholar]
- Patel S., Rauf A., Khan H., Abu-Izneid T. Renin-angiotensin-aldosterone (RAAS): the ubiquitous system for homeostasis and pathologies. Biomed. Pharmacother. 2017;94:317–325. doi: 10.1016/j.biopha.2017.07.091. [DOI] [PubMed] [Google Scholar]
- Patel S.K., Juno J.A., Lee W.S., Wragg K.M., Hogarth P.M., Kent S.J., Burrell L.M. Plasma ACE2 activity is persistently elevated following SARS-CoV-2 infection: implications for COVID-19 pathogenesis and consequences. Eur. Respir. J. 2021;57:2003730. doi: 10.1183/13993003.03730-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phetsouphanh C., Darley D., Howe A., Mee C., Munier L., Patel S.K., Juno J.A., Burrell L.M., Kent S.J., Dore G.J., Kelleher A.D., Matthews G., Kelleher A. 2021. Immunological dysfunction persists for 8 months following initial mild-moderate SARS-CoV-2 infection. medRxiv. 2021 doi: 10.1101/2021.06.01.21257759. 06.01.21257759. [DOI] [PubMed] [Google Scholar]
- Pirola C.J., Sookoian S. Estimation of renin-angiotensin-aldosterone-system (RAAS)-Inhibitor effect on COVID-19 outcome: a meta-analysis. J. Infect. 2020;81:276–281. doi: 10.1016/j.jinf.2020.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plum L.M., Rink L., Hajo H. The essential toxin: impact of zinc on human health. Int. J. Environ. Res. Publ. Health. 2010 doi: 10.3390/ijerph7041342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pucci F., Bogaerts P., Rooman M. Modeling the molecular impact of sars-cov-2 infection on the renin-angiotensin system. Viruses. 2020;12:1367. doi: 10.3390/v12121367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puray-Chavez M., LaPak K.M., Schrank T.P., Elliott J.L., Bhatt D.P., Agajanian M.J., Jasuja R., Lawson D.Q., Davis K., Rothlauf P.W., Liu Z., Jo H., Lee N., Tenneti K., Eschbach J.E., Mugisha C.S., Cousins E.M., Cloer E.W., Vuong H.R., VanBlargan L.A., Bailey A.L., Gilchuk P., Crowe J.E., Diamond M.S., Hayes D.N., Whelan S.P.J., Horani A., Brody S.L., Goldfarb D., Major M., Ben Kutluay S.B. Systematic analysis of SARS-CoV-2 infection of an ACE2-negative human airway cell. Cell Rep. 2021 doi: 10.1101/2021.03.01.433431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radzikowska U., Ding M., Tan G., Zhakparov D., Peng Y., Wawrzyniak P., Wang M., Li S., Morita H., Altunbulakli C., Reiger M., Neumann A.U., Lunjani N., Traidl-Hoffmann C., Nadeau K.C., O'Mahony L., Akdis C., Sokolowska M. Distribution of ACE2, CD147, CD26, and other SARS-CoV-2 associated molecules in tissues and immune cells in health and in asthma, COPD, obesity, hypertension, and COVID-19 risk factors. Allergy Eur. J. Allergy Clin. Immunol. 2020;75:2829–2845. doi: 10.1111/all.14429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramadori G. 2020. Hypoalbuminemia: an underestimated, vital characteristic of hospitalized COVID-19 positive patients? Hepatoma Res. 2020 doi: 10.20517/2394-5079.2020.43. [DOI] [Google Scholar]
- Ramchand J., Patel S.K., Srivastava P.M., Farouque O., Burrell L.M. Elevated plasma angiotensin converting enzyme 2 activity is an independent predictor of major adverse cardiac events in patients with obstructive coronary artery disease. PloS One. 2018;13 doi: 10.1371/journal.pone.0198144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramya K., Suresh R., Kumar H.Y., Kumar B.R.P., Murthy N.B.S. Decades-old renin inhibitors are still struggling to find a niche in antihypertensive therapy. A fleeting look at the old and the promising new molecules. Bioorg. Med. Chem. 2020;28 doi: 10.1016/j.bmc.2020.115466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy R., Asante I., Liu S., Parikh P., Liebler J., Borok Z., Rodgers K., Baydur A., Louie S.G. Circulating angiotensin peptides levels in Acute Respiratory Distress Syndrome correlate with clinical outcomes: a pilot study. PloS One. 2019;14 doi: 10.1371/journal.pone.0213096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehman S.U., Tabish M. Alternative splicing of ACE2 possibly generates variants that may limit the entry of SARS-CoV-2: a potential therapeutic approach using SSOs. Clin. Sci. 2020;134:1143–1150. doi: 10.1042/CS20200419. [DOI] [PubMed] [Google Scholar]
- Reindl-Schwaighofer R., Hödlmoser S., Eskandary F., Poglitsch M., Bonderman D., Strassl R., Aberle J.H., Oberbauer R., Zoufaly A., Hecking M. ACE2 elevation in severe COVID-19. Am. J. Respir. Crit. Care Med. 2021 doi: 10.1164/rccm.202101-0142LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren L., Yu S., Xu W., Overton J.L., Chiamvimonvat N., Thai P.N. Lack of association of antihypertensive drugs with the risk and severity of COVID-19: a meta-analysis. J. Cardiol. 2020;77 doi: 10.1016/j.jjcc.2020.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds H.R., Adhikari S., Pulgarin C., Troxel A.B., Iturrate E., Johnson S.B., Hausvater A., Newman J.D., Berger J.S., Bangalore S., Katz S.D., Fishman G.I., Kunichoff D., Chen Y., Ogedegbe G., Hochman J.S. Renin–angiotensin–aldosterone system inhibitors and risk of covid-19. N. Engl. J. Med. 2020;382:2441–2448. doi: 10.1056/nejmoa2008975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riccelli M.G., Goldoni M., Poli D., Mozzoni P., Cavallo D., Corradi M. Welding fumes, a risk factor for lung diseases. Int. J. Environ. Res. Publ. Health. 2020 doi: 10.3390/ijerph17072552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzo E. Ivermectin, antiviral properties and COVID-19: a possible new mechanism of action. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2020;393:1153–1156. doi: 10.1007/s00210-020-01902-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues-Machado M.G., Magalhães G.S., Cardoso J.A., Kangussu L.M., Murari A., Caliari M.V., Oliveira M.L., Cara D.C., Noviello M.L.M., Marques F.D., Pereira J.M., Lautner R.Q., Santos R.A.S., Campagnole-Santos M.J. AVE 0991, a non-peptide mimic of angiotensin-(1-7) effects, attenuates pulmonary remodelling in a model of chronic asthma. Br. J. Pharmacol. 2013;170:835–846. doi: 10.1111/bph.12318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Röhrborn D., Eckel J., Sell H. Shedding of dipeptidyl peptidase 4 is mediated by metalloproteases and up-regulated by hypoxia in human adipocytes and smooth muscle cells. FEBS Lett. 2014;588:3870–3877. doi: 10.1016/j.febslet.2014.08.029. [DOI] [PubMed] [Google Scholar]
- Rubino F., Amiel S.A., Zimmet P., Alberti G., Bornstein S., Eckel R.H., Mingrone G., Boehm B., Cooper M.E., Chai Z., Del Prato S., Ji L., Hopkins D., Herman W.H., Khunti K., Mbanya J.-C., Renard E. New-onset diabetes in covid-19. N. Engl. J. Med. 2020;383:789–790. doi: 10.1056/nejmc2018688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sama I.E., Ravera A., Santema B.T., Van Goor H., Ter Maaten J.M., Cleland J.G.F., Rienstra M., Friedrich A.W., Samani N.J., Ng L.L., Dickstein K., Lang C.C., Filippatos G., Anker S.D., Ponikowski P., Metra M., Van Veldhuisen D.J., Voors A.A. Circulating plasma concentrations of angiotensin-converting enzyme 2 inmen and women with heart failure and effects of renin-angiotensin-aldosterone inhibitors. Eur. Heart J. 2020;41:1810–1817. doi: 10.1093/eurheartj/ehaa373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos R.A.S., Ferreira A.J., Verano-Braga T., Bader M. Angiotensin-converting enzyme 2, angiotensin-(1-7) and Mas: new players of the renin-angiotensin system. J. Endocrinol. 2013;216 doi: 10.1530/JOE-12-0341. [DOI] [PubMed] [Google Scholar]
- Sathish T., Kapoor N., Cao Y., Tapp R.J., Zimmet P. Proportion of newly diagnosed diabetes in COVID-19 patients: a systematic review and meta-analysis. Diabetes Obes. Metabol. 2021 doi: 10.1111/dom.14269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer A.K., Pfaender S., Hagmeyer S., Tarana L., Mattes A.K., Briel F., Küry S., Boeckers T.M., Grabrucker A.M. Characterization of zinc amino acid complexes for zinc delivery in vitro using Caco-2 cells and enterocytes from hiPSC. Biometals. 2017;30:643–661. doi: 10.1007/s10534-017-0033-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmaier A.H. The kallikrein-kinin and the renin-angiotensin systems have a multilayered interaction. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003 doi: 10.1152/ajpregu.00535.2002. [DOI] [PubMed] [Google Scholar]
- Schülke S. Induction of interleukin-10 producing dendritic cells as a tool to suppress allergen-specific T helper 2 responses. Front. Immunol. 2018;9 doi: 10.3389/fimmu.2018.00455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenzato L., Botton J., Drouin J., Baricault B., Vabre C., Cuenot F., Penso L., Herlemont P., Sbidian E., Weill A., Dray-Spira R., Zureik M. Antihypertensive drugs and COVID-19 risk: a cohort study of 2 million hypertensive patients. Hypertension. 2021;77:833–842. doi: 10.1161/HYPERTENSIONAHA.120.16314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senchenkova E.Y., Russell J., Almeida-Paula L.D., Harding J.W., Granger D.N. Angiotensin II-mediated microvascular thrombosis. Hypertension. 2010;56:1089–1095. doi: 10.1161/HYPERTENSIONAHA.110.158220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma N., Anders H.J., Gaikwad A.B. Fiend and friend in the renin angiotensin system: an insight on acute kidney injury. Biomed. Pharmacother. 2019 doi: 10.1016/j.biopha.2018.12.018. [DOI] [PubMed] [Google Scholar]
- Shen X.Z., Okwan-Duodu D., Blackwell W.L., Ong F.S., Janjulia T., Bernstein E.A., Fuchs S., Alkan S., Bernstein K.E. Myeloid expression of angiotensin-converting enzyme facilitates myeloid maturation and inhibits the development of myeloid-derived suppressor cells. Lab. Invest. 2014;94:536–544. doi: 10.1038/labinvest.2014.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenoy V., Gjymishka A., Jarajapu Y.P., Qi Y., Afzal A., Rigatto K., Ferreira A.J., Fraga-Silva R.A., Kearns P., Douglas J.Y., Agarwal D., Mubarak K.K., Bradford C., Kennedy W.R., Jun J.Y., Rathinasabapathy A., Bruce E., Gupta D., Cardounel A.J., Mocco J., Patel J.M., Francis J., Grant M.B., Katovich M.J., Raizada M.K. Diminazene attenuates pulmonary hypertension and improves angiogenic progenitor cell functions in experimental models. Am. J. Respir. Crit. Care Med. 2013;187:648–657. doi: 10.1164/rccm.201205-0880OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simões E Silva A.C., Silveira K.D., Ferreira A.J., Teixeira M.M. ACE2, angiotensin-(1-7) and Mas receptor axis in inflammation and fibrosis. Br. J. Pharmacol. 2013 doi: 10.1111/bph.12159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skidgel R.A., Erdös E.G. The broad substrate specificity of human angiotensin i converting enzyme. Clin. Exp. Hypertens. A9. 1987:243–259. doi: 10.3109/10641968709164184. [DOI] [PubMed] [Google Scholar]
- Sodhi C.P., Wohlford-Lenane C., Yamaguchi Y., Prindle T., Fulton W.B., Wang S., McCray P.B., Chappell M., Hackam D.J., Jia H. Attenuation of pulmonary ACE2 activity impairs inactivation of des-arg9 bradykinin/BKB1R axis and facilitates LPS-induced neutrophil infiltration. Am. J. Physiol. Lung Cell Mol. Physiol. 2018;314:L17–L31. doi: 10.1152/ajplung.00498.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soler M.J., Wysocki J., Ye M., Lloveras J., Kanwar Y., Batlle D. ACE2 inhibition worsens glomerular injury in association with increased ACE expression in streptozotocin-induced diabetic mice. Kidney Int. 2007;72:614–623. doi: 10.1038/sj.ki.5002373. [DOI] [PubMed] [Google Scholar]
- Song W., Gui M., Wang X., Xiang Y. Cryo-EM structure of the SARS coronavirus spike glycoprotein in complex with its host cell receptor ACE2. PLoS Pathog. 2018;14 doi: 10.1371/journal.ppat.1007236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soro-Paavonen A., Gordin D., Forsblom C., Rosengard-Barlund M., Waden J., Thorn L., Sandholm N., Thomas M.C., Groop P.H. Circulating ACE2 activity is increased in patients with type 1 diabetes and vascular complications. J. Hypertens. 2012;30:375–383. doi: 10.1097/HJH.0b013e32834f04b6. [DOI] [PubMed] [Google Scholar]
- Su J.B. Different cross-talk sites between the renin-angiotensin and the kallikrein-kinin systems. JRAAS - J. Renin-Angiotensin-Aldosterone Syst. 2014 doi: 10.1177/1470320312474854. [DOI] [PubMed] [Google Scholar]
- Sul J.W., Kim T.Y., Yoo H.J., Kim J., Suh Y.A., Hwang J.J., Koh J.Y. A novel mechanism for the pyruvate protection against zinc-induced cytotoxicity: mediation by the chelating effect of citrate and isocitrate. Arch Pharm. Res. (Seoul) 2016;39:1151–1159. doi: 10.1007/s12272-016-0814-9. [DOI] [PubMed] [Google Scholar]
- Suryadevara N., Shrihari S., Gilchuk P., VanBlargan L.A., Binshtein E., Zost S.J., Nargi R.S., Sutton R.E., Winkler E.S., Chen E.C., Fouch M.E., Davidson E., Doranz B.J., Chen R.E., Shi P.Y., Carnahan R.H., Thackray L.B., Diamond M.S., Crowe J.E. Neutralizing and protective human monoclonal antibodies recognizing the N-terminal domain of the SARS-CoV-2 spike protein. Cell. 2021;184:2316–2331. doi: 10.1016/j.cell.2021.03.029. e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suter F., Consolaro E., Pedroni S., Moroni C., Pastò E., Paganini M.V., Pravettoni G., Cantarelli U., Rubis N., Perico N., Perna A., Peracchi T., Ruggenenti P., Remuzzi G. A simple, home-therapy algorithm to prevent hospitalisation for COVID-19 patients: a retrospective observational matched-cohort study. EClinicalMedicine. 2021 doi: 10.1016/j.eclinm.2021.100941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi S., Yoshiya T., Yoshizawa-Kumagaye K., Sugiyama T. Nicotianamine is a novel angiotensin-converting enzyme 2 inhibitor in soybean. Biomed. Res. 2015;36:219–224. doi: 10.2220/biomedres.36.219. [DOI] [PubMed] [Google Scholar]
- Thomson E.C., Rosen L.E., Shepherd J.G., Spreafico R., da Silva Filipe A., Wojcechowskyj J.A., Davis C., Piccoli L., Pascall D.J., Dillen J., Lytras S., Czudnochowski N., Shah R., Meury M., Jesudason N., De Marco A., Li K., Bassi J., O’Toole A., Pinto D., Colquhoun R.M., Culap K., Jackson B., Zatta F., Rambaut A., Jaconi S., Sreenu V.B., Nix J., Zhang I., Jarrett R.F., Glass W.G., Beltramello M., Nomikou K., Pizzuto M., Tong L., Cameroni E., Croll T.I., Johnson N., Di Iulio J., Wickenhagen A., Ceschi A., Harbison A.M., Mair D., Ferrari P., Smollett K., Sallusto F., Carmichael S., Garzoni C., Nichols J., Galli M., Hughes J., Riva A., Ho A., Schiuma M., Semple M.G., Openshaw P.J.M., Fadda E., Baillie J.K., Chodera J.D., Rihn S.J., Lycett S.J., Virgin H.W., Telenti A., Corti D., Robertson D.L., Snell G. Circulating SARS-CoV-2 spike N439K variants maintain fitness while evading antibody-mediated immunity. Cell. 2021;184:1171–1187.e20. doi: 10.1016/j.cell.2021.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tikellis C., Bialkowski K., Pete J., Sheehy K., Su Q., Johnston C., Cooper M.E., Thomas M.C. ACE2 deficiency modifies renoprotection afforded by ACE inhibition in experimental diabetes. Diabetes. 2008;57:1018–1025. doi: 10.2337/db07-1212. [DOI] [PubMed] [Google Scholar]
- Tobaiqy M., Elkout H., Maclure K. Analysis of thrombotic adverse reactions of covid-19 astrazeneca vaccine reported to eudravigilance database. Vaccines. 2021;9 doi: 10.3390/vaccines9040393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolouian R., Vahed S.Z., Ghiyasvand S., Tolouian A., Ardalan M. COVID-19 interactions with angiotensin-converting enzyme 2 (ACE2) and the kinin system; looking at a potential treatment. J. Ren. Inj. Prev. 2020;9 doi: 10.34172/jrip.2020.19. e19–e19. [DOI] [Google Scholar]
- Towler P., Staker B., Prasad S.G., Menon S., Tang J., Parsons T., Ryan D., Fisher M., Williams D., Dales N.A., Patane M.A., Pantoliano M.W. ACE2 X-ray structures reveal a large hinge-bending motion important for inhibitor binding and catalysis. J. Biol. Chem. 2004;279:17996–18007. doi: 10.1074/jbc.M311191200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trask A.J., Groban L., Westwood B.M., Varagic J., Ganten D., Gallagher P.E., Chappell M.C., Ferrario C.M. Inhibition of angiotensin-converting enzyme 2 exacerbates cardiac hypertrophy and fibrosis in ren-2 hypertensive rats. Am. J. Hypertens. 2010;23:687–693. doi: 10.1038/ajh.2010.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner A.J. ACE2 cell biology, regulation, and physiological functions. Prot. Arm Renin Angiotensin Syst. Funct. Asp. Ther. Implic. 2015:185–189. doi: 10.1016/B978-0-12-801364-9.00025-0. [DOI] [Google Scholar]
- Úri K., Fagyas M., Siket I.M., Kertész A., Csanádi Z., Sándorfi G., Clemens M., Fedor R., Papp Z., Édes I., Tóth A., Lizanecz E. New perspectives in the renin-angiotensin-aldosterone system (RAAS) IV: circulating ACE2 as a biomarker of systolic dysfunction in human hypertension and heart failure. PloS One. 2014;9 doi: 10.1371/journal.pone.0087845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Úri K., Fagyas M., Kertész A., Borbély A., Jenei C., Bene O., Csanádi Z., Paulus W.J., Édes I., Papp Z., Tóth A., Lizanecz E. Circulating ACE2 activity correlates with cardiovascular disease development. JRAAS - J. Renin-Angiotensin-Aldosterone Syst. 2016;17 doi: 10.1177/1470320316668435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaduganathan M., Vardeny O., Michel T., McMurray J.J.V., Pfeffer M.A., Solomon S.D. Renin–angiotensin–aldosterone system inhibitors in patients with covid-19. N. Engl. J. Med. 2020;382:1653–1659. doi: 10.1056/nejmsr2005760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidya A., Williams J.S. The relationship between vitamin D and the renin-angiotensin system in the pathophysiology of hypertension, kidney disease, and diabetes. Metabolism. 2012 doi: 10.1016/j.metabol.2011.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Veerdonk F.L., Netea M.G., van Deuren M., van der Meer J.W.M., de Mast Q., Brüggemann R.J., van der Hoeven H. Kallikrein-kinin blockade in patients with covid-19 to prevent acute respiratory distress syndrome. Elife. 2020;9 doi: 10.7554/ELIFE.57555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan D.E., Lazos S.A., Tong K. Angiotensin II regulates the expression of plasminogen activator inhibitor-1 in cultured endothelial cells: a potential link between the renin-angiotensin system and thrombosis. J. Clin. Invest. 1995;95:995–1001. doi: 10.1172/JCI117809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velez J.C.Q., Ierardi J.L., Bland A.M., Morinelli T.A., Arthur J.M., Raymond J.R., Janech M.G. Enzymatic processing of angiotensin peptides by human glomerular endothelial cells. Am. J. Physiol. Ren. Physiol. 2012;302 doi: 10.1152/ajprenal.00087.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickers C., Hales P., Kaushik V., Dick L., Gavin J., Tang J., Godbout K., Parsons T., Baronas E., Hsieh F., Acton S., Patane M., Nichols A., Tummino P. Hydrolysis of biological peptides by human angiotensin-converting enzyme-related carboxypeptidase. J. Biol. Chem. 2002;277:14838–14843. doi: 10.1074/jbc.M200581200. [DOI] [PubMed] [Google Scholar]
- Wallentin L., Lindbäck J., Eriksson N., Hijazi Z., Eikelboom J.W., Ezekowitz M.D., Granger C.B., Lopes R.D., Yusuf S., Oldgren J., Siegbahn A. Angiotensin-converting enzyme 2 (ACE2) levels in relation to risk factors for COVID-19 in two large cohorts of patients with atrial fibrillation. Eur. Heart J. 2020;41:4037–4046. doi: 10.1093/eurheartj/ehaa697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters T.E., Kalman J.M., Patel S.K., Mearns M., Velkoska E., Burrell L.M. Angiotensin converting enzyme 2 activity and human atrial fibrillation: increased plasma angiotensin converting enzyme 2 activity is associated with atrial fibrillation and more advanced left atrial structural remodelling. Europace. 2017;19:1280–1287. doi: 10.1093/europace/euw246. [DOI] [PubMed] [Google Scholar]
- Xie F., Zhang X., Xie L. Prognostic value of serum zinc levels in patients with acute HC/zinc chloride smoke inhalation. Med. Times. 2017;96 doi: 10.1097/MD.0000000000008156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu P., Sriramula S., Lazartigues E. ACE2/ANG-(1-7)/Mas pathway in the brain: the axis of good. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011 doi: 10.1152/ajpregu.00222.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue J., Moyer A., Peng B., Wu J., Hannafon B.N., Ding W.Q. Chloroquine is a zinc ionophore. PloS One. 2014;9 doi: 10.1371/journal.pone.0109180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagisawa M., Foster P.J., Hanaki H., Ōmura S. 2021. Global Trends in Clinical Studies of Ivermectin in COVID-19. [Google Scholar]
- Yan R., Zhang Y., Li Y., Xia L., Guo Y., Zhou Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science. 2020;367:1444–1448. doi: 10.1126/science.abb2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G., Tan Z., Zhou L., Yang M., Peng L., Liu J., Cai J., Yang R., Han J., Huang Y., He S. Effects of angiotensin II receptor blockers and ACE (Angiotensin-Converting enzyme) inhibitors on virus infection, inflammatory status, and clinical outcomes in patients with COVID-19 and hypertension: a single-center retrospective study. Hypertension. 2020;76:51–58. doi: 10.1161/HYPERTENSIONAHA.120.15143. [DOI] [PubMed] [Google Scholar]
- Yaseen M.M., Abuharfeil N.M., Darmani H., Daoud A. Mechanisms of immune suppression by myeloid-derived suppressor cells: the role of interleukin-10 as a key immunoregulatory cytokine. Open Biol. 2020 doi: 10.1098/rsob.200111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye M., Wysocki J., Gonzalez-Pacheco F.R., Salem M., Evora K., Garcia-Halpin L., Poglitsch M., Schuster M., Batlle D. Murine recombinant angiotensin-converting enzyme 2: effect on angiotensin II-dependent hypertension and distinctive angiotensin-converting enzyme 2 inhibitor characteristics on rodent and human angiotensin-converting enzyme 2. Hypertension. 2012;60:730–740. doi: 10.1161/HYPERTENSIONAHA.112.198622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung M.L., Teng J.L.L., Jia L., Zhang C., Huang C., Cai J.P., Zhou R., Chan K.H., Zhao H., Zhu L., Siu K.L., Fung S.Y., Yung S., Chan T.M., To K.K.W., Chan J.F.W., Cai Z., Lau S.K.P., Chen Z., Jin D.Y., Woo P.C.Y., Yuen K.Y. Soluble ACE2-mediated cell entry of SARS-CoV-2 via interaction with proteins related to the renin-angiotensin system. Cell. 2021;184:2212–2228. doi: 10.1016/j.cell.2021.02.053. e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C.M., Wong R.S.M., Wu E.B., Kong S.L., Wong J., Yip G.W.K., Soo Y.O.Y., Chiu M.L.S., Chan Y.S., Hui D., Lee N., Wu A., Leung C.B., Sung J.J.Y. Cardiovascular complications of severe acute respiratory syndrome. Postgrad. Med. 2006;82:140–144. doi: 10.1136/pgmj.2005.037515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Y., Cao D., Zhang Y., Ma J., Qi J., Wang Q., Lu G., Wu Y., Yan J., Shi Y., Zhang X., Gao G.F. Cryo-EM structures of MERS-CoV and SARS-CoV spike glycoproteins reveal the dynamic receptor binding domains. Nat. Commun. 2017;8 doi: 10.1038/ncomms15092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan S., Wang R., Chan J.F.W., Zhang A.J., Cheng T., Chik K.K.H., Ye Z.W., Wang S., Lee A.C.Y., Jin L., Li H., Jin D.Y., Yuen K.Y., Sun H. Metallodrug ranitidine bismuth citrate suppresses SARS-CoV-2 replication and relieves virus-associated pneumonia in Syrian hamsters. Nat. Microbiol. 2020;5:1439–1448. doi: 10.1038/s41564-020-00802-x. [DOI] [PubMed] [Google Scholar]
- Zamai L. The yin and Yang of ACE/ACE2 pathways: the rationale for the use of renin-angiotensin system inhibitors in COVID-19 patients. Cells. 2020 doi: 10.3390/cells9071704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamai L. Unveiling human non-random genome editing mechanisms activated in response to chronic environmental changes: I. Where might these mechanisms come from and what might they have led to? Cells. 2020 doi: 10.3390/cells9112362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamai L. Upregulation of the renin-angiotensin system pathways and SARS-CoV-2 infection: the rationale for the administration of zinc-chelating agents in COVID-19 patients. Cells. 2021;10:506. doi: 10.3390/cells10030506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamai L., Rocchi M.B.L. Hypothesis: possible influence of antivector immunity and SARS-CoV-2 variants on efficacy of ChAdOx1 nCoV-19 vaccine. Br. J. Pharmacol. bph. 2021;15620 doi: 10.1111/BPH.15620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanza C., Racca F., Longhitano Y., Piccioni A., Franceschi F., Artico M., Abenavoli L., Maiese A., Passaro G., Volonnino G., Russa R. La. Risk management and treatment of coagulation disorders related to COVID-19 infection. Int. J. Environ. Res. Publ. Health. 2021 doi: 10.3390/ijerph18031268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S., Liu Y., Wang X., Yang L., Li H., Wang Y., Liu M., Zhao X., Xie Y., Yang Y., Zhang Shenghui, Fan Z., Dong J., Yuan Z., Ding Z., Zhang Y., Hu L. SARS-CoV-2 binds platelet ACE2 to enhance thrombosis in COVID-19. J. Hematol. Oncol. 2020;13 doi: 10.1186/s13045-020-00954-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R., Wu Y., Zhao M., Liu C., Zhou L., Shen S., Liao S., Yang K., Li Q., Wan H. Role of HIF-1α in the regulation ACE and ACE2 expression in hypoxic human pulmonary artery smooth muscle cells. Am. J. Physiol. Lung Cell Mol. Physiol. 2009;297 doi: 10.1152/ajplung.90415.2008. [DOI] [PubMed] [Google Scholar]
- Zhang Xue, Yu J., Pan L., ya Jiang H.yin. ACEI/ARB use and risk of infection or severity or mortality of COVID-19: a systematic review and meta-analysis. Pharmacol. Res. 2020 doi: 10.1016/j.phrs.2020.104927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Xiaoqing, Zhang X., Li S., Niu S. ACE2 and COVID-19 and the resulting ARDS. Postgrad. Med. 2020 doi: 10.1136/postgradmedj-2020-137935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P., Zhu L., Cai J., Lei F., Qin J.J., Xie J., Liu Y.M., Zhao Y.C., Huang Xuewei, Lin L., Xia M., Chen M.M., Cheng X., Zhang X., Guo D., Peng Y., Ji Y.X., Chen J., She Z.G., Wang Y., Xu Q., Tan R., Wang H., Lin J., Luo P., Fu S., Cai H., Ye P., Xiao B., Mao W., Liu L., Yan Y., Liu M., Chen M., Zhang X.J., Wang X., Touyz R.M., Xia J., Zhang B.H., Huang Xiaodong, Yuan Y., Rohit L., Liu P.P., Li H. Association of inpatient use of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers with mortality among patients with hypertension hospitalized with COVID-19. Circ. Res. 2020;126:1671–1681. doi: 10.1161/CIRCRESAHA.120.317134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Qin L., Zhang P., Li K., Liang L., Sun J., Xu B., Dai Y., Li X., Zhang C., Peng Y., Feng Y., Li A., Hu Z., Xiang H., Ogg G., Ho L.P., McMichael A., Jin R., Knight J.C., Dong T., Zhang Y. Longitudinal COVID-19 profiling associates IL-1RA and IL-10 with disease severity and RANTES with mild disease. JCI Insight. 2020;5 doi: 10.1172/jci.insight.139834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H., Chen Y., Zhang Shuyuan, Niu P., Qin K., Jia W., Huang B., Zhang Senyan, Lan J., Zhang L., Tan W., Wang X. Structural definition of a neutralization epitope on the N-terminal domain of MERS-CoV spike glycoprotein. Nat. Commun. 2019;10 doi: 10.1038/s41467-019-10897-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler C.G.K., Allon S.J., Nyquist S.K., Mbano I.M., Miao V.N., Tzouanas C.N., Cao Y., Yousif A.S., Bals J., Hauser B.M., Feldman J., Muus C., Wadsworth M.H., Kazer S.W., Hughes T.K., Doran B., Gatter G.J., Vukovic M., Taliaferro F., Mead B.E., Guo Z., Wang J.P., Gras D., Plaisant M., Ansari M., Angelidis I., Adler H., Sucre J.M.S., Taylor C.J., Lin B., Waghray A., Mitsialis V., Dwyer D.F., Buchheit K.M., Boyce J.A., Barrett N.A., Laidlaw T.M., Carroll S.L., Colonna L., Tkachev V., Peterson C.W., Yu A., Zheng H.B., Gideon H.P., Winchell C.G., Lin P.L., Bingle C.D., Snapper S.B., Kropski J.A., Theis F.J., Schiller H.B., Zaragosi L.E., Barbry P., Leslie A., Kiem H.P., Flynn J.A.L., Fortune S.M., Berger B., Finberg R.W., Kean L.S., Garber M., Schmidt A.G., Lingwood D., Shalek A.K., Ordovas-Montanes J., Banovich N., Brazma A., Desai T., Duong T.E., Eickelberg O., Falk C., Farzan M., Glass I., Haniffa M., Horvath P., Hung D., Kaminski N., Krasnow M., Kuhnemund M., Lafyatis R., Lee H., Leroy S., Linnarson S., Lundeberg J., Meyer K., Misharin A., Nawijn M., Nikolic M.Z., Pe’er D., Powell J., Quake S., Rajagopal J., Tata P.R., Rawlins E.L., Regev A., Reyfman P.A., Rojas M., Rosen O., Saeb-Parsy K., Samakovlis C., Schiller H., Schultze J.L., Seibold M.A., Shepherd D., Spence J., Spira A., Sun X., Teichmann S., Theis F., Tsankov A., van den Berge M., von Papen M., Whitsett J., Xavier R., Xu Y., Zhang K. Kinins and chymase: The forgotten components of the renin-angiotensin system and their implications in COVID-19 disease. Am. J. Physiol. - Lung Cell. Mol. Physiol. 2021 doi: 10.1152/AJPLUNG.00548.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoufaly A., Poglitsch M., Aberle J.H., Hoepler W., Seitz T., Traugott M., Grieb A., Pawelka E., Laferl H., Wenisch C., Neuhold S., Haider D., Stiasny K., Bergthaler A., Puchhammer-Stoeckl E., Mirazimi A., Montserrat N., Zhang H., Slutsky A.S., Penninger J.M. Human recombinant soluble ACE2 in severe COVID-19. Lancet Respir. Med. 2020;8:1154–1158. doi: 10.1016/S2213-2600(20)30418-5. [DOI] [PMC free article] [PubMed] [Google Scholar]