Abstract
Viruses cause widely transmitted diseases resulting in pandemic conditions. Currently, the world is being hit by the Covid-19 pandemic caused by the SAR-CoV-2 infection. Countries in the world are competing to develop antivirals to overcome this problem. Diterpene compounds derived from natural ingredients (plants, corals, algae, fungi, sponges) and synthesized products have potential as antivirals. This article summarizes the different types of diterpenes such as daphnane, tiglilane, kaurane, abietane, pimarane, labdane, dollabelane, jatrophane, dolastane, prenylated guaiane, tonantzitlolone, casbane, have antivirals activity such as targeting HIV, Coxsackie virus, herpes virus, hepatitis virus, influenza virus, Chikungunya virus, Zika virus, dengue virus, and SARS-CoV. Some compounds such as andrographolide and its derivatives show promising activity in inhibiting the influenza virus. Additionally, compounds such as pineolidic acid, forskolin, sugiol, and many other diterpene compounds showed anti-SAR-CoV activity. The diterpene compound class's high antivirals potential does not rule out the possibility that these compounds can also act as anti-SAR-CoV-2 drugs in the future.
Keywords: Antivirals, Covid-19, Diterpenes, Pandemic, SARS-CoV-2, Infectious diseases
Antivirals, Covid-19, Diterpenes, Pandemic, SARS-CoV-2, Infectious diseases.
1. Introduction
Many of the infectious diseases epidemics which becomes a pandemic, which occur due to viral infections. Throughout history, there have been many disease outbreaks caused by viral infections, including Spanish Flu Pandemic (1918–1920), Smallpox (1972), HIV epidemic (1981), SARS (2003), H1N1 Pandemic (2009), Ebola Virus (2014–2016), Zika Virus (2015–2016) (Huremović, 2019). Moreover, in December 2019 there were new cases of pneumonia, and the first reported cases in China were caused by infection with a new virus, novel coronavirus (SARS-CoV-2) known as novel coronavirus disease 2019 (COVID-19) by WHO (Lai et al., 2020). In general, viruses are intracellular parasites containing single-stranded or double-stranded, linear or circular RNA or DNA genomes (Gelderblom, 1996; Gohar et al., 2021).
Pandemics have a direct impact on health and can also adversely affect economic, social, and political stability. The pandemic has infected millions of people and caused thousands of deaths. Travel is strictly restricted, the closure of schools, markets, and specific sectors that will indirectly affect economic, social, security growth, etc (Qiu et al., 2017). The problem of a pandemic is a global problem, and every country is racing to overcome it. The development of antivirals is one of the efforts to accelerate recovery from normal conditions. One of the antiviral compounds that have been widely observed is the terpene group which can be obtained from natural or synthetic materials.
Diterpenes are the product of the mevalonic acid biosynthesis pathway (Singh and Sharma, 2015). Some diterpenes indicated to have antivirals activity, such as kirkinine, excoecariatoxin (anti-HIV) (Olivon et al., 2015), jiadifenoic acids JP (anti-Coxsackie virus) (Zhang et al., 2014), briaexcavatolide U, briaexcavatin L (anti-HCMV) (Yeh et al., 2012), genkwanine P, laurifolioside A (anti-HBV) (Li et al., 2018), linearol, isosidol (anti-HPIV-2) (Kilic et al., 2020), debromoaplysiatoxin (anti-CHIKV) (Gupta et al., 2014) and others. Additionally, several subclasses of diterpene compounds have potential antiviral activity, such as daphnane (Figure 1), tiglane (Figure 2), kaurene (Figure 3), abietane (Figure 4), pimarane (Figure 5), labdane (Figure 6), dolabellane (Figure 7), jatrophan (Figure 8), tonantzitlolone (Figure 9), and miscellaneous (Figures 10 and 11), which are the main focus of this article. The purpose of this review article is to examine the terpenes compounds that have the potential in the development of antivirals to overcome the current pandemic.
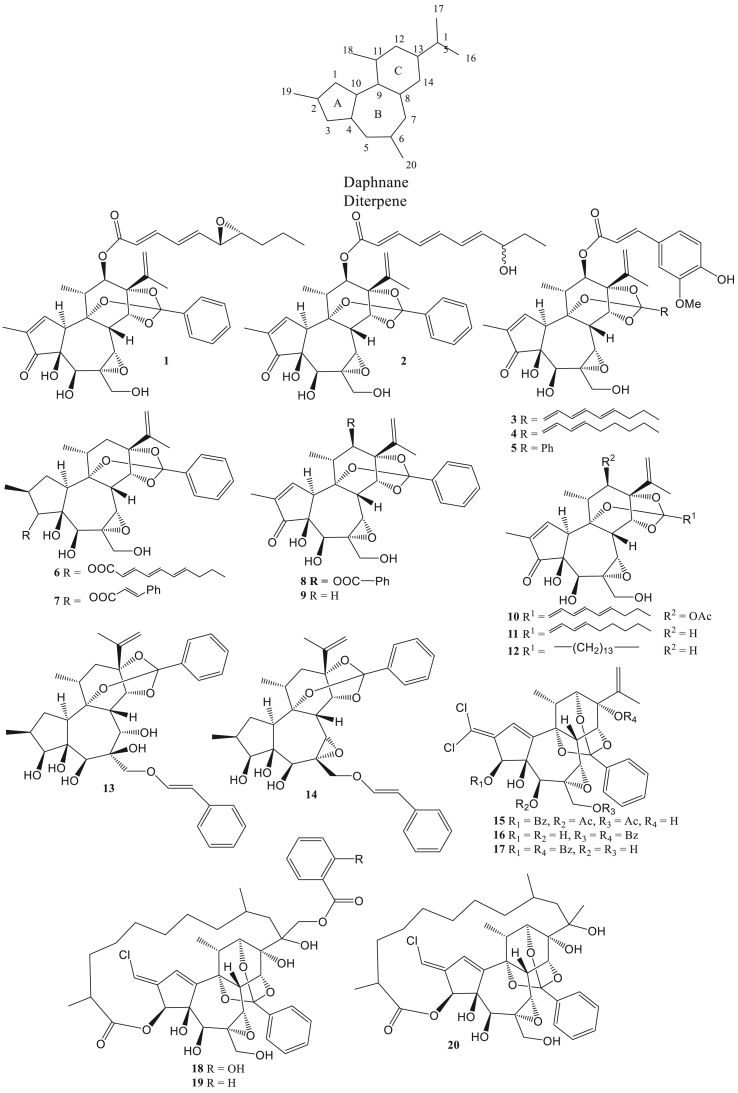
Figure 1.
Daphnane diterpen as antivirals.
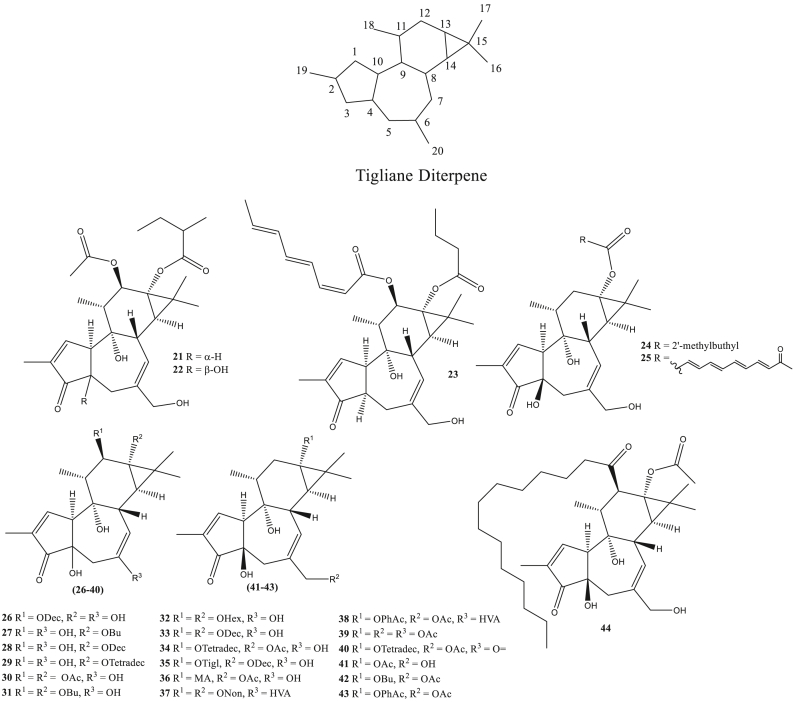
Figure 2.
Tiglilane diterpene as antivirals.
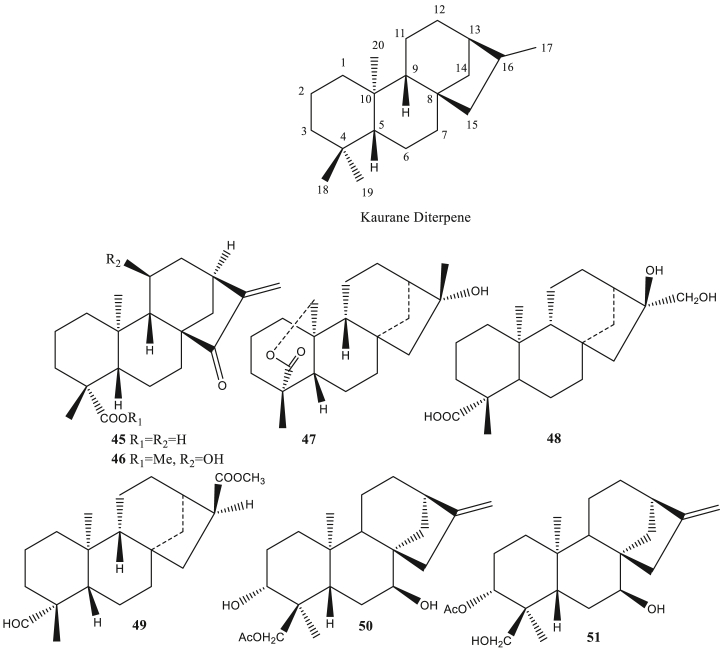
Figure 3.
Kaurene diterpene as antivirals.
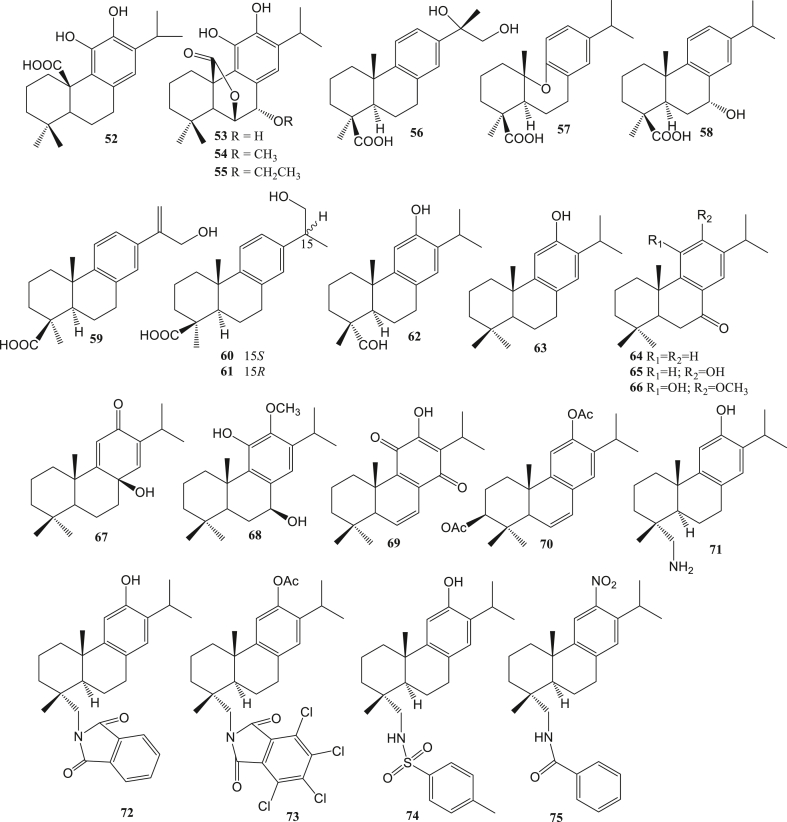
Figure 4.
Abietane diterpene as antivirals.
Figure 5.
Pimarane diterpene as antivirals.
Figure 6.
Labdane diterpene as antivirals.
Figure 7.
Dolabellane diterpene as antivirals.
Figure 8.
Jatrophane (132–137), Dolastane (138–142), and Prenylated guaiane diterpene (143–144) as antivirals.
Figure 9.
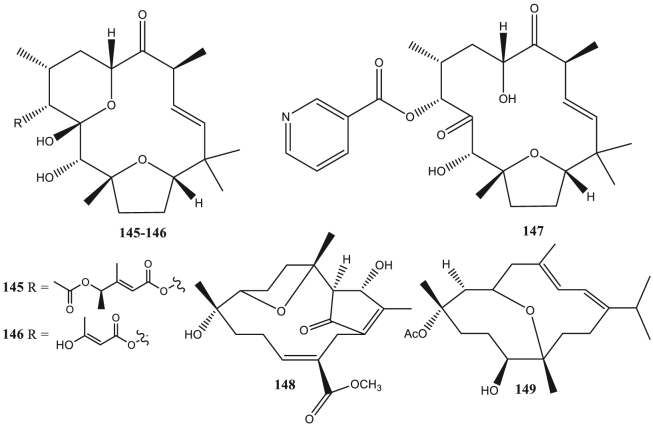
Tonantzitlolone (145–147), and Casbane (148–149) diterpene as antivirals.
Figure 10.
Miscellaneous diterpene as antivirals.
Figure 11.
Miscellaneous diterpene as antivirals (continuation).
2. Type of diterpenes
Diterpenes are chemical compounds consisting of two isoprene units. These compounds are found in animals, plants, fungi, algae, coral, and others. Several types of diterpene compounds such as daphnane, tiglilane, kaurene, abietane, pimarane, labdane, dolabellane, jatrophane, dolastane, prenylated guaiane diterpene, tonantzitlolone, casbane, and miscellaneous. Diterpene compounds have pharmacological activities such as antivirals. Daphnane diterpene (1–20) is one type of diterpene that generally in the form of a 5/7/6-tricyclic ring equipped with a polyhydroxyl group at positions C3, C4, C5, C9, C13, C14, or C20. Additionally, several diterpene daphnane groups have orthoester groups that bind to C9, C13, and C14 (Jin et al., 2019). Daphnane diterpenes such as acutilobins A, wikstchalide W, Trigocherrins A, and others. The type of daphnane diterpene has antivirals activity such as anti-HIV ((Huang et al., 2012), anti-HBV (Li et al., 2018), anti-DENV, and anti-CHIKV (Allard et al., 2012).
Tiglilane diterpene (21–44) is a macrocyclic diterpene that has a basic structure similar to the diterpene daphnane. Besides, it has the addition of ring 3 bound to C13 and C14. Tigliane diterpenes are composed of a 5/6/7/3-tetracyclic ring. Tiglilane diterpene group compounds such as phorbol-12-decanoate, phorbol-12,13,20-triacetate, 12-O-tetradecanoylphorbol-13-acetate, and others. Compounds of this type had been reported antivirals activities such as anti-HIV-1 and anti-HIV-2 (Nothias-Scaglia et al., 2015; Olivon et al., 2015).
Kaurane diterpene (45–51) is one of the diterpenes that belong to the tetracyclic type. The kauren structure is composed of 4 rings consisting of 3 cyclohexane rings that fuse to form a perhydrofenanthrenic structure and 1 cyclopentane ring formed by a two carbon bridge between C8 and C13 (García et al., 2007). Compounds of this type include ent-15-oxo-kaur-16-en-19-oic acid, tripterifordin, linearol and others. This type has Antivirals activity, such as anti-HIV (Yan et al., 2018), and anti-HPIV-2 (Dang et al., 2015).
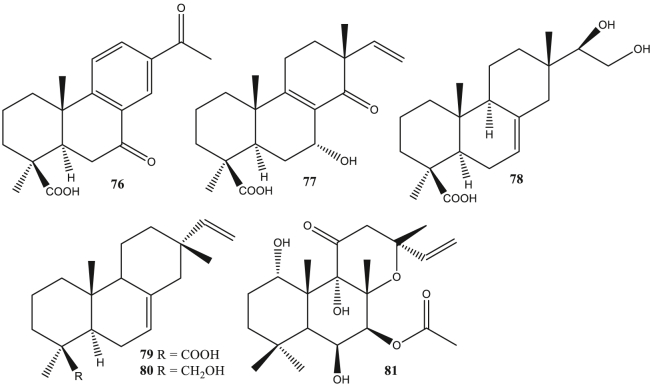
Abietane diterpene (52–75) is a tricyclic diterpene consisting of about 20 carbons. Compounds belonging to this type include rosmanol, jiadifenoic acid C, sugiol, and others. Antivirals of this type, among others, as anti-HIV (Pariš et al., 1993), anti-Coxsackie (González and Zaragozá, 2014), anti-ZIKV (Sousa et al., 2020), anti-DENV (Sousa et al., 2020), and anti-SAR-CoV (Wen et al., 2007). Pimarane diterpene (76–81) is a type of tricyclic diterpene. Pimarane diterpene-type compounds include jiadifenoic acids L, 4-epi-isopiric acid, forskolin, and others. The reported antivirals for this type are anti-Coxsackie (Zhang et al., 2013) and anti-SAR-CoV (Wen et al., 2007).
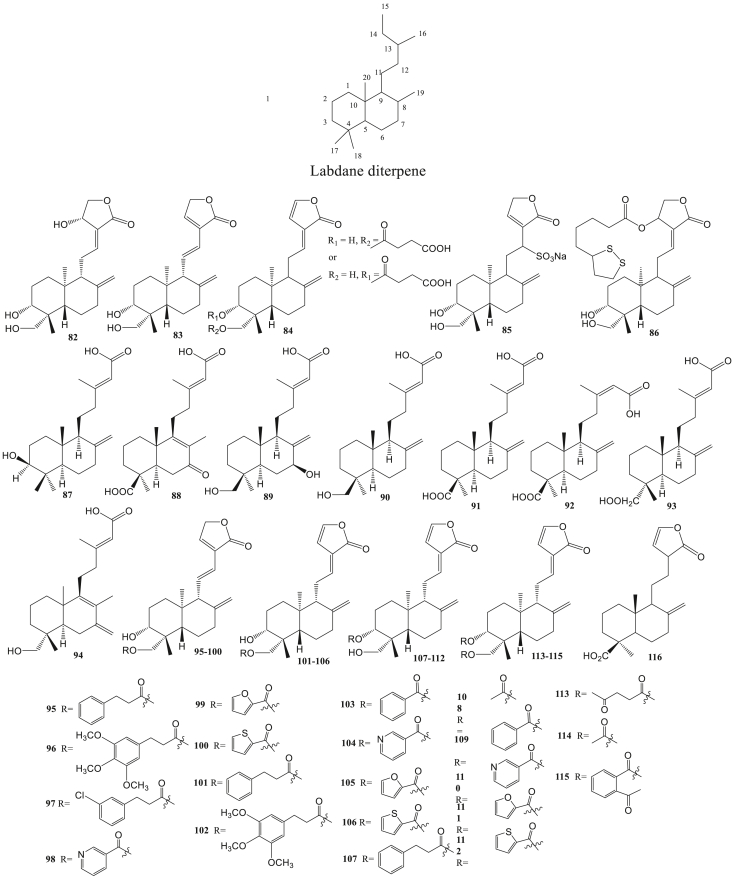
Labdane diterpene (82–116) is a bicyclic diterpene. The structure is composed of 4 isoprene units. The basic structure consists of two parts, namely two cyclohexene rings (C1–C10) which are joined to form a decalin system and five-carbon chains (C11–C16) bonded to C-9. There are five methyl branches attached to C4 (for C18 and C19), C-8 (for C17), C10 (for C20), and C13 (for C16) respectively (Tran et al., 2017). The types of labdane diterpenes include andrographolide, forsyshiyanins A, pineolidic acid, and others. This type of antivirals activity is like anti-HIV (Niranjan Reddy et al., 2005), anti-HBV (Chen et al., 2014), anti-DENV (Panraksa et al., 2017), and anti-influenza (Chen et al., 2009).
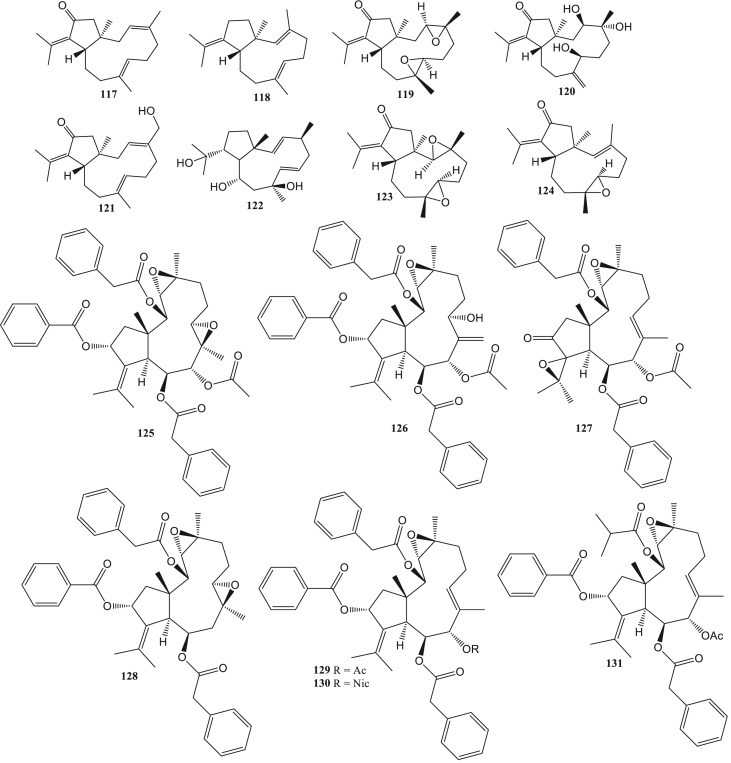
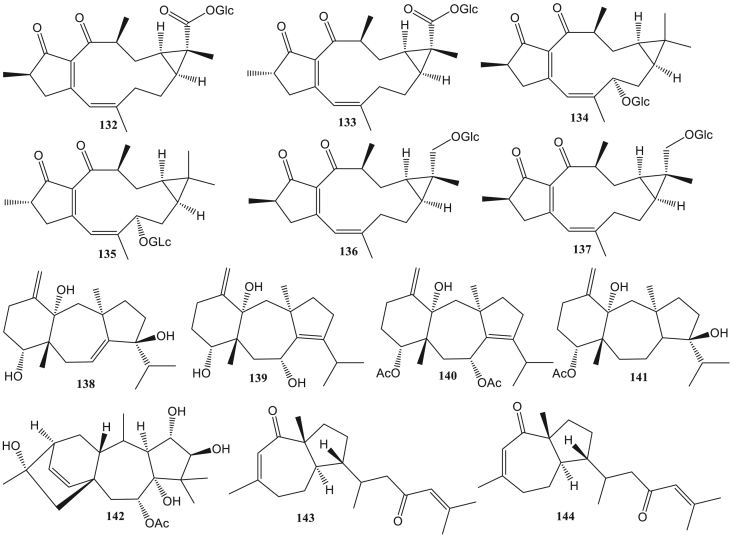
Dollabelane diterpene (117–131) is one of the diterpenes whose basic structure complies with the 5/11-bicyclic system. This type has anti-HIV (Pardo-Vargas et al., 2014) and anti-HSV (Ogawa et al., 2018). Jatrophane diterpene (132–137) is a bicyclic pentadecan diterpene. The basic structure of the diterpene jatrophane consists of four isoprene units forming a ring with a 5/12-bicyclic system. The structure is usually equipped with several substituents such as acyl groups, acetyl, ptopanoyl, butanoyl, isobutyryl, benzoyl, and others (Zhao et al., 2020). Jatrophane diterpenes had reported having antiviral activity such as anti-HBV (Li et al., 2018). Dolastane diterpene (138–142) is a diterpene composed of a 5/7/6-tricyclic ring system (Tuckett et al., 1998). This type of antivirals activity is anti-HSV (Vallim et al., 2010), anti-HHV, anti-BoHV-5 (Pinto et al., 2019), anti-CHIKV, and anti-ZIKV (Cirne-Santos et al., 2020).
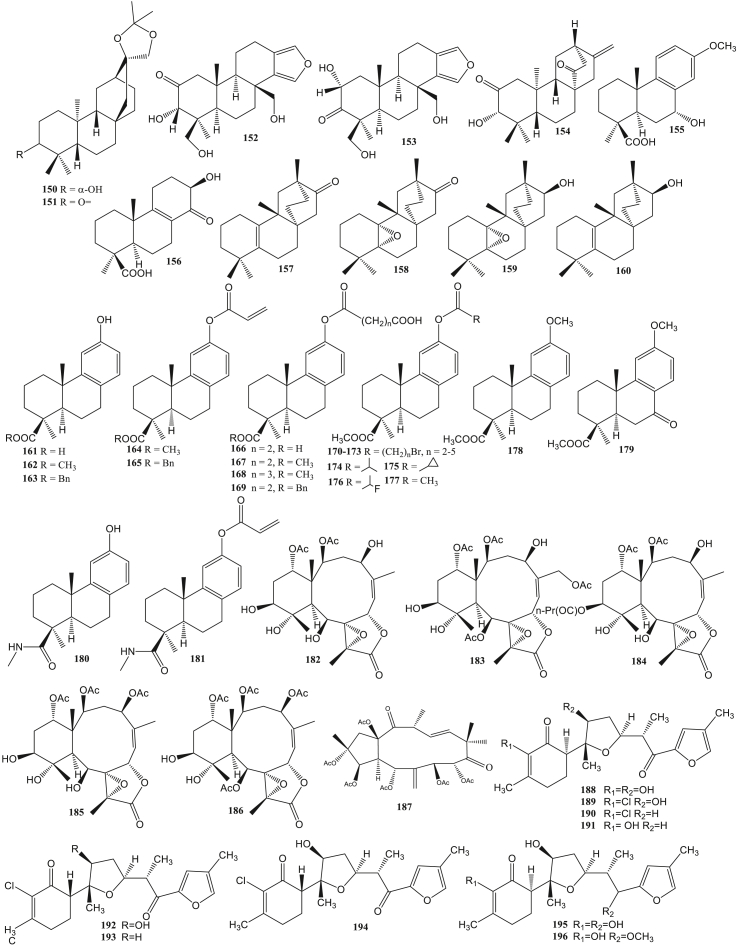
Prenylated guaiane diterpene (143–144) has been reported to be anti-HSV (Kashman et al., 1987). Tonantzitlolone diterpene (145–147) can act as an anti-CHIKV (Olivon et al., 2015). Casbane diterpene (148–149) has anti-HCMV activity (Wang et al., 2013a, Wang et al., 2013b). Miscellaneous diterpene (150–209) is the most common type of diterpene reported as antivirals in this report. This type is reported to be anti-HIV (Pereira et al., 2004), anti-Coxsackie (Gu et al., 2014), anti-HSV (González et al., 2010), anti-influenza virus (Dang et al., 2015), anti-HCMV (Wang et al., 2013a, Wang et al., 2013b), and anti-SAR-CoV (Wen et al., 2007) (Table S1).
3. Human Immunodeficiency Virus (HIV)
Human Immunodeficiency Virus (HIV) is a disease caused by a virus that affects the body's immune system which can lead to AIDS disease. It is recorded that until 2019 there were 75.7 million people infected with HIV from the start of the epidemic with a death rate of 32.7 million people with AIDS (HIV/AIDS, 2020). HIV belongs to the Retroviridae family and the genus Lentivirus, shaped like a ball with a diameter of 100 nm. The virus has two copies of single strain RNA and can transcribe (Lefkowitz et al., 2018). There are two types of Human Immunodeficiency Virus (HIV): Human Immunodeficiency Virus types 1 and 2 (HIV-1 and HIV-2). HIV-1 is known to spread throughout the world, while HIV-2 is spread in West Africa and India (Luzuriaga, 2018). HIV is cytotoxic to CD4+ cells. Infection of CD4+ cells causes a decrease in CD4 cell counts in HIV patients. HIV can be transmitted through sexual contact, blood transfusion, maternal and infant contact, after or before delivery (Levy and Castelli, 2019). HIV enters the host cell through the interaction between the virus's glycoprotein envelope of the receptor cells on the host cell. The viral RNA genome is inserted into the host cell through membrane fusion. The viral RNA genome undergoes a reverse transcription process with the help of the reverse transcriptase enzyme (Giese et al., 2016). Several diterpene compounds have been indicated to have anti-HIV activity both from the natural product and synthetic.
Daphnane diterpenes such as acutilobins A–G (1–7), daphnetoxin (8), genkwanine VIII (9), kirkinine (10), excoecariatoxin (11), and 14′-ethyltetrahydrohuratoxin (12), isolated from Daphne acutiloba Rehd rods had an anti-HIV-1 activity with an EC50 of less than 2 nM (Huang et al., 2012). The leaves of Stilingia lineata are indicated to contain 12-O-acetyl-4α-deoxyphorbol-13(2″-methyl)-butyrate (21), 12-O-acetylphorbol-13(2″-methyl)-butyrate (22), 12β-O-[nona-2Z,4E,6E-trienoyl]-4α-deoxyphorbol-13-butyrate (23), 12-deoxyphorbol-13(2″-methyl)butyrate (24), and 12-deoxyphorbol-13-[8′-oxo-hexadeca-2E,4E,6E-trienoate] (25) which functions actively as anti-HIV. Compounds 21–25 can inhibit the outgrowth of HIV-1 and HIV-2 in the strong category, with an EC50 value of less than 1 μM (Olivon et al., 2015).
Anti-HIV-1 and anti-HIV-2 activity through inhibition of viral replication has been indicated from the terpenic compounds of the Euphorbiaceae species. Phorbol ester derivative compounds, phorbol-12-decanoate (26), phorbol-13-butyrate (27), phorbol-13-decanoate (28), phorbol-13-tetradecanoate (29), phorbol-12,13-diacetate (30), phorbol-12,13-dibutyrate (31), phorbol-12,13-dihexanoate (32), phorbol-12,13-didecanoate (33), 12-O-tetradecanoylphorbol-13-acetate (34), 12-O-tiglylphorbol-13-decanoate (35), 12-O-(N-methylanthranilate)-phorbol-13-acetate (sapintoxin D) (36), 12,13-O,O′-dinonanoylphorbol-20-homovanillate (37), 12-O-phenylacetyl-13-O-acetylphorbol-20-homovanillate (38), phorbol-12,13,20-triacetate (39), and 12-O-tetradecanoyl-20-oxo-20-deoxyphorbol-13-acetate (40) has anti-HIV-1 activity with IC50 values ranging from 0.9 nM - 27.4 μM, with compound 29 having the best activity. 12-deoxy phorbol derivative, 12-deoxyphorbol-13-acetate (prostratin) (41), 13-O-isobutyryl-12-deoxyphorbol-20-acetate (42), and 13-O-phenylacetyl-12-deoxyphorbol-20-acetate (43), also acts as anti-HIV-1 with an IC50 value of 0.3 μM–1.9 μM. Ingerol ester derivative compounds, ingenol-3-mebutate (ingenol-3-angelate) (202), and ingenol-3,20-dibenzoate (203) able to inhibit HIV-1 activity with IC50 values 17 nM and 27 nM. Apart from being active as anti-HIV-1, compounds 27–44 and 202–203 also have anti-HIV-2 activity. Compounds 29, 31–38, 43, 202, and 203 have HIV-2 inhibitory activity with IC50 values ranging from 0.2 nM - 80 nM, with the best inhibitory activity shown by compounds 23 and 35 (IC50 0.2 nM). The anti-HIV mechanism is thought to be related to the activation of kinase C isoenzymes (PKC) (Nothias-Scaglia et al., 2015). Furthermore, anti-PKC beta antibodies inhibited phorbol-12-myristate acetate (44) induced activation of NF-ĸB and HIV-1 (Kim et al., 1996).
Diterpenes from sponge Hyattella intestinalis, spongiatriol (152), and isospongiatriol (153) were significantly able to inhibit HIV growth with IC50 values less than 20 μg/ml. The two diterpenes act as inhibitors against the activation of NF-κB (Nuclear Factor kappa light chain enhancer of activated B cells) (Ahmadi et al., 2017). NF-κB is one of the transcription factors that is activated by a viral infection. NF-κB is required to express IFNB (Interferon Beta), induced by viruses (Balachandran and Beg, 2011). Ent-3S-hydroxy-atis-16(17)-en-1,14-dione (154) isolated from Homalanthus nutans, ent-15-oxo-kaur-16-en-19-oic acid (45) and ent-15-oxo-kaur-16-en-19-oic acid methyl ester (46) isolated from Chrysobalanus icaco has anti-HIV activity with EC50 value 19, 1.58 and 50 μM respectively (Gustafson et al., 1991). Euphorneroid D (150) and ent-3-oxoatisan-16a,17-acetonide (151) from the bark of Euphorbia neriifolia showed anti-HIV-1 activity with EC50 34 μM and 24 μM (Yan et al., 2018).
Tripterygium wilfordii root contains tripterifordin (47) also has potential as an anti-HIV-1 (Chen et al., 1992, 1995). 16β,17-Dihydroxy-ent-kauran-19-oic acid (48) from Annona squamosal (Wu et al., 1996) and methyl-16α-hydro-19-al-ent-kauran-17-oate (49) from Annona glabra (Chang et al., 1998) able to inhibit HIV-1 replication with EC50 value 2,38 μM and 15 μM, respectively, in H9 lymphocytes. Carnosalic acid (52) from Rosmarinus afficinalis can inhibit HIV-1 protease by 90%. While the derivatives, rosmanol (53), 7-O-methylrosmanol (54), and 7-O-ethylrosmanol (55) also have anti-HIV-1 activity. The existence of a benzilic group CH2 and a carboxylic group performs in the inhibiting of HIV-1 protease from carnosic acid (Pariš et al., 1993).
The dolabellane diterpenes of the octocoral Caribbean Eunicea laciniata are 13-keto-1(R),11(S)-dolabella-3(E),7(E),12(18)-triene (117), and β–Araneosene (118) exhibits HIV-1 inhibitory activity. In addition, the derivatives of β–Araneosene, (1R,11S,3S,4S,7S,8S)13-keto-di-3,4:7,8-epoxy-dolabella-12(18)-ene (119), (1R,3R,4R,7S,11S)13-keto-3,4,7-trihydroxy-dolabella-8(17),12(18)-diene (120) and (1R,3E,7E,11S)-13-keto-16-hydroxy-dolabella-3,7,12(18)-triene (121) showed an impressive increase in anti-HIV-1 activity compared to compound 117 with EC50 value 0.73 μM, 3.9 μM and 0.69 μM (Pardo-Vargas et al., 2014). Additionally, the compound of 8,10,18-trihydroxy-2,6-dolabelladiene (122) from the algae Dictyota pfaffii was indicated to have anti-HIV activity. This compound acts as a non-competitive inhibitor of the HIV-1 reverse transcriptase enzyme so that it can interfere with HIV-1 replication (Cirne-Santos et al., 2006, 2008). Reverse transcriptase is a multifunctional enzyme such as RNA-dependent DNA polymerase, DNA-dependent DNA polymerase, and RNase H activities (Pomerantz and Horn, 2003).
Andrographolide (82) can reduce the amount of p24 antigen on MT2 cells so that it has the potential to be an anti-HIV drug (Niranjan Reddy et al., 2005). This compound can also inhibit HIV-induced cell-cycle dysregulation, this effect in a significant increase in the concentration of CD4+ lymphocytes so that it can reduce replication. HIV (Calabrese et al., 2000). Dehydroandrographolide succinic acid monoester (84) compound has HIV-1 and HIV-2 inhibitory activity by inhibiting HIV-induced cell fusion (Chang et al., 1991).
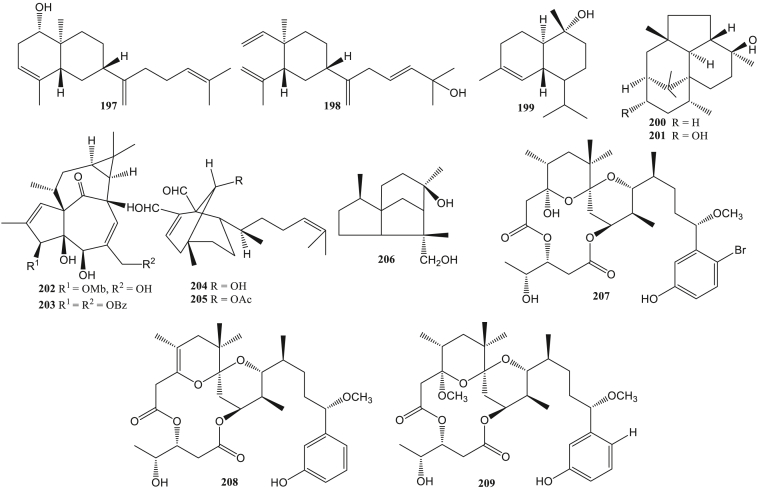
The SJ23B (187) compound isolated from Euphorbia hyberna is classified as a jatrophane diterpenes capable of inhibiting HIV-1 infection with an IC50 value of 2 nM. The compounds’ mechanism is to induce the latent reactivation process of HIV-1 by activating NF-ĸB and downregulating the expression of HIV-1 receptors such as CD4, CXCR4, and CCR5, thereby preventing infection of other CD4 + cells (Bedoya et al., 2009). Two diterpenes, (6R)-6-hydroxydichotoma-3,14-diene-1,17-dial (204) and (6R)-6-acetoxidichotoma-3,14-diene-1,17-dial (205) from Dictyota menstrualis have anti-HIV-1 activity by inhibiting the replication of the virus. The diterpene compound can inhibit the viral replication process by acting as an inhibitor of the HIV-1 reverse transcriptase enzyme (Pereira et al., 2004).
4. Coxsackievirus
Coxsackievirus is part of the enterovirus genus of the Picornaviridae family. This virus is 30 nm in size and has single-stranded RNA. Furthermore, there are two types of Coxsackievirus, including Coxsackievirus A and B (Oberste, 2008). This virus can be transmitted through respiratory droplets, blisters, or feces (Ang et al., 2009). This disease causes hand, foot, and mouth disease (HFMD) since 1997 (Lum et al., 1998). The Study of compounds diterpenes as anti-Coxsackie viruses have long been used.
The terpenic abiatane compound from Illicium jiadifengpi has anti-Coxsackie activity. Jiadifenoic acids J-K (56–57), jiadifenoic acids L-N (76–78), and jiadifenoic acids O–P (155–156) have anti-Coxsackie virus type B3 activity. The IC50 value of compounds 77, 78, and 156 against Coxsackievirus inhibition were less than 20 μmol/mL (Zhang et al., 2014). 7α-hydroxycallitrisic acid (58), 4-epi-isomapiric acid (79), and isopimara-7,15-dien-19-ol (80) significantly showed antivirals activity against Coxsackievirus types B2, B3, B4, and B6 with an IC50 value of less than 25 μmol/mL (Zhang et al., 2013). Abietane diterpenes (+)-jiadifenoic acid C (59) which is a semisynthetic product of callitrisic acid has anti-Coxsackie B virus (González and Zaragozá, 2014). The root of Illicium majus contains 15S-majusanic acid E (60) and 15R-majusanic acid E (61). These compounds had anti-Coxsackie B3 virus activity with IC50 values of 17.4 μg/mL and 12.8 μg/mL (Wang et al., 2013a, Wang et al., 2013b). The terplisabolane compound from Claoxylon polot, namely claoxylones A-I (188–196) showed anti-Coxsackie B3 virus activity with an IC50 value of 6.0–33.3 μM (Gu et al., 2014).
5. Herpes virus
Herpes Simplex Virus (HSV) belongs to the Herpesviruses family and the genus Simplexviruses (Knipe and Whitley, 2020). There are two types of herpes simplex virus (HSV), herpes simplex virus types 1 and 2 (HSV-1 and HSV-2). HSV-1 infects the orolabial area, whereas HSV-2 targets the genital area (Widener and Whitley, 2014). HSV-1 and HSV-2 are frequent, long-term infections, often asymptomatic (Tronstein et al., 2011). It is estimated that in 2016 there were 491 million cases of HSV-2 that infected people aged 15–49 years. HSV-1, it is estimates that there are 192 million cases (James et al., 2020). Several articles have observed anti-HSV activity in several terpenes.
The scopadurosane (157) derivative compound, the compound 158–160 has anti-HSV-2 activity. The presence of a hydroxy group (-OH) on C-13 in compound 158–160 affects the compound's anti-HSV-2 strength (González et al., 2010). Dolabellane diterpenes, (1R, 3R, 4R, 7R, 8R, 11S)-di-3,4:7,8-epoxy-13-keto-dolabell-12(18)-ene (123) from Eunicea laciniata and (1R, 3E, 4R, 7R, 8R, 11S)-di-7,8-epoxy-13-keto-dolabell-3,12(18)-diene (124) from Eunicea sperula has anti-HSV-1 activity with inhibition values of 42.6% and 73.7% (Amaya-Garcia et al., 2017). Nigella damascene seeds contain dolabellane diterpenes, damasterpenes I-III (129–131), and damasterpenes V-VIII (125–128). Compounds 125, 126, and 130 showed HSV-1 inhibitory activity at a concentration of 10 μM with 20–35% inhibition. Meanwhile, compounds 127, 128, 129, and 131 showed less than 20% (Ogawa et al., 2018).
Two dolastane diterpenes from the brown algae Canistrocarpus cervicornis, 4-hydroxy-9,14-dihydroxydolasta-1(15),7-diene (138) and 4,7,14-trihydroxydolasta-1(15),8-diene (139) can inhibit HSV-1 infection with 90% and 99% inhibition. Both of these compounds can become anti-HSV-1 agents because their inhibition values are similar to acyclovir (inhibition = 99%) (Vallim et al., 2010). Anti-herpesvirus activity in two diterpenes dolastane diterpenes (4R,7R,14S)-4α,7α-diacetoxy-14-hydroxydolast-1(15),8-diene) (140) and (4R,9S,14S)-4α-acetoxy-9β,14α-dihidroxydolast-1(15),7-diene) (141) from Canistrocarpus cervicornis. Compound 140 was reported to have much better inhibitory activity on human alphaherpesvirus-1 (HHV) than compound 141 with EC50 values 6.25 μM and 120 μM. Additionally, these compounds can also inhibit Bovine alphaherpesvirus 5 (BoHV-5) (Pinto et al., 2019). Dolastane diterpenes compounds can inhibit the HSV replication process by inhibiting DNA polymerase activity (Vallim et al., 2010). Meanwhile, two prenylated guaiane compounds from Epipolasis reiswiq, reiswigins A and B (143–144) were also indicated to have anti-HSV-1 activity(Kashman et al., 1987).
Briareum excavatum has been reported to contain briacavatolides A-C (182–184), briaexcavatolide U (185), and briaexcavatin L (186). Anti-HCMV evaluation showed that the briacavatolide C (184) compound had the greatest inhibitory activity with an IC50 value of 18 μM (Yeh et al., 2012). Ehrenbergol C and asetil ehrenberoksida B (148–149) from soft coral Sarcophyton ehrenbergi had anti-human cytomegalovirus (HCMV) activity with EC50 values 20 and 8 μg/mL (Wang et al., 2013a, Wang et al., 2013b). HCMV is beta herpes that causes lifelong infection in humans. This virus is a significant cause of defects in infants and immune disorders. Infants can be infected with this virus through the placenta, labor, and breastfeeding (Landolfo et al., 2003). The gyrosanols A (197) and gyrosanols B (198) from soft coral Sinularia gyrosa have anti-HCMV activity with IC50 values 2.6 dan 3.7 μg/mL. Also, compounds 197 and 198 show significant anti-inflammatory activity by reducing the COX-2 protein (Cheng et al., 2010).
6. Hepatitis virus
Hepatitis virus is the major cause of inflammatory liver disease. There are five types of hepatitis viruses, including hepatitis A (HAV), B (HBV), C (HCV), D (HDV), and E (HEV) viruses. Hepatitis A (HAV) and hepatitis E (HEV) are transmitted orally or through feces. Both viruses cause acute illness. Hepatitis B (HBV) and hepatitis C (HCV) are transmitted parenterally and cause chronic hepatitis. Meanwhile, hepatitis D (HDV) is pathogenic when combined with HBV (Lefkowitch, 2021). All types of hepatitis belong to different families. HBA belongs to the Piconaviridae family, HBV from the Hepadnaviridae family, HCV from the Flaviviridae family, and HEV from the Hepeviridae family (Ryu, 2016). Hepatitis virus has caused 1.34 million deaths in 2015, of the total deaths caused by HBV (66%), HCV (30%), HAV (0.8%), and HEV (3.3%) (WHO, 2017).
Wikstroemia Chamaedaphne has been reported to contain diterpene compounds, wikstchalide W (13), genkwanine P (14), laurifolioside A (132), 2-epi-laurifolioside A (133), laurifolioside B (134), 2-epi-laurifolioside B (135), laurifolioside (136), and 2-epi-laurifolioside (137). Compounds (14) and (132) have anti-HBV activity by inhibiting HBsAg surface antecedents with IC50 values of 46.5 and 88.3 μg/mL. While compounds 13, 133–137 were able to inhibit HBV replication in the range of 2%–33% (Li et al., 2018).
Labdane diterpene, andrographolide (82) can inhibit HCV replication by regulating heme oxygenase-1 expression, thereby increasing levels of biliverdin metabolites. This promotes the antivirals response of IFN and inhibits NS3/4A protease activity. Besides, andrographolide can activate the phosphorylation of p38 MAPK, which stimulates the expression of heme oxygenase-1 mediated by nuclear factor erythroid 2 (Nrf2) (Lee et al., 2014). NS3/4A protease is an enzyme responsible for the selective cleavage of polyproteins into individual viral proteins. This protease plays an essential role in HCV replication, one of the three HCV drug targets (Meewan et al., 2019).
Andrographolide (82) and dehydroandrographolide (83) from Andrographis paniculata can inhibit HBV DNA replication with IC50 value 22.6 μM and 54.1 μM. A total of 48 derivatives of the two compounds were evaluated for anti-HBV activity. There were 21 derivatives of dehydroandrographolide and andrographolide (95–115) with IC50 values less than 50 μM with anti-HBV mechanisms by inhibiting of viral DNA replication (Chen et al., 2014). Compounds 95, 99, and 112 had anti-HBV activity by inhibiting HBsAg secretion with IC50 value is less than 50 μM (Chen et al., 2014). HBsAg (Hepatitis B surface antigen), is a hepatitis B virus surface antigen consisting of lipids and proteins on the surface of the virion and is produced excessively during the virus life cycle (Blumberg and Alter, 1965; Howard, 1986). The mechanism for inhibiting HBeAg secretion is shown by compound 95 and 100 with IC50 values less than 50 μM (Chen et al., 2014). Hepatitis B e antigen (HBeAg) is a product of the nucleocapsid (core) HBV gene secreted in serum. HBeAg is a marker of viral replication associated with liver infectivity and damage (Karayiannis et al., 1985).
7. Influenza virus
Influenza is an RNA virus that includes in the Orthomyxoviridae family. Influenza viruses consist of three types include influenza A, B, and C. This virus has three glycoprotein envelopes: hemagglutinin (HA), neuraminidase (NA), and matrix protein (M1 and M2). The influenza virus has eight RNA strands that allow the formation of new virus subtypes. For example, the H1N1 influenza virus is a structural variation between HA and NA. Influenza virus infects the respiratory tract and can cause acute respiratory distress syndrome (ARDS) (Rao et al., 2019). Influenza is an RNA virus that belongs to the Orthomyxoviridae family (Rosário-Ferreira et al., 2020). Influenza virus has a single-stranded RNA genome, negative sense, and ribonucleoproteins of different sizes (Zhang and Lamb, 1996).
Antivirals activity against Human Parainfluenza Virus Type 2 (HPIV-2) was also investigated in the ent-kaurene diterpenes. Linearol (50) and isosidol (51) compounds obtained from the aerial part of Sideritis Lycia have anti-HPIV-2 activity with an antiviral index value (CD50/ED50) of less than 2 μM (Kilic et al., 2020). Phenolic derivatives of diterpenes (+)-acids podocarpic (161) and (+)-totarol (181) have anti-influenza A virus activity. Evaluation of (+)-podocarpic acid derivatives (161–181) can inhibit the influenza virus. Methyl podocarpate (162) and its O-acetyl derivative (177) can increase the anti-influenza virus activity of podocarpic acid (161) by 100 times stronger. Meanwhile, benzyl ester (163) can increase anti-influenza virus activity 10 times stronger. Totarol has 5 times stronger activity to inhibit influenza virus (PR8) compared to podocarpic acid. The totarol was able to inhibit about 12 times stronger VR1679 virus (H3N2) than compound 170. However, compound 170 inhibited PR8 virus (H1N1) 15 times stronger than totarol. Compound 164 has anti-influenza virus (PR8) activity through hemolysis inhibition mediated by hemagglutinin. Hemagglutinin protein is essential for virus entry (Dang et al., 2015).
Andrographolide (82), 14-dehydroxyandrographolide-12-sulfonic acid sodium salt (85), and 14-α-lipoyl andrographolide (86) can inhibit various kinds of influenza viruses such as H5N1, H1N1, and H9N2 (Chen et al., 2009). Andrographolide can block the interaction pathway between the retinoic acid-inducible gene-1 (RIG-1) receptors induced by the influenza virus. In this process, there was an improvement in cell death caused by influenza virus infection (Yu et al., 2014). The 14-α-lipoyl andrographolide (86) was able to inhibit virus adsorption to red blood cells with a minimum inhibitory concentration (MIC) of 5.3 mM (H1N1), 7.1 mM (H9N2), and 16.8 mM (H5N1). This shows that the compound can interfere with viral hemagglutinin so that it can block binding to cellular receptors (Chen et al., 2009).
Some labdane diterpene isolated from the fruit of Forsythia suspense have been indicated to have anti-infuenza activity type H1N1 virus. 3β-hydroxy-8(17),13E-labdadien-15-oic acid (87), forsyshiyanins A-B (88–89), 19-hydroxy-ent-labda-8(17),13E-dien-15-oic acid (90), ent-linda-8(17),13E-dien-15,19-dioic acid (91), ent-linda-8(17),13Z-dien-15,19-dioic acid (92), enantio-labd-8(20),13-dien-15,18-dioic acid (93), and 18-hydroxy-7-oxolabda-8(9),13(E)-dien-15-oic acid (94), all of these compounds have IC50 values of less than 30 μM (Zhao et al., 2020).
Rhodomollin B (142) from Rhododendron molle fruits showed inhibitory activity of Influenza A95/359 virus with an IC50 value of 19.24 μM (Li et al., 2016). Trichoderma atroviride containing wickerols A and B (200–201) had anti-influenza virus H1N1 strain with IC50 values 5.0 μg/ml and 0.07 μg/ml. Both compounds can inhibit DNA polymerase activity in viruses so that the viral replication process is inhibited (Yamamoto et al., 2012).
8. Chikungunya virus (CHIKV)
Chikungunya virus (CHIKV) is a virus, including in the Flaviviridae family. The Aedes aegypti mosquito spreads the virus. The incubation period for CHIKV is relatively short, 2–4 days (Halstead, 2018). CHIKV disease is characterized by high fever, pain in one or several joints, and a rash on the face (De Ranitz et al., 1965). CHIKV infection is widespread in various countries in Asia (Jamal and Sam, 2018), Europe (Zannoli et al., 2017), America (Vasconcelos et al., 2018), and Africa (Diallo et al., 2018).
Several diterpenes have indicated antivirals activity against CHIKV. Daphnane diterpenes from Trigonostemon cherrieri, trigocherrins A-B (15–16), trigocherrin F (17), and trigocherriolides A-C (18–20) can inhibit CHIKV virus replication better than chloroquine (Allard et al., 2012). Daphnane diterpenes can reduce viral protein gene expression so that CHIKV RNA production decreases. The decreased production of CHIKV RNA was also caused by blocking the interaction between viral DNA and the reverse transcriptase enzyme so that the viral replication process was inhibited (Kaur et al., 2013). Meanwhile, tonantzitlolone B, tonantzitlolone C, and tonantzitlolone F (145–147) from Stilingia lineata leaves can inhibit CHIKV by EC50 value less than 25 μM (Olivon et al., 2015).
The Anti-CHIKV activity of several terpenes are from the marine cyanobacterium Trichodesmium erythraeum, debromoaplysiatoxin (207), anhydrodebromoaplysiatoxin (208), and 3-methoxydebromoaplysiatoxin (209). Compound 207 can inhibit CHIKV infection better than compounds 208 and 209. These three compounds inhibit the virus replication process after the virus enters the host cell by inhibiting viral DNA polymerase activity, which has a necessary role in the viral replication process (Gupta et al., 2014). Dolastane (141) from Canistrocarpus cervicornis had an anti-CHIKV activity with an EC50 value of 1.28 μM (Cirne-Santos et al., 2020).
9. Zika virus (ZIKV)
Zika virus (ZIKV) is a virus from the Flaviviridae family that can be transmitted through Aedes bite. Aegypti and Aedes albopictus. The disease caused by ZIKV infection is almost the same as the dengue virus, West Nile virus, Japanese encephalitis virus, and yellow fever virus but neurological complications can lead to Guillain-Barré syndrome and congenital malformations that cause microcephaly (Plourde and Bloch, 2016; Noorbakhsh et al., 2019). Zika infection is sometimes asymptomatic, but some cause clinical symptoms non-specifics such as headache, low-grade fever, arthralgia, rash, and conjunctivitis (Lr, 2016). Globally, it is estimated that there were 3–4 million cases of Zika in 2016 (WHO, 2016).
Semi-synthetic derivatives of abietane diterpene, 18-aminoferruginol (71), 12-hydroxy-N,N-phthaloydehydroabietylamine (72), 12-acetoxy-N,N-(tetrachloro)phthaloydehydroabietyl amine (73), 12-hydroxy-N-tosyl-dehydroabietylamine (74), 18-oxoferruginol (62), and 12-nitro-N-benzoyl-dehydroabietylamine (75) are potential anti-ZIKV agents. The best ZIKV inhibitory activity was compound 75 with EC50 0.67 μM, while the anti-ZIKV activity of compound 62, 71–74 with EC50 2.60–18.57 μM (Sousa et al., 2020). Dolastane (141) from Canistrocarpus cervicornis had an anti-ZIKV activity with an EC50 value of 0.75 μM (Cirne-Santos et al., 2020).
10. Dengue virus (DENV)
Dengue virus (DENV) is a virus, including in the flavivirus genus and the Flaviviridae family. DENV can cause dengue to dengue shock syndrome (Murugesan and Manoharan, 2020). This virus is spread in tropical areas. There are five types of dengue viruses, including DENV-1, DENV-2, DENV-3, DENV-4, and DENV-5 (Gubler and John, 2014). It is estimated that around 390 million dengue fever cases per year (Bhatt et al., 2013). There is an increase in dengue fever cases annually from 2000 (505,430 cases) to 2019 (4.2 million cases), with deaths increasing from 960 cases (2000) to 4032 cases (2015) (WHO, 2014).
Andrographolide (82) has anti-DENV-2 activity with EC50 21.30 μM (HepG2) and 22.74 μM (HeLa) (Panraksa et al., 2017). Anti-DENV andrographolide activity is associated with GRP78 and the unfolded protein response (Paemanee et al., 2019). The compound of Trigonostemon cherrieri is trigocherrins A (15), and trigocherriolides A-B (18–19) can inhibit DENV. The IC50 of the three compounds ranged from 3.1 to 16.0 μM (Allard et al., 2012). The anti-DENV activity was also shown by (+)-ferruginol (63) and 18-oxoferruginol (62), with inhibition values of 1.4 and 5.0 μM (Sousa et al., 2020).
11. Coronavirus (SARS-CoV)
SARS-CoV (Severe Acute Respiratory Syndrome-Coronavirus) is one of the first infectious diseases discovered in China. This disease is one of the causes of global problems because it spreads in 26 countries on five continents. More than 8,000 SARS cases and 744 death cases have been recorded (Peiris et al., 2003). This virus comes from the Coronaviridae family and has 90 single-stranded RNA genetic material. SARS-CoV attacks the respiratory system. The virus can be transmitted through body fluids (mouth, nose, or eyes). SARS-CoV binds to the ACE2 receptor and introduces its genetic material to host cells by endocytosis. Patients infected with this virus are characterized by a high fever (more than 38 °C), headaches, body aches, and respiratory infections. Symptoms of the disease will appear after 2–14 days after infection (Freeman and Kenez, 2016).
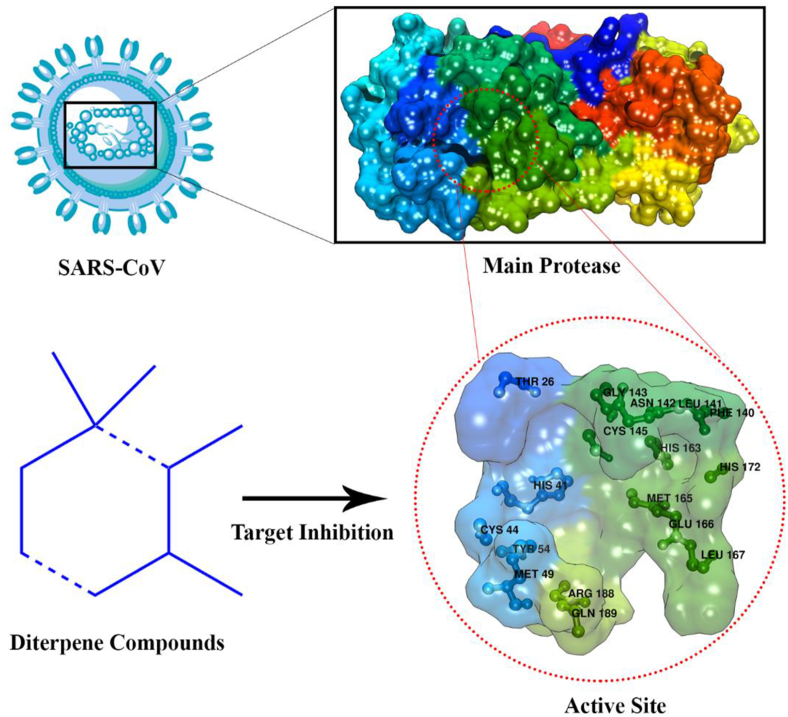
Several diterpenes have the potential to be anti-SARS-CoV. Ferruginol (63), dehydroabieta-7-one (64), sugiol (65), cryptojaponol (66), [8β-hydroxyabieta-9(11),13-dien-12-one)] (67), 7β-hydroxydeoxycryptojaponol (68), 6,7-dehydroroyleanone (69), 3β,12-diacetoxyabieta-6,8,11,13-tetraene (70), pinusolidic acid (116), forskolin (81), cedrane-3α,12-diol (206), and α-cadinol (199) had anti-SARS-CoV activity. Compounds 63–65, 67, 69, 81, 116 obtained from the wood core of Chamaecyparis obtuse var. formosana, compound 66 obtained from the wood core of Cryptomeria japonica, compound 70, 206, and 199 obtained from the wood core of Juniperus formosana. Compounds 63, 65, 68, and 70 showed significant anti-SARS-CoV activity with EC50 values less than 1.5 μM and better than valinomicyn. Meanwhile, compounds 64, 69, 116, 68, and 206 showed moderate activity against SARS-CoV with EC50 values between 3.8-7.5 μM. These compounds act as competitive inhibitors against the SARS-CoV 3CL protease by blocking the SARS-CoV 3CL protease activity, thereby disrupting the viral replication cycle. The SARS-CoV 3CL protease is the main target in anti-SARS-CoV treatment. The SARS-CoV 3CL protease has an important role in the virus replication process because it plays a role in viral cell division (Wen et al., 2007).
Inhibition of SARS-CoV by diterpenes at the molecular level through the mechanism of inhibition of the main protease enzyme active site (Figure 12). The targeting of the active site is expected to provide an overview of the inhibition of the main protease 3CL through the interaction between diterpene compounds (ligand) and amino acid residues on the active site of the protein (receptor) (Wen et al., 2007). This interaction is expected to change the conformation of the complex structure (ligand-receptor) so that the protein becomes inactive and stops virus replication. More specifically, the mechanism of inhibition occurs through binding to amino acids on the active site of the main protease as a receptor in the form of hydrogen bonds.
Figure 12.
Diterpenes as inhibitor targeting the active site of SARS-CoV main protease (PDB code: 1UK4).
12. Conclusions
Diterpenes are compounds that can be obtained from the natural product or the synthesis process. The source of diterpenes from the natural product can obtain from plants (Daphne acutiloba, Stilingia lineata, Illicium jiadifengpi, Wikstroemia chamaedaphne, etc), porifera (Hyattella intestinalis), coral (Eunicea laciniata, Eunicea laciniata, Briareum excavatum, etc), algae (Dictyota pfaffii, Canistrocarpus cervicornis, Trichodesmium erythraeum, etc), and fungi (Trichoderma atroviride). Diterpenes antivirals activity can inhibit HIV, Coxsackievirus, HSV, HHV, BoHV-5, HCMV, Hepatitis virus, HPIV, H3N2, H1N1, PR8, H5N1, H9N2, CHIKV, ZIKV, DENV, and SAR-CoV. This class of compounds may be an alternative in developing anti-SARS-CoV-2 drugs to tackle the Coronavirus disease 2019 (Covid-19) pandemic.
Declarations
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
This work was supported by Hibah Riset Mandat Kolaborasi Mitra Luar NegeriUniversitas Airlangga 2021, Contract number 800/UN3.15/PT/2021.
Data availability statement
No data was used for the research described in the article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- Ahmadi P., Haruyama T., Kobayashi N., de Voogd N.J., Tanaka J. Spongian diterpenes from the sponge Hyattella aff. Intestinalis. Chem. Pharm. Bull. 2017;65(9):874–877. doi: 10.1248/cpb.c17-00297. [DOI] [PubMed] [Google Scholar]
- Allard P.-M., Leyssen P., Martin M.-T., Bourjot M., Dumontet V., Eydoux C., Guillemot J.-C., Canard B., Poullain C., Guéritte F. Antiviral chlorinated daphnane diterpenoid orthoesters from the bark and wood of Trigonostemon cherrieri. Phytochemistry. 2012;84:160–168. doi: 10.1016/j.phytochem.2012.07.023. [DOI] [PubMed] [Google Scholar]
- Amaya-Garcia F., Sánchez Nunez M.L., Ramos F.A., Puyana M., Nunes de Palmer Paixao I.C., Laneuville Teixeira V., Castellanos L. Dolabellane diterpenes from the Caribbean soft corals Eunicea laciniata and Eunicea asperula and determination of their anti HSV-1 activity. Rev. Colomb. Quím. 2017;46(1):5–12. [Google Scholar]
- Ang L.W., Koh B.K., Chan K.P., Chua L.T., James L., Goh K.T. Epidemiology and control of hand, foot and mouth disease in Singapore. Ann. Acad. Med. Singapore. 2009;38(2):106–112. [PubMed] [Google Scholar]
- Balachandran S., Beg A. Defining emerging roles for NF-kB in antivirus responses: revisiting the interferon-β enhanceosome paradigm. PLoS Pathog. 2011;7(10) doi: 10.1371/journal.ppat.1002165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedoya L.M., Márquez N., Martínez N., Gutiérrez-Eisman S., Álvarez A., Calzado M.A., Rojas J.M., Appendino G., Muñoz E., Alcamí J. SJ23B, a jatrophane diterpene activates classical PKCs and displays strong activity against HIV in vitro. Biochem. Pharmacol. 2009;77(6):965–978. doi: 10.1016/j.bcp.2008.11.025. [DOI] [PubMed] [Google Scholar]
- Bhatt S., Gething P.W., Brady O.J., Messina J.P., Farlow A.W., Moyes C.L., Drake J.M., Brownstein J.S., Hoen A.G., Sankoh O. The global distribution and burden of dengue. Nature. 2013;496(7446):504–507. doi: 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg B.S., Alter H.J. A new antigen in leukemia sera. JAMA. 1965;191(7):541–546. doi: 10.1001/jama.1965.03080070025007. [DOI] [PubMed] [Google Scholar]
- Calabrese C., Berman S.H., Babish J.G., Ma X., Shinto L., Dorr M., Wells K., Wenner C.A., Standish L.J. A phase I trial of andrographolide in HIV positive patients and normal volunteers. Phytother Res. 2000;14(5):333–338. doi: 10.1002/1099-1573(200008)14:5<333::aid-ptr584>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Chang F.-R., Yang P.-Y., Lin J.-Y., Lee K.-H., Wu Y.-C. Bioactive kaurane diterpenoids from Annona glabra. J. Nat. Product. 1998;61(4):437–439. doi: 10.1021/np970497z. [DOI] [PubMed] [Google Scholar]
- Chang R.S., Ding L., Gai-Qing C., Qi-Choa P., Ze-Lin Z., Smith K.M. Dehydroandrographolide succinic acid monoester as an inhibitor against the human immunodeficiency virus. PSEBM (Proc. Soc. Exp. Biol. Med.) 1991;197(1):59–66. doi: 10.3181/00379727-197-43225. [DOI] [PubMed] [Google Scholar]
- Chen H., Ma Y.-B., Huang X.-Y., Geng C.-A., Zhao Y., Wang L.-J., Guo R.-H., Liang W.-J., Zhang X.-M., Chen J.-J. Synthesis, structure–activity relationships and biological evaluation of dehydroandrographolide and andrographolide derivatives as novel anti-hepatitis B virus agents. Bioorg. Med. Chem. Lett. 2014;24(10):2353–2359. doi: 10.1016/j.bmcl.2014.03.060. [DOI] [PubMed] [Google Scholar]
- Chen J.-X., Xue H.-J., Ye W.-C., Fang B.-H., Liu Y.-H., Yuan S.-H., Yu P., Wang Y.-Q. Activity of andrographolide and its derivatives against influenza virus in vivo and in vitro. Biol. Pharm. Bull. 2009;32(8):1385–1391. doi: 10.1248/bpb.32.1385. [DOI] [PubMed] [Google Scholar]
- Chen K., Shi Q., Fujioka T., Nakano T., Hu C.-Q., Jin J.-Q., Kilkuskie R.E., Lee K.-H. Anti-AIDs agents—XIX. Neotripterifordin, a novel anti-HIV principle from Tripterygium wilfordii: isolation and structural elucidation. Bioorg. Med. Chem. 1995;3(10):1345–1348. doi: 10.1016/0968-0896(95)00114-v. [DOI] [PubMed] [Google Scholar]
- Chen K., Shi Q., Fujioka T., Zhang D.-C., Hu C.-Q., Jin J.-Q., Kilkuskie R.E., Lee K.-H. Anti-AIDS agents, 4. Tripterifordin, a novel anti-HIV principle from Tripterygium wilfordii: isolation and structural elucidation. J. Nat. Product. 1992;55(1):88–92. doi: 10.1021/np50079a013. [DOI] [PubMed] [Google Scholar]
- Cheng S.-Y., Chuang C.-T., Wang S.-K., Wen Z.-H., Chiou S.-F., Hsu C.-H., Dai C.-F., Duh C.-Y. Antiviral and anti-inflammatory diterpenoids from the soft coral Sinularia gyrosa. J. Nat. Product. 2010;73(6):1184–1187. doi: 10.1021/np100185a. [DOI] [PubMed] [Google Scholar]
- Cirne-Santos C.C., de Souza Barros C., de Oliveira M.C., Rabelo V.W.-H., Azevedo R.C., Teixeira V.L., Ferreira D.F., de Palmer Paixão I.C.N. In vitro studies on the inhibition of replication of Zika and chikungunya viruses by dolastane isolated from seaweed Canistrocarpus cervicornis. Sci. Rep. 2020;10(1):1–10. doi: 10.1038/s41598-020-65357-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirne-Santos C.C., Souza T.M.L., Teixeira V.L., Fontes C.F.L., Rebello M.A., Castello-Branco L.R.R., Abreu C.M., Tanuri A., Frugulhetti I.C., Bou-Habib D.C. The dolabellane diterpene Dolabelladienetriol is a typical noncompetitive inhibitor of HIV-1 reverse transcriptase enzyme. Antivir. Res. 2008;77(1):64–71. doi: 10.1016/j.antiviral.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Cirne-Santos C.C., Teixeira V.L., Castello-Branco L.R., Frugulhetti I.C., Bou-Habib D.C. Inhibition of HIV-1 replication in human primary cells by a dolabellane diterpene isolated from the marine algae Dictyota pfaffii. Planta Med. 2006;72(4):295–299. doi: 10.1055/s-2005-916209. [DOI] [PubMed] [Google Scholar]
- Dang Z., Jung K., Zhu L., Xie H., Lee K.-H., Chen C.-H., Huang L. Phenolic diterpenoid derivatives as anti-influenza a virus agents. ACS Med. Chem. Lett. 2015;6(3):355–358. doi: 10.1021/ml500533x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Ranitz C., Myers R., Varkey M., Isaac Z., Carey D. Clinical impressions of chikungunya in Vellore gained from study of adult patients. Indian J. Med. Res. 1965;53(8):756–763. [PubMed] [Google Scholar]
- Diallo D., Dia I., Diagne C.T., Gaye A., Diallo M. Elsevier; 2018. Emergences of Chikungunya and Zika in Africa, Chikungunya and Zika Viruses; pp. 87–133. [Google Scholar]
- Freeman D., Kenez E. SARS-CoV attack (severe acute respiratory syndrome) Ciottone's Disaster Med. 2016:782. [Google Scholar]
- García P.A., De Oliveira A.B., Batista R. Occurrence, biological activities and synthesis of kaurane diterpenes and their glycosides. Molecules. 2007;12(3):455–483. doi: 10.3390/12030455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelderblom H.R. fourth ed. Medical Microbiology; 1996. Structure and Classification of Viruses. [Google Scholar]
- Giese S., Pelchen-Matthews A., Marsh M. 2016. HIV–The Cell Biology of Virus Infection and Replication. [Google Scholar]
- Gohar U.F., Iqbal I., Shah Z., Mukhtar H., Zia-Ul-Haq M. COVID-19: recent developments in therapeutic approaches. In: Zia-Ul-Haq M., Bin-Jumah M.N., Alothamn S.I., Henidi H.A., editors. Alternative Medicine Interventions for COVID-19. 2021. pp. 249–274. [Google Scholar]
- González M.A., Agudelo L., Betancur-Galvis L. Synthesis and antiviral activity of scopadulane-rearranged diterpenes. Antivir. Res. 2010;85(3):562–565. doi: 10.1016/j.antiviral.2010.01.001. [DOI] [PubMed] [Google Scholar]
- González M.A., Zaragozá R.n.J. Semisynthesis of the antiviral abietane diterpenoid jiadifenoic acid C from callitrisic acid (4-epidehydroabietic acid) isolated from sandarac resin. J. Nat. Product. 2014;77(9):2114–2117. doi: 10.1021/np500569y. [DOI] [PubMed] [Google Scholar]
- Gu H.-S., Ma S.-G., Li Y.-H., Wang Y.-D., Liu Y.-B., Li L., Li Y., Qu J., Lv H.-N., Chen X.-G. Claoxylones A–I, prenylbisabolane diterpenoids with anti-Coxsackie B virus activity from the branches and leaves of Claoxylon polot. Tetrahedron. 2014;70(41):7476–7483. [Google Scholar]
- Gubler D.J., John A.S. 2014. Dengue Viruses. [Google Scholar]
- Gupta D.K., Kaur P., Leong S.T., Tan L.T., Prinsep M.R., Chu J.J.H. Anti-Chikungunya viral activities of aplysiatoxin-related compounds from the marine cyanobacterium Trichodesmium erythraeum. Mar. Drugs. 2014;12(1):115–127. doi: 10.3390/md12010115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafson K.R., Munro M.H., Blunt J.W., Cardellina J.H., II, McMahon J.B., Gulakowski R.J., Cragg G.M., Cox P.A., Brinen L.S., Clardy J. HIV inhibitory natural products. 3. Diterpenes from Homalantus acuminatus and Chrysobalanus icaco. Tetrahedron. 1991;47(26):4547–4554. [Google Scholar]
- Halstead S.B. Elsevier; 2018. Chikungunya and Zika Disease, Chikungunya and Zika Viruses; pp. 69–85. [Google Scholar]
- HIV/AIDS, J. U. N. P. o . 2020. Global HIV & AIDS Statistics—2020 Fact Sheet. [Google Scholar]
- Howard C.R. The biology of hepadnaviruses. J. Gen. Virol. 1986;67(7):1215–1235. doi: 10.1099/0022-1317-67-7-1215. [DOI] [PubMed] [Google Scholar]
- Huang S.Z., Zhang X.J., Li X.Y., Kong L.M., Jiang H.Z., Ma Q.Y., Liu Y.Q., Hu J.M., Zheng Y.T., Li Y. Daphnane-type diterpene esters with cytotoxic and anti-HIV-1 activities from Daphne acutiloba Rehd. Phytochemistry. 2012;75:99–107. doi: 10.1016/j.phytochem.2011.11.013. [DOI] [PubMed] [Google Scholar]
- Huremović D. Springer; 2019. Brief History of Pandemics (Pandemics throughout History), Psychiatry of Pandemics; pp. 7–35. [Google Scholar]
- Jamal I., Sam C. Elsevier; 2018. Chikungunya and Zika Virus in Asia, Chikungunya and Zika Viruses; pp. 135–192. [Google Scholar]
- James C., Harfouche M., Welton N.J., Turner K.M., Abu-Raddad L.J., Gottlieb S.L., Looker K.J. Herpes simplex virus: global infection prevalence and incidence estimates, 2016. Bull. World Health Organ. 2020;98(5):315. doi: 10.2471/BLT.19.237149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y.-X., Shi L.-L., Zhang D.-P., Wei H.-Y., Si Y., Ma G.-X., Zhang J. A review on daphnane-type diterpenoids and their bioactive studies. Molecules. 2019;24(9):1842. doi: 10.3390/molecules24091842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karayiannis P., Fowler M.J., Lok A.S., Greenfield C., Monjardino J., Thomas H.C. Detection of serum HBV-DNA by molecular hybridisation: correlation with HBeAg/anti-HBe status, racial origin, liver histology and hepatocellular carcinoma. J. Hepatol. 1985;1(2):99–106. doi: 10.1016/s0168-8278(85)80759-5. [DOI] [PubMed] [Google Scholar]
- Kashman Y., Hirsch S., Koehn F., Cross S. Reiswigins A and B, novel antiviral diterpenes from a deepwater sponge. Tetrahedron Lett. 1987;28(45):5461–5464. doi: 10.1016/S0040-4039(00)96754-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur P., Thiruchelvan M., Lee R.C.H., Chen H., Chen K.C., Ng M.L., Chu J.J.H. Inhibition of chikungunya virus replication by harringtonine, a novel antiviral that suppresses viral protein expression. Antimicrob. Agents Chemother. 2013;57(1):155–167. doi: 10.1128/AAC.01467-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilic T., Topcu G., Goren A.C., Aydogmus Z., Karagoz A., Yildiz Y.K., Aslan I. Ent-kaurene diterpenoids from sideritis lycia with antiviral and cytotoxic activities. Record Nat. Prod. 2020;14(4) [Google Scholar]
- Kim C.H., Gollapudi S., Kim A., Lee T., Gupta S. Role of protein kinase C-β isozyme in activation of latent human immunodeficiency virus type 1 in promonocytic U1 cells by phorbol-12-myristate acetate. AIDS Res. Hum. Retrovir. 1996;12(14):1361–1366. doi: 10.1089/aid.1996.12.1361. [DOI] [PubMed] [Google Scholar]
- Knipe D.M., Whitley R. 2020. Herpesviridae: Herpes Simplex Virus 1 and 2 (HSV-1, HSV-2) [Google Scholar]
- Lai C.-C., Shih T.-P., Ko W.-C., Tang H.-J., Hsueh P.-R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): the epidemic and the challenges. Int. J. Antimicrob. Agents. 2020;55(3):105924. doi: 10.1016/j.ijantimicag.2020.105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landolfo S., Gariglio M., Gribaudo G., Lembo D. The human cytomegalovirus. Pharmacol. Ther. 2003;98(3):269–297. doi: 10.1016/s0163-7258(03)00034-2. [DOI] [PubMed] [Google Scholar]
- Lee J.C., Tseng C.K., Young K.C., Sun H.Y., Wang S.W., Chen W.C., Lin C.K., Wu Y.H. Andrographolide exerts anti-hepatitis C virus activity by up-regulating haeme oxygenase-1 via the p38 MAPK/N rf2 pathway in human hepatoma cells. Br. J. Pharmacol. 2014;171(1):237–252. doi: 10.1111/bph.12440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefkowitch J.H. Vol. 89. 2021. Acute Viral Hepatitis. Scheuer's Liver Biopsy Interpretation. [Google Scholar]
- Lefkowitz E.J., Dempsey D.M., Hendrickson R.C., Orton R.J., Siddell S.G., Smith D.B. Virus taxonomy: the database of the international committee on taxonomy of viruses (ICTV) Nucleic Acids Res. 2018;46(D1):D708–D717. doi: 10.1093/nar/gkx932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy J.A., Castelli J.C. 2019. HIV (Human Immunodeficiency Virus) [Google Scholar]
- Li S.-F., Jiao Y.-Y., Zhang Z.-Q., Chao J.-B., Jia J., Shi X.-L., Zhang L.-W. Diterpenes from buds of Wikstroemia chamaedaphne showing anti-hepatitis B virus activities. Phytochemistry. 2018;151:17–25. doi: 10.1016/j.phytochem.2018.01.021. [DOI] [PubMed] [Google Scholar]
- Li Y., Liu Y.-B., Yan H.-M., Liu Y.-L., Li Y.-H., Lv H.-N., Ma S.-G., Qu J., Yu S.-S. Rhodomollins A and B, two diterpenoids with an unprecedented backbone from the fruits of Rhododendron molle. Sci. Rep. 2016;6:36752. doi: 10.1038/srep36752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lr P. Jamieson DJ, powers AM, honein MA. Zika virus. N. Engl. J. Med. 2016;374(16):1552–1563. doi: 10.1056/NEJMra1602113. [DOI] [PubMed] [Google Scholar]
- Lum L.C., Wong K., Lam S., Chua K., Goh A. Neurogenic pulmonary oedema and enterovirus 71 encephalomyelitis. Lancet. 1998;352(9137):1391. doi: 10.1016/s0140-6736(05)60789-1. [DOI] [PubMed] [Google Scholar]
- Luzuriaga K. Elsevier; 2018. Human Immunodeficiency Virus, Principles and Practice of Pediatric Infectious Diseases; pp. 1198–1200. e1191. [Google Scholar]
- Meewan I., Zhang X., Roy S., Ballatore C., O’Donoghue A.J., Schooley R.T., Abagyan R. Discovery of new inhibitors of hepatitis C virus NS3/4A protease and its D168A mutant. ACS Omega. 2019;4(16):16999–17008. doi: 10.1021/acsomega.9b02491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murugesan A., Manoharan M. Elsevier; 2020. Dengue Virus, Emerging and Reemerging Viral Pathogens; pp. 281–359. [Google Scholar]
- Niranjan Reddy V., Malla Reddy S., Ravikanth V., Krishnaiah P., Venkateshwar Goud T., Rao T., Siva Ram T., Gonnade R.G., Bhadbhade M., Venkateswarlu Y. A new bis-andrographolide ether from Andrographis paniculata nees and evaluation of anti-HIV activity. Nat. Prod. Res. 2005;19(3):223–230. doi: 10.1080/14786410410001709197. [DOI] [PubMed] [Google Scholar]
- Noorbakhsh F., Abdolmohammadi K., Fatahi Y., Dalili H., Rasoolinejad M., Rezaei F., Salehi-Vaziri M., Shafiei-Jandaghi N.Z., Gooshki E.S., Zaim M. Zika virus infection, basic and clinical aspects: a review article. Iran. J. Public Health. 2019;48(1):20. [PMC free article] [PubMed] [Google Scholar]
- Nothias-Scaglia L.-F., Pannecouque C., Renucci F., Delang L., Neyts J., Roussi F., Costa J., Leyssen P., Litaudon M., Paolini J. Antiviral activity of diterpene esters on chikungunya virus and HIV replication. J. Nat. Product. 2015;78(6):1277–1283. doi: 10.1021/acs.jnatprod.5b00073. [DOI] [PubMed] [Google Scholar]
- Oberste M.S. Group B Coxsackieviruses; 2008. Comparative Genomics of the coxsackie B Viruses and Related Enteroviruses; pp. 33–47. [DOI] [PubMed] [Google Scholar]
- Ogawa K., Nakamura S., Hosokawa K., Ishimaru H., Saito N., Ryu K., Fujimuro M., Nakashima S., Matsuda H. New diterpenes from Nigella damascena seeds and their antiviral activities against herpes simplex virus type-1. J. Nat. Med. 2018;72(2):439–447. doi: 10.1007/s11418-017-1166-6. [DOI] [PubMed] [Google Scholar]
- Olivon F., Palenzuela H., Girard-Valenciennes E., Neyts J., Pannecouque C., Roussi F., Grondin I., Leyssen P., Litaudon M. Antiviral activity of flexibilane and tigliane diterpenoids from Stillingia lineata. J. Nat. Product. 2015;78(5):1119–1128. doi: 10.1021/acs.jnatprod.5b00116. [DOI] [PubMed] [Google Scholar]
- Paemanee A., Hitakarun A., Wintachai P., Roytrakul S., Smith D.R. A proteomic analysis of the anti-dengue virus activity of andrographolide. Biomed. Pharmacother. 2019;109:322–332. doi: 10.1016/j.biopha.2018.10.054. [DOI] [PubMed] [Google Scholar]
- Panraksa P., Ramphan S., Khongwichit S., Smith D.R. Activity of andrographolide against dengue virus. Antivir. Res. 2017;139:69–78. doi: 10.1016/j.antiviral.2016.12.014. [DOI] [PubMed] [Google Scholar]
- Pardo-Vargas A., Ramos F.A., Cirne-Santos C.C., Stephens P.R., Paixão I.C.P., Teixeira V.L., Castellanos L. Semi-synthesis of oxygenated dolabellane diterpenes with highly in vitro anti-HIV-1 activity. Bioorg. Med. Chem. Lett. 2014;24(18):4381–4383. doi: 10.1016/j.bmcl.2014.08.019. [DOI] [PubMed] [Google Scholar]
- Pariš A., Štrukelj B., Renko M., Turk V., Pukl M., Umek A., Korant B. Inhibitory effect of carnosolic acid on HIV-1 protease in cell-free assays. J. Nat. Product. 1993;56(8):1426–1430. doi: 10.1021/np50098a031. [DOI] [PubMed] [Google Scholar]
- Peiris J.S., Yuen K.Y., Osterhaus A.D., Stöhr K. The severe acute respiratory syndrome. N. Engl. J. Med. 2003;349(25):2431–2441. doi: 10.1056/NEJMra032498. [DOI] [PubMed] [Google Scholar]
- Pereira H., Leão-Ferreira L., Moussatché N., Teixeira V., Cavalcanti D., Costa L., Diaz R., Frugulhetti I. Antiviral activity of diterpenes isolated from the Brazilian marine alga Dictyota menstrualis against human immunodeficiency virus type 1 (HIV-1) Antivir. Res. 2004;64(1):69–76. doi: 10.1016/j.antiviral.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Pinto A.M.V., Marinho R.d.S.S., Vieira M.C.R., Barros C.d.S., Cirne-Santos C.C., Leite J.P.G., Teixeira V.L., Piaxao I.C. N.d.P. Antiviral activity of diterpenes from Canistrocarpus cervicornis against human herpesvirus 1 and bovine herpesvirus 5. Arch. Biomed. Eng. Biotechnol. 2019;3(2):1–6. [Google Scholar]
- Plourde A.R., Bloch E.M. A literature review of Zika virus. Emerg. Infect. Dis. 2016;22(7):1185. doi: 10.3201/eid2207.151990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomerantz R.J., Horn D.L. Twenty years of therapy for HIV-1 infection. Nat. Med. 2003;9(7):867–873. doi: 10.1038/nm0703-867. [DOI] [PubMed] [Google Scholar]
- Qiu W., Rutherford S., Mao A., Chu C. The pandemic and its impacts. Health Cult. Soc. 2017;9:1–11. [Google Scholar]
- Rao S., Nyquist A.-C., Stillwell P.C. Elsevier; 2019. Influenza, Kendig's Disorders of the Respiratory Tract in Children; pp. 460–465. e462. [Google Scholar]
- Rosário-Ferreira N., Preto A.J., Melo R., Moreira I.S., Brito R.M. The central role of non-structural protein 1 (NS1) in influenza biology and infection. Int. J. Mol. Sci. 2020;21(4):1511. doi: 10.3390/ijms21041511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu W.-S. Academic Press; 2016. Molecular Virology of Human Pathogenic Viruses. [Google Scholar]
- Singh B., Sharma R.A. Plant terpenes: defense responses, phylogenetic analysis, regulation and clinical applications. 3 Biotech. 2015;5(2):129–151. doi: 10.1007/s13205-014-0220-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa F.T., Nunes C., Romano C.M., Sabino E.C., González-Cardenete M.A. Revista do Instituto de Medicina Tropical de São Paulo; 2020. Anti-Zika Virus Activity of Several Abietane-type Ferruginol Analogues; p. 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran Q.T.N., Wong W.S.F., Chai C.L.L. Labdane diterpenoids as potential anti-inflammatory agents. Pharmacol. Res. 2017;124:43–63. doi: 10.1016/j.phrs.2017.07.019. [DOI] [PubMed] [Google Scholar]
- Tronstein E., Johnston C., Huang M.-L., Selke S., Magaret A., Warren T., Corey L., Wald A. Genital shedding of herpes simplex virus among symptomatic and asymptomatic persons with HSV-2 infection. JAMA. 2011;305(14):1441–1449. doi: 10.1001/jama.2011.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuckett M.W., Watkins W.J., Whitby R.J. Rapid access to tricyclic compounds using zirconium chemistry and intramolecular Diels-Alder reactions: synthesis of the pisiferanol and dolastane skeletons. Tetrahedron Lett. 1998;39(1-2):123–126. [Google Scholar]
- Vallim M.A., Barbosa J.E., Negratilde D., De-Paula J.C., Galvatilde V.A.G., Teixeira V.L., de Palmer Paixatilde I.C.N. In vitro antiviral activity of diterpenes isolated from the Brazilian brown alga Canistrocarpus cervicornis. J. Med. Plants Res. 2010;4(22):2379–2382. [Google Scholar]
- Vasconcelos P.F., Powers A.M., Hills S. Elsevier; 2018. The Emergence of Chikungunya and Zika Viruses in the Americas, Chikungunya and Zika Viruses; pp. 215–235. [Google Scholar]
- Wang S.-K., Hsieh M.-K., Duh C.-Y. New diterpenoids from soft coral Sarcophyton ehrenbergi. Mar. Drugs. 2013;11(11):4318–4327. doi: 10.3390/md11114318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y.-D., Zhang G.-J., Qu J., Li Y.-H., Jiang J.-D., Liu Y.-B., Ma S.-G., Li Y., Lv H.-N., Yu S.-S. Diterpenoids and sesquiterpenoids from the roots of Illicium majus. J. Nat. Product. 2013;76(10):1976–1983. doi: 10.1021/np400638r. [DOI] [PubMed] [Google Scholar]
- Wen C.-C., Kuo Y.-H., Jan J.-T., Liang P.-H., Wang S.-Y., Liu H.-G., Lee C.-K., Chang S.-T., Kuo C.-J., Lee S.-S. Specific plant terpenoids and lignoids possess potent antiviral activities against severe acute respiratory syndrome coronavirus. J. Med. Chem. 2007;50(17):4087–4095. doi: 10.1021/jm070295s. [DOI] [PubMed] [Google Scholar]
- WHO . Regional Office for the Eastern Mediterranean. 2014. Dengue and severe dengue, world health organization. [Google Scholar]
- WHO . 2016. Situation Report: Zika Virus, Microcephaly, Guillain-Barré Syndrome. [Google Scholar]
- WHO . World Health Organization; 2017. Global Hepatitis Report 2017. [Google Scholar]
- Widener R.W., Whitley R.J. Vol. 123. Elsevier; 2014. Herpes Simplex Virus, Handbook of Clinical Neurology; pp. 251–263. [DOI] [PubMed] [Google Scholar]
- Wu Y.-C., Hung Y.-C., Chang F.-R., Cosentino M., Wang H.-K., Lee K.-H. Identification of ent-16β, 17-dihydroxykauran-19-oic acid as an anti-HIV principle and isolation of the new diterpenoids annosquamosins A and B from Annona squamosa. J. Nat. Product. 1996;59(6):635–637. doi: 10.1021/np960416j. [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Izumi N., Ui H., Sueki A., Masuma R., Nonaka K., Hirose T., Sunazuka T., Nagai T., Yamada H. Wickerols A and B: novel anti-influenza virus diterpenes produced by Trichoderma atroviride FKI-3849. Tetrahedron. 2012;68(45):9267–9271. [Google Scholar]
- Yan S.-L., Li Y.-H., Chen X.-Q., Liu D., Chen C.-H., Li R.-T. Diterpenes from the stem bark of Euphorbia neriifolia and their in vitro anti-HIV activity. Phytochemistry. 2018;145:40–47. doi: 10.1016/j.phytochem.2017.10.006. [DOI] [PubMed] [Google Scholar]
- Yeh T.-T., Wang S.-K., Dai C.-F., Duh C.-Y. Briacavatolides A–C, new briaranes from the Taiwanese octocoral Briareum excavatum. Mar. Drugs. 2012;10(5):1019–1026. doi: 10.3390/md10051019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu B., Dai C.-q., Jiang Z.-y., Li E.-q., Chen C., Wu X.-l., Chen J., Liu Q., Zhao C.-l., He J.-x. Andrographolide as an anti-H1N1 drug and the mechanism related to retinoic acid-inducible gene-I-like receptors signaling pathway. Chin. J. Integr. Med. 2014;20(7):540–545. doi: 10.1007/s11655-014-1860-0. [DOI] [PubMed] [Google Scholar]
- Zannoli S., Morotti M., Denicolò A., Tassinari M., Chiesa C., Pierro A., Sambri V. Global epidemiology of Zika and Chikungunya virus human infections. Microbiol. Med. 2017;32(3) [Google Scholar]
- Zhang G.-J., Li Y.-H., Jiang J.-D., Yu S.-S., Qu J., Ma S.-G., Liu Y.-B., Yu D.-Q. Anti-Coxsackie virus B diterpenes from the roots of Illicium jiadifengpi. Tetrahedron. 2013;69(3):1017–1023. [Google Scholar]
- Zhang G.-J., Li Y.-H., Jiang J.-D., Yu S.-S., Wang X.-J., Zhuang P.-Y., Zhang Y., Qu J., Ma S.-G., Li Y. Diterpenes and sesquiterpenes with anti-Coxsackie virus B3 activity from the stems of Illicium jiadifengpi. Tetrahedron. 2014;70(30):4494–4499. [Google Scholar]
- Zhang J., Lamb R.A. Characterization of the membrane association of the influenza virus matrix protein in living cells. Virology. 1996;225(2):255–266. doi: 10.1006/viro.1996.0599. [DOI] [PubMed] [Google Scholar]
- Zhao L., Xiang K.-L., Liu R.-X., Xie Z.-P., Zhang S.-M., Dai S.-J. Anti-inflammatory and anti-viral labdane diterpenoids from the fruits of Forsythia suspensa. Bioorg. Chem. 2020;96:103651. doi: 10.1016/j.bioorg.2020.103651. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No data was used for the research described in the article.