Abstract
Background
The relationship between vitamin D (VitD) and insulin sensitivity and secretion in type 2 diabetes mellitus (T2D) has been shown to be different amongst different ethnic populations. In Saudi Arabia, where both T2D and VitD deficiency are highly prevalent health concerns, little is known about the relationship between VitD, insulin sensitivity, resistance and the relative importance of ethnicity. Our primary aim in this study was to investigate influence of ethnicity on VitD association with glycaemic profile and to measures of obesity as a secondary outcome, among multiethnic postmenopausal women with T2D in Saudi Arabia.
Methods
A cross-sectional study was conducted at King Fahad Medical Research Center, King Abdulaziz University, Jeddah, Saudi Arabia. Postmenopausal females (n = 173, age ≥ 50 years) with T2D were randomly selected in this study. Anthropometric measures and fasting blood samples were obtained for all study participants. Several biochemical parameters were measured including 25-hydroxyvitamin D (25(OH)D), glycosylated hemoglobin (HbA1c), insulin, glucose and c-peptide. Surrogate markers for insulin resistance were calculated using Homeostasis Model Assessment 2 for insulin resistance and beta cell activity (HOMA2-IR, HOMA2-β).
Results
Overall, 25(OH)D was inversely associated with fasting glucose (r=-0.165, P = 0.037), insulin (r=-0.184, P = 0.02), C-peptide (r=-0.19, P = 0.015) and HOMA2- IR C-peptide (r=-0.23, P = 0.004). Additionally, serum 25 (OH)D showed a negative correlation with body weight (r=-0.173 P = 0.028), waist and hip circumferences (r=-0.167, P = 0.033; r=-0.22, P = 0.004 respectively) but not with body mass index (BMI) or waist hip ratio (WHR).
In the white ethnic group but not in black or Asian population groups, 25(OH)D level was also associated with only serum fasting C-peptide and HOMA2-IR C-peptide and BMI (P < 0.05).
Conclusions
Insulin resistance and obesity were associated with VitD status in T2D in this cohort. Our findings also suggest that these VitD associations in women from white ethnic background are different than in those from black/Asian ethnic backgrounds. Whether VitD supplements are able to improve either obesity and/or insulin sensitivity should be further investigated in different ethnic population groups.
Keywords: Postmenopausal, Vitamin D, Deficiency, Type 2 diabetes, Insulin Sensitivity, Insulin resistance, Saudi Arabia
Background
Vitamin D (VitD) has a pivotal role in the regulation of calcium (Ca) concentrations in blood through its influence on intestinal absorption and bone metabolism and through its interaction with calciotropic hormones [1]. The influence of VitD on extra-skeletal tissue is gaining increasing prominence in the literature, thought to contribute to insulin resistance, pathology of pancreatic β -cell and systemic inflammation and ultimately type 2 diabetes mellitus (T2D) risk [2–7]. It is proposed that VitD can influence the progression and control of T2D either directly by binding to its own receptor (VitD receptor) on β-cells of the pancreas or indirectly by regulating extracellular Ca or Ca influx to pancreatic β-cells [8, 9].
VitD deficiency/insufficiency, which is assessed by circulating blood 25-hydroxyvitamin D 25(OH)D concentration, is one of the most globally widespread health concerns among postmenopausal women [10] and has been suspected as a risk factor for T2D in Europeans, African-Americans and south Asians [4–7]. It has also been reported that an inverse relationship exists between VitD status and risk of T2D and metabolic syndrome [11].
In several observational studies, VitD deficiency has been linked to insulin sensitivity and secretion which can both be impaired in T2D; however the role of ethnicity has not been fully examined [2, 12, 13]. A large cross-sectional study [14] in the US revealed that the Homeostatic Model Assessment of Insulin Resistance (HOMA-IR) and β-cell secretion (HOMA-%β) showed no association with serum 25(OH)D level in non-Hispanic black individuals. However, the same study revealed a correlation between 25(OH)D levels and HOMA-IR in non-Hispanic whites and Mexican Americans. This finding can be interpreted as due to a lower responsiveness to VitD and parathyroid hormone (PTH) in blacks in comparison to whites [14].
Saudi Arabia has a multi-ethnic population and both T2D and VitD deficiency are highly prevalent and of widespread concerns [15, 16].
There are few data in the literature from Saudi Arabia concerning the association/relationship between VitD, insulin sensitivity and resistance with consideration of ethnicity. The aim of this study therefore was to investigate the effects of ethnicity on VitD (25(OH)D) associations with insulin sensitivity, diabetic control and measures of obesity in postmenopausal women in Saudi Arabia with T2D; to target prospectively the ethnic group with stronger VitD associations for VitD dosing treatment.
Methods
Study design and recruitment
This cross-sectional study was conducted on 173 postmenopausal women with T2D, aged between 50 and 87 years, living in the western region of Saudi Arabia (Jeddah). We assessed VitD status (25(OH)D) in the participants and its association with: (1) glycaemic parameters (HbA1c, fasting glucose, fasting insulin, fasting C-peptide and insulin sensitivity indices); (2) bone related parameters (intact PTH, Ca, albumin, phosphorus (PO4) and magnesium (Mg)); (3) anthropometric measures (weight, height, waist hip ratio (WHR) and BMI); (4) lifestyle factors (physical activity, smoking, dietary VitD intake, veiling and sun exposure); (5) Socio-demographic factors including skin tone and ethnicity.
Subjects for this study were sequentially recruited from seven primary health care centers (PHCCs) distributed in Jeddah (a PHCC from each of the seven geographical sectors of Jeddah area was chosen to represent a randomly selected adult population). A multi-stage sampling technique was implemented. In stage 1, one PHCC was chosen from each of the seven sectors of the Jeddah area. In stage 2, random selection of samples was conducted from the selected PHCCs to select female files of the registered population. In stage 3, all women in the selected age group (≥ 50 years) among selected files were contacted for possible recruitment to the study based on the predefined criteria of inclusion. The number of women randomly selected from each center was proportionally identified according to the number of the registered women in each center. Subjects willing to participate in this study were referred to a clinic at the Centre of Excellence for Osteoporosis Research (CEOR) in King Fahd Medical Research Centre (KFMRC), King Abdulaziz University (KAU), Jeddah. Each participant provided written informed consent relating to participation in this study. Following the ethical standards in Declaration of Helsinki, ethical approval of this study was obtained from the Research Ethics Committee, the Faculty of Medicine, KAU (ref no.179 − 16, Oct/2017).
The recruitment and selection of patients was based on specific inclusion and exclusion criteria. Inclusion criteria included postmenopausal women: Last menstrual period (LMP) ≥ 1year and follicular stimulating hormone (FSH) > 15 IU/L), previously diagnosed with T2D according to the criteria of the American Diabetes Association ( HbA1c ≥ 48 mmol/mol or fasting plasma glucose ≥ 7 mmol/L) [17]. Women with history of chronic liver or renal disease, cancer, malabsorption syndrome, rheumatoid arthritis, hyperthyroidism, other endocrinal disorders that might affect bone (e.g. Hyperparathyroidism) or history of intake of medications with possible effects on VitD (e.g. VitD supplements, glucocorticoids and anticonvulsants) were excluded from the study. Sample size was calculated as follows: The total number of females aged between 50 and 79 years living in western region of Saudi Arabia is approx. 430,739, according to the latest demographic survey of the general Saudi authority for statistics (https://www.stats.gov.sa/en). The prevalence of VitD insufficiency (serum 25(OH)D < 20 ng/ml) in Saudi Arabia is 87.8 % [18]. The maximum expected prevalence of VitD insufficiency and deficiency is 90 %. The level of confidence is 95 % with study power of 0.80. Accordingly, the calculated sample size was 150 females, determined by a validated sample size determinant program (Epi-Info, version 6, GA, USA). The sample size was increased by 15 % to guard against drop out and to increase the validity of the study. Following multiple stages of exclusion (Fig. 1), a sample size of 173 was included in this study.
Fig. 1.
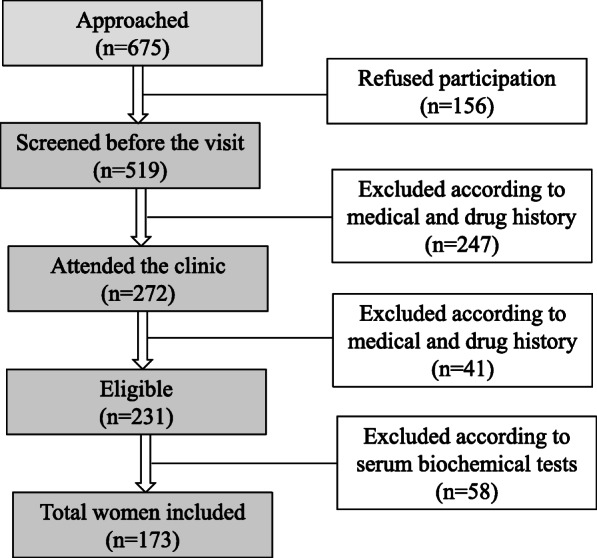
Flow chart of the study postmenopausal participants with T2D
Initially, all participants answered a questionnaire (completed by the researcher), which requested information including socio-demographic factors, dietary VitD intake (semi-quantitative Food Frequency Questionnaire (SFFQ) [19], lifestyle history including smoking habits and physical activity, medical history, menstrual history and drug history. Each participant underwent anthropometric and blood pressure measurements.
Skin tone was recorded for each participant based on the Fitzpatrick skin tone classification [20]. Duration (number of hours) of weekly exposure to outdoor sunlight in the last month was noted in the participants’ questionnaires as well as the use of sunscreen. Due to cultural or religious reasons, most women in Saudi Arabia, especially the elderly, wear a veil and cape. Women participating in the study who cover their head and body, with face and hands exposed were considered as partially covered, while participating women covering their whole body and face, with only the eyes and hands exposed were considered as totally covered.
The daily VitD intake from food was estimated using a semi-quantitative Food Frequency Questionnaire (SFFQ). The SFFQ used in the study was adapted from a validated SFFQ in Saudi Arabia [19]. Items included the most commonly VitD rich food consumed in the region; salmon, tuna, sardines, milk, laban (buttermilk), yogurt, egg and beef liver. The frequency of this food intake was expressed as number of servings per day/week/month. The daily VitD intake in IU was then calculated and compared to their estimated average requirement (EAR) (600–800 IU/day based on the IOM recommendation for women aged 50 y and over [21].
Serum measurements of 25(OH)D and other hormones
Serum 25(OH)D and intact PTH levels were measured by direct competitive chemiluminescence immunoassay (CLIA), using a LIASON auto-analyzer (DiaSorin Inc, Stillwater, MN, USA). The intra and inter-assay coefficient of variations (CV) of serum samples were < 8 %. VitD deficiency was defined based on Institute of Medicine (IOM) guidelines [21] as the 25(OH) D level below 12 ng/ml (< 30 nmol/l) and VitD insufficiency as the 25(OH)D level of 12–19 ng/ml (30–49 nmol/l), and VitD sufficiency between 20 and 50 ng/ml (50–125 nmol/L). FSH and Thyroid function test (TFT) including thyroid-Stimulating Hormone (TSH), free thyroxin (T3) and free triiodothyronine (T4) were measured in serum by immunoassays, using VITROS ECiQ (Ortho-Clinical Diagnostics Inc., Rochester, NY, USA) to exclude any women with hyperthyroidism or not postmenopausal.
Serum measurements of liver enzymes, renal function, high-sensitivity C-reactive protein, lipid and bone profile
Liver enzymes (including Aspartate Aminotransferase (AST), Alanine Aminotransferase (ALT), and Alkaline Phosphatase (ALP)), creatinine, total cholesterol, direct high density lipoprotein cholesterol (HDL-C), triglycerides, low density lipoprotein cholesterol (LDL-C), very low density lipoprotein cholesterol (VLDL-C) albumin, Ca, PO4 and Mg) were all measured in serum by reflectance spectrophotometry, employing the colorimetric method using a VITROS 250 Clinical Chemistry Auto-analyzer (Ortho-Clinical Diagnostics Inc., Rochester, NY, USA). The intra and inter-assay CV of serum samples were 4.1 and 4.5 % respectively. LDL-C concentrations in serum were directly calculated by the analyzer, based on standardized calculations (Friedewald equation) [22] dependent on the level of total cholesterol, direct HDL-C and triglyceride measured by the same analyzer. VLDL-C serum levels were estimated by dividing the triglyceride by 2.2. Subjects with high liver enzymes were excluded (the normal clinical level of serum being AST ˂45 U/L; ALT ˂50 U/L and ALP between 80 and 280 U/L). Samples with creatinine levels higher than normal were excluded (i.e. a normal level of serum creatinine in females ˂105µmol/L).
High sensitive C-reactive protein (hs-CRP) was measured in serum by immunoassay, using a VITROS 5,1 FS chemistry auto-analyzer (Ortho-Clinical Diagnostics Inc., Rochester, NY, USA). The intra-assay and inter-assay CV of serum samples were 3.5 and 4 % respectively.
Measurements of glycaemic control parameters
Glycosylated hemoglobin (HbA1c) was determined using a VITROS 5,1 FS chemistry auto-analyzer (Ortho-Clinical Diagnostics Inc., Rochester, NY, USA). Fasting glucose in serum was measured by means of a colorimetric method, using a VITROS 250 Clinical Chemistry Auto-analyzer (Ortho-Clinical Diagnostics Inc., Rochester, NY, USA). The intra and inter-assay CV for HbA1c and fasting glucose samples were both < 5 %. Insulin and c-peptide (a consequent product produced when insulin is secreted) were measured in serum by a sandwich CLIA using a LIAISON autoanalyzer (DiaSorin Inc, Stillwater, MN, USA). The intra and inter-assay CV for insulin and C-peptide serum samples were both < 6 %. Fasting insulin and Homeostasis Model Assessment 2 (HOMA2) [23] were measured for all women, with the exception of those on insulin therapy, due to the influence of insulin intake on these measures.
HOMA2 was used to estimate insulin resistance and β-cell function. HOMA2 indices [24] (HOMA2-IR and HOMA2-%β) were calculated from fasting glucose, fasting insulin and fasting c-peptide in a steady-state condition (fasting glucose: 3–25 mmol/L, fasting insulin: 2.88–43.16 mIU/L and fasting c-peptide: 0.6–10.5 µU/ml) using an updated computer HOMA calculator software (version 2.2.3) issued by University of Oxford Diabetes Trials Unit, available at https://www.dtu.ox.ac.uk/homacalculator/ .
Statistical analysis
The statistical analysis was performed using SPSS program (v.20 SPSS Chicago Inc). Normality of data was tested by Kolmogorov-Smirnov test. All numerical parametric results were expressed as means ± SD, while numerical non-parametric results were presented as median (IQR). Descriptive results were expressed as a percentage of the total sample number. Correlations between different parameters were obtained using Pearson correlation for normally distributed data and Spearman correlation for non-normally distributed data. The non-parametric test, Kruskal-Wallis H test, was used based on non-normally distributed data to compare between groups. A probability value ≤ 0.05 was considered statistically significant. Multiple linear regression analysis (stepwise) was used for independent variables that showed significant correlations at the bivariate level (P ≤ 0.05) to determine independent predictors of the dependent variable.
Results
Participants’ general characteristics are summarized in (Table 1).
Table 1.
General characteristics of the participating women
| Variables | (N = 173) Results |
|---|---|
| • Age (years) | 59.6 ± 6.8 |
| • Age at menopause (years) | 49.7 ± 4.2 |
| • Years since menopause | 9.8 ± 7.2 |
| • Age at T2DM diagnosis (years) | 46.7 ± 9.3 |
| • Years since T2D | 12 (6–20) |
| • T2D therapy mode | |
| Diet | 5 (3 %) |
| OHD | 91 (53 %) |
| Diet + OHD | 3 (2 %) |
| Insulin | 15 (9 %) |
| Insulin + OHD | 59 (34 %) |
| • Use of statin therapy | 84 (49 %) |
| • Hypertensive (according to medical records) | |
| Yes | 125 (72 %) |
| No | 48 (28 %) |
| • SBP (mmHg) | 144 ± 23 |
| • DBP (mmHg) | 82 (76–90) |
| • Marital status | |
| Single | 1 (1 %) |
| Married | 113 (65 %) |
| Divorced | 10 (6 %) |
| Widow | 49 (28 %) |
| • Education | |
| Illiterate | 73 (42 %) |
| Elementary | 36 (21 %) |
| Intermediate | 29 (17 %) |
| Secondary | 25 (14 %) |
| University | 10 (6 %) |
| Postgraduate | 0 (0 %) |
| • Occupation | |
| Housewife | 160 (93 %) |
| Governmental employed | 0 (0 %) |
| Privately employed | 4 (2 %) |
| Self-employed | 0 (0 %) |
| Retired | 9 (5 %) |
| • Ethnicity | |
| White (Arabic) | 126 (73 %) |
| Black (African) | 30 (17 %) |
| South Asian (Pakistani) | 17 (10 %) |
| • Skin tone (Fitzpatrick)a | |
| Type I (light, pale white) | 0 (0 %) |
| Type II (white, fair) | 24 (14 %) |
| Type III (medium white to olive) | 68 (39 %) |
| Type IV (olive, mid brown) | 50 (29 %) |
| Type V (brown, dark brown) | 31 (18 %) |
| Type VI (very dark brown, black) | 0 (0 %) |
| • Sun exposure | |
| < 1 h/week | 85 (49 %) |
| 1–2 h/week | 48 (28 %) |
| 2–3 h/week | 21 (12 %) |
| > 3 h/week | 19 (11 %) |
| • Veiling type | |
| Totally covered (use of niqab: eyes exposed only) | 125 (72 %) |
| Partially covered (face exposed) | 48 (28 %) |
| • Use of sunscreen | 0 (0 %) |
| • Subjects consuming dietary VitD above EARb | 0 (0 %) |
| • Physical activity | |
| Yes | 53 (31 %) |
| No | 120 (69 %) |
| • Smoking | |
| Yes | 3 (2 %) |
| No | 170 (98 %) |
| • Serum total cholesterol (mmol/L) | 4.2 ± 1.3 |
| • Serum triglyceride (mmol/L) | 1.4 (0.99–2.2) |
| • Serum HDL-C (mmol/L) | 1.0 (0.8–1.3) |
| • Serum LDL-C (mmol/L) | 2.15 (1.7-3.0) |
| • Serum VLDL-C (mmol/L) | 0.62 (0.45-1.0) |
| • Serum hs-CRP (mg/L) | 5.4 (2.8–9.9) |
The numerical data are presented as mean ± SD if normally distributed and as median (IQR) if non-normally distributed. Descriptive data are presented as n (%).T2D is Type 2 Diabetes Mellitus. OHD is Oral Hypoglycemic Drugs. BMI represents Body Mass Index, WHR Waist Hip Ratio, SBP Systolic Blood pressure, DBP Diastolic Blood Pressure
aFitzpatrick scale [20]. EAR is estimated average requirement
bEAR for women aged 50 y and over based on IOM recommendation (600-800 IU/day) [21]. 25(OH)D is 25-hydroxyvitamin D; PTH is Parathyroid Hormone; Ca is Calcium; PO4 is Phosphate; and Mg is Magnesium. HDL-C is high lipoprotein cholesterol; LDL-C is low density lipoprotein cholesterol; VLDL-C is very low density lipoprotein cholesterol; and hs-CRP is high sensitive C-reactive protein
Serum 25(OH)D levels and dietary VitD intake in all participating women and in sub-classified ethnic groups are shown in (Table 2); showing overall serum 25(OH)D mean (± SD) of 14.2 ± 9.2 ng/ml and non-significant differences in median of serum 25(OH) D levels and dietary VitD intake between ethnic groups.
Table 2.
Serum 25(OH)D levels and dietary VitD daily intake among the participants as classified by ethnicity
| Ethnicity | 25 (OH)D (ng/ml) | P | Dietary VitD intake (IU/day) | P |
|---|---|---|---|---|
| Overall (n=173) | 14.2±9.2 | 110 (53.5-180) | ||
| White-Arabic (n=126) | 13.1 (7.6-19.2) | 0.70 | 110 (62-168) | 0.38 |
| Black-African (n=30) | 10.8 (8.2-17.3) | 114 (73-218) | ||
| Asian-Pakistani (n=17) | 12 (6-17.6) | 100 (60-176) |
Numerical data are presented as mean ± SD or median (IQR). 25(OH)D is 25-hydroxyvitamin D. Differences in VitD between different ethnic groups were tested by Kruskal-Wallis H test
According to IOM [19] guidelines for VitD status classification, 47 % were VitD deficient, 31 % were VitD insufficient, and 22 % demonstrated optimal levels of VitD.
In the full cohort, the relationship between serum 25 (OH)D level showed an inverse association with body weight (P = 0.028), waist and hip circumferences (P = 0.033, P = 0.004 respectively). Conversely, BMI and WHR did not show any association with total 25 (OH)D (Table 3). When the relationships were investigated within each ethnic group independently, no statistical significant correlation was found except for BMI, which was positively correlated with 25(OH)D in white postmenopausal women (r=-0.250, P = 0.009, Pearson correlation, 2-tailed).
Table 3.
The serum 25(OH)D correlation with anthropometric measures and bone related parameters in whole group
| Variable | Results (N=173) | Correlation with 25(OH)D | |
|---|---|---|---|
| r | P | ||
| Weight (kg) | 79.3 ± 18 | -0.173* | 0.028* |
| Height (cm) | 154.7 ± 6 | -0.020 | >0.1 |
| BMI (kg/m²)(a) | 49.5 (43.3-58) | -0.120 | >0.1 |
| Waist circumference (cm) | 100.2 ± 12.7 | -0.167* | 0.033* |
| Hip circumference (cm) | 113.5 ± 13.4 | -0.220* | 0.004* |
| WHR | 0.9 ± 0.06 | 0.086 | >0.1 |
| Serum Intact PTH (pg/ml)(a) | 47.9 (33.3-61.9) | -0.340* | <0.0001* |
| Serum Ca (mmol/L)(a) | 2.25 (2.07-2.42) | 0.009 | >0.1 |
| Serum PO4(mmol/L) | 1.19±0.2 | -0.060 | >0.1 |
| Serum Mg (mmol/L)(a) | 0.7 (0.6-0.8) | -0.110 | >0.1 |
Results are presented as mean ±SD or median (IQR)
PTH parathyroid hormone, Ca calcium, PO4 phosphorus, Mg magnesium
aSpearman Correlation (2-tailed). The rest of correlations are Pearson correlations (2-tailed)
*Significant correlation (p<0.05)
The correlation between 25(OH)D and bone related parameters were non-significant except for serum intact PTH which showed an inverse relationship with 25(OH)D (p < 0.0001) (Table 3). No independent effect of ethnicity was found.
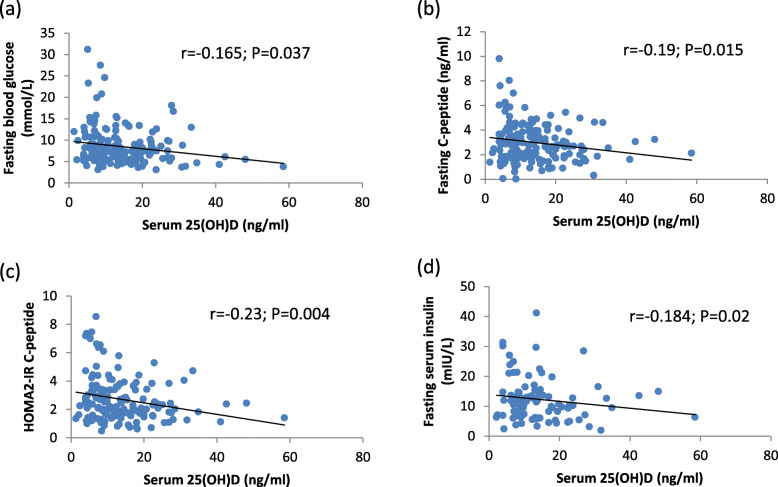
When correlations were assessed between serum 25(OH)D and the glycaemic control parameters, a significant negative correlation was found between 25(OH)D and: fasting glucose (P = 0.037), fasting insulin (P = 0.02) fasting C-peptide (P = 0.015), HOMA2- IR C-peptide (P = 0.004) (Fig. 2). The correlations between 25(OH) and the remaining glycaemic control parameters (including HbA1c, HOMA2- IR insulin, HOMA2-%β) were not significant.
Fig. 2.
The relationship between 25(OH)D and glycaemic parameters. a The relationship between total 25(OH)D and fasting glucose (n = 173,2-tailed Spearmen correlation). b The relationship between total 25(OH)D and fasting C-peptide (n = 173, 2-tailed Pearson correlation). c The relationship between total 25(OH)D and HOMA2-IR C-peptide (n = 173, 2-tailed Spearmen correlation). d The relationship between total 25(OH)D and fasting insulin (n = 99*, 2-tailed Pearson correlation). *Subjects not taking insulin
After sub-dividing the group according to ethnicity, 25(OH)D was associated with serum fasting C-peptide and HOMA2-IR C-peptide in white group only, while it was not associated with any parameters in either black and Asian groups (Table 4). Additionally, 25(OH)D levels did not show any significant correlation with skin tones, sun exposure, dietary VitD intake, veiling type, age, duration of diabetes and menopause, ethnicity, presence of hypertension, diabetes treatment, BMI class, smoking, marital status, occupation or education (data not shown).
Table 4.
Correlations between 25(OH) D and glycaemic parameters among different ethnic groups of the study participants
| Variable | Results (N=173) | White (n=126) | Black (n=30) | Asian (n=17) | |||
|---|---|---|---|---|---|---|---|
| r | P | r | P | r | P | ||
| Fasting insulin (pmol/L)a | 93.05 ± 72.9 | -0.14 | >0.1 | -0.27 | 0.27 | -0.24 | >0.1 |
| Fasting c-peptide (nmol/L) | 0.98 ± 0.51 | -0.23* | 0.012* | -0.03 | 0.86 | -0.21 | >0.1 |
| Fasting glucose (mmol/L) | 7.4 (5.6-10.2) | -0.17 | 0.065 | -0.13 | 0.57 | 0.075 | >0.1 |
| HbA1c (mmol/mol) | 64 ± 864 ± 8 | -0.03 | >0.1 | -0.21 | 0.29 | 0.003 | >0.1 |
| HOMA2-IR insulina | 2.69 ± 1.54 | 0.20 | >0.1 | -0.23 | 0.35 | -0.20 | >0.1 |
| HOMA2-%β insulina | 79.9 (46.1-136.9) | 0.10 | >0.1 | 0.045 | 0.86 | -0.10 | >0.1 |
| HOMA2-IR C-peptide | 2.7 ± 8.56 | -0.25* | 0.009* | -0.23 | 0.27 | -0.26 | >0.1 |
| HOMA2-%β C-peptide | 69.5 (35.3-102.6) | 0.066 | >0.1 | 0.12 | 0.57 | -0.23 | >0.1 |
Correlations in white group are Pearson correlation (2-tailed). Correlations in black and Asian group are Spearman correlations (2-tailed)
aMeasured in subjects not taking exogenous insulin, total (n=99): white (n=68), black (n=21) and Asian (n=10). HOMA2-IR is homeostatic assessment 2 for insulin resistance. HOMA2-%β is homeostatic assessment 2 for β-cell function; HOMA2-IR/% β C-peptide was calculated using fasting glucose and C-peptide; HOMA2- IR/% β insulin was calculated using fasting glucose and fasting insulin
*Significant correlation (p<0.05)
Multiple linear regression analysis results for the study subjects are shown in (Table 5). Hip circumference and HOMA2-IR C-peptide were the only independent variables showing statistical significance as predictors for serum 25(OH)D levels, explaining 8.5 % of variability in serum vitD levels.
Table 5.
Stepwise multiple regression analysis between serum 25(OH)D level and independent variables (N=173)
| Dependent variable | Independent variables | β | p | 95% CI for β | |
|---|---|---|---|---|---|
| lower limit | upper limit | ||||
| Serum 25(OH)D | Hip circumference (cm) | -0.150 | 0.007 | -0.258 | -0.041 |
| HOMA2-IR C-peptide | -1.252 | 0.008 | -2.175 | -0.328 | |
| Total R2= 0.085 | |||||
Stepwise variable inclusion with p < 0.05 and exclusion with p > 0.10
95% CI confidence intervals, ß unstandardized regression coefficient, R2 percent variance explained by each variable
Discussion
Prevalence of VitD deficiency between 40 and 100 % were previously identified in studies in elderly US and European cohorts [25–36]. In the Middle East including Saudi Arabia, 25(OH)D deficiency has also been documented previously despite the abundance of sunlight, with almost half of the study subjects (47 %) having VitD deficiency and 31 % VitD insufficiency. This high prevalence of VitD deficiency or insufficiency found in our study subjects was as expected due to several factors influencing negatively on VitD status including lack of adequate sunlight exposure (due to veiling and extreme hot weather) and inadequate dietary VitD intake.
The current study is the first to investigate insulin resistance and sensitivity (including HOMA-2) in multi-ethnic groups in Saudi Arabia (Jeddah). VitD in this study was found to be significantly correlated (p < 0.05) with fasting insulin, fasting glucose, fasting C-peptide and insulin resistance indices (HOMA2-IR C-peptide). This finding was consistent with what was reported by Forouhi et al. [37], Hahn et al. [38], Weiler et al. [39] and Dutta et al. [40]. These associations between VitD and glycaemic parameters in our study can be attributed to the biological mechanism whereby VitD has a direct effect on the pancreatic β-cell by binding to VDR or an indirect effect through its role in regulation of extracellular Ca and Ca flux into the pancreatic β-cell [41]. Our results show that ethnicity can modify the associations between VitD and glycaemic markers as 25(OH)D was associated with serum fasting C-peptide and HOMA2-IR C-peptide in the white group (n = 126), but not associated with any diabetic parameter in either the black (n = 30) and Asian (n = 17) groups. This finding confirms results from a large cross-sectional US study in non-Hispanic whites and blacks carried by Scragg et al., where 25(OH)D was associated with HOMA-IR in whites but not in blacks [14]. The mechanism underlying this absence of an association between 25(OH)D and insulin resistance in blacks is unclear. This observed differences in the relationship between VitD and T2D between blacks and whites might be due to the variation in the threshold at which VitD takes effect in different ethnicities and a possible decreased responsiveness to VitD and PTH in blacks [14]. In addition, ethnic differences in factors such as culture, genetic background, lifestyle and diet might also contribute to the variation in the VitD relationship with T2D. Further studies are needed to clarify this ethnic discrepancy in VitD action, which could only be achieved using a supplementation protocol, thus providing novel insight into potential preventive mechanisms linked to VitD for these specific groups.
Our data failed to show any association between VitD and glycemic control (HbA1c) which was in line with what has been reported [42, 43]. Moreover, a meta-analysis of fifteen dietary intervention trials demonstrated that, in patients with type 1 and 2 diabetes (or patients with impaired glucose intolerance), VitD had no impact on reducing HbA1c [44]. However, the situation is far from clear, with several studies finding an association with HbA1c, including a cohort study from Saudi Arabia in 1000 patients with type1 and 2 diabetes which demonstrated an inverse correlation [45–48]. Diabetes is a heterogenous disease with multiple treatment modalities and so discrepancies between studies are to be expected, a problem which can only be addressed by more defined and larger populations within studies.
In the present study, we explored VitD association with measures of obesity in T2D and we reported that VitD was inversely related to weight, waist and hip circumferences. This is not unanticipated, as obesity has an adverse effect on VitD status due to of 25(OH)D being sequestered within adipose tissue [49, 50]. However, VitD was not related to BMI or WHR (conventional measures of overall obesity and central obesity respectively), which is in contrast with what some other studies have observed [49, 51, 52]. However, when ethnicity was taken into account, 25(OH)D was associated with BMI in white women. In comparison, 25(OH)D was not associated with anthropometric measures in the other two ethnic groups (black and Asian). These findings are in agreement with findings of other studies that has shown that ethnicity might modify the relationship between adiposity (including BMI and WHR) and serum 25(OH)D. In prior studies, either a lack of association has been observed between these variables in single multi-ethnic study groups or, different associations are found between different ethnicites [14, 53, 54]. These observations question whether VitD supplementation will have the same effect on obesity and insulin resistance among individuals from different ethnic groups. This also highlights a future need to investigate anthropometric and glycaemic responsiveness to VitD supplementation in multiple ethnicities, and could suggests ethnically personalized VitD recommendations against obesity and/or insulin resistance.
Recently, several studies have examined the relationship between 25(OH)D, obesity and diabetes, reporting lower 25(OH)D [55, 56]. Obesity is considered as a primary risk factor for T2D and so it challenging to disentangle whether low 25(OH)D is related directly to T2D, or indirectly through obesity [56–58]. In the present study, waist circumference and HOMA2-IR C-peptide remained predictors of vitD status after multiple regressions supporting vitD association with obesity and insulin resistance.
Overall, there is a need for postmenopausal women with T2D in Saudi Arabia to increase their VitD levels (which can be approached by economical approaches including VitD supplementation and increasing sunlight exposure). In addition, further studies are required to explore potential VitD protective mechanisms for T2D and adiposity in various ethnic cohorts, to understand observed disparity of VitD impact between different ethnicities. Derivation of an appropriate cut-off level for 25(OH)D for improving insulin resistance would need to be considered in the future study.
The present study has several limitations that have to be taken into account or consideration when interpreting the results. Sample sizes of subcategorized black and Asian ethnic groups were small and would need to be increased in order to confirm these findings related to ethnicity. Another limitation is that this study is cross-sectional in nature, therefore, causality of temporal VitD associations in T2D cannot be confirmed. Furthermore, variations in diabetic treatment regimens and duration of T2D amongst participating women might contribute to the VitD relationship with glycaemic control parameters. Physical activity and VitD dietary intake were both assessed by self-report. Although, the present study relied on single measurements of vitD and did not consider seasonal variation of vitD level, the abundance of sunshine throughout the years in Saudi Arabia makes the seasonal aspect less relevant than in other cohorts.
Conclusions
VitD deficiency (serum 25(OH)D < 12 ng/ml) is highly prevalent among postmenopausal women with T2D. Our findings confirm the inverse VitD relationship with some measures of obesity and insulin sensitivity in T2D, however this association was only observed in white subjects but not in those from black or Asian origin. Further studies are required to understand the underlying mechanism responsible for this ethnic variation and to explore and compare VitD supplementation responses in black, and white and Asian ethnic groups.
Acknowledgements
We are thankful for all the participants of this study.
Abbreviations
- 25(OH)D
25-hydoxyvitamin D
- ALP
Alkaline phosphatase
- ALT
Alanine aminotransferase
- AST
Aspartate aminotransferase
- Ca
Calcium
- CEOR
Centre of Excellence for Osteoporosis Research
- CLIA
Competitive chemiluminescence immunoassay
- CV
Coefficient of variation
- FSH
Follicular stimulating hormone
- Hb
Hemoglobin
- HbA1c
Glycosylated hemoglobin percentage
- HDL-C
High density lipoprotein cholesterol
- HEGC
Hyperinsulinemic euglycemic clamp
- HOMA-%β
Homeostatic model assessment β-cell secretion
- HOMA-IR
Homeostatic model assessment of insulin resistance
- Hs-CRP
High sensitive C-reactive protein
- IQR
Interquartile range
- KAU
King Abdul-Aziz University
- KAUH
King Abdul-Aziz University Hospital
- KFMRC
King Fahad Medical Research Centre
- LDL-C
Low density lipoprotein cholesterol
- LMP
Last menstrual period
- Mg
Magnesium
- PHCC
Primary health care center
- PO4
Phosphate
- PTH
Parathyroid hormone
- QUICK-I
Quantitative insulin sensitivity check index
- T2D
Type 2 diabetes mellitus
- T3
Thyroxin
- T4
Triiodothyronine
- TFT
Thyroid function test
- TSH
Thyroid-stimulating hormone
- VDBP
Vitamin D binding protein
- VitD
Vitamin D
- VLDL-C
Very low density lipoprotein cholesterol
- WC
Waist circumference
- WHR
Waist-hip ratio
Authors’ contributions
SA contributed to the study design and execution, data analysis and manuscript drafting. EA contributed to data analysis, writing review and supervision. MDR contributed to supervision, writing review and editing. MIN contributed to writing review. AC and SL-N contributed to supervision. All authors read and approved the final manuscript.
Funding
Joint supervision programme, Department of Physiology, Faculty of Medicine, KAU, Jeddah, Saudi Arabia. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Ethical approval of this study was obtained from the Research Ethics Committee, the Faculty of Medicine, KAU (ref no.179 − 16, Oct/2017). Fully informed, written consent was obtained from the participants.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Shatha Alharazy, Email: smalharazy@kau.edu.sa.
Eman Alissa, Email: ealissa@kau.edu.sa.
Susan Lanham-New, Email: s.lanham-new@surrey.ac.uk.
Muhammad Imran Naseer, Email: minaseer@kau.edu.sa.
Adeel G. Chaudhary, Email: chaudhary@kau.edu.sa
M Denise Robertson, Email: m.robertson@surrey.ac.uk.
References
- 1.DeLuca HF. Overview of general physiologic features and functions of vitamin D. Am J Clin Nutr. 2004;80(6):1689S–1696S. doi: 10.1093/ajcn/80.6.1689S. [DOI] [PubMed] [Google Scholar]
- 2.Lips P, Eekhoff M, van Schoor N, Oosterwerff M, de Jongh R, Krul-Poel Y, et al. Vitamin D and type 2 diabetes. J Steroid Biochem Mol Biol. 2017;173:280–285. doi: 10.1016/j.jsbmb.2016.11.021. [DOI] [PubMed] [Google Scholar]
- 3.Mezza T, Muscogiuri G, Sorice G, Prioletta A, Salomone E, Pontecorvi A, et al. Vitamin D deficiency: a new risk factor for type 2 diabetes. Ann Nutr Metab. 2012;61(4):337–348. doi: 10.1159/000342771. [DOI] [PubMed] [Google Scholar]
- 4.Grimnes G, Figenschau Y, Almås B, Jorde R, Vitamin D. insulin secretion, sensitivity, and lipids results from a case-control study and a randomized controlled trial using hyperglycemic clamp technique. Diabetes. 2011;60(11):2748–2757. doi: 10.2337/db11-0650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deleskog A, Hilding A, Brismar K, Hamsten A, Efendic S, Östenson C-G. Low serum 25-hydroxyvitamin D level predicts progression to type 2 diabetes in individuals with prediabetes but not with normal glucose tolerance. Diabetologia. 2012;55(6):1668–1678. doi: 10.1007/s00125-012-2529-x. [DOI] [PubMed] [Google Scholar]
- 6.Harris SS. Does vitamin D deficiency contribute to increased rates of cardiovascular disease and type 2 diabetes in African Americans? Am J Clin Nutr. 2011;93(5):1175S–1178S. doi: 10.3945/ajcn.110.003491. [DOI] [PubMed] [Google Scholar]
- 7.Boucher B. Hypovitaminosis D and risk of type 2 diabetes in British South Asians. Diabetic Med. 2006;23(3):336. doi: 10.1111/j.1464-5491.2006.01825a.x. [DOI] [PubMed] [Google Scholar]
- 8.Lee S, Clark SA, Gill RK, Christakos S. 1, 25-Dihydroxyvitamin D3 and pancreatic beta-cell function: vitamin D receptors, gene expression, and insulin secretion. Endocrinology. 1994;134(4):1602–1610. doi: 10.1210/endo.134.4.8137721. [DOI] [PubMed] [Google Scholar]
- 9.Xuan Y, Hy Z, Liu JM. Vitamin D. Type 2 diabetes mellitus (维生素 D 与 2 型糖尿病) J Diabetes. 2013;5(3):261–267. doi: 10.1111/1753-0407.12024. [DOI] [PubMed] [Google Scholar]
- 10.Kuchuk NO, van Schoor NM, Pluijm SM, Chines A, Lips P, Vitamin D. Vitamin D status, parathyroid function, bone turnover, and BMD in postmenopausal women with osteoporosis: global perspective. J Bone Mineral Res. 2009;24(4):693–701. doi: 10.1359/jbmr.081209. [DOI] [PubMed] [Google Scholar]
- 11.Khan H, Kunutsor S, Franco OH, Chowdhury R. Vitamin D, type 2 diabetes and other metabolic outcomes: a systematic review and meta-analysis of prospective studies. Proc Nutr Soc. 2013;72(01):89–97. doi: 10.1017/S0029665112002765. [DOI] [PubMed] [Google Scholar]
- 12.Gulseth HL, Wium C, Angel K, Eriksen EF, Birkeland KI. Effects of vitamin D supplementation on insulin sensitivity and insulin secretion in subjects with type 2 diabetes and vitamin D deficiency: a randomized controlled trial. Diabetes Care. 2017;40(7):872–878. doi: 10.2337/dc16-2302. [DOI] [PubMed] [Google Scholar]
- 13.Mitri J, Pittas AG. Vitamin D and diabetes. Endocrinol Metab Clin. 2014;43(1):205–232. doi: 10.1016/j.ecl.2013.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scragg R, Sowers M, Bell C. Serum 25-hydroxyvitamin D, diabetes, and ethnicity in the Third National Health and Nutrition Examination Survey. Diabetes Care. 2004;27(12):2813–2818. doi: 10.2337/diacare.27.12.2813. [DOI] [PubMed] [Google Scholar]
- 15.Al-Alyani H, Al-Turki HA, Al-Essa ON, Alani FM, Sadat-Ali M. Vitamin D deficiency in Saudi Arabians: A reality or simply hype: A meta-analysis (2008–2015) J Fam Commu Med. 2018;25(1):1. doi: 10.4103/jfcm.JFCM_73_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meo SA. Prevalence and future prediction of type 2 diabetes mellitus in the Kingdom of Saudi Arabia: A systematic review of published studies. J Pakistan Med Assoc. 2016;66(6):722–725. [PubMed] [Google Scholar]
- 17.Association AD Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33(Supplement 1):S62–S69. doi: 10.2337/dc10-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ardawi M-S, Sibiany A, Bakhsh T, Qari M, Maimani A. High prevalence of vitamin D deficiency among healthy Saudi Arabian men: relationship to bone mineral density, parathyroid hormone, bone turnover markers, and lifestyle factors. Osteoporosis Int. 2012;23(2):675–686. doi: 10.1007/s00198-011-1606-1. [DOI] [PubMed] [Google Scholar]
- 19.Zareef TA, Jackson RT, Alkahtani AA. Vitamin D intake among premenopausal women living in Jeddah: food sources and relationship to demographic factors and bone health. J Nutri Metab. 2018;2018:8570986. doi: 10.1155/2018/8570986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fitzpatrick T. Peau et soleil. J Med Esthet. 1975;2:33–34. [Google Scholar]
- 21.Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96(1):53–58. doi: 10.1210/jc.2010-2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502. doi: 10.1093/clinchem/18.6.499. [DOI] [PubMed] [Google Scholar]
- 23.Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27(6):1487–1495. doi: 10.2337/diacare.27.6.1487. [DOI] [PubMed] [Google Scholar]
- 24.Levy JC, Matthews DR, Hermans MP. Correct homeostasis model assessment (HOMA) evaluation uses the computer program. Diabetes Care. 1998;21(12):2191–2192. doi: 10.2337/diacare.21.12.2191. [DOI] [PubMed] [Google Scholar]
- 25.Holick MF. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc. 2006;81(3):353–373. doi: 10.4065/81.3.353. [DOI] [PubMed] [Google Scholar]
- 26.Bischoff-Ferrari HA, Giovannucci E, Willett WC, Dietrich T, Dawson-Hughes B. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr. 2006;84(1):18–28. doi: 10.1093/ajcn/84.1.18. [DOI] [PubMed] [Google Scholar]
- 27.Malabanan A, Veronikis I, Holick M. Redefining vitamin D insufficiency. Lancet. 1998;351(9105):805–806. doi: 10.1016/S0140-6736(05)78933-9. [DOI] [PubMed] [Google Scholar]
- 28.Thomas MK, Lloyd-Jones DM, Thadhani RI, Shaw AC, Deraska DJ, Kitch BT, et al. Hypovitaminosis D in medical inpatients. N Engl J Med. 1998;338(12):777–783. doi: 10.1056/NEJM199803193381201. [DOI] [PubMed] [Google Scholar]
- 29.Chapuy M-C, Preziosi P, Maamer M, Arnaud S, Galan P, Hercberg S, et al. Prevalence of vitamin D insufficiency in an adult normal population. Osteoporosis Int. 1997;7(5):439–443. doi: 10.1007/s001980050030. [DOI] [PubMed] [Google Scholar]
- 30.Holick MF, Siris ES, Binkley N, Beard MK, Khan A, Katzer JT, et al. Prevalence of vitamin D inadequacy among postmenopausal North American women receiving osteoporosis therapy. J Clin Endocrinol Metab. 2005;90(6):3215–3224. doi: 10.1210/jc.2004-2364. [DOI] [PubMed] [Google Scholar]
- 31.Glerup H, Mikkelsen K, Poulsen L, Hass E, Overbeck S, Thomsen J, et al. Commonly recommended daily intake of vitamin D is not sufficient if sunlight exposure is limited. J Intern Med. 2000;247(2):260–268. doi: 10.1046/j.1365-2796.2000.00595.x. [DOI] [PubMed] [Google Scholar]
- 32.Boonen S, Bischoff-Ferrari H, Cooper C, Lips P, Ljunggren O, Meunier P, et al. Addressing the musculoskeletal components of fracture risk with calcium and vitamin D: a review of the evidence. Calcified Tissue Int. 2006;78(5):257–270. doi: 10.1007/s00223-005-0009-8. [DOI] [PubMed] [Google Scholar]
- 33.Lips P. Vitamin D deficiency and secondary hyperparathyroidism in the elderly: consequences for bone loss and fractures and therapeutic implications. Endocrine Rev. 2001;22(4):477–501. doi: 10.1210/edrv.22.4.0437. [DOI] [PubMed] [Google Scholar]
- 34.Bakhtiyarova S, Lesnyak O, Kyznesova N, Blankenstein M, Lips P. Vitamin D status among patients with hip fracture and elderly control subjects in Yekaterinburg, Russia. Osteoporosis Int. 2006;17(3):441–446. doi: 10.1007/s00198-005-0006-9. [DOI] [PubMed] [Google Scholar]
- 35.McKenna MJ. Differences in vitamin D status between countries in young adults and the elderly. Am J Med. 1992;93(1):69–77. doi: 10.1016/0002-9343(92)90682-2. [DOI] [PubMed] [Google Scholar]
- 36.Larsen ER, Mosekilde L, Foldspang A. Vitamin D and calcium supplementation prevents osteoporotic fractures in elderly community dwelling residents: a pragmatic population-based 3‐year intervention study. J Bone Mineral Res. 2004;19(3):370–378. doi: 10.1359/JBMR.0301240. [DOI] [PubMed] [Google Scholar]
- 37.Forouhi NG, Luan JA, Cooper A, Boucher BJ, Wareham NJ. Baseline Serum 25-Hydroxy Vitamin D Is Predictive of Future Glycemic Status and Insulin Resistance. The Medical Research Council Ely Prospective Study 1990–2000. Diabetes. 2008;57(10):2619–2625. doi: 10.2337/db08-0593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hahn S, Haselhorst U, Tan S, Quadbeck B, Schmidt M, Roesler S, et al. Low Serum 25-Hydroxyvitamin D Concentrations are Associated with Insulin Resistance and Obesity in Women with Polycystic Ovary Syndrome. Exp Clin Endocrinol Diabetes. 2006;114(10):577–583. doi: 10.1055/s-2006-948308. [DOI] [PubMed] [Google Scholar]
- 39.Weiler HA, Lowe J, Krahn J, Leslie WD. Osteocalcin and vitamin D status are inversely associated with homeostatic model assessment of insulin resistance in Canadian Aboriginal and white women: the First Nations Bone Health Study. J Nutr Biochem. 2013;24(2):412–418. doi: 10.1016/j.jnutbio.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 40.Dutta D, Maisnam I, Shrivastava A, Sinha A, Ghosh S, Mukhopadhyay P, et al. Serum vitamin-D predicts insulin resistance in individuals with prediabetes. Indian J Med Res. 2013;138(6):853. [PMC free article] [PubMed] [Google Scholar]
- 41.Pittas AG, Lau J, Hu FB, Dawson-Hughes B. The role of vitamin D and calcium in type 2 diabetes. A systematic review and meta-analysis. J Clin Endocrinol Metab. 2007;92(6):2017–2029. doi: 10.1210/jc.2007-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Almetwazi MS, Noor AO, Almasri DM, Popovici I, Alhawassi T, Alburikan KA, et al. The association of vitamin D deficiency and glucose control among diabetic patients. Saudi Pharm J. 2017;25(8):1179–1183. doi: 10.1016/j.jsps.2017.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sheth JJ, Shah A, Sheth FJ, Trivedi S, Lele M, Shah N, et al. Does vitamin D play a significant role in type 2 diabetes? BMC Endocrine Disord. 2015;15(1):5. doi: 10.1186/s12902-015-0003-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.George P, Pearson E, Witham M. Effect of vitamin D supplementation on glycaemic control and insulin resistance: a systematic review and meta-analysis. Diabetic Med. 2012;29(8):e142–e150. doi: 10.1111/j.1464-5491.2012.03672.x. [DOI] [PubMed] [Google Scholar]
- 45.Buhary BM, Almohareb O, Aljohani N, Alrajhi S, Elkaissi S, Sherbeeni S, et al. Association of Glycosylated Hemoglobin Levels With Vitamin D Status. J Clin Med Res. 2017;9(12):1013–1018. doi: 10.14740/jocmr3227w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Darraj H, Badedi M, Poore KR, Hummadi A, Khawaji A, Solan Y, et al. Vitamin D deficiency and glycemic control among patients with type 2 diabetes mellitus in Jazan City, Saudi Arabia. Diabetes Metab Synd Obesity. 2019;12:853. doi: 10.2147/DMSO.S203700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ahmadieh H, Azar ST, Lakkis N, Arabi A. Hypovitaminosis d in patients with type 2 diabetes mellitus: a relation to disease control and complications. ISRN Endocrinol. 2013;2013:641098. doi: 10.1155/2013/641098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Saif-Elnasr M, Ibrahim IM, Alkady MM. Role of Vitamin D on glycemic control and oxidative stress in type 2 diabetes mellitus. J Res Med Sci. 2017;22:22. doi: 10.4103/1735-1995.200278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr. 2000;72(3):690–693. doi: 10.1093/ajcn/72.3.690. [DOI] [PubMed] [Google Scholar]
- 50.Arunabh S, Pollack S, Yeh J, Aloia JF. Body fat content and 25-hydroxyvitamin D levels in healthy women. J Clin Endocrinol Metab. 2003;88(1):157–161. doi: 10.1210/jc.2002-020978. [DOI] [PubMed] [Google Scholar]
- 51.Devaraj S, Jialal G, Cook T, Siegel D, Jialal I. Low vitamin D levels in Northern American adults with the metabolic syndrome. Hormone Metab Res. 2011;43(01):72–74. doi: 10.1055/s-0030-1268485. [DOI] [PubMed] [Google Scholar]
- 52.Al-Daghri N, Al-Attas O, Alokail M, Alkharfy K, Al-Othman A, Draz H, et al. Hypovitaminosis D associations with adverse metabolic parameters are accentuated in patients with Type 2 diabetes mellitus: a body mass index-independent role of adiponectin? J Endocrinol Investig. 2013;36(1):1–6. doi: 10.3275/8183. [DOI] [PubMed] [Google Scholar]
- 53.Nesby-O’Dell S, Scanlon KS, Cogswell ME, Gillespie C, Hollis BW, Looker AC, et al. Hypovitaminosis D prevalence and determinants among African American and white women of reproductive age: third National Health and Nutrition Examination Survey, 1988–1994. Am J Clin Nutr. 2002;76(1):187–192. doi: 10.1093/ajcn/76.1.187. [DOI] [PubMed] [Google Scholar]
- 54.Renzaho AM, Halliday JA, Nowson C. Vitamin D, obesity, and obesity-related chronic disease among ethnic minorities: a systematic review. Nutrition. 2011;27(9):868–879. doi: 10.1016/j.nut.2010.12.014. [DOI] [PubMed] [Google Scholar]
- 55.Kabadi SM, Lee BK, Liu L. Joint effects of obesity and vitamin D insufficiency on insulin resistance and type 2 diabetes: results from the NHANES 2001–2006. Diabetes Care. 2012;35(10):2048–2054. doi: 10.2337/dc12-0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Goldner WS, Stoner JA, Thompson J, Taylor K, Larson L, Erickson J, McBride C. Prevalence of vitamin D insufficiency and deficiency in morbidly obese patients: a comparison with non-obese controls. Obesity Surg. 2008;18(2):145–150. doi: 10.1007/s11695-007-9315-8. [DOI] [PubMed] [Google Scholar]
- 57.Hyppönen E, Power C. Vitamin D status and glucose homeostasis in the 1958 British birth cohort: the role of obesity. Diabetes Care. 2006;29(10):2244–2246. doi: 10.2337/dc06-0946. [DOI] [PubMed] [Google Scholar]
- 58.Tzotzas T, Papadopoulou FG, Tziomalos K, Karras S, Gastaris K, Perros P, Krassas GE. Rising serum 25-hydroxy-vitamin D levels after weight loss in obese women correlate with improvement in insulin resistance. J Clin Endocrinol Metab. 2010;95(9):4251–4257. doi: 10.1210/jc.2010-0757. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.