Abstract
The use of small interfering RNAs (siRNAs) has been under investigation for the treatment of several unmet medical needs, including acute lung injury/acute respiratory distress syndrome (ALI/ARDS) wherein siRNA may be implemented to modify the expression of pro-inflammatory cytokines and chemokines at the mRNA level. The properties such as clear anatomy, accessibility, and relatively low enzyme activity make the lung a good target for local siRNA therapy. However, the translation of siRNA is restricted by the inefficient delivery of siRNA therapeutics to the target cells due to the properties of naked siRNA. Thus, this review will focus on the various delivery systems that can be used and the different barriers that need to be surmounted for the development of stable inhalable siRNA formulations for human use before siRNA therapeutics for ALI/ARDS become available in the clinic.
KEY WORDS: Pulmonary administration, Drug delivery, Nanoparticles, siRNA, Cellular uptake, Endosomal escape, Inflammatory diseases, ALI/ARDS
Abbreviations: AAV, adeno-associated virus; Ago-2, argonaute 2; ALI/ARDS, acute lung injury/acute respiratory distress syndrome; AM, alveolar macrophage; ATI, alveolar cell type I; ATII, alveolar cell type II; AV, adenovirus; CFDA, China Food and Drug Administration; COPD, chronic obstructive pulmonary disease; CPP, cell-penetrating peptide; CS, cigarette smoke; CXCR4, C–X–C motif chemokine receptor type 4; DPI, dry powder inhaler; DAMPs, danger-associated molecular patterns; DC-Chol, 3β-(N-(N′,N′-dimethylethylenediamine)-carbamoyl) cholesterol; DODAP, 1,2-dioleyl-3-dimethylammonium-propane; DODMA, 1,2-dioleyloxy-N,N-dimethyl-3-aminopropane; DDAB, dimethyldioctadecylammonium bromide; DOGS, dioctadecyl amido glycin spermine; DOPC, 1,2-dioleoyl-sn-glycero-3-phosphocholine; DOPE, 1,2-dioleoyl-l-α-glycero-3-phosphatidylethanolamine; DOSPA, 2,3-dioleyloxy-N-[2-(sperminecarboxamido)ethyl]-N,N-dimethyl-1-propanaminium; DOTAP, 1,2-dioleoyl-3-trimethylammonium-propane; DOTMA, N-[1-(2,3-dioleoyloxy)propyl]-N,N,N-trimethylammonium; DPPC, 1,2-dipalmitoyl-sn-glycero-3-phosphocholine; dsDNA, double-stranded DNA; dsRNA, double-stranded RNA; EC, endothelial cell; eggPG, l-α-phosphatidylglycerol; EpiC, epithelial cell; EPC, egg phosphatidylcholine; EXOs, exosomes; FDA, US Food and Drug Administration; HALI, hyperoxic acute lung injury; Hem-CLP, hemorrhagic shock followed by cecal ligation and puncture septic challenge; HMGB1, high-mobility group box 1; HMVEC, human primary microvascular endothelial cell; HNPs, hybrid nanoparticles; ICAM-1, intercellular adhesion molecule-1; IFN, interferons; LPS, lipopolysaccharides; MEND, multifunctional envelope-type nano device; MIF, macrophage migration inhibitory factor; miRNA, microRNA; mRNA, messenger RNA; Myd88, myeloid differentiation primary response 88; N/P ratio, nitrogen /phosphate ratio; NETs, neutrophil extracellular traps; NF-κB, nuclear factor kappa B; NPs, nanoparticles; PAI-1, plasminogen activator inhibitor-1; PAMAM, polyamidoamine; PAMPs, pathogen-associated molecular patterns; PD-L1, programmed death ligand-1; PDGFRα, platelet-derived growth factor receptor-α; pDNA, plasmid DNA; PEEP, positive end-expiratory pressure; PEG, polyethylene glycol; PEI, polyethyleneimine; PF, pulmonary fibrosis; PFC, perfluorocarbon; PLGA, poly(d,l-lactic-co-glycolic acid); PMs, polymeric micelles; PRR, pattern recognition receptor; PS, pulmonary surfactant; RIP2, receptor-interacting protein 2; RISC, RNA-induced silencing complex; RNAi, RNA interference; ROS, reactive oxygen species; shRNA, short RNA; siRNA, small interfering RNA; SLN, solid lipid nanoparticle; SNALP, stable nucleic acid lipid particle; TGF-β, transforming growth factor-β; TLR, Toll-like receptor; TNF-α, tumor necrosis factor-α; VALI, ventilator-associated lung injury; VILI, ventilator-induced lung injury
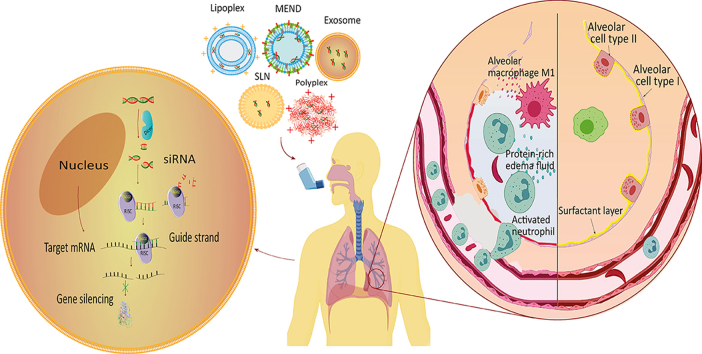
Graphical abstract
The pulmonary administration of siRNA in an appropriate delivery vector seems promising to treat undruggable lung diseases such as acute lung injury/acute respiratory distress syndrome ALI/ARDS.
1. Introduction
Acute lung injury (ALI) or its more severe clinical manifestation, acute respiratory distress syndrome (ARDS), is an acute inflammatory lung disease that leads to high rates of morbidity and mortality annually1, 2, 3. Recently, ARDS has been divided into mild, moderate, and severe by the Berlin definition according to the ratio of the partial pressure of arterial oxygen to the fraction of inspired oxygen, and therefore excluded the term ALI, since it is analogous to mild ARDS4. ALI/ARDS originates from a wide variety of direct (pulmonary) lung injuries such as pneumonia, or indirect (extrapulmonary) insults such as sepsis (Table 1)5, 6, 7. Regardless of the differences between the pulmonary and extrapulmonary forms of ALI/ARDS, the terminal pathophysiological characteristics are remarkably similar, including dysfunction of the alveolar-capillary membrane, enhanced inflammation, and decreased alveolar fluid clearance, resulting in pulmonary edema, impaired gas exchange, and hypoxemia8, 9, 10.
Table 1.
ALI/ARDS risk factors.
| Frequency | Direct (pulmonary) lung injury | Indirect (extrapulmonary) lung injury |
|---|---|---|
| Common | Pneumonia Aspiration of gastric contents |
Non-pulmonary sepsis Major trauma |
| Less common | Lung contusion Inhalation of toxic gases (NO2, ammonia, phosgene) Near drowning Embolism (fat embolism, amniotic fluid embolism) Reperfusion edema following lung transplantation |
Transfusion of blood products Acute pancreatitis Hemorrhagic shock or reperfusion injury Drug overdose (narcotics, sedatives, aspirin, thiazides) |
Despite the extensive studies and the multiple possible targets of ALI/ARDS, there are still no specific pharmacological treatments. To date, the best practice against ALI/ARDS remains protective ventilation, including low tidal volumes and high positive end-expiratory pressures (PEEP) levels11,12. Nevertheless, the mortality remains unchanged at approximately 40%, besides the long-term use of mechanical ventilation can aggravate lung damage [ventilator-associated lung injury (VALI)] or even cause ventilator-induced lung injury (VILI)8,10,13,14. Prone position ventilation, neuromuscular blockade, or extracorporeal membrane oxygenation can be used in the short term to manage patients with severe hypoxemia. Also, adjuvant therapies such as exogenous surfactants, inhaled nitric oxide may improve arterial oxygenation. However, none of these supportive therapies can reduce inflammation in the lungs nor have been efficiently demonstrated to move forward the overall clinical outcomes of ALI/ARDS1,15. Steroid anti-inflammatory drugs were supposed to reduce the associated pulmonary inflammation, though their efficacy in ALI/ARDS is debated and the clinical outcomes are controversial16,17. Accordingly, ALI/ARDS represents an unmet medical need that requires innovative therapeutic strategies to decrease mortalities by treating the underlying molecular mechanisms besides the supportive treatment. For instance, the blockade of signaling pathways that amplify the inflammation associated with this devastating disease may present an attractive approach to limit lung injury18. Gene silencing may present the best paradigm that can specifically and efficiently inhibit these mechanisms and open up new treatment opportunities for ALI/ARDS. Specifically, gene silencing via short-interfering RNA (siRNA) may be implemented to modify the expression of pro-inflammatory mediators in ALI/ARDS at the mRNA level19. Interestingly, the design and the identification of a potent siRNA could be done more easily and rapidly as compared to conventional drug development20. Typically, siRNA can trigger one specific gene. By contrast, other RNA interference therapeutics such as microRNAs (miRNAs) bind imperfectly to mRNA and can degrade multiple mRNA targets, causing toxicity21, 22, 23, 24. Further, siRNA can be easily synthesized, does not require genome integration, and thereby avoids the gene therapy-related risks of mutation and teratogenicity25, in contrast to other gene therapies, such as the plasmid-based short RNAs (shRNA) and plasmid DNA (pDNA) Besides, siRNA has a small size, higher transfection efficacy, potency and specificity, fewer obstacles, and lower immune response, making them the best fit for RNAi therapeutics22,23.
The characteristics, including clear anatomy, accessibility, relative immune privilege, and relatively low enzyme activity, make the lung a good target for local siRNAs therapy26. Nevertheless, its translation into the clinic is hampered by an inefficient delivery to the target cells owing to several unfavorable physicochemical properties of naked siRNA. Thus, there have been significant efforts to design efficient vector systems to overcome the shortcomings in siRNA delivery and to develop stable siRNA formulations for pulmonary administration pushing forward towards the clinical availability of siRNA therapeutics for ALI/ARDS.
In this review, we will first introduce the pathophysiological mechanisms of ALI/ARDS as the first step to determine the possible targets for siRNA. The potential of siRNA therapeutic as a novel approach to combat ALI/ARDS is then emphasized. Then, we highlight the privileges of the pulmonary route for the treatment of ALI/ARDS and we discuss the different intracellular and extracellular barriers that siRNA or siRNA carriers have to overcome to reach their targets. Finally, the different delivery vectors that can be applied for siRNA pulmonary delivery in ALI/ARD are presented.
2. Pathogenesis of ALI
ALI/ARDS is divided into 3 phases: exudative, proliferative, and fibrotic. Initially, the injured lung goes by an acute/early or exudative phase characterized by damage of the alveolar barrier and pulmonary edema. Then, the exudative phase evolves into a proliferative or organizing phase after approximately 1–2 weeks to begin the process of lung repair, and finally, it may progress to an irreversible fibrotic phase in some patients7,27, 28, 29, 30.
2.1. Exudative phase
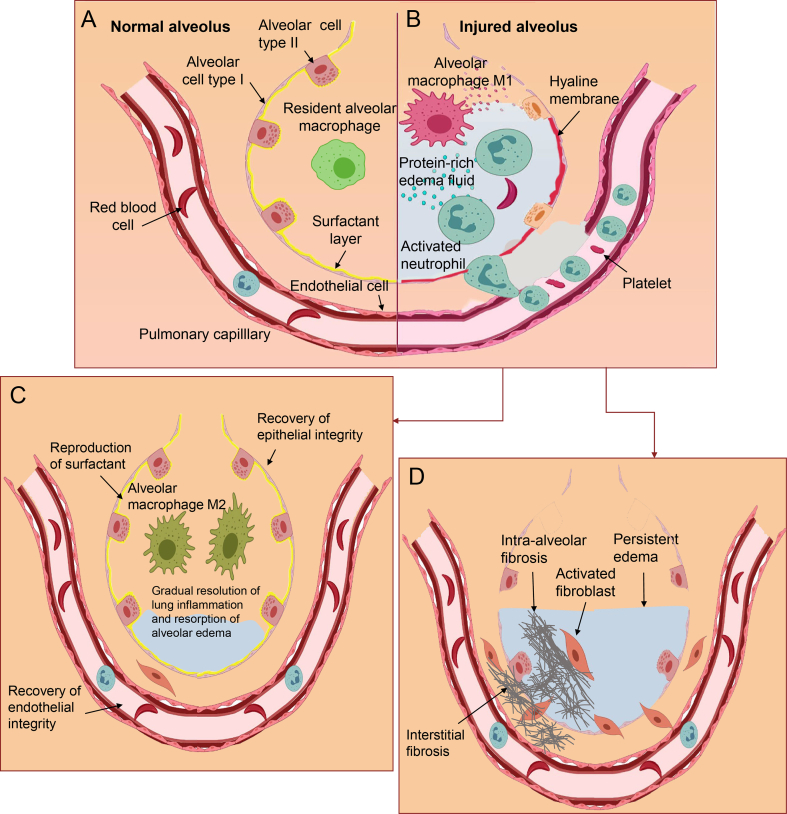
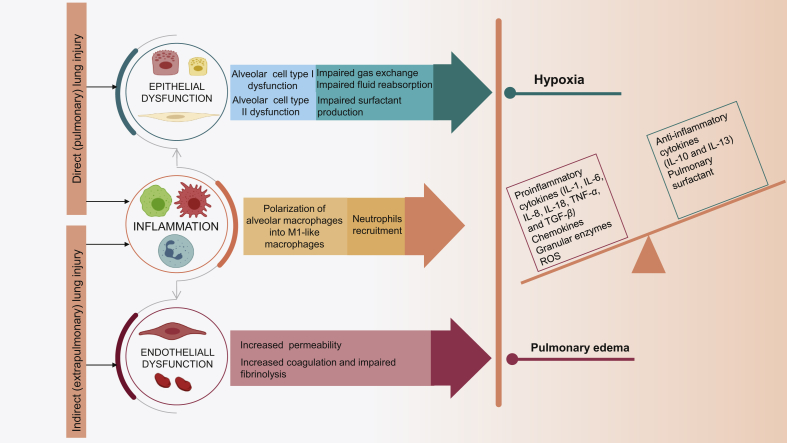
This phase starts with the disruption of alveolar-capillary integrity by pulmonary or extrapulmonary injuries. Initially, resident alveolar macrophages (AMs) recognize nonendogenous pathogen-associated molecular patterns (PAMPs), such as lipopolysaccharides (LPS) and lipoteichoic acid, or endogenous danger-associated molecular patterns (DAMPs), such as histones and high-mobility group box 1 (HMGB1), via pattern recognition receptors (PRRs) such as Toll-like receptors (TLRs). This leads to NF-κB-dependent polarization of AMs into M1-like macrophages, that release pro-inflammatory mediators and chemokines contributing to the excessive recruitment of neutrophils and monocytic cells30, 31, 32, 33, 34, 35, 36, 37. The aggregation of neutrophils within the pulmonary vasculature can also result from the complement activation and release of C5a7. In the lung, activated neutrophils release numerous cytotoxic substances, including reactive oxygen species (ROS), various proinflammatory cytokines, granular enzymes, and neutrophil extracellular traps (NETs). In ALI/ARDS, AMs and neutrophils are the primary sources of proinflammatory cytokines. Consequently, the balance between proinflammatory (IL-1, IL-6, IL-8, IL-18, TNF-α, and TGF-β) and anti-inflammatory mediators (IL-10 and IL-13) is broken13,30,35,38. This inflammatory dysfunction leads to the disruption of alveolar epithelium constituted of alveolar cells type (ATI), that cover the majority of the alveolar surface area playing a primary role in gas exchange and fluid reabsorption, as well as type II alveolar cells (ATII) playing a critical role in alveolar fluid clearance, the production of pulmonary surfactant, and the repopulation of the damaged ATI10,39. The endothelial injury also contributes to the flooding of airspaces, but epithelium injury is more involved39. Eventually, the resultant increase of alveolar-capillary permeability, surfactant dysfunction, and decreased alveolar fluid clearance lead to pulmonary edema, impaired gas exchange, and hypoxemia10. Furthermore, the irreversible damage of ATI and the subsequent replacement of the denuded space by the deposition of proteins, fibrin, and cellular debris, contribute to the formation of hyaline membranes (Fig. 1A and B)29.
Figure 1.
Schematic illustration of normal alveolus (A), injured alveolus during the exudative (B), proliferative (C), and fibrotic (D) phases in ALI/ARDS.
Alveolar epithelial cell (EpiCs) death, either apoptotic or necrotic, is the most extreme form of alveolar barrier injury observed in ALI/ARDS, and one of the most important therapeutic targets for ALI/ARDS. Besides the inflammatory-induced epithelial injury, epithelial cell death can be directly caused by acid aspiration, hyperoxia, and physical forces13,39. The disruption of the endothelial barrier leads to interstitial and alveolar immune cells infiltration and plasma extravasation, facilitated by the injury of alveolar epithelium increasing the pulmonary edema. Besides, the activation of endothelial cells (ECs) by stimuli such as hypoxia and inflammatory cytokines lead to their shift to prothrombotic, proinflammatory, and proadhesive phenotype promoting the procoagulant environment in ALI/ARDS (Fig. 2)10,40.
Figure 2.
The pathogenesis of ALI/ARDS.
2.2. Proliferative phase
Overlapping with the exudative phase, the proliferative phase starts. In this phase, ATII cells proliferate and differentiate into ATI cells to replace injured and necrotic cells, leading to the reabsorption of alveolar edema and restoration of alveolar architecture and function7,15,27. Besides the epithelial integrity, endothelial integrity must also be restored, but this process is less well understood. Interestingly, it has been suggested that broncho-alveolar stem cells also have a role in this resolution41. Simultaneously, resolution of inflammation involves termination of pro-inflammatory signaling and clearance of apoptotic neutrophils41. AMs shift from M1 to M2 macrophages able to produce anti-inflammatory cytokines such as IL-4 and IL-13. This results in a decrease of proinflammatory cytokines production and removal of neutrophils, necrotic cells, and debris from the alveolar space (Fig. 1C)34,42. Also, it has been reported that T-regulatory lymphocytes may suppress cytokines production and neutrophil cell death contributing to the resolution of lung inflammation41,42.
2.3. Fibrotic phase
Pulmonary fibrosis is a more frequent complication in ALI/ARDS of primary pulmonary origin28, which may start in parallel with the previous phases in some patients. It is marked by extensive basement membrane damage, persistent edema, loss of elastic tissue, and obliteration of alveolar spaces with a dense irregular matrix27,34,43. This is mainly due to the proliferation and accumulation of activated fibroblasts, secreting extracellular matrix proteins such as collagen, predominantly the fibrillar collagen type I, but also type III collagen43, resulting in the development of interstitial and intra-alveolar fibrosis that may slowly resolve or cause irreversible lung destruction. The observed fibroblast activation may be regulated by growth factors such as TGF-β, coagulation cascade proteins, and proinflammatory cytokines44. This phase does not happen in all ALI/ARDS patients but has been associated with mechanical, biochemical, and histological evidences of fibrosis with a doubling of lung collagen, progressive hypoxia, multiorgan failure, and increased mortality (Fig. 1D)27,34,44.
3. siRNAs as promising therapeutics for ALI/ARDS
RNA interference (RNAi) is a natural cellular process that silences gene expression by promoting the degradation of mRNA24. RNAi mechanism was uncovered in Caenorhabditis elegans by Fire and Mello in 199845, but it was until 2006 when it gained momentum as they won the Nobel Prize in Physiology or Medicine46. RNAi is a post-transcriptional gene regulation process wherein double-stranded endogenous or exogenous noncoding RNA molecules, including small interfering RNAs (siRNA), microRNAs (miRNAs), short RNAs (shRNAs), or piwi-interacting RNAs (piRNAs), induce silencing of homologous messenger RNA (mRNA) in a sequence-specific way47. Initially, the process starts by breaking down the double-stranded RNA (dsRNA) into smaller RNA duplexes, known as siRNA, by the enzyme Dicer. The resulting 21‒23 nucleotide dsRNA, or the directly transfected synthetic siRNA, are then incorporated into the RNA-induced silencing complex (RISC). Subsequently, the endonuclease argonaute 2 (Ago-2) component of the RISC unwinds the duplex into two single-stranded RNAs, the “passenger or sense” that will be released, and the “guide or antisense” strand that will guide the complex RISC to the complementary target mRNA sequence. Finally, the complex cleaves the target mRNA, mediated by Ago-2, leading to inhibition of mRNA translation and the knockdown of the target gene47, 48, 49.
Synthetic siRNAs are directly delivered to the cytosol, however, the plasmid-based shRNAs need to cross the cellular membrane to penetrate the nucleus where they are transcribed before being exported to the cytosol to be processed into active siRNAs47, and has therefore attracted less attention as a pharmaceutical agent in gene therapy50. Typically, siRNA can trigger specific gene silencing since the antisense strand only binds to mRNA that is perfectly complementary to it. By contrast, miRNA that has a stem-loop and whose biogenesis is initially started in the nucleus binds imperfectly to mRNA and can degrade multiple mRNA targets causing toxicity21, 22, 23, 24. Accordingly, RNAi therapy has unlimited choices of targets and it is possible to design effective RNAi drug targeting for any disease-promoting gene according to the mRNA sequence25,51. siRNA is more favorable for therapeutic use over other gene therapies because it can be easily synthesized, does not require genome integration, and thereby avoids the gene therapy-related risks of mutation and teratogenicity25, with no problem of permanent modification since siRNA therapy can be stopped and controlled at any time and stage of treatment52. The delivery of siRNA is easier compared with shRNA plasmid or pDNA delivery since its site of action is the cytosol. Furthermore, siRNA has a small size, higher transfection efficacy, potency and specificity, fewer obstacles, and lower immune response, making them the best fit for RNAi therapeutics22,23.
Since the discovery of the Nobel prize-winning mechanism of RNAi fifteen years ago46, it has become a promising drug target for treating a myriad of diseases, including genetic diseases, autoimmune and inflammatory diseases, cancer, and viral infection53. To date, three siRNA-based therapeutics are already in the pharmaceutical market. The first is ONPATTRO® (patisiran), a lipid nanoparticle administered intravenously once every 3 weeks, approved by the US Food and Drug Administration (FDA) in August 2018, to treat hereditary transthyretin amyloidosis (hATTR). The second is GIVLAARI™ (givosiran) approved in the USA in November 2019 to treat acute hepatic porphyria. The third is Oxlumo™ (lumasiran) approved in November 2020 by the EU and FDA to treat primary hyperoxaluria type 1. Both givosiran and lumasiran are administered subcutaneously as N-acetylgalactosamine (GalNAc) conjugates54,55. Beyond the 3 siRNAs-based drugs already approved, there are another 7 in phase 3 trials. For instance, nedosiran is developed to treat primary hyperoxaluria, vutrisiran for the treatment of hATTR like patisiran, teprasiran for the prevention of acute kidney injury after transplant or cardiovascular surgery, fitusiran, for hemophilia A and B, tivanisiran to treat ocular pain and dry eye disease, and cosdosiran to treat nonarteritic anterior ischemic optic neuropathy. Also, siRNA therapy was expanded from orphan and rare diseases to more prevalent conditions, such as inclisiran for hypercholesterolemia56. Most of these drug candidates are GalNAc conjugates, except teprasiran, cosdosiran, and tivanisiran, which were not delivered with a carrier or conjugated to a targeting ligand.
Notably, siRNAs have been previously reported to be an effective nucleic acid-based therapy in respiratory diseases57, 58, 59, 60, including ALI/ARDS, by targeting the essential inflammatory pathways involved in this disease61,62. Much work has been done to find potential therapeutic targets of ALI/ARDS, such as those involved in epithelial-endothelial dysfunction, immune cell recruitment, or fibroblast activation; to reinforce lung defense mechanisms and enhance lung repair process19,63. Nevertheless, these studies need to be accompanied by simultaneous research about the best delivery strategies of siRNA therapy of ALI/ARDS to achieve rapid successful outcomes.
4. Pulmonary delivery of siRNA for treatment of ALI/ARDS: Advantages and barriers
4.1. Advantages of siRNA pulmonary delivery
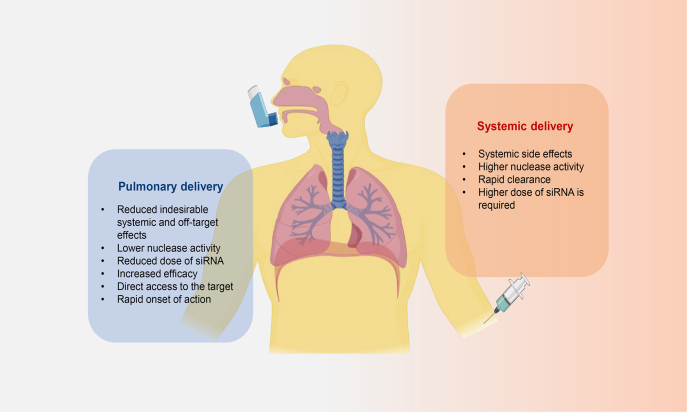
The delivery of drugs to the lung can be achieved directly via the pulmonary route or indirectly by systemic administration. However, nucleic acids can be degraded by serum nucleases in the bloodstream, hurdling the systemic delivery for siRNA19. In contrast, the local administration of siRNAs offers several important benefits over systemic delivery, such as minimized undesirable systemic effects, as well as reduced dose of siRNAs due to the high bioavailability at the target site and the lower lung nuclease activity improving siRNA stability and efficacy (Fig. 3)20,23,58,62,64. Most importantly, the pulmonary route allows direct access to lung EpiCs, key target cells for gene knockdown in ALI/ARDS21.
Figure 3.
Pulmonary versus systemic delivery of siRNA to the lung.
Although inhalation of siRNAs is clinically the most common and non-invasive method, the majority of in vivo studies were conducted intratracheally or intranasally due to their simplicity and ease of administration to animals compared to the inhalation formulations21,58,65. Clinically, intranasal instillations are easy to perform. However, the particles are vastly filtered out in the human nasal cavity. Similarly, intratracheal administration is relatively invasive and not suitable for human use57.
It has been reported that the most appropriate method for pulmonary administration of siRNA-based therapeutics is inhalation using pressurized metered dose inhalers (pMDIs), requiring aerosolization of the drug by suspension or dissolution in a propellant57. The two other inhalation devices, nebulizers and dry powder inhalers (DPIs) are less common owing to the higher chance of siRNA degradation by the applied stress of nebulizers, and the difficulty to formulate siRNA-containing powders or preserving the integrity and bioactivity of siRNA, which hinders the use of DPIs19. However, the use of liquid formulations is not suitable for long-term storage. Accordingly, DPIs seem to be the more promising option because they are more stable, and have a lower risk of contamination extending their shelf-life26,63,66, 67, 68.
4.2. Barriers of siRNA pulmonary delivery
Despite all the advantages of siRNA pulmonary delivery over systemic delivery, it still faces several barriers. This includes extracellular barriers that can be anatomical, physiological, metabolic, and immunological due to the inherent properties of the lung as well as intracellular barriers, related to the cellular uptake and endosomal escape69,70, all with considering the disease pathological changes. Overcoming these barriers is indispensable to develop a successful inhalable siRNA against ALI/ARDS61.
4.2.1. Extracellular barriers
To reach its target, siRNA needs first to overcome the undermentioned extracellular barriers.
4.2.1.1. Anatomical barriers
The human respiratory tract includes 23 groups of branching airways. The first 16 generations from the nasal cavity to the bronchi constitute the region of the conducting airways responsible for the conduction of gases, and the next 17–23 generations extending from the bronchioles to alveoli compose the respiratory airways responsible for the gaseous exchange71. This branched structure of the airways with different diameters and lengths presents an initial hurdle that siRNA must pass along to reach the deep lung area57,66. Here, the site of deposition mainly depends on the size of particles. For deep penetration into the alveolar region, particles should have an optimal aerodynamic diameter between 1 and 5 μm, smaller enough to not deposit in the upper airways and larger enough for not being exhaled during normal breathing62,72, with maximum deposition in alveoli region between 1 and 2 μm73. However, 100 nm particles have been stated to settle successfully into the alveolar space due to the Brownian diffusion mechanism57,62,66,73. To avoid the exhalation of nanoparticles and improve their lung deposition, the “Trojan microparticles” or nanoparticles-in-microparticles, have been proposed to deliver them as dissociable microparticles having desired aerodynamic range (1–5 μmol/L in size) but can disassemble into nanoparticles upon lung deposition. Simultaneously, this strategy provides the aerodynamic behavior of microparticles and the ability of nanoparticles to avoid clearance74,75. Accordantly, a previous study conducted in a rodent model using near-infrared (NIR) fluorescent NPs reported a negligible systemic absorption (less than 5% after 1 h) of particles bigger than 100 nm after intratracheal administration. This study showed also that particles smaller than 34 nm were rapidly removed to the lymph and those smaller than 6 nm were quickly excreted in the urine76. These findings support the use of 100‒200 nm as the optimal nanoscale for pulmonary route74. Typically, the extrapulmonary distribution of NPs is neglected in this nanoscale range. However, it may be enhanced by the loss of alveolar-capillary barrier integrity associated with ALI/ARDS75.
4.2.1.2. Physiological and metabolic barriers
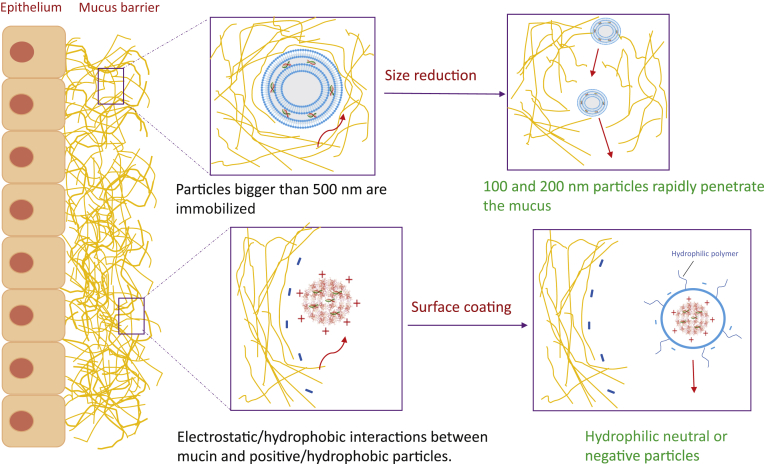
After lung deposition, the next hurdle for siRNA pulmonary delivery to overcome is the clearance of particles. Clearance mechanisms depend on the site of deposition in the lung. The particles are mainly removed by the mucociliary clearance in the conducting airways and the AMs in the respiratory region75,77. The continuous turnover of mucus, the mucociliary clearance driven by the physical action of the ciliated EpiCs, and the possible interactions (electrostatic, hydrophilic, hydrophobic) with particles, constitute a major pulmonary delivery barrier72,78. The interaction of pulmonary delivered particles with mucus and their mucociliary clearance are both surface and particle-size-dependent75. It was found that particles larger than 500 nm were sterically immobilized. In contrast, 100‒200 nm diameter particles rapidly penetrated the mucus of human respiratory systems79. Also, positively charged particles will be more trapped in the mucus rich in anionic mucin glycoproteins than the negatively charged particles. Yet, the commonly utilized siRNA delivery vehicles are polycationic which may hurdle their penetration to the mucus barrier, and therefore their therapeutic efficacy80. In contrast, neutral particles exhibited higher average transport rates75. The hydrophilic and neutral surfaces can be achieved by coating particles with hydrophilic and neutrally charged polymers, such as polyethylene glycol (PEG), or charge reversible peptides. In addition to the glycosylated regions, the mucin peptides may also trap hydrophobic particulates (Fig. 4)72,78, 79, 80, 81. Favorably, an enhanced mucoadhesion could prolong the lung retention time of pulmonary delivered particles, leading to an improved and pharmacokinetic profile75. The impaired mucociliary clearance in ALI/ARDS can further prolong this retention.
Figure 4.
Strategies to overcome the mucosal barrier.
Besides the respiratory mucus, the alveolar surface is covered by pulmonary surfactant (PS), a complex mixture of lipids and proteins82. PS can strongly interact with drugs hindering their effectiveness19,57. Fortunately, this may not have a great impact on ALI/ARDS since surfactant production is disrupted. Formerly, PS has been considered as a siRNA delivery barrier to the respiratory target cells. Lately, a beneficial role of PS in local pulmonary siRNA delivery has been illustrated, hypothesizing that the lung surfactant proteins may act as an “endogenous” delivery vector82.
On the other hand, the ALI/ARDS-increased alveolar fluid can hamper the drug molecules from reaching the epithelium. Moreover, the possible interaction of the cationic siRNA complexes with the anionic constituents of alveolar liquid, including phospholipids and proteins, especially in protein-rich edema fluid in ALI/ARDS constitutes another barrier that can hinder their diffusion. Also, the consolidated or collapsed alveoli further hinder the transducing of acutely injured lungs. Likewise, an associated cough can increase the removal of siRNA from the airways19,61. Despite the lower metabolic capacity of the lung and its negligible nuclease activity, naked siRNA or siRNA carriers may undergo enzymatic degradation following pulmonary delivery, mediated by enzymes such as antitrypsin and proteases61,75.
4.2.1.3. Immunological barriers
siRNA molecules deposited in the respiratory region are primarily cleared by AMs77, further accumulated in the airspace in ALI/ARDS, to be either degraded or removed to the lymphatic system74. It has been speculated that particles smaller than 150 nm can well escape AMs uptake72. Cationic particles can be bound to the anionic sialic acid present on the surface of AMs and thus preferentially phagocytosed compared with the negatively charged ones75. Similar to macrophages, neutrophils that are extensively recruited to the lungs in ALI/ARDS may contribute to siRNA clearance. Also, possible electrostatic interactions between siRNA delivery complexes and cytokines, chemokines, immunoglobulins, and complement components, remarkably increased in the ALI/ARDS alveolar space, can hinder their effectiveness78.
4.2.1.4. Therapeutic barriers
Mechanical ventilation (MV) largely impacts the aerosolization efficiency and deposition within the respiratory tracts of the pulmonary delivered siRNA/siRNA carriers83,84. Harmfully, the presence of heat and humidity in the ventilator circuit can reduce the efficiency of drug delivery83. In patients receiving mechanical ventilation, it is difficult to ensure that the aerosolized particles are efficiently delivered to distal airways85. It has been reported that the aerosol generated by nebulizers in mechanically ventilated patients was mostly deposited in proximal airways with a lung deposition lower than 20% of the nominal dose84,86. Thus, inhalation devices and administration procedures have to be improved for patients under MV84. Positively, the time required to deliver a specified amount of drug to its target and the proportion of drugs delivered could be better managed by controlling the ventilator settings (tidal volume, airflow, inspiratory time/total duration of the breath)83,85.
4.2.2. Intracellular barriers
After overcoming the aforementioned extracellular barriers, siRNA has to bypass a set of intracellular barriers to reach RNAi machinery in the cytoplasm.
4.2.2.1. Cellular uptake
The anionic cell membrane is another barrier to siRNA delivery. Naked siRNA does not easily cross the cell membrane due to its negative charge and high molecular weight87. Thus, effective delivery strategies may be used to facilitate siRNA cellular uptake. The major cell uptake pathway associated with the non-viral delivery of siRNA is endocytosis72. Generally, the cationic siRNA-carrier interacts with anionic proteoglycans and enters the cells by endocytosis88. Clathrin-dependent endocytosis is the best characterized and the most common route involved in the cellular uptake of siRNA during pulmonary delivery, particularly if the diameter is less than 200 nm61,66. Particles under 150 nm can both avoid macrophage uptake and be efficiently endocytosed. Besides, caveolae-dependent endocytosis may also mediate siRNA cellular entry, especially for sizes greater than 500 nm66.
4.2.2.2. Endosomal escape
After cellular entry, the entrapped siRNA/siRNA-carrier vesicles are transported in early endosomes that mature into late endosomes (pH 5–6), and then into lysosomes (pH ~4.5) containing nucleases and other degradative enzymes. Thereby, siRNAs must escape into the cytosol (pH ~7.4) at an early stage to avoid lysosomal degradation66,88. The endosomal escape is the rate-limiting step for efficient siRNA delivery88. Several strategies have been investigated to promote the endosomal escape, including the incorporation of polymers such as polyethyleneimine (PEI) due to their high buffering capacity causing an increased osmolarity and endosome disruption, fusogenic lipids such DOPE (1,2-dioleoyl-l-α-glycero-3-phosphatidylethanolamine) or pH-sensitive fusogenic peptides such as GALA (a repeat of glutamic acid-alanine-leucine-alanine, WEAALAEALAEALAEHLAEALAEALEALAA) owing to their ability to undergo conformational changes in low pH, causing a destabilization of endosomal membrane66,70. Notably, it has been reported that even 0.1% endosomal escape in the cytosol of target cells may be enough for effective RNAi performance since siRNA can amplify itself within the cell88. For caveolae-mediated endocytosis, vectors are entrapped in nonacidic vesicles, transported to the Golgi or endoplasmic reticulum, and in that way escaping the lysosomal degradation66,89.
5. siRNA delivery systems for treatment of ALI/ARDS
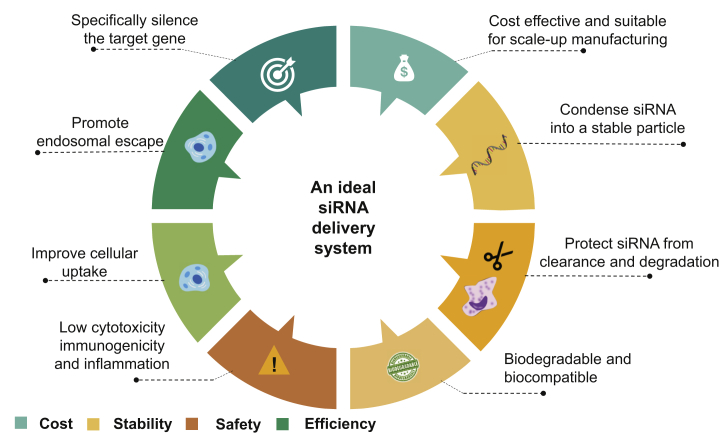
Although the use of siRNAs seems to be a powerful approach against ALI/ARDS, its clinical application is still hurdled by many obstacles as discussed earlier. Similar to other nucleic acid-based therapeutics, this includes off-target effects, triggering of interferon responses, poor stability, and inefficient in vivo delivery, which have been the main challenge to applying siRNA-based therapeutics in humans20,53,88. The properties of suitable delivery systems for siRNAs as potential therapeutic agents include the ability to: (1) entrap siRNA into a stable particle, (2) effectively protect siRNA from enzymatic degradation, (3) avoid pulmonary extracellular barriers, (4) possess high transfection efficiency to facilitate its cellular uptake, (5) have good intracellular trafficking properties to promote the endosomal escape and the cytosolic release of siRNA. Also, an optimal siRNA delivery vector should be biodegradable, biocompatible, with negligible toxicity and immunogenicity, and should not cause inflammation. Also, it should be suitable for large-scale manufacturing (Fig. 5)72,88,90. Owing to the similarities between DNA and RNA, many vectors tested for DNA delivery have been explored for drug delivery of siRNA to the lung against ALI/ARDS (Table 2)91, 92, 93, 94, 95, 96, 97, 98, 99, 100.
Figure 5.
The ideal delivery system for siRNA.
Table 2.
Respiratory siRNA-based preclinical studies against ALI/ARDS.
| Cargo/target gene | Delivery system | Administration route | ALI model | Year | Ref. |
|---|---|---|---|---|---|
| PD-L1 siRNA | Naked siRNA/liposomal siRNA | Intratracheal/i.v. | Shock/sepsis | 2020 | 91 |
| Rip2 siRNA | Lipofectamine | Intratracheal | CS | 2019 | 92 |
| Myd88 siRNA | Serum-derived exosomes | Intratracheal | LPS | 2018 | 93 |
| Paxillin siRNA | JetPEI polyplexes | Intratracheal | LPS | 2015 | 94 |
| CXCR4 antagonist PAI-1 siRNA |
PFC nanoemulsion polyplexes | Intratracheal | LPS | 2019 | 95 |
| TNF-α siRNA | PAMAM dendrimers | Intranasal | LPS | 2020 | 96 |
| TNF-α siRNA | Cationic phosphorus dendrimers | Intranasal | LPS | 2017 | 97 |
| S1PLyase siRNA HMGB1A |
R3V6 cationic peptide | Intratracheal | LPS | 2014 | 98 |
| TNF-α siRNA | Fluorinated cationic polypeptides | Intratracheal | LPS | 2020 | 99 |
| TNF-α siRNA | Cationic dextran nanogel coated with surfactant protein B and DOPC:eggPG | Oro-tracheal | LPS | 2018 | 100 |
CS, cigarette smoke; CXCR4, C–X–C motif chemokine receptor type 4; DOPC, 1,2-dioleoyl-sn-glycero-3-phosphocholine; eggPG, l-α-phosphatidylglycerol; HMGB1A, high-mobility group box 1A; LPS, lipopolysaccharides; Myd88, myeloid differentiation primary response 88; PAI-1, plasminogen activator inhibitor-1; PAMAM, polyamidoamine; PD-L1, programmed death ligand-1; PEI, polyethyleneimine; PFC, perfluorocarbon; Rip2, receptor-interacting protein 2; siS1PLyase, sphingosine-1-phosphate lyase siRNA; TNF-α, tumor necrosis factor-α.
5.1. Viral vectors
Virus-based delivery vectors, such as adenoviruses, adeno-associated viruses, retroviruses, lentiviruses, were used to introduce genetic material into host cells after removing their virulence genes and inserting engineered genes into their genomes101,102.
5.1.1. Adenovirus
Adenovirus (AV), a non-enveloped double-stranded DNA (dsDNA) virus, was approved for clinical trials in 1990 as the first viral gene delivery vector101. Gendicine™, which is an AV-based system loaded with mutated p53 gene, was approved by the CFDA in 2003 for treatment of head and neck squamous cell carcinoma as the first-ever gene therapy103. AV is widely used in lung-targeted gene therapy104,105. However, AV-mediated gene delivery is limited by AV-induced immunological responses104, inflammation, and non-specificity of cell targeting105. Further, AV-mediated gene transfer is relatively inefficient in the absence of epithelial damage. However, this is not a limitation in the case of ALI/ARDS which is characterized by epithelial barrier dysfunction104.
5.1.2. Adeno-associated virus (AAV)
AAV is a small single-stranded DNA nonpathogenic parvovirus that is replication-defective and needs a helper virus to complete its lytic life cycle106,107. AVVs are safer and less immunogenic compared to AV104,105, which makes them an attractive candidate for gene delivery. However, they have lower transfection efficiency101. The AAV serotypes have different tissue tropisms due to different capsid proteins. The serotype AAV6 has been reported to be the most effective vector in infecting pulmonary epithelium101,104. Interestingly, AVV capsid has been modified to improve both the safety and efficacy of these vectors for their application in gene therapy of several human diseases especially lung diseases107. Recently, Duncan et al.108 highlighted the mucus-penetrating ability of AAV6 as a potential mechanism to achieve robust in vivo lung gene transfer and effective inhaled gene therapy. Successfully, AAV6 was used in a recent study to deliver miRNA-21-5p intending to inhibit apoptosis of ATII cells in hyperoxic acute lung injury (HALI) rats’ model108,109. Encouragingly, two AAV-based therapies have been approved by the FDA, luxturna (AAV2-based drug) indicated for rare eye disease and zolgensma, (AAV9-based drug) for spinal muscular atrophy, in 2017 and 2019, respectively106,110,111.
5.1.3. Retroviruses
Retroviruses are enveloped RNA viruses characterized by their ability to reverse transcribing single-stranded RNA into dsDNA, before its insertion into the genome21,101,112. The non-specificity, the insertional mutagenesis, and the risk of tumorigenesis are their major drawbacks101,104. The most common type of retroviruses is lentivirus. Lentiviral vectors have been reported to have lower genotoxicity risk compared to other retroviral vectors112.
Generally, despite the high transduction efficiency of viral vectors, their clinical application is limited by cytotoxicity, high immunogenicity, high potential of harmful insertional mutagenesis in the host genome, and even carcinogenesis113. Besides, viral delivery systems are more suitable for RNAi therapeutics that require transport into the nucleus such as miRNA and shRNA but not for siRNA that acts in the cytoplasm.
5.2. Non-viral vectors
Non-viral carriers have been developed to avoid the immune system stimulation associated with viral vectors25. Despite their relative safety and low-cost production61, these vectors generally do not possess the high level of tissue tropism and transfection efficiency of viral vectors114. The non-viral vectors applied for the pulmonary delivery of siRNA include various delivery systems, such as lipid-based, polymer-based, peptide-based delivery systems, and hybrid nanoparticles, etc.
5.2.1. Lipid-based delivery vectors
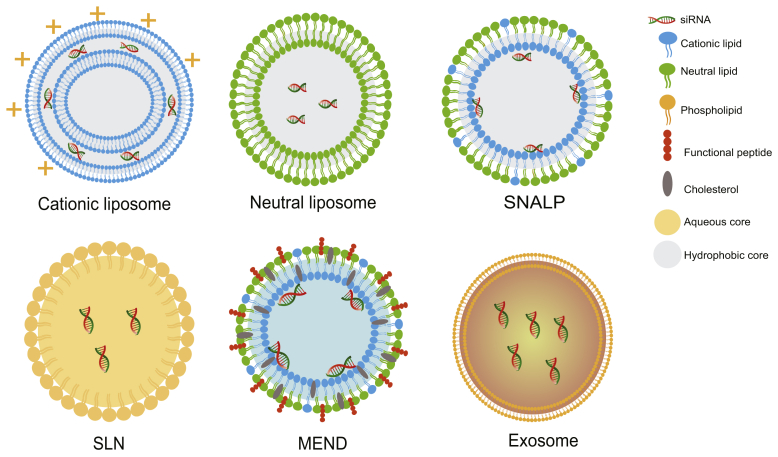
Commonly, lipid-based systems are the most investigated vectors for siRNA delivery. These include liposomes, stable nucleic acid lipid particles (SNALPs), solid lipid nanoparticles (SLNs), pH-responsive lipids, and exosomes (Table 3 and Fig. 6)115, 116, 117, 118, 119, 120, 121, 122, 123, 124, 125, 126, 127, 128, 129, 130, 131, 132, 133, 134, 135.
Table 3.
Summary of the advantages and drawbacks of different lipid-based delivery carriers that can be used for siRNA pulmonary delivery against ALI/ARDS.
| siRNA delivery system | Features and advantage | Disadvantage | Delivery mechanism |
|---|---|---|---|
| Cationic liposomes115, 116, 117, 118, 119 | Good transfection efficiency Efficient endosomal escape Liposomes are the only NPs approved by the FDA for inhalation |
Poor stability Cytotoxicity Immunogenicity |
Complexation of siRNA into lipoplexes through electrostatic interaction |
| Neutral liposomes120, 121, 122, 123 | Fusogenic lipids are used to mediate the endosomal release | Low encapsulation efficiency | Encapsulation of siRNA into their aqueous core |
| Stable nucleic acid lipid particles (SNALPs)124,125 | Have both the advantages of cationic and neutral liposomes Good stability |
Large-scale manufacturing challenges | siRNAs are loaded in the interior of liposomes (electrostatic interaction with cationic lipids) The surface charge is nearly neutral |
| Solid lipid nanoparticles (SLNs)116,124,126,127 | Longer stability Less toxicity than liposomes |
Low encapsulation efficiency Good transfection efficiency |
siRNA is incorporated into the hydrophobic core hydrophobic ion-pairing approach and coated with a lipid membrane |
| Multifunctional envelope-type nano device (MEND)116,128,129 | Improved cellular uptake and endosomal escape Targeted delivery |
Large-scale manufacturing challenges | siRNA is encapsulated in the inner phase of the lipid bilayer |
| Exosomes130, 131, 132, 133, 134, 135 | Native biocompatibility Low toxicity Low immunogenicity High cellular uptake and endosomal escape efficiency |
Purification techniques of exosomes are tedious Expensive for large-scale manufacturing |
Intrinsic ability to encapsulate nucleic acids siRNA can be loaded into exosomes through electroporation |
Figure 6.
Schematic representation of different lipid-based vectors used for siRNA delivery against ALI/ARDS.
5.2.1.1. Lipoplexes (cationic liposomes)
So far, liposomes are the only nanomedicine approved by the FDA for inhalation75. Two types of liposomes could deliver siRNA to the lung, cationic liposomes by complexation and neutral liposomes by encapsulation136. Cationic liposomes are considered the most attractive nanocarriers for siRNA delivery. Typically, the positively charged head of cationic lipids, such as DOTAP (1,2-dioleoyl-3-trimethylammonium-propane), DOTMA (N-[1-(2,3-dioleoyloxy)propyl]-N,N,N-trimethylammonium), DOSPA (2,3-dioleyloxy-N-[2-(sperminecarboxamido)ethyl]-N,N-dimethyl-1-propanaminium), DDAB (dimethyldioctadecylammonium bromide), DOGS (dioctadecyl amido glycin spermine), and DC-Chol (3β-(N-(N′,N′-dimethylethylenediamine)-carbamoyl) cholesterol), binds with the anionic phosphate groups of the nucleic acid to form lipoplexes of 50–200 nm in diameter through spontaneous electrostatic interaction115,121,122,125. Lipoplexes are easy to prepare, protect siRNA from degradation, and have high transfection efficiency102,115.
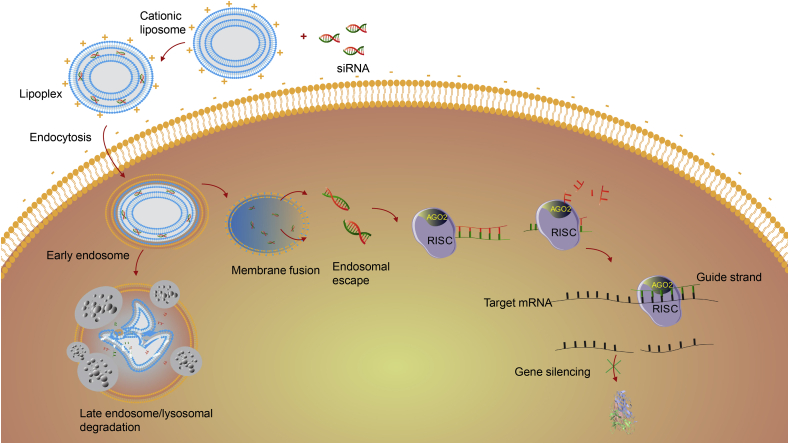
The cationic lipids can directly fuse with anionic membranes and release their payload into the cytosol. Besides this direct fusion, lipoplexes are most commonly internalized via clathrin-mediated endocytosis. Subsequently, the positive charge of cationic lipids increases due to the low endosomal/lysosomal pH which in turn augments the affinity between the anionic endosomal/lysosomal membrane and the lipoplex. Finally, the exchange of lipids between lipoplexes and biological membranes, enhanced by the fusogenic character of liposomes, promotes the release of siRNA into the cytosol (Fig. 7)66,88,137,138. The transfection efficiency can be further improved by optimizing the ratio of lipid:siRNA or N/P ratio (Nitrogen to phosphate ratio) to obtain a slightly positive charged lipoplex102.
Figure 7.
Schematic illustration of cellular uptake and intracellular trafficking of lipoplexes.
Various modifications have been made to protect cationic liposomes from non-specific interactions, reduce their immunological responses and cellular toxicity, and promote endosomal escape. This was achieved by the addition of PEG on their surface to form “stealth liposomes”57,136. However, PEGylation may be more advantageous in the systemic delivery challenged with the reticuloendothelial clearance system than the pulmonary administration since it can hinder their interaction with the cell membrane, and thereby reduce the transfection efficiency136. The inclusion of fusogenic lipids, such as DOPE in cationic liposomes is thought to increase the interactions between the liposomal and endosomal membranes facilitating the release of siRNA125,136,139. Many commercially available transfection agents such as Lipofectin (DOTMA/DOPE), or lipofectamine (DOSPA/DOPE) which are based on cationic liposomes, were implemented for siRNA transfection62,122.
Despite the several advantages of cationic liposomes, including efficient in vitro transfection, high loading capacity, structural flexibility, excellent biocompatibility, biodegradability, and ease of large-scale production, their advancement is still hindered by some drawbacks. These include safety concerns, nonspecific tissue uptake, and premature siRNA release due to their interaction with proteins, lipoproteins, and the extracellular matrix116, 117, 118, 119. Furthermore, cationic liposomes are highly toxic to immune cells and can induce their clearance by macrophages through activation of the complement cascade115,118. Contrarily to their neutral and negatively charged counterparts, cationic liposomes can contribute to lung inflammation and toxicity by prompting the production of ROS117. Besides the size and surface charge, cationic liposome-induced toxicity is influenced by the nature and concentration of cationic lipid, as well as lipids:siRNA ratio. These characteristics should be thoroughly considered to develop safe and efficient lipoplexes122,123,140. It has been stated that the monovalent cationic lipids such as DOTAP are less toxic than the multivalent cationic lipids such as Lipofectamine®, but also less efficient in packing and binding nucleic acids115,125,141.
Recently, Xu et al.91 used both naked siRNA and liposomal encapsulated siRNA by intratracheal and i.v. delivery, respectively, to silence PD-L1 (Programmed death ligand-1) expression significantly up-regulated in pulmonary ECs and/or EpiCs after hemorrhagic shock followed by cecal ligation and puncture septic challenge (Hem-CLP)-induced ALI in mice model. Interestingly, treatment with naked siRNA mainly affected EpiCs but did not target ECs. In contrast, PD-L1 siRNA delivery by liposome mostly targeted ECs, but not EpiCs. Consequently, i.v. administration of liposomes loading the same siRNA attenuated the development of Hem-CLP induced ALI. These results were not observed when naked siRNA was given intratracheally, due to the key role of pulmonary ECs in mediating the PD-1: PD-L1 pathway mainly targeted by i.v. delivery of the liposomal form91. In another study conducted by Dong et al.92, Lipofectamine loaded with siRNA was administered intratracheally to suppress Receptor-interacting protein 2 (RIP2) upregulated in cigarette smoke (CS)-induced ALI mouse model. Rip2 knockdown successfully ameliorated CS-induced inflammation compared with the control siRNA92. Intravenously administered Lipofectamine siRNA was also used to achieve targeted protein and cell-specific knockdown in AMs in lung ischemia-reperfusion injury rat model. Toll-like receptor-4 (TLR4) siRNA was specifically taken up by AMs, but not by pulmonary artery ECs nor ATII cells. Consequently, TLR4 siRNA-specific knockdown could significantly ameliorate lung injury. Furthermore, neither siRNA nor the lipid vector administration was associated with interferon production142.
5.2.1.2. Neutral liposomes
Neutral liposomes composed of neutral lipids, such as cholesterol and its derivatives, DOPC (1,2-dioleoyl-sn-glycero-3-phosphocholine), and DOPE; have also been tested for siRNA delivery by the encapsulation of siRNA into the aqueous core of the liposome121, 122, 123. The loss of net charge improves the safety profile of lipid vectors, but it hinders their interaction with siRNA resulting in a relatively low siRNA entrapment compared to their cationic counterparts (less than 10%). Hence, neutral lipids are often used as transfection enhancers with other vectors123,136. Frequently, DOPE is used as a fusogenic lipid to mediate endosomal release due to its ability to shift to an inverted hexagonal phase at the acidic environment of endosomes supporting the fusion between the nanocarrier and the endosomal membrane120.
5.2.1.3. Stable nucleic acid lipid particles (SNALPs)
SNALPs were first developed in 2001 as a new generation of cationic liposomes. SNALPs are composed of a mixture of an ionizable positively charged phospholipid, such as 1,2-dioleyloxy-N,N-dimethyl-3-aminopropane (DODMA) or 1,2-dioleyl-3-dimethylammonium-propane (DODAP), as well as a neutral helper lipid (including Chol and fusogenic lipids), to overcome the two siRNA intracellular barriers (cellular uptake and endosomal release), with a PEG-coating to prevent degradation and non-specific interactions122,125.
5.2.1.4. Solid lipid nanoparticles (SLNs)
Besides the liposomal formulations, SLNs have been adapted for siRNA delivery to the lung to avoid stability issues associated with liposomes. SLNs are composed of a neutral hydrophobic core, where siRNA can be incorporated by the hydrophobic ion-pairing approach and coated with a lipid membrane116,125. Usually, lipids such as fatty acids/esters/alcohols, triglycerides, waxes, and cholesterol, are used in formulating SLNs127. Most recently, aerosolizable TNF-α siRNA-encapsulated SLNs were successfully prepared for potential pulmonary delivery, suggesting the possibility to deliver the siRNA-SLNs dry powder into the lung to silence the gene expression of a gene of interest in cells, such as AMs or lung EpiCs126.
5.2.1.5. Multifunctional envelope-type nano device (MEND)
MEND is a nanoparticle in which siRNA is entrapped in the inner phase of a lipid bilayer modified with functional molecules such as peptides. Also, pH-responsive lipids can be incorporated into the lipid membranes to control their intracellular traficking116. For example, a GALA-MEND has been recently designed and optimized by incorporating a pH-responsive lipid (YSK05) to deliver CD31 siRNA to the pulmonary endothelium based on GALA peptide targeting129. The incorporated YSK05 enhanced the endosomal release of the optimized MEND resulting in an improved knockdown in the lung endothelium by comparison with DOTMA or DOTAP-MENDs when intravenously injected into mice. Furthermore, the incorporated helper lipids such as DOPE synergistically enhanced both the selectivity and efficiency of the designed MEND129. Thus, GALA modified MEND may be a potential carrier of siRNA in curing various lung diseases including ALI/ARDS128.
5.2.1.6. Exosomes (EXOs)
EXOs are endogenous extracellular nanovesicles deriving from the endosomal system with a particle size ranging from 30 to 150 nm. EXOs are released by various types of cells into the extracellular environment as nature's nano delivery system of lipids, proteins, and nucleic acids, playing an important role in intercellular communication133, 134, 135,143.
Interestingly, Zhang et al.93 used serum-derived exosomes as vehicles to deliver siRNA against Myd88 (involved in innate immune signaling) to the lung. siRNAs were first loaded into serum EXOs via modified calcium-mediated transfection and then were intratracheally instilled in LPS-induced ALI mouse model. Efficiently, the siRNA-carriers were internalized by AMs, achieved specific gene silencing, and modulated LPS-induced inflammation. More interestingly, serum-derived EXOs themselves were neither inflammogenic nor immunogenic when delivered into the lung. Curiously, the intratracheally instilled EXOs were only taken up by AMs, but not by LPS-activated neutrophils. Accordingly, the delivery of EXO-based siRNA to other lung cells, such as EpiCs, requires the development of new methods to avoid their uptake by AMs93.Another study suggests that exosomes of human induced pluripotent stem cells with a size of 122 nm isolated by differential centrifugation, could be used as a natural delivery system of ICAM-1 siRNA to inhibit the intercellular adhesion molecule (ICAM-1) protein expression in HMVECs (Human primary pulmonary microvascular endothelial cells). Exosomes were successfully internalized by LPS activated HMVECs, leading to selective suppression of ICAM-1 surface expression in vitro which may alleviate ALI in vivo144. Based on those findings, EXOs seem like an attractive candidate for siRNA delivery because of their natural potential to carry genetic material, their native biocompatibility with low inherent immunogenicity and toxicity in comparison with exogenous carriers130, 131, 132. Furthermore, they can potentially evade the endosomal pathway and lysosomal degradation134. However, their purification techniques are cumbersome and expensive for large-scale manufacturing131,133.
5.2.2. Polymer-based delivery vectors
Polymer-based vectors are attractive candidates for siRNA delivery systems because of their facile synthesis and lower immunogenicity compared to liposomes145. Generally, polymer-based carriers can be separated into two categories: polycations and polymeric nanoparticles.
5.2.2.1. Polycations
Similar to lipoplexes, polyplexes or dendriplexes are formed by electrostatic interactions between their cationic amine (N) groups and the anionic phosphate (P) groups of nucleic acids. They include synthetic polycations such as polyethylenimine (PEI), polyamidoamine (PAMAM) dendrimers, and natural polycations such as chitosan146. The transfection efficiency of cationic polymers is influenced by their structure, size, and surface charge. Also, the degree of complexation quantified by the N/P ratio affects the net charge, size, and stability of the delivery system147. Typically, an excess of the polymer is needed to generate stable cationic vehicles and facilitate their internalization148. However, an excessively high positive charge may cause cytotoxicity149. Hence, an appropriate N/P ratio should be found for each system to assure the balance between transfection efficiency and cytotoxicity147. The polycations internalized by the clathrin-dependent pathway use the “proton sponge” effect to escape from endosomes150. The proton sponges cationic polymers, such as PEI and PAMAM, prevent acidification of endocytic vesicles owing to their high buffering capacity inciting the membranes H+-ATPases to actively translocate H+ from the cytoplasm into the endosome to maintain the desired pH. The increased protonation causes an influx of chloride and water into the endosome leading to disruption of the endosomal membrane, and eventually endosomal escape of the endocytosed polyplexes into the cytosol150, 151, 152.
Although PEI has an excellent gene condensation ability, high buffering capacity leading to great endosome escape activity, and high transfection efficiency153, it suffers from molecular weight-dependent cytotoxicity and nonbiodegradability hindering its application as an effective delivery system154. The positive charge of PEI has been incriminated in necrotic and apoptotic cell death both in vitro and in vivo152. Also, it has been demonstrated that linear PEIs are less toxic than their branched counterparts155.
5.2.2.1.1. Polyplexes
In vivo-jetPEI™ is a linear PEI that has low toxicity and high efficiency for local gene and siRNA delivery compared to the other cationic lipids and polymers156. Fu et al.94 used JetPEI polyplex as a transfecting agent to deliver paxillin siRNA into the mouse lung by intratracheal instillation to suppress LPS-induced paxillin accumulation which is hypothesized to mediate the associated endothelial hyperpermeability. Resultantly, in vivo knockdown of paxillin attenuated LPS-induced pulmonary endothelial hyperpermeability and injury. Further, other pulmonary cells might be affected by this knockdown94.
In a previous study, dexamethasone-conjugated PEI (DEXA-PEI) was utilized as a siRNA vehicle into lung tissue for the knockdown of macrophage migration inhibitory factor (MIF) overexpressed in particulate matter (PM)-induced airway inflammation. The results showed that DEXA-PEI/MIF siRNA reduced the PM-induced MIF expression in PM-activated EpiCs compared to naked MIF siRNA after intratracheal application in mice. The maximized reduction of inflammation compared to dexamethasone (which itself, with or without scrambled siRNA, has an anti-inflammatory effect) suggested the synergistic effect between siRNA and the developed delivery system157.
Fluorination may increase the stability of polycations, lower their toxicity, and improve cellular siRNA delivery through enhancement of cellular uptake and endosomal escape158. Interestingly, perfluorocarbon (PFC), a biocompatible polymer widely used in pulmonary therapies due to its high oxygen dissolving capacity, has been demonstrated to attenuate ARDS in both humans and animals159. For example, it has been demonstrated that lung delivery of vaporized PFC improved the gas exchange in a detergent-induced lung injury160. Another study also stated that inhalation of PFC may protect from blast lung injury159. Similarly, it was reported that i.v. administration of PFC emulsion significantly improved LPS-induced ALI in rat's model161. Therefore, PFC may represent an excellent candidate for siRNA delivery of ALI/ARDS. To achieve combined inhibition of CXCR4 (C–X–C motif chemokine receptor type 4) and PAI-1 (plasminogen activator inhibitor-1) which are overexpressed in ALI, Wang et al.95 prepared PFC emulsion polyplexes containing a fluorinated polymeric CXCR4 antagonist for the delivery of siRNA against PAI-1. The resultant polyplex emulsions protected siRNA from degradation and boosted siRNA uptake and endosomal escape with no significant cytotoxicity observed. After intratracheal instillation, PFC emulsion polyplexes showed strong lung retention and improved therapeutic outcomes in LPS-induced ALI model in mice95. However, some reports indicated that the presence of fluorine can negatively influence the pulmonary distribution of siRNA polyplexes. It was found that siRNA-nonfluorinated polyplexes were mainly accumulated in the lungs after i.v. delivery in contrast to their fluorinated counterparts that were majorly distributed to the liver. Beneficially, the fluorine modification did remarkably reduce PEI cytotoxicity without altering its binding affinity and improved the in vitro siRNA delivery potency162.
5.2.2.1.2. Polymeric micelles (PMs)
PMs are nanocarriers (usually <100 nm) formed of a hydrophilic polymeric shell, such as PEG, and a hydrophobic core, such as PEI163,164. Their biocompatibility, low toxicity, and relatively high stability make them attractive delivery systems165. Cationic micelle-forming block polymers can be used to trap negatively charged siRNAs in their core via electrostatic interaction166. Interestingly, PMs can be engineered to achieve active targeting and improved cellular uptake by their conjugation with specific targeting ligands or stimuli-responsive components167. However, they are limited by low loading efficiency and complexity in transport through the cell membrane166.
Most recently, Hou J et al.168 developed PMs composed of branched PEI modified with PEGs and loaded with siRNAs against PTPN13 (protein tyrosine phosphatase-N13) and NADPH oxidase-4, which are promoters of pulmonary fibrosis (PF) that constitutes a severe complication in ALI/ARDS. The fibroblast-targeting was achieved by the anti-platelet-derived growth factor receptor-α (PDGFRα) antibody onto the carrier. The developed carrier achieved remarkable antifibrotic effects and successfully suppressed the development of bleomycin-induced PF in murine model168. The same PMs loaded with siRNA against runt-related transcription factor-1 and decorated with an anti-stem-cell antigen-1 antibody could successfully inhibit PF in mice by preventing the differentiation of lung resident mesenchymal stem cells into myofibroblast169.
5.2.2.1.3. Dendriplexes
Polycationic dendrimers, characterized by their unique three-dimensional structure, can also be used to condense siRNA into dendriplexes170. Cationic PAMAM and phosphorus dendrimers were widely tested for siRNA delivery owing to their positive charge, their high degree of surface functionality, their excellent buffering capacity, and high transfection efficiency149.
PAMAM dendrimers were applied to locally deliver TNF-α siRNA to the lung. Compared with non-complexed siRNAs, the cationic dendriplexes with a size between 127 and 153 nm displayed a high siRNA condensation, low cytotoxicity, increased cellular uptake, and transfection performance, and efficiently silence TNF-α in vitro in LPS-activated macrophages. The intranasal pre-administration of PAMAM dendriplexes in a murine LPS-induced lung inflammation model resulted in improved performance 4 h after the LPS challenge. However, PAMAM dendriplexes showed lower efficacy than non-complexed TNF-α siRNA on the 3rd day, requiring more frequent administrations. This could be explained by the higher stability of the non-complexed siRNA and their deep penetration into the lung retarding their removal compared to dendriplexes that undergo fast mucociliary clearance96. Interestingly, Agnoletti et al.171 engineered inhalable dry powders of TNF-α siRNA-loaded G4 PAMAM dendriplexes embedded in microparticles formed of a saccharide matrix, aiming to avoid the unfavorable exhalation of nanoparticles and facilitate their transport. The designed system was well-tolerated and showed an enhanced cellular uptake of siRNA leading to efficient silencing of TNF-α in LPS-activated macrophages. Favorably, the suitability of this system for inhalation therapy was confirmed by the efficient knockdown and the preserved siRNA integrity upon spray drying in the reconstituted NPs171.
Comparatively, it has been reported that cationic phosphorus dendrimers have better delivery efficiency of siRNA than PAMAM dendrimers172. Further, these dendrimers themselves could possess a potent anti-inflammatory action173. A previous study conducted by Bohr et al.97 inspected the potential of TNF-α siRNA cationic phosphorus dendrimers to modulate LPS-induced ALI both in vitro and in vivo. In their research, phosphorus third-generation (G3) dendrimers were functionalized with either cationic pyrrolidinium (DP) or morpholinium (DM) terminal groups. Results showed a strong binding between siRNA and both DP and DM dendrimers, with stronger binding for DP. This was attributed to the different pKa values between DP (8.1) and DM (7.1) resulting in higher ionization of DP in the pH of complexation. However, DP dendriplexes exhibited higher cytotoxicity against macrophages. This could be partly related to their higher surface charge. Similarly, DP dendriplexes showed better cellular uptake and improved in vitro silencing efficiency of TNF-α in LPS-activated macrophages than DM dendriplexes and/or non-complexed siRNA. In correlation with in vitro results, the nasal administration of DP dendriplexes in LPS-induced ALI murine model strongly inhibited TNF-α expression, much higher than DM dendriplexes and free siRNA97. Accordingly, in implementing dendriplexes for siRNA delivery, the dose should be carefully adjusted to compromise between achieving the highest efficacy and at the same time avoiding carrier-associated toxicity.
Poly-l-lysine (PLL) is a well-known cationic polymer that has been widely studied as a non-viral gene delivery vector owing to its strong positive charge74. Recently, Yang J et al.80 used dendritic PLL to deliver TNF-α siRNA to the lung. Interestingly, they have used charge-reversible pro-peptide of RAGE-binding peptide modified with cis-aconitic anhydride for the surface-coating of the delivery nanocomplex and giving it a negative surface charge. This allowed an efficient mucosal penetration of the developed nanosystem after intratracheal delivery. Then, the positive charge was recovered in the mild acidity of the inflamed alveolar space (pH ≈ 6.8), allowing efficient transfection of AMs and TNF-α silencing80.
5.2.2.2. Polymeric nanoparticles
The polyplex formation depends on electrostatic interactions and the anionic nature of the RNA payload. However, their superstructure may be compromised by the competitive interactions with other physiological polyanions147. For this reason, polymers capable of forming neutral or anionic NPs have been suggested. Unlike polycations, siRNA is either dispersed throughout the polymeric matrix or adsorbed on the NPs surface. Appropriate surfactants or cationic polymers such as PEI or chitosan can be added to the matrix174. Practically, the delivery of siRNA by these materials is challenged by their relatively low nucleic acid loading and transfection efficiency147,148.
NPs based on hydrophobic polymers such as PLGA [poly(d,l-lactic-co-glycolic acid)] have attracted more attention to deliver siRNA57,174,175. The endosomal escape of PLGA NPs is due to the surface charge reversal caused by the pH change from anionic (in the physiological and alkaline pH) to cationic (in the endosome/lysosome acidic pH)147,174. After the endosomal escape, PLGA nanoparticles may remain in the cytosol for more than 14 days, allowing a slow release of the payload174. Further, combination with cationic polymers or cationic lipids can improve its transfection efficiency and intracellular trafficking176. Frede et al.177 designed a multi-shell nanoparticle (size of 145 nm and zeta potential of + 23 mV), consisting of a calcium phosphate core, coated with siRNAs, encapsulated within PLGA, and finally coated with a final outer layer of PEI, as a vehicle for local treatment of pulmonary inflammatory disorders. Successfully, multiple nasal instillations of the calcium phosphate PLGA NPs loaded with siRNAs against different pro-inflammatory mediators (CCL-2, IP-10, and IFN-γ) to mice suffering from TH1 cell-mediated lung inflammation showed fruitful internalization (mainly endocytosed by macrophages and dendritic cells and less by EpiCs and lymphocytes), protected siRNA molecules, and decreased gene expression, thereby mitigated the inflammatory responses in the lung177.
5.2.3. Peptide-based delivery vectors
Peptides are other possible carriers that can be applied for siRNA delivery because of their stability, low cytotoxicity and immunogenicity, biocompatibility, and biodegradability179. Peptides can be used alone or as an assistant component to other systems, such as fusogenic peptides used to bypass the endosomal entrapment barrier and cell-penetrating peptides (CPPs) used to facilitate the cellular entry180.
5.2.3.1. Fusogenic peptides
Fusogenic peptides are short peptides that can promote endosomal release by increasing interactions with endosomal membrane178. GALA is a synthetic pH-sensitive fusogenic peptide able to convert from a random coil structure at pH˃6 to an amphipathic a-helical structure able to insert into membranes and cause membrane leakage at pH˂6, promoting the endosomal escape181,182. As abovementioned, GALA-modified MEND has been proposed as a siRNA carrier to take advantage of the dual role of GALA, enhancing the endosomal release, and targeting the lung endothelium129.
5.2.3.2. Cell-penetrating peptides (CPPs)
CPPs are short chains of about 30 or fewer amino acids. Based on differences in physicochemical characters, CPPs can be classified into three classes: cationic, hydrophobic, and amphipathic183, 184, 185. Internalization of siRNA conjugated with CPPs can be mediated via endocytic or nonendocytic pathways depending on the particle size, peptide type, and siRNA loading method186. However, some CPPs have limitations due to non-specificity and easy proteolysis182. Moreover, CPP-mediated delivery of siRNAs is majorly challenged by endosomal entrapment187. It has been reported that CPPs remain localized in the endosome and are further captured by lysosomes rather than undergoing endosomal escape188. Therefore, various modifications have been used with CPPs to promote endosomal escape and improve RNAi efficiency. This includes the attachment of fusogenic peptides such as HA2 peptide derived from influenza virus hemagglutinin, fusogenic lipids such as DOPE, or the use of buffering agents such as chloroquine178. Generally, CPPs can either vectorize siRNA by direct covalent conjugation through a linker or by non-covalent conjugation through electrostatic and hydrophobic interactions between CPPs and siRNA, typically forming nanoparticle structures184,189. So far, no CPPs or CPPs/cargo complexes have been approved by the FDA185.
5.2.3.2.1. Covalent CPPs delivery of siRNA
Besides the activation of innate immunity, the covalent conjugation of siRNA to CPPs can sterically hinder the interaction between siRNA and RISC reducing its efficiency. Additionally, conjugates with charged nucleic acids are unstable and difficult to purify187,190,191. Hence, covalent modification is most suitable for charge-neutral oligonucleotides than charged molecules such as siRNA120.
5.2.3.2.2. Non-covalent CPP delivery of siRNA
The formation of nanoparticle complexes between anionic nucleic acids and CPPs is mainly driven by electrostatic and/or hydrophobic interactions120,189,190. Nevertheless, CPPs have poor encapsulating efficiency, and the nanoparticles formed are more heterogeneous and less defined than their CPP/siRNA conjugate counterparts186,187. Further, CPPs have relatively low transfection efficiency requiring additional modifications to form efficient and stable CPP polyplexes because of their low molecular weight, weak electron charge density, inefficient gene complexation capability, limited capacity for endosomal escape, and rapid degradation under physiological conditions. Generally, CPPs are not used alone but as assistant components in lipoplexes and polymeric polyplexes178.
Interestingly, CPP can be used for the co-delivery of genes and drugs186. For example, R3V6, a cationic amphiphilic peptide was used as a carrier for the delivery of the combinational therapy of sphingosine-1-phosphate lyase siRNA (siS1PLyase), reported to be involved in acute pulmonary inflammation, and recombinant HMGB1A, an antagonist of the pro-inflammatory cytokine HMGB1. The ternary NPs of siS1PLyase-HMGB1A-R3V6 has been designed to test the synergistic effect of siS1PLyase-HMGB1A against LPS-induced ALI model98,192. Remarkably, the ternary NPs and siRNA/R3V6 nanocomplexes displayed higher stability compared with naked siRNA or siRNA/PEI, confirming that R3V6 could protect siRNA from nucleases and proteases. The designed nanocomplex exhibited similar delivery efficiency into the lung EpiCs compared to siRNA-HMGB1A-PEI and siRNA-HMGB1A-Lipofectamine. Moreover, it was shown to be less toxic than both of them with higher anti-inflammatory potency against LPS-activated macrophages. Consistently, the intratracheal administration of siS1PLyase-HMGB1A-R3V6 NPs efficiently reduced the S1PLyase level, the inflammatory response, and apoptosis in lung tissue. These findings confirmed the advantages of the peptide R3V6 over other types of carriers in terms of toxicity and combined delivery of siS1PLyase and HMGB1A98.
Later, Ge et al.99 developed guanidinated and fluorinated bifunctional polypeptides for the lung delivery of TNF-α siRNA into AMs aiming to simultaneously enhance siTNF-α polyplexes cell membrane penetration by the guanidine groups, and enable their transmucus penetration by fluorocarbon modification. Resultantly, the cellular uptake of fluorinated siTNF-α-loaded cationic polypeptides by macrophages was more effective than free siRNA, nonfluorinated, and PEI-siRNA polyplexes. Remarkably, the fluorine modification of the cationic polypeptides potentiated the mucus permeation by 240 folds. Compatibly, the TNF-α knockdown of all the fluorinated polypeptides in LPS-activated macrophages was more effective than the non-fluorinated and PEI-siRNA polyplexes. This was confirmed in vivo by a potent TNF-α silencing, reduced pro-inflammatory cytokines, recovered pulmonary ventilation function, and alleviated tissue damage in the LPS-ALI mice model after intratracheal administration99.
5.2.4. Hybrid nanoparticles (HNPs)
Hybrid lipid-polymer NPs combine both the natural biocompatibility and low immunogenicity of lipids, and the high binding affinity for RNA of polymeric nanocarriers90,193. In this respect, Angelo et al.194 designed hybrid lipid-polymer nanoparticles (size of 150 nm, and zeta potential close to −25mV) as a pulmonary delivery carrier of siRNA to the human airway EpiCs. They used PLGA and dipalmitoylphosphatidylcholine (DPPC), with or without PEI as the third component in their formulations194. DPPC, the principal lipid constituent of pulmonary surfactant, was expected to moderate HNPs interactions in the airways195, while the inclusion of PEI would likely enhance siRNA entrapment inside the HNPs. Resultantly, the optimized siRNA-loaded HNPs displayed an optimal in vitro aerodynamic behavior after administration with a vibrating mesh nebulizer. They exhibited good stability in artificial mucus, excellent penetration inside extracellular lung barriers, and efficient internalization inside the airway EpiCs without inducing any proinflammatory or cytotoxic effects194.
Recently, Dormenval et al.196 aimed to design physiochemically stable inhalable solid dosage forms for siRNA delivery suitable for large-scale production. Therefore, they prepared spray-dried TNF-α siRNA-loaded lipidoid-PLGA hybrid nanoparticles (LPNs) supposed to treat chronic obstructive pulmonary disease (COPD)-associated inflammation. The prepared TNF-α siRNA-loaded LPNs (size of 197.1 nm, PDI of 0.098, and zeta potential of 18.1 mV) were maintained in the same range till 10-fold scaled up formulations. Favorably, the spray drying neither affected the siRNA integrity, nor the in vitro release and thereby efficient gene silencing was maintained196.
Kusumoto et al.197 designed a GALA-modified MEND constituted of an anionic core of siRNA-PEI coated with a cationic liposomal envelope of DOTMA, EPC (egg phosphatidylcholine), and cholesterol. They aimed to study the impact of GALA surface modification which was supposed to enhance the endosomal release and target the pulmonary ECs. GALA/MEND loaded with siRNA against CD31 could efficiently silence the endothelial marker gene CD31 compared to the unmodified MEND indicating the high potency of this system to deliver siRNA to the lung endothelium, one of the promising targets to treat ALI/ARDS197.
Interestingly, Merckx et al.100 designed a hybrid nanoparticle composed of siRNA-loaded cationic dextran nanogel core coated with a shell of the hydrophobic surfactant protein B (SP-B) and incorporated in a phospholipid mixture of DOPC and eggPG (LL-α-phosphatidylglycerol), or DPPC and eggPG. The proteolipid coated siRNA-loaded nanogels were then administered by tracheal aspiration to silence TNF-α in LPS-induced ALI murine model. Remarkably, the incorporation of SP-B model in the DPPC:eggPG coated formulation improved cytosolic siRNA delivery to the respiratory immune cells leading to a significant gene silencing compared to other nanocomposites. However, the administration of these nanocomposites to the naïve mice increased the inflammatory markers100. Most recently, the same group has demonstrated that the proteolipid coated siRNA-loaded nanogels can be freeze-dried and then reconstituted before nebulization for inhalation therapy without influencing their integrity or efficiency198.
6. Conclusions and outlook
The field of siRNA therapy is attracting great interest due to its potential therapeutic to silence any gene of interest including the deadliest diseases, such as ALI/ARDS. Despite the extensive studies and the multiple possible targets of ALI/ARDS, there are still no specific pharmacological treatments. To date, the best practice against ALI/ARDS remains protective ventilation that can aggravate lung damage or even cause lung injury. Therefore, gene silencing via siRNA can be implemented to open up new treatment opportunities for ALI/ARDS. Many preclinical reports are supporting the suitability of siRNA-based therapies for ALI/ARDS by recovering the integrity of lung epithelial and/or endothelial barriers or reinforcing the pulmonary defense mechanisms. However, the majority of these studies focus only on the target of siRNA instead of looking into the delivery perspective. The pulmonary route seems to be the best choice to deliver siRNA into the lung effectively and safely. Despite the advantages of naked siRNA delivery, namely its simplicity and reduced toxicity, the results obtained from the local administration of free siRNA were controversial between different studies. Multiple designed vectors for gene delivery are used in several diseases. However, only some of them have been tested for siRNA delivery against ALI/ARDS. Most of the relevant studies used the available commercial transfection agents such as Lipofectamine, known for their inherent toxicity and instability. Hence, to be feasible and viable in the clinic, the development of an efficient and safe delivery system suitable for the pulmonary delivery of siRNA against ALI/ARS seems promising. This has been proofed by a lot of recent studies that demonstrated the possibility to maintain the integrity of siRNA while formulating inhalable forms.
Both viral and non-viral vector systems have been designed or adapted to protect siRNA and deliver it to its cytosolic target after bypassing the different extracellular and intracellular barriers. Given the inherent immunogenicity of viral vectors, non-viral vectors are increasingly used to deliver nucleic acids. Although non-viral delivery systems are less efficient than viral vectors, they are less immunogenic, less expensive, and easier to produce in large amounts. Exosomes have also been proposed to deliver siRNA into the lung as nature's nano delivery system. However, exosome-mediated delivery is mainly hindered by purification techniques. Comparatively, liposomal delivery systems showed good transfection efficiency, polymer-based nanocarriers displayed sustained release profiles and higher cellular internalization potential, and peptide vectors exhibited enhanced endosomal escape. Interestingly, hybrid nanocarriers seem to be the best delivery system since it allows to combine the advantages of these different carriers into one single vector. Nevertheless, sophisticated approaches usually have characterization and manufacturing scale-up difficulties that may hinder their clinical translation.
Strategies such as the use of a cocktail of siRNAs silencing different mRNAs might be used in combination for targeting different pathways to provide a synergistic silencing effect given the complex pathological mechanisms and pathways involved in ALI/ARDS. Also, the use of targeting peptides to maximize uptake of vectors into alveolar cells or the codelivery of siRNA with other therapeutic agents may further improve the therapeutic outcomes. Innovative nebulization technologies are also needed to improve the delivery of siRNA/carrier to the distal lung while maintaining its integrity.
Besides the delivery issues, there still exist many limitations to overcome. These include safety issues, manufacturing scale-up, the development of inhalation devices that can both maintain siRNA integrity and be suitable for patients under mechanical ventilation. Further, the extrapolation of results from animal studies to human applications is another bottleneck regarding the big differences in the airways between rodents and humans. Various injury models should be combined to create a similar human ALI/ARDS model such as cecal ligation and puncture followed by hemorrhage rather than LPS injury alone. Moreover, in some studies, siRNA treatment was given preventively or in the early phase of the disease which is not practical in the clinical application where the diagnosis and treatment of ARDS are delayed. Frequently, the studies assessed only the inflammatory mediators’ levels without histopathological investigations which is an important point to consider especially when using nanoparticles since the inhalation of NPs itself can cause inflammation. Additionally, the fibroproliferation process may present another promising target for siRNA therapy besides the inflammatory process. Remarkably, most of the studies that investigated the efficiency of delivery vectors in ALI/ARDS used limited targets such as anti-TNF-α siRNA. Similarly, studies that investigated the potential therapeutic targets for siRNA in ALI/ARDS did not focus on the delivery strategies. However, for successful outcomes, both the targets and the delivery strategies should be considered while developing a siRNA therapy for ALI/ARDS. Despite the encouraging preclinical results, the authorities do not list any clinical trial on siRNA delivery against ALI/ARDS. Therefore, more work is needed to develop the appropriate carrier delivering siRNA to the right target in the suitable device to see the first inhalable siRNA-based therapy against ALI/ARDS in the near future.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (Nos. 81872823, and 82073782), the Shanghai Science and Technology Committee (No. 19430741500), the Key Laboratory of Modern Chinese Medicine Preparation of Ministry of Education of Jiangxi University of Traditional Chinese Medicine (TCM-201905, China).
Footnotes
Peer review under responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Author contributions
Wei He conceived and designed the work. Makhloufi Zoulikha, Qingqing Xiao, and George Frimpong Boafo co-wrote the paper. Wei He, Zhongjian Chen, and Marwa A. Sallam commented and corrected the paper. All of the authors have read and approved the final manuscript.
Conflicts of interest
All the authors declare that this article content has no conflict of interest.
References
- 1.Ostwani W., Shanley T.P. In: Pediatric critical care medicine. Wheeler D., Wong H., Shanley T., editors. Springer-Verlag London Ltd; London: 2014. Acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) pp. 101–126. [Google Scholar]
- 2.McNicholas B.A., Rooney G.M., Laffey J.G. Lessons to learn from epidemiologic studies in ARDS. Curr Opin Crit Care. 2018;24:41–48. doi: 10.1097/MCC.0000000000000473. [DOI] [PubMed] [Google Scholar]
- 3.Mart M.F., Ware L.B. The long-lasting effects of the acute respiratory distress syndrome. Expert Rev Respir Med. 2020;14:577–586. doi: 10.1080/17476348.2020.1743182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matthay M.A., Arabi Y.M., Siegel E.R., Ware L.B., Bos L.D.J., Sinha P., et al. Phenotypes and personalized medicine in the acute respiratory distress syndrome. Intensive Care Med. 2020;46:2136–2152. doi: 10.1007/s00134-020-06296-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matthay M.A., Zemans R.L., Zimmerman G.A., Arabi Y.M., Beitler J.R., Mercat A., et al. Acute respiratory distress syndrome. Nat Rev Dis Prim. 2019;5:18. doi: 10.1038/s41572-019-0069-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lumb A.B. In: Nunn's applied respiratory physiology. Lumb A.B., editor. Elsevier; Amsterdam: 2017. Acute lung injury. 439–49.e1. [Google Scholar]
- 7.Weinberger S.E., Cockrill B.A., Mandel J. Principles of pulmonary medicine. Elsevier; Amsterdam: 2019. Acute respiratory distress syndrome; pp. 357–369. [Google Scholar]
- 8.Xu B., Chen S si, Liu M zhuo, Gan C xia, Li J qi, Guo G hua. Stem cell derived exosomes-based therapy for acute lung injury and acute respiratory distress syndrome: a novel therapeutic strategy. Life Sci. 2020;254:117766. doi: 10.1016/j.lfs.2020.117766. [DOI] [PubMed] [Google Scholar]
- 9.Kaku S., Nguyen C.D., Htet N.N., Tutera D., Barr J., Paintal H.S., et al. Acute respiratory distress syndrome: etiology, pathogenesis, and summary on management. J Intensive Care Med. 2020;35:723–737. doi: 10.1177/0885066619855021. [DOI] [PubMed] [Google Scholar]
- 10.Vellingiri V., Thirusangu P., Din I. In: Chronic lung diseases. Rayees S., Din I., Singh G., Malik F.A., editors. Springer; Singapore: 2020. Acute respiratory distress syndrome: therapeutics, pathobiology, and prognosis; pp. 143–156. [Google Scholar]
- 11.Menk M., Estenssoro E., Sahetya S.K., Neto A.S., Sinha P., Slutsky A.S., et al. Current and evolving standards of care for patients with ARDS. Intensive Care Med. 2020;46:2157–2167. doi: 10.1007/s00134-020-06299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coudroy R., Boissier F., Thille A.W. In: Acute respiratory distress syndrome. Chiumello D., editor. Springer International Publishing; Cham: 2017. Acute respiratory distress syndrome (ARDS): definition, incidence, and outcome; pp. 1–13. [Google Scholar]
- 13.Aranda-Valderrama P., Kaynar A.M. The basic science and molecular mechanisms of lung injury and acute respiratory distress syndrome. Int Anesthesiol Clin. 2018;56:1–25. doi: 10.1097/AIA.0000000000000177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith L.S. In: Pediatric acute respiratory distress syndrome: a clinical guide. Shein S.L., Rotta A.T., editors. Springer International Publishing; Cham: 2020. Pathobiology of pediatric acute respiratory distress syndrome; pp. 19–32. [Google Scholar]
- 15.Sweeney R.M., McAuley D.F. Acute respiratory distress syndrome. Lancet. 2016;388:2416–2430. doi: 10.1016/S0140-6736(16)00578-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mokra D., Mikolka P., Kosutova P., Mokry J. Corticosteroids in acute lung injury: the dilemma continues. Int J Mol Sci. 2019;20:4765. doi: 10.3390/ijms20194765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Villar J., Ferrando C., Martínez D., Ambrós A., Muñoz T., Soler J.A., et al. Dexamethasone treatment for the acute respiratory distress syndrome: a multicentre, randomised controlled trial. Lancet Respir Med. 2020;8:267–276. doi: 10.1016/S2213-2600(19)30417-5. [DOI] [PubMed] [Google Scholar]
- 18.Patel V.J., Biswas Roy S., Mehta H.J., Joo M., Sadikot R.T. Alternative and natural therapies for acute lung injury and acute respiratory distress syndrome. Biomed Res Int. 2018;2018:2476824. doi: 10.1155/2018/2476824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jagrosse M.L., Dean D.A., Rahman A., Nilsson B.L. RNAi therapeutic strategies for acute respiratory distress syndrome. Transl Res. 2019;214:30–49. doi: 10.1016/j.trsl.2019.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saw P.E., Song E.W. siRNA therapeutics: a clinical reality. Sci China Life Sci. 2020;63:485–500. doi: 10.1007/s11427-018-9438-y. [DOI] [PubMed] [Google Scholar]
- 21.Fujita Y., Takeshita F., Kuwano K., Ochiya T. RNAi therapeutic platforms for lung diseases. Pharmaceuticals. 2013;6:223–250. doi: 10.3390/ph6020223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu B., Zhong L., Weng Y., Peng L., Huang Y., Zhao Y., et al. Therapeutic siRNA: state of the art. Signal Transduct Target Ther. 2020;5:101. doi: 10.1038/s41392-020-0207-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sajid M.I., Moazzam M., Kato S., Yeseom Cho K., Tiwari R.K. Overcoming barriers for siRNA therapeutics: from bench to bedside. Pharmaceuticals. 2020;13:294. doi: 10.3390/ph13100294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lam J.K.W., Chow M.Y.T., Zhang Y., Leung S.W.S. siRNA versus miRNA as therapeutics for gene silencing. Mol Ther Nucleic Acids. 2015;4:e252. doi: 10.1038/mtna.2015.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu C.F., Wang J. Delivery systems for siRNA drug development in cancer therapy. Asian J Pharm Sci. 2015;10:1–12. [Google Scholar]
- 26.Kandil R., Merkel O.M. Pulmonary delivery of siRNA as a novel treatment for lung diseases. Ther Deliv. 2019;10:203–206. doi: 10.4155/tde-2019-0009. [DOI] [PubMed] [Google Scholar]
- 27.Thompson B.T., Chambers R.C., Liu K.D. Acute respiratory distress syndrome. N Engl J Med. 2017;377:562–572. doi: 10.1056/NEJMra1608077. [DOI] [PubMed] [Google Scholar]
- 28.Kornecki A., Singh R.N. In: Kendig's disorders of the respiratory tract in children. Wilmott R.W., Deterding R., Li A., Ratjen F., Sly P., Zar hj, Bush A., editors. Elsevier; Philadelphia: 2019. Acute respiratory distress syndrome. 606–14.e3. [Google Scholar]
- 29.Udobi K.F., Childs E., Touijer K. Acute respiratory distress syndrome. Am Fam Physician. 2003;67:315–322. [PubMed] [Google Scholar]
- 30.Butt Y., Kurdowska A., Allen T.C. Acute lung injury: a clinical and molecular review. Arch Pathol Lab Med. 2016;140:345–350. doi: 10.5858/arpa.2015-0519-RA. [DOI] [PubMed] [Google Scholar]
- 31.Aghasafari P., George U., Pidaparti R. A review of inflammatory mechanism in airway diseases. Inflamm Res. 2019;68:59–74. doi: 10.1007/s00011-018-1191-2. [DOI] [PubMed] [Google Scholar]
- 32.Lian J., Lin J., Zakaria N., Yahaya B.H. Acute lung injury: disease modelling and the therapeutic potential of stem cells. Adv Exp Med Biol. 2020;1298:149–166. doi: 10.1007/5584_2020_538. [DOI] [PubMed] [Google Scholar]
- 33.Lee J.H., Park J., Lee J.W. Therapeutic use of mesenchymal stem cell–derived extracellular vesicles in acute lung injury. Transfusion. 2019;59:876–883. doi: 10.1111/trf.14838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang X., Xiu H., Zhang S., Zhang G. The role of macrophages in the pathogenesis of ALI/ARDS. Mediators Inflamm. 2018;2018:1264913. doi: 10.1155/2018/1264913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Silva P.L., Rocco P.R.M. In: Acute respiratory distress syndrome. Chiumello D., editor. Springer International Publishing; Cham: 2017. Pathophysiology of acute respiratory distress syndrome; pp. 15–27. [Google Scholar]
- 36.He W., Kapate N., Shields C.W., Mitragotri S. Drug delivery to macrophages: a review of targeting drugs and drug carriers to macrophages for inflammatory diseases. Adv Drug Deliv Rev. 2020;165–166:15–40. doi: 10.1016/j.addr.2019.12.001. [DOI] [PubMed] [Google Scholar]
- 37.Teng C., Lin C., Huang F., Xing X., Chen S., Ye L., et al. Intracellular codelivery of anti-inflammatory drug and anti-miR 155 to treat inflammatory disease. Acta Pharm Sin B. 2020;10:1521–1533. doi: 10.1016/j.apsb.2020.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kellner M., Noonepalle S., Lu Q., Srivastava A., Zemskov E., Black S.M. ROS signaling in the pathogenesis of Acute Lung Injury (ALI) and acute respiratory distress syndrome (ARDS) Adv Exp Med Biol. 2017;967:105–137. doi: 10.1007/978-3-319-63245-2_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zemans R.L. In: Lung epithelial biology in the pathogenesis of pulmonary disease. Sidhaye V.K., Koval M., editors. Academic Press; London: 2017. Acute respiratory distress syndrome; pp. 185–209. [Google Scholar]
- 40.Heidemann S.M., Nair A., Bulut Y., Sapru A. Pathophysiology and management of acute respiratory distress syndrome in children. Pediatr Clin North Am. 2017;64:1017–1037. doi: 10.1016/j.pcl.2017.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ware L.B. In: Oxford textbook of critical care. Webb A., Angus D., Finfer S., Gattinoni L., Singer M., editors. Oxford University Press; Oxford: 2016. Pathophysiology of acute respiratory distress syndrome; pp. 498–500. [Google Scholar]
- 42.Noone P.M., Reddy S.P. Recent advances in dead cell clearance during acute lung injury and repair. Fac Rev. 2021;10:33. doi: 10.12703/r/10-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marshall R., Bellingan G., Laurent G. The acute respiratory distress syndrome: fibrosis in the fast lane. Thorax. 1998;53:815–817. doi: 10.1136/thx.53.10.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marshall R.P., Bellingan G., Webb S., Puddicombe A., Goldsack N., McAnulty R.J., et al. Fibroproliferation occurs early in the acute respiratory distress syndrome and impacts on outcome. Am J Respir Crit Care Med. 2000;162:1783–1788. doi: 10.1164/ajrccm.162.5.2001061. [DOI] [PubMed] [Google Scholar]
- 45.Fire A., Xu S., Montgomery M.K., Kostas S.A., Driver S.E., Mello C.C. Potent and specific genetic interference by double-stranded RNA in caenorhabditis elegans. Nature. 1998;391:806–811. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- 46.The Nobel Prize in Physiology or Medicine 2006. Available from: https://www.nobelprize.org/prizes/medicine/2006/summary/[accessed February 14, 2021].
- 47.Sioud M. RNA and CRISPR interferences: past, present, and future perspectives. Methods Mol Biol. 2020;2115:1–22. doi: 10.1007/978-1-0716-0290-4_1. [DOI] [PubMed] [Google Scholar]
- 48.Birchler J.A. In: RNA interference: application to drug discovery and challenges to pharmaceutical development. Johnson P.H., editor. John Wiley & Sons; Hoboken: 2011. RNA interference: what is it? pp. 1–11. [Google Scholar]
- 49.Abdurakhmonov I.Y. In: RNA interference. Abdurakhmonov I., editor. 2016. RNA Interference — a hallmark of cellular function and gene manipulation. Available from: [DOI] [Google Scholar]
- 50.Kheiriabad S., Ghaffari M., Dolatabadi J.E.N., Hamblin M.R. In: RNA interference and CRISPR technologies: technical advances and new therapeutic opportunities. Sioud M., editor. Springer; New York: 2020. PAMAM dendrimers as a delivery system for small interfering RNA; pp. 91–106. [Google Scholar]
- 51.Hu B., Weng Y., Xia X., Liang X., Huang Y. Clinical advances of siRNA therapeutics. J Gene Med. 2019;21 doi: 10.1002/jgm.3097. [DOI] [PubMed] [Google Scholar]
- 52.Tatiparti K., Sau S., Kashaw S.K., Iyer A.K. siRNA delivery strategies: a comprehensive review of recent developments. Nanomaterials. 2017;7:77. doi: 10.3390/nano7040077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nikam R.R., Gore K.R. Journey of siRNA: clinical developments and targeted delivery. Nucleic Acid Ther. 2018;28:209–224. doi: 10.1089/nat.2017.0715. [DOI] [PubMed] [Google Scholar]
- 54.Scott L.J. Givosiran: first approval. Drugs. 2020;80:335–339. doi: 10.1007/s40265-020-01269-0. [DOI] [PubMed] [Google Scholar]
- 55.Scott L.J., Keam S.J. Lumasiran: first approval. Drugs. 2021;81:277–282. doi: 10.1007/s40265-020-01463-0. [DOI] [PubMed] [Google Scholar]
- 56.Zhang M.M., Bahal R., Rasmussen T.P., Manautou J.E., Zhong X.B. The growth of siRNA-based therapeutics: updated clinical studies. Biochem Pharmacol. 2021;189:114432. doi: 10.1016/j.bcp.2021.114432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lam J.K.W., Liang W., Chan H.K. Pulmonary delivery of therapeutic siRNA. Adv Drug Deliv Rev. 2012;64:1–15. doi: 10.1016/j.addr.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dua K., Wadhwa R., Singhvi G., Rapalli V., Shukla S.D., Shastri M.D., et al. The potential of siRNA based drug delivery in respiratory disorders: recent advances and progress. Drug Dev Res. 2019;80:714–730. doi: 10.1002/ddr.21571. [DOI] [PubMed] [Google Scholar]
- 59.Ballarín-González B., Thomsen T.B., Howard K.A. Clinical translation of RNAi-based treatments for respiratory diseases. Drug Deliv Transl Res. 2013;3:84–99. doi: 10.1007/s13346-012-0098-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thanki K., Blum K.G., Thakur A., Rose F., Foged C. Formulation of RNA interference-based drugs for pulmonary delivery: challenges and opportunities. Ther Deliv. 2018;9:731–749. doi: 10.4155/tde-2018-0029. [DOI] [PubMed] [Google Scholar]
- 61.Qiu Y., Lam J.K.W., Leung S.W.S., Liang W. Delivery of RNAi therapeutics to the airways — from bench to bedside. Molecules. 2016;21:1249. doi: 10.3390/molecules21091249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Merkel O.M., Rubinstein I., Kissel T. SiRNA Delivery to the lung: what's new? Adv Drug Deliv Rev. 2014;75:112–128. doi: 10.1016/j.addr.2014.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liang W., Chow M.Y.T., Chow S.F., Chan H.K., Kwok P.C.L., Lam J.K.W. Using two-fluid nozzle for spray freeze drying to produce porous powder formulation of naked siRNA for inhalation. Int J Pharm. 2018;552:67–75. doi: 10.1016/j.ijpharm.2018.09.045. [DOI] [PubMed] [Google Scholar]
- 64.McCarthy S.D., González H.E., Higgins B.D. Future trends in nebulized therapies for pulmonary disease. J Pers Med. 2020;10:37. doi: 10.3390/jpm10020037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Guo Y., Bera H., Shi C., Zhang L., Cun D., Yang M. Pharmaceutical strategies to extend pulmonary exposure of inhaled medicines. Acta Pharm Sin B. 2021;8:2565–2584. doi: 10.1016/j.apsb.2021.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bardoliwala D., Patel V., Javia A., Ghosh S., Patel A., Misra A. Nanocarriers in effective pulmonary delivery of siRNA: current approaches and challenges. Ther Deliv. 2019;10:311–332. doi: 10.4155/tde-2019-0012. [DOI] [PubMed] [Google Scholar]
- 67.Chow M.Y.T., Qiu Y., Liao Q., Kwok P.C.L., Chow S.F., Chan H.K., et al. High siRNA loading powder for inhalation prepared by co-spray drying with human serum albumin. Int J Pharm. 2019;572:118818. doi: 10.1016/j.ijpharm.2019.118818. [DOI] [PubMed] [Google Scholar]
- 68.Peng T., Lin S., Niu B., Wang X., Huang Y., Zhang X., et al. Influence of physical properties of carrier on the performance of dry powder inhalers. Acta Pharm Sin B. 2016;6:308–318. doi: 10.1016/j.apsb.2016.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang J., Lu Z., Wientjes M.G., Au J.L.S. Delivery of siRNA therapeutics: barriers and carriers. AAPS J. 2010;12:492–503. doi: 10.1208/s12248-010-9210-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dominska M., Dykxhoorn D.M. Breaking down the barriers: siRNA delivery and endosome escape. J Cell Sci. 2010;123:1183–1189. doi: 10.1242/jcs.066399. [DOI] [PubMed] [Google Scholar]
- 71.Ahookhosh K., Pourmehran O., Aminfar H., Mohammadpourfard M., Sarafraz M.M., Hamishehkar H. Development of human respiratory airway models: a review. Eur J Pharm Sci. 2020;145:105233. doi: 10.1016/j.ejps.2020.105233. [DOI] [PubMed] [Google Scholar]
- 72.Ding L., Tang S., Wyatt T.A., Knoell D.L., Oupický D. Pulmonary siRNA delivery for lung disease: review of recent progress and challenges. J Control Release. 2021;330:977–991. doi: 10.1016/j.jconrel.2020.11.005. [DOI] [PubMed] [Google Scholar]
- 73.Shekunov B. In: Inhalation aerosols. Hickey A.J., Mansour H.M., editors. CRC Press; Boca Raton: 2019. Physicochemical properties of respiratory particles and formulations; pp. 3–30. [Google Scholar]
- 74.Rachel G., Ramsey J.M., Heise A., Cryan S.A., Greene C.M. In: Design of nanostructures for versatile therapeutic applications. Grumezescu A.M., editor. William Andrew Publishing; Norwich, NY: 2018. Nanotechnology approaches to pulmonary drug delivery: targeted delivery of small molecule and gene-based therapeutics to the lung; pp. 221–253. [Google Scholar]
- 75.Liu Q., Guan J., Qin L., Zhang X., Mao S. Physicochemical properties affecting the fate of nanoparticles in pulmonary drug delivery. Drug Discov Today. 2020;25:150–159. doi: 10.1016/j.drudis.2019.09.023. [DOI] [PubMed] [Google Scholar]
- 76.Choi H.S., Ashitate Y., Lee J.H., Kim S.H., Matsui A., Insin N., et al. Rapid translocation of nanoparticles from the lung airspaces to the body. Nat Biotechnol. 2010;28:1300–1303. doi: 10.1038/nbt.1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Garcia-Contreras L. In: Inhalation aerosols. Hickey A.J., Mansour H.M., editors. CRC Press; Boca Raton: 2019. Bioavailability of inhaled compounds; pp. 71–105. [Google Scholar]
- 78.Youngren-Ortiz S.R., Gandhi N., España-Serrano, Chougule M.B. Aerosol Delivery of siRNA to the lungs. Part 1: rationale for gene delivery systems. KONA Powder Part J. 2016;33:63–85. doi: 10.14356/kona.2016014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schuster B.S., Suk J.S., Woodworth G.F., Hanes J. Nanoparticle diffusion in respiratory mucus from humans without lung disease. Biomaterials. 2013;34:3439–3446. doi: 10.1016/j.biomaterials.2013.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yang J., Duan S., Ye H., Ge C., Piao C., Chen Y., et al. Pro-peptide-reinforced, mucus-penetrating pulmonary siRNA delivery mitigates cytokine storm in pneumonia. Adv Funct Mater. 2021;31:2008960. [Google Scholar]
- 81.McCright J.C., Maisel K. Engineering drug delivery systems to overcome mucosal barriers for immunotherapy and vaccination. Tissue Barriers. 2020;8:1695476. doi: 10.1080/21688370.2019.1695476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.De Backer L., Cerrada A., Pérez-Gil J., De Smedt S.C., Raemdonck K. Bio-inspired materials in drug delivery: exploring the role of pulmonary surfactant in siRNA inhalation therapy. J Control Release. 2015;220:642–650. doi: 10.1016/j.jconrel.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 83.Dhand R. How should aerosols be delivered during invasive mechanical ventilation? Respir Care. 2017;62:1343–1367. doi: 10.4187/respcare.05803. [DOI] [PubMed] [Google Scholar]
- 84.Montigaud Y., Georges Q., Pourchez J., Leclerc L., Goy C., Clotagatide A., et al. Aerosol delivery during invasive mechanical ventilation: development of a preclinical ex vivo respiratory model for aerosol regional deposition. Sci Rep. 2019;9:17930. doi: 10.1038/s41598-019-54480-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Artigas A., Camprubí-Rimblas M., Tantinyà N., Bringué J., Guillamat-Prats R., Matthay M.A. Inhalation therapies in acute respiratory distress syndrome. Ann Transl Med. 2017;5:293. doi: 10.21037/atm.2017.07.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dugernier J., Ehrmann S., Sottiaux T., Roeseler J., Wittebole X., Dugernier T., et al. Aerosol delivery during invasive mechanical ventilation: a systematic review. Crit Care. 2017;21:264. doi: 10.1186/s13054-017-1844-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bi Q., Song X., Hu A., Luo T., Jin R., Ai H., et al. Magnetofection: magic magnetic nanoparticles for efficient gene delivery. Chin Chem Lett. 2020;31:3041–3046. [Google Scholar]
- 88.Subhan M.A., Torchilin V.P. siRNA based drug design, quality, delivery and clinical translation. Nanomedicine. 2020;29:102239. doi: 10.1016/j.nano.2020.102239. [DOI] [PubMed] [Google Scholar]
- 89.He W., Xing X., Wang X., Wu D., Wu W., Guo J., et al. Nanocarrier-mediated cytosolic delivery of biopharmaceuticals. Adv Funct Mater. 2020;30:1910566. [Google Scholar]
- 90.Marquez A.R., Madu C.O., Lu Y. An Overview of various carriers for siRNA delivery. Oncomedicine. 2018;3:48–58. [Google Scholar]
- 91.Xu S., Yang Q., Bai J., Tao T., Tang L., Chen Y., et al. Blockade of endothelial, but not epithelial, cell expression of PD-L1 following severe shock attenuates the development of indirect acute lung injury in mice. Am J Physiol Lung Cell Mol Physiol. 2020;318:L801–L812. doi: 10.1152/ajplung.00108.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dong J., Liao W., Tan L.H., Yong A., Peh W.Y., Wong W.S.F. Gene silencing of receptor-interacting protein 2 protects against cigarette smoke-induced acute lung injury. Pharmacol Res. 2019;139:560–568. doi: 10.1016/j.phrs.2018.10.016. [DOI] [PubMed] [Google Scholar]
- 93.Zhang D., Lee H., Wang X., Rai A., Groot M., Jin Y. Exosome-mediated small RNA delivery: a novel therapeutic approach for inflammatory lung responses. Mol Ther. 2018;26:2119–2130. doi: 10.1016/j.ymthe.2018.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fu P., Usatyuk P.V., Lele A., Harijith A., Gregorio C.C., Garcia J.G.N., et al. c-Abl mediated tyrosine phosphorylation of paxillin regulates LPS-induced endothelial dysfunction and lung injury. Am J Physiol Lung Cell Mol Physiol. 2015;308:L1025–L1038. doi: 10.1152/ajplung.00306.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang Y., Ding L., Li Z., Chen G., Sun M., Oupicky D. Treatment of acute lung injury and early- and late-stage pulmonary fibrosis with combination emulsion siRNA polyplexes. J Control Release. 2019;314:12–24. doi: 10.1016/j.jconrel.2019.10.030. [DOI] [PubMed] [Google Scholar]
- 96.Bohr A., Tsapis N., Foged C., Andreana I., Yang M., Fattal E. Treatment of acute lung inflammation by pulmonary delivery of anti-TNF-α siRNA with PAMAM dendrimers in a murine model. Eur J Pharm Biopharm. 2020;156:114–120. doi: 10.1016/j.ejpb.2020.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bohr A., Tsapis N., Andreana I., Chamarat A., Foged C., Delomenie C., et al. Anti-inflammatory effect of anti-TNF-α siRNA cationic phosphorus dendrimer nanocomplexes administered intranasally in a murine acute lung injury model. Biomacromolecules. 2017;18:2379–2388. doi: 10.1021/acs.biomac.7b00572. [DOI] [PubMed] [Google Scholar]
- 98.Oh B., Lee M. Combined delivery of HMGB-1 box A peptide and S1PLyase siRNA in animal models of acute lung injury. J Control Release. 2014;175:25–35. doi: 10.1016/j.jconrel.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 99.Ge C., Yang J., Duan S., Liu Y., Meng F., Yin L. Fluorinated α-helical polypeptides synchronize mucus permeation and cell penetration toward highly efficient pulmonary siRNA delivery against acute lung injury. Nano Lett. 2020;20:1738–1746. doi: 10.1021/acs.nanolett.9b04957. [DOI] [PubMed] [Google Scholar]
- 100.Merckx P., De Backer L., Van Hoecke L., Guagliardo R., Echaide M., Baatsen P., et al. Surfactant protein B (SP-B) enhances the cellular siRNA delivery of proteolipid coated nanogels for inhalation therapy. Acta Biomater. 2018;78:236–246. doi: 10.1016/j.actbio.2018.08.012. [DOI] [PubMed] [Google Scholar]
- 101.Goswami R., Subramanian G., Silayeva L., Newkirk I., Doctor D., Chawla K., et al. Gene therapy leaves a vicious cycle. Front Oncol. 2019;9:297. doi: 10.3389/fonc.2019.00297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wechsler M.E., Clegg J.R., Peppas N.A. In: Encyclopedia of tissue engineering and regenerative medicine. Reis R.L., editor. Academic Press; Oxford: 2019. The interface of drug delivery and regenerative medicine; pp. 77–86. [Google Scholar]
- 103.Zhang W.W., Li L., Li D., Liu J., Li X., Li W., et al. The first approved gene therapy product for cancer Ad-p53 (Gendicine): 12 years in the clinic. Hum Gene Ther. 2018;29:160–179. doi: 10.1089/hum.2017.218. [DOI] [PubMed] [Google Scholar]
- 104.Devaney J., Contreras M., Laffey J.G. Clinical Review: gene-based therapies for ALI/ARDS: where are we now? Crit Care. 2011;15:1–12. doi: 10.1186/cc10216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lin X., Dean D.A. Gene therapy for ALI/ARDS. Crit Care Clin. 2011;27:705–718. doi: 10.1016/j.ccc.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kili S., Chaffman M. In: Second generation cell and gene-based therapies. Vertès A.A., Smith D.M., Qureshi N., Dowden N.J., editors. Academic Press; Oxford: 2020. Development and deployment of gene therapies: an ADA-SCID case study; pp. 15–39. [Google Scholar]
- 107.Büning H., Srivastava A. Capsid modifications for targeting and improving the efficacy of AAV vectors. Mol Ther Methods Clin Dev. 2019;12:248–265. doi: 10.1016/j.omtm.2019.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Duncan G.A., Kim N., Colon-Cortes Y., Rodriguez J., Mazur M., Birket S.E., et al. An adeno-associated viral vector capable of penetrating the mucus barrier to inhaled gene therapy. Mol Ther Methods Clin Dev. 2018;9:296–304. doi: 10.1016/j.omtm.2018.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Qin S., Wang H., Liu G., Mei H., Chen M. miR-21-5p ameliorates hyperoxic acute lung injury and decreases apoptosis of AEC II cells via PTEN/AKT signaling in rats. Mol Med Rep. 2019;20:4953–4962. doi: 10.3892/mmr.2019.10779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Smalley E. First AAV gene therapy poised for landmark approval. Nat Biotechnol. 2017;35:998–999. doi: 10.1038/nbt1117-998. [DOI] [PubMed] [Google Scholar]
- 111.Keeler A.M., Flotte T.R. Recombinant adeno-associated virus gene therapy in light of Luxturna (and Zolgensma and Glybera): where are we, and how did we get here? Annu Rev Virol. 2019;6:601–621. doi: 10.1146/annurev-virology-092818-015530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Marquez Loza L.I., Yuen E.C., McCray P.B. Lentiviral vectors for the treatment and prevention of cystic fibrosis lung disease. Genes. 2019;10:218. doi: 10.3390/genes10030218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Shirley J.L., de Jong Y.P., Terhorst C., Herzog R.W. Immune responses to viral gene therapy vectors. Mol Ther. 2020;28:709–722. doi: 10.1016/j.ymthe.2020.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Dunn A., Shi D. Polymeric vectors for strategic delivery of nucleic acids. Nano Life. 2017;7:1730003. [Google Scholar]
- 115.Ozpolat B., Sood A.K., Lopez-Berestein G. Liposomal siRNA nanocarriers for cancer therapy. Adv Drug Deliv Rev. 2014;66:110–116. doi: 10.1016/j.addr.2013.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yonezawa S., Koide H., Asai T. Recent advances in siRNA delivery mediated by lipid-based nanoparticles. Adv Drug Deliv Rev. 2020;154–155:64–78. doi: 10.1016/j.addr.2020.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhu Y., Meng Y., Zhao Y., Zhu J., Xu H., Zhang E., et al. Toxicological exploration of peptide-based cationic liposomes in siRNA delivery. Colloids Surf B Biointerfaces. 2019;179:66–76. doi: 10.1016/j.colsurfb.2019.03.052. [DOI] [PubMed] [Google Scholar]
- 118.Gujrati M., Lu Z.R. In: Gene therapy of cancer. Lattime E.C., Gerson S.L., editors. Academic Press; San Diego: 2014. Targeted systemic delivery of therapeutic siRNA; pp. 47–65. [Google Scholar]
- 119.Xiao Q., Li X., Li Y., Wu Z., Xu C., Chen Z., et al. Biological drug and drug delivery-mediated immunotherapy. Acta Pharm Sin B. 2020;11:941–960. doi: 10.1016/j.apsb.2020.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ruseska I., Zimmer A. Internalization mechanisms of cell-penetrating peptides. Beilstein J Nanotechnol. 2020;11:101–123. doi: 10.3762/bjnano.11.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hattori Y., Nakamura A., Arai S., Kawano K., Maitani Y., Yonemochi E. SiRNA delivery to lung-metastasized tumor by systemic injection with cationic liposomes. J Liposome Res. 2015;25:279–286. doi: 10.3109/08982104.2014.992024. [DOI] [PubMed] [Google Scholar]
- 122.Petrilli R., Eloy J.O., de Souza M.C., Abriata Barcellos J.P., Marchetti J.M., Yung B., et al. In: Nanobiomaterials in drug delivery. Grumezescu A.M., editor. William Andrew Publishing; Norwich: 2016. Lipid nanoparticles as non-viral vectors for siRNA delivery: concepts and applications; pp. 75–109. [Google Scholar]
- 123.Lechanteur A., Sanna V., Duchemin A., Evrard B., Mottet D., Piel G. Cationic liposomes carrying siRNA: impact of lipid composition on physicochemical properties, cytotoxicity and endosomal escape. Nanomaterials. 2018;8:270. doi: 10.3390/nano8050270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Estanqueiro M., Vasconcelos H., Lobo J.M.S., Amaral H. In: Drug targeting and stimuli sensitive drug delivery systems. Grumezescu A.M., editor. William Andrew Publishing; Norwich: 2018. Delivering miRNA modulators for cancer treatment; pp. 517–565. [Google Scholar]
- 125.Semple S.C., Klimuk S.K., Harasym T.O., Dos Santos N., Ansell S.M., Wong K.F., et al. Efficient encapsulation of antisense oligonucleotides in lipid vesicles using ionizable aminolipids: formation of novel small multilamellar vesicle structures. Biochim Biophys Acta Biomembr. 2001;1510:152–166. doi: 10.1016/s0005-2736(00)00343-6. [DOI] [PubMed] [Google Scholar]
- 126.Wang J.L., Hanafy M.S., Xu H., Leal J., Zhai Y., Ghosh D., et al. Aerosolizable siRNA-encapsulated solid lipid nanoparticles prepared by thin-film freeze-drying for potential pulmonary delivery. Int J Pharm. 2021;596:120215. doi: 10.1016/j.ijpharm.2021.120215. [DOI] [PubMed] [Google Scholar]
- 127.Tekade R.K., Maheshwari R., Tekade M., Chougule M.B. In: Nanotechnology-based approaches for targeting and delivery of drugs and genes. Mishra V., Kesharwani P., Mohd Cairul Iqbal Mohd Amin M.C.I., Iyer A., editors. Elsevier; Amsterdam: 2017. Solid lipid nanoparticles for targeting and delivery of drugs and genes; pp. 256–286. [Google Scholar]
- 128.Hayashi Y., Hatakeyama H., Kajimoto K., Hyodo M., Akita H., Harashima H. Multifunctional envelope-type nano device: evolution from nonselective to active targeting system. Bioconjug Chem. 2015;26:1266–1276. doi: 10.1021/acs.bioconjchem.5b00184. [DOI] [PubMed] [Google Scholar]
- 129.Abd Elwakil M.M., Khalil I.A., Elewa Y.H.A., Kusumoto K., Sato Y., Shobaki N., et al. Lung-endothelium-targeted nanoparticles based on a pH-sensitive lipid and the GALA peptide enable robust gene silencing and the regression of metastatic lung cancer. Adv Funct Mater. 2019;29:1807677. [Google Scholar]
- 130.Jafari D., Shajari S., Jafari R., Mardi N., Gomari H., Ganji F., et al. Designer exosomes: a new platform for biotechnology therapeutics. BioDrugs. 2020;34:567–586. doi: 10.1007/s40259-020-00434-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Jiang X.C., Gao J.Q. Exosomes as novel bio-carriers for gene and drug delivery. Int J Pharm. 2017;521:167–175. doi: 10.1016/j.ijpharm.2017.02.038. [DOI] [PubMed] [Google Scholar]
- 132.Shao J., Zaro J., Shen Y. Advances in exosome-based drug delivery and tumor targeting: from tissue distribution to intracellular fate. Int J Nanomed. 2020;15:9355–9371. doi: 10.2147/IJN.S281890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Patil S.M., Sawant S.S., Kunda N.K. Exosomes as drug delivery systems: a brief overview and progress update. Eur J Pharm Biopharm. 2020;154:259–269. doi: 10.1016/j.ejpb.2020.07.026. [DOI] [PubMed] [Google Scholar]
- 134.Ha D., Yang N., Nadithe V. Exosomes as therapeutic drug carriers and delivery vehicles across biological membranes: current perspectives and future challenges. Acta Pharm Sin B. 2016;6:287–296. doi: 10.1016/j.apsb.2016.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Bunggulawa E.J., Wang W., Yin T., Wang N., Durkan C., Wang Y., et al. Recent advancements in the use of exosomes as drug delivery systems. J Nanobiotechnol. 2018;16:81. doi: 10.1186/s12951-018-0403-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Arruda D.C., Hoffmann C., Charrueau C., Bigey P., Escriou V. In: Nanostructures for novel therapy: synthesis, characterization and applications. Ficai D., Grumezescu A.M., editors. Elsevier; Amsterdam: 2017. Innovative nonviral vectors for small-interfering RNA delivery and therapy; pp. 713–740. [Google Scholar]
- 137.Do Minh A., Sharon D., Chahal P., Kamen A.A. In: Comprehensive biotechnology. Moo-Young M., editor. Oxford; Pergamon: 2019. Cell transfection; pp. 383–390. [Google Scholar]
- 138.He W., Turkeshi A., Li X., Zhang H. Progress in systemic co-delivery of microRNAs and chemotherapeutics for cancer treatment by using lipid-based nanoparticles. Ther Deliv. 2020;11:591–603. doi: 10.4155/tde-2020-0052. [DOI] [PubMed] [Google Scholar]
- 139.Hattori Y., Tamaki K., Ozaki K.I., Kawano K., Onishi H. Optimized combination of cationic lipids and neutral helper lipids in cationic liposomes for siRNA delivery into the lung by intravenous injection of siRNA lipoplexes. J Drug Deliv Sci Technol. 2019;52:1042–1050. [Google Scholar]
- 140.Youngren-Ortiz S.R., Gandhi N.S., España-Serrano L., Chougule M.B. Aerosol delivery of siRNA to the lungs. Part 2: nanocarrier-based delivery systems. Kona. 2017;34:44–69. doi: 10.14356/kona.2017005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Dokka S., Toledo D., Shi X., Castranova V., Rojanasakul Y. Oxygen radical-mediated pulmonary toxicity induced by some cationic liposomes. Pharm Res. 2000;17:521–525. doi: 10.1023/a:1007504613351. [DOI] [PubMed] [Google Scholar]
- 142.Merry H.E., Phelan P., Hwang B., Mulligan M.S. Validating the use of short interfering RNA as a novel technique for cell-specific target gene knockdown in lung ischemia-reperfusion injury. J Thorac Cardiovasc Surg. 2016;151:499–506. doi: 10.1016/j.jtcvs.2015.09.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zhao Y., Li X., Zhang W., Yu L., Wang Y., Deng Z., et al. Trends in the biological functions and medical applications of extracellular vesicles and analogues. Acta Pharm Sin B. 2021;8:2114–2135. doi: 10.1016/j.apsb.2021.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ju Z., Ma J., Wang C., Yu J., Qiao Y., Hei F. Exosomes from iPSCs delivering siRNA attenuate intracellular adhesion molecule-1 expression and neutrophils adhesion in pulmonary microvascular endothelial cells. Inflammation. 2017;40:486–496. doi: 10.1007/s10753-016-0494-0. [DOI] [PubMed] [Google Scholar]
- 145.Rai R., Alwani S., Badea I. Polymeric nanoparticles in gene therapy: new avenues of design and optimization for delivery applications. Polymers. 2019;11:745. doi: 10.3390/polym11040745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sarvari R., Nouri M., Agbolaghi S., Roshangar L., Sadrhaghighi A., Seifalian A.M., et al. A summary on non-viral systems for gene delivery based on natural and synthetic polymers. Int J Polym Mater Polym Biomater. 2020:1–20. [Google Scholar]
- 147.Kargaard A., Sluijter J.P.G., Klumperman B. Polymeric siRNA gene delivery—transfection efficiency versus cytotoxicity. J Control Release. 2019;316:263–291. doi: 10.1016/j.jconrel.2019.10.046. [DOI] [PubMed] [Google Scholar]
- 148.Piotrowski-Daspit A.S., Kauffman A.C., Bracaglia L.G., Saltzman W.M. Polymeric vehicles for nucleic acid delivery. Adv Drug Deliv Rev. 2020;156:119–132. doi: 10.1016/j.addr.2020.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Rai D.B., Pooja D., Kulhari H. In: Pharmaceutical applications of dendrimers. Chauhan A., Kulhari H., editors. Elsevier; Amsterdam: 2019. Dendrimers in gene delivery; pp. 211–231. [Google Scholar]
- 150.Zhou D., Zeng M., Gao Y., Sigen A., Lyu J., Wang W. In: Nucleic acid nanotheranostics: biomedical applications. Filice M., Ruiz-Cabello J., editors. Elsevier; Amsterdam: 2019. Advanced polymers for nonviral gene delivery; pp. 311–364. [Google Scholar]
- 151.Nimesh S. In: Gene therapy potential applications of nanotechnology. Nimesh S., editor. Woodhead Publishing; Cambridge: 2013. Theory and limitations to gene therapy; pp. 89–111. [Google Scholar]
- 152.Yuan W., Li H. In: Nanostructures for drug delivery. Andronescu E., Grumezescu A.M., editors. Elsevier; Amsterdam: 2017. Polymer-based nanocarriers for therapeutic nucleic acids delivery; pp. 445–460. [Google Scholar]
- 153.Sung Y.K., Kim S.W. Recent advances in polymeric drug delivery systems. Biomater Res. 2020;24:12. doi: 10.1186/s40824-020-00190-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Itani R., Al Faraj A. SiRNA conjugated nanoparticles—a next generation strategy to treat lung cancer. Int J Mol Sci. 2019;20:6088. doi: 10.3390/ijms20236088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Nimesh S. In: Gene therapy potential applications of nanotechnology. Nimesh S., editor. Woodhead Publishing; Cambridge: 2013. Polyethylenimine nanoparticles; pp. 197–223. [Google Scholar]
- 156.Civenni G. In: Methods in molecular biology. Napoli S., editor. Springer; New York: 2017. Targeting promoter-associated noncoding RNA in vivo; pp. 259–270. [DOI] [PubMed] [Google Scholar]
- 157.Choi M., Lee M., Rhim T. Dexamethasone-conjugated polyethylenimine/MIF siRNA complex regulation of particulate matter-induced airway inflammation. Biomaterials. 2013;34:7453–7461. doi: 10.1016/j.biomaterials.2013.05.082. [DOI] [PubMed] [Google Scholar]
- 158.Chen G., Wang K., Hu Q., Ding L., Yu F., Zhou Z., et al. Combining fluorination and bioreducibility for improved siRNA polyplex delivery. ACS Appl Mater Interfaces. 2017;9:4457–4466. doi: 10.1021/acsami.6b14184. [DOI] [PubMed] [Google Scholar]
- 159.Zhang Z., Li H., Liang Z., Li C., Yang Z., Li Y., et al. Vaporized perfluorocarbon inhalation attenuates primary blast lung injury in canines by inhibiting mitogen-activated protein kinase/nuclear factor-κB activation and inducing nuclear factor, erythroid 2 like 2 pathway. Toxicol Lett. 2020;319:49–57. doi: 10.1016/j.toxlet.2019.10.019. [DOI] [PubMed] [Google Scholar]
- 160.Wang X., Zhang J., Li X., Liu Y., Yang H., Zhao X., et al. Sustained improvement of gas exchange and lung mechanics by vaporized perfluorocarbon inhalation in piglet acute lung injury model. Clin Respir J. 2014;8:160–166. doi: 10.1111/crj.12053. [DOI] [PubMed] [Google Scholar]
- 161.Hou S., Ding H., Lv Q., Yin X., Song J., Landén N.X., et al. Therapeutic effect of intravenous infusion of perfluorocarbon emulsion on LPS-induced acute lung injury in rats. PLoS One. 2014;9 doi: 10.1371/journal.pone.0087826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Xue L., Yan Y., Kos P., Chen X., Siegwart D.J. PEI fluorination reduces toxicity and promotes liver-targeted siRNA delivery. Drug Deliv Transl Res. 2021;11:255–260. doi: 10.1007/s13346-020-00790-9. [DOI] [PubMed] [Google Scholar]
- 163.Galetti M., Rossi S., Caffarra C., Gerboles A.G., Miragoli M. In: Exposure to engineered nanomaterials in the environment. Marmiroli N., White J.C., Song J., editors. Elsevier; Amsterdam: 2019. Innovation in nanomedicine and engineered nanomaterials for therapeutic purposes; pp. 235–262. [Google Scholar]
- 164.Rana V., Sharma R. In: Applications of targeted nano drugs and delivery systems. Mohapatra S.S., Ranjan S., Dasgupta N., Mishra R.K., Thomas S., editors. Elsevier; Amsterdam: 2019. Recent advances in development of nano drug delivery; pp. 93–131. [Google Scholar]
- 165.Yadav H.K.S., Almokdad A.A., Shaluf S.I.M., Debe M.S. In: Nanocarriers for drug delivery. Mohapatra S.S., Ranjan S., Dasgupta N., Mishra R.K., Thomas S., editors. Elsevier; Amsterdam: 2019. Polymer-based nanomaterials for drug-delivery carriers; pp. 531–556. [Google Scholar]
- 166.Yousefpour Marzbali M., Yari Khosroushahi A. Polymeric micelles as mighty nanocarriers for cancer gene therapy: a review. Cancer Chemother Pharmacol. 2017;79:637–649. doi: 10.1007/s00280-017-3273-1. [DOI] [PubMed] [Google Scholar]
- 167.Wang X., Mohammad I.S., Fan L., Zhao Z., Nurunnabi M., Sallam M.A., et al. Delivery strategies of amphotericin B for invasive fungal infections. Acta Pharm Sin B. 2021;8 doi: 10.1016/j.apsb.2021.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Hou J., Ji Q., Ji J., Ju S., Xu C., Yong X., et al. Co-delivery of siPTPN13 and siNOX4 via (myo)-fibroblasttargeting polymeric micelles for idiopathic pulmonary fibrosis therapy. Theranostics. 2021;11:3244–3261. doi: 10.7150/thno.54217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Ji Q., Hou J., Yong X., Gong G., Muddassir M., Tang T., et al. Targeted dual small interfering ribonucleic acid delivery via non-viral polymeric vectors for pulmonary fibrosis therapy. Adv Mater. 2021;33 doi: 10.1002/adma.202007798. [DOI] [PubMed] [Google Scholar]
- 170.Bhatt P., Trehan S., Inamdar N., Mourya V.K., Misra A. In: Applications of polymers in drug delivery. Misra A., Shahiwala A., editors. Elsevier; Amsterdam: 2021. Polymers in drug delivery: an update; pp. 1–42. [Google Scholar]
- 171.Agnoletti M., Bohr A., Thanki K., Wan F., Zeng X., Boetker J.P., et al. Inhalable siRNA-loaded nano-embedded microparticles engineered using microfluidics and spray drying. Eur J Pharm Biopharm. 2017;120:9–21. doi: 10.1016/j.ejpb.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 172.Shcharbin D., Bryszewska M., Mignani S., Shi X., Majoral J.P. Phosphorus dendrimers as powerful nanoplatforms for drug delivery, as fluorescent probes and for liposome interaction studies: a concise overview. Eur J Med Chem. 2020;208:112788. doi: 10.1016/j.ejmech.2020.112788. [DOI] [PubMed] [Google Scholar]
- 173.Mignani S., Shi X., Ceña V., Shcharbin D., Bryszewska M., Majoral J.P. In vivo therapeutic applications of phosphorus dendrimers: state of the art. Drug Discov Today. 2021;26:677–689. doi: 10.1016/j.drudis.2020.11.034. [DOI] [PubMed] [Google Scholar]
- 174.Nimesh S. In: Gene therapy potential applications of nanotechnology. Nimesh S., editor. Woodhead Publishing; Cambridge: 2013. Poly(d,l-lactide-co-glycolide)-based nanoparticles; pp. 309–329. [Google Scholar]
- 175.Zakharova L., Pashirova T., Kashapov R., Gabdrakhmanov D., Sinyashin O. In: Nanostructures for drug delivery. Andronescu E., Grumezescu A.M., editors. Elsevier; Amsterdam: 2017. Drug delivery mediated by confined nanosystems: structure–activity relations and factors responsible for the efficacy of formulations; pp. 749–806. [Google Scholar]
- 176.Beg S., Almalki W.H., Khatoon F., Malik A., Afaq S., Choudhry H., et al. Lipid/polymer-based nanocomplexes in nucleic acid delivery as cancer vaccines. Drug Discov Today. 2021;S1359–6446(21) doi: 10.1016/j.drudis.2021.02.013. 00096-9. [DOI] [PubMed] [Google Scholar]
- 177.Frede A., Neuhaus B., Knuschke T., Wadwa M., Kollenda S., Klopfleisch R., et al. Local delivery of siRNA-loaded calcium phosphate nanoparticles abates pulmonary inflammation. Nanomedicine. 2017;13:2395–2403. doi: 10.1016/j.nano.2017.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Kang Z., Meng Q., Liu K. Peptide-based gene delivery vectors. J Mater Chem B. 2019;7:1824–1841. doi: 10.1039/c8tb03124j. [DOI] [PubMed] [Google Scholar]
- 179.Yin D., Zhang M., Chen J., Huang Y., Liang D. Shear-responsive peptide/siRNA complexes as lung-targeting gene vectors. Chin Chem Lett. 2021;32:1731–1736. [Google Scholar]
- 180.So W.H., Wong C.T.T., Xia J. Peptide photocaging: a brief account of the chemistry and biological applications. Chin Chem Lett. 2018;29:1058–1062. [Google Scholar]
- 181.Singh T., Murthy A.S.N., Yang H.J., Im J. Versatility of cell-penetrating peptides for intracellular delivery of siRNA. Drug Deliv. 2018;25:1996–2006. doi: 10.1080/10717544.2018.1543366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Zhang D., Wang J., Xu D. Cell-penetrating peptides as noninvasive transmembrane vectors for the development of novel multifunctional drug-delivery systems. J Control Release. 2016;229:130–139. doi: 10.1016/j.jconrel.2016.03.020. [DOI] [PubMed] [Google Scholar]
- 183.Taylor R.E., Zahid M. Cell penetrating peptides, novel vectors for gene therapy. Pharmaceutics. 2020;12:225. doi: 10.3390/pharmaceutics12030225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Gessner I., Neundorf I. Nanoparticles modified with cell-penetrating peptides: conjugation mechanisms, physicochemical properties, and application in cancer diagnosis and therapy. Int J Mol Sci. 2020;21:2536. doi: 10.3390/ijms21072536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Xie J., Bi Y., Zhang H., Dong S., Teng L., Lee R.J., et al. Cell-penetrating peptides in diagnosis and treatment of human diseases: from preclinical research to clinical application. Front Pharmacol. 2020;11:697. doi: 10.3389/fphar.2020.00697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Khan M.M., Filipczak N., Torchilin V.P. Cell penetrating peptides: a versatile vector for co-delivery of drug and genes in cancer. J Control Release. 2020;330:1220–1228. doi: 10.1016/j.jconrel.2020.11.028. [DOI] [PubMed] [Google Scholar]
- 187.Lehto T., Ezzat K., Wood M.J.A., EL Andaloussi S. Peptides for nucleic acid delivery. Adv Drug Deliv Rev. 2016;106:172–182. doi: 10.1016/j.addr.2016.06.008. [DOI] [PubMed] [Google Scholar]
- 188.Bansal S.K., Rajpoot K., Sreeharsha N., Youngren-Ortiz S.R., Anup N., Tekade R.K. In: The future of pharmaceutical product development and research. Tekade R.K., editor. Academic Press; London: 2020. Endosomal escape tendency of drug delivery systems to mediate cytosolic delivery of therapeutics; pp. 227–258. [Google Scholar]
- 189.McClorey G., Banerjee S. Cell-penetrating peptides to enhance delivery of oligonucleotide-based therapeutics. Biomedicines. 2018;6:51. doi: 10.3390/biomedicines6020051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Vaissière A., Aldrian G., Konate K., Lindberg M.F., Jourdan C., Telmar A., et al. A retro-inverso cell-penetrating peptide for siRNA delivery. J Nanobiotechnol. 2017;15:34. doi: 10.1186/s12951-017-0269-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Roberts T.C., Langer R., Wood M.J.A. Advances in oligonucleotide drug delivery. Nat Rev Drug Discov. 2020;19:673–694. doi: 10.1038/s41573-020-0075-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Tarvirdipour S., Huang X., Mihali V., Schoenenberger C.A., Palivan C.G. Peptide-based nanoassemblies in gene therapy and diagnosis: paving the way for clinical application. Molecules. 2020;25:1–37. doi: 10.3390/molecules25153482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Helal H.M., Mortada S.M., Sallam M.A. Paliperidone-loaded nanolipomer system for sustained delivery and enhanced intestinal permeation: superiority to polymeric and solid lipid nanoparticles. AAPS PharmSciTech. 2017;18:1946–1959. doi: 10.1208/s12249-016-0657-1. [DOI] [PubMed] [Google Scholar]
- 194.D'Angelo I., Costabile G., Durantie E., Brocca P., Rondelli V., Russo A., et al. Hybrid lipid/polymer nanoparticles for pulmonary delivery of siRNA: development and fate upon in vitro deposition on the human epithelial airway barrier. J Aerosol Med Pulm Drug Deliv. 2018;31:170–181. doi: 10.1089/jamp.2017.1364. [DOI] [PubMed] [Google Scholar]
- 195.Yu Z., Liu X., Chen H., Zhu L. Naringenin-loaded dipalmitoylphosphatidylcholine phytosome dry powders for inhaled treatment of acute lung injury. J Aerosol Med Pulm Drug Deliv. 2020;33:194–204. doi: 10.1089/jamp.2019.1569. [DOI] [PubMed] [Google Scholar]
- 196.Dormenval C., Lokras A., Cano-Garcia G., Wadhwa A., Thanki K., Rose F., et al. Identification of factors of importance for spray drying of small interfering RNA-loaded lipidoid-polymer hybrid nanoparticles for inhalation. Pharm Res. 2019;36:142. doi: 10.1007/s11095-019-2663-y. [DOI] [PubMed] [Google Scholar]
- 197.Kusumoto K., Akita H., Ishitsuka T., Matsumoto Y., Nomoto T., Furukawa R., et al. Lipid envelope-type nanoparticle incorporating a multifunctional peptide for systemic siRNA delivery to the pulmonary endothelium. ACS Nano. 2013;7:7534–7541. doi: 10.1021/nn401317t. [DOI] [PubMed] [Google Scholar]
- 198.Merckx P., Lammens J., Nuytten G., Bogaert B., Guagliardo R., Maes T., et al. Lyophilization and nebulization of pulmonary surfactant-coated nanogels for siRNA inhalation therapy. Eur J Pharm Biopharm. 2020;157:191–199. doi: 10.1016/j.ejpb.2020.09.011. [DOI] [PubMed] [Google Scholar]