Abstract
Background:
Computer assisted three-dimensional (3D) printing of anatomic models using advanced imaging has wide applications within orthopaedics. The purpose of this study is to evaluate the 3D printing accuracy of carpal bones.
Methods:
Seven cadaveric wrists underwent CT scanning, after which select carpal bones (scaphoid, capitate, lunate, and trapezium) were dissected in toto. Dimensions including length, circumference, and volume were measured directly from the cadaver bones. The CT images were converted into 3D printable stereolithography (STL) files. The STL files were converted into solid prints using a commercially available 3D printer. The 3D printed models’ dimensions were measured and compared to those of the cadaver bones. A paired t-test was performed to determine if a statistically significant difference existed between the mean measurements of the cadavers and 3D printed models. The intraclass correlation coefficients (ICC) between the two groups were calculated to measure the degree of agreement.
Results:
On average, the length and circumference of the 3D printed models were within 2.3 mm and 2.2 mm, respectively, of the cadaveric bones. There was a larger discrepancy in the volume measured, which on average was within 0.65 cc (15.9%) of the cadaveric bones. These differences were not statistically significant (P > 0.05). There was strong agreement between all measurements except the capitate’s length and lunate’s volume.
Conclusion:
3D printing can add value to patient care and improve outcomes. This study demonstrates that 3D printing can both accurately and reproducibly fabricate boney models that closely resemble the corresponding cadaveric anatomy.
Key Words: Cadaver, Carpal bones, Computed tomography (CT), Three-dimensional (3D)
Introduction
Three-dimensional (3D) printing is a process which involves fabricating an object based on a computerized model, a concept which was first introduced in the 1980s and since has become one of the most efficient methods for fabricating custom-designed products for various uses. These models can be generated de novo using computerized aided design (CAD) software. More recently, data sources from advanced medical imaging techniques such as computed tomography (CT) and magnetic resonance imaging (MRI), have gained popularity. The radiological scan, which is most often generated in a Digital Imaging and Communications in Medicine (DICOM) file format, can be processed and converted into a Standard Tessellation Language (STL) file which is recognized by 3D printers (1).
Computer assisted 3D printing of anatomical models using advanced imaging has wide applications in the medical field and specifically within orthopaedics (2). These anatomical models can be printed on-demand based on a medical image, thus becoming increasingly popular for their use in medical education, pre-surgical planning and surgical training, and the creation of patient-specific guides, implants, and prosthetics. An anatomical 3D printed model can aid in pre-surgical planning by allowing the surgeon to visualize tissue anatomy, simulate the surgical process, select and manipulate surgical equipment, and demonstrate these techniques to patients preoperatively affording them a better understanding of their pathology (3).
George et al describe the inaccuracies that occur during each step of the 3D printing process: imaging, segmentation, STL generation, STL post-processing, 3D printing, and cleaning/preparation of the anatomic model (4). A major contributor to this inaccuracy is the difference in attenuation thresholds of the imaged bone and adjacent soft tissue structures during segmentation, which leads to imprecise bone dimensions (4). An inaccurate 3D printed model of a patient’s anatomy can potentially result in an inappropriate treatment plan leading to negative consequences for the patient and clinician (4). Previous work by Ogden et al and Wu et al has sought to determine the dimensional accuracy of these 3D printed anatomical models compared to the imaging studies and actual cadaveric bones themselves (5, 6). Nonetheless, there is limited literature present on the accuracy of 3D printed anatomical models compared to cadaveric specimens, specifically in the carpus.
The purpose of this study is to investigate how accurate 3D printed anatomical models of select carpal bones are to their corresponding cadaveric specimens. We hypothesize that the 3D printed models will be very accurate and show no statistically significant differences in measured length, circumference, and volume compared to that of the cadaveric bones.
Materials and Methods
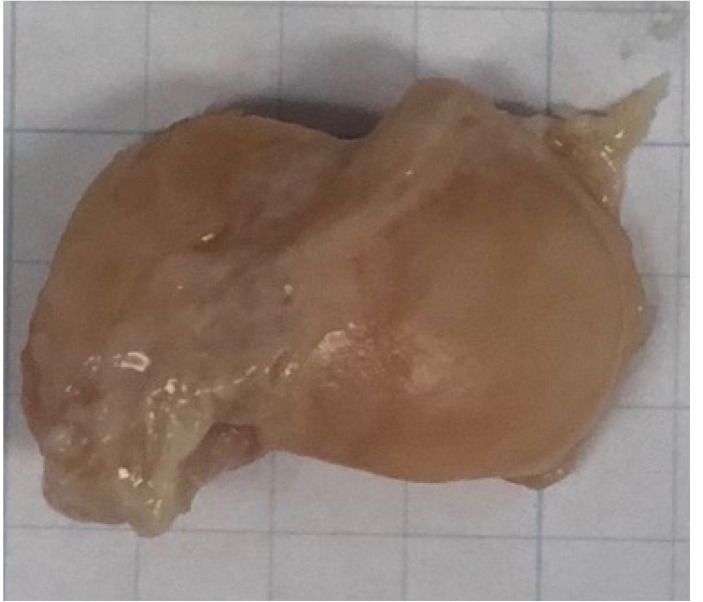
A basic science, cadaveric study was performed. Eight adult cadaveric wrists from Science Care were scanned on a 16-slice multi-detector CT unit (Siemens Somatom Emotion, Siemens Medical) using a bone algorithm consisting of: 130 kVp, 200 mAs, and 1 mm slice thickness with no gap or overlap. These source data were used to generate 0.75 mm axial reconstructions at 0.4 mm increments (i.e. - 0.35 mm overlap) and 1 mm sagittal and coronal reconstructions, with no overlap or gap, in both bone and soft tissue windows. The imaging protocol described above is consistent with that used in standard clinical practice (7). Cadavers with evidence of bony injury or previous surgical intervention were excluded, leaving seven cadaveric wrists as the basis for this study. After the scans were obtained, each cadaver was dissected and the scaphoid, capitate, lunate, and trapezium were removed, yielding 28 bony specimens for evaluation. Dissection was carefully done to remove as much of the soft tissue as possible on each of the bones, while maintaining the native cartilage. Two authors (JM and CL) performed the dissections. A cadaveric scaphoid after dissection is shown in Figure 1.
Figure 1.
A cadaveric scaphoid shown after dissection in toto
Each bone was measured for volume (cc), length (mm), and circumference (mm) by one of three authors (JM, CL, and PB), yielding a total of 84 cadaveric measurements as follows: (a) The volume of each bone was measured using a volume displacement technique. This was done by measuring the displaced water with a 1.00 cc tuberculin syringe after the carpal bone was placed in a glass that was filled with the maximum amount of water, leaving a meniscus at the top. The measurements of water displacement were performed immediately, not allowing the bone to absorb water and affect the volume measurement; (b) The length of each bone was considered its longest dimension and was measured using a digital caliper. The length of the scaphoid bone was considered the distance from the tip of the proximal pole to the tip of the distal pole. The length of the capitate was considered its length from the 3rd metacarpal articulation to the lunate articulation (distal pole to proximal pole). The length of the lunate was considered the distance spanning the capitate articulation in the sagittal plane. The length of the trapezium was considered its length from the 2nd metacarpal articulation to the tip of the ridge adjacent to the groove for the flexor carpi radialis tendon; (c) The circumference of each bone was measured by wrapping a silk suture around specific portions of each carpal bone. The circumference of the scaphoid was measured around the mid-portion of its waist. The circumference of the capitate was measured around the mid-portion of its body in the axial plane. The circumference of the lunate was measured around its capitate articulation and its radius articulation. The circumference of the trapezium was measured around its trapezoid articulation and its 1st metacarpal articulation. Figure 2 is an illustration of each carpal bone and a representation of where the length (points A to B) and circumference (continuous X’s) were measured.
Figure 2.
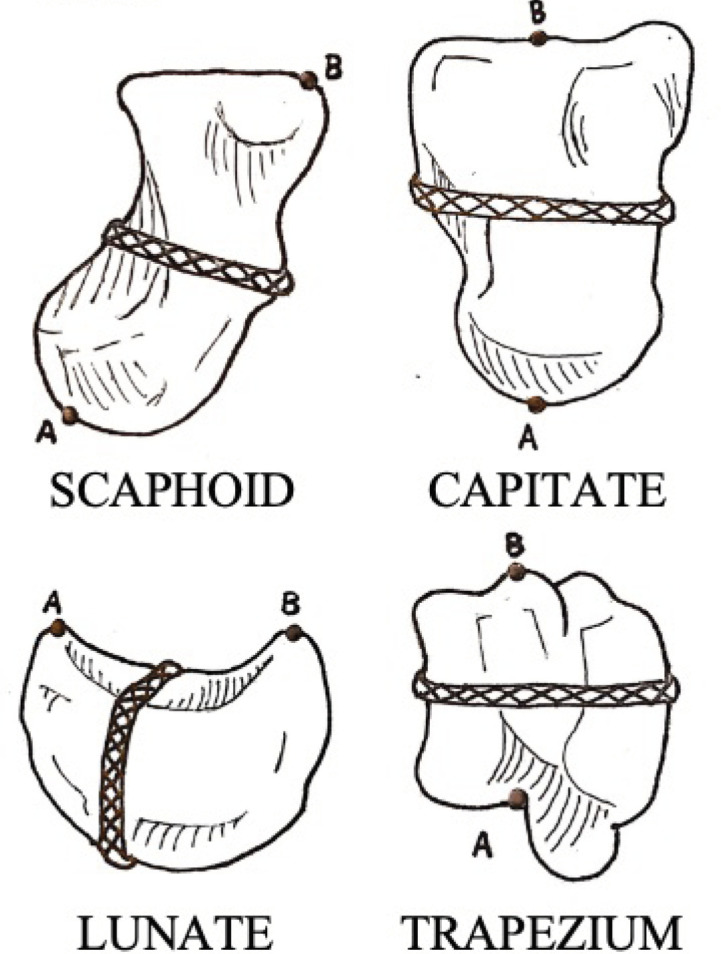
An illustration of where length (points A to B) and circumference (continuous X’s) were measured on each carpal bone
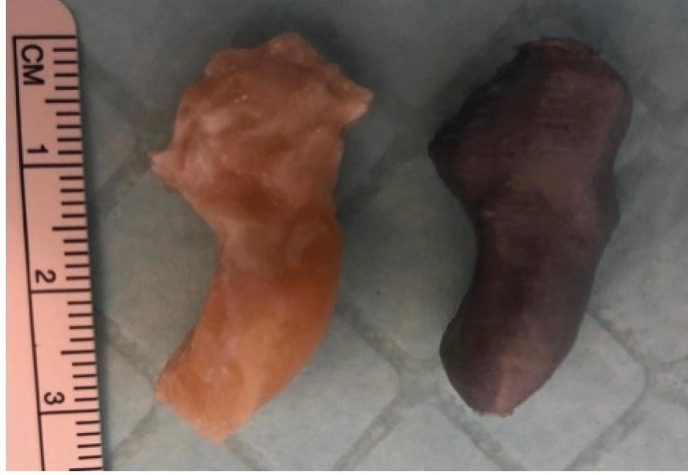
The process of converting CT images into 3D printed anatomical models is described as follows. The DICOM data from each CT scan was processed and manually converted into a STL file with segmentation, threshold editing, island isolation, and smoothing. 3D Slicer (www.slicer.org), a medical informatics, open source software platform was used for the processing of the DICOM data. The resulting STL file for each carpal bone was then edited and prepared for 3D printing using Meshmixer (www.meshmixer.com) and Netfabb (www.autodesk.com/netfabb), open source modeling software. No modification (e.g. smoothing) was performed as this would have altered the dimensions. The 3D modeled individual carpal bones were then printed using a commercially available, fused deposition modeling (FDM) 3D printer (www.ultimaker.com) with a layer resolution of 20-200 microns and an XYZ accuracy of 12.5/12.5/2.5 microns. The 3D printer uses a 3.0 mm diameter, 0.75 kg weight spool of polylactic acid (PLA) filament. The filament cost approximately $0.10-0.20 per 3D print. The CT data acquisition, the reconstruction software, and the 3D printer were all either used commonly in clinical practice, free ware, or commercially available, therefore, our methodology can be replicated in any clinical setting. Figure 3 displays a cadaveric scaphoid compared to its 3D printed anatomical model. Each of the 28 3D printed models were measured for length, circumference, and volume in the same fashion as the cadaver bones [Figure 2].
Figure 3.
A cadaveric scaphoid (left) shown alongside its 3D printed anatomical model (right) in the same plane
In total, 56 specimens (28 cadavers and 28 3D printed models) were evaluated with 3 measurements each, yielding a total of 168 measurements. The dimensions of the cadaver bones were compared to those of their corresponding 3D printed models in order to evaluate the accuracy of the conversion and printing process. The absolute difference in measurements was calculated and a percent error (% error) between these measurements was calculated using the formula: % error = [(|cadaver-3D|)/3D]x100. A paired t-test was performed to determine if a statistically significant difference existed between the mean measurements of the cadavers and 3D printed models. A p-value of less than 0.05 was considered statistically significant. The intraclass correlation coefficients (ICC) between the two groups were calculated to measure the degree of agreement. A power analysis was performed and in order to reach power of 80%, a total of 160 cadaveric wrists (80 per group) would be required.
Results
Table 1 displays the cadaver bones’ and 3D printed models’ mean measurements, standard deviations, absolute differences, and percent error. The absolute difference between the cadavers’ and 3D printed models’ measurement was calculated. This value was then divided by the 3D printed model’s measurement and multiplied by 100 to determine the percent error between the cadaver’s and 3D printed model’s measurement. The percent error is one way to quantify the discrepancy between measurements. On average, the cadaver capitates were 0.50 cc larger in volume than their corresponding 3D printed models, and the cadaver trapeziums were 2.5 mm longer than their corresponding 3D printed models. Otherwise, all 3D printed models’ measurements on average were larger than their corresponding cadaver measurements. The average length and circumference of the 3D printed bones were within 2.3 mm and 2.2 mm, respectively, of the cadaveric bone dimensions. The average volume of the 3D printed bones was within 0.65 cc of the cadaveric bones, which was a larger discrepancy compared to length and circumference, corresponding to an error of 15.9%. The p-value displayed in Table 1 is the result of the paired t-test performed to determine if a statistically significant difference existed between the means of cadaver and 3D printed model measurements. None of these mean values were significantly different (P>0.05). All measurements except for the capitate’s length and lunate’s volume demonstrate strong agreement between the two groups represented by high ICC values shown in Table 2.
Table 1.
Cadaver bone and 3D printed model measurements of volume, length, and circumfer-ence. The mean and standard deviates for each specimen’s measurements are included. The abso-lute difference and percent error between the two groups’ measurements are listed
| Cadavers |
Capitate | Lunate | Scaphoid | Trapezium | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| V(cc) | L(mm) | C (mm) | V(cc) | L(mm) | C (mm) | V(cc) | L(mm) | C (mm) | V(cc) | L(mm) | C (mm) | |
| Mean | 4.5 | 24.2 | 54.5 | 3.1 | 19.2 | 49.0 | 3.9 | 29.7 | 42.8 | 3.8 | 21.1 | 44.6 |
| SD | 1.0 | 2.1 | 4.5 | 0.5 | 2.8 | 7.2 | 1.1 | 4.4 | 4.6 | 1.0 | 4.0 | 5.9 |
| 3D Printed | ||||||||||||
| Mean | 4.3 | 26.1 | 55.0 | 3.6 | 20.0 | 49.2 | 4.4 | 29.8 | 44.5 | 3.9 | 21.0 | 44.9 |
| SD | 1.0 | 2.7 | 4.8 | 1.1 | 3.4 | 8.9 | 1.1 | 3.8 | 4.6 | 1.1 | 3.1 | 6.4 |
| Absolute difference | 0.2 | 1.9 | 0.5 | 0.5 | 0.8 | 0.2 | 0.5 | 0.1 | 1.7 | 0.1 | 0.1 | 0.3 |
| % error | 4.7 | 7.3 | 0.9 | 13.9 | 4.0 | 0.4 | 11.4 | 0.3 | 3.8 | 2.6 | 0.5 | 0.7 |
| p -value | 0.3 | 0.1 | 0.7 | 0.2 | 0.3 | 0.8 | 0.1 | 0.9 | 0.1 | 0.6 | 0.7 | 0.7 |
Abbreviations: V, volume; L, length; C, circumference; cc, cubic centimeter; mm, millimeter; SD, standard deviation; % error, percent error
Table 2.
Intraclass correlation coefficients representing the agreement between cadaver and 3D printed model measurements for each volume, length, and circumference with reported 95% confidence intervals
| Variable | ICC Value | 95% CI | Relationship |
|---|---|---|---|
| Capitate | |||
| Volume | 0.835 | 0.377 – 0.969 | Strong Relationship |
| Length | 0.406 | 0.000 – 0.848 | Weak Relationship |
| Circumference | 0.870 | 0.429 – 0.976 | Strong Relationship |
| Lunate | |||
| Volume | 0.288 | 0.000 – 0.817 | Very Weak Relationship |
| Length | 0.776 | 0.220 – 0.957 | Strong Relationship |
| Circumference | 0.940 | 0.692 – 0.989 | Strong Relationship |
| Scaphoid | |||
| Volume | 0.794 | 0.133 – 0.948 | Strong Relationship |
| Length | 0.726 | 0.000 – 0.948 | Strong Relationship |
| Circumference | 0.836 | 0.320 – 0.970 | Strong Relationship |
| Trapezium | |||
| Volume | 0.816 | 0.262 – 0.966 | Strong Relationship |
| Length | 0.750 | 0.031 – 0.953 | Strong Relationship |
| Circumference | 0.946 | 0.727 – 0.990 | Strong Relationship |
Abbreviations: ICC, intraclass correlation coefficient; CI, confidence interval
Discussion
Currently in the field of orthopaedics, the use of 3D printing technology is gaining much popularity; however, the knowledge is limited, the learning curve is steep, and the cost is high, all of which have prevented its multitude of applications from becoming commonplace in clinical practice (8). Operative complications in the majority of complex orthopaedic cases are due to prolonged operative and anesthesia time, excessive intraoperative bleeding, and the administration of high dose medications, some of which can be attributed to inadequate and imprecise pre-operative planning (9). Increasing evidence has shown that the use of 3D printed models for surgical planning has led to an increase surgical success rate, decreased operative time, and enhanced physician-patient communication (10,11). 3D printing could add tremendous value to patient care and improve outcomes. For this reason, 3D printing should be accurate, reproducible, and efficient; therefore, studies are needed to confirm the reliability of 3D printing in order to justify its everyday use in clinical practice.
In the present study, we compared dimensions (length, circumference, and volume) of select cadaver carpal bones (scaphoid, capitate, lunate, and trapezium) to their corresponding 3D printed models that were based on CT scans of the cadaveric wrists. Our results indicate that there is no statistically significant difference between measurements of the cadaver bones and those of their paired 3D printed models, suggesting that 3D printing based on STL files generated from CT scans is accurate. It was unanticipated that nearly all the 3D printed models’ measurements were larger on average than their corresponding carpal bones’ measurements. Soft tissue and cartilage are poorly visualized and differentiated from one another on CT imaging. In contrast, CT is highly effective in delineating boney structures. Therefore, since the models were printed based on CT scans, one can imagine that a 3D printed model is itself a purely boney representation of the cadaver bone stripped of its surrounding connective tissue such as cartilage, ligament, and tendon. If this were true, measurements of length, circumference, and volume on average would likely be smaller in the 3D printed models given that the cadaver specimens had remaining soft tissue attachments despite the authors’ best effort to skeletonize the bone during cadaveric dissection in toto.
There are only a few articles on biomedical 3D printing that describe the accuracy and/or reproducibility, which are key elements that must be addressed by both the researchers and practicing radiologists who play a large role in producing 3D printed models for patient care. There is the potential for error in each step of the 3D printing process, which includes imaging, segmentation, STL generation, STL postprocessing, 3D printing, and cleaning/preparation of the anatomic model (4). Huotilainen et al aimed to demonstrate the imprecision of the DICOM to STL conversion step (12). In their study, three different institutions converted an identical DICOM data set of a single patient’s skull into an STL file using their preferred software, none of which were the same. Using the same 3D printer, these STL files were subsequently used to fabricate 3 individual medical skull models. The three fabricated skulls were scanned and differences in the model geometries were evaluated utilizing CAD inspection software. The authors concluded that medical models of the same individual can vary greatly depending on the DICOM to STL conversion software and technical parameters used. The cumulative errors that occur during each step of the workflow protocol are often overlooked due to an overreliance on the underlying technologies. As the use of 3D printing becomes more widespread, radiologists will need to validate their techniques by using standardized accuracy and reproducibility metrics, and all those involved in clinical 3D printing will have to abide by reporting guidelines.
Specific to hand surgery, Schweizer et al evaluated the accuracy of reductions of surgically reconstructed scaphoid nonunions or fractures using patient-specific 3D printed reduction guides compared to a freehand technique (13). Preoperatively, 3D surface models of the injured and uninjured scaphoid were generated from CT scans. The uninjured scaphoid served as a reconstruction template and ‘mirror-model’. Postoperatively, a 3D surface model of the healed scaphoid was generated from a CT scan obtained at fracture union. Translational differences in the flexion-extension, ulnar-radial, and pronation-supination planes between the superimposed ‘mirror-model’ and postoperative healed 3D surface model were recorded. When comparing the average residual displacement between scaphoids in the reduction guide group and the freehand group, there was a statistically significant difference of 7o versus 26o, respectively. The authors concluded that although the scaphoid is small, custom 3D printed reduction guides lead to a significantly more anatomic reconstruction compared to that resulting from a freehand technique (13).
There were several limitations to our study. First, two authors performed the cadaveric wrist dissection, which was operator dependent without an exact technique followed. Because of this, varying amounts of soft tissue remained on the cadaver bones with the potential to skew measurements. In contrast, the 3D printed models represented a purely boney structure without soft tissue or cartilage given the different signal densities of these tissues on CT scan. Second, the aforementioned protocol used to measure length and circumference is imperfect due to the normal anatomical variation that existed amongst the seven cadaveric wrists. The measurement of each cadaver bone and model was performed in an operator dependent fashion by multiple authors. Previous work by Heinzelmann et al established morphometric data for the scaphoid by defining its length and width measured from the proximal pole to the distal articular surface and around the waist, respectively (14). Similarly, Vaezi et al evaluated normal radiographic indexes of the wrist including capitate length, which was defined as the distance from its distal pole to proximal pole on an AP x-ray of the wrist (15). These established anatomic dimensions of the scaphoid and capitate correspond to those utilized in our study; however, there remains a paucity of data on the accepted length and circumference of the trapezium and lunate to guide our determination of these dimensions. It is questionable if the length and circumference were measured in the exact location for both the cadaver bone and 3D printed model. Markers on the carpal bones to indicate locations for measuring circumference and length were deemed unnecessary as these placed by one observer would have biased the second observer. Only one set of data points for each measurement exists, and therefore, actual kappa coefficients cannot be calculated. Our methodology utilizing multiple authors to repeatedly measure the same specimen serves as a proxy to inter- and intra-rater reliability. Third, though not statistically significant, the 15.9% difference in average volume between cadaver and 3D printed models is likely clinically significant, especially when creating custom implants for carpal bone fixation using 3D printed models as a template. The difference in average volume can be attributed to the inconsistency of the measuring technique and our study’s limited sample size (n=7). The volume displacement technique utilized allows for a great deal of inconsistency, specifically in the amount of fluid that is drawn up with the tuberculin syringe. It is very likely that not all of the displaced fluid was measured with some being left at the glass-bowl interface. Future studies should work to increase the sample size, which in theory would decrease the standard deviation and lessen the effects of human error on the difference in measurements between cadaver bones and 3D printed models. Lastly, 3D Slicer was used because it is ubiquitous and free, but this technology has not been validated.
Within the field of Orthopaedics, 3D printing gives way patient-specific instrumentation, which is not only useful, but also cost effective (16). Despite this, inaccurate 3D printed models can potentially result in inappropriate preoperative planning, ultimately leading to complications for the patient and clinician (4). This study demonstrates that the process of 3D printing can both accurately and reproducibly fabricate boney models that closely resemble the corresponding cadaveric anatomy.
Acknowledgements
The authors wish to thank CT technologist Shanna Zimran, BS, for her time and assistance in generating the CT images.
References
- 1.Eltorai AEM, Nguyen E, Daniels AH. Three-Dimensional Printing in Orthopedic Surgery. Orthopedics. 2015;38(11):684–687. doi: 10.3928/01477447-20151016-05. [DOI] [PubMed] [Google Scholar]
- 2.Liaw CY, Guvendiren M. Current and emerging applications of 3D printing in medicine. Biofabri-cation. 2017;9(2):024102. doi: 10.1088/1758-5090/aa7279. [DOI] [PubMed] [Google Scholar]
- 3.Galvez M, Asahi T, Baar A, Carcuro G, Cuchacovich N, Fuentes JA, et al. Use of Three-dimensional Printing in Orthopaedic Surgical Planning. J Am Acad Orthop Surg Glob Res Rev. 2018;2(5):e071. doi: 10.5435/JAAOSGlobal-D-17-00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.George E, Liacouras P, Rybicki FJ, Mitsouras D. Measuring and Establishing the Accuracy and Reproducibility of 3D Printed Medical Models. Radiographics. 2017;37(5):1424–1450. doi: 10.1148/rg.2017160165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ogden KM, Aslan C, Ordway N, Diallo D, Tillapaugh-Fay G, Soman P. Factors Affecting Dimensional Accuracy of 3-D Printed Anatomical Structures Derived from CT Data. J Digit Imaging. 2015;28(6):654–663. doi: 10.1007/s10278-015-9803-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu AM, Shao ZX, Wang JS, Yang XD, Weng WQ, Wang XY, et al. The accuracy of a method for printing three-dimensional spinal models. PLoS ONE. 2015;10(4):e0124291. doi: 10.1371/journal.pone.0124291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fishman, EK. Siemens Sensation 16 - CT Protocols - CTisus.com CT Scanning. CTisus. 1999. https:// www.ctisus.com/responsive/protocols/siemens?sensation-16.
- 8.Vaish A, Vaish R. 3D printing and its applications in Orthopedics. J Clin Orthop Trauma. 2018;9(Suppl 1):S74–S75. doi: 10.1016/j.jcot.2018.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zambouri A. Preoperative evaluation and prepa-ration for anesthesia and surgery. Hippokratia. 2007;11(1):13–21. [PMC free article] [PubMed] [Google Scholar]
- 10.Guo F, Dai J, Zhang J, Ma Y, Zhu G, Shen J, et al. Individualized 3D printing navigation template for pedicle screw fixation in upper cervical spine. PLoS ONE. 2017;12:e0171509. doi: 10.1371/journal.pone.0171509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zheng W, Su J, Cai L, Lou Y, Wang J, Guo X, et al. Application of 3D-printing technology in the treatment of humeral intercondylar fractures. Orthop Traumatol Surg Res. 2018;104:83–8. doi: 10.1016/j.otsr.2017.11.012. [DOI] [PubMed] [Google Scholar]
- 12.Huotilainen E, Jaanimets R, Valášek J, Marcian P, Salmi M, Tuomi J, et al. Inaccuracies in additive manufactured medical skull models caused by the DICOM to STL conversion process. J Craniomaxillofac Surg. 2014;42(5):e259–265. doi: 10.1016/j.jcms.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 13.Schweizer A, Mauler F, Vlachopoulos L, Nagy L, Fürnstahl P. Computer-Assisted 3-Dimensional Reconstructions of Scaphoid Fractures and Nonunions With and Without the Use of Patient-Specific Guides: Early Clinical Outcomes and Postoperative Assessments of Reconstruction Accuracy. J Hand Surg Am. 2016;41(1):59–69. doi: 10.1016/j.jhsa.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 14.Heinzelmann AD, Archer G, Bindra RR. Anthro-pometry of the human scaphoid. J Hand Surg Am. 2007;32(7):1005–1008. doi: 10.1016/j.jhsa.2007.05.030. [DOI] [PubMed] [Google Scholar]
- 15.Vaezi T, Hassankhani GG, Ebrahimzadeh MH, Moradi A. Evaluation of Normal Ranges of Wrist Radiologic Indexes in Mashhad Population. Arch Bone Jt Surg. 2017;5(6):451–458. [PMC free article] [PubMed] [Google Scholar]
- 16.Ballard DH, Mills P, Duszak R, Weisman JA, Rybicki FJ, Woodard PK. Medical 3D Printing Cost-Savings in Orthopedic and Maxillofacial Surgery: Cost Analysis of Operating Room Time Saved with 3D Printed Anatomic Models and Surgical Guides. Academic Radiology. 2020;27:1103–13. doi: 10.1016/j.acra.2019.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]