Abstract
Background:
Immunosuppression strategies have changed over time in pediatric heart transplantation (PHT). Thus, comorbidity profiles may have evolved. CTOTC-04 is a multicenter, prospective, cohort study assessing the impact of pre-transplant sensitization on outcomes after PHT. This sub study reports one year outcomes among recipients without pre-transplant donor-specific antibodies (DSA).
Methods:
We recruited consecutive candidates (<21 years) at 8 centers. Sensitization status was determined by a core laboratory. Immunosuppression was standardized: thymoglobulin induction with tacrolimus/mycophenolate mofetil maintenance. Steroids were not used beyond 1 week. Rejection surveillance was by serial biopsy.
Results:
There were 240 transplants. Subjects for this sub-study (n=186) were non-sensitized (n=108) or had no DSA (n=78). Median age was 6 years, 48.4% male, and 38.2% had congenital heart disease. Patient survival was 94.5% (95% CI: 90.1–97.0). Freedom from any type of rejection was 67.5%. Risk factors for rejection were older age at transplant and presence of non-DSA pre-transplant. Freedom from infection requiring hospitalization/intravenous antimicrobials was 75.4%. Freedom from rehospitalization was 40.3%. New onset diabetes mellitus and post-transplant lymphoproliferative disorder (PTLD) occurred in 1.6% and 1.1% of subjects respectively. There was no decline in renal function over the first year. Corticosteroids were used in 14.5% at 1 year.
Conclusions:
PHT recipients without DSA at transplant, and managed with a steroid avoidance regimen, have excellent short-term survival and low risk of first year diabetes mellitus, and PTLD. Rehospitalization remains common. These contemporary observations allow for improved caregiver/patient counseling and provide the necessary outcomes data to help design future randomized controlled trials.
Introduction
Steroid avoidance regimens are becoming increasingly popular after pediatric solid organ transplantation and have been used in a few pediatric heart transplant centers since the 1980’s (1–3). Nonetheless, contemporary outcomes of such regimens in pediatric heart transplantation are limited to retrospective studies (4,5) and multi-center registries (6,7). We developed a prospective, multi-institutional observational cohort study (CTOTC-04) to assess the impact of pre-transplant sensitization on pre- and post-transplant outcomes in pediatric heart candidates following listing for transplantation (8). The use of a central core laboratory for anti-HLA antibody determination has enabled us to identify a large cohort of subjects who had no evidence of donor specific antibody (DSA) at the time of transplantation. This cohort, considered to be “low immunologic risk”, was managed with a uniform immunosuppressive regimen that included no routine use of corticosteroids beyond the first week after transplantation (8). In addition, there was standardized rejection surveillance using serial endomyocardial biopsy (EMB). The main objective of this current report is to evaluate detailed outcomes for children managed by this strategy in the absence of pre-transplant DSA. These contemporary observations will allow for improved counseling of patients and their families about the expected post-transplant clinical course. Furthermore, these data should provide the necessary outcomes to help design future randomized controlled trials for pediatric heart recipients.
Methods
The National Institutes of Health (NIH)–sponsored Clinical Trials in Organ Transplantation in Children (CTOTC) “Alloantibodies in Pediatric Heart Transplantation” (www.ctotc.org) is a prospective, observational cohort study which includes 8 North American pediatric transplantation centers. This study entailed approximately 3 years of accrual and a minimum of 1 year follow up from February 2011 through December 31, 2014. Consecutive subjects < 21 years of age listed for heart transplantation were enrolled and were excluded only if they were listed for multiple organ transplants, < 1 year of follow up was anticipated, were participating in another research study that would interfere with the scientific integrity or safety of this study, or were unwilling to consent. Full details of study design, including overall study design, organization and duration, study sites, inclusion and exclusion criteria, subject population, study definitions, primary and secondary endpoints, immunosuppression management, rejection surveillance, study visits with ‘schedule of events’, and details of the various study core laboratories are provided elsewhere (8). All study activities were approved by institutional review boards at each of the participating centers.
Sensitization status
Inclusion criteria for this sub-study included all CTOTC-04 subjects who were non-sensitized at transplantation or had evidence of HLA antibodies but without donor-specificity. Subjects were considered sensitized when one or more class I and/or class II HLA antibodies with median fluorescence intensity (MFI) >1,000 were detected using Luminex® LABScreen™ single antigen beads (One Lambda; Canoga Park, CA) based on analysis of the sample closest to, but before, transplantation. This was usually obtained within 24 hours prior to transplantation. Donor-specificity of HLA antibody was determined at the Core Alloantibody Laboratory by a single expert in histocompatibility (A. Zeevi, University of Pittsburgh) as previously described (8). All subjects in this sub-study had a negative donor-specific cytotoxicity crossmatch.
Immunosuppression management and clinical care guidelines
The standardized immunosuppression protocol has been previously described (8). In brief, all patients received thymoglobulin induction therapy (total cumulative dose 7.5 mg/kg) and maintenance immunosuppression with tacrolimus and mycophenolate mofetil (MMF). Corticosteroids were used prior to the administration of each dose of thymoglobulin, and routine maintenance corticosteroids were not given. Centers followed local site clinical care guidelines for infectious disease prophylactic medication and surveillance monitoring for cytomegalovirus and Epstein-Barr virus. Concomitant use of other medications was defined by each center’s standard of care, with no prohibited medications (8).
Rejection surveillance and definitions
Rejection surveillance by EMB was standard clinical practice at all participating centers. Clinical care guidelines were established for surveillance at approximately the following time points during the first year: weeks 1–2, 4, 8 and months 3, 4, 6, 9, 12. Rejection diagnosis was based on local site interpretation of clinical findings, diagnostic test results and local EMB pathology laboratory reports. Rejection on EMB was classified according to the guidelines of the International Society for Heart and Lung Transplantation (ISHLT) (9, 10). Acute cellular rejection was defined as ISHLT grade 2R or greater. Acute antibody-mediated rejection was also defined according to the criteria of the ISHLT (10). Acute mixed rejection was defined according to the criteria of the ISHLT as concurrent evidence of both the presence of acute cellular rejection (ISHLT ≥ grade 2R) and the histopathologic and/or immunopathologic characteristics of antibody-mediated rejection (10). Acute clinical rejection was defined as augmentation of immunosuppression based on clinical findings in the absence of histologic confirmation. Acute rejection with hemodynamic compromise was defined as echocardiographic fractional shortening of < 26 % with ± >5% decrease from the last echocardiogram and/or new onset heart failure and was deemed severe when there was concomitant use of inotropic agents (8).
Outcomes analyzed
Definitions of all outcomes for CTOTC-04 are provided elsewhere (8). Outcomes for this study included death and re-transplantation, acute rejection (including cellular rejection, antibody mediated rejection, rejection with hemodynamic compromise, and clinical rejection), infection (with or without laboratory documentation of a pathogen) requiring hospital admission or intravenous antimicrobial therapy, rehospitalization (longer than 24 hours), new onset diabetes mellitus, post-transplant lymphoproliferative disease, and renal function. New onset diabetes was defined as new onset of insulin dependency or the need for oral hypoglycemic agents lasting > 30 consecutive days post-transplant. Infections were broadly categorized by microorganism type, when available, and the Schwartz formula (GFR= 0.413 * Ht/Cr serum) was used to determine estimated glomerular filtration rate (GFR)(11). For this report, all outcomes were analyzed up to one year post-transplant. Factors analyzed to assess increased risk for acute rejection included: age, gender, race, ethnicity, diagnosis of congenital heart disease, ABO incompatibility, UNOS status at transplant, history of prior sensitizing event, and presence of non-DSA antibodies.
Statistical considerations
For the current report, data are summarized using descriptive statistics for categorical (counts and percentages) and continuous (medians and IQRs) variables. Survival curves were estimated using the Kaplan-Meier method with corresponding 95% confidence intervals. Logistic regression models were developed to estimate probabilities of acute cellular rejection and any acute rejection, separately. Risk factors, from the list specified in the preceding paragraph, were identified using backwards elimination variable selection with α=0.10 threshold for model inclusion. All statistical analyses were performed using SAS Version 9.4 (SAS Institute, Inc., Cary, NC).
Results
Participant characteristics
Among the 240 heart transplant recipients in CTOTC-04, 237 (98.8%) had ≥1 antibody sample available at the core laboratory for testing. Of these, 186 subjects were non-sensitized [108 (58.1%)] or had no DSA [78 (41.9%)]. Baseline characteristics of the cohort are shown in Table 1. Median age for the cohort was 6 years (IQR 1, 14) with non-black race being younger at transplant (5 vs 11.5 years), 90 (48.4%) were males, and 71 (38.2%) had congenital heart disease (CHD). Subjects of non-black race were more likely to have CHD 42% vs 16.7% (p=0.0052). One-hundred sixty-one (86.6%) recipients were status 1A at transplant with 27 (14.5%) on a ventilator and 42 (22.6%) on mechanical circulatory support [35 (18.8%) VAD and 7 (3.8%) ECMO]. There was no difference between non-black and black race for the presence of non-DSA HLA antibodies versus having no HLA antibodies.
Table 1.
Baseline characteristics for the 186 subjects who were non-sensitized (n=108) or who were sensitized without DSA (n=78).
| Total (N=186) |
|
|---|---|
| Median Age at Listing (IQR) | 6 (1, 14) |
| Median Age at Transplant (IQR) | 6 (1, 14) |
| Median Weight at Listing (IQR) | 16.6 (7.5, 45.9) |
| Median Weight at Transplant (IQR) | 17.2 (8.1, 48.9) |
| Diagnosis | |
| Cardiomyopathy | 112 (60.2) |
| Congenital Heart Disease | 71 (38.2) |
| Other | 3 (1.6) |
| Race | |
| White | 104 (55.9) |
| Black or African American | 36 (19.4) |
| Non-White/Non-Black | 15 (8.1) |
| Unknown or Not Reported | 31 (16.7) |
| Ethnicity | |
| Hispanic or Latino | 24 (12.9) |
| Not Hispanic or Latino | 123 (66.1) |
| Unknown or Not Reported | 39 (21.0) |
| Male | 90 (48.4) |
| Blood Type | |
| A | 62 (33.3) |
| AB | 6 (3.2) |
| B | 30 (16.1) |
| O | 88 (47.3) |
| UNOS Status at Listing | |
| 1A | 130 (69.9) |
| 1B | 32 (17.2) |
| 2 | 24 (12.9) |
| UNOS Status at Transplant | |
| 1A | 161 (86.6) |
| 1B | 19 (10.2) |
| 2 | 6 (3.2) |
| Prior Sensitizing Event | |
| Surgery | 87 (46.8) |
| Blood Transfusion | 89 (47.8) |
| VAD | 44 (23.7) |
| ECMO | 18 (9.7) |
| Any MCS | 54 (29.0) |
| Homograft | 17 (9.1) |
| Prior Transplant | 7 (3.8) |
| Pregnancy | 1 (0.5) |
| Hospitalized at Listing | 136 (73.1) |
| ICU at Listing | 84 (45.2) |
| Ventilator at Listing | 28 (15.1) |
| ECMO at Listing | 2 (1.1) |
| VAD at Listing | 21 (11.3) |
| MCS at Listing | 23 (12.4) |
| Hospitalized at Transplant | 140 (75.3) |
| ICU at Transplant | 84 (45.2) |
| Ventilator at Transplant | 27 (14.5) |
| ECMO at Transplant | 7 (3.8) |
| VAD at Transplant | 35 (18.8) |
| MCS at Transplant | 42 (22.6) |
Immunosuppression and endomyocardial biopsy
There were 182 subjects that survived to hospital discharge and 181 of them were discharged on tacrolimus. Five subjects (2.8%) switched to cyclosporine over the first 12 months. MMF was the sole adjunct immunosuppression in 155 (85.2%) subjects throughout the first year, with 27 using azathioprine at some time-point during this period. The number and percentage of subjects on corticosteroids at the time of discharge and scheduled study visits is listed in Table 2. The proportion on corticosteroids at the time of scheduled visits was approximately constant throughout the first year; 15.6% at initial discharge and 14.5% at one year. Overall, fifty-seven (30.6%) subjects were on prednisone at discharge or at some time point in the first year. Of these, 36 (63.2%) experienced rejection. In the remaining 21 subjects, 10 were on steroids at one visit, 2 at 2 visits, 2 at 3 visits, 1 at 4 visits, and 6 were on steroids at all five study visits. There were 9 subjects on a calcineurin inhibitor and corticosteroid without MMF or azathioprine at some time point after discharge. Reason for corticosteroid usage beyond acute rejection was not routinely collected for this protocol.
Table 2.
Corticosteroid usage at the time of scheduled study visits in the first year post-transplant.
| Total (N=186) | |
|---|---|
| Steroid Usage | |
| Visit 3/Discharge | 29 (15.6) |
| Visit 4/Month 1 | 30 (16.1) |
| Visit 5/Month 3 | 32 (17.2) |
| Visit 6/Month 6 | 30 (16.1) |
| Visit 7/Month 12 | 27 (14.5) |
The indications for endomyocardial biopsy for the biopsy proven rejection episodes were: 65 (86.7%) surveillance, 5 (10.7%) non-specific symptoms, 3 (4.0%) other and 2 (2.7%) graft dysfunction diagnosed on echocardiogram.
Death and retransplantation
Probability of patient survival at one year was 94.5% (CI: 90.1%, 97.0%). Ten (5.4%) subjects died in the first year at a median of 27 days post-transplantation (IQR 6, 185 days). Causes of death prior to 28 days were: primary graft failure (3), bleeding (1), infection (1) and multi-organ system failure (1). Deaths after 28 days were due to: acute cellular rejection (1), pulmonary hemorrhage (1), respiratory failure (1), and cerebrovascular accident (1). There was one re-transplant at 216 days due to coronary artery disease.
Rejection
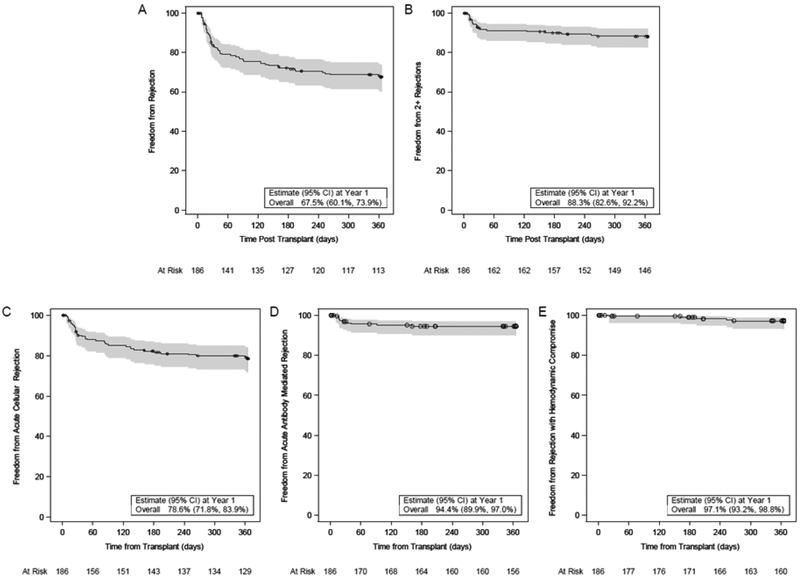
Fifty-eight (31.2%) subjects had an acute rejection event in the first year post-transplant and 21 (11.3%) had recurrent rejection (2 or more episodes). Freedom from any type of acute rejection in the first year post-transplant was 67.5% (CI: 60.1%, 73.9%) and freedom from recurrent rejection was 88.3% (CI: 82.6%, 92.2%). Freedom from acute cellular rejection was 78.6%, antibody mediated rejection 94.4% and rejection with hemodynamic compromise 97.1% (Figure 1). Although most rejection episodes were confirmed by EMB (75 episodes), rejection was clinically diagnosed and treated in 15 subjects (8.1%; 16 episodes). Among the 16 episodes of clinically diagnosed rejection, the diagnosis was based on history, physical examination and electrocardiogram in 9, (including two in overt heart failure), on new-onset cardiac dysfunction observed on echocardiogram in 4, and other in 3. A multivariable logistic regression model identified risk factors for acute cellular rejection that included older age (p=0.0055) and non-black race (p=0.059) while a separate multivariable logistic model identified risk factors for any rejection that included older age (p=0.0117), non-black race (p=0.0043) and presence of non-DSA antibodies before transplant (p=0.0527) (Table 3).
Figure 1.
Probability of freedom from any type of rejection (A) recurrent rejection (2 or more episodes) (B), acute cellular rejection (C), antibody mediated rejection (D) and rejection with hemodynamic compromise (E) in the first year post-transplant with corresponding 95% confidence interval. The number of participants at risk is presented at select time points along the x-axis. Censored data is shown as circles.
Table 3:
Risk factors for any acute rejection event in the first year post-transplant identified using multivariable logistic regression.
| Parameter | P-value | Odds Ratio | 95% Wald Confidence Limit |
|---|---|---|---|
| Age at Transplant (years)* | 0.0117 | 1.066 | (1.014, 1.121) |
| Race (Non-black vs. black) | 0.0043 | 3.181 | (1.239, 8.166) |
| (Unknown vs. black) | 0.4145 | 1.160 | (0.331, 4.071) |
| Sensitization (non-DSA ab vs. no ab) | 0.0527 | 1.923 | (0.992, 3.725) |
Note: Variables were selected using backwards elimination with α=0.10 threshold for inclusion Potential variables included age, gender, race, ethnicity, diagnosis of congenital heart disease, ABO incompatibility, UNOS status at transplant, history of prior sensitizing event, and presence of non - DSA antibodies.
Odds ratio represents the increase in odds for a one year increase in age.
Infection
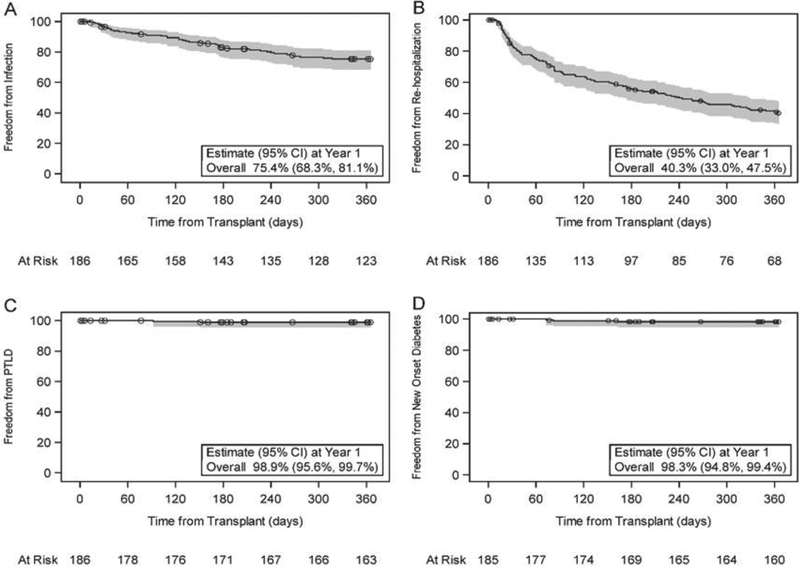
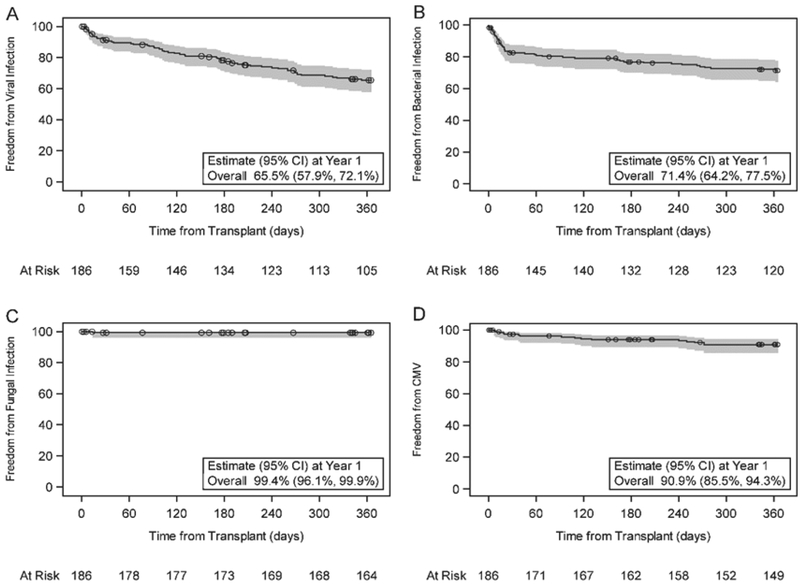
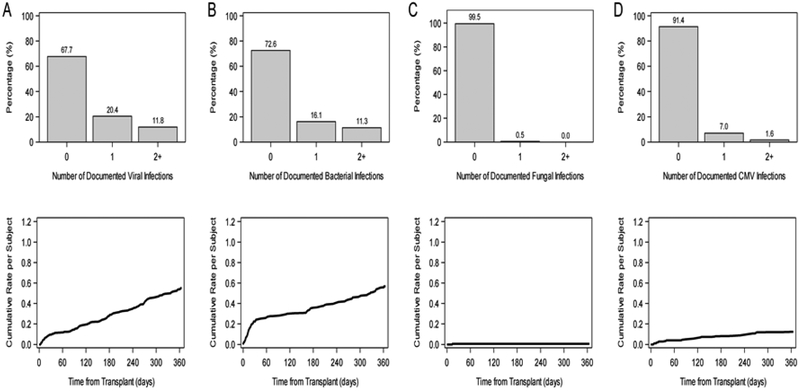
There were 69 infections requiring hospital admission and/or the use of intravenous antimicrobial agents in 43 subjects in the first year post transplant. Freedom from infection in the first year post-transplant was 75.4% (CI: 68.3%, 81.1%) (Figure 2). The average length of infection-related hospital stay was 7.5 ± 11.5 days, ranging from 1 to 64 days. Freedom from laboratory documented viral infection (65.5%; CI: 57.9%, 72.1%), bacterial infection (71.4%; CI: 64.2%, 77.5%) and fungal infection (99.4%; CI: 96.1%, 99.9%) are shown in Figure 3 (panels a, b, and c respectively). Of note, there was an approximately constant hazard for risk of viral infection throughout the first year (Figure 3a), but bacterial infections occurred mainly in the first 30 days (Figure 3b). Since time-to-event analyses do not reflect total burden of infection over time, we also expressed documented viral and bacterial infection as proportion of subjects with none, one, or more than one infection as well as by the cumulative rate per subject (Figure 4).
Figure 2.
Probability of freedom from Infection requiring hospitalization or intravenous antimicrobials (A), Re-hospitalization (B), PTLD (C) and New Onset Diabetes (D) in the first year post-transplant with corresponding 95% confidence interval. The number of participants at risk is presented at select time points along the x-axis. Censored data is shown as circles. One participant with diabetes pre-transplant is excluded from panel D.
Figure 3.
Probability of freedom from laboratory documented infection in the first year post-transplant with corresponding 95% confidence interval by type: Viral (A), Bacterial (B), Fungal (C), and CMV (D). The number of participants at risk is presented at select time points along the x-axis. Censored data is shown as circles.
Figure 4.
Burden of infection assessed by proportion of subjects with 0, 1 or 2 or more laboratory documented infection episodes (upper panels) and by average number of infection episodes per subject (lower panels) by infection type: viral (A), bacterial (B), fungal (C), and CMV (D).
Cytomegalovirus (CMV) invasive disease or syndrome occurred in 16 subjects (8.6%) with the median time to infection of 101 days (IQR 20, 245) post-transplant (Figure 4d). In patients who developed CMV disease, the majority were CMV negative at transplantation and received organs from CMV positive donors (10 of 14 cases with documented serology). None died during the acute CMV infectious episode, though 4 of these 16 subjects died between 339 and 549 days post transplantation. Cause of death in these 4 patients was coronary artery disease (1), respiratory failure (1), sudden cardiac death (1), and cerebrovascular accident (1).
Other post transplant outcomes
There were 226 re-hospitalizations in 105 (56.4%) subjects in the first year post transplant. Freedom from hospitalization in the first year was 40.3% (CI: 33.0%, 47.5%) (Figure 2). Two (1.1%) subjects developed post-transplant lymphoproliferative disease and 3 (1.6%) subjects developed new onset diabetes in the first year post-transplant (Figure 2, panel c and d). There were 40 subjects without pre-existing or new onset diabetes in whom HbA1c data was available post-transplantation. The median HbA1c at one year post-transplant was 5.1 % (IQR 4.7, 5.4). There was no significant change in GFR over the first year (p=0.11). Median estimated GFR was 93 ml/min/1.73m2 (IQR 72.2, 120.6) at transplant (n=172) and 105 ml/min/1.73m2 (IQR 82.6, 122.8) at one year post-transplant (n=156). At 1 year post-transplant, all patients had a serum creatinine of ≤2 mg/dl and no subject was on dialysis. However, one patient underwent renal transplantation between months 6 and 12 post-transplantation.
Discussion
In this sub-analysis of the CTOTC-04 observational cohort study, we identified a large cohort of subjects who had no evidence of DSA at the time of transplantation. These subjects with no HLA antibody at transplantation, or antibody that is not donor-specific, are generally considered to be at “low immunologic risk” and are increasingly managed with steroid avoidance regimens. Our cohort, identified by using a core alloantibody laboratory and a single expert in histocompatibility, was managed with a steroid avoidance regimen that included no routine use of corticosteroids beyond the first week after transplantation, routine induction therapy with thymoglobulin, and standardized maintenance immunosuppression with tacrolimus and mycophenolate mofetil. Furthermore, rejection surveillance was standardized using serial EMB, and we utilized standardized study definitions and well-defined clinical endpoints. On-site monitoring of study performance and sources of data added to the study integrity. This prospective study design allowed us to evaluate clinical and laboratory outcomes with much greater reliability than has been traditionally associated with transplant registries. This has enabled us to produce a detailed picture of contemporary outcomes after pediatric transplantation.
Since the 1980’s, single center series have reported low rejection rates and comparable survival outcomes to pediatric registry data using steroid avoidance maintenance regimens (1–7) Each of these reports, however, had different immunosuppression protocols - some varied over time within the same center and some used echocardiography as the primary surveillance tool for detection of rejection. Moderate cellular rejection by EMB is not necessarily associated with echocardiographic changes, and therefore may underestimate acute cellular rejection (12). Singh and colleagues reported on 55 consecutive patients from 2 centers who received a comparable steroid avoidance regimen to our study and with rejection surveillance using EMB at frequent intervals during the first-year post-transplant (4). Interestingly, they reported 16 % of the study population was started on steroids after a variable duration of a steroid free regimen. This percentage is remarkably similar to our findings. Singh reported the reasons for the reintroduction of steroids included treatment of rejection and persistent protein-losing enteropathy. In this analysis the reason for corticosteroid use beyond treatment for rejection was not collected. However, one could speculate that steroids may be used during the first year for acute or chronic lung disease or as additional immunosuppression in patients on single drug maintenance therapy.
Singh reported an 87% freedom from rejection at 1 year, significantly less than reported in our cohort. (4) More recently, a retrospective, single center study compared historical controls using conventional triple immunosuppression (cyclosporine, azathioprine and steroids), to patients who received a similar steroid avoidance regimen to the one used in our study. Incidence of acute rejection in the first year after transplant in the conventional immunosuppression group was 58% at one year, compared to 38% in the steroid avoidance group. (5) This incidence of rejection at one year in the steroid avoidance group was similar to our findings of 31%. Large database studies have reported a decline in 1st year rejection over time from a high of approximately 55% to 15% in the present era (13, 14). It is difficult to compare these results with our findings for several reasons. First, the diagnosis of rejection in registries is not consistently dependent on endomyocardial pathology. Surveillance EMB may lead to diagnosis of more acute rejection (15). Second, there is the possibility of underreporting in large, multicenter databases. Lastly, the ISHLT report was limited to those treated for rejection between discharge and 1 year and therefore did not capture rejection episodes that occurred during the index hospitalization (14).
Even though our cohort was deemed low immunologic risk, there was still a considerable amount of acute cellular rejection in the first year. The risk of acute rejection increased with older age at transplant, as has been previously reported in multiple prior studies including the registry of ISHLT and studies from the Pediatric Heart Transplant Study, (14,16–17) and may reflect the more robust nature of the immune response in later childhood / early adult life. Of particular note, there was more rejection in non-black race subjects in this cohort. Prior studies have reported black race as a risk factor for repeat rejection, late rejection and lower survival (17–21). However, similar to our study, Singh et al reported the incidence of cumulative rejection episodes per patient to be almost identical among racial groups during the first year post-transplant (22). It may be that black race is no longer a risk factor for first year rejection, but remains a risk factor for recurrent, late, and hemodynamically significant rejection. Ongoing analysis of the CTOTC-04 cohort will determine if early risk factors change during long-term follow-up. Additionally, subjects with non-DSA antibodies pre-transplant were 1.9 times more likely to have cellular rejection than those with no HLA antibodies. One plausible explanation is that those with non-DSA antibody pre transplant are more likely to have historical DSA pre-transplant that is not detected at the time of transplant due to waning serum concentrations over time as the sensitizing events become more remote. Alternatively, as we have previously demonstrated within CTOTC-04, DSA at transplant may have been present at levels below the definition of sensitization (i.e. below an MFI of 1000 for this study). Following transplantation, patients in both of these scenarios are at risk for memory responses and the development of high strength antibody in the post-transplant period (23). This raises the important question as to whether patients with non-DSA HLA antibody should be managed with the same immunosuppressive regimen as those with no HLA antibody. This requires further investigation.
Freedom from any infection at one year was 75.4%. This finding was better than that reported by a Pediatric Heart Transplant Study (PHTS) registry study in 2011 looking at the impact of induction therapy on infection and malignancy. In this prior study, freedom from any infection at 12 months was approximately 67% with no difference in freedom from infection at one year whether induction therapy was used or not. However, the analysis included all classes of induction therapy. (24) Marshall et al. also found a similar incidence of infection between their conventional triple therapy and the steroid avoidance cohorts. (5) Unlike the PHTS analysis, more viral than bacterial infections were found in our cohort and likely reflects the closer surveillance that occurs in a prospective observational study compared to a registry. Only two patients developed PTLD in the first year of our study. This finding is perhaps not surprising. A PHTS analysis in 2006 showed a probability of freedom from PTLD of 98% at 1 year and 92% at 5 years (25). In the same study cited above evaluating the impact of induction therapy on the development of infection and malignancy (24), the use of induction therapy (with the exception of OKT3) was associated with a lower risk of developing PTLD compared to no induction. As the authors suggest, the total immunosuppression exposure rather than induction use alone may be a more accurate determinant of PTLD risk. Ongoing follow-up of the CTOTC-04 study will determine if our steroid avoidance protocol using induction therapy is associated with low-risk of late-onset PTLD.
New onset diabetes was also rare in our cohort, being observed in only 3 subjects (1.6%). This is similar to that recently reported by Sehgal et al. who identified the incidence of new onset diabetes in pediatric heart transplant patients using the OPTN database as 2.4%, 9.0%, and 10.4% at one, five and 10 years after transplant, respectively (26). These authors did find a lower incidence of diabetes after 2000 and speculated that less maintenance steroid use and lower incidence of rejection requiring steroid treatment in the present era may account for some of the difference. A recent report in solid organ transplants in Canada documented a 2% incidence of diabetes in their heart transplant population, using a steroid avoidance protocol in the majority of patients. (27) Although tacrolimus is known to be a diabetogenic agent (28), it seems likely that avoidance or minimization of corticosteroid usage in many pediatric heart transplant centers in the current era is contributing to the low incidence of post-transplant new-onset diabetes mellitus.
In conclusion, using the first large scale, multi-institutional prospective study of pediatric heart recipients in which standardized care (including immunosuppression and rejection surveillance) was achieved, we demonstrated that recipients without donor-specific antibody at transplant, and managed with a steroid avoidance regimen, have excellent short-term survival and low risk of first year diabetes mellitus and post-transplant lymphoproliferative disorder. Rehospitalization remains common, driven by acute rejection and infection. These contemporary observations allow for improved caregiver/patient counseling and provide the necessary outcomes data to help design future randomized controlled trials. Ongoing follow-up of the CTOTC cohorts will allow us to determine the medium and long-term impact of this steroid avoidance protocol on pediatric heart transplant outcomes.
Acknowledgment:
This research was performed as a project of the Clinical Trials in Organ Transplantation in Children, a collaborative clinical research project headquartered at the National Institute of Allergy and Infectious Diseases. The work was supported by Grant U01AI077867 “Alloantibodies in Cardiac Transplantation - Intervention, Outcomes and Mechanisms” from the Division of Allergy, Immunology and Transplantation of the National Institutes of Health. Statistical and clinical coordinating was performed by Rho, Inc. (Chapel Hill, NC). The study was monitored by an external Data Safety and Monitoring Board appointed by NIAID. The study is registered at https://clinicaltrials.gov/ ( NCT01005316).
Funding information: National Institute of Allergy and Infectious Diseases, Grant/Award Number: U01AI077867
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
None of the authors have a financial relationship with a commercial entity that has an interest in the subject of the presented manuscript or other conflicts of interest to disclose.
References
- 1.Dionigi B, Razzouk AJ, Hasaniya NW, Chinnock RE, Bailey LL. Late outcomes of pediatric heart transplantation are independent of pre-transplant diagnosis and prior cardiac surgical intervention. J Heart Lung Transplant 2008;27:1090–5. [DOI] [PubMed] [Google Scholar]
- 2.Smith RR, Wray J, Khaghani A, Yacoub M. Ten year survival after paediatric heart transplantation: a single centre experience. Eur J Cardiothorac Surg 2005;27:790–4. [DOI] [PubMed] [Google Scholar]
- 3.Leonard H, Hornung T, Parry G, Dark JH. Pediatric cardiac transplant: results using a steroid-free maintenance regimen. Pediatr Transplant 2003;7:59–63. [DOI] [PubMed] [Google Scholar]
- 4.Singh TP, Faber C, Blume ED et al. Safety and early outcomes using a corticosteroid-avoidance immunosuppression protocol in pediatric heart transplant recipients. J Heart Lung Transplant 2010;29:517–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marshall CD, Richmond ME, Singh RK et al. A comparison of traditional versus contemporary immunosuppressive regimens in pediatric heart recipients. J Pediatr 2013;163:132–6. [DOI] [PubMed] [Google Scholar]
- 6.Auerbach SR, Gralla J, Campbell DN, Miyamoto SD, Pietra BA. Steroid avoidance in pediatric heart transplantation results in excellent graft survival. Transplantation 2014;97:474–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Auerbach SR, Kukreja M, Gilbert D et al. Maintenance steroid use at 30 days post-transplant and outcomes of pediatric heart transplantation: A propensity matched analysis of the Pediatric Heart Transplant Study database. J Heart Lung Transplant 2015;34:1066–72. [DOI] [PubMed] [Google Scholar]
- 8.Zuckerman WA, Zeevi A, Mason KL et al. Study rationale, design and pre-transplant alloantibody status: a first report of Clinical Trials in Organ Transplantation in children-04 (CTOTC-04) in pediatric heart ransplantation. Am J Transplant 2018;18:2135–2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stewart S, Winters GL, Fishbein MC et al. Revision of the 1990 working formulation for the standardization of nomenclature in the diagnosis of heart rejection. J Heart Lung Transplant 2005;24:1710–20. [DOI] [PubMed] [Google Scholar]
- 10.Berry GJ, Burke MM, Andersen C, et al. The 2013 International Society for Heart and Lung Transplantation working formulation for the standardization of nomenclature in the pathologic diagnosis of antibody-mediated rejection in heart transplantation. J Heart Lung Transplant 2013;32:1147–1162. [DOI] [PubMed] [Google Scholar]
- 11.Schwartz GJ, Munoz A, Schneider MF et al. New equation to estimate GFR in children with CKD. J Am Soc Nephrol. 2009;20:629–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosenthal DN, Chin C, Nishimura K et al. Identifying cardiac transplant rejection in children: diagnostic utility of echocardiography, right heart catheterization and endomyocardial biopsy data. J Heart Lung Transplant 2004;23:323–9. [DOI] [PubMed] [Google Scholar]
- 13.Butts RJ, Savage AJ, Atz AM et al. Validation of a simple score to determine risk of early rejection after pediatric heart transplantation. JACC Heart Fail. 2015;3:670–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rossano JW, Cherikh WS, Chambers DC et al. The Registry of the International Society for Heart and Lung Transplantation: twentieth pediatric heart transplantation report-2017; focus theme: allograft ischemic time. J Heart Lung Transplant 2017;36:1060–1069. [DOI] [PubMed] [Google Scholar]
- 15.Godown J, Pruitt E, Dodd DA et al. Practice variation in the diagnosis of acute rejection among pediatric heart transplant centers: an analysis of the pediatric heart transplant study (PHTS) database. J Heart Lung Transplant. 2018;37:S191. [DOI] [PubMed] [Google Scholar]
- 16.Gossett JG, Canter CE, Zheng J et al. Decline in rejection in the first year after pediatric cardiac transplantation: a multi-institutional study. J Heart Lung Transplant 2010;29:625–32. [DOI] [PubMed] [Google Scholar]
- 17.Ameduri RK, Zheng J, Schechtman KB et al. Has late rejection decreased in pediatric heart transplantation in the current era? A mult-institutional study. J Heart Lung Transplant 2012;31:980–6. [DOI] [PubMed] [Google Scholar]
- 18.Everitt MD, Pahl E, Schechtman KB et a;. Rejection with hemodynamic compromise in the current era of pediatric heart transplantation: a multi-institutional study. J Heart Lung Transplant 2011;30:282–8. [DOI] [PubMed] [Google Scholar]
- 19.Chin C, Naftel DC, Singh TP et al. Risk factors for recurrent rejection in pediatric heart transplantation: a multicenter expereince. J Heart Lung Transplant 2004;23:178–85. [DOI] [PubMed] [Google Scholar]
- 20.Felkel TO, Smith AL, Reichenspurner HCet al.. Survival and incidence of acute rejection in heart transplant recipients undergoing successful withdrawal from steroid therapy. J Heart Lung Transplant 2002;21:530–539 [DOI] [PubMed] [Google Scholar]
- 21.Girnita DM, Brooks MM, Webber SA et al. Genetic polymorphisms impact the risk of acute rejection in pediatric heart transplantation: a multi-institutional study. Transplantation 2008;85:1632–1639. [DOI] [PubMed] [Google Scholar]
- 22.Singh TP, Naftel DC, Addonizio L et al. Association of race and socioeconomic position with outcomes in pediatric heart transplant recipients. Am J Transplant 2010;10:2116–2123. [DOI] [PubMed] [Google Scholar]
- 23.Dipchand AI, Webber S, Mason K et al. Incidence, characterization and impact of newly detected donor specific anti-HLA antibody in the first year after pediatric heart transplantation: a report from the CTOTC-04 study. Am J Transplant 2018;18:2135–2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gajarski RJ, Blume ED, Urschel S et al. Infection and malignancy after pediatric heart transplantation: The role of induction therapy. J Heart Lung Transplant 2011;30:299–308. [DOI] [PubMed] [Google Scholar]
- 25.Webber SA, Naftel DC, Fricker FJ et al. Lymphoproliferative disorders after paediatric heart transplantation: a multi-institutional study. Lancet 2006;367:233–9. [DOI] [PubMed] [Google Scholar]
- 26.Sehgal S, Bock MJ, Louks Palac H et al. New-onset diabetes mellitus after heart transplantation in children - Incidence and risk factors. Pediatr Transplant 2016, 20:963–969. [DOI] [PubMed] [Google Scholar]
- 27.Chanchlani R, Joseph Kim S, Kim ED et al. Incidence of hyperglycemia and diabetes and association with electrolyte abnormalities in pediatric solid organ transplant recipients. Nephrol Dial Transplant 2017;32:1579–1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hathout EH, Chinnock RE, Johnston JK et al. Pediatric post-transplant diabetes: data from a large cohort of pediatric heart-transplant recipients. Am J Transplant 2003;3:994. [DOI] [PubMed] [Google Scholar]