Abstract
Background:
Time limited eating (TLE) has been shown to be effective for weight loss and improvement of glycemic control in adults with obesity and type 2 diabetes (T2D), but has not been well studied in adolescents. TLE may be a more feasible, flexible and effective dietary intervention for adolescents because it removes the need for intensive counting of calories or macronutrients, and emphasizes eating during a specified time period.
Objectives:
The aim of this study is to assess the feasibility of a TLE approach in adolescents with obesity using a continuous glucose monitor (CGM) to promote adherence to the intervention.
Methods:
We propose a prospective, randomized controlled trial, in 60 adolescents (ages 14–18) with obesity (BMI% ≥ 95th percentile). Youth will be randomized to one of three treatment groups for a 12-week intervention: Group 1) Low sugar and carbohydrate education (LSC, 5% of total daily calories from sugar (< 35 g)/day; < 90 g carbohydrate (CHO)/day) + blinded CGM (used to monitor adherence and glycemic outcomes without real time feedback), Group 2) LSC + TLE (16-h fast/8-h feed for 5 days per week) + blinded CGM, and Group 3) LSC + TLE+ real time feedback via CGM (to evaluate effect of providing CGM data on intervention efficacy). Outcomes will include change in total body fat (TBF) percentage measured on DEXA scan, BMI status and fasting blood glucose at 12 weeks compared to baseline.
Conclusions:
TLE is a potentially powerful lifestyle intervention that could be readily integrated into pediatric weight management programs to optimize their impact and accelerate healthy changes.
Clinical trial registration:
ClinicalTrials.gov identifier: NCT03954223
Keywords: Time limited eating, Continuous glucose monitoring, Obesity, Pediatrics
1. Introduction
The majority of adolescents with obesity demonstrate declining beta cell (β-cell) function and progressive insulin resistance over their lifetime. [1,2,3] In our population of lower income minority adolescents, 1 in 3 has obesity or severe obesity, and of those 30–50% go on to develop pre-diabetes (PD) or type 2 diabetes (T2D) as young adults. [4,5] Although diet and increased adiposity play a significant role in the pathogenesis of PD and T2D, the standard treatment model of intensive lifestyle modifications often result in modest decreases in adiposity and BMI z-score. [6] Therefore further explorations into innovative dietary strategies that reduce adiposity and promote euglycemia are needed to disrupt the natural progression of disease in this high-risk patient population.
Novel dietary approaches like time limited eating (TLE) have been shown to be effective for weight loss and improve glycemic control in adults with obesity and T2D but have not been well studied in adolescents. [7-11] One TLE approach involves interspersing normal daily caloric intake with 16-h periods of calorie restriction/fasting several times a week. [11,12] TLE may actually be more feasible, non-stigmatizing, flexible, and effective for adolescents than alternative dietary approaches, such as severe caloric restriction. The TLE approach removes the need for intensive counting of daily caloric intake or macronutrient content and focuses on a straightforward task of consuming food during a pre-specified time period. [13,14]
One major limitation to implementing any dietary intervention in pediatric populations is concern for poor adherence and difficulty in reliably assessing compliance. [14-16] We aim to overcome these issues with the use of continuous glucose monitoring (CGM) to monitor and promote adherence to the intervention and thus improve overall efficacy. [17] In addition, the use of CGM will provide important outcome data related to overall glycemic response.
In this manuscript, the authors describe the design and rationale for a prospective randomized trial of an innovative, dietary intervention incorporating nutrition education, TLE, and CGM use for the management of adolescents with obesity. The objective of this clinical trial is to assess the feasibility and efficacy of a TLE approach in this population and determine whether the use of CGM promotes adherence to the intervention. We will also evaluate whether providing individual feedback to participants based on their CGM data enhances efficacy.
2. Methods
2.1. Overall study design and location
This study was designed as a prospective, randomized controlled pilot study in 60 adolescents (ages 14–18) with obesity (BMI% ≥ 95th percentile) recruited from clinical programs at Children's Hospital Los Angeles (CHLA) (See Fig. 1). Patients will be randomized to one of three treatment groups for a 12-week intervention:
Fig. 1.
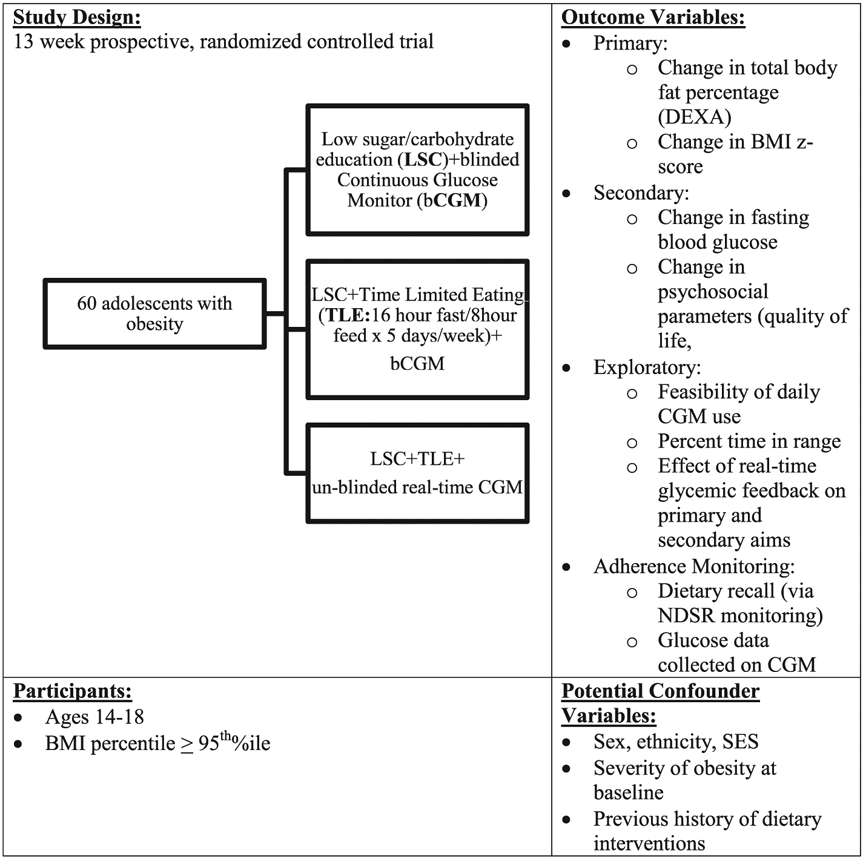
Study design overview and enrollment flow.
Group 1): Control - Low sugar and carbohydrate educational intervention (LSC, < 5% of total daily calories from sugar/day; < 90 g carbohydrate (CHO)/day) + blinded CGM (used to monitor adherence and glycemic outcomes without real time feedback).
Group 2): LSC + TLE (16-h fast/8-h feed for 5 days per week) + blinded CGM.
Group 3): LSC + TLE+ real time feedback via CGM (to evaluate the effect of providing CGM data on intervention efficacy).
The protocol was designed to build on prior evidence in adult populations while being culturally relevant and sensitive to the type and severity of behavioral symptoms related to weight management in adolescents. [18-20]
Study procedures were approved by the CHLA Institutional Review Board and is in accordance with the Helsinki Declaration of 1975, as revised in 2008. The study will be reported according to the Consolidated Standards of Reporting Trials (CONSORT) statement for randomized trials of nonpharmacological treatments and is registered with ClinicalTrials.gov (NCT03954223).
2.2. Intervention design (see Fig. 1)
2.2.1. Participant recruitment and eligibility criteria
Clinical research coordinators (CRC) will introduce the study to the youth/family member dyad recruited over the phone, in-person or by email. Youth interested in participating will be scheduled for a face-to-face visit in which written informed consent will be obtained from the adolescent and one parent, guardian or family member (for participants ages 14–17 years). Additionally, written informed consent is required for participating family member. Eligible participants are adolescents, ages 14–18 years with BMI ≥95th percentile for age and sex, with a personal smart phone, and at least one parent, legal guardian or family member willing to participate in the 12-week intervention (Table 1). Youth will be excluded if they: 1) have previous diagnosis of Prader Willi Syndrome, brain tumor or hypothalamic obesity; 2) have serious developmental or intellectual disability or previously diagnosed eating disorder or positive screen at consent visit; 3) are unable to participate in the assessments (e.g. inability to wear CGM, inability to be in the imaging modality without sedation); 4) previously underwent bariatric surgery; 5) currently use medication that impacts weight or executive functioning (e.g., antipsychotics, sedatives, hypnotics, off-label obesity medication, insulin); 6) currently participate in psychotherapy regarding weight or eating behavior, including diagnosis of binge eating disorder; or 7) currently participate in other interventional studies or clinical treatment related to weight management. In our experience, children younger than 14 years of age and older than 18 years would require different intervention/counseling strategies. Therefore, we can develop a more consistent “age-neutral” approach if we limit the age range to 14–18 years.
Table 1.
Schedule of events.
| Weeks | −1 | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| In Person Visit Number: | 1 | 2 | 3 | 4 | 5 | |||||||||
| Study Window (days) | 1 | 3 | 3 | 3 | ||||||||||
| Consent Completed | x | |||||||||||||
| CGM Education Session | x | |||||||||||||
| CGM Placed | x | |||||||||||||
| Randomized | x | |||||||||||||
| Dietary Education | x | |||||||||||||
| Weekly phone calls: | x | x | x | x | x | x | x | x | x | |||||
| Surveys | ||||||||||||||
| Demographic information | x | |||||||||||||
| Pediatric Quality of Life | x | x | x | x | x | |||||||||
| Physical Activity | x | x | x | x | x | |||||||||
| Binge Eating Disorder | x | x | x | x | x | |||||||||
| Center for Epidemiological Studies Depression Scale | x | x | x | x | x | |||||||||
| Perceived Stress Scale | x | x | x | x | x | |||||||||
| Satisfaction Survey Teen | x | x | x | |||||||||||
| NSDR: 24-h dietary recall (3 days: weekday, weekend, TLE day for Group 2/3) | x | x | x | |||||||||||
| Anthropometrics: teen | x | x | x | x | x | |||||||||
| Anthropometrics: family | x | x | ||||||||||||
| Imaging: | ||||||||||||||
| DEXA Scan: Total body fat % | x | x | ||||||||||||
| CGM Data: | ||||||||||||||
| Average fasting blood glucose | x | x | x | x | x | |||||||||
| Time in range | x | x | x | x | x | |||||||||
| Adherence to TLE-number of glycemic excursions of > 80 mg/dL during reported fasting periods | x | x | x | x | x | |||||||||
| Labs: | ||||||||||||||
| Metabolic biomarkers- Hemoglobin A1c, Insulin, c-peptide, Lipid Panel, Liver Function Tests, and Fasting glucose, c-reactive protein (CRP), uric acid, leptin | x | x | ||||||||||||
| Compensation: | x | x | x | x | x |
Participants will be recruited from: 1) clinical programs at CHLA, 2) the surrounding community and 3) a direct mailing campaign. The research team will partner with community centers and programs such as libraries and recreational centers and place recruitment flyers in these locations. A direct mailing campaign will be utilized to send 10,000 recruitment letters to families with adolescents ages 14–18 years across 40 neighborhoods in Los Angeles County.
2.2.2. Randomization
Randomization will be at the level of the youth utilizing block (block size = 3 and 6) and stratified randomization to ensure the groups are balanced in terms of number of participants and the distribution of potential confounding variables such as sex and ethnicity. Adolescents (n = 60) will be randomly assigned 1:1:1 to one of the intervention arms.
2.3. Intervention components
Table 2 outlines the components of the three intervention arms. All participants will receive nutrition education delivered by the CRC emphasizing a LSC approach. The education session will occur at the week 0 study visit and last approximately 120 min. Participants will be randomized to LSC alone or LSC in addition to TLE at the week 0 study visit. Participants will choose and pay for their own food for the entire intervention. Research visits will be scheduled at 0, 4, 8 and 12 weeks and last 120 min (5 total visits, including initial consent visit; See Table 1). The CRC will document BMI status and participants will complete surveys at each visit. Additionally, the CRC will encourage adherence to the intervention using a coaching model designed to improve dietary compliance, lifestyle change and weight loss. The CRC will discuss the study components and participant barriers to adherence in scheduled weekly phone calls. The CRC will download CGM data and review percent wear, glucose time in range and average fasting blood glucose levels for all 3 groups. Participants in group 3 will have access to real time CGM data for their review. All participants will receive standard advice for physical activity, screen and sleep time as per the American Academy of Pediatrics age-appropriate recommendations [21].
Table 2.
Intervention arm components.
| LSCa + bCGMb | LSC + TLEc + bCGM | LSC + TLE + rCGMd | |
|---|---|---|---|
| Education covering reducing total daily added sugar consumption | x | x | x |
| Limiting sugar-sweetened beverages | x | x | x |
| Education covering reducing total daily carbohydrate intake | x | x | x |
| Motivational interviewing | x | x | x |
| Time Limited Eating (8 h eating period for 5 days/ week) | x | x | |
| Participant compensation | x | x | x |
| Family member involvement | x | x | x |
| Wearing continuous glucose monitor (CGM) | x | x | x |
| Access to real-time glycemic data from CGM | x |
Low sugar and carbohydrate educational intervention (LSC): < 35 g per day, approximately 5% of total daily calories from sugar/day; < 90 g carbohydrate (CHO)/day.
blinded Continuous Glucose Monitor (bCGM).
Time Limited Eating (TLE): 16-h fast/8-h feed for 5 days per week.
CRCs will be required to have a Bachelor's degree and at least one year experience in clinical trial coordination and complete a structured training program. CRCs will come from a variety of backgrounds and professional experiences. CRCs are not required to have achieved or maintained significant weight loss themselves as previous research on coaching interventions suggests that characteristics such as communication skills and empathy are more crucial to success in this role. To assess intervention fidelity, each month the PI will observe phone conversations and in-person study visits on 5 participants from each intervention group.
2.3.1. Participant CRC interactions
The CRC and adolescent will interact via monthly face-to-face visits (approximately 2 h each) and 12 weekly phone calls (15 min each). Participants will complete validated surveys at each visit and CRCs will use a qualitative semi-structured interview utilizing motivational interviewing (MI) techniques to elicit both positive and negative impacts on weight management, the TLE approach and CGM use, and to identify barriers such as emotional eating, poor coping skills for life stressors and social challenges.
Each CRC will have a cell phone used solely for the purpose of this study so that participants may directly contact their CRC directly regarding any questions or concerns. Phone calls and text messages will be utilized for appointment reminders, scheduling weekly phone meetings, providing emotional support, and following up on items discussed in a prior visit or phone call [5]. All interactions will be documented in a database created for the study, including date, time, type, and duration of contact, topics discussed, and any relevant notes.
The CRCs delivering the curriculum will receive 10 h of training over a 4-week period from the principal investigator and dietitian. The training will cover: 1) the importance, rationale and education about the TLE approach, 2) the concept of behavior change, patient-centered approach and motivational-interviewing (MI) techniques, and 3) practical advice specific to each stage of the intervention and ways to assist the youth with common barriers and triggers. The curriculum will cover active listening, non-judgmental communications, MI techniques, and creating self-management goals. In addition, the CRCs will be required to demonstrate understanding of the LSC curriculum, TLE approach and CGM use through simulated role-plays and observations of CRC-Participant sessions.
2.3.2. Low sugar and carbohydrate education curriculum
The LSC curriculum is modeled after two nutrition curriculums: 1) Kids N Fitness© (KNF) an evidence-based, weight management program for children with overweight and obesity and their families [22-24] and 2) the Healthy Eating through Reduction of Excess Sugar (HEROES) intervention (NIH R01 MD010358-A1) which is currently used by Dr. Michael Goran's lab at CHLA as a method to reduce sugar intake in diets of youth with fatty liver disease. The intervention consists of recommendations for low added sugars (< 35 g per day, approximately equal to 5% of daily energy for the average teenager and roughly equivalent to the recommendations from the American Heart Association) [25] and a moderate carbohydrate-restricted (< 90 g per day) daily diet [26]. No specific caloric restriction will be recommended and participants will not be required to keep logs of daily consumptions. Participants will be provided with general recommendations for low sugar and low carbohydrate food options to reference. Recommendations will be made for the avoidance of sugar-sweetened beverages, including juices, and unhealthy food options high in added sugars (Table 2).
2.3.3. Time limited eating
In this intervention arm, participants will receive the LSC education with additional instructions to consume all of their food in an eight hour time window (i.e. from 11 AM to 7 PM) five days per week. Participants will select their eating window based on feasibility and normal daily routine. They will be required to set the time window at the consent visit. Non-caloric beverages (water, tea, coffee) will be allowed during the fasting period. No energy restriction will be used, but participants will be given the LSC guidelines based on age-appropriate dietary guidelines (Table 2).
2.3.4. CGM training
Participants will wear Dexcom G6 continuous glucose monitors (CGM, Dexcom, San Diego, CA, USA) continuously for 13 weeks (week –1 to week 12 study period). All participants will be blinded to CGM for seven days prior to commencement of the dietary intervention for baseline data collection, and then randomized to one of the three intervention arms. Participants and their family member will be educated on the use of the Dexcom G6 Mobile CGM system at the first visit. Participants will receive training on the system using prepared training materials. All initial sensor insertions will be performed at the clinic by participants and/or family member, under the supervision of the CRC, using the automated sensor applicator. The sensor will be changed every 10 days. Diurnal glucose profiles, derived from interstitial fluid readings performed every 5 min over back-to-back 10-day cycles for 91 days will be collected using the CGM device. The CGM data will be uploaded to the cloud platform, Clarity, into a research clinic group to be reviewed by the research team.
The Dexcom app will be downloaded to the subject and family members' personal smart phone and an account will be created with a pseudonym of the subjects' choice. Participants in Group 1 and 2 will be blinded to the CGM data. Participants in Group 3 will utilize a personal smart phone with Bluetooth to provide real time blood glucose levels throughout the 12-week intervention period. Remote monitoring will be set up for all participants and their family member. The CRC will have access to this data and analyze trends to evaluate adherence, which will be reviewed with the participants and their family members at face to face visits. Any glycemic excursions noted during self-reported periods of fasting will be reviewed and discussed to evaluate barriers to adherence. Participants will be instructed to contact the CRC for any concerns with the device. If blood glucose levels are higher than the current diagnostic criteria for diabetes in adolescents (fasting blood glucose ≥126 mg/dL or random blood glucose > 200 mg/dL with symptoms of polyuria or polydipsia), the families will be instructed to take their adolescents to their primary provider or the emergency department for appropriate evaluation and endocrine referral as needed.
2.4. Measurements
CRCs will conduct all assessments at baseline, 4, 8 and 12 weeks post consent. All data will be collected and stored in REDCap.
2.4.1. Primary outcomes
2.4.1.1. Assessment of total body fat content.
Total body fat (TBF) will be measured by total body dual-energy x-ray absorptiometry (DEXA scan) using a Hologic QDR 5400 densitometer (Hologic, Inc., Bedford, MA). TBF will be expressed as fat mass (kg) and percentage of total body mass (BF%). DEXA also provides measures of total lean mass and bone mass.
2.4.1.2. Participant's weight change.
Height and weight will be assessed at all in-person visits with the on-site CRC for all participants. Height will be measured using a Quick Medical stadiometer, accurate to 0.1 cm (Quick Medical, Issaquah, WA). Weight will be measured on a self-calibrating Mobile Stand Digital Scale, accurate to 0.1 kg. Participants will wear minimal clothing during the height and weight measurements. BMI will be calculated as kilograms per meter squared from which zBMI and percent over the 95th percentile (%BMIp95) will be determined utilizing the CDC growth charts.
2.4.2. Secondary outcomes
2.4.2.1. Adherence. Nutrient Data System Recall (NDSR) 24 Hour Dietary Recall [27-29]
Twenty-four-hour dietary recalls will be conducted for all participants at 3 time points throughout the study. For those in group 1, one weekday and one weekend day will be collected. For those in group 2 and 3, one TLE day and one non-TLE day will be collected. One recall will be completed at each study visit, in-person, for the day prior to the visit and the second recall will be completed over the phone to ensure the recalls are capturing the 24-h period prior to completion of the recall. The procedures used in the 1985–86 United States Department of Agriculture Continuing Survey of Food Intakes of Individuals (USDA-CSFII) will be followed and all recalls will be collected in a personal interview using a standardized protocol based on the “multiple pass” method which was developed and tested by the USDA for use in the 1994–1996 CSFII in an effort to limit the extent of under-reporting. Data will be collected and compiled using the most current version of the Nutrient Data System Recall (NDSR). NDSR, which was developed by the Nutrition Coordinating Center, University of Minnesota, is considered the ‘gold standard' of nutrition databases for 24-h recall dietary assessment. The NDSR time-related database updates analytic data while maintaining nutrient profiles true to the version used for data collection, thereby reflecting the marketplace throughout the study. The CRC will ask the participant to list in sequence all foods and beverages consumed during the previous day, review the list with the participant to identify anything not report in the initial list, and then obtain details (e.g., portion sizes, brand names, preparation methods) for each item.
2.4.2.2. Retention.
Data relating to participant attendance with scheduled research visits and phone calls will be tracked remotely and stored on HIPAA-compliant central servers. Data will be reported on the three key components specific to the intervention: 1) adherence with dietary intervention group, 2) adherence with use of CGM, and 3) satisfaction with intervention. We will also use strategies to increase retention, including: incentives for participation and adherence; frequent contacts with participants to maintain engagement and foster open communication; email and text reminders about face-to-face visits; and recording contact information of relatives or friends to be able to reach participants.
2.4.2.3. Change in metabolic parameters.
The following laboratory samples will be obtained after an 8-h overnight fast at baseline and 12 weeks: hemoglobin A1c, insulin, c-peptide, c-reactive protein (CRP), uric acid, leptin, fasting blood glucose (FBG), lipid profile, alanine aminotransferase (ALT), and aspartate aminotransferase (AST). FBG, total cholesterol, high density lipoprotein (HDL) cholesterol, and triglycerides (TG) will be measured via Vitros 960 colorimetric assay, and low density lipoprotein (LDL) cholesterol will be calculated. Fasting insulin level will be obtained and the homeostatic model assessment of insulin resistance will be calculated. Hemoglobin A1c will be measured using a DCA 2000 (Bayer Corporation, Elkhart, IN) [30,31].
2.4.2.4. Continuous glucose monitor data evaluation.
Dexcom G6 Mobile CGM system will be utilized. The automated sensor applicator measures 65.7 × 115.8 × 49.8mm (H × W × L) and features a single button that deploys the sensor and retracts the introducer needle when pressed. All participants will use the G6 system for 10-day wear periods (up to 240h). CGM data will be downloaded at each face-to-face visit by the CRC. CGM data from the 7 days prior to the intervention commencement and the final 7 days of the intervention will be used as the pre-post study outcomes respectively. This data will be utilized to compute the following measures: mean, maximum, and minimum glucose levels, standard deviation of glucose, mean amplitude of glycemic excursion, and overall percent of total time spent in euglycemic range.
2.4.3. Covariates
2.4.3.1. Demographics and medical history.
At the consent visit, we will assess participant demographics, completed by the family member, including family member's age, family member and adolescent's race/ethnicity, household composition, socioeconomic status (education, income), as well as family and adolescent's medical history. These variables will be entered as covariates in the analysis.
2.4.3.2. Family member's weight.
One family member's weight and height will be measured at consent and 12 weeks using a Quick Medical stadiometer, accurate to 0.1 cm (Quick Medical, Issaquah, WA). Weight will be measured on a self-calibrating Mobile Stand Digital Scale, accurate to 0.1 kg. BMI will be calculated as kilograms per meter squared.
2.4.4. Moderators
2.4.4.1. Psychosocial and behavioral metrics of disease.
Participants will complete a battery of self-reported questionnaires, described below.
2.4.4.2. Pediatric quality of life scale (PedsQL).
Quality of life will be measured utilizing the Pediatric Quality of Life Scale (PedsQL) which is a 10-item questionnaire designed to assess quality of life parameters for youth under 19 years of age [32]. The PedsQL is a brief, standardized, generic assessment instrument that systematically assesses patients' and parents' perceptions of health-related quality of life (HRQOL) in pediatric patients. The PedsQL is based on a modular approach to measuring HRQOL and consists of a 15-item core measure of global HRQOL and eight supplemental modules assessing specific symptom or treatment domains. [32]
2.4.4.3. Patient reported outcomes measurement information system (PROMIS®): physical activity.
PROMIS was a National Institutes of Health initiative to create patient-reported outcomes (PROs) to assess domains of physical, psychological, social health, and quality of life [33,34]. The PROMIS® instruments were developed using rigorous qualitative and quantitative methods [17] and standardized to a reference population. [18] To date, the pediatric PROMIS® instruments have been implemented in various pediatric populations [33].
The PROMIS® measures for children have been found to demonstrate feasibility, internal consistency, construct validity, and responsiveness to change in a clinical setting. The Physical Activity Questionnaire component of PROMIS® will be utilized to evaluate self-reported levels of physical activity in all study participants. PROMIS® pediatric instruments have been developed and validated to assess domains of health in children less than 18 years of age. The physical activity survey is a self-administered, 7-day recall instrument developed to assess general levels of physical activity of children and adolescents [33,35].
2.4.4.4. The center for epidemiologic studies depression scale (CES-DC).
The Center for Epidemiological Studies Depression Scale for Children (CES-DC) will be utilized to measure baseline depression and anxiety and monitor any changes associated with the intervention. The CES-DC has been developed and validated in children younger than 18 years of age. The CES-DC is a 20-item self-report scale designed to measure depressive symptoms in children and adolescents [36]. The CES-DC has been reported to be a highly reliable and valid for assessing depressive symptoms across ethnic, gender and age groups [37]. Cronbach Alpha coefficient for the scale was reported to be 0.85 for the general population. In the recent studies Cronbach Alphas have been reported to range from 0.79 to 0.91 [37]. Test-retest reliability of the scale was reported to be 0.79 in 15-year-old Guatemalan adolescents [38]. Concurrent, divergent and convergent validity of the CES-DC has also been reported in numerous studies [39].
2.4.4.5. The perceived stress scale.
The Perceived Stress Scale (PSS) is widely used for measuring psychological distress. PSS measures the participant's subjective perceptions of distress in life situations. Based on Lazarus' transactional model of stress and coping, the PSS assesses how well a person feels they can cope with stressors, rather than measuring the nature of the stressors themselves. The measure has demonstrated strong reliability (r > 0.80) and validity in social and health science studies [40].
2.4.4.6. Binge eating disorder screener (BEDS).
Given that adolescents with obesity are at high risk of binge-eating disorder (BED) symptoms; our goal is to screen participants at baseline to ensure appropriate referrals are made in a timely manner. In addition, we aim to monitor for BED symptomatology throughout the study period. Binge Eating Disorder Screener (BEDS-7) is a brief, valid, patient-reported screening tool for use in primary care and general psychiatry settings to identify individuals most likely to have BED and to facilitate further evaluation or referral to specialists. It is validated in youth ages 12–21 years [41,42].
2.4.4.7. Focus groups.
Upon conclusion of the study period, all participants will participate in a one-time, thirty minute focus group conducted by a behavioral psychologist and/or CRC. We aim to conduct three focus group sessions with 5–6 adolescents per group. The aim of the focus group session will be to explore participants' knowledge, perspective and attitudes towards their experience in the study. The focus group will include three core topics: 1) the TLE based approach; 2) CGM use in youth with obesity; 3) general perceptions of intervention-focused weight management strategies in youth with obesity. The sessions will be audio recorded and transcribed and pertinent themes will be identified.
2.4.4.8. Intervention compensation.
Each youth participant, regardless of which group they are assigned to, will receive compensation in the form of gift cards. Each youth participant will receive $25 at each study visit they attend (5 visits in total, for a total of $125 per participant). Parents/guardians/family members will also receive $15 gift card for each study visit they attend for a total of $75 for participation in the study to account for time and effort required to support their participant. Parking validation will be provided on an as-needed basis upon request. All families who do not have a car will be offered free ride-sharing transportation via UBER to- and from study visits upon request.
2.5. Analytic plan and power analysis
2.5.1. Power and sample size estimates
The study aims to examine three main outcomes: (1) the difference in the mean change of TBF, as measured by DEXA scan, from baseline to post evaluation at 12 weeks across all three groups, (2) the difference in mean change of zBMI and excess percent of the 95th percentile (%BMIp95) from baseline to post evaluation at 12 weeks across all three groups, and (3) the difference in mean change of TBF and BMI status (zBMI/%BMIp95) between Groups 2 and 3.
This study will enroll 60 participants and randomize via block randomization between the three intervention arms. All calculations utilize an estimate of standard deviation of change based on a difference of 0.5 between pre- and post-measurement within each group [13,43,44]. For Aim 1, based on Analysis of Variance (ANOVA) with two-sided Type I error of 5%, this study has 85% power to detect an effect size of 0.50 with the variance means of 2.67 across three study groups to evaluate the difference in the mean change of TBF as measured by DEXA from baseline to 12 weeks. For Aim 2, based on ANOVA with two-sided Type I error of 5%, this study has 81% power to detect an effect size of 0.47 with the variance of the group means of 0.0004 across three study groups for the mean change of zBMI and %BMIp95 from baseline to 12 weeks. Aim 3 is an exploratory aim which seeks to assess the differences in mean TBF percentage and zBMI/%BMIp95 change from baseline to post intervention between Group 2 and 3. Although the proposed sample size does not have sufficient study power of 80% to detect this difference, it will be valuable information to capture at least a 10% change in zBMI from baseline to 12 weeks between these two groups. Assuming 20% attrition rate, this study will achieve the desired study power of 80% for study aims 1 and 2 (nQuery + nTerim 4.0 and Stata/SE 15.1).
2.5.2. Statistical analysis
To evaluate baseline comparability, the three randomized groups will be compared with respect to demographics, lifestyle, and clinical characteristics using t-tests (or non-parametric comparisons) and Chi-Square tests. The primary objective of this analysis is to assess the difference in TBF percentage and zBMI/%BMIp95 between baseline and the end of the intervention (12 weeks) using intention to treat (ITT) and per protocol approaches. The mean change of these endpoints will be compared between baseline and 12 weeks across three study groups by using ANOVA. Multivariable regression analysis using a mixed-effects model will be utilized to appropriately account for repeated measures and assess the effect of the possible factors in the mean change in TBF percentage and zBMI/%BMIp95 accounting for demographic characteristics and other measured clinical parameters. Additionally, the moderator effect of ethnicity and sex will be also evaluated by including the interaction terms between ethnicity and treatment arms into the model to assess whether the change in the endpoints between the two treatment arms depends on ethnicity as well as sex. The statistical significance is set with a two-sided test at 5% level throughout the analysis.
3. Discussion
This research study represents a unique opportunity to introduce youth with obesity to a novel clinical application of TLE that is straightforward, flexible, non-stigmatizing and feasible to implement in a real-life setting. In addition, a TLE approach has the potential to change the trajectory of their underlying disease process. This pilot study will provide essential formative information that could change the way we educate and treat adolescents with obesity regarding eating as part of their medical regimen. This research will generate new knowledge that can readily be integrated into pediatric weight management programs to optimize their impact and accelerate healthy changes for all youth with obesity.
To our knowledge, this study is the first randomized controlled trial performed in the United States to explore the feasibility and efficacy of a TLE based approach in adolescents. In 2019, Jebeile et al. investigated the feasibility, effectiveness, and acceptability of intermittent energy restriction (IER) in 45 adolescents (aged 12–17 years) with obesity in Australia [13]. The results demonstrated that after the initial intervention phase, participants self-selected to stay in the IER dietary intervention, indicating feasibility, and that %BMIp95 was reduced at 12 and 26 weeks compared to baseline. The current study will contribute to the understanding of the metabolic impact of a TLE approach on TBF, weight and metabolic factors impacting youth with obesity in Southern California. The collection of psychosocial factors including feasibility, satisfaction and impact of the approach on quality of life will provide important information about the feasibility, tolerability and safety of this intervention approach, which will help guide dissemination into clinical practice.
Moreover, by utilizing the data collected from CGM, this study has the potential to uncover unknown data regarding the glycemic excursions seen in youth with obesity prior to the diagnosis of T2D and assess how dietary interventions can delay β-cell deterioration. Although CGM are utilized in routine clinical care for youth with type 1 diabetes, this research represents an unparalleled incubator to test the use of CGM as an intervention tool for monitoring adherence and promoting sustained engagement. This is a novel method to utilize glycemic excursion as a metric of dietary intervention adherence in youth with obesity. In addition, the immediate biofeedback obtained from the CGM may improve the efficacy of the dietary intervention by providing the participant with immediate feedback relating to their food consumption.
There are several potential challenges and considerations related to this work. First, as this is only the second RCT to assess a TLE based approach in adolescents with obesity, we considered excluding teens with severe obesity, given that recent literature has demonstrate this group of adolescents are the most refractory to dietary intervention [45]. However, the researchers felt that as this is a pragmatic study design, all youth should be encouraged to participate regardless of their baseline BMI status as the targeted behaviors are relevant and beneficial for all youth with obesity, including those with severe obesity.
Secondly, the study cannot be adequately powered to analyze the difference in outcomes between those receiving blinded and un-blinded CGM data (groups 2 vs. 3). The study is powered to assess the primary outcome of interest which is the comparison of change in TBF percentage and BMI status between groups 1 and 2. Therefore the evaluation of the impact of real time feedback from the CGM will be utilized to inform future implementation research.
Third, we recognize that the adherence verification model of utilizing dietary recall matched to CGM data is not without its limitations. Due to feasibility and logistical implementation components the dietary recall will only be performed at three time points and is limited by the participants' recall. Additionally, although CGM data should be able to capture large glycemic excursions that occur with meals, meals low in carbohydrates may be hard to confirm based on glycemic excursion alone and therefore accuracy of intervention adherence in that case will be left to participant report. It is difficult to accurately track adherence to dietary interventions in the pediatric population. It is our hope that by utilizing the combination of dietary recall and CGM data, we will be able to provide a reasonable estimate of adherence in our population which will strengthen our outcome analysis and conclusions.
Finally, the proposed study design does not include a group of age-matched adolescents who do not receive a weight management intervention. This type of control group is difficult to identify and follow longitudinally. However, the current literature of pediatric obesity interventions and the natural trajectory for youth with obesity suggest that the weight velocity of many youth not receiving any intervention continues to increase. [46]
4. Conclusions
There is a high likelihood that adolescents with obesity will grow into adults with obesity. Unfortunately, there remains a dearth of intervention options for adolescents with obesity and a continued lack of understanding as to how best to personalize obesity interventions to optimize outcomes. Positive results from this study will have important implications for the management of youth with obesity. The proposed TLE based intervention removes many barriers that impact adherence and sustainability (e.g. need for consistent caloric counting or macronutrient monitoring) in conventional dietary recommendations. Furthermore, this approach strategically incorporates the underlying developmentally and socially acceptable eating window for many adolescents to better support habit formation in youth in their day-to-day environment.
Acknowledgements
This work was supported in part by grants 1) UL1TR001855 from the National Center for Advancing Translational Science (NCATS) of the U.S. National Institutes of Health and 2) NIH/NCRR SC-CTSI Grant Number UL1 TR000130. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Funding source
The Saban Research Institute of Children's Hospital Los Angeles.
UL1TR001855 from the National Center for Advancing Translational Science (NCATS) of the U.S. National Institutes of Health.
NIH/NCRR SC-CTSI Grant Number UL1 TR000130.
Abbreviations:
- TLE
Time Limited Eating
- CGM
Continuous Glucose Monitor
- LSC
Low Sugar Carbohydrates
- T2D
Type 2 Diabetes
- PD
Pre-Diabetes
- BMI
Body mass index
- zBMI
Body mass index Z-score
- %BMIp95
Percent over the 95th percentile
- Coef
Coefficient
- CI
Confidence Interval
Footnotes
Financial disclosure
The authors have no financial relationships or conflict of interest relevant to this article to disclose.
Declaration of Competing Interest
The authors have no financial relationships or conflict of interest relevant to this article to disclose.
References
- [1].Fox CK, Gross AC, Bomberg EM, Ryder JR, Oberle MM, Bramante CT, Kelly AS, Severe obesity In the pediatric population: current concepts In clinical care, Curr. Obes. Rep 8 (3) (2019) 201–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Salsberry P, Tanda R, Anderson SE, Kamboj MK, Pediatric type 2 diabetes: prevention and treatment through a life course health development framework, In: Halfon N, Forrest CB, Lerner RM, Faustman EM (Eds.), Handbook of Life Course Health Development, Springer, 2018, pp. 197–236 Copyright 2018, The author(s). Cham (CH). [PubMed] [Google Scholar]
- [3].Cree-Green M, Wiromrat P, Stuppy JJ, Thurston J, Bergman BC, Baumgartner AD, Bacon S, Scherzinger A, Pyle L, Nadeau KJ, Youth with type 2 diabetes have hepatic, peripheral, and adipose Insulin resistance, Am. J. Physiol. Endocrinol. Metab 316 (2) (2019) E186–e195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Viner R, White B, Christie D, Type 2 diabetes In adolescents: a severe phenotype posing major clinical challenges and public health burden, Lancet 389 (10085) (2017) 2252–2260. [DOI] [PubMed] [Google Scholar]
- [5].Twig G, Reichman B, Afek A, Derazne E, Hamiel U, Furer A, Gershovitz L, Bader T, Cukierman-Yaffe T, Kark JD, Pinhas-Hamiel O, Severe obesity and cardio-metabolic comorbidities: a nationwide study of 2.8 million adolescents, Int. J. Obes 43 (7) (2019) 1391–1399. [DOI] [PubMed] [Google Scholar]
- [6].Inge TH, Laffel LM, Jenkins TM, Marcus MD, Leibel NI, Brandt ML, Haymond M, Urbina EM, Dolan LM, Zeitler PS, Comparison of surgical and medical therapy for type 2 diabetes in severely obese adolescents, JAMA Pediatr. 172 (5) (2018) 452–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].de Cabo R, Mattson MP, Effects of Intermittent fasting on health, Aging, Disease, N Engl J Med 381 (26) (2019) 2541–2551. [DOI] [PubMed] [Google Scholar]
- [8].Gabel K, Hoddy KK, Varady KA, Safety of 8-h time restricted feeding In adults with obesity, Appl. Physiol. Nutr. Metab 44 (1) (2019) 107–109. [DOI] [PubMed] [Google Scholar]
- [9].Fitzgerald KC, Vizthum D, Henry-Barron B, Schweitzer A, Cassard SD, Kossoff E, Hartman AL, Kapogiannis D, Sullivan P, Baer DJ, Mattson MP, Appel LJ, Mowry EM, Effect of Intermittent vs. daily calorie restriction on changes In weight and patient-reported outcomes In people with multiple sclerosis, Mult Scler Relat Disord 23 (2018) 33–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ganesan K, Habboush Y, Sultan S, Intermittent fasting: the choice for a healthier lifestyle, Cureus 10 (7) (2018) e2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Gabel K, Hoddy KK, Haggerty N, Song J, Kroeger CM, Trepanowski JF, Panda S, Varady KA, Effects of 8-hour time restricted feeding on body weight and metabolic disease risk factors in obese adults: a pilot study, Nutr Health. Aging 4 (4) (2018) 345–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Wilkinson MJ, Manoogian ENC, Zadourian A, Lo H, Fakhouri S, Shoghi A, Wang X, Fleischer JG, Navlakha S, Panda S, Taub PR, Ten-hour time-restricted eating reduces weight, blood pressure, and atherogenic lipids in patients with metabolic syndrome, Cell Metab. 31 (1) (2020) 92–104.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Jebeile H, Gow ML, Lister NB, Mosalman Haghighi M, Ayer J, Cowell CT, Baur LA, Garnett SP, Intermittent energy restriction is a feasible, effective, and acceptable intervention to treat adolescents with obesity, J. Nutr 149 (7) (2019) 1189–1197. [DOI] [PubMed] [Google Scholar]
- [14].Payne JE, Turk MT, Kalarchian MA, Pellegrini CA, Defining adherence to dietary self-monitoring using a mobile app: a narrative review, J. Acad. Nutr. Diet 118 (11) (2018) 2094–2119. [DOI] [PubMed] [Google Scholar]
- [15].Sevilla-Villanueva B, Gibert K, Sanchez-Marre M, Fito M, Covas MI, Evaluation of adherence to nutritional intervention through trajectory analysis, IEEE J Biomed Health Inform 21 (3) (2017) 628–634. [DOI] [PubMed] [Google Scholar]
- [16].Desroches S, Lapointe A, Ratte S, Gravel K, Legare F, Turcotte S, Interventions to enhance adherence to dietary advice for preventing and managing chronic diseases in adults, Cochrane Database Syst. Rev 2 (2013) Cd008722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Taylor PJ, Thompson CH, Luscombe-Marsh ND, Wycherley TP, Wittert G, Brinkworth GD, Efficacy of real-time continuous glucose monitoring to improve effects of a prescriptive lifestyle intervention in type 2 diabetes: a pilot study, Diabetes Ther 9 (4) (2019) 713–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Goran MI, Plows JF, Ventura EE, Effects of consuming sugars and alternative sweeteners during pregnancy on maternal and child health: evidence for a secondhand sugar effect, Proc. Nutr. Soc (2018) 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Shearrer GE, O’Reilly GA, Belcher BR, Daniels MJ, Goran MI, Spruijt-Metz D, Davis JN, The impact of sugar sweetened beverage intake on hunger and satiety in minority adolescents, Appetite 97 (2016) 43–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Shih M, Dumke KA, Goran MI, Simon PA, The association between community-level economic hardship and childhood obesity prevalence in Los Angeles, Pediatr Obes 8 (6) (2013) 411–417. [DOI] [PubMed] [Google Scholar]
- [21].Barlow SE, Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report, Pediatrics 120 (Suppl. 4) (2007) S164–S192. [DOI] [PubMed] [Google Scholar]
- [22].Dreimane D, Safani D, MacKenzie M, Halvorson M, Braun S, Conrad B, Kaufman F, Feasibility of a hospital-based, family-centered intervention to reduce weight gain in overweight children and adolescents, Diabetes Res. Clin. Pract 75 (2) (2007) 159–168. [DOI] [PubMed] [Google Scholar]
- [23].Wright K, Suro Z, Using community–academic partnerships and a comprehensive school-based program to decrease health disparities in activity in school-aged children, J Prev Interv Community 42 (2) (2014) 125–139. [DOI] [PubMed] [Google Scholar]
- [24].Wright K, Giger JN, Norris K, Suro Z, Impact of a nurse-directed, coordinated school health program to enhance physical activity behaviors and reduce body mass index among minority children: a parallel-group, randomized control trial, Int. J. Nurs. Stud 50 (6) (2013) 727–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kelly AS, Barlow SE, Rao G, Inge TH, Hayman LL, Steinberger J, Urbina EM, Ewing LJ, Daniels SR, Severe obesity in children and adolescents: identification, associated health risks, and treatment approaches: a scientific statement from the American Heart Association, Circulation 128 (15) (2013) 1689–1712. [DOI] [PubMed] [Google Scholar]
- [26].Zeitler P, Arslanian S, Fu J, Pinhas-Hamiel O, Reinehr T, Tandon N, Urakami T, Wong J, Maahs DM, ISPAD clinical practice consensus guidelines 2018: type 2 diabetes mellitus in youth, Pediatr. Diabetes 19 (Suppl. 27) (2018) 28–46. [DOI] [PubMed] [Google Scholar]
- [27].Johnson RK, Driscoll P, Goran MI, Comparison of multiple-pass 24-hour recall estimates of energy intake with total energy expenditure determined by the doubly labeled water method in young children, J. Am. Diet. Assoc 96 (11) (1996) 1140–1144. [DOI] [PubMed] [Google Scholar]
- [28].Feskanich D, Sielaff BH, Chong K, Buzzard IM, Computerized collection and analysis of dietary intake information, Comput. Methods Prog. Biomed 30 (1) (1989) 47–57. [DOI] [PubMed] [Google Scholar]
- [29].Sievert YA, Schakel SF, Buzzard IM, Maintenance of a nutrient database for clinical trials, Control. Clin. Trials 10 (4) (1989) 416–425. [DOI] [PubMed] [Google Scholar]
- [30].Wallace TM, Levy JC, Matthews DR, Use and abuse of HOMA modeling, Diabetes Care 27 (6) (2004) 1487–1495. [DOI] [PubMed] [Google Scholar]
- [31].Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC, Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man, Diabetologia 28 (7) (1985) 412–419. [DOI] [PubMed] [Google Scholar]
- [32].Varni JW, Seid M, Rode CA, The PedsQL: measurement model for the pediatric quality of life inventory, Med. Care 37 (2) (1999) 126–139. [DOI] [PubMed] [Google Scholar]
- [33].Cella D, Riley W, Stone A, Rothrock N, Reeve B, Yount S, Amtmann D, Bode R, Buysse D, Choi S, Cook K, Devellis R, DeWalt D, Fries JF, Gershon R, Hahn EA, Lai JS, Pilkonis P, Revicki D, Rose M, Weinfurt K, Hays R, The patient-reported outcomes measurement information system (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005-2008, J. Clin. Epidemiol 63 (11) (2010) 1179–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Cella D, Yount S, Rothrock N, Gershon R, Cook K, Reeve B, Ader D, Fries JF, Bruce B, Rose M, The patient-reported outcomes measurement information system (PROMIS): progress of an NIH roadmap cooperative group during its first two years, Med. Care 45 (5 Suppl 1) (2007) S3–s11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Cook KF, Bamer AM, Amtmann D, Molton IR, Jensen MP, Six patient-reported outcome measurement information system short form measures have negligible age- or diagnosis-related differential item functioning in individuals with disabilities, Arch. Phys. Med. Rehabil 93 (7) (2012) 1289–1291. [DOI] [PubMed] [Google Scholar]
- [36].Cosco TD, Prina M, Stubbs B, Wu YT, Reliability and validity of the center for epidemiologic studies depression scale in a population-based cohort of Middle-Aged U.S. Adults, J. Nurs. Meas 25 (3) (2017) 476–485. [DOI] [PubMed] [Google Scholar]
- [37].Faulstich ME, Carey MP, Ruggiero L, Enyart P, Gresham F, Assessment of depression in childhood and adolescence: an evaluation of the center for epidemiological studies depression scale for children (CES-DC), Am. J. Psychiatry 143 (8) (1986) 1024–1027. [DOI] [PubMed] [Google Scholar]
- [38].Berganza CE, Aguilar G, Depression in guatemalan adolescents, Adolescence 27 (108) (1992) 771–782. [PubMed] [Google Scholar]
- [39].Siu AL, Screening for depression in children and adolescents: US preventive services task force recommendation statement, Pediatrics 137 (3) (2016) e20154467. [DOI] [PubMed] [Google Scholar]
- [40].Nielsen MG, Ornbol E, Vestergaard M, Bech P, Larsen FB, Lasgaard M, Christensen KS, The construct validity of the perceived stress scale, J. Psychosom. Res 84 (2016) 22–30. [DOI] [PubMed] [Google Scholar]
- [41].Ivezaj V, White MA, Grilo CM, Examining Binge-Eating Disorder and Food Addiction in Adults with Overweight and Obesity, Obesity (Silver Spring), (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Herman BK, Deal LS, DiBenedetti DB, Nelson L, Fehnel SE, Brown TM, Development of the 7-item binge-eating disorder screener (BEDS-7), Prim. Care. Companion CNS Disord 18 (2) (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Wilkinson MJ, Manoogian EN, Zadourian A, Lo H, Fakhouri S, Shoghi A, Wang X, Fleischer JG, Navlakha S, Panda S, Ten-hour time-restricted eating reduces weight, blood pressure, and atherogenic lipids in patients with metabolic syndrome, Cell Metab. 31 (1) (2020) 92–104. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Chow LS, Manoogian ENC, Alvear A, Fleischer JG, Thor H, Dietsche K, Wang Q, Hodges JS, Esch N, Malaeb S, Harindhanavudhi T, Nair KS, Panda S, Mashek DG, Time-restricted eating effects on body composition and metabolic measures in humans who are overweight: a feasibility study, Obesity (Silver Spring) 28 (5) (2020) 860–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Ryder JR, Fox CK, Kelly AS, Treatment options for severe obesity in the pediatric population: current limitations and future opportunities, Obesity (Silver Spring) 26 (6) (2018) 951–960. [DOI] [PubMed] [Google Scholar]
- [46].Styne DM, Arslanian SA, Connor EL, Farooqi IS, Murad MH, Silverstein JH, Yanovski JA, Pediatric obesity-assessment, Treatment, and Prevention: An Endocrine Society Clinical Practice Guideline, J. Clin. Endocrinol. Metab 102 (3) (2017) 709–757. [DOI] [PMC free article] [PubMed] [Google Scholar]


