Abstract
Background:
Stromal CD8+ tumor-infiltrating lymphocytes (TILs) are an important prognostic and predictive indicator in non-small cell lung cancer (NSCLC). In this study, we aimed to develop and test the feasibility of a digital image analysis (DIA) workflow for estimating stromal CD8+ TIL density.
Methods:
A DIA workflow developed in a software platform (QuPath) was applied to a specified region of interest (ROI) within the stromal compartment of dual PD-L1/CD8 immunostained slides from 50 lung adenocarcinoma patients. A random tree classifier was trained from 25 training cases and applied to 25 test cases. The DIA-estimated CD8+ TIL densities were compared to manual estimates of three pathologists, who independently quantitated the percentage of CD8+ TILs from predefined ROIs in QuPath.
Results:
The average estimated total stromal cell count per case was 520 (range: 282–816) by QuPath and 551 (range: 265–744) by pathologists. The DIA-estimated CD8+ TIL density (mean = 16.9%) was comparable to pathologists' manual estimates (mean = 15.9%). A paired t-test showed no statistically significant difference between DIA and pathologist estimates of CD8+ TIL density among both training (n = 25, P = 0.55) and test (n = 25, P = 0.34) cases. There was an almost perfect agreement between QuPath and each pathologist's estimates of CD8+ TIL density (κ = 0.85–0.86).
Conclusions:
These findings demonstrate the feasibility of applying a DIA workflow for estimating stromal CD8+ TIL density in NSCLC. DIA has the potential to provide an efficient and standardized approach for estimating stromal CD8+ TIL density.
Keywords: CD8, digital image analysis, lung adenocarcinoma, tumor-infiltrating lymphocytes
INTRODUCTION
Checkpoint inhibitor immunotherapies targeting the programmed cell death 1/programmed death ligand 1 death-ligand 1 (PD-1) axis have become standard of care for the management of advanced non-small cell lung cancer (NSCLC).[1,2,3,4] Tumor cell PD-L1 expression levels as measured by PD-L1 immunohistochemistry (IHC) have been used as a predictive biomarker to select patients for anti-PD-1/PD-L1 therapies. However, utilizing PD-L1 expression alone as a predictive biomarker may exclude patients who may benefit from checkpoint blockade and only 45% of those with high PD-L1 expression (>50% of tumor cells) will respond to anti-PD-1 monotherapy.[5] In addition, the prognostic role of PD-L1 expression is less clear.
There is growing evidence that CD8+ tumor-infiltrating lymphocytes (TILs) play a key role in the response to anti-PD-1/PD-L1 therapies.[6] In addition, studies have demonstrated that stromal CD8+ TILs are important predictive as well as prognostic indicators in NSCLC.[7,8,9] Thus, we have developed PD-L1/CD8 dual IHC for clinical practice to improve the predictivity of response to anti-PD-1/PD-L1 therapies. The assessment of tumor-associated CD8+ TILs has been shown to be prognostic as well as predictive of response to neoadjuvant, adjuvant, and systemic therapies with or without immune checkpoint inhibitors (ICI) in a variety of tumor types. In particular, immunoscore based on the assessment of CD3+ and CD8+ T cells in two distinct tumor areas was recently introduced into ESMO Clinical Practice Guidelines for gastrointestinal cancer and into the WHO classification of the digestive system tumors.[6,10,11,12] In NSCLC, there have been multiple studies reporting the utility of CD8+ T cell scoring to predict patient outcomes after resection[8,9] and response to ICIs.[7]
While the manual counting of PD-L1+ tumor cells is clinically used for PD-L1 assessment, a scoring system for CD8+ TILs has not yet been established.[13] A bottleneck of clinical implementation of a CD8+ T cell assessment is due in part to the lack of a universally accepted scoring system in NSCLC. In addition, manual TIL evaluation is subject to high interobserver variability.[14,15] Therefore, we aimed to evaluate a role of a digital platform in scoring stromal CD8+ T cell infiltration that was originally proposed as a manual assessment by Donnem et al.[8,9] Digital image analysis (DIA) has the potential to provide an accurate, efficient and standardized approach for estimating CD8+ TIL density. Several studies have used QuPath, an open-source image analysis software, to enumerate CD8+ T cells.[7,16,17] However, there are no studies comparing DIA-estimated CD8+ density with pathologists' estimates. In this study, we developed a DIA workflow for estimating stromal CD8+ TIL density in accordance with the quantitative approach developed by Al-Shibli et al.[8,18] and evaluated the concordance between DIA and manual estimates.
METHODS
After obtaining IRB approval, 50 dual PD-L1/CD8-stained tissue slides from 50 lung adenocarcinoma patients were obtained from the pathology archive. The cases were selected to represent a broad spectrum of CD8+ T cell density and PD-L1 expression. The dual immunohistochemical stain included PD-L1 (clone E1 L3N, Cell Signaling Technology, Danvers, MA; antibody dilution: 1:200; ethylenediaminetetraacetic acid, pH 9.0, incubation for 20 min) and CD8 (Leica Biosystems; ready to use; ethylenediaminetetraacetic acid, pH 9.0, incubation for 20 min).
Slides stained for PD-L1 and CD8 were digitized at 400x magnification (0.2214 μm pixel width and height) using the Nano Zoomer S360 digital slide scanner (Hamamatsu Photonics, Hamamatsu, Japan). A DIA workflow was developed in QuPath v. 0.1.3 (Belfast, UK)[19] and comprised of stain estimation, cell detection, feature computation, and stain intensity classification. In each case, a region of interest (ROI) in the tumor stroma containing at least 200 nucleated cells was selected to include nontumor cells and CD8+ TILs in the stroma. Each ROI was selected from a unique scanned slide to capture variability in background immunohistochemical staining intensities. The cell detection parameters were optimized by applying the cell detection across all cases iteratively, resulting in the following final QuPath parameters: optical density sum detection image, pixel size: 0.5 μm, intensity threshold 0.1, nucleus parameters with background radius 6 μm, minimum area 6 μm2, maximum area 1200 μm2, sigma 1.5. For each detected candidate cell, 55 morphologic and intensity-based features were computed. A random tree classifier was trained within the specified ROIs in 25 randomly selected “training” cases. A subset of these training cases was utilized to validate the classifier against manual pathologist estimates of stromal cells and CD8+ cells. The classifier was subsequently applied to the remaining 25 “test” cases that were not used for training or validation to identify nucleated stromal cells and classify them as CD8 positive or negative. Three pathologists (D. S., Y. H., M. M.-K.) independently quantitated the total number of nucleated cells and the percentage of CD8+ TILs from the aforementioned predefined stromal ROIs of digitized slides of training and test cases using annotation tools in QuPath.
Statistical analyses were performed using R version 3.5.3 (R Core Team, Vienna, Austria)[20] and Graphpad Prism v5.0 (GraphPad Software, San Diego, CA, USA)[21] to compare DIA-estimated stromal cell counts and CD8+ TIL densities to the pathologists' manual estimates. Pearson's correlation coefficient was estimated in R to examine the relationship between the DIA results and each pathologist's manual estimates. A paired t-test was conducted in Graphpad Prism to determine whether a statistically significant difference exists between DIA and all three pathologists' estimates. Cohen's weighted Kappa (κ) coefficient was estimated in R to assess the concordance of CD8+ TIL densities between the three pathologists and QuPath, using an ordinal variable for CD8+ TIL density with 5, 10, 15, 20, and 25% as cutoffs.
RESULTS
A DIA workflow was applied in the stromal compartments of 50 dual PD-L1/CD8 stained slide images of lung adenocarcinoma (mean area: 83,932 μm2) [Figure 1]. The cell detection algorithm for detecting the red-stained CD8+ cells was significantly improved by utilizing the optical density sum compared to the hematoxylin optical density transformed image in QuPath. A cell classifier trained from 25 training cases classified the detected candidate cells most commonly as stromal cells, with the remaining candidate cells being artifact resulting from background brown PD-L1 staining.
Figure 1.
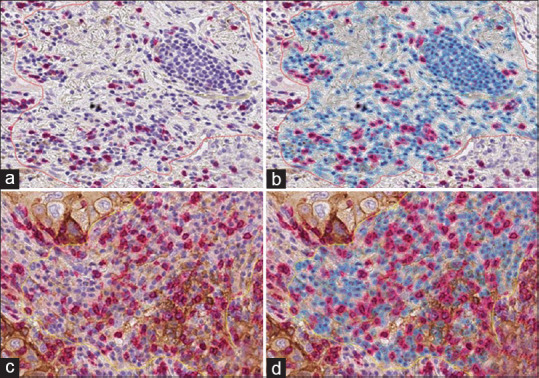
Illustration of digital image analysis workflow applied to a region of interest in a PD-L1/CD8 dual stain slide image. Cells are color-coded by classification: red (stroma, CD8+), blue (stroma, CD8-), and gray (artifact). Examples of cases where concordance between pathologists and QuPath is high (a and b) and low (c and d)
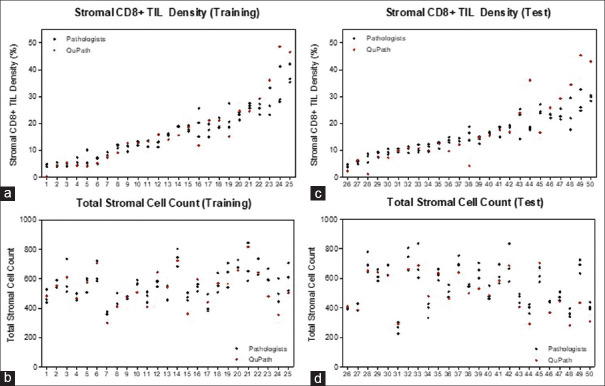
The average total stromal cell counts estimated was 519 (range: 282–816) by QuPath and 551 (range: 226–845) by pathologists [Table 1 and Figure 2], amounting to a total of 27,550 cells annotated on average by each pathologist. The DIA-estimated CD8+ TIL density (mean = 16.9%) was comparable to pathologists' manual estimates (mean = 15.9%). There was no statistically significant difference between DIA and pathologist estimates of CD8+ TIL density among both training (n = 25, P = 0.55) and test (n = 25, P = 0.34) cases. Among training cases, there was no statistically significant difference between DIA and pathologist estimates of total stromal cell count (P = 0.11). Among test cases, DIA-estimated stromal cell counts were lower than the pathologists' by 39.8 cells on average (95% confidence interval [CI]: 11.3–68.4, P < 0.05). This is as a result of the underdetection of cells particularly in slides with high background PD-L1 staining, where identification of cell borders is challenging [Figure 1].
Table 1.
Summary statistics and comparison of the pathologist and digital image analysis-estimated CD8+ tumor-infiltrating lymphocytes density and total stromal cell count
| Mean(minimum-maximum) | |||
|---|---|---|---|
|
| |||
| Training (n=25) | Test (n=25) | Overall (n=50) | |
| CD8+ TIL density(%) | |||
| Pathologist 1 | 13.9(2.3-28.2) | 15.0(3.9-35.4) | 14.5(2.3-35.4) |
| Pathologist 2 | 16.3(4.7-29.8) | 16.0(4.6-36.6) | 16.1(4.6-36.6) |
| Pathologist 3 | 16.5(3.8-32.6) | 17.7(4.1-42.1) | 17.1(3.8-42.1) |
| Pathologists’ average | 16.2 | 15.6 | 15.9 |
| QuPath | 17.0(1.2-45.3) | 16.9(0.2-48.6) | 16.9(0.2-48.6) |
| Mean of differences(95% CI) | −0.6(−2.7-1.5) | −1.4(−4.4-1.6) | −1.0(−2.7-0.7) |
| Paired t-test(P) | 0.55 | 0.34 | 0.26 |
| Total cell count | |||
| Pathologist 1 | 534(267-724) | 556(356-803) | 545(267-803) |
| Pathologist 2 | 522(226-746) | 527(359-683) | 524(226-746) |
| Pathologist 3 | 575(302-837) | 590(374-845) | 583(302-845) |
| Pathologists’ average | 558 | 544 | 551 |
| QuPath | 504(282-706) | 535(300-816) | 519(282-816) |
| Mean of differences(95% CI) | 22.5(−5.6-50.5) | 39.8(11.3-68.4) | 31.2(11.7-50.6) |
| Paired t-test(P) | 0.11 | <0.05 | <0.05 |
TIL: Tumor-infiltrating lymphocytes, CI: Confidence interval
Figure 2.
Distribution of pathologists (black) and QuPath (red) estimates of CD8+ TIL density (a and c) and total stromal cell count (b and d) for training (n = 25) and test (n = 25) cases, arranged from lowest to highest CD8+ TIL density
There was a high concordance of CD8+ TIL density estimates between QuPath and pathologists, with the weighted κ coefficient ranging from 0.85 to 0.86 (a κ coefficient >0.8 is considered almost perfect agreement) [Table 2]. Concordance between pathologists was also high, with κ coefficients ranging from 0.88 to 0.94. The Pearson's correlation coefficients between pathologists and QuPath were greater for CD8+ TIL density estimates (0.87–0.91) than total stromal cell counts (0.77–0.87). Similarly, between the three pathologists, higher correlations were observed for CD8+ TIL density (0.93–0.96) than for total stromal cell counts (0.79–0.88). In one case, the difference in cell counts between pathologists was as high as 259 cells (586 vs. 845 cells). These findings suggest greater variation both among pathologists and between pathologists and QuPath in determining what qualifies as a single “cell” compared to the intensity threshold for CD8 positivity.
Table 2.
Interobserver and pathologist-QuPath agreement and correlation coefficient
| Training (n=25) | Test (n=25) | All cases (n=50) | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| QuPath | Path 1 | Path 2 | QuPath | Path 1 | Path 2 | QuPath | Path 1 | Path 2 | |
| CD8+ TIL density | |||||||||
| Cohen’s weighted kappa(5, 10, 15, 20, 25% cutoffs) | |||||||||
| Path 1 | 0.88 | 0.80 | 0.86 | ||||||
| Path 2 | 0.88 | 0.92 | 0.79 | 0.80 | 0.85 | 0.88 | |||
| Path 3 | 0.86 | 0.88 | 0.94 | 0.82 | 0.85 | 0.92 | 0.85 | 0.88 | 0.94 |
| Correlation coefficient | |||||||||
| Path 1 | 0.90 | 0.85 | 0.87 | ||||||
| Path 2 | 0.91 | 0.97 | 0.82 | 0.95 | 0.87 | 0.96 | |||
| Path 3 | 0.96 | 0.93 | 0.95 | 0.87 | 0.93 | 0.94 | 0.91 | 0.93 | 0.94 |
| Total cell count | |||||||||
| Correlation coefficient | |||||||||
| Path 1 | 0.69 | 0.83 | 0.77 | ||||||
| Path 2 | 0.75 | 0.81 | 0.81 | 0.92 | 0.78 | 0.88 | |||
| Path 3 | 0.86 | 0.69 | 0.79 | 0.89 | 0.85 | 0.92 | 0.87 | 0.79 | 0.87 |
TIL: Tumor-infiltrating lymphocytes
CONCLUSIONS
As DIA becomes increasingly utilized in the quantitative evaluation of tissue-based biomarkers, methods to validate the DIA estimates against the “gold standard” are important to develop. While several studies have utilized DIA for quantitating CD8+ T cells, none have explicitly demonstrated how DIA estimates compare with manual pathologist estimates. In this study, we directly compared DIA estimates with pathologists' quantification of stromal cell count and CD8+ density that were generated on the same software platform utilized for image analysis. This made it possible to gather quantitative manual estimates, standardize the ROIs being evaluated, and standardize slide evaluation modality. As a result, these minimized variables that could confound the comparison between DIA and pathologist estimates.
Under the standardized digital environment of our study, there was high concordance among pathologists, despite the known challenges of interobserver variability in stromal TIL evaluation.[14,15] Interestingly, compared to CD8+ density, there was greater interpathologist variability in the stromal cell count, particularly among CD8-negative stromal cells. The discordance in cell count among pathologists as well as with QuPath was largely due to differences in morphologic and intensity thresholds for cell identification. Even a seemingly simple and objective task of counting cells can be subject to significant interobserver variability, which can pose a challenge for defining a “gold standard” by which to compare DIA results.
A notable challenge included variation in cell detection adequacy among cases. Across all 50 cases, we applied the same cell detection algorithm, which optimized the detection of CD8+ TILs at the cost of lowering the sensitivity for detecting CD8-negative stromal cells. As we estimated CD8+ density as a proportion of CD8-positive cells among all stromal cells, the differential sensitivity in cell detection had the greatest impact in cases with the highest proportion of CD8+ TILs (≥25%). In addition, several cases with high discordance had high background PD-L1 staining, where detection of individual cells and identification of cell borders were challenging. This issue may be addressed by performing individual PD-L1 and CD8 stains on separate slides. These findings demonstrate the importance of identifying potential biases introduced through tradeoffs made in the process of developing image analysis algorithms, as they can disproportionately impact performance quality in certain cases more so than others (e.g., high vs. low CD8 density). In addition, this demonstrates the importance of preanalytical processes affecting the quality of digital images used for applying a DIA workflow. In our study, we attempted to capture variability in slide staining characteristics and background staining intensities by selecting each ROI from a different scanned slide. Further studies are needed to assess potential differences in the performance of a DIA workflow that is applied to digital images obtained from multiple slide scanners.
Despite these challenges, the high correlation and almost perfect concordance between DIA and pathologists' estimates of CD8+ density show that DIA is a feasible alternative to manual quantification, which is time-consuming, subjective, and difficult to standardize. Significant gains in efficiency can be achieved, as a task that took pathologists 15–30 min can be accomplished in under 5 min through DIA. In this study, we curated ROIs in the stromal compartment that did not contain any tumor cells in order to maximize the accuracy of the stromal cell classifier and minimize factors that can contribute to discrepancies between DIA and pathologist estimates. In this controlled digital setting, we demonstrated excellent agreement between DIA and pathologists for estimating CD8+ density. Therefore, DIA can be applied to achieve cell quantification and biomarker evaluation that are comparable to those of pathologists. Given the growing evidence on stromal CD8+ TILs as important predictive and prognostic indicators in NSCLC, DIA poses a promising tool to generate an accurate, efficient, and standardized estimate of stromal CD8+ TILs that can be used to predict response to anti-PD-1/PD-L1 therapies.
Financial support and sponsorship
This study was financially supported by a research grant from the Department of Pathology, Massachusetts General Hospital.
Conflicts of interest
MMK has served as a compensated consultant for H3 Biomedicine and AstraZeneca and received a research grant (through institution) from Novartis. The other authors have no conflicts of interest to disclose.
Footnotes
Available FREE in open access from: http://www.jpathinformatics.org/text.asp?2021/12/1/28/320703
REFERENCES
- 1.Hellmann MD, Ciuleanu TE, Pluzanski A, Lee JS, Otterson GA, Audigier-Valette C, et al. Nivolumab plus Ipilimumab in lung cancer with a high tumor mutational burden. N Engl J Med. 2018;378:2093–104. doi: 10.1056/NEJMoa1801946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hellmann MD, Paz-Ares L, Bernabe Caro R, Zurawski B, Kim SW, Carcereny Costa E, et al. Nivolumab plus ipilimumab in advanced non-small-cell lung cancer. N Engl J Med. 2019;381:2020–31. doi: 10.1056/NEJMoa1910231. [DOI] [PubMed] [Google Scholar]
- 3.Forde PM, Chaft JE, Smith KN, Anagnostou V, Cottrell TR, Hellmann MD, et al. Neoadjuvant PD-1 blockade in resectable lung cancer. N Engl J Med. 2018;378:1976–86. doi: 10.1056/NEJMoa1716078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gandhi L, Rodríguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378:2078–92. doi: 10.1056/NEJMoa1801005. [DOI] [PubMed] [Google Scholar]
- 5.Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csłszi T, Fülöp A, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375:1823–33. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 6.Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515:568–71. doi: 10.1038/nature13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fumet JD, Richard C, Ledys F, Klopfenstein Q, Joubert P, Routy B, et al. Prognostic and predictive role of CD8 and PD-L1 determination in lung tumor tissue of patients under anti-PD-1 therapy. Br J Cancer. 2018;119:950–60. doi: 10.1038/s41416-018-0220-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Donnem T, Hald SM, Paulsen EE, Richardsen E, Al-Saad S, Kilvaer TK, et al. Stromal CD8+ T-cell density a promising supplement to TNM staging in non-small cell lung cancer. Clin Cancer Res. 2015;21:2635–43. doi: 10.1158/1078-0432.CCR-14-1905. [DOI] [PubMed] [Google Scholar]
- 9.Donnem T, Kilvaer TK, Andersen S, Richardsen E, Paulsen EE, Hald SM, et al. Strategies for clinical implementation of TNM-Immunoscore in resected nonsmall-cell lung cancer. Ann Oncol. 2016;27:225–32. doi: 10.1093/annonc/mdv560. [DOI] [PubMed] [Google Scholar]
- 10.Kirilovsky A, Marliot F, El Sissy C, Haicheur N, Galon J, Pagès F. Rational bases for the use of the Immunoscore in routine clinical settings as a prognostic and predictive biomarker in cancer patients. Int Immunol. 2016;28:373–82. doi: 10.1093/intimm/dxw021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baxevanis CN, Sofopoulos M, Fortis SP, Perez SA. The role of immune infiltrates as prognostic biomarkers in patients with breast cancer. Cancer Immunol Immunother. 2019;68:1671–80. doi: 10.1007/s00262-019-02327-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nassif EF, Thibault C, Oudard S, Galon J. Precision immunity: Immunoscore and neoadjuvant treatment in bladder cancer. Oncoimmunology. 2021;10:1888488. doi: 10.1080/2162402X.2021.1888488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hendry S, Salgado R, Gevaert T, Russell PA, John T, Thapa B, et al. Assessing tumor-infiltrating lymphocytes in solid tumors: A practical review for pathologists and proposal for a standardized method from the international immuno-oncology biomarkers working group: Part 2: TILs in melanoma, gastrointestinal tract carcinom. Adv Anat Pathol. 2017;24:311–35. doi: 10.1097/PAP.0000000000000161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O'Loughlin M, Andreu X, Bianchi S, Chemielik E, Cordoba A, Cserni G, et al. Reproducibility and predictive value of scoring stromal tumour infiltrating lymphocytes in triple-negative breast cancer: A multi-institutional study. Breast Cancer Res Treat. 2018;171:1–9. doi: 10.1007/s10549-018-4825-8. [DOI] [PubMed] [Google Scholar]
- 15.Dano H, Altinay S, Arnould L, Bletard N, Colpaert C, Dedeurwaerdere F, et al. Interobserver variability in upfront dichotomous histopathological assessment of ductal carcinoma in situ of the breast: The DCISion study. Mod Pathol. 2020;33:354–66. doi: 10.1038/s41379-019-0367-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ledys F, Klopfenstein Q, Truntzer C, Arnould L, Vincent J, Bengrine L, et al. RAS status and neoadjuvant chemotherapy impact CD8+ cells and tumor HLA class I expression in liver metastatic colorectal cancer. J Immunother Cancer. 2018;6:123. doi: 10.1186/s40425-018-0438-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu B, Arakawa Y, Yokogawa R, Tokunaga S, Terada Y, Murata D, et al. PD-1/PD-L1 expression in a series of intracranial germinoma and its association with Foxp3+ and CD8+ infiltrating lymphocytes. PLoS One. 2018;13:e0194594. doi: 10.1371/journal.pone.0194594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Al-Shibli KI, Donnem T, Al-Saad S, Persson M, Bremnes RM, Busund LT. Prognostic effect of epithelial and stromal lymphocyte infiltration in non-small cell lung cancer. Clin Cancer Res. 2008;14:5220–7. doi: 10.1158/1078-0432.CCR-08-0133. [DOI] [PubMed] [Google Scholar]
- 19.Bankhead P, Loughrey MB, Fernández JA, Dombrowski Y, McArt DG, Dunne PD, et al. QuPath: Open source software for digital pathology image analysis. Sci Rep. 2017;7:16878. doi: 10.1038/s41598-017-17204-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.R Development Core Team. R. A Language and Environment for Statistical Computing. 2016. [Last accessed on 2019 Jan 04]. Available from: http://www.r-project.org .
- 21.GraphPad Software. GraphPad Prism. [Last accessed on 2019 Jan 05]. Available from: http://www.graphpad.com .