Abstract
The autonomic nervous system (ANS) can be affected by COVID-19, and dysautonomia may be a possible complication in post-COVID individuals. Orthostatic hypotension (OH) and postural tachycardia syndrome (POTS) have been suggested to be common after SARS-CoV-2 infection, but other components of ANS function may be also impaired. The Composite Autonomic Symptom Scale 31 (COMPASS-31) questionnaire is a simple and validated tool to assess dysautonomic symptoms. The aim of the present study was to administer the COMPASS-31 questionnaire to a sample of post-COVID patients with and without neurological complaints. Participants were recruited among the post-COVID ambulatory services for follow-up evaluation between 4 weeks and 9 months from COVID-19 symptoms onset. Participants were asked to complete the COMPASS-31 questionnaire referring to the period after COVID-19 disease. Heart rate and blood pressure were manually taken during an active stand test for OH and POTS diagnosis. One-hundred and eighty participants were included in the analysis (70.6% females, 51 ± 13 years), and OH was found in 13.8% of the subjects. Median COMPASS-31 score was 17.6 (6.9–31.4), with the most affected domains being orthostatic intolerance, sudomotor, gastrointestinal and pupillomotor dysfunction. A higher COMPASS-31 score was found in those with neurological symptoms (p < 0.01), due to more severe orthostatic intolerance symptoms (p < 0.01), although gastrointestinal (p < 0.01), urinary (p < 0.01), and pupillomotor (p < 0.01) domains were more represented in the non-neurological symptoms group. This study confirms the importance of monitoring ANS symptoms as a possible complication of COVID-19 disease that may persist in the post-acute period.
Keywords: COVID-19, Long-COVID, Autonomic dysfunction, COMPASS-31, Orthostatic intolerance
Introduction
Novel coronavirus disease (COVID-19) has been suggested to not only affect health during the acute phase of the SARS-CoV-2 infection, but some manifestations have been consistently reported in several studies also after the recovery. Such phenomenon has been named “long-COVID” or “post-acute COVID-19” depending on the time course definition of the persisting or novel symptoms [1], the latter being defined as a syndrome characterized by persistent symptoms and/or delayed or long-term complications beyond 4 weeks from the onset of COVID-19 [2]. These complications include myocarditis, pulmonary fibrosis, encephalitis, thromboembolic events, psychiatric illness, and persisting symptoms such as dyspnea, cough, and fatigue [3, 4]. Among these clinical manifestations, neurological symptoms may be also common, as cognitive deficits have been reported in 36% of patients two to four months after COVID-19 [5, 6], and the reported fatigue and dysexecutive syndrome may depend on cortical reorganization [7]. Despite few data are present and further investigations are recommended, age, higher BMI and female sex have been proposed as risk factors for post-COVID [8]. Some recent papers have pointed a rationale for autonomic dysfunction following COVID-19, suggesting a role of the virus infection and/or of the related immune response on the autonomic nervous system (ANS), often resulting in orthostatic intolerance (OI), including orthostatic hypotension (OH) and postural tachycardia syndrome (POTS) [9, 10]. OH and POTS can be common in the elderly, particularly in geriatrics outpatients [11], as well as in individuals with other dysautonomic features like in neurological diseases (e.g., Parkinson or dementia) [12] or diabetes [13], the latter due to the possible presence of neuropathies affecting baroceptors and increased arterial stiffness [13, 14]. Such symptoms are often disabling and affect people’s quality of life; additionally, they can increase the risk of further events and falls. A recent case series reported typical features of orthostatic intolerance, fatigue, and activity intolerance in post-COVID individuals, and autonomic testing found heterogeneous responses, including OH and POTS [15]. As of this time, reports of COVID-19-related dysautonomia have been limited, but some pathophysiological mechanisms have been suggested, as para- or post-infectious immune-mediated process [15], supported by the presence of dysautonomia in Guillain-Barré syndrome and acute autoimmune autonomic neuropathy [16, 17]. For these reasons, patients with persisting or novel symptoms after COVID-19 should be assessed for autonomic disorders, including an active stand test measuring blood pressure and heart rate [8, 9] following standard procedures [18]. Due to the hypothesized influence of SARS-CoV-2 infection on the autonomic nervous system, dysautonomia manifestations other than orthostatic syndrome might be supposed. The Composite Autonomic Symptom Scale 31 (COMPASS-31) questionnaire is a widely validated tool to assess symptoms of ANS dysfunction, and it evaluates six domains related to ANS function: OI, vasomotor, secretomotor, gastrointestinal, urinary, and pupillomotor [19].
The aim of this study was to assess the prevalence of ANS dysfunction, evaluated with the COMPASS-31 questionnaire and an active stand test, in a real-life setting in consecutive patients referred to the post-COVID ambulatory service, and to compare the patients who presented neurological symptoms with those without neurological manifestations.
Materials and methods
This prospective observational study included patients who referred to the post-COVID ambulatory service of the University Hospital and Health Services of Trieste (ASUGI) between the 15.02 and 15.05 of 2021. All the procedures were performed according to the Declaration of Helsinki and the local institutional review board and ethics committee (CEUR-FVG) approved the study. To be included in the study, participants had to present persistent symptoms and/or delayed or long-term complications between 4 weeks and 9 months from the onset of COVID-19 confirmed by a nasopharyngeal swab. Participants were excluded if before COVID-19 they suffered from ANS dysfunctions or cognitive impairment, or if they were prescribed medications that might alter ANS as: antidepressants, beta-blockers, ACE inhibitors, calcium channel blockers, alpha-1 blockers, antihistamines, cholinesterase inhibitors, and central antihypertensives. Those who reported a cognitive deficit as a post-COVID symptom received the Montreal Cognitive Assessment (MoCA) and neuropsychological evaluation, and if the corrected score was lower than 15 or the neuropsychological evaluation suggested a remarkable cognitive deficit, they were excluded from the analysis. Participants were grouped between those presenting post-COVID neurological symptoms based on the medical check performed by an infectious disease specialist working in the post-COVID ambulatory service and then confirmed by the neurologist according to the clinical and instrumental (where possible, e.g., nerve conduction study) evaluation.
ANS dysfunction evaluation
During the medical check, all the participants who met the inclusion and exclusion criteria were invited to complete the validated Italian version of the COMPASS-31 questionnaire [20]. To better assess post-COVID autonomic dysfunction, all questions were adapted to refer to the period after COVID-19, and improvements/worsening were evaluated comparing the time of the visit with the time when the dysautonomic symptoms appeared. A pilot testing was conducted on 10 post-COVID patients (not included in this study) on two separate occasions 14 days apart, according to previous surveys development [21–23] to investigate the reliability of the adapted version of the questionnaire employing a test–retest correlation that confirmed the appropriate reliability (Pearson’s r > 0.900, p < 0.01). The total score of COMPASS-31 and domains score were computed according to the original instructions [19]. COMPASS-31 score was presented as a continuous variable (0–100) and a cutoff of 13.25 was used to suggest ANS dysfunction, as previously defined for diagnosis of multiple system atrophy with predominant parkinsonism [24]. Domains scores were presented as continuous variables; the maximum weighted scores for each subdomain are as follows: 40 for orthostatic intolerance, 5 for vasomotor dysfunction, 15 for secretomotor dysfunction, 25 for gastrointestinal (GI) dysfunction, 10 for urinary dysfunction, and 5 for pupillomotor dysfunction [19].
Although not present in the original COMPASS-31 questions, additional items were included for a better understanding of other possible features of ANS dysfunction following COVID-19. These questions included the subjective evaluation of (i) thermal/environmental comfort (patients were asked if after COVID-19 they felt more uncomfortable while staying in hot, cold, humid, or windy environments), (ii) heat and cold sensation (if they were more/less sensible to contact heat and cold), and (iii) sexual dysfunction (e.g., impotence and reduced lubrication). These items were scored with a yes/no answer.
The active stand test was performed at the end of the visit, following guidelines [18]. Measures were manually taken with a sphygmomanometer [25] by a trained neurologist in OI evaluation, asking the patient to rest in the supine position for 5 min before the first measure, and then 3 min after standing. OH was defined as a fall of > 20 mmHg systolic and > 10 mmHg diastolic after standing for 3 min [18], and corrected in case of supine hypertension [26], while POTS is characterized by orthostatic symptoms (in the absence of orthostatic hypotension) with an increase in heart rate of 30 beats per minute or more when standing for more than 30 s [18, 27].
Statistical analysis
All analyses were conducted using IBM SPSS Statistics (v.22.0, Chicago, IL, USA). Kolmogorov–Smirnov test of normality was performed. Categorical variables are presented as counts and percentages (%), while normally distributed continuous variables are presented as means and standard deviations (SDs) and non-normally distributed continuous variables are presented as medians (25th–75th percentile). To determine whether the associations between disease features and severity and the total COMPASS-31 score resulted from global autonomic symptoms across multiple subdomains, we grouped patients by the number of positive subdomains (score > 0 in any category) of the COMPASS-31: (1) the “global autonomic symptoms” group included all patients with five or more positive subdomains, and (2) the “limited autonomic symptoms” group included patients with fewer than five positive subdomains [28]. Post-COVID patients who presented neurological symptoms were compared to post-COVID patients without neurological symptoms, and further subgroup analysis compared the neurological patients according to their prevalent symptom. Between-groups comparisons were performed with the Mann–Whitney U test and the Fisher’s exact test. A univariate analysis was performed to investigate the association of different factors, including age, sex, and clinical characteristics, with the COMPASS-31 score. Factors that were associated with this score with p < 0.10 were included in the stepwise multivariable model and were confirmed as significant if p < 0.05, as previously described [29–31]. A binary logistic regression was performed between the COMPASS-31 total score and the OI domain, with the presence of OH and POTS as evaluated during the stand test. A value of p < 0.05 was considered significant.
Results
Demographics and clinical characteristics of the sample
One-hundred and ninety-two patients were first included in data collection; after excluding those who had a remarkable cognitive impairment, 180 participants were included in the final analysis (70.6% females, 51 ± 13 years). Among these, 97 were characterized by neurological manifestations (myalgia/asthenia 22.7%, headache 13.4%, hyposmia/hypogeusia 37.1%, dizziness 7.2%, sleep disturbances 10.3%, “brain fog”/cognitive deficit 42.3%), while 83 did not presented neurological symptoms but reported other post-COVID complications (61.3% exertional dyspnea, 29.1% arthralgia, 9.6% other) (Table 1). The median time from COVID-19 onset to the visit was 59 (31–175 days).
Table 1.
COMPASS-31 score, domains, and additional items in post-COVID patients with and without neurological symptoms
| Neuro n = 97 |
Others n = 83 |
Significance | |
|---|---|---|---|
| Age (y) | 53 ± 14 | 49 ± 11 | 0.073 |
| Females [n (%)] | 67 (69.1) | 60 (72.3) | 0.743 |
| COMPASS-31 | |||
| Score [0–100] | 20.7 (5.4–32.2) | 14.2 (8.2–30.3) | 0.002 |
| > 13.25 [n (%)] | 55 (56.7) | 45 (55.4) | 0.654 |
| Orthostatic intolerance | |||
| Score [0–40] | 12.0 (0.0–20.0) | 0.0 (0.0–20.0) | < 0.001 |
| > 0 [n (%)] | 53 (54.6) | 39 (47.0) | 0.369 |
| Vasomotor | |||
| Score [0–5] | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.226 |
| > 0 [n (%)] | 22 (22.7) | 20 (24.1) | 0.860 |
| Secretomotor | |||
| Score [0–15] | 2.0 (0.0–6.0) | 2.1 (0.0–6.4) | 0.227 |
| > 0 [n (%)] | 53 (54.6) | 45 (54.2) | 1.000 |
| Gastrointestinal | |||
| Score [0–24] | 3.0 (0.0–6.1) | 4.5 (2.7–7.1) | < 0.001 |
| > 0 [n (%)] | 66 (68.0) | 77 (92.8) | < 0.001 |
| Urinary | |||
| Score [0–10] | 0.0 (0.0–1.0) | 0.0 (0.0–1.1) | < 0.001 |
| > 0 [n (%)] | 26 (26.8) | 38 (45.8) | 0.012 |
| Pupillomotor | |||
| Score [0–6] | 1.0 (0.0–2.0) | 1.3 (0.3–2.3) | < 0.001 |
| > 0 [n (%)] | 59 (60.8) | 64 (77.1) | 0.024 |
| Global autonomic symptoms [n (%)] | 23 (23.7) | 24 (28.9) | 0.496 |
| Environmental intolerance [n (%)] | |||
| Heat | 17 (17.5) | 23 (27.7) | 0.109 |
| Cold | 33 (34.0) | 34 (41.0) | 0.356 |
| Humid | 8 (8.2) | 20 (24.1) | 0.004 |
| Wind | 4 (4.1) | 9 (10.8) | 0.093 |
| Subj. thermal sensation [n (%)] | |||
| Heat | 7 (7.2) | 12 (14.5) | 0.145 |
| Cold | 5 (5.2) | 16 (19.3) | 0.005 |
| Sexual impairment [n (%)] | 13 (13.4) | 13 (15.7) | 0.677 |
Means ± standard deviations, medians (25th–75th percentile) and proportions are reported
ANS dysfunction
The median COMPASS-31 total score was 17.6 (6.9–31.4) and was higher than 13.25 in 61.1% of the sample (Fig. 1). The OI domain score was 6.0 (0.0–20.0) and was higher than 0 in 51.1% of the sample. The vasomotor domain score was 0.0 (0.0–0.0) and was higher than 0 in 23.3% of the sample. The secretomotor domain score was 2.0 (0.0–6.0) and was higher than 0 in 54.4% of the sample. The GI domain score was 4.0 (1.5–7.0) and was higher than 0 in 79.4% of the sample. The urinary domain score was 0.0 (0.0–1.1) and was higher than 0 in 35.6% of the sample. The pupillomotor domain score was 1.1 (0.0–2.2) and was higher than 0 in 68.3% of the sample. “Global autonomic symptoms” were found in 26.1% of the sample. Reduced tolerance to environmental conditions was found for heat (22.2%), cold (37.2%), humidity (15.6%) and wind (7.2%). Subjectively altered contact thermal sensation was found for heat (10.6%) and cold (11.7%). Sexual impairment was reported by 14.4% of the sample. Active stand test suggested the prevalence of OH in 13.8% of the sample, while POTS was found in none of the subjects. In particular, those with OH were mainly characterized by decreased diastolic blood pressure after 3 min standing (− 15 ± 3 mmHg).
Fig. 1.
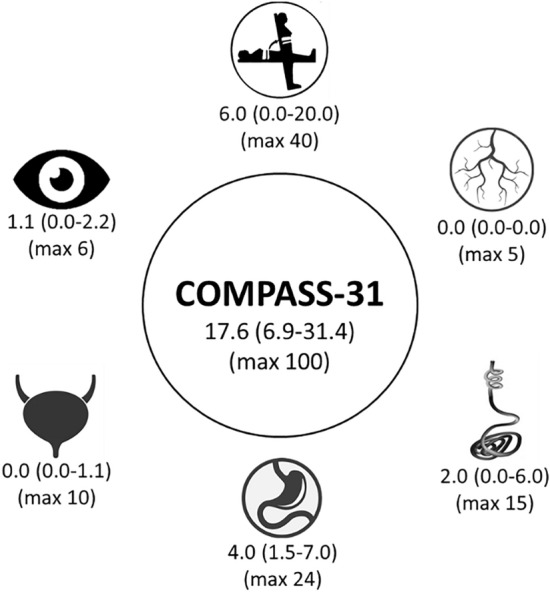
Graphical representation of median (25th–75th percentile) COMPASS-31 total score and subdomains scores, with maximal score for each domain, in post-COVID individuals (n = 180). From top, clockwise: orthostatic intolerance, vasomotor, sudomotor, gastrointestinal, urinary, pupillomotor domains
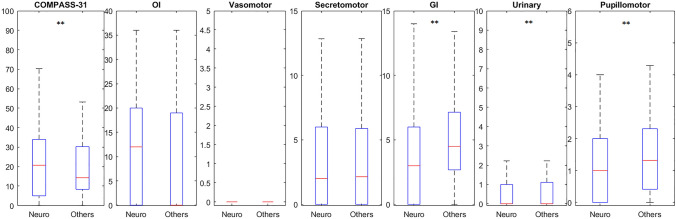
Subgroups analysis between post-COVID patients with neurological symptoms and post-COVID patients without neurological symptoms (Table 1) suggested that, despite no significant differences in demographics or prevalence of ANS dysfunction (COMPASS-31 > 13.25), the post-COVID patients with neurological symptoms reported a higher overall COMPASS-31 score (p < 0.01) and OI score (p < 0.01), and less GI (p < 0.01), urinary (p < 0.05) and pupillomotor scores (p < 0.05) (Fig. 2). Similarly, intolerance to humidity (p < 0.01) and subjective cold sensation (p < 0.01) were less prevalent in the neurological symptoms group.
Fig. 2.
Boxplots showing the comparison between patients with neurological symptoms (neuro) and patients without neurological symptoms (others) at the time of the visit. COMPASS-31 score and subdomains with corresponding maximal score: orthostatic intolerance (OI), vasomotor, secretomotor, gastrointestinal (GI), urinary, and pupillomotor. **p < 0.01
Factors associated with ANS dysfunction
A trend for a negative association between age and COMPASS-31 score > 13.25 was found at the multivariate analysis (p < 0.072), suggesting younger subjects might present more dysautonomic symptoms, while sex was found significantly associated with COMPASS-31, suggesting females have a higher probability to develop ANS dysfunction (OR 2.771, 95% CI 1.091–7.035; p = 0.032). Binary logistic regression failed to show any association between the OI domain and OH assessed at the active stand test (p = 0.262). At the univariate analysis, both subjective altered heat sensation (OR 3.936, 95% CI 1.087–14.253; p = 0.39) and cold sensation (OR 4.609, 95% CI 1.289–16.477; p = 0.019) were significantly associated with COMPASS-31 score > 13.25. However, only cold sensation was significantly associated with the presence of positive responses in the OI domain (OR 4.007, 95% CI 1.271–12.631; p = 0.018). In the subgroup analysis among the patients with neurological symptoms at the visit, the regression analysis failed to show any specific association between the reported symptom(s) and ANS dysfunction or OH, and non-significant difference was found in the OI subdomain score between those with and without OH at the active stand test.
Discussion
This study confirms and highlights the importance of assessing and monitoring ANS symptoms as possible long-term complications of SARS-CoV-2 infection. The main finding is the remarkable prevalence of ANS dysfunction in most post-COVID individuals, as shown by COMPASS-31 median and > 13.25 score, independent of the presence or absence of neurological symptoms. The most affected domains were orthostatic, secretomotor, urinary, and pupillomotor impairment, and these findings are in line with preliminary reports using COMPASS-31 in post-COVID patients [32], with a prevalence of a “global dysautonomia” in about 1/4 of the sample. Compared with previously published healthy controls from the literature [28, 33, 34], present data suggest a higher COMPASS-31 score in the post-COVID patients and ANS involvement. The absence of a significant association between age and COMPASS-31 score may depend on the specific questions asking for new dysautonomic symptoms after COVID-19, which might suggest an age-independent ANS response to the disease. Females were more commonly characterized by a higher COMPASS-31 score, in line with previous findings suggesting a higher prevalence of OI [35, 36] and symptomatic dysautonomia [37] in women. Comparison between patients with and without neurological complaining at the time of the visit suggested only minor differences, with a greater overall COMPASS-31 score in the neurological patients characterized by a higher OI domain score (despite no differences in the proportion of participants reporting at least one OI symptom) partially compensated by less GI, urinary and pupillomotor symptoms. Due to the absence of objective measures of autonomic function in the different domains, it is possible only to speculate that those with neurological symptoms post-COVID may suffer from more severe orthostatic impairment (despite some degree of lightheadedness may be present in all post-COVID participants), whereas those without neurological complications may be principally characterized by digestive and urinary alterations. The association between orthostatic impairment and neurological symptoms might be hypothesized due to the high prevalence of self-reported cognitive impairment that might be associated to altered postural vascular responses [38–40]. A common characteristic of the participants was the presence of fatigue, which can be a common post-COVID complication; however, no pathological differences between fatigued and non-fatigued patients on autonomic testing were previously found [41].
Despite few data have been published yet on dysautonomic symptoms and post-COVID, the rationale behind an ANS involvement during and after SARS-CoV-2 infection supports this study’s observations [9, 10, 42, 43]. Some of the mechanisms that have been hypothesized to influence ANS in COVID-19 include the sympathetic activation inducing pro-inflammatory cytokines that are at the base of the cytokine response [44]. As such, overactivation of the sympathetic nervous system may underlie the development of some responses that have been suggested to influence COVID-19 symptoms and recovery. In addition, the immune response may influence ANS function [45], as previously reported for OH and POTS being associated with antibodies (e.g., α-/β-adrenoceptors and muscarinic receptors), previous infections and autoimmune disorders [46–48]. Despite none of the patients was diagnosed with POTS, and OH was present in about one individual on 10, about one-half of the sample reported at least one symptom, although mild, of OI. This discrepancy, and the absence of a significant association between the OI component of COMPASS-31 and the active stand test, are not surprising: indeed, blood pressure and heart rate measurements were manually taken at a conventional time of 3 min, and this is known to potentially affect the assessment validity due to the measurement method and the different time courses of the responses [49]. Nevertheless, many questions still need to be clarified to understand the mechanisms underlying COVID-19 and vasovagal dysfunction, including the influence of SARS-CoV-2 on cerebral blood flow, possible hypoxia secondary to residual lung damage, prolonged bed rest, and the development of neuropathies [10, 50–55]. The present study included specific questions about subjective alterations in contact heat and cold sensations, which were reported to be altered in around 10% of the sample and were associated with higher COMPASS-31 total and OI domain scores. As such, these results support the hypothesis of a possible sensory or autonomic neuropathy involvement, although further studies including peripheral neurophysiology investigations are needed.
SARS-CoV-2 has been suggested to affect microcirculation, resulting in endothelial cell swelling and damage (endotheliitis), microscopic blood clots (microthrombosis), capillary congestion, and damage to pericytes, tissue repair angiogenesis, and scar formation [56]. In addition, a correlation is present between endothelial function and ANS [57], and vasomotor activity is a component of dysautonomia. However, symptoms of the vasomotor domain were the less commonly reported alteration in this sample (about 1/4), suggesting a minor impact of COVID-19 on vascular responses, or minor attention towards the related clinical manifestations (e.g., changes in hands and feet color).
About half of post-COVID individuals have reported secretomotor and sweating abnormalities. Together with the impaired environmental tolerance and thermal sensation, these observations suggest possible thermoregulatory alterations. However, since these findings were not supported by previously published objective measures (e.g., quantitative sensory testing—QST, or thermoregulatory responses in hot/cold environments), it is not possible to confirm the hypothesis of impaired temperature regulation. Findings from a not yet published case report from our group found altered heat and cold sensation and pain in one patient who developed neuropathy after COVID-19. Heat, in combination with the COVID-19 pandemic, has been raised as a global concern due to the interference of cooling strategies and heat risk prevention strategies, with the recommendation to stay at home [58], and might be particularly relevant for the most vulnerable populations. Among these populations, people with cognitive disorders, reduced mobility, and autonomic dysfunction might be at a higher risk of heat risk [59]. Since most of these risk factors might be present in post-COVID patients, future actions should include this category of individuals among those at risk of heat-related illnesses.
GI symptoms can be present at COVID-19 onset, alone or in combination with other symptoms. Indeed, the angiotensin-converting enzyme 2 (ACE2) receptors, where the SARS-CoV-2 spike protein binds, is highly expressed in the gut [60], and viral invasion has been suspected due to the presence of the virus in the colonic tissue and feces [61, 62]. A previous study found the prevalence of GI symptoms during the post-COVID period in nearly half of the interviewed individuals [63]. Some of these sequelae, as abnormal appetite, nausea, and diarrhea, are also part of the GI domain of the COMPASS-31 score. However, the proportion of post-COVID patients who reported at least one GI symptom was far greater in our sample (more than 3/4).
Inflammation and demyelination of the pudendal nerve are associated with the possible development of bladder incontinence and urinary symptoms, with pudendal neuropathy being a possible feature of COVID-19 as also reported in previous viral infections in animals and humans [64, 65]. In the acute phase, increased urinary frequency has been previously reported in 1/8 of male patients, and suggested to be secondary to viral cystitis due to underlying COVID-19 disease [66]. However, despite these potential mechanisms, a large cohort study found bladder dysfunction not significantly increased in the 2–5 months after COVID‐19 infection [67]. In our sample, 1/4 to 1/2 of the sample reported some sort of urinary dysfunction, including both involuntary voids and voiding difficulty. Sexual impairment, in particular erectile dysfunction, has been suggested to be possible after COVID-19 due to both endothelial and autonomic components of male erection [68, 69]. In addition, anosmia and ageusia, possible post-COVID complications, may participate in impaired sexual responses [70]. Nevertheless, it is not possible to reconduct sexual impairment only to ANS dysfunction, as psychogenic might play a fundamental role during a pandemic [71]. Our sample was characterized by similar proportions of reported sexual impairment in males and females; nevertheless, this is not surprising as sexual dysfunction in females was reported after COVID-19 disease [72].
Vision abnormalities have been reported in clinical practice by several COVID-19 and post-COVID individuals, including sore eyes and light sensitivity [73, 74]. Despite the possible influence of acute SARS-CoV-2 infection and hospitalization on ocular manifestations described in this sample, none of the participants reported eye complaints (e.g., acute conjunctivitis) during the acute phase of the disease, and an ANS involvement might be suspected. Indeed, pupil responses assessed with an automatic pupillometer were found impaired in patients recovering from COVID-19 [75]. Although this impairment might be present already during the acute phase in critically ill COVID-19 patients [76], controversial results have been found since ANS responses might be attenuated in the intensive care unit (ICU) population by ICU-related interventions, such as the administration of anesthetics, analgesics and inotropic medications that may have a prolonged effect [77]. In our sample, about one-half of the participants reported ocular impairments, and light sensitivity was the most common complaint.
This study has some limitations that need to be discussed. First, the primary ANS dysfunction evaluation was performed with a questionnaire that, despite a wide validation in different samples, suffers from possible subjective interpretation and emotional components. Indeed, the symptoms screened in the questionnaire and suggestive of ANS dysfunction are non-specific (e.g., diarrhea or urinary incontinence), and in absence of well-known condition with autonomic impairment characteristics, they might be a manifestation of disorders besides dysautonomia. Despite some studies are suggestive of an autonomic involvement after COVID-19 [9, 15], caution should be taken when considering COMPASS-31 results in this sample. The time reference for the symptoms was modified to adapt to the post-COVID period and did not affect the test–retest performance; nevertheless, some of the participants might have underrated some features that were already present before COVID-19 (e.g., GI or ocular symptoms). Second, the objective measure of OI was performed with a manual collection of heart rate and blood pressure during the active stand test, a procedure that is known to have a limited validity. Although continuous beat-to-beat monitoring should be preferred, such devices are still not common in many hospital and ambulatory services, and this data collection was performed during the first period of a dedicated ambulatory service for post-COVID manifestations. The authors are aware that objective and standard protocols for dysautonomic symptoms should be recommended; however, ANS dysfunction is often overlooked and patients are referred to dedicated centers only when persisting and severe symptoms are present. Even mild ANS impairment can be disabling and influence individuals’ quality of life.
Unfortunately, we were not able to further investigate the reported symptoms with objective techniques due to the absence of local dedicated services with the appropriate devices and protocols, and patients were referred to national centers for dysautonomic disorders. At this time, we are not able to provide findings about the diagnostic accuracy of our screening due to the absence of further follow-up and a possible selection bias (only some individuals might have had the opportunity to reach the dysautonomia center for instrumental investigations). However, although possibly overestimated, this study provides evidence of self-reported common dysautonomic manifestations in post-COVID individuals and recommends ANS screening and subsequent instrumental diagnosis as part of follow-up monitoring.
Conclusions
This study conducted in post-COVID individuals with and without neurological manifestations revealed that most of the sample was characterized by dysautonomic symptoms, as reported with the COMPASS-31 score. Orthostatic intolerance, sudomotor, gastrointestinal, and pupillomotor abnormalities were commonly reported as complications of COVID-19. After an active stand test, about 10% of the individuals were characterized by a fall in blood pressure suggestive of orthostatic hypotension. Other dysfunctions include reduced tolerance to environmental conditions and sexual impairments. Taken together, these findings confirm the hypothesis of an ANS involvement after COVID-19, and recommend further clinical and research evaluations in post-COVID individuals with and without neurological symptoms.
Acknowledgements
The authors want to thank the post-COVID ambulatory staff members and the participants. The authors thank Matteo di Franza for editorial assistance and proofreading.
Funding
Open access funding provided by Università degli Studi di Trieste within the CRUI-CARE Agreement. No funding was received for conducting this study.
Declarations
Conflicts of interest
The authors have no conflicts of interest to declare.
Ethical approval
All procedures performed in study were approved by the CEUR-FVG ethical committee.
Informed consent
Informed consent was obtained from all individual participants included in the study.
References
- 1.Alwan NA, Johnson L. Defining long COVID: going back to the start. Med (New York, N.Y.) 2021;2:501–504. doi: 10.1016/j.medj.2021.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nalbandian A, Sehgal K, Gupta A, et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27:601–615. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cabrera Martimbianco AL, Pacheco RL, Bagattini ÂM, Riera R. Frequency, signs and symptoms, and criteria adopted for long COVID: a systematic review. Int J Clin Pract. 2021 doi: 10.1111/ijcp.14357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lund LC, Hallas J, Nielsen H, et al. Post-acute effects of SARS-CoV-2 infection in individuals not requiring hospital admission: a Danish population-based cohort study. Lancet Infect Dis. 2021 doi: 10.1016/S1473-3099(21)00211-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blazhenets G, Schröter N, Bormann T, et al. Slow but evident recovery from neocortical dysfunction and cognitive impairment in a series of chronic COVID-19 patients. J Nucl Med. 2021 doi: 10.2967/jnumed.121.262128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van den Borst B, Peters JB, Brink M, et al. Comprehensive health assessment three months after recovery from acute COVID-19. Clin Infect Dis Off Publ Infect Dis Soc Am. 2020 doi: 10.1093/cid/ciaa1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Versace V, Sebastianelli L, Ferrazzoli D, et al. Intracortical GABAergic dysfunction in patients with fatigue and dysexecutive syndrome after COVID-19. Clin Neurophysiol Off J Int Fed Clin Neurophysiol. 2021;132:1138–1143. doi: 10.1016/j.clinph.2021.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sudre CH, Murray B, Varsavsky T, et al. Attributes and predictors of Long-COVID: analysis of COVID cases and their symptoms collected by the Covid Symptoms Study App. medRxiv. 2020 doi: 10.1101/2020.10.19.20214494. [DOI] [Google Scholar]
- 9.Dani M, Dirksen A, Taraborrelli P, et al. Autonomic dysfunction in “long COVID”: rationale, physiology and management strategies. Clin Med. 2021;21:e63–e67. doi: 10.7861/clinmed.2020-0896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raj SR, Arnold AC, Barboi A, et al. Long-COVID postural tachycardia syndrome: an American autonomic society statement. Clin Auton Res Off J Clin Auton Res Soc. 2021 doi: 10.1007/s10286-021-00798-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tran J, Hillebrand SL, Meskers CGM, et al. Prevalence of initial orthostatic hypotension in older adults: a systematic review and meta-analysis. Age Ageing. 2021 doi: 10.1093/ageing/afab090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bengtsson-Lindberg M, Larsson V, Minthon L, et al. Lack of orthostatic symptoms in dementia patients with orthostatic hypotension. Clin Auton Res Off J Clin Auton Res Soc. 2015;25:87–94. doi: 10.1007/s10286-014-0244-z. [DOI] [PubMed] [Google Scholar]
- 13.Kozakova M, Palombo C. Diabetes mellitus, arterial wall, and cardiovascular risk assessment. Int J Environ Res Public Health. 2016;13:201. doi: 10.3390/ijerph13020201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takahashi M, Miyai N, Nagano S, et al. Orthostatic blood pressure changes and subclinical markers of atherosclerosis. Am J Hypertens. 2015;28:1134–1140. doi: 10.1093/ajh/hpu301. [DOI] [PubMed] [Google Scholar]
- 15.Goodman BP, Khoury JA, Blair JE, Grill MF. COVID-19 dysautonomia. Front Neurol. 2021;12:624968. doi: 10.3389/fneur.2021.624968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chakraborty T, Kramer CL, Wijdicks EFM, Rabinstein AA. Dysautonomia in Guillain-Barré syndrome: prevalence, clinical spectrum, and outcomes. Neurocrit Care. 2020;32:113–120. doi: 10.1007/s12028-019-00781-w. [DOI] [PubMed] [Google Scholar]
- 17.Young RR, Asbury AK, Corbett JL, Adams RD. Pure pan-dysautonomia with recovery. Description and discussion of diagnostic criteria. Brain. 1975;98:613–636. doi: 10.1093/brain/98.4.613. [DOI] [PubMed] [Google Scholar]
- 18.Lahrmann H, Cortelli P, Hilz M, et al. EFNS guidelines on the diagnosis and management of orthostatic hypotension. Eur J Neurol. 2006;13:930–936. doi: 10.1111/j.1468-1331.2006.01512.x. [DOI] [PubMed] [Google Scholar]
- 19.Sletten DM, Suarez GA, Low PA, et al. COMPASS 31: a refined and abbreviated composite autonomic symptom score. Mayo Clin Proc. 2012;87:1196–1201. doi: 10.1016/j.mayocp.2012.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pierangeli G, Turrini A, Giannini G, et al. Translation and linguistic validation of the composite autonomic symptom score COMPASS 31. Neurol Sci Off J Ital Neurol Soc Ital Soc Clin Neurophysiol. 2015;36:1897–1902. doi: 10.1007/s10072-015-2278-y. [DOI] [PubMed] [Google Scholar]
- 21.Buoite Stella A, Francescato MP, Sims ST, Morrison SA. Fluid intake behavior in athletes during typical training bouts. J Sports Med Phys Fitness. 2017;57:1504–1512. doi: 10.23736/S0022-4707.16.06722-0. [DOI] [PubMed] [Google Scholar]
- 22.Buoite Stella A, Yardley J, Francescato MP, Morrison SA. Fluid intake habits in type 1 diabetes individuals during typical training bouts. Ann Nutr Metab. 2018;73:10–18. doi: 10.1159/000489823. [DOI] [PubMed] [Google Scholar]
- 23.Buoite Stella A, Ajčević M, Furlanis G, et al. Smart technology for physical activity and health assessment during COVID-19 lockdown. J Sports Med Phys Fitness. 2020 doi: 10.23736/S0022-4707.20.11373-2. [DOI] [PubMed] [Google Scholar]
- 24.Kim Y, Seok JM, Park J, et al. The composite autonomic symptom scale 31 is a useful screening tool for patients with Parkinsonism. PLoS One. 2017;12:e0180744. doi: 10.1371/journal.pone.0180744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McDonagh STJ, Mejzner N, Clark CE. Prevalence of postural hypotension in primary, community and institutional care: a systematic review and meta-analysis. BMC Fam Pract. 2021;22:1. doi: 10.1186/s12875-020-01313-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Metzler M, Duerr S, Granata R, et al. Neurogenic orthostatic hypotension: pathophysiology, evaluation, and management. J Neurol. 2013;260:2212–2219. doi: 10.1007/s00415-012-6736-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Freeman R, Wieling W, Axelrod FB, et al. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin Auton Res Off J Clin Auton Res Soc. 2011;21:69–72. doi: 10.1007/s10286-011-0119-5. [DOI] [PubMed] [Google Scholar]
- 28.Adler BL, Russell JW, Hummers LK, McMahan ZH. Symptoms of autonomic dysfunction in systemic sclerosis assessed by the COMPASS-31 questionnaire. J Rheumatol. 2018;45:1145–1152. doi: 10.3899/jrheum.170868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Furlanis G, Ajcevic M, Buoite Stella A, et al. Wake-up stroke: thrombolysis reduces ischemic lesion volume and neurological deficit. J Neurol. 2020;267:666–673. doi: 10.1007/s00415-019-09603-7. [DOI] [PubMed] [Google Scholar]
- 30.Buoite Stella A, Ajčević M, Furlanis G, et al. A physiological perspective of the associations between hydration status and CTP neuroimaging parameters in hyper-acute ischaemic stroke patients. Clin Physiol Funct Imaging. 2021 doi: 10.1111/cpf.12690. [DOI] [PubMed] [Google Scholar]
- 31.Ajcevic M, Furlanis G, Stella AB, et al. A CT perfusion based model predicts outcome in wake-up stroke patients treated with recombinant tissue plasminogen activator. Physiol Meas. 2020 doi: 10.1088/1361-6579/ab9c70. [DOI] [PubMed] [Google Scholar]
- 32.Kedor C, Freitag H, Meyer-Arndt L, et al. Chronic COVID-19 Syndrome and Chronic Fatigue Syndrome (ME/CFS) following the first pandemic wave in Germany—a first analysis of a prospective observational study. medRxiv. 2021 doi: 10.1101/2021.02.06.21249256. [DOI] [Google Scholar]
- 33.Greco C, Di Gennaro F, D’Amato C, et al. Validation of the composite autonomic symptom score 31 (COMPASS 31) for the assessment of symptoms of autonomic neuropathy in people with diabetes. Diabet Med. 2017;34:834–838. doi: 10.1111/dme.13310. [DOI] [PubMed] [Google Scholar]
- 34.Treister R, O’Neil K, Downs HM, Oaklander AL. Validation of the composite autonomic symptom scale 31 (COMPASS-31) in patients with and without small fiber polyneuropathy. Eur J Neurol. 2015;22:1124–1130. doi: 10.1111/ene.12717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anjum I, Sohail W, Hatipoglu B, Wilson R. Postural orthostatic tachycardia syndrome and its unusual presenting complaints in women: a literature minireview. Cureus. 2018;10:e2435. doi: 10.7759/cureus.2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Low PA, Sandroni P, Joyner M, Shen W-K. Postural tachycardia syndrome (POTS) J Cardiovasc Electrophysiol. 2009;20:352–358. doi: 10.1111/j.1540-8167.2008.01407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Foschi M, Giannini G, Merli E, et al. Frequency and characteristics of dysautonomic symptoms in multiple sclerosis: a cross-sectional double-center study with the validated italian version of the composite autonomic symptom score-31. Neurol Sci Off J Ital Neurol Soc Ital Soc Clin Neurophysiol. 2021;42:1395–1403. doi: 10.1007/s10072-020-04620-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Novak P. Post COVID-19 syndrome associated with orthostatic cerebral hypoperfusion syndrome, small fiber neuropathy and benefit of immunotherapy: a case report. eNeurologicalSci. 2020;21:100276. doi: 10.1016/j.ensci.2020.100276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miller AJ, Sheehan T, Bourne KM, et al. Attention and executive function are impaired during active standing in postural tachycardia syndrome. Auton Neurosci. 2020;227:102692. doi: 10.1016/j.autneu.2020.102692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wells R, Malik V, Brooks AG, et al. Cerebral blood flow and cognitive performance in postural tachycardia syndrome: insights from sustained cognitive stress test. J Am Heart Assoc. 2020;9:e017861. doi: 10.1161/JAHA.120.017861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Townsend L, Moloney D, Finucane C, et al. Fatigue following COVID-19 infection is not associated with autonomic dysfunction. PLoS One. 2021;16:e0247280. doi: 10.1371/journal.pone.0247280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goldstein DS. The extended autonomic system, dyshomeostasis, and COVID-19. Clin Auton Res Off J Clin Auton Res Soc. 2020;30:299–315. doi: 10.1007/s10286-020-00714-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goldstein DS. The possible association between COVID-19 and postural tachycardia syndrome. Hear Rhythm. 2021;18:508–509. doi: 10.1016/j.hrthm.2020.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fudim M, Qadri YJ, Ghadimi K, et al. Implications for neuromodulation therapy to control inflammation and related organ dysfunction in COVID-19. J Cardiovasc Transl Res. 2020;13:894–899. doi: 10.1007/s12265-020-10031-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guilmot A, Maldonado Slootjes S, Sellimi A, et al. Immune-mediated neurological syndromes in SARS-CoV-2-infected patients. J Neurol. 2021;268:751–757. doi: 10.1007/s00415-020-10108-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Blitshteyn S, Brinth L, Hendrickson JE, Martinez-Lavin M. Autonomic dysfunction and HPV immunization: an overview. Immunol Res. 2018;66:744–754. doi: 10.1007/s12026-018-9036-1. [DOI] [PubMed] [Google Scholar]
- 47.Ruzieh M, Batizy L, Dasa O, et al. The role of autoantibodies in the syndromes of orthostatic intolerance: a systematic review. Scand Cardiovasc J. 2017;51:243–247. doi: 10.1080/14017431.2017.1355068. [DOI] [PubMed] [Google Scholar]
- 48.Watari M, Nakane S, Mukaino A, et al. Autoimmune postural orthostatic tachycardia syndrome. Ann Clin Transl Neurol. 2018;5:486–492. doi: 10.1002/acn3.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Breeuwsma AC, Hartog LC, Kamper AM, et al. Diagnosing orthostatic hypotension with continuous and interval blood pressure measurement devices. J Hum Hypertens. 2018;32:831–837. doi: 10.1038/s41371-018-0091-9. [DOI] [PubMed] [Google Scholar]
- 50.Manganotti P, Bellavita G, Tommasini V, et al. Cerebrospinal fluid and serum interleukins 6 and 8 during the acute and recovery phase in COVID-19 neuropathies patients. J Med Virol. 2021 doi: 10.1002/jmv.27061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Buoite Stella A, Ajčević M, Furlanis G, Manganotti P. Neurophysiological adaptations to spaceflight and simulated microgravity. Clin Neurophysiol. 2021;132:498–504. doi: 10.1016/j.clinph.2020.11.033. [DOI] [PubMed] [Google Scholar]
- 52.Manganotti P, Bellavita G, D’Acunto L, et al. Clinical neurophysiology and cerebrospinal liquor analysis to detect Guillain-Barré syndrome and polyneuritis cranialis in COVID-19 patients: a case series. J Med Virol. 2021;93:766–774. doi: 10.1002/jmv.26289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chippa V, Aleem A, Anjum F (2021) Post acute coronavirus (COVID-19) syndrome. In: StatPearls. StatPearls Publishing, Treasure Island, FL [PubMed]
- 54.Su XW, Palka SV, Rao RR, et al. SARS-CoV-2-associated Guillain-Barré syndrome with dysautonomia. Muscle Nerve. 2020;62:E48–E49. doi: 10.1002/mus.26988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ghosh R, Roy D, Sengupta S, Benito-León J. Autonomic dysfunction heralding acute motor axonal neuropathy in COVID-19. J Neurovirol. 2020;26:964–966. doi: 10.1007/s13365-020-00908-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Østergaard L. SARS CoV-2 related microvascular damage and symptoms during and after COVID-19: consequences of capillary transit-time changes, tissue hypoxia and inflammation. Physiol Rep. 2021;9:e14726. doi: 10.14814/phy2.14726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cieślik-Guerra UI, Fila M, Kamiński M, et al. Correlation between the activity of the autonomic nervous system and endothelial function in patients with acute coronary syndrome. Pol Arch Med Wewn. 2014;124:509–515. doi: 10.20452/pamw.2456. [DOI] [PubMed] [Google Scholar]
- 58.Daanen H, Bose-O’Reilly S, Brearley M, et al. COVID-19 and thermoregulation-related problems: practical recommendations. Temp (Austin, Tex) 2020;8:1–11. doi: 10.1080/23328940.2020.1790971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Buoite Stella A, Filingeri D, Ravanelli N, et al. Heat risk exacerbation potential for neurology patients during the COVID-19 pandemic and related isolation. Int J Biometeorol. 2021;65:627–630. doi: 10.1007/s00484-020-02044-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mao R, Qiu Y, He J-S, et al. Manifestations and prognosis of gastrointestinal and liver involvement in patients with COVID-19: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2020;5:667–678. doi: 10.1016/S2468-1253(20)30126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wu Y, Guo C, Tang L, et al. Prolonged presence of SARS-CoV-2 viral RNA in faecal samples. Lancet Gastroenterol Hepatol. 2020;5:434–435. doi: 10.1016/S2468-1253(20)30083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xiao F, Tang M, Zheng X, et al. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology. 2020;158:1831–1833.e3. doi: 10.1053/j.gastro.2020.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Weng J, Li Y, Li J, et al. Gastrointestinal sequelae 90 days after discharge for COVID-19. Lancet Gastroenterol Hepatol. 2021;6:344–346. doi: 10.1016/S2468-1253(21)00076-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.McMillan MT, Pan X-Q, Smith AL, et al. Coronavirus-induced demyelination of neural pathways triggers neurogenic bladder overactivity in a mouse model of multiple sclerosis. Am J Physiol Renal Physiol. 2014;307:F612–F622. doi: 10.1152/ajprenal.00151.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pourfridoni M, Pajokh M, Seyedi F. Bladder and bowel incontinence in COVID-19. J Med Virol. 2021;93:2609–2610. doi: 10.1002/jmv.26849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mumm J-N, Osterman A, Ruzicka M, et al. Urinary frequency as a possibly overlooked symptom in COVID-19 patients: does SARS-CoV-2 cause viral cystitis? Eur Urol. 2020;78:624–628. doi: 10.1016/j.eururo.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Welk B, Richard L, Braschi E, Averbeck MA. Is coronavirus disease 2019 associated with indicators of long-term bladder dysfunction? Neurourol Urodyn. 2021 doi: 10.1002/nau.24682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sansone A, Mollaioli D, Ciocca G, et al. Addressing male sexual and reproductive health in the wake of COVID-19 outbreak. J Endocrinol Invest. 2021;44:223–231. doi: 10.1007/s40618-020-01350-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sansone A, Mollaioli D, Ciocca G, et al. “Mask up to keep it up”: preliminary evidence of the association between erectile dysfunction and COVID-19. Andrology. 2021 doi: 10.1111/andr.13003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bertolo R, Cipriani C, Bove P. Anosmia and ageusia: a piece of the puzzle in the etiology of COVID-19-related transitory erectile dysfunction. J Endocrinol Invest. 2021;44:1123–1124. doi: 10.1007/s40618-021-01516-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Duran MB, Yildirim O, Kizilkan Y, et al. Variations in the number of patients presenting with andrological problems during the coronavirus disease 2019 pandemic and the possible reasons for these variations: a multicenter study. Sex Med. 2021;9:100292. doi: 10.1016/j.esxm.2020.100292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kaya Y, Kaya C, Tahta T, et al. Examination of the effect of COVID-19 on sexual dysfunction in women. Int J Clin Pract. 2021;75:e13923. doi: 10.1111/ijcp.13923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Domínguez-Varela IA, Rodríguez-Gutiérrez LA, Morales-Mancillas NR, et al. COVID-19 and the eye: a review. Infect Dis (London, England) 2021;53:399–403. doi: 10.1080/23744235.2021.1882697. [DOI] [PubMed] [Google Scholar]
- 74.Pardhan S, Vaughan M, Zhang J, et al. Sore eyes as the most significant ocular symptom experienced by people with COVID-19: a comparison between pre-COVID-19 and during COVID-19 states. BMJ Open Ophthalmol. 2020;5:e000632. doi: 10.1136/bmjophth-2020-000632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Karahan M, Demirtaş AA, Hazar L, et al. Autonomic dysfunction detection by an automatic pupillometer as a non-invasive test in patients recovered from COVID-19. Graefe’s Arch Clin Exp Ophthalmol: Albr von Graefes Arch fur Klin und Exp Ophthalmol. 2021;9:8–6. doi: 10.1007/s00417-021-05209-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Battaglini D, Santori G, Chandraptham K, et al. Neurological complications and noninvasive multimodal neuromonitoring in critically ill mechanically ventilated COVID-19 patients. Front Neurol. 2020;11:602114. doi: 10.3389/fneur.2020.602114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vrettou CS, Korompoki E, Sarri K, et al. Pupillometry in critically ill patients with COVID-19: a prospective study. Clin Auton Res Off J Clin Auton Res Soc. 2020;30:563–565. doi: 10.1007/s10286-020-00737-7. [DOI] [PMC free article] [PubMed] [Google Scholar]