Abstract
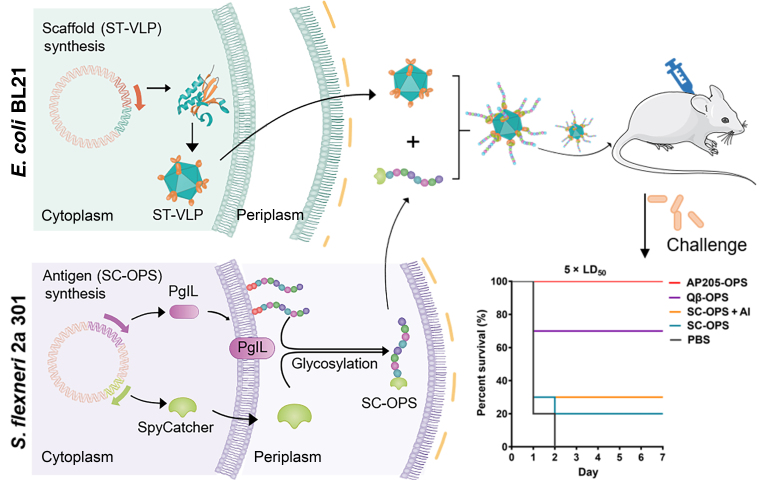
Conjugate vaccines represent one of the most effective means for controlling the occurrence of bacterial diseases. Although nanotechnology has been greatly applied in the field of vaccines, it is seldom used for conjugate vaccine research because it is very difficult to connect polysaccharides and nanocarriers. In this work, an orthogonal and modular biosynthesis method was used to produce nanoconjugate vaccines using the SpyTag/SpyCatcher system. When SpyTag/SpyCatcher system is combined with protein glycosylation technology, bacterial O-polysaccharide obtained from Shigela flexneri 2a can be conjugated onto the surfaces of different virus-like particles (VLPs) in a biocompatible and controlled manner. After confirming the excellent lymph node targeting and humoral immune activation abilities, these nanoconjugate vaccines further induced efficient prophylactic effects against infection in a mouse model. These results demonstrated that natural polysaccharide antigens can be easily connected to VLPs to prepare highly efficient nanoconjugate vaccines. To the best of the researchers’ knowledge, this is the first time VLP-based nanoconjugate vaccines are produced efficiently, and this strategy could be applied to develop various pathogenic nanoconjugate vaccines.
Electronic Supplementary Material
Supplementary material (Figs. S1–S9) is available in the online version of this article at 10.1007/s12274-021-3713-4.
Keywords: Shigela flexneri 2a, O-polysaccharide, virus-like particle, SpyTag/SpyCatcher system, nanoconjugate vaccines
Electronic Supplementary Material
Orthogonal modular biosynthesis of nanoscale conjugate vaccines for vaccination against infection
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Nos. 81930122 and U20A20361) and the National Key Research and Development Project of China (No. 2021YFC2102101).
Contributor Information
Chao Pan, Email: panchaosunny@163.com.
Hengliang Wang, Email: wanghl@bmi.ac.cn.
Li Zhu, Email: jewly54@bmi.ac.cn.
References
- [1].Irvine D J, Swartz M A, Szeto G L. Engineering synthetic vaccines using cues from natural immunity. Nat. Mater. 2013;12:978–990. doi: 10.1038/nmat3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Karch C P, Burkhard P. Vaccine technologies: From whole organisms to rationally designed protein assemblies. Biochem. Pharmacol. 2016;120:1–14. doi: 10.1016/j.bcp.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Schmidt S T, Foged C, Korsholm K S, Rades T. Liposome-based adjuvants for subunit vaccines: Formulation strategies for subunit antigens and immunostimulators. Pharmaceutics. 2016;8:7. doi: 10.3390/pharmaceutics8010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Poteet E, Lewis P, Chen C Y, Ho S O, Do T, Chiang S, Labranche C, Montefiori D, Fujii G, Yao Q Z. Toll-like receptor 3 adjuvant in combination with virus-like particles elicit a humoral response against HIV. Vaccine. 2016;34:5886–5894. doi: 10.1016/j.vaccine.2016.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Vartak A, Sucheck S J. Recent advances in subunit vaccine carriers. Vaccines. 2016;4:12. doi: 10.3390/vaccines4020012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Dowling D J, Scott E A, Scheid A, Bergelson I, Joshi S, Pietrasanta C, Brightman S, Sanchez-Schmitz G, van Haren S D, Ninkovic J, et al. Toll-like receptor 8 agonist nanoparticles mimic immunomodulating effects of the live BCG vaccine and enhance neonatal innate and adaptive immune responses. J. Allergy Clin. Immunol. 2017;140:1339–1350. doi: 10.1016/j.jaci.2016.12.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Lynn G M, Laga R, Darrah P A, Ishizuka A S, Balaci A J, Dulcey A E, Pechar M, Pola R, Gerner M Y, Yamamoto A, et al. In vivo characterization of the physicochemical properties of polymer-linked TLR agonists that enhance vaccine immunogenicity. Nat. Biotechnol. 2015;33:1201–1210. doi: 10.1038/nbt.3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ebrahimian M, Hashemi M, Maleki M, Hashemitabar G, Abnous K, Ramezani M, Haghparast A. Co-delivery of dual toll-like receptor agonists and antigen in poly(lactic-Co-glycolic) acid/polyethylenimine cationic hybrid nanoparticles promote efficient in vivo immune responses. Front. Immunol. 2017;8:1077. doi: 10.3389/fimmu.2017.01077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Christensen D. Vaccine adjuvants: Why and how. Hum. Vaccin. Immunother. 2016;12:2709–2711. doi: 10.1080/21645515.2016.1219003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Banday A H, Jeelani S, Hruby V J. Cancer vaccine adjuvants-recent clinical progress and future perspectives. Immunopharmacol. Immunotoxicol. 2015;37:1–11. doi: 10.3109/08923973.2014.971963. [DOI] [PubMed] [Google Scholar]
- [11].Xu H, Huang J, Liu Z L, Li X, Wang K F, Feng E L, Wu J, Zhu L, Yao K H, Pan C, et al. Expression of Bordetella pertussis antigens fused to different vectors and their effectiveness as vaccines. Vaccines (Basel) 2021;9:542. doi: 10.3390/vaccines9060542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Reddy S T, van der Vlies A J, Simeoni E, Angeli V, Randolph G J, O’Neil C P, Lee L K, Swartz M A, Hubbell J A. Exploiting lymphatic transport and complement activation in nanoparticle vaccines. Nat. Biotechnol. 2007;25:1159–1164. doi: 10.1038/nbt1332. [DOI] [PubMed] [Google Scholar]
- [13].Irvine D J, Hanson M C, Rakhra K, Tokatlian T. Synthetic nanoparticles for vaccines and immunotherapy. Chem. Rev. 2015;115:11109–11146. doi: 10.1021/acs.chemrev.5b00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Molino N M, Neek M, Tucker J A, Nelson E L, Wang S W. Display of DNA on nanoparticles for targeting antigen presenting cells. ACS Biomater. Sci. Eng. 2017;3:496–501. doi: 10.1021/acsbiomaterials.7b00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Bachmann M F, Jennings G T. Vaccine delivery: A matter of size, geometry, kinetics and molecular patterns. Nat. Rev. Immunol. 2010;10:787–796. doi: 10.1038/nri2868. [DOI] [PubMed] [Google Scholar]
- [16].Win S J, Ward V K, Dunbar P R, Young S L, Baird M A. Cross-presentation of epitopes on virus-like particles via the MHC I receptor recycling pathway. Immunol. Cell Biol. 2011;89:681–688. doi: 10.1038/icb.2010.161. [DOI] [PubMed] [Google Scholar]
- [17].Lin A Y, Lunsford J, Bear A S, Young J K, Eckels P, Luo L, Foster A E, Drezek R A. High-density sub-100-nm peptide-gold nanoparticle complexes improve vaccine presentation by dendritic cells in vitro. Nanoscale Res. Lett. 2013;8:72. doi: 10.1186/1556-276X-8-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].An M, Li M, Xi JC, Liu H P. Silica nanoparticle as a lymph node targeting platform for vaccine delivery. ACS Appl. Mater. Interfaces. 2017;9:23466–23475. doi: 10.1021/acsami.7b06024. [DOI] [PubMed] [Google Scholar]
- [19].Kang S, Ahn S, Lee J, Kim J Y, Choi M, Gujrati V, Kim H, Kim J, Shin E C, Jon S. Effects of gold nanoparticle-based vaccine size on lymph node delivery and cytotoxic T-lymphocyte responses. J. Control. Release. 2017;256:56–67. doi: 10.1016/j.jconrel.2017.04.024. [DOI] [PubMed] [Google Scholar]
- [20].Rahimian S, Kleinovink J W, Fransen M F, Mezzanotte L, Gold H, Wisse P, Overkleeft H, Amidi M, Jiskoot W, Lowik C W, et al. Near-infrared labeled, ovalbumin loaded polymeric nanoparticles based on a hydrophilic polyester as model vaccine: In vivo tracking and evaluation of antigen-specific CD8+ T cell immune response. Biomaterials. 2015;37:469–477. doi: 10.1016/j.biomaterials.2014.10.043. [DOI] [PubMed] [Google Scholar]
- [21].Wen R, Umeano A C, Chen P P, Farooqi A A. Polymer-based drug delivery systems for cancer. Crit. Rev. Ther. Drug Carrier Syst. 2018;35:521–553. doi: 10.1615/CritRevTherDrugCarrierSyst.2018021124. [DOI] [PubMed] [Google Scholar]
- [22].De Gregorio E, Rappuoli R. From empiricism to rational design: A personal perspective of the evolution of vaccine development. Nat. Rev. Immunol. 2014;14:505–514. doi: 10.1038/nri3694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Pushko P, Pumpens P, Grens E. Development of virus-like particle technology from small highly symmetric to large complex virus-like particle structures. Intervirology. 2013;56:141–165. doi: 10.1159/000346773. [DOI] [PubMed] [Google Scholar]
- [24].Smith M T, Hawes A K, Bundy B C. Reengineering viruses and virus-like particles through chemical functionalization strategies. Curr. Opin. Biotechnol. 2013;24:620–626. doi: 10.1016/j.copbio.2013.01.011. [DOI] [PubMed] [Google Scholar]
- [25].Mateu M G. Virus engineering: Functionalization and stabilization. Protein Eng. Des. Sel. 2011;24:53–63. doi: 10.1093/protein/gzq069. [DOI] [PubMed] [Google Scholar]
- [26].Strable E, Finn M G. Chemical modification of viruses and viruslike particles. In: Manchester M, Steinmetz M F, editors. Viruses and Nanotechnology. Berlin: Springer; 2009. pp. 1–21. [Google Scholar]
- [27].Sletten E M, Bertozzi C R. Bioorthogonal chemistry: Fishing for selectivity in a sea of functionality. Angew. Chem., Int. Ed. 2009;48:6974–6998. doi: 10.1002/anie.200900942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Zakeri, B.; Fierer, J. O.; Celik, E.; Chittock, E. C.; Schwarz-Linek, U.; Moy, V. T.; Howarth, M. Peptide tag forming a rapid covalent bond to a protein, through engineering a bacterial adhesin. Proc. Natl. Acad. Sci. USA2012, 109, E690–E697. [DOI] [PMC free article] [PubMed]
- [29].Li L, Fierer J O, Rapoport T A, Howarth M. Structural analysis and optimization of the covalent association between SpyCatcher and a peptide Tag. J. Mol. Biol. 2014;426:309–317. doi: 10.1016/j.jmb.2013.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Brune K D, Leneghan D B, Brian I J, Ishizuka A S, Bachmann M F, Draper S J, Biswas S, Howarth M. Plug-and-display: Decoration of virus-like particles via isopeptide bonds for modular immunization. Sci. Rep. 2016;6:19234. doi: 10.1038/srep19234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Janitzek C M, Matondo S, Thrane S, Nielsen M A, Kavishe R, Mwakalinga S B, Theander T G, Salanti A, Sander A F. Bacterial superglue generates a full-length circumsporozoite protein virus-like particle vaccine capable of inducing high and durable antibody responses. Malar. J. 2016;15:545. doi: 10.1186/s12936-016-1574-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Brune K D, Buldun C M, Li Y Y, Taylor I J, Brod F, Biswas S, Howarth M. Dual plug-and-display synthetic assembly using orthogonal reactive proteins for twin antigen immunization. Bioconjugate Chem. 2017;28:1544–1551. doi: 10.1021/acs.bioconjchem.7b00174. [DOI] [PubMed] [Google Scholar]
- [33].Thrane S, Janitzek C M, Matondo S, Resende M, Gustavsson T, de Jongh W A, Clemmensen S, Roeffen W, van de Vegte-Bolmer M, van Gemert G J, et al. Bacterial superglue enables easy development of efficient virus-like particle based vaccines. J. Nanobiotechnol. 2016;14:30. doi: 10.1186/s12951-016-0181-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Sáfadi M A, Bettinger J A, Maturana G M, Enwere G, Borrow R, Global Meningococcal I. Evolving meningococcal immunization strategies. Exp. Rev. Vaccin. 2015;14:505–517. doi: 10.1586/14760584.2015.979799. [DOI] [PubMed] [Google Scholar]
- [35].Martin L B, Simon R, MacLennan C A, Tennant S M, Sahastrabuddhe S, Khan M I. Status of paratyphoid fever vaccine research and development. Vaccine. 2016;34:2900–2902. doi: 10.1016/j.vaccine.2016.03.106. [DOI] [PubMed] [Google Scholar]
- [36].Bröker M, Berti F, Costantino P. Factors contributing to the immunogenicity of meningococcal conjugate vaccines. Hum. Vacc. Immunother. 2016;12:1808–1824. doi: 10.1080/21645515.2016.1153206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Kuberan B, Linhardt R J. Carbohydrate based vaccines. Curr. Org. Chem. 2000;4:635–677. doi: 10.2174/1385272003376111. [DOI] [Google Scholar]
- [38].Pan C, Wu J, Qing S, Zhang X, Zhang L L, Yue H, Zeng M, Wang B, Yuan Z, Qiu Y F, et al. Biosynthesis of self-assembled proteinaceous nanoparticles for vaccination. Adv. Mater. 2020;32:2002940. doi: 10.1002/adma.202002940. [DOI] [PubMed] [Google Scholar]
- [39].Peng, Z. H.; Wu, J.; Wang, K. F.; Li, X.; Sun, P.; Zhang, L. L.; Huang, J.; Liu, Y.; Hua, X. T.; Yu, Y. S. et al. Production of a promising biosynthetic self-assembled nanoconjugate vaccine against Klebsiella pneumoniae serotype O2 in a general Escherichia coli host. Adv. Sci., in press, DOI: 10.1002/advs.202100549. [DOI] [PMC free article] [PubMed]
- [40].Pan C, Sun P, Liu B, Liang H Y, Peng Z H, Dong Y, Wang D S, Liu X K, Wang B, Zeng M, et al. Biosynthesis of conjugate vaccines using an O-linked glycosylation system. mBio. 2016;7:e00443–16. doi: 10.1128/mBio.00443-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Hanson C M, George A M, Sawadogo A, Schreiber B. Is freezing in the vaccine cold chain an ongoing issue? A literature review. Vaccine. 2017;35:2127–2133. doi: 10.1016/j.vaccine.2016.09.070. [DOI] [PubMed] [Google Scholar]
- [42].Paulis L E, Mandal S, Kreutz M, Figdor C G. Dendritic cell-based nanovaccines for cancer immunotherapy. Curr. Opin. Immunol. 2013;25:389–395. doi: 10.1016/j.coi.2013.03.001. [DOI] [PubMed] [Google Scholar]
- [43].Vinuesa C G, Linterman M A, Yu D, MacLennan I C M. Follicular helper T cells. Annu. Rev. Immunol. 2016;34:335–368. doi: 10.1146/annurev-immunol-041015-055605. [DOI] [PubMed] [Google Scholar]
- [44].McHeyzer-Williams L J, Milpied P J, Okitsu S L, McHeyzer-Williams M G. Class-switched memory B cells remodel BCRs within secondary germinal centers. Nat. Immunol. 2015;16:296–305. doi: 10.1038/ni.3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Orthogonal modular biosynthesis of nanoscale conjugate vaccines for vaccination against infection