Abstract
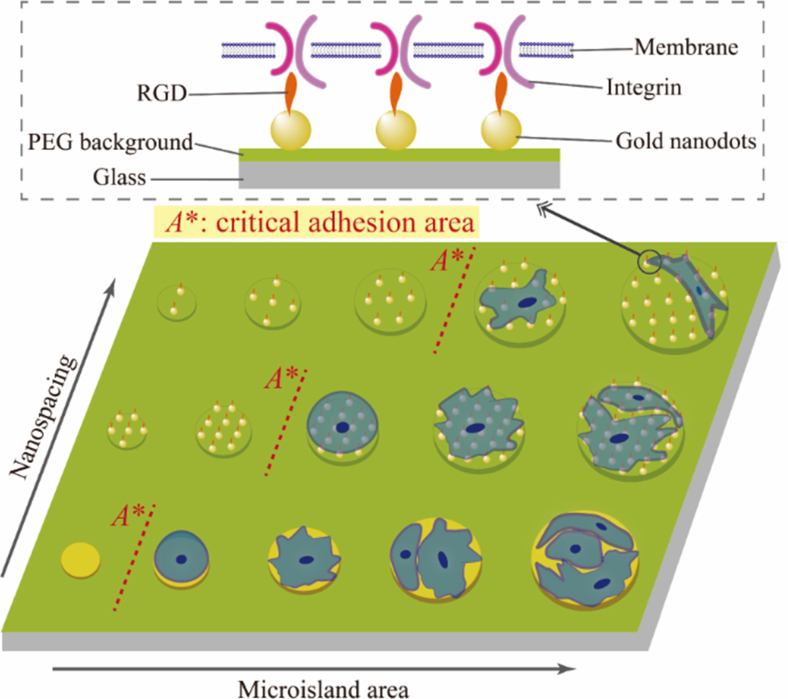
Cell adhesion to extracellular matrices (ECM) is critical to physiological and pathological processes as well as biomedical and biotechnological applications. It has been known that a cell can adhere on an adhesive microisland only over a critical size. But no publication has concerned critical adhesion areas of cells on microislands with nanoarray decoration. Herein, we fabricated a series of micro-nanopatterns with different microisland sizes and arginine—glycine—aspartate (RGD) nanospacings on a nonfouling poly(ethylene glycol) background. Besides reproducing that nanospacing of RGD, a ligand of its receptor integrin (a membrane protein), significantly influences specific cell adhesion on bioactive nanoarrays, we confirmed that the concept of critical adhesion area originally suggested in studies of cells on micropatterns was justified also on the micro-nanopatterns, yet the latter exhibited more characteristic behaviors of cell adhesion. We found increased critical adhesion areas of human mesenchymal stem cells (hMSCs) on nanoarrayed microislands with increased RGD nanospacings. However, the numbers of nanodots with respect to the critical adhesion areas were not a constant. A unified interpretation was then put forward after combining nonspecific background adhesion and specific cell adhesion. We further carried out the asymptotic analysis of a series of micro-nanopatterned surfaces to obtain the effective RGD nanospacing on unpatterned free surfaces with densely grafted RGD, which could be estimated nonzero but has never been revealed previously without the assistance of the micro-nanopatterning techniques and the corresponding analysis.
Electronic Supplementary Material
Supplementary materials and methods (details of fabrication of micro-nanopatterns), and supplementary results (selective adhesion or localization of hMSCs on nanoarrayed microislands with non-fouling background, calculation of critical number of integrin-ligand binding N*, etc.) are available in the online version of this article at 10.1007/s12274-021-3711-6.
Keywords: biomaterial, surface patterning, cell adhesion, arginine—glycine—aspartate (RGD) nanospacing, poly(ethylene glycol), critical adhesion area
Electronic Supplementary Material
Critical adhesion areas of cells on micro-nanopatterns
Acknowledgements
This work was financially supported by the National Key R&D Program of China (No. 2016YFC1100300) and the National Natural Science Foundation of China (Nos. 21961160721 and 21704018).
References
- [1].Ravasio A, Le A P, Saw T B, Tarle V, Ong H T, Bertocchi C, Mège R M, Lim C T, Gov N S, Ladoux B. Regulation of epithelial cell organization by tuning cell-substrate adhesion. Integr. Biol. 2015;7:1228–1241. doi: 10.1039/C5IB00196J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Davidson M D, Burdick J A, Wells R G. Engineered biomaterial platforms to study fibrosis. Adv. Healthc. Mater. 2020;9:1901682. doi: 10.1002/adhm.201901682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Salber J, Gräter S, Harwardt M, Hofmann M, Klee D, Dujic J, Jinghuan H, Ding J D, Kippenberger S, Bernd A, et al. Influence of different ECM mimetic peptide sequences embedded in a nonfouling environment on the specific adhesion of human-skin keratinocytes and fibroblasts on deformable substrates. Small. 2007;3:1023–1031. doi: 10.1002/smll.200600596. [DOI] [PubMed] [Google Scholar]
- [4].Chen Q, Yu S, Zhang D H, Zhang W J, Zhang H D, Zou J C, Mao Z W, Yuan Y, Gao C Y, Liu R H. Impact of antifouling PEG layer on the performance of functional peptides in regulating cell behaviors. J. Am. Chem. Soc. 2019;141:16772–16780. doi: 10.1021/jacs.9b07105. [DOI] [PubMed] [Google Scholar]
- [5].Crosby C O, Zoldan J. Mimicking the physical cues of the ECM in angiogenic biomaterials. Regen. Biomater. 2019;6:61–73. doi: 10.1093/rb/rbz003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Yalak G, Shiu J Y, Schoen I, Mitsi M, Vogel V. Phosphorylated fibronectin enhances cell attachment and upregulates mechanical cell functions. PLoS One. 2019;14:e0218893. doi: 10.1371/journal.pone.0218893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Chen Q, Zhang D H, Zhang W J, Zhang H D, Zou J C, Chen M J, Li J, Yuan Y, Liu R H. Dual mechanism β-amino acid polymers promoting cell adhesion. Nat. Commun. 2021;12:562. doi: 10.1038/s41467-020-20858-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Xu B B, Feng C, Hu J H, Shi P, Gu G X, Wang L, Huang X Y. Spin-casting polymer brush films for stimuli-responsive and antifouling surfaces. ACS Appl. Mater. Interfaces. 2016;8:6685–6692. doi: 10.1021/acsami.5b12820. [DOI] [PubMed] [Google Scholar]
- [9].Silva J M, García J R, Reis R L, García A J, Mano J F. Tuning cell adhesive properties via layer-by-layer assembly of chitosan and alginate. Acta Biomater. 2017;51:279–293. doi: 10.1016/j.actbio.2017.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Li J, Di Russo J, Hua X M, Chu Z Q, Spatz J P, Wei Q. Surface immobilized E-cadherin mimetic peptide regulates the adhesion and clustering of epithelial cells. Adv. Healthc. Mater. 2019;8:1801384. doi: 10.1002/adhm.201801384. [DOI] [PubMed] [Google Scholar]
- [11].Gao J M, Ding X Q, Yu X Y, Chen X B, Zhang X Y, Cui S Q, Shi J Y, Chen J, Yu L, Chen S, et al. Cell-free bilayered porous scaffolds for osteochondral regeneration fabricated by continuous 3D-printing using nascent physical hydrogel as ink. Adv. Healthc. Mater. 2021;10:2001404. doi: 10.1002/adhm.202001404. [DOI] [PubMed] [Google Scholar]
- [12].Xie Y J, Hu C, Feng Y, Li D F, Ai T, Huang Y L, Chen X, Huang L J, Tan J L. Osteoimmunomodulatory effects of biomaterial modification strategies on macrophage polarization and bone regeneration. Regen. Biomater. 2020;7:233–245. doi: 10.1093/rb/rbaa006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Chopra A, Kutys M L, Zhang K H, Polacheck W J, Sheng C C, Luu R J, Eyckmans J, Hinson J T, Seidman J G, Seidman C E, et al. Force generation via β-cardiac myosin, titin, and α-actinin drives cardiac sarcomere assembly from cell-matrix adhesions. Dev. Cell. 2018;44:87–96.e5. doi: 10.1016/j.devcel.2017.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Doss B L, Pan M, Gupta M, Grenci G, Mège R M, Lim C T, Sheetz M P, Voituriez R, Ladoux B. Cell response to substrate rigidity is regulated by active and passive cytoskeletal stress. Proc. Natl. Acad. Sci. USA. 2020;117:12817–12825. doi: 10.1073/pnas.1917555117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kong F, Li Z H, Parks W M, Dumbauld D W, García A J, Mould A P, Humphries M J, Zhu C. Cyclic mechanical reinforcement of integrin-ligand interactions. Mol. Cell. 2013;49:1060–1068. doi: 10.1016/j.molcel.2013.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Oria R, Wiegand T, Escribano J, Elosegui-Artola A, Uriarte J J, Moreno-Pulido C, Platzman I, Delcanale P, Albertazzi L, Navajas D, et al. Force loading explains spatial sensing of ligands by cells. Nature. 2017;552:219–224. doi: 10.1038/nature24662. [DOI] [PubMed] [Google Scholar]
- [17].Elloumi-Hannachi I, García J R, Shekeran A, García A J. Contributions of the integrin β1 tail to cell adhesive forces. Exp. Cell Res. 2015;332:212–222. doi: 10.1016/j.yexcr.2014.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Zhou D W, Lee T T, Weng S, Fu J P, García A J. Effects of substrate stiffness and actomyosin contractility on coupling between force transmission and vinculin-paxillin recruitment at single focal adhesions. Mol. Biol. Cell. 2017;28:1901–1911. doi: 10.1091/mbc.e17-02-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Yu L X, Hou Y, Xie W Y, Camacho J L C, Cheng C, Holle A, Young J, Trappmann B, Zhao W F, Melzig M F, et al. Ligand diffusion enables force-independent cell adhesion via activating α5β1 integrin and initiating Rac and RhoA signaling. Adv. Mater. 2020;32:2002566. doi: 10.1002/adma.202002566. [DOI] [PubMed] [Google Scholar]
- [20].Schaufler V, Czichos-Medda H, Hirschfeld-Warnecken V, Neubauer S, Rechenmacher F, Medda R, Kessler H, Geiger B, Spatz J P, Cavalcanti-Adam E A. Selective binding and lateral clustering of α5β1 and αvβ3 integrins: Unraveling the spatial requirements for cell spreading and focal adhesion assembly. Cell Adhes. Migr. 2016;10:505–515. doi: 10.1080/19336918.2016.1163453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kechagia J Z, Ivaska J, Roca-Cusachs P. Integrins as biomechanical sensors of the microenvironment. Nat. Rev. Mol. Cell Biol. 2019;20:457–473. doi: 10.1038/s41580-019-0134-2. [DOI] [PubMed] [Google Scholar]
- [22].Sales A, Ende K, Diemer J, Kyvik A R, Veciana J, Ratera I, Kemkemer R, Spatz J P, Guasch J. Cell type-dependent integrin distribution in adhesion and migration responses on protein-coated microgrooved substrates. ACS Omega. 2019;4:1791–1800. doi: 10.1021/acsomega.8b03608. [DOI] [Google Scholar]
- [23].Liu Q, Zheng S, Ye K, He J H, Shen Y, Cui S Q, Huang J L, Gu Y X, Ding J D. Cell migration regulated by RGD nanospacing and enhanced under moderate cell adhesion on biomaterials. Biomaterials. 2020;263:120327. doi: 10.1016/j.biomaterials.2020.120327. [DOI] [PubMed] [Google Scholar]
- [24].Kang H, Wong D S H, Yan X H, Jung H J, Kim S, Lin S, Wei K C, Li G, Dravid V P, Bian L M. Remote control of multimodal nanoscale ligand oscillations regulates stem cell adhesion and differentiation. ACS Nano. 2017;11:9636–9649. doi: 10.1021/acsnano.7b02857. [DOI] [PubMed] [Google Scholar]
- [25].Pallarola D, Platzman I, Bochen A, Cavalcanti-Adam E A, Axmann M, Kessler H, Geiger B, Spatz J P. Focal adhesion stabilization by enhanced integrin-cRGD binding affinity. BioNanoMaterials. 2017;18:20160014. doi: 10.1515/bnm-2016-0014. [DOI] [Google Scholar]
- [26].Li J C, Chen Y, Kawazoe N, Chen G P. Ligand density-dependent influence of arginine—glycine—aspartate functionalized gold nanoparticles on osteogenic and adipogenic differentiation of mesenchymal stem cells. Nano Res. 2018;11:1247–1261. doi: 10.1007/s12274-017-1738-5. [DOI] [Google Scholar]
- [27].Kafi M A, Aktar K, Todo M, Dahiya R. Engineered chitosan for improved 3D tissue growth through Paxillin-FAK-ERK activation. Regen. Biomater. 2020;7:141–151. doi: 10.1093/rb/rbz034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Wong S H D, Wong W K R, Lai C H N, Oh J, Li Z, Chen X Y, Yuan W H, Bian L M. Soft polymeric matrix as a macroscopic cage for magnetically modulating reversible nanoscale ligand presentation. Nano Lett. 2020;20:3207–3216. doi: 10.1021/acs.nanolett.9b05315. [DOI] [PubMed] [Google Scholar]
- [29].Massia S P, Hubbell J A. An RGD spacing of 440 nm is sufficient for integrin alpha V beta 3-mediated fibroblast spreading and 140 nm for focal contact and stress fiber formation. J. Cell Biol. 1991;114:1089–1100. doi: 10.1083/jcb.114.5.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Cavalcanti-Adam E A, Volberg T, Micoulet A, Kessler H, Geiger B, Spatz J P. Cell spreading and focal adhesion dynamics are regulated by spacing of integrin ligands. Biophys. J. 2007;92:2964–2974. doi: 10.1529/biophysj.106.089730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Graeter S V, Huang J, Perschmann N, López-García M, Kessler H, Ding J D, Spatz J P. Mimicking cellular environments by nanostructured soft interfaces. Nano Lett. 2007;7:1413–1418. doi: 10.1021/nl070098g. [DOI] [PubMed] [Google Scholar]
- [32].Cheng Z A, Zouani O F, Glinel K, Jonas A M, Durrieu M C. Bioactive chemical nanopatterns impact human mesenchymal stem cell fate. Nano Lett. 2013;13:3923–3929. doi: 10.1021/nl4020149. [DOI] [PubMed] [Google Scholar]
- [33].Lagunas A, Castaño A G, Artés J M, Vida Y, Collado D, Pérez-Inestrosa E, Gorostiza P, Claros S, Andrades J A, Samitier J. Large-scale dendrimer-based uneven nanopatterns for the study of local arginine-glycine-aspartic acid (RGD) density effects on cell adhesion. Nano Res. 2014;7:399–409. doi: 10.1007/s12274-014-0406-2. [DOI] [Google Scholar]
- [34].Tsimbouri P, Gadegaard N, Burgess K, White K, Reynolds P, Herzyk P, Oreffo R, Dalby M J. Nanotopographical effects on mesenchymal stem cell morphology and phenotype. J. Cell. Biochem. 2014;115:380–390. doi: 10.1002/jcb.24673. [DOI] [PubMed] [Google Scholar]
- [35].Li S Y, Wang X, Cao B, Ye K, Li Z H, Ding J D. Effects of nanoscale spatial arrangement of arginine—glycine—aspartate peptides on dedifferentiation of chondrocytes. Nano Lett. 2015;15:7755–7765. doi: 10.1021/acs.nanolett.5b04043. [DOI] [PubMed] [Google Scholar]
- [36].Mashinchian O, Turner L A, Dalby M J, Laurent S, Shokrgozar M A, Bonakdar S, Imani M, Mahmoudi M. Regulation of stem cell fate by nanomaterial substrates. Nanomedicine. 2015;10:829–847. doi: 10.2217/nnm.14.225. [DOI] [PubMed] [Google Scholar]
- [37].Ye K, Cao L P, Li S Y, Yu L, Ding J D. Interplay of matrix stiffness and cell-cell contact in regulating differentiation of stem cells. ACS Appl. Mater. Interfaces. 2016;8:21903–21913. doi: 10.1021/acsami.5b09746. [DOI] [PubMed] [Google Scholar]
- [38].Lagunas A, Tsintzou I, Vida Y, Collado D, Pérez-Inestrosa E, Pereira C R, Magalhaes J, Andrades J A, Samitier J. Tailoring RGD local surface density at the nanoscale toward adult stem cell chondrogenic commitment. Nano Res. 2017;10:1959–1971. doi: 10.1007/s12274-016-1382-5. [DOI] [Google Scholar]
- [39].Zhang M, Sun Q, Liu Y L, Chu Z Q, Yu L X, Hou Y, Kang H, Wei Q, Zhao W F, Spatz J P, et al. Controllable ligand spacing stimulates cellular mechanotransduction and promotes stem cell osteogenic differentiation on soft hydrogels. Biomaterials. 2021;268:120543. doi: 10.1016/j.biomaterials.2020.120543. [DOI] [PubMed] [Google Scholar]
- [40].Arnold M, Cavalcanti-Adam E A, Glass R, Blümmel J, Eck W, Kantlehner M, Kessler H, Spatz J P. Activation of integrin function by nanopatterned adhesive interfaces. ChemPhysChem. 2004;5:383–388. doi: 10.1002/cphc.200301014. [DOI] [PubMed] [Google Scholar]
- [41].Huang J H, Gräter S V, Corbellini F, Rinck S, Bock E, Kemkemer R, Kessler H, Ding J D, Spatz J P. Impact of order and disorder in RGD nanopatterns on cell adhesion. Nano Lett. 2009;9:1111–1116. doi: 10.1021/nl803548b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Liu Y, Medda R, Liu Z, Galior K, Yehl K, Spatz J P, Cavalcanti-Adam E A, Salaita K. Nanoparticle tension probes patterned at the nanoscale: Impact of integrin clustering on force transmission. Nano Lett. 2014;14:5539–5546. doi: 10.1021/nl501912g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Ye K, Wang X, Cao L P, Li S Y, Li Z H, Yu L, Ding J D. Matrix stiffness and nanoscale spatial organization of cell-adhesive ligands direct stem cell fate. Nano Lett. 2015;15:4720–4729. doi: 10.1021/acs.nanolett.5b01619. [DOI] [PubMed] [Google Scholar]
- [44].Deng J, Zhao C S, Spatz J P, Wei Q. Nanopatterned adhesive, stretchable hydrogel to control ligand spacing and regulate cell spreading and migration. ACS Nano. 2017;11:8282–8291. doi: 10.1021/acsnano.7b03449. [DOI] [PubMed] [Google Scholar]
- [45].Gallant N D, Capadona J R, Frazier A B, Collard D M, García A J. Micropatterned surfaces to engineer focal adhesions for analysis of cell adhesion strengthening. Langmuir. 2002;18:5579–5584. doi: 10.1021/la025554p. [DOI] [Google Scholar]
- [46].Yao X, Peng R, Ding J D. Cell-material interactions revealed via material techniques of surface patterning. Adv. Mater. 2013;25:5257–5286. doi: 10.1002/adma.201301762. [DOI] [PubMed] [Google Scholar]
- [47].Estévez M, Martínez E, Yarwood S J, Dalby M J, Samitier J. Adhesion and migration of cells responding to microtopography. J. Biomed. Mater. Res. 2015;103:1659–1668. doi: 10.1002/jbm.a.35293. [DOI] [PubMed] [Google Scholar]
- [48].Sonam S, Sathe S R, Yim E K F, Sheetz M P, Lim C T. Cell contractility arising from topography and shear flow determines human mesenchymal stem cell fate. Sci. Rep. 2016;6:20415. doi: 10.1038/srep20415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Cao B, Peng Y M, Liu X N, Ding J D. Effects of functional groups of materials on nonspecific adhesion and chondrogenic induction of mesenchymal stem cells on free and micropatterned surfaces. ACS Appl. Mater. Interfaces. 2017;9:23574–23585. doi: 10.1021/acsami.7b08339. [DOI] [PubMed] [Google Scholar]
- [50].Cho D H, Xie T, Truong J, Stoner A C, Hahm J I. Recent advances towards single biomolecule level understanding of protein adsorption phenomena unique to nanoscale polymer surfaces with chemical variations. Nano Res. 2020;13:1295–1317. doi: 10.1007/s12274-020-2735-7. [DOI] [Google Scholar]
- [51].Liu R L, Ding J D. Chromosomal repositioning and gene regulation of cells on a micropillar array. ACS Appl. Mater. Interfaces. 2020;12:35799–35812. doi: 10.1021/acsami.0c05883. [DOI] [PubMed] [Google Scholar]
- [52].Yao X, Ding J D. Effects of microstripe geometry on guided cell migration. ACS Appl. Mater. Interfaces. 2020;12:27971–27983. doi: 10.1021/acsami.0c05024. [DOI] [PubMed] [Google Scholar]
- [53].Zhao P, Li X, Fang Q, Wang F L, Ao Q, Wang X H, Tian X H, Tong H, Bai S L, Fan J. Surface modification of small intestine submucosa in tissue engineering. Regen. Biomater. 2020;7:339–348. doi: 10.1093/rb/rbaa014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Yao X, Wang X L, Ding J D. Exploration of possible cell chirality using material techniques of surface patterning. Acta Biomater. 2021;126:92–108. doi: 10.1016/j.actbio.2021.02.032. [DOI] [PubMed] [Google Scholar]
- [55].Chen C S, Mrksich M, Huang S, Whitesides G M, Ingber D E. Geometric control of cell life and death. Science. 1997;276:1425–1428. doi: 10.1126/science.276.5317.1425. [DOI] [PubMed] [Google Scholar]
- [56].Yan C, Sun J G, Ding J D. Critical areas of cell adhesion on micropatterned surfaces. Biomaterials. 2011;32:3931–3938. doi: 10.1016/j.biomaterials.2011.01.078. [DOI] [PubMed] [Google Scholar]
- [57].Glass R, Möller M, Spatz J P. Block copolymer micelle nanolithography. Nanotechnology. 2003;14:1153–1160. doi: 10.1088/0957-4484/14/10/314. [DOI] [Google Scholar]
- [58].Arnold M, Schwieder M, Blümmel J, Cavalcanti-Adam E A, López-Garcia M, Kessler H, Geiger B, et al. Cell interactions with hierarchically structured nano-patterned adhesive surfaces. Soft Matter. 2009;5:72–77. doi: 10.1039/B815634D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Wang X, Li S Y, Yan C, Liu P, Ding J D. Fabrication of RGD micro/nanopattern and corresponding study of stem cell sifferentiation. Nano Lett. 2015;15:1457–1467. doi: 10.1021/nl5049862. [DOI] [PubMed] [Google Scholar]
- [60].Wang L Y, Cai P Q, Luo J, Zhang F L, Liu J, Chen Y P, Zhu Z P, Song Y Y, Yang B Q, Liu X, et al. Engineering subcellular-patterned biointerfaces to regulate the surface wetting of multicellular spheroids. Nano Res. 2018;11:5704–5715. doi: 10.1007/s12274-018-2117-6. [DOI] [Google Scholar]
- [61].Dai J, Yao Y. Adaptive ordering and filament polymerization of cell cytoskeleton by tunable nanoarrays. Nano Res. 2021;14:620–627. doi: 10.1007/s12274-020-3076-2. [DOI] [Google Scholar]
- [62].Yao X, Liu R L, Liang X Y, Ding J D. Critical areas of proliferation of single cells on micropatterned surfaces and corresponding cell type dependence. ACS Appl. Mater. Interfaces. 2019;11:15366–15380. doi: 10.1021/acsami.9b03780. [DOI] [PubMed] [Google Scholar]
- [63].Xiong J P, Stehle T, Diefenbach B, Zhang R G, Dunker R, Scott D L, Joachimiak A, Goodman S L, Arnaout M A. Crystal structure of the extracellular segment of integrin αVβ3. Science. 2001;294:339–345. doi: 10.1126/science.1064535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Xiong J P, Stehle T, Zhang R G, Joachimiak A, Frech M, Goodman S L, Arnaout M A. Crystal structure of the extracellular segment of integrin αVβ3 in complex with an Arg-Gly-Asp ligand. Science. 2002;296:151–155. doi: 10.1126/science.1069040. [DOI] [PubMed] [Google Scholar]
- [65].Jiang Y H, Jahagirdar B N, Reinhardt R L, Schwartz R E, Keene C D, Ortiz-Gonzalez X R, Reyes M, Lenvik T, Lund T, Blackstad M, et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41–49. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- [66].Even-Ram S, Artym V, Yamada K M. Matrix control of stem cell fate. Cell. 2006;126:645–647. doi: 10.1016/j.cell.2006.08.008. [DOI] [PubMed] [Google Scholar]
- [67].Peng R, Yao X, Ding J D. Effect of cell anisotropy on differentiation of stem cells on micropatterned surfaces through the controlled single cell adhesion. Biomaterials. 2011;32:8048–8057. doi: 10.1016/j.biomaterials.2011.07.035. [DOI] [PubMed] [Google Scholar]
- [68].Peng Y M, Liu Q J, He T L, Ye K, Yao X, Ding J D. Degradation rate affords a dynamic cue to regulate stem cells beyond varied matrix stiffness. Biomaterials. 2018;178:467–480. doi: 10.1016/j.biomaterials.2018.04.021. [DOI] [PubMed] [Google Scholar]
- [69].Saburi E, Abazari M F, Hassannia H, Mansour R N, Eshaghi-Gorji R, Gheibi M, Rahmati M, Enderami S E. The use of mesenchymal stem cells in the process of treatment and tissue regeneration after recovery in patients with Covid-19. Gene. 2021;777:145471. doi: 10.1016/j.gene.2021.145471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Mao T J, He Y N, Gu Y X, Yang Y Q, Yu Y, Wang X L, Ding J D. Critical frequency and critical stretching rate for reorientation of cells on a cyclically stretched polymer in a microfluidic chip. ACS Appl. Mater. Interfaces. 2021;13:13934–13948. doi: 10.1021/acsami.0c21186. [DOI] [PubMed] [Google Scholar]
- [71].Haubner R, Gratias R, Diefenbach B, Goodman S L, Jonczyk A, Kessler H. Structural and functional aspects of RGD-containing cyclic pentapeptides as highly potent and selective integrin αvβ3 antagonists. J. Am. Chem. Soc. 1996;118:7461–7472. doi: 10.1021/ja9603721. [DOI] [Google Scholar]
- [72].Hersel U, Dahmen C, Kessler H. RGD modified polymers: Biomaterials for stimulated cell adhesion and beyond. Biomaterials. 2003;24:4385–4415. doi: 10.1016/S0142-9612(03)00343-0. [DOI] [PubMed] [Google Scholar]
- [73].Cui S Q, Yu L, Ding J D. Strategy of “Block Blends” to generate polymeric thermogels versus that of one-component block copolymer. Macromolecules. 2020;53:11051–11064. doi: 10.1021/acs.macromol.0c02488. [DOI] [Google Scholar]
- [74].Cui S Q, Chen L, Yu L, Ding J D. Synergism among polydispersed amphiphilic block copolymers leading to spontaneous physical hydrogelation upon heating. Macromolecules. 2020;53:7726–7739. doi: 10.1021/acs.macromol.0c01430. [DOI] [Google Scholar]
- [75].Lehnert D, Wehrle-Haller B, David C, Weiland U, Ballestrem C, Imhof B A, Bastmeyer M. Cell behaviour on micropatterned substrata: Limits of extracellular matrix geometry for spreading and adhesion. J. Cell Sci. 2004;117:41–52. doi: 10.1242/jcs.00836. [DOI] [PubMed] [Google Scholar]
- [76].Gallant N D, Michael K E, García A J. Cell adhesion strengthening: Contributions of adhesive area, integrin binding, and focal adhesion assembly. Mol. Biol. Cell. 2005;16:4329–4340. doi: 10.1091/mbc.e05-02-0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Coyer S R, Singh A, Dumbauld D W, Calderwood D A, Craig S W, Delamarche E, García A J. Nanopatterning reveals an ECM area threshold for focal adhesion assembly and force transmission that is regulated by integrin activation and cytoskeleton tension. J. Cell Sci. 2012;125:5110–5123. doi: 10.1242/jcs.108035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Wang R, Lin T S, Johnson J A, Olsen B D. Kinetic monte carlo simulation for quantification of the gel point of polymer networks. ACS Marco Lett. 2017;6:1414–1419. doi: 10.1021/acsmacrolett.7b00586. [DOI] [PubMed] [Google Scholar]
- [79].Guo M, Pegoraro A F, Mao A, Zhou E H, Arany P R, Han Y L, Burnette D T, Jensen M H, Kasza K E, Moore J R, et al. Cell volume changethrough water efflux impacts cell stiffness and stem cell fate. Proc. Natl. Acad. Sci. USA. 2017;114:E8618–E8627. doi: 10.1073/pnas.1705179114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Chen C S, Alonso J L, Ostuni E, Whitesides G M, Ingber D E. Cell shape provides global control of focal adhesion assembly. Biochem. Biophys. Res. Commun. 2003;307:355–361. doi: 10.1016/S0006-291X(03)01165-3. [DOI] [PubMed] [Google Scholar]
- [81].Jiun K, Hong S Y, Park H S, Kim D S, Lee W. Structure and function of RGD peptides derived from disintegrin proteins. Mol. Cells. 2005;19:205–211. [PubMed] [Google Scholar]
- [82].Takagi J, Petre B M, Walz T, Springer T A. Global conformational rearrangements in integrin extracellular domains in outside-in and inside-out signaling. Cell. 2002;110:599–611. doi: 10.1016/S0092-8674(02)00935-2. [DOI] [PubMed] [Google Scholar]
- [83].Craig D, Gao M, Schulten K, Vogel V. Structural insights into how the MIDAS ion stabilizes integrin binding to an RGD peptide under force. Structure. 2004;12:2049–2058. doi: 10.1016/j.str.2004.09.009. [DOI] [PubMed] [Google Scholar]
- [84].Wong S H D, Yin B H, Yang B G, Lin S E, Li R, Feng Q, Yang H R, Zhang L, Yang Z M, Li G, et al. Anisotropic nanoscale presentation of cell adhesion ligand enhances the recruitment of diverse integrins in adhesion structures and mechanosensing-dependent differentiation of stem cells. Adv. Funct. Mater. 2019;29:1806822. doi: 10.1002/adfm.201806822. [DOI] [Google Scholar]
- [85].Meyer R K, Aebi U. Bundling of actin filaments by α-Actinin depends on its molecular length. J. Cell Biol. 1990;110:2013–2024. doi: 10.1083/jcb.110.6.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Arnold M, Hirschfeld-Warneken V C, Lohmüller T, Heil P, Blümmel J, Cavalcanti-Adam E A, López-García M, Walther P, Kessler H, Geiger B, et al. Induction of cell polarization and migration by a gradient of nanoscale variations in adhesive ligand spacing. Nano Lett. 2008;8:2063–2069. doi: 10.1021/nl801483w. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Critical adhesion areas of cells on micro-nanopatterns