Abstract
Pathogens adapted to sub‐lethal acidic conditions could increase the virulence and survival ability under lethal conditions. In the aquaculture industry, feed acidifiers have been used to increase the growth of aquatic animals. However, there is limited study on the effects of acidic condition on the virulence and survival of pathogens in aquaculture. In this study, we investigated the survival ability of Vibrio parahaemolyticus at lethal acidic pH (4.0) after adapted the bacteria to sub‐lethal acidic pH (5.5) for 1 hr. Our results indicated that the adapted strain increased the survival ability at lethal acidic pH invoked by an inorganic (HCl) or organic (citric) acid. RNA‐sequencing (RNA‐seq) results revealed that 321 genes were differentially expressed at the sub‐lethal acidic pH including cadC, cadBA and groES/groEL relating to acid tolerance response (ATR), as well as genes relating to outer membrane, heat‐shock proteins, phosphotransferase system and flagella system. Quantitative real‐time polymerase chain reaction (qRT‐PCR) confirmed that cadC and cadBA were upregulated under sub‐lethal acidic conditions. The CadC protein could directly regulate the expression of cadBA to modulate the ATR in V. parahaemolyticus. RNA‐seq data also indicated that 113 genes in the CadC‐dependent way and 208 genes in the CadC‐independent way were differentially expressed, which were related to the regulation of ATR. Finally, the motility and cytotoxicity of the sub‐lethal acidic adapted wild type (WT) were significantly increased compared with the unadapted strain. Our results demonstrated that the dietary acidifiers may increase the virulence and survival of V. parahaemolyticus in aquaculture.
Keywords: Acid tolerance response, CadC‐CadBA, RNA‐seq, Vibrio parahaemolyticus
1. INTRODUCTION
The outbreak of vibriosis diseases has a negative effect on the sustainable development of the aquaculture industry and also causes the economic loss to aquaculture industry (Baker‐Austin et al., 2018; De Schryver et al., 2014; Stentiford et al., 2017). Vibrio parahaemolyticus, Vibrio anguillarum, Vibrio alginolyticus and Vibrio harveyi are the most common pathogens for aquatic animals, in which V. parahaemolyticus has been frequently involved in disease outbreaks worldwide (Baker‐Austin et al., 2018; Bauer et al., 2021; Hickey & Lee, 2017; Mohamad et al., 2019). Vibrio parahaemolyticus is a Gram‐negative bacterium in various aqueous environments including marine, coastal and freshwater, which could cause infectious diseases in oysters, shrimp and fish (Johnson et al., 2012; McCarter, 1999; Yang et al., 2019). The infection of V. parahaemolyticus could cause the external haemorrhages and tail rot in fish, the white spots on foot of abalones and the acute hepatopancreatic necrosis disease in shrimp (Alcaide et al., 1999; Cai et al., 2007; Imaizumi et al., 2018). Furthermore, V. parahaemolyticus is also an important foodborne pathogen in humans, which could cause the acute gastroenteritis with diarrhoeal by the consumption of contaminated raw seafood (Soumaré et al., 2007). Therefore, the contamination of V. parahaemolyticus in aquatic products should be taken a great concern due to its negative effects on the aquatic production and public health.
In 1996, V. parahaemolyticus serotype O3:K6 was identified in India for the first time, which was then frequently reported to cause diarrhoeal outbreaks in humans worldwide (Okuda et al., 1997; Velazquez‐Roman et al., 2014). Vibrio parahaemolyticus serotypes O3:K6 has been known as the most prevalent serotype in the clinical samples, which carries tdh and/or trh genes as the main virulence factors (Guerrero et al., 2017; Raghunath, 2015). Vibrio parahaemolyticus serotype O3:K6 has been detected in 56.8% of fish samples and 26.3% of clinical samples in India, respectively (Guin et al., 2019). In Mexico, V. parahaemolyticus was detected in 15% of sea water, oyster and fish samples from 2001 to 2002 (Cabrera‐García et al., 2004). Vibrio parahaemolyticus has been reported in 16.2% of freshwater fish samples and 42.0% of seafood samples in China, while 80.7%, 15.0% and 4.3% of shellfish, crustaceans and fish samples were contaminated by V. parahaemolyticus, respectively (Chen et al., 2021; Su & Chen, 2020). Thus, the continuous monitoring of V. parahaemolyticus in aquatic animals and products is important for the aquatic food safety.
The dietary acidifiers were applied in the feed to improve the growth, survival and feed utilization of aquatic animals (Asriqah et al., 2018). Previous studies showed that the supplement of citric acid, sorbic acid or formic acid in feed could increase the bioavailability of minerals in rainbow trout and Pacific white shrimp (He et al., 2017; Vielma & Lall, 1997). Moreover, short‐chain organic acids could also provide energy for renewing the intestinal epithelia and maintaining the gut health (Abu Elala & Ragaa, 2015). However, the broodstock and fresh feed were identified as the important sources of V. parahaemolyticus (Yingkajorn et al., 2014). Previous studies demonstrated that the preadaptation of pathogens to sub‐lethal acidic conditions could increase the bacterial survival ability under lethal acidic conditions (Cakar et al., 2019; Kuper & Jung, 2005; Lee et al., 2007). This phenomenon is known as the acid tolerance response (ATR). Additionally, ATR has been related to the enhancement of bacterial virulence (Merrell & Camilli, 1999; O'Driscoll et al., 1996). The above observations have indicated that the acidic feed may increase the virulence and survival ability of pathogens, which may increase the risk of V. parahaemolyticus causing infectious diseases in both aquatic animals and humans.
Vibrio parahaemolyticus is an important gastrointestinal pathogen, which could colonize in the small intestine of the host. A previous study indicated that CadBA is responsible for ATR in V. parahaemolyticus (Tanaka et al., 2008). This study aimed to assess how acid influences the virulence and survival ability of V. parahaemolyticus. Genes involved in ATR were identified by RNA‐seq, and the role of CadC in the modulation of the ATR was further investigated. Our study also demonstrated how sub‐lethal acidic conditions could affect the motility and cytotoxicity of V. parahaemolyticus.
2. MATERIALS AND METHODS
2.1. Bacterial strains, plasmids and culture conditions
The strains, plasmids and primers used in this study are listed in Tables 1 and 2. The V. parahaemolyticus RIMD2210633 strain and derivatives (mutants and complementary) were grown at 37°C in Luria–Bertani (LB) medium with 3% NaCl, and the Escherichia coli strains were grown at 37°C in LB medium.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant characteristics | Reference |
|---|---|---|
| Escherichia coli | ||
| DH5α λpir | Host for π requiring plasmids | Laboratory collection |
| SM10 λpir | Host for π requiring plasmids, conjugal donor | Liang et al. (2003) |
| BL21(DE3) | Host strain for protein expression | Novagen |
| pET28a‐cadC/BL21 | BL21, pET28a carrying the cadC N‐terminal, Kmr | This study |
| Vibrio parahaemolyticus | ||
| RIMD2210633 | Clinical isolate. Carbr | Laboratory collection |
| ΔcadA | RIMD2210633, in‐frame deletion in cadA, Carbr | This study |
| ΔcadB | RIMD2210633, in‐frame deletion in cadB, Carbr | This study |
| ΔcadC | RIMD2210633, in‐frame deletion in cadC, Carbr | This study |
| cadC + | ΔcadC, pMMB207 expressing cadC‐his, Carbr, Cmr | This study |
| Plasmids | ||
| pDM4 | Suicide vector, pir dependent, R6K, SacBR, Cmr | Wang et al. (2002) |
| pMMB207 | IncQ lacIq Δbla Ptac‐lac lacZa, Cmr | Morales et al. (1991) |
| pET28a | Expressing vector, Kmr | Novagen |
| pDM4‐cadA | pDM4 with cadA fragment deleted 4 to 2,130 nt, Cmr | This study |
| pDM4‐cadB | pDM4 with cadB fragment deleted 4 to 1,338 nt, Cmr | This study |
| pDM4‐cadC | pDM4 with cadC fragment deleted 4 to 450 nt, Cmr | This study |
| pMMB207‐cadC‐his | pMMB207 carrying the cadC‐his, Cmr | This study |
| pET28a‐cadC | pET28a carrying the cadC N‐terminal, Kmr | This study |
TABLE 2.
The primers used in this study
| Primer name | Primer sequence (5′ to 3′) |
|---|---|
| cadA‐up‐F | GAGCGGATAACAATTTGTGGAATCCCGGGAGACTGCACCATTCGTATCAGCATT |
| cadA‐up‐R | AGAGGAACTTACATTTGGACATCTCCAAGGCGAATG |
| cadA‐down‐F | GATGTCCAAATGTAAGTTCCTCTCGAAGATGACGCC |
| cadA‐down‐R | AGCGGAGTGTATATCAAGCTTATCGATACCAGCAATCACTTTGTGTATATACCA |
| cadA‐out‐F | TGTACCGCTAGCAACCATGCTGGG |
| cadA‐out‐R | CGTCTCACTCCGTGTCGGTGGATG |
| cadB‐up‐F | GCTTATCGATACCGTCGACCCTCGAGAAGACATATCCGGTTTGCTTCTC |
| cadB‐up‐R | TGGGGATTACATAACATCTCCAAATCTATTCTAC |
| cadB‐down‐F | GGAGATGTTATGTAATCCCCAAACGCTGGCACTTCGG |
| cadB‐down‐R | CACTAGTGACGCGTACTCGAGCTTTAGTGAATGGAGGCATGATGG |
| cadB‐out‐F | CAGGTATCTACTTCATAGCATC |
| cadB‐out‐R | ACCACTTCGTAGCCCGCTTTCT |
| cadC‐up‐F | GCTTATCGATACCGTCGACCCTCGAAGTTGGCAAAACGCTGTGGTGT |
| cadC‐up‐R | GAAACTGCTTGATTTGAAAATAGACTCCAACC |
| cadC‐down‐F | TTTTCAAATCAAGCAGTTTCGTCAAGGCATCG |
| cadC‐down‐R | CACTAGTGACGCGTACTCGATCGAACGACAGAAGGGTGAACA |
| cadC‐out‐F | CGGCAAGTAGGCGTGCTGTAC |
| cadC‐out‐R | TTGACCCACATCAATATTCATC |
| cadC ‐com‐F | TCGGTACCCGGGGATCCTCTAGTAAGGAGGTAGGATAATAATGGTTGGAGTCTATTTTCA |
| cadC ‐com‐R | TCCGCCAAAACAGCCAAGCTTTAGTGATGATGATGATGATGTTAAAGCATTCGAACGACA |
| cadC ‐pET28a‐F | TGGTGGACAGCAAATGGGTCGCGGATCCATGGTTGGAGTCTATTTTCA |
| cadC ‐pET28a‐R | CCGGATCTCAGTGGTGGTGGTGGTGGTGCATCAGTCCCAAGCGCCACGG |
| PcadBA‐F | TGCCTGCAGGTCGACGATGTTGATGAGAGTTTATCTATC |
| PcadBA‐R | AACATCTCCAAATCTATTCTA |
| EMSA‐FAM | TGCCTGCAGGTCGACGAT |
| cadA‐RT‐F | ATGCCGTATGCGTTACGAGT |
| cadA‐RT‐R | ATCGTGGGCATGTACTCAGC |
| cadB‐RT‐F | CATACCGATGCAATCGTCGC |
| cadB‐RT‐R | ACGGGCGTGCTTTATTACCA |
| cadC‐RT‐F | AGTAACGGCAGGTGCGATAG |
| cadC‐RT‐R | CGAAACGTGGCTACAAGCTG |
| gyrB‐RT‐F | TTACCGTCATGGTGAGCCTG |
| gyrB‐RT‐R | CACGCAGACGTTTTGCTAGG |
2.2. Construction of the deletion mutant and complementary strains
The deletion mutant strains of V. parahaemolyticus cadA, cadB and cadC were constructed as previously described (Zhou et al., 2010). Briefly, the upstream and downstream fragments of the cadA, cadB and cadC genes were amplified using PCR with specific primers (Table 2) and cloned into the suicide plasmid pDM4. The recombinant plasmids were transformed into E. coli SM10 λpir, conjugated into the WT and then selected in the LB medium with carbenicillin and chloramphenicol. The second cross‐over recombination products were selected in LB medium with 10% sucrose. The mutant strains were verified using PCR with specific primers (cadA‐out‐F/R, cadB‐out‐F/R, cadC‐out‐F/R), followed by sequencing.
The ribosome‐binding site (RBS) and open reading frame (ORF) regions of cadC were amplified using PCR with specific primers (Table 2) and cloned into the pMMB207 plasmid. The recombinant plasmid pMMB207‐cadC was transformed into E. coli SM10 λpir and conjugated to the ∆cadC. Polymerase chain reaction and sequencing confirmed the complementary strain, which was named as cadC+ .
2.3. ATR analysis
The overnight cultured strains WT, ΔcadA, ΔcadB, ΔcadC and cadC + were diluted into fresh LB medium and incubated at 37°C until they reached an optical density (OD600) of 0.2–0.3. The strains were resuspended in LB medium at different pH values (pH 7.5 or pH 5.5) and cultured at 37°C for 1 hr. Subsequently, the strains cultured at pH 7.5 or pH 5.5 LB medium were transferred into LB medium at pH 4.0. An aliquot was taken from each culture at the indicated time points and diluted appropriately. The bacteria were placed onto the LB agar plates, and the surviving bacteria were counted. Each experiment was repeated 3 times.
2.4. RNA‐sequencing analysis
The WT strain was cultured overnight and then diluted 1:100 into fresh LB medium. After culturing for 3 hr, half of the culture was treated with LB medium at pH 5.5 (HCl) for 2 hr. The remaining bacteria were further cultured during this period. One millilitre of bacterial cells was collected; their total RNA was extracted using an RNA Easy kit (Qiagen). The RNA samples were treated with DNase I (Invitrogen) to remove any genomic DNA (gDNA) contamination and sequenced by the Illumina HiSeq (GENEWIZ). The quality control and filtered data were processed by Cutadapt version 1.9.1 (Martin, 2011); the filtered data were then aligned to the reference genome via software Bowtie 2 version 2.2.6 (Langmead & Salzberg, 2012). HTSeq version 0.6.1p1 was used to estimate gene expression levels from the clean data (Anders et al., 2015). The different expression analysis was conducted using the DESeq 2 Bioconductor package (Love et al., 2014).
2.5. Quantitative real‐time reverse transcription PCR (qRT‐PCR)
The WT strain was cultured and exposed to sub‐lethal acidic pH as described above in RNA‐sequencing analysis. One mL of bacterial cells from each of the exposed and unexposed cultures was collected; their total RNA was extracted using the RNA Easy kit (Qiagen). The total RNA samples were treated with DNase I to remove gDNA. Equal amounts of RNA (1 μg) were used to generate the first‐strand complementary DNA (cDNA) (Takara) using random primers. Three independent qRT‐PCR experiments were performed with specific primer pairs listed in Table 2. The reaction mixture was run on the Applied Biosystems 7500 Real‐Time System (Applied Biosystems). The messenger RNA (mRNA) levels were normalized to the gyrB mRNA level in each sample using the 2−ΔΔCt method.
2.6. Electrophoretic mobility shift assay
The DNA‐binding domain of cadC was amplified using PCR (Table 2), cloned into the pET28a plasmid and purified the CadC‐his protein. Electrophoretic mobility shift assay (EMSA) analysis was performed as previously described (Gu et al., 2016). The DNA probes were amplified and purified by a DNA Gel Purification Kit (TIANGEN). The EMSA reaction mixture (20 μl) was mixed with 10 ng of the DNA probes, 1 μg of poly‐dIdC, 4 μl of 5 × binding buffer (10 mM NaCl, 0.1 mM DTT, 0.1 mM EDTA and 10 mM Tris, pH 7.4), and different concentrations of CadC‐his protein. The reaction mixture was then incubated at 25°C for 30 min. Subsequently, 2 μl of 10 × EMSA/Gel‐shift loading buffer was added to each sample, and the samples were resolved using native PAGE (6% gel) with 0.5 × TBE buffer for 2 hr at 100 V. The gel was scanned by a Typhoon FLA 9500 (GE Healthcare).
2.7. Motility analysis
The WT strain was cultured and exposed to sub‐lethal acidic pH as described above in RNA‐sequencing analysis. Two microlitres of the diluted culture (OD600 = 1.0), alongside the unexposed control, was spotted on LB plates containing 0.3% agar and incubated at 37°C for 12 hr to investigate the swimming capacity. The experiments were repeated three times.
2.8. Cytotoxicity analysis
The WT strain was cultured and exposed to sub‐lethal acidic pH as described above in RNA‐sequencing analysis. The culture, along with the unexposed control, was centrifuged with 4,000 g and washed twice by Dulbecco's modified Eagle's medium (DMEM). The pellet was resuspended in DMEM containing 10% foetal bovine serum (FBS). The bacteria were diluted 1:108, and each bacterial suspension was inoculated into a well of a 24‐well plate containing 5 × 105 HeLa cells/well to achieve a multiplicity of infection (MOI) of 100. Following infection, the lactate dehydrogenase (LDH) activity of the supernatants was measured at the indicated times using the CyQUANT™ LDH Cytotoxicity Assay Kit (Invitrogen) according to the manufacturer's protocol.
3. RESULTS
3.1. Acid tolerance of Vibrio parahaemolyticus
The ATR of V. parahaemolyticus RIMD2210633 was determined by using the inorganic acid HCl and the organic acid citric acid. The WT strain did not survive after an incubation period of 1 hr at pH 4.0, which is lethally acidic for the bacteria unless preincubation in the LB medium with pH 5.5 for 1 hr (Figure 1a). Overall, 16.65% and 1.80% of the bacteria preincubated in sub‐lethal acidic (HCl, pH 5.5) could survive with the lethal acidic challenge (HCl, pH 4.0) of 0.5 hr and 1 hr, respectively (Figure 1b). A similar result was also found when citric acid was used (Figure 1c). These results indicate that the adaptation to sub‐lethal acidic pH enhanced the survival of V. parahaemolyticus at the lethal acidic pH invoked both by an inorganic acid (HCl) and organic acid (citric acid).
FIGURE 1.
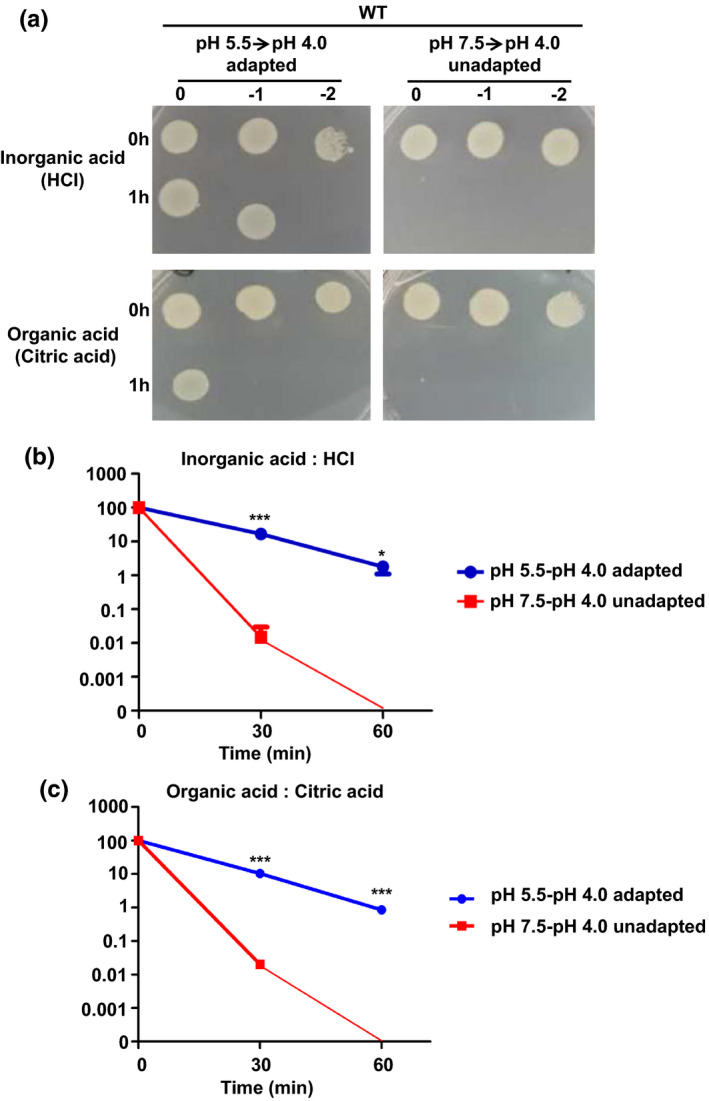
Acid tolerance response assays on Vibrio parahaemolyticus strains. (a) The survival of V. parahaemolyticus. The WT strain was exposed to the lethal acidic pH (4.0) for 1 hr with or without preadaptation to sub‐lethal acidic pH (5.5) for 1 hr. The bacteria were then serially diluted (10‐fold) and spotted onto LB agar plates. Both an inorganic acid (HCl) and organic acid (citric acid) were tested. The survival of V. parahaemolyticus with inorganic acid (HCl) stress (b) and organic acid (citric acid) stress (c). Survival percentage was obtained by dividing the surviving populations by the initial populations, which correspond to 100%. *p < .05, ***p < .001, t test [Colour figure can be viewed at wileyonlinelibrary.com]
3.2. The genes responsible for the ATR in Vibrio parahaemolyticus
In this study, we compared the transcriptomes of V. parahaemolyticus exposed to sub‐lethal acidic pH conditions with those of unexposed bacteria using RNA‐seq to gain further insight into the genes responsible for the ATR (Table S1). The results showed that 321 genes showed significantly different expression levels, with 234 genes upregulated and 87 downregulated (Figure 2a). Furthermore, a volcano plot of the RNA‐seq data was generated to visualize the differentially expressed genes at the sub‐lethal acidic pH and assess the quality and comparability of the hybridizations (Figure 2b).
FIGURE 2.
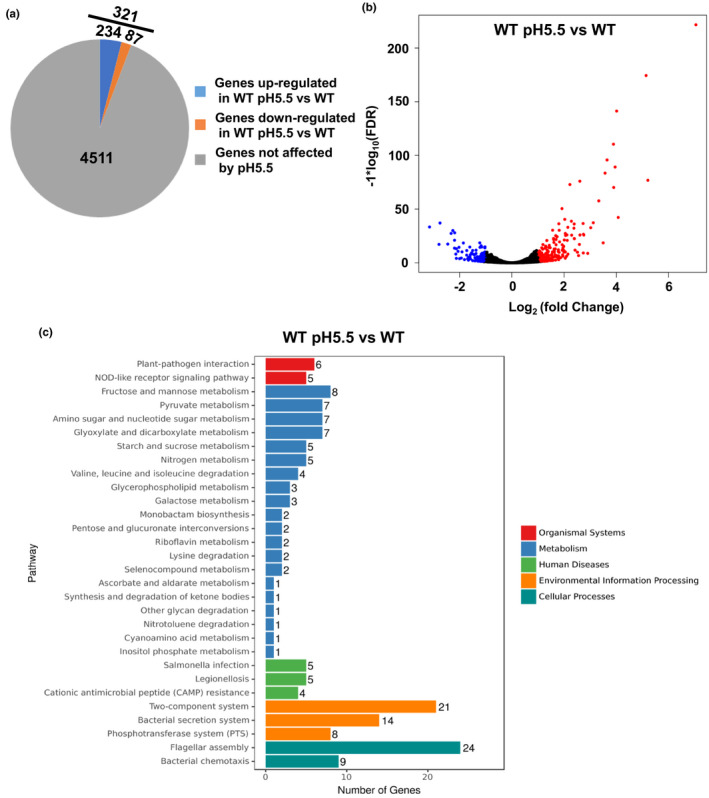
The differentially expressed genes at the sub‐lethal acidic pH (5.5, HCl). (a) Pie charts representing the differentially transcribed genes between the WT strain cultured at pH 5.5 and that cultured at pH 7.5. (b) A volcano plot was generated to visualize the differentially expressed genes at the sub‐lethal acidic pH (5.5). The x‐axis represents the log2 of the fold change plotted against the −log10 of the adjusted false discovery rate. Red and blue points indicate the up‐ and downregulated genes, respectively. (c) KEGG analysis of the pathways regulated under the sub‐lethal acidic condition. The number on each bar represents the number of differentially expressed genes in each pathway [Colour figure can be viewed at wileyonlinelibrary.com]
Based on the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis, the differentially expressed genes were divided into organismal systems (n = 11), metabolism (n = 65), human diseases (n = 14), environmental information processing (n = 43) and cellular processes (n = 33; Figure 2c). Two heat‐shock protein genes (VP0651 and VP0821), VPA0586/VPA0287 (groES/groEL), outer membrane protein genes (ompU and ompV) and Na+/H+ antiporter protein (VP1229) were significantly upregulated under sub‐lethal acidic conditions. It was also established that the DNA‐binding transcriptional activator (cadC), lysine decarboxylase (cadA) and lysine/cadaverine antiporter (cadB) genes were upregulated in lethal acidic pH conditions. In addition, virulence‐associated genes, including phosphotransferase system (PTS), flagellar assembly and bacterial chemotaxis, were significantly upregulated under the sub‐lethal acidic conditions, while the type III secretion system and the two‐component system CpxRA (VPA0148/VPA0149) was downregulated. It was speculated that the ATR could influence bacterial pathogenesis in addition to enhancing survival.
3.3. CadBA modulates the ATR in Vibrio parahaemolyticus
RNA‐seq analysis indicated that the cadBA genes were upregulated when the cells were exposed to the sub‐lethal acidic pH (5.5) (Figure 3a), and qRT‐PCR confirmed this result (Figure 3b). The location of cadBA‐cadC genes and their predicted promoter are shown in Figure 3c. The RT‐PCR product of the expected size was obtained with the primers spanning the regions from cadA to cadB. No product was obtained using RNA as the template, and a product of the same size was observed when the genomic DNA was used as the template (Figure 3c), indicating that the cadB and cadA genes are contranscribed.
FIGURE 3.
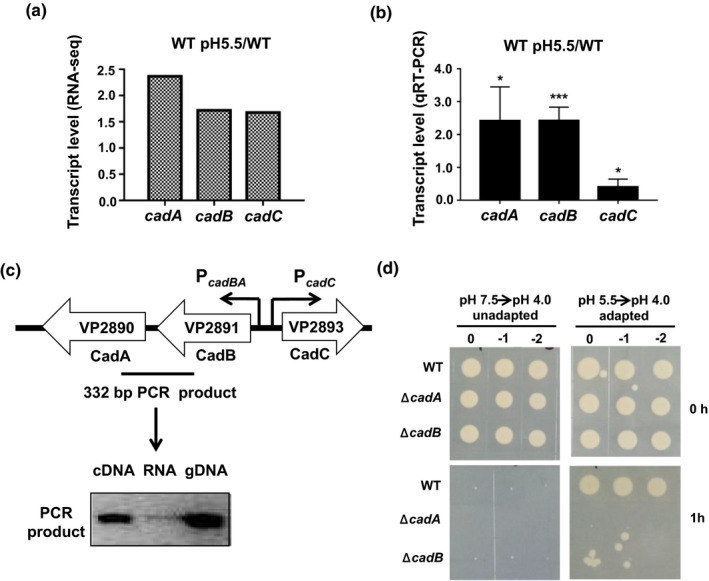
CadBA is responsible for the ATR in Vibrio parahaemolyticus. (a) The cadBA and cadC mRNA levels based on the RNA‐seq. (b) qRT‐PCR analysis of the cadBA and cadC mRNA levels under the sub‐lethal acidic condition. The results are shown normalized to the gyrB mRNA levels and were determined by the ΔΔCT method. The differences are shown relative to the levels at pH 7.5. *p < .05, ***p < .001, t test. (c) The genetic environment of the cadBA‐cadC genes in V. parahaemolyticus chromosome. Arrows indicate the predicted promoter regions and RT‐PCR was used to determine whether cadA and cadB are co‐transcribed. (d) The survival of V. parahaemolyticus. The WT, ΔcadA, and ΔcadB strains were exposed to the lethal acidic pH (4.0, HCl) for 1 hr with or without preadaptation at the sub‐lethal acidic pH (5.5, HCl) for 1 hr. The bacteria were then serially diluted (10‐fold) and spotted onto LB agar plates [Colour figure can be viewed at wileyonlinelibrary.com]
The deletion mutant strains of ΔcadB and ΔcadA were constructed and assessed to better understand the function of cadBA in the ATR. The results showed that the WT, ΔcadB and ΔcadA strains could not survive in lethal acidic conditions without adaptation to the sub‐lethal acidic conditions. When the strains were incubated at the sub‐lethal acidic pH for 1 hr, the WT strain could survive at the lethal acidic pH; however, the ΔcadB and ΔcadA strains could not (Figure 3d), demonstrating that the CadBA protein is necessary for the survival of V. parahaemolyticus under the extremely acidic conditions.
3.4. CadC directly regulates the expression of cadBA to mediate ATR
RNA‐seq and qRT‐PCR indicated that the cadC mRNA level was increased when cells were exposed to sub‐lethal acidic pH (Figure 3a,b). The transcript level of cadB and cadA genes was decreased in the ΔcadC strain compared with the levels in WT, and the complementary strain cadC + restored the levels to the WT (Figure 4a), indicating that the CadC protein regulates the expression of cadBA in V. parahaemolyticus. EMSA determined the CadC protein directly bound to the promoter region of cadBA, and poly(dI‐dC) was added as a nonspecific competitor. As expected, the CadC protein could directly bind to the promoter of cadBA in a concentration‐dependent manner (Figure 4b). Additionally, the gyrB promoter was employed as a negative control, and the CadC protein at the highest concentration, could not bind to the negative control DNA (Figure 4b).
FIGURE 4.
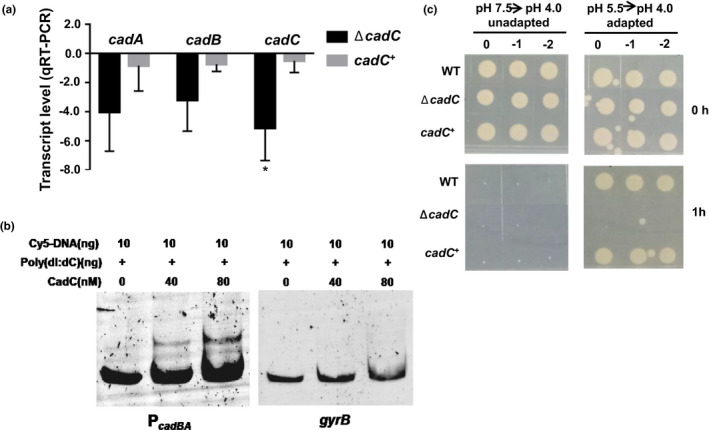
CadC directly regulates the expression of cadBA to modulate the ATR in Vibrio parahaemolyticus. (a) qRT‐PCR analysis of the cadBA and cadC mRNA levels in the WT, ΔcadC and cadC + strains. Total RNA was isolated from the WT, ΔcadC and cadC + strains cultured in the LB medium at pH 7.5 for 5 hr. The results are shown normalized to the gyrB mRNA levels and were determined by the ΔΔCT method. The differences are shown relative to the levels in the WT. *p < .05, t test. (b) EMSA assays were performed with the CadC protein and cadBA promoter. The CadC protein at the indicated concentration and 20 ng of FAM‐labelled PcadBA DNA probe was used. The shifts were verified by a nonspecific competitor DNA [poly(dI:dC)], and the gyrB promoter was used as a negative control. (c) The survival of V. parahaemolyticus. The WT, ΔcadC and cadC + strains were exposed to the lethal acidic pH (4.0, HCl) for 1 hr with or without preadaptation at the sub‐lethal acidic pH (5.5, HCl) for 1 hr. The bacteria were then serially diluted (10‐fold) and spotted onto LB agar plates [Colour figure can be viewed at wileyonlinelibrary.com]
The ATR of ΔcadC strain was also investigated. Results showed that the WT, ΔcadC, and cadC + strains could not survive at the lethal acidic pH (4.0) without adaptation. In contrast, the WT and cadC + strains could survive at the lethal acidic pH after adaptation to the sub‐lethal acidic pH (5.5) for 1 hr. The ΔcadC strain could not survive at the lethal acidic pH after preadaptation (Figure 4c), indicating that the CadC protein is involved in regulating the ATR. These results suggest that CadC protein could directly bind to the promoter region of cadBA and regulate the expression of cadBA to modulate the ATR in V. parahaemolyticus.
3.5. CadC‐dependent and CadC‐independent genes responsible for the ATR in Vibrio parahaemolyticus
In the sub‐lethal acid (pH 5.5, HCl), 321 genes were differentially expressed by the RNA‐seq (Figure 2a). CadC protein as a transcription factor could directly regulate the expression of cadBA to modulate the ATR. RNA‐seq was used to identify the genes regulated by the CadC protein to determine the CadC‐dependent and CadC‐independent genes responsible for the ATR. The transcriptomes of ΔcadC and WT were compared to identify the changes in gene expression patterns in the absence of a functional CadC protein (Table S2). Results showed that 565 genes were expressed differently in ΔcadC; 292 genes were upregulated, while 273 genes were downregulated (Figure 5a). The volcano plot of the RNA‐seq data was generated to visualize the differentially expressed genes of the ΔcadC strain and to assess the quality and comparability of the hybridizations (Figure 5b).
FIGURE 5.
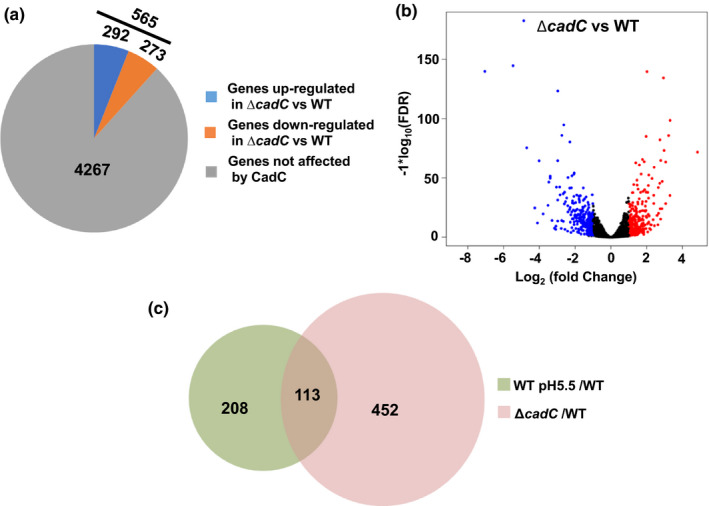
The regulon of CadC protein assessed using RNA‐seq. (a) Pie charts represent differentially transcribed genes between the WT and ΔcadC strains cultured in LB at pH 7.5. (b) A volcano plot was generated to visualize the differentially expressed genes in ΔcadC. The x‐axis represents the log2 of the fold change plotted against the −log10 of the adjusted false discovery rate. Red and blue points indicate the up‐ and downregulated genes, respectively. (c) Venn diagrams showing the genes whose expression is co‐regulated by sub‐lethal acidic pH (5.5, HCl) and CadC protein [Colour figure can be viewed at wileyonlinelibrary.com]
The RNA‐seq data of the sub‐lethal acid and ΔcadC were compared, 113 genes were identified as co‐regulated (Figure 5c and Table S3). These genes could be considered responsible for the adaptation to the sub‐lethal acidic pH through CadC (113 genes) and independently of CadC (208 genes). Among the CadC‐dependent genes, a Na+/H+ antiporter protein (VP1229) was upregulated at the sub‐lethal acidic pH and downregulated in the ΔcadC strain; the CadC protein may regulate the expression of vp1229 to modulate the ATR. Furthermore, cadB and cadA genes were downregulated by CadC and induced at the sub‐lethal acidic pH; these results were confirmed using qRT‐PCR (Figure 3b and Figure 4a). CadC regulates the expression of formate dehydrogenase, D‐lactate dehydrogenase, glycerol‐3‐phosphate dehydrogenase, and 6 PTS proteins. The two heat‐shock protein genes (VP0651 and VP0821) and groES/groEL (VPA0586/VPA0287) were downregulated in the ΔcadC strain. These data suggested that adaptation to sub‐lethal acidic pH increases the expression of the genes that modulate the ATR through CadC‐dependent or CadC‐independent way in V. parahaemolyticus.
3.6. Motility and cytotoxicity were induced in sub‐lethal pH conditions
Motility and cytotoxicity were identified as the main virulence factors of V. parahaemolyticus (Gu et al., 2019; Zhang & Orth, 2013). RNA‐seq data showed that polar flagellar genes were upregulated under sub‐lethal acidic conditions (Figure 6a). The swimming motility and cytotoxicity of V. parahaemolyticus were assessed after the adaptation to a sub‐lethal acidic pH (5.5) for 1 hr. The results showed that the swimming motility of WT was significantly increased post‐adaptation to the sub‐lethal acids, including the inorganic acid (HCl) and organic acid (citric acid) (Figure 6b,c). The cytotoxicity of WT strain on HeLa cells was also significantly increased after the cells adapted to the sub‐lethal acidic pH (Figure 6d). These results indicated that adaption to sub‐lethal acidic conditions could increase the virulence of V. parahaemolyticus.
FIGURE 6.
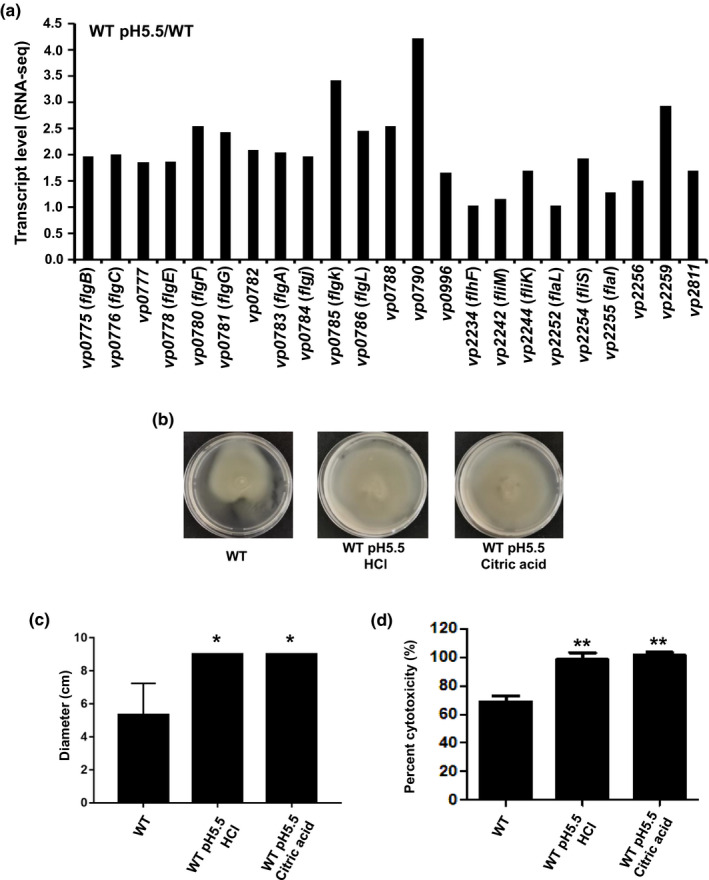
Preadaptation to sub‐lethal acidic pH enhances the motility and cytotoxicity of Vibrio parahaemolyticus. (a) The transcriptional levels of polar flagellar genes based on the RNA‐seq (b) The swimming analysis of V. parahaemolyticus. The WT strain was adapted to the sub‐lethal acidic pH (5.5, HCl, or citric acid) for 1 hr. After normalization to OD600 of 1.0, the bacteria were spotted onto LB plates with 0.3% agar and cultured at 37°C. (c) The diameter of the swimming capacity of the WT strain under various culture conditions. *, p < .05, t test. (d) The cytotoxicity of WT strain adapted to the sub‐lethal acidic pH (5.5, HCl, or citric acid). **, p < .01, t test [Colour figure can be viewed at wileyonlinelibrary.com]
4. DISCUSSION
Vibrio parahaemolyticus were highly prevalent in shrimp, fish and seafood products (Lee et al., 2019; Lei et al., 2020; Li et al., 2020; Tan et al., 2020). A previous study indicated that V. parahaemolyticus was detected in 3.6% of natural feed samples and 83.3% of fresh feed samples, respectively (Yingkajorn et al., 2014). The feed acidifiers have been used to increase the growth of aquatic animals, but there is limited knowledge of how acid influences the virulence and survival ability of pathogens in aquatic animals. Our results showed that the survival ability of V. parahaemolyticus was increased at the lethal acidic pH (4.0) after adaptation to sub‐lethal acidic conditions (pH 5.5), including the treatment of both the inorganic acids and organic acids (Figure 1). The CadC‐CadBA was identified as a critical factor involved in regulating the acid tolerance response, which could increase bacterial resistance to both inorganic acids and organic acids. Furthermore, we identified 321 differentially expressed genes under sub‐lethal acidic conditions, in which 113 genes were dependent to CadC, while 208 genes were independent to CadC (Figure 5). Besides, we also showed that the swimming motility and cytotoxicity were significantly increased after adapting V. parahaemolyticus to the sub‐lethal acid (pH 5.5; Figure 6).
The upregulation of virulence genes under the acid stress conditions was also detected in V. cholerae, S. Typhimurium, and Listeria monocytogenes indicating that the environmental stress could induce the expression of virulence genes and facilitate the colonization of these pathogens in the host (Cakar et al., 2018; Conte et al., 2000; Lamas et al., 2019). Our results showed that the motility and cytotoxicity of V. parahaemolyticus were significantly increased after adapting to the sub‐lethal acid conditions (Figure 6). In addition, RNA‐seq results also showed that the expression of two heat‐shock protein genes (VP0651 and VP0821) was significantly increased under the sub‐lethal acidic condition, which may enhance the survival ability of V. parahaemolyticus at high temperature. The previous study showed that the high‐salt adaptation of V. parahaemolyticus could increase its survival under lethal stress (Kalburge et al., 2014). It is necessary to investigate how acidic could influence the virulence and survival of V. parahaemolyticus under environmental stress conditions in further studies.
Previous study revealed that the supplementation of organic acids in diet could improve the feed conversion ratio and the survival rate of Oreochromis niloticus and red hybrid tilapia (Asriqah et al., 2018). Fulvic acids in feed supplement could also improve the growth performance and intestinal health of rainbow trout, loach and crayfish (Gao et al., 2017; Lieke et al., 2021; Zhang, 2018). The application of citric acid in the feed supplement could mitigate the distal intestine of juvenile turbot, while formic acid could increase the bioavailability of minerals in rainbow trout (Vielma & Lall, 1997; Zhao et al., 2019). Our results indicated that the sub‐lethal acid conditions could increase the survival ability and virulence of V. parahaemolyticus, in which the CadC‐CadBA plays an essential role in the regulation of ATR. This phenomenon is reported in the other pathogens including Salmonella and V. cholerae (Lee et al., 2008; Merrell & Camilli, 1999). These results indicated that the supplementation of acidifiers in the feed may increase the virulence and survival of V. parahaemolyticus in the aquaculture.
The CadC‐CadBA system was confirmed to be involved in the ATR, which could protect bacteria against acidic conditions. This study identified the CadC‐CadBA gene cluster in the V. parahaemolyticus and further identified the expression difference of ATR genes in CadC‐dependent or CadC‐independent ways by RNA‐seq. Under sub‐lethal acidic conditions, 321 genes were significantly differentially expressed, including the heat‐shock protein, outer membrane protein, metabolism, two‐component system and virulence (Figure 2). Two genes encoding heat‐shock proteins (VP0651 and VP0821) were significantly upregulated under sub‐lethal acidic conditions, which was agreed to a previous study revealed that adaptation to sub‐lethal acidic conditions could enhance the survival of V. parahaemolyticus at high temperatures (Chiang et al., 2014). In this study, the heat‐shock‐related genes VPA0586/VPA0287 (GroES/GroEL) were significantly upregulated when the cells were adapted to the sub‐lethal acidic pH, which is consistent with a previous study result in Campylobacter jejuni (Varsaki et al., 2015). The VPA0586/VPA0287 genes were responded to acidic stress in a CadC‐dependent manner and significantly downregulated in the ΔcadC strain (Table S3). Two outer membrane protein genes (ompU and ompV) were upregulated under sub‐lethal acidic conditions, which was known to involve in the response to environmental stress (Kao et al., 2009; Merrell et al., 2001; Wu et al., 2006). Twenty‐one genes related to the two‐component system were significantly differentially expressed under sub‐lethal acidic conditions including the CpxRA, which was known to involve in the growth of E. coli under acidic pH (Xu et al., 2020). The genes responsible for the ATR were expressed in a CadC‐independent manner.
The virulence‐associated genes involved in the bacterial secretion system, flagellar assembly and bacterial chemotaxis were significantly differentially expressed under sub‐lethal acidic conditions (Figure 2). Twenty‐four flagellar assembly genes and nine bacterial chemotaxis genes were upregulated under sub‐lethal acidic conditions. Previous studies showed that low motility was associated with acid stress in E. coli and S. Typhimurium (Ryan et al., 2015; Soutourina et al., 2002), whereas another study indicated that the expression of flagellar genes was induced at low pH in E. coli, which was consistent with our results by RNA‐seq (Maurer et al., 2005). The cytotoxicity of pathogens was reported to be induced after adapting to sub‐lethal acidic conditions (Yeung & Boor, 2004). Our study demonstrated that the motility and the cytotoxicity of V. parahaemolyticus were significantly increased after the adaptation to sub‐lethal acidic conditions (Figure 6). Further studies are required to investigate the mechanism of how low pH conditions could influence the regulation of motility in V. parahaemolyticus.
This study confirmed that CadC and CadBA could modulate the ATR system in V. parahaemolyticus. The CadC protein could directly bind to the promoter of cadBA and regulate the expression of CadBA (Figure 7). Additionally, RNA‐seq data showed that 113 genes were involved in ATR in a CadC‐dependent way, whereas 208 genes modulated the ATR independently to CadC. Motility and cytotoxicity analysis indicated that adaptation to a sub‐lethal acidic pH could increase the virulence of V. parahaemolyticus (Figure 7). This study demonstrated that the adaptation of V. parahaemolyticus under the sub‐lethal acidic conditions could increase its virulence and survival ability, which increase the potential risk of disease outbreaks in aquaculture.
FIGURE 7.
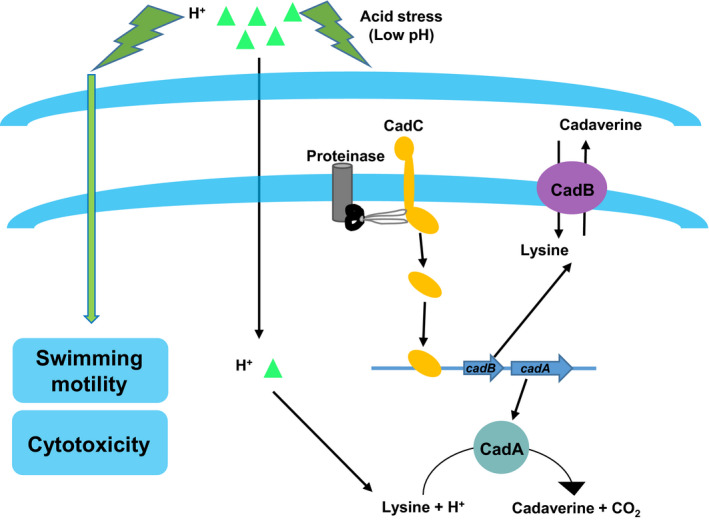
Working model of the mechanism that modulates the ATR and virulence in Vibrio parahaemolyticus. As the bacteria adapt to the sub‐lethal acidic pH, they upregulate cadBA and cadC, which are responsible for the ATR. The motility and cytotoxicity of V. parahaemolyticus were increases after adapt to the sub‐lethal acidic pH [Colour figure can be viewed at wileyonlinelibrary.com]
CONFLICTS OF INTEREST
The authors declare no conflict of interest.
Supporting information
Table S1
Table S2
Table S3
Gu D, Wang K, Lu T, Li L, Jiao X. Vibrio parahaemolyticus CadC regulates acid tolerance response to enhance bacterial motility and cytotoxicity. J Fish Dis.2021;44:1155–1168. 10.1111/jfd.13376
Funding information
This work was supported by the National Natural Science Foundation of China (31700122) and China Postdoctoral Science Foundation (2018M632388); the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
DATA AVAILABILITY STATEMENT
The data sets supporting the results of this article are included within the article and its additional files. The RNA‐Seq data in fastq format have been deposited to the European Nucleotide Archive with the accession number PRJEB38562.
REFERENCES
- Abu Elala, N. M., & Ragaa, N. M. (2015). Eubiotic effect of a dietary acidifier (potassium diformate) on the health status of cultured Oreochromis niloticus . Journal of Advance Research, 6(4), 621–629. 10.1016/j.jare.2014.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcaide, E., Amaro, C., Todolí, R., & Oltra, R. (1999). Isolation and characterization of Vibrio parahaemolyticus causing infection in Iberian toothcarp Aphanius iberus . Diseases of Aquatic Organisms, 35, 77–80. 10.3354/dao035077 [DOI] [PubMed] [Google Scholar]
- Anders, S., Pyl, P. T., & Huber, W. (2015). HTSeq–a Python framework to work with high‐throughput sequencing data. Bioinformatics, 31(2), 166–169. 10.1093/bioinformatics/btu638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asriqah, A., Nugroho, R. A., & Aryani, R. (2018). Effect of various organic acid supplementation diets on Clarias gariepinus BURCHELL, 1822: Evaluation of growth, survival and feed utilization. F1000Research, 7, 1465. 10.12688/f1000research.15954.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker‐Austin, C., Oliver, J. D., Alam, M., Ali, A., Waldor, M. K., Qadri, F., & Martinez‐Urtaza, J. (2018). Vibrio spp. infections. Nature Reviews Disease Primers, 4(1), 8. 10.1038/s41572-018-0005-8 [DOI] [PubMed] [Google Scholar]
- Bauer, J., Teitge, F., Neffe, L., Adamek, M., Jung, A., Peppler, C., Steinhagen, D., & Jung‐Schroers, V. (2021). Impact of a reduced water salinity on the composition of Vibrio spp. in recirculating aquaculture systems for Pacific white shrimp (Litopenaeus vannamei) and its possible risks for shrimp health and food safety. Journal of Fish Diseases, 44(1), 89–105. 10.1111/jfd.13270 [DOI] [PubMed] [Google Scholar]
- Cabrera‐García, M. E., Vázquez‐Salinas, C., & Quiñones‐Ramírez, E. I. (2004). Serologic and molecular characterization of Vibrio parahaemolyticus strains isolated from seawater and fish products of the Gulf of Mexico. Applied Environmental Microbiology, 70(11), 6401–6406. 10.1128/AEM.70.11.6401-6406.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai, J., Li, J., Thompson, K. D., Li, C., & Han, H. (2007). Isolation and characterization of pathogenic Vibrio parahaemolyticus from diseased post‐larvae of abalone Haliotis diversicolor supertexta . Journal of Basic Microbiology, 47(1), 84–86. 10.1002/jobm.200610192 [DOI] [PubMed] [Google Scholar]
- Cakar, F., Zingl, F. G., Moisi, M., Reidl, J., & Schild, S. (2018). In vivo repressed genes of Vibrio cholerae reveal inverse requirements of an H(+)/Cl(‐) transporter along the gastrointestinal passage. Proceedings of the National Academy of Sciences of the United States of America, 115(10), E2376–E2385. 10.1073/pnas.1716973115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cakar, F., Zingl, F. G., & Schild, S. (2019). Silence is golden: Gene silencing of V. cholerae during intestinal colonization delivers new aspects to the acid tolerance response. Gut Microbes, 10(2), 228–234. 10.1080/19490976.2018.1502538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, H., Dong, S., Yan, Y., Zhan, L., Zhang, J., Chen, J., Zhang, Z., Zhang, Y., & Mei, L. (2021). Prevalence and population analysis of Vibrio parahaemolyticus isolated from freshwater fish in Zhejiang province, China. Foodborne Pathogens and Disease, 18(2), 139–146. 10.1089/fpd.2020.2798 [DOI] [PubMed] [Google Scholar]
- Chiang, M. L., Chen, H. C., Wu, C., & Chen, M. J. (2014). Effect of acid adaptation on the environmental stress tolerance of three strains of Vibrio parahaemolyticus . Foodborne Pathogens and Disease, 11(4), 287–294. 10.1089/fpd.2013.1641 [DOI] [PubMed] [Google Scholar]
- Conte, M. P., Petrone, G., Di Biase, A. M., Ammendolia, M. G., Superti, F., & Seganti, L. (2000). Acid tolerance in Listeria monocytogenes influences invasiveness of enterocyte‐like cells and macrophage‐like cells. Microbial Pathogenesis, 29(3), 137–144. 10.1006/mpat.2000.0379 [DOI] [PubMed] [Google Scholar]
- De Schryver, P., Defoirdt, T., & Sorgeloos, P. (2014). Early mortality syndrome outbreaks: A microbial management issue in shrimp farming? PLoS Path, 10(4), e1003919. 10.1371/journal.ppat.1003919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao, Y., He, J., He, Z., Li, Z., Zhao, B., Mu, Y., Lee, J. Y., & Chu, Z. (2017). Effects of fulvic acid on growth performance and intestinal health of juvenile loach Paramisgurnus dabryanus (Sauvage). Fish and Shellfish Immunology, 62, 47–56. 10.1016/j.fsi.2017.01.008 [DOI] [PubMed] [Google Scholar]
- Gu, D., Guo, M., Yang, M., Zhang, Y., Zhou, X., & Wang, Q. (2016). A σE‐mediated temperature gauge controls a switch from LuxR‐mediated virulence gene expression to thermal stress adaptation in Vibrio alginolyticus . PLoS Path, 12(6), e1005645. 10.1371/journal.ppat.1005645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu, D., Meng, H., Li, Y., Ge, H., & Jiao, X. (2019). A GntR family transcription factor (VPA1701) for swarming motility and colonization of Vibrio parahaemolyticus . Pathogens, 8(4), 235. 10.3390/pathogens8040235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero, A., Lizárraga‐Partida, M. L., Gómez Gil Rodríguez, B., Licea‐Navarro, A. F., Revilla‐Castellanos, V. J., Wong‐Chang, I., & González‐Sánchez, R. (2017). Genetic analysis of Vibrio parahaemolyticus O3:K6 strains that have been isolated in Mexico since 1998. PLoS One, 12(1), e0169722. 10.1371/journal.pone.0169722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guin, S., Saravanan, M., Anjay, , Chowdhury, G., Pazhani, G. P., Ramamurthy, T., & Chandra Das, S. (2019). Pathogenic Vibrio parahaemolyticus in diarrhoeal patients, fish and aquatic environments and their potential for inter‐source transmission. Heliyon, 5(5), e01743. 10.1016/j.heliyon.2019.e01743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, W., Rahimnejad, S., Wang, L., Song, K., Lu, K., & Zhang, C. (2017). Effects of organic acids and essential oils blend on growth, gut microbiota, immune response and disease resistance of Pacific white shrimp (Litopenaeus vannamei) against Vibrio parahaemolyticus . Fish and Shellfish Immunology, 70, 164–173. 10.1016/j.fsi.2017.09.007 [DOI] [PubMed] [Google Scholar]
- Hickey, M. E., & Lee, J. L. (2017). A comprehensive review of Vibrio (Listonella) Anguillarum: Ecology, pathology and prevention. Reviews in Aquaculture, 10(3), 585–610. 10.1111/raq.12188 [DOI] [Google Scholar]
- Imaizumi, K., Tinwongger, S., Kondo, H., & Hirono, I. (2018). Disinfection of an EMS/AHPND strain of Vibrio parahaemolyticus using ozone nanobubbles. Journal of Fish Diseases, 41(4), 725–727. 10.1111/jfd.12783 [DOI] [PubMed] [Google Scholar]
- Johnson, C. N., Bowers, J. C., Griffitt, K. J., Molina, V., Clostio, R. W., Pei, S., Laws, E., Paranjpye, R. N., Strom, M. S., Chen, A., Hasan, N. A., Huq, A., Noriea, N. F., Grimes, D. J., & Colwell, R. R. (2012). Ecology of Vibrio parahaemolyticus and Vibrio vulnificus in the coastal and estuarine waters of Louisiana, Maryland, Mississippi, and Washington (United States). Applied Environmental Microbiology, 78(20), 7249–7257. 10.1128/AEM.01296-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalburge, S. S., Whitaker, W. B., & Boyd, E. F. (2014). High‐salt preadaptation of Vibrio parahaemolyticus enhances survival in response to lethal environmental stresses. Journal of Food Protection, 77(2), 246–253. 10.4315/0362-028X.JFP-13-241 [DOI] [PubMed] [Google Scholar]
- Kao, D., Cheng, Y., Kuo, T., Lin, S., Lin, C., Chow, L., & Chen, W. (2009). Salt‐responsive outer membrane proteins of Vibrio Anguillarum serotype O1 as revealed by comparative proteome analysis. Journal of Applied Microbiology, 106(6), 2079–2085. 10.1111/j.1365-2672.2009.04178.x [DOI] [PubMed] [Google Scholar]
- Kuper, C., & Jung, K. (2005). CadC‐mediated activation of the cadBA promoter in Escherichia coli . Journal of Molecular Microbiology and Biotechnology, 10(1), 26–39. 10.1159/000090346 [DOI] [PubMed] [Google Scholar]
- Lamas, A., Regal, P., Vázquez, B., Cepeda, A., & Franco, C. M. (2019). Short chain fatty acids commonly produced by gut microbiota influence Salmonella enterica motility, biofilm formation, and gene expression. Antibiotics, 8(4), 265. 10.3390/antibiotics8040265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langmead, B., & Salzberg, S. L. (2012). Fast gapped‐read alignment with Bowtie 2. Nature Methods, 9(4), 357–359. 10.1038/nmeth.1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, Y., Choi, Y., Lee, S., Lee, H., Kim, S., Ha, J., Lee, J., Oh, H., Kim, Y., & Yoon, Y. (2019). Occurrence of pathogenic Vibrio parahaemolyticus in seafood distribution channels and their antibiotic resistance profiles in S. Korea. Letters in Applied Microbiology, 68(2), 128–133. 10.1111/lam.13099 [DOI] [PubMed] [Google Scholar]
- Lee, Y. H., Kim, J. H., Bang, I. S., & Park, Y. K. (2008). The membrane‐bound transcriptional regulator CadC is activated by proteolytic cleavage in response to acid stress. Journal of Bacteriology, 190, 5120–5126. 10.1128/JB.00012-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, Y. H., Kim, B. H., Kim, J. H., Yoon, W. S., Bang, S. H., & Park, Y. K. (2007). CadC has a global translational effect during acid adaptation in Salmonella enterica Serovar Typhimurium. Journal of Bacteriology, 189(6), 2417–2425. 10.1128/JB.01277-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei, T., Jiang, F., He, M., Zhang, J., Zeng, H., Chen, M., Pang, R., Wu, S., Wei, L., Wang, J., Ding, Y., & Wu, Q. (2020). Prevalence, virulence, antimicrobial resistance, and molecular characterization of fluoroquinolone resistance of Vibrio parahaemolyticus from different types of food samples in China. International Journal of Food Microbiology, 317, 108461. 10.1016/j.ijfoodmicro.2019.108461 [DOI] [PubMed] [Google Scholar]
- Li, Y., Xie, T., Pang, R., Wu, Q., Zhang, J., Lei, T., Xue, L., Wu, H., Wang, J., Ding, Y., Chen, M., Wu, S., Zeng, H., Zhang, Y., & Wei, X. (2020). Food‐borne Vibrio parahaemolyticus in China: Prevalence, antibiotic susceptibility, and genetic characterization. Frontiers in Microbiology., 11, 1670. 10.3389/fmicb.2020.01670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang, W., Wang, S., Yu, F., Zhang, L., Qi, G., Liu, Y., Gao, S., & Kan, B. (2003). Construction and evaluation of a safe, live, oral Vibrio cholerae vaccine candidate, IEM108. Infection and Immunity, 71(10), 5498–5504. 10.1128/IAI.71.10.5498-5504.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieke, T., Steinberg, C. E. W., Pan, B., Perminova, I. V., Meinelt, T., Knopf, K., & Kloas, W. (2021). Phenol‐rich fulvic acid as a water additive enhances growth, reduces stress, and stimulates the immune system of fish in aquaculture. Scientific Reports, 11(1), 174. 10.1038/s41598-020-80449-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love, M. I., Huber, W., & Anders, S. (2014). Moderated estimation of fold change and dispersion for RNA‐seq data with DESeq2. Genome Biology, 15(12), 550. 10.1186/s13059-014-0550-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin, M. (2011). Cutadapt removes adapter sequences from high‐throughput sequencing reads. EMBnet Journal, 17(1), 10. 10.14806/ej.17.1.200 [DOI] [Google Scholar]
- Maurer, L. M., Yohannes, E., Bondurant, S. S., Radmacher, M., & Slonczewski, J. L. (2005). pH regulates genes for flagellar motility, catabolism, and oxidative stress in Escherichia coli K‐12. Journal of Bacteriology, 187(1), 304–319. 10.1128/JB.187.1.304-319.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarter, L. (1999). The multiple identities of Vibrio parahaemolyticus . Journal of Molecular Microbiology and Biotechnology, 1(1), 51–57. 10.1109/16.405277 [DOI] [PubMed] [Google Scholar]
- Merrell, D. S., Bailey, C., Kaper, J. B., & Camilli, A. (2001). The ToxR‐mediated organic acid tolerance response of Vibrio cholerae requires OmpU. Journal of Bacteriology, 183(9), 2746–2754. 10.1128/JB.183.9.2746-2754.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrell, D. S., & Camilli, A. (1999). The cadA gene of Vibrio cholerae is induced during infection and plays a role in acid tolerance. Molecular Microbiology, 34(4), 836–849. 10.1046/j.1365-2958.1999.01650.x [DOI] [PubMed] [Google Scholar]
- Mohamad, N., Mohd Roseli, F. A., Azmai, M. N. A., Saad, M. Z., Md Yasin, I. S., Zulkiply, N. A., & Nasruddin, N. S. (2019). Natural concurrent infection of Vibrio harveyi and V. alginolyticus in cultured hybrid groupers in Malaysia. Journal of Aquatic Animal Health, 31, 88–96. 10.1002/aah.10055 [DOI] [PubMed] [Google Scholar]
- Morales, V. M., Bäckman, A., & Bagdasarian, M. (1991). A series of wide‐host range low‐copy‐number vectors that allow direct screening for recombinants. Gene, 97(1), 39–47. 10.1016/0378-1119(91)90007-X [DOI] [PubMed] [Google Scholar]
- O'Driscoll, B., Gahan, C. G., & Hill, C. (1996). Adaptive acid tolerance response in Listeria monocytogenes: Isolation of an acid‐tolerant mutant which demonstrates increased virulence. Applied and Environmental Microbiology, 62(5), 1693–1698. 10.1007/BF00399626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda, J., Ishibashi, M., Hayakawa, E., Nishino, T., Takeda, Y., Mukhopadhyay, A. K., Garg, S., Bhattacharya, S. K., Nair, G. B., & Nishibuchi, M. (1997). Emergence of a unique O3:K6 clone of Vibrio parahaemolyticus in Calcutta, India, and isolation of strains from the same clonal group from Southeast Asian travelers arriving in Japan. Journal of Clinical Microbiology, 35(12), 3150–3155. 10.1128/JCM.35.12.3150-3155.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghunath, P. (2015). Roles of thermostable direct hemolysin (TDH) and TDH‐related hemolysin (TRH) in Vibrio parahaemolyticus . Frontiers in Microbiology., 5(805), 805. 10.3389/fmicb.2014.00805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan, D., Pati, N. B., Ojha, U. K., Padhi, C., Ray, S., Jaiswal, S., Singh, G. P., Mannala, G. K., Schultze, T., Chakraborty, T., & Suar, M. (2015). Global transcriptome and mutagenic analyses of the acid tolerance response of Salmonella enterica serovar Typhimurium. Applied Environmental Microbiology, 81(23), 8054–8065. 10.1128/AEM.02172-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soumaré, M., Seydi, M., Gbabangai, E., Diop, S. A., Ndour, C. T., Sow, A. I., Diop, B. M., & Sow, P. S. (2007). Acute Vibrio parahaemolyticus gastroenteritis in Dakar. Medecine et Maladies Infectieuses, 37(10), 673–677. 10.1016/j.medmal.2007.07.002 [DOI] [PubMed] [Google Scholar]
- Soutourina, O. A., Krin, E., Laurent‐Winter, C., Hommais, C. F., Danchin, A., & Bertin, P. N. (2002). Regulation of bacterial motility in response to low pH in Escherichia coli: The role of H‐NS protein. Microbiology, 148(5), 1543–1551. 10.1099/00221287-148-5-1543 [DOI] [PubMed] [Google Scholar]
- Stentiford, G. D., Sritunyalucksana, K., Flegel, T. W., Williams, B. A. P., Withyachumnarnkul, B., Itsathitphaisarn, O., & Bass, D. (2017). New paradigms to help solve the global aquaculture disease crisis. PLoS Path, 13(2), e1006160. 10.1371/journal.ppat.1006160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su, C., & Chen, L. (2020). Virulence, resistance, and genetic diversity of Vibrio parahaemolyticus recovered from commonly consumed aquatic products in Shanghai, China. Marine Pollution Bulletin, 160(3), 111554. 10.1016/j.marpolbul.2020.111554 [DOI] [PubMed] [Google Scholar]
- Tan, C. W., Rukayadi, Y., Hasan, H., Thung, T. Y., Lee, E., Rollon, W. D., Hara, H., Kayali, A. Y., Nishibuchi, M., & Radu, S. (2020). Prevalence and antibiotic resistance patterns of Vibrio parahaemolyticus isolated from different types of seafood in Selangor, Malaysia. Saudi Journal of Biological Sciences, 27(6), 1602–1608. 10.1016/j.sjbs.2020.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka, Y., Kimura, B., Takahashi, H., Watanabe, T., Obata, H., Kai, A., Morozumi, S., & Fujii, T. (2008). Lysine decarboxylase of Vibrio parahaemolyticus: Kinetics of transcription and role in acid resistance. Journal of Applied Microbiology, 104(5), 1283–1293. 10.1111/j.1365-2672.2007.03652.x [DOI] [PubMed] [Google Scholar]
- Varsaki, A., Murphy, C., Barczynska, A., Jordan, K., & Carroll, C. (2015). The acid adaptive tolerance response in Campylobacter jejuni induces a global response, as suggested by proteomics and microarrays. Microbial Biotechnology, 8(6), 974–988. 10.1111/1751-7915.12302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velazquez‐Roman, J., León‐Sicairos, N., de Jesus Hernández‐Díaz, L., & Canizalez‐Roman, A. (2014). Pandemic Vibrio parahaemolyticus O3:K6 on the American continent. Frontiers in Cellular and Infection Microbiology, 3(110), 110. 10.3389/fcimb.2013.00110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vielma, J., & Lall, S. (1997). Dietary formic acid enhances apparent digestibility of minerals in rainbow trout, Oncorhynchus mykiss (Walbaum). Aquaculture Nutrition, 3(4), 265–268. 10.1111/j.1365-2095.1997.00041.x [DOI] [Google Scholar]
- Wang, S., Lauritz, J., Jass, J., & Milton, D. L. (2002). A ToxR homolog from Vibrio anguillarum serotype O1 regulates its own production, bile resistance, and biofilm formation. Journal of Bacteriology, 184(6), 1630–1639. 10.1128/JB.184.6.1630-1639.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, L., Lin, X., Wang, F., Ye, D., Xiao, X., Wang, S., & Peng, X. (2006). OmpW and OmpV are required for NaCl regulation in Photobacterium Damsela . Journal of Proteome Research, 5(9), 2250–2257. 10.1021/pr060046c [DOI] [PubMed] [Google Scholar]
- Xu, Y., Zhao, Z., Tong, W., Ding, Y., Liu, B., Shi, Y., Wang, J., Sun, S., Liu, M., Wang, Y., Qi, Q., Xian, M., & Zhao, G. (2020). An acid‐tolerance response system protecting exponentially growing Escherichia coli . Nature Communications, 11, 1496. 10.1038/s41467-020-15350-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, C., Zhang, X., Fan, H., Li, Y., Hu, Q., Yang, R., & Cui, Y. (2019). Genetic diversity, virulence factors and X farm‐to‐table spread pattern of Vibrio parahaemolyticus food‐associated isolates. Food Microbiology, 84, 103270. 10.1016/j.fm.2019.103270 [DOI] [PubMed] [Google Scholar]
- Yeung, P. S., & Boor, K. J. (2004). Effects of acid stress on Vibrio parahaemolyticus survival and cytotoxicity. Journal of Food Protection, 67(7), 1328–1334. 10.1002/jctb.1063 [DOI] [PubMed] [Google Scholar]
- Yingkajorn, M., Mitraparp‐Arthorn, P., Nuanualsuwan, S., Poomwised, R., Kongchuay, N., Khamhaeng, N., & Vuddhakul, V. (2014). Prevalence and quantification of pathogenic Vibrio parahaemolyticus during shrimp culture in Thailand. Diseases of Aquatic Organisms, 112(2), 103–111. 10.3354/dao02800 [DOI] [PubMed] [Google Scholar]
- Zhang, J. (2018). Modulation of growth performance and nonspecific immunity of red swamp crayfish Procambarus clarkia upon dietary fulvic acid supplementation. Fish and Shellfish Immunology, 83, 158–161. 10.1016/j.fsi.2018.09.012 [DOI] [PubMed] [Google Scholar]
- Zhang, L., & Orth, K. (2013). Virulence determinants for Vibrio parahaemolyticus infection. Current Opinion in Microbiology, 16(1), 70–77. 10.1016/j.mib.2013.02.002 [DOI] [PubMed] [Google Scholar]
- Zhao, S., Chen, Z., Zheng, J., Dai, J., Ou, W., Xu, W., Ai, Q., Zhang, W., Niu, J., Mai, K., & Zhang, Y. (2019). Citric acid mitigates soybean meal induced inflammatory response and tight junction disruption by altering TLR signal transduction in the intestine of turbot, Scophthalmus maximus L. Fish and Shellfish Immunology, 92, 181–187. 10.1016/j.fsi.2019.06.004 [DOI] [PubMed] [Google Scholar]
- Zhou, X., Konkel, M. E., & Call, D. R. (2010). Vp1659 is a Vibrio parahaemolyticus type III secretion system 1 protein that contributes to translocation of effector proteins needed to induce cytolysis, autophagy, and disruption of actin structure in HeLa cells. Journal of Bacteriology, 192(13), 3491–3502. 10.1128/JB.01493-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Table S2
Table S3
Data Availability Statement
The data sets supporting the results of this article are included within the article and its additional files. The RNA‐Seq data in fastq format have been deposited to the European Nucleotide Archive with the accession number PRJEB38562.


