Abstract
Objectives
This study aimed to determine the effect of bariatric surgery‐induced weight loss on bone marrow adipose tissue (BMAT) and bone mineral density (BMD) in postmenopausal, nondiabetic women.
Methods
A total of 14 postmenopausal, nondiabetic women with obesity who were scheduled for laparoscopic Roux‐en‐Y gastric bypass surgery (RYGB) were included in this study. Vertebral bone marrow fat signal fraction was determined by quantitative chemical shift magnetic resonance imaging, and vertebral volumetric BMD (vBMD) was determined by quantitative computed tomography before surgery and 3 and 12 months after surgery. Data were analyzed by linear mixed model.
Results
Body weight [mean (SD)] decreased after surgery from 108 (13) kg at baseline to 89 (12) kg at 3 months and 74 (11) kg at 12 months (P < 0.001). BMAT decreased after surgery from 51% (8%) at baseline to 50% (8%) at 3 months and 46% (7%) at 12 months (P = 0.004). vBMD decreased after surgery from 101 (26) mg/cm3 at baseline to 94 (28) mg/cm3 at 3 months (P = 0.003) and 94 (28) mg/cm3 at 12 months (P = 0.035). Changes in BMAT and vBMD were not correlated (ρ = −0.10 and P = 0.75). Calcium and vitamin D concentrations did not change after surgery.
Conclusions
RYGB decreases both BMAT (after 12 months) and vBMD (both after 3 months and 12 months) in postmenopausal, nondiabetic women. Changes in BMAT and vBMD were not correlated. These findings suggest that BMAT does not contribute to bone loss following RYGB.
Study Importance.
What is already known?
-
►
Bariatric surgery is associated with increased fracture risk.
-
►
Bone marrow adipose tissue (BMAT) is associated with low bone mineral density (BMD) and increased fracture risk.
-
►
Studies on the effect of bariatric surgery on BMAT have shown conflicting results, possibly caused by heterogeneous study populations and confounding effects of type 2 diabetes mellitus.
What does this study add?
-
►
We show that, after Roux‐en‐Y gastric bypass, both BMAT (after 12 months) and volumetric BMD (both after 3 months and 12 months) decrease in postmenopausal, nondiabetic women, and that changes in BMAT and volumetric BMD are not correlated.
-
►
This suggests that BMAT does not contribute to bone loss following gastric bypass surgery in this clinically relevant study population of postmenopausal, nondiabetic women.
Introduction
Obesity is becoming more prevalent in our society, and consequently, there is an increase in the bariatric surgery rate (1). Besides the positive effect of bariatric surgery on obesity and its associated morbidity, bariatric surgery has a negative effect on bone health, decreasing bone mineral density (BMD) and increasing fracture risk (2). Especially in postmenopausal women (who are already at risk of developing osteoporosis and sustaining fractures), the effect of bariatric surgery on bone health needs to be addressed.
High bone marrow adipose tissue (BMAT) is associated with low BMD (3) and vertebral fractures (4), and this latter association exists even independently of bone mass in some studies (5, 6). There are several theories on the etiology of the negative association between bone volume and BMAT. First, as bone marrow adipocytes and osteoblasts share a common progenitor, bone marrow stromal cells (7, 8), an increase in bone marrow adipocytes would be accompanied by a decrease in osteoblasts and, therefore, bone formation. Second, bone marrow adipocytes secrete fatty acids and adipokines, which could influence bone metabolism in a paracrine manner (9). However, the exact interaction between BMAT and bone in vivo remains elusive.
Recently, interest has risen on the interaction between bone and BMAT during extensive weight loss after bariatric surgery. It was reported that patients suffering from anorexia nervosa have increased BMAT with low bone mass (10), and animal studies have shown more BMAT and less bone during caloric restriction (11, 12). So far, studies on the association between vertebral BMAT and bone after bariatric surgery have shown inconsistent results (13, 14, 15). A possible explanation for these inconsistent results could be the heterogeneity of participants included in these studies and the chosen time point for measurement. The studies included pre‐ and postmenopausal women and patients with and without diabetes. Estrogen has anabolic effects on bone, and estradiol is able to reduce BMAT (16, 17). Furthermore, BMAT significantly changes during the menstrual cycle, likely because of effects of sex hormones (17), and BMAT and bone both change substantially after menopause. In addition, patients with type 2 diabetes mellitus have an increased risk of fractures (18), and BMAT seems to be associated with glycemic control (19, 20). Owing to the fact that bariatric surgery affects both the hormonal status and glycemic control of patients, the presence of diabetes before surgery may influence the effect of bariatric surgery on BMAT. Furthermore, in the aforementioned studies, measurements were taken at 6 months after surgery, when patients have achieved approximately 75% of the expected weight loss, whereas other studies performed measurements at 12 months after surgery, when body weight has usually reached its nadir. As the rate of weight loss is the largest within the first 3 months following surgery (i.e., approximately 50% of the expected weight loss), we were also interested in determining changes that occur in a shorter time frame following surgery. Therefore, we aimed to determine the effects of weight loss caused by laparoscopic Roux‐en‐Y gastric bypass surgery (RYGB) on BMAT and volumetric BMD (vBMD) in postmenopausal, nondiabetic women shortly after RYGB, at 3 months, and after 1 year.
Methods
Participants
Participants were recruited from a population of women scheduled for laparoscopic RYGB surgery at the MC Slotervaart in Amsterdam, the Netherlands. All participants were older than 50 years old and were postmenopausal. Exclusion criteria were having diabetes mellitus (defined by either a mentioning of diabetes mellitus in their medical history, taking antidiabetes medication, or having fasting plasma glucose ≥ 7.0 mmol/L or hemoglobin A1c ≥ 48 mmol/mol) and being on medication known to have an influence on bone metabolism, including estrogens, corticosteroids, and bone‐resorption inhibitors. Inclusions took place between June 12, 2015, and July 28, 2017. Four visits to the Amsterdam University Medical Center at the Academic Medical Center location were scheduled for radiological examinations and laboratory tests. Two visits were scheduled before surgery, one between 3 months and 2 weeks before surgery, and one between 2 weeks and 1 day before surgery. Two visits were scheduled after surgery: 3 months after surgery and 1 year after surgery. Primary outcome parameters were changes in vertebral bone marrow fat fraction, which was measured by quantitative chemical shift imaging (QCSI) with magnetic resonance imaging (MRI), and changes in vertebral vBMD, which were measured by quantitative computed tomography (QCT). Secondary outcome parameters were changes in bone‐turnover markers (procollagen type 1 N‐terminal propeptide [P1NP] and C‐terminal crosslinking telopeptides of collagen type 1 [CTx]), calcium, phosphorus, 25‐hydroxyvitamin D (25OH‐vitD), and parathyroid hormone (PTH). According to protocol, all participants were prescribed omeprazole (for 3 months) and calcium carbonate/cholecalciferol supplementation after RYGB. Postmenopausal status was defined as the last menstrual bleeding occurring more than 1 year ago and was confirmed with an increased follicle stimulating hormone (FSH; FSH > 20 IU/L). Two participants had unknown last menopausal bleeding dates due to uterus extirpations but had FSH measurements > 20 IU/L.
This study was carried out in compliance with the World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects and was approved by the medical ethics committee of the Amsterdam University Medical Center, located at the Academic Medical Center in Amsterdam. The study was registered at the Netherlands Trial Register (NTR5056). Written informed consent was obtained prior to the first visit.
Quantification of BMAT by QCSI MRI
In order to quantify the bone marrow fat signal fraction (i.e., BMAT), an in‐house‐developed turbo spin echo‐Dixon sequence was used as described previously (17, 21). MRI scans were made on a 1.5‐T MAGNETOM Avanto (Siemens Healthineers AG, Erlangen, Germany). Five participants had their last QCSI measurement on the upgraded 1.5‐T MAGNETOM Avantofit (Siemens Healthineers AG). The upgraded version of the MRI was cross calibrated by scanning three healthy volunteers (intraclass correlation coefficient = 0.89; P = 0.12). Robustness of the QCSI method was previously tested over four scanner changes (22), and exclusion of the scans made on the upgraded Avanto fit did not change the results. BMAT was measured at the level of the L3‐L5 vertebrea in a coronal plane. The Dixon technique is based on the different resonance frequencies of the water and fat protons within the bone marrow, and it measures the fat signal fraction (i.e., the fat signal divided by the sum of the fat and the water signal). The in‐phase (IP) image is acquired when the water and fat signal are in phase, and the out‐of‐phase (OP) image is acquired when the water and fat signals are out of phase. Water‐only and fat‐only images can be reconstructed from the IP and OP images. Imaging parameters were as follows: repetition time (TR) = 2,500 millseconds, echo time (TE) = 22.3 millseconds, slice thickness = 4 mm, matrix size = 256 × 256, number of excitations = 1, and field of view = 350 × 350 mm2 with a turbo factor of 4, resulting in an acquisition time of 5 minutes and 20 seconds. Paracoronal IP, OP, and magnitude images were acquired of a single slice through the dorsal third of the vertebral bodies of L3‐L5. A midsagittal localizer image was used for slice positioning of the measurement. A small flex coil was used to detect the MR signal for all participants to avoid motion and respiration artifacts. Regions of interest were drawn on the individual vertebral bodies (Figure 1A), and three erosions were applied to ensure that only the trabecular bone area within the vertebral bodies was included in the regions of interest (Figure 1B). Complex data and a region‐growing algorithm were used to construct a fat signal fraction map (Figure 1C).
Figure 1.
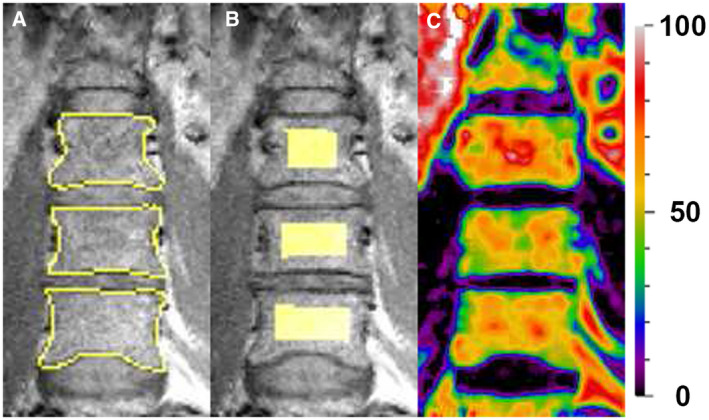
(A) Outline of vertebral bodies L3‐L5. (B) Region of interest derived from the outline of the vertebral bodies L3‐L5. (C) Fatsignalfraction map with color coding ranging from 0% fat to 100% fat.
QCT
QCT was used to quantify vBMD within the vertebral bodies (L3‐L4) using Mindways QCT Pro Bone Investigational Toolkit (BIT; Mindways Software Inc., Austin, Texas). Images were acquired on a Brilliance CT 64‐slice (Philips, Best, the Netherlands), and the CT scanner was calibrated prior to the QCT measurements.
Visceral adipose tissue
Visceral adipose tissue (VAT) was quantified in a single slice at the level of L4, using the CT images acquired for the vBMD assessment. Semiautomated prototype software was used to quantify VAT (Cardiac Risk Assessment package, syngo.via, Siemens Healthineers AG). The internal abdominal wall was traced in a single slice, at the center of L4, and a threshold was set at −50 to −200 Hounsfield units to detect pixels with a value in the adipose tissue range (23).
Biochemical measurements
Morning fasted blood samples were drawn to determine the bone‐formation marker P1NP and bone‐resorption marker CTx. Additionally, calcium, phosphorus, 25OH‐vitD, and PTH concentrations were determined. FSH, hemoglobin A1c, and fasted glucose were determined at baseline. We measured serum concentrations of calcium, phosphate, and PTH on a Hitachi Modular 8000 (C602 and C702; Roche Diagnostics, Mannheim, Germany), and 25OH‐vitD was measured using an automated immunoassay (LIAISON analyzer; DiaSorin Inc., Stillwater, Minnesota). Serum concentrations of CTx and P1NP were measured using an immunoassay (Modular Analytics E 170, Roche Diagnostics and Aidian, Espoo, Finland, respectively). Interassay coefficients of variation for CTx and P1NP were 3% and 8%, respectively.
Statistics
This study was powered to detect a difference in bone marrow fat signal fraction of 5% with a variance of 2.4%. A 5% difference is likely to represent a clinically relevant change, owing to the fact that we previously showed that BMAT decreases by 5% after 2 weeks of 17β‐estradiol treatment in postmenopausal women (17). In addition, BMAT increases with age by an estimated 7% for each decade (24). The variance is based on our previous study in postmenopausal women (17). The sample size was calculated using nQuery Advisor (version 7.0; GraphPad Software, San Diego, California), and the power analysis was approved by the ethics committee of the Amsterdam UMC. Outlier analysis was performed; significant outliers were excluded from further analysis, but this had no effect on the results. SPSS Statistics for Windows (version 22; IBM Corp., Armonk, New York) was used for statistical analysis. Graphs were constructed using GraphPad Prism (version 7.0). Mean and standard deviations (SD) or median and interquartile ranges (IQR) are reported, depending on the distribution of the data. A linear mixed model was used to determine the changes in the primary outcome parameters over time and to account for missing data. Covariance structure was chosen based on the Akaike information criterion and the Schwarz’s Bayesian criterion. We used the Spearman rank correlation test to determine correlations between variables.
Results
A total of 17 postmenopausal, nondiabetic Caucasian women who were scheduled for laparoscopic RYGB were enrolled in this study. Three participants were excluded, as vBMD was incorrectly measured in L1‐L2 instead of L3‐L4, leaving 14 participants subject to further analysis. One participant retracted informed consent after the first MRI because of physical complaints during the scan (i.e., headache). Two participants missed their second visit because their surgery was rescheduled last minute to an earlier date. One participant had 9 months between her second visit and the surgery, as her RYGB surgery was postponed because of complications of earlier knee replacement surgery.
Baseline characteristics are shown in Table 1. All participants had significant weight loss following RYGB and, to lesser extent, between the two baseline visits (mean body weight for visit 1 = 108 [13] kg; visit 2 = 104 [15] kg, P = 0.003 compared with visit 1; visit 3 = 89 [12] kg, P ≤ 0.001 compared with visit 1; visit 4 = 74 [11] kg, P ≤ 0.001 compared with visit 1; Figure 2A).
TABLE 1.
Patient characteristics at baseline (N = 14)
| Age (y) | 58 ± 4 |
| Body weight (kg) | 108 ± 13 |
| BMI (kg/m2) | 38 ± 4 |
| Time postmenopausal (y) | 4 (1‐15) |
| Serum FSH (IU/L) | 59 ± 18 |
| Fasting plasma glucose (mmol/L) | 5.8 ± 0.7 |
| HbA1c (mmol/mol) | 38 ± 2 |
Data are expressed as mean ± SD or median (interquartile range).
FSH, follicle stimulating hormone; HbA1c, hemoglobin A1c.
Figure 2.
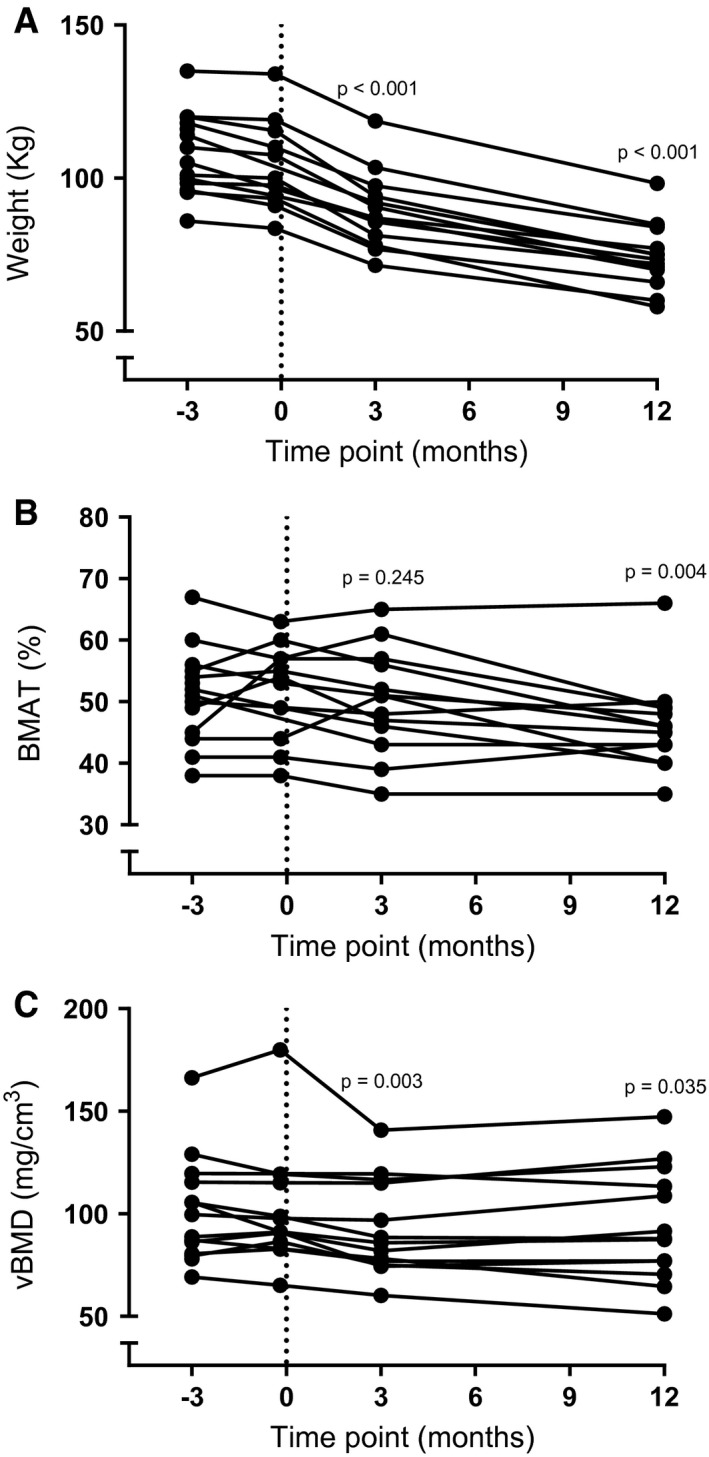
(A) Weight loss following Roux‐en‐Y gastric bypass surgery. (B) Changes in vertebralbonemarrow fat fraction and (C) volumetric bone mineral density (vBMD) following weight loss due to Roux‐en‐Y gastric bypass. P value indicates a difference compared with baseline measurement at visit 1. BMAT, bone marrow adipose tissue.
BMAT
BMAT remained stable during the two baseline measurements (mean BMAT for visit 1 = 51% [8%]; visit 2 = 52% [8%]) and 3 months after surgery (mean BMAT for visit 3 = 50% [8%]) but decreased 12 months after surgery (mean BMAT for visit 4 = 46% [7%], P = 0.004 compared with visit 1; Figure 2B). Changes in BMAT and changes in body weight at visit 4 compared with visit 1 tended to be positively correlated, possibly suggesting that participants who lost more body weight also lost more BMAT (Figure 3B; ρ = 0.55 and P = 0.052). BMAT and body weight were not correlated at visit 1 or visit 4 (ρ = 0.43 and P = 0.12; ρ = 0.12 and P = 0.69, respectively).
Figure 3.
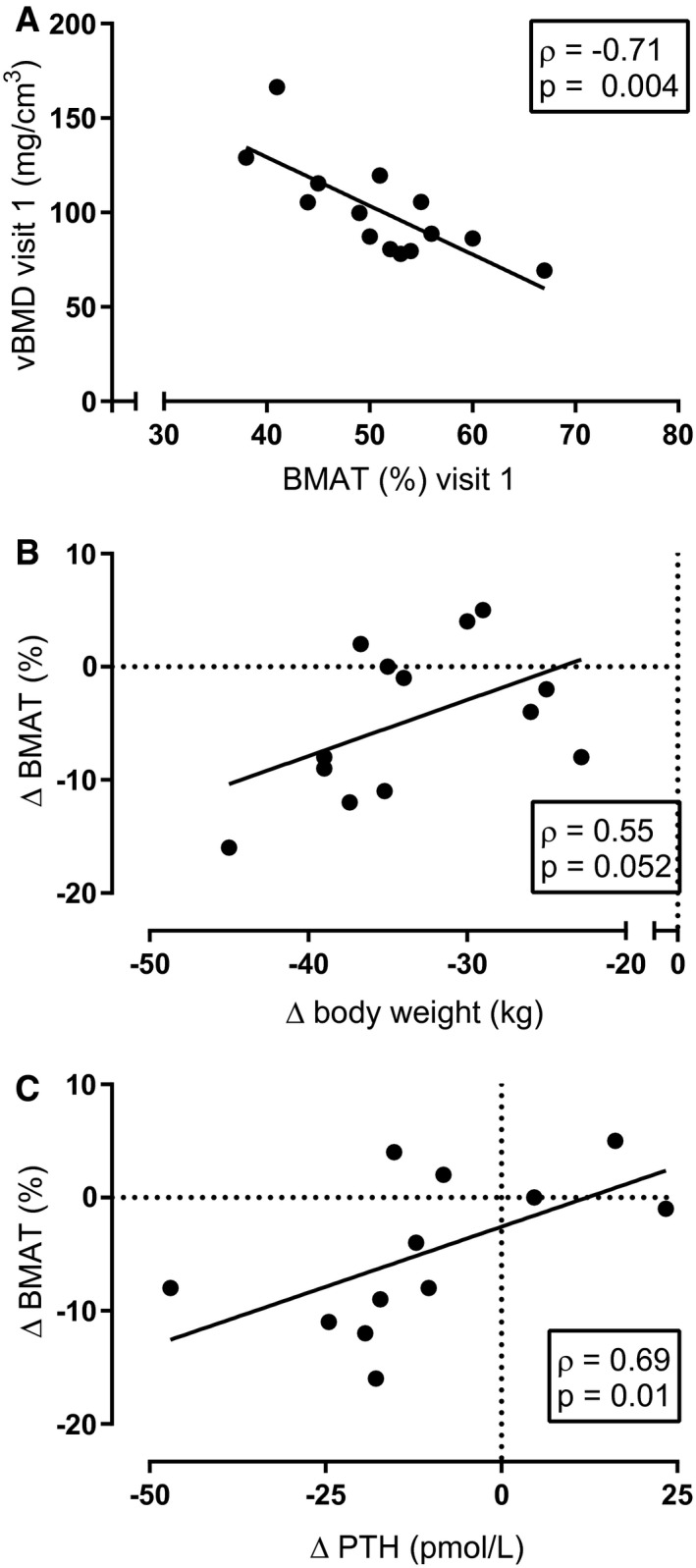
(A) At baseline (visit 1), vertebral bone marrow adipose tissue (BMAT) and volumetric bone mineral density (vBMD) were negatively correlated. (B) Changes in weight loss at visit 4 tended to be positively correlated with changes in BMAT at visit 4. (C) Changes in BMAT were positively correlated with changes in parathyroid hormone (PTH).
vBMD
vBMD remained stable during the two baseline visits (mean vBMD for visit 1 = 101 [26] mg/cm3; visit 2 = 102 [30] mg/cm3) but decreased 3 months and 12 months after surgery (mean vBMD visit 3 = 93 [23] mg/cm3, P = 0.003 compared with visit 1; mean vBMD for visit 4 = 94 [28] mg/cm3, P = 0.035 compared with baseline visit 1; Figure 2C). At baseline, BMAT and vBMD were inversely correlated (Figure 3A; ρ = −0.71 and P = 0.004), although this correlation was lost at 12 months. Changes in vBMD and body weight (ρ = 0.20 and P = 0.52) and changes in vBMD and BMAT were not associated (ρ = −0.10 and P = 0.75).
VAT
At baseline, VAT was not associated with BMAT or vBMD (ρ = 0.06 and P = 0.84; ρ = −0.02 and P = 0.94, respectively). VAT significantly decreased following RYGB surgery (mean VAT in single slice at level L4 for visit 1 = 188 [68] cm2; visit 2 = 187 [74] cm2; visit 3 = 122 [48] cm2, P < 0.001 compared with visit 1; visit 4 = 62 [24] cm2, P < 0.001 compared with visit 1). No association was found between changes in BMAT or vBMD and changes in VAT (ρ = 0.16 and P = 0.96; ρ = −0.19 and P = 0.53, respectively).
Bone turnover
Both the bone‐formation marker P1NP and the bone‐resorption marker CTx remained stable during the two baseline measurements (mean P1NP for visit 1 = 53 [16] μg/L and visit 2 = 52 [15] μg/L; mean CTx for visit 1 = 271 [93] pg/L and visit 2 = 299 [99] pg/L) and increased significantly following RYGB (mean P1NP for visit 3 = 82 [24] μg/L, P < 0.001 compared with visit 1; visit 4 = 99 [29] μg/L, P < 0.001 compared with visit 1; mean CTx for visit 3 = 690 [191] pg/L, P < 0.001 compared with visit 1; visit 4 = 851 [203] pg/L, P < 0.001 compared with visit 1; Figures 4A‐4B, respectively). PTH, vitamin D, and calcium concentrations did not change during the study period (median PTH for visit 1 = 4.78 [2.3] pmol/L; visit 2 = 4.66 [1.8] pmol/L; visit 3 = 4.91 [1.4] pmol/L; and visit 4 = 4.59 [1.4] pmol/L, Figure 4C; mean 25OH‐vitD2+3 for visit 1 = 75 [30] nmol/L; visit 2 = 69 [31] nmol/L; visit 3 = 76 [15] nmol/L; visit 4 = 81 [16] nmol/L, Figure 4D; and mean calcium for visit 1 = 2.39 [0.06] mmol/L; visit 2 = 2.40 [0.08] mmol/L; visit 3 = 2.39 [0.06] mmol/L; visit 4 = 2.37 [0.08] mmol/L, Figure 4E). Phosphate significantly increased during the study (mean phosphate for visit 1 = 0.99 [0.13] mmol/L; visit 2 = 1.01 [0.08] mmol/L, P = 0.04 compared with visit 1; visit 3 = 1.06 [0.11] mmol/L, P < 0.001 compared with visit 1; and visit 4 = 1.16 [0.09] mmol/L, P < 0.001 compared to visit 1; Figure 4F).
Figure 4.
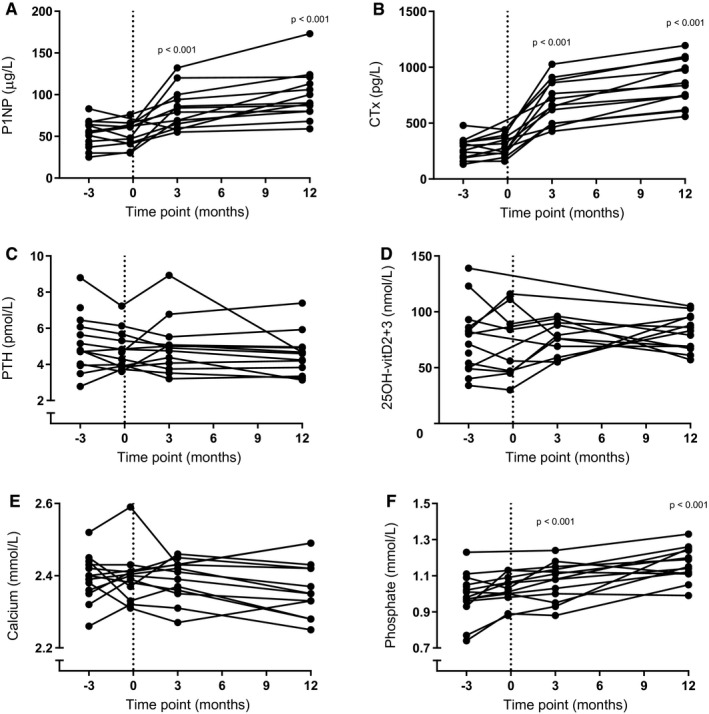
(A) Bone‐formation marker procollagen type 1 N‐terminal propeptide (P1NP) and (B) bone‐resorption marker C‐terminal crosslinking telopeptides of collagen type I (CTx) increased following Roux‐en‐Y gastric bypass surgery. (C) Parathyroid hormone (PTH), (D) vitamin D (24OH‐vitD2+3), and (E) calcium concentrations did not change during the study period. (F) Phosphate significantly increased during the study period. P value indicates a difference compared with baseline measurement at visit 1
Discussion
In this prospective longitudinal study, we show that both BMAT and vBMD decrease after RYGB in postmenopausal, nondiabetic women.
BMAT was decreased 12 months after RYGB in this homogenous, clinically relevant population of postmenopausal, nondiabetic female participants. Our results are consistent with a recent study by Blom‐Høgestøl and colleagues (25) that showed a decrease in BMAT in iliac crest biopsies of female individuals 1 year after RYGB surgery compared with baseline. However, in the study by Blom‐Høgestøl and colleagues, only eight out of eighteen female individuals were postmenopausal, and the study also included individuals with diabetes. Other studies have not shown changes in BMAT following RYGB (13, 14, 15). This lack of change is likely related to the more heterogeneous study populations, including male and female individuals, pre‐ and postmenopausal women, and individuals both with and without diabetes. Another difference between these studies and our current study is that we used QCSI, whereas other studies (13, 14, 15) used MR spectroscopy to quantify BMAT, which limits direct comparison. MR spectroscopy is considered the gold‐standard method for quantification of BMAT; however, good agreement has been shown between QCSI and MR spectroscopy (26, 27, 28).
In addition to a decrease in BMAT, we also show a decrease in vBMD both 3 months and 12 months after RYGB. This implies that the decrease in vBMD precedes the decrease in BMAT. At baseline, BMAT and vBMD showed the well‐known inverse relationship that is also present in women with osteoporosis (16, 29, 30, 31). However, 1 year after RYGB, the association between BMAT and vBMD was lost, suggesting that BMAT is not functionally linked to bone mass following RYGB. Weight loss is most rapid within the first 3 months after surgery, which could be a possible explanation for the rapid increase in bone turnover with a negative balance, leading to a decrease in vBMD. The observed increases in bone‐turnover markers P1NP and CTx are consistent with previous studies (14, 15). Although calcium remained unchanged, we observed an increase in phosphate after RYGB. The latter is most likely related to the catabolic state induced by bariatric surgery and is consistent with previous research (32). Vitamin D and PTH remained unchanged after RYGB, which suggests that the decrease in vBMD is not caused by deficiencies, although we cannot exclude small changes that were not detected in this study that may have had an effect. Interestingly, we did find a positive correlation between changes in BMAT and changes in PTH, whereas BMAT and PTH were not correlated at baseline or after RYGB. Bone marrow adipocytes are responsive to PTH, and intermittent administration of teriparatide decreases BMAT (30, 33). However, to our knowledge, we are the first study to report these longitudinal results on changes in BMAT and PTH following RYGB, and further research is necessary to interpret these findings.
Changes in BMAT and changes in body weight tended to be positively associated in our study, which could indicate that participants who lost more weight also lost more BMAT. However, VAT was not associated with BMAT, and changes in VAT were not associated with changes in BMAT. These results are consistent with previous research which has shown no association between VAT and BMAT in a group of individuals with morbid obesity who were scheduled for bariatric surgery (20) and in a group of postmenopausal, nondiabetic women with obesity (19). However, other studies have shown an association between VAT and BMAT in a group of premenopausal women (34), as well as changes in BMAT and changes in VAT following RYGB (14). Hormonal status and glycemic control in these individuals could possibly explain these differences because both are associated with changes in BMAT, as well as changes in body composition and VAT (35). Furthermore, we did not find a negative association between VAT and vBMD as described previously (36, 37). A possible explanation could be the relatively small range in VAT in our participants, as morbid obesity is the indication for RYGB surgery and, consequently, one of the inclusion criteria of our study.
Our participant lost some weight prior to surgery, as they were advised to lose weight if possible, with the guidance of a dietician as part of the program and to reduce the risks of surgery. However, we observed no changes in BMAT and BMD between the baseline measurements prior to surgery. Our results are in line with a short‐term diet interventional study by Cordes and colleagues that showed no changes in BMAT after 4 weeks of diet intervention (38). However, our results are inconsistent with a longer study of 12‐week‐long diet‐induced weight loss that showed a decrease in BMAT and an increase in BMD (39) but no relationship between changes in BMAT and changes in BMD. The discrepancy with our study is likely related to the shorter interval (59 d) and limited weight loss (3 kg) between the baseline visits in our study.
As aforementioned, the baseline data confirm the well‐known inverse association between BMAT and vBMD. However, after RYGB, BMAT and vBMD were no longer associated. Furthermore, we did not find an association between changes in BMAT and changes in vBMD. These findings implicate that changes in BMAT and bone metabolism following RYGB are different from changes following administration of estrogen (16), teriparatide (29, 30), or bisphosphonates (31), in which there is an association between decreases in BMAT and increases in vBMD. Decreases in both BMAT and BMD are also described in animal studies on lactating mice (40) and in mice after cold exposure (41). A possible explanation could be that the effects of bariatric surgery on bone and BMAT separately are stronger than the effects of BMAT on bone, and this might also be the case for lactation and cold exposure.
Our study has several strengths and limitations. We used QCSI to measure bone marrow adiposity, whereas other studies have used MR spectroscopy. The QCSI method is well validated and considered the gold standard for quantification of bone marrow fat in patients with Gaucher disease (22). The QCSI method measures the fat signal fraction, as it does not correct for T1 and T2 effects. However, T2 effects are minimized by using a short echo time (23 ms), and T1 bias is minimized by the long repetition time of the sequence (2,500 ms). A limitation of our study is that we did not include a control group. Therefore, we cannot distinguish between the effects of bariatric surgery and weight loss per se. We performed two baseline measurements to show that vBMD and BMAT were stable before surgery. Therefore, the effects observed after surgery are more likely to be caused by surgery and weight loss, and not by other factors. Calcium carbonate was used as calcium supplement. Calcium carbonate is best absorbed in an acidic environment, and absorption is possibly lower after RYGB (42, 43), although other studies have shown no difference between calcium carbonate and calcium citrate supplementation on PTH and BMD after RYGB (44, 45). Generalizability of our results is limited, as we only included Caucasian women. Finally, our study has a relatively small sample size, but we included only postmenopausal women without diabetes to exclude effects of concurrent changes in estrogen status and glucose homeostasis on BMAT. Other studies on the effects of bariatric surgery on BMAT (13, 14, 15, 25) included lower numbers of postmenopausal, nondiabetic participants, often too small for subgroup analysis, which makes our study the first, to our knowledge, to report BMAT and vBMD changes in this clinically relevant study population.
Conclusion
RYGB decreases BMAT 12 months after surgery and decreases vBMD both 3 months and 12 months after surgery in postmenopausal, nondiabetic women, and changes in BMAT and vBMD are not correlated. These findings suggest that BMAT does not contribute to bone loss following gastric bypass surgery.
Funding agencies
This study has been funded by an Alliance Grant provided by Amsterdam University Medical Center/Amsterdam Movement Sciences (Grant 2013‐01). Funding sources had no influence on the study design, the interpretation of the results, or the manuscript.
Disclosure
The authors declared no conflicts of interest.
Author contributions
Study design: KB, NB, PB, AV, MH, and MM; study conduction: KB, EA, and GS; data collection: KB, VG, and YA; data analysis: KB, EA, and GS; data interpretation: KB, NB, and PB; drafting manuscript: KB and PB. All authors were involved in writing the paper and had final approval of the submitted and published versions.
Clinical trial registration
Netherlands Trial Register identifier NL4951 (NTR5056).
Acknowledgments
The authors would like to thank study participants for their contribution, Thomas Boerlage, Floris Westerink, Paula Wolvers, Stijn Meijnikman, Sylke Haal and Maimoena Guman for their help selecting the participants, and Raschel van Luijk, Ruud Wellenberg, Tessel Boertien and Marieke Tebbens for their assistance during the study visits.
References
- 1.Angrisani L, Santonicola A, Iovino P, Formisano G, Buchwald H, Scopinaro N. Bariatric surgery worldwide 2013. Obes Surg 2015;25:1822‐1832. [DOI] [PubMed] [Google Scholar]
- 2.Rousseau C, Jean S, Gamache P, et al. Change in fracture risk and fracture pattern after bariatric surgery: nested case‐control study. BMJ 2016;354:i3794. doi: 10.1136/bmj.i3794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Justesen J, Stenderup K, Ebbesen EN, Mosekilde L, Steiniche T, Kassem M. Adipocyte tissue volume in bone marrow is increased with aging and in patients with osteoporosis. Biogerontology 2001;2:165‐171. [DOI] [PubMed] [Google Scholar]
- 4.Wehrli FW, Hopkins JA, Hwang SN, Song HK, Snyder PJ, Haddad JG. Cross‐sectional study of osteopenia with quantitative MR imaging and bone densitometry. Radiology 2000;217:527‐538. [DOI] [PubMed] [Google Scholar]
- 5.Schwartz AV, Sigurdsson S, Hue TF, et al. Vertebral bone marrow fat associated with lower trabecular BMD and prevalent vertebral fracture in older adults. J Clin Endocrinol Metab 2013;98:2294‐2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beekman KM, Veldhuis‐Vlug AG, den Heijer M, et al. The effect of raloxifene on bone marrow adipose tissue and bone turnover in postmenopausal women with osteoporosis. Bone 2019;118:62‐68. [DOI] [PubMed] [Google Scholar]
- 7.Tencerova M, Kassem M. The bone marrow‐derived stromal cells: commitment and regulation of adipogenesis. Front Endocrinol (Lausanne) 2016;7:127. doi: 10.3389/fendo.2016.00127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science 1999;284:143‐147. [DOI] [PubMed] [Google Scholar]
- 9.Elbaz A, Wu X, Rivas D, Gimble JM, Duque G. Inhibition of fatty acid biosynthesis prevents adipocyte lipotoxicity on human osteoblasts in vitro. J Cell Mol Med 2010;14:982‐991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bredella MA, Fazeli PK, Miller KK, et al. Increased bone marrow fat in anorexia nervosa. J Clin Endocrinol Metab 2009;94:2129‐2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Devlin MJ, Cloutier AM, Thomas N, et al. Caloric restriction leads to high marrow adiposity and low bone mass in growing mice. J Bone Miner Res 2010;25:2078‐2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cawthorn WP, Scheller EL, Learman BS, et al. Bone marrow adipose tissue is an endocrine organ that contributes to increased circulating adiponectin during caloric restriction. Cell Metab 2014;20:368‐375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim TY, Schwartz AV, Li X, et al. Bone marrow fat changes after gastric bypass surgery are associated with loss of bone mass. J Bone Miner Res 2017;32:2239‐2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bredella MA, Greenblatt LB, Eajazi A, Torriani M, Yu EW. Effects of Roux‐en‐Y gastric bypass and sleeve gastrectomy on bone mineral density and marrow adipose tissue. Bone 2016;95:85‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ivaska KK, Huovinen V, Soinio M, et al. Changes in bone metabolism after bariatric surgery by gastric bypass or sleeve gastrectomy. Bone 2017;95;47‐54. [DOI] [PubMed] [Google Scholar]
- 16.Syed FA, Oursler MJ, Hefferanm TE, Peterson JM, Riggs BL, Khosla S. Effects of estrogen therapy on bone marrow adipocytes in postmenopausal osteoporotic women. Osteoporos Int 2008;19:1323‐1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Limonard EJ, Veldhuis‐Vlug AG, van Dussen L, et al. Short‐term effect of estrogen on human bone marrow fat. J Bone Miner Res 2015;30:2058‐2066. [DOI] [PubMed] [Google Scholar]
- 18.Wang H, Ba Y, Xing Q, Du J‐L. Diabetes mellitus and the risk of fractures at specific sites: a meta‐analysis. BMJ Open 2019;9:e024067. doi: 10.1136/bmjopen-2018-024067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baum T, Yap SP, Karampinos DC, et al. Does vertebral bone marrow fat content correlate with abdominal adipose tissue, lumbar spine bone mineral density, and blood biomarkers in women with type 2 diabetes mellitus? J Magn Reson Imaging 2012;35:117‐124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu EW, Greenblatt L, Eajazi A, Torriani M, Bredella MA. Marrow adipose tissue composition in adults with morbid obesity. Bone 2017;97:38‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maas M, Akkerman EM, Venema HW, Stoker J, Den Heeten GJ. Dixon quantitative chemical shift MRI for bone marrow evaluation in the lumbar spine: a reproducibility study in healthy volunteers. J Comput Assist Tomogr 2001;25:691‐697. [DOI] [PubMed] [Google Scholar]
- 22.van Dussen L, Akkerman EM, Hollak CEM, Nederveen AJ, Maas M. Evaluation of an imaging biomarker, Dixon quantitative chemical shift imaging, in Gaucher disease: lessons learned. J Inherit Metab Dis 2014;37:1003‐1011. [DOI] [PubMed] [Google Scholar]
- 23.Yoshizumi T, Nakamura T, Yamane M, et al. Abdominal fat: standardized technique for measurement at CT. Radiology 1999;211:283‐286. [DOI] [PubMed] [Google Scholar]
- 24.Griffith JF, Yeung DKW, Ma HT, Leung JCS, Kwok TCY, Leung PC. Bone marrow fat content in the elderly: a reversal of sex difference seen in younger subjects. J Magn Reson Imaging 2012;36:225‐230. [DOI] [PubMed] [Google Scholar]
- 25.Blom‐Høgestøl IK, Mala T, Kristinsson JA, et al. Changes in bone marrow adipose tissue one year after Roux‐en‐Y gastric bypass: a prospective cohort study. J Bone Miner Res 2019;34:1815‐1823. [DOI] [PubMed] [Google Scholar]
- 26.Shen W, Gong X, Weiss J, Jin Y. Comparison among T1‐weighted magnetic resonance imaging, modified Dixon method, and magnetic resonance spectroscopy in measuring bone marrow fat. J Obes 2013;2013:298675. doi: 10.1155/2013/298675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li G, Xu Z, Gu H, et al. Comparison of chemical shift‐encoded water‐fat MRI and MR spectroscopy in quantification of marrow fat in postmenopausal females. J Magn Reson Imaging 2017;45:66‐73. [DOI] [PubMed] [Google Scholar]
- 28.Ruschke S, Pokorney A, Baum T, et al. Measurement of vertebral bone marrow proton density fat fraction in children using quantitative water‐fat MRI. MAGMA 2017;30:449‐460. [DOI] [PubMed] [Google Scholar]
- 29.Cohen A, Stein EM, Recker RR, et al. Teriparatide for idiopathic osteoporosis in premenopausal women: a pilot study. J Clin Endocrinol Metab 2013;98:1971‐1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang Y, Luo X, Xie X, et al. Influences of teriparatide administration on marrow fat content in postmenopausal osteopenic women using MR spectroscopy. Climacteric 2016;19:285‐291. [DOI] [PubMed] [Google Scholar]
- 31.Yang YI, Luo X, Yan F, et al. Effect of zoledronic acid on vertebral marrow adiposity in postmenopausal osteoporosis assessed by MR spectroscopy. Skeletal Radiol 2015;44:1499‐1505. [DOI] [PubMed] [Google Scholar]
- 32.Muschitz C, Kocijan R, Haschka J, et al. The impact of vitamin D, calcium, protein supplementation, and physical exercise on bone metabolism after bariatric surgery: the BABS study. J Bone Miner Res 2016;31:672‐682. [DOI] [PubMed] [Google Scholar]
- 33.Fan YI, Hanai J‐I, Le PT, et al. Parathyroid hormone directs bone marrow mesenchymal cell fate. Cell Metab 2017;25:661‐672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bredella MA, Torriani M, Ghomi RH, et al. Vertebral bone marrow fat is positively associated with visceral fat and inversely associated with IGF‐1 in obese women. Obesity (Silver Spring) 2011;19:49‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ambikairajah A, Walsh E, Tabatabaei‐Jafari H, Cherbuin N. Fat mass changes during menopause: a metaanalysis. Am J Obstet Gynecol 2019;15‐17. [DOI] [PubMed] [Google Scholar]
- 36.Bredella MA, Torriani M, Ghomi RH, et al. Determinants of bone mineral density in obese premenopausal women. Bone 2011;48:748‐754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sheu Y, Cauley JA. The role of bone marrow and visceral fat on bone metabolism. Curr Osteoporos Rep 2011;9:67‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cordes C, Dieckmeyer M, Ott B, et al. MR‐detected changes in liver fat, abdominal fat, and vertebral bone marrow fat after a four‐week calorie restriction in obese women. J Magn Reson Imaging 2015;48:748‐754. [DOI] [PubMed] [Google Scholar]
- 39.Bosy‐Westphal A, Later W, Schautz B, et al. Impact of intra‐ and extra‐osseous soft tissue composition on changes in bone mineral density with weight loss and regain. Obesity (Silver Spring) 2011;19:1503‐1510. [DOI] [PubMed] [Google Scholar]
- 40.Bornstein S, Brown SA, Le PT, et al. FGF‐21 and skeletal remodeling during and after lactation in C57BL/6J mice. Endocrinology 2014;155:3516‐3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Motyl KJ, Bishop KA, Demambro VE, et al. Altered thermogenesis and impaired bone remodeling in Misty mice. J Bone Miner Res 2013;28:1885‐1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Recker RR. Calcium absorption and achlorhydria. N Engl J Med 1985;313:70‐73. [DOI] [PubMed] [Google Scholar]
- 43.Tondapu P, Provost D, Adams‐Huet B, Sims T, Chang C, Sakhaee K. Comparison of the absorption of calcium carbonate and calcium citrate after Roux‐en‐Y gastric bypass. Obes Surg 2009;19:1256‐1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ring Madsen L, Espersen R, Rejnmark L, Richelsen B. Effect of calcium citrate vs calcium carbonate on elevated parathyroid hormone after Roux‐en‐Y gastric bypass. A double‐blinded, randomized trial. Clin Endocrinol (Oxf) 2018;89:734‐741. [DOI] [PubMed] [Google Scholar]
- 45.Baretta GAP, Cambi MPC, Rodrigues AL, Mendes SA. Secondary hyperparathyroidism after bariatric surgery: treatment is with calcium carbonate or calcium citrate? Arq Bras Cir Dig 2015;28:43‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]


