Abstract
Analysis of long-term potentiation (LTP) provides a powerful window into cellular mechanisms of learning and memory. Prior work shows late LTP (L-LTP), lasting >3 hr, occurs abruptly at postnatal day 12 (P12) in the stratum radiatum of rat hippocampal area CA1. The goal here was to determine the developmental profile of synaptic plasticity leading to L-LTP in the mouse hippocampus. Two mouse strains and two mutations known to affect synaptic plasticity were chosen: C57BL/6J and Fmr1−/y on the C57BL/6J background, and 129SVE and Hevin−/− (Sparcl1−/−) on the 129SVE background. Like rats, hippocampal slices from all of the mice showed test pulse-induced depression early during development that was gradually resolved with maturation by 5 weeks. All the mouse strains showed a gradual progression between P10-P35 in the expression of short-term potentiation (STP), lasting ≤1 hr. In the 129SVE mice, L-LTP onset (>25% of slices) occurred by 3 weeks, reliable L-LTP (>50% slices) was achieved by 4 weeks, and Hevin−/− advanced this profile by 1 week. In the C57BL/6J mice, L-LTP onset occurred significantly later, over 3–4 weeks, and reliability was not achieved until 5 weeks. Although some of the Fmr1−/y mice showed L-LTP before 3 weeks, reliable L-LTP also was not achieved until 5 weeks. L-LTP onset was not advanced in any of the mouse genotypes by multiple bouts of theta-burst stimulation at 90 or 180 min intervals. These findings show important species differences in the onset of STP and L-LTP, which occur at the same age in rats but are sequentially acquired in mice.
Keywords: development, maturation, synaptic plasticity, theta-burst potentiation
1 |. INTRODUCTION
The hippocampus is critical for spatial navigation and processing of new information. It is the main brain region used to study long-term potentiation (LTP), a cellular mechanism of learning and memory. Knowing the maturational profile of synaptic plasticity provides a basis for investigating abnormalities leading to intellectual disabilities and other neurodevelopmental disorders. LTP has been most rigorously studied in stratum radiatum of hippocampal area CA1; hence, the excitatory synapses in this subfield are the focus of this and many prior studies. In Sprague Dawley rats, LTP begins to consolidate in hippocampal area CA1 around postnatal day 21 (P21) (Kramar & Lynch, 2003). Our previous work in Long–Evans rats revealed test pulse depression that lasts until P21 (Cao & Harris, 2012), replicating earlier findings (Abrahamsson, Gustafsson, & Hanse, 2007, 2008). Theta-burst stimulation (TBS) reversed the test pulse depression at P8–P11, but no potentiation was produced above the initial naïve response. At P12, the TBS reliably induced enduring LTP lasting more than 3 hr (late LTP [L-LTP]). When multiple episodes of TBS were delivered, the onset age of L-LTP was advanced to P10 (Cao & Harris, 2012). Here, our goal was to extend these studies to mouse hippocampus.
Mice are a widely used model system to test the effects of genetic manipulations on normal behavior and physiology (Ellenbroek & Youn, 2016; Homberg, Wohr, & Alenina, 2017). Little is known about the developmental profile of synaptic plasticity in mice. Two commonly used wild-type mouse strains, C57BL/6J and 129SVE, were chosen together with Fmr1−/y and Hevin−/− (Sparcl1−/−), which are known for producing aberrations in synaptic plasticity and development. Fmr1−/y on the C57BL/6J background is a common model of fragile X syndrome (FXS) for mental retardation and autism (He & Portera-Cailliau, 2013; Pfeiffer & Huber, 2009). The cause of FXS is the mutation preventing the synthesis of FMRP, an RNA-binding protein selectively expressed in neurons and responsible for mRNA transport and local protein synthesis in dendrites (Wang et al., 2016). Hevin is a protein released by astrocytes and interneurons that is critical for synapse formation and rearrangement (Mongredien et al., 2019). The synaptogenic activity of Hevin promotes glutamatergic synapse maturation and refines cortical connectivity and plasticity (Risher et al., 2014; Singh et al., 2016).
We tested for the impact of these two strains and two key mutations on the developmental profile of synaptic plasticity in hippocampal area CA1. The outcomes provide a foundation for investigating genetic effects on synaptic plasticity and may help to explain the inter-species variance in synaptogenesis and developmental capacity for learning and memory.
2 |. MATERIALS AND METHODS
2.1 |. Ethical approval
Procedures were approved by the University of Texas at Austin Institutional Animal Care and Use Committee and complied with all NIH requirements for the humane care and use of laboratory mice (protocol # AUP-2012–00127, AUP-2012–00056, and their successor protocols).
2.2 |. Animals
Breeding pairs of C57BL/6J (RRID:IMSR_JAX:000664) and Fmr1−/y (RRID:MGI:5703659) on this background were kindly donated by Dr D. Brager (Center for Learning and Memory, University of Texas at Austin) who received the founder pair from Dr K. Huber (University of Texas Southwestern). Breeding pairs of 129SVES6 (129SVE) mice were obtained from a supplier (Taconic, Rensselaer, NY; RRID: IMSR_TAC:129sve), and Hevin−/− (Sparcl1−/−, allelic composition Sparcl1tm1Pmc/Sparcl1tm1Pmc, RRID:MGI:4454665) were on this background. We will be referring to this knock-out as Hevin−/−. The generation of Fmr1−/y and Hevin−/− (Sparcl1−/−) has been described before (Barker et al., 2005; McKinnon, McLaughlin, Kapsetaki, & Margolskee, 2000; The Dutch-Belgian Fragile X Consortium, 1994).
Animals were co-housed and provided with food and water ad libitum on a 12 hr light–dark cycle. The experimental design was originally optimized for the Fmr1−/y mice, in which the males have the strongest phenotypes; hence, for the appropriate comparison with Fmr1−/y data, males were used for all the experiments. The exact age of each animal was known. Since the developmental profiles were more gradual in the mice than in rats, and for ease of graphical presentation, data from the mice were grouped by age as P10–13 (<2 weeks), P13–17 (2 weeks), P18–23 (3 weeks), P26–31 (4 weeks), and P32–37 (5 weeks).
2.3 |. Slice preparation
Hippocampal slices were prepared from mouse pups at P8–P38 as previously described (Bourne, Kirov, Sorra, & Harris, 2007). Animals were decapitated under isoflurane anesthesia when appropriate (age older than P33). The brain was removed, and the left hippocampus was dissected out and rinsed with room temperature artificial cerebrospinal fluid (aCSF) containing (in mM) 117 NaCl, 5.3 KCl, 26 NaHCO3, 1 NaH2PO4, 2.5 CaCl2, 1.3 MgSO4, and 10 glucose, pH 7.4, and bubbled with 95% O2/5% CO2. Four slices (400 μm thick) from the middle third of the hippocampus were cut at 70° transverse to the long axis on a tissue chopper (Stoelting, Wood Dale, IL) and transferred to four individual interface chambers in the Synchroslice system (Lohmann Research Equipment, Castrop-Rauxel, Germany). The slices were placed on a net at the liquid–gas interface between aCSF and humidified 95% O2/5% CO2 atmosphere held at 32–33°C. The entire dissection and slice preparation took 5–7 min. The slices recovered in the chambers for 3 hr before the recordings commenced.
2.4 |. Electrophysiology
The stimulation and data acquisition were obtained using the SynchroBrain software (Lohmann Research Equipment). A concentric bipolar stimulating electrode (FHC Inc., Bowdoin, ME) was positioned near the CA3 side, and a metal recording electrode (Thomas Recording, Geissen, Germany) was placed ∼400 μm away from the stimulating electrode, also in the middle of CA1 stratum radiatum. Stimuli consisted of 200 μs biphasic current and each stimulus was applied every 5 min. Response measurements included the amplitude (mV) of the fiber volley (FV) and slope (mV/ms) of the field excitatory postsynaptic potential (fEPSP). The maximum positive amplitude of the FV was measured where it was clearly separated from the stimulus artifact and onset of the negative slope of the fEPSP. Each fEPSP slope was estimated by linear regression in the middle of the initial phase of the fEPSP over an interval ranging from 0.2 to 0.4 ms, depending on response magnitude. The stimulus intensity and response analysis time frames were kept constant for each slice throughout the duration of each experiment. Stimulus intensities were set to obtain about 50% of the maximum response levels typical for each age group of animals. Stimulus intensities were set to obtain about 50% of the maximum response levels typical for each age group of animals. This setting was found to be within 40–60% of the maximum response obtained by delivering increasing stimulus intensities (ranging from 100 to 500 μA). This partial I/O was done at the end of each experiment to avoid response plasticity at higher stimulus intensities before the experiment.
The various TBS paradigms are described in the Results and figure legends. Briefly, the 8T TBS paradigm consisted of eight trains with 30 s intervals with each train containing 10 bursts at 5 Hz and each burst containing four pulses at 100 Hz. The 1T TBS paradigm consisted of one train of the same stimulation pattern. The fEPSP slope is expressed as a percentage of the naïve fEPSP or the averaged baseline response obtained 30 min before delivering the TBS paradigm as indicated in the Results and figure legends. Baseline responses were recorded for 60 min before the delivery of the TBS paradigm. Experiments within an age/genotype were grouped depending on the success of inducing potentiation with a threshold set at 120% of the naïve fEPSP slope. fEPSP slope changes normalized to the first naïve response or the baseline were averaged across the slices for each genotype.
2.5 |. Statistics
Statistical analyses were performed using Prism (GraphPad Software Inc., San Diego, CA). Data are presented throughout as the mean ± SEM. The minimal level of significance was set at p < .05. The outliers were detected and removed using Grubbs’ test (no more than one data point per dataset). The total number of animals and slices of each cohort (genotype and age) used in the 8T LTP experiments presented in Figures 1–8 are presented in Table 1. In other experiments, the number of slices is indicated in parenthesis in Figures 4, 5c, 8g, 9. The specific tests and the outcomes are indicated in the figure legends.
FIGURE 1.
Test pulse-induced depression was resolved by 4–5 weeks for all strains and genotypes. (Top row) Electrode positions in hippocampal area CA1 and slice paradigm to test for late long-term potentiation (L-LTP) and experimental design. Experimental design: Slices were recovered for 3 hr without stimulation (tan frame). Then the stimulus intensity was set to obtain the approximate half-maximal response (40–60%). The stimulus was repeated at this intensity at 5 min intervals for 60 min to obtain the baseline responses. The 8T consisted of eight trains at 30 s intervals with 10 bursts at 5 Hz of four pulses each at 100 Hz. Then, the responses were monitored for 180 min and L-LTP was determined by averaging the response slope over the last 155–180 min. All field excitatory postsynaptic potential (fEPSP) slopes are normalized to the first (naïve) response at time 0. (a–d) Baseline responses to the test pulse stimulation for the first 1 hr of the experiments show an age-dependent decrease in test pulse-induced depression for all four genotypes (two-way analysis of variance [ANOVA], interaction: F (9,208) = 0.427 (p = .92), age: F (3,208) = 24.03 (p < .0001), genotype: F (3,208) = 1.70 (p = .17)). All genotypes revealed significant differences between ages before or at 2 weeks and 4–5 weeks old (Dunnet’s post hoc tests). Data from 4 and 5 week old mice did not differ and are plotted together for Hevin−/−
FIGURE 8.
A second episode of 8T separated in time augments the initial late long-term potentiation (L-LTP). (a) Experimental design: Baseline, first 8T (black arrowhead), delivery of second 8T (red arrowhead) 180 min after the first. (b–e) Changes in field excitatory postsynaptic potential (fEPSP) slopes were normalized relative to the naïve baseline response and averaged across the slices before and after the onset ages of L-LTP (red dotted line at 120% baseline) and plotted versus time for each genotype. (f) Within each slice, the initial L-LTP was averaged over 155–180 min after the first 8T (orange frame). Next, the L-LTP was considered to be augmented if the fEPSP slope after the second 8T (averaged over 225–260 min, black frame) was greater than the initial L-LTP (orange frame) by at least 10% (red dotted line, C57BL/6J: t = 6.335, df = 5, partial η2 = 0.889, **p = .0014; Fmr1−/y: t = 4.646, df = 5, partial η2 = 0.812; *p = .0056; 129SVE: t = 6.221, df = 5, partial η2 = 0.886, **p = .0016; Hevin−/−: t = 5.184, df = 5, partial η2 = 0.843, **p = .0035). (For Hevin−/−, the data from the after onset age group at 3 weeks were separated from 4 weeks because the augmentation of L-LTP at 3 weeks did not reach criterion but did reach criterion at 4 weeks.) (g) Slices from 4 week old animals that had no initial L-LTP also showed no augmentation, in response after the second 8T (C57BL/6: t = 12.11, df = 5, partial η2 = 0.967, ***p < .0001; Hevin−/−: t = 7.369, df = 5, partial η2 = 0.916, ***p = .0007)
TABLE 1.
Total number of slices and animals in each strain and age group for Figures 1–8. For Figures 9 and 10, the number of slices in each condition is indicated in parenthesis in the figures themselves
| <2 weeks | 2 weeks | 3 weeks | 4 weeks | 5 weeks | |
|---|---|---|---|---|---|
| Animals | |||||
| C57BL/6J | 8 | 7 | 10 | 8 | 6 |
| Fmr1−/y | 6 | 4 | 6 | 4 | 7 |
| 129SVE | 6 | 6 | 6 | 6 | |
| Hevin−/− | 5 | 5 | 6 | 7 | 4 |
| Slices | |||||
| C57BL/6J | 14 | 11 | 18 | 11 | 16 |
| Fmr1−/y | 9 | 10 | 10 | 10 | 17 |
| 129SVE | 10 | 11 | 10 | 10 | |
| Hevin−/− | 11 | 12 | 13 | 14 | 10 |
FIGURE 4.
Demonstration that 8T is a robust induction paradigm for late long-term potentiation (L-LTP) for all strains and genotypes. Reducing the number of trains in the theta-burst stimulation (TBS) paradigm from 8T to 1–4T resulted in the same magnitude and endurance of L-LTP in (a) C57BL/6, (b) Fmr1−/y, (c) 129SVE, and (d) Hevin−/−. Dotted red line is at 120% naïve. The slices were obtained from animals (a–c) at 4 weeks or (d) at 5 weeks, all were after the onset age of L-LTP for 8T
FIGURE 5.
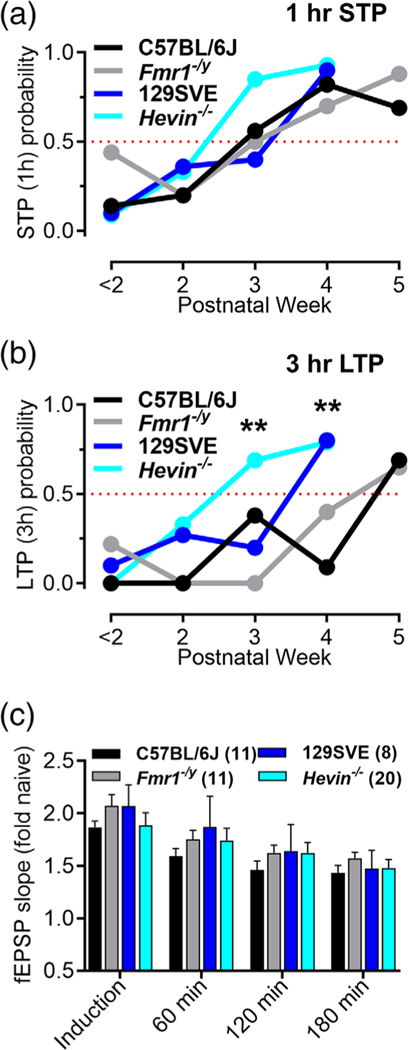
Differences among strains in the probability of late long-term potentiation (L-LTP). The probability of short-term potentiation (STP) (a) and L-LTP (b) by strain and genotype across postnatal age. The probabilities were calculated as the ratio of the number of successful STP or L-LTP experiments relative to the total number of experiments for each condition and age group. No significant differences were detected for STP. Significant differences were detected for the probability of L-LTP (b) at postnatal Week 3 (χ2 = 13.16, df = 3; **p = .0043) and postnatal Week 4 (χ2 = 16, df = 3; **p = .0012). 129SVE strain had a significantly higher probability of L-LTP than C57BL/6J at 4 weeks (χ2 = 12, df = 3; ***p = .0006). Within backgrounds, a significant difference was detected at 3 weeks between C57BL/6J and Fmr1−/y (χ2 = 4.875, df = 1; *p = .0272) and between 129SVE and Hevin−/− (χ2 = 5.490, df = 1; *p = .0191). Both genotype pairs were equal at their corresponding after onset ages. (c) The magnitude of field excitatory postsynaptic potential (fEPSP) potentiation at different time periods post theta-burst stimulation (TBS) did not differ significantly across strains or genotypes in the after onset age groups for each strain or genotype
FIGURE 9.
Second episode of 8T did not produce late long-term potentiation (L-LTP) in slices lacking initial L-LTP in 4-week-old C57BL/6J mice. (a) Experimental design: Each slice was subjected to two identical theta-burst stimulation (TBS) paradigms consisting of either one or eight trains that were spaced by 90 min (1T light blue, 8T pink arrows) or 180 min (1T navy, 8T red arrows). Initial L-LTP was calculated by averaging responses at 60–85 min after the first 8T (gray time frame) or 155–180 min for the 180 min 8T interval (orange time frame). The effect of the second 8T was calculated at 135–160 min for the 90 min interval (green time frame) or at 225–260 min for the 180 min interval (black time frame). (b) Summary of the mean changes in field excitatory postsynaptic potential (fEPSP) slope normalized to the 30 min averaged baseline responses with 8T episodes spaced 90 min (pink) or 180 min (red). (c) Quantification of the experiments at the representative time frames for the experiments in (b). No significant differences were detected at any of the time points for the different separations in 8T. (d) Summary of the mean changes in fEPSP slope normalized to the 30 min of the averaged baseline responses with 1T theta-burst stimulation (TBS) episodes spaced 90 min (blue) or 180 min (navy). (e) Quantification of the experiments at the representative time frames for the experiments in (d). No significant differences were detected between the levels of potentiation at orange and black intervals (pre and post second 1T spaced 180 min after the first 1T, one-way analysis of variance [ANOVA], F(3,9) = 2.736, p = .267). A significant difference was detected by two-way RM ANOVA between 1T-90 m-1T and 1T-180 m-8T by TBS spacing factor (F(1,7) = 6.762; *p = .0354)
3 |. RESULTS
3.1 |. Developmental test pulse depression
In immature rat hippocampus (<P20), test pulse stimulation produces a marked depression of the fEPSPs (Abrahamsson et al., 2007, 2008; Cao & Harris, 2012; Xiao, Wasling, Hanse, & Gustafsson, 2004). Both tetanic stimulation and TBS can reverse this test pulse-induced depression, but they produce no potentiation above the first naïve response at these young ages. To calculate the magnitude of test pulse depression and discern between the reversal of depression and LTP, all responses were normalized relative to the first naïve response. Here, test pulses were given at the lowest frequency of 1 pulse per 5 min that was tested in rats (Figure 1). For mice aged ≤2 weeks, the test pulse depression was significant at 55 min (Table 2). By 4–5 weeks, the depression was gone, and such age dependence was similar between all four mouse strains and genotypes. Thus, like in rats, test pulse depression in mice was age dependent.
TABLE 2.
The magnitude of test pulse depression across ages and genotypes. The responses were normalized to the first naïve slope and the difference between the normalized average slopes at Time 0 and 55 min is shown with means and 95% confidence interval (upper and lower limits)
| C57BL/6J | Fmr1 −/y | 129SVE | Hevin −/− | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||||||
| Mean | Upper limit | Lower limit | Mean | Upper limit | Lower limit | Mean | Upper limit | Lower limit | Mean | Upper limit | Lower limit | |
| <2 weeks | −0.19 | −0.15 | −0.23 | −0.17 | −0.12 | −0.23 | −0.16 | −0.09 | −0.24 | −0.17 | −0.10 | −0.24 |
| 2 weeks | −0.16 | −0.08 | −0.23 | −0.18 | −0.12 | −0.24 | −0.17 | −0.12 | −0.21 | −0.14 | −0.05 | −0.23 |
| 3 weeks | −0.08 | −0.01 | −0.15 | −0.07 | 0.02 | −0.15 | −0.09 | −0.06 | −0.11 | −0.02 | 0.06 | −0.10 |
| 4 weeks | −0.06 | 0.00 | −0.13 | 0.02 | 0.12 | −0.08 | −0.03 | 0.08 | −0.14 | 0.09 | 0.19 | −0.01 |
| 5 weeks | −0.04 | 0.02 | −0.11 | 0.00 | 0.05 | −0.06 | −0.02 | 0.09 | −0.13 | |||
| 4–5 weeks | −0.05 | −0.01 | −0.10 | 0.00 | 0.05 | −0.04 | 0.05 | 0.12 | −0.03 | |||
3.2 |. Strain- and genotype-specific differences in developmental onset of STP and L-LTP
Eight trains of TBS (8T) reliably produced L-LTP at P12 in rats (Cao & Harris, 2012) and young adult C57BL/6J mice (Cao & Harris, 2014). Hence, we used this 8T protocol to determine the onset age of L-LTP in acute slices from mouse hippocampal area CA1 (Figure 1, top right). The LTP threshold was set at 120% of the naïve response. For each age, the number of experiments was tabulated where 8T failed (none) or succeeded in producing short-term potentiation lasting 1 hr (STP) or L-LTP lasting at least 3 hr (LTP). Age groupings are shown on weekly basis (Figures 1–5), and also as the relative frequency of slices showing L-LTP (Figures 6–9, Supplementary Figures S1 and S2). The before onset groups comprised ages where L-LTP was induced in 25% or less of tested slices. The onset groups had 25–50% success rate among tested slices. The after onset groups had L-LTP in more than 50% of tested slices. The percentage was calculated by dividing the number of slices that showed only STP (gray sectors) or L-LTP (red sectors) by the total number of slices tested in the corresponding age group as shown in pie charts in Figures 2 and 3 (and Supplementary Figures S1 and S2).
FIGURE 6.
Magnitude of starting naïve responses did not predict success of late long-term potentiation (L-LTP) across ages, strains, or genotypes. Slices in each age group lacking L-LTP (black dots, gray bars) or producing 3 hr L-LTP (red dots and bars). Age groups are indicated relative to when L-LTP could first be produced as before onset, onset, and after onset for each genotype. No significant differences were detected across the genotypes or developmental stages of L-LTP onset for the before onset age group, or for the onset versus after onset age groups (two-way analysis of variance [ANOVA], interaction: F(7,127) = 0.691 (p = .680), age: F (1,127) = 0.0722 (p = .789), genotype: F(1,127) = 1.57 (p = .151)). Individual slice values are plotted as dots. Only three slices showed L-LTP in the before onset group, so these were not included in the statistical analyses
FIGURE 2.
Week-by-week analysis of short-term potentiation (STP) and late long-term potentiation (L-LTP) in the C57BL/6J (a1–a5) and Fmr1−/y (b1–b5) mice. In time course plots and pie charts, the experiments with no potentiation (none) are colored black, those with STP lasting less than 1 hr (STP 1 hr) are colored gray, and those with LTP lasting 3 hr (LTP 3 hr) are colored red. Representative waveforms for pre-theta-burst stimulation (TBS) baseline responses are colored gray, for 3 hr post-TBS are colored black for no potentiation and red for L-LTP at 3 hr. The pie charts show the relative fractions with the actual number of slices in each fraction for each age. The numbers of all animals and slices are also listed in Table 1
FIGURE 3.
Week-by-week analysis of 1 and 3 hr LTP in the 129SVE (a1–4) and Hevin−/− (b1–4) mice. The same color and labeling schemes as in Figure S2
In mice from the C57BL/6J strain and Fmr1−/y on C57BL/6J background, 8T reversed test pulse depression in all slices before 2 weeks of age (Figure 2 a1,b1, Supplementary Figure S1a1,b1). By 2 weeks, about 16% of slices from the C57BL/6J wild-type mice showed STP, but none had L-LTP (Figure 2a1,2, Supplementary Figure S1a1). By 3 weeks, 31% of slices from the Fmr1−/y mutant mice showed only STP and 7% showed L-LTP (Figure 2b1–3, Supplementary Figure S1b1). Between 3 and 4 weeks, 41% of slices produced STP and 26% produced L-LTP in the C57BL/6J wild-type mice (Figure 2a3,4, Supplementary Figure S1a2); whereas, in the Fmr1−/y mutants, at 4 weeks 30% of slices produced STP and 40% produced L-LTP (Figure 2b4). At 5 weeks, L-LTP was reliably produced in more than 50% of slices from both the C57BL/6J wild-type mice and Fmr1−/y mutants (Figure 2a5,b5). These findings suggest a gradual onset for the production of STP and L-LTP in the C57BL/6J strain, an effect that was apparently delayed in Fmr1−/y.
Next, we tested mice from the 129SVE strain and Hevin−/− on the 129SVE background. Before 2 weeks, all slices showed reversal of test pulse depression, 10% of slices from 129SVE mice showed minimal L-LTP (Figure 3a1), and 9% of slices from Hevin−/− mice showed subtle STP (Figure 3b1), but most slices showed no potentiation at all. Between 2 and 3 weeks, 38% of slices showed either STP or L-LTP in the 129SVE mice (14% STP and 24% L-LTP, Figure 3a2,3, Supplementary Figure S2a2), contrasting with 33% of slices from Hevin−/− showing L-LTP a week earlier by 2 weeks (Figure 3b2). At 4 weeks, 80% of slices showed L-LTP and 10% had STP in the 129SVE mice (Figure 3a4) versus 74% and 15% in Hevin−/− between 3 and 4 weeks (Figure 3b3,4, Supplementary Figure S2b3). Thus, reliable L-LTP occurred at 4 weeks in the 129SVE strain (Figure 3a4) and even earlier, at 3 weeks, for Hevin−/− (Figure 3b3).
In young adult rats and C57BL/6J mice (7–9 weeks old), L-LTP is saturated by eight trains of TBS (8T) delivered in stratum radiatum of hippocampal area CA1 (Cao & Harris, 2014). Saturating in this context means that another episode of TBS given 5 min after the first episode produces no additional LTP. We performed additional experiments demonstrating that 1T, 2T, and 8T produced L-LTP of the same magnitude and endurance at 4–5 weeks (Figure 4). Hence, the 8T paradigm used here produced saturating L-LTP, consistent with prior experiments (Abraham & Huggett, 1997; Cao & Harris, 2014; Kramar et al., 2012).
Both Fmr1−/y and Hevin−/− accelerated L-LTP onset relative to their wild-type backgrounds.
The probability of producing at least STP (including STP and L-LTP slices) or L-LTP was compared across ages, strains, and genotypes (Figure 5). More slices showed STP in Hevin−/− mice by Week 3 than other strains; however, this effect was not statistically significant, and all strains had ≥50% of slices showing STP at this age (Figure 5a). The onset age for L-LTP was significantly earlier at 3 weeks for the Hevin−/− (corresponds to the LTP probability between 50 and 75%) and at 4 weeks for Fmr1−/y versus C57BL/6J (Figure 5b). The after onset age at 4 weeks in 129SVE and the Hevin−/− (LTP probability ≥75%) was earlier when compared with 5 weeks in C57BL/6J and the Fmr1−/y (Figure 5b). The differences between C57BL/6J and Fmr1−/y and between 129SVE and Hevin−/− were also significant at 3 weeks (Figure 5b). Once established, there were no significant differences in the magnitude of L-LTP between mouse strains or genotypes across time post-induction (Figure 5c).
Another measure of developmental onset would be the coincidence of no potentiation and potentiation occurring in different slices from the same animal. This coincidence was calculated as the percentage of animals that had slices showing both no potentiation and potentiation lasting for at least 1 hr out of the total number of animals at the onset age for each genotype (Table 3). More than 60% of animals at the L-LTP onset age showed this coincidence in support of the hypothesis that these were indeed the relative onset ages for each genotype.
TABLE 3.
The percent of animals at the onset age of L-LTP from each genotype that had some slices showing no potentiation and other slices from the same animal showing at least 1 hr of potentiation (coincidence)
| Genotype | Coincidence (%) |
|---|---|
| C57BL/6J | 66 |
| Fmr1 −/y | 75 |
| 129SVE | 75 |
| Hevin −/− | 60 |
Abbreviation: L-LTP, late long-term potentiation.
3.3 |. The magnitude of the naïve fEPSP did not predict the developmental onset of L-LTP
The naïve fEPSP slopes were compared across experiments to test their potential effect in determining when L-LTP was first produced. In each of the three key age groups (before onset, onset, and after onset ages of L-LTP), the slices were divided as having no L-LTP at 3 hr or having L-LTP at 3 hr. No significant differences in the naïve slopes were detected across strains, genotypes, or age groups (Figure 6). Thus, the magnitude of the naïve responses did not determine the occurrence of L-LTP in these experiments.
3.4 |. Age dependence of basal synaptic transmission
To test for age- and genotype-dependent effects on synaptic transmission we measured the relationship between FV amplitudes and fEPSP slopes during baseline stimulation (Figure 7). In both the C57BL/6J and Fmr1−/y mice, the ratio between FV and fEPSP was significantly less before than after the onset age of LTP, with no significant differences between these genotypes (Figure 7a–c). Similarly, this ratio was less prior to LTP onset in the Hevin−/− and trended less in the 129SVE mice with no differences between the genotypes (Figure 7d–f). Thus, baseline synaptic transmission increases in parallel with the onset of L-LTP.
FIGURE 7.
Developmental change in relationship of fiber volley (FV) to field excitatory postsynaptic potential (fEPSP) slope. The fEPSP slope and FV values were averaged during baseline recordings from each slice. (a–c) The FV-slope relationship is stronger with age for C57BL/6J and Fmr1−/y (two-way analysis of variance [ANOVA], interaction: F(1,42) = 0.002 (p > .05), age: F (1,42) = 21.3 (***p < .0001), Tukey’s post hoc age: (**p = .009 for C57BL/6J and *p = .01 for Fmr1−/y), for both genotypes (F (1,42) = 3.52 (p > .05)). (d–f) The FV-slope ratio increased significantly across the two age groups for Hevin−/− (two-way analysis of variance [ANOVA], interaction: F (1,35) = 0.96 (p > .05), age: F(1,35) = 11.35 (**p = .002), Tukey’s post hoc test p = .014) and did not differ significantly from its 129SVE background genotype (F (1,35) = 0.37 (p > .05)). The averages from each slice included 2–3 baseline measurements before onset and up to 12 baseline measurements after late long-term potentiation (L-LTP) onset to avoid test pulse-induced depression
3.5 |. Age dependence of the augmentation of L-LTP
Prior work in the rat hippocampus revealed that multiple time-separated episodes of TBS result in additional potentiation or augmentation of L-LTP. The timing of this effect is strain and age-related in the rat hippocampus (Bowden, Abraham, & Harris, 2012; Cao & Harris, 2012, 2014; Kramar et al., 2012; Manahan-Vaughan, 2000; Manahan-Vaughan & Schwegler, 2011). We tested whether a pair of 8T episodes spaced 180 min apart would augment L-LTP in mouse hippocampus (Figure 8a), as it did in rat. Two criteria were set for augmentation. First, the initial potentiation had to be ≥120% of the naïve response at 3 hr following the first 8T. Then, the second 8T had to elevate the fEPSP slope ≥10% above the initial L-LTP, and last for at least 70 min. Longer monitoring of the responses in slices from developing animals could become unreliable after 11 hr in vitro so experiments were terminated by 9 hr (i.e., 3 hr recovery plus 6 hr of recording).
Slices from the different mouse genotypes were compared in their respective age groups relative to the onset age when L-LTP first occurred. The time course of L-LTP and augmentation of L-LTP is illustrated for all four genotypes (Figure 8b–e). At the onset age of L-LTP, some slices from the Fmr1−/y mutant mice that had threshold levels of initial L-LTP, produced augmentation of L-LTP; however, none of the other genotypes met the 10% augmentation criterion at their onset ages of L-LTP (Figure 8f). After the onset age of L-LTP, slices from all four genotypes produced augmentation of L-LTP (Figure 8b–f). To illustrate the age-dependent differences for the Hevin−/− mutants, slices tested for augmentation of L-LTP at 3 weeks are shown with the onset group and 4 weeks with the after onset group (Figure 8f).
Even after the onset age of L-LTP, some slices showed no initial L-LTP. In those 4 week old slices, the second 8T also produced no potentiation and hence no augmentation (Figure 8g). In fact, for slices without initial L-LTP in the C57BL/6J and Hevin−/− mice, the second 8T episode resulted in a significant reduction in fEPSP slope 45–70 min later (Figure 8g). This depression could reflect the failure to reverse the ongoing decline in the fEPSP slope normally observed over time in slices from developing animals that fail to produce initial L-LTP.
3.6 |. Two episodes of TBS do not enable L-LTP in slices initially lacking production of L-LTP
Since two episodes of 8T produced no potentiation in slices lacking initial L-LTP, further testing was done varying the timing (90 vs. 180 min intervals) and strength (8T vs. 1T) of the episodes (Figure 9a). Four-week-old C57BL/6J mice were chosen as this age was at the onset age when a single episode of 8T did not reliably produce L-LTP. When two episodes of 8T were spaced by 90 or 180 min, and STP was present initially for 1 hr, no additional STP was produced after the second 8T and the response dropped back to baseline or below by 3 hr (Figure 9b,c). When the gentler 1T episodes were spaced by 90 min, substantial potentiation was induced after both the first and the second 1T episodes; however, it did not last (Figure 9d,e). If the 1T episodes were spaced by 180 min, the second episode produced much less potentiation than the first, and it also did not last (Figure 9d,e). Thus, neither change in timing or strength produced reliable initial L-LTP nor augmentation of STP to produce L-LTP at the earlier developmental stage.
4 |. DISCUSSION
In the past, two major induction protocols have been used to discern the developmental onset of synaptic plasticity in the rat hippocampus. Repeated tetanic stimulation (three times at 100 Hz for 1 s each) first produced L-LTP at P15 (Harris & Teyler, 1984; Jackson, Suppes, & Harris, 1993), while the 8T paradigm produced L-LTP earlier, at P12 (Cao & Harris, 2012). Hence, the more efficient 8T paradigm was adopted here to investigate the developmental onset of plasticity in the mouse hippocampus. In rats, STP and L-LTP developmental onset occurred at the same time. In contrast, three stages of plasticity emerged in mouse hippocampus. First, STP gradually emerged between P10 and P28 in all four mouse genotypes. Second, L-LTP onset (25–50% of slices) occurred at different ages depending on mouse genotype. Third, the after onset age of L-LTP (>50% of slices) also depended on mouse genotype. In the C57BL/6J mice, L-LTP onset occurred between 3 and 4 weeks, and reliable L-LTP was not achieved until 5 weeks. Although some Fmr1−/y mice showed L-LTP before 3 weeks, reliable L-LTP was also not achieved until 5 weeks in this genotype. In the 129SVE mice, L-LTP onset occurred by 3 weeks, reliable L-LTP was achieved by 4 weeks, and Hevin−/− advanced this profile by 1 week.
4.1 |. Differential effects of development on basal synaptic transmission
The relationship of presynaptic FV amplitude to the slope of fEPSP provides a measure of the strength of synaptic transmission needed to evoke a postsynaptic response. The absolute magnitude of the naïve fEPSP slope used during TBS did not differ across strains or genotypes. In addition, for all strains and genotypes tested here, the ratio between fEPSP slope and the presynaptic FV was lower prior to, than after the onset of L-LTP. Earlier observations showed that the Fmr1/Fxr2 double knock-out disrupted this relationship, and the single Fmr1−/y had no effect (Zhang, Hou, Klann, & Nelson, 2009), consistent with our findings. Thus, the differences in L-LTP onset ages were not explained by differences in the initial strength of activation or basal synaptic transmission in mice reported here, or previously in rats (Cao & Harris, 2012).
Test pulse depression, indicated by a gradual decline in fEPSP response to test pulses, has been ascribed to reversible silencing of AMPARs (Abrahamsson et al., 2007, 2008). In rats and all four mouse genotypes, test pulse depression no longer occurred at 5 weeks, coincident with the latest onset age of L-LTP in mice, but well after L-LTP onset in rats. Thus, differences between species, strains, and genotypes were not explained by a capacity to avoid test pulse depression during development.
4.2 |. Developmental metaplasticity
Some patterns of stimulation have no direct effect on synaptic strength but instead modulate the subsequent expression of plasticity, a phenomenon known as metaplasticity (Abraham & Bear, 1996; Abraham & Tate, 1997; Young & Nguyen, 2005). Spaced learning produces longer memories than massed learning, and the efficacy of memory is dependent on the interval between episodes of learning (Ebbinghaus, 1885; Fields, 2005). Similarly, spacing episodes of plasticity induction is considered to be a good model for understanding the cellular mechanisms of spaced learning (Kramar et al., 2012; Lynch & Gall, 2013; Lynch, Kramar, Babayan, Rumbaugh, & Gall, 2013). Regarding LTP, sufficient time must pass between the TBS episodes to augment LTP after a second episode of TBS. In adult rat, augmentation of previously saturated LTP was first observed at a 90 min spacing between bouts of 8T, and prolonging the time between TBS episodes increased the probability of augmentation (Cao & Harris, 2014). The delay between episodes of TBS reflects the time needed to enlarge the postsynaptic area after the initial induction of LTP in adult rats (Bell et al., 2014). In adults, this synaptic enlargement is also homeostatically balanced by stalled spine outgrowth that reflects temporal dynamics of resource reallocation to clusters of potentiated synapses (Bell et al., 2014; Bourne & Harris, 2007; Chirillo, Waters, Lindsey, Bourne, & Harris, 2019).
The patterns of augmentation of L-LTP are also developmentally regulated. In rats, a second episode of 8T delivered 90 min after the first episode produced L-LTP at P10-P11 but not at P8-P9. In the C57BL/6J mice, applying a second 8T episode 90 or 180 min after the first did not produce L-LTP even at 4 weeks of age, when STP could be produced in most slices. Instead, in mouse hippocampus, augmentation could be achieved only after L-LTP was reliably established for C57BL/6J, 129SVE, and Hevin−/− genotypes at after onset age. Curiously, the augmentation of L-LTP was observed earlier in Fmr1−/y mice than other genotypes, in slices that had initial L-LTP. These observations suggest that the development of L-LTP and metaplasticity involve processes that depend on species, strain, and genotype, but are independent from those processes that result in STP.
4.3 |. Genetic differences between mice and rats
Such striking differences between mice and rats in their developmental profiles of synaptic plasticity are consistent with genetic analysis. Almost half of the ∼1 K genes tested so far also show differential expression between mouse and rat hippocampal dendrites, with much less divergence in the other tissues (Francis et al., 2014). There are also large differences between rat and mouse adult hippocampal neurogenesis, a process that is especially important for learning and memory (Lazarov & Hollands, 2016; Snyder et al., 2009). Rats have more adult-born, death-resistant neurons, and these neurons mature faster in rats than in mice. The young neurons show a much higher contribution to fear learning tasks in rats than mice (Miller & Hen, 2015). These genetic and functional differences are consistent with rats having an earlier and more discrete onset age of L-LTP than mice.
4.4 |. Contrasting onset ages of L-LTP and spinogenesis in rat and mouse hippocampus
In this work, we chose gene manipulations that had been reported to alter dendritic spines and synaptic plasticity. Fmr1−/y neurons have been characterized by an overproduction of underdeveloped spines that might not support the plasticity events (He & Portera-Cailliau,−2013). Treatment of neonatal Fmr1−/y mice with the antibiotic minocycline resulted in better learning outcomes along with enhanced spine maturation (Bilousova et al., 2009). Furthermore, in adult Fmr1−/ y mice (3–5 months old), spaced trials rescued learning deficits (Seese, Wang, Yao, Lynch, & Gall, 2014). However, in all the mouse genotypes tested here, including the developing Fmr1−/y mice, STP was not augmented to produce L-LTP upon spaced bouts of 8T in slices that had no initial L-LTP. Thus, future work will be needed to know whether those spaced learning effects resulted from the augmentation of L-LTP or other processes.
Hevin is required for the development of thalamocortical connectivity between P14 and P25 mouse cortex (Risher et al., 2014). Moreover, when Hevin is absent, cortical dendritic spines show significant immaturity demonstrated by fewer but longer spines and a distinct refinement problem. In the second week of development, cortical spines often receive innervations from one cortical and one thalamic axon. By P25, these multiply innervated spines are refined to receive either a thalamocortical or intracortical synapse in wild-type (129SVE) mice. In the Hevin−/− mice this pruning effect does not occur uniformly and the ratio between thalamocortical and intracortical inputs is altered, retaining more of the intracortical synapses at the expense of thalamocortical connections. The role of Hevin in refining hippocampal dendritic spines is unknown; however, its absence in Hevin−/− mice appears to advance the developmental onset age of L-LTP. This finding is consistent with the hypothesis that lack of refinement of CA3-CA1 synapses by Hevin promotes the earlier maturation of plasticity. Application of Hevin protein to autaptic cortical neurons results in a robust induction of NR2B containing NMDAR activity (Singh et al., 2016). Perhaps, the lack of regulation of the NR2B subunit in the Hevin−/− mice stimulates the maturation of synapses at an earlier developmental stage.
In rats, the developmental onset of L-LTP at P12 is coincident with the emergence of dendritic spines, suggesting dendritic spines are necessary for sustained synaptic plasticity (Cao & Harris, 2012; Fiala, Feinberg, Popov, & Harris, 1998; Kirov, Goddard, & Harris, 2004). Initial 3D reconstructions in perfusion-fixed rat hippocampus show evidence for mature dendritic spines at P12, but not at P8 or P10 (Smith, 2019). Preliminary data from rat hippocampal slices showed that 90 min after the initial 8T, dendritic spines were not produced at P8 (Harris, Watson, Kuwajima, & Cao, 2012). In rat hippocampal slices at P10–11, preliminary data suggest that spines were produced 90 min after the initial 8T (Smith, 2019). Thus, a shift from shaft synapses and filopodia to spines might account for this developmental shift in L-LTP onset for rat hippocampus.
The onset of L-LTP in Fmr1−/y at 4 weeks was delayed relative to the background C57BL/6J strain at 3 weeks. This pattern contrasted with the earlier onset age in Hevin−/− by 2 weeks, relative to its background 129SVE strain between 2 and 3 weeks. All genotypes showed reliable L-LTP by 5 weeks. These findings contrast with the developmental onset ages of dendritic spines. Mature dendritic spines have been reported by P15 in C57BL/6J mouse hippocampus (Bilousova et al., 2009), well before the onset age of reliable L-LTP at 5 weeks. Confocal microscopy studies reveal a few mushroom spines by 9–12 days in organotypic slices from mouse hippocampus (Parnass, Tashiro, & Yuste, 2000). Similarly, at 14 days in organotypic slices from C57BL/6J and Fmr1−/y more than 40% of the protrusions were classified as mushroom spines, although less than 10% had mature heads with a diameter greater than 0.5 μm (Bilousova et al., 2009). Reconstructions from serial section EM show mature spines by P24 in the C57BL/6J hippocampus (Nikonenko et al., 2013). Thus, the onset of L-LTP appears to be later than the onset of dendritic spines in mouse hippocampus, suggesting that spines might be necessary but not sufficient.
4.5 |. Other factors that may influence variation in the developmental onset of L-LTP
The wide variance in L-LTP onset ages among individual mice may reflect divergence in many factors that lead to the maturation of neurons. In rats, the discrete onset age of L-LTP may reflect less variation in these factors between animals. Prior work also showed a high rate of failure of LTP induction in P16-P30 C57BL/6J mice when five tetanic stimuli were used in normal calcium concentration (Adesnik & Nicoll, 2007). The success rate of LTP induction was improved by increasing the calcium concentration. One possible explanation is the involvement of different mechanisms of LTP over developmental stages. There is evidence that in 2-week-old mice of mixed 129SVE-C57BL/6J background the initial LTP is mediated by postsynaptic insertion of GluR2-lacking subunits which are later exchanged for GluR2-containing AMPA receptors that are less permeable to calcium (Jia et al., 1996; Plant et al., 2006; Purkey et al., 2018; Sanderson, Gorski, & Dell’Acqua, 2016). LTP in young mice is less dependent on the phosphorylation of GluR1 under mild stimulation conditions (Adesnik & Nicoll, 2007; Lee et al., 2003; Lu et al., 2007; Wikstrom, Matthews, Roberts, Collingridge, & Bortolotto, 2003). Thus, in addition to age, the exact induction protocol may influence the success of LTP.
Another factor that could influence the exact onset age of LTP is the maturation of the inhibitory system and the resulting excitation/inhibition (E/I) balance (Ben-Ari, Khalilov, Kahle, & Cherubini, 2012; Eichler & Meier, 2008). The GABAergic system is depolarizing early during development but switches with maturation to hyperpolarizing. Varying the inter-stimulus interval shows that paired-pulse inhibition first occurs in the developing rat hippocampus at P6 (Harris & Teyler, 1983). Patch-clamp experiments, in hippocampus from both mice and rats, reveal that prior to the maturation of the GABAergic system, the degree of postsynaptic depolarization needed to induce LTP is less (Meredith, Floyer-Lea, & Paulsen, 2003). Furthermore, the Fmr1−/y mice show a dramatic E/I imbalance mediated by reduced GABAergic inhibition in both hippocampus and subiculum (Cea-Del Rio & Huntsman, 2014; Curia, Papouin, Seguela, & Avoli, 2009; Eichler & Meier, 2008; Paluszkiewicz, Martin, & Huntsman, 2011; Sabanov et al., 2017). The saturating TBS protocol used here would overcome inhibitory effects at all ages; hence, the potential E/I imbalance would not explain the gradual onset of enduring LTP in mice (Pike, Meredith, Olding, & Paulsen, 1999; Thomas, Watabe, Moody, Makhinson, & O’Dell, 1998).
The gradual onset of L-LTP in mice could also stem from variation in the rate of maturation of neurons along the septal temporal axis (Altman, 1966; Altman & Das, 1966; Angevine, 1965; Bayer, 1980a, 1980b; Bayer & Altman, 1975). Taking four slices from the middle of the mouse hippocampus might overlap this developmental axis, whereas the larger rat hippocampus does not. This hypothesis is further supported by our finding that at onset, slices from the same mouse hippocampus could express no potentiation or potentiation lasting ≥1 hr. This coincidence occurred in a majority of mice from all genotypes at the onset ages of L-LTP. Such a finding might also reflect coincidental natural variation between neighboring hippocampal slices in the capacity for L-LTP and spine maturity. Thus, future experiments will need to measure both the capacity for L-LTP and spine structure in individual mouse slices to address the necessity and sufficiency of dendritic spines for L-LTP and the impact of these mutations on that process.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Dr Darrin Brager for providing the breeding pairs of Fmr1−/y mice, and Mr Clayton Smith and Ms Amanda Heatherly for maintaining the mouse colony. This work was supported by National Institutes of Health (grant Nos. NS021184, NS033574, NS074644, MH-095980, MH-104319); the National Science Foundation (NSF) (grant No. 1707356 to K. M. H.); the Texas Emerging Technologies Fund; NIH/NIDA R01 (DA031833); and NIH/NINDS R01 (NS102237) to C. E.
Funding information
National Institutes of Health, Grant/Award Numbers: MH-095980, MH-104319, NS021184, NS033574, NS074644; National Institutes of Health/NIDA, Grant/Award Numbers: DA031833, NS102237; National Science Foundation, Grant/Award Number: 1707356; Texas Emerging Technology Fund
Footnotes
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of this article.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Abraham WC, & Bear MF (1996). Metaplasticity: The plasticity of synaptic plasticity. Trends in Neurosciences, 19(4), 126–130. [DOI] [PubMed] [Google Scholar]
- Abraham WC, & Huggett A. (1997). Induction and reversal of long-term potentiation by repeated high-frequency stimulation in rat hippocampal slices. Hippocampus, 7(2), 137–145. [DOI] [PubMed] [Google Scholar]
- Abraham WC, & Tate WP (1997). Metaplasticity: A new vista across the field of synaptic plasticity. Progress in Neurobiology, 52(4), 303–323. [DOI] [PubMed] [Google Scholar]
- Abrahamsson T, Gustafsson B, & Hanse E. (2007). Reversible synaptic depression in developing rat CA3 CA1 synapses explained by a novel cycle of AMPA silencing-unsilencing. Journal of Neurophysiology, 98(5), 2604–2611. 10.1152/jn.00602.2007 [DOI] [PubMed] [Google Scholar]
- Abrahamsson T, Gustafsson B, & Hanse E. (2008). AMPA silencing is a prerequisite for developmental long-term potentiation in the hippocampal CA1 region. Journal of Neurophysiology, 100(5), 2605–2614. 10.1152/jn.90476.2008 [DOI] [PubMed] [Google Scholar]
- Adesnik H, & Nicoll RA (2007). Conservation of glutamate receptor 2-containing AMPA receptors during long-term potentiation. The Journal of Neuroscience, 27(17), 4598–4602. 10.1523/JNEUROSCI.0325-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman J. (1966). Proliferation and migration of undifferentiated precursor cells in the rat during postnatal gliogenesis. Experimental Neurology, 16 (3), 263–278. 10.1016/0014-4886(66)90063-x [DOI] [PubMed] [Google Scholar]
- Altman J, & Das GD (1966). Autoradiographic and histological studies of postnatal neurogenesis. I. A longitudinal investigation of the kinetics, migration and transformation of cells incorporating tritiated thymidine in neonate rats, with special reference to postnatal neurogenesis in some brain regions. The Journal of Comparative Neurology, 126(3), 337–389. 10.1002/cne.901260302 [DOI] [PubMed] [Google Scholar]
- Angevine JB Jr. (1965). Time of neuron origin in the hippocampal region. An autoradiographic study in the mouse. Experimental Neurology, 2, 1–70. [PubMed] [Google Scholar]
- Barker TH, Framson P, Puolakkainen PA, Reed M, Funk SE, & Sage EH (2005). Matricellular homologs in the foreign body response: Hevin suppresses inflammation, but hevin and SPARC together diminish angiogenesis. The American Journal of Pathology, 166 (3), 923–933. 10.1016/S0002-9440(10)62312-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer SA (1980a). Development of the hippocampal region in the rat. I. Neurogenesis examined with 3H-thymidine autoradiography. The Journal of Comparative Neurology, 190(1), 87–114. [DOI] [PubMed] [Google Scholar]
- Bayer SA (1980b). Development of the hippocampal region in the rat. II. Morphogenesis during embryonic and early postnatal life. The Journal of Comparative Neurology, 190(1), 115–134. [DOI] [PubMed] [Google Scholar]
- Bayer SA, & Altman J. (1975). The effects of X-irradiation on the postnatally-forming granule cell populations in the olfactory bulb, hippocampus, and cerebellum of the rat. Experimental Neurology, 48(1), 167–174. 10.1016/0014-4886(75)90231-9 [DOI] [PubMed] [Google Scholar]
- Bell ME, Bourne JN, Chirillo MA, Mendenhall JM, Kuwajima M, & Harris KM (2014). Dynamics of nascent and active zone ultrastructure as synapses enlarge during long-term potentiation in mature hippocampus. The Journal of Comparative Neurology, 522 (17), 3861–3884. 10.1002/cne.23646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ari Y, Khalilov I, Kahle KT, & Cherubini E. (2012). The GABA excitatory/inhibitory shift in brain maturation and neurological disorders. The Neuroscientist, 18(5), 467–486. 10.1177/1073858412438697 [DOI] [PubMed] [Google Scholar]
- Bilousova TV, Dansie L, Ngo M, Aye J, Charles JR, Ethell DW, & Ethell IM (2009). Minocycline promotes dendritic spine maturation and improves behavioural performance in the fragile X mouse model. Journal of Medical Genetics, 46(2), 94–102. 10.1136/jmg.2008.061796 [DOI] [PubMed] [Google Scholar]
- Bourne J, & Harris KM (2007). Do thin spines learn to be mushroom spines that remember? Current Opinion in Neurobiology, 17(3), 381–386. 10.1016/j.conb.2007.04.009 [DOI] [PubMed] [Google Scholar]
- Bourne JN, Kirov SA, Sorra KE, & Harris KM (2007). Warmer preparation of hippocampal slices prevents synapse proliferation that might obscure LTP-related structural plasticity. Neuropharmacology, 52 (1), 55–59. [DOI] [PubMed] [Google Scholar]
- Bowden JB, Abraham WC, & Harris KM (2012). Differential effects of strain, circadian cycle, and stimulation pattern on LTP and concurrent LTD in the dentate gyrus of freely moving rats. Hippocampus, 22(6), 1363–1370. 10.1002/hipo.20972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao G, & Harris KM (2012). Developmental regulation of the late phase of long-term potentiation (L-LTP) and metaplasticity in hippocampal area CA1 of the rat. Journal of Neurophysiology, 107(3), 902–912. 10.1152/jn.00780.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao G, & Harris KM (2014). Augmenting saturated LTP by broadly spaced episodes of theta-burst stimulation in hippocampal area CA1 of adult rats and mice. Journal of Neurophysiology, 112(8), 1916–1924. 10.1152/jn.00297.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cea-Del Rio CA, & Huntsman MM (2014). The contribution of inhibitory interneurons to circuit dysfunction in fragile X syndrome. Frontiers in Cellular Neuroscience, 8, 245. 10.3389/fncel.2014.00245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirillo MA, Waters MS, Lindsey LF, Bourne JN, & Harris KM (2019). Local resources of polyribosomes and SER promote synapse enlargement and spine clustering after long-term potentiation in adult rat hippocampus. Scientific Reports, 9(1), 3861. 10.1038/s41598-019-40520-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curia G, Papouin T, Seguela P, & Avoli M. (2009). Downregulation of tonic GABAergic inhibition in a mouse model of fragile X syndrome. Cerebral Cortex, 19(7), 1515–1520. 10.1093/cercor/bhn159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebbinghaus H. (1885). Über das Gedächnis: Untersuchungen zur experimentellen psychologie. Leipzig, Germany: Veit & Co. [Google Scholar]
- Eichler SA, & Meier JC (2008). E-I balance and human diseases—From molecules to networking. Frontiers in Molecular Neuroscience, 1, 2. 10.3389/neuro.02.002.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellenbroek B, & Youn J. (2016). Rodent models in neuroscience research: Is it a rat race? Disease Models & Mechanisms, 9(10), 1079–1087. 10.1242/dmm.026120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiala JC, Feinberg M, Popov V, & Harris KM (1998). Synaptogenesis via dendritic filopodia in developing hippocampal area CA1. The Journal of Neuroscience, 18(21), 8900–8911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields RD (2005). Making memories stick. Scientific American, 292(2), 75–81. [PubMed] [Google Scholar]
- Francis C, Natarajan S, Lee MT, Khaladkar M, Buckley PT, Sul JY, … Kim J. (2014). Divergence of RNA localization between rat and mouse neurons reveals the potential for rapid brain evolution. BMC Genomics, 15, 883. 10.1186/1471-2164-15-883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris KM, & Teyler TJ (1983). Evidence for late development of inhibition in area CA1 of the rat hippocampus. Brain Research, 268(2), 339–343. [DOI] [PubMed] [Google Scholar]
- Harris KM, & Teyler TJ (1984). Developmental onset of long-term potentiation in area CA1 of the rat hippocampus. The Journal of Physiology, 346, 27–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris KM, Watson DJ, Kuwajima M, & Cao G. (2012). Shift in synapse structure and location advances the onset age of late-phase LTP (Vol. 145.03), Washington, DC: Society for Neuroscience Abstracts: Society for Neuroscience. [Google Scholar]
- He CX, & Portera-Cailliau C. (2013). The trouble with spines in fragile X syndrome: Density, maturity and plasticity. Neuroscience, 251, 120–128. 10.1016/j.neuroscience.2012.03.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homberg JR, Wohr M, & Alenina N. (2017). Comeback of the rat in biomedical research. ACS Chemical Neuroscience, 8(5), 900–903. 10.1021/acschemneuro.6b00415 [DOI] [PubMed] [Google Scholar]
- Jackson PS, Suppes T, & Harris KM (1993). Stereotypical changes in the pattern and duration of long-term potentiation expressed at postnatal days 11 and 15 in the rat hippocampus. Journal of Neurophysiology, 70(4), 1412–1419. [DOI] [PubMed] [Google Scholar]
- Jia Z, Agopyan N, Miu P, Xiong Z, Henderson J, Gerlai R, … Roder J. (1996). Enhanced LTP in mice deficient in the AMPA receptor GluR2. Neuron, 17(5), 945–956. 10.1016/s0896-6273(00)80225-1 [DOI] [PubMed] [Google Scholar]
- Kirov SA, Goddard CA, & Harris KM (2004). Age-dependence in the homeostatic upregulation of hippocampal dendritic spine number during blocked synaptic transmission. Neuropharmacology, 47(5), 640–648. [DOI] [PubMed] [Google Scholar]
- Kramar EA, Babayan AH, Gavin CF, Cox CD, Jafari M, Gall CM, … Lynch G. (2012). Synaptic evidence for the efficacy of spaced learning. Proceedings of the National Academy of Sciences of the United States of America, 109(13), 5121–5126. 10.1073/pnas.1120700109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramar EA, & Lynch G. (2003). Developmental and regional differences in the consolidation of long- term potentiation. Neuroscience, 118(2), 387–398. [DOI] [PubMed] [Google Scholar]
- Lazarov O, & Hollands C. (2016). Hippocampal neurogenesis: Learning to remember. Progress in Neurobiology, 138–140, 1–18. 10.1016/j.pneurobio.2015.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HK, Takamiya K, Han JS, Man H, Kim CH, Rumbaugh G, … Huganir RL (2003). Phosphorylation of the AMPA receptor GluR1 subunit is required for synaptic plasticity and retention of spatial memory. Cell, 112(5), 631–643. [DOI] [PubMed] [Google Scholar]
- Lu Y, Allen M, Halt AR, Weisenhaus M, Dallapiazza RF, Hall DD, … Hell JW (2007). Age-dependent requirement of AKAP150-anchored PKA and GluR2-lacking AMPA receptors in LTP. The EMBO Journal, 26(23), 4879–4890. 10.1038/sj.emboj.7601884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch G, & Gall CM (2013). Mechanism based approaches for rescuing and enhancing cognition. Frontiers in Neuroscience, 7, 143. 10.3389/fnins.2013.00143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch G, Kramar EA, Babayan AH, Rumbaugh G, & Gall CM (2013). Differences between synaptic plasticity thresholds result in new timing rules for maximizing long-term potentiation. Neuropharmacology, 64, 27–36. 10.1016/j.neuropharm.2012.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manahan-Vaughan D. (2000). Long-term depression in freely moving rats is dependent upon strain variation, induction protocol and behavioral state. Cerebral Cortex, 10(5), 482–487. [DOI] [PubMed] [Google Scholar]
- Manahan-Vaughan D, & Schwegler H. (2011). Strain-dependent variations in spatial learning and in hippocampal synaptic plasticity in the dentate gyrus of freely behaving rats. Frontiers in Behavioral Neuroscience, 5(7), 1–9. 10.3389/fnbeh.2011.00007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinnon PJ, McLaughlin SK, Kapsetaki M, & Margolskee RF (2000). Extracellular matrix-associated protein Sc1 is not essential for mouse development. Molecular and Cellular Biology, 20(2), 656–660. 10.1128/mcb.20.2.656-660.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith RM, Floyer-Lea AM, & Paulsen O. (2003). Maturation of long-term potentiation induction rules in rodent hippocampus: Role of GABAergic inhibition. The Journal of Neuroscience, 23(35), 11142–11146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller BR, & Hen R. (2015). The current state of the neurogenic theory of depression and anxiety. Current Opinion in Neurobiology, 30, 51–58. 10.1016/j.conb.2014.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mongredien R, Erdozain AM, Dumas S, Cutando L, del Moral AN, Puighermanal E, … Vialou V. (2019). Cartography of hevin-expressing cells in the adult brain reveals prominent expression in astrocytes and parvalbumin neurons. Brain Structure & Function, 224(3), 1219–1244. 10.1007/s00429-019-01831-x [DOI] [PubMed] [Google Scholar]
- Nikonenko I, Nikonenko A, Mendez P, Michurina TV, Enikolopov G, & Muller D. (2013). Nitric oxide mediates local activity-dependent excitatory synapse development. Proceedings of the National Academy of Sciences of the United States of America, 110(44), E4142–E4151. 10.1073/pnas.1311927110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paluszkiewicz SM, Martin BS, & Huntsman MM (2011). Fragile X syndrome: The GABAergic system and circuit dysfunction. Developmental Neuroscience, 33(5), 349–364. 10.1159/000329420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parnass Z, Tashiro A, & Yuste R. (2000). Analysis of spine morphological plasticity in developing hippocampal pyramidal neurons. Hippocampus, 10(5), 561–568. [DOI] [PubMed] [Google Scholar]
- Pfeiffer BE, & Huber KM (2009). The state of synapses in fragile X syndrome. The Neuroscientist, 15(5), 549–567. 10.1177/1073858409333075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike FG, Meredith RM, Olding AW, & Paulsen O. (1999). Rapid report: Postsynaptic bursting is essential for ‘Hebbian’ induction of associative long-term potentiation at excitatory synapses in rat hippocampus. The Journal of Physiology, 518(Pt 2), 571–576. 10.1111/j.1469-7793.1999.0571p.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plant K, Pelkey KA, Bortolotto ZA, Morita D, Terashima A, McBain CJ, … Isaac JT (2006). Transient incorporation of native GluR2-lacking AMPA receptors during hippocampal long-term potentiation. Nature Neuroscience, 9(5), 602–604. 10.1038/nn1678 [DOI] [PubMed] [Google Scholar]
- Purkey AM, Woolfrey KM, Crosby KC, Stich DG, Chick WS, Aoto J, & Dell’Acqua ML (2018). AKAP150 palmitoylation regulates synaptic incorporation of Ca(2+)-permeable AMPA receptors to control LTP. Cell Reports, 25(4), 974–987 e974. 10.1016/j.celrep.2018.09.085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risher WC, Patel S, Kim IH, Uezu A, Bhagat S, Wilton DK, … Eroglu C. (2014). Astrocytes refine cortical connectivity at dendritic spines. eLife, 3, 1–24. 10.7554/eLife.04047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabanov V, Braat S, D’Andrea L, Willemsen R, Zeidler S, Rooms L, … Balschun D. (2017). Impaired GABAergic inhibition in the hippocampus of Fmr1 knockout mice. Neuropharmacology, 116, 71–81. 10.1016/j.neuropharm.2016.12.010 [DOI] [PubMed] [Google Scholar]
- Sanderson JL, Gorski JA, & Dell’Acqua ML (2016). NMDA receptor-dependent LTD requires transient synaptic incorporation of Ca(2) (+)-permeable AMPARs mediated by AKAP150-anchored PKA and calcineurin. Neuron, 89(5), 1000–1015. 10.1016/j.neuron.2016.01.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seese RR, Wang K, Yao YQ, Lynch G, & Gall CM (2014). Spaced training rescues memory and ERK1/2 signaling in fragile X syndrome model mice. Proceedings of the National Academy of Sciences of the United States of America, 111(47), 16907–16912. 10.1073/pnas.1413335111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh SK, Stogsdill JA, Pulimood NS, Dingsdale H, Kim YH, Pilaz LJ, … Eroglu C. (2016). Astrocytes assemble thalamocortical synapses by bridging NRX1alpha and NL1 via hevin. Cell, 164(1–2), 183–196. 10.1016/j.cell.2015.11.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. (2019). In Haines C, Cao G, Ventura SL, Drake MH, Kuwajima M, & Harris KM (Eds.), Shift in synapse structure and location advances the onset age of late-phase LTP (Vol. 037.08), Washington, DC: Society for Neuroscience Abstracts: Society for Neuroscience. [Google Scholar]
- Snyder JS, Choe JS, Clifford MA, Jeurling SI, Hurley P, Brown A, … Cameron HA (2009). Adult-born hippocampal neurons are more numerous, faster maturing, and more involved in behavior in rats than in mice. The Journal of Neuroscience, 29(46), 14484–14495. 10.1523/JNEUROSCI.1768-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Dutch-Belgian Fragile X Consortium. (1994). Fmr1 knockout mice: A model to study fragile X mental retardation. Cell, 78(1), 23–33. [PubMed] [Google Scholar]
- Thomas MJ, Watabe AM, Moody TD, Makhinson M, & O’Dell TJ (1998). Postsynaptic complex spike bursting enables the induction of LTP by theta frequency synaptic stimulation. The Journal of Neuroscience, 18(18), 7118–7126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ET, Taliaferro JM, Lee JA, Sudhakaran IP, Rossoll W, Gross C, … Bassell GJ (2016). Dysregulation of mRNA localization and translation in genetic disease. The Journal of Neuroscience, 36(45), 11418–11426. 10.1523/JNEUROSCI.2352-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikstrom MA, Matthews P, Roberts D, Collingridge GL, & Bortolotto ZA (2003). Parallel kinase cascades are involved in the induction of LTP at hippocampal CA1 synapses. Neuropharmacology, 45(6), 828–836. 10.1016/s0028-3908(03)00336-8 [DOI] [PubMed] [Google Scholar]
- Xiao MY, Wasling P, Hanse E, & Gustafsson B. (2004). Creation of AMPA-silent synapses in the neonatal hippocampus. Nature Neuroscience, 7(3), 236–243. 10.1038/nn1196 [DOI] [PubMed] [Google Scholar]
- Young JZ, & Nguyen PV (2005). Homosynaptic and heterosynaptic inhibition of synaptic tagging and capture of long-term potentiation by previous synaptic activity. The Journal of Neuroscience, 25(31), 7221–7231. 10.1523/JNEUROSCI.0909-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Hou L, Klann E, & Nelson DL (2009). Altered hippocampal synaptic plasticity in the FMR1 gene family knockout mouse models. Journal of Neurophysiology, 101(5), 2572–2580. 10.1152/jn.90558.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.