Abstract
Objective
Leukocyte morphological parameters known as CPD (cell population data) is detected by hematology analyzer UniCel DxH800 with VCS technology. This study aimed to investigate the diagnostic efficacy of morphological changes in CPD parameters in distinguishing active tuberculosis from community-acquired pneumonia.
Methods
From October 2018 to February 2019, 88 patients with active tuberculosis, 78 patients with community-acquired pneumonia, and 89 healthy controls were enrolled in this study. CPD was obtained using Unicel DxH800 analyzer for all whole blood samples, one-way ANOVA (non-parametric) and area analysis under ROC curve were performed.
Results
The neutrophil mean conductivity (NMC), monocyte mean volume (MMV), monocyte mean conductivity (MMC), lymphocyte percentage (LY%), and monocyte percentage (MO%) were significantly higher in the active tuberculosis group than in the community-acquired pneumonia group. The white blood cell (WBC) count and neutrophil percentage (NE%) were significantly lower in the active tuberculosis group than in the community-acquired pneumonia group. The analysis of the area under the ROC curve proved that WBC count, neutrophil percentage (NE%), lymphocyte percentage (LY%), and monocyte percentage (MO%) did not achieve a higher area under the curve (AUC: 0.63, 0.71, 0.62, and 0.7, respectively). However, the AUC of NMC, MMV, and MMC in the CPD parameters was 0.951, 0.877, 0.98, respectively, and the simultaneous measurement of the three parameters was 0.99. The sensitivity and specificity were 98.5% and 91.1%, respectively.
Conclusion
The combined diagnosis of NMC, MMV, and MMC could assist the clinical diagnosis of active tuberculosis and community-acquired pneumonia.
Keywords: active tuberculosis, cell population data, community-acquired pneumonia, VCS technology, CPD parameters
1. Introduction
Active tuberculosis (ATB) and community-acquired pneumonia (CAP) have similar symptoms, such as fever, cough, and expectoration; also, similarities are found in the imaging findings of pulmonary inflammation [1]. When the two diseases are combined, the patient’s condition will become more complicated, increasing the difficulty of identification and diagnosis. The two diseases often cover each other’s symptoms. The symptoms of the first disease are more obvious, which may cause missed diagnosis and misdiagnosis of another disease. There are many cases of tuberculosis as the first disease with secondary pneumonia, while the cases of pneumonia as the first, causing neglected treatment of tuberculosis reports are seldom. Currently, distinguishing the two diseases clinically is still difficult [2]. Therefore, a simple, fast, and accurate method is needed to distinguish ATB from CAP. The cell population data (CPD) can measure the inherent biological characteristics of more than 8,000 white blood cells simultaneously using the VCS technology of the blood analyzer UniCel DxH800. The VCS technology uses the direct current impedance to detect cell volume (V), the exact size of all cell types, and the conductivity of the internal structure of the cell (C) by radiofrequency opacity. Multiple angles light scatter (S) includes upper medium angle light scatter (UMALS), medium angle light scatter (MALS), lower medium angle light scatter (LMALS), and lower angle light scatter (LALS) to detect cell particle size and membrane morphology and axial light loss (AL2) so as to analyze cell transparency. Leukocytes are detected and analyzed by simultaneously obtaining three morphological parameters directly related to cell morphology. In three-dimensional space, a series of parameters can be obtained by comprehensively measuring the cell volume, size, and particle size, which can be accurately and quickly used to perform a microscopic evaluation of the WBC morphology [3,4,5]. In this study, the morphological changes in leukocyte CPD parameters were evaluated to investigate its diagnostic efficacy in distinguishing active tuberculosis from community-acquired pneumonia.
2. Material and methods
2.1. Case selection
This study included 88 patients with ATB from Chongqing Infectious Disease Medical Center from October 2018 to February 2019, 78 patients including CAP inpatients in the Department of Laboratory Medicine, Daping Hospital, Army Medical University, and 89 healthy controls who visited the physical examination center for health checkups.
The inclusion criteria for patients with ATB, in accordance with the “Public Health Organization Standards of the People’s Republic of China for the diagnosis of tuberculosis WS 288-2017,” were as follows: sputum culture, sputum antacid staining, at least one positive molecular diagnosis, and chest X-ray findings consistent with TB imaging lesions. Selected patients were all newly diagnosed patients with no history of anti-TB treatment.
The admission criteria for CAP and the diagnosis complied with the guidelines for the diagnosis and treatment of acquired pneumonia issued by the Respiratory Branch of the Chinese Medical Association in 2016 [6]. The criteria were as follows: (1) community incidence; (2) pneumonia-related clinical manifestations: (i) new symptoms of cough, sputum, or pre-existing respiratory disease worsened, with or without chest pain, and purulent sputum, (ii) signs of consolidation of the lungs and/or smell of wet rales, (iii) fever, (iv) the number of peripheral blood leukocytes >10 × 109/L or <4 × 109/L, with or without a nuclear shift to the left; and (3) chest imaging showing newly appearing patchy infiltrates, lobes, or segments with consolidated shadows, ground-glass opacity, or interstitial changes with or without pleural effusion. Patients with any of the aforementioned criteria who were diagnosed with bacterial pneumonia through bacterial staining and culture, excluding lung tumors, TB, noninfectious pulmonary interstitial disease, pulmonary embolism, pulmonary edema, atelectasis, pulmonary eosinophil infiltration, and pulmonary vasculitis.
The inclusion criteria for the healthy control group were as follows: individuals who went to the hospital for physical examination during the same period; no history of exposure to patients with TB; normal chest X-ray; negative results for interferon-gamma release assay T-SPOT; and no fever and cough. The liver function, kidney function, hepatitis B, and routine blood tests were normal.
The exclusion criteria were as follows: age <18 years; pregnant women; HIV infection; cancer; patients with severe abnormalities in liver and kidney function; patients with autoimmune diseases; patients who received immunosuppressants such as glucocorticoids; or patients who received anti-TB treatment.
Ethical approval: The study protocol was approved by the hospital ethics committee.
2.2. Testing method
2.2.1. CPD parametric analysis
All samples were collected in EDTA-anticoagulated tubes by the experienced phlebotomists and analyzed within 4 h after specimen collection using UniCel DxH800 blood analyzer except coagulation and hemolysis. The following parameters were evaluated: WBC count, neutrophil percentage (NE%), lymphocyte percentage (LY%), monocyte percentage (MO%), and CPD parameters (V, V-SD, C, C-SD, UMALS, UMALS-SD, MALS, MALS-SD, LMALS, LMALS-SD, LALS, LALS-SD, AL2, and AL2-SD).
2.3. Statistical methods
The GraphPad Prism 8.0 statistical software (GraphPad Software, San Diego, CA, USA) was used for all data processing. After specimen testing, we obtained the data of all specimens and calculated the average value of testing results for all parameters in different groups. Data were exhibited by the mean ± standard deviation depending on the data characteristics, and the differences were evaluated using the independent sample t test in comparisons between two groups. Comparison among three means was performed using the one-way ANOVA (and nonparametric). The parameters with the statistical difference between the ATB and CAP were analyzed by the receiver operating characteristic (ROC) curve and appraised the diagnostic value. The cutoff value was defined based on the max You-den’s index. A p-value <0.05 was considered a statistically significant difference.
3. Results
3.1. Basic information of each parameter
This study retrospectively analyzed 88 patients with ATB (mean age 49.8 ± 14.5 years; male/female: 42/46 years), 78 patients with community-acquired pneumonia (age: 50.9 ± 19.1 years; male/female: 35/43 years), and 89 healthy controls (age: 51.9 ± 19.1 years; male/female: 41/48 years). The values of the following 27 indicators were significantly higher in the ATB group compared with the CAP group (LY%, MO%, NMV-SD, NMC, NMC-SD, N-MALS, N-MALS-SD, N-UMALS, N-LMALS-SD, N-LALS-SD, N-AL2, LMV-SD, LMC, LMC-SD, L-MALS,L-MALS-SD, L-UMALS, L-UMALS-SD, L-LMALS-SD, L-LALS-SD, L-AL2-SD, MMV, MMV-SD, MMC, MMC-SD, M-MALS, M-UMALS; P < 0.05). The values of 11 parameters (WBC, NE%, N-LALS, L-AL2, M-MALS-SD, M-UMALS-SD, M-LMALS-SD, M-LALS, M-LALS-SD, M-AL2, and M-AL2-SD; P < 0.05) was significantly lower in the ATB group than in the CAP group, while the values of NMV, N-UMALS-SD, N-LMALS, N-AL2-SD, LMV, L-LMALS, L-LALS, and M-LMALS were not statistically significantly different between active TB and community-acquired pneumonia groups (Table A1).
3.2. Sensitivity and specificity of CPD parameters to identify active TB and community-acquired pneumonia
The area under the ROC curve of CPD parameters had significant differences between ATB and CAP groups. WBC, NE%, LY%, and MO% did not show high sensitivity and specificity, and their area under the curve (AUC, 0.63, 0.71, 0.62, and 0.7) was less than 0.75 (Table 1). Conversely, in the analysis of CPD parameters, there are 13 parameters with AUC area above 0.8 (Table A2), of which three parameters (NMC, MMV, and MMC) achieved higher AUC compared with other parameters, which were 0.951, 0.877, and 0.98, respectively (Table 1). Combined measurement of NMC, MMV, and MMC could achieve an AUC as high as 0.99, and its sensitivity and specificity to distinguish ATB from CAP group were 98.5% and 91.1%, respectively (Table 1 and Figure 1).
Table 1.
Sensitivity and specificity of cut-off values for each parameter in the analysis of active tuberculosis and community-acquired pneumonia groups
| Parameters | AUC | Cut-off value | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|
| WBC | 0.63 | >8.450 | 38.46 | 85.57 |
| NE% | 0.71 | >66.70 | 69.23 | 64.95 |
| LY% | 0.62 | <13.08 | 39.74 | 85.57 |
| MO% | 0.70 | <9.550 | 85.9 | 52.58 |
| NMC | 0.951 | <149.5 | 83.33 | 93.81 |
| MMV | 0.877 | <169.5 | 71.79 | 89.69 |
| MMC | 0.98 | <129.5 | 92.31 | 89.69 |
| NMC + MMV + MMC | 0.99 | — | 98.5 | 91.1 |
WBC: white blood cells, NE%: neutrophil percentage, LY%: lymphocyte percentage, MO%: monocyte percentage, NMC: neutrophil mean conductivity, MMV: monocyte mean volume, MMC: monocyte mean conductivity.
Figure 1.
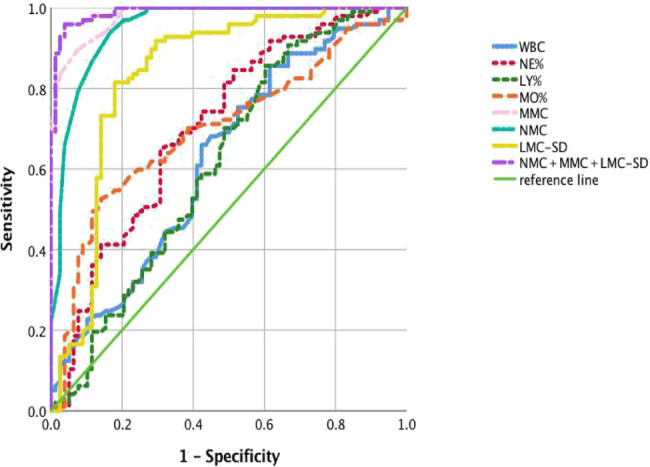
Analysis each parameter between ATB and CAP which under the ROC curve. Diagnostic value was appraised by the receiver operating characteristic (ROC) curve. Cutoff value was defined based on the max You-den’s index, which is the value when the difference between the sensitivity and 1-specificity of all points on the ROC curve is the largest. Compared with the WBC count and three of WBC conventional differential count, the CPD parameter achieved a higher AUC, and the maximum area under the AUC curve obtained by combining MNC, MMV, and MMC was 0.99. WBC, white blood cells; NE%, neutrophil percentage; LY%, lymphocyte percentage; MO%, monocyte percentage; NMC, neutrophil mean conductivity; MMV, monocyte mean volume; MMC, monocyte mean conductivity.
4. Discussion
Pneumonia is an infectious disease characterized by the inflammation of the terminal airways, alveoli, and interstitial lungs, affecting people worldwide, including developed and developing countries. In Asian countries, 1 million adults are affected by community-acquired pneumonia alone [7]. Pneumonia can be caused by pathogenic microorganisms, immune damage, physical and chemical factors, allergies, and drugs. The common pathogens of infectious pneumonia include bacteria, viruses, mycoplasma, fungi, rickettsia, chlamydia, and protozoa. Among these, bacterial pneumonia is the most common cause of pneumonia, accounting for 80% of all types of adult infectious pneumonia. Globally, pneumonia is a serious public health concern and a major cause of mortality and morbidity [8]. In recent years, with the aging of the population, the abuse of antibacterial drugs, the increase in immune damage, and the changes in the human living environment have led to great difficulty in the diagnosis and treatment of pneumonia [9,10,11,12]. Rapid and accurate diagnosis, timely treatment and management of the patients, and proper use of antibiotics need urgent attention.
TB has long been a global health problem. According to WHO global tuberculosis report in 2019 that there were 10 million new people suffer from TB, and the number of deaths caused by TB has been higher than that caused by HIV/AIDS in the last 5 years. China is 1 of the 22 countries with the largest TB epidemic burden in the world; the number of patients with TB is second to that of India [13]. However, if diagnosed and treated correctly, most patients with TB can be cured. The diagnosis of TB is mainly based on medical history, clinical manifestations, chest X-ray examination, and examination of sputum TB bacteria; however, the bacteriological examination is time consuming [14,15]. The clinical manifestations of TB, such as cough, fever, and the diversity of chest X-ray results, account for the lack of specificity. Hence, it is easily confused with community-acquired pneumonia, leading to the failure to diagnose and treat in time.
In this study, the VCS technology of the UniCel DxH800 blood analyzer was used to directly examine the peripheral blood of patients with active TB and community-acquired pneumonia. The results confirmed statistically significant differences in NMC, MMV, and MMC between the two groups (P < 0.0001; Table 1 and Figure 1). These three parameters were combined to obtain the area under the ROC curve of 0.99, the sensitivity and specificity were 98.5% and 91.1%, respectively. Compared with the WBC count and WBC differential percentage, the combination of NMC, MMV, and MMC had higher sensitivity and specificity with higher diagnostic efficacy for distinguishing ATB from CAP.
Studies have shown that phagocytes, such as monocytes, macrophages, and multinuclear neutrophils, are the first line of defense against the invasion of bacterial pathogens. During bacterial invasion, macrophages are identified by surface exposure, and vesicle or cytoplasmic pattern recognition receivers recognize bacteria and initiate phagocytic cells for phagocytosis. Neutrophils leave the circulation and migrate to inflammatory lesions to deal with the infection. Therefore, after the body is infected by bacteria, there are changes in the size of the white blood cell subpopulation, the number of particles, the nuclear-to-cytoplasmic ratio, and the cell membrane. These differences might vary in different diseases. The results of this study showed that among all CPD parameters, neutrophils, and monocytes, had statistically significant differences in the identification of active TB and community-acquired pneumonia. These changes indicated that the volume of activated cells and volume heterogeneity increase after infection. The nuclear/cytoplasmic ratio was high (the nucleus was more naïve). In the anti-TB immune response, various cytokines are secreted, the number of particles increases in the cytoplasm, and the membrane morphology changes, resulting in a significant change in the electrical conductivity of the cells.
In conclusion, leukocyte community parameters were easily obtained from blood CPD parameters, and the detection cost was low. These parameters could quickly, accurately, and objectively reflect the inherent biological characteristics of cells, thus having potential clinical application value in providing quick and easy reference indicators of ATB and CAP.
Appendix
Table A1.
parameters in active tuberculosis and community-acquired pneumonia and healthy control (Mean ± SD)
| Parameter | 1 | 2 | 3 | P | ||
|---|---|---|---|---|---|---|
| ATB (N = 88) | CAP (N = 78) | HC (N = 89) | 1 vs 2 | 1 vs 3 | 2 vs 3 | |
| WBC | 6.614 ± 2.224 | 8.175 ± 4.097 | 7.086 ± 2.746 | 0.0208 | Ns | Ns |
| NE% | 64.18 ± 9.191 | 71.31 ± 13.1 | 68.74 ± 12.46 | <0.0001 | 0.0075 | Ns |
| LY% | 20.7 ± 8.047 | 18.04 ± 13.6 | 21.23 ± 10.25 | 0.0302 | Ns | 0.0348 |
| MO% | 9.899 ± 3.522 | 7.945 ± 2.992 | 7.466 ± 2.579 | <0.0001 | <0.0001 | Ns |
| neutrophil CPD parameter | ||||||
| NMV | 144.2 ± 6.266 | 145.2 ± 7.995 | 145.7 ± 7.233 | Ns | Ns | Ns |
| NMV-SD | 20.32 ± 3.177 | 18.81 ± 2.633 | 17.85 ± 2.107 | 0.0059 | <0.0001 | Ns |
| NMC | 153.4 ± 2.645 | 139.8 ± 7.314 | 151.5 ± 3.526 | <0.0001 | 0.0003 | <0.0001 |
| NMC-SD | 9.042 ± 3.948 | 5.863 ± 1.734 | 5.536 ± 1.051 | <0.0001 | <0.0001 | Ns |
| N-MALS | 134.3 ± 5.684 | 128.99.347 | 136.6 ± 4.945 | 0.0006` | 0.0262 | <0.0001 |
| N-MALS-SD | 14.95 ± 5.118 | 12.73 ± 2.612 | 11.05 ± 1.572 | 0.0305 | <0.0001 | <0.0001 |
| N-UMALS | 137.8 ± 4.474 | 124.2 ± 11.22 | 136.3 ± 5.342 | <0.0001 | Ns | <0.0001 |
| N-UMALS-SD | 14.5 ± 4.385 | 16.82 ± 6.577 | 11.85 ± 1.68 | Ns | <0.0001 | <0.0001 |
| N-LMALS | 126.8 ± 9.128 | 127.3 ± 11.23 | 133 ± 6.441 | Ns | <0.0001 | 0.0016 |
| N-LMALS-SD | 19.16 ± 6.887 | 16.26 ± 4.386 | 14.05 ± 2.624 | 0.0008 | <0.0001 | 0.0004 |
| N-LALS | 155.2 ± 20.28 | 163.6 ± 18 | 170.9 ± 13.64 | 0.011 | <0.0001 | Ns |
| N-LALS-SD | 41.1 ± 6.712 | 34.27 ± 7.237 | 33.03 ± 6.652 | <0.0001 | <0.0001 | Ns |
| N-AL2 | 142.8 ± 3.893 | 140.7 ± 4.248 | 140.7 ± 5.399 | 0.0023 | 0.0048 | Ns |
| N-AL2-SD | 15.72 ± 4.343 | 15.73 ± 3.459 | 11.56 ± 1.843 | Ns | <0.0001 | <0.0001 |
| lymphocyte CPD parameter | ||||||
| LMV | 89.19 ± 4.457 | 89.1 ± 4.323 | 87.49 ± 3.956 | Ns | 0.0409 | Ns |
| LMV-SD | 17.55 ± 3.883 | 15.38 ± 2.686 | 15.31 ± 2.534 | 0.0011 | <0.0001 | Ns |
| LMC | 119.6 ± 3.204 | 111.7 ± 6.74 | 120.4 ± 3.021 | <0.0001 | Ns | <0.0001 |
| LMC-SD | 13.09 ± 2.558 | 9.642 ± 3.156 | 9.136 ± 1.923 | <0.0001 | <0.0001 | Ns |
| L-MALS | 61.27 ± 6.314 | 51.29 ± 13.23 | 64.53 ± 7.477 | <0.0001 | 0.0029 | <0.0001 |
| L-MALS-SD | 19.65 ± 2.614 | 17.42 ± 1.702 | 17.59 ± 1.808 | <0.0001 | <0.0001 | Ns |
| L-UMALS | 64.13 ± 8.007 | 44.54 ± 20.61 | 66 ± 9.197 | <0.0001 | Ns | <0.0001 |
| L-UMALS-SD | 21.91 ± 2.516 | 19.99 ± 2.875 | 19.41 ± 1.508 | <0.0001 | <0.0001 | Ns |
| L-LMALS | 54.03 ± 5.828 | 51.71 ± 7.47 | 58.43 ± 6.191 | Ns | <0.0001 | <0.0001 |
| L-LMALS-SD | 21.67 ± 2.818 | 20.19 ± 1.55 | 20.36 ± 1.664 | <0.0001 | <0.0001 | Ns |
| L-LALS | 39.85 ± 4.814 | 38.87 ± 2.894 | 37.65 ± 2.692 | Ns | <0.0001 | 0.0207 |
| L-LALS-SD | 14.94 ± 4.987 | 12.38 ± 1.96 | 11.69 ± 1.637 | <0.0001 | <0.0001 | Ns |
| L-AL2 | 75.76 ± 3.785 | 79.28 ± 3.407 | 75.23 ± 2.807 | <0.0001 | Ns | <0.0001 |
| L-AL2-SD | 12.78 ± 3.313 | 11.91 ± 2.354 | 10.94 ± 1.366 | 0.0223 | <0.0001 | 0.0195 |
| monocyte CPD parameter | ||||||
| MMV | 182.4 ± 11.35 | 166 ± 10.34 | 166.8 ± 7.935 | <0.0001 | <0.0001 | Ns |
| MMV-SD | 21.96 ± 2.533 | 20.51 ± 3.783 | 18.95 ± 3.034 | 0.0004 | <0.0001 | 0.0108 |
| MMC | 132.5 ± 2.389 | 119.4 ± 6.561 | 128.9 ± 3.146 | <0.0001 | <0.0001 | <0.0001 |
| MMC-SD | 7.266 ± 3.161 | 6.811 ± 2.953 | 6.087 ± 1.876 | 0.0216 | <0.0001 | Ns |
| M-MALS | 84.27 ± 3.353 | 74.19 ± 12.72 | 86.59 ± 5.088 | 0.0002 | 0.0002 | <0.0001 |
| M-MALS-SD | 10.91 ± 1.396 | 13.31 ± 2.944 | 11.74 ± 1.295 | <0.0001 | <0.0001 | 0.0012 |
| M-UMALS | 94.48 ± 5.368 | 76.29 ± 18.02 | 94.69 ± 6.135 | <0.0001 | Ns | <0.0001 |
| M-UMALS-SD | 11.87 ± 1.99 | 16.48 ± 4.544 | 12.73 ± 1.858 | <0.0001 | 0.0015 | <0.0001 |
| M-LMALS | 71.01 ± 2.845 | 67.46 ± 9.699 | 75.58 ± 5.032 | Ns | <0.0001 | <0.0001 |
| M-LMALS-SD | 14.07 ± 3.399 | 16.77 ± 3.094 | 14.73 ± 1.617 | <0.0001 | 0.0001 | <0.0001 |
| M-LALS | 81.4 ± 11.04 | 87.42 ± 14.32 | 90.58 ± 12.19 | 0.0001 | <0.0001 | Ns |
| M-LALS-SD | 23.19 ± 4.195 | 25.94 ± 3.796 | 26.43 ± 3.898 | <0.0001 | <0.0001 | Ns |
| M-AL2 | 131.6 ± 5.154 | 134.2 ± 4.587 | 131.3 ± 4.686 | 0.0003 | Ns | 0.0001 |
| M-AL2-SD | 12.45 ± 2.246 | 14.71 ± 3.824 | 12.64 ± 2.588 | <0.0001 | Ns | <0.0001 |
ATB: Active Tuberculosis, CAP: Community-acquired pneumonia, HC: Health control, WBC: White blood count, NE%: neutrophil percentage, LY%: lymphocyte percentage, MO%: monocyte percentage, NMV: Neutrophil mean volume,NMV-SD: Neutrophil mean volume standard deviation, NMC: Neutrophil mean conductivity, NMC-SD: Neutrophil mean conductivity standard deviation, N-MALS: Neutrophil medium angle light scatter, N-MALS-SD: Neutrophil medium angle light scatter standard deviation: N-UMALS: Neutrophil upper medium angle light scatter, N-UMALS-SD: Neutrophil upper medium angle light scatter standard deviation, N-LMALS: Neutrophil lower medium angle light scatter, N-LMALS-SD: Neutrophil lower medium angle light scatter standard deviation, N-LALS: Neutrophil lower angle light scatter, N-LALS-SD: Neutrophil lower angle light scatter standard deviation, N-AL2: Neutrophil axial light loss measurement, N-AL2-SD: Neutrophil axial light loss measurement standard deviation, LMV: Lymphocyte mean volume, LMV-SD: Lymphocyte mean volume standard deviation, LMC: Lymphocyte mean conductivity,LMC-SD: Lymphocyte mean conductivity standard deviation, L-UMALS:Lymphocyte upper median angle light scatter, L-UMALS-SD: Lymphocyte upper median angle light scatter standard deviation, L-MALS: Lymphocyte medium angle light scatter, L-MALS-SD: Lymphocyte medium angle light scatter standard deviation, L-LMALS: Lymphocyte lower median angle light scatter, L-LMALS-SD: Lymphocyte lower median angle light scatter standard deviation, L-LALS: Lymphocyte lower angle light scatter, L-LALS-SD: Lymphocyte lower angle light scatter standard deviation, L-AL2: Lymphocyte axial light loss measurement, L-AL2-SD: Lymphocyte axial light loss measurement standard deviation, MMV: Monocyte mean volume, MMV-SD: Monocyte mean volume standard deviation, MMC: Monocyte mean conductivity, MMC-SD: Monocyte mean conductivity standard deviation, M-UMALS: Monocyte upper median angle light scatter, M-UMALS-SD: Monocyte upper median angle light scatter standard deviation, M-MALS: Monocyte medium angle light scatter, M-MALS-SD: Monocyte medium angle light scatter standard deviation, M-LMALS: Monocyte lower median angle light scatter, M-LMALS-SD: Monocyte lower median angle light scatter standard deviation, M-LALS: Monocyte lower angle light scatter, M-LALS-SD: Monocyte lower angle light scatter standard deviation, M-AL2: Monocyte axial light loss measurement, M-AL2-SD: Monocyte axial light loss measurement standard deviation.
Table A2.
Sensitivity and specificity at the designated cut-off values of parameters for differentiating active tuberculosis from community-acquired pneumonia
| Parameter | AUC | Cutoff value | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|
| NMC | 0.951 | <149.5 | 83.33 | 93.81 |
| NMC-SD | 0.807 | <6.475 | 79.49 | 69.07 |
| N-UMALS | 0.874 | <132.5 | 75.64 | 88.66 |
| LMC | 0.816 | <114.4 | 64.1 | 96.91 |
| LMC-SD | 0.836 | <10.88 | 82.05 | 81.44 |
| L-MALS-SD | 0.809 | <18.04 | 76.92 | 79.38 |
| L-AL2 | 0.803 | >76.5 | 83.33 | 64.95 |
| MMV | 0.877 | <169.5 | 71.79 | 89.69 |
| MMC | 0.98 | <129.5 | 92.31 | 89.69 |
| M-MALS-SD | 0.806 | >11.82 | 66.67 | 84.54 |
| M-UMALS | 0.803 | <87.5 | 65.38 | 97.94 |
| M-UMALS-SD | 0.846 | >12.29 | 80.77 | 74.23 |
| M-LMALS-SD | 0.829 | >15.06 | 71.79 | 86.6 |
Footnotes
Funding information: This study was supported by Chongqing Municipal Social People’s Livelihood Technology Innovation Project (CSTC2015SHMSZX120102) and 973 project sub-topics (K2015CB755401) and Major Project (2018XLC2027) of Army Medical University.
Conflict of interest: The authors declare that there are no conflicts of interest in this work.
Data availability statements: All data generated or analyzed during this study are included in this published article.
Contributor Information
Jing Wang, Email: 403413860@qq.com.
Shaoli Deng, Email: dengshaoli@tmmu.edu.cn.
Qing Huang, Email: qinghuang@tmmu.edu.cn.
References
- [1].Cavallazzi R, Wiemken T, Christensen D, Peyrani P, Blasi F, Levy G, et al. Predicting mycobacterium tuberculosis in patients with community-acquired pneumonia. Eur Respir J. 2014;43(1):17884. 10.1183/09031936.00017813. [DOI] [PubMed]; Cavallazzi R, Wiemken T, Christensen D, Peyrani P, Blasi F, Levy G. et al. Predicting mycobacterium tuberculosis in patients with community-acquired pneumonia. Eur Respir J. 2014;43(1):17884. doi: 10.1183/09031936.00017813. [DOI] [PubMed] [Google Scholar]
- [2].Garcia R. Community-acquired pneumonia due to streptococcus pneumoniae: when to consider coinfection with active pulmonary tuberculosis. Case Rep Infect Dis. 2019;2019:4618413. 10.1155/2019/4618413. [DOI] [PMC free article] [PubMed]; Garcia R. Community-acquired pneumonia due to streptococcus pneumoniae: when to consider coinfection with active pulmonary tuberculosis. Case Rep Infect Dis. 2019;2019:4618413. doi: 10.1155/2019/4618413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Dheda K, Udwadia ZF, Huggett JF, Johnson MA, Rook GA. Utility of the antigen-specific interferon-gamma assay for the management of tuberculosis. Curr Opin Pulm Med. 2005;11(3):195–202. 10.1097/01.mcp.0000158726.13159.5e. [DOI] [PubMed]; Dheda K, Udwadia ZF, Huggett JF, Johnson MA, Rook GA. Utility of the antigen-specific interferon-gamma assay for the management of tuberculosis. Curr Opin Pulm Med. 2005;11(3):195–202. doi: 10.1097/01.mcp.0000158726.13159.5e. [DOI] [PubMed] [Google Scholar]
- [4].Sun T, Wu B, Wang J, Yuan T. Evaluation of the diagnostic efficacy of monocyte parameters and MCP-1 to distinguishing active tuberculosis from latent tuberculosis. Clin Lab. 2019;65(7). 10.7754/Clin.Lab.2018.181115. [DOI] [PubMed]; Sun T, Wu B, Wang J, Yuan T. Evaluation of the diagnostic efficacy of monocyte parameters and MCP-1 to distinguishing active tuberculosis from latent tuberculosis. Clin Lab. 2019;65(7) doi: 10.7754/Clin.Lab.2018.181115. [DOI] [PubMed] [Google Scholar]
- [5].Sun T, Li J, Wu B, Huang H, Deng SJ. Effects of blood storage on cell population data. Clin Lab. 2020;66(8). 10.7754/Clin.Lab.2020.191135. [DOI] [PubMed]; Sun T, Li J, Wu B, Huang H, Deng SJ. Effects of blood storage on cell population data. Clin Lab. 2020;66(8) doi: 10.7754/Clin.Lab.2020.191135. [DOI] [PubMed] [Google Scholar]
- [6].Chinese Medical Association Respiratory Branch. Guidelines for the diagnosis and treatment of community-acquired pneumonia in Chinese adults (2016 edition). Chin J Tuberc Respir Med. 2016;39(4):253–79.; Chinese Medical Association Respiratory Branch. Guidelines for the diagnosis and treatment of community-acquired pneumonia in Chinese adults (2016 edition) Chin J Tuberc Respir Med. 2016;39(4):253–79. [Google Scholar]
- [7].Peto L, Nadjm B, Horby P, Ngan T, Doorn RV, Kinh NV, et al. The bacterial aetiology of adult community-acquired pneumonia in Asia: a systematic review. Trans R Soc Trop Med Hyg. 2014;108(6):326. 10.1093/trstmh/tru058. [DOI] [PMC free article] [PubMed]; Peto L, Nadjm B, Horby P, Ngan T, Doorn RV, Kinh NV. et al. The bacterial aetiology of adult community-acquired pneumonia in Asia: a systematic review. Trans R Soc Trop Med Hyg. 2014;108(6):326. doi: 10.1093/trstmh/tru058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Catia C, Ignacio ML, Carolina GV, Alicia SJ, Antoni TJ. Microbial etiology of pneumonia: epidemiology, diagnosis and resistance patterns. Int J Mol Sci. 2016;17(12):2120. 10.3390/ijms17122120. [DOI] [PMC free article] [PubMed]; Catia C, Ignacio ML, Carolina GV, Alicia SJ, Antoni TJ. Microbial etiology of pneumonia: epidemiology, diagnosis and resistance patterns. Int J Mol Sci. 2016;17(12):2120. doi: 10.3390/ijms17122120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Gupta D, Agarwal R, Aggarwal AN, Singh N, Mishra N, Khilnani G, et al. Guidelines for diagnosis and management of community- and hospital-acquired pneumonia in adults: joint ICS/NCCP(I) recommendations. Lung India. 2012;29(6):S27–62. 10.4103/0970-2113.99248. [DOI] [PMC free article] [PubMed]; Gupta D, Agarwal R, Aggarwal AN, Singh N, Mishra N, Khilnani G. et al. Guidelines for diagnosis and management of community- and hospital-acquired pneumonia in adults: joint ICS/NCCP(I) recommendations. Lung India. 2012;29(6):S27–62. doi: 10.4103/0970-2113.99248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Bahlis LF, Diogo LP, Kuchenbecker RD, Fuchs SC. Clinical, epidemiological, and etiological profile of inpatients with community-acquired pneumonia in a public hospital in the interior of Brazil. J Bras Pneumol. 2018;44:261–6. 10.1590/S1806-37562017000000434. [DOI] [PMC free article] [PubMed]; Bahlis LF, Diogo LP, Kuchenbecker RD, Fuchs SC. Clinical, epidemiological, and etiological profile of inpatients with community-acquired pneumonia in a public hospital in the interior of Brazil. J Bras Pneumol. 2018;44:261–6. doi: 10.1590/S1806-37562017000000434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kksal I, Ozlü T, Bayraktar O, Ylmaz G, Sucu N. Etiological agents of community-acquired pneumonia in adult patients in Turkey; a multicentric, cross-sectional study. Tuberk Toraks. 2010;58(2):119–27. [PubMed]; Kksal I, Ozlü T, Bayraktar O, Ylmaz G, Sucu N. Etiological agents of community-acquired pneumonia in adult patients in Turkey; a multicentric, cross-sectional study. Tuberk Toraks. 2010;58(2):119–27. [PubMed] [Google Scholar]
- [12].Bruns A, Oosterheert JJ, Cucciolillo MC, Moussaoui RE, Groenwold R, Prins JM, et al. Cause‐specific long‐term mortality rates in patients recovered from community‐acquired pneumonia as compared with the general Dutch population. Clin Microbiol Infect. 2011;17(5):763–8. 10.1111/j.1469-0691.2010.03296.x. [DOI] [PubMed]; Bruns A, Oosterheert JJ, Cucciolillo MC, Moussaoui RE, Groenwold R, Prins JM. et al. Cause‐specific long‐term mortality rates in patients recovered from community‐acquired pneumonia as compared with the general Dutch population. Clin Microbiol Infect. 2011;17(5):763–8. doi: 10.1111/j.1469-0691.2010.03296.x. [DOI] [PubMed] [Google Scholar]
- [13].WHO. WHO global tuberculosis report; 2019. https://www.who.int/tb/publications/global_report/en/; WHO. WHO global tuberculosis report. 2019. https://www.who.int/tb/publications/global_report/en/ [Google Scholar]
- [14].Alsdurf H, Hill PC, Matteelli A, Getahun H, Menzies D. The cascade of care in diagnosis and treatment of latent tuberculosis infection: a systematic review and meta-analysis. 2016;16(11):1269–78. 10.1016/S1473-3099(16)30216-X. [DOI] [PubMed]; Alsdurf H, Hill PC, Matteelli A, Getahun H, Menzies D. The cascade of care in diagnosis and treatment of latent tuberculosis infection: a systematic review and meta-analysis. 2016;16(11):1269–78. doi: 10.1016/S1473-3099(16)30216-X. [DOI] [PubMed] [Google Scholar]
- [15].Khawcharoenporn T, Apisarnthanarak A, Sungkanuparph S, Woeltje KF, Fraser VJ. Tuberculin skin test and isoniazid prophylaxis among health care workers in high tuberculosis prevalence areas. Int J Tuberc Lung Dis. 2011;15(1):14–23. [PubMed]; Khawcharoenporn T, Apisarnthanarak A, Sungkanuparph S, Woeltje KF, Fraser VJ. Tuberculin skin test and isoniazid prophylaxis among health care workers in high tuberculosis prevalence areas. Int J Tuberc Lung Dis. 2011;15(1):14–23. [PubMed] [Google Scholar]


