Abstract
Aim
To gain further insights into the efficacy of SAR425899, a dual glucagon‐like peptide‐1/glucagon receptor agonist, by providing direct comparison with the glucagon‐like peptide‐1 receptor agonist, liraglutide, in terms of key outcomes of glucose metabolism.
Research Design and Methods
Seventy overweight to obese subjects with type 2 diabetes (T2D) were randomized to receive once‐daily subcutaneous administrations of SAR425899 (0.12, 0.16 or 0.20 mg), liraglutide (1.80 mg) or placebo for 26 weeks. Mixed meal tolerance tests were conducted at baseline (BSL) and at the end of treatment (EOT). Metabolic indices of insulin action and secretion were assessed via Homeostasis Model Assessment (HOMA2) and oral minimal model (OMM) methods.
Results
From BSL to EOT (median [25th, 75th] percentile), HOMA2 quantified a significant improvement in basal insulin action in liraglutide (35% [21%, 74%]), while secretion enhanced both in SAR425899 (125% [63%, 228%]) and liraglutide (73% [43%, 147%]). OMM quantified, both in SAR425899 and liraglutide, a significant improvement in insulin sensitivity (203% [58%, 440%] and 36% [21%, 197%]), basal beta‐cell responsiveness (67% [34%, 112%] and 40% [16%, 59%]), and above‐basal beta‐cell responsiveness (139% [64%, 261%] and 69% [−15%, 120%]). A significant delay in glucose absorption was highlighted in SAR425899 (37% [52%,18%]).
Conclusions
SAR425899 and liraglutide improved postprandial glucose control in overweight to obese subjects with T2D. A significantly higher enhancement in beta‐cell function was shown by SAR425899 than liraglutide.
Keywords: beta‐cell function, disposition index, dual agonist, glucagon, glucagon‐like peptide‐1, insulin sensitivity, liraglutide, mixed meal tolerance test, oral minimal model
1. INTRODUCTION
Type 2 diabetes (T2D) is a complex metabolic disease characterized by concomitant insulin resistance and impaired beta‐cell function leading to chronic hyperglycaemia, which is the hallmark of the disease.1, 2 The increase in its prevalence is both rapid and progressive, with obesity being one of the most important risk factors associated with the development of the disease,3, 4, 5 while its long‐term complications are the major causes of morbidity, mortality and exceptional healthcare costs.6, 7
Treatment of T2D generally consists of a combination of changes in lifestyle, comprising diet and exercise, as well as pharmacological treatments. In particular, many oral antidiabetic drugs, such as metformin, sulphonylureas, sodium‐glucose co‐transporter‐2 inhibitors, as well as injectable drugs, including glucagon‐like peptide‐1 receptor agonists (GLP‐1 RAs) and insulin, have been developed, and their use is targeted on a patient‐by‐patient basis.8 Among the injectable drugs, liraglutide is a well‐known GLP‐1 RA, with once‐daily administration, and it was shown to be efficacious in weight loss and glycaemic control,9, 10 acting mainly by reducing appetite and caloric intake as well as by increasing insulin secretion.11
Recently, the combination of multiple agents has become another treatment option, but this raised some limitations because of possible interactions between drugs.12, 13 A promising pharmacological treatment is represented by the combination of glucagon‐like peptide‐1 and glucagon receptor agonists (GLP‐1/GCG RAs) in one compound, which were shown to be safe and effective in the treatment of T2D, also providing effective weight loss.14, 15, 16 Among these drugs,17 a novel dual GLP‐1/GCG RA, SAR425899, showed significant reductions in body weight (BW), fasting plasma glucose (FPG) and HbA1c after 4 weeks of treatment in overweight to obese patients with T2D.18 In a recent work,19 the mode of action of SAR425899 was investigated using the oral minimal model (OMM) method,20 and it was found that SAR425899 was able to improve postprandial glycaemic control by significantly enhancing beta‐cell function and slowing the glucose absorption rate.19 However, results reported in Visentin et al.19 were based on data from a 4‐week phase 1 study with a limited number of patients and no comparison with an active comparator was available.18
Here, the aim is to give further insights into the quantification of SAR425899 effects, and to compare it with the active comparator liraglutide in postprandial glucose control using data from a 26‐week phase 2b clinical study conducted in a larger overweight/obese T2D population.
2. RESEARCH DESIGN AND METHODS
2.1. Database and protocol
Two hundred and ninety‐six overweight/obese subjects with T2D were enrolled in a multicentre, randomized, double‐blind, placebo‐controlled, parallel‐group phase 2b study assessing the dose–response relationship of SAR425899 versus placebo or open‐label active comparator liraglutide (NCT02973321). Subjects were randomized to receive a once‐daily subcutaneous injection of placebo (PBO, N = 33), one of three dose regimens of SAR425899 (SAR 0.12 mg, N = 66; SAR 0.16 mg, N = 66; SAR 0.20 mg, N = 64), or 1.80 mg of liraglutide (Lira 1.80 mg, N = 67) for 26 weeks of treatment.
A subset of 75 subjects underwent two standardized mixed‐meal tolerance tests (MMTT), that is, at baseline (BSL, day −1) and at the end of treatment (EOT, week 26), with measurement samples available for both visits. The meal was consumed at time 0, within 15 minutes, consisting of 78 g of carbohydrates (600 kcal composed of 50%‐55% carbohydrates, 15%‐20% proteins and 25%‐30% fat), and was served 4 hours after the morning dose. During each MMTT, blood samples were drawn at time t = 0, 10, 20, 30, 60, 90, 120, 180 and 240 minutes, for measurement of plasma glucose, insulin and C‐peptide with standard techniques. A total of 70 subjects (mean ± SD: age = 56.1 ± 9.6 years, BW = 96.8 ± 17.5 kg [BSL] vs. 91.7 ± 16.8 kg [EOT], body mass index = 34.1 ± 5.1 kg/m2 [BSL] vs. 32.2 ± 4.8 kg/m2 [EOT], HbA1c = 8.2% ± 0.9% [BSL] vs. 6.6% ± 0.9% [EOT]) were eligible for the analysis (disaggregated subjects' characteristics are reported in Table S1). In particular, 5/75 subjects were excluded because of issues concerning data/protocol: four subjects, belonging to the SAR425899 cohorts, were not able to follow the programmed titration rule to achieve the final stable dose, while one subject, belonging to the SAR425899 0.16 mg cohort, had issues with glucose and insulin measurements at the EOT visit. Finally, of note, distribution among cohorts was not assessed as per protocol but according to stable dose after 8 weeks from BSL to EOT and corresponds to PBO (N = 7), SAR 0.12 mg (N = 21), SAR 0.16 mg (N = 15), SAR 0.20 mg (N = 10) and Lira 1.80 mg (N = 17). Only metformin was allowed as an additional glucose‐lowering agent and was kept stable throughout the study with a distribution among the cohorts as PBO (N = 5), overall SAR doses (N = 38) and liraglutide (N = 15). Plasma glucose, insulin and C‐peptide time courses are reported in Figure 1.
FIGURE 1.
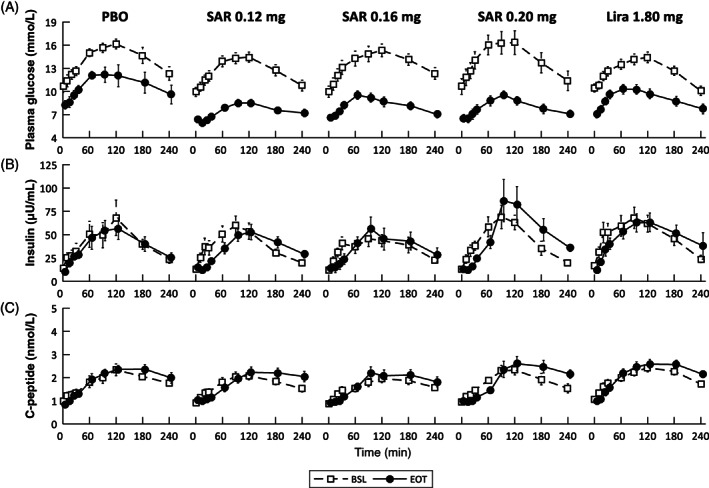
Mean ± standard error (SE) time courses of (A) plasma glucose, (B) insulin and (C) C‐peptide measured at day ‐1 (baseline [BSL], dashed line and white squares), and after 26 weeks (end of treatment [EOT], continuous line and black circles) of placebo (PBO), SAR425899 at 0.12 mg (SAR 0.12 mg), SAR425899 at 0.16 mg (SAR 0.16 mg), SAR425899 at 0.20 mg (SAR 0.20 mg) and liraglutide at 1.80 mg (Lira 1.80 mg) administration
2.2. Safety and tolerability
Adverse events (AEs) were assessed and reported throughout the study from screening to the follow‐up period. Safety outcomes comprised hypoglycaemia, physical examination, vital signs, ECG, safety laboratory values, Holter monitor and antidrug antibodies.
2.3. Calculations
Basal values and incremental area under the curve (iAUC) of postprandial plasma glucose (G), insulin (I) and C‐peptide (Cp) concentrations were calculated from the data using the trapezoidal rule. In particular, basal value was considered as the premeal concentration value, that is, at t = 0 minutes.
Models were used to assess glucose control in all cohorts. In particular, the Homeostasis Model Assessment (HOMA2)21 was used to assess insulin action and beta‐cell responsivity in basal (premeal) conditions (HOMA2 %S and HOMA2 %B, respectively) from FPG, insulin and C‐peptide data. The OMM method,20, 22, 23, 24 which combines the so‐called oral glucose23 and C‐peptide minimal models,24 was used to assess insulin action, beta‐cell function and gastrointestinal glucose absorption during the meal from postprandial glucose, insulin and C‐peptide data. Briefly, the oral glucose minimal model23 describes plasma glucose dynamics using the plasma insulin concentration and carbohydrate content of the meal as known inputs and provides an estimate of insulin sensitivity (SI), an outcome quantifying the ability of insulin to suppress endogenous glucose production and promote glucose disposal, as well as an estimate of the time profile of meal glucose rate of appearance (Ra).23 In particular, such outcomes are simultaneously estimated (SI and the time profile of Ra), thus allowing us to intrinsically account for any changes in the time profile of Ra on SI estimation. Note that Ra was estimated assuming that the meal was completely absorbed within 360 minutes, as performed in.19, 25 In addition, to better quantify the potential effect of the drug on meal glucose absorption, the AUC of model‐predicted Ra in the first 120 minutes after meal ingestion, normalized by the total orally absorbed glucose, was calculated (AUC [Ra0‐120]).
The C‐peptide minimal model24 describes the plasma C‐peptide concentration in relation to the observed changes in glucose concentration and provides an estimate of the overall beta‐cell responsivity to glucose (Φtot). Specifically, such an index is given by a combination of two components: the dynamic (Φdynamic) and static (Φstatic) responsivity indices. In particular, Φdynamic quantifies the secretion of promptly releasable insulin and is assumed to be stimulated by the rate of increase in glucose concentration, while Φstatic quantifies the delayed (by a time constant T) provision of new releasable insulin above a certain threshold level. In addition, to complete the picture of beta‐cell responsivity, the index of basal beta‐cell responsivity (Φb) was also calculated, from fasting plasma C‐peptide and glucose data, as the ratio of basal secretion per unit of basal glucose concentration.24
Finally, the disposition index (DI), defined as Φtot × SI, was calculated to evaluate beta‐cell function in light of the prevailing SI.26, 27 A more detailed description of the OMM method is provided in the supporting information.
2.4. Statistical analysis
Variables are reported as median [25th, 75th] percentile for each outcome, unless otherwise stated. Differences among visits (BSL vs. EOT) were assessed using a paired Student's t‐test, for normally distributed variables, or a Wilcoxon signed rank test otherwise. Based upon distributions, differences among cohorts were assessed using one‐way analysis of variance (ANOVA) or a Kruskal–Wallis test on the percentage deviation between BSL versus EOT values, that is, (EOT – BSL)/BSL, and post hoc analysis was performed using correction for multiple comparisons, that is, Tukey–Kramer for ANOVA or Dunn–Sidak for Kruskal–Wallis, respectively. Normality of distributions was assessed by the Lilliefors test. A P value of less than .05 was considered statistically significant. In case a zero value was found in BSL within the same cohort, the calculated percentage deviation for that index has been removed from the analysis.
All SAR425899 dose regimens tested in this study achieved similar effects on postprandial glucose, insulin and C‐peptide. Thus, an equivalence test28 was performed among SAR425899 doses on basal concentration values as well as above‐basal AUCs for glucose, insulin and C‐peptide data. In particular, for each outcome, we calculated the standard deviations in each SAR425899 dose group and the maximum was set as the equivalence margin, and the 90% confidence interval around the mean difference was calculated for each comparison (SAR 0.12 vs. SAR 0.16 mg, SAR 0.12 vs. SAR 0.20 mg, SAR 0.16 vs. SAR 0.20 mg). As shown in the Results section, the hypothesis of similarity among the SAR dose regimens was confirmed by our data, allowing us to group them together (as SAR cohort), thus increasing the statistical power both when comparing within (BSL vs. EOT visit) and between (SAR vs. PBO and Lira) cohorts. Nevertheless, all comparisons were also repeated regarding all SAR cohorts separately (see the Discussion section).
3. RESULTS
3.1. Plasma glucose, insulin and C‐peptide concentrations
The time course data of postprandial plasma glucose, insulin and C‐peptide concentrations are shown in Figure 1, while basal (at t = 0 minutes) concentrations and the iAUC of plasma glucose, insulin and C‐peptide excursions are reported in Table 1 for the PBO, SAR 0.12 mg, SAR 0.16 mg, SAR 0.20 mg and Lira 1.80 mg cohorts.
TABLE 1.
Basal concentration and incremental area under the curve (iAUC) of glucose, insulin and C‐peptide
| Cohort | |||||||
|---|---|---|---|---|---|---|---|
| Outcome | Visit | PBO | SAR 0.12 mg | SAR 0.16 mg | SAR 0.20 mg | Lira 1.80 mg | |
| (N = 7) | (N = 21) | (N = 15) | (N = 10) | (N = 17) | |||
| Gb [mmol/L] | BSL | 10.3 [9.8, 12.0] | 9.6 [8.0, 11.1] | 8.8 [7.8, 12.0] | 10.0 [8.0, 11.9] | 10.5 [8.5, 11.3] | |
| EOT | 9.0 [7.9, 9.2] | 5.8 [5.1, 6.5] | 6.6 [5.4, 7.4] | 6.3 [5.7, 6.9] | 6.5 [5.9, 8.1] | ||
| P value | .02 | <.001 | <.001 | .002 | <.001 | ||
| Ib [μU/mL] | BSL | 11.3 [8.4, 18.5] | 11.2 [7.0, 16.6] | 11.3 [6.7, 14.6] | 10.5 [10.2, 15.4] | 11.7 [8.1, 25.0] | |
| EOT | 9.8 [6.8, 12.2] | 11.2 [6.3, 18.0] | 9.9 [7.1, 15.3] | 11.0 [8.3, 17.6] | 11.3 [4.9, 17.7] | ||
| P value | NS | NS | NS | NS | .028 | ||
| Cpb [nmol/L] | BSL | 1.11 [0.81, 1.24] | 0.81 [0.63, 1.28] | 0.72 [0.64, 1.09] | 0.94 [0.77, 1.13] | 1.07 [0.76, 1.23] | |
| EOT | 0.87 [0.81, 0.95] | 0.89 [0.70, 1.33] | 0.85 [0.71, 1.07] | 0.82 [0.72, 1.27] | 0.88 [0.69, 1.20] | ||
| P value | NS | .041 | NS | NS | NS | ||
| iAUC (G) [102 mmol/L*min] | BSL | 8.2 [7.5, 9.0] | 7.5 [4.9, 1.03] | 9.7 [7.2, 1.06] | 9.3 [6.9, 1.23] | 5.7 [4.4, 7.1] | |
| EOT | 6.4 [2.9, 11.1] | 3.0 [1.8, 5.1] | 5.1 [2.0, 7.0] | 3.7 [2.5, 5.4] | 4.1 [2.4, 8.4] | ||
| P value | NS | <.001 | <.001 | .003 | NS | ||
| iAUC (I) [103 μU/mL*min] | BSL | 6.0 [3.8, 10.6] | 5.0 [3.3, 6.4] | 4.1 [3.3, 6.4] | 7.1 [4.4, 9.2] | 7.2 [3.7, 11.2] | |
| EOT | 6.2 [4.9, 8.3] | 4.0 [2.7, 7.5] | 4.3 [2.4, 9.2] | 8.9 [5.1, 10.8] | 6.9 [4.2, 13.2] | ||
| P value | NS | NS | NS | NS | NS | ||
| iAUC (Cp) [102 nmol/L*min] | BSL | 2.2 [2.1, 2.4] | 1.8 [1.6, 2.7] | 2.0 [1.2, 2.4] | 2.0 [1.3, 3.4] | 2.3 [1.6, 3.2] | |
| EOT | 2.6 [2.2, 3.5] | 1.8 [1.2, 2.4] | 2.1 [1.2, 2.7] | 2.5 [1.8, 3.3] | 2.9 [2.2, 3.8] | ||
| P value | NS | NS | NS | NS | NS | ||
Note: Median [25th, 75th] percentile of basal glucose, insulin and C‐peptide concentrations (Gb, Ib and Cpb, respectively) and iAUC of glucose (iAUC (G)), insulin (iAUC (I)) and C‐peptide (iAUC (Cp)) excursions. For each cohort and metric, a comparison was performed between baseline (BSL) versus end of treatment (EOT) visits based on outcomes' distribution: a paired t‐test for normally distributed values, otherwise a Wilcoxon signed‐rank test (P < .05 was considered statistically significant).
Glucose: Comparing BSL with EOT visits, basal glucose concentration (Gb) was significantly lower in the PBO, SAR 0.12 mg, SAR 0.16 mg, SAR 0.20 mg and Lira 1.80 mg cohorts, while iAUC (G) was only significantly reduced in the SAR 0.12, SAR 0.16 and SAR 0.20 mg cohorts at the EOT versus BSL visits.
Insulin: Comparing BSL with EOT visits, the Lira 1.80 mg cohort showed a reduction in basal insulin concentration (Ib) at EOT versus BSL visits, while no significant changes were found for all cohorts in iAUC (I).
C‐peptide: Comparing BSL with EOT visits, SAR 0.12 mg showed a slight, but significantly higher basal concentration (Cpb) at EOT versus BSL visits, while no significant changes were found in all cohorts in iAUC (Cp).
3.1.1. Pooling SAR cohorts
To assess differences among cohorts, the percentage deviations of both basal and iAUCs of glucose, insulin and C‐peptide were calculated. First, equivalence tests were performed on both basal concentration values as well as above‐basal AUCs of glucose, insulin and C‐peptide data. For each tested variable, results showed equivalence among all SAR cohorts. Therefore, we decided to group the SAR cohorts when comparing the SAR with the PBO and Lira cohorts. Then, one‐way ANOVA was performed to assess differences among the PBO, SAR and Lira cohorts, revealing statistically significant differences in iAUC (G) (P = .0013), Ib (P = .026) and Cpb (P = .016). In particular, post hoc analysis highlighted statistically significant differences between SAR versus Lira cohorts, with iAUC (G) significantly lower (−54% [−78%, −34%] vs. –20% [−40%, 31%]; P = .0012), while Ib (8% [−23%, 58%] vs. –23% [−37%, −11%]; P = .011) and Cpb (10% [−7%, 33%] vs. –11% [−18%, −5%]; P = .005) were significantly higher.
3.2. Insulin action, glucose absorption and beta‐cell responsivity
Figure 2 shows the mean ± standard error (SE) of HOMA2 indices of insulin action and beta‐cell responsivity (A and B, respectively), as well as OMM indices of insulin action (C), glucose absorption (D), beta‐cell responsivity indices (E and F) and DI (G), at BSL and EOT, for the PBO, SAR and Lira cohorts. The percentage deviation between EOT versus BSL visits for each outcome and cohort is reported in Figure 3.
FIGURE 2.
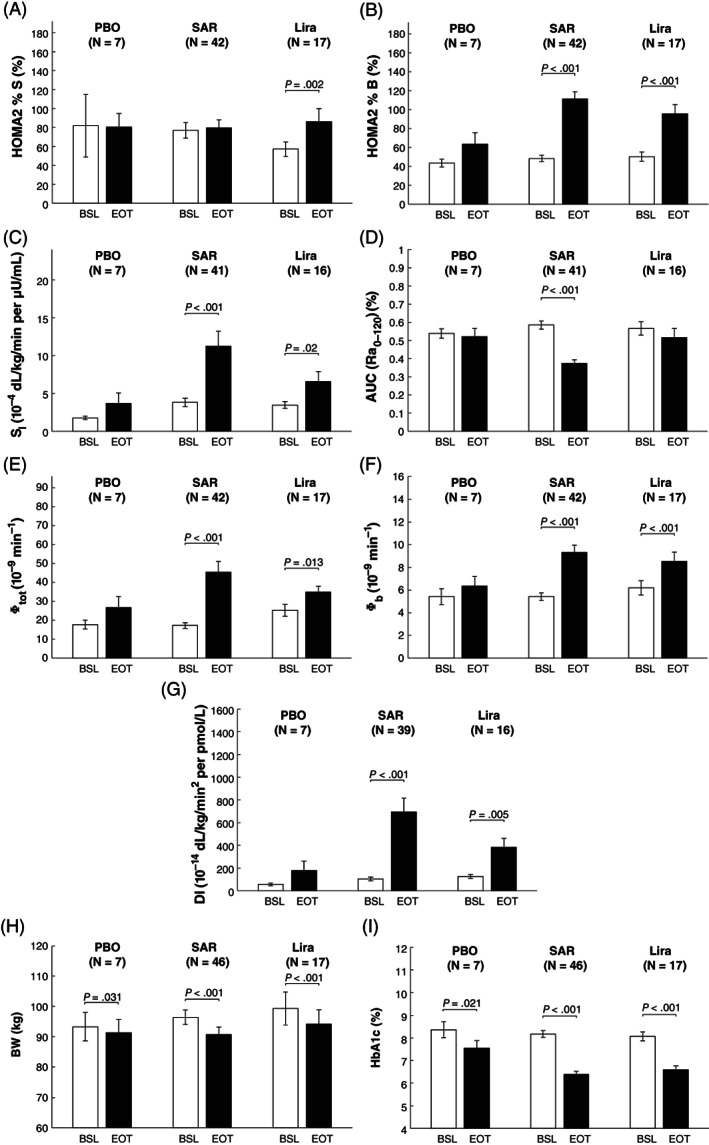
Mean ± standard error (SE) of Homeostasis Model Assessment (HOMA2) indices of insulin action (HOMA2 %S, panel A) and beta‐cell responsivity (HOMA2 %B, panel B), as well as Oral Minimal Model (OMM) indices of insulin sensitivity (SI, panel C), fractional glucose absorption 2 hours after meal ingestion (AUC(Ra0‐120), panel D), overall above basal and basal β‐cell responsiveness (Φtot, and Φb, panels E and F, respectively), disposition index (DI, panel G), together with body weight (BW, panel H) and HbA1c (panel I). Indices were calculated at day ‐1 (baseline [BSL], white bars) and after 26 weeks (end of treatment [EOT], black bars) of placebo (PBO), all SAR425899 dose regimens (SAR 0.12, 0.16 and 0.20 mg) and liraglutide (Lira) administration. Comparison between BSL vs. EOT was performed using paired T‐test, for normally distributed variables, and Wilcoxon Signed‐Rank test otherwise (P‐value <.05 was considered statistically significant). Of note, the number of subjects (N) may change among outcomes due to missing/unreliable samples limiting model outcome estimation
FIGURE 3.
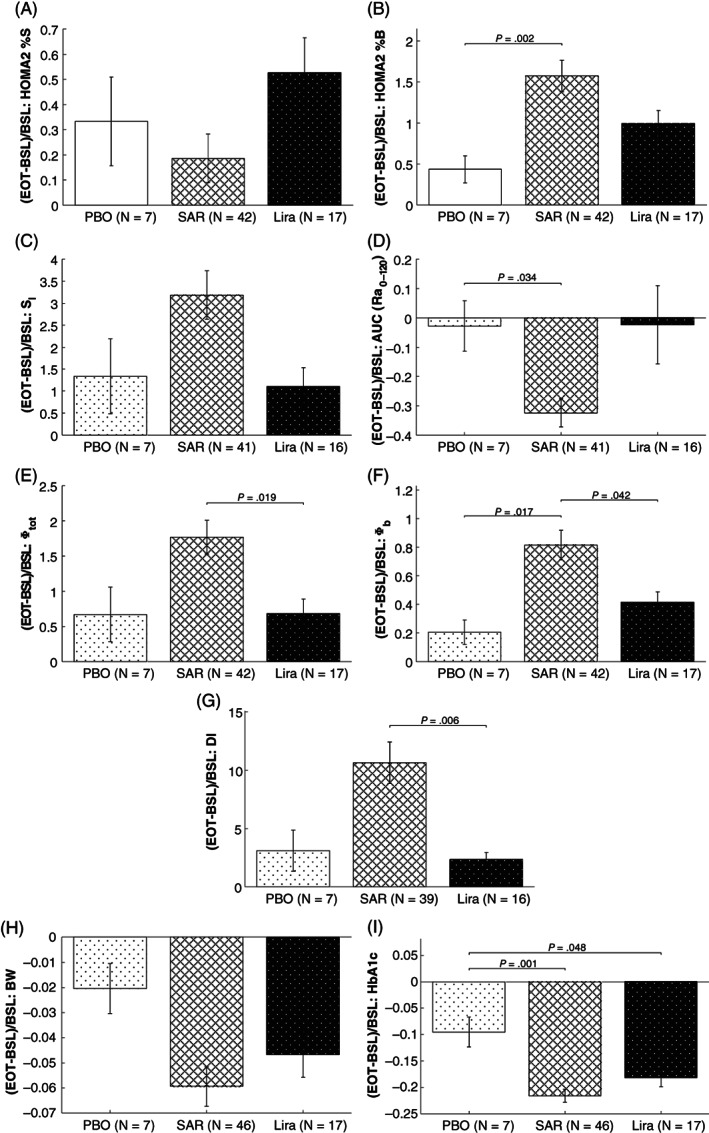
Percent deviation between BSL vs. EOT values, that is (EOT – BSL)/BSL, of Homeostasis Model Assessment (HOMA2) index of insulin action (HOMA2 %S, panel A) and beta‐cell responsivity (HOMA2 %B, panel B), as well as Oral Minimal Model (OMM) indices of insulin sensitivity (SI, panel C), fractional glucose absorption 2 hours after meal ingestion (AUC(Ra0‐120), panel D), overall above basal and basal β‐cell responsiveness (Φtot, and Φb, panels E and F, respectively), disposition index (DI, panel G), together with body weight (BW, panel H) and HbA1c (panel I). Indices were calculated for placebo (PBO, white with dots), all SAR425899 dose regimens (SAR 0.12, 0.16 and 0.20 mg, white with lines) and liraglutide (Lira, black with dots) cohorts. Comparison between cohorts was performed by one‐way analysis of variance (ANOVA) followed by post‐hoc analysis using Tukey‐Kramer correction for multiple comparisons, for normally distributed variables, and Kruskall‐Wallis test followed by post‐hoc analysis using Dunn‐Sidak correction for multiple comparisons otherwise (P‐value<.05 was considered statistically significant). Of note, the number of subjects (N) may change among outcomes due to missing/unreliable samples limiting model outcome estimation
3.2.1. Insulin action
Comparing BSL with EOT visits, from basal (premeal) data, a significant improvement in insulin action (HOMA2 %S) was only found in the Lira cohort (60% [25%, 77%] vs. 69% [41%, 146%]; P = .002), while no statistically significant difference was observed between visits in the PBO and SAR cohorts (Figure 2A). In contrast, from postprandial excursions data, a significant improvement in insulin sensitivity (SI) was found in the SAR (2.3 [1.5, 4.7] vs. 7.7 [4.3, 12.1] 10−4 dL/kg/min per μU/mL; P < .001) and Lira cohorts (3.1 [1.9, 4.9] vs. 4.9 [2.3, 9.6] 10−4 dL/kg/min per μU/mL; P = .02), while no statistically significant difference was observed between visits in the PBO cohort (Figure 2C). Percentage deviation in the PBO, SAR and Lira cohorts was 43% [37%, 72%], 4% [−31%, 47%] and 35% [21%, 74%] for HOMA2 %S, while 62% [−52%, 254%], 203% [58%, 440%] and 36% [21%, 197%] for SI, respectively (Figure 3A,C, respectively). ANOVA detected a statistically significant difference in the percentage deviation among cohorts in SI (P = .041) but post hoc analysis did not confirm this result.
3.2.2. Glucose absorption
The pattern of the meal glucose rate of appearance (Ra), estimated via the oral glucose minimal model,20, 23 is shown in Figure 4. SAR delayed meal glucose absorption from BSL to the EOT visit, as quantified by the fractional AUC (Ra0‐120) (59% [49%, 70%] to 39% [27%, 48%]; P < .001; Figure 2D). No statistically significant difference was found in both the PBO and Lira cohorts. Percentage deviation in fractional AUC (Ra0‐120) was −7% [−19%, 17%], −37% [−52%, −18%] and −18% [−39%, 15%] in the PBO, SAR and Lira cohorts, respectively (Figure 3D). According to ANOVA, the difference among cohorts was significant (P = .013) and post hoc analysis highlighted that this was a result of the significant difference between SAR versus PBO cohorts (−37% [−52%, −18%] vs. –7% [−19%, 17%]; P = .034) only.
FIGURE 4.
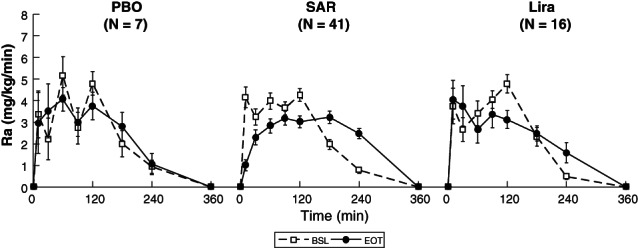
Mean ± standard error (SE) time courses of estimated meal glucose rate of appearance (Ra) at day ‐1 (baseline [BSL]) and after 26 weeks (end of treatment [EOT]) for placebo (PBO, left panel), SAR425899 (SAR, middle panel) and liraglutide (Lira, right panel) cohorts. Ra is assumed to be completely absorbed within 6 hours after meal ingestion. Of note, the number of subjects (N) may change among outcomes due to missing/unreliable samples limiting model parameter estimation
3.2.3. Beta‐cell responsivity
Comparing BSL with EOT visits, a significant improvement in basal beta‐cell responsivity was quantified in the SAR and Lira cohorts by both HOMA2 %B (Figure 2B; SAR: 44% [33.5%, 55%] vs. 106% [80%, 130%], P < .001, and Lira: 51% [31%, 64%] vs. 92% [66%, 115%], P < .001) and Φb (Figure 2F; SAR: 5.1 [4.0, 6.9] to 8.6 [6.5, 11.0] minutes−1, P < .001, and Lira: 5.5 [4.0, 8.3] to 8.3 [5.8, 11.6] minutes−1, P < .001), but not in the PBO cohort. Percentage deviation in the PBO, SAR and Lira cohorts was 42% [16%, 66%], 125% [63%, 228%] and 73% [43%, 147%] for HOMA2 %B, while 26%,20, 29 67% [34%, 112%] and 40% [16%, 59%] in Φb, respectively (Figure 3B,F, respectively). A statistically significant difference among cohorts was found by ANOVA both in HOMA2 %B (P = .0006), with post hoc analysis highlighting a statistically significant difference between PBO versus SAR only (42% [16%, 66%] vs. 125% [63%, 228%], P = .002), and in Φb (P = .003), with post hoc analysis highlighting a statistically significant difference of SAR (67% [34%, 112%]) against both PBO (26% [22%, 31%], P = .017) and Lira cohorts (40% [16%, 59%], P = .042).
Total above‐basal beta‐cell responsivity (Φtot) significantly increased from BSL to the EOT visit in both the SAR (15.6 [9.8, 23.7] to 33.7 [23.1, 55.9] 10−9 minutes−1, P < .001) and Lira (25.6 [15.9, 30.6] to 36.9 [26.7, 45.2] 10−9 minutes−1, P = .013) cohorts, while no statistically significant difference was observed in the PBO cohort (Figure 2E). Percentage deviation in Φtot was 31% (−13%, 161%), 139% (64%, 261%) and 69% (−15%, 120%) in the PBO, SAR and Lira cohorts, respectively (Figure 3E). ANOVA highlighted a statistically significant difference among cohorts (P = .009) and post hoc analysis found that this was because of a significant difference between SAR versus Lira cohorts (139% [64%, 261%] vs. 69% [−15%, 120%], P = .019).
3.2.4. Disposition index
The DI, as quantified by Φtot × SI, significantly increased from BSL to the EOT in both the SAR (58.5 [32.0, 159.2] to 429.1 [267.3, 1011.5] 10−14 dL/kg/minutes per pmol/L, P < .001) and Lira (115.5 [71.7, 180.0] to 252.5 [118.3, 512.9] 10−14 dL/kg/minutes per pmol/L, P = .005) cohorts, but not in the PBO group (Figure 2G). Percentage deviation in DI was 42% (−33%, 724%), 662% (177%, 1598%) and 154% (33%, 443%) in the PBO, SAR and Lira cohorts, respectively (Figure 3G). A statistically significant difference among cohorts was detected by ANOVA (P = .002) and post hoc analysis highlighted a statistically significant difference between SAR versus Lira cohorts (662% [177%, 1598%] vs. 154% [33%, 443%], P = .006).
3.3. Body weight
After 26 weeks of administration, within the same MMTT substudy data, a statistically significant reduction in BW was observed in both the SAR (92.9 [83.3, 112.1] vs. 89.7 [76.3, 102.2] kg, P < .001) and Lira (103.9 [83.1, 120.5] vs. 98.0 [81, 112.1] kg, P < .001) cohorts, and, despite being to a lower extent, also in the PBO cohort (89.7 [83.2, 103.9] vs. 87.4 [81.6, 97.7] kg, P = .031; Figure 2H). Percentage deviation in BW was −1% [−3%, 0%], −4% [−10%, −2%] and −5% [−8%, −3%] in the PBO, SAR and Lira cohorts, respectively, but no statistically significant difference among cohorts was found (Figure 3H).
3.4. HbA1c
After 26 weeks of administration, within the same MMTT substudy data, a statistically significant reduction in HbA1c was observed in both the SAR (8.1% [7.4%, 8.6%] vs. 6.2% [5.7%, 6.9%], P < .001) and Lira (8.0% [7.7%, 8.55] vs. 6.6% [6.0%, 7.0%], P < .001) cohorts, and, paralleling the reduction in BW, also to a lower extent in the PBO group (8.3% [7.6%, 9.3%] vs. 7.1% [6.9%, 8.4%], P = .021; Figure 2I). Percentage deviation in HbA1c was −7% [−15%, −6%], −22% [−28%, −16%] and −18% [−24%, −13%] in the PBO, SAR and Lira cohorts, respectively (Figure 3I). A statistically significant difference among cohorts was detected by ANOVA (P = .0014) and post hoc analysis highlighted a statistically significant difference between PBO (−7% [−15%, −6%]) versus SAR (−22% [−28%, −16%], P = .001) and Lira cohorts (−18% [−24%, −13%], P = .048).
3.5. Safety and tolerability
SAR425899 was generally well tolerated except for gastrointestinal AEs and lipase elevations (GLP‐1 class effect). Gastrointestinal AEs, that is, nausea and vomiting, and effects on increase in heart rate observed in this study were similar for Lira and SAR and comparable with known effects of the GLP‐1 class.
4. DISCUSSION
In this work, data from a 26‐week, phase 2b study in overweight/obese patients with T2D were used to quantify the effects of SAR425899 (SAR), a novel dual GLP‐1/GCG RA, on glycaemic control and to compare it with Lira 1.80 mg, a well‐known selective GLP‐1 RA, which is used for treatment of T2D. In particular, this drug has been developed for the treatment of overweight/obese patients with T2D based on the simultaneous activation of GLP‐1 and glucagon receptors that are expected to enhance weight loss. In fact, GLP‐1 is known to enhance satiety, with subsequent reduction in food intake, as well as beta‐cell secretion in a glucose‐dependent manner, suppressing glucagon secretion and slowing gastric emptying. In addition, glucagon also has an anoretic effect, suppresses food intake and increases energy expenditure.
By protocol, a single‐dose regimen was tested for Lira 1.80 mg, while three dose regimens were tested for SAR425899 (0.12, 0.16 and 0.20 mg). Firstly, data showed similar effects on postprandial glucose, insulin and C‐peptide for the three dose regimens of SAR425899. Therefore, we performed an equivalence test among the SAR dose regimens in our data in a model‐independent way, that is, using as metrics basal values as well as above‐basal AUCs of glucose, insulin and C‐peptide data. Results confirmed the hypothesis of equivalent effects for all SAR dose regimens in all the metrics. This allowed the grouping of all SAR dose regimens in one single SAR cohort with the advantage of increasing the statistical power when comparing within (BSL vs. EOT visit) and between (SAR vs. PBO and Lira) cohorts. Then, for both SAR and Lira, as well as the PBO cohort, model‐based quantification of basal insulin action and beta‐cell responsivity by means of HOMA methodology,21 as well as postprandial insulin action, gastrointestinal glucose absorption, together with beta‐cell function, by means of the OMM method,20 were performed.
Comparing BSL with EOT visits, the HOMA2 index of basal insulin action (HOMA2 %S) was only improved in the Lira cohort, while the HOMA2 index of basal beta‐cell responsivity (HOMA2 %B) was improved in the SAR and Lira cohorts, as its OMM counterpart Φb. In particular, the slight, but significant improvement in the HOMA2 %S index reflects the complementary reduction in both Gb and Ib within the Lira cohort (Table 1). This would suggest an effect of liraglutide also on basal insulin action, as reported in28 but not in30. Hence, further studies are needed to confirm such a finding and possibly clarify the mechanisms of drug action. OMM indices of insulin sensitivity (SI), basal (Φb) and above‐basal (Φtot) beta‐cell responsivity indices and DI, a measure for overall beta‐cell function in light of the prevailing insulin sensitivity, were significantly improved in both the SAR and Lira cohorts. In addition, as previously reported,19, 31 results showed a significant delay in glucose absorption (AUC (Ra0‐120)) in the SAR cohort, while this was not observed for liraglutide. It is worth noting that similar results would also be obtained if groups receiving different SAR doses were treated separately (results not shown). In addition, concerning the two indices of basal beta‐cell responsivity, that is HOMA2 %B and Φb, a good correlation was shown, with R = 0.89 and 0.86 for BSL and EOT visits, respectively. Finally, a significant reduction in BW, as well as in HbA1c, was observed in both the SAR and Lira cohorts, confirming previously reported results,10, 32 and, to a lesser extent, also in the PBO cohort. In addition, a statistically significant reduction in basal glucose concentration was also observed in all (including the PBO) cohorts (Table 1). Focusing on the PBO cohort, because no changes in diabetes therapy occurred during the study, this could be, at least in part, associated with the observed reduction in BW within this group (Figure 2). This may be related to a higher compliance of the subjects with standard diabetes therapy because of the participation in the study, but many other complementary effects could also have a role. Nevertheless, a comparison among cohorts was performed on the percentage deviation between BSL versus EOT visits to account for these differences.
Cross‐cohort comparisons indicated significantly improved OMM‐based beta‐cell responsivity and disposition indices for SAR versus Lira. Moreover, SAR significantly improved basal beta‐cell responsivity, for both OMM and HOMA2 indices, as well as delayed glucose absorption compared with PBO. Of note, in parallel with these results, HbA1c was significantly reduced in the SAR and Lira cohorts compared with the PBO cohort. No other outcomes were significantly different in the PBO versus the SAR or Lira cohorts. This could be, at least in part, attributed to both the low number of subjects (N = 7) and the high variability in the PBO cohort. Finally, if one repeated the analysis keeping the SAR cohorts separated by dose regimens, ANOVA still detected a statistically significant difference in the percentage deviation of glucose absorption (AUC (Ra0‐120)), basal and above‐basal beta‐cell responsivity and DI, together with HbA1c. However, post hoc analysis could only find a significantly higher DI in SAR 0.20 mg versus Lira, OMM basal beta‐cell responsivity in SAR 0.12 mg versus PBO, and the HbA1c of all SAR cohorts against PBO (results not shown). Again, the low number of subjects in each cohort and the higher correction for multiple comparisons might preclude the possibility of detecting more significant differences among groups.
The number of subjects and their unbalanced distribution among cohorts represents a limitation of the study, which could possibly affect the assessment of differences within and between cohorts. However, even with such limitations, significant differences between BSL versus EOT visits as well as in the percentage deviation among cohorts were highlighted. In addition, only one dose was used for the active comparator, potentially limiting the comparison. However, the dose selected for the active comparator, that is Lira 1.80 mg, is the one generally used as a standard treatment regimen for the population under study. It is important to point out that the modelling approach allows quantification of the overall effect on insulin secretion, hence because of both glucose and GLP‐1. To discriminate the different contributions on insulin secretion, for example, from glucose and GLP‐1, GLP‐1 data would be needed during the experiment. In addition, glucagon was not measured, which prevented an assessment of its contribution to glucose control. Such data were not available because the aim of this study was to assess the overall effect of the two compounds on postprandial glucose metabolism. Further studies are needed to assess the contribution of GLP‐1 action (or GLP‐1/glucagon) to glucose metabolism and insulin secretion after administration of the two compounds.
The OMM method provided useful insights into both the SAR425899 and liraglutide mechanisms of action in this population of overweight/obese patients with T2D. For instance, it highlighted that the significant improvement (BSL vs. EOT visits) in the overall beta‐cell responsivity index (Φtot), in both the SAR and Lira cohorts, was mainly driven by an increase in the static (Φstatic), rather than the dynamic (Φdynamic), component of beta‐cell responsiveness (Figure S1). This could be a result of their mechanisms of action and, especially for the SAR cohort, also because of the delayed gastrointestinal absorption, which perhaps limited the contribution of Φdynamic to Φtot.
In conclusion, here we present the first comparison between SAR425899, a dual GLP‐1/GCG RA, and liraglutide, a selective GLP‐1 RA, providing new insights into their mechanisms of action on postprandial glucose metabolism in overweight/obese patients with T2D. The dual GLP‐1/GCG RA agonist SAR425899 showed an improvement in postprandial glycaemic control versus the selective GLP‐1 RA active comparator liraglutide in terms of iAUC (G) (−54% [−78%, −34%] vs. –20% [−40%, 31%], P = .0012), at least within the dose ranges and population studied in this trial. In parallel, that is, by means of minimal model analysis, this improvement was confirmed by the higher increase in the DI in SAR versus Lira (662% [177%, 1598%] vs. 154% [33%, 443%], P = .006). This was shown to be mainly because of a significant enhancement of beta‐cell responsiveness in SAR versus the selective GLP‐1 RA liraglutide (139% [64%, 261%] vs. 69% [−15%, 120%], P = .019).
CONFLICT OF INTEREST
BG, TK and MR are Sanofi employees. No other potential conflicts of interest relevant to this article are reported.
AUTHOR CONTRIBUTIONS
All authors reviewed and approved the final version of the manuscript. MS performed the analysis, contributed to the discussion and wrote the manuscript. RV performed the analysis, contributed to the discussion and edited the manuscript. BG, MR and TK provided the data, contributed to the discussion and edited the manuscript. CC and CDM reviewed data analysis, contributed to results interpretation and edited the manuscript. CDM is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Supporting information
Appendix S1 Supporting Information
ACKNOWLEDGEMENTS
The authors wish to thank the SAR425899 project team for providing data and input to the analyses. This study was supported by Sanofi (NCT02973321) and MIUR (Italian Minister for Education) under the initiative ‘Departments of Excellence’ (Law 232/2016).
Schiavon M, Visentin R, Göbel B, et al. Improved postprandial glucose metabolism in type 2 diabetes by the dual glucagon‐like peptide‐1/glucagon receptor agonist SAR425899 in comparison with liraglutide. Diabetes Obes Metab. 2021;23:1795–1805. 10.1111/dom.14394
Funding information MIUR (Italian Minister for Education), Grant/Award Number: “Departments of Excellence” (Law 232/2016); Sanofi, Grant/Award Number: NCT02973321
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request
REFERENCES
- 1.Holt RIG, Cockram CS, Flyvbjerg A, Goldstein BJ. Textbook of Diabetes. 4th ed.Oxford, UK: Blackwell Publishing Ltd; 2010. [Google Scholar]
- 2.Stumvoll M, Goldstein BJ, van Haeften TW. Type 2 diabetes: principles of pathogenesis and therapy. Lancet. 2005;9467:1333‐1346. [DOI] [PubMed] [Google Scholar]
- 3.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047‐1053. [DOI] [PubMed] [Google Scholar]
- 4.Hossain P, Kawar B, El Nahas M. Obesity and diabetes in the developing world – a growing challenge. N Engl J Med. 2007;356:213‐215. [DOI] [PubMed] [Google Scholar]
- 5.Ma R, Chan J. Metabolic complications of obesity. In: Williams G, Fruhbeck G, eds. Obesity: Science to Practice. John Wiley & Sons Ltd; 2009:235‐270. [Google Scholar]
- 6.Guariguata L, Whiting DR, Hambleton I, Beagley J, Linnenkamp U, Shaw JE. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract. 2014;103:137‐149. [DOI] [PubMed] [Google Scholar]
- 7.Krolewski AS, Warram JH, Freire FB. Epidemiology of late diabetic complications. A basis for the development and evaluation of preventive programs. Endocrinol Metab Clin North Am. 1996;2:217‐242. [DOI] [PubMed] [Google Scholar]
- 8.Marín‐Peñalver JJ, Martín‐Timón I, Sevillano‐Collantes C, del Cañizo‐Gómez FJ. Update on the treatment of type 2 diabetes mellitus. World J Diabetes. 2016;7(17):354‐395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blonde L, Russell‐Jones D. The safety and efficacy of liraglutide with or without oral antidiabetic drug therapy in type 2 diabetes: an overview of the LEAD 1‐5 studies. Diabetes Obes Metab. 2009;11(suppl 3):26‐34. [DOI] [PubMed] [Google Scholar]
- 10.Davies JM, Bergenstal R, Bode B, et al. Efficacy of Liraglutide for weight loss among patients with type 2 diabetes: the SCALE diabetes randomized clinical trial. JAMA. 2015;315(7):687‐699. [DOI] [PubMed] [Google Scholar]
- 11.van Can J, Sloth B, Jensen CB, Flint A, Blaak EE, Saris WHM. Effects of the once‐daily GLP‐1 analog liraglutide on gastric emptying, glycemic parameters, appetite and energy metabolism in obese, non‐diabetic adults. Int J Obes. 2014;38(6):784‐793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Finan B, Clemmensen C, Muller TD. Emerging opportunities for the treatment of metabolic diseases: glucagon‐like peptide‐1 based multi‐agonists. Mol Cell Endocrinol. 2015;418(1):42‐54. [DOI] [PubMed] [Google Scholar]
- 13.Tschöp MH, Finan B, Clemmensen C, et al. Unimolecular polypharmacy for treatment of diabetes and obesity. Cell Metab. 2016;24(1):51‐62. [DOI] [PubMed] [Google Scholar]
- 14.Heppner KM, Perez‐Tilve D. GLP‐1 based therapeutics: simultaneously combating T2DM and obesity. Front Neurosci. 2015;9:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Habegger KM, Heppner KM, Geary N, Bartness TJ, DiMarchi RD, Tschöp MH. The metabolic actions of glucagon revisited. Nat Rev Endocrinol. 2010;6(12):689‐697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sanchez‐Garrido MA, Brandt SJ, Clemmensen C, Muller TD, DiMarchi RD, Tschöp MH. GLP‐1/glucagon receptor co‐agonism for treatment of obesity. Diabetologia. 2017;60(10):1851‐1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salem V, Izzi‐Engbeaya C, Coello C, et al. Glucagon increases energy expenditure independently of brown adipose tissue activation in humans. Diabetes Obes Metab. 2016;18:72‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tillner J, Posch MG, Wagner F, et al. A novel dual glucagon‐like peptide and glucagon receptor agonist SAR425899: results of randomized, placebo‐controlled first‐in‐human and first‐in‐patient trials. Diabetes Obes Metab. 2019;21(1):120‐128. [DOI] [PubMed] [Google Scholar]
- 19.Visentin R, Schiavon M, Göbel B, et al. Man. Dual glucagon‐like peptide‐1 receptor/glucagon receptor agonist SAR425899 improves beta‐cell function in type 2 diabetes. Diabetes Obes Metab. 2020;22:640‐647. [DOI] [PubMed] [Google Scholar]
- 20.Cobelli C, Dalla Man C, Toffolo G, Basu R, Vella A, Rizza R. The oral minimal model method. Diabetes. 2014;63(4):1203‐1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levy JC, Matthews DR, Hermans MP. Correct homeostasis model assessment (HOMA) evaluation uses the computer program. Diab Care. 1998;21(12):2191‐2192. [DOI] [PubMed] [Google Scholar]
- 22.Basu R, Dalla Man C, Campioni M, et al. Effects of age and sex on postprandial glucose metabolism differences in glucose turnover, insulin secretion, insulin action, and hepatic insulin extraction. Diabetes. 2006;55(7):2001‐2014. [DOI] [PubMed] [Google Scholar]
- 23.Dalla Man C, Caumo A, Cobelli C. The oral glucose minimal model: estimation of insulin sensitivity from a meal test. IEEE Trans Biomed Eng. 2002;49(5):419‐429. [DOI] [PubMed] [Google Scholar]
- 24.Breda E, Cavaghan K, Toffolo G, Polonsky KS, Cobelli C. Oral glucose tolerance test minimal model indexes of β‐cell function and insulin sensitivity. Diabetes. 2001;50:150‐158. [DOI] [PubMed] [Google Scholar]
- 25.Konopka AR, Esponda RR, Robinson MM, et al. Hyperglucagonemia mitigates the effect of metformin on glucose production in prediabetes. Cell Rep. 2016;15:1394‐1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bergman RN, Phillips LS, Cobelli C. Physiologic evaluation of factors controlling glucose tolerance in man: measurement of insulin sensitivity and beta‐cell glucose sensitivity from the response to intravenous glucose. J Clin Invest. 1981;68:1456‐1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cobelli C, Toffolo G, Dalla Man C, et al. Assessment of beta‐cell function in humans, simultaneously with insulin sensitivity and hepatic extraction, from intravenous and oral glucose tests. Am J Physiol Endocrinol Metab. 2007;293:E1‐E15. [DOI] [PubMed] [Google Scholar]
- 28.Walker E, Nowacki AS. Understanding equivalence and noninferiority testing. J Gen Intern Med. 2011;26(2):192‐196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Díaz‐Soto G, de Luis DA, Conde‐Vicente R, Izaola‐Jauregui O, Ramos C, Romero E. Beneficial effects of liraglutide on adipocytokines, insulin sensitivity parameters and cardiovascular risk biomarkers in patients with type 2 diabetes: a prospective study. Diabetes Res Clin Pract. 2014;104:92‐96. [DOI] [PubMed] [Google Scholar]
- 30.Anholm C, Kumarathurai P, Pedersen LR, et al. Liraglutide effects on beta‐cell, insulin sensitivity and glucose effectiveness in patients with stable coronary artery disease and newly diagnosed type 2 diabetes. Diabetes Obes Metab. 2017;19:850‐857. [DOI] [PubMed] [Google Scholar]
- 31.Kapitza C, Zdravkovic M, Hindsberger C, Flint A. The effect of the once‐daily human glucagon‐like petptide 1 analog Liraglutide on the pharmacokinetics of acetaminophen. Adv Ther. 2011;28(8):650‐660. [DOI] [PubMed] [Google Scholar]
- 32.Visentin R, Klabunde T, Man CD, Cobelli C. Modeling the effect of Liraglutide in type 2 diabetes mellitus with the type 2 diabetes mellitus simulator: a paradigm for in silico trials. J Diabetes Sci Technol. 2016;10(2):476‐611.28264166 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Supporting Information
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request


