Abstract
Background
Perihilar cholangiocarcinoma (pCCA) is a rare tumour that requires complex multidisciplinary management. All known data are almost exclusively derived from expert centres. This study aimed to analyse the outcomes of patients with pCCA in a nationwide cohort.
Methods
Data on all patients diagnosed with pCCA in the Netherlands between 2010 and 2018 were obtained from the Netherlands Cancer Registry. Data included type of hospital of diagnosis and the received treatment. Outcomes included the type of treatment and overall survival.
Results
A total of 2031 patients were included and the median overall survival for the overall cohort was 5.2 (95% CI 4.7‐5.7) months. Three‐hundred‐ten (15%) patients underwent surgical resection, 271 (13%) underwent palliative systemic treatment, 21 (1%) palliative local anti‐cancer treatment and 1429 (70%) underwent best supportive care. These treatments resulted in a median overall survival of 29.6 (95% CI 25.2‐34.0), 12.2 (95% CI 11.0‐13.3), 14.5 (95%CI 8.2‐20.8) and 2.9 (95% CI 2.6‐3.2) months respectively. Resection rate was 13% in patients who were diagnosed in non‐academic and 32% in academic centres (P < .001), which resulted in a survival difference in favour of academic centres. Median overall survival was 9.7 (95% CI 7.7‐11.7) months in academic centres compared to 4.9 (95% CI 4.3‐5.4) months in non‐academic centres (P < .001).
Conclusions
In patients with pCCA, resection rate and overall survival were higher for patients who were diagnosed in academic centres. These results show population‐based outcomes of pCCA and highlight the importance of regional collaboration in the treatment of these patients.
Keywords: cholangiocarcinoma, klatskin tumour, treatment outcome
Lay Summary/Key points.
In a nationwide analysis on 2031 patients with perihilar cholangiocarcinoma, median overall survival was poor with 5.2 (95% CI 4.7‐5.7) months. Surgical resection is the only curative treatment and was performed more frequently in patients diagnosed in academic hospitals (32%) compared to those in non‐academic centres 13%. Median overall survival was 29.6 (95% CI 25.2‐34.0) months in the 310 (15%) patients who underwent surgical resection, 12.2 (95% CI 11.0‐13.3) months in the 271 (13%) who underwent palliative systemic treatment, and 2.9 (95% CI 2.6‐3.2) months in the 1429 (70%) who underwent best supportive care only.
1. INTRODUCTION
Perihilar cholangiocarcinoma (pCCA) is a tumour of biliary origin that arises between the segmental bile ducts and the cystic duct. The anatomic location of the tumour usually leads to biliary obstruction and consequently most patients present with obstructive cholestasis and jaundice.1, 2 Without treatment, survival is poor with a median overall survival of 5‐10 months.3, 4, 5, 6 In the absence of metastases, complete resection of the extrahepatic bile duct, usually combined with major liver resection is the only curative treatment and chance for long‐term survival. However, most patients present with tumours that are unresectable because they are locally advanced or metastatic. Palliative systemic chemotherapy can offer some survival benefit in these patients.7, 8 In addition, liver transplantation can be an alternative curative option in selected patients.9, 10
Randomized clinical trials on pCCA are very rare, mostly because of the rarity of the disease. Consequently, the current treatment strategies and outcomes are derived from a few high volume centres with a special interest in the disease, where the outcomes are likely different from most other centres. In addition, the majority of literature is focused on patients who undergo a curative resection, who represent a minority of all patients diagnosed with pCCA. Reliable data on resection rates and the proportion of patients who receive systemic therapy are unavailable. Systemic therapy has only been part of standard treatment for unresectable cholangiocarcinoma since the completion of the ABC‐02 trial in 2010.11 In addition, real‐life data on survival outcomes of all patients with pCCA on a national level are limited.
In 2014, the Dutch Hepatocellular & Cholangiocarcinoma Group (DHCG) was initiated, one of its’ goals is to (further) improve treatment and outcomes of pCCA. As of 2020 the Dutch Foundation for Oncologic Collaboration (SONCOS) requires that all patients with pCCA are discussed in a regional multidisciplinary meeting in which sufficient experience with the disease is present and that the treatment of these patients should be centralized in a few experienced centres. These efforts are all aimed to improve care for pCCA, yet there are no reports on the outcomes for all patients diagnosed with pCCA.
The objective of this study was to analyse the treatment and outcomes of all patients registered with the diagnosis of pCCA at the nationwide cancer registry in the Netherlands.
2. MATERIALS AND METHODS
All patients registered with perihilar cholangiocarcinoma in the Netherlands Cancer Registry (NCR) between 2010 and 2018 were included. Perihilar cholangiocarcinoma was defined as a malignant lesion of biliary origin arising proximal to the cystic duct and distal to the segmental bile ducts, but the correct tumour origin may be hard to determine. Patients were identified for inclusion in the NCR through the national pathology archives (PALGA) and the hospital discharge register (HDR) and were verified in patient records in all Dutch hospitals by trained data clerks 9 months later when further data were gathered and coded. Tumour location is coded according to the International Classification of Diseases for Oncology (ICD‐O‐3) with cholangiocarcinoma being C24.0 and subdivided on the perihilar location as C24.4 (local code).12 The NCR data are considered to have a high degree of accuracy.13 The study protocol was evaluated and approved by the DHCG. The Institutional Medical Ethics committee of the Amsterdam University Medical Center waived the need for ethical approval and individual informed consent.
Patient characteristics included in the NCR were: age, gender, socioeconomic status and previous diagnosis of a malignancy. Socioeconomic state was coded by linking the patients’ postal code at the time of diagnosis to data from the Netherlands Institute for Social Research. The socioeconomic score was based on income, employment and education, and scores were divided into the tertiles low, middle and high. A higher score indicated higher income, employment and/or education. All malignancies except for basal cell carcinoma were defined as a previous malignancy. Tumour characteristics were cTNM stage (UICC‐TNM) and the location of metastases (ICD‐O‐3). Outcome data included the type of cancer treatment and survival data. The hospital of first diagnosis was defined as the type of hospital at which the patient first presented and was diagnosed (regular, teaching or academic hospital), irrespective of the hospital in which the patient underwent treatment. The Netherlands has eight academic hospitals. Teaching hospitals are the Dutch STZ hospitals, which are non‐academic teaching hospitals. The remaining hospitals were defined as regular. Follow‐up data were collected by linkage of the NCR with the Dutch civil municipal registry and was last updated on February 1st 2019. Patients who had unresectable tumours at surgical exploration were included in the best supportive care, palliative systemic therapy or other palliative anti‐cancer therapy group according to the treatment applied in these patients.
2.1. Statistical analysis
Categorical variables were presented as numbers with percentages and differences between variables were tested using either Fisher's exact or chi‐square tests. Continuous variables were presented as median with range and differences were tested using Kruskal‐Wallis tests. Survival and follow‐up data were presented as medians with 95% confidence intervals. Survival curves were generated according to the Kaplan‐Meier methods and differences between groups were tested using log‐rank tests. Statistical analyses were performed using SPSS Version 26 (IBM, Chicago, IL) and figures were generated using Graphpad Prism (Graphpad Inc, La Jolla, CA).
3. RESULTS
In the study period a total of 2036 patients were diagnosed with pCCA which resulted in a median annual incidence of 224 (range 196‐265). The incidence increased over time with a median of 212 cases over the years 2010‐2012 and 246 cases during 2016‐2018 (P =.032). These absolute Dutch incidences translate to 1.37 new diagnoses of pCCA per 100.000 inhabitants per year in the study period. Follow‐up data were missing for five patients, these were excluded for the analyses. Baseline characteristics are shown in Table 1.
TABLE 1.
Baseline characteristics of the entire cohort and for subgroups according to the received treatment
|
All N = 2031 |
Best supportive care N = 1429 |
Chemotherapy N = 271 |
Resection N = 310 |
Other palliative anti‐cancer therapy* N = 21 |
P value | |
|---|---|---|---|---|---|---|
| Age, years, median (IQR) | 72 (64‐80) | 76 (68‐82) | 63 (56‐70) | 67 (58‐73) | 66 (57‐71) | <.001 |
| ≥ 70 years old, n (%) | 1222 (60) | 1020 (71) | 70 (26) | 126 (41) | 6 (29) | <.001 |
| ≥ 75 years old, n (%) | 872 (43) | 786 (55) | 28 (10) | 56 (18) | 2 (10) | <.001 |
| Male sex, n (%) | 1057 (52) | 719 (50) | 138 (51) | 188 (61) | 12 (57) | .010 |
| Inclusion period, n (%) | .007 | |||||
| 2010‐2012 | 645 (32) | 487 (34) | 76 (28) | 75 (24) | 7 (33) | |
| 2013‐2015 | 638 (31) | 450 (32) | 86 (32) | 97 (31) | 5 (24) | |
| 2016‐2018 | 748 (37) | 492 (34) | 109 (40) | 138 (45) | 9 (43) | |
| Hospital of first diagnosis, n (%) | <.001 | |||||
| Regular | 744 (37) | 543 (38) | 96 (35) | 97 (31) | 8 (38) | |
| Teaching | 1040 (51) | 755 (53) | 139 (51) | 135 (44) | 11 (52) | |
| Academic | 245 (12) | 129 (9) | 36 (13) | 78 (25) | 2 (10) | |
| Socioeconomic status, n (%) | .398 | |||||
| ‐ Low | 643 (32) | 462 (32) | 81 (30) | 93 (30) | 7 (33) | |
| ‐ Middle | 801 (40) | 574 (40) | 99 (37) | 122 (39) | 6 (29) | |
| ‐ High | 586 (29) | 392 (28) | 91 (34) | 95 (31) | 8 (38) | |
| Previous malignancy, n (%) | 386 (19) | 276 (19) | 41 (15) | 64 (21) | 5 (24) | .194 |
| T‐stage, n (%) | <.001 | |||||
| cT1 | 138 (7) | 77 (5) | 10 (4) | 50 (16) | 1 (5) | |
| cT2 | 190 (9) | 108 (8) | 23 (9) | 35 (18) | 4 (19) | |
| cT3 | 349 (17) | 204 (14) | 66 (24) | 75 (24) | 4 (19) | |
| cT4 | 297 (15) | 209 (15) | 66 (24) | 14 (5) | 8 (38) | |
| cTx | 1057 (52) | 831 (58) | 106 (39) | 116 (36) | 4 (19) | |
| N stage, n (%) | <.001 | |||||
| cN0 | 937 (46) | 578 (40) | 107 (40) | 244 (79) | 8 (38) | |
| cN+ | 595 (29) | 409 (29) | 127(45) | 48 (16) | 11 (52) | |
| cNx | 500 (25) | 342 (31) | 37 (6) | 17 (6) | 2 (10) | |
| Metastases at presentation, n (%) | 729 (36) | 545 (38) | 171 (63) | 6 (2) | 7 (33) | <.001 |
Not included in the statistical testing due to the low number of patients.
Median follow‐up time was 61.1 (95%CI 53.8‐68.3) months. Median overall survival was 5.2 (95%CI 4.7‐5.7) months, with 1‐, 3‐ and 5‐year survival rates of 31%, 10% and 6% respectively. Overall survival was dependent of age, gender, the presence of metastases, treatment and socioeconomic status (Figure 1).
FIGURE 1.
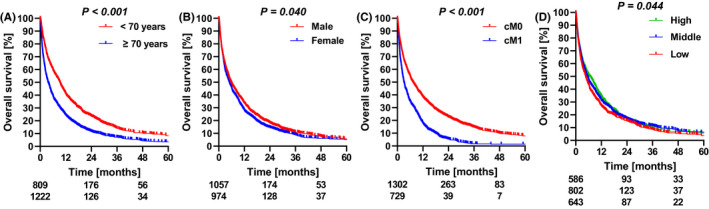
Overall survival according to (A) age, (B) gender, (C) metastasis at presentation and (D) socio‐economic state. Depicted below the graphs are the numbers at risk at 0, 24 and 48 months
3.1. Treatment and survival
Overall survival differed according to the type of treatment (Figure 2). In total, 479 (24%) patients underwent surgical exploration, of which 169 (35%) patients were unresectable. Surgical exploration was associated with a median overall survival of 20.8 (95% CI 18.0‐23.6) months and survival was 12.2 (95% CI 11.1‐13.2) months in the subgroup of patients with unresectable tumours. A curative‐intent resection was performed in 310 patients (15% of all patients) and median overall survival in these patients was 29.6 (95% CI 25.2‐34.0) months. 271 patients (13%) received any form of palliative systemic therapy which resulted in a median overall survival of 12.2 (95% CI 11.0‐13.3) months. Median overall survival in the 1429 patients (70%) who underwent only best supportive case was 2.9 (95% CI 2.6‐3.2) months. Resection was associated with a 5‐year overall survival rate of 27%, while patients who received systemic therapy or best supportive care had negligible 5‐survival rates, which were 1.8% and 1.6% respectively. The remaining 21 patients (1%) underwent other palliative anti‐cancer therapies, including irreversible electroporation (nine patients), photodynamic therapy (five patients), radio‐embolization (three patients), external beam radiotherapy (one patient), radio‐frequent ablation (one patient), and an unspecified treatment in the remaining two patients. Median overall survival was 14.5 (95% CI 8.2‐20.8) months in these 21 patients.
FIGURE 2.
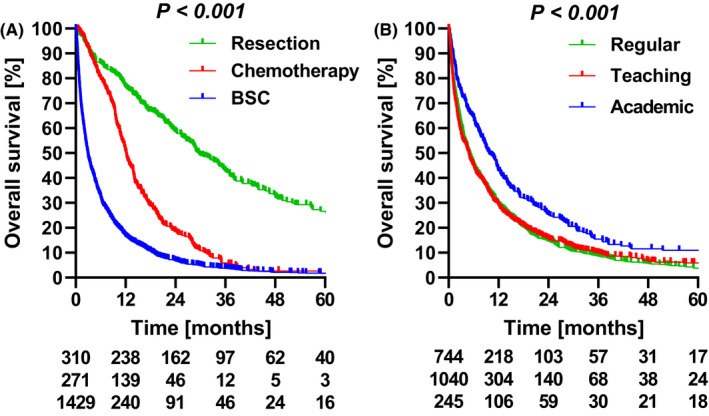
Overall survival according to (A) treatment and (B) hospital of diagnosis. Depicted below the graphs are the numbers at risk
3.2. Hospital of diagnosis
The hospital of first diagnosis was a general hospital for 744 patients (37%), a teaching hospital for 1040 patients (51%) and for 245 patients (12%) an academic medical center. The remaining two patients were diagnosed in a hospital outside of the Netherlands, and were not included in the subsequent analyses. Overall survival was similar between patients diagnosed in regular and teaching hospitals (P =.918), but was higher in patients diagnosed in an academic center (P <.001). Median overall survival was 9.7 (95%CI 7.7‐11.7) months in patients diagnosed with pCCA in academic centres compared to 4.9 (95%CI 4.3‐5.4) months in patients diagnosed in non‐academic centres (P <.001). Patients who were initially diagnosed in academic centres were younger and had higher socioeconomic status compared to patients in other hospitals (Table 2).
TABLE 2.
Baseline characteristics for subgroups according to the hospital of first diagnosis
|
Regular N = 744 |
Teaching N = 1040 |
Academic N = 245 |
P value | |
|---|---|---|---|---|
| Age, years, median (IQR) | 74 (66‐81) | 73 (64‐80) | 67 (58‐75) | <.001 |
| ≥ 70 years old, n (%) | 475 (64) | 635 (61) | 111 (45) | <.001 |
| ≥ 75 years old, n (%) | 363 (49) | 442 (43) | 67 (27) | <.001 |
| Male sex, n (%) | 391 (53) | 514 (49) | 151 (62) | .007 |
| Cancer treatment, n (%) | <.001 | |||
| Best supportive care | 543 (73) | 755 (73) | 129 (53) | |
| Chemotherapy | 96 (13) | 139 (13) | 36 (15) | |
| Resection | 97 (13) | 135 (13) | 78 (32) | |
| Other palliative anti‐cancer therapy | 8 (1) | 11 (1) | 2 (1) | |
| Socioeconomic status, n (%) | <.001 | |||
| Low | 223 (30) | 330 (32) | 89 (36) | |
| Middle | 332 (45) | 401 (39) | 67 (27) | |
| High | 189 (25) | 308 (30) | 89 (36) | |
| Previous malignancy, n (%) | 132 (18) | 206 (20) | 47 (19) | .546 |
| T‐stage, n (%) | <.001 | |||
| cT1 | 53 (7) | 62 (6) | 23 (9) | |
| cT2 | 58 (8) | 100 (10) | 32 (13) | |
| cT3 | 134 (18) | 167 (16) | 48 (20) | |
| cT4 | 86 (12) | 159 (15) | 52 (21) | |
| cTx | 413 (56) | 552 (53) | 90 (37) | |
| N stage, n (%) | .002 | |||
| cN0 | 328 (44) | 481 (46) | 128 (52) | |
| cN+ | 215 (29) | 297 (29) | 82 (34) | |
| cNx | 201 (27) | 262 (25) | 35 (14) | |
| Metastases at presentation, n (%) | 275 (37) | 370 (36) | 83 (34) | .796 |
The resection rate in patients who were first diagnosed in regular hospitals was 13% (97/744), and 13% (135/1040) in teaching hospitals (P =.473), while the resection rate was 32% (78/245) in academic centres (P <.001). In accordance with the resection rate, surgical exploration was performed more frequently, with 45% (110/245) in academic centres compared to 21% in regular and teaching hospitals (371/1784) (P <.001). The resection rate in all patients diagnosed in non‐academic centres increased from 10% (91/917) in 2010‐2014 to 16% (141/867) in 2015‐2018. However. the absolute difference in resection rate with that in patients diagnosed in academic centres remained similar, because the resection rate in all patients diagnosed in academic centres increased from 27% (40/140) to 36% (38/105).
After exclusion of all patients who underwent surgical resection, median overall survival remained higher in academic (7.2 months (95%CI 5.6‐8.8)) compared to non‐academic centres (3.7 months (95%CI 3.3‐4.1), P =.066). Of the patients diagnosed in non‐academic centres who did not undergo resection, 16% (254/1552) received any form of systemic chemotherapy, compared to 23% (38/167) of patients diagnosed in academic centres (P =.040). While the survival in the palliative chemotherapy group was similar between academic and non‐academic centres (12.5 (95%CI 9.9‐15.1) vs 12.2 (95%CI 10.8‐13.6) months, (P =.174), survival with best supportive care was higher in academic (4.7 (95%CI 2.9‐6.5) months) vs non‐academic centres (2.8 (95%CI 2.5‐3.1) months, (P =.012).
After adjustment for age, gender, socioeconomic status, cN category and metastases at presentation, a diagnosis at an academic medical center remained associated with a higher resection rate. Corrected for age, gender, socioeconomic status, cN category, metastases at presentation and treatment, the diagnosis at an academic medical center remained associated with improved overall survival (Table 3).
TABLE 3.
Multivariable analysis for resection rate and for overall survival
| Multivariable analysis | ||||
|---|---|---|---|---|
| Resection rate | Overall survival | |||
| Odds ratio (95% CI) | P value | Hazard ratio (95%CI) | P value | |
| Age, continuous | 0.92 (0.91‐0.94) | <.001 | 1.02 (1.01‐1.02) | <.001 |
| Male gender | 1.10 (0.82‐1.49) | .520 | 1.03 (0.94‐1.14) | .521 |
| Socioeconomic status | ||||
| Low | Reference | Reference | ||
| Middle | 1.05 (0.73‐1.50) | .799 | 0.86 (0.77‐0.96) | .010 |
| High | 1.00 (0.69‐1.48) | .969 | 0.83 (0.74‐0.94) | .003 |
| cN category | ||||
| cN0 | Reference | Reference | ||
| cN+ | 0.26 (0.17‐0.38) | <.001 | 1.19 (1.06‐1.33) | .004 |
| cNx | 0.24 (0.13‐0.41) | <.001 | 1.27 (1.11‐1.45) | <.001 |
| Metastases at presentation | 0.02 (0.01‐0.04) | <.001 | 1.72 (1.54‐1.91) | <.001 |
| Diagnosis at academic center | 2.26 (1.52‐3.36) | <.001 | 0.86 (0.74‐0.99) | .043 |
| Cancer treatment | ||||
| Best supportive care | Reference | |||
| Chemotherapy | 0.49 (0.42‐0.57) | <.001 | ||
| Resection | 0.29 (0.25‐0.35) | <.001 | ||
| Other palliative anti‐cancer therapy | 0.50 (0.31‐0.79) | .003 | ||
Median overall survival of patients who underwent a resection was similar for those diagnosed in academic centres (28.9 months (95%CI 18.4‐39.3) compared to those diagnosed in non‐academic centres (29.7 months (95%CI 24.5‐34.9), P =.845). The majority of resections were performed in academic centres (n = 292, 94%), 7 (2%) in non‐academic centres and 11 (4%) in hospitals outside of the Netherlands. Forty‐two patients (14%) died within 90 days after surgery. The majority of patients (n = 241, 78%) underwent major liver resection with bile duct resection which was associated with a 15% mortality rate. Another 16 patients (5%) underwent minor liver resection without any 90‐day mortality. Bile duct resection only was performed in 36 patients, (12%) of which two patients (6%) died within 90 days. Pancreatoduodenectomy in addition to bile‐duct resection was performed in 11 patients of whom two patients died within 90 days. The remaining five patients (2%) underwent an unspecified procedure without any reported 90‐day mortality. Age was an important factor in 90‐day mortality after surgery, patients aged 70 or older had a 19% (24/126) mortality and patients aged 75 or older a 25% (14/56) mortality rate, compared to 10% (18/184) in patients aged under 70.
In the group of 1429 patients who underwent best supportive care, 821 (57%) underwent biliary drainage which was associated with higher overall survival (3.6 (95%CI 3.2‐4.1) vs 2.1 (95%CI 1.8‐2.4) months, P <.001).
4. DISCUSSION
This is the first nationwide study that included all patients diagnosed with perihilar cholangiocarcinoma with as main result that the resection rate (overall 15%) was more than two‐fold higher in patients first diagnosed in academic centres vs non‐academic centres (32% vs 13%, adjusted OR 2.26). In the 2031 patients diagnosed in a 9‐year time span median overall survival was 5.2 months; in patients who underwent resection, received systemic chemotherapy and received best supportive care, median overall survival was 29.6, 12.2 and 2.9 months respectively. The overall survival benefit of patients diagnosed in academic centres can be partially attributed to the difference in resection rate between patients diagnosed in academic and non‐academic centres.
The 32% resection rate in patients first diagnosed in academic centres is similar to previously published resection rates in series from experienced centres that range from 30% to 34%.4, 14 The overall resection rate of 15% is lower than usually reported, however, no other nationwide series including all patients are currently available for comparison. Most series include only patients referred as candidate for surgery and therefore usually do not include patients with metastatic disease, patients with rapidly progressive disease, and patients who do not wish to receive any anti‐cancer treatment. Therefore, it is likely the resection rates of perihilar cholangiocarcinoma are usually overestimated.
The more than twofold higher resection rate in patients diagnosed in academic centres compared to non‐academic centres suggests that some patients were eligible but not considered and not referred for surgery. A previous study in an tertiary referral center already observed that the assessment of resectability at the referring center was not always accurate.15 The study found that one‐third of patients referred with initially presumed unresectable disease were considered resectable at the tertiary center. However, since the study only included referred patients the problem was likely underestimated, which is demonstrated by the difference in resection rate in the present nationwide study. The rarity of the disease and differences in work‐up and staging across centres in the absence of uniform resection criteria inevitably leads to difficulties in the diagnostic process and selection for the most appropriate treatment.16 With 1427 registered radiologists and 631 gastroenterologist in the Netherlands, the number of patients with perihilar cholangiocarcinoma, most of the physicians encounter less than1 patient every 2 years. Although this study did not include data on the number of centres that perform PHC surgery, a previous Dutch nationwide study on liver surgery reported that 14 centres performed these resections.17 Considering the 310 resections performed over 9 years in this study, the number of pCCA per center per year is estimated at 2 to 3 procedures. Since there are a few Dutch centres that report an annual higher volume of pCCA resections,18, 19 the number of resections in some centres is likely much less that the annual 2 to 3. Mortality rate within 90‐days after surgery was 14% in this nationwide study, which corresponds to the mortality reported in series from experienced Western centres.19, 20 Since at least 100 of the 310 resections were performed at a single center with a mortality rate around 9%,20 mortality rates are consequently higher in some other centres. Although there is no data to support that outcomes after resection improve with increasing hospital volume specific to pCCA, there is supporting data from overall liver resection cohorts and from liver transplantation for pCCA.21, 22, 23
The high mortality after resection, especially in elderly patients is in line with previous literature.24 However, expert centres have shown that surgery for pCCA can be performed with acceptable mortality and survival.25, 26 Therefore, age itself should not be a contraindication for surgery, despite the 25% mortality rate in patients aged 75 or older in this study. Patient selection is likely key, especially in older patients and care must be taken to reduce other factors that are associated with adverse outcomes, such as biliary drainage and preoperative remnant liver volume modulation.20, 24, 27, 28 In addition, the extent of surgery should be kept as minimal as possible.25, 26
Implementation of multidisciplinary meetings did exist in the study period, and as of 2020 discussion of all patients with pCCA in these meetings is mandatory according to a new Dutch guideline. Discussion of these patients with regional experts in might increase the accuracy of staging, currently about half of all patients who undergo explorative surgery are found to have unresectable disease.18, 29, 30 Regional multidisciplinary meetings might help to increase the number of patients referred for curative resection, but also help select patients that might be eligible for palliative systemic therapy or other experimental palliative local therapies. Further centralization of the resection for pCCA that are currently performed across 14 centres to a handful of hospitals might help to decease the 90‐day mortality rate. Overall survival rate among resected patients did not differ between patients diagnosed in academic vs those diagnosed in non‐academic centres. Although this result is encouraging, it is likely resected patients who were diagnosed in non‐academic centres have less advanced disease, and these patients have withstood a ‘test of time’ because of the time that is often associated with a tertiary referral. These factors are likely associated with improved survival. In contrast, patients diagnosed at an academic centre were more likely to undergo resection and therefore are very likely to have more advanced disease. This is likely associated with impaired survival. The exact interplay of these factors could be only partially addressed in this study, and while there was no survival difference, there might be differences in patient and disease characteristics that were not recorded in this study.
In this study, 271 (13%) underwent palliative systemic therapy. Patients without resection were more likely to undergo systemic therapy in academic centres compared to non‐academic centres (23% vs 16%). As a result of the higher resection rate in academic centres, the patients receiving systemic therapy in academic centres likely have more extensive disease compared to non‐academic centres. Nevertheless overall survival in these patients was similar. Although the proportion of patients receiving chemotherapy increased during the study period, the proportion of patients that receive chemotherapy seems low, which might be caused by a reluctance to administer chemotherapy in these patients who usually have some liver dysfunction and hyperbilirubinemia.31, 32, 33 Chemotherapy trials usually exclude patients with liver dysfunction, some chemotherapeutic agents are not recommended in case of liver dysfunction, some require dose‐adjustment, and some can cause additional liver dysfunction, which all can cause reluctance to administer chemotherapy to patients with pCCA.34 Data from the ABC‐02 trial showed that chemotherapy is safe and feasible in jaundiced patients with a good performance score.35 Also second‐line treatment has been established 36, 37 and several trials are currently investigating additional first‐line regimens.38 Regional multidisciplinary meeting could also help to select patients for palliative systemic therapy, as well as help to improve supportive care. Patients with a diagnosis in an academic center that underwent supportive care had higher survival rates compared to those diagnosed in non‐academic centres. Although data that can explain this difference is lacking, it is likely drainage strategies at least can be partially attributed.39, 40, 41, 42 Also the available expertise on treatment of biliary and periampullary tumours present in academic centres, often with dedicated physicians with regular multidisciplinary meetings is likely beneficiary for every aspect of pCCA treatment, including best supportive care. Regional meetings could help non‐academic centre to access this expertise in order toenhance knowledge on pCCA treatment and to improve supportive care.
This study has some limitations that mainly reflect the retrospective study design. However, this nationwide cohort including all patients diagnosed with pCCA from the Netherlands Cancer Registry is likely more reflective of the real‐life outcomes compared to expert series and the follow‐up data are very complete because of the linkage with the municipal registry. Nevertheless the number of variables available in the registry is limited and therefore in depth analyses were not possible. Therefore, the survival difference in favour of patients diagnosed in academic centres could in part be because of selection bias. For instance, well‐educated patients without comorbidity might be more likely to present at an academic center when suspected for pCCA, who likely have a more favourable prognosis. Furthermore, misclassification of cholangiocarcinoma is quite common and therefore could also have occurred in the present data.38 Finally, a liver transplantation program for pCCA has been available in the Netherlands as of 2011 and a relatively small number of transplants have been performed for pCCA.9 In this database, no patients were transplanted indicating these patients were classified as resections or the tumours were misclassified.
Despite an increase in resection rate over the study years, the difference in resection rate remains substantial between patients diagnosed in academic and non‐academic centres. Awareness of cholangiocarcinoma is increasing, with many national, European and global initiatives to increase the knowledge of the disease and its treatment options, and in order to facilitate research initiatives for this rare disease entity.38 Many initiatives were started during the study period, but the results demonstrate that further increasing awareness might directly benefit some patients. The results illustrate that all initiatives should be employed in all hospitals in which these patients can present.
In conclusion, this nationwide study on the outcomes of pCCA highlights a persistent difference in resection rate across patients diagnosed in academic and non‐academic centres, as well as differences associated with palliative treatment. These results illustrate that the required multidisciplinary expertise and awareness on this rare disease is not sufficiently available across all hospitals and warrants both further regional collaboration and centralization of management of patients with pCCA.
CONFLICTS OF INTEREST
The authors report no relevant funding and no conflicts of interest. The need for ethical approval and individual informed consent was waived by the ethics committee. Permission to reproduce material from other sources is not applicable.
ACKNOWLEDGEMENTS
The authors report no acknowledgements
van Keulen A‐M, Franssen S, van der Geest LG, et al. Nationwide treatment and outcomes of perihilar cholangiocarcinoma. Liver Int. 2021;41:1945–1953. 10.1111/liv.14856
Handling Editor: Pierre Nahon
REFERENCES
- 1.Rizvi S, Gores GJ. Pathogenesis, diagnosis, and management of cholangiocarcinoma. Gastroenterology. 2013;145(6):1215‐1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Razumilava N, Gores GJ. Cholangiocarcinoma. Lancet. 2014;383(9935):2168‐2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liberato MJ, Canena JM. Endoscopic stenting for hilar cholangiocarcinoma: efficacy of unilateral and bilateral placement of plastic and metal stents in a retrospective review of 480 patients. BMC Gastroenterol. 2012;12:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jarnagin WR, Fong Y, DeMatteo RP, et al. Staging, resectability, and outcome in 225 patients with hilar cholangiocarcinoma. Ann Surg. 2001;234(4):507–517; discussion 17–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nordback IH, Pitt HA, Coleman J, et al. Unresectable hilar cholangiocarcinoma: percutaneous versus operative palliation. Surgery. 1994;115(5):597‐603. [PubMed] [Google Scholar]
- 6.Gaspersz MP, Buettner S, van Vugt JLA, et al. Conditional survival in patients with unresectable perihilar cholangiocarcinoma. HPB (Oxford). 2017;19(11):966‐971. [DOI] [PubMed] [Google Scholar]
- 7.Valle JW, Borbath I, Khan SA, et al. Biliary cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow‐up. Ann Oncol. 2016;27(suppl 5):v28‐v37. [DOI] [PubMed] [Google Scholar]
- 8.Khan SA, Davidson BR, Goldin RD, et al. Guidelines for the diagnosis and treatment of cholangiocarcinoma: an update. Gut. 2012;61(12):1657‐1669. [DOI] [PubMed] [Google Scholar]
- 9.Vugts J, Gaspersz M, Roos E, et al. Eligibility for liver transplantation in patients with perihilar cholangiocarcinoma. Ann Surg Oncol. 2020;46(2):e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Darwish Murad S, Kim WR, Harnois DM, et al. Efficacy of neoadjuvant chemoradiation, followed by liver transplantation, for perihilar cholangiocarcinoma at 12 US Centers. Gastroenterology. 2012;143(1):88–98.e3; quiz e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362(14):1273‐1281. [DOI] [PubMed] [Google Scholar]
- 12.Fritz A, Percy C, Jack A, Shanmugaratnam K, Sobin LH, Maxwell Parkin D, Whelan SL; World Health Organization . International classification of diseases for oncology; 2000. [Google Scholar]
- 13.Schouten LJ, Hoppener P, van den Brandt PA, Knottnerus JA, Jager JJ. Completeness of cancer registration in Limburg. The Netherlands. Int J Epidemiol. 1993;22(3):369‐376. [DOI] [PubMed] [Google Scholar]
- 14.Chaiteerakij R, Harmsen WS, Marrero CR, et al. A new clinically based staging system for perihilar cholangiocarcinoma. Am J Gastroenterol. 2014;109(12):1881‐1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coelen RJS, Huiskens J, Olthof PB, et al. Compliance with evidence‐based multidisciplinary guidelines on perihilar cholangiocarcinoma. United European Gastroenterol J. 2017;5(4):519‐526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chaudhary RJ, Higuchi R, Nagino M, et al. Survey of preoperative management protocol for perihilar cholangiocarcinoma at 10 Japanese high‐volume centers with a combined experience of 2,778 cases. J Hepatobiliary Pancreat Sci. 2019;26(11):490‐502. [DOI] [PubMed] [Google Scholar]
- 17.Olthof PB, Elfrink AKE, Marra E, et al. Volume‐outcome relationship of liver surgery: a nationwide analysis. Br J Surg. 2020;107(7):917–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gaspersz MP, Buettner S, Roos E, et al. A preoperative prognostic model to predict surgical success in patients with perihilar cholangiocarcinoma. J Surg Oncol. 2018;118(3):469‐476. [DOI] [PubMed] [Google Scholar]
- 19.Franken LC, Schreuder AM, Roos E, et al. Morbidity and mortality after major liver resection in patients with perihilar cholangiocarcinoma: a systematic review and meta‐analysis. Surgery. 2019;165(5):918‐928. [DOI] [PubMed] [Google Scholar]
- 20.Franken LC, Rassam F, van Lienden KP, et al. Effect of structured use of preoperative portal vein embolization on outcomes after liver resection of perihilar cholangiocarcinoma. BJS Open. 2020;4(3):449–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Filmann N, Walter D, Schadde E, et al. Mortality after liver surgery in Germany. Br J Surg. 2019;106(11):1523‐1529. [DOI] [PubMed] [Google Scholar]
- 22.Farges O, Goutte N, Bendersky N, Falissard B, Group AC‐FHS . Incidence and risks of liver resection: an all‐inclusive French nationwide study. Ann Surg. 2012;256(5):697–704;discussion ‐5. [DOI] [PubMed] [Google Scholar]
- 23.Kitajima T, Hibi T, Moonka D, Sapisochin G, Abouljoud MS, Nagai S. Center experience affects liver transplant outcomes in patients with hilar cholangiocarcinoma. Ann Surg Oncol. 2020;27(13):5209‐5221. [DOI] [PubMed] [Google Scholar]
- 24.Wiggers JK, Groot Koerkamp B, Cieslak KP, et al. Postoperative mortality after liver resection for perihilar cholangiocarcinoma: development of a risk score and importance of biliary drainage of the future liver remnant. J Am Coll Surg. 2016;223(2):321‐31 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takahashi Y, Ebata T, Yokoyama Y, Igami T, Sugawara G, Nagino M. Surgical treatment of perihilar cholangiocarcinoma in octogenarians: a single center experience. J Hepatobiliary Pancreat Sci. 2013;20(3):324‐331. [DOI] [PubMed] [Google Scholar]
- 26.Akashi K, Ebata T, Mizuno T, et al. Surgery for perihilar cholangiocarcinoma from a viewpoint of age: Is it beneficial to octogenarians in an aging society? Surgery. 2018;164(5):1023‐1029. [DOI] [PubMed] [Google Scholar]
- 27.Olthof PB, Wiggers JK, Groot Koerkamp B, et al. Postoperative liver failure risk score: identifying patients with resectable perihilar cholangiocarcinoma who can benefit from portal vein embolization. J Am Coll Surg. 2017;225(3):387‐394. [DOI] [PubMed] [Google Scholar]
- 28.Olthof PB, Aldrighetti L, Alikhanov R, et al. Portal vein embolization is associated with reduced liver failure and mortality in high‐risk resections for perihilar cholangiocarcinoma. Ann Surg Oncol. 2020;27(7):2311‐2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matsuo K, Rocha FG, Ito K, et al. The Blumgart preoperative staging system for hilar cholangiocarcinoma: analysis of resectability and outcomes in 380 patients. J Am Coll Surg. 2012;215(3):343‐355. [DOI] [PubMed] [Google Scholar]
- 30.Coelen RJS, Ruys AT, Wiggers JK, et al. Development of a risk score to predict detection of metastasized or locally advanced perihilar cholangiocarcinoma at staging laparoscopy. Ann Surg Oncol. 2016;23(Suppl 5):904‐910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Venook AP, Egorin MJ, Rosner GL, et al. Phase I and pharmacokinetic trial of gemcitabine in patients with hepatic or renal dysfunction: cancer and Leukemia Group B 9565. J Clin Oncol. 2000;18(14):2780‐2787. [DOI] [PubMed] [Google Scholar]
- 32.Donelli MG, Zucchetti M, Munzone E, D'Incalci M, Crosignani A. Pharmacokinetics of anticancer agents in patients with impaired liver function. Eur J Cancer. 1998;34(1):33‐46. [DOI] [PubMed] [Google Scholar]
- 33.Eklund JW, Trifilio S, Mulcahy MF. Chemotherapy dosing in the setting of liver dysfunction. Oncology (Williston Park). 2005;19(8):1057‐1063; discussion 63‐4, 69. [PubMed] [Google Scholar]
- 34.Gong J, Cho M, Fakih M. Chemotherapy in patients with hepatobiliary cancers and abnormal hepatic function. J Gastrointest Oncol. 2017;8(2):314‐323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lamarca A, Benafif S, Ross P, Bridgewater J, Valle JW. Cisplatin and gemcitabine in patients with advanced biliary tract cancer (ABC) and persistent jaundice despite optimal stenting: effective intervention in patients with luminal disease. Eur J Cancer. 2015;51(13):1694‐1703. [DOI] [PubMed] [Google Scholar]
- 36.Rizzo A, Ricci AD, Tober N, et al. Second‐line treatment in advanced biliary tract cancer: today and tomorrow. Anticancer Res. 2020;40(6):3013‐3030. [DOI] [PubMed] [Google Scholar]
- 37.Lamarca A, Palmer DH, Wasan HS, et al. ABC‐06 | A randomised phase III, multi‐centre, open‐label study of active symptom control (ASC) alone or ASC with oxaliplatin / 5‐FU chemotherapy (ASC+mFOLFOX) for patients (pts) with locally advanced / metastatic biliary tract cancers (ABC) previously‐treated with cisplatin/gemcitabine (CisGem) chemotherapy. J Clin Oncol. 2019;37(15_suppl):4003. [Google Scholar]
- 38.Banales JM, Marin JJG, Lamarca A, et al. Cholangiocarcinoma 2020: the next horizon in mechanisms and management. Nat Rev Gastroenterol Hepatol. 2020;17(9):557‐588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Abraham NS, Barkun JS, Barkun AN. Palliation of malignant biliary obstruction: a prospective trial examining impact on quality of life. Gastrointest Endosc. 2002;56(6):835‐841. [DOI] [PubMed] [Google Scholar]
- 40.Arvanitakis M, Van Laethem JL, Pouzere S, Le Moine O, Deviere J. Predictive factors for survival in patients with inoperable Klatskin tumors. Hepatogastroenterology. 2006;53(67):21‐27. [PubMed] [Google Scholar]
- 41.Iwasaki A, Kubota K, Kurita Y, et al. The placement of multiple plastic stents still has important roles in candidates for chemotherapy for unresectable perihilar cholangiocarcinoma. J Hepatobiliary Pancreat Sci. 2020;27(10):700‐711. [DOI] [PubMed] [Google Scholar]
- 42.Park J, Kim M‐H, Kim K‐P, et al. Natural history and prognostic factors of advanced cholangiocarcinoma without surgery, chemotherapy, or radiotherapy: a large‐scale observational study. Gut Liv. 2009;3(4):298‐305. [DOI] [PMC free article] [PubMed] [Google Scholar]


