Abstract
The prenatal developmental toxicity potential of alkyl dimethyl benzyl ammonium chloride (ADBAC) and didecyl dimethyl ammonium chloride (DDAC) was evaluated in regulatory‐compliant studies. Pregnant female CD rats (25/group) and New Zealand White rabbits (16/group) were administered ADBAC (0, 10, 30, or 100 mg/kg/day and 0, 1, 3, or 9 mg/kg/day, respectively), or DDAC (0, 1, 10, or 20 mg/kg/day and 0, 1, 3, or 10 mg/kg/day, respectively), by oral gavage on gestation days (GD) 6–15 for rats and GD 6–18 for rabbits. At scheduled termination (GD 21 for rats; GD 29 for rabbits), maternal necropsies were conducted and live fetuses were weighed and examined for external, visceral, and skeletal malformations and variations. Clinical signs of maternal toxicity were observed in rats and rabbits dosed with ADBAC, resulting in no‐observed‐adverse‐effect levels (NOAELs) of 10 and 3 mg/kg/day, respectively. Despite the treatment‐related maternal toxicity of ADBAC, the NOAEL for prenatal developmental toxicity was 100 and 9 mg/kg/day for rats and rabbits, respectively, the highest doses evaluated. Repeated oral doses of DDAC resulted in maternal toxicity in both species at the top two doses, with 25% mortality noted in rabbits at 10 mg/kg/day. No teratogenic effects were observed at any dose for either species. However, increased incidence of dead fetuses per litter and decreased fetal body weights were observed in rabbits at the maternally lethal dose of 10 mg/kg/day. The NOAEL for maternal toxicity of DDAC was 1 mg/kg/day for both species and the NOAEL for prenatal developmental toxicity was 20 and 3 mg/kg/day, for rats and rabbits, respectively.
Keywords: alkyl dimethyl benzyl ammonium chloride, didecyl dimethyl ammonium chloride, prenatal developmental toxicity, quaternary ammonium compounds
Abbreviations
- ADBAC
alkyl dimethyl benzyl ammonium chloride
- ANOVA
analysis of variance
- DDAC
didecyl dimethyl ammonium chloride
- ECHA
European Chemical Agency
- EPA
Environmental Protection Agency
- FIFRA
Federal Insecticide Fungicide and Rodenticide Act
- GD
gestation day
- NOAEL
no‐observed‐adverse‐effect level
- NZW
New Zealand White
- OECD
Organisation for Economic Co‐operation and Development
- QACs or Quats
quaternary ammonium compounds
1. INTRODUCTION
Quaternary ammonium compounds are substances characterized by the presence of a positively charged nitrogen covalently bonded to methyl or alkyl substituents. Based on their chemical nature and intrinsic property of disrupting microbial membrane structure and function at relatively low concentrations, they have been recognized as possessing antimicrobial properties for decades. The chemical structures of alkyl dimethyl benzyl ammonium chloride (ADBAC; CAS RN 68424‐85‐1) and didecyl dimethyl ammonium chloride (DDAC; CAS RN 7173‐51‐5) are shown in Figure 1. The alkyl chains found in ADBAC and DDAC are derived from naturally occurring raw materials (plant oils) resulting in characteristic ratios of mixtures of varying chain length. Typically, antimicrobial activity is associated with alkyl chain lengths from 10 to 16 carbons.
FIGURE 1.
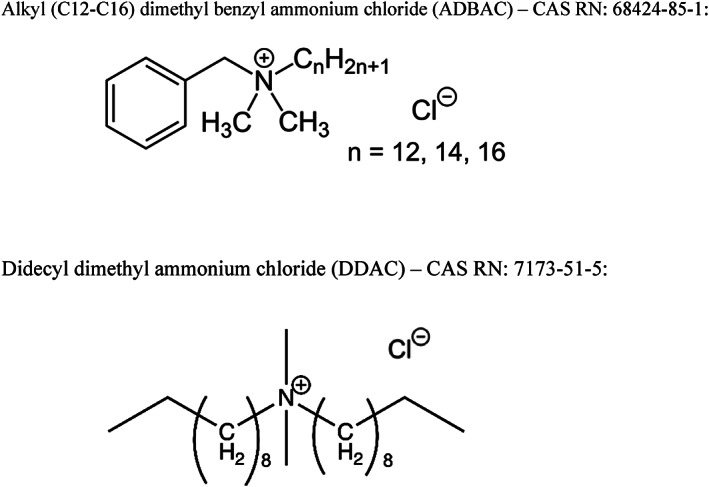
Representative structure diagrams for alkyl (C12‐C16) dimethyl benzyl ammonium chloride (ADBAC) and didecyl dimethyl ammonium chloride (DDAC). ADBAC – CAS RN: 68424‐85‐1: DDAC – CAS RN: 7173‐51‐5
Depending on the structure, quaternary ammonium compounds can have surfactant‐type activity; however, when used to control the growth of microorganisms, they are regulated in the United States by the Environmental Protection Agency (EPA) as antimicrobial pesticides. The EPA defines antimicrobial pesticides as substances “intended to disinfect, sanitize, reduce, or mitigate growth or development of microbiological organisms or to protect inanimate objects, industrial processes or systems, surfaces, water, or other chemical substances from contamination, fouling or deterioration caused by bacteria, viruses, fungi, protozoa, algae, or slime” (EPA, 2019).
In 1988, EPA issued guidance and rationale for grouping or “clustering” of quaternary ammonium compounds based on chemistry and on the strength of evidence that they possess a common mechanism of action (EPA, 1988). Indeed, although ADBAC and DDAC belong to different EPA clusters, they behave similarly in biological systems and are prototype substances for applying read‐across principles to data development programs. There are numerous examples of effect and no‐effect doses or concentrations of ADBAC and DDAC in mammalian and aquatic species that are in close agreement.
Registration requirements for all EPA registered antimicrobial pesticides includes an extensive evaluation of product chemistry, product performance (antimicrobial efficacy), and data from studies to determine potential hazards to humans, domestic animals, and the environment. In the case of both ADBAC and DDAC, extensive datasets have been developed to address acute, subchronic, chronic, prenatal developmental, and reproductive toxicity potential. Included in these requirements are the necessity to conduct classic prenatal developmental toxicity evaluations, following EPA and Organisation for Economic Cooperation and Development (OECD) guidelines for study design and conduct. The studies described herein are those conducted in response to these requirements and were submitted to the EPA for review. Because ADBAC and DDAC are considered “active substances” under the principle European regulatory scheme now known as the Biocidal Product Regulation (EU (European Union), 2012), these studies are also a key part of the dataset supporting a wide range of biocidal uses in Europe. This work was sponsored by a consortium of quaternary ammonium compound manufacturers1 and overseen by a third party toxicology and regulatory consulting company. They were initiated and completed during the period of 1988–1992 at the Bushy Run Research Center (Export, PA) by an experienced team of developmental and reproductive scientists. All of the studies were conducted according to regulatory guidelines and EPA Good Laboratory Practices and were subject to internal and external audits by EPA. The studies complied with all applicable sections of the Final Rules of the Animal Welfare Act regulations (Code of Federal Regulations, Title 9) in effect at the time of the study, with compliance assured by an Institutional Animal Care and Use Committee (US e‐CFR (United States Electronic Code of Federal Regulations), 2020) at Bushy Run Research Center.
2. MATERIALS AND METHODS
2.1. Test substances and dose preparation
ADBAC (CAS RN 68424‐85‐1) and DDAC (CAS RN 7173‐51‐5) were provided by the sponsor as 80% technical concentrates as produced at manufacture. ADBAC had an alkyl chain length distribution of 40% C12, 50% C14, and 10% C16. Each dosing solution was prepared by dissolving the appropriate amount of ADBAC or DDAC with deionized (Milli‐Q) water. Concentrations of the dosing solutions were adjusted for percent active ingredient of each test substance (ADBAC = 81.09%; DDAC = 80.8%). Dosing solutions were prepared at appropriate intervals for each study based on stability data and stored at room temperature. Each dose solution formulation was analyzed for test substance content prior to use and homogeneity and stability were determined.
2.2. Vehicle, analysis of dose formulations, and dose administration
The vehicle used in preparation of the test substance formulations and for administration to the control group was with deionized (Milli‐Q) water (prepared on‐site). ADBAC and DDAC were orally administered to the test animals via gavage at the following target concentrations: for ADBAC studies, 10, 30, or 100 mg/kg/day for rats and 1, 3, or 9 mg/kg/day for rabbits; and for DDAC studies 1, 10, or 20 mg/kg/day for rats and 1, 3, or 10 mg/kg/day for rabbits. The test substances were administered to rats and rabbits once daily from gestation day (GD) 6–15 for rats and GD 6–18 for rabbits. The selected route of administration for these studies was oral because it, along with the dermal route, are potential routes of exposure to ADBAC and DDAC for humans. In addition, the oral route is the preferred route of exposure for this study design. See Table 1 for study design and group assignments for each prenatal developmental toxicity study.
TABLE 1.
Study design and group assignments for prenatal developmental toxicity studies with ADBAC and DDAC in rats and rabbits
| Test species | Group number | Treatment | Target dosage level (mg/kg/day) | Nominalconcentration (mg/ml) | Dose volume (ml/kg) | No. females |
|---|---|---|---|---|---|---|
| CD rats | 1 | Vehicle controla | 0 | 0 | 5 | 25 |
| 2 | ADBAC | 10 | 2.0 | 5 | 25 | |
| 3 | ADBAC | 30 | 6.0 | 5 | 25 | |
| 4 | ADBAC | 100 | 20.0 | 5 | 25 | |
| New Zealand White rabbits | 1 | Vehicle controla | 0 | 0 | 2 | 16 |
| 2 | ADBAC | 1 | 0.5 | 2 | 16 | |
| 3 | ADBAC | 3 | 1.5 | 2 | 16 | |
| 4 | ADBAC | 9 | 4.5 | 2 | 16 | |
| CD rats | 1 | Vehicle controla | 0 | 0 | 5 | 25 |
| 2 | DDAC | 1 | 0.2 | 5 | 25 | |
| 3 | DDAC | 10 | 2.0 | 5 | 25 | |
| 4 | DDAC | 20 | 4.0 | 5 | 25 | |
| New Zealand White Rabbits | 1 | Vehicle controla | 0 | 0 | 2 | 16 |
| 2 | DDAC | 1 | 0.5 | 2 | 16 | |
| 3 | DDAC | 3 | 1.5 | 2 | 16 | |
| 4 | DDAC | 10 | 5.0 | 2 | 16 |
The vehicle was deionized (Milli‐Q) water.
Abbreviations: ADBAC, alkyl dimethyl benzyl ammonium chloride; DDAC, didecyl dimethyl ammonium chloride.
Dosage levels for the prenatal developmental toxicity studies were selected based on the results of previous range‐finding studies in which pregnant female rats and rabbits (five animals per dose group) were administered ADBAC or DDAC from GD 6 to 15 (rats) or GD 6 to 18 (rabbits). Each range‐finding study consisted of five dose groups plus a vehicle control group. The dose ranges for each study were as follows: 25–400 mg ADBAC/kg/day for rats; 1–60 mg ADBAC/kg/day for rabbits; 1–50 mg DDAC/kg/day for rats; and 1–100 mg DDAC/kg/day for rabbits.
In the range‐finding studies with ADBAC, 100% mortality was noted in rats at ≥200 mg/kg/day and in rabbits at 60 mg/kg/day. In addition, 40% mortality was noted in rabbits administered ADBAC at 30 mg/kg/day. Clinical signs were noted in the nonlethal doses in rats at 100 mg/kg/day (perioral wetness only) and rabbits at 10 mg/kg/day (audible respiration). Rabbits dosed with 10 mg/kg/day also exhibited body weight loss and decreased food consumption. There were no treatment‐related effects on gestational parameters (e.g., number of corpora lutea, number and viability of implantation sites and fetal survival), fetal body weights or fetal external exams noted at any treatment level (≥25 mg/kg/day or ≥1 mg/kg/day in rats and rabbits, respectively).
In the range‐finding studies with DDAC, excessive mortality was noted in the rabbits at 100 and 30 mg/kg/day (100 and 80% mortality, respectively) and 20% mortality (one in five rabbits) occurred at 3 mg/kg/day. Maternal mortality also occurred in rats at ≥37.5 mg/kg/day (40%) and 25 mg/kg/day (20%). Other signs of maternal toxicity including reduced body weights and food consumption and/or clinical signs were also observed at ≥12.5 mg/kg/day in rats and ≥3 mg/kg/day in rabbits. There were no effects on gestational parameters in either species at any dose level. Fetal weights were slightly reduced in the rats at the maternally lethal dose of 37.5 mg/kg/day and there was no effect on rabbit fetal body weights in rabbits at less than or equal to the maternally lethal dose of 30 mg/kg/day. No external malformations or treatment‐related variations were observed in the fetuses at any dose level in either rats or rabbits.
ADBAC and DDAC were found to be stable in the vehicle over the range of concentrations used in each study, for at least 12 days at room temperature storage (data not presented). Test substances were not detected or quantifiable in the analyzed vehicle formulations that were administered to the control groups. Dose formulations were analyzed to determine homogeneity and test substance concentration using high performance liquid chromatography for ADBAC formulations and gas chromatography equipped with a nitrogen‐phosphorus detector for DDAC formulations. Dose formulation concentrations (Table 2) and homogeneity (data not presented) were analytically confirmed as being well within the laboratory's acceptance criteria of ±15% of target concentrations.
TABLE 2.
Mean measured concentration of ADBAC and DDAC in the dose formulations
| Test species | Group number | Treatment | Nominal concentration (mg/ml) | Mean measured concentrations (mg/ml)a | Mean measured concentrations (% of nominal)a |
|---|---|---|---|---|---|
| CD rats | 1 | Vehicle controla | 0 | <MDLb | — |
| 2 | ADBAC | 2.0 | 1.89 | 94.5 | |
| 3 | ADBAC | 6.0 | 6.06 | 101.0 | |
| 4 | ADBAC | 20.0 | 21.00 | 104.8 | |
| New Zealand White rabbits | 1 | Vehicle controla | 0 | <MDLb | — |
| 2 | ADBAC | 0.5 | 0.47 | 94.9 | |
| 3 | ADBAC | 1.5 | 1.42 | 94.7 | |
| 4 | ADBAC | 4.5 | 4.18 | 93.0 | |
| CD rats | 1 | Vehicle controla | 0 | <MDLc | — |
| 2 | DDAC | 0.2 | 0.195 and 0.206 | 97.7 and 102.8 | |
| 3 | DDAC | 2.0 | 1.94 and 2.06 | 97.0 and 103.0 | |
| 4 | DDAC | 4.0 | 4.16 and 3.91 | 103.8 and 97.8 | |
| New Zealand White rabbits | 1 | Vehicle controla | 0 | <MDLc | — |
| 2 | DDAC | 0.5 | 0.47 | 94.0 | |
| 3 | DDAC | 1.5 | 1.52 | 101.4 | |
| 4 | DDAC | 5.0 | 4.74 | 95.2 |
Abbreviations: ADBAC, alkyl dimethyl benzyl ammonium chloride; DDAC, didecyl dimethyl ammonium chloride; MDL, minimum detection limit.
The vehicle was deionized (Milli‐Q) water.
MDL for ADBAC was 0.05 mg/L.
MDL for DDAC was 0.01 mg/L.
2.3. Animals, housing, and environmental conditions
Sexually mature, virgin female and virgin male Crl:CDBR rats (155‐160/sex/study), were obtained from Charles River Breeding Laboratories, Inc., Portage, MI. Females were approximately 56 days old upon arrival and males were approximately 63 days old upon arrival and all animals were acclimated for at least 14 days. During acclimation, females were housed two per cage in stainless steel wire mesh cages and males were housed two per cage during the first week of acclimation and then one to two per cage during the remainder of the acclimation period. Certified ground rodent chow and tap water were provided ad libitum throughout the study.
At the conclusion of the acclimation period, rats were paired one male to one female in stainless steel wire mesh cages and remained together until evidence of successful mating (vaginal or dropped copulation plugs). The day successful mating was confirmed was designated GD 0. After successful mating females were housed individually in stainless steel wire mesh cages until study completion.
Virgin female New Zealand White (NZW) rabbits, approximately 5.5 months old were received from Hazleton Research Products, Inc., Denver, PA. Females were individually housed in stainless steel cages from arrival through study completion. Rabbits were provided certified pelleted feed and tap water ad libitum for the duration of the study. Females were acclimated for at least 14 days prior to being naturally bred (one male to one female) to male rabbits from the laboratory's male breeding colony. The day successful mating was observed was designated GD 0.
Each rat and rabbit was identified by an ear tag with a unique animal number. All animals were housed in environmentally controlled rooms with a 12‐hr light–dark cycle. The study animal rooms were routinely maintained at a temperature range of 66–77 °F for rats and 61–72 °F for rabbits with a humidity range of 40–70% for rats and 40–60% for rabbits.
2.4. Study design
These studies were conducted in compliance with the U.S. EPA Pesticide Assessment Guidelines (EPA, 1984a; EPA, 1988), the OECD Test Guideline 414 (OECD, 1981), and EPA, Federal Insecticide, Fungicide and Rodenticide Act (FIFRA) Good Laboratory Practice Standards (EPA (United States Environmental Protection Agency), 1983; EPA, 1984b; EPA, 1989).
For the two studies in rats, 25 bred female Crl:CDBR rats per group were administered the test substance by oral gavage once daily from GD 6 to 15. Dosage levels were 10, 30, or 100 mg/kg/day for ADBAC and 1, 10, or 20 mg/kg/day for DDAC, each administered at a dose volume of 5 ml/kg based on the dam's body weight on GD 6 (ADBAC) or the most recent body weight (DDAC). A concurrent control group, composed of 25 bred females per study received the vehicle (deionized water) on a comparable regimen.
For the two studies in rabbits, 16 naturally bred female NZW rabbits per group were administered the test substance by oral gavage once daily from GD 6 to 18. Dosage levels were 1, 3, or 9 mg/kg/day for ADBAC and 1, 3, or 10 mg/kg/day for DDAC, each administered at a dose volume of 2 ml/kg based on the animal's body weight on GD 6 (ADBAC) or the most recent body weight (DDAC). A concurrent control group composed of 16 bred females per study received the vehicle (deionized water) on a comparable regimen.
On each day of successful mating for rats and rabbits (GD 0), all successfully mated females were allocated to the four test groups within their respective studies by a computer‐generated randomization procedure stratified by body weight on GD 0, such that all groups within each study were equivalent in both mean body weight and mean body weight range. An overview of the group allocation and study design is presented in Table 1.
The female rats were 11–12 weeks of age and female rabbits were approximately 6 months old at the initiation of dose administration (GD 6). On the day of necropsy (GD 21 for rats and GD 29 for rabbits), a laparohysterectomy was performed on each female. For both species, a final body weight and gravid uterine weights were recorded, the uteri, placentae, and ovaries were examined, and the numbers of fetuses, early and late resorptions, total implantations, and corpora lutea were recorded. The fetuses were weighed, sexed, and examined for external, visceral, and skeletal malformations and prenatal developmental variations.
2.5. Parameters evaluated
2.5.1. Maternal observations
All animals were observed twice daily, once in the morning and once in the afternoon, for moribundity and mortality. Individual clinical observations were recorded at least once daily throughout gestation (prior to dose administration during the treatment period).
Individual maternal body weights were recorded on GD 0, 6, (prior to the onset of dosing) and periodically throughout gestation and on the day of necropsy (GD 21 for rats or GD 29 for rabbits). Food consumption was also measured periodically throughout gestation for all studies except the definitive study in rabbits dosed with DDAC. Gravid uterine weight was collected and net body weight (the final body weight on the day on necropsy exclusive of the weight of the uterus and contents) and net body weight change (the net body weight on the day of necropsy minus the GD 0 body weight) were calculated and presented for each gravid female at the scheduled laparohysterectomy. Rats were euthanized by carbon dioxide inhalation and rabbits were euthanized by injection of sodium pentobarbital or T‐61 euthanasia solution prior to laparohysterectomies and macroscopic examinations.
2.6. Necropsy and pathology
2.6.1. Maternal laparohysterectomy
At the time of necropsy for both rats and rabbits, the contents of the thoracic, abdominal, and pelvic cavities were examined and any abnormalities were recorded. The liver was weighed and ovaries were examined for the number of corpora lutea. Each uterus was externally examined for signs of hemorrhage, removed from the peritoneal cavity, weighed and dissected longitudinally to expose the contents. The number and location of all fetuses, early and late resorptions, the total number of implantation sites, and the individual uterine distribution of implantation sites were recorded. The placentae were also examined. Uteri from females with no macroscopic evidence of implantation sites were placed in a 10% ammonium sulfide solution for detection of early resorptions (Salewski, 1964).
2.6.2. Fetal morphological examination
Each viable fetus was examined externally, weighed, and anesthetized by hypothermia (rats) or euthanized by an intraperitoneal injection of sodium pentobarbital (rabbits). The sex of each fetus was determined (externally for rats with confirmation at internal exam; internally for rabbits).
One‐half of the fetuses from each litter were subjected to a visceral examination, fresh dissection technique which included evaluation of the heart and major blood vessels (Staples, 1974). After visceral examination, these fetuses were eviscerated and decapitated. The heads of these fetuses were fixed in Bouin's solution and the craniofacial structures were subsequently examined by free‐hand sectioning techniques (Wilson, 1965, 1973 and/or van Julsingha & Bennett, 1977). The remaining intact fetuses were eviscerated and all fetuses (intact and decapitated) were processed and stained with alizarin red S (Crary, 1962) for skeletal evaluations. The intact rat fetuses (approximately one‐half of each litter) and all rabbit fetuses (intact and decapitated) were evaluated for skeletal malformations and prenatal developmental variations.
External, visceral, and skeletal findings were recorded as prenatal developmental variations (alterations in anatomic structure that are considered to have no significant biological effect on animal health or body conformity and/or occur at high incidence, representing slight deviations from normal) or malformations (structural anomalies that alter general body conformity, disrupt or interfere with normal body function, or may be incompatible with life).
2.6.3. Necropsy of females that died, aborted, or delivered early
A gross necropsy was performed on all animals that did not survive to the scheduled necropsy in an attempt to determine the cause of death. Pregnancy status was determined and uterine contents were identified as described in Section 2.6.1. Any female showing signs of abortion or premature delivery were euthanized and examined for grossly evident morphological changes. Products from premature deliveries were retained in 10% neutral buffered formalin for possible future analysis.
2.7. Statistical methods
The unit of comparison was the pregnant female or the litter (Weil, 1970). Analyses were conducted using two‐tailed tests for minimum significance levels of 1 and 5%, comparing each test substance‐treated group to the control group. Data obtained from nongravid animals and animals that delivered early were excluded from statistical analyses.
For each study, results of the quantitative continuous variables (e.g., maternal body weights, organ weights, fetal weights, etc.) were intercompared for the three test substance‐exposed groups and vehicle control group by use of Levene's test for equal variances (Levene, 1960), analysis of variance (ANOVA), and t tests for pairwise comparisons. When Levene's test indicated homogeneous variances, and the ANOVA was significant, the pooled t test was used. When Levene's test indicated heterogeneous variances, all groups were compared by an ANOVA for unequal variances (Brown & Forsythe, 1974) followed, when necessary, by the separate variance t test.
Nonparametric data were statistically evaluated using the Kruskal–Wallis test (Sokal & Rohlf, 1969) followed by the Mann–Whitney U test (Sokal & Rohlf, 1969) when appropriate. Incidence data were compared using Fisher's exact test (Sokal & Rohlf, 1969). For all statistical tests, the probability value of <.05 (two‐tailed) was used as the criterion for significance.
3. RESULTS
The use of the terms “significant” or “significantly” indicates a statistically significant change from the corresponding control value at p < .05 or p < .01. Summary data are tabulated for statistically significant and/or test substance‐related differences, unless otherwise noted.
3.1. ADBAC prenatal developmental toxicity studies in rats and rabbits
3.1.1. Maternal survival and clinical observations
Animal fate and reproductive performance is summarized in Table 3. All animals survived to the scheduled necropsy on GD 21 (rats) or GD 29 (rabbits). One female rat in the 30 mg/kg/day group delivered early on GD 21 prior to scheduled necropsy and was removed from study as the assigned GD 0 may have not been accurate. Administration of ADBAC to rats and rabbits did not affect survival or the pregnancy rate noted at terminal necropsy.
TABLE 3.
Fate and reproductive performance of female rats and rabbits administered ADBAC by oral gavage
| CD rats | NZW rabbits | |||||||
|---|---|---|---|---|---|---|---|---|
| Parameter evaluated | ADBAC dose level (mg/kg/day) | ADBAC dose level (mg/kg/day) | ||||||
| 0 (control) | 10 | 30 | 100 | 0 (control) | 1 | 3 | 9 | |
| No. females on study | 25 | 25 | 25 | 25 | 16 | 16 | 16 | 16 |
| No. females that died | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| No. females that delivered earlya | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| No. females that aborted | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| No. females examined at laparotomy | 25 | 25 | 24 | 25 | 16 | 16 | 16 | 16 |
| No. nonpregnant (%) | 0 (0.0)b | 0 (0.0) | 1 (4.2) | 4 (16.0) | 3 (18.8) | 1 (6.3) | 2 (12.5) | 1 (6.3) |
| No. pregnant (%) | 25 (100) | 25 (100) | 23 (95.8) | 21 (84.0) | 13 (81.3) | 15 (93.8) | 14 (87.5) | 15 (93.8) |
| No. females with nonviable implants only | 1 (4.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| No. females with viable implants | 24 (96.0) | 25 (100) | 23 (100) | 21 (100) | 13 (100) | 15 (100) | 14 (100) | 15 (100) |
| Total number pregnant females on study | 25 (100) | 25 (100) | 24 (96.0) | 21 (84.0) | 13 (81.3) | 15 (93.8) | 14 (87.5) | 15 (93.8) |
Abbreviations: ADBAC, alkyl dimethyl benzyl ammonium chloride.
Female that delivered early was removed from study.
Values are presented as number of females and percent (%) in parentheses.
Treatment‐related clinical signs were noted in rats and rabbits at the highest dose (100 mg/kg/day or 9 mg/kg/day, respectively) and in rats at the mid‐dose (30 mg/kg/day) (Table 4). The observations in rats at 100 mg/kg/day included: perioral wetness in 14 rats (a significant increase from controls); audible respiration during and subsequent to treatment in three rats; and one rat with dehydration, unkempt appearance, loose feces, urine stains and perioral wetness. The observations noted in rats at 30 mg/kg/day were limited to two rats with audible respiration with one of the animals also exhibiting urine stains, gasping, perinasal encrustation, loose feces and perioral wetness. The treatment‐related observations noted in rabbits at 9 mg/kg/day were limited to two animals: hypoactivity and labored respiration in one rabbit and audible respiration in another. There were no treatment‐related clinical observation noted in the rats at 10 mg/kg/day or in the rabbits at ≤3 mg/kg/day.
TABLE 4.
Clinical observation of pregnant female rats and rabbits administered ADBAC by oral gavage
| CD rats | NZW rabbits | |||||||
|---|---|---|---|---|---|---|---|---|
| Clinical observation | ADBAC dose level (mg/kg/day) | ADBAC dose level (mg/kg/day) | ||||||
| 0 (control) | 10 | 30 | 100 | 0 (control) | 1 | 3 | 9 | |
| No. pregnant females at scheduled necropsy | 25 | 25 | 23 | 21 | 13 | 15 | 14 | 15 |
| Dehydrated/unkempt | 0a | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Audible/labored respiration | 0 | 0 | 2 | 5b | 0 | 0 | 0 | 2 |
| Gasping | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 |
| Perioral wetness | 0 | 0 | 1 | 14b | 0 | 0 | 0 | 0 |
| Hypoactive | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
Abbreviations: ADBAC, alkyl dimethyl benzyl ammonium chloride; NZW, New Zealand White.
Data are presented as number of animals exhibiting finding at least once.
Significantly different from control group (p < .05).
3.1.2. Maternal body weights and food consumption
Mean maternal body weights (Figures 2 and 4) and body weight gains (Figures 3 and 5) in all treatment groups for rats and rabbits were unaffected by test substance administration. Differences in absolute maternal body weight and body weight gain between treated and control groups for each species were slight and not statistically significant.
FIGURE 2.

Maternal body weights (g; mean ± SD) during a prenatal developmental toxicity study of alkyl dimethyl benzyl ammonium chloride (ADBAC) in rats
FIGURE 4.
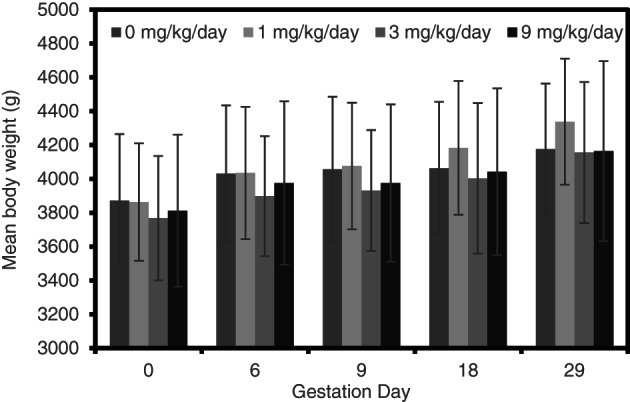
Maternal body weights (g; mean ± SD) during a prenatal developmental toxicity study of alkyl dimethyl benzyl ammonium chloride (ADBAC) in rabbits
FIGURE 3.
Maternal body weight change (g; mean ± SD) during a prenatal developmental toxicity study of alkyl dimethyl benzyl ammonium chloride (ADBAC) in rats
FIGURE 5.
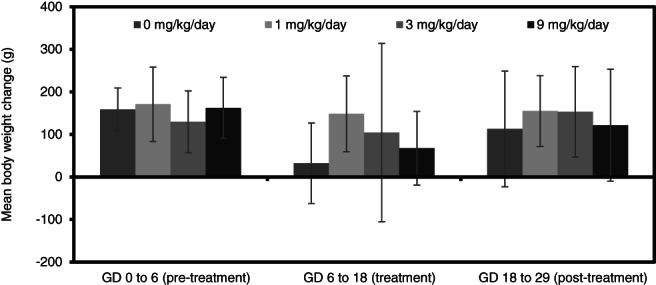
Maternal body weight change (g; mean ± SD) during a prenatal developmental toxicity study of alkyl dimethyl benzyl ammonium chloride (ADBAC) in rabbits
Mean maternal food consumption in rats at 100 mg/kg/day was significantly (p < .05) lower compared to the control group (Table 5), during the first 3 days of treatment (GD 6–9). This transient effect was not considered to be adverse as food consumption (g/animal/day) was similar to the control group during the entire dosing period (GD 6–15) and at all other intervals throughout the study (Table 5). A transient reduction in maternal food consumption was also noted in the 30 mg/kg/day rats during GD 12–15. This reduction was not considered adverse as it was not seen in a dose‐related manner and all other intervals were not significantly different from controls. There was no reduction of food consumption in rats receiving oral doses of ADBAC at 10 mg/kg/day. In fact, food consumption increased on GD 6–9 and GD 9–12 in these animals. There were no statistically significant effects on food consumption (g/animal/day) noted in the rabbits administered ADBAC at ≤9 mg/kg/day throughout the study (Table 6).
TABLE 5.
Food consumption (g/animal/day; mean ± SD) of pregnant female rats administered ADBAC by oral gavage
| ADBAC dose level (mg/kg/day) | Gestation days | |||||
|---|---|---|---|---|---|---|
| 0–6 (pretreatment) | 6–9 | 9–12 | 12–15 | 6–15 (treatment) | 15–21 (posttreatment) | |
| 0 (control) | 23.51 ± 1.68 | 24.30 ± 1.39 | 25.04 ± 2.11 | 26.67 ± 2.44 | 25.34 ± 1.72 | 28.64 ± 2.96 |
| 10 | 24.41 ± 2.96 | 26.40 ± 3.75a | 27.07 ± 2.68a | 27.11 ± 2.70 | 26.73 ± 2.66 | 29.49 ± 2.45 |
| 30 | 23.76 ± 1.89 | 22.87 ± 5.02 | 24.87 ± 2.60 | 24.37 ± 3.1b | 24.04 ± 2.59 | 27.47 ± 1.83 |
| 100 | 23.71 ± 2.41 | 21.94 ± 3.88a | 24.09 ± 3.94 | 26.03 ± 2.78 | 24.02 ± 3.18 | 28.09 ± 6.17 |
Note: N = 25, 25, 24, 21 rats in the 0, 10, 30, 100 mg/kg/day groups, respectively, with slight variations due to weighing error, food spillage, and so forth; nongravid female weights not included in calculation of mean.
Abbreviation: ADBAC, alkyl dimethyl benzyl ammonium chloride.
Significantly different from control at p < .05.
Significantly different from control at p < .01.
TABLE 6.
Food consumption (g/animal/day; mean ± SD) of pregnant female rabbits administered ADBAC by oral gavage
| ADBAC dose level (mg/kg/day) | 0–6 (pretreatment) | 6–18 (treatment) | 18–29 (posttreatment) |
|---|---|---|---|
| 0 (control) | 214.65 ± 29.75 | 161.20 ± 32.76 | 142.52 ± 27.91 |
| 1 | 211.79 ± 32.08 | 190.62 ± 34.60 | 161.07 ± 9.71 |
| 3 | 201.88 ± 39.48 | 182.36 ± 40.87 | 161.75 ± 34.29 |
| 9 | 205.52 ± 39.88 | 180.74 ± 40.69 | 143.57 ± 30.79 |
Note: N = 13, 15, 14, 15 rabbits in the 0, 1, 3, 9 mg/kg/day groups, respectively, with slight variations due to weighing error, food spillage, and so forth; nongravid female weights not included in calculation of mean.
Abbreviation: ADBAC, alkyl dimethyl benzyl ammonium chloride.
3.1.3. Maternal necropsy, laparohysterectomy, and gravid uterine weights
At the scheduled necropsy of the rats on GD 21, a single female rat dosed with ADBAC at 100 mg/kg/day had the following macroscopic findings: gas‐filled gastrointestinal tract, ulceration in the stomach, color changes in the liver and lymph nodes and a small spleen. During the in‐life phase, this female showed audible respiration, perioral wetness and perioral encrustation. One female rat at 30 mg/kg/day was observed with a swollen liver. This female had no clinical observations during the study. There were no effects of treatment on terminal body weight, gravid uterine weight, net body weight, net body weight change or absolute and relative (to net body weight) liver weights (data not provided).
At the scheduled necropsy of the female rabbits on GD 29, there were no treatment‐related gross macroscopic findings. The terminal body weight, gravid uterine weight, net body weight, net body weight change, and liver weight (absolute and relative to corrected body weight) were unaffected by treatment (data not provided).
Intrauterine growth and survival (as determined by preimplantation loss, total number implants, viable implants, nonviable implants, percent live fetuses, mean fetal body weights, and fetal sex ratios), were similar across all groups (Table 7).
TABLE 7.
Summary of gestational parameters (mean ± SD; per litter) for female rats and rabbits administered ADBAC by oral gavage
| CD rats | NZW rabbits | |||||||
|---|---|---|---|---|---|---|---|---|
| Parameter evaluated | ADBAC dose level (mg/kg/day) | ADBAC dose level (mg/kg/day) | ||||||
| 0 (control) | 10 | 30 | 100 | 0 (control) | 1 | 3 | 9 | |
| Pregnant females at scheduled necropsy | 24 | 25 | 23 | 21 | 13 | 15 | 14 | 15 |
| Corpora lutea | 16.3 ± 2.28 | 18.0 ± 2.09 | 17.7 ± 2.69 | 17.1 ± 1.67 | 9.5 ± 1.71 | 10.0 ± 2.24 | 9.4 ± 2.37 | 9.3 ± 1.40 |
| Total implants | 15.0 ± 2.81 | 16.5 ± 1.33 | 15.9 ± 1.95 | 16.0 ± 1.53 | 9.2 ± 1.17 | 9.1 ± 2.33 | 8.1 ± 2.54 | 8.9 ± 1.51 |
| Percent preimplantation lossa | 5.9 ± 6.92 | 8.3 ± 7.29 | 9.8 ± 9.29 | 6.1 ± 7.25 | 3.3 ± 7.30 | 7.6 ± 17.81 | 13.6 ± 19.56 | 5.3 ± 10.08 |
| Viable implants | 14.3 ± 3.51 | 15.6 ± 1.50 | 15.2 ± 2.17 | 15.4 ± 1.40 | 8.6 ± 1.56 | 8.4 ± 2.16 | 7.9 ± 2.51 | 8.3 ± 0.96 |
| Nonviable implants | 0.7 ± 0.98 | 0.9 ± 0.91 | 0.7 ± 0.97 | 0.6 ± 0.80 | 0.6 ± 1.19 | 0.7 ± 1.10 | 0.3 ± 0.61 | 0.6 ± 0.74 |
| Percent live fetuses | 92.3 ± 19.81 | 94.4 ± 5.55 | 95.6 ± 5.98 | 96.3 ± 4.58 | 93.5 ± 13.01 | 92.6 ± 10.41 | 96.7 ± 6.97 | 94.1 ± 6.87 |
| Sex ratio (% male fetuses) | 53.4 ± 13.82 | 57.0 ± 16.04 | 51.1 ± 17.12 | 45.5 ± 13.60 | 38.9 ± 16.75 | 52.7 ± 17.59 | 56.3 ± 16.01 | 54.0 ± 20.65 |
Abbreviations: ADBAC, alkyl dimethyl benzyl ammonium chloride; NZW, New Zealand White.
Percent preimplantation loss = ([corpora lutea − total implants]/corpora lutea) × 100.
3.1.4. Fetal morphological data
The numbers of fetuses (litters) available for morphological evaluation were 357 (24), 389 (25), 350 (23), and 324 (21) in the control 10, 30, and 100 mg/kg/day groups, respectively, for rats and 112 (13), 126 (15), 110 (14), and 124 (15) in the control, 1, 3, and 9 mg/kg/day groups, respectively, for rabbits.
No treatment‐related fetal malformations were observed in the rats or rabbits after oral administration of ADBAC (Tables 8 and 9, respectively). The following external malformations were observed in the rat fetuses: one rat fetus in the 100 mg/kg/day group had gastroschisis and one fetus in this group had thread‐like tail and imperforate anus. There were no external malformations noted in the rabbits. Fetal visceral malformations in rats were limited to absent innominate artery in one rat fetus at 10 mg/kg/day and hydronephrosis and/or hydroureter noted in 1 (1), 2 (2), 8 (3), and 5 (2) rat fetuses (litters) in the control, 10, 30, and 100 mg/kg/day groups. The following visceral malformations were observed in the rabbit fetuses: herniated diaphragm in one fetus at 1 mg/kg/day and dilated lateral ventricle (brain) with tissue depression in 2 (2) and 2 (1) rabbit fetuses (litters) in the 3 and 9 mg/kg/day treatment groups.
TABLE 8.
Incidence of fetuses/litters with malformation in the rat after maternal oral administration of ADBAC
| Fetal malformations | ADBAC dose level (mg/kg/day) | |||
|---|---|---|---|---|
| 0 (control) | 10 | 30 | 100 | |
| Number examined externally | 357/24a | 389/25 | 350/23 | 324/21 |
| Gastroschisis | 0/0 | 0/0 | 0/0 | 1/1 |
| Thread‐like tail and imperforate anus | 0/0 | 0/0 | 0/0 | 1/1 |
| Number examined viscerally | 185/24 | 200/25 | 182/23 | 169/21 |
| Hydroureter and/or hydronephrosis | 1/1 | 2/2 | 8/3 | 5/2 |
| Absent innominate artery | 0/0 | 1/1 | 0/0 | 0/0 |
| Number examined skeletally | 172/24 | 189/25 | 168/23 | 155/21 |
| No skeletal malformations | ||||
| Total number with malformations | 1/1 | 3/3 | 8/3 | 7/4 |
Abbreviation: ADBAC, alkyl dimethyl benzyl ammonium chloride.
Data are presented as number of fetuses/number of litters.
TABLE 9.
Incidence of fetuses/litters with malformations in the rabbit after maternal oral administration of ADBAC
| Fetal malformations | ADBAC dose level (mg/kg/day) | |||
|---|---|---|---|---|
| 0 (control) | 1 | 3 | 9 | |
| Number examined externally | 112/13a | 126/15 | 110/14 | 124/15 |
| No external malformations | ||||
| Number examined viscerally | 112/13 | 126/15 | 110/14 | 124/15 |
| Dilated lateral ventricle (brain) with tissue depression | 0/0 | 0/0 | 2/2 | 2/1 |
| Herniated diaphragm | 0/0 | 1/1 | 0/0 | 0/0 |
| Number examined skeletally | 112/13 | 126/15 | 110/14 | 124/15 |
| Fused thoracic centra (#10–11) and fused ribs (#7–8) | 0/0 | 0/0 | 0/0 | 1/1 |
| Absent lumbar centrum and arches #6 | 1/1 | 0/0 | 0/0 | 0/0 |
| Extra rib between #10 and #11 | 0/0 | 0/0 | 0/0 | 1/1 |
| Total number with malformations | 1/1 | 1/1 | 2/2 | 4/2 |
Abbreviation: ADBAC, alkyl dimethyl benzyl ammonium chloride.
Data are presented as number of fetuses/number of litters.
Skeletal malformations were observed in one control rabbit fetus with absent lumbar centrum and arches (#6) and one rabbit litter in the 9 mg/kg/day group with one fetus exhibiting fused thoracic centra (#10 and 11) and ribs (#7 and 8) and another fetus presenting an extra rib between ribs #10 and #11. There were no skeletal malformations observed in the rat fetuses in any dose group.
No test substance‐related external, visceral or skeletal prenatal developmental variations were noted. A few fetal variations noted with mean litter proportions significantly different from the concurrent controls were not observed in a dose‐related manner and/or the values were within the ranges of the laboratory's historical control database.
3.2. DDAC prenatal developmental toxicity studies in rats and rabbits
3.2.1. Maternal survival and clinical observations
The fate and reproductive performance of rats and rabbits administered DDAC by oral gavage is summarized in Table 10. All rats survived to the scheduled necropsy on GD 21. Oral gavage administration of DDAC up to 20 mg/kg/day did not affect survival or the pregnancy rate of rats.
TABLE 10.
Fate and reproductive performance of female rats and rabbits administered DDAC by oral gavage
| CD rats | NZW rabbits | |||||||
|---|---|---|---|---|---|---|---|---|
| Parameter evaluated | DDAC dose level (mg/kg/day) | DDAC dose level (mg/kg/day) | ||||||
| 0 (control) | 1 | 10 | 20 | 0 (control) | 1 | 3 | 10 | |
| No. females on study | 25 | 25 | 25 | 25 | 16 | 16 | 16 | 16 |
| No. females that died | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 |
| No. pregnant | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 |
| No. females that delivered earlya | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 1 |
| No. females that aborted | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| No. females examined at laparotomy | 25 | 25 | 25 | 25 | 16 | 14 | 16 | 11 |
| No. nonpregnant | 0 (0.0)b | 1 (4.0) | 1 (4.0) | 2 (8.0) | 2 (12.5) | 1 (7.1) | 1 (6.2) | 2 (18.2) |
| No. pregnant | 25 (100) | 24 (96.0) | 24 (96.0) | 23 (92.0) | 14 (87.5) | 13 (92.9) | 15 (93.7) | 9 (81.8) |
| No. females with nonviable implants only | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| No. females with viable implants | 25 (100) | 24 (100) | 24 (100) | 23 (100) | 14 (100) | 13 (100) | 15 (100) | 9 (100) |
| Total number pregnant females on study | 25 (100) | 24 (96.0) | 24 (96.0) | 23 (92.0) | 14 (87.5) | 15 (93.7) | 15 (93.7) | 14 (87.5) |
Abbreviations: ADBAC, alkyl dimethyl benzyl ammonium chloride; DDAC, didecyl dimethyl ammonium chloride; NZW, New Zealand White.
Females that delivered early were removed from study and data were not tabulated.
Values are presented as number of females and percent (%) in parentheses.
Mortality (29%) occurred between GD 9 and 11 in the pregnant rabbits that were administered DDAC at 10 mg/kg/day. The necropsy observations of the four rabbits that died prior to scheduled termination included: sloughing of the esophageal lining; sloughing, hemorrhage and/or distention of the glandular and nonglandular portion of the stomach.
Two rabbits in the 1 mg/kg/day group and one rabbit in the 10 mg/kg/day group delivered early on GD 29 prior to scheduled necropsy and were removed from study. No rabbits aborted.
Treatment‐related clinical signs, indicative of respiratory irritation following oral administration of a locally acting irritant (e.g., labored respiration, audible respiration, and/or gasping), were noted in rats and rabbits at the top‐ and mid‐dose groups (Table 11). A significant increase in the number of animals exhibiting audible respiration was noted at 10 and 20 mg/kg/day in the rat and at 10 mg/kg/day in the rabbits. There were no treatment‐related clinical observations noted in the rats or rabbits at 1 mg/kg/day.
TABLE 11.
Clinical observation of pregnant female rats and rabbits administered DDAC by oral gavage
| CD rats | NZW rabbits | |||||||
|---|---|---|---|---|---|---|---|---|
| Clinical observation | DDAC dose level (mg/kg/day) | DDAC dose level (mg/kg/day) | ||||||
| 0 (control) | 1 | 10 | 20 | 0 (control) | 1 | 3 | 10 | |
| No. pregnant females at termination | 25 | 24 | 24 | 23 | 14 | 13 | 15 | 13 |
| Labored respirationa | 0b | 0 | 0 | 0 | 0 | 0 | 0 | 4 |
| Audible respiration | 0 | 0 | 6c | 15d | 0 | 0 | 3 | 7d |
| Gasping | 0 | 0 | 0 | 4 | 0 | 0 | 0 | 2 |
Abbreviations: ADBAC, alkyl dimethyl benzyl ammonium chloride; DDAC, didecyl dimethyl ammonium chloride; NZW, New Zealand White.
Includes all pregnant females at scheduled necropsy and the pregnant rabbits that died prior to scheduled necropsy.
Data are presented as number of animals exhibiting finding at least once.
Significantly different from control group (p < .05).
Significantly different from control group (p < .01).
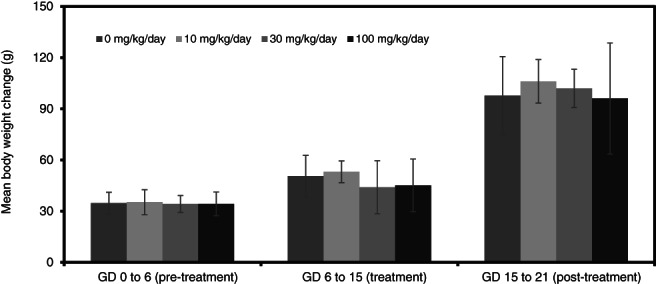
3.2.2. Maternal body weights and food consumption
While not statistically significant, mean maternal body weights and body weight gains in rats (Figures 6 and 7) were slightly reduced at 20 mg/kg/day during dosing (GD 6–15). There were no differences in body weight or body weight gain at 1 and 10 mg/kg/day compared to controls.
FIGURE 6.
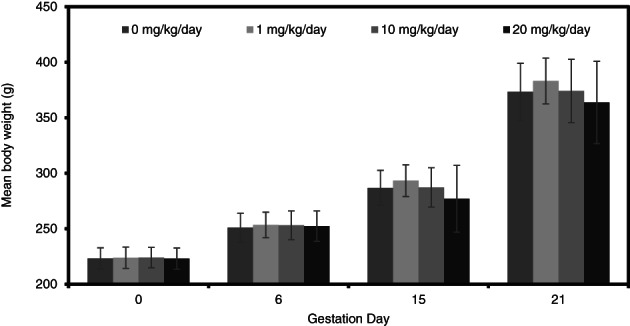
Maternal body weights (g; mean ± SD) during a prenatal developmental toxicity study of didecyl dimethyl ammonium chloride (DDAC) in rats
FIGURE 7.
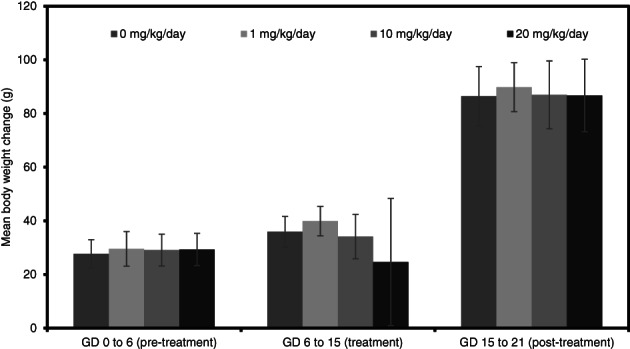
Maternal body weight changes (g; mean ± SD) during a prenatal developmental toxicity study of didecyl dimethyl ammonium chloride (DDAC) in rats
In rabbits, there were no statistically significant differences among groups in absolute body weights throughout the study (Figure 8). However, maternal weight gains were significantly reduced compared to control at 3 and 10 mg/kg/day during dosing period (Figure 9). The body weight gain of rabbits at 10 mg/kg/day was significantly increased compared to the control group for one post‐dosing period (GD 24–29). There were no effects on absolute body weight or body weight gain in the 1 mg/kg/day treatment group.
FIGURE 8.
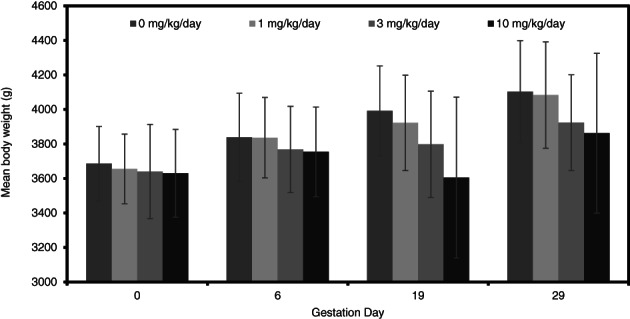
Maternal body weights (g; mean ± SD) during a prenatal developmental toxicity study of didecyl dimethyl ammonium chloride (DDAC) in rabbits
FIGURE 9.
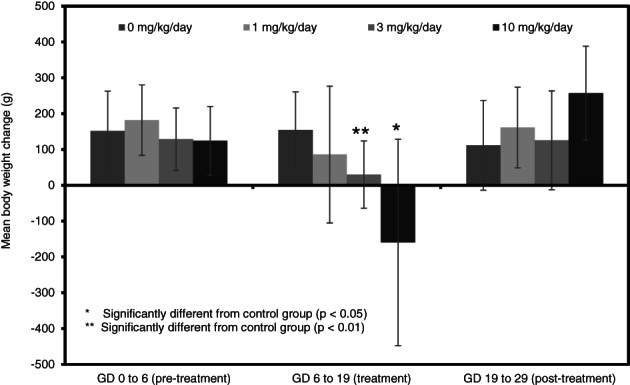
Maternal body weight changes (g; mean ± SD) during a prenatal developmental toxicity study of didecyl dimethyl ammonium chloride (DDAC) in rabbits
Mean maternal food consumption was similar in the rats across all dose group. Food consumption was not measured in the rabbits.
3.2.3. Maternal necropsy, laparohysterectomy, and gravid uterine weights
At the scheduled necropsy of the rats on GD 21, two female rats dosed with DDAC at 20 mg/kg/day had ulcerations in the stomach and gas‐filled gastrointestinal tract. These macroscopic findings were considered to be related to treatment. There were no effects of treatment on terminal body weight, gravid uterine weight, net body weight, net body weight change, or absolute and relative (to net body weight) liver weights (data not provided).
At the scheduled necropsy of the female rabbits on GD 29, two rabbits each in the 3 and 10 mg/kg/day group exhibited reddened glandular stomach that is expected to be a result of oral gavage dosing of DDAC. There were no treatment‐related gross macroscopic findings noted in the 1 mg/kg/day animals. Net body weights in all treatment groups were not significantly different from the control group. However, net body weight gain appeared to be reduced in the 3 and 10 mg/kg/day group although only significantly reduced at 3 mg/kg/day. Absolute and relative (to net body weight) were unaffected by treatment (data not provided).
In the rats, intrauterine growth and survival (as determined by preimplantation loss, total number implants, viable implants, nonviable implants, percent live fetuses, mean fetal body weights, and fetal sex ratios), were similar across all groups (Table 12). In the rabbits, the number of dead fetuses in the 10 mg/kg/day group was significantly increased compared to the controls. No other statistical significant difference in litter survival and growth were noted across group. However, the male and female rabbit fetal body weights in the 10 mg/kg/day group were slightly reduced (approximately 12–14%) compared to the controls.
TABLE 12.
Summary of gestational parameters and fetal body weights (mean ± SD; per litter) for female rats and rabbits administered DDAC by oral gavage
| CD rats | NZW rabbits | |||||||
|---|---|---|---|---|---|---|---|---|
| Parameter evaluated | DDAC dose level (mg/kg/day) | DDAC dose level (mg/kg/day) | ||||||
| 0 (control) | 1 | 10 | 20 | 0 (control) | 1 | 3 | 10 | |
| Pregnant females at scheduled necropsy | 25 | 24 | 24 | 23 | 13 | 13 | 15 | 9 |
| Corpora lutea | 14.2 ± 1.93 | 14.7 ± 1.90 | 14.4 ± 1.91 | 14.4 ± 1.70 | 11.1 ± 2.02 | 10.5 ± 1.27 | 10.7 ± 1.50 | 10.9 ± 1.62 |
| Total implants | 13.7 ± 1.25 | 14.3 ± 1.69 | 14.1 ± 1.73 | 14.0 ± 1.51 | 9.2 ± 2.36 | 9.6 ± 1.26 | 9.9 ± 1.98 | 10.1 ± 1.90 |
| Percent preimplantation lossa | 3.8 ± 6.39 | 3.0 ± 7.14 | 2.9 ± 6.32 | 4.6 ± 7.02 | 16.5 ± 15.81 | 8.5 ± 8.67 | 7.2 ± 12.38 | 7.2 ± 8.36 |
| Viable implants | 13.5 ± 1.23 | 14.0 ± 1.73 | 13.3 ± 2.05 | 13.5 ± 1.53 | 8.6 ± 2.93 | 9.0 ± 1.47 | 9.5 ± 1.81 | 9.0 ± 1.41 |
| Nonviable implants | 0.2 ± 0.41 | 0.3 ± 0.55 | 0.8 ± 1.34 | 0.5 ± 0.59 | 0.6 ± 0.84 | 0.6 ± 0.65 | 0.4 ± 1.06 | 1.1 ± 1.27 |
| Dead fetuses | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.20 | 0.0 ± 0.00 | 0.1 ± 0.27 | 0.3 ± 0.48 | 0.1 ± 0.26 | 0.7 ± 0.87b |
| Percent live fetuses | 98.6 ± 2.91 | 98.0 ± 3.76 | 94.2 ± 9.52 | 96.6 ± 4.16 | 90.8 ± 13.44 | 93.3 ± 7.62 | 96.7 ± 8.81 | 90.0 ± 11.05 |
| Sex ratio (% male fetuses) | 51.8 ± 13.94 | 52.2 ± 14.58 | 47.8 ± 14.23 | 47.2 ± 13.34 | 48.2 ± 17.42 | 56.7 ± 20.69 | 45.5 ± 18.51 | 46.4 ± 15.49 |
| Fetal body weights (g): Litter weight | 5.150 ± 0.314 | 5.177 ± 0.266 | 5.209 ± 0.210 | 4.988 ± 0.622 | 40.58 ± 3.44 | 40.84 ± 3.97 | 40.20 ± 3.98 | 35.49 ± 7.61 |
| Male fetuses | 5.296 ± 0.334 | 5.320 ± 0.275 | 5.321 ± 0.257 | 5.133 ± 0.645 | 41.53 ± 3.17 | 42.08 ± 4.32 | 39.94 ± 3.73 | 35.77 ± 6.80 |
| Female fetuses | 4.981 ± 0.341 | 5.026 ± 0.255 | 5.118 ± 0.178 | 4.872 ± 0.613 | 40.18 ± 4.48 | 40.24 ± 5.13 | 40.40 ± 4.49 | 34.88 ± 9.01 |
Note: Malformation tables taken from the posters.
Abbreviations: ADBAC, alkyl dimethyl benzyl ammonium chloride; DDAC, didecyl dimethyl ammonium chloride; NZW, New Zealand White.
Percent preimplantation loss = ([corpora lutea − total implants]/corpora lutea) × 100.
Significantly different from control group (p < .05).
3.2.4. Fetal morphological data
The numbers of fetuses (litters) available for morphological evaluation were 337 (25), 337 (24), 319 (24), and 311 (23) in the control 1, 10, and 20 mg/kg/day groups, respectively, for rats and 120 (14), 117 (13), 143 (15), and 81 (9) in the control, 1, 3, and 10 mg/kg/day groups, respectively, for rabbits.
The external, visceral, and skeletal prenatal developmental malformations observed in the rat and rabbit fetuses were not considered to be treatment‐related as the malformations occurred in single fetuses/litter, in a similar percentage of control and treated fetuses/liters, or did not occur in a dose‐related manner (Tables 13 and 14, respectively).
TABLE 13.
Incidence of fetuses/litters with malformations in the rat after maternal oral administration of DDAC
| Fetal malformations | DDAC dose level (mg/kg/day) | |||
|---|---|---|---|---|
| 0 (control) | 1 | 10 | 20 | |
| Number examined externally | 337/25a | 337/24 | 319/24 | 311/23 |
| Thread‐like tail and imperforate anus | 1/1b | 0/0 | 0/0 | 0/0 |
| Umbilical hernia | 0/0 | 0/0 | 0/0 | 1/1 |
| Micrognathia | 0/0 | 0/0 | 0/0 | 1/1c |
| Fetus edematous | 0/0 | 0/0 | 1/1 | 0/0 |
| Shortened hindpaw | 1/1d | 0/0 | 0/0 | 0/0 |
| Number examined viscerally | 176/25 | 175/24 | 164/24 | 162/23 |
| Hydroureter and/or hydronephrosis | 3/3 | 6/4 | 14/7 | 6/5 |
| Number examined skeletally | 161/25 | 162/24 | 155/24 | 149/23 |
| Ribs, centra, and arches absent (cervical, thoracic, lumber, and sacral) | 1/1b | 0/0 | 0/0 | 0/0 |
| One cervical or lumbar arch and/or centrum absent | 1/1 | 0/0 | 0/0 | 1/1 |
| Rib #13 absent—unilateral | 0/0 | 0/0 | 2/2 | 0/0 |
| Total number with malformations | 6/5 | 6/4 | 16/9 | 9/6 |
Abbreviation: DDAC, didecyl dimethyl ammonium chloride.
Data are presented as number of fetuses/number of litters.
The same fetus exhibited the external and skeletal malformations.
The eye bulge was also absent during external examination and a skeletal examination of this fetus revealed that the nasal, premaxillary, and mandible bones were malformed.
The skeletal examination of this fetus revealed that the proximal and distal phalanges of the hindpaw were absent.
TABLE 14.
Incidence of fetuses/litters with malformations in the rabbit after oral administration of DDAC
| Fetal malformations | DDAC dose level (mg/kg/day) | |||
|---|---|---|---|---|
| 0 (control) | 1 | 3 | 10 | |
| Number examined externally | 120/14a | 117/13 | 143/15 | 81/9 |
| Lateral scoliosis, clubbed hindlimbs, short tail | 0/0 | 0/0 | 0/0 | 1/1b |
| Forelimb joint rigid | 0/0 | 0/0 | 0/0 | 2/1 |
| Gastroschisis | 0/0 | 1/1 | 1/1 | 0/0 |
| Dome‐shaped head and microglossia | 0/0 | 0/0 | 1/1 | 0/0 |
| Number examined viscerally | 120/14 | 117/13 | 143/15 | 81/9 |
| Dilated lateral ventricle (brain) with tissue depression | 1/1 | 4/1 | 5/5 | 1/1b |
| Kidney absent (unilateral) | 0/0 | 0/0 | 0/0 | 1/1b |
| Urinary bladder absent | 1/1 | 0/0 | 0/0 | 0/0 |
| Gall bladder absent | 0/0 | 2/1c | 1/1 | 0/0 |
| Uterine horn and ovary absent | 0/0 | 1/1c | 0/0 | 0/0 |
| Number examined skeletally | 120/14 | 117/13 | 143/15 | 81/9 |
| Vertebral segments (thoracic to caudal) and ribs fused, absent, and/or malformed | 0/0 | 0/0 | 0/0 | 1/1b |
| Lateral scoliosis without associated vertebral and rib malformations | 2/2d | 0/0 | 0/0 | 1/1 |
| Sternebrae duplicated | 1/1d | 0/0 | 1/1 | 0/0 |
| Total number with malformations | 11/6 | 7/3 | 13/7 | 7/5 |
Abbreviation: DDAC, didecyl dimethyl ammonium chloride.
Data are presented as number of fetuses/number of litters.
The same fetus exhibited the external, visceral, and skeletal malformations.
Findings occurred in the same litter/fetus.
Findings occurred in the same litter/fetus.
The incidence of external, visceral and skeletal prenatal developmental variations in the rat and rabbit fetuses was not significantly altered by test substance administration (data not provided).
4. DISCUSSION AND CONCLUSION
Beginning in the 1980s, ADBAC and DDAC were evaluated for their potential to cause adverse effects in a series of classic and comprehensive hazard assessment protocols covering environmental fate and effects, ecotoxicity, toxicity (including acute, subchronic, chronic, prenatal developmental, and reproductive toxicity), among other endpoints. This extensive testing program fulfilled the U.S. EPA requirements of registration as antimicrobial pesticides under FIFRA as well as the requirements for actives substances under the Biocidal Products Directive (now Regulation; BPR) in Europe. These compounds and other quaternary ammonium compounds in various clusters identified by EPA demonstrate a remarkably similar profile in biological activity. ADBAC and DDAC have been shown to be acute irritants and corrosive in concentrate form, at the point of contact, independent of species or route of administration. This direct acting irritant mode of action has key regulatory implications as current risk assessment guidance in the United States and Europe deals with direct acting irritants differently from other substances. For example, it has been demonstrated and the regulatory authorities agree that a 10‐fold uncertainty factor to account for interspecies extrapolation is not necessary because there is no significant difference in the sensitivity to ADBAC and DDAC in their direct‐acting irritation potential between humans and test species. When administered via the diet in subchronic and chronic studies, ADBAC and DDAC are well tolerated up to a clear threshold dose or concentration, after which they produce a dose‐ and time‐related gastric/gastrointestinal irritation, weight loss and occasional secondary clinical chemistry changes. Robust toxicological data sets that meet regulatory standards provide substantial evidence that ADBAC and DDAC do not produce adverse effects in target organs distant from the site of administration and absorption.
In the reported prenatal developmental toxicity studies, the principle adverse findings noted at necropsy were ulceration of the stomach, sloughing of the esophageal lining and hemorrhage in the stomach, hallmark indicators of irritation and/or corrosion. Gastric irritation or corrosion was the probable key adverse outcome that initiated the corresponding clinical observations, reduction in food consumption, body weights, and body weight gain in both rats and rabbits and which produced significant maternal morbidity and mortality in the dose range finding studies.
Administration of ADBAC by oral gavage produced maternal clinical signs of toxicity at 100 mg/kg/day and possibly at 30 mg/kg/day in CD rats and at 9 mg/kg/day in NZW rabbits. Body weights and body weight changes were unaffected in both species and only transient reduction in food consumption in rats were observed. There was no evidence of treatment‐related prenatal developmental toxicity or embryotoxicity in either species at any dose level evaluated. The no‐observed‐adverse‐effect level (NOAEL) for maternal toxicity was 10 mg/kg/day in the rat and 3 mg/kg/day in the rabbit. The NOAEL for prenatal developmental toxicity was 100 and 9 mg/kg/day in the rat and rabbit, respectively.
Administration of DDAC by oral gavage produced clinical signs of maternal toxicity (labored respiration, audible respiration and/or gasping) in rats at 10 and 20 mg/kg/day. Maternal toxicity, including mortality, was observed in the rabbit after oral gavage doses at 10 mg/kg/day and nonlethal indications of maternal toxicity (clinical signs and reduced body weight) were observed at 10 and 3 mg/kg/day. There was no evidence of treatment‐related prenatal developmental toxicity or embryotoxicity in the rat at doses up to 20 mg/kg/day. In the rabbit, an increased incidence of dead fetuses per litter and reduced fetal body weight per litter was observed, but only at the maternally lethal dose of 10 mg/kg/day. As these effects were attributed to excessive maternal toxicity, which resulted in maternal body weight loss during treatment, they are not considered classic prenatal developmental toxicity effects. There was no evidence of teratogenicity in either species at any administered dose. The NOAEL for maternal toxicity was 1 mg/kg/day in both the rat and rabbit. The NOAEL for prenatal developmental toxicity was 20 mg/kg/day in the rat and 3 mg/kg/day in the rabbit. The rabbit NOAEL represents a conservative value as the adverse effects were associated with clear excessive maternal toxicity including death.
Published in 2006, the U.S. EPA's Reregistration Eligibility Decision documents for ADBAC (EPA, 2006a) and DDAC (EPA, 2006b) provide a concise overview of the potential human health and environmental effects of these substances and the regulatory basis for their continued broad range of registered uses. In addition, there have been specific regulatory actions and decisions, including tolerance exemptions, that affirm the adequacy of the ADBAC and DDAC toxicological databases to support safe food contact uses. The tolerance exemptions and the nature of the reviews supporting them are discussed in detail in the EPA 2017 Final Work Plans for these substances (EPA, 2017a; EPA, 2017b). In evaluating tolerance exemptions for ADBAC, EPA cited several key elements which led to their final decision that the toxicological evidence associated with such uses was sufficient and therefore, an additional safety factor, typically required by the Food Quality Protection Act (FQPA (Food Quality Protection Act), 1996), was not necessary. A justification for this decision by the Agency was the fact that “there is no evidence of increased susceptibility to the fetus following in utero exposure in the prenatal developmental toxicity studies.” (EPA, 2006a). In a more recent review of the supporting dataset, EPA confirmed the adequacy of the current database for prenatal developmental toxicity, which includes the studies that are the subject of this paper, as being acceptable for risk assessment purposes (EPA, 2017a). EPA reached the same conclusions for DDAC (EPA, 2017b).
Additionally, EPA determined the Margin of Exposure (MOE), defined as the ratio of the NOAEL from animal toxicology studies to a predicted or estimated human exposure level or dose, to assess the safety of ADBAC and DDAC for human exposure. From the recent EPA Final Work Plans (EPA, 2017a; EPA, 2017b), the target MOE for ADBAC and DDAC for acute and chronic dietary exposure was 100. Therefore, an MOE of less than 100 indicates concern. Estimates of human dietary exposures from food contact uses, as determined by EPA, are very low. For example, ADBAC exposures resulting from use of a typical disinfecting solution (4,900 ppm) on food contact surfaces are 0.0202 mg/kg/day for the general population and 0.0159 mg/kg/day for females 13–49 years of age. Taking the prenatal developmental toxicity NOAELs for ADBAC of 100 and 9 mg/kg/day (rats and rabbits, respectively), the calculated MOEs (100/0.0159 and 9/0.0159) are 6,289 and 566 for women of childbearing age, well above the targeted value of 100. Margins of exposure for DDAC for prenatal developmental toxicity are similarly large for women of childbearing age (1,258 and 189 for rats and rabbits, respectively). Finally, the Biocidal Products Committee concluded that neither ADBAC nor DDAC cause prenatal developmental effects at doses that do not produce excessive maternal toxicity (ECHA (European Chemicals Agency), 2015a; ECHA (European Chemicals Agency), 2015b).
In spite of the existence of guideline‐compliant prenatal developmental toxicity studies with ADBAC and DDAC, upon which EPA and ECHA have based decisions regarding safe uses, there has been at least one recent report of a nonguideline study in which Hrubec et al. (2017) reported that specific effects on development in mice were attributed to an ADBAC‐DDAC mixture (Hrubec et al., 2017). Neural tube defects were reported following dosing of pregnant mice and “ambient” exposures to an ADBAC‐DDAC mixture used for cage cleaning. No description was provided for how the disinfection solutions were mixed and applied, nor were any attempts made to analyze or even estimate “ambient” concentrations of ADBAC/DDAC in the caged animals' immediate environment. Thus, actual exposures to ADBAC/DDAC in the study are unknown. The authors did not account for several possible confounding factors related to neural tube defects in mice, including maternal stress from handling and transporting bred mice, low humidity, noise, and odors (Brown, Johnston, & Murphy, 1974; Brown, Johnston, & Niswander, 1972; Bruce, 1961; Hostetler, 2018; Hrubec et al., 2017; Kimmel, Cook, & Staples, 1976). Moreover, the reported increase in neural tube defects were only noted around the time of neural tube closure (GD 10), and not observed closer to term, such as at GD 18. Thus, by only reporting observations at the early time point, uncertainty remains about whether the affected fetuses in the Hrubec et al. (2017) would have been viable. In addition, the observed neural tube findings may have reflected a delay in normal development rather than a defect. Importantly, no other structural abnormalities were observed.
The present publication describes four studies in rats and rabbits, evaluating ADBAC and DDAC, which are considered definitive studies for evaluating potential prenatal developmental toxicants by EPA and by ECHA. While the studies were conducted relatively early in the registration process, between 1988 and 1992, they represent detailed, robust, guideline‐compliant evaluations supported by well‐conducted dose range finding studies to identify maternally toxic doses. The reproductive toxicity potential of ADBAC and DDAC has also been evaluated in guideline‐compliant multigenerational studies in rats, as part of the EPA registration testing program (EPA, 2019) and the Biocidal Products Regulation review program (EU (European Union), 2012). As was the case with the prenatal developmental toxicity studies with these substances, EPA and ECHA accepted these studies as strong evidence that ADBAC and DDAC are not reproductive toxicants. A companion paper describing these studies in detail is in preparation.
In conclusion, ADBAC and DDAC have been thoroughly evaluated in guideline‐compliant studies that identified clear maternally toxic doses, and importantly, doses that were without effects on developing embryos. These substances are classic direct acting irritants and do not produce target organ toxicity distant from their point of contact. The regulatory authorities responsible for the registration of antimicrobial pesticides or biocidal active substances (EPA and ECHA, respectively) have concluded that the toxicological testing databases supporting ADBAC and DDAC are adequate for risk assessment and that neither of these substances is considered a prenatal developmental toxicant. Additionally, MOEs for all relevant populations using EPA‐modeled exposure data greatly exceed the EPA target value of 100. Based on the overwhelming evidence supporting the safety of ADBAC and DDAC, any reported research on these substances that ignores existing data deserves critical evaluation to determine its relevance for human risk assessment.
CONFLICTS OF INTEREST
K.A. H., L.C. F., and B. L. B. are consultants to the ADBAC and DDAC Issues Steering Committees, the studies' sponsor. L. C. F. was employed at and participated in the collection and tabulation of the data for these studies conducted by Bushy Run Research Center, which received funding from the ADBAC and DDAC Issues Steering Committees to conduct these studies.
Hostetler KA, Fisher LC, Burruss BL. Prenatal developmental toxicity of alkyl dimethyl benzyl ammonium chloride and didecyl dimethyl ammonium chloride in CD rats and New Zealand White rabbits. Birth Defects Research. 2021;113:925–944. 10.1002/bdr2.1889
ENDNOTE
The ADBAC and DDAC Issues Steering Committees (ISCs) operate under the auspices of the Household and Commercial Products Association. Member companies are Lonza, Inc.; Mason Chemical, a subsidiary of Pilot Chemical; and Stepan Company. Toxicology Regulatory Services has provided toxicology services related to these substances for more than 25 years and is under contract by the ADBAC and DDAC ISCs and the Household and Commercial Products Association.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- Brown, K. S., Johnston, M. C., & Murphy, P. F. (1974). Isolated cleft palate in A/J mice after transitory exposure to drinking‐water deprivation and low humidity in pregnancy. Teratology, 9(2), 151–158. [DOI] [PubMed] [Google Scholar]
- Brown, K. S., Johnston, M. C., & Niswander, J. D. (1972). Isolated cleft palate in mice after transportation during pregnancy. Teratology, 5, 119–124. [DOI] [PubMed] [Google Scholar]
- Brown, M. B., & Forsythe, A. B. (1974). The small sample behavior of some statistics which test the equality of several means. Technometrics, 16, 129–132. [Google Scholar]
- Bruce, H. M. (1961). Time relations in the pregnancy‐block induced in mice by strange males. Journal of Reproduction and Fertility, 2(2), 138–142. [Google Scholar]
- Crary, D. D. (1962). Modified benzyl alcohol clearing of alizarin‐stained specimens without loss of flexibility. Stain Technology, 37, 124–125. [DOI] [PubMed] [Google Scholar]
- ECHA (European Chemicals Agency) . (2015a). Assessment report—Didecyldemethylammonium chloride product‐type 8 (wood preservative). European Chemicals Agency.
- ECHA (European Chemicals Agency) . (2015b). Assessment report—Alkyl C12‐16_dimtehylbenzyl ammonium chloride product‐type 8 (wood preservative). European Chemicals Agency.
- EPA . (1984a). Federal Insecticide, Fungicide and Rodenticide Act (FIFRA). Pesticide Assessment Guidelines (Subdivision F, Hazard Evaluation): Human and Domestic Animals, Section 83‐3.
- EPA . (1984b). Federal Insecticide, Fungicide and Rodenticide Act (FIFRA). Good laboratory practice standards: 40 CFR part 160. Final rule. Federal Register, 49(86), 18738–18739. [Google Scholar]
- EPA . (1988). Pesticide registration notice 88‐2: Clustering of quaternary ammonium compounds. Retrieved from https://www.epa.gov/sites/production/files/2015-09/documents/pr88-2.pdf.
- EPA . (1989). Federal Insecticide, Fungicide and Rodenticide Act (FIFRA). Good laboratory practice standards: 40 CFR part 160. Final rule. Federal Register, 54(158), 34052–34074. [Google Scholar]
- EPA . (2006a). Reregistration eligibility decision for alkyl dimethyl benzyl ammonium chloride (ADBAC). Case Number 0350. Office of Prevention, Pesticides and Toxic Substances (7510C). EPA739‐R‐06‐009.
- EPA . (2006b). Reregistration eligibility decision for aliphatic alkyl quaternaries (DDAC). Case Number 3003. Office of Prevention, Pesticides and Toxic Substances (7510P). EPA739‐R‐06‐008.
- EPA . (2017a). Alkyl dimethyl benzyl ammonium chloride (ADBAC) Final Work Plan. Registration Review: Initial Docket Case Number 0350. Docket Number EPA‐HQ‐OPP‐2015‐0737.
- EPA . (2017b). Didecyl dimethyl ammonium chloride (DDAC) Final Work Plan. Registration Review: Initial Docket Case Number 3003. Docket Number EPA‐HQ‐OPP‐2015‐0740.
- EPA . (2019). Antimicrobial pesticide registration. 04 April 2019. Retrieved from https://www.epa.gov/pesticide-registration/antimicrobial-pesticide-registration#what.
- EPA (United States Environmental Protection Agency) . (1983). Part IV Environmental Protection Agency pesticide programs; good laboratory practice standards: 40 CFR part 160. Final rule. Federal Register, 48(230), 53946–53969. [Google Scholar]
- EU (European Union) . (2012). Regulation (EU) No. 528/2012 of the European Parliament and of the Council of 22 May 2012 concerning the making available on the market and use of biocidal products. OJ L 167/1, 27.6.2012.
- FQPA (Food Quality Protection Act) . (1996). Public Law 104‐170.
- Hostetler, K. A. (2018). Letter to the editor: Comments on “ambient and dosed exposure to quaternary ammonium disinfectants causes neural tube defects in rodents”. Birth Defects Research, 110(6), 543–544. 10.1002/bdr2.1194 [DOI] [PubMed] [Google Scholar]
- Hrubec, T. C., Melin, V. E., Shea, C. S., Ferguson, E. E., Garofola, C., Repine, C. M., … Hunt, P. A. (2017). Ambient and dosed exposure to quaternary ammonium disinfectants causes neural tube defects in rodents. Birth Defects Research, 109(14), 1166–1178. 10.1002/bdr2.1064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel, C. A., Cook, R. O., & Staples, R. E. (1976). Teratogenic potential of noise in mice and rats. Toxicology and Applied Pharmacology, 36(2), 239–245. [DOI] [PubMed] [Google Scholar]
- Levene, H. (1960). Robust tests for equality of variance. In Olkin I., Ghurye S. G., Hoeffding W., Madow W. G. & Mann H. B., (Eds.), Contributions to probability and statistics (pp. 278–292). Stanford, CA: Stanford University Press. [Google Scholar]
- OECD . (1981). Organisation for Economic Co‐operation and Development Guideline for Testing of Chemicals. Section 4 ‐ Health Effects: Test No. 414. Prenatal Developmental Toxicity Study.
- Salewski, E. (1964). Farbemethode zum makroskopischen nachweis van implantations‐stellen am uterus der ratte. Naunyn‐Schmiedebergs, Archiv für Experimentalle Pathologie und Pharmakologie, 247, 367. [Google Scholar]
- Sokal, R. R., & Rohlf, F. J. (1969). Biometry (pp. 369–371). San Francisco, CA: W.H. Freeman and Co. [Google Scholar]
- Staples, R. E. (1974). Detection of visceral alterations in mammalian fetuses. Teratology, 9, A37. [Google Scholar]
- US e‐CFR (United States Electronic Code of Federal Regulations) . (2020). Title 9—Animals and Animal Products, Chapter I—Animal and Plant Health Inspection Service, Department of Agriculture, Subchapter A—Animal Welfare Part 3—Standards. Retrieved from https://www.ecfr.gov/cgi-bin/text-idx?tpl=/ecfrbrowse/Title09/9cfrv1_02.tpl.
- van Julsingha, E. B., & Bennett, C. G. (1977). A dissecting procedure for the detection of anomalies in the rabbit foetal head. In Neubert D., Merker H. J., & Kwasigroch T. E. (Eds.), Methods in prenatal toxicology (pp. 126–144). Littleton, MA: PSG Publishing Company. [Google Scholar]
- Weil, C. S. (1970). Selection of the valid number of sampling units and a consideration of their combination in toxicological studies involving reproduction, teratogenesis or carcinogenesis. Food and Cosmetics Toxicology, 8, 177–182. [DOI] [PubMed] [Google Scholar]
- Wilson, J. G. (1965). Embryological considerations in teratology. Annals of the New York Academy of Sciences, 123(1), 219–227. 10.1111/j.1749-6632.1965.tb12260.x [DOI] [PubMed] [Google Scholar]
- Wilson, J. G. (1973). Environment and birth defects. New York, NY: Academic Press. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


