Abstract
This review discusses the influence of gut microbiota dysbiosis on diabetic kidney disease through metabolite profile changes and immune and inflammatory mechanisms. We also elaborate on the mechanism of dysbiosis in the onset and development of other kidney diseases.
Keywords: diabetic kidney disease, gut microbiota, immune reaction, mechanism, metabolism
SUMMARY AT A GLANCE
This review presents scientific evidence on the pathophysiologic links between gut microbiota and diabetic kidney disease (DKD), highlighting the influence of gut microbiota dysbiosis on DKD through metabolite profile changes and immunologic mechanisms.
1. INTRODUCTION
Gut microbiota, comprised of trillions of microbes, are of great significance to the human body. They coevolve with humans for mutual and beneficial coexistence and play vital roles in maintaining overall health and in preventing the development of diseases.1 Gut microbiota are relatively stable and participate in various physiological processes in the human body.2 They maintain host homeostasis in multiple ways, with their roles including modulating metabolism, maintaining the integrity of the intestinal barrier, inhibiting pathogenic bacterial invasion and regulating physiological and immune responses.3 In contrast, dysbiosis, or a state of imbalance in gut microbial composition and function, is closely linked to the occurrence and development of many chronic diseases, both within and outside the gastrointestinal tract.4 We have briefly summarized the known extraintestinal diseases related to gut microbiota (Figure 1).
FIGURE 1.
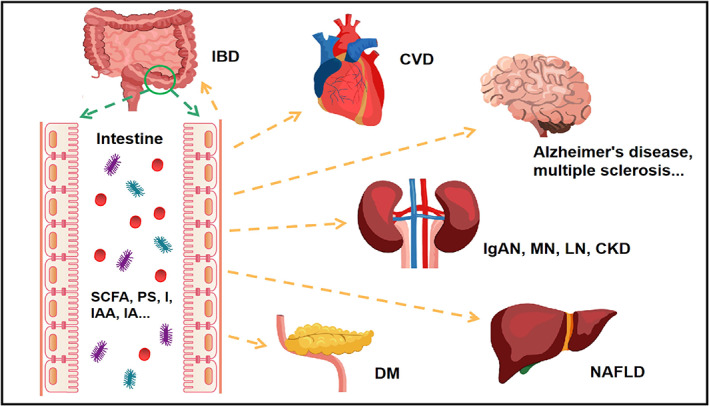
The relationship between gut microbiota and local/distal organs. IBD (inflammatory bowel disease), CVD (cardiovascular diseases), IgAN (immunoglobulin A nephropathy), MN (membranous nephropathy), LN (lupus nephropathy), CKD (chronic kidney disease), NAFLD (nonalcoholic fatty liver disease), SCFA (short‐chain fatty acids), PS (phenyl sulphate), IAA (indole‐3‐acetic acid), IA (indole acetate)
Diabetic kidney disease (DKD), or diabetic nephropathy (DN)—a deleterious complication of diabetes mellitus (DM)—is an important cause of chronic kidney disease, and its prevalence in China has risen rapidly in recent years.5 Patients often experience complications related to cardiovascular and neurological diseases and are characterized by having an increased risk for the progression of end‐stage renal disease (ESRD). This increases both patient mortality and the social burden associated with DKD. Despite the impact of this, the condition's pathogenic mechanism remains unclear. However, recent studies have indicated that gut microbiota dysbiosis is one of the most characteristic changes in DKD.6
To provide a theoretical reference for DKD pathogenesis from the perspective of gut microbiota, we reviewed the role of gut microbiota in DKD and related mechanisms.
2. GUT MICROBIOTA, THE DRIVERS OF KIDNEY DISEASES
Immunoglobulin A nephropathy (IgAN), lupus nephropathy (LN) and membranous nephropathy (MN) are characterized as immune disturbances, with emerging evidence showing that they are related to gut microbiota dysbiosis.
Gut microbiota surface antigens stimulate dendritic cells to produce B‐cell activating factor (BAFF), whose function is to promote the class switching of B cells to IgA+ B cells, whether the process is mediated by T cells.7 Abnormally produced gut‐derived IgA can leak into the bloodstream and lead to immunocomplex deposition in the kidney, thereby promoting the occurrence and development of IgAN. Studies have also shown that five metabolites of gut microbiota are related to increased serum BAFF levels in patients with IgAN.8
LN is an autoimmune disease characterized by the production of pathogenic autoantibodies. Studies have found that supplementing Lactobacillus spp. in LN mice can alleviate kidney disorders. Possible mechanisms behind this effect include supplementation improving intestinal barrier integrity, increasing IL‐10, and reducing IL‐6 and IgG2, thereby decreasing the incidence of kidney diseases.9
Patients with MN had higher Fusobacteria and Proteobacteria but lower Firmicutes populations than those of individuals in the healthy control (HC) group.10 Our research revealed that lipopolysaccharide (LPS)‐producing bacteria were enriched in MN and DKD. Increased LPS leaks into the bloodstream through the damaged intestinal barrier, activates the NF‐κB pathway and triggers the release of inflammatory factors, resulting in the aggravation of kidney damage.11
In recent years, emerging studies have shown that gut microbiota affect CKD progression via uremic toxin production, interactions with chronic inflammation and immune response regulation. Gut microbiota dysbiosis and intestinal barrier destruction in CKD lead to bacteria, or their metabolites, entering the bloodstream.12 Many previous studies have shown that metabolites produced by dysbiotic gut bacterial communities can destroy the intestinal mucosal barrier and cause kidney damage.13, 14
Current studies have revealed that traditional Chinese medicine aids in the management of kidney diseases. Traditional Chinese medicine can improve the intestinal barrier, regulate intestinal flora and inhibit microinflammation induced by gut‐derived metabolites in order to exert its renoprotective effects.15, 16 Zheng et al identified the keystone microbiome markers in CKD.17 As a result of in‐depth study of the gut‐kidney axis, diet therapy has become a hot topic in CKD treatment.18 After evaluating the gut microbiota of kidney transplant donors and recipients, it was found that microflora similarity may be related to the prognosis of recipients after transplantation.19 However, relevant studies have shown that gut microbiota dysbiosis and impaired intestinal barrier function exist in oxalosis‐related CKD, but microbiota‐targeted therapy cannot prevent its progression.20 Therefore, the precise molecular mechanism of the gut microbiota in kidney diseases remains unclear.
Numerous studies have also revealed that enterotypical differences in gut microbiota exist between various kidney diseases, and the dominant bacteria in different kidney diseases overlap. Therefore, further study is needed regarding whether the virulence factors of these dominant bacteria differ.21
3. GUT MICROBIOTA, THE CENTRAL REGULATORS OF DKD
3.1. Gut microbiota are altered in DM
As early as 2010, it was found that type 2 diabetes mellitus (T2DM) was linked to gut microbiota alterations.22 In recent years, emerging evidence from experiments in both humans and mice suggest that the gut microbiota of patients with diabetes are altered significantly as compared to that of healthy individuals,23 even in the pre‐diabetes state.24 As for the composition of gut microbiota, a higher Firmicutes/Bacteroidetes ratio was observed in patients with T2DM,23 which was reported to be positively correlated with increases in plasma glucose levels and the occurrence of metabolic disorders.25 Patients with T2DM had higher abundances of Enterobacteriaceae, Collinsella, Streptococcus, Lactobacillus and Lachnospiraceae/Ruminococcus than those of healthy individuals. These bacteria may be involved in low‐grade inflammation and can aggravate insulin resistance.23, 26 However, short‐chain fatty acid (SCFA)‐producing probiotics, including Bacteroides, Prevotella, Lachnospira, Roseburia and Faecalibacterium, were markedly depleted in DM as compared to those in healthy individuals.26, 27 Butyrate is an SCFA that acts as an energy resource for colonic epithelial cells. However, the examination of altered T2DM gut microbiota revealed the depletion of several butyrate‐producing bacteria such as Akkermansia, Bacteroides and Faecalibacterium. These bacteria, and their metabolite, butyrate, help improve glucose metabolism.28 Thus, depletion of the butyrate‐producing bacteria mentioned above, and the resulting decreased levels of butyrate, may participate in DM pathogenesis. Members of the genus Blautia decreased significantly in Japanese patients with T2DM as compared to those in control subjects. Blautia was reported to be an SCFA producer, whose abundance was negatively correlated with haemoglobin A1c and fasting plasma glucose levels.29 In summary, the alteration of DM gut microbiota usually manifests as a decreased beneficial bacteria and an increased harmful bacteria.
In the DM state, the functions of intestinal bacteria also change to some extent. It has been reported that the profiles of gut microbial functions, such as tyrosine and butyrate production, are altered in T2DM as compared to those in controls.30 Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways, including the insulin signalling pathway and glycolysis/gluconeogenesis, were upregulated in patients with DM.29 Many antidiabetic drugs can prevent DM progression, or alleviate symptoms by regulating the gut microbiota and reducing endotoxin‐ and microbe‐mediated inflammation. Metformin can treat DM by regulating the structure and function of gut microbiota.31 After metformin use, positive connections among microbial genera are strengthened, and abundances of Escherichia, Bifidobacterium and A. muciniphila increased, whereas that of Intestinibacter decreased. Bifidobacteriaceae can act as a probiotic to improve endotoxemia and glucose tolerance,32 and A. muciniphila can relieve T2DM by reducing oxidative stress and inflammation.33 Dapagliflozin can also improve DM by adjusting gut microflora. Using an Sprague–Dawley(SD) rat diabetic model, Mei Yang found that, while similar blood glucose‐lowering effects were observed after treatments with dapagliflozin and metformin, the dominant enterotypes in the dapagliflozin group were Ruminococcaceae, whereas the dominant types in the metformin group were both Ruminococcaceae and Muribaculaceae. Dapagliflozin increased Desulfovibrionaceae (Proteobacteria), reduced Bacteroidetes/Proteobacteria and decreased the abundance of Actinobacteria and Spirochaetes, compared with those of metformin treatment.32
Interestingly, a study performed on Zucker diabetic fatty rats found that Roux‐en‐Y gastric bypass surgery (RYGB) and sleeve gastrectomy (SG) induced similar glucose profile improvement, and increased microbiota diversity, compared with those of sham‐operated groups. However, RYGB resulted in a higher abundance of Proteobacteria, Gammaproteobacteria, Betaproteobacteria, Fusobacteria and Clostridium than that of control groups. SG, on the other hand, resulted in more abundant Actinobacteria than that of the control groups.34 Clostridium and Fusobacterium were also negatively correlated with serum insulin levels.35 Overall, gut microbiota affect host metabolism, the state of which is also determined by many other intrinsic and extrinsic factors.
3.2. Gut microbiota are related to DKD
DKD is one of the most serious complications of DM. At present, several studies on gut microbiota in DKD exist, mainly focusing on how gut microbiota change. This includes the effects of such changes, their involvement in the mechanisms behind the development of DKD, and the effects of medications meant to treat DKD by regulating gut microbiota (Table 1).
TABLE 1.
Summary of current research on DN and gut microbiota
| Types | Published studies | Year | Author | Sample and methods | Subgroups | Conclusions |
|---|---|---|---|---|---|---|
| Animal studies | Gut microbiota dysbiosis‐induced activation of the intrarenal renin‐angiotensin system is involved in kidney injuries in rat diabetic nephropathy. | 2020 | Lu CC et al. |
Kidney Histology—PAS Plasma—Measurement of acetate, Measurement of circulating RAS Faeces—16S rDNA sequencing, RT‐PCR |
Healthy male Sprague–Dawley rats were divided into three groups, the latter two groups were injected with 65 mg/kg/d STZ to build DM model (1) the control group (2) DM group (3) DM + AB |
The excessive acetic acid produced by the gut microbiota may be involved in the early DN kidney damage by activating the RAS in the kidney |
| Dysbiosis of intestinal microbiota mediates tubulointerstitial injury in diabetic nephropathy via the disruption of cholesterol homeostasis. | 2020 | Hu ZB et al. |
Blood/Urine—biochemical saasy for some indicators of renal function Faeces—16 s rDNA sequencing, FMT Renal tissues—PAS, transmission electron microscopy Measurement of lipid accumulation Measurement of acetic acid concentration |
(1) WT rats treated with drinking water; (2) DM rats treated with drinking water (3) DM rats treated with an antibiotic |
Acetate‐producing bacteria in the intestine mediate the imbalance of cholesterol homeostasis by activating GPR43, resulting in tubulointerstitial damage in DN | |
| The potential role of the gut microbiota in modulating renal function in experimental diabetic nephropathy murine models established in same environment. | 2020 | Li Y et al. |
Faeces—16S rRNA gene sequences, FMT, qPCR, GC–MS Serum—ELISA Urine—biochemical saasy for some indicators of renal function |
17 male SPF C57BL/6 mice were injected with 80 mg/kg/d STZ to build DM model. Then they were divided into 2 groups (1) severe proteinuria group (SP) (2) mild proteinuria group (MP) |
Allobaculum and Anaerosporobacter may worsen renal function, while Blautia may be a protective factor in DN | |
| Exploring the role of the metabolite‐sensing receptor GPR109a in diabetic nephropathy. | 2019 | Snelson M et al. |
Blood—ELISA Urine—ELISA Kidney Histology—PAS, RT‐PCR Ileum Histology—H&E, RT‐PCR |
(1) Gpr109a−/− mice treated with a control diet (2) Gpr109a−/− mice treated with a high fibre diet (3) WT mice treated with a control diet (4) WT mice treated with a high fibre diet |
This study shows that GPR109A does not play a key role in the intestinal homeostasis of T1DM or the occurrence and development of DN | |
| Gut microbiome‐derived phenyl sulphate contributes to albuminuria in diabetic kidney disease. | 2019 | Kikuchi K et al. |
Renal tissues—PAS, qPCR Blood—Untargeted metabolomics analysis, LC/MS/MS, ELISA Urine—biochemical saasy for some indicators of renal function Faeces—16S rRNA gene sequences |
SLCO4C1‐Tg rats, C57BL6 mice, KKAy mice and db/db mice which were induced by STZ 50 mg/kg/d | Gut microbiome‐derived phenyl sulphate can increase proteinuria by inducing podocyte damage in DN. Therefore, PS can become a biomarker for early diagnosis of DN and a potential therapeutic target | |
| Human studies | Gut microbiota profile and selected plasma metabolites in type 1 diabetes without and with stratification by albuminuria. | 2020 | Winther SA et al. |
Faeces—16S rRNA gene sequences Serum—ultra HPLC coupled to MS/MS |
161 individuals with type 1 diabetes and 50 healthy control individuals. Individuals with type 1 diabetes were divided into(1) normoalbuminuria (<3.39 mg/mmol); (2) microalbuminuria (3.39–33.79 mg/mmol); (3) macroalbuminuria (≥33.90 mg/mmol) | In type 1 diabetic patients with macroaibuminuria, compared with those with microalbuminuria and normoalbumuria, the plasma concentrations of indoxyl sulphate, L‐citrulline and L‐kynurenine are higher, but the level of tryptophan is lower |
| Utility of plasma concentration of trimethylamine N‐oxide in predicting cardiovascular and renal complications in individuals with type 1 diabetes. | 2019 | Winther SA et al. |
Serum—concentration determination of TMAO, biochemical saasy for some indicators of renal function Urine—biochemical saasy for some indicators of renal function |
1159 individuals with type 1 diabetes | Intestinal‐derived TMAO may be a marker of renal function, and its higher concentration is associated with CVD events and poor renal prognosis | |
| Understanding the gut‐kidney axis among biopsy‐proven diabetic nephropathy, type 2 diabetes mellitus and healthy controls: an analysis of the gut microbiota composition. | 2019 | Tao S et al. | Faeces—16S rRNA gene sequences | 14 DNs, 14 T2DMs without renal diseases (DM), 14 healthy controls (HC) and household contacts (HH) of DM group |
DM versus HC: g_Prevotella_9 DN versus DM: the variables of g_Escherichia‐Shigella and g_Prevotella_9 |
|
| Probiotic supplementation in diabetic haemodialysis patients has beneficial metabolic effects. | 2017 | Soleimani A et al. | Serum—biomarkers of inflammation and oxidative stress |
60 diabetic patients on haemodialysis were randomly divided into 2 groups (1) treatment group: take a capsule containing the probiotics Lactobacillus acidophilus, Lactobacillus casei and Bifidobacterium bifidum for 12 weeks (2) control group: take a capsule containing placebo for 12 weeks |
Supplementation of probiotics in diabetic haemodialysis patients for 12 weeks has a beneficial effect on blood glucose homeostasis parameters and some biomarkers of inflammation and oxidative stress. | |
| Research on Chinese Medicine | Resveratrol Modulates the Gut Microbiota and Inflammation to Protect Against Diabetic Nephropathy in Mice. | 2020 | Cai TT et al. |
Serum—biochemical saasy for some indicators of renal function, ELISA Kidney tissue—PAS, RT‐PCR Small intestine tissue—HE Faeces—16S rRNA gene sequences, FMT |
Male C57BL/KsJ diabetic db/db mice db/m mice |
After resveratrol treatment, diabetic mice have a greater change in gut microbiota than db/m mice. And the intestinal mucosal barrier is enhanced, permeability and inflammation are reduced |
| Gut Microbial Changes in Diabetic db/db Mice and Recovery of Microbial Diversity upon Pirfenidone Treatment. | 2020 | Singh H et al. |
Cecum tissue—16 s rDNA sequencing Urine—GC–MS |
(1) db/m mice (2) db/db mice (3) db/db + short‐acting PFD (4) db/db + long‐acting PFD (5) db/db + low‐dose CCK (6) db/db + high‐dose CCK |
PFD has a beneficial effect on db/db mice, and this effect is achieved by adjusting the abundance and diversity of gut microbiota |
Abbreviations: BCP, Bupleurum chinense DC; BHID, Bekhogainsam decoction; BPS, Bupleurum smithii var. parvifolium; CCK, cholecystokinin; CCP, Cordyceps cicadae polysaccharides; CCPH, CCP high‐dose group (300 mg/kg BW); CCPL, CCP low‐dose group (75 mg/kg BW); CCPM, CCP middle‐dose group (150 mg/kg BW); CVD, cadiovascular disease; DJP, D. loddigesii; DM + AB, diabetic rats treated with antibiotics; DM, diabetic mellitus; DMBG, dimethyl biguanide; DMBG, dimethyl biguanide group (100 mg/kg BW); DN group diabetic nephropathy group; ELISA, enzyme linked immunosorbent assay; FMT, faecal microbiota transplantation; GC‐MS, gas chromatography‐mass spectrometer; PFD, pirfenidone; PI3K/Akt, phosphatidylinositol‐3‐kinase/protein kinase B; PS, phenyl sulphate; RAS, renin‐angiotensin system; SPF, specific pathogen‐free; STZ, streptozotocin; TMAO, trimethylamine N‐oxide; WT, wild type.
3.2.1. Changes of gut microbiota composition in patients with DKD
Like DM, DKD is associated with abnormal gut microbiota. A study from China recruited DNs diagnosed using renal biopsy, T2DMs without renal diseases (T2DM) and HCs. The research revealed that faecal samples from patients with DM contained higher abundances of Firmicutes and Proteobacteria than did those of HCs and T2DMs. According to the authors, Proteobacteria can increase the level of circulating LPS, resulting in persistent inflammation. The richness of gut microbiota, based on operational taxonomic unit (OTUs), was significantly lower in DNs than in T2DMs, and there were obvious disparities between the microbiota compositions of patients with HC, DM and DN. Escherichia‐Shigella was significantly increased in the DN group, whereas Prevotella_9 decreased, and the richness of the two genera can allow for effective distinguishment between DN and DM subjects.36
A study on CKD suggested that the abundances of Escherichia‐Shigella spp. were positively related to disease progression. The genera Escherichia‐Shigella were enriched in the faecal samples of patients with advanced CKD and played a role in renal impairment by generating additional indoxyl sulphate (IS).37 Escherichia‐Shigella are also conditional pathogenic bacteria that can penetrate the intestinal epithelial barrier and aggravate gut leakiness.36 In a C57BL/6 mouse model, with DN induced by streptozotocin (STZ) and a high‐fat diet, some differences in gut microbiota composition existed between mice with severe and mild proteinuria (SP and MP, respectively). For example, Allobaculum and Anaerosporobacter were enriched in SP mice, whereas Blautia were enriched in MP mice. In SP subjects, the genus Allobaculum was positively related to body weight and blood sugar, and the genus Anaerosporobacter was positively related to 24 h urine protein. Conversely, in mice with MP, Blautia was found to be negatively correlated with 24 h urine protein. Intriguingly, after the mice received faecal microbiota transplants (FMT) from DKD subjects with SP and MP, FMT‐SP mice experienced more severe proteinuria after STZ injection than did FMT‐MP mice.38 Collectively, these findings indicate that gut microorganisms are linked to DKD development.
3.2.2. Gut microbiota aid in DKD diagnosis
Our previous study found that patients with DKD presented with higher richness of Bacteroidetes and lower Firmicutes/Bacteroidetes ratios as compared with those of patients with MN. Peptostreptococcus, a potential pathogen, is one of the genera enriched in patients with DKD. There were significant differences in gut microbiota composition between patients with DKD and those with MN, and microbiota profiles based on four OTUs could distinguish MN from DKD with high accuracy. As for microbial functions, modules related to the metabolic pathways of some amino acids, carbohydrates, energy and lipids were increased in the gut microbiota of DKDs.11 Therefore, gut microbiota may also aid in the diagnosis, and differential diagnosis, of DKD.
3.2.3. Gut microbiota provide potential targets for DKD treatment
To date, studies have focused on the effects of traditional Chinese medicine in treating DKD by modulating the composition or function of gut microbiota, such as Cordyceps cicadae polysaccharides, Tangshen formula and Shenyan Kangfu tablets.39, 40, 41 Recently, researchers found that QiDiTangShen (QDTS) granules, a traditional Chinese medicine composed of seven herbs, could reverse mucosal injury, reduce proteins in urine and KIM‐1 and relieve pathological damage to the kidney. However, QDTS granules did not impact body weight, blood glucose, or urea nitrogen. QDTS treatment improved serum BA profiles and reduced abnormally elevated Lactobacillus, Bacteroides and Roseburia levels in DM mice, wherein the above bacteria regulate bile acid metabolism. KEGG pathway analysis revealed that primary bile acid biosynthesis and secondary bile acid biosynthesis were lower in the DMQD group. In brief, QDTS effectively reduced urine protein levels and alleviated renal injuries in mice with DN through the gut microbiota‐bile acid axis.42 Thus, gut microbiota may be a potential target for future management of DKD.
4. MECHANISMS BEHIND THE EFFECTS OF GUT MICROBIOTA ON DKD
Through the gut‐kidney axis, gut microbiota may promote the development and progression of DKD in many ways (Figure 2).
FIGURE 2.
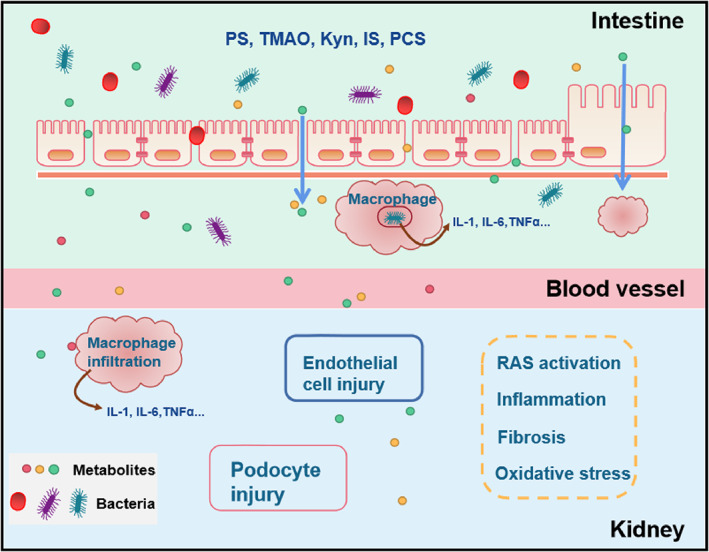
The mechanism of gut microbiota on the kidney. The gut microbiota itself and its metabolites can enter the Interstitial fluid through the increased permeability of the intestinal mucosa. Macrophages can phagocytose bacteria and release inflammatory factors. Metabolites and inflammatory factors enter the blood, reach the kidneys and cause damage to endothelial cells and podocytes through the activation of the RAS system, inflammation and oxidative stress. phenyl sulphate (PS); Trimethylamine‐N‐oxide (TMAO); Kynurenine (Kyn); Indoxyl Sulphate (IS); p‐cresol sulphate (PCS); Interlrukin 1 (IL‐1); Interlrukin 6 (IL‐6); Tumour Necrosis Factor (TNFα); Renin‐angiotensin System (RAS)
4.1. Gut microbiota can promote DKD progression through metabolite production
Gut microbiota can affect the host by producing numerous metabolites. Levels of phenyl sulphate (PS), a derivative of dietary L‐tyrosine metabolism in the gastrointestinal tract, have been reported to be obviously correlated with the basal and predicted 2‐year progression of albuminuria in patients with DKD. After db/db mice were treated with PS, foot process effacement and glomerular basement membrane thickening became apparent, and mRNA levels of Tnfa, Ccl2, Emr1 and Fn1 in kidney tissues increased. Meanwhile, the albuminuria level in db/db mice treated with PS was higher than that in controls. PS is toxic to human podocytes in vitro43 and can cause podocyte injury and promote renal inflammation and fibrosis in DM.
In SD rats with STZ‐induced DM, plasma acetate concentration and abnormal gut flora increased, accompanied by RAS system activation and renal function impairment. Nonetheless, these phenomenon can be reversed using broad‐spectrum antibiotics, indicating that the presence of abnormal intestinal flora in DM can lead to renal injury by activating the RAS system through metabolites.44 Tryptophan can be metabolized into kynurenine (Kyn) by tryptophan 2,3‐dioxygenase (TDO) and indoleamine (2,3)‐dioxygenase (IDO).45 The kynurenine pathway is influenced by gut microbiota.46 Kyn, and its derivatives, can promote vascular endothelial dysfunction and atherosclerosis in patients with CKD.47 Kyn was found to be positively correlated with the urinary albumin‐to‐creatinine ratio (UACR) and negatively correlated with estimated glomerular filtration rates (eGFR) in DKD. However, the molecular mechanisms behind these relationships remain unclear.48
4.2. Gut microbiota increase intestinal permeability and promote inflammation in DKD
Additionally, gut microbiota can promote the occurrence of DKD by inducing or aggravating inflammation. Growing evidence has shown that, during DM and DKD, the intestinal mucosal barrier becomes more permeable owing to structural and functional abnormalities. This can even occur before the onset of DM itself. Intestinal bacteria may pass through the leaky intestinal barrier and act as dietary pathogens, or antigens, to elicit an immune response.49, 50 According to an experiment performed on db/db mice, a high‐fat diet could alter gut microbiota composition and reduce the expression of epithelial tight junction proteins (ZO‐1 and occludin). These changes increased intestinal permeability and enhanced LPS absorption, thereby promoting visceral adipose tissue inflammation, oxidative stress and macrophage infiltration. However, such pathological changes can be reversed using antibiotic therapy.50 In fact, some agents can improve the intestinal mucosal barrier, lower the intestinal permeability, reduce inflammation by regulating gut microbiota and alleviating diabetes or DKD.40, 51 However, the underlying association between intestinal flora and inflammation needs to be investigated in detail.
4.3. Gut microbiota promote DKD by activating the immune response
The human immune system and gut microbes interact with each other.52 Gut microbiota can participate in the development of DKD by disturbing the immune system. The activation of complement C5, which was enhanced in db/db mice, resulted in the increased expression of inflammatory and fibrogenic factors in renal tissues and promoted both oxidative stress and renal damage. However, despite its effect on renal tissues, C5a was enriched in the gut. After using C5aR antagonist (C5aRA), decreased abundances of Proteobacteria and Epsilonbacteraeota were restored at the phylum level in the gut microbiota of db/db mice. Meanwhile, the reduced abundance of Desulfovibrio and the increased abundances of Bacteroides, Eubacterium and Roseburia were reversed at the genus level. C5aRA also restored the reduced production of SCFAs.
Overactivation of C5 can induce gut microbiota dysbiosis and reduce SCFA production by intestinal microbiota. C5 can promote DKD by activating the STAT3 pathway and inducing inflammation in the kidneys, but SCFAs can partially offset its injurious effects.53 A gut microbiome‐immune axis exists in the human body. Gut microbiota‐derived metabolites can pass through the intestinal epithelium and accumulate in the circulatory system. They can also be recognized by the immune system and cause a wide variety of consequences in the body through various pathways.54 Intestinal flora, such as Firmicutes, Proteobacteria, Clostridium asparagiforme, Escherichia fergusonii and Desulfovibrio desulfuricans, can produce trimethylamine N‐oxide (TMAO).55 Escherichia coli can metabolize tryptophan into indole, which can, in turn, be converted into IS and Kyn in the liver.56 Bacteroides, Bifidobacterium, Lactobacillus and Clostridium are all related to p‐cresyl sulphate (PCS) generation.57 PS, TMAO, Kyn, IS and PCS are uremic toxins that increase in the DKD state.43, 48, 58 It has been reported that their accumulation continuously stimulates the immune system, which could result in increased inflammatory factor production and renal damage.52, 59, 60, 61 In conclusion, there are various mechanisms by which bacteria affect the progression of DKD.
5. SUMMARY AND PROSPECTS
This article reviews the relationship between gut microbiota dysbiosis and the onset and development of kidney diseases. The most common pathogenetic mechanism is the presence of abnormal intestinal flora that cause intestinal inflammation and destroy the intestinal mucosal barrier, allowing live bacteria or their metabolites enter the blood. This can damage local organs and elicit systemic inflammation. Maintaining gut microbiota and keeping the intestinal environment in a stable and healthy state can help reduce activity leading to the onset and progression of kidney diseases. In DKD, gut microbiota dysbiosis can promote DKD through mechanisms such as metabolite changes, inflammatory responses and immune activation. Altered intestinal flora do exist in DKD mice with different proteinuria stratifications, suggesting that gut microbiota may be potential indicators for predicting disease stratification of DKD. In the future, we should further study the mechanisms through which gut microbiota promote DKD, with the goal of providing insights for improving the clinical diagnosis and treatment of the condition.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ACKNOWLEDGEMENTS
This work was supported by the National Natural Science Foundation of China (Grant Nos. 81873611 and 81700633), Science and Technology Innovation Team of Henan (Grant No. 17IRTSTHN020); Foundation for Leading Personnel of Central Plains of China (Grant No. 194200510006). 2020 Key Project of Medical Science and Technology (Grant No. SBGJ202002056). The material of our pictures comes from software of Word Processing System.
Wang P, Wang T, Zheng X, Cui W, Shang J, Zhao Z. Gut microbiota, key to unlocking the door of diabetic kidney disease. Nephrology. 2021;26:641–649. 10.1111/nep.13874
Funding information 2020 Key Project of Medical Science and Technology, Grant/Award Number: SBGJ202002056; Foundation for Leading Personnel of Central Plains of China, Grant/Award Number: 194200510006; National Natural Science Foundation of China, Grant/Award Numbers: 81700633, 81873611; Science and Technology Innovation Team of Henan, Grant/Award Number: 17IRTSTHN020
Contributor Information
Jin Shang, Email: fccshangj2@zzu.edu.cn.
Zhanzheng Zhao, Email: zhanzhengzhao@zzu.edu.cn.
REFERENCES
- 1.Anders HJ, Andersen K, Stecher B. The intestinal microbiota, a leaky gut, and abnormal immunity in kidney disease. Kidney Int. 2013;83:1010‐1016. [DOI] [PubMed] [Google Scholar]
- 2.Sonnenburg JL, Backhed F. Diet‐microbiota interactions as moderators of human metabolism. Nature. 2016;535:56‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shahi SK, Freedman SN, Mangalam AK. Gut microbiome in multiple sclerosis: the players involved and the roles they play. Gut Microbes. 2017;8:607‐615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clemente JC, Ursell LK, Parfrey LW, Knight R. The impact of the gut microbiota on human health: an integrative view. Cell. 2012;148:1258‐1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xiong YB, Zhou LL. The signaling of cellular senescence in diabetic nephropathy. Oxidative Med Cell Longev. 2019;7495629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eftekhari A, Vahed SZ, Kavetskyy T, et al. Cell junction proteins: crossing the glomerular filtration barrier in diabetic nephropathy. Int J Biol Macromol. 2020;148:475‐482. [DOI] [PubMed] [Google Scholar]
- 7.Nasr SH, Dogan A, Larsen CP. Leukocyte cell‐derived chemotaxin 2‐associated amyloidosis: a recently recognized disease with distinct clinicopathologic characteristics. Clin J Am Soc Nephrol. 2015;10:2084‐2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sallustio F, Curci C, Chaoul N, et al. High levels of gut‐homing immunoglobulin A‐positive+B lymphocytes support the pathogenic role of intestinal mucosal hyperresponsiveness in immunoglobulin a nephropathy patients. Nephrol Dial Transplant. 2020.36(3):452–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mu Q, Zhang H, Liao X, et al. Control of lupus nephritis by changes of gut microbiota. Microbiome. 2017;5:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang J, Luo D, Lin Z, et al. Dysbiosis of gut microbiota in adult idiopathic membranous nephropathy with nephrotic syndrome. Microb Pathog. 2020;147:104359. [DOI] [PubMed] [Google Scholar]
- 11.Yu W, Shang J, Guo R, et al. The gut microbiome in differential diagnosis of diabetic kidney disease and membranous nephropathy. Ren Fail. 2020;42:1100‐1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee YK, Menezes JS, Umesaki Y, Mazmanian SK. Proinflammatory T‐cell responses to gut microbiota promote experimental autoimmune encephalomyelitis. P Natl Acad Sci USA. 2011;108:4615‐4622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen L, Chen DQ, Liu JR, et al. Unilateral ureteral obstruction causes gut microbial dysbiosis and metabolome disorders contributing to tubulointerstitial fibrosis. Exp Mol Med. 2019;51(3):1‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen YY, Chen DQ, Chen L, et al. Microbiome‐metabolome reveals the contribution of gut‐kidney axis on kidney disease. J Transl Med. 2019;17(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tu Y, Fang QJ, Sun W, et al. Total flavones of Abelmoschus manihot remodels gut microbiota and inhibits microinflammation in chronic renal failure progression by targeting autophagy‐mediated macrophage polarization. Front Pharmacol. 2020;11:566611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ji C, Deng Y, Yang A, et al. Rhubarb enema improved colon mucosal barrier injury in 5/6 nephrectomy rats may associate with gut microbiota modification. Front Pharmacol. 2020;11:1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zheng L, Chen S, Wang F, et al. Distinct responses of gut microbiota to Jian‐pi‐Yi‐Shen decoction are associated with improved clinical outcomes in 5/6 Nephrectomized rats. Front Pharmacol. 2020;11:604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feng YL, Cao G, Chen DQ, et al. Microbiome‐metabolomics reveals gut microbiota associated with glycine‐conjugated metabolites and polyamine metabolism in chronic kidney disease. Cell Mol Life Sci. 2019;76(24):4961‐4978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim JE, Kim HE, Cho H, et al. Effect of the similarity of gut microbiota composition between donor and recipient on graft function after living donor kidney transplantation. Sci Rep. 2020;10(1):18881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Konrad L, Andersen K, Kesper MS, Kumar SV, Mulay SR, Anders HJ. The gut flora modulates intestinal barrier integrity but not progression of chronic kidney disease in hyperoxaluria‐related nephrocalcinosis. Nephrol Dial Transplant. 2020;35(1):86‐97. [DOI] [PubMed] [Google Scholar]
- 21.Coussement J, Argudín MA, Heinrichs A, et al. Host and microbial factors in kidney transplant recipients with Escherichia coli acute pyelonephritis or asymptomatic bacteriuria: a prospective study using whole‐genome sequencing. Nephrol Dial Transplant. 2019;34(5):878‐885. [DOI] [PubMed] [Google Scholar]
- 22.Larsen N, Vogensen FK, van den Berg FWJ, et al. Gut microbiota in human adults with type 2 diabetes differs from non‐diabetic adults. PLoS One. 2010;5:e9085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Remely M, Hippe B, Zanner J, Aumueller E, Brath H, Haslberger AG. Gut microbiota of obese, type 2 diabetic individuals is enriched in faecalibacterium prausnitzii, akkermansia muciniphila and peptostreptococcus anaerobius after weight loss. Endocr Metab Immune Disord Drug Targets. 2016;16:99‐106. [DOI] [PubMed] [Google Scholar]
- 24.Wu H, Tremaroli V, Schmidt C, et al. The gut microbiota in prediabetes and diabetes: a population‐based cross‐sectional study. Cell Metab. 2020;32:379‐390. [DOI] [PubMed] [Google Scholar]
- 25.Wu R, Zhao D, An R, et al. Linggui Zhugan formula improves glucose and lipid levels and alters gut microbiota in high‐fat diet‐induced diabetic mice. Front Physiol. 2019;10:918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Candela M, Biagi E, Soverini M, et al. Modulation of gut microbiota dysbioses in type 2 diabetic patients by macrobiotic Ma‐pi 2 diet. Brit J Nutr. 2016;116:80‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ma Q, Li Y, Wang J, et al. Investigation of gut microbiome changes in type 1 diabetic mellitus rats based on high‐throughput sequencing. Biomed Pharmacother. 2020;124:109873. [DOI] [PubMed] [Google Scholar]
- 28.Song Y, Wu MS, Tao G, Lu MW, Lin J, Huang JQ. Feruloylated oligosaccharides and ferulic acid alter gut microbiome to alleviate diabetic syndrome. Food Res Int. 2020;137:109410. [DOI] [PubMed] [Google Scholar]
- 29.Inoue R, Ohue‐Kitano R, Tsukahara T, et al. Prediction of functional profiles of gut microbiota from 16S rRNA metagenomic data provides a more robust evaluation of gut dysbiosis occurring in Japanese type 2 diabetic patients. J Clin Biochem Nutr. 2017;61:217‐221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li Q, Chang Y, Zhang K, Chen H, Tao S, Zhang Z. Implication of the gut microbiome composition of type 2 diabetic patients from northern China. Sci Rep. 2020;10:5450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu H, Esteve E, Tremaroli V, et al. Metformin alters the gut microbiome of individuals with treatment‐naive type 2 diabetes, contributing to the therapeutic effects of the drug. Nat Med. 2017;23:850‐858. [DOI] [PubMed] [Google Scholar]
- 32.Yang M, Shi FH, Liu W, et al. Dapagliflozin modulates the fecal microbiota in a type 2 diabetic rat model. Front Endocrinol. 2020;11:635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang L, Qin Q, Liu M, Zhang X, He F, Wang G. Akkermansia muciniphila can reduce the damage of gluco/lipotoxicity, oxidative stress and inflammation, and normalize intestine microbiota in streptozotocin‐induced diabetic rats. Pathog Dis. 2018;76:fty028. [DOI] [PubMed] [Google Scholar]
- 34.Guo Y, Liu CQ, Shan CX, et al. Gut microbiota after roux‐en‐Y gastric bypass and sleeve gastrectomy in a diabetic rat model: increased diversity and associations of discriminant genera with metabolic changes. Diabetes Metab Res Rev. 2017;33:e2857. [DOI] [PubMed] [Google Scholar]
- 35.Chen Y, Li Z, Hu S, et al. Gut metagenomes of type 2 diabetic patients have characteristic single‐nucleotide polymorphism distribution in Bacteroides coprocola. Microbiome. 2017;5:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tao S, Li L, Li L, et al. Understanding the gut‐kidney axis among biopsy‐proven diabetic nephropathy, type 2 diabetes mellitus and healthy controls: an analysis of the gut microbiota composition. Acta Diabetol. 2019;56:581‐592. [DOI] [PubMed] [Google Scholar]
- 37.Wu IW, Lin CY, Chang LC, et al. Gut microbiota as diagnostic tools for mirroring disease progression and circulating nephrotoxin levels in chronic kidney disease: discovery and validation study. Int J Biol Sci. 2020;6:420‐434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li Y, Su X, Gao Y, et al. The potential role of the gut microbiota in modulating renal function in experimental diabetic nephropathy murine models established in same environment. Biochim Biophys Acta Mol basis Dis. 2020;1866:165764. [DOI] [PubMed] [Google Scholar]
- 39.Yang J, Dong H, Wang Y, et al. Cordyceps cicadae polysaccharides ameliorated renal interstitial fibrosis in diabetic nephropathy rats by repressing inflammation and modulating gut microbiota dysbiosis. Int J Biol Macromol. 2020;163:442‐456. [DOI] [PubMed] [Google Scholar]
- 40.Zhao TT, Zhang HJ, Yin X, et al. Tangshen formula modulates gut microbiota and reduces gut‐derived toxins in diabetic nephropathy rats. Biomed Pharmacother. 2020;129:110325. [DOI] [PubMed] [Google Scholar]
- 41.Chen Q, Ren DW, Wu JQ, et al. Shenyan Kangfu tablet alleviates diabetic kidney disease through attenuating inflammation and modulating the gut microbiota. J Nat Med. 2020;75:84‐98. [DOI] [PubMed] [Google Scholar]
- 42.Wei HL, Wang L, An ZC, et al. QiDiTangShen granules modulated the gut microbiome composition and improved bile acid profiles in a mouse model of diabetic nephropathy. Biomed Pharmacother. 2021;111061:133. [DOI] [PubMed] [Google Scholar]
- 43.Kikuchi K, Saigusa D, Kanemitsu Y, et al. Gut microbiome‐derived phenyl sulfate contributes to albuminuria in diabetic kidney disease. Nat Commun. 2019;10:1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lu CC, Hu ZB, Wang R, et al. Gut microbiota dysbiosis‐induced activation of the intrarenal renin‐angiotensin system is involved in kidney injuries in rat diabetic nephropathy. Acta Pharmacol Sin. 2020;41:1111‐1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu JR, Miao H, Deng DQ, et al. Gut microbiota‐derived tryptophan metabolism mediates renal fibrosis by aryl hydrocarbon receptor signaling activation. Cell Mol Life Sci. 2020;78:909–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wiedlocha M, Marcinowicz P, Janoska‐Jazdzik M, et al. Gut microbiota, kynurenine pathway and mental disorders‐review. Prog Neuro‐Psychopharmacol Biol Psychiatry. 2021;110145:106. [DOI] [PubMed] [Google Scholar]
- 47.Pawlak K, Mysliwiec M, Pawlak D. Kynurenine pathway ‐ a new link between endothelial dysfunction and carotid atherosclerosis in chronic kidney disease patients. Adv Med Sci. 2010;55:196‐203. [DOI] [PubMed] [Google Scholar]
- 48.Hirayama A, Nakashima E, Sugimoto M, et al. Metabolic profiling reveals new serum biomarkers for differentiating diabetic nephropathy. Anal Bioanal Chem. 2012;404:3101‐3109. [DOI] [PubMed] [Google Scholar]
- 49.Joesten WC, Short AH, Kennedy MA. Spatial variations in gut permeability are linked to type 1 diabetes development in non‐obese diabetic mice. BMJ Open Diabetes Res Care. 2019;7:e000793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cani PD, Bibiloni R, Knauf C, et al. Changes in gut microbiota control metabolic endotoxemia‐induced inflammation in high‐fat diet‐induced obesity and diabetes in mice. Diabetes. 2008;57:1470‐1481. [DOI] [PubMed] [Google Scholar]
- 51.Cai TT, Ye XL, Li RR, et al. Resveratrol modulates the gut microbiota and inflammation to protect against diabetic nephropathy in mice. Front Pharmacol. 2020;11:1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Glorieux G, Gryp T, Perna A. Gut‐derived metabolites and their role in immune dysfunction in chronic kidney disease. Toxins. 2020;12:245.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li L, Wei T, Liu S, et al. Complement C5 activation promotes type 2 diabetic kidney disease via activating STAT3 pathway and disrupting the gut‐kidney axis. J Cell Mol Med. 2020;25:960–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ma N, Ma X. Dietary amino acids and the gut‐microbiome‐immune axis: physiological metabolism and therapeutic prospects. Compr Rev Food Sci Food Saf. 2019;18:221‐242. [DOI] [PubMed] [Google Scholar]
- 55.Tanase DM, Gosav EM, Neculae E, et al. Role of gut microbiota on onset and progression of microvascular complications of type 2 diabetes (T2DM). Nutrients. 2020;12:3719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Matsumoto T, Kojima M, Takayanagi K, Taguchi K, Kobayashi T. Role of s‐equol, indoxyl sulfate, and trimethylamine n‐oxide on vascular function. Am J Hypertens. 2020;33:793‐803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wing MR, Patel SS, Ramezani A, Raj DS. Gut microbiome in chronic kidney disease. Exp Physiol. 2016;101:471‐477. [DOI] [PubMed] [Google Scholar]
- 58.Atoh K, Itoh H, Haneda M. Serum indoxyl sulfate levels in patients with diabetic nephropathy: relation to renal function. Diabetes Res Clin Pract. 2009;83:220‐226. [DOI] [PubMed] [Google Scholar]
- 59.Al‐Obaide MAI, Singh R, Datta P, et al. Gut microbiota‐dependent trimethylamine‐N‐oxide and serum biomarkers in patients with T2DM and advanced CKD. J Clin Forensic Med. 2017;6:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sun CY, Hsu HH, Wu MS. P‐cresol sulfate and indoxyl sulfate induce similar cellular inflammatory gene expressions in cultured proximal renal tubular cells. Nephrol Dial Transplant. 2013;28:70‐78. [DOI] [PubMed] [Google Scholar]
- 61.Wakamatsu T, Yamamoto S, Ito T, et al. Indoxyl sulfate promotes macrophage IL‐1β production by activating aryl hydrocarbon receptor/NF‐κ/MAPK cascades, but the NLRP3 inflammasome was not activated. Toxins. 2018;10:124. [DOI] [PMC free article] [PubMed] [Google Scholar]


